Abstract
Background
This study aimed to undertake a network pharmacology analysis to identify the active compounds of the herbal extract Christina Loosestrife, or Lysimachia Christinae (Jin Qian Cao), in the treatment of nephrolithiasis.
Material/Methods
The active components of Christina Loosestrife were identified from the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database and analysis platform and the online Taiwan TCM database. The potentially active compounds were screened based on their parenteral bioavailability identified from the TCMSP database. The PharmMapper integrated pharmacophore matching platform was used for target identification of active compounds in nephrolithiasis. The identified active compounds were validated by molecular docking using the systemsDock network pharmacology website. Biological functions and pathway outcomes of effective targets were analyzed using the Metascape gene annotation resource. The results were used to construct the pharmacological networks, which were visualized and integrated using Cytoscape software.
Results
There were 16 active compounds of Christina Loosestrife and 11 nephrolithiasis-associated targets that were obtained. Functional enrichment analysis showed that Christina Loosestrife might exert its therapeutic effects by regulating pathways that included purine salvage, interleukin-4 (IL-4) and IL-13 signaling, and neutrophil degranulation.
Conclusions
Network pharmacology analysis of the herbal extract, Christina Loosestrife, identified multiple active compounds, targets, and pathways involved in the effects on nephrolithiasis.
MeSH Keywords: Drug Delivery Systems, Molecular Docking Simulation, Nephrolithiasis, Pharmacology, Primulaceae
Background
Nephrolithiasis is the clinical term used for the formation of stones in the renal pelvis and ureter. Intermittent renal colic and hematuria are the main clinical symptoms of nephrolithiasis, which can lead to chronic kidney disease and loss of renal function. In the adult population in China, between 2013 and 2014, the prevalence of nephrolithiasis was 5.8%, and the estimated number of patients with nephrolithiasis was 1.1 billion [1]. Nephrolithiasis has a high recurrence rate of between 6.12% and 34.17% at one year and five years, respectively [2].
Developments in the clinical management of kidney stones have resulted in the development of extracorporeal shock wave lithotripsy, which is now the first-line approach to the treatment of nephrolithiasis. However, lithotripsy is expensive and is followed by a high recurrence rate of kidney stones in approximately 50% of cases at between 5–10 years, which increases to 75% in 20 years [3]. Therefore, approaches to the prevention of the formation of kidney stones are required. Chinese herbal medicine has a long history of nephrolithiasis treatment, even before Western medicine. Chinese herbal medicine, such as takusya, jin qian cao, desmodyum styracyfolium, and wulingsan, can increase the excretion of urinary citrate, reduce urinary calcium and oxalic acid excretion, and have diuretic effects, that can prevent nephrolithiasis [4].
Christina Loosestrife, or Lysimachia Christinae (Jin Qian Cao), is a traditional Chinese medicine used in the treatment of nephrolithiasis [5,6]. A flavonoid extract of Christina Loosestrife has been shown to inhibit the formation of calcium oxalate crystals in a rat model of hyperoxaluria by interfering with calcium metabolism [7]. The total flavonoid content of Christina Loosestrife increased in the urine of rats, and an increase in urinary prothrombin fragment 1 (UPTF1) was associated with reduced urinary calcium and uric acid [8]. Christina Loosestrife has an inhibitory effect on the formation of calcium oxalate crystals in human urine [9]. It can be used in patients with urinary calculi after extracorporeal shock wave lithotripsy and has been shown to reduce the recurrence rate of urinary calculi [10]. However, the pharmacological mechanisms of Christina Loosestrife remain unknown.
Therefore, this study aimed to undertake a network pharmacology analysis to identify the active compounds of the herbal extract Christina Loosestrife, or Lysimachia Christinae (Jin Qian Cao), in the treatment of nephrolithiasis.
Material and Methods
Screening for the effective chemical components of Christina Loosestrife
The active components of Christina Loosestrife, or Lysimachia Christinae (Jin Qian Cao), were identified from the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database and analysis platform (http://ibts.hkbu.edu.hk/LSP/tcmsp.php) [11], and the online Taiwan TCM database (http://tcm.cmu.edu.tw/) [12]. The chemical components were identified using search keywords that included Jin Qian Cao. Lian Qian Cao, and Christina Loosestrife, using PubMed, CNKI, and the Wanfang databases. Because the flavone glycoside may be hydrolyzed to glycosides in the gut by intestinal enzymes, both flavonoid glycosides and glycosides were identified.
The molecular structure of the compounds was confirmed using the PubChem or ChemSpider chemical structure database platforms. Based on the absorption, distribution, metabolism, excretion, and toxicity (ADME/T) value calculated by the TCMSP database, compounds with an oral bioavailability (OB) <30%, a drug-like index (DL) <0.18, or low concentration were omitted. Compounds with an OB ≥30% and a DL ≥0.18 were selected as active compounds for further investigation, and their structural diagrams obtained from the database were stored in two formats, the MOL and SDF formats. These two-dimensional (2D) structures of the components were converted to three-dimensional (3D) structure diagrams using ChemDraw Professional version 16.0 software (PerkinElmer, Waltham, MA, USA), and saved as MOL2 format files.
Reverse target prediction
The main active ingredients of the Christina Loosestrife were uploaded to the PharmMapper server (http://59.78.98.102/pharmmapper/) [13–15] in MOL2 format. The search term, Human Protein Targets Only for Select Targets Set was the default setting for the remaining parameters. The Protein Data Bank identity (PDB ID) of the filtered protein target was imported into the UniProt (https://www.uniprot.org/) database, and the prediction targets of the active ingredients of Christina Loosestrife were obtained by retrieval and transformation.
Screening of nephrolithiasis-associated targets
The keywords, kidney stone or nephrolithiasis were used to search the Online Mendelian Inheritance in Man (OMIM) database [16] (http://omim.org/), the MalaCards integrated annotation database [17], and PubMed, to obtain the reported genes associated with nephrolithiasis. After removing repetitive genes and false-positive genes, nephrolithiasis-associated targets were collected.
Molecular docking and binding affinity
Molecular docking is often used to study the interaction between active small molecules with key network targets [18,19]. The active compound (MOL2 format) and the target protein PDB ID were uploaded to the systemsDock version 2.0 network pharmacology website (http://systemsdock.unit.oist.jp), an online molecular docking program [20]. The smaller the binding free energy, the more stable the ligand-receptor binding, and the larger the docking score, the more stable the ligand-receptor binding. A docking score >4.25 indicated binding affinity between the molecule and the target. A docking score >5.0 indicated that the molecule had a good binding affinity to the target. A docking score >7.0 indicated a strong binding affinity [21]. The binding affinity between the core target and the active compound was evaluated based on the proportion of the active compound with a docking score of ≥4.25, which verified the validity of the potential core target.
Biological functions and pathway outcomes analysis of the targets
Biological functions and pathway outcomes of effective targets were analyzed using the Metascape gene annotation platform (http://metascape.org/) [22], in which the input as species and the analysis as species were selected as H. sapiens, and the threshold was set as P<0.01. Gene Ontology (GO) annotation and Reactome signaling pathway analysis were performed. The results were sorted according to the number of targets involved in each pathway, and the top biological processes and signaling pathways were selected and visualized using GraphPad Prism version 7.0 software (GraphPad Software, La Jolla, CA, USA).
Network construction
The compound-target network and target-pathway network were constructed and merged into the compound-target-pathway network using Cytoscape version 3.6.1 (https://cytoscape.org/) to achieve a systematic understanding of the complex relationships among compounds, targets, and nephrolithiasis [23].
Results
Screening for the effective chemical components of Christina Loosestrife and target prediction
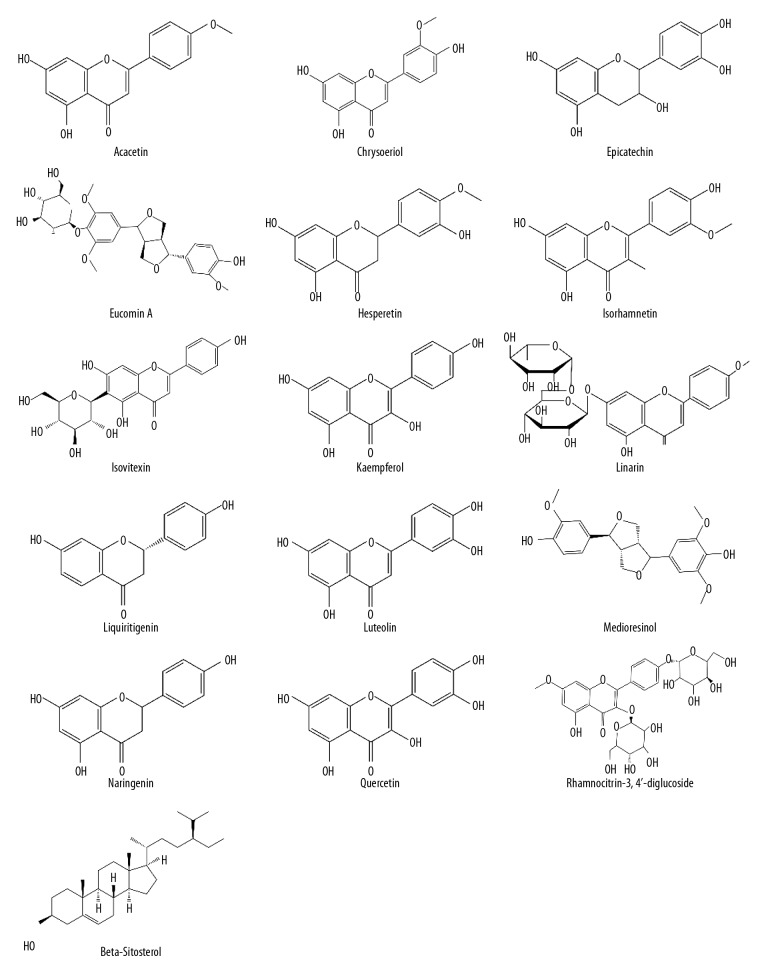
A total of 188 compounds were identified for Christina Loosestrife from the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database, the online Taiwan TCM database, and the literature search. Following the absorption, distribution, metabolism, excretion, and toxicity (ADME/T) calculation, 16 compounds with an oral bioavailability (OB) ≥30%, and a drug-like index (DL) ≥0.18 were identified as effective active compounds (Figure 1, Table 1). These chemical compounds were searched using the PharmMapper integrated pharmacophore matching platform for reverse prediction, and 414 targets were obtained. There were 155 nephrolithiasis-associated targets identified after screening using the Online Mendelian Inheritance in Man (OMIM), the MalaCards integrated annotation database and the PubMed database. There were 11 common targets for Christina Loosestrife and nephrolithiasis (Table 1).
Figure 1.
The structure of the 16 active compounds in Christina Loosestrife.
Table 1.
The main active ingredients and protein targets in Christina Loosestrife.
| Compound | Pubchem CID | OB (%) | DL | Gene | Uniprot ID | Target |
|---|---|---|---|---|---|---|
| Quercetin | 5280343 | 46.43 | 0.28 | AGXT | P21549 | Serine pyruvate aminotransferase |
| APRT | P07741 | Adenine phosphoribosyltransferase | ||||
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| HPRT1 | P00492 | Hypoxanthine-guanine phosphoribosyltransferase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| LCN2 | P80188 | Neutrophil gelatinase-associated lipocalin | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| VDR | P11473 | Vitamin D3 receptor | ||||
| Epicatechin | 182232 | 48.96 | 0.24 | AGXT | P21549 | Serine pyruvate aminotransferase |
| APRT | P07741 | Adenine phosphoribosyltransferase | ||||
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| HPRT1 | P00492 | Hypoxanthine-guanine phosphoribosyltransferase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| LCN2 | P80188 | Neutrophil gelatinase-associated lipocalin | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| VDR | P11473 | Vitamin D3 receptor | ||||
| Hesperetin | 72281 | 70.31 | 0.27 | AGXT | P21549 | Serine--pyruvate aminotransferase |
| APRT | P07741 | Adenine phosphoribosyltransferase | ||||
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| HPRT1 | P00492 | Hypoxanthine-guanine phosphoribosyltransferase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| LCN2 | P80188 | Neutrophil gelatinase-associated lipocalin | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| VDR | P11473 | Vitamin D3 receptor | ||||
| Naringenin | 932 | 59.29 | 0.21 | AGXT | P21549 | Serine pyruvate aminotransferase |
| APRT | P07741 | Adenine phosphoribosyltransferase | ||||
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| HPRT1 | P00492 | Hypoxanthine-guanine phosphoribosyltransferase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| LCN2 | P80188 | Neutrophil gelatinase-associated lipocalin | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| VDR | P11473 | Vitamin D3 receptor | ||||
| Luteolin | 5280445 | 36.16 | 0.25 | AGXT | P21549 | Serine--pyruvate aminotransferase |
| APRT | P07741 | Adenine phosphoribosyltransferase | ||||
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| HPRT1 | P00492 | Hypoxanthine-guanine phosphoribosyltransferase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| LCN2 | P80188 | Neutrophil gelatinase-associated lipocalin | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| VDR | P11473 | Vitamin D3 receptor | ||||
| Medioresinol | 181681 | 87.19 | 0.62 | AGXT | P21549 | Serine pyruvate aminotransferase |
| APRT | P07741 | Adenine phosphoribosyltransferase | ||||
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| HPRT1 | P00492 | Hypoxanthine-guanine phosphoribosyltransferase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| LCN2 | P80188 | Neutrophil gelatinase-associated lipocalin | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| VDR | P11473 | Vitamin D3 receptor | ||||
| Kaempferol | 5280863 | 41.88 | 0.24 | AGXT | P21549 | Serine pyruvate aminotransferase |
| APRT | P07741 | Adenine phosphoribosyltransferase | ||||
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| HPRT1 | P00492 | Hypoxanthine-guanine phosphoribosyltransferase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| VDR | P11473 | Vitamin D3 receptor | ||||
| Eucommin A | 442836 | 30.51 | 0.85 | AGXT | P21549 | Serine-pyruvate aminotransferase |
| APRT | P07741 | Adenine phosphoribosyltransferase | ||||
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| LCN2 | P80188 | Neutrophil gelatinase-associated lipocalin | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| VDR | P11473 | Vitamin D3 receptor | ||||
| Isorha-mnetin | 5281654 | 49.6 | 0.31 | AGXT | P21549 | Serine pyruvate aminotransferase |
| APRT | P07741 | Adenine phosphoribosyltransferase | ||||
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| HPRT1 | P00492 | Hypoxanthine-guanine phosphoribosyltransferase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| VDR | P11473 | Vitamin D3 receptor | ||||
| Acacetin | 5280442 | 34.97 | 0.24 | APRT | P07741 | Adenine phosphoribosyltransferase |
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| HPRT1 | P00492 | Hypoxanthine-guanine phosphoribosyltransferase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| LCN2 | P80188 | Neutrophil gelatinase-associated lipocalin | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| VDR | P11473 | Vitamin D3 receptor | ||||
| Chrysoeriol | 5280666 | 35.85 | 0.27 | AGXT | P21549 | Serine--pyruvate aminotransferase |
| APRT | P07741 | Adenine phosphoribosyltransferase | ||||
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| HPRT1 | P00492 | Hypoxanthine-guanine phosphoribosyltransferase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| VDR | P11473 | Vitamin D3 receptor | ||||
| Isovitexin | 162350 | 31.29 | 0.72 | APRT | P07741 | Adenine phosphoribosyltransferase |
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| HPRT1 | P00492 | Hypoxanthine-guanine phosphoribosyltransferase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| VDR | P11473 | Vitamin D3 receptor | ||||
| Linarin | 5317025 | 39.84 | 0.71 | AGXT | P21549 | Serine pyruvate aminotransferase |
| APRT | P07741 | Adenine phosphoribosyltransferase | ||||
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| Rhamno-citrin-3,4′-digluco-side | – | 32.52 | 0.64 | APRT | P07741 | Adenine phosphoribosyltransferase |
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| GBA | P04062 | Beta-glucocerebrosidase | ||||
| HPRT1 | P00492 | Hypoxanthine-guanine phosphoribosyltransferase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| Liquiriti-genin | 114829 | 32.76 | 0.18 | AGXT | P21549 | Serine pyruvate aminotransferase |
| BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 | ||||
| F2 | P00734 | Prothrombin | ||||
| HPRT1 | P00492 | Hypoxanthine-guanine phosphoribosyltransferase | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| MMP9 | P14780 | 67 kDa matrix metalloproteinase-9 | ||||
| REG1A | P05451 | Lithostathine-1-alpha | ||||
| VDR | P11473 | Vitamin D3 receptor | ||||
| β-Sitosterol | 222284 | 36.91 | 0.75 | BIRC7 | Q96CA5 | Baculoviral IAP repeat-containing protein 7 |
| F2 | P00734 | Prothrombin | ||||
| JAK2 | O60674 | Tyrosine-protein kinase JAK2 | ||||
| LCN2 | P80188 | Neutrophil gelatinase-associated lipocalin | ||||
| VDR | P11473 | Vitamin D3 receptor |
OB – oral bioavailability; DL – drug-like index; CID – compound identity number.
Molecular docking
Docking of the 16 active compounds and 11 targets was simulated using systemsDock, with the default parameters. The docking scores of the candidate compounds are shown in Table 2. The molecular docking results identified 13 (81.3%) active compounds associated with JAK2 (PDBID: 3UGC), which had a docking score >4.25. There were 10 (62.5%) active compounds associated with BIRC7 (PDBID: 2I3H), with a docking score of >4.25. There were 8 (50%) active compounds associated with F2 (PDBID: 4UD9), with a docking score of >4.25. There were 10 (62.5%) active compounds with MMP9 (PDBID: 6ESM), with a docking score of >4.25. There were 9 (56.3%) active compounds with VDR (PDBID: 3B0T), with a docking score of >4.25. There were 8 (50.0%) active compounds with GBA (PDBID: 2NT0), with a docking score of >4.25. There were 11 (68.8%) active compounds with APRT (PDBID: 4X45), with a docking score of >4.25. There were 11 (68.8%) active compounds with HPRT1 (PDBID: 4RAO), with a docking score of >4.25. There were12 active compounds (75.0%) with AGXT (PDBID: 5F9S), with a docking score of >4.25. There were 8 (50.0%) active components with LCN2 (PDBID: 4MVK), with a docking score of >4.25. Due to the lack of a crystal structure, molecular docking was not performed on REG1A. The molecular docking results showed that most of the active constituents of Christina Loosestrife had good binding ability to the key targets in the network.
Table 2.
Molecular docking of targets for Christina Loosestrife.
| Number | Target | PDB ID | Compound | Docking score |
|---|---|---|---|---|
| 1 | JAK2 | 3UGC | Endogenic ligand | 5.180 |
| Luteolin | 6.361 | |||
| Epicatechin | 6.294 | |||
| Quercetin | 6.370 | |||
| Isorhamnetin | NA | |||
| β-Sitosterol | NA | |||
| Kaempferol | 6.357 | |||
| Acacetin | 4.620 | |||
| Linarin | 8.288 | |||
| Liquiritigenin | 6.330 | |||
| Isovitexin | 6.610 | |||
| Hesperetin | 4.655 | |||
| Chrysoeriol | 4.655 | |||
| Naringenin | 6.626 | |||
| Rhamnocitrin-3, 4′-diglucoside | 8.245 | |||
| Eucommin A | NA | |||
| Medioresinol | 6.853 | |||
| 2 | BIRC7 | 2I3H | Endogenic ligand | 4.490 |
| Luteolin | 6.730 | |||
| Epicatechin | 6.760 | |||
| Quercetin | 6.775 | |||
| Isorhamnetin | NA | |||
| β-Sitosterol | NA | |||
| Kaempferol | 6.736 | |||
| Acacetin | 3.139 | |||
| Linarin | 5.262 | |||
| Liquiritigenin | 6.633 | |||
| Isovitexin | 5.518 | |||
| Hesperetin | 3.377 | |||
| Chrysoeriol | 3.338 | |||
| Naringenin | 6.630 | |||
| Rhamnocitrin-3, 4′-diglucoside | 5.254 | |||
| Eucommin A | NA | |||
| Medioresinol | 5.511 | |||
| 3 | F2 | 4UD9 | Endogenic ligand | 4.790 |
| Luteolin | 5.899 | |||
| Epicatechin | NA | |||
| Quercetin | NA | |||
| Isorhamnetin | NA | |||
| β-Sitosterol | NA | |||
| Kaempferol | 6.017 | |||
| Acacetin | 3.660 | |||
| Linarin | 6.120 | |||
| Liquiritigenin | 5.856 | |||
| Isovitexin | 6.064 | |||
| Hesperetin | 4.001 | |||
| Chrysoeriol | 4.031 | |||
| Naringenin | 5.936 | |||
| Rhamnocitrin-3, 4′-diglucoside | 6.218 | |||
| Eucommin A | NA | |||
| Medioresinol | 6.100 | |||
| 4 | MMP9 | 6ESM | Endogenic ligand | 4.640 |
| Luteolin | 6.788 | |||
| Epicatechin | NA | |||
| Quercetin | NA | |||
| Isorhamnetin | 4.453 | |||
| β-Sitosterol | NA | |||
| Kaempferol | 6.683 | |||
| Acacetin | 4.116 | |||
| Linarin | 7.096 | |||
| Liquiritigenin | 6.981 | |||
| Isovitexin | 6.419 | |||
| Hesperetin | 4.166 | |||
| Chrysoeriol | 4.284 | |||
| Naringenin | 6.844 | |||
| Rhamnocitrin-3, 4′-diglucoside | 7.102 | |||
| Eucommin A | NA | |||
| Medioresinol | 6.385 | |||
| 5 | VDR | 3B0T | Endogenic ligand | 8.500 |
| Luteolin | NA | |||
| Epicatechin | NA | |||
| Quercetin | NA | |||
| Isorhamnetin | NA | |||
| β-Sitosterol | NA | |||
| Kaempferol | 6.052 | |||
| Acacetin | 4.700 | |||
| Linarin | 8.331 | |||
| Liquiritigenin | 6.152 | |||
| Isovitexin | NA | |||
| Hesperetin | 4.748 | |||
| Chrysoeriol | 4.731 | |||
| Naringenin | 6.138 | |||
| Rhamnocitrin-3, 4′-diglucoside | 8.347 | |||
| Eucommin A | NA | |||
| Medioresinol | 7.421 | |||
| 6 | GBA | 2NT0 | Endogenic ligand | 5.180 |
| Luteolin | 5.762 | |||
| Epicatechin | 6.229 | |||
| Quercetin | 5.809 | |||
| Isorhamnetin | 3.542 | |||
| β-Sitosterol | 5.376 | |||
| Kaempferol | 5.577 | |||
| Acacetin | 3.147 | |||
| Linarin | 5.543 | |||
| Liquiritigenin | 5.400 | |||
| Isovitexin | 5.658 | |||
| Hesperetin | 3.331 | |||
| Chrysoeriol | 3.478 | |||
| Naringenin | NA | |||
| Rhamnocitrin-3, 4′-diglucoside | NA | |||
| Eucommin A | NA | |||
| Medioresinol | NA | |||
| 7 | APRT | 4X45 | Endogenic ligand | 5.700 |
| Luteolin | 7.171 | |||
| Epicatechin | 7.095 | |||
| Quercetin | 7.135 | |||
| Isorhamnetin | 4.444 | |||
| β-Sitosterol | 7.528 | |||
| Kaempferol | 7.160 | |||
| Acacetin | 4.214 | |||
| Linarin | 6.232 | |||
| Liquiritigenin | 7.154 | |||
| Isovitexin | 6.220 | |||
| Hesperetin | 4.334 | |||
| Chrysoeriol | 4.323 | |||
| Naringenin | NA | |||
| Rhamnocitrin-3, 4′-diglucoside | NA | |||
| Eucommin A | NA | |||
| Medioresinol | NA | |||
| 8 | HPRT1 | 4RAO | Endogenic ligand | 5.370 |
| Luteolin | 7.118 | |||
| Epicatechin | 7.092 | |||
| Quercetin | 7.038 | |||
| Isorhamnetin | 4.493 | |||
| β-Sitosterol | 7.625 | |||
| Kaempferol | 7.178 | |||
| Acacetin | 4.236 | |||
| Linarin | 6.630 | |||
| Liquiritigenin | 7.156 | |||
| Isovitexin | 6.391 | |||
| Hesperetin | 4.294 | |||
| Chrysoeriol | 4.311 | |||
| Naringenin | NA | |||
| Rhamnocitrin-3, 4′-diglucoside | NA | |||
| Eucommin A | NA | |||
| Medioresinol | NA | |||
| 9 | AGXT | 5F9S | Endogenic ligand | 5.220 |
| Luteolin | 6.161 | |||
| Epicatechin | 6.398 | |||
| Quercetin | 6.349 | |||
| Isorhamnetin | 5.428 | |||
| β-Sitosterol | 8.408 | |||
| Kaempferol | 6.329 | |||
| Acacetin | 5.736 | |||
| Linarin | 8.406 | |||
| Liquiritigenin | 6.207 | |||
| Isovitexin | 7.780 | |||
| Hesperetin | 5.741 | |||
| Chrysoeriol | 5.455 | |||
| Naringenin | NA | |||
| Rhamnocitrin-3, 4′-diglucoside | NA | |||
| Eucommin A | NA | |||
| Medioresinol | NA | |||
| 10 | LCN2 | 4MVK | Endogenic ligand | 5.190 |
| Luteolin | 6.684 | |||
| Epicatechin | 6.732 | |||
| Quercetin | 6.668 | |||
| Isorhamnetin | 3.984 | |||
| β-Sitosterol | 6.616 | |||
| Kaempferol | 6.735 | |||
| Acacetin | 3.567 | |||
| Linarin | 5.553 | |||
| Liquiritigenin | 6.565 | |||
| Isovitexin | 5.435 | |||
| Hesperetin | 3.642 | |||
| Chrysoeriol | 3.643 | |||
| Naringenin | NA | |||
| Rhamnocitrin-3, 4′-diglucoside | NA | |||
| Eucommin A | NA | |||
| Medioresinol | NA |
NA – not available; PDB – Protein Data Bank.
The analysis of the target biological functions and pathways of Christina Loosestrife
Gene Ontology (GO) functional enrichment analysis and pathway functional analysis were performed for the 11 effective targets that were identified by molecular docking. For biological process, terms such as adenine salvage, regulation of body fluid levels, and cellular response to oxidative stress were enriched (Figure 2A). For the cellular component, the terms including secretory granule lumen, cytoplasmic vesicle lumen, and vesicle lumen were enriched (Figure 2B). Terms that included purine phosphoribosyltransferase activity, cofactor binding, and serine-type endopeptidase activity were enriched for molecular function (Figure 2C). The reaction pathway analysis showed that most targets were enriched in neutrophil degranulation, interleukin-4 (IL-4) and IL-13 signaling, and purine salvage (Figure 2D).
Figure 2.
Enrichment analysis of potential targets of the active compounds of Christina Loosestrife in nephrolithiasis. Analysis of the Gene Ontology (GO) terms for biological process (A), cellular component (B), and molecular function (C), or analysis of the Reactome pathways (D) are shown. LogP is the log-value of the P-value. P<0.05 is considered to be significant. To show the results more intuitively, the results of the enrichment analysis are shown by LogP. The count represents the number of genes.
Network construction and analysis
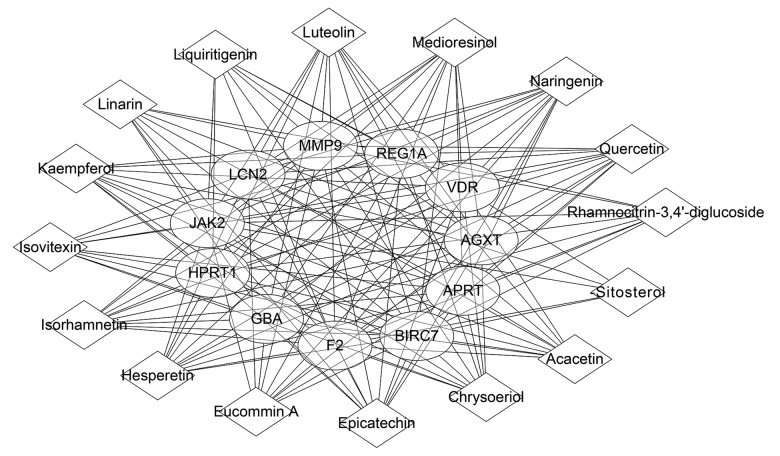
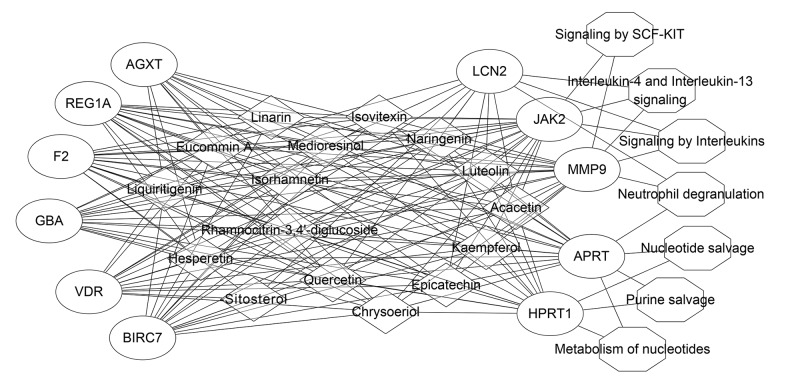
Based on the above findings, the nephrolithiasis-associated compound-target-pathway network of Christina Loosestrife was validated. The effective target proteins, chemical constituents, and pathways were imported into Cytoscape software to construct the compound-target component (Figure 3), target-pathway (Figure 4) and compound-target-pathway network diagrams (Figure 5). Targets and compounds with high node degrees are shown in Table 3. In the compound-target network, there were 154 sides and 27 nodes. In the target-pathway network, there were 17 sides and 12 nodes. In the compound-target-pathway network, there were 171 sides and 34 nodes. These results indicated the complex relationship between compounds, targets, and pathways of Christina Loosestrife in nephrolithiasis.
Figure 3.
The compound target network associated with Christina Loosestrife in nephrolithiasis. The square represents the component; the circle represents the target; the size of the node represents the size of the node degree.
Figure 4.
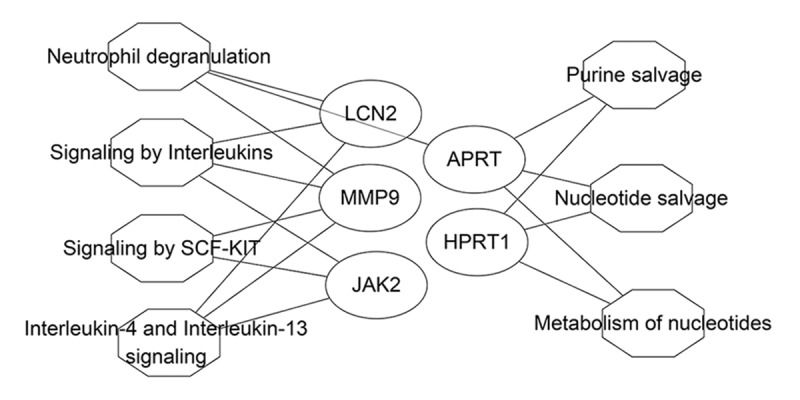
The compound target network associated with Christina Loosestrife in nephrolithiasis. The circle represents the target; the octagon represents the tumor-related pathway; the size of the node represents the size of the node degree.
Figure 5.
The compound target network associated with Christina Loosestrife in nephrolithiasis. The square represents the component; the circle represents the target; the octagon represents the tumor-related pathway; the size of the node represents the size of its node degree.
Table 3.
Important targets and ingredients with a high node degree for Christina Loosestrife.
| Ingredients | Degree | Targets | Target | Degree | Ingredients |
|---|---|---|---|---|---|
| Epicatechin | 11 | AGXT, APRT, BIRC7, F2, GBA, HPRT1, JAK2, LCN2, MMP9, REG1A, VDR | JAK2 | 16 | Acacetin, Chrysoeriol, Epicatechin, Eucommin A, Hesperetin, Isorhamnetin, Isovitexin, Kaempferol, Linarin, Liquiritigenin, Luteolin, Medioresinol, Naringenin, Quercetin, Rhamnocitrin-3,4′-diglucoside, β-Sitosterol |
| Hesperetin | 11 | AGXT, APRT, BIRC7, F2, GBA, HPRT1, JAK2, LCN2, MMP9, REG1A, VDR | MMP9 | 15 | Acacetin, Chrysoeriol, Epicatechin, Eucommin A, Hesperetin, Isorhamnetin, Isovitexin, Kaempferol, Linarin, Liquiritigenin, Luteolin, Medioresinol, Naringenin, Quercetin, Rhamnocitrin-3,4′-diglucoside |
| Luteolin | 11 | AGXT, APRT, BIRC7, F2, GBA, HPRT1, JAK2, LCN2, MMP9, REG1A, VDR | APRT | 14 | Acacetin, Chrysoeriol, Epicatechin, Eucommin A, Hesperetin, Isorhamnetin, Isovitexin, Kaempferol, Linarin, Luteolin, Medioresinol, Naringenin, Quercetin, Rhamnocitrin-3,4′-diglucoside |
| Medioresinol | 11 | AGXT, APRT, BIRC7, F2, GBA, HPRT1, JAK2, LCN2, MMP9, REG1A, VDR | HPRT1 | 13 | Acacetin, Chrysoeriol, Epicatechin, Hesperetin, Isorhamnetin, Isovitexin, Kaempferol, Luteolin, Liquiritigenin, Medioresinol, Naringenin, Quercetin, Rhamnocitrin-3,4′-diglucoside |
| Naringenin | 11 | AGXT, APRT, BIRC7, F2, GBA, HPRT1, JAK2, LCN2, MMP9, REG1A, VDR | LCN2 | 9 | Acacetin, Epicatechin, Eucommin A, Hesperetin, Luteolin, Medioresinol, Naringenin, Quercetin, β-Sitosterol |
| Quercetin | 11 | AGXT, APRT, BIRC7, F2, GBA, HPRT1, JAK2, LCN2, MMP9, REG1A, VDR | |||
| Chrysoeriol | 10 | AGXT, APRT, BIRC7, F2, GBA, HPRT1, JAK2, MMP9, REG1A, VDR | |||
| Eucommin A | 10 | AGXT, APRT, BIRC7, F2, GBA, JAK2, LCN2, MMP9, REG1A, VDR | |||
| Isorhamnetin | 10 | AGXT, APRT, BIRC7, F2, GBA, HPRT1, JAK2, MMP9, REG1A, VDR | |||
| Kaempferol | 10 | AGXT, APRT, BIRC7, F2, GBA, HPRT1, JAK2, MMP9, REG1A, VDR |
Discussion
The present study aimed to investigate the active compounds of the herbal extract Christina Loosestrife, or Lysimachia Christinae (Jin Qian Cao), in the treatment of nephrolithiasis using network pharmacology. Several pathways were identified, including neutrophil degranulation, interleukin-4 (IL-4), and IL-13 signaling, and purine recovery.
The glycoside, Eucommin A is the main lignan component of Eucommia ulmoides. Eucommin A has significant effects against free radical effects in vivo and in vitro, but there is no direct evidence to support a relationship between Eucommia A and antioxidant activity [24]. Hesperetin is a natural flavanon-glycoside derived from the citrus fruits of the Rutaceae family. Hesperetin can scavenge 2,2-diphenylpicrylhydrazyl (DPPH) free radicals and hydroxyl radicals [25]. Also, medioresinol is a furofuran type lignan that has strong antioxidant activity [26]. Flavonoids that include nepicatechin, luteolin, naringenin, quercetin, chrysoeriol, isorhamnetin, and kaempferol had a high degree of molecular docking, indicating that flavonoids are the main active compounds associated with the effects of Christina Loosestrife. This finding is supported by those from a previous study [27].
Among the flavonoids present in Christina Loosestrife, quercetin has effects in reducing oxidation and uric acid levels and has anti-inflammatory and diuretic effects [28]. Quercetin can also reduce kidney damage by increasing the activity of superoxide dismutase (SOD) and catalase. The antioxidant and anti-inflammatory effects of quercetin are associated with increased serum levels of paraoxonase/arylesterase 1 (PON1), and its effects on renal calculus formation may be associated with the inhibition of deposition of calcium oxalate crystals [29,30]. Catechin is another important antioxidant found in plants, including tea and grapeseed [31]. Catechin may exert its antioxidant activity by removing free radicals and by metal chelation and regulating transcription factors and enzymes [32]. Catechin has previously been shown to regulate the expression of osteopontin (OPN), malondialdehyde (MDA), and 8-hydroxy-2′-deoxyguanosine (8-OHdG) in a rat model of nephrolithiasis [33]. Catechin also increased the activity of SOD activity in NRK-52E renal tubular epithelial cells treated with calcium oxalate in vitro to restore mitochondrial membrane potentials and degrade caspase-3 [33].
In the present study, several targets were identified that showed a high degree of molecular docking, including the tyrosine-protein kinase Janus kinase 2 (JAK2) (degree, 16), the 67 kDa matrix metalloproteinase-9 (MMP9) (degree, 15), adenine phosphoribosyltransferase (APRT) (degree, 14), hypoxanthine phosphoribosyl-transferase 1 (HPRT1) (degree, 13), alanine-glyoxylate and serine-pyruvate aminotransferase (AGXT) (degree, 12), and lipocalin-2 (LCN2) (degree, 9). These findings supported the roles of these compounds in the compound-target interactions. Previous studies have shown that the AGXT gene, which encodes alanine/glyoxylate aminotransferase, transfers glyoxylic acid to glycine in the liver, and deficiency leads to calcium oxalate deposits in multiple tissues [34,35]. HPRT and APRT are key enzymes in the purine and pyrimidine nucleotide salvage pathway [36]. HPRT and APRT catalyze the salvage of the adenine and guanine into their respective monophosphate nucleosides, resulting in increased serum levels of uric acid [37]. LCN2, MMP9, and JAK2 are mainly involved in the regulation of oxidative stress and the immune response [37–40].
In the present study, Gene Ontology (GO) functional enrichment analysis identified several terms, including adenine salvage, the regulation of body fluid levels, and the cellular response to oxidative stress. Further pathway analysis showed that most targets were enriched in IL-4 and IL-13 signaling, purine salvage, and neutrophil degranulation. These findings are supported by those from a previous study that showed increased urinary uric acid levels were associated with the formation of urinary calculi in a rat model [41]. Hyperoxaluria plays an important role in promoting supersaturation of calcium oxalate calculi and is one of the three main factors in the formation of uric acid calculi [42]. Christina Loosestrife was shown in this study to have a possible role in reducing hyperoxaluria by regulating key factors, including hypoxanthine-guanine phosphoribosyltransferase (HPRT) and adenine phosphoribosyltransferase (APRT) in the purine salvage pathway. The inflammatory networks formed by these factors are important regulators for the formation of nephrolithiasis [43]. Exposure of epithelial cells to high concentrations of oxalic acid and calcium oxalate crystals can induce high levels of reactive oxygen species (ROS) and reduce the activity of superoxide dismutase (SOD) [44], and lead to cell apoptosis or necrosis [45]. Excessive ROS can induce renal epithelial cells to produce a series of cytokines, triggering an inflammatory response [46]. The findings from these previous studies support the findings from the present study hat Christina Loosestrife may reduce the inflammatory responses induced by oxalic acid and calcium oxalate crystals through the JAK2, LCN2, and MMP9 and inflammatory and immune-related pathways.
This study had several limitations. This network pharmacology study relied on data available from databases that included the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database and analysis platform and the online Taiwan TCM database, which may not have included sufficient data. This study focused on the composition of the compounds identified. However, the study did not investigate the effects of the concentrations of these compounds, the interactions between them, and the in vivo metabolic processes involved. Therefore, this network pharmacology analysis of the herbal extract, Christina Loosestrife, may have included bias in the identification of active compounds, targets, and pathways involved in the effects on nephrolithiasis, or may have missed some of the compounds involved. Future functional studies should be undertaken to validate the findings from this network pharmacology study.
Conclusions
This study aimed to undertake a network pharmacology study to identify the active compounds of the herbal extract Christina Loosestrife, or Lysimachia Christinae (Jin Qian Cao), in the treatment of nephrolithiasis. This study identified 16 active compounds of Christina Loosestrife and 11 nephrolithiasis-associated targets, which were enriched in several processes and pathways, including flavonoids and their glycosides, which are involved in purine metabolism and oxidative stress pathways.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Zeng G, Mai Z, Xia S, et al. Prevalence of kidney stones in China: An ultrasonography based cross-sectional study. BJU Int. 2017;120(1):109–16. doi: 10.1111/bju.13828. [DOI] [PubMed] [Google Scholar]
- 2.Huang WY, Chen YF, Carter S, et al. Epidemiology of upper urinary tract stone disease in a Taiwanese population: A nationwide, population based study. J Urol. 2013;189(6):2158–63. doi: 10.1016/j.juro.2012.12.105. [DOI] [PubMed] [Google Scholar]
- 3.Zhou J, Jin J, Li X, et al. Total flavonoids of Desmodium styracifolium attenuates the formation of hydroxy-L-proline-induced calcium oxalate urolithiasis in rats. Urolithiasis. 2018;46(3):231–41. doi: 10.1007/s00240-017-0985-y. [DOI] [PubMed] [Google Scholar]
- 4.Miyaoka R, Monga M. Use of traditional Chinese medicine in the management of urinary stone disease. Int Braz J Urol. 2009;35(4):396–405. doi: 10.1590/s1677-55382009000400002. [DOI] [PubMed] [Google Scholar]
- 5.Chinese Pharmacopoeia Commission. Pharmacopoeia of the People’s Republic of China (2015 edition) China Medical Science and Technology Press; Beijing, China: 2015. [Google Scholar]
- 6.Liu XF, Li TB. [Medication rule of TCM treatment for urinary lithiasis based on literature]. Chinese Journal of Information on Traditional Chinese Medicine. 2013;8:26–28. [in Chinese] [Google Scholar]
- 7.Zou ZH, Cui WQ, Chen HP, et al. [Effect of flavonoids extracted from Lysimachia hristinae hance on renal calcium oxalate stones in rats]. Chinese Journal of Experimental Traditional Medical Formulae. 2013;4:195–99. [in Chinese] [Google Scholar]
- 8.Tao TT, Lv BD, Huang XJ, et al. [Study on the total flavone extract of lysimachia on calcium oxalate stone formation in rats]. Zhong Guo Xian Dai Yi Sheng. 2016;18:30–33. [in Chinese] [Google Scholar]
- 9.Wang P, Shen YH, Xie AJ, et al. [The effect of extract of Lysimachia christinae Hance on calcium oxalate crystal growth in healthy urine]. An Hui Da Xue Xue Bao (Zi Ran Ke Xue Ban) 2006;1:80–84. [in Chinese] [Google Scholar]
- 10.Wang QY, Gao X, Wang XL, Zhang LY. [Clinical observation on the effiect of traditional Chinese medicine with extracorporeal shock wave lithotripsy for urolithiasis]. Zhuan Hua Yi Xue Za Zhi. 2015;1:49–50. [ in Chinese] [Google Scholar]
- 11.Ru JL, Peng L, Wang JN, et al. TCMSP: A database of systems pharmacology for drug discovery from herbal medicines. J Cheminform. 2014;6(1):13. doi: 10.1186/1758-2946-6-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen CY. TCM Database@Taiwan: The world’s largest traditional Chinese medicine database for drug screening in silico. PLoS One. 2012;6(1):e15939. doi: 10.1371/journal.pone.0015939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu X, Ouyang S, Yu B, et al. PharmMapper server: A web server for potential drug target identification using pharmacophore mapping approach. Nucleic Acids Res. 2010;38(Web Server issue):W609–14. doi: 10.1093/nar/gkq300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang X, Pan C, Gong J, et al. Enhancing the enrichment of pharmacophore-based target prediction for the polypharmacological profiles of drugs. J Chem Inf Model. 2016;56(6):1175–83. doi: 10.1021/acs.jcim.5b00690. [DOI] [PubMed] [Google Scholar]
- 15.Wang X, Shen Y, Wang S, et al. PharmMapper 2017 update: A web server for potential drug target identification with a comprehensive target pharmacophore database. Nucleic Acids Res. 2017;45(W1):W356–60. doi: 10.1093/nar/gkx374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amberger JS, Bocchini CA, Schiettecatte F, et al. OMIM.org: Online Mendelian Inheritance in Man (OMIM(R)), an online catalog of human genes and genetic disorders. Nucleic Acids Res. 2015;43(Database issue):D789–98. doi: 10.1093/nar/gku1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rappaport N, Twik M, Plaschkes I, et al. MalaCards: An amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res. 2017;45(D1):D877–87. doi: 10.1093/nar/gkw1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feng W, Ao H, Yue S, et al. Systems pharmacology reveals the unique mechanism features of Shenzhu Cap-sule for treatment of ulcerative colitis in comparison with synthetic drugs. Sci Rep. 2018;8:1–12. doi: 10.1038/s41598-018-34509-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu D, Gao Y, Xiang H, et al. Exploration into mechanism of antidepressant of Bupleuri radix based on network pharmacology. Acta Pharm Sin. 2018;53:210–19. [Google Scholar]
- 20.Hsin KY, Matsuoka Y, Asai Y, et al. systemsDock: A web server for network pharmacology-based prediction and analysis. Nucleic Acids Res. 2016;44(W1):W507–13. doi: 10.1093/nar/gkw335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsin KY, Ghosh S, Kitano H. Combining machine learning systems and multiple docking simulation packages to improve docking prediction reliability for network pharmacology. PLoS One. 2013;8(12):e83922. doi: 10.1371/journal.pone.0083922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tripathi S, Pohl MO, Zhou Y, et al. Meta- and orthogonal integration of influenza “OMICs” data defines a role for UBR4 in virus budding. Cell Host Microbe. 2015;18(6):723–35. doi: 10.1016/j.chom.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shannon P, Markiel A, Ozier O, et al. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13(11):2498–504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang JJ, Qin XM, Gao XX, et al. Research progress on chemical compounds, pharmacological action, and quality status of Eucommia ulmoides. Chinese Traditional and Herbal Drugs. 2017;48(15):3228–37. [Google Scholar]
- 25.Chao TL, Wang QQ. New advances in research of pharmacological effects of hesperetin and its derivatives. Chinese Traditional and Herbal Drugs. 2018;49(14):3446–51. [Google Scholar]
- 26.Huang S-W, Qiao J-W, Sun X, et al. Secoiridoids and lignans from the leaves of Diospyros kaki Thunb. with antioxidant and neuroprotective activities. Journal of Functional Foods. 2016;24:183–95. [Google Scholar]
- 27.Xie HJ, Gao HW, Wang J, et al. [The mechanism of total flavonoids of Lysimachiae herba on kidney injury induced by calcium oxalate crystallization]. Zhong Guo Zhong Xi Yi Jie He Wai Ke Za Zhi. 2018;24(1):58–63. [in Chinese] [Google Scholar]
- 28.Zhu W, Xu YF, Feng Y, et al. Prophylactic effects of quercetin and hyperoside in a calcium oxalate stone forming rat model. Urolithiasis. 2014;42(6):519–26. doi: 10.1007/s00240-014-0695-7. [DOI] [PubMed] [Google Scholar]
- 29.Amengual-Cladera E, Nadal-Casellas A, Gomez-Perez Y, et al. Phytotherapy in a rat model of hyperoxaluria: The antioxidant effects of quercetin involve serum paraoxonase 1 activation. Exp Biol Med (Maywood) 2011;236(10):1133–38. doi: 10.1258/ebm.2011.011090. [DOI] [PubMed] [Google Scholar]
- 30.Park HK, Jeong BC, Sung MK, et al. Reduction of oxidative stress in cultured renal tubular cells and preventive effects on renal stone formation by the bioflavonoid quercetin. J Urol. 2008;179(4):1620–26. doi: 10.1016/j.juro.2007.11.039. [DOI] [PubMed] [Google Scholar]
- 31.Mendoza-Wilson AM, Glossman-Mitnik D. Theoretical study of the molecular properties and chemical reactivity of (+)-catechin and (−)-epicatechin related to their antioxidant ability. Journal of Molecular Structure: THEOCHEM. 2006;761(1):97–106. [Google Scholar]
- 32.Higdon JV, Frei B. Tea catechins and polyphenols: Health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43(1):89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 33.Zhai W, Zheng J, Yao X, et al. Catechin prevents the calcium oxalate monohydrate induced renal calcium crystallization in NRK-52E cells and the ethylene glycol induced renal stone formation in rat. BMC Complement Altern Med. 2013;13:228. doi: 10.1186/1472-6882-13-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cochat P, Fargue S, Bacchetta J, et al. Primary hyperoxaluria. Nephrol Ther. 2011;7(4):249–59. doi: 10.1016/j.nephro.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 35.Li GM, Shen Q, Xu H, et al. [Primary hyperoxaluria type 1 in one child and literature review]. Zhong Guo Dun Zheng Er Ke Za Zhi. 2013;6:453–57. [in Chinese] [Google Scholar]
- 36.Li Q. The effect of heroin on purine nucleotide metabolism and the therapeutic effect of purine nucleotides. Ji Lin Da Xue. 2010;15(9):775–78. [in Chinese] [Google Scholar]
- 37.Kardakos IS, Volanis DI, Kalikaki A, et al. Evaluation of neutrophil gelatinase-associated lipocalin, interleukin-18, and cystatin C as molecular markers before and after unilateral shock wave lithotripsy. Urology. 2014;84(4):783–88. doi: 10.1016/j.urology.2014.05.034. [DOI] [PubMed] [Google Scholar]
- 38.Vittori M, Baroni S, Ferraro PM, et al. Neutrophil gelatinase-associated lipocalin (NGAL) value changes before and after shock wave lithotripsy. Urolithiasis. 2017;45(4):347–51. doi: 10.1007/s00240-016-0932-3. [DOI] [PubMed] [Google Scholar]
- 39.Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10(5):1045–56. doi: 10.1016/s1097-2765(02)00710-4. [DOI] [PubMed] [Google Scholar]
- 40.Van den Steen PE, Proost P, Wuyts A, et al. Neutrophil gelatinase B poten-tiates interleukin-8 tenfold by aminoterminal processing, whereas it degrades CTAP-III, PF-4, and GRO-alpha and leaves RANTES and MCP-2 intact. Blood. 2000;96:2673–81. [PubMed] [Google Scholar]
- 41.Khan SR, Glenton PA. Experimental induction of calcium oxalate nephrolithiasis in mice. J Urol. 2010;184(3):1189–96. doi: 10.1016/j.juro.2010.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pak CY, Adams-Huet B, Poindexter JR, et al. Rapid communication: Relative effect of urinary calcium and oxalate on saturation of calcium oxalate. Kidney Int. 2004;66(5):2032–37. doi: 10.1111/j.1523-1755.2004.00975.x. [DOI] [PubMed] [Google Scholar]
- 43.Liu X, Chen J, Su HW. [Research progress on oxygen free radicals, inflammatory reaction and formation of kidney stones]. Shan Dong Yi Yao. 2016;56(33):111–13. [in Chinese] [Google Scholar]
- 44.Ouyang JM, Yao XQ, Tan J, Wang FX. Renal epithelial cell injury and its promoting role in formation of calcium oxalate monohydrate. J Biol Inorg Chem. 2011;16(3):405–16. doi: 10.1007/s00775-010-0738-7. [DOI] [PubMed] [Google Scholar]
- 45.Tuncdemir M, Demirkesen O, Ozturk M, et al. Antiapoptotic effect of angiotensin-II type-1 receptor blockade in renal tubular cells of hyperoxaluric rats. Urol Res. 2010;38(2):71–80. doi: 10.1007/s00240-010-0255-8. [DOI] [PubMed] [Google Scholar]
- 46.Toblli JE, Cao G, Casas G, et al. NF-kappaB and chemokine-cytokine expression in renal tubulointerstitium in experimental hyperoxaluria. Role of the renin-angiotensin system. Urol Res. 2005;33(5):358–67. doi: 10.1007/s00240-005-0484-4. [DOI] [PubMed] [Google Scholar]