Abstract
Background
SCORing for Atopic Dermatitis (SCORAD) is a tool developed by the European Task Force on Atopic Dermatitis (AD) which is used by physicians to assess AD severity during consultations with their patients. Patient‐Oriented SCORAD (PO‐SCORAD) is a self‐assessment tool for use by patients which has been validated in a study performed in European countries. However, there is currently no adapted tool for evaluating AD severity in black skin.
Objective
To evaluate the performance of the version of the PO‐SCORAD specifically adapted for black skin patients (children and adults) with AD.
Methods
In this multicenter, cross‐sectional and non‐interventional study, children and adults with AD were recruited during regular consultations. This international study was performed in seven sub‐Saharan countries (Benin, Burkina Faso, Cameroon, Ivory Coast, Gabon, Mali and Senegal). During the consultation, AD severity was assessed by the physician using SCORAD score and by the patients or parents using PO‐SCORAD.
Results
One hundred and thirteen patients were included, 72 children and 41 adults, mainly females (61.6%). SCORAD assessed by physicians and PO‐SCORAD assessed by patients/parents were well correlated (r = 0.66, P < 0.0001). Correlation coefficients for SCORAD and PO‐SCORAD subscale scores were also good, except for symptom intensity criteria.
Conclusion
Altogether, these data indicate that PO‐SCORAD for black skin correlates well with SCORAD and is therefore a valuable tool, which requires no specific level of education, for use by black skin patients with AD.
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disease evolving through a pattern of flares and remission, which affects 5–20% of children under 11 years old1, 2, 3 and 1–17% of adults.4, 5, 6 In sub‐Saharan Africa, AD prevalence in children aged 6–7 years is about 5%, and in children aged 13–14 years is between 8% and 19%.7, 8
The SCORing for AD (SCORAD) index, first described in 1993 by the European Task Force on AD,9 was developed in order to assess the severity of the disease and improve AD management. The SCORAD index, which has been validated in clinical studies,10 has proven useful in evaluating AD severity at specific time points (i.e. during consultations with the physician) but does not provide information regarding the evolution of AD symptoms between visits. A self‐assessment scale for patients, the Patient‐Oriented SCORAD (PO‐SCORAD) index, was derived from the SCORAD to allow close monitoring of AD symptoms between consultations.11 The PO‐SCORAD uses the same evaluation criteria as the SCORAD but has been designed to be easy to use by patients and their family, for instance by including visual support to help them assess the different items composing the score. The PO‐SCORAD was initially validated against the SCORAD index in 471 patients with AD in Europe12 and in a large observational study, including 4222 patients from 12 European countries, displaying good correlation with the SCORAD measured by physicians.13 More recently, the validity of PO‐SCORAD has also been demonstrated for assessing AD severity in adults.14 However, the majority of patients included in PO‐SCORAD validation studies were only Caucasian. To fill the gap, a version of PO‐SCORAD was adapted by members of the Fondation pour la Dermatite Atopique (https://www.fondation-dermatite-atopique.org) for use in black skin patients (infants, children and adults) with AD (version 5.0; https://www.poscorad.com/#/poscorad/uk). The adapted version focuses, in particular, on objective signs such as erythema and lichenification (Fig. 1) which are the most difficult signs to be assessed properly in such patients.
Figure 1.
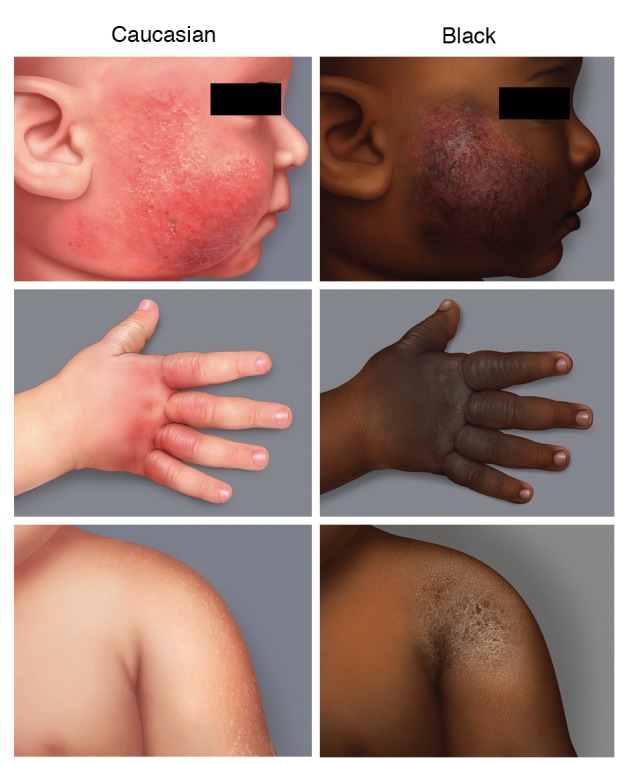
Standardized images of atopic dermatitis symptoms for scoring in individuals with Caucasian or Black skin.
The main objective of the current study was to assess the consistency between SCORAD measured by physicians and the adapted PO‐SCORAD for black skin measured by patients/parents.
Materials and methods
Study design
This was a multicenter, cross‐sectional and non‐interventional study in a population of children and adults with AD. This international study was performed between August 2017 and March 2018 in seven sub‐Saharan countries (Benin, Burkina Faso, Cameroon, Ivory Coast, Gabon, Mali and Senegal) in accordance with Good Clinical Practice and was approved by the corresponding Ethics Committees. Patients provided written informed consent, and parents provided consent for their child's participation.
Patients diagnosed with AD were included during a regular consultation with their physician. The SCORAD and its subcomponents were evaluated by the physician during the consultation. PO‐SCORAD was evaluated independently by patients, if adult, or by one parent (or legal guardian) if the patient was a child, in a specific room away from the presence of the physician, and placed in a sealed envelope.
Patient population
Eligible patients were children (aged < 18 years) or adults (≥18 years) consulting for AD diagnosed according to UK Working Party criteria.15 Patients were excluded from the study if they had any other inflammatory dermatosis, any infectious dermatosis or any ichthyosis, bullous or congenital dermatosis, or were unable to read or to understand a written document in French or in English (patient/parent(s)/legal guardian or third person).
Assessments
The SCORAD and its subcomponents (extent and intensity criteria for lesions, and subjective symptoms composed of pruritus and sleep loss scores) were calculated by the physician. The SCORAD score range is between 0 and 103 points and defines three classes of AD severity (i.e. mild if SCORAD <25, moderate if 25 ≤ SCORAD ≤ 50 and severe if SCORAD > 50).
The PO‐SCORAD considers the same items as the SCORAD. Patients or parents completed the questionnaire by coloring the area of skin affected by AD on a body scheme, answered questions about the intensity of eczema lesions (i.e. redness, swelling/papulation, dryness, oozing/scabs, scratch marks and skin thickening) with the help of a visual tool with drawings of lesions and completed visual analog scales for pruritus and sleep loss evaluation. In case of difficulties understanding or completing the PO‐SCORAD, the patient could rely on a third person. An independent reader measured the extent of the lesions drawn on the scheme and calculated the score.
Statistical analysis
Spearman's correlation coefficient between PO‐SCORAD and SCORAD subscale and total scores for the total population and for each age subgroup (<18 years and ≥18 years), and between SCORAD subscale and total score, was computed along with their 95% confidence interval (CI) and P‐value. There was no imputation for missing data (one for SCORAD subscale C and one for PO‐SCORAD subscale B).
Results
Population
A total of 113 patients were enrolled in the study, 72 children (63.7%) and 41 adults (36.3%). All patients were included in the analysable population (Fig. 2). The number of patients varied from 10 (Gabon) to 19 (Burkina Faso, Ivory Coast and Mali) per country. Although skin phototype was not assessed, all patients were presumed to be phototype VI (Fitzpatrick).
Figure 2.
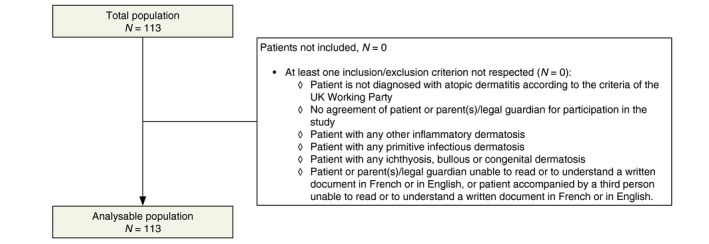
Patient flow chart.
Most patients were female (61.6%). Overall mean age was 16.2 ± 17.2 years (5.4 ± 4.7 years for children and 35.1 ± 14.7 years for adults). The median age of children was 4 years (Q1–Q3: 2 and 7 years, respectively; 4 children were aged ≥15 years). Mean time since AD diagnosis was 34.9 months, varying from 4.7 months (Cameroon) to 113.8 months (Ivory Coast).
SCORing for AD total score assessed by the physician during the consultation was 37.7. AD severity, based on SCORAD score, was moderate in 55.5% of patients, mild and severe in 22.3% each. Similarly, AD severity based on PO‐SCORAD score was mild, moderate or severe in 21.4%, 55.4% and 23.2% of patients, respectively. AD severity was similar in children and adults.
Description and correlation between SCORAD and PO‐SCORAD total scores
Patient‐oriented‐SCORAD and SCORAD total scores showed a positive relationship (Fig. 3). A significant correlation was observed between PO‐SCORAD evaluated by patients or parents and SCORAD evaluated by physicians, with a Spearman's correlation coefficient of r = 0.66 (95% CI: 0.54; 0.75, P < 0.0001). This positive correlation was also observed in subgroups of patients: Spearman's coefficient was 0.67 in children (95% CI: 0.52; 0.78, P < 0.0001) and 0.64 in adults (95% CI: 0.40; 0.79, P < 0.0001).
Figure 3.
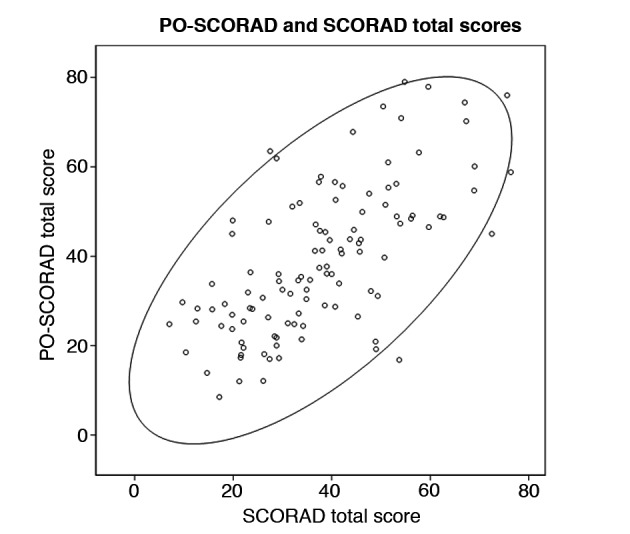
Relationship between Patient‐Oriented‐SCORing for Atopic Dermatitis (PO‐SCORAD) and SCORAD total scores in the overall population.
Description and correlation between SCORAD and PO‐SCORAD subscale scores
Patient‐oriented‐SCORAD and SCORAD subscale correlation coefficients were higher for subjective symptoms (r = 0.81) and extent of AD (r = 0.69) than for intensity of AD symptoms (r = 0.49; Table 1). Considering the intensity of AD symptoms, redness and swelling/papulation were underestimated by patients or parents compared to physicians’ evaluations (Table 2). Conversely, scratch marks and thickening of the skin were overestimated by patients or parents compared to physicians, while both evaluations were similar for dryness and oozing/scabs (Table 2).
Table 1.
Spearman's correlation coefficient and 95% confidence intervals between Patient‐Oriented‐SCORing for Atopic Dermatitis (PO‐SCORAD) and SCORAD subscale scores in the overall population
| n | Spearman coefficient (95% CI) | P‐value | |
|---|---|---|---|
| Subscale A score: extent | 113 | 0.6853 (0.5711; 0.7710) | <0.0001 |
| Subscale B score: intensity | 112 | 0.4849 (0.3270; 0.6138) | <0.0001 |
| Subscale C score: subjective symptoms | 112 | 0.8103 (0.7336; 0.8648) | <0.0001 |
Table 2.
Percentage of patients in each intensity subscore category for the different atopic dermatitis symptoms evaluated by patients or parents (PO‐SCORAD) or by physicians (SCORAD)
| SCORAD intensity subscore (N = 113) | PO‐SCORAD intensity subscore (N = 113) | |||||||
|---|---|---|---|---|---|---|---|---|
| Absent | Light | Moderate | Severe | Absent | Light | Moderate | Severe | |
|
Redness n (%) |
18 (15.9) | 42 (37.2) | 46 (40.7) | 7 (6.2) | 36 (31.9) | 38 (33.6) | 27 (23.9) | 12 (10.6) |
|
Swelling/papulation n (%) |
36 (31.9) | 32 (28.3) | 38 (33.6) | 7 (6.2) | 61 (54.5) | 27 (24.1) | 15 (13.4) | 9 (8) |
|
Dryness n (%) |
8 (7.1) | 38 (33.6) | 50 (44.2) | 17 (15) | 14 (12.4) | 42 (37.2) | 38 (33.6) | 19 (16.8) |
|
Oozing/scabs n (%) |
54 (47.8) | 29 (25.7) | 23 (20.4) | 7 (6.2) | 50 (44.6) | 29 (25.9) | 22 (19.6) | 11 (9.8) |
|
Scratch marks n (%) |
30 (26.5) | 44 (38.9) | 31 (27.4) | 8 (7.1) | 21 (18.6) | 28 (24.8) | 30 (26.5) | 34 (30.1) |
|
Skin thickening n (%) |
51 (45.1) | 31 (27.4) | 25 (22.1) | 6 (5.3) | 35 (31.3) | 37 (32.1) | 27 (24.1) | 14 (12.5) |
PO‐SCORAD, Patient‐Oriented‐SCORing for Atopic Dermatitis.
Discussion
Self‐assessment tools are important in chronic diseases such as AD to allow better monitoring of disease status between consultations and to provide help adapting the therapeutic strategy. PO‐SCORAD is a validated self‐assessment scale, part of which is based on the intensity of six clinical signs. Some of these clinical signs are the same regardless of skin color (i.e. skin thickening or edema, oozing or crusts, excoriations, xerosis). However, some of the other clinical signs, such as erythema and lichenification, present differently on white and black skin. To evaluate this issue, a new version of the PO‐SCORAD, designed specifically for black skin patients with AD, has been developed and tested in black African patients.
This study demonstrated that, in 113 black skin patients with AD, there was a good correlation between PO‐SCORAD (evaluated by patients or parents) and SCORAD (evaluated by physicians). Spearman's correlation coefficients were homogenous in the two age groups, thus validating the use of PO‐SCORAD in both children and adult patients with black skin. Interestingly, when studying the correlation between PO‐SCORAD for black skin and SCORAD subscale scores in more detail, a good correlation was observed for extent criterion and subjective symptoms, but a poorer correlation was described for symptom intensity criteria. This can be explained by an underestimation of swelling, papulation and redness by patients/parents, and an overestimation of scratch marks (excoriation) and thickening (lichenification) of the skin, compared to physicians’ evaluations. Indeed, training of patients or parents may need to focus on evaluating the intensity level of symptoms. However, despite these limited differences in the evaluation of the severity of signs of AD, the correlation between PO‐SCORAD for black skin and SCORAD total scores was similar to the correlation between PO‐SCORAD and SCORAD which was published previously in a large study of patients from 12 European countries.13
This study also presents some limitations. First, the sample size is limited for subgroup analyses and did not allow a comparison between SCORAD and PO‐SCORAD according to the different disease severity subclasses. Second, patients or parents completed PO‐SCORAD independently but, if necessary, could ask for some help. Also, to avoid miscalculations by patients/parents, an independent reader was in charge of measuring the extent of the lesions drawn on the schemes and for calculating the score. Therefore, transposition of these results during real‐life use of PO‐SCORAD in the general population should be considered with caution. Finally, a single time point has been evaluated in this study, and it would be interesting to measure the correlation between SCORAD and PO‐SCORAD over a longer time period to assess whether changes in AD severity level over time are also well correlated.
The diversity of the recruited patients (severity of disease, gender, geographic origin) strengthens the results of this study. Furthermore, even if evaluation of erythema by patients/parents as well as by physicians was quite difficult, the correlation between SCORAD and PO‐SCORAD was good.
In conclusion, this study has demonstrated that PO‐SCORAD for black skin is a simple tool that allows patients and parents to easily self‐assess AD severity. PO‐SCORAD for black skin may be a valuable tool to monitor disease severity between physician visits and to involve patients in the management of their disease. The tool requires no specific level of parent/patient education in order to successfully perform assessments (which are aided by standardized drawings of objective symptoms), and it can also be helpful during consultation with the physician to report disease evolution since the last visit. Finally, further development of PO‐SCORAD may also be performed in order to use it as a tool to support the need for patient referral to a specialist.
Acknowledgements
The authors thank all physicians, patients and parents who participated in this study. We also thank Ylana Chalem and Marie Auges from the sponsor study team for their support in analysing the data, including inferential statistical analyses, and Claire Laurens for providing medical writing support. Additional editorial support was provided by David P. Figgitt PhD, ISMPP CMPP™, Content Ed Net, with funding from Pierre Fabre Dermatologie.
Conflicts of interest
OF, APMN, SD, SC, PAN, FA, PYY and MTD received investigator fees from Pierre Fabre Dermatologie. AZ, CC, FZ and AD are employed by Pierre Fabre.
Funding sources
This study was funded by Pierre Fabre Dermatologie.
References
- 1. Fennessy M, Coupland S, Popay J, Naysmith K. The epidemiology and experience of atopic eczema during childhood: a discussion paper on the implications of current knowledge for health care, public health policy and research. J Epidemiol Community Health 2000; 54: 581–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Williams H, Robertson C, Stewart A et al Worldwide variations in the prevalence of symptoms of atopic eczema in the International Study of Asthma and Allergies in Childhood. J Allergy Clin Immunol 1999; 103(1 Pt 1): 125–138. [DOI] [PubMed] [Google Scholar]
- 3. Stensen L, Thomsen SF, Backer V. Change in prevalence of atopic dermatitis between 1986 and 2001 among children. Allergy Asthma Proc 2008; 29: 392–396. [DOI] [PubMed] [Google Scholar]
- 4. Leung DY, Bieber T. Atopic dermatitis. Lancet 2003; 361: 151–160. [DOI] [PubMed] [Google Scholar]
- 5. Sacotte R, Silverberg JI. Epidemiology of adult atopic dermatitis. Clin Dermatol 2018; 36: 595–605. [DOI] [PubMed] [Google Scholar]
- 6. Barbarot S, Auziere S, Gadkari A et al Epidemiology of atopic dermatitis in adults: results from an international survey. Allergy 2018; 73: 1284–1293. [DOI] [PubMed] [Google Scholar]
- 7. Asher MI, Montefort S, Bjorksten B et al Worldwide time trends in the prevalence of symptoms of asthma, allergic rhinoconjunctivitis, and eczema in childhood: ISAAC Phases One and Three repeat multicountry cross‐sectional surveys. Lancet 2006; 368: 733–743. [DOI] [PubMed] [Google Scholar]
- 8. Ait‐Khaled N, Odhiambo J, Pearce N et al Prevalence of symptoms of asthma, rhinitis and eczema in 13‐ to 14‐year‐old children in Africa: the International Study of Asthma and Allergies in Childhood Phase III. Allergy 2007; 62: 247–258. [DOI] [PubMed] [Google Scholar]
- 9. European Task Force on Atopic Dermatitis . Severity scoring of atopic dermatitis: the SCORAD index. Consensus Report of the European Task Force on Atopic Dermatitis. Dermatology 1993; 186: 23–31. [DOI] [PubMed] [Google Scholar]
- 10. Kunz B, Oranje AP, Labreze L, Stalder JF, Ring J, Taieb A. Clinical validation and guidelines for the SCORAD index: consensus report of the European Task Force on Atopic Dermatitis. Dermatology 1997; 195: 10–19. [DOI] [PubMed] [Google Scholar]
- 11. Vourc'h‐Jourdain M, Barbarot S, Taieb A et al Patient‐oriented SCORAD: a self‐assessment score in atopic dermatitis. A preliminary feasibility study. Dermatology 2009; 218: 246–251. [DOI] [PubMed] [Google Scholar]
- 12. Stalder JF, Barbarot S, Wollenberg A et al Patient‐oriented SCORAD (PO‐SCORAD): a new self‐assessment scale in atopic dermatitis validated in Europe. Allergy 2011; 66: 1114–1121. [DOI] [PubMed] [Google Scholar]
- 13. Coutanceau C, Stalder JF. Analysis of correlations between patient‐oriented SCORAD (PO‐SCORAD) and other assessment scores of atopic dermatitis severity and quality of life. Dermatology 2014; 229: 248–255. [DOI] [PubMed] [Google Scholar]
- 14. Silverberg JI, Margolis DJ, Boguniewicz M et al Validation of five patient‐reported outcomes for atopic dermatitis severity in adults. Br J Dermatol 2019; 10.1111/bjd.18002 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 15. Williams HC, Burney PG, Pembroke AC, Hay RJ, The UK. Working party's diagnostic criteria for atopic dermatitis. III. Independent hospital validation. Br J Dermatol 1994; 131: 406–416. [DOI] [PubMed] [Google Scholar]


