Abstract
Background
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are used for pain relief following tonsillectomy in children. However, as they inhibit platelet aggregation and prolong bleeding time they could cause increased perioperative bleeding. The overall risk remains unclear. This review was originally published in 2005 and was updated in 2010 and in 2012.
Objectives
The primary objective of this review was to assess the effects of NSAIDs on bleeding with paediatric tonsillectomy. Our secondary outcome was to establish whether NSAIDs affect the incidence of other postoperative complications when compared to other forms of analgesia.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2012, Issue 10); MEDLINE (inception until October 2012); EMBASE (inception until October 2012); Current Problems (produced by the UK Medicines Control Agency), MedWatch (produced by the US Food and Drug Administration) and the Australian Adverse Drug Reactions Bulletins (to May 2010). The original search was performed in August 2004. We also contacted manufacturers and researchers in the field.
Selection criteria
We included randomized controlled trials assessing NSAIDs in children, up to and including 16 years of age, undergoing elective tonsillectomy or adenotonsillectomy.
Data collection and analysis
Two authors independently assessed trial quality and extracted the data. We contacted study authors for additional information, where necessary.
Main results
We included 15 studies that involved 1101 children in this updated review. One study was added as a result of our 2012 search, another previously included study was removed due to lack of randomization. Fourteen included studies compared NSAIDs with other analgesics or placebo and reported on bleeding requiring surgical intervention. The use of NSAIDs was associated with a non‐significant increase in the risk of bleeding requiring surgical intervention: Peto odds ratio (OR) 1.69 (95% confidence interval (CI) 0.71 to 4.01). Ten studies involving 365 children reported perioperative bleeding requiring non‐surgical intervention. NSAIDs did not significantly alter the number of perioperative bleeding events requiring non‐surgical intervention: Peto OR 0.99 (95% CI 0.41 to 2.40) but the confidence intervals did not exclude an increased risk. Thirteen studies involving 1021 children reported postoperative vomiting. There was less vomiting when NSAIDs were used as part of the analgesic regime than when NSAIDs were not used: Mantel Haenszel (M‐H) risk ratio (RR) 0.72 (95% CI 0.61 to 0.85).
Authors' conclusions
There is insufficient evidence to exclude an increased risk of bleeding when NSAIDs are used in paediatric tonsillectomy. They do however confer the benefit of a reduction in vomiting.
Keywords: Adolescent; Child; Child, Preschool; Humans; Infant; Anti‐Inflammatory Agents, Non‐Steroidal; Anti‐Inflammatory Agents, Non‐Steroidal/adverse effects; Pain, Postoperative; Pain, Postoperative/drug therapy; Postoperative Hemorrhage; Postoperative Hemorrhage/chemically induced; Postoperative Nausea and Vomiting; Postoperative Nausea and Vomiting/prevention & control; Randomized Controlled Trials as Topic; Tonsillectomy; Tonsillectomy/adverse effects
Plain language summary
Do nonsteroidal anti‐inflammatory drugs (NSAIDs) increase the risk of bleeding in children having their tonsils out?
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are used for pain relief following tonsillectomy in children. Bleeding is a recognized complication of this procedure and NSAIDs can interfere with blood clotting, so there has been concern that these drugs will increase the risk of bleeding. If bleeding is severe this may result in the child being re‐admitted to hospital, having a blood transfusion or returning to theatre. It was therefore important to establish whether these drugs are safe to use in children having their tonsils out. The review focused on clinically significant bleeding that results in the child requiring additional treatment rather than the measured blood loss. We also wanted to establish whether NSAIDs affect the incidence of other postoperative complications such as nausea and vomiting when compared to other forms of analgesia. Additionally we aimed to investigate whether different types of NSAIDs were more likely to lead to bleeding.
The main limitation of our updated review was that bleeding following tonsillectomy is an uncommon event (occurring in 3% to 5% of children). We found all the data from randomized controlled trials that are currently available (15 trials studying approximately 1000 children). Our results were consistent with both an increased and decreased risk of bleeding. There were insufficient data to compare the risk of bleeding with each individual type of NSAID. However, we were able to compare ketorolac, which has been perceived as having a greater risk of bleeding, with the other NSAIDs and found no increased risk of bleeding. There was less nausea and vomiting when NSAIDs were used as part of the pain relief regime than when NSAIDs were not used.
There is insufficient evidence to exclude an increased risk of bleeding when NSAIDs are used in paediatric tonsillectomy. They do, however, confer the benefit of a reduction in vomiting.
Summary of findings
Summary of findings for the main comparison. Nonsteroidal anti‐inflammatory drugs for paediatric tonsillectomy.
| Nonsteroidal anti‐inflammatory drugs for paediatric tonsillectomy | ||||||
| Patient or population: patients with paediatric tonsillectomy Settings: Intervention: nonsteroidal anti‐inflammatory drugs | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Nonsteroidal anti‐inflammatory drugs | |||||
| Perioperative bleeding requiring surgical intervention | Moderate1 | OR 1.69 (0.71 to 4.01) | 1044 (14 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 20 per 1000 | 33 per 1000 (14 to 76) | |||||
| Perioperative bleeding requiring non‐surgical intervention | Moderate1 | OR 0.99 (0.41 to 2.4) | 745 (10 studies) | ⊕⊕⊕⊝ moderate2 | ||
| 50 per 1000 | 50 per 1000 (21 to 112) | |||||
| Vomiting | Moderate3 | RR 0.72 (0.61 to 0.85) | 1021 (13 studies) | ⊕⊕⊕⊕ high | ||
| 357 per 1000 | 257 per 1000 (218 to 303) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Based on Marret 2003. 2 Confidence interval crosses no effect and is inconsistent with an increased risk of bleeding. 3 Median control group risk from included studies.
Background
Description of the condition
Tonsillectomy is one of the most common surgical procedures for children, both in the UK and globally. The most recent figures are set at over 45,000 tonsillectomies per year in the UK (www.hesonline.nhs.uk). Indications for tonsillectomy are recurrent throat infections, recurrent tonsillitis, peritonsillar abscess or obstructive sleep apnoea.
Description of the intervention
Effective pain relief is important for the management of the paediatric tonsillectomy patient and opioid analgesics are often selected for this purpose. However, opioids may increase the occurrence of nausea and vomiting, respiratory depression and excessive sedation, and urinary retention.
Nonsteroidal anti‐inflammatory drugs (NSAIDs) are also used for pain relief following tonsillectomy in children. They are proven analgesics (Langford 2006) and reviews (Kokki 2003; Romsing 1997) have shown that they are effective in the management of mild to moderate postoperative pain in children. NSAIDs may also reduce postoperative nausea and vomiting (Öztekin 2002) as well as the time to adequate oral intake and time to discharge (Tawalbeh 2001).
How the intervention might work
NSAIDs work by interfering with cyclo‐oxygenase, which reduces the production of prostaglandins leading to a reduction in swelling and pain. Used as an alternative to opioids for pain relief they can avoid side effects such as nausea and vomiting, which could make NSAIDs a more suitable choice of analgesic for the paediatric tonsillectomy patient.
However, NSAIDs also inhibit platelet aggregation and may prolong bleeding time. This potential increased risk of bleeding has been a concern in the postoperative use of NSAIDs.
Why it is important to do this review
Bleeding after tonsillectomy is a well recognized and potentially serious complication. Estimates of the incidence of bleeding requiring treatment vary from 2% to 10%, and bleeding requiring re‐operation from 1% to 5.5% (Marret 2003). This has raised concerns that NSAID use may lead to increased perioperative bleeding following tonsillectomy and concerns about increased rates of primary haemorrhage (bleeding within 24 hours of surgery) that may require operative intervention.
Two meta‐analyses (Marret 2003; Moiniche 2003) reviewed the use of NSAIDs and the risk of bleeding after tonsillectomy in both adult and paediatric patients. Marret et al concluded that NSAIDs increased the risk of re‐operation for haemostasis after tonsillectomy but Moiniche et al concluded that NSAIDs should be used cautiously until further data were available.
Our original review (Cardwell 2005) concluded that there is currently no evidence that using NSAIDs caused any statistically significant increase in bleeding requiring further clinical intervention. However, as bleeding requiring further surgical intervention following tonsillectomy is an uncommon event (Collison 2000), a large number of participants are required to provide an adequate number of events to give a significant result. The numbers required are increased further because this is a non‐inferiority research question, with the aim being to show that the NSAIDs are not worse than other analgesic methods. It remains unknown whether the various NSAIDs may have different tendencies to cause bleeding following tonsillectomy in children.
Objectives
The primary objective of this review was to assess the effects of NSAIDs on bleeding with paediatric tonsillectomy. Our secondary outcome was to establish whether NSAIDs affect the incidence of other postoperative complications when compared to other forms of analgesia.
As discussed in the background, there is good evidence (Kokki 2003; Romsing 1997) to show that NSAIDs are effective analgesics in children. It was not the remit of our review to question this but rather to assess the risk of bleeding when using NSAIDs for pain relief following paediatric tonsillectomy.
Methods
Criteria for considering studies for this review
Types of studies
We included randomized controlled trials (RCTs). We only included those studies which reported results for the bleeding outcomes as below (Types of outcome measures).
Types of participants
We included children, aged up to and including 16 years of age, who underwent elective tonsillectomy or adenotonsillectomy. We included all indications for tonsillectomy and all surgical techniques. We excluded studies that were for adenoidectomy only.
We excluded patients with a bleeding tendency and those with contraindications to the use of NSAIDs (asthma, renal disease).
Types of interventions
We included studies in which patients had been given NSAIDs compared to either a placebo or other analgesics. NSAIDS could have been given pre‐, intra‐ or postoperatively and by any route. Doses prescribed were on a mg/kg basis, as recommended by the British National Formulary (BNF 2010). We excluded any trials that included aspirin as aspirin is no longer recommended for use in children. We did not include the cyclo‐oxygenase‐2 (COX‐2) inhibitors because these are known to have no effect on platelets (Meade 1993). Also, none of the COX‐2 inhibitors are approved for use in children. We excluded trials for lozenges and local (intratonsillar) injections.
Types of outcome measures
Our original review outcomes were the following.
Bleeding requiring further surgical intervention.
Need for blood transfusion.
Nausea or vomiting, or both.
Prolonged hospital stay.
Postoperative pain scores in the first 24 hours.
Other side effects of NSAIDs.
We had to adjust our outcome measures from those in the protocol as some were not specifically mentioned in the included trials. We had wanted to look at the need for blood transfusion but as this was not specifically reported we altered our second outcome measure to 'bleeding requiring non‐surgical intervention'. This included intravenous fluid therapy, prolonged observation and non‐surgical haemostasis.
Our third outcome measure was nausea or vomiting, or both. In the majority of papers postoperative nausea and vomiting were measured as the number of patients having at least one emetic episode, although vomiting is unlikely to occur without nausea. Some papers (Kokki 2002; Öztekin 2002; Rawlinson 2011; St Charles 1997) attempted to separate nausea and vomiting, with nausea being measured by nurse questioning or observation, or discussion with the primary care giver. In the interests of clarity we changed our third outcome measure to 'vomiting'.
The outcome measure of increased hospital stay was not specifically reported, although it is implied by the need for further intervention. Our pain data were not fully comprehensive as we only included those papers that had bleeding as an outcome. Also, pain was measured in a variety of different ways making direct comparisons impossible. There is already good evidence (Kokki 2003; Romsing 1997) to show that NSAIDs provide good postoperative analgesia. After discussion with the Cochrane Anaesthesia Review Group we decided to exclude pain from our results and focus this meta‐analysis on the risk of bleeding associated with the use of NSAIDs in paediatric tonsillectomy. Other side effects of NSAIDs were not mentioned in any of the included trials.
Therefore, our revised outcome measures were the following.
Primary outcomes
1. Perioperative bleeding requiring surgical intervention
Secondary outcomes
2. Perioperative bleeding requiring non‐surgical intervention
3. Vomiting
Search methods for identification of studies
Electronic searches
We searched MEDLINE (from inception to October 2012) and EMBASE (from inception until October 2012) on all fields using the search strategies found in Appendix 1 and Appendix 2.
In the 2012 update, we searched www.clinicaltrials.gov and www.controlled‐trials.com for relevant ongoing studies using the search term 'tonsillectomy'. We also did forward citation searches using Web of Science®; for this search we used the review's included studies published from 2000 onwards (Antila 2006; Keidan 2004; Kokki 2002; Pickering 2002; Rawlinson 2011; Öztekin 2002).
We did not impose language restrictions on any of our searches.
Searching other resources
We performed an advanced search of the Cochrane Central Register of Controlled Trials (CENTRAL) using the search strategy found in Appendix 3 and looked at all trials under the term 'tonsil*' (The Cochrane Library 2012, Issue 10). The Cochrane Anaesthesia Review Group Trials Search Co‐ordinator performed handsearching as required. Our original search was performed in August 2004 and the search was repeated in 2012.
We also searched Current Problems (produced by the UK Medicines Control Agency), MedWatch (produced by the US Food and Drug Administration) and the Australian Adverse Drug Reactions Bulletins (to May 2010). This search was not done in the 2012 update.
We contacted all the pharmaceutical companies that manufacture NSAIDs and sought conference proceedings and sources of ongoing and unpublished studies. We also checked references of all identified RCTs to identify potentially relevant citations. This was not done in the 2012 update.
Data collection and analysis
Selection of studies
Two authors independently screened titles and abstracts identified from the electronic searches and handsearches (Dr M Cardwell and Dr G Siviter in the original review and 2010 update; Professor A Smith and Mrs S Lewis (AS and SL) in the 2012 update), following the eligibility criteria. The full papers were sourced for all those studies identified at this stage. Two authors, Dr A Nicholson (AN) and SL, then independently assessed all the full papers for eligibility and recorded their decisions on study eligibility forms. Descriptions of these decisions are included in the Characteristics of included studies and Characteristics of excluded studies. We resolved disagreements by discussion between the authors.
Data extraction and management
Two authors (AN and SL) independently extracted the data from all eligible studies including those from the original review. Any disagreement was resolved by consensus. If agreement was not obtained we sought independent expert advice (Professor A Smith). We contacted the study authors for clarification, when necessary.
A copy of the data extraction form used in the 2012 update is included in Appendix 4.
Assessment of risk of bias in included studies
Due to changes to the 'Risk of bias' tool in RevMan 5.2 since the previous update, we reconsidered the risk of bias for all included studies. These changes included separation of blinding of participants and personnel from blinding of outcome assessors. We considered each individual outcome for performance and detection bias (see the data extraction form in Appendix 4). Each included study was appraised according to the criteria described below.
For each of the following criteria, judgments were made as to 'Low' or 'High' risk of bias and 'Unclear', meaning that insufficient information was available to make a judgment.
1. Random sequence generation
Low: adequate sequence generation was reported using computer‐generated random numbers, codes or sealed envelopes.
High: a system was used which was generated by a non‐random approach, e.g. odd or even date of birth.
Unclear: the trial report did not describe one of the adequate methods but mentioned randomization.
2. Allocation concealment
Low: a randomization method was described that would not allow an investigator or participant to know or influence allocation to an intervention group before an eligible participant entered the study, such as masked drug prepared by a pharmacist not otherwise involved in the study.
High: an inadequate method of allocation was used, such as alternate medical record numbers or unsealed envelopes; or there was information in the study report indicating that investigators or participants were aware of group allocation.
Unclear: the trial report mentioned randomization but there was no information on the method used, or a method was reported that was not clearly adequate.
3. Blinding of participants and personnel
This item was graded as 'Low' if participants and personnel were blinded, 'High' for unblinded participants and personnel and 'Unclear' if the relevant information was not stated in the trial report.
4. Blinding of outcome assessors
This item was graded as 'Low' for blinded outcome assessment, 'High' for unblinded outcome assessment and 'Unclear' if the relevant information was not stated in the trial report.
5. Incomplete outcome data
Low: numbers of withdrawals or exclusions per group, with reasons, were provided; or it was clear from the report that there were no withdrawals or exclusions; or sufficiently low numbers of withdrawals and exclusions to not have a clinically relevant impact on the results.
High: some withdrawal was evident but the numbers per group and reasons were not provided.
Unclear: unclear from trial report whether there were any withdrawals.
We defined an intention‐to‐treat (ITT) analysis as having been conducted when all trial participants were analysed in the group to which they were randomized, regardless of which (or how much) of the treatment they actually received and regardless of other protocol irregularities, such as ineligibility. Where necessary, and possible, we contacted authors to establish the outcomes of withdrawn or excluded participants in order to include them in the ITT analysis. Participants were only included for the ITT analysis if outcome data were available.
6. Selective reporting bias
This item was graded 'Low' if all outcomes were reported, 'High' if outcomes were measured but not reported and 'Unclear' if there was insufficient information to permit judgement of high or low reporting bias.
7. Free of other sources of bias
In this section we considered, in particular, whether:
the trial had been stopped early;
the allocation groups were similar at baseline, and whether the type of surgery (tonsillectomy or adenotonsillectomy) performed in the two groups was similar;
rescue analgesia was available, whether it included opioids, and if the use of rescue analgesia differed between the two groups.
Low: the trial appeared to be free of other components that could put it at risk of bias.
High: there were other factors in the trial that could put it at risk of bias.
Unclear: it was unclear whether the trial was or was not free of other components that could put it at risk of bias.
Measures of treatment effect
All our outcomes were dichotomous and we entered total and numbers of events, respectively, into Revman 5.2 (RevMan 5.2). Where studies presented combined results for nausea and vomiting it was assumed that these events could all be categorized under the 'vomiting' outcome. Vomiting was measured at various time points in each study. We took the data as the number of patients having at least one vomiting event within the first 24 hours. Where hours were not specified, data were taken from vomiting events in the ward or during the hospital stay, which could be assumed to be no more than 24 hours from the information within the texts of all but one paper (Thiagarajan 1993). For bleeding outcomes we used Peto odds ratios with 95% confidence intervals (CI) as a measure of effect as the outcome was rare and there were zero counts in some cells. For the more frequent vomiting outcome we used Mantel‐Haenzsel (M‐H) risk ratios.
If data had been presented in other forms such as hazard ratios and we had been unable to obtain the required tabular data from the study authors, we would have recorded effect estimates and used these in the analyses.
Unit of analysis issues
We included studies that reported more than one comparison. Antila (Antila 2006) compared the intervention drug against both placebo and another analgesic (tramadol). In other studies (Kokki 2002; Romsing 1998) the intervention drug was administered both pre‐ and postoperatively. We chose to include all of these results in the update, which allowed for subgroup analysis by timing of administration. Where these results needed to be presented in one comparison we divided the control group or combined the groups into a single pair‐wise comparison (Section 16.5.4, Higgins 2011).
Dealing with missing data
We contacted the authors for clarification where data were missing or unclear. Some participants that were enrolled were excluded for a variety of reasons. In these cases we attempted to include them in our figures in order to follow the ITT principle and only if outcome data could be obtained from the authors.
Assessment of heterogeneity
We assessed heterogeneity between studies using the Chi2 test and I2 statistic. Important heterogeneity (Chi2 P < 0.1 or I2 > 50%) was investigated using subgroup analyses. This heterogeneity may be due to:
type of NSAID used;
timing of administration;
placebo or other treatment.
Assessment of reporting biases
If 10 or more studies were included in any one meta‐analysis, funnel plots were examined to visually assess the presence of publication bias and the Egger's test was used to test for asymmetry.
Data synthesis
We attempted meta‐analysis of the outcomes for which we had comparable effect measures and where measures of heterogeneity indicated that pooling of results was appropriate. The extent of the heterogeneity was considered before any meta‐analysis was attempted. The presence of any I2 values in excess of 80% for any group of studies argued against an overall estimate being presented. For studies similar enough to support meta‐analysis, we performed the analyses in RevMan 5.2 (RevMan 5.2) using fixed‐effect models.
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis on the:
type of NSAID;
timing of administration;
surgical technique.
In the papers we identified with respect to the different NSAIDs, we were unable to perform subgroup analysis on all the individual NSAIDs as there were insufficient trials to do this. We were particularly interested in ketorolac as it is perceived to have a greater risk of bleeding and is not recommended for intraoperative use. Therefore, a subgroup analysis was performed on this drug alone, as there were sufficient papers to do so. All doses of drugs that fell within the recommended limits were included.
In the 2012 update we performed a subgroup analysis based on whether the intervention was administered pre‐ or postoperatively. We looked at the data both between studies and within studies, where possible. Our definition of preoperative was if the intervention was given after induction of anaesthesia and before surgery. Our definition of postoperative was if the intervention was given after surgery and before extubation.
We were unable to look at the surgical technique or underlying indication for tonsillectomy and the effects on bleeding outcomes as there were insufficient papers assessing these variables.
In the 2012 update we also chose to consider subgroup analysis on whether the intervention was compared against another analgesic treatment or against placebo.
Our subgroup analysis was therefore presented for each outcome as:
type of NSAID;
timing of administration;
type of control.
Clinical heterogeneity was assessed through careful evaluation of the populations, interventions and outcomes within each study. The Chi2 test and I2 statistic were used to estimate the extent of statistical heterogeneity.
Sensitivity analysis
A sensitivity analysis was performed by removing studies that had a high or unclear risk of bias in each domain. We compared these results with studies that had a low risk of bias (including baseline imbalances) for each outcome.
We also considered the use of different effect measures, for example M‐H risk ratio versus Peto odds ratio, in sensitivity analysis.
Results
Description of studies
Results of the search
The original search resulted in 13 studies being eligible for inclusion (Cardwell 2005). A further two studies were added in the 2010 update (Cardwell 2010). One was from the repeated search (2004 to 2010) (Antila 2006) and one had been incorrectly excluded from the original review (Pickering 2002). Therefore, 15 studies were included in the 2010 published update; see the study flow diagram (Figure 1).
1.
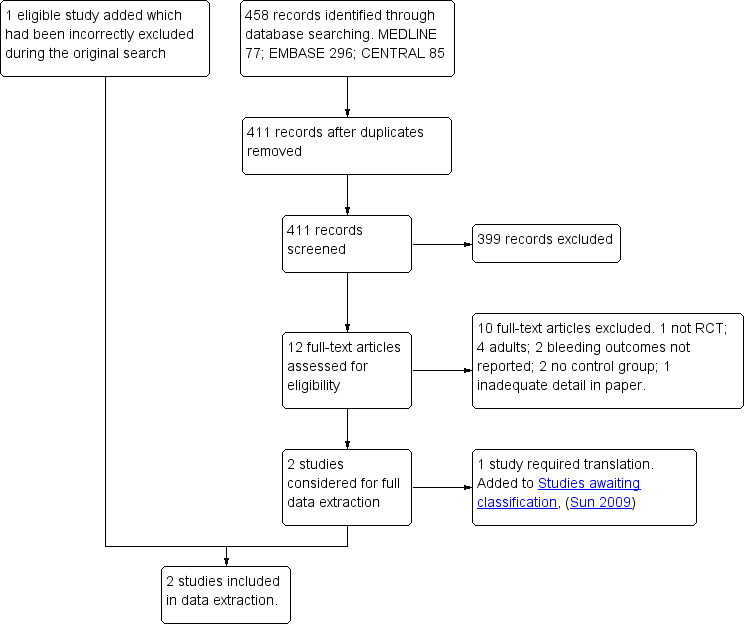
Study flow diagram for the results of the 2010 update database search.
During an updated search (2010 to 2012) we identified 395 references through searches of electronic databases, 105 from the forward citation search and 185 from searching ongoing trial databases. We identified three possible eligible studies from the ongoing trial databases, which have been included in Characteristics of ongoing studies. Of these, one is completed but as yet it is not published. We removed duplicates from our electronic database and forward citation searches, which resulted in 419 unique references. We excluded 384 studies on the basis of the titles and abstracts and then considered eligibility for 35 studies, from which two studies were added (Platzer 2011; Rawlinson 2011). Platzer 2011 (Platzer 2011) was later excluded during the data extraction process as no bleeding outcomes were reported. See the study flow diagram for the 2012 update (Figure 2).
2.
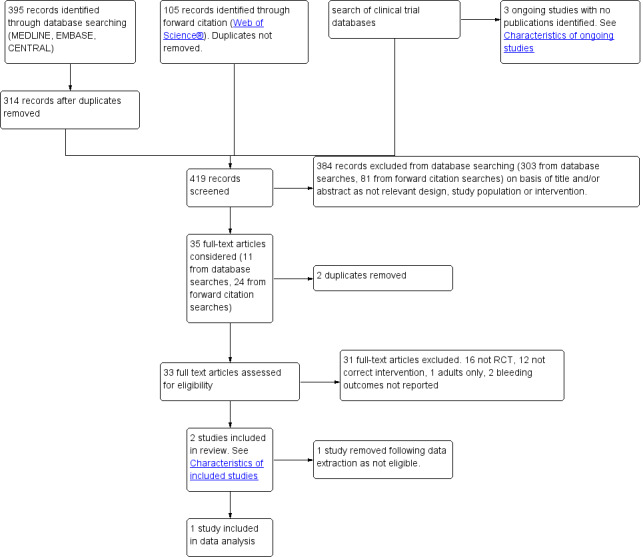
Study flow diagram of May 2010 to October 2012 update search.
During data extraction one previously eligible study was excluded (Tawalbeh 2001) as there was no evidence of randomization. One study which had been awaiting classification (Sun 2009) was also excluded as it did not measure relevant outcomes. We therefore added one study but removed one study, so we included 15 studies in this update.
Included studies
There were 15 studies with 1101 patients, aged 1 to 16 years, that met our inclusion criteria. All children were scheduled for tonsillectomy, with some studies also including patients scheduled for additional surgery, for example adenoidectomy (Antila 2006; Harley 1998; Keidan 2004; Pickering 2002; Rawlinson 2011; Romsing 1998; Rusy 1995; Splinter 1996) or other procedures, or both (Öztekin 2002; St Charles 1997; Sutherland 1998). All patients were ASA I or I‐II, with the exception of St Charles 1997 which did not provide this information.
Ketorolac was the intervention drug in six studies (Gunter 1995; Keidan 2004; Romsing 1998; Rusy 1995; Splinter 1996; Sutters 1995), administered at a dose of 1 mg/kg. There were three studies that had ibuprofen as the intervention drug (Harley 1998; Pickering 2002; St Charles 1997) at a dose of 5 mg/kg. There were three studies that had diclofenac as the intervention drug (Öztekin 2002; Rawlinson 2011; Thiagarajan 1993) at a dose of 1 mg/kg. There were two studies that had ketoprofen as the intervention drug, Antila 2006 at a dose of 2 mg/kg and Kokki 2002 at a dose of 0.5 mg/kg followed by a 3 mg/kg infusion. Tenoxicam at a dose of 0.75 mg/kg was the intervention drug for Sutherland 1998.
There were 10 studies in which the intervention drug was given preoperatively (Antila 2006; Keidan 2004; Kokki 2002; Öztekin 2002; Pickering 2002; Romsing 1998; Rusy 1995; Splinter 1996; Sutherland 1998; Thiagarajan 1993). Of these, all but one study (Pickering 2002) gave the intervention drug after induction of anaesthesia and before surgery. In Pickering 2002 the intervention drug was given one hour before surgery. There were seven studies in which the intervention drug was given postoperatively (Antila 2006; Harley 1998; Kokki 2002; Romsing 1998; St Charles 1997; Sutters 1995). For these, the intervention drug was given immediately following surgery and before the end of anaesthesia (Antila 2006; Gunter 1995; Romsing 1998; Sutters 1995) or whilst in the postanaesthesia care unit (PACU), the ward or at home. For both Kokki 2002 and Romsing 1998 there were two comparison arms of pre‐ and postoperative administration of the intervention drug. In Antila 2006 the intervention drug was given both pre‐ and postoperatively to all patients in the intervention group. In Rawlinson 2011 the intervention drug was described as being given perioperatively.
There were 14 studies that reported perioperative bleeding requiring surgical intervention. Of these, some studies did not have perioperative bleeding requiring surgical intervention as a primary outcome (Antila 2006; Rawlinson 2011; Sutherland 1998) however they did report on this outcome and were therefore included. Keidan 2004 did not report the primary outcome but was included in the review as it reported data for the outcome perioperative bleeding requiring non‐surgical intervention.
The 15 eligible studies are summarized in the Characteristics of included studies table.
Excluded studies
We excluded studies which had an incorrect design, population, intervention or did not include bleeding as an outcome (see Characteristics of excluded studies for more information). Many studies were retrospective; some looked at adults only, or a mixture of adults and children where the data from the children could not be extracted despite contacting the authors (Courtney 2001; Dommerby 1984; Petrusen 1991; Schmidt 2001); in other studies the intervention was either not an NSAID or was a COX‐2 inhibitor. The references for the excluded studies contain those excluded in the original review and the earlier update.
Risk of bias in included studies
A summary of the 'Risk of bias' results can be found in Figure 3.
3.
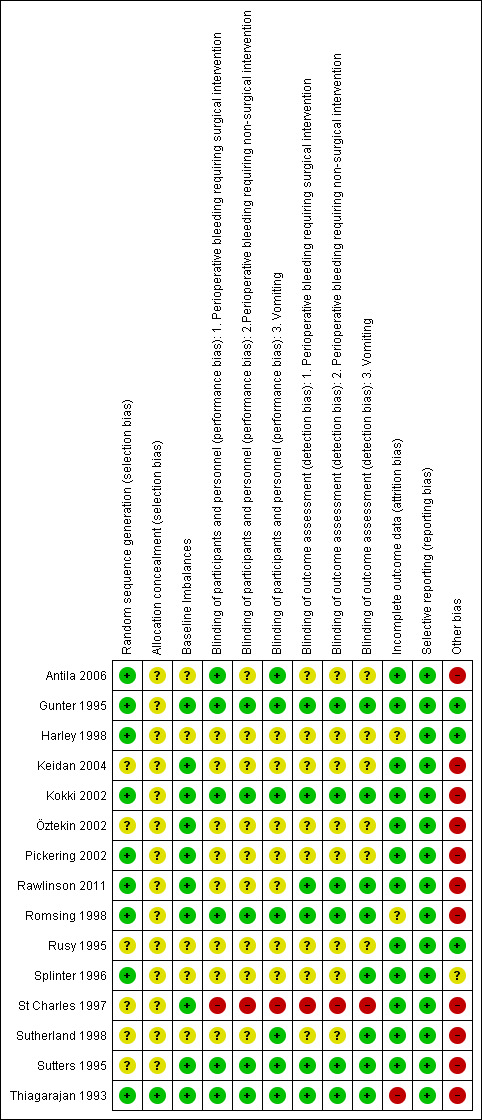
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
All studies were described as randomized however for several papers it was not clear how randomization was performed (Keidan 2004; Öztekin 2002; Rusy 1995; St Charles 1997; Sutherland 1998; Sutters 1995). Only one study (Thiagarajan 1993) had a low risk of bias for allocation concealment. Methods for allocation concealment were not reported in the other studies.
Blinding
Only one study (St Charles 1997) was assessed as having a high risk of blinding for performance and detection bias. All other studies were either described as single‐ or double‐blinded in the abstract or within the text of the journal report. There were, however, few studies that provided a full or adequate description of blinding of either participants and personnel or outcome assessment (Gunter 1995; Kokki 2002; Romsing 1998; Sutters 1995; Thiagarajan 1993).
Incomplete outcome data
There was potential for attrition bias in some of the papers (Gunter 1995; Keidan 2004; Kokki 2002; Pickering 2002; Rawlinson 2011; Romsing 1998; Sutters 1995; Thiagarajan 1993) as some participants that were enrolled were excluded for a variety of reasons. Details of the reasons for these exclusions are given in the 'Risk of bias' tables in the Characteristics of included studies. We successfully contacted some authors (Kokki 2002; Sutters 1995) to obtain our required additional information regarding patient outcomes and we therefore included the data in an ITT analysis. We were unable to obtain further information on the remaining exclusions, despite attempts to contact the authors, and we therefore did not include these missing patients in the analysis.
Selective reporting
There was no risk of bias identified in any of the studies. All studies reported the expected outcome data.
Other potential sources of bias
Two studies (Gunter 1995; Splinter 1996) were terminated early due to risks of excessive bleeding, indicating a high risk for early stopping bias. Pickering (Pickering 2002) had an early stop to recruitment in the placebo group due to an increased demand for analgesics. One paper stopped the study prematurely because of excessive bleeding (Romsing 1998), which was found to be caused by one particular surgeon. This surgeon was then excluded from the trial and the trial continued. In the previous update it was decided that the data were taken only after the trial resumed. However, in this update we decided to include data from this surgeon to provide the most conservative results.
Another risk of bias considered was that of surgery type. Along with baseline imbalances we considered whether the authors reported a balance between tonsillectomies and other additional, related surgeries. Antila 2006, Harley 1998, Rusy 1995, Splinter 1996 and Sutherland 1998 had not provided sufficient detail to judge this domain as at a low risk of bias.
The use of additional opioid analgesics in the majority of studies, in both the intervention and control groups, had the potential to bias the results of the vomiting outcome. Only Harley (Harley 1998) did not describe any rescue analgesics and it was assumed that none were given. Gunter (Gunter 1995) reported that more patients in the intervention group received additional morphine as a rescue analgesic. However, in this paper the investigators stated that there was no difference in emesis rates for those patients who received more morphine. No other studies reported on whether the use of rescue analgesics had effected the vomiting data. The effect of this risk of bias was considered further in a Sensitivity analysis.
Summary of risk of bias
There were no studies that were judged as low risk of bias in all domains. This was often due to an unclear risk assessment, reflecting omission of detail in the paper, rather than an assessment of high risk of bias.
Effects of interventions
See: Table 1
Outcome 1: perioperative bleeding requiring surgical intervention
Fourteen studies involving 1044 children compared NSAIDs with either another analgesic or placebo and assessed perioperative bleeding requiring surgical intervention. The incidence of bleeding was higher in the intervention group (16/576, 2.8%) than in the control group (7/468, 1.3%). Although the confidence interval of the effect estimate (Peto OR 1.69, 95% CI 0.71 to 4.01, P = 0.24) crossed no effect it did not exclude an increased risk of bleeding requiring surgical intervention of up to four times in the group treated with NSAIDs (see Figure 4).
4.
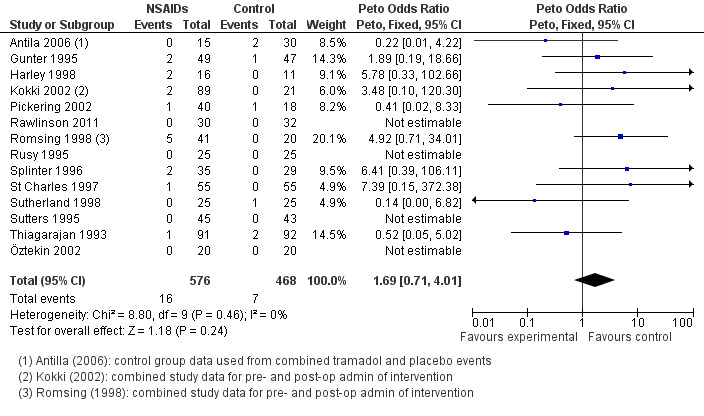
Forest plot of comparison: 1 Nonsteroidal versus control (analgesics or placebo), outcome: 1.1 Perioperative bleeding requiring surgical intervention.
A funnel plot of 10 studies contributing data showed no evidence of asymmetry. This suggests that publication bias did not affect the results (see Figure 5). There was no evidence of heterogeneity between these studies (I2 = 0%, P = 0.46).
5.
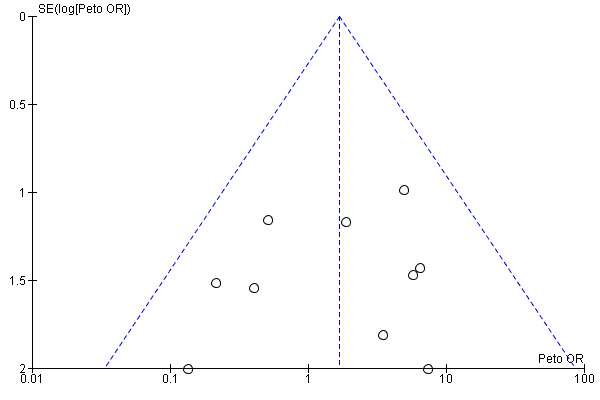
Funnel plot of comparison: 1 Nonsteroidal versus control (analgesics or placebo), outcome: 1.1 Perioperative bleeding requiring surgical intervention.
Outcome 2: perioperative bleeding requiring non‐surgical intervention
Ten studies involving 745 children compared NSAIDs with either another analgesic or placebo and assessed perioperative bleeding requiring non‐surgical intervention (see Analysis 1.2). NSAIDs did not significantly alter bleeding requiring non‐surgical intervention (Peto OR 0.99, 95% CI 0.41 to 2.40) but again this finding did not exclude an increased risk of bleeding. However, there was evidence of moderate heterogeneity between studies for this outcome (I2 = 61%, P = 0.05).
1.2. Analysis.
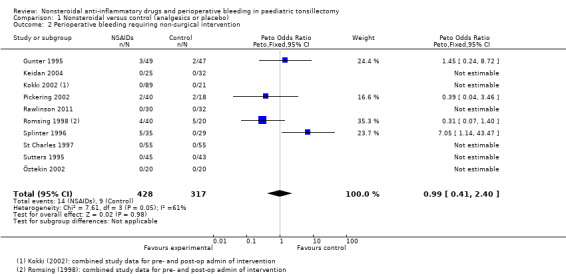
Comparison 1 Nonsteroidal versus control (analgesics or placebo), Outcome 2 Perioperative bleeding requiring non‐surgical intervention.
Outcome 3: vomiting
Thirteen studies involving 1021 children compared NSAIDs with either another analgesic or placebo and assessed vomiting. NSAIDs were shown to significantly reduce the risk of vomiting (M‐H risk ratio (RR) 0.72, 95% CI 0.61 to 0.85, P = 0.0001) (see Figure 6). Heterogeneity was low between these studies (I2 = 26%, P = 0.18).
6.
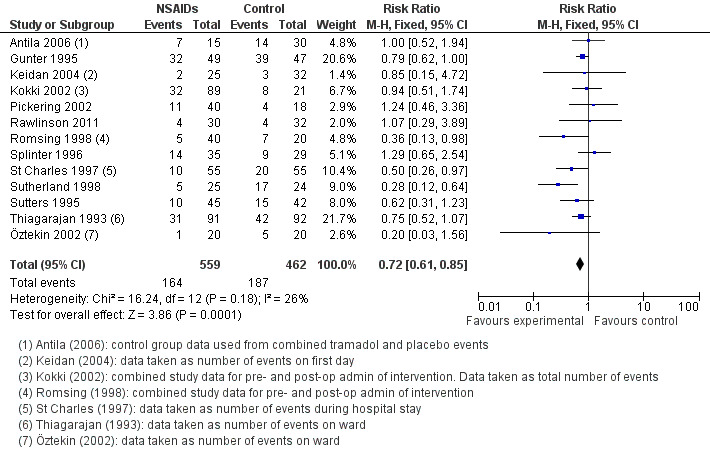
Forest plot of comparison: 1 Nonsteroidal versus control (analgesics or placebo), outcome: 1.3 Vomiting.
Subgroup analyses
Type of NSAID
In our protocol we stated that if there were enough data we would create subgroups for individual drugs. After our search we found that we had inadequate numbers of papers to create subgroups for all the NSAIDs. However, we were able to do this for ketorolac. Five studies involving 359 children compared ketorolac against another analgesic or placebo and assessed Outcome 1, perioperative bleeding requiring surgical intervention (see Analysis 2.1). The children in the intervention group had an increased risk of bleeding with a Peto OR of 3.82 (95% CI 1.03 to 14.10). The nine studies using other NSAIDs had a lower risk of bleeding with a Peto OR of 0.89 (95% CI 0.28 to 2.83). The statistical test for differences between subgroups was not significant (P = 0.1)
2.1. Analysis.
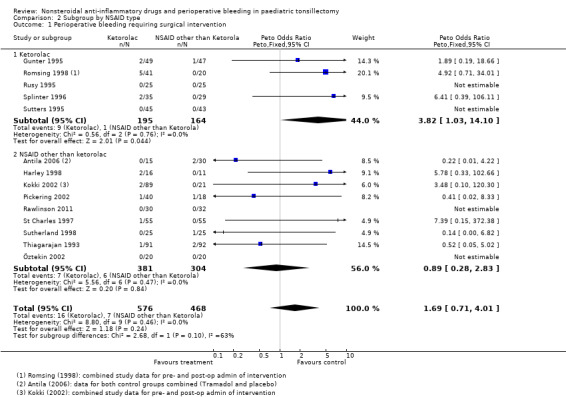
Comparison 2 Subgroup by NSAID type, Outcome 1 Perioperative bleeding requiring surgical intervention.
For Outcome 2, perioperative bleeding requiring non‐surgical intervention, the difference in the effect estimates between ketorolac and other NSAIDs was smaller (see Analysis 2.2) and again there was no statistical evidence of a difference between subgroups (P = 0.36). Analysis 2.3shows that the effect on vomiting was almost identical for ketorolac and the other NSAIDs.
2.2. Analysis.
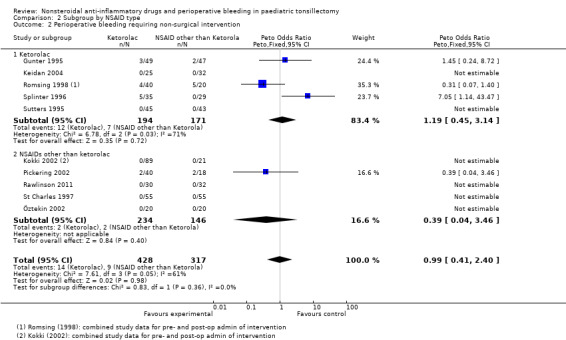
Comparison 2 Subgroup by NSAID type, Outcome 2 Perioperative bleeding requiring non‐surgical intervention.
2.3. Analysis.
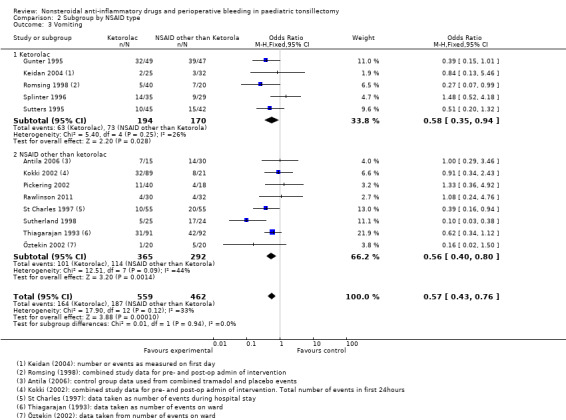
Comparison 2 Subgroup by NSAID type, Outcome 3 Vomiting.
Timing of administration
The subgroup analysis by timing of administration for Outcome 1, risk of bleeding requiring surgical intervention, showed a non‐significant increased risk when NSAIDs were given postoperatively (Peto OR 3.18, 95% CI 0.65 to 15.58) (see Analysis 3.1) but differences between the timing subgroups were not significant for this outcome (P = 0.27); similarly for Outcomes 2 and 3 (P = 0.62 and P = 0.60, respectively) (Analysis 3.2; Analysis 3.3).
3.1. Analysis.
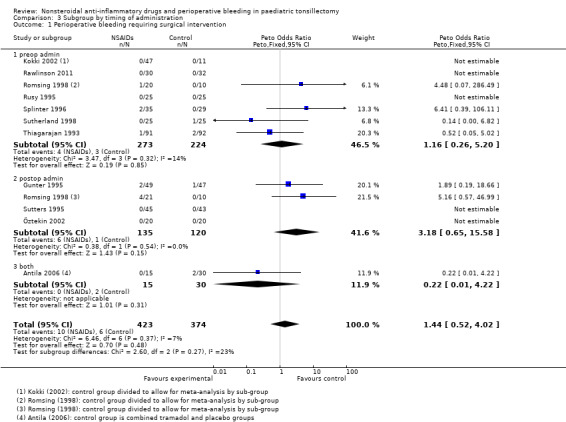
Comparison 3 Subgroup by timing of administration, Outcome 1 Perioperative bleeding requiring surgical intervention.
3.2. Analysis.
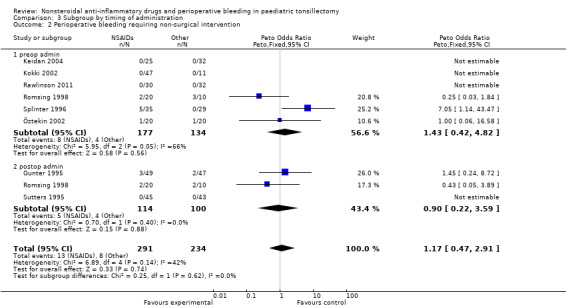
Comparison 3 Subgroup by timing of administration, Outcome 2 Perioperative bleeding requiring non‐surgical intervention.
3.3. Analysis.
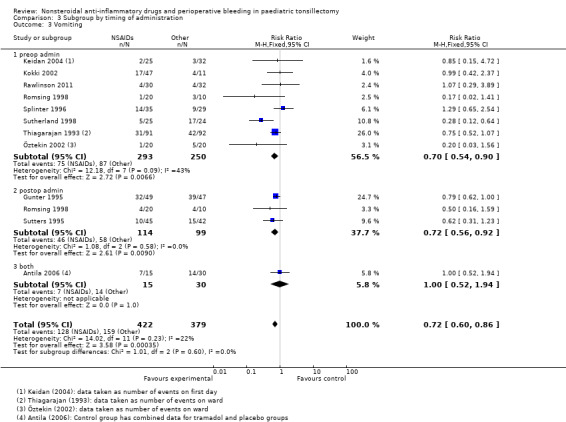
Comparison 3 Subgroup by timing of administration, Outcome 3 Vomiting.
We excluded four studies from these subgroup analyses. One study (Pickering 2002) gave the drug preoperatively at one hour before surgery and three studies (Harley 1998; Kokki 2002; St Charles 1997) administered the intervention drug in the PACU or later. The time of administration was unclear in Rawlinson (Rawlinson 2011). We included this in the preoperative group but removing it made no difference to the results.
Type of control
The subgroup analysis by type of control, that is placebo or other analgesic treatment, for Outcome 1, risk of bleeding requiring surgical intervention, showed no difference between groups (P = 0.83, I2 = 0%) (see Analysis 4.1). Similarly, there was no difference between placebo and other treatments for Outcome 3 (P = 0.88, I2 = 0%) (see Analysis 4.3). For Outcome 2 there was evidence of heterogeneity between groups (P = 0.02, I2 = 81.2%), with a higher odds ratio for bleeding in the other treatment control group compared with placebo, but even in this group the results were consistent with the possibility of both an increased and decreased risk of bleeding (OR 3.16, 95% CI 0.88 to 11.33).
4.1. Analysis.
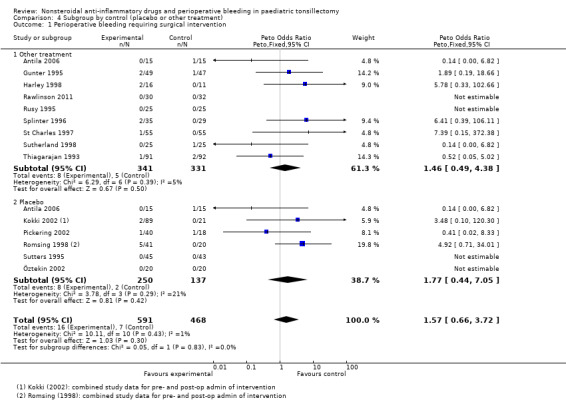
Comparison 4 Subgroup by control (placebo or other treatment), Outcome 1 Perioperative bleeding requiring surgical intervention.
4.3. Analysis.
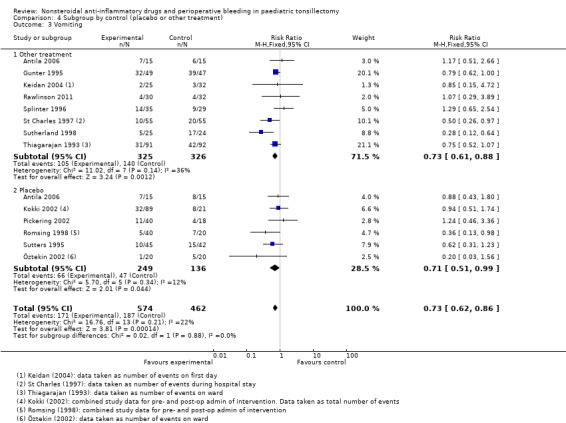
Comparison 4 Subgroup by control (placebo or other treatment), Outcome 3 Vomiting.
Sensitivity analyses
Risk of bias
Sensitivity analysis was performed based on selection bias, by removing those six studies with an unclear method of randomization. The results remained the same for the bleeding outcomes: Outcome 1 Peto OR 1.78 (95% CI 0.72 to 4.44); Outcome 2 Peto OR 0.99 (95% CI 0.41 to 2.40). The smaller sample size affected the results for the vomiting outcome in this sensitivity analysis: M‐H RR 0.84 (95% CI 0.70 to 1.01).
We carried out similar sensitivity analyses for performance and detection bias and baseline imbalances by removing studies with an unclear or high risk of bias. The results were unchanged: six studies for performance bias from Analysis 1.1, Peto OR 1.51 (95% CI 0.51 to 4.47); eight studies for detection bias from Analysis 1.1, Peto OR 2.04 (95% CI 0.63 to 6.56); and five studies for baseline imbalances from Analysis 1.1, Peto OR 1.84 (95% CI 0.64 to 5.26).
1.1. Analysis.
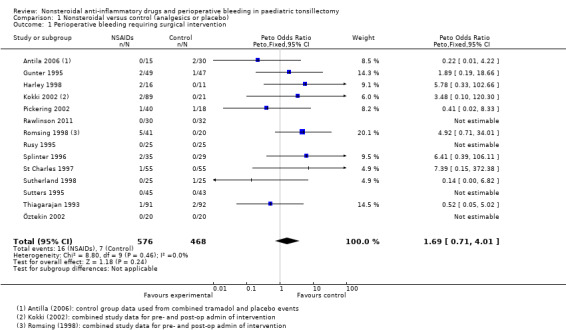
Comparison 1 Nonsteroidal versus control (analgesics or placebo), Outcome 1 Perioperative bleeding requiring surgical intervention.
Effect measures
A sensitivity analysis was also performed on the different effect measures used. The Peto OR was used for bleeding outcomes, which was deemed appropriate for rare events. Using a M‐H RR as an alternative gave the result as 1.42 (95% CI 0.64 to 3.15) for Outcome 1, still showing a non‐significant risk of perioperative bleeding with the use of NSAIDs. Using a M‐H RR as an alternative for Outcome 2 did not affect the results, M‐H RR of 0.99 (95% CI 0.47 to 2.07), still showing that NSAIDs did not affect bleeding requiring non‐surgical intervention. For the vomiting outcome we looked at using Peto OR and again found that this did not alter the interpretation of the results (Peto OR 0.57, 95% CI 0.43 to 0.75) suggesting that NSAIDs reduced the risk of vomiting.
Early stopping
Our process of meta‐analysis could not take account of the early stopping of some of the trials. We noted that the decision was made to stop two of the trials (Gunter 1995; Splinter 1996) before completion due to the higher number of bleeding events in the intervention group. Recruitment to the placebo group was stopped early in Pickering 2002 due to an increased need for analgesics in this group. These studies potentially introduced bias to the review, which may have increased the adverse effect of NSAIDs. Removing these studies did not, however, affect the results for Outcome 1 (Peto OR 1.62, 95% CI 0.57 to 6.64) nor Outcome 3 (M‐H RR 0.64, 95% CI 0.51 to 0.79). Meta‐analysis was not possible for Outcome 3 as removing these studies left only one remaining study (Romsing 1998).
Surgery type
A sensitivity analysis was performed by removing those studies with an unclear risk of bias for surgery type (Antila 2006; Harley 1998; Rusy 1995; Splinter 1996; Sutherland 1998), that is where there was no detail in the papers to demonstrate if the different surgeries performed were balanced between the intervention and control groups. This did not affect the results for any of the outcomes: Outcome 1 Peto OR 1.84 (95% CI 0.64 to 5.26); Outcome 2 Peto OR 0.54 (95% CI 0.20 to 1.49); Outcome 3 M‐H RR 0.72 (95% CI 0.60 to 0.86).
Opioid rescue analgesic
To address the potential bias introduced by the use of rescue analgesics in both groups, we removed those studies in which patients in the control group were given more rescue analgesic than in the intervention group (Antila 2006; Kokki 2002; and the preoperative group for Romsing 1998). These three studies used opioid rescue analgesics (morphine, oxycodone and fentanyl, respectively). This sensitivity analysis did not affect the results for the vomiting outcome: M‐H RR 0.70 (95% CI 0.58 to 0.84).
Discussion
Summary of main results
The focus of our review was clinically relevant outcomes that affect patients, and to investigate whether there was an increased risk of bleeding associated with the use of NSAIDS at the time of paediatric tonsillectomy. Our first outcome was bleeding requiring surgical intervention and our results are consistent with both a decreased and an increased risk of bleeding in the intervention group. Our second outcome was bleeding requiring non‐surgical intervention. This included methods of non‐ surgical haemostasis, intravenous fluid therapy and increased hospital stay.
As for bleeding requiring further surgical intervention, our results do not exclude an increased risk of bleeding associated with NSAID use. We therefore cannot draw any conclusions about the effect of NSAIDs on bleeding. Our subgroup analyses suggested an increased risk of bleeding requiring surgical intervention with ketorolac but the statistical test for differences between subgroups was not significant and this pattern was not seen with Outcome 2, bleeding requiring non‐surgical intervention. There was some evidence of a difference in Outcome 2 with an increased risk of bleeding for those studies comparing NSAIDs against a placebo, rather than another analgesic treatment, but again this difference was not significant.
Our third outcome measure was vomiting. Our results suggest that children who receive NSAIDs are 25% less likely to vomit postoperatively than children who did not receive NSAIDs. The difference was statistically significant (P ≤ 0.001). There are two possible mechanisms for this observed effect, both related to better pain control in the NSAIDs group. Many of the included studies had opioids available as a rescue analgesic. If children on NSAIDs received less opioid analgesic due to better pain control, this might explain the reduction in vomiting since opioids are known to lead to nausea and vomiting. If an opioid‐sparing effect is assumed then in those studies in which less rescue analgesic was given to the intervention group the results would show a larger reduction in vomiting. Our sensitivity analysis showed no difference in the results when studies were removed in which less rescue analgesic was given.
Apart from an opioid‐sparing mechanism, it is also possible that the reduction in vomiting seen in the NSAID group could be explained by a reduction in pain for those patients in the intervention group, since pain can lead to vomiting and nausea. We did not consider pain in this review due to the differences in reporting between studies, and therefore we were unable to consider a possible correlation between vomiting and pain.
Overall completeness and applicability of evidence
The objective of our review was to assess the effects of NSAIDs on bleeding with paediatric tonsillectomy. We were able to identify 15 studies with 1101 patients undergoing tonsillectomy surgery from our searches. All of these studies compared the use of NSAIDs with either another analgesic or a placebo. Bleeding, either requiring surgical or non‐surgical intervention, was considered as an outcome in all the studies.
Bleeding requiring further surgical intervention is an uncommon event following tonsillectomy. Assessing the pooled numerical values for the number of events, we have approximately 500 patients in each group and only a very small number of bleeding events. The results of the pooled data, shown on the forest plot in Figure 4, showed no statistically significant increase in bleeding. However, power calculations indicated that 1469 patients in each group would be needed to detect a risk ratio of 1.5, a 5% assumed risk of bleeding in the control group and a 7.5% assumed risk in the NSAID group, with 80% power (α 0.05, two‐tailed). Since this a non‐inferiority research question, perhaps the more relevant power calculation is to exclude a 2.5% difference in bleeding with a one‐sided α 0.025 and this would require 1194 patients in each group. This review, therefore, had insufficient numbers to be able to exclude an increased risk of bleeding associated with the use of NSAIDs in paediatric tonsillectomy.
We considered whether NSAIDs affect the incidence of postoperative vomiting, and 13 of our included studies reported on this outcome. Postoperative nausea and vomiting is a much more common event and this was reflected in our results. Again we had approximately 500 patients in each group and our results demonstrated a significant reduction in the risk of vomiting for the NSAIDs group. Power calculations for this outcome indicated that 354 patients in each group would be needed to detect a risk ratio of 0.75, a 40% assumed risk of vomiting in the control group and a 30% risk of bleeding in the NSAIDs group, with a power of 80% (α 0.05, two‐tailed). This review, therefore, had sufficient numbers to potentially demonstrate that the use of NSAIDs for paediatric tonsillectomy reduces the risk of vomiting.
Quality of the evidence
We considered the quality of the studies using the 'Risk of bias' assessment tools. There were no studies that had a low risk of bias in all the domains, however our sensitivity analysis suggested that these biases did not affect the overall result. A funnel plot (see Figure 5) suggests an absence of bias across 10 included studies for our first comparison (Analysis 1.1). No outcome was graded down for quality in the 'Summary of findings' table.
The included studies date back to 1995 and we considered the directness of evidence and whether the interventions tested were still clinically relevant. Three papers studied ibuprofen (Harley 1998; Pickering 2002; St Charles 1997) but used doses of 5 mg/kg. However, ibuprofen is now prescribed to children in a dose of 10 mg/kg so the doses studied are half that now given, which may have an effect on the potential to cause bleeding.
Another study (Thiagarajan 1993) used papaveretum as the comparison drug, which is no longer commonly used for children. Five studies (Rawlinson 2011; Splinter 1996; Sutherland 1998; Sutters 1995; Thiagarajan 1993) administered either the intervention or comparison drug, or both, intramuscularly, which is also no longer commonly used for children. However, these are not variables that are likely to affect the results and we did not downgrade the evidence for indirectness.
Potential biases in the review process
This review considered studies of paediatric tonsillectomy patients. Although we attempted to account for different surgery types and to aim for balance, it must be acknowledged that some patients also underwent additional surgeries (commonly adenoidectomy) and this could have potentially affected the bleeding outcomes. We attempted to address this in the sensitivity analysis. There were also different surgical techniques used between studies, as well as different anaesthetics, which we did not consider in our subgroup analysis. These differing techniques and drugs could affect bleeding outcomes.
We also did not consider age or gender in our review, for which there may be risks associated with postoperative haemorrhage (Tomkinson 2011).
For our analysis we combined studies comparing different NSAIDs (ketorolac, ibuprofen, diclofenac, ketoprofen and tenoxicam) against different controls (placebo, morphine, fentanyl, codeine, acetominophen, tramadol and papaveretum). We considered subgroup analysis according to the intervention drug but we were only able to consider ketorolac for subgroup analysis. Within this subgroup ketorolac was considered against a variety of controls. The intervention and control drugs were also given by different routes (intravenously, intramuscularly or orally).
Our second subgroup analysis considered the timing of administration of the intervention drug. We were unable to perform this meta‐analysis using data within study populations as there were only two studies for which this would have been possible. We chose to combine the data between those studies in which the intervention drug was given preoperatively and those studies in which it was given postoperatively. The differences between estimates may therefore be due to differences in the study populations or trial design, unrelated to timing of NSAID administration. However, we did not find any differences between the timing subgroups.
We limited our included study eligibility criteria to those studies which reported on perioperative bleeding requiring surgical or non‐surgical interventions, as justified by our main objective to consider the effect of NSAIDs on bleeding. However, this meant that some studies were excluded from our review which could have potentially been included in our meta‐analysis of the effect of NSAIDs on the incidence of vomiting. We did not include a measure of millilitres of blood as this was less relevant in paediatric cases. However, there were studies that measured such intraoperative blood loss and our definitions of perioperative bleeding requiring surgical or non‐surgical bleeding did not allow for this. Our review did not, therefore, consider whether there may be a difference in the amount of bleeding that is subclinical.
Agreements and disagreements with other studies or reviews
Two meta‐analyses (Marret 2003; Moiniche 2003) reviewed the use of NSAIDs and the risk of bleeding after tonsillectomy in both adult and paediatric patients. Marret et al concluded that NSAIDs increase the risk of re‐operation for haemostasis after tonsillectomy. Moiniche et al concluded that NSAIDs should be used cautiously until further data are available.
The first review (Marret 2003) studied paediatric and adult data but only included trials published in English. The paper has not been updated. Our review includes a further two papers which have been published since that review. The outcome measures in that review were the need for surgical electrocautery to stop the bleeding and postoperative bleeding requiring a change in postoperative management. The primary outcome, which required a return to theatre, was different from ours; and the need for surgical electrocautery probably overestimated the bleeding as this can be part of the primary surgical procedure. The papers included in the review were not exactly the same as ours as the review included two that only studied adult participants and the review did not include eight papers which we included. It is unclear from the review why these were not included but three papers (Kokki 2002; Öztekin 2002; Pickering 2002) do not appear to have been reviewed at all as they are not mentioned or referenced. We considered all three of these studies to be of sufficient quality to include in our review.
In the second review (Moiniche 2003) the papers reviewed were similar to ours except adult studies were also included and no papers published after December 2001 were included. The significance of their results was dependent on the method used. They found that re‐operation because of bleeding occurred more often with NSAIDs. The Peto OR was statistically significant (Peto OR 2.33, 95% CI 1.12 to 4.83) and was of borderline significance using the method described by Shadish and Haddock (Sadish 1994) (OR 1.92, 95% CI 1.00 to 3.71); the NNT was 60 (95% CI 34 to 277). However, they did not find interoperative blood loss, postoperative blood loss or hospital admission to be more common with NSAIDs and therefore concluded that NSAIDs could be used cautiously until further data became available.
A Cochrane systematic review (Standing 2009) considered diclofenac for acute pain in children (perioperative pain, migraine, renal colic and soft tissue injury and fractures). This paper included two studies from our review (Öztekin 2002; Thiagarajan 1993) and included outcomes of bleeding requiring surgical intervention and nausea or vomiting, or both. The authors concluded that diclofenac did not appear to increase the incidence of perioperative bleeding (Mantel‐Haenzel RR 1.25, 95% CI 0.31 to 4.97). They also conclude that there is a reduction in nausea and vomiting (Mantel‐Haenzel RR 0.58, 95% CI 0.47 to 0.73).
Authors' conclusions
Implications for practice.
The objective of this review was to assess the effects of NSAIDs on bleeding with paediatric tonsillectomy. From the data available to date, there is no evidence that using NSAIDs caused any statistically significant increase in bleeding that required further clinical intervention. Bleeding requiring further surgical intervention is an uncommon event following tonsillectomy. Although not statistically significant, our results showed an increased risk of bleeding requiring surgical intervention with a large confidence interval (95% CI 0.71 to 4.01). This indicates that the total number of participants studied is inadequate and further studies need to be performed. This is also shown in our power calculations. This limitation of our original review remains as only one paper was added to this updated review. There was less vomiting when NSAIDs were used as part of the analgesic regime compared to when NSAIDs were not used.
We therefore conclude that there is insufficient evidence to exclude an increased risk of bleeding when NSAIDs are used in paediatric tonsillectomy. They do, however, confer the benefit of a reduction in vomiting.
Implications for research.
Further studies are required assessing the impact of NSAIDs on bleeding in paediatric tonsillectomy. Future studies should be sufficiently powered to consider the relatively uncommon risk of bleeding. They should be sufficiently blinded and avoid the bias introduced by opioid rescue analgesics, different surgical techniques and surgeries in addition to tonsillectomy. Stratifying by age and gender would also be considered for this potential difference in bleeding outcomes.
Currently none of the selective COX‐2 inhibitors are approved for use in children and there remains limited information to support their use in treating pain following paediatric tonsillectomy. This would be a further potential topic for research in this field.
What's new
| Date | Event | Description |
|---|---|---|
| 1 July 2013 | New search has been performed | A 'Summary of findings' table has been included. |
| 1 July 2013 | New search has been performed | We reconsidered a previously excluded subgroup analysis of timing of administration of the intervention drug. We were able to conduct this subgroup analysis by considering the timing between studies as well as within studies. |
| 1 July 2013 | New citation required but conclusions have not changed | We updated our search from 2010 to 2012. We did a full paper review of 12 new studies of which we excluded 11. We included Rawlinson 2011. We arranged translation of Sun 2009, which was then excluded. We reconsidered Tawalbeh 2001 as not being eligible and removed this study from the included studies. Due to changes to the Cochrane risk of bias tables we chose to re‐extract data from all existing studies as well as the new study. This resulted in some minor alterations to the data. Where studies had provided both pre and postoperative data (Kokki 2002; Romsing 1998), we included data in the analysis from both groups. We reconsidered and chose to include data from a particular surgeon (in Romsing 1998) in order to show any potential increased risk of bleeding. |
| 1 July 2013 | New search has been performed | We made revisions to the text and to the 'Risk of bias' tables in light of alterations as a result of data extraction. We made changes to the outcomes in order to improve clarity. For the vomiting outcome we also defined the timing in the text as being within 24 hours. This was then reflected in minor alterations to the data. |
| 1 July 2013 | New search has been performed | Additional authors were added to the review. |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 2, 2005
| Date | Event | Description |
|---|---|---|
| 24 August 2010 | Amended | We included a study (Pickering 2002) which had been incorrectly excluded from our previous review (Cardwell 2005). This study had been excluded because it studied COX‐2 inhibitors; but it also had an ibuprofen group and a placebo group, so data were used from these two groups. |
| 24 August 2010 | Amended | We completed a risk of bias table, risk of bias graph and risk of bias summary. |
| 24 August 2010 | New search has been performed | We updated our search from 2004 to 2010. We did a full paper review of 11 new studies from our updated search of which we excluded 10 (Bhattacharya 2005; Bhattacharya 2009; Hardy 2010; Heaney 2007; Hiller 2004; Jeyakumar 2008; Kedek 2005; Louizos 2006; McKean 2008; Nikanne 2005) and included one (Antila 2006). An additional paper is awaiting further assessment as it needs to be translated (Sun 2009). This new study does not change our conclusions. |
| 1 August 2008 | New search has been performed | Converted to new review format. |
Acknowledgements
We would like to thank Dr Toni Tan who translated Sun 2009 and assessed its eligibility.
We would like to thank Dr Mina Nishimori who translated the two papers by Azuma et al (Azuma 1982) and Tamura et al (Tamura 1972) from Japanese to English.
We would like to thank Mrs D Dunton, a librarian at North Manchester General Hospital, who provided support for our searches.
We would like to thank Drs Iveta Simera and Igor Burceff for help with the translation of the paper by Gonchar et al (Gonchar 1974).
We would like to thank Dr Nicola Petrucci for help with the translation of the study by Calvet et al (Calvet 1969).
We would like to thank William Pollard for the translation of the study by Lacomme et al (Lacomme 1978).
We would also like to thank Dr Jane Ballantyne, Prof Nathan Pace, Dr A E Pickering, Dr Allan Cyna and Amy Godfrey Arkle, Kathie Godfrey, Janet Wale and Iveta Simera for their help and editorial advice during the preparation of the original review (Cardwell 2005). CARG's consumer panel helped to write the plain language summary section.
Appendices
Appendix 1. Search strategy for MEDLINE (OvidSP)
1. exp tonsillectomy/ or tonsil*.ti,ab. 2. exp Anti‐inflammatory agents/ or exp Analgesics‐non‐narcotic/ or exp cyclooxygenase‐inhibitors/ or (Ketorolac or Ibuprofen or Diclofenac or Naproxen or Piroxicam or ketoprofen or indomethacin).mp. or exp Postoperative‐Hemorrhage/ or (postoperative adj3 h?emorrhage).mp. 3. 1 and 2 4. ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or drug therapy.fs. or randomly.ab. or trial.ab. or groups.ab.) not (animals not (humans and animals)).sh. 5. 3 and 4
Appendix 2. Search strategy for EMBASE (OvidSP)
1 exp tonsillectomy/ or tonsil*.ti,ab. 2 exp antiinflammatory agent/ or exp analgesic‐agent/ or exp postoperative‐hemorrhage/ or (Ketorolac or Ibuprofen or Diclofenac or Naproxen or Piroxicam or ketoprofen or indomethacin).mp. 3 1 and 2 4 (randomized‐controlled‐trial/ or randomization/ or controlled‐study/ or multicenter‐study/ or phase‐3‐clinical‐trial/ or phase‐4‐clinical‐trial/ or double‐blind‐procedure/ or single‐blind‐procedure/ or (random* or cross?over* or factorial* or placebo* or volunteer* or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*))).ti,ab.) not (animals not (humans and animals)).sh. 5 3 and 4
Appendix 3. Search strategy for CENTRAL (The Cochrane Library)
#1 MeSH descriptor Tonsillectomy explode all trees #2 tonsil* #3 (#1 OR #2) #4 MeSH descriptor Anti‐Inflammatory Agents explode all trees #5 MeSH descriptor Analgesics, Non‐Narcotic explode all trees #6 Ketorolac or Ibuprofen or Diclofenac or Naproxen or Piroxicam or ketoprofen or indomethacin #7 MeSH descriptor Cyclooxygenase Inhibitors explode all trees #8 (#4 OR #5 OR #6 OR #7) #9 MeSH descriptor Postoperative Hemorrhage explode all trees #10 (#3 AND ( #8 OR #9 ))
Appendix 4. Data extraction form 2012 update
Data Collection Form
| Review title or ID |
| |
| Study ID(surname of first author and year first full report of study published e.g. Smith 2001) |
| |
| Report IDs of other reports of this study(e.g. duplicate publications, follow‐up studies) |
| |
1. General Information
| Date form completed (dd/mm/yyyy) | |
| Name/ID of person extracting data | |
|
Report title (title of paper/ abstract/ report that data extracted from) |
|
|
Reference details |
|
| Report author contact details | |
|
Publication type (e.g. full report, abstract, letter) |
|
| Study funding sources (including role of funders) | |
| Possible conflicts of interest (for study authors) |
2. Study Eligibility
| Study Characteristics |
Eligibility criteria |
Yes No Unclear |
Location in text (pg & ¶ /fig / table) |
| Type of study | Randomized Controlled Trials | ||
| Participants | Children <16yrs, who underwent elective tonsillectomy or adenotonsillectomy |
||
| Types of intervention | NSAIDs. Any route of administration. | ||
| Drug exclusions | Does trial include aspirin? Does trial include COX‐2 inhibitors? |
||
| Types of outcome measures | Bleeding requiring further surgical intervention Bleeding not requiring further surgical intervention Nausea or vomiting, or both |
||
| INCLUDE EXCLUDE | |||
| Reason for exclusion | |||
DO NOT PROCEED IF EXCLUDED FROM REVIEW
3. Population and setting
|
Description (include comparative information for each group (i.e. intervention and controls) if available |
Location in text (pg & ¶ /fig / table) |
||
|
Population and description (from which study participants are drawn) |
|||
|
Setting (including location and social context) |
|||
|
Inclusion criteria |
|||
|
Exclusion criteria |
|||
| Method/s of recruitment of participants | |||
|
Informed consent obtained |
Yes/No/Unclear | ||
4. Methods
| Descriptions as stated in report/paper |
Location in text (pg & ¶ /fig / table |
||
| Aim of study | |
||
| Design(e.g. parallel, crossover, cluster) | |||
|
Unit of allocation (by individuals, cluster /groups or body parts) |
|||
|
Start date |
|||
|
End date |
|||
|
Total study duration |
|||
| Ethical approval needed/obtained for study | Yes/No/Unclear | ||
5. Risk of Bias assessment
| Domain |
Risk of bias Low/ High/ Unclear |
Support for judgement |
Location in text (pg & ¶ /fig / table |
|
Random sequence generation (selection bias) |
|||
|
Allocation concealment (selection bias) |
|||
|
Baseline Imbalances |
|||
|
Blinding of participants and personnel (performance bias) |
Outcome: Bleeding requiring surgical intervention | ||
|
Outcome: Bleeding not requiring surgical intervention |
|||
|
Outcome: Nausea or vomiting, or both |
|||
|
Blinding of outcome assessment (detection bias) |
Outcome: Bleeding requiring surgical intervention |
||
|
Outcome: Bleeding not requiring surgical intervention |
|||
|
Outcome: Nausea or vomiting, or both |
|||
|
Incomplete outcome data (attrition bias) |
Outcome: Bleeding requiring surgical intervention |
||
|
Outcome: Bleeding not requiring surgical intervention |
|||
|
Outcome: Nausea or vomiting, or both |
|||
|
Selective outcome reporting (reporting bias) |
|||
|
Other bias |
6. Participants
Provide overall data and, if available, comparative data for each intervention or comparison group.
| Description as stated in report/paper | Location in text (pg & ¶ /fig / table | ||
|
Total no. randomized |
|||
|
Clusters (if applicable, no., type, no. people per cluster) |
|||
|
Baseline imbalances |
|||
|
Withdrawals and exclusions (if not provided below by outcome) |
|||
|
Age age range (mean) |
Intervention | Comparison | |
|
|
|||
|
Sex Male/Female |
Intervention | Comparison | |
|
|
|||
|
Race/Ethnicity |
|||
|
Severity of illness |
|||
|
Co‐morbidities |
|||
|
Anaesthetics used |
|||
|
Rescue analgesics given (and at what stage) |
|||
|
Rescue anti‐emetics given (and at what stage) |
|||
|
Other drugs given (and at what stage) |
|||
| Other relevant sociodemographics | |||
|
Subgroups measured |
|||
|
Subgroups reported |
|||
7.1 Intervention group
|
Description as stated in report/paper |
Location in text (pg & ¶ /fig / table |
|
| Intervention | NSAID |
|
|
Type of surgical procedure and method (e.g. tonsillectomy by electrocautery) |
||
|
No. randomized to group (specify whether no. people or clusters) |
||
|
Description (type, dose) |
||
|
Duration of treatment period |
||
|
Timing (e.g. pre‐operatively, intra‐operatively, post‐operatively) |
||
|
Delivery (e.g. mechanism, medium, intensity) |
||
|
Providers (e.g. no., profession, training, ethnicity etc. if relevant) |
||
|
Co‐interventions |
7.2 Comparison groups – repeated as required
|
Description as stated in report/paper |
Location in text (pg & ¶ /fig / table |
|
|
Comparison group type (placebo, no treatment, different drug) |
|
|
|
Type of surgical procedure and method (e.g. tonsillectomy by electrocautery) |
||
|
No. randomized to group (specify whether no. people or clusters) |
||
|
Description (type, dose) |
||
|
Duration of treatment period |
||
|
Timing (e.g. pre‐operatively, intra‐operatively, post‐operatively) |
||
|
Delivery (e.g. mechanism, medium, intensity) |
||
|
Providers (e.g. no., profession, training, ethnicity etc. if relevant) |
||
|
Co‐interventions |
7.3 Comparison groups
|
Description as stated in report/paper |
Location in text (pg & ¶ /fig / table |
|
|
Comparison group type (placebo, no treatment, different drug) |
|
|
|
Type of surgical procedure and method (e.g. tonsillectomy by electrocautery) |
||
|
No. randomized to group (specify whether no. people or clusters) |
||
|
Description (type, dose) |
||
|
Duration of treatment period |
||
|
Timing (e.g. pre‐operatively, intra‐operatively, post‐operatively) |
||
|
Delivery (e.g. mechanism, medium, intensity) |
||
|
Providers (e.g. no., profession, training, ethnicity etc. if relevant) |
||
|
Co‐interventions |
8.1 Outcomes (repeat for each outcome)
| Description as stated in report/paper |
Location in text (pg & ¶ /fig / table |
|
|
Outcome name |
||
|
Time points measured |
||
|
Time points reported |
||
|
Outcome definition (with diagnostic criteria if relevant) |
||
|
Person measuring/reporting |
||
|
Unit of measurement |
||
|
Scales: upper and lower limits (indicate whether high or low score is good) |
||
|
Is outcome tool validated? |
Yes/No/Unclear | |
|
Imputation of missing data (e.g. assumptions made for ITT analysis) |
||
|
Assumed risk estimate (e.g. baseline or population risk noted in Background) |
||
|
Power |
9.1 Results (repeat for each outcome)
|
Description as stated in report/paper |
Location in text (pg & ¶ /fig / table |
||||||
|
Comparison |
|||||||
|
Outcome |
|||||||
|
Subgroup |
|||||||
|
Timepoint (specify whether from start or end of intervention) |
|||||||
| Results | Intervention | Comparison | |||||
| No. events/no. participants | No. events/no.participants | ||||||
| No. missing participants and reasons | |||||||
| No. participants moved from other group and reasons | |||||||
|
Any other results reported |
|||||||
|
Unit of analysis (by individuals, cluster/ groups or body parts) |
|||||||
| Statistical methods used & appropriateness of these methods(e.g. adjustment for correlation) | |||||||
|
Reanalysis required? (specify) |
Yes/No/Unclear | ||||||
|
Reanalysis possible? |
Yes/No/Unclear | ||||||
| Reanalysed results |
|
||||||
10. Applicability
| Have important population groups been excluded from the study?(consider disadvantaged populations, and possible differences in the intervention effect) | Yes No Unclear | |
|
Is the intervention likely to be aimed at disadvantaged groups? (e.g. lower socioeconomic groups) |
Yes No Unclear | |
|
Does the study directly address the review question? (any issues of partial or indirect applicability) |
Yes No Unclear |
11. Other information
|
Description as stated in report/paper |
Location in text (pg & ¶ /fig / table |
|
|
Key conclusion of study authors |
|
|
|
References to other relevant studies |
||
|
Correspondence required for further study information (from whom, what and when) |
END
Data and analyses
Comparison 1. Nonsteroidal versus control (analgesics or placebo).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perioperative bleeding requiring surgical intervention | 14 | 1044 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [0.71, 4.01] |
| 2 Perioperative bleeding requiring non‐surgical intervention | 10 | 745 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.41, 2.40] |
| 3 Vomiting | 13 | 1021 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.61, 0.85] |
1.3. Analysis.
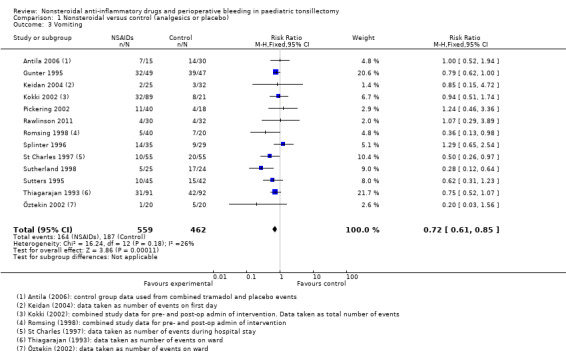
Comparison 1 Nonsteroidal versus control (analgesics or placebo), Outcome 3 Vomiting.
Comparison 2. Subgroup by NSAID type.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perioperative bleeding requiring surgical intervention | 14 | 1044 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [0.71, 4.01] |
| 1.1 Ketorolac | 5 | 359 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.82 [1.03, 14.10] |
| 1.2 NSAID other than ketorolac | 9 | 685 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.28, 2.83] |
| 2 Perioperative bleeding requiring non‐surgical intervention | 10 | 745 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.41, 2.40] |
| 2.1 Ketorolac | 5 | 365 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.45, 3.14] |
| 2.2 NSAIDs other than ketorolac | 5 | 380 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.39 [0.04, 3.46] |
| 3 Vomiting | 13 | 1021 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.43, 0.76] |
| 3.1 Ketorolac | 5 | 364 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.35, 0.94] |
| 3.2 NSAID other than ketorolac | 8 | 657 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.40, 0.80] |
Comparison 3. Subgroup by timing of administration.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perioperative bleeding requiring surgical intervention | 11 | 797 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.52, 4.02] |
| 1.1 preop admin | 7 | 497 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.16 [0.26, 5.20] |
| 1.2 postop admin | 4 | 255 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.18 [0.65, 15.58] |
| 1.3 both | 1 | 45 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.01, 4.22] |
| 2 Perioperative bleeding requiring non‐surgical intervention | 8 | 525 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.17 [0.47, 2.91] |
| 2.1 preop admin | 6 | 311 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [0.42, 4.82] |
| 2.2 postop admin | 3 | 214 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.22, 3.59] |
| 3 Vomiting | 11 | 801 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.86] |
| 3.1 preop admin | 8 | 543 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.54, 0.90] |
| 3.2 postop admin | 3 | 213 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.56, 0.92] |
| 3.3 both | 1 | 45 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.52, 1.94] |
Comparison 4. Subgroup by control (placebo or other treatment).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Perioperative bleeding requiring surgical intervention | 14 | 1059 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.57 [0.66, 3.72] |
| 1.1 Other treatment | 9 | 672 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.46 [0.49, 4.38] |
| 1.2 Placebo | 6 | 387 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.77 [0.44, 7.05] |
| 2 Perioperative bleeding requiring non‐surgical intervention | 9 | 687 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.19 [0.45, 3.14] |
| 2.1 Other treatment | 5 | 389 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.16 [0.88, 11.33] |
| 2.2 Placebo | 4 | 298 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.31 [0.07, 1.40] |
| 3 Vomiting | 13 | 1036 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.62, 0.86] |
| 3.1 Other treatment | 8 | 651 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.61, 0.88] |
| 3.2 Placebo | 6 | 385 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.51, 0.99] |
4.2. Analysis.
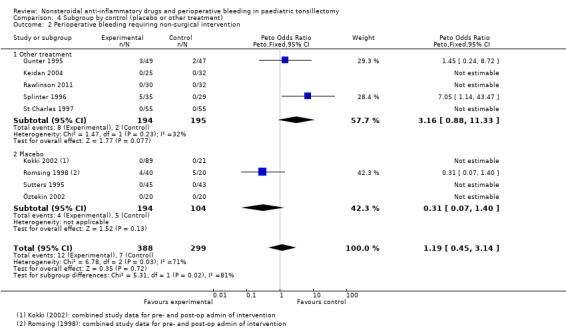
Comparison 4 Subgroup by control (placebo or other treatment), Outcome 2 Perioperative bleeding requiring non‐surgical intervention.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Antila 2006.
| Methods | Randomized, parallel group trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | The study had three groups (placebo, ketoprofen and tramadol). Placebo and tramadol group were combined and compared with ketoprofen group for data analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Baseline Imbalances All outcomes | Unclear risk | Although baseline imbalances appear comparable in Table 1, there are no details on types of surgery and whether this is balanced between groups |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Low risk | Nurse, not otherwise participating in treatment of patient prepared the coded study treatment syringes and infusions. Assumed coded means not labelled with contents, i.e. double blind. Assumed surgeon unaware of group allocation |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Unclear risk | N/a |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Low risk | Nurse, not otherwise participating in treatment of patient prepared the coded study treatment syringes and infusions. Assumed coded means not labelled with contents, i.e. double blind. Assumed surgeon unaware of group allocation |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Assumed surgeon unaware of allocation but assessment of bleeding may have been made on the ward by nurses ‐ unclear if ward nurses are blinded. Not clear who decided return to theatre is required |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Unclear risk | N/a |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Unclear risk | Unclear who measured this and whether they were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias All outcomes | High risk | Ketoprofen group had less fentanyl than tramadol group which could affect vomiting results |
Gunter 1995.
| Methods | Randomized, parallel group trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | No analgesia given in theatre. Paracetamol 20 mg/kg given in recovery to all patients. Morphine 0.05 mg/kg twice in recovery as rescue analgesia. One withdrawal from the morphine group due to an intraoperative arterial bleed. ITT analysis not performed as no details available on patient outcome. Study terminated early due to an increased incidence of major bleeding in ketorolac group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Baseline Imbalances All outcomes | Low risk | Type of surgery similar in both groups |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Low risk | Subjects, families, anaesthesiologists, nurses and investigators were blinded to study drug assignment |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Low risk | Subjects, families, anaesthesiologists, nurses and investigators were blinded to study drug assignment |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Low risk | Subjects, families, anaesthesiologists, nurses and investigators were blinded to study drug assignment |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Low risk | Subjects, families, anaesthesiologists, nurses and investigators were blinded to study drug assignment |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Low risk | Subjects, families, anaesthesiologists, nurses and investigators were blinded to study drug assignment |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Low risk | Subjects, families, anaesthesiologists, nurses and investigators were blinded to study drug assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Study terminated at 97 patients after interim analysis revealed excessive bleeding in the study group. One exclusion not included in the data as no outcome details available |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias All outcomes | Low risk | Some patients received extra morphine ‐ but two doses more common in ketorolac group. Investigators state that no difference in emesis rates for those who received more |
Harley 1998.
| Methods | Randomized, parallel group trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Dose of paracetamol was low | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization code |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Baseline Imbalances All outcomes | Unclear risk | No breakdown of how many surgical interventions per group. Also no details of demographic data available |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Otolaryngology, operating‐room and recovery room staff members all blinded to randomization codes. Not clear if parents were blinded. Intervention occurred after discharge, variable lengths of drug administration, reliability of parents recording data |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Unclear risk | N/a |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Unclear risk | N/a |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Not clear who assessed need for return to OR or presentation to hospital. Not clear if parents were blinded ‐ their knowledge of intervention may have influenced return to hospital |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Unclear risk | N/a |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Unclear risk | N/a |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss to follow‐up on three patients (10%) ‐ not clear if these were lost before or after randomization. Also no statistical data for some patients ‐ "As a result of factors such as follow‐up compliance not all patients were available for each statistical comparison" |
| Selective reporting (reporting bias) | Low risk | Nausea and vomiting data probably not collected |
| Other bias All outcomes | Low risk | No rescue analgesics described, assumed not given |
Keidan 2004.
| Methods | Randomized, parallel group trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Received by random assignment. No further details given |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Baseline Imbalances All outcomes | Low risk | All had same surgical procedures. More boys in Ketorolac group and more history of motion sickness |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Unknown if surgeon or anaesthetist blinded |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Unclear risk | Unknown if surgeon or anaesthetist blinded |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Unclear risk | Unknown if surgeon or anaesthetist blinded |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Unclear who is assessing this outcome |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Unclear risk | Unknown if surgeon or anaesthetist blinded |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Unclear risk | Unknown if surgeon or anaesthetist blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three patients excluded from analysis due to lack of data (no further explanation given) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias All outcomes | High risk | Extra fentanyl given as rescue analgesic, levels not given, could affect results for vomiting |
Kokki 2002.
| Methods | Randomized, parallel group trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study had two ketoprofen groups. Have included both the pre‐ and post‐ketoprofen groups to the analysis against the divided control group. Placebo control group is small, not explained by study authors, could be due to poor randomization or allocation concealment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | Prescription in an envelope but no further details given |
| Baseline Imbalances All outcomes | Low risk | Demographic data all similar. All tonsillectomies. However, small placebo group |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Low risk | Drug syringes prepared by nurses not otherwise involved in study |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Low risk | Drug syringes prepared by nurses not otherwise involved in study |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Low risk | Drug syringes prepared by nurses not otherwise involved in study |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Low risk | Assume nurses assessing outcomes were blinded |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Low risk | Assume nurses assessing outcomes were blinded |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Low risk | Assume nurses assessing outcomes were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient excluded but e‐mail contact with authors confirm outcomes and so included in order to follow ITT principle |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias All outcomes | High risk | Oxycodone higher in placebo group and this not allowed for in analyses |
Pickering 2002.
| Methods | Randomized, parallel group trial | |
| Participants |
adenotonsillectomy
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study had three groups. Data from the ibuprofen and placebo groups used in the review. Recruitment to placebo group finished early due to increased need for analgesics in this group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number table |
| Allocation concealment (selection bias) | Unclear risk | Hospital pharmacist allocated children randomly to groups. But no further details on this |
| Baseline Imbalances All outcomes | Low risk | Demographic details comparable and no large differences in surgery types between groups. However, recruitment to placebo group finished early |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Patients and investigators blinded to group assignment. No mention of whether surgeon, anaesthetist or nurses blinded |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Unclear risk | Patients and investigators blinded to group assignment. No mention of whether surgeon, anaesthetist or nurses blinded |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Unclear risk | Patients and investigators blinded to group assignment. No mention of whether surgeon, anaesthetist or nurses blinded |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Not clear who "investigators" are and whether they are assessing outcomes or not |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Unclear risk | Not clear who "investigators" are and whether they are assessing outcomes or not |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Unclear risk | Not clear who "investigators" are and whether they are assessing outcomes or not |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three exclusions: one patient from placebo group given diclofenac during surgery; two patients from ibuprofen group ‐ one given diclofenac during surgery and one was too young. Small number of missing patients (5%) unlikely to affect results |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias All outcomes | High risk | Rescue analgesics used in both groups is same as intervention drug |
Rawlinson 2011.
| Methods | Randomized, parallel group trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random list |
| Allocation concealment (selection bias) | Unclear risk | Numbered envelopes. Does not give further details |
| Baseline Imbalances All outcomes | Low risk | Similar number of tonsillectomies and adenotonsillectomies |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Not clear if surgical team/nursing staff were blinded |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Unclear risk | Not clear if surgical team/nursing staff were blinded |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Unclear risk | Not clear if surgical team/nursing staff were blinded |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Low risk | Investigator blinded to allocation made all postoperative observations |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Low risk | Investigator blinded to allocation made all postoperative observations |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Low risk | Investigator blinded to allocation made all postoperative observations |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three missing patients: two due to protocol violations and one due to cancellation of surgery. Not clear if randomized. Small number of missing patients (<10%) unlikely to affect results |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Other bias All outcomes | High risk | Codeine group received ibuprofen postoperatively, Ibuprofen group received fentanyl |
Romsing 1998.
| Methods | Randomized, parallel group trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Pain was primary outcome. Study stopped after first 15 children because of worries about a surgeon causing excess bleeding. Data used in 2012 update includes this surgeon. Also, included data from patient who had been excluded from the authors' results due to bleeding requiring immediate reoperation. Have included both the pre‐ and post‐ketoprofen groups to the analysis against the divided control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | No details given as to how allocation was concealed |
| Baseline Imbalances All outcomes | Low risk | Distribution of tonsillectomies and adenotonsillectomies appear equivalent. Other demographic data also comparable |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Low risk | All patients, parents, personnel involved in patient management and data collection were unaware of drug group assignment |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Low risk | All patients, parents, personnel involved in patient management and data collection were unaware of drug group assignment |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Low risk | All patients, parents, personnel involved in patient management and data collection were unaware of drug group assignment |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Low risk | All patients, parents, personnel involved in patient management and data collection were unaware of drug group assignment |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Low risk | All patients, parents, personnel involved in patient management and data collection were unaware of drug group assignment |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One patient excluded from Pre‐ group as given a different anaesthetic. Two patients excluded from Post‐ group: one given diclofenac; one needed re‐operation due to bleeding. We included the patient who required bleeding in the results |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias All outcomes | High risk | Distribution groups of excluded surgeon not given. More fentanyl given in post‐ketaprofen and placebo group |
Rusy 1995.
| Methods | Randomized, parallel group trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear how randomization was performed |
| Allocation concealment (selection bias) | Unclear risk | Not enough detail in paper |
| Baseline Imbalances All outcomes | Unclear risk | No distribution given for tonsillectomies and adenotonsillectomies. Not data for other demographics |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Assume that patients is blinded but no details of whether surgeon and other personnel blinded |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Unclear risk | Assume that patients is blinded but no details of whether surgeon and other personnel blinded |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Unclear risk | N/a |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Only reports that investigator responsible for bleeding times is blinded. Unclear if other personnel are blinded |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Unclear risk | Only reports that investigator responsible for bleeding times is blinded. Unclear if other personnel are blinded |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Unclear risk | N/a |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data complete |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias All outcomes | Low risk | More acetaminophen used in comparison group, however vomiting not measured in this study, so not likely to affect results |
Splinter 1996.
| Methods | Randomized, parallel group trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study designed to look at nausea and vomiting. Study terminated early at 64 patients because of excessive bleeding during interim analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Baseline Imbalances All outcomes | Unclear risk | No demographic data given, nor balance of surgical groups |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Assumed anaesthetist not blinded. No details whether surgeon blinded |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Unclear risk | Assumed anaesthetist not blinded. No details whether surgeon blinded |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Unclear risk | Assumed anaesthetist not blinded. No details whether surgeon blinded |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Not clear who assessed bleeding |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Unclear risk | Not clear who assessed bleeding |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Low risk | Assumed nurses and parents assessing vomiting were blind to intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses reported |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias All outcomes | Unclear risk | Distribution of rescue analgesics and anti‐emetics between groups not described ‐ these were given during observation period and would affect vomiting |
St Charles 1997.
| Methods | Randomized, parallel group trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Surgical technique included electro‐dissection or cold dissection and snare techniques. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly assigned to one of two groups. No details given |
| Allocation concealment (selection bias) | Unclear risk | No details given. |
| Baseline Imbalances All outcomes | Low risk | Lots of surgical techniques but balanced. Demographic data clearly reported and largely equivalent |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | High risk | Assumed no‐one blinded |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | High risk | Assumed no‐one blinded |
| Blinding of participants and personnel (performance bias) 3. Vomiting | High risk | Assumed no‐one blinded |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | High risk | Inconsistencies in how data collected (some retrospectively from charts). No blinding. Not clear how bleeding detected. Parents in intervention group given free samples to take home |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | High risk | Inconsistencies in how data collected (some retrospectively from charts). No blinding. Not clear how bleeding detected. Parents in intervention group given free samples to take home |
| Blinding of outcome assessment (detection bias) 3. Vomiting | High risk | Nurses and parents not blinded ‐ responsible for reporting and recording any signs of PONV |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No incomplete data |
| Selective reporting (reporting bias) | Low risk | All expected outcome measures reported |
| Other bias All outcomes | High risk | Extra analgesics given in OR and during observation period. May affect vomiting outcomes. Inconsistencies mean that treatment was barely standardized |
Sutherland 1998.
| Methods | Randomized, parallel group trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Study originally designed to look at pain. One withdrawal from morphine group included in our analysis so an ITT analysis could be performed. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomized but no further details given |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes used but no further details given |
| Baseline Imbalances All outcomes | Unclear risk | Demographic data comparable. But no details given of surgery type |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Unclear if surgeon blinded |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Unclear risk | N/a |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Low risk | Nursing staff blinded |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Unclear who is assessing patients |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Unclear risk | N/a |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Low risk | Nurses assessing patients were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for excluded child included in order to follow ITT principle |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias All outcomes | High risk | Tenoxicam group required more morphine. Could affect vomiting results |
Sutters 1995.
| Methods | Randomized, parallel group trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Fentanyl iv given in repeated doses 0.5 µ/kg in recovery. Child from control group excluded by authors due to postoperative oedema but included so an ITT analysis could be performed following e‐mail confirmation from authors of no bleeding outcomes. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details given |
| Allocation concealment (selection bias) | Unclear risk | Group assignment was sealed in a manila envelope. No further details |
| Baseline Imbalances All outcomes | Low risk | Well documented and no differences in demographic or surgery type |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Low risk | Anaesthetist aware of group allocation. But drug given after surgery. Not clear whether surgeon knew allocation but operations similar between groups |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Low risk | Anaesthetist aware of group allocation. But drug given after surgery. Not clear whether surgeon knew allocation but operations similar between groups |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Low risk | Anaesthetist aware of group allocation. But drug given after surgery. Not clear whether surgeon knew allocation but operations similar between groups |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Low risk | Identity of injection not revealed to PACU nurse, the parents, or to investigator who performed clinical assessments. Not clear who did assessment of bleeding in PACU/DSA ‐ but probably nurses or investigator |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Low risk | Identity of injection not revealed to PACU nurse, the parents, or to investigator who performed clinical assessments. Not clear who did assessment of bleeding in PACU/DSA ‐ but probably nurses or investigator |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Low risk | Identity of injection not revealed to PACU nurse, the parents, or to investigator who performed clinical assessments. Not clear who did assessment of bleeding in PACU/DSA ‐ but probably nurses or investigator |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One exclusion because child had severe postop oedema. This patient was included in order to follow the ITT principle following e‐mail contact with study author |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias All outcomes | High risk | Higher use of fentanyl in ketorolac group. Could affect vomiting results |
Thiagarajan 1993.
| Methods | Randomized, parallel group trial | |
| Participants |
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | Papaveretum now not commonly used for children. Intramuscular analgesia now considered inappropriate for children. 15 withdrawals mentioned in paper, unable to get in touch with author to account for these. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Trial numbers were randomized in blocks |
| Allocation concealment (selection bias) | Low risk | Randomization code was held by the hospital pharmacy |
| Baseline Imbalances All outcomes | Low risk | Report on different surgical techniques but do not give information on male/female ratios |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Low risk | Syringes, identified only by the patient's name were prepared by the pharmacy on day of operation. Therefore assumed all personnel blinded |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Low risk | Syringes, identified only by the patient's name were prepared by the pharmacy on day of operation. Therefore assumed all personnel blinded |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Low risk | Syringes, identified only by the patient's name were prepared by the pharmacy on day of operation. Therefore assumed all personnel blinded |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Low risk | Syringes, identified only by the patient's name were prepared by the pharmacy on day of operation. Therefore assumed all personnel blinded |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Low risk | Syringes, identified only be the patient's name were prepared by the pharmacy on day of operation. Therefore assumed all personnel blinded |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Low risk | Syringes, identified only be the patient's name were prepared by the pharmacy on day of operation. Therefore assumed all personnel blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Fifteen exclusions; five from diclofenac group and four from papaveretum group not given drugs because anaesthetist unaware of their participation in trial; one patient had obstructive sleep apnoea; five records lost or incomplete. Unable to account for withdrawals as unable to get in touch with the author |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias All outcomes | High risk | Papaveretum given to more patients in intervention group. May affect vomiting results |
Öztekin 2002.
| Methods | Randomized, parallel group trial | |
| Participants |
adenoidectomy or myringotomy
|
|
| Interventions |
|
|
| Outcomes |
|
|
| Notes | No placebo suppository given. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not clear how randomization was performed |
| Allocation concealment (selection bias) | Unclear risk | No details given |
| Baseline Imbalances All outcomes | Low risk | All demographic details given |
| Blinding of participants and personnel (performance bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Not clear if surgeons and staff on wards were blinded |
| Blinding of participants and personnel (performance bias) 2.Perioperative bleeding requiring non‐surgical intervention | Unclear risk | Not clear if surgeons and staff on wards were blinded |
| Blinding of participants and personnel (performance bias) 3. Vomiting | Unclear risk | Not clear if surgeons and staff on wards were blinded |
| Blinding of outcome assessment (detection bias) 1. Perioperative bleeding requiring surgical intervention | Unclear risk | Parents and patients blinded. Aware that nurse measuring pain is blinded but no mention of other nurses being blinded |
| Blinding of outcome assessment (detection bias) 2. Perioperative bleeding requiring non‐surgical intervention | Unclear risk | Parents and patients blinded. Aware that nurse measuring pain is blinded but no mention of other nurses being blinded |
| Blinding of outcome assessment (detection bias) 3. Vomiting | Unclear risk | Parents and patients blinded. Aware that nurse measuring pain is blinded but no mention of other nurses being blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data complete |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported |
| Other bias All outcomes | High risk | Placebo group used more rescue morphine, both in PACU and ward, could affect vomiting results |
ASA = American Society of Anesthesiologists; DSA = Day surgery area; ITT = Intention‐to‐treat; iv = Intravenously; M/F = Male/female; N/a = Not applicable; OR = Operating room; PACU = Postanaesthesia care unit; PCA ‐ Patient‐controlled analgesia; PONV = Postoperative nausea and vomiting; VAS = Visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agrawal 1999 | Retrospective study |
| Aho 2003 | Adult participants |
| Azuma 1982 | Not a randomized controlled study |
| Bailey 1997 | Adult participants |
| Bhattacharya 2005 | Does not look at bleeding |
| Bhattacharya 2009 | No control group |
| Bone 1988 | Does not look at bleeding |
| Calvet 1969 | An uncontrolled study |
| Courtney 2001 | A mixture of adult and paediatric participants. We obtained the original data from the authors to separate the two types of participants but it was not possible to determine exactly who had experienced bleeding. |
| De Lima Pontes 1985 | Bleeding not assessed |
| Dommerby 1984 | A mixture of adult and paediatric data. We wrote to the authors to try to separate the data but did not receive a reply |
| Elshammaa 2011 | NSAIDs not studied |
| Fassolt 1974 | Adult participants |
| Francois 1994 | Bleeding not assessed |
| Gallagher 1995 | Retrospective study |
| Geva 2011 | NSAIDs not studied |
| Gonchar 1974 | Not a randomized controlled trial |
| Hardy 2010 | Bleeding not studied |
| Heaney 2007 | No control group |
| Hiller 2004 | Adult participants |
| Ismail 2010 | Adult participants |
| Jeyakumar 2008 | Retrospective study |
| Judkins 1996 | Retrospective study |
| Kedek 2005 | No detail on bleeding in paper. We emailed the author but did not receive a reply |
| Knudsen 1995 | Adult participants |
| Kristensen 1986 | Adult participants. No control group |
| Lacomme 1978 | Bleeding not assessed |
| Lee 1996 | Postoperative survey |
| Lindgren 1985 | A second drug was included with trial drug, therefore no proper control |
| Louizos 2006 | Adult participants |
| Mahadevan 1995 | Audit |
| Martinez‐Gallardo 1997 | Adult participants |
| Mather 1995 | Blood loss not studied |
| McKean 2008 | Adult participants |
| Mendham 1996 | No control group |
| Nakayama 1982 | Only one study group |
| Nikanne 2005 | Adult participants |
| Nordbladh 1991 | Adult participants |
| Parker 1986 | Adult participants |
| Pestieau 2011 | NSAIDs not studied |
| Petrusen 1991 | Mainly adult participants with a few older children. We were unable to separate out the paediatric and adult data |
| Platzer 2011 | Majority of adenoidectomy patients. Bleeding not assessed |
| Purday 1995 | Re‐analysis of a retrospective study |
| Robb 1995 | Re‐analysis of a retrospective study |
| Robinson 1994 | Retrospective study |
| Romsing 2000 | All participants received diclofenac during the surgery. Randomization to either paracetamol or diclofenac occurred on the first day postoperatively |
| Rorarius 1993 | Adult participants |
| Salonen 2001 | Adult participants |
| Salonen 2002 | All patients received ketoprofen. No control group |
| Schmidt 2001 | Unable to separate adult and paediatric data |
| Smith 1999 | Retrospective study |
| Stage 1988 | Studied aspirin, which is no longer used in children |
| Stewart 2012 | Not an RCT |
| Sun 2009 | Bleeding not assessed |
| Swanepoel 1999 | Did not compare a nonsteroidal anti‐inflammatory drug with a control. Both groups took diclofenac: one orally and one rectally |
| Tamura 1972 | Bleeding not assessed |
| Tawalbeh 2001 | Not an RCT |
| Virtaniemi 1999 | Adult patients |
| Watters 1988 | Bleeding not assessed |
| Yaman 2011 | Retrospective study |
| Zhang ‐ AFT | Ongoing trial. Not tonsillectomy |
| Özkiris 2012 | Not an RCT |
NSAIDs = Nonsteroidal anti‐inflammatory drugs; RCT = Randomized controlled trial.
Characteristics of ongoing studies [ordered by study ID]
Hartnick.
| Trial name or title | Postoperative Ibuprofen and the Risk of Bleeding After Tonsillectomy with or without Adenoidectomy |
| Methods | Randomized, double‐blinded interventional study |
| Participants | Children aged 2 ‐18 yrs undergoing tonsillectomy with or without adenoidectomy |
| Interventions | Ibuprofen vs acetaminophen |
| Outcomes | Primary outcome level 3 postoperative haemorrhage |
| Starting date | May 2012 |
| Contact information | Christopher Hartnick, Massachusetts Eye and Ear Infirmary. NCT01605903 |
| Notes | Estimated enrolment of 1066 patients |
KITS Study.
| Trial name or title | KITS Study ‐ Ketorolac in Tonsillectomy Surgery: a Double Blinded, Randomized Clinical Trial |
| Methods | Double‐blinded, randomized clinical trial |
| Participants | Children aged 3 ‐ 15 yrs scheduled for tonsillectomy for sleep disordered breathing |
| Interventions | Ketorolac vs saline |
| Outcomes | Primary outcome: pain |
| Starting date | December 2011 |
| Contact information | Ban Tsui, University of Alberta. NCT01498796 |
| Notes |
Wheeler.
| Trial name or title | Safety and Efficacy of Intravenous Ibuprofen for Treatment of Pain in Pediatric Patients undergoing Tonsillectomy |
| Methods | Multi‐centre, randomized, double‐blind, placebo controlled |
| Participants | Children 6 ‐ 17 yrs old scheduled to undergo tonsillectomy |
| Interventions | Intravenous ibuprofen versus saline |
| Outcomes |
|
| Starting date | April 2011 |
| Contact information | Art P Wheeler, Cumberland Pharmaceuticals Inc. NCT01332253 |
| Notes | Study has been completed as of September 2012. Not yet published |
Differences between protocol and review
In our protocol, we stated that if there were enough data we would create subgroups for individual drugs. After our search, we found that we had inadequate numbers of papers to do this for all the NSAIDs. However, we were able to do this for ketorolac.
In the 2012 update we renamed the original outcomes. In particular the second outcome was changed from 'bleeding not requiring surgical intervention' to 'perioperative bleeding requiring non‐surgical intervention'. Although we considered the same types of examples given in the original protocol (intravenous fluid therapy, prolonged observation and non‐surgical haemostasis), this change in the wording improved clarity for data extraction purposes. We also changed the third outcome to only include reported data on vomiting and added the time to event as within 24 hours.
Contributions of authors
Updated review
Sharon R Lewis (SL), Amanda Nicholson (AN), Mary E Cardwell (MC), Gretchen Siviter (GS), Andrew F Smith (AFS)
Conceiving the original review: MC, GS
Designing the original review: MC, Dr G Siviter, AFS
Co‐ordinating the 2012 update: SL
Undertaking manual searches: SL, AN
Screening search results: SL, MC, AFS
Organizing retrieval of papers: SL
Screening retrieved papers against inclusion criteria: MC, SL
Appraising quality of papers: SL, AN
Extracting data from papers: SL, AN
Writing to authors of papers for additional information: SL
Providing additional data about papers: MC
Obtaining and screening data on unpublished studies: SL, AN
Data management for the review: AN, SL
Entering data into Review Manager (RevMan 5.2): SL, AN
RevMan statistical data: AN, SL
Double entry of data: (data entered by person one: SL; data entered by person two: AN)
Interpretation of data: AN, SL, AFS
Statistical inferences: AN, SL, AFS
Writing the review: SL, AN
Providing guidance on the review: AFS
Securing funding for the review: AFS
Guarantor for the review (one author): AFS
Person responsible for reading and checking review before submission: AN
Original review (Cardwell 2005)
GS and MC conceived the idea for the review and wrote the protocol. AFS helped to design the protocol.
SL and AN wrote the review update with advice and editing from AFS and MC.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute of Health Research (NIHR), UK.
This review forms part of the NIHR Cochrane Collaboration Programme Grant 10/40001/04 entitled Enhancing the safety, quality and productivity of perioperative care
Declarations of interest
Sharon R Lewis: see Sources of support
Amanda Nicholson: AN worked for the Cardiff Research Consortium, which provided research and consultancy services to the pharmaceutical industry, from March to Septmber 2011. Cardiff Research Consortium has no connection with AN's work with The Cochrane Collaboration. AN's husband has small direct holdings in several drug and biotech companies as part of a wider balanced share portfolio
Mary E Cardwell: none known
Gretchen Siviter: none known
Andrew F Smith: see Sources of support
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Antila 2006 {published data only}
- Antila H, Manner T, Kuurila K, Salantera S, Kujala R, Aantaa R. Ketoprofen and tramadol for analgesia during early recovery after tonsillectomy in children. Paediatric Anaesthesia 2006;16:548‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gunter 1995 {published data only}
- Gunter JB, Varughese AM, Harrington JF, Wittkugel EP, Patankar SS, Matar MM, et al. Recovery and complications after tonsillectomy in children: a comparison of ketorolac and morphine. Anesthesia and Analgesia 1995;81(6):1136‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harley 1998 {published data only}
- Harley EH, Dattolo RA. Ibuprofen for tonsillectomy pain in children: Efficacy and complications. Otolaryngology ‐ Head and Neck Surgery 1998;119(5):492‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Keidan 2004 {published data only}
- Keidan I, Zaslansky R, Eviatar E, Segal S, Sarfaty SM. Intraoperative ketorolac is an effective substitute for fentanyl in children undergoing outpatient adenotonsillectomy. Paediatric Anaesthesia 2004;14:318‐23. [EMBASE: 2004187894] [DOI] [PubMed] [Google Scholar]
Kokki 2002 {published and unpublished data}
- Kooki H, Salonen A. Comparison of pre‐and postoperative administration of ketoprofen for analgesia after tonsillectomy in children. Paediatric Anaesthesia 2002;12:162‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Öztekin 2002 {published data only}
- Oztekin S, Hepaguslar H, Kar AA, Ozzeybek D, Artikaslan O, Elar Z. Preemptive diclofenac reduces morphine use after remifentanil‐based anaesthesia for tonsillectomy. Paediatric Anaesthesia 2002;12(8):694‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pickering 2002 {published data only}
- Pickering AE, Bridge HS, Nolan J, Stoddart PA. Double‐blind placebo controlled analgesic study of ibuprofen or rofecoxib in combination with paracetamol for tonsillectomy in children. British Journal of Anaesthesia 2002;88(1):72‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rawlinson 2011 {published data only}
- Rawlinson E, Walker A, Skone R, Thillaivasan A, Bagshaw O. A randomised controlled trial of two analgesic techniques for paediatric tonsillectomy*. Anaesthesia 2011;66(10):919‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Romsing 1998 {published data only}
- Romsing J, Ostergaard S, Walther‐Larsen, Valentin N. Analgesic efficacy and safety of preoperative versus postoperative ketorolac in paediatric tonsillectomy. Acta Anaesthesiologica Scandinavica 1998;42:770‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rusy 1995 {published data only}
- Rusy LM, Houck CS, Sullivan LJ, Ohlms LA, Jones DT, McGill TJ, et al. A double blind evaluation of ketorolac tromethamine versus acetaminophen in paediatric tonsillectomy: analgesia and bleeding. Anesthesia and Analgesia 1995;80:226‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Splinter 1996 {published data only (unpublished sought but not used)}
- Splinter WM, Rhine EJ, Roberts DW, Reid CW, MacNeil HB. Preoperative ketorolac increases bleeding after tonsillectomy in children. Canadian Journal of Anaesthesia 1996;43(6):560‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
St Charles 1997 {published data only}
- Charles CS, Matt BH, Hamilton MM, Katz BP. A comparison of ibuprofen versus acetaminophen with codeine in the young tonsillectomy patient. Otolaryngology ‐ Head and Neck Surgery 1997;117:76‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sutherland 1998 {published data only}
- Sutherland CJ, Montgomery JE, Kestin IG. A comparison of intramuscular tenoxicam with intramuscular morphine for pain relief following tonsillectomy in children. Paediatric Anaesthesia 1998;8(4):321‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sutters 1995 {published and unpublished data}
- Sutters KA, Levine JD, Dibble S, Savedra M, Miaskowski C. Analgesic efficacy and safety of single‐dose intramuscular ketorolac for postoperative pain management in children following tonsillectomy. Pain 1995;61(1):145‐53. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thiagarajan 1993 {published data only}
- Thiagarajan J, Bates, Hitchcock M, Morgan‐Hughes J. Blood loss following tonsillectomy in children. A blind comparison of diclofenac and papaveretum. Anaesthesia 1993;47:132‐5. [MEDLINE: ] [PubMed] [Google Scholar]
References to studies excluded from this review
Agrawal 1999 {published data only}
- Agrawal A, Gerson CR, Seligman I, Dsida RM. Postoperative haemorrhage after tonsillectomy: use of ketorolac tromethamine. Otolaryngology ‐ Head and Neck Surgery 1999;120(3):335‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Aho 2003 {published data only}
- Aho M, Kokki H, Nikanne E. Nimesulide versus ibuprofen for postoperative tonsillectomy pain. Clinical Drug Investigation 2003;23(10):651‐60. [EMBASE: 2003424478] [DOI] [PubMed] [Google Scholar]
Azuma 1982 {published data only}
- Azuma F. The clinical efficacy and plasma concentration of a 25 mg suppository of diclofenac sodium in children with post‐tonsillectomy pain and inflammation. Practica Otologica 1982;75(6):1445‐53. [EMBASE: 1982200723] [Google Scholar]
Bailey 1997 {published data only}
- Bailey R, Sinha C, Burgess LP. Ketorolac tromethamine and haemorrhage in tonsillectomy: A prospective, randomized, double‐blind study. The Laryngoscope 1997;107(2):166‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bhattacharya 2005 {published data only}
- Bhattachcharya D, Mohan CM, Dasgupta S, Chakraborty R. A comparison of rectal diclofenac with intravenous pethidine for pain relief following tonsillectomy and adenoidectomy in children. Journal of Anaesthesiology Clinical Pharmacology 2005;21(2):143‐6. [EMBASE: 2007207227] [Google Scholar]
Bhattacharya 2009 {published data only}
- Bhattacharya D, Mazumdar S, Chowdhury S, Basu S, Saha S. Single dose IV dexamethasone with preemptive transdermal diclofenac patch reduces opioid requirement and postoperative morbidity following tonsillectomy. Journal of Anaesthesiology Clinical Pharmacology 2009;25(1):29‐32. [Google Scholar]
Bone 1988 {published data only}
- Bone ME, Fell D. A comparison of rectal diclofenac with intramuscular papaveretum placebo for pain relief following tonsillectomy. Anaesthesia 1998;43:277‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Calvet 1969 {published data only}
- Calvet J, Torossian F. Use of phenylbutazone combined with deglycerated liquorice juice in postoperative care following tonsillectomy and adenotonsillectomy [Sull'uso del fenilbutazone associato al liquirizia deglicirrizzinizzato nel trattamento dei tonsillectomizzati e degli adenoidectomizzati]. La Clinica Otorinolaringoiatrica 1969;21(5):319‐25. [MEDLINE: ] [PubMed] [Google Scholar]
Courtney 2001 {published data only}
- Courtney MJ, Cabraal D. Tramadol vs diclofenac for post tonsillectomy analgesia. Archives of Otolaryngology ‐ Head and Neck Surgery 2001;127:385‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
De Lima Pontes 1985 {published data only}
- Lima Pontes PA, Gregorio LC. Double‐blind clinical trial: naproxen sodium suspension vs placebo in post‐operative adeno‐tonsillectomy in children [Ensaio clinico duplo‐cego de naproxen sodico suspensao vs placebo no pos‐operatorio de adenoamigdalectomias em criancas]. Revista Brasileira de Medicina 1985;42(3):57‐61. [EMBASE: 1985185817] [Google Scholar]
Dommerby 1984 {published data only}
- Dommerby H, Rasmussen OR. Diclofenac (Voltaren). Pain‐relieving effect after tonsillectomy. Acta Otolaryngology 1984;98:185‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Elshammaa 2011 {published data only}
- Elshammaa N, Chidambaran V, Housny W, Thomas J, Zhang X, Michael R. Ketamine as an adjunct to fentanyl improves postoperative analgesia and hastens discharge in children following tonsillectomy ‐ a prospective, double‐blinded, randomized study. Paediatric Anaesthesia. 2011/05/18 2011; Vol. 21, issue 10:1009‐14. [MEDLINE: ] [DOI] [PubMed]
Fassolt 1974 {published data only}
- Fassolt A. The control of pain after tonsillectomy with mefenamic acid [Mefenaminsaure zur kontrolle des wundschmerzes nach tonsillektomie]. Schweizerische Rundschau Fur Medizin Praxis 1974;63(34):1040‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Francois 1994 {published data only}
- Francois M, Consten L, Rezvani Y, Gaudemar I, Aboucaya J‐P, Florant A, Narcy P. The efficacy and tolerance of tiaprofenic acid versus paracetamol after tonsillectomy in children [Etude comparative de l'efficacite et de la tolerance de l'acide tiaprofenique et du paracetamol dans les suites d'amygdalectomie chez l'enfant]. Journal Francais d'Oto‐Rhino‐Laryngologie 1994;43(6):437‐40. [EMBASE: 1995001032] [Google Scholar]
Gallagher 1995 {published data only}
- Gallagher JE, Blauth J, Fornadley JA. Perioperative ketorolac tromethamine and postoperative hemorrhage in cases of tonsillectomy and adenoidectomy. The Laryngoscope 1995;105(6):606‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Geva 2011 {published data only}
Gonchar 1974 {published data only}
- Gonchar D I. Nitrous oxide analgesia in combination with seduxen, analgin and fentanyl in tonsillectomy [Anal'geziia zakis'iu azota v sochetanii s seduksenom, anal'ginom i fentanilom pri tonzillektomii]. Vestnik Otorinolaringologii 1974;0(4):70‐1. [MEDLINE: ] [PubMed] [Google Scholar]
Hardy 2010 {published data only}
- Hardy M.Z.R, Zayuah M.S, Baharudin A, Wan Aasim W.A, Shamsul K.H, Hashimah I, et al. The Effects of topical viscous lignocaine 2% versus per‐rectal diclofenac in early post‐tonsillectomy pain in children. International Journal of Pediatric Otorhinolaryngology 2010;74(4):374‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Heaney 2007 {published data only}
- Heaney M, Looney Y, Mckinstry C, O'Hare B. Sequential clot strength analyses following diclofenac in pediatric adenotonsillecomy. Paediatric Anaesthesia 2007;17(11):1078‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hiller 2004 {published data only}
- Hiller A, Silvanto M, Savolainen S, Tarkkila P. Proparacetamol and diclofenac alone and in combination for analgesia after elective tonsillectomy. Acta Anaesthesiologica Scandinavica 2004;48(9):1185‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ismail 2010 {published data only}
Jeyakumar 2008 {published data only}
- Jeyaumar A, Brickman TM, Williamson ME, Hirose K, Krakovitz P, Whittemore K, et al. Nonsteroidal anti‐inflammatory drugs and postoperative bleeding following adenotonsillecomy in pediatric patients. Archives of Otolaryngology ‐ Head and Neck Surgery 2008;134(1):24‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Judkins 1996 {published data only}
- Judkins JH, Dray TG, Hubbell RN. Intraoperative ketorolac and post tonsillectomy bleeding. Archives of Otolaryngology ‐ Head and Neck Surgery 1996 September;122(9):937‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kedek 2005 {published data only}
- Kedek A, Derbent A, Uyar M, Bilgen C, Uyar M, Kirazli T, et al. Pre‐emptive effects of ibuprofen syrup and lidocaine infiltration on post‐operative analgesia in children undergoing tonsillectomy. The Journal of International Medical Research 2005;33:188‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Knudsen 1995 {published data only}
- Knudsen KE, Brofeldt S, Mikkelsen S, Bille M, Brennum J, Dahl JB. Peritonsillar infiltration with low‐dose tenoxicam after tonsillectomy. British Journal of Anaesthesia 1995;75:286‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kristensen 1986 {published data only}
- Kristensen S, Tveteras K, Outzen KE, Poulsen HB. Treatment of pain after tonsillectomy. Comparison between naproxen and acetylsalicylic acid [Smertebehandling efter tonsillektomi. En sammenlignende undersogelse af naproxen ( Naprosyn) og acetylsalicylsyre (Kalcatyl)]. Ugeskrift for Laeger 1986;148(44):2832‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Lacomme 1978 {published data only}
- Lancomme Y, Pessey J‐J. Effects of a niflumic acid derivative in tonsil surgery in children ‐ controlled study [Effet d'un derive de l'acide influmique dans la chiruriqie amygdalienne chez l'enfant ‐essai controle]. Revue de Laryngologie ‐ Otologie ‐ Rhinologie 1978;99(1‐2):159‐63. [MEDLINE: ] [PubMed] [Google Scholar]
Lee 1996 {published data only}
- Lee WC, Sharpe JF. Complications of paediatric tonsillectomy post‐discharge. Journal of Laryngology and Otology 1996;110(2):136‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lindgren 1985 {published data only}
- Lindgren L, Saarnivaara L. Comparison of paracetamol and aminophenazone plus diazepam suppositories for anxiety and pain relief after tonsillectomy in children. Acta Anaesthesiologica Scandinavica 1985;29:679‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Louizos 2006 {published data only}
- Louizos AA, Pandazi AB, Koraka CP, Davilis DI, Georgiou LG. Preoperative administration of rofecoxib versus ketoprofen for pain relief after tonsillectomy. Annals of Otology, Rhinology and Laryngology 2006;115(3):201‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mahadevan 1995 {published data only}
- Mahadevan M, Bartley J. Paediatric day case tonsillectomy: Early green lane experience. Australian Journal of Otolaryngology 1995;2(2):150‐2. [EMBASE: 1995328062] [Google Scholar]
Martinez‐Gallardo 1997 {published data only}
- Martinez‐Gallardo F. Post tonsillectomy analgesia with diclofenac sodium [Analgesia postamigdalectomia con diclofenac sodico]. Prensa Medica Mexicana 1977;42(5‐6):264‐6. [EMBASE: 1978246256] [Google Scholar]
Mather 1995 {published data only}
- Mather ST, Peutrell JM. Postoperative morphine requirements, nausea and vomiting following anaesthesia for tonsillectomy. Comparison of intravenous morphine and non opioid techniques. Paediatric Anaesthesia 1995;5:185‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
McKean 2008 {published data only}
- Mckean SA, Lee MS, Hussain SS. Comparative study of posttonsillectomy hemorrhage with the use of diclofenac versus dihydrocodeine for postoperative analgeisa and review of the literature. Otolaryngology ‐ Head and Neck Surgery 2008;37(4):577‐81. [MEDLINE: ] [PubMed] [Google Scholar]
Mendham 1996 {published data only}
- Mendham JE, Mather SJ. Comparison of diclofenac and tenoxicam for postoperative analgesia with and without fentanyl in children undergoing adenotonsillectomy or tonsillectomy. Paediatric Anaesthesia 1996;6:467‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nakayama 1982 {published data only}
- Nakayama K. Analgesic effect of voltaren suppository following tonsillectomy in children. Practica Otologica 1982;75:1455‐60. [EMBASE: 1982200724] [Google Scholar]
Nikanne 2005 {published data only}
- Nikanne E, Kokki H, Salo J, Linna T‐J. Celecoxib and ketoprofen for pain management during tonsillectomy: a placebo‐controlled clinical trial. Otolaryngology ‐ Head and Neck Surgery 2005;132:287‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nordbladh 1991 {published data only}
- Nordbladh I, Ohlander B, Bjorkman R. Analgesia in tonsillectomy: A double‐blind study on pre and post‐operative treatment with diclofenac. Clinical Otolaryngology and Allied Sciences 1991;16:554‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Özkiris 2012 {published data only}
Parker 1986 {published data only}
- Parker DA, Gibbin KP, Noyelle RM. Syrup formulations for post‐tonsillectomy analgesia. The Journal of Laryngology and Otology 1986;100:1055‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pestieau 2011 {published data only}
- Pestieau SR, Quezado ZM, Johnson YJ, Anderson JL, Cheng YI, McCarter RJ, et al. High‐dose dexmedetomidine increases the opioid‐free interval and decreases opioid requirement after tonsillectomy in children. Canadian Journal of Anaesthesia. 2011/04/05 2011; Vol. 58, issue 6:540‐50. [MEDLINE: ] [DOI] [PubMed]
Petrusen 1991 {published data only}
- Petrusen. The analgesic effect of diclofenac (voltaren) after tonsillectomy [Den smartstillande effekten av diclofenac (Voltaren) efter tonsillectomi]. OPMEAR 1991;36:65‐7. [EMBASE: 1991316041] [Google Scholar]
Platzer 2011 {published data only}
Purday 1995 {published data only}
- Purday J, Telford R, Saunders M, Pringle M. Diclofenac and post‐tonsillectomy haemorrhage. Anaesthesia 1995;50(9):827‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Robb 1995 {published data only}
- Robb PJ, Rollin AM, Saunders DA. Diclofenac and post‐tonsillectomy haemorrhage. Clinical Otolaryngology and Allied Sciences 1995;20(5):483‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Robinson 1994 {published data only}
- Robinson PM, Ahmed I. Diclofenac and post‐tonsillectomy haemorrhage. Clinical Otolaryngology and Allied Sciences 1994;19(4):344‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Romsing 2000 {published data only}
- Romsing J, Ostergaard D, Drozdziewicz D, Schultz P, Ravin G. Diclofenac or acetaminophen for analgesia in paediatric tonsillectomy outpatients. Acta Anaesthesiologica Scandinavica 2000;44(3):291‐5. [EMBASE: 2000089118] [DOI] [PubMed] [Google Scholar]
Rorarius 1993 {published data only}
- Rorarius MG, Baer GA, Siirtola M, Lahti T, Laippala P. Effect of intravenous diclofenac or indomethacin on the emergence from anaesthesia for tonsillectomy. Acta Anaesthesiologica Scandinavica 1993;37(6):616‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salonen 2001 {published data only}
- Salonen A, Kokki H, Tuovinen K. I.v. ketoprofen for analgesia after tonsillectomy: Comparison of pre‐ and post‐operative administration. British Journal of Anaesthesia 2001;86(3):377‐81. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Salonen 2002 {published data only}
- Salonen A, Kokki H, Nuutinen J. The effect of ketoprofen on recovery after tonsillectomy in children: a 3‐week follow‐up study. International Journal of Pediatric Otorhinolaryngology 2002;62(2):143‐50. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schmidt 2001 {published data only}
- Schmidt A, Bjorkman S, Akeson J. Preoperative rectal diclofenac versus paracetamol for tonsillectomy: effects on pain and blood loss. Acta Anaesthesiologica Scandinavica 2001;45:48‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Smith 1999 {published data only}
- Smith I, Wilde A. Secondary tonsillectomy haemorrhage and non‐steroidal anti‐inflammatory drugs. The Journal of Laryngology and Otology 1999;113(1):28‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stage 1988 {published data only}
- Stage J, Jensen JH, Bonding P. Post‐tonsillectomy haemorrhage and analgesics. A comparative study of acetylsalicylic acid and paracetamol. Clinical Otolaryngology and Allied Sciences 1988;13(3):201‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stewart 2012 {published data only}
Sun 2009 {published data only}
- Sun Y, Xu W‐Y, Hu J, Wang Y‐T, Bai J. Effects of diclofenac sodium suppositories on emergence agitation after sevoflurane maintenance in children undergoing adenotonsillectomy. Journal of Shanghai Jiaotong University (Medical Science) 2009;29(7):842‐4. [Google Scholar]
Swanepoel 1999 {published data only}
- Swanepoel A, Semple P. Oral versus rectal diclofenac for postoperative tonsillectomy pain in children. Anaesthesia 1999;54(3):298‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tamura 1972 {published data only}
- Tamura M, Yamamura M, Yoshioka A, Makimoto K, Kotera A. On the effect of a non‐steroid antipyretic GP 45 840 [In Japanese]. Jibiinkoka‐Rinsho 1972;65(6):583‐9. [Google Scholar]
Tawalbeh 2001 {published data only}
- Tawalbeh MI, Nawashreh OO, Busban AM. Comparative study of diclofenac sodium and paracetamol for treatment of pain after adenotonsillectomy in children. Saudi Medical Journal 2001;22(2):121‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Virtaniemi 1999 {published data only}
- Virtaniemi J, Kokki H, Nikanne E, Aho M. Ketoprofen and fentanyl for pain after uvulopalatopharyngoplasty and tonsillectomy. Laryngoscope 1999;109(12):1950‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Watters 1988 {published data only}
- Watters CH, Patterson CC, Matthews HMC, Campbell W. Diclofenac sodium for post‐tonsillectomy pain in children. Anaesthesia 1988;43:641‐3. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yaman 2011 {published data only}
Zhang ‐ AFT {published data only}
- Zhang J. AFT Pharmaceuticals Ltd. A study comparing the effects of a combination of paracetamol and ibuprofen with paracetamol alone or ibuprofen alone or placebo. Australian New Zealand Clinical Trials Registry. [ACTRN12611000952943]
References to ongoing studies
Hartnick {published data only}
- Postoperative Ibuprofen and the Risk of Bleeding After Tonsillectomy with or without Adenoidectomy. Ongoing study May 2012.
KITS Study {published data only}
- KITS Study ‐ Ketorolac in Tonsillectomy Surgery: a Double Blinded, Randomized Clinical Trial. Ongoing study December 2011.
Wheeler {published data only}
- Safety and Efficacy of Intravenous Ibuprofen for Treatment of Pain in Pediatric Patients undergoing Tonsillectomy. Ongoing study April 2011.
Additional references
BNF 2010
- British Medical Association and Royal Pharmaceutical Society of Great Britain. British National Formulary. British Medical Association and Royal Pharmaceutical Society of Great Britain, March 2010. [ISBN‐10: 0853699291] [Google Scholar]
Collison 2000
- Collison PJ, Mettler B. Factors associated with post‐tonsillectomy haemorrhage. Ear Nose and Throat Journal 2000;79(8):640‐9. [PUBMED: 10969475] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kokki 2003
- Kokki H. Nonsteroidal anti‐inflammatory drugs for postoperative pain: a focus on children. Paediatric Drugs 2003;5(2):103‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Langford 2006
- Langford RM, Mehta V. Selective cyclooxygenase inhibition: its role in pain and anaesthesia. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie 2006;60(7):323‐8. [PUBMED: 16837162] [DOI] [PubMed] [Google Scholar]
Marret 2003
- Marret E, Flahault A, Samama C‐M, Bonnet F. Effects of postoperative, nonsteroidal, antiinflammatory drugs on bleeding risk after tonsillectomy. Anesthesiology 2003;98:1497‐502. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Meade 1993
- Meade EA, Smith WL, DeWitt DL. Differential inhibition of prostaglandin endoperoxide synthase (cyclo‐oxygenase) isozymes by aspirin and other non‐steroidal anti‐inflammatory drugs. Journal of Biological Chemistry 1993;268:6610‐4. [EMBASE: 1993099364] [PubMed] [Google Scholar]
Moiniche 2003
- Moiniche S, Romsing J, Dahl JB, Tramer MR. Nonsteroidal antiinflammatory drugs and the risk of operative site bleeding after tonsillectomy: a quantitative systematic review. Anesthesia and Analgesia 2003;96:68‐77. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
RevMan 5.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2010.
Romsing 1997
- Romsing J, Walther‐Larsen S. Peri‐operative use of nonsteroidal anti‐inflammatory drugs in children: analgesic efficacy and bleeding. Anaesthesia 1997;52:673‐83. [EMBASE: 1997230788] [DOI] [PubMed] [Google Scholar]
Sadish 1994
- Sadish WR, Haddock CK. In: Cooper H, Hedges LV editor(s). The handbook of research synthesis. 1st Edition. New York: The Russell Sage Foundation, 1993. [ISBN 10 0871542269; ISBN 13 9780871542267] [Google Scholar]
Standing 2009
Tomkinson 2011
- Tomkinson A, Harrison W, Owens D, Harris S, McClure V, Temple M. Risk factors for postoperative hemorrhage following tonsillectomy. The Laryngoscope 2011;121:279‐88. [DOI] [PubMed] [Google Scholar]
Web of Science® [Computer program]
- Thomson Reuters. Web of Science. Philadelphia, PA: Institute for Scientific Information. Thomson Reuters, launch date 2002.
www.clinicaltrials.gov [Computer program]
- National Library of Medicine (NLM) at the National Institutes of Health (NIH). www.clinicaltrials.gov. National Library of Medicine (NLM) at the National Institutes of Health (NIH), launch date 2008.
www.controlled‐trials.com [Computer program]
- Springer Science + Business Media. www.controlled‐trials.com. Springer Science + Business Media, launch date 1998.
References to other published versions of this review
Cardwell 2005
- Cardwell ME, Siviter G, Smith AF. Non‐steroidal anti‐inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003591.pub2] [DOI] [PubMed] [Google Scholar]
Cardwell 2010
- Cardwell ME, Siviter G, Smith AF. Nonsteroidal anti‐inflammatory drugs and perioperative bleeding in paediatric tonsillectomy. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD003591.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


