Abstract
Background
Various methods have been used to try to protect kidney function in patients undergoing surgery. These most often include pharmacological interventions such as dopamine and its analogues, diuretics, calcium channel blockers, angiotensin‐converting enzyme (ACE) inhibitors, N‐acetyl cysteine (NAC), atrial natriuretic peptide (ANP), sodium bicarbonate, antioxidants and erythropoietin (EPO).
Objectives
This review is aimed at determining the effectiveness of various measures advocated to protect patients' kidneys during the perioperative period.
We considered the following questions: (1) Are any specific measures known to protect kidney function during the perioperative period? (2) Of measures used to protect the kidneys during the perioperative period, does any one method appear to be more effective than the others? (3) Of measures used to protect the kidneys during the perioperative period,does any one method appear to be safer than the others?
Search methods
In this updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library, Issue 2, 2012), MEDLINE (Ovid SP) (1966 to August 2012) and EMBASE (Ovid SP) (1988 to August 2012). We originally handsearched six journals (Anesthesia and Analgesia, Anesthesiology, Annals of Surgery, British Journal of Anaesthesia, Journal of Thoracic and Cardiovascular Surgery, and Journal of Vascular Surgery) (1985 to 2004). However, because these journals are properly indexed in MEDLINE, we decided to rely on electronic searches only without handsearching the journals from 2004 onwards.
Selection criteria
We selected all randomized controlled trials in adults undergoing surgery for which a treatment measure was used for the purpose of providing renal protection during the perioperative period.
Data collection and analysis
We selected 72 studies for inclusion in this review. Two review authors extracted data from all selected studies and entered them into RevMan 5.1; then the data were appropriately analysed. We performed subgroup analyses for type of intervention, type of surgical procedure and pre‐existing renal dysfunction. We undertook sensitivity analyses for studies with high and moderately good methodological quality.
Main results
The updated review included data from 72 studies, comprising a total of 4378 participants. Of these, 2291 received some form of treatment and 2087 acted as controls. The interventions consisted most often of different pharmaceutical agents, such as dopamine and its analogues, diuretics, calcium channel blockers, ACE inhibitors, NAC, ANP, sodium bicarbonate, antioxidants and EPO or selected hydration fluids. Some clinical heterogeneity and varying risk of bias were noted amongst the studies, although we were able to meaningfully interpret the data. Results showed significant heterogeneity and indicated that most interventions provided no benefit.
Data on perioperative mortality were reported in 41 studies and data on acute renal injury in 44 studies (all interventions combined). Because of considerable clinical heterogeneity (different clinical scenarios, as well as considerable methodological variability amongst the studies), we did not perform a meta‐analysis on the combined data.
Subgroup analysis of major interventions and surgical procedures showed no significant influence of interventions on reported mortality and acute renal injury. For the subgroup of participants who had pre‐existing renal damage, the risk of mortality from 10 trials (959 participants) was estimated as odds ratio (OR) 0.76, 95% confidence interval (CI) 0.38 to 1.52; the risk of acute renal injury (as reported in the trials) was estimated from 11 trials (979 participants) as OR 0.43, 95% CI 0.23 to 0.80. Subgroup analysis of studies that were rated as having low risk of bias revealed that 19 studies reported mortality numbers (1604 participants); OR was 1.01, 95% CI 0.54 to 1.90. Fifteen studies reported data on acute renal injury (criteria chosen by the individual studies; 1600 participants); OR was 1.03, 95% CI 0.54 to 1.97.
Authors' conclusions
No reliable evidence from the available literature suggests that interventions during surgery can protect the kidneys from damage. However, the criteria used to diagnose acute renal damage varied in many of the older studies selected for inclusion in this review, many of which suffered from poor methodological quality such as insufficient participant numbers and poor definitions of end points such as acute renal failure and acute renal injury. Recent methods of detecting renal damage such as the use of specific biomarkers and better defined criteria for identifying renal damage (RIFLE (risk, injury, failure, loss of kidney function and end‐stage renal failure) or AKI (acute kidney injury)) may have to be explored further to determine any possible benefit derived from interventions used to protect the kidneys during the perioperative period.
Keywords: Adult; Humans; Creatinine; Creatinine/urine; Postoperative Complications; Postoperative Complications/prevention & control; Randomized Controlled Trials as Topic; Renal Insufficiency; Renal Insufficiency/prevention & control; Surgical Procedures, Operative; Surgical Procedures, Operative/adverse effects; Urine
Plain language summary
No evidence indicates that any of the measures used to protect patients' kidneys during the perioperative period are beneficial
The kidneys may be damaged during an operation as a result of direct and indirect insult. The reasons for this are multiple and include changes to physiology brought on by the surgery and by the body’s response to such insult. Damage to kidneys during the perioperative period is associated with significant morbidity and mortality. This updated Cochrane review looked at 72 randomized controlled trials (RCTs) with 4378 participants (search data until August 2012); interventions most often included pharmacological interventions (administration of dopamine and its analogues, diuretics, calcium channel blockers, angiotensin‐converting enzyme (ACE) inhibitors, N‐acetyl cysteine, atrial natriuretic peptide, sodium bicarbonate, antioxidants and erythropoietin) or selected hydration fluids. We attempted to identify any possible damage to the kidneys by evaluating kidney function up to seven days after the operation.
No clear evidence from available RCTs suggests that any of the measures used to protect the kidneys during the perioperative period are beneficial. These findings held true in 14 studies of patients with pre‐existing renal damage and in 24 studies that were considered of good methodological quality. The primary outcomes of these studies were mortality and acute renal injury. Reported mortality in studies with low risk of bias was not different between intervention and control groups (odds ratio (OR) 1.01, 95% confidence interval (CI) 0.52 to 1.97) or for acute renal injury (OR 1.05, 95% CI 0.55 to 2.03). The summary of findings revealed a similar picture. So we conclude that evidence suggests that none of the interventions used currently are helpful in protecting the kidneys during the perioperative period, nor do they cause increased harm.
Summary of findings
Summary of findings for the main comparison. Interventions in patients with pre‐existing renal dysfunction.
| Interventions for protecting renal function in patients with pre‐existing renal impairment who are undergoing surgery | ||||||
|
Patient or population: patients with pre‐existing renal impairment Settings: perioperative period (7 days) Intervention: interventions to protect the kidneys during the perioperative period Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo or no intervention | Various interventions | |||||
|
Mortality in patients with pre‐existing renal impairment As reported in the included trials Folliow‐up: 7 days |
Study population | OR 0.74 (0.36 to 1.52) | 959 (10 studies) | ⊕⊝⊝⊝ very lowa,b,c,d | Evidence is not strong and is of poor quality | |
| 38 per 1000 | 29 per 1000 (15 to 56) | |||||
| Moderate | ||||||
| 20 per 1000 | 15 per 1000 (8 to 30) |
|||||
|
Acute renal injury in patients with pre‐existing renal impairment As reported in the included trials Follow‐up: 1 to 7 days |
Study population | OR 0.40 (0.22 to 0.76) | 979 (11 studies) | ⊕⊝⊝⊝ very lowe,f,g,h | Evidence is not strong and is of poor quality (although it might give a statistical edge) | |
| 62 per 1000 | 28 per 1000 (15 to 50) | |||||
| Moderate | ||||||
| 40 per 1000 | 18 per 1000 (9 to 32) |
|||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio. | ||||||
| GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aOnly six of the 10 studies showed low risk of bias. bSignificant clinical heterogeneity between studies was noted. cClinical heterogeneity and indications varied across the chosen studies. dThe numbers of events and the total numbers of cases studied were small. eOnly six of the 11 included studies were assessed as having low risk of bias. fSsignificant clinical heterogeneity amongst the included studies was noted. gClinical scenarios in the included studies varied. hReported incidences were low and the numbers of participants in the included studies were small.
Summary of findings 2. Interventions to protect the kidneys in the perioperative period in patients undergoing surgery: low ROB studies only.
| Interventions to protect the kidneys during the perioperative period in patients undergoing surgery: low ROB studies only | ||||||
|
Patient or population: patients undergoing surgery
Settings: perioperative period (7 days)
Intervention: interventions to protect the kidneys in patients undergoing surgery: low ROB studies only Comparison: placebo or no intervention | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control |
Interventions to protect the kidneys: low ROB cases only |
|||||
| Reported mortality, low risk of bias studies only Follow‐up: mean 7 days | Study population | OR 1.01 (0.52 to 1.97) | 1604 (19 studies) | ⊕⊝⊝⊝ very lowa,b,c,d | Evidence is not strong and is of poor quality | |
| 23 per 1000 | 23 per 1000 (12 to 42) | |||||
| Moderate | ||||||
| 20 per 1000 | 20 per 1000 (11 to 37) | |||||
| Acute renal injury, low‐risk studies only | Study population | OR 1.05 (0.55 to 2.03) | 1550 (16 studies) | ⊕⊝⊝⊝ very lowe,f,g,h | Evidence is not strong and is of poor quality (although it might give a statistical edge) | |
| 23 per 1000 | 24 per 1000 (13 to 45) | |||||
| Moderate | ||||||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in the footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; OR: Odds ratio; ROB: Risk of bias. | ||||||
| GRADE Working Group grades of evidence: High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
aOnly six of the 10 studies showed low risk of bias. bSignificant clinical heterogeneity between studies was noted. cClinical heterogeneity and indications varied across the chosen studies. dThe numbers of events and the total numbers of cases studied were small. eOnly six of the 11 included studies were assessed as having low risk of bias. fSignificant clinical heterogeneity amongst the included studies was noted. gClinical scenarios in the included studies varied. hReported incidences were low and the numbers of participants in the included studies were small.
Background
Description of the condition
Intraoperative changes in renal blood flow and glomerular filtration rate are common. Postoperative renal dysfunction is mainly attributed to adverse events that occur during the perioperative period, including hypotension, hypovolaemia and sepsis, or it may be due to perioperative administration of contrast material (Morcos 2004). The reported risk of perioperative renal failure varies according to aetiology, definition and type of surgery; acute renal failure during the perioperative period is a serious complication associated with considerable morbidity and mortality. When postoperative renal dysfunction occurs, it is generally thought to be multi‐factorial in nature.
Description of the intervention
Over the past few decades, attempts have been made to protect the kidneys both during surgery and in the immediate postoperative period (Wang 2003). Various regimens, such as low‐dose dopamine, dopexamine, fenoldopam or diuretics, have been tried. The results are somewhat uncertain, hence a number of other measures have been tried. These include N‐acetyl cysteine (Adabag 2008; Barr 2008; Burns 2005; Fischer 2005; Hynninen 2006; Prasad 2010; Ristikankare 2006), atrial natriuretic peptide (ANP) (Chen 2007; Mitaka 2008; Sezai 2000; Sezai 2009) and erythropoietin (EPO) (Song 2009). Amongst nephrologists, considerable enthusiasm has surrounded the potential for EPO to provide some renal protection (Johnson 2006).
Different tests, some simple and some complicated, are used with varying success to detect acute kidney injury (AKI) in the perioperative period. Measurement of urine output over a 24‐hour period after surgery is one of the simpler tests. A commonly used measure is creatinine clearance, which is often examined by using the Cockcroft‐Gault formula, which takes into consideration the age, body weight and sex of the individual, as well as serum creatinine levels. Glomurular filtration rate (GFR) can be measured, as can renal plasma flow. Other tests include assessment of the ability of kidneys to clear a water load (free water clearance) and to excrete sodium (fractional excretion of sodium).
Several newer tests are used as markers of renal damage. The ratio in urine of microalbumin to creatinine (Hynninen 2006; Turner 2008) has been used but is considered an index of kidney damage, most often in chronic kidney disease. Urinary N‐acetyl‐beta‐D‐glucosaminidase (U‐NAG) to creatinine ratio (Hynninen 2006; Mitaka 2008), retinol‐binding protein (RBP) to creatinine ratio and urinary neutrophil gelatinase‐associated lipocalin (NGAL) to creatinine ratio are newer methods that can be used to detect renal damage; we have looked at these tests in preparing this update of the review. Plasma cystatin C (CysC) (Chen 2007; Haase 2007; Harten 2008; Hynninen 2006) is another available marker. In a recent study (Endre 2011), glutamytranspeptidase (GGT), alkaline phosphatase (ALP), NGAL, CysC, kidney injury molecule‐1 (KIM‐1) and interleukin‐18 (IL‐18) were used in intensive care units as biomarkers of acute kidney injury.
How the intervention might work
Over the past few decades, several strategies have been used to attempt to protect the kidneys both during surgery and in the immediate postoperative period on a physiological basis (maintaining adequate cardiac output, maintaining renal vasodilatation, suppressing renal vasoconstriction and maintaining renal tubular flow). Various pharmacological regimens, such as use of low‐dose dopamine, dopexamine, calcium channel blockers, angiotensin‐converting enzyme (ACE) inhibitors or diuretics, have been tried. Some success has been reported with such interventions (Welch 1995) but no clear evidence of success (Renton 2005) or of deterioration in renal function has been found (Lassnigg 2000).
Dopamine, an endogenous catecholamine, given at a dose of 2 µg·kg·min to 5 µg·kg·min (low‐dose or renal dopamine), causes renal vasodilatation with a dose‐dependent increase in renal blood flow (McDonald 1964; Seri 1988); at higher doses, dopamine augments renal blood flow by increasing cardiac output through β‐adrenergic stimulation. The effect of dopamine has been studied extensively over the years (ANZICS CTG 2000). The net sum of these actions is seen as an increase in renal blood flow, an increase in GFR, diuresis and natriuresis. Mannitol, an osmotic diuretic, attenuates ischaemic reperfusion injury through multiple mechanisms, including maintenance of glomerular filtration pressure, prevention of tubular obstruction by cellular casts, scavenging of hydroxyl free radicals and prevention of cellular swelling (Schrier 1984). Furosemide, a loop diuretic, blocks ion transport activity in the medullary thick ascending loop of Henle and enhances tubular oxygen balance by decreasing tubular oxygen demand and consumption. However, loop diuretics also cause renal cortical vasodilatation, resulting in redistribution of blood flow, which could undermine the benefit previously described (Moitra 2009). Calcium channel blockers appear to confer protection against intracellular calcium injury in ischaemic reperfusion injury (Schrier 1991). Rapid administration of fluids results in expansion of intravascular volume, leading to an increase in cardiac output. ACE inhibitors alter the balance between the vasoconstrictive and salt‐retentive properties of angiotensin ІІ and between the vasodilatory and natriuretic properties of bradykinin (Brown 1998). In the kidneys, ACE inhibitors decrease glomerular capillary pressure by decreasing arterial pressure and by selectively dilating efferent arterioles (Anderson 1986).
N‐acetyl cysteine (NAC) has a variety of biological actions. It is an antioxidant (Zafarullah 2003); it stimulates endothelium‐derived relaxing factor, thereby improving microvascular flow (Kiefer 2000); and it increases cyclic guanosine monophosphate (GMP) levels, thereby acting as a vasodilator and as an inhibitor of platelet aggregation. These various clinical actions might have led several investigators to focus on the use of NAC for the prevention of contrast‐induced nephropathy (CIN) (Kay 2003; Tepel 2000). The natriuretic peptides play an important role in cardiovascular, renal and endocrine homeostasis. The natriuretic and diuretic actions of ANP are due to renal haemodynamic and direct tubular actions (Levin 1998). ANP increases GFR by increasing pressure within the glomerular capillaries (Marin‐Grez 1986). ANP also inhibits angiotensin II-stimulated sodium and water transport in proximal tubules (Harris 1987), vasopressin‐stimulated water transport in the collecting tubules (Dillingham 1986) and sodium absorption in the inner medullary collecting duct (Sonnenberg 1986). The combined effect of all of this consists of natriuresis and diuresis. Thus ANP has been used to try to counteract the two proposed pathophysiological mechanisms of decreased GFR in AKI, namely, reduced glomerular perfusion and tubular obstruction (Edelstein 1997). Accumulating evidence indicates that EPO has tissue protective or pleiotropic effects (Chatterjee 2005; Maiese 2005) that may be useful in preventing or treating AKI. The protective mechanisms are multi‐factorial and involve inhibition of apoptotic cell death, stimulation of cellular regeneration, inhibition of deleterious pathways and promotion of recovery (Moore 2011).
Why it is important to do this review
The previously published version of this review (Zacharias 2008) was unable to detect much benefit derived from various interventions to protect the kidneys during the perioperative period. The efficacy of dopamine and its analogues, diuretics, calcium channel blockers, ACE inhibitors, NAC, ANP, sodium bicarbonate, EPO and antioxidants has yet to be proved in the capacity of reversing or preventing AKI during the perioperative period. In this updated review, we are taking a fresh look at the current status of this important topic.
Objectives
This review is aimed at determining the effectiveness of various measures advocated to protect patients' kidneys during the perioperative period.
We considered the following questions: (1) Are any specific measures known to protect kidney function during the perioperative period? (2) Of measures used to protect the kidneys during the perioperative period, does any one method appear to be more effective than the others? (3) Of measures used to protect the kidneys during the perioperative period,does any one method appear to be safer than the others?
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomized controlled trials of any intervention (dopamine and its analogues, diuretics, calcium channel blockers, ACE inhibitors, N‐acetyl cysteine, atrial natriuretic peptides, hydration fluids or any other interventions) versus control (placebo or no intervention), published in any language.
Types of participants
We included participants undergoing all types of major surgery during which a specified intervention was used to protect the kidneys from possible damage during surgery. We did not include studies that specifically considered a paediatric population. We did not include studies with participants undergoing transplant surgery (heart, liver or kidney) because of the complexity of the surgery and the postoperative management required for these participants.
Types of interventions
We included studies that used the following interventions to maintain or protect kidney function during anaesthesia and surgery.
Dopamine and its analogues.
Diuretics.
Calcium channel blockers.
Angiotensin‐converting enzyme (ACE) inhibitors.
Hydration fluids.
N‐acetyl cysteine.
Atrial natriuretic peptide.
Erythropoietin (EPO).
Any other measures.
Types of outcome measures
Primary outcomes
Postoperative adverse outcomes. These included significant adverse outcomes: acute renal failure or death.
Secondary outcomes
Any changes in perioperative renal function. These included the following measures.
Urine output.
Creatinine clearance (or glomerular filtration rate).
Renal plasma flow (or renal blood flow).
Free water clearance.
Fractional excretion of sodium.
Urinary NAG/creatinine ratio.
Urinary RBP/creatinine ratio.
Plasma cystatin C.
Urinary NGAL/creatinine ratio.
Search methods for identification of studies
Electronic searches
In our updated review, we searched the following electronic databases: Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 2, 2012); MEDLINE (Ovid SP) (1966 to August 2012); and EMBASE (Ovid SP) (1988 to August 2012). We used the search strategies given in Appendix 1.
Searching other resources
We originally handsearched six major journals in anaesthesia and vascular or thoracic surgery (1985 to 2004).
Anesthesia and Analgesia.
Anesthesiology.
Annals of Surgery.
British Journal of Anaesthesia.
Journal of Thoracic and Cardiovascular Surgery.
Journal of Vascular Surgery.
However, because these journals are properly indexed in MEDLINE, we decided to rely on the electronic searches only without handsearching these journals from 2004 onwards.
Data collection and analysis
Selection of studies
We evaluated for appropriateness of inclusion all studies obtained by the search methods described above, as well as their abstracts and summaries. We obtained full publications for those studies, which required further assessment. Two of the review authors (MZ, NPC or PS or MM) evaluated these studies without prior consideration of the results, and consensus was reached on the final selection. Colleagues at the local hospital or university translated some of the selected studies into English.
Data extraction and management
We used specifically designed data extractions forms to extract the relevant data (Appendix 2). Two authors (MZ, NC or PS or MM) separately extracted and compared data. We resolved differences by discussion and reaching consensus. Wherever we considered it necessary, we attempted to contact the authors for further clarification, data, or both, with limited success.
The data collected included the following.
Reported mortality or acute renal failure.
Nature of the surgical intervention.
Nature of the intervention used.
Methodological quality (risk of bias) of the study.
Presence of pre‐existing renal damage.
Any other relevant information.
Results of the individual studies were reported in many different ways, including means, standard deviations (SDs), standard errors of the mean (SEMs), median or interquartile ranges (IQRs) or ranges. We converted standard errors of the mean and interquartile ranges to standard deviations using appropriate formulae. We considered the interquartile range to be 1.35 times the standard deviation (for the purpose of this review, we assumed that the data were normally distributed). We calculated the standard deviation as the square root of the sample size times the standard error of the mean.
We considered creatinine clearance as a surrogate measure of GFR. When data involved weights, we made an assumption of 70 kg to convert the data. When data were presented in graphical form, we extracted the numerical data from the graphs as accurately as possible. We converted all data to uniform measurements; thus urine output, creatinine clearance, renal plasma flow and free water clearance were expressed in mL/min and fractional excretion of sodium as a percentage, urinary microalbumin/creatinine ratio as mg/mg, U‐NAG (N‐acetyl‐beta‐D‐glucosaminidase)/creatinine ratio as mcmol/mmol, urinary retinol‐binding protein/creatinine ratio as mcg/mmol, urinary NGAL (urinary neutrophil gelatinase‐associated lipocalin)/creatinine ratio as ng/mmol and plasma cystatin C as mg/L.
In this review, we chose to look at data for the various renal function tests at 24 hours, two to three days and five to seven days, because these were the times that the results were most frequently reported in the selected studies. We were reluctant to look at data collected earlier than 24 hours because these would have shown acute changes brought on by anaesthesia and surgery. Even though it is not specified in most of the publications, we assumed that data on urine output at 24 hours reflect the average reading for urine output in the first 24 hours after surgery; the same applies to urine output results at two to four days and five to seven days postoperatively. For measurements such as creatinine clearance, renal plasma flow, free water clearance and fractional excretion of sodium, we assumed that the data were obtained at the specified time.
Assessment of risk of bias in included studies
At least two review authors independently assessed each included study for methodological quality on the basis of assessment of the following domains of quality: randomization, concealment of allocation, blinding and acknowledgement of dropouts. An overall quality assessment was rated as good, moderately good or poor (see Table 3).
1. Methodological quality of included studies.
| Study ID | Randomization | Allocation concealment | Blinding | Withdrawals recorded | Overall quality |
| Adabag 2008 | Randomly assigned by the investigational pharmacist Block randomization (blocks of 10) |
Participants, researchers and clinicians blinded to treatment assignment |
Participants, researchers and clinicians (including data collecting nurse) blinded Drug packets matched in volume, colour, consistency and transparency and given mixed with fruit juice to mask taste |
Not reported | Good |
| Amano 1994 | ‘Randomly assigned’ into two groups | None described | None described; control group had no treatment | Not described | Poor |
| Amano 1995 | ‘Patients were randomized into either diltiazem or no treatment' | Not described | None described; control group had no treatment | Not described | Poor |
| Ascione 1999 | ‘Prospectively randomized by card allocation’ | ‘Prospectively randomized by card allocation’ | Not used | Not discussed | Moderate |
| Barr 2008 | Randomization done by pharmacy department; method of randomization uncertain Not sure about adequacy of randomization |
No specific mention of allocation concealment except to say ‘double‐blinded’ Allocation concealment inadequate |
No specific mention of who all were blinded; ‘double‐blinded’, placebo‐controlled trial Not sure whether blinding was adequate |
One withdrawal from study reported | Moderate |
| Berendes 1997 | ‘Placebo controlled prospective study’; no randomization | None described | None described | Not described | Poor |
| Bergman 2002 | ‘Consented and were randomized’ | Not described | Not used | 3 participants (2, 1) not operated upon; 2 participants excluded from final analysis because of clinical management changes | Poor |
| Burns 2005 | Randomization done by pharmacy trial co‐ordinator using a permitted block strategy | Allocation concealment using central randomization with drugs prepared by pharmacy | Quadruple‐blinded (participants, clinicians, data collectors and data analyst) placebo‐controlled study | Clearly accounted for (5 in intervention group and 2 in control group) | Good |
| Carcoana 2003 | ‘Prospective randomized double‐blinded and placebo‐controlled study’ Computer‐generated random number tables |
Not specifically described, but quite likely it was concealed allocation | Blinded manner; drug or saline supplied by the department investigational pharmacy in a blinded manner + additive for the CPB circuit prime (mannitol or saline, supplied similarly) | All allocated participants completed the trial (withdrawals before allocation) | Good |
| Chen 2007 | ‘Randomized’; no details provided ‘Double‐blind, placebo‐controlled proof of concept trial’ |
No details provided ‘Double‐blind, placebo‐controlled proof of concept trial’ |
No details provided ‘Double‐blind, placebo‐controlled proof of concept trial’ |
Four withdrawals from trial reported |
Poor |
| Cho 2009 | Computer‐generated randomization method used | Computer allocation, no further details given | Not described except by the statement, ‘investigator blinded to the study group evaluated the postoperative data’ | Not reported | Moderate |
| Cogliati 2007 | Randomization from a computer list, in an envelope | Sealed envelope used ‘All personnel and patients were blinded to the assignment’ |
Blinded nurse, not involved with study, prepared the drug/ placebo in identical 50 mL filled syringes ‘All personnel and patients were blinded to the assignment’ |
1 participant | Good |
| Colson 1990 | Allocated in a randomized double‐blind fashion to 2 groups No details on randomization method |
Allocated in a randomized double‐blind fashion to 2 groups No description of allocation concealment |
No details on blinding except ‘double‐blind fashion’ | Not given | Poor |
| Colson 1992 | Allocated in a randomized double‐blind fashion to 2 groups No details on randomization method |
Allocated in a randomized double‐blind fashion to 2 groups No description of allocation concealment |
No details on blinding except ‘double‐blind fashion’ | Not given | Poor |
|
Costa 1990 |
Participants with renal dysfunction (CCl < 50 mL/min) ‘Randomly divided into 3 groups’; no description of randomization |
No description of allocation | No description of blinding | Not given | Poor |
| Cregg 1999 | ‘Randomly allocated’ into 3 groups; no description of randomization | No description of allocation | No description of blinding | Not given | Poor |
| Dawidson 1991 | ‘Randomized to either treatment group’ by pulling a card from a previously prepared deck | No description of allocation concealment | No details on blinding | Not given | Poor |
| de Lasson 1995 | ‘Randomly allocated into infusion of dopamine or placebo’ by one of the authors, who was unaware of the treatment allocation |
‘Randomly allocated into infusion of dopamine or placebo’ by one of the authors, who was unaware of the treatment allocation; no description of allocation concealment |
No blinding described | Not given | Poor |
| de Lasson 1997 | Randomization and drug or placebo preparation provided by drug company; method not described, but likely to be good | Not sure of any allocation concealment, but likely possible | Possible, but blinded tables not described | 1 participant had additional drugs but was not excluded | Moderate |
| Dehne 2001 | Randomly allocated into 2 groups, randomization method not described | Allocation concealment not described | Blinding not mentioned | All participants accounted for in calculations | Poor |
| Donmez 1998 | ‘Randomly allocated into 3 groups’; method of randomization not described | ‘Randomly allocated into 3 groups’; method of allocation concealment not described | ‘Randomly allocated into 3 groups’; method of blinding not described | Dropouts not described | Poor |
| Dural 2000 | ‘Randomly allocated into 3 groups’; method of randomization not described | ‘Randomly allocated into 3 groups’; method of allocation concealment not described | ‘Randomly allocated into 3 groups’; method of blinding not described | Dropouts not described | Poor |
| Durmaz 2003 | Randomization done by the last digit of the medical record number of participant (quasi‐randomization) | ‘Patients were prospectively allocated into 2 groups’ No details given |
Not given | Not given | Poor |
| Fischer 2005 | Retrospective chart review of a randomized trial in 2003, which used computer‐generated allocation list (randomly permuted blocks of random size) provided by department of Medical Statistics | Computer‐generated allocation list (randomly permuted blocks of random size) provided by department of Medical Statistics | Drugs supplied in identical looking glass vials containing drug or placebo | Exclusions described in text | Good |
|
Gubern 1988 |
’Prospectively randomized’; no details of method of randomization | ’Prospectively randomized’; no details of method of allocation | ’Prospectively randomized’; no details of method of blinding | Fate of participants discussed | Poor |
| Haase 2007 | Random assignment of participants using Microsoft Excel‐based random number generation to create a randomization list, in blocks of 10 | Allocation concealment ensured by quadruple‐blinding (participants, clinicians, data collectors and data analysers were unaware of groups or treatment) | Quadruple‐blinding (participants, clinicians, data collectors and data analysers were blinded) | 0 participants | Good |
| Haase 2009 | Microsoft Excel‐based random number generation, with blocks of 10; central randomization by department of pharmacy | Allocation concealment achieved by central randomization, blinding to all researchers, participants and others. Allocation revealed only after data analysis | Both fluids in separate shrink‐wrapped black plastic bags that were identical in appearance (blinded to participants, anaesthetists, surgeons, ICU personnel, nurses and others) | 1 in each group | Good |
| Halpenny 2002 | ‘Random allocation used’; method not given | ‘Random allocation used’; method not given | ‘Random allocation used’; method not given | 1 participant excluded from the trial | Poor |
| Harten 2008 | ‘Randomized’, but no details given | Allocated to control and intervention groups using opaque envelopes immediately before surgery; not sure whether allocation was maintained | No blinding | 1 died before operation (intervention group) | Poor |
| Hynninen 2006 | Random assignment in blocks of 10 done by hospital pharmacy, no details given |
Allocation done by hospital pharmacy Clinical and study personnel not aware of study allocation |
Blinding quite likely, although not detailed in text | 1 participant withdrew from study intraoperatively (does not mention which group, although most likely the intervention group-1 less in that group) |
Moderate |
| Kaya 2007 | Computer‐generated randomization done by statistician | Sequentially numbered, sealed envelopes |
SNP and saline in uniformly appearing 50 mL syringes, blinded to surgeons, perfusionists and nurses; investigators did not know the details | None | Good |
| Kleinschmidt 1997 | Randomization by computer | Not described in detail | Not described in detail | No detailed description | Poor |
| Kramer 2002 | Participants randomly assigned to receive 1 of 2 treatments | No details given | No details given | Early termination of study in 33 of 56 participants; ITT used | Poor |
| Kulka 1996 | Allocated into 2 groups in a double‐blinded random fashion; no details of randomization given | Allocated into 2 groups in a double‐blinded random fashion; no details of allocation given | Allocated into 2 groups in a double‐blinded random fashion; no details of blinding given | 2 participants excluded | Poor |
| Lassnigg 2000 | Placebo‐controlled randomized double‐blind trial; block randomization done and sealed envelopes used | Placebo‐controlled randomized double‐blind trial; block randomizations done with the use of sealed envelopes; no further details on allocation concealment provided | Placebo‐controlled randomized double‐blind trial; no other details of blinding provided | 3 participants excluded from analysis | Moderate |
| Lau 2001 | ‘Recruited patients were allocated to one of 2 groups’; no details on randomization | ‘Recruited patients were allocated to one of 2 groups’; no details on allocation concealment | ‘Recruited patients were allocated to one of 2 groups’; no details on blinding provided | 2 participants accounted for | Poor |
| Licker 1996 | 'Patients were allocated in a randomized double‐blind manner’; no details of randomization given | 'Patients were allocated in a randomized double‐blind manner’; no details of allocation concealment given | 'Patients were allocated in a randomized double‐blind manner’; no details of blinding given | 2 participants excluded from the trial | Poor |
| Loef 2004 | ‘Randomized in a double‐blind fashion’; no details of randomization given | ‘Randomized in a double‐blind fashion’; no details of allocation given | ‘Randomized in a double‐blind fashion’; no details of blinding given | All participants completed the trial | Poor |
| Marathias 2006 | Used a 2:1 ratio in randomization process, participants randomly assigned into groups; no other details of randomization given | Participants randomly assigned into groups; no other details of allocation given | Participants randomly assigned into groups; no other details of blinding given | Not given | Poor |
| Mitaka 2008 | ‘Patients were randomized into 2 groups’; not sure what method of randomization was used | Not sure how allocation was performed | ‘Blind infusion was performed’; not sure about blinding | None indicated | Poor |
| Morariu 2005 | Designed as a prospective double‐blind placebo‐controlled randomized trial; no other details of randomization provided | Prospective double‐blind placebo‐controlled randomized trial; no other details of allocation concealment provided | Prospective double‐blind placebo‐controlled randomized trial; no other details of blinding provided | All participants competed the trial | Poor |
| Morgera 2002 | ‘Patients were randomized’; no other details given | ‘Patients were randomized’; no other details given | ‘Patients were randomized’; no other details given | 2 participants excluded from analysis | Poor |
| Myles 1993 | Randomly assigned with the use of a table of random numbers; ‘prospective double‐blind randomized trial’ | Coded 50 mL syringes from the pharmacy, with contents remaining unknown to investigators until the end of the trial; allocation concealed | Coded 50 mL syringes from the pharmacy, with contents remaining unknown to investigators until the end of the trial; blinded | 3 withdrawals before start of trial | Good |
| Nicholson 1996 | ‘Prospective randomized trial’; no further details on randomization | ‘Prospective randomized trial’; no further details on allocation | ‘Prospective randomized trial’; no details on blinding | None reported | Poor |
| Nouri‐Majalan 2009 | ‘Patients were randomized’; no further details | No indication of allocation concealment, but for statement, ‘To prevent bias, surgeons, nurses, and lab technicians were blinded to patient assignment’ | Possible: ‘To prevent bias, surgeons, nurses, and lab technicians were blinded to patient assignment’ |
None indicated in text | Poor |
| O'Hara 2002 | ‘Prospective randomized study’; no further details on randomization given | ‘Prospective randomized study’; no further details on allocation | ‘Prospective randomized study’; no further details on blinding | 11 of 35 excluded | Poor |
| Parks 1994 | ‘Patients were randomly allocated into 2 groups’; no further details on randomization | ‘Patients were randomly allocated into 2 groups’; no further details on allocation | ‘Patients were randomly allocated into 2 groups’; no further details on blinding | Not disclosed | Poor |
| Perez 2002 | Randomization performed by aleatorized numbers prepared in closed envelopes | No details on concealment of allocation except ‘Randomization performed by aleatorized numbers prepared in closed envelopes’ | Drug or placebo given with an identical container in a double‐blind manner with the same volume of drug or saline | 4 participants excluded | Moderate |
| Prasad 2010 | Randomized, prospective, open‐label study Random number generated from a random number table |
No concealment of assignment | No blinding | 4 excluded after randomization? | Poor |
| Prowle 2012 | Random assignment by the hospital pharmacy clinical trials co‐ordinator Microsoft Excel–based random number generator permuted block strategy with blocks of 10 |
Allocation stratified into 2 groups based on pre‐op use of statins Allocation concealed to participants, anaesthetists, cardiac surgeons, intensive care specialists, bedside nurses and investigators |
'Double‐blind'. Atorvastatin or placebo medication prepared in capsules of identical appearance |
8 in intervention group and 7 in control group | Good |
| Pull Ter Gunne 1990 | Random assignment into 2 groups; no further details | Random assignment into 2 groups; no further details | Random assignment into 2 groups; no further details; the anaesthesiologist was aware of the allocation and treatment received | No details provided | Poor |
| Ristikankare 2006 | ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’ | ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’, but no details of allocation concealment provided | ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’; no details of blinding provided | 3 participants excluded | Moderate |
| Ryckwaert 2001 | ‘Patients were allocated in a randomized double‐blind fashion to 2 groups’; no further details of randomization given | ‘Patients were allocated in a randomized double‐blind fashion to 2 groups’; no further details of allocation given | ‘Patients were allocated in a randomized double‐blind fashion to 2 groups’; no further details of blinding given | No dropouts detailed in text | Poor |
| Sezai 2000 | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; no other details on randomization method | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; no other details on allocation method | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; no other details on blinding | Not described, but study probably had no dropouts | Poor |
| Sezai 2009 | Randomly allocated into 2 groups by drawing lots | ‘Randomly allocated by drawing lots’ No other details |
No evidence of blinding | No mention in the text | Poor |
| Sezai 2011 | Randomly allocated into 2 groups by lottery method | 'Randomly allocated into 2 groups'; no evidence of concealment of allocation | No blinding discussed | Dropouts discussed | Poor |
| Shackford 1983 | Participants were assigned by random number to 1 of 2 groups; no details on randomization given | Participants were assigned by random number to 1 of 2 groups; no details on concealment of allocation given | Participants assigned by random number to 1 of 2 groups; no details on blinding | No dropouts | Poor |
| Shim 2007 | Participants randomly allocated to 1 of 2 groups with use of a computer‐generated randomization table | Participants randomly allocated to 1 of 2 groups with use of a computer‐generated randomization table; no further details on allocation concealment given | All medical personnel involved in the study blinded to the contents of the infusion bottle | No dropouts recorded | Moderate |
| Song 2009 | Block randomization developed by research centre Randomization stratified by serum creatinine levels | Allocation via Internet using predetermined randomization | Participants, healthcare clinicians and researchers blinded | None | Good |
| Tang 1999 | Prospectively randomly assigned | No details on allocation provided in text | No details of blinding provided in text | No dropouts recorded | Poor |
| Tang 2002 | Participants randomly assigned; no further details on randomization given | Participants randomly assigned; no further details on allocation given | 2 different types of procedures; no blinding possible | 5 participants subsequently excluded from trial | Poor |
| Thompson 1986 | ‘Patients were randomized’; no more details | ‘Patients were randomized’; no more details | No details provided | ‘There were no withdrawals’ | Poor |
| Turner 2008 | Random assignment done with use of computer‐generated randomization list | Computer‐generated randomization list placed in sealed envelopes and opened in numerical order by a third party, who prepared the study infusion | Third party prepared the infusion. Infusions were such that volumes were equal in the bag and of identical colour, and contents of the bag were indistinguishable; the infusion was done over 30 minutes to avoid haemodynamic effects of treatment | Yes, none lost | Good |
| Urzua 1992 | Participants randomly assigned into 1 of 2 groups, according to the last digit of their clinical history number (quasi‐randomization) | No description of concealment of allocation | No report of blinding | All participants completed | Poor |
| Wahbah 2000 | ‘Patients were randomly allocated into 4 equal groups’; no further details on randomization | Patients were randomly allocated into 4 equal groups’; no further details on allocation | No description of blinding | None described | Poor |
| Welch 1995 | ‘Patients were randomly assigned’; no further details on randomization method used | ‘Patients were randomly assigned’; no further details on allocation method used | No description of blinding | None described | Poor |
| Wijnen 2002 | ‘Patients were randomized’; no further details on method of randomization used | ‘Patients were randomized’; no details on method of allocation used | No details on blinding | One death described | Poor |
| Witczak 2008 | Participants were ‘randomized’ It appears that the anaesthesiologist ‘randomly drew an envelope with the assigned treatment’ |
Allocation concealment was possible only for participants and the statistician | No; control received no treatment Participants and the statistician were blinded |
Not described | Poor |
| Woo 2002 | ‘Patients were randomized’; no further details on method of randomization used | ‘Patients were randomized’; no details on method of allocation used | No details on blinding | 8 participants excluded because of death or major complications | Poor |
| Yavuz 2002A | ‘Patients were prospectively randomized’; no details on method of randomization used | ‘Patients were prospectively randomized’; no details on method of allocation used | No description of blinding | States no deaths; no description of dropouts | Poor |
| Yavuz 2002B | ‘Patients randomized into 4 groups’; no further details on randomization given | ‘Patients randomized into 4 groups’; no description of allocation used | No description of blinding | No mortality described, but no suggestion of dropouts | Poor |
| Zanardo 1993 | ‘Randomly assigned’; no further details of randomization given | ‘Randomly assigned’; no further details of allocation given | No blinding described | No dropouts described | Poor |
Measures of treatment effect
We pooled continuous outcomes with mean differences (MDs) and 95% confidence intervals (CIs). Initially, we pooled the results using a fixed‐effect model, but substantial heterogeneity existed in many analyses, and we explored the reasons for this. Because of considerable heterogeneity seen in the results, we opted to present the data using a random‐effects model.
Dichotomous outcomes (acute renal failure and mortality) were very rare events, hence we have presented these as odds ratios (ORs), using the Peto method.
Unit of analysis issues
We found no unit of analysis issues.
Dealing with missing data
We attempted to contact the authors of the publications to ask for information related to missing data, as well as further information on risk of bias.
Assessment of heterogeneity
Heterogeneity was assessed by visual inspection of forest plots, the test for heterogeneity and I2. We suspected significant heterogeneity on the basis of the I2 tests; values of I2 greater than 25% were regarded as moderate heterogeneity and values of I2 greater than 75% as significant heterogeneity) (Higgins 2008). Clinical heterogeneity was determined on the basis of clinical and demographic data provided in the studies.
Assessment of reporting biases
Reporting biases were assessed by using funnel plots constructed from the data.
Data synthesis
We used RevMan 5.1 for the synthesis of data (Deeks 2008). Continuous data are presented as MDs with 95% CIs. Because substantial heterogeneity was suspected, these results were pooled using a random‐effects model. For dichotomous outcome data (primary outcomes, mortality and acute renal injury), we used a fixed‐effect model because the incidence rate was very low; results are presented as ORs, using the Peto method.
Subgroup analysis and investigation of heterogeneity
We undertook subgroup analyses for the following situations.
Methods used for renal protection.
Types of operation.
Studies on participants with pre‐existing renal dysfunction.
Sensitivity analysis
We undertook sensitivity analyses for randomized controlled trials using only studies with low or moderate risk of bias.
Results
Description of studies
Details of studies can be found in 'Characteristics of included studies' and 'Characteristics of excluded studies'.
Results of the search
In the 2008 update (Zacharias 2008), we identified 136 studies from the MEDLINE search, 113 studies from the EMBASE search and 177 from CENTRAL (426 studies in total). We searched reference lists and bibliographical data from all retrieved articles and reviews for additional, relevant material. We sought information from authors of published studies and contacted recognized experts on this topic about any unpublished data. We identified a further 25 studies by this method. Thus in 2008, a total of 451 studies were considered potentially eligible for this review.
The extended search strategy in 2011 yielded 655 studies, and four studies were obtained from other sources. Of these, we obtained full papers for 35 studies and included 26 additional studies in the review. A further search in August 2012 provided a further 318 studies. From this group, we added one study to the review, one conference presentation to the list of studies awaiting further analysis and one exclusion due to duplicity. In this updated review, we have included 72 studies (Figure 1).
1.
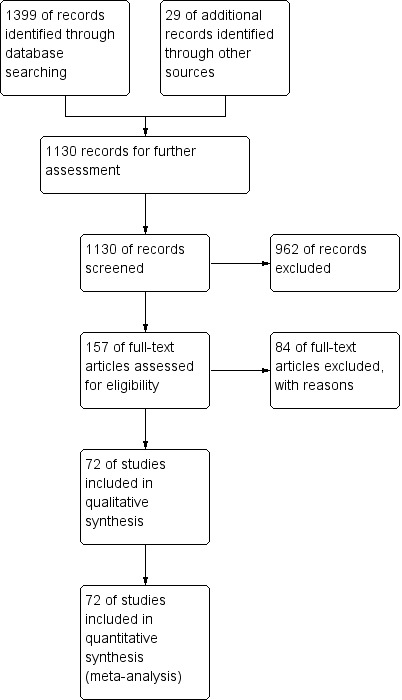
Study flow diagram, as of December 2012.
Included studies
See 'Characteristics of included studies'. The 72 included studies comprised a total of 4378 participants; 2291 of these received some form of intervention to protect the kidneys, and 2087 acted as controls. Of the 72 included studies, 13 had multiple arms (Barr 2008; Berendes 1997; Carcoana 2003; Colson 1992; Costa 1990; Dehne 2001; Donmez 1998; Dural 2000; Kleinschmidt 1997; Lassnigg 2000; Wahbah 2000; Yavuz 2002B; Zanardo 1993). We used the data from each arm separately for analysis of the interventions; whenever we did this, we adjusted (reduced) the numbers in the control groups in the appropriate sections. Barr 2008 had three arms; one arm used fenoldopam, one arm N‐acetyl cysteine and another arm a combination of the two (the latter was excluded from the review). Berendes 1997 had three treatment arms with increasing strengths of dopexamine in cardiac surgery; another arm acted as control. We combined the three treatment groups for the purpose of analysis. Carcoana 2003 had three treatment arms and a control arm; one treatment group received dopamine infusion during the surgery, one group received a bolus of mannitol in the pump prime and the third group received both treatments. We excluded the third arm of the study. Colson 1992 had two treatment arms and one control arm; one treatment group received a calcium channel blocker, and the second group received an angiotensin‐converting enzyme (ACE) inhibitor. Costa 1990 also had three arms-two treatment arms and one control arm; we excluded the arm that used multiple interventions (dopamine and SNP). Dehne 2001 had two control groups-one for participants with normal renal function and one for participants with pre‐existing renal dysfunction. Intervention groups in this study (two) used dopexamine and matched the control for the presence or absence of pre‐existing renal failure. Donmez 1998 used two intervention groups-one received verapamil and the other received nimodipine. Dural 2000 had three arms; one arm received dopamine, another arm received mannitol and the third arm was the control group. Kleinschmidt 1997 had two intervention groups-pentoxyfilline and gamma hydroxybutyrate-and one control group. Lassnigg 2000 had three arms-two active (dopamine or furosemide) and one control. In the case of Wahbah 2000, we used only one of three treatment arms because two arms combined multiple interventions (dopamine and mannitol, or dopamine and furosemide). This study contained a fourth control arm. Yavuz 2002B had three treatment arms and one control arm; one treatment arm used two interventions simultaneously, and we excluded this arm in the appropriate sections. Zanardo 1993 used two doses of dopamine by infusion; we combined the two groups in the analysis.
We identified three studies that were published both in abstract form and as full papers. We were unable to confirm whether they were duplicate papers and hence considered only the full papers for inclusion in the analysis (Kulka 1996; O'Hara 2002; Ryckwaert 2001) for this review. We have referred to the abstract publications (Kulka 1993; O'Hara 2002A; Ryckwaert 1995) in the table 'Characteristics of excluded studies'. The details of participants' sex were not available for all studies, so we did not attempt to separately document this information. All included studies except one (Cregg 1999) involved adult populations; the Cregg study involved correction of scoliosis surgery and included a younger age group.
Forty‐nine studies involved participants undergoing cardiac surgery (Adabag 2008; Amano 1994; Amano 1995; Ascione 1999; Barr 2008; Berendes 1997; Bergman 2002; Burns 2005; Carcoana 2003; Chen 2007; Cogliati 2007; Colson 1990; Costa 1990; Cregg 1999; Dehne 2001; Donmez 1998; Dural 2000; Durmaz 2003; Fischer 2005; Haase 2007; Haase 2009; Kaya 2007; Kleinschmidt 1997; Kramer 2002; Kulka 1996; Lassnigg 2000; Loef 2004; Marathias 2006; Morariu 2005; Morgera 2002; Myles 1993; Nouri‐Majalan 2009; Prasad 2010; Prowle 2012; Ristikankare 2006; Ryckwaert 2001; Sezai 2000; Sezai 2009; Sezai 2011; Shim 2007; Song 2009; Tang 1999; Tang 2002; Urzua 1992; Witczak 2008; Woo 2002; Yavuz 2002A; Yavuz 2002B; Zanardo 1993). Fifteen trials included participants undergoing abdominal aortic surgery (for aortic aneurysm and occlusive arterial disease) (Colson 1992; Dawidson 1991; de Lasson 1995; de Lasson 1997; Halpenny 2002; Hynninen 2006; Lau 2001; Licker 1996; Mitaka 2008; Nicholson 1996; Pull Ter Gunne 1990; Shackford 1983; Turner 2008; Welch 1995; Wijnen 2002). Four trials consisted of participants undergoing biliary surgery (Gubern 1988; Parks 1994; Thompson 1986; Wahbah 2000); one involved laparoscopic colorectal surgery (Perez 2002); one partial nephrectomy (O'Hara 2002); and one correction of scoliosis (Cregg 1999). Fourteen studies involved participants with pre‐existing renal dysfunction or those with increased risk of renal dysfunction as a result of the surgery (Adabag 2008; Burns 2005; Chen 2007; Cogliati 2007; Dehne 2001; Durmaz 2003; Haase 2007; Haase 2009; Marathias 2006; Nouri‐Majalan 2009; Prasad 2010; Prowle 2012; Ristikankare 2006; Witczak 2008).
Various treatment measures were used in the different trials to protect the kidneys during the perioperative period. Interventions included dopamine and its analogue or agonist (dopexamine or fenoldopam) in 22 studies (Barr 2008; Berendes 1997; Carcoana 2003; Cogliati 2007; Costa 1990; Cregg 1999; de Lasson 1995; Dehne 2001; Dural 2000; Halpenny 2002; Lassnigg 2000; Myles 1993; O'Hara 2002; Parks 1994; Perez 2002; Tang 1999; Wahbah 2000; Welch 1995; Woo 2002; Yavuz 2002A; Yavuz 2002B; Zanardo 1993); diuretics (mannitol, furosemide) in six trials (Carcoana 2003; Dural 2000; Gubern 1988; Lassnigg 2000; Nicholson 1996; Shim 2007); calcium channel blockers (diltiazem, nicardipine, felodipine, verapamil, nimodipine) in nine trials (Amano 1995; Bergman 2002; Cho 2009; Colson 1992; de Lasson 1997; Donmez 1998; Witczak 2008; Yavuz 2002B; Zanardo 1993); ACE inhibitors (captopril, enalapril) in four trials (Colson 1990; Colson 1992; Licker 1996; Ryckwaert 2001); N‐acetyl cysteine in seven trials (Adabag 2008; Barr 2008; Burns 2005; Fischer 2005; Hynninen 2006; Prasad 2010; Ristikankare 2006); atrial natriuretic peptide in five trials (Chen 2007; Mitaka 2008; Sezai 2000; Sezai 2009; Sezai 2011); and, in one trial each, glutathione (Amano 1994), prostaglandin (Morgera 2002), theophylline (Kramer 2002), clonidine (Kulka 1996), dexamethasone (Loef 2004; Morariu 2005), pentoxifylline (Kleinschmidt 1997), gamma hydroxybutyrate (Kleinschmidt 1997), antioxidant therapy (Wijnen 2002), phenylephrine (Urzua 1992), ursodeoxycholic acid (Thompson 1986) and preoperative haemodialysis (Durmaz 2003); and surgical measures such as off‐pump cardiac surgery (Ascione 1999; Tang 2002) and an extraperitoneal approach to aortic surgery (Lau 2001). Five studies looked at the effects of hydration fluids (Dawidson 1991; Harten 2008; Marathias 2006; Pull Ter Gunne 1990; Shackford 1983). One trial (Song 2009) used erythropoietin (EPO) as the intervention. We have included this because it is currently an area of interest in this field.
We have conducted subgroup analyses of trials to observe the effects of different interventions on renal protection in the perioperative period. These subgroups included dopamine and its analogue or agonist; diuretics; calcium channel blockers; ACE inhibitors; atrial natriuretic peptide; N‐acetyl cysteine; EPO; and hydration fluids. We also undertook subgroup analyses to observe the effects of the type of surgery; these included cardiac surgery, abdominal aortic surgery and biliary surgery. We performed a limited subgroup analysis of studies with pre‐existing renal impairment. We also completed a limited sensitivity analysis on studies with low risk of bias.
Excluded studies
We provide the reasons for excluding studies in the table 'Characteristics of excluded studies'. Studies published in languages other than English (German, Turkish, Serbian, Russian and Japanese) were translated with the help of volunteers. All of these studies were available as full publications. We have had only limited success in receiving adequate information and feedback from the authors whom we attempted to contact, in spite of repeated attempts. All included and excluded studies were published between 1976 and 2012. We did not include three studies in the analysis because we could not confirm that they were not duplicate publications (see below). We did not include three studies authored by Boldt et al because of issues surrounding the reliability of studies from that group of researchers.
Risk of bias in included studies
Even though we included 72 studies in the review, the overall methodological quality of the studies was poor. We used a quality assessment system (see 'Methodological quality of studies' Table 3) based on method of randomization, allocation concealment, blinding and reporting of dropouts as the criterion. We scored the methodological quality of selected studies as good, moderately good or poor. When randomization, allocation concealment and blinding (participants, researchers, care givers and nurses) were adequately described and appropriately done, we classified the study as a good quality study. When randomization, allocation concealment and blinding (participants, researchers, care givers and nurses) were stated to have been done but no details were given in the publication, we classified the study as moderately good. When we found no evidence of allocation concealment and blinding, we classified the study as having poor methodological quality. The risk of bias information is given in Figure 2 and Figure 3. The methodological quality assessment identified twelve studies of good quality (Adabag 2008; Burns 2005; Carcoana 2003; Cogliati 2007; Fischer 2005; Haase 2007; Haase 2009; Kaya 2007; Myles 1993; Prowle 2012; Song 2009; Turner 2008) and another nine studies for which the methodological quality was considered moderately good (Ascione 1999; Barr 2008; Cho 2009; de Lasson 1997; Hynninen 2006; Lassnigg 2000; Perez 2002; Ristikankare 2006; Shim 2007). Most of the studies that we assessed (51 studies) were classified as having poor methodological quality (see 'Methodological quality of studies', additional Table 3). We had no success in obtaining data on concealment of allocation, blinding and method of randomization from most of the trial authors. Some trials were old, and we had very little chance of contacting these authors. We received replies from only five authors.
2.
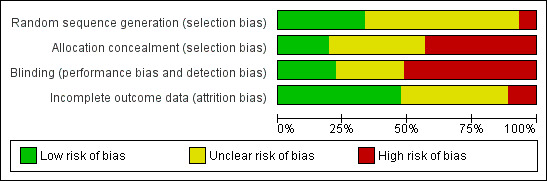
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across 78 included studies.
3.
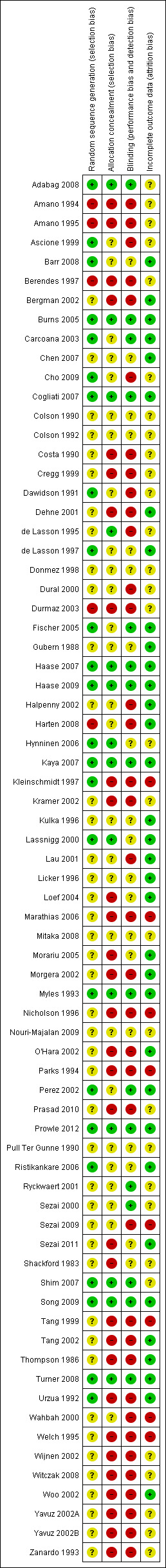
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
See 'Characteristics of included studies' and Table 3 for details.
Blinding
See 'Characteristics of included studies' and Table 3 for details.
Incomplete outcome data
Data are available in 'Characteristics of included studies'.
Selective reporting
Funnel plots for primary outcomes, reported mortality and reported acute renal failure showed no evidence of selective reporting (see Analysis 1.1 and Analysis 1.2).
1.1. Analysis.
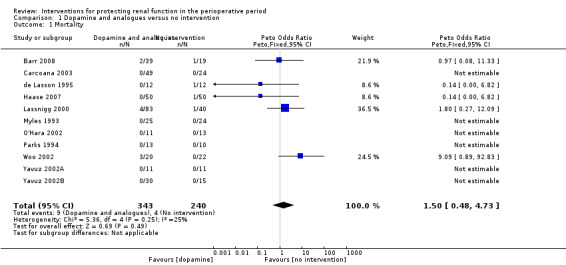
Comparison 1 Dopamine and analogues versus no intervention, Outcome 1 Mortality.
1.2. Analysis.
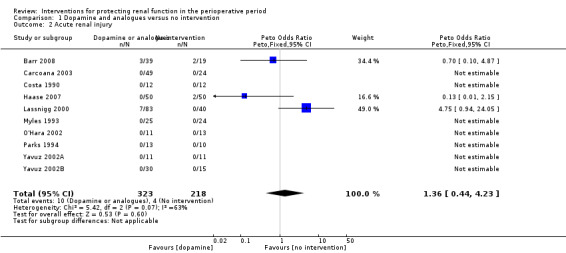
Comparison 1 Dopamine and analogues versus no intervention, Outcome 2 Acute renal injury.
Other potential sources of bias
None of the publications mentioned any conflict of interest with respect to the choice of drugs used. The following studies acknowledged pharmaceutical company sponsorships: de Lasson 1995; de Lasson 1997; Halpenny 2002; Kramer 2002; Lassnigg 2000; and Thompson 1986.
We constructed funnel plots to detect publication bias in primary outcomes, mortality and acute renal injury (failure) but employed this approach only in studies that looked at participants with pre‐existing renal damage and studies that were assessed to have low risk of bias.
Effects of interventions
We collected and analysed data from the selected 72 studies.
The dichotomous data (mortality and acute renal failure) consisted of rare events, so we used the Peto method of analysis and reported these results as Peto odds ratios (ORs) with 95% confidence intervals (CIs). We presented all continuous data results as mean differences (MDs) with 95% CIs. Results were plagued by heterogeneity throughout the analyses, so we used a random‐effects model instead of a fixed‐effect model. We undertook subgroup analysis for treatment measures and type of surgery. We were able to conduct only limited subgroup analysis for studies with pre‐existing renal impairment because of the inadequate number of trials identified. A limited sensitivity analysis was done for studies with low risk of bias. To make the review less cumbersome for the reader, study results are listed in the text only when they were considered essential; references to appropriate 'Data and Analysis' tables are given.
Mortality
Data on perioperative mortality were reported in 41 studies, which included 3116 participants. Many cases of mortality were due to a combination of factors, including surgical causes and pathology. The risk of mortality was very low, and we did not perform a meta‐analysis because of the significant clinical heterogeneity noted.
Acute renal injury
Acute renal injury (renal damage) in the postoperative period was reported in 44 studies. Many studies did not specify the criteria used to diagnose acute renal failure (ARF), hence we obtained information from these studies on the numbers of participants with acute renal injury requiring renal replacement therapy, in both treatment and control groups. Reported incidences were very low, and because of the significant clinical heterogeneity observed, we did not perform a meta‐analysis of the data.
Effectiveness of measures used for renal protection
In this section, we tried to combine the data from the 72 identified studies to ascertain the effectiveness of treatments provided to protect the kidneys during the perioperative period compared with findings in the control population. Again, because of clinical heterogeneity in the form of different procedures and populations and decades of reporting such data, we decided to refrain from combining the data for a meta‐analysis.
Effect of various interventions on renal protection
Most studies looked at dopamine and its analogues, although some trials used other measures to protect the kidneys during the perioperative period.
Dopamine or its analogues
Infusions of dopamine or its analogue (dopexamine) or agonist (fenoldopam) were used as treatment in 22 studies (Barr 2008; Berendes 1997; Carcoana 2003; Cogliati 2007; Costa 1990; Cregg 1999; de Lasson 1995; Dehne 2001; Dural 2000; Haase 2007; Halpenny 2002; Lassnigg 2000; Myles 1993; O'Hara 2002; Parks 1994; Perez 2002; Tang 1999; Wahbah 2000; Welch 1995; Woo 2002; Yavuz 2002A; Yavuz 2002B).
Mortality was reported in 11 trials (583 participants, OR 1.50, 95% CI 0.48 to 4.73, I2 = 25%; see Analysis 1.1), and acute renal injury was reported in 10 trials (541 participants, OR 1.36, 95% CI 0.44 to 4.23, I2 = 63%; see Analysis 1.2).
Urine output at 24 hours after operation was studied in 13 trials (see Analysis 1.3.1). Considerable heterogeneity was observed (I2 = 92%), and no difference was noted between intervention and control groups (MD 0.18 mL/min, 95% CI ‐0.19 to 0.54). Urine flow at two to three days showed no significant increase with the intervention in seven studies (see Analysis 1.3.2); the urine flow difference was 0.51 mL/min (95% CI 0.04 to 0.97), and heterogeneity was high (I2 = 95%). On the fifth to seventh day, treatment did not offer any advantages in five trials (see Analysis 1.3.3) (MD 0.23 mL/min, 95% CI ‐0.06 to 0.51, I2 = 72%).
1.3. Analysis.
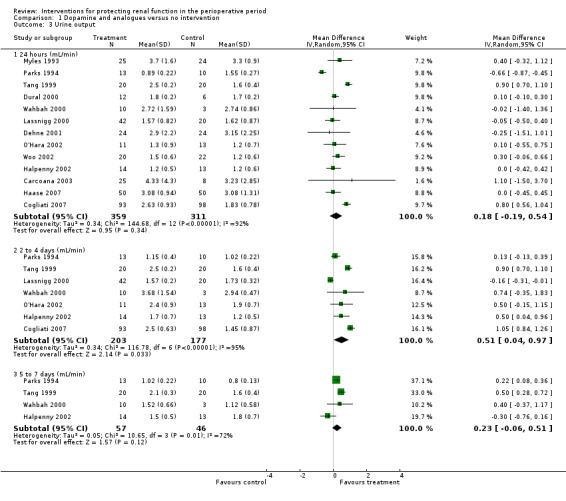
Comparison 1 Dopamine and analogues versus no intervention, Outcome 3 Urine output.
Creatinine clearance was studied in 15 trials after dopamine or its analogues were administered (see Analysis 1.4). Fourteen trials reported creatinine clearance at 24 hours; nine studies at two to three days; and five studies at five to seven days after an operation. Analysis showed no significant difference between intervention and control groups at 24 hours (616 participants, MD 7.17 mL/min, 95% CI ‐5.53 to 19.86) and considerable heterogeneity (I2 = 91%); one study, in particular, favoured the treatment group (Berendes 1997). No differences were reported at two to four days (MD 7.31 mL/min, 95% CI ‐6.19 to 20.82, I2 = 94%) or at five to seven days after the operation (MD ‐3.33 mL/min, 95% CI ‐13.63 to 6.98, I2 = 18%).
1.4. Analysis.
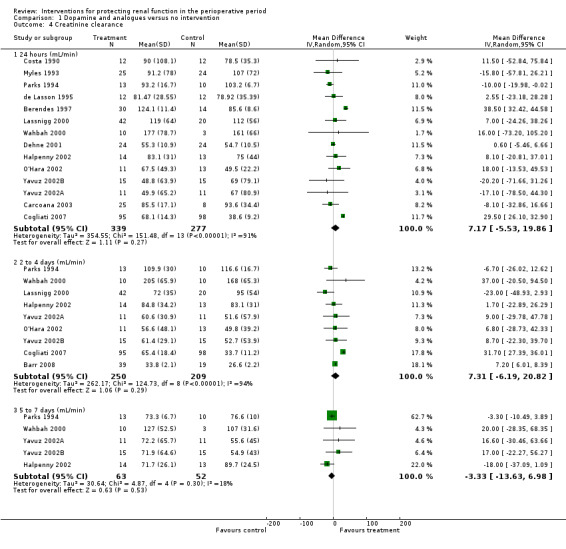
Comparison 1 Dopamine and analogues versus no intervention, Outcome 4 Creatinine clearance.
Free water clearance in mL/min was looked at 24 hours after surgery in six trials (see Analysis 1.5). Results showed no difference between treatment and control groups (MD 0.03 mL/min, 95% CI ‐0.17 to 0.22, I2 = 0%). Fractional excretion of sodium at 24 hours was reported in five trials (see Analysis 1.6). As in the previous section, we did not analyse these data.
1.5. Analysis.
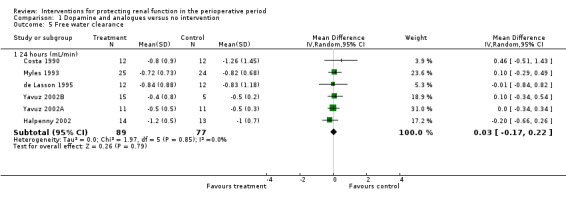
Comparison 1 Dopamine and analogues versus no intervention, Outcome 5 Free water clearance.
1.6. Analysis.
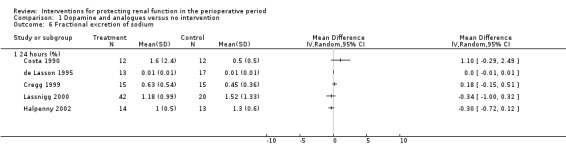
Comparison 1 Dopamine and analogues versus no intervention, Outcome 6 Fractional excretion of sodium.
Renal blood flow in mL/min was studied at 24 hours after surgery in only two trials (see Analysis 1.7) (de Lasson 1995; O'Hara 2002). No difference was noted (48 participants, MD 75.36 mL/min, 95% CI ‐63.27 to 213.98, I2 = 45%).
1.7. Analysis.
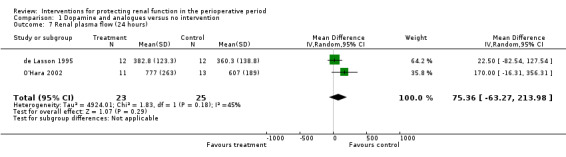
Comparison 1 Dopamine and analogues versus no intervention, Outcome 7 Renal plasma flow (24 hours).
Diuretics
Mortality was reported in four trials (255 participants, OR 2.49, 95% CI 0.50 to 7.74, I2 = 0%; see Analysis 2.1), and acute renal injury was reported in five trials (305 participants, OR 2.39, 95% CI 0.68 to 8.47, I2 = 35%; see Analysis 2.2).
2.1. Analysis.
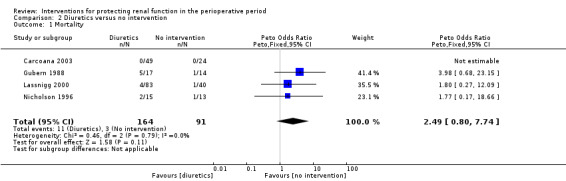
Comparison 2 Diuretics versus no intervention, Outcome 1 Mortality.
2.2. Analysis.
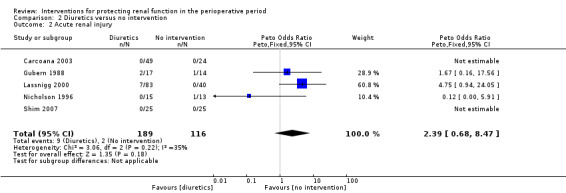
Comparison 2 Diuretics versus no intervention, Outcome 2 Acute renal injury.
Mannitol or furosemide was used as treatment in six studies. Data were available for only five studies (see Analysis 2.3). Urine output did not show a significant difference between groups at 24 hours in four studies (MD 0.10 mL/min, 95% CI ‐0.12 to 0.33, I2 = 0%; see Analysis 2.3.1); at two to three days in three studies (MD 0.17 mL/min, 95% CI ‐0.06 to 0.40, I2 = 0%). No significant differences were noted on any occasion.
2.3. Analysis.
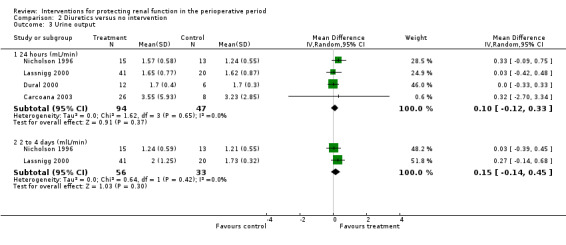
Comparison 2 Diuretics versus no intervention, Outcome 3 Urine output.
Creatinine clearance was measured at 24 hours in three studies. This measure showed no statistically significant differences (see Analysis 2.4.1; MD ‐18.02 mL/min, 95% CI ‐41.78 to 5.75, I2 = 55%). The same was true on the second to fourth day (see Analysis 2.4.2; MD 2.33 mL/min, 95% CI ‐14.76 to 19.42, I2 = 0%).
2.4. Analysis.
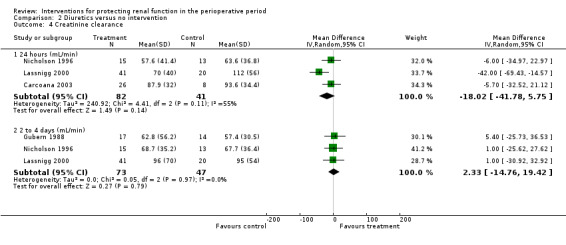
Comparison 2 Diuretics versus no intervention, Outcome 4 Creatinine clearance.
Calcium channel blockers
Mortality was reported in two trials (68 participants), and acute renal injury was reported in six trials (172 participants, OR 0.11, 95% CI 0.01 to 1.17; see Analysis 3.1 and Analysis 3.2, respectively).
3.1. Analysis.
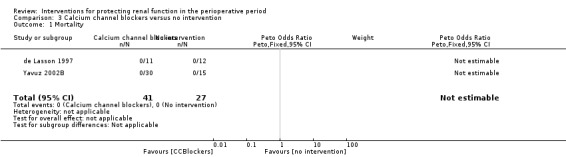
Comparison 3 Calcium channel blockers versus no intervention, Outcome 1 Mortality.
3.2. Analysis.
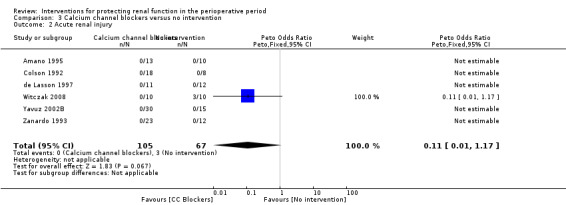
Comparison 3 Calcium channel blockers versus no intervention, Outcome 2 Acute renal injury.
Calcium channel blockers such as diltiazem, nicardipine and felodipine were used in nine studies. Four studies looked at urine output at 24 hours after treatment; no difference was observed between treatment and control groups (MD 0.23 mL/min, 95% CI 0.02 to 0.45, I2 = 0%; see Analysis 3.3.1). Five studies looked at creatinine clearance at 24 hours postoperatively (see Analysis 3.4.1). No advantage was derived from treatment (MD 4.74 mL/min, 95% CI ‐3.30 to 12.77, I2 = 57%). A total of 251 participants were included in the four studies.
3.3. Analysis.
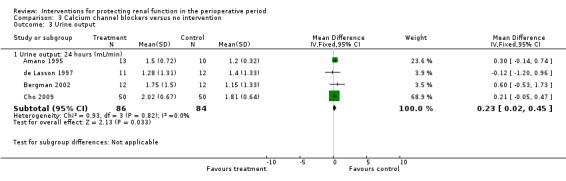
Comparison 3 Calcium channel blockers versus no intervention, Outcome 3 Urine output.
3.4. Analysis.
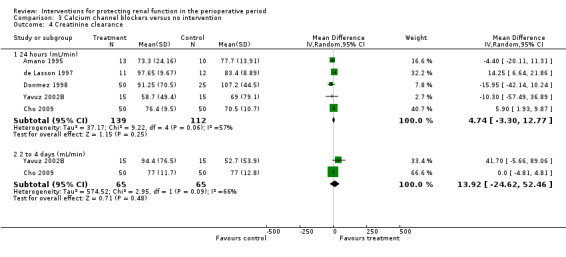
Comparison 3 Calcium channel blockers versus no intervention, Outcome 4 Creatinine clearance.
Three studies measured free water clearance at 24 hours (see Analysis 3.5.1) and reported no difference (MD ‐0.09 mL/min, 95% CI ‐0.47 to 0.29, I2 = 43%).
3.5. Analysis.
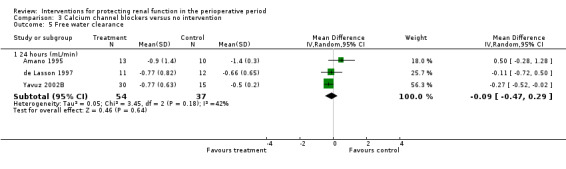
Comparison 3 Calcium channel blockers versus no intervention, Outcome 5 Free water clearance.
ACE inhibitors
Data were insufficient for calculations of risk of mortality and acute renal injury in this intervention group (see Analysis 4.1 and Analysis 4.2).
4.1. Analysis.
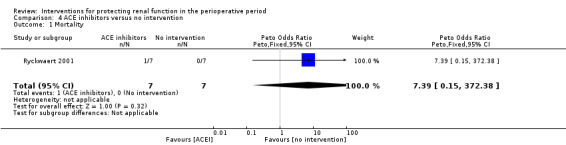
Comparison 4 ACE inhibitors versus no intervention, Outcome 1 Mortality.
4.2. Analysis.
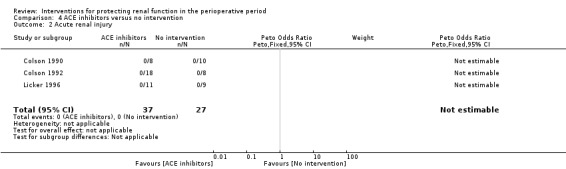
Comparison 4 ACE inhibitors versus no intervention, Outcome 2 Acute renal injury.
Four trials (Colson 1990; Colson 1992; Licker 1996; Ryckwaert 2001) looked at the usefulness of ACE inhibitors (enalapril or captopril) as renal protective agents. Data from three studies show that renal plasma flow in mL/min at the end of the operation (see Analysis 4.3.1) was not significantly different (MD 46.37 mL/min, 95% CI ‐68.61 to 161.34, I2 = 0%).
4.3. Analysis.
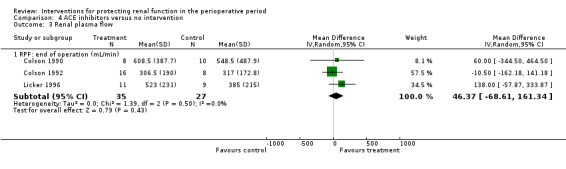
Comparison 4 ACE inhibitors versus no intervention, Outcome 3 Renal plasma flow.
Atrial Natriuretic Peptide
Mortality was reported in three trials (825 participants, OR 0.52, 95% CI 0.19 to 1.44, I2 = 48%; see Analysis 5.1), and acute renal injury was reported in four trials (865 participants, OR 0.23, 95% CI 0.08 to 0.64, I2 = 0%; see Analysis 5.2).
5.1. Analysis.
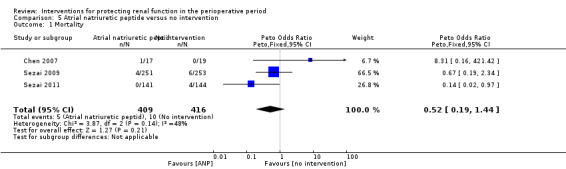
Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 1 Mortality.
5.2. Analysis.
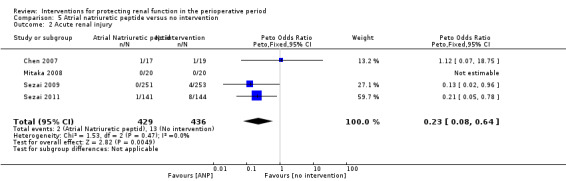
Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 2 Acute renal injury.
Five trials produced evidence for the use of atrial natriuretic peptide (ANP) (Chen 2007; Mitaka 2008; Sezai 2000; Sezai 2009; Sezai 2011). Much larger numbers of participants were included in Sezai 2009 and Sezai 2011 (789 participants), and this considerably influenced the meta‐analysis.
Urine output at 24 hours showed no significant change in three studies; this favoured the intervention (4.82 mL/min, 95% CI ‐2.74 to 12.38, I2 = 100%; see Analysis 5.3). Creatinine clearance at 24 hours in five studies showed similar results, favouring treatment by 4.31 mL/min (95% CI 0.34 to 8.28, I2= 99%; see Analysis 5.4). Creatinine clearance at 2 to 3 days in four studies favoured the intervention (13.11 mL/min, 95% CI 13.11 to 13.76, but with I2 = 100%; see Analysis 5.5). The poor methodological quality and the large heterogeneity (I2=100%) of the two dominant studies (Sezai 2009; Sezai 2011) make any conclusions worthless.
5.3. Analysis.
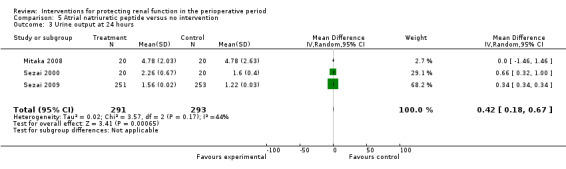
Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 3 Urine output at 24 hours.
5.4. Analysis.
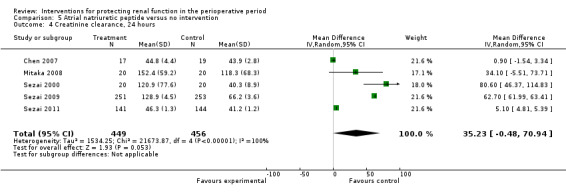
Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 4 Creatinine clearance, 24 hours.
5.5. Analysis.
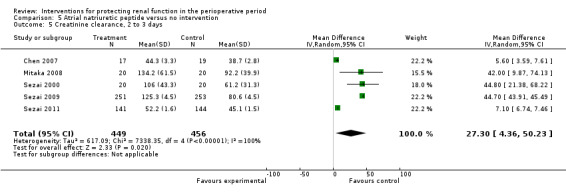
Comparison 5 Atrial natriuretic peptide versus no intervention, Outcome 5 Creatinine clearance, 2 to 3 days.
N‐Acetyl Cysteine
Mortality was reported in six trials (641 participants, OR 1.01, 95% CI 0.42 to 2.42, I2 = 0%; see Analysis 6.1), and acute renal injury was reported in five trials (601 participants, OR 0.91, 95% CI 0.32 to 2.62; I2 = 0%; see Analysis 6.2).
6.1. Analysis.
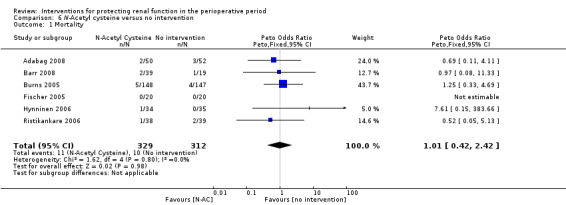
Comparison 6 N‐Acetyl cysteine versus no intervention, Outcome 1 Mortality.
6.2. Analysis.
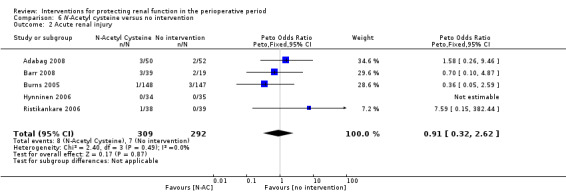
Comparison 6 N‐Acetyl cysteine versus no intervention, Outcome 2 Acute renal injury.
Seven trials used administration of N‐acetyl cysteine (NAC) as a measure to protect the kidneys intraoperatively (Adabag 2008; Barr 2008;Burns 2005; Fischer 2005; Haase 2007; Hynninen 2006; Prasad 2010; Ristikankare 2006). Urine output at 24 hours was estimated in two studies (146 participants). No differences were reported (0.23 mL/min, 95% CI ‐0.21 to 0.68, I2 = 47%).
Erythropoietin (EPO)
Recognition of EPO as a possible drug for renal protection is increasing, hence we reported data from the single available study. One study (Song 2009) used this drug in participants undergoing cardiac surgery, some of whom had pre‐existing raised creatinine. The data are given in Analysis 7.3, Analysis 7.4 and Analysis 7.5.
7.3. Analysis.
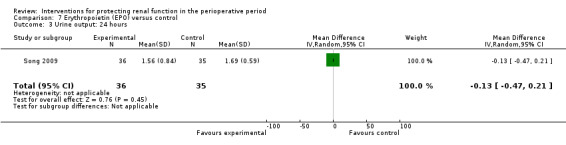
Comparison 7 Erythropoietin (EPO) versus control, Outcome 3 Urine output: 24 hours.
7.4. Analysis.
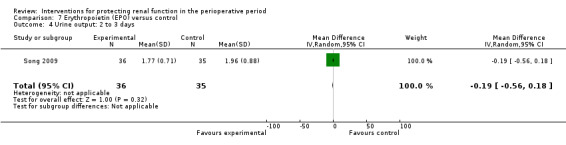
Comparison 7 Erythropoietin (EPO) versus control, Outcome 4 Urine output: 2 to 3 days.
7.5. Analysis.
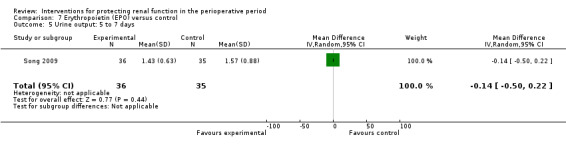
Comparison 7 Erythropoietin (EPO) versus control, Outcome 5 Urine output: 5 to 7 days.
We estimated the risk of mortality with this intervention in one study (71 participants, OR 0.13, 95% CI 0.00 to 6.63; see Analysis 7.1). The risk of acute renal injury was not estimable from this single intervention study (see Analysis 7.2).
7.1. Analysis.
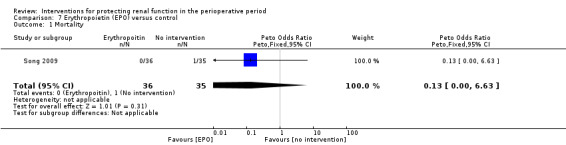
Comparison 7 Erythropoietin (EPO) versus control, Outcome 1 Mortality.
7.2. Analysis.
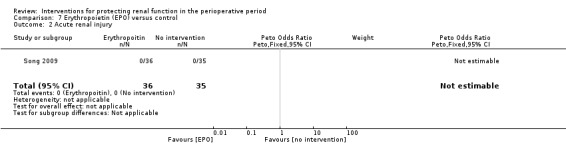
Comparison 7 Erythropoietin (EPO) versus control, Outcome 2 Acute renal injury.
Intravenous fluid
Mortality was reported in four trials (152 participants, OR 0.75, 95% CI 0.16 to 3.42, I2 = 0%; see Analysis 8.1), and acute renal injury was reported in three trials (123 participants, OR 0.22, 95% CI 0.05 to 0.96, I2 = 56%; see Analysis 8.2).
8.1. Analysis.
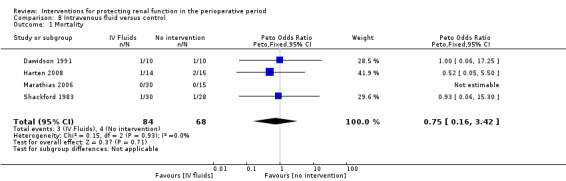
Comparison 8 Intravenous fluid versus control, Outcome 1 Mortality.
8.2. Analysis.
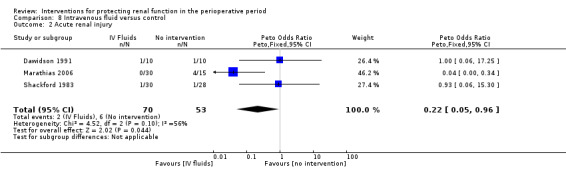
Comparison 8 Intravenous fluid versus control, Outcome 2 Acute renal injury.
Five trials studied the role of intravenous fluids such as colloids and hypertonic saline (Dawidson 1991; Harten 2008; Marathias 2006; Pull Ter Gunne 1990; Shackford 1983). Two studies (Pull Ter Gunne 1990; Shackford 1983) looked at creatinine clearance at 24 hours (see Analysis 8.3.1), and no difference was noted (MD ‐10.34 mL/min, 95% CI ‐29.57 to 8.88, I2 = 0%).
8.3. Analysis.
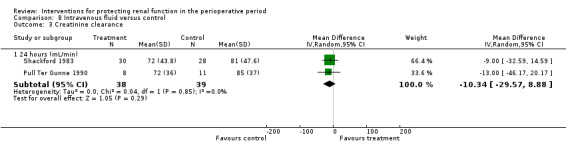
Comparison 8 Intravenous fluid versus control, Outcome 3 Creatinine clearance.
Cardiac surgery
Forty‐eight studies looked at the influence of different interventions in protecting the kidneys during cardiac surgery, and various measures were attempted.
Mortality
The risk of mortality (as reported in the trials) was estimated from 26 trials (2390 participants, OR 0.96, 95% CI 0.56 to 1.64, I2 = 10%; see Analysis 9.1).
9.1. Analysis.
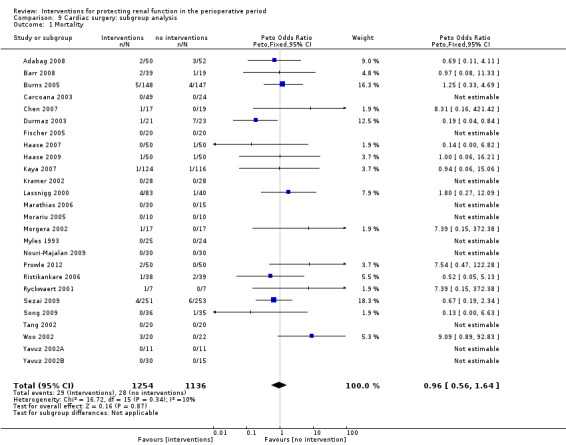
Comparison 9 Cardiac surgery: subgroup analysis, Outcome 1 Mortality.
Acute renal injury
The risk of acute renal injury (as reported in the trials) was estimated from 31 trials (2504 participants, OR 0.55, 95% CI 0.32 to 0.92, I2 = 49%; see Analysis 9.2).
9.2. Analysis.
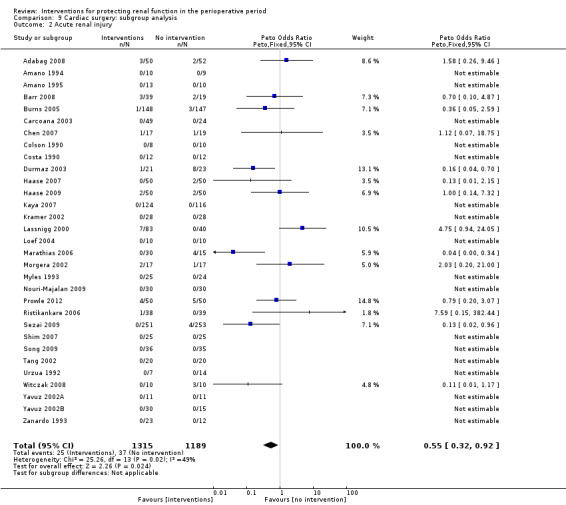
Comparison 9 Cardiac surgery: subgroup analysis, Outcome 2 Acute renal injury.
Urine output
Seventeen studies (774 participants in the intervention groups and 701 participants in the control groups) looked at the influence of different interventions on urine output after cardiac surgery at 24 hours (see Analysis 9.3.1). Urine output increased by 0.26 mL/min in the treatment group (95% CI 0.17 to 0.36) but with significant heterogeneity in the results (I2 = 86%). Nine studies (see Analysis 9.3.2) looked at urine output two to three days after surgery. This measurement also showed no difference between intervention and control groups (MD 0.21 mL/min, 95% CI ‐0.13 to 0.54, I2 = 100%).
9.3. Analysis.
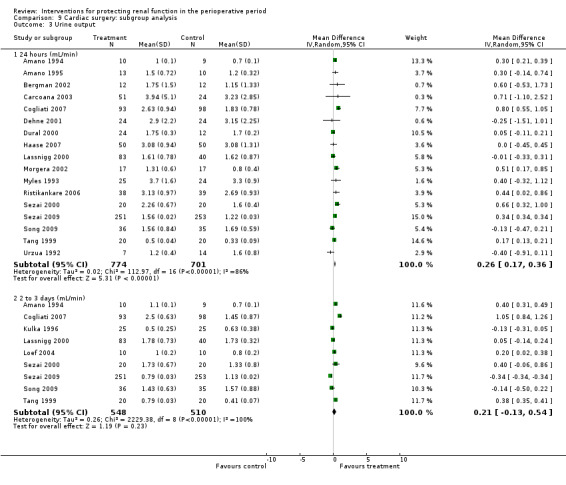
Comparison 9 Cardiac surgery: subgroup analysis, Outcome 3 Urine output.
Creatinine clearance
Twenty‐four studies (see Analysis 9.4.1) looked at creatinine clearance after cardiac surgery at 24 hours in 1120 participants in the intervention groups and in 1016 participants in the control groups. The results suggested no significant improvement in creatinine clearance with treatment compared with control (MD 9.38 mL/min, 95% CI ‐5.99 to 24.74). Heterogeneity was high (I2 = 100%). Creatinine clearance was reported in 17 studies at two to three days after cardiac surgery (see Analysis 9.4.2) and in seven studies on the fifth to seventh day (see Analysis 9.4.3). Any evidence of beneficial effects of treatment in the later postoperative days was not clear because of the high heterogeneity of the results. Creatinine clearance improved on the second to third postoperative days (MD 14.21 mL/min, 95% CI 3.58 to 24.85, I2 = 100%) and on the fifth to seventh postoperative days (MD 14.99 mL/min, 95% CI 0.84 to 29.13, I2 = 97%). However, it is worthwhile noting that Sezai 2000, Sezai 2009 and Sezai 2011 (using atrial natriuretic peptide infusion as the treatment) estimated the GFR, which considerably favoured the treatment groups; the latter two studies included disproportionately high numbers of participants (789) and showed poor methodological quality.
9.4. Analysis.
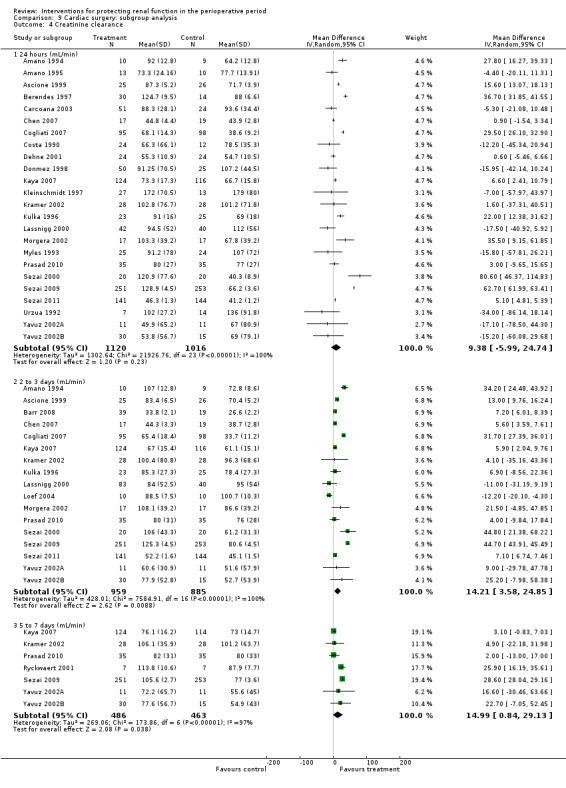
Comparison 9 Cardiac surgery: subgroup analysis, Outcome 4 Creatinine clearance.
Free water clearance
Free water clearance in mL/min was measured at 24 hours in seven studies (see Analysis 9.5.1). The results suggested less water clearance with treatment at 24 hours (MD ‐1.81 mL/min, 95% CI ‐2.02 to ‐1.60), with significant heterogeneity (I2 = 98%). Again, Sezai 2009 has largely influenced the results. A difference was reported from the second to the third day in four studies (MD ‐3.55 mL/min, 95% CI ‐3.89 to ‐03.21) but with significant heterogeneity (I2 = 99%).
9.5. Analysis.
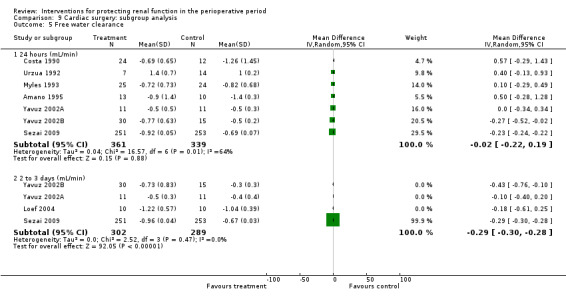
Comparison 9 Cardiac surgery: subgroup analysis, Outcome 5 Free water clearance.
Fractional excretion of sodium
Eight studies documented fractional excretion of sodium at 24 hours, and three studies at two to three days postoperatively (see Analysis 9.6).
9.6. Analysis.
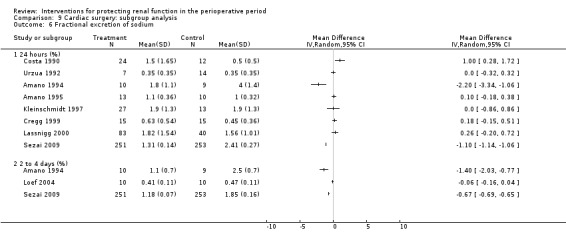
Comparison 9 Cardiac surgery: subgroup analysis, Outcome 6 Fractional excretion of sodium.
Aortic surgery
Mortality
The risk of mortality (as reported in the trials) was estimated from eight trials (236 participants, OR 0.76, 95% CI 0.20 to 2.89, I2 = 5%; see Analysis 10.1).
10.1. Analysis.
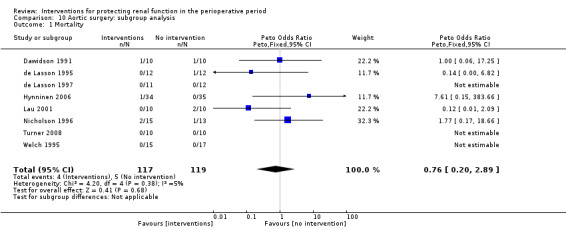
Comparison 10 Aortic surgery: subgroup analysis, Outcome 1 Mortality.
Acute renal injury
The risk of acute renal injury (as reported in the trials) was estimated from eight trials (284 participants, OR 0.62, 95% CI 0.11 to 3.70, I2 = 0%; see Analysis 10.2).
10.2. Analysis.
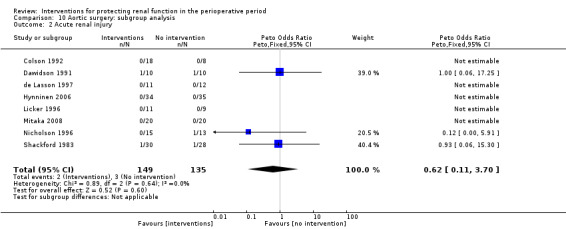
Comparison 10 Aortic surgery: subgroup analysis, Outcome 2 Acute renal injury.
Urine output
Seven studies measured urine output at 24 hours after elective aortic surgery. No demonstrable benefit was derived from treatment (see Analysis 10.3.1; MD ‐0.04 mL/min, 95% CI ‐0.10 to 0.19, I2 = 0%). Two trials measured urine output on second and third postoperative days and on fifth and seventh postoperative days (see Analysis 10.3.2 and Analysis 10.3.3) and showed no difference with treatment (MD 0.26 mL/min, 95% CI ‐0.06 to 0.58, I2 = 12% and MD ‐0.09 mL/min, 95% CI ‐0.39 to 0.21, I2 = 23%, respectively).
10.3. Analysis.
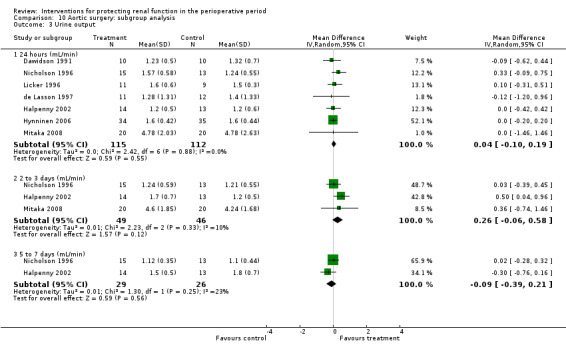
Comparison 10 Aortic surgery: subgroup analysis, Outcome 3 Urine output.
Creatinine clearance
Ten studies (see Analysis 10.4) looked at creatinine clearance after elective aortic surgery. All ten studies estimated creatinine clearance at 24 hours after surgery (see Analysis 10.4.1), and the results suggested no benefit resulting from treatment (MD 7.99 mL/min, 95% CI ‐0.77 to 16.74, I2 = 22%). The same conclusions were drawn from five trials that measured creatinine clearance on the second to third postoperative days and on the fifth to seventh postoperative days (see Analysis 10.4.2 and Analysis 10.4.3). The MD were 11.62 mL/min (95% CI ‐6.13 to 29.37, I2 = 46%) and ‐12.85 mL/min (95% CI ‐26.41 to 0.72, I2 = 5%), respectively. It is interesting to note that heterogeneity was moderate in these results.
10.4. Analysis.
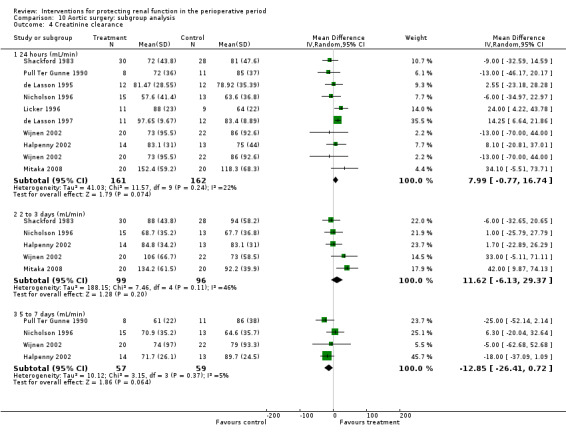
Comparison 10 Aortic surgery: subgroup analysis, Outcome 4 Creatinine clearance.
Free water clearance
Five trials studied this outcome at 24 hours after aortic surgery (see Analysis 10.5.1). Results showed no significant benefit derived from treatment (MD ‐0.25 mL/min, 95% CI ‐0.51 to 0.01, I2 = 0%). Two studies looked at free water clearance on the second to fourth postoperative days and on the fifth to seventh postoperative days (see Analysis 10.5.2 and Analysis 10.5.3). No differences were noted (MD 0.37 mL/min, 95% CI ‐0.12 to 0.85, I2 = 0% and MD 0.24 mL/min, 95% CI ‐0.13 to 0.61, I2 = 0%, respectively).
10.5. Analysis.
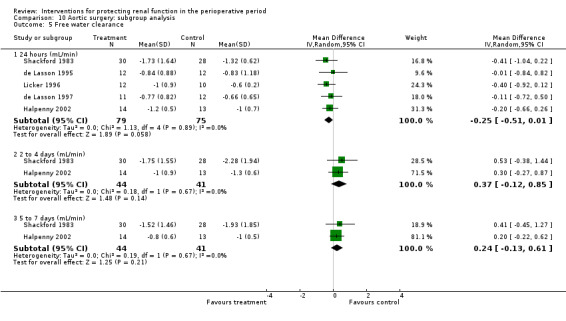
Comparison 10 Aortic surgery: subgroup analysis, Outcome 5 Free water clearance.
Fractional excretion of sodium
Five studies reported this outcome (see Analysis 10.6.1) at 24 hours, and two studies (see Analysis 10.6.2) reported the results on the second to fourth postoperative days.
10.6. Analysis.
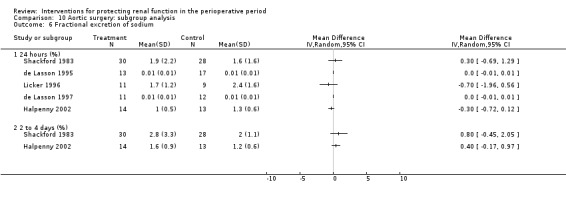
Comparison 10 Aortic surgery: subgroup analysis, Outcome 6 Fractional excretion of sodium.
Renal plasma flow
Four trials estimated renal plasma flow after aortic surgery (see 'Data and analyses'; Analysis 10.7). Analysis of results at the end of the operation in two studies (see Analysis 10.7.1) showed no statistically significant difference between treatment and control groups (MD 50.29 mL/min, 95% CI ‐92.83 to 193.40, I2 = 28%). The same held true for renal plasma flow at 24 hours after operation in two studies (see Analysis 10.7; MD 45.86 mL/min, 95% CI ‐18.64 to 110.36, I2 = 0%).
10.7. Analysis.
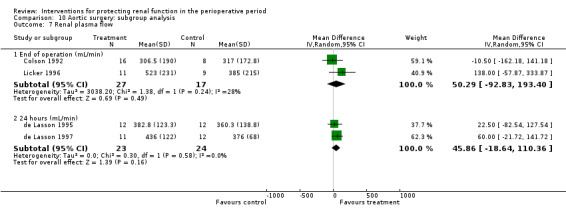
Comparison 10 Aortic surgery: subgroup analysis, Outcome 7 Renal plasma flow.
Biliary surgery
Urine output
Only two trials looked at urine output at 24 hours, two to four days, and five to seven days after biliary surgery (see Analysis 11.1). Some evidence from these two studies showed that urine output was less at 24 hours after an intervention was used (MD ‐0.59 mL/min, 95% CI ‐0.99 to ‐0.19, I2 = 18%), but it was not different at two to four days (MD 0.24 mL/min, 95% CI ‐0.22 to 0.69, I2 = 26%) and favoured control at five to seven days (MD 0.23 mL/min, 95% CI 0.09 to 0.37, I2 = 0%).
11.1. Analysis.
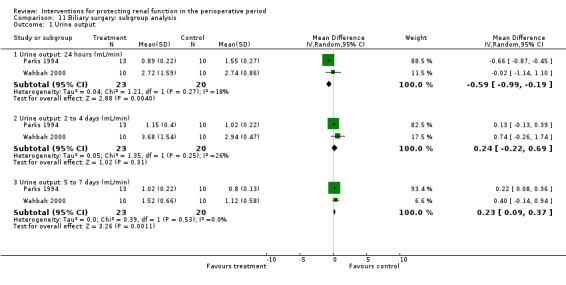
Comparison 11 Biliary surgery: subgroup analysis, Outcome 1 Urine output.
Creatinine clearance
Three trials measured creatinine clearance in mL/min at 24 hours (see Analysis 11.2.1). No benefit was derived from the use of an intervention (MD ‐2.84 mL/min, 95% CI ‐14.07 to 8.39, I2 = 46%). The same was true for creatinine clearance values from three trials measuring creatinine clearance on the second to fourth postoperative days (see Analysis 11.2.2; MD 0.42 mL/min, 95% CI ‐16.68 to 17.52, I2 = 8%) and from two trials at five to seven days postoperatively (MD 0.58 mL/min, 95% CI ‐16.43 to 17.60, I2 = 28%).
11.2. Analysis.
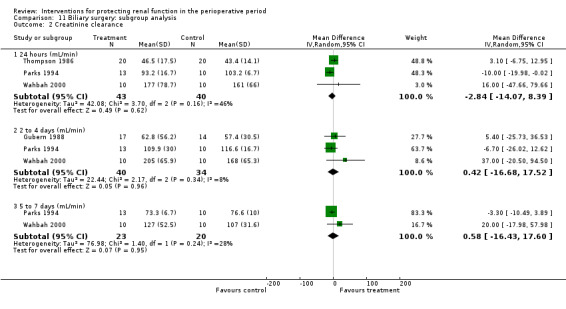
Comparison 11 Biliary surgery: subgroup analysis, Outcome 2 Creatinine clearance.
Pre‐existing renal impairment
Fourteen studies included participants with pre‐existing renal impairment or high risk of renal damage, although different criteria were used (Adabag 2008; Burns 2005; Chen 2007; Cogliati 2007; Dehne 2001; Durmaz 2003; Haase 2007; Haase 2009; Marathias 2006; Nouri‐Majalan 2009; Prasad 2010; Prowle 2012; Ristikankare 2006; Witczak 2008). All of these trials involved participants undergoing cardiac surgery. Bergman 2002 used diltiazem infusions; Costa 1990 used dopamine or dopexamine infusions; Durmaz 2003 used preoperative haemodialysis; and Marathias 2006 used preoperative hydration. We identified an additional ten studies with pre‐existing renal damage.
Ten studies looked at mortality. No differences were noted between treatment groups and control groups (OR 0.74, 95% CI 0.36 to 1.52, I2= 20%; see Analysis 12.1). Participants who developed acute renal injury requiring renal dialysis were reported in eleven studies (OR 0.40, 95% CI 0.22 to 0.76, I2 = 37%; see Analysis 12.2). Both of these analyses used the fixed‐effect model because the incidence rate was very low. Studies were sufficient for the construction of funnel plots (Figure 4 and Figure 5); both suggested no significant publication bias.
12.1. Analysis.
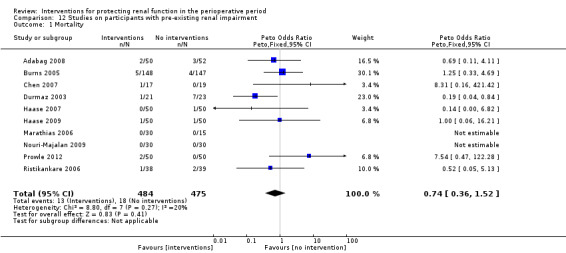
Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 1 Mortality.
12.2. Analysis.
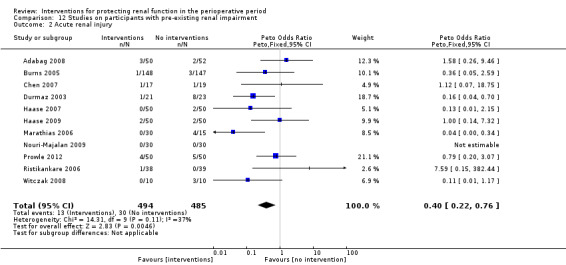
Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 2 Acute renal injury.
4.
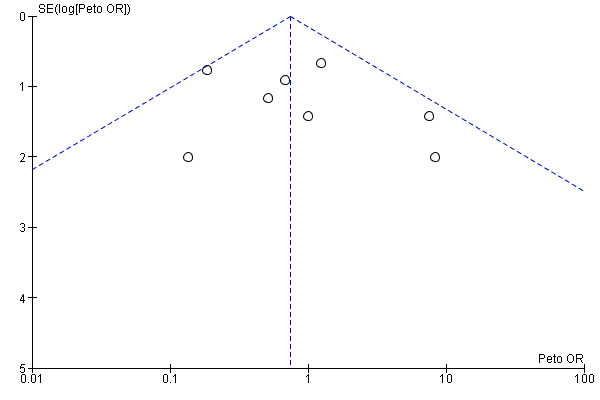
Funnel plot of comparison: 12 Studies on participants with pre‐existing renal impairment, outcome: 12.1 Mortality.
5.
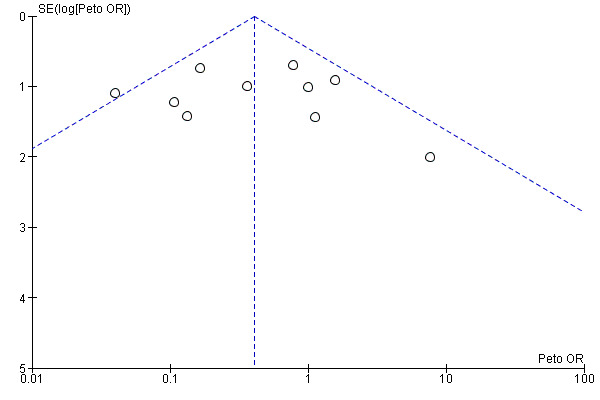
Funnel plot of comparison: 12 Studies on participants with pre‐existing renal impairment, outcome: 12.2 Acute renal injury.
Four studies looked at urine output at 24 hours. The heterogeneity of these studies was large and hence precludes any conclusions; at 24 hours, the difference between intervention groups and control groups was 0.55 mL/min (95% CI 0.37 to 0.74 mL, I2 = 76%; see Analysis 12.3.1), and at 2 to 3 days postoperatively, the difference was 0.48 mL/min (95% CI 0.32 to 0.64, I2 = 98%; see Analysis 12.3.2). Four studies also looked at creatinine clearance at 24 hours, and the difference was insignificant (0.65 mL/min, 95% CI ‐0.75 to 2.06, I2 = 97%; see Analysis 12.4.1). On postoperative days 2 to 3, the difference was 1.33 mL/min in three studies (95% CI ‐0.02 to 2.68, I2 = 95%; see Analysis 12.4.2).
12.3. Analysis.
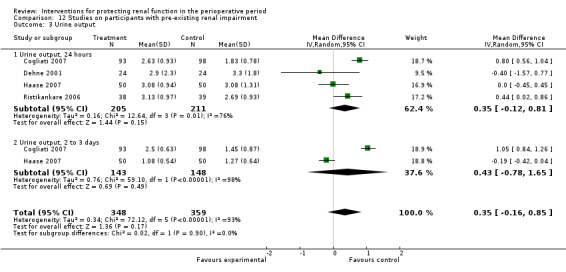
Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 3 Urine output.
12.4. Analysis.
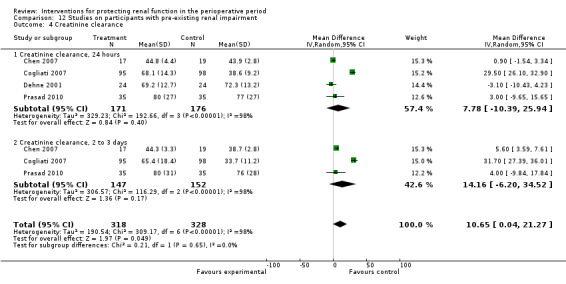
Comparison 12 Studies on participants with pre‐existing renal impairment, Outcome 4 Creatinine clearance.
Low risk of bias studies
In this update, we identified 24 studies as having low risk of bias (Additional Table 3, Characteristics of included studies), 12 of them being assessed as good (Adabag 2008; Burns 2005; Cogliati 2007; Fischer 2005; Haase 2007; Haase 2009; Kaya 2007; Myles 1993; Prowle 2012; Shim 2007; Song 2009; Turner 2008) and 12 assessed as moderately good (Ascione 1999; Barr 2008; Carcoana 2003; Cho 2009; Dawidson 1991; de Lasson 1995; de Lasson 1997; Hynninen 2006; Lassnigg 2000; Morariu 2005; Perez 2002; Ristikankare 2006). Data from these studies were subjected to sensitivity analysis. Unfortunately, reports on Perez 2002 contained data that were unsuitable for analysis, and this was confirmed by contact with the authors. We performed sensitivity analyses on the low risk of bias studies, and available data enabled us to report on mortality, acute renal impairment requiring renal supportive measures (dialysis) and urine output and creatinine clearance at 24 hours.
Nineteen studies reported mortality numbers (1604 participants,OR 1.01, 95% CI 0.52 to 1.97, I2= 0%; see Analysis 13.1). Fifteen studies reported data on acute renal injury requiring dialysis (1600 participants, OR 1.05, 95% CI 0.55 to 2.03, I2= 1%; see Analysis 13.2). Studies were sufficient for construction of funnel plots (Figure 6 and Figure 7); both suggested no significant publication bias.
13.1. Analysis.
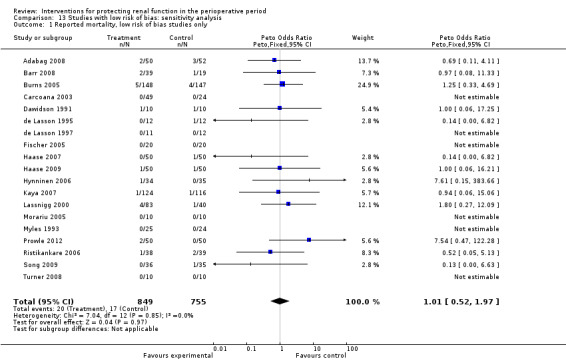
Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 1 Reported mortality, low risk of bias studies only.
13.2. Analysis.
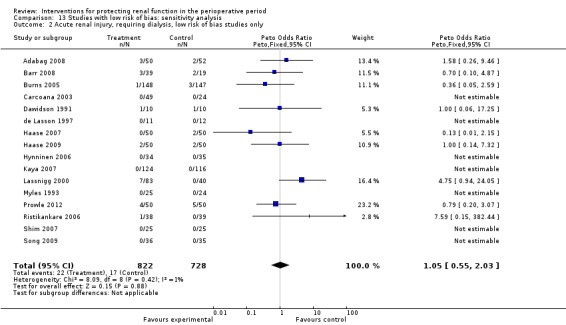
Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 2 Acute renal injury, requiring dialysis, low risk of bias studies only.
6.
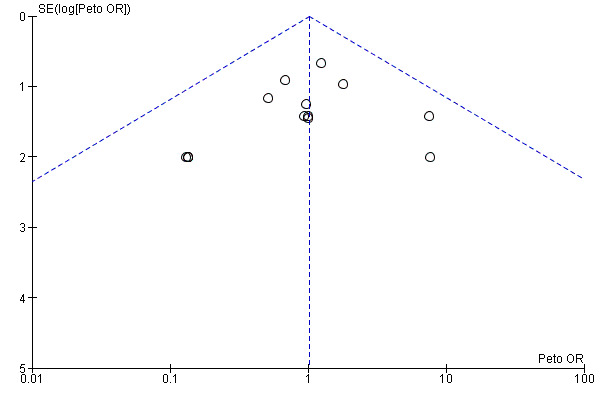
Funnel plot of comparison: 13 Studies with low risk of bias: sensitivity analysis, outcome: 13.1 Reported mortality, low risk of bias studies only.
7.
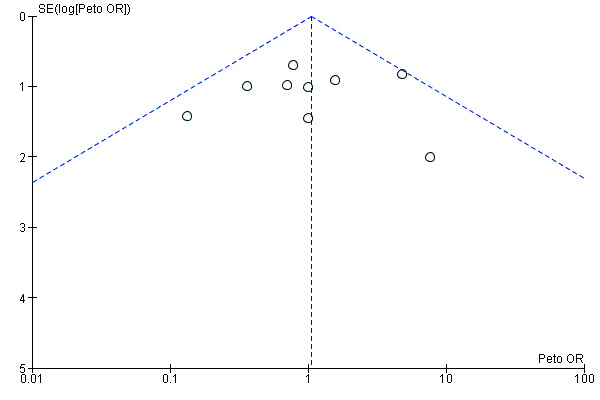
Funnel plot of comparison: 13 Studies with low risk of bias: sensitivity analysis, outcome: 13.2 Acute renal injury, requiring dialysis, low risk of bias studies only.
Urine output at 24 hours was reported in 11 studies; the difference was 0.20 mL/min (95% CI ‐0.04 to 0.44 mL/min, I2 = 71%; see Analysis 13.3). Creatinine clearance at 24 hours was reported in nine studies, and the difference was 6.59 mL/min (95% CI ‐3.53 to 16.72 mL/min, I2 = 94%; see Analysis 13.4).
13.3. Analysis.
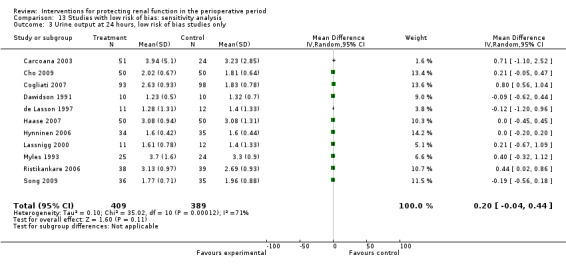
Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 3 Urine output at 24 hours, low risk of bias studies only.
13.4. Analysis.
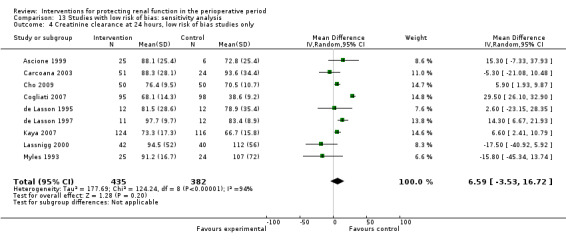
Comparison 13 Studies with low risk of bias: sensitivity analysis, Outcome 4 Creatinine clearance at 24 hours, low risk of bias studies only.
Results suggested that even the low risk of bias studies, although few, showed no overall advantage for treatment versus control groups.
Assessment of small study bias
Funnel plots were examined for meta‐analyses with 10 or more studies. Results showed no evidence of small sample biases (graphs not shown).
Summary of findings
A summary of findings (SoF) table was developed using GradePro on studies with pre‐existing renal impairment (Table 1). The results suggest no advantage of interventions for mortality, but statistical evidence suggests that interventions might be helpful for reducing acute renal injury. However, it should be noted that the evidence is of low quality because clinical heterogeneity was considerable. Studies with low risk of bias (Table 2) show no significant changes in reported perioperative mortality and in acute renal injury between intervention and control groups. Both SoF analyses suffer from low quality of evidence because of clinical heterogeneity and the small numbers of participants and events included in each study.
Discussion
Summary of main results
Renal dysfunction after major surgery is one of the causes of postoperative morbidity and mortality. The cause of renal injury in the postoperative period is thought to be multi‐factorial. Over the past three or four decades, many studies have tried to identify interventions that could provide renal protection during the perioperative period. Many different interventions have been tried, such as continuous infusions of dopamine or its analogues, use of diuretics such as mannitol and diligent use of ACE inhibitors and calcium channel blockers, to name a few. Drugs such as N‐acetyl cysteine (NAC), atrial natriuretic peptide (ANP), sodium bicarbonate, antioxidants and erythropoietin (EPO) are some of the more recent interventions. None of these interventions appears to have a good evidence base.
We were unable to combine the whole data because of significant heterogeneity among selected studies. Given the large range of treatments, operation types and methods used to protect the kidneys, it is not surprising that heterogeneity is large. Heterogeneity could have been the result of multiple causes, including differences in the nature of treatment, the duration of treatment or participants' conditions, and of course it could have been related to the methodological quality of the studies. We used subgroup analyses to explore this further.
We performed subgroup analyses for the different interventions that were studied. Dopamine and its analogues showed no improvement in urine output or in other tests such as creatinine clearance, free water clearance or fractional excretion of sodium. Overall, on the basis of available studies, it appears that dopamine and its analogues do not offer much protection to the kidneys. It is worth noting that in a multi‐centre study of participants in intensive care units (ANZICS CTG 2000), no significant benefit of dopamine was noted for these seriously ill individuals.
A 'Summary of findings' table, which used only low risk of bias studies, showed no benefit derived from interventions for domains of mortality and acute renal injury requiring dialysis. We looked at the effects of some of the major interventions (dopamine and its analogues, diuretics, calcium channel blockers, ACE inhibitors, atrial natriuretic peptide, N‐acetyl cysteine, IV fluids and EPO) and major surgical procedures (cardiac surgery and abdominal aortic surgery), as well as groups of participants identified as having pre‐existing renal damage; none of these groups showed a significant change in reported mortality or renal injury (as reported in the individual studies) in treatment groups versus control groups.
Overall completeness and applicability of evidence
Many physiological and biochemical variables can be used as markers of change in renal function. The various trials included in this review used numerous different markers as indicators of altered renal function. Each test has significant limitations, and results of the analysis must be interpreted in the context of these limitations.
Plasma creatinine is the most frequently measured marker of renal function. It is assumed that plasma creatinine remains constant and that clearance of creatinine occurs solely by glomerular filtration. Thus plasma creatinine is an indirect determinant of glomerular filtration rate (GFR). However, a greater than 50% reduction in GFR is needed before a change in plasma creatinine is seen. Plasma creatinine also reflects an individual's muscle mass, and alterations in muscle mass influence the plasma creatinine concentration, which does not reflect changes in GFR. A small amount of tubular excretion of creatinine occurs, which, in terms of normal GFR, is insignificant. However, with severe renal impairment, tubular secretion of creatinine has a greater role, and therefore plasma creatinine does not accurately reflect GFR. GFR is usually determined by the clearance of an inert substance, which is freely filtered at the glomerulus and has no tubular secretion or reabsorption. The gold standard has been estimation of inulin clearance. Creatinine clearance correlates well with GFR. For accuracy, it is essential that creatinine clearance is determined correctly. This requires a timed and complete collection of urine, along with a plasma creatinine determination. A variety of formulae have been derived that are based on plasma creatinine, body weight and age and are to estimate creatinine clearance and hence GFR. When renal function is stable, these estimates correlate well with measured GFR (r = 0.9).
Urine output is a non‐specific measure of renal function. Clearly, if no urine is produced, then no glomerular filtration occurs. However, urine output can be influenced by several factors that regulate renal tubular handling of water. Oliguria (<400 mL urine/24 h) may just reflect excess salt and water retention by the kidney due to low fluid intake, not necessarily impaired renal function or the effects of increased antidiuretic hormone (ADH) release-a normal response to surgery or stress.
In clinical practice, renal blood flow is rarely determined, but this can be done by using clearance techniques using the Fick principle.
Free water clearance measures urinary concentrating ability. Any form of damage to the kidney impairs urinary concentrating ability. With renal tubular injury, free water clearance is impaired. Likewise, free water clearance is modified by diuretic therapy.
The fractional excretion of sodium has been used as a marker of renal function. More correctly, it reflects renal tubular reabsorption of sodium. The normal physiological response to a reduction in renal perfusion and glomerular filtration is to activate the tubular glomerular feedback mechanism, leading to increased reabsorption of sodium, along with water. The net effect consists of increased blood pressure and hence renal perfusion. In the acute situation (the first 24 hours after an event that affects renal function), low fractional sodium excretion (FeNa < 1%) indicates impaired renal perfusion. With any form of established renal damage, or the use of diuretics, the fractional excretion of sodium is increased and becomes impossible to interpret. It is, therefore, essential that the changes in markers of renal function that were recorded in the analysed papers are examined critically for the variables that influence reported measurements. Conclusions drawn from the results should be closely examined for the validity of the renal function measures that were used. An inability to correctly interpret the results prompted us to refrain from analysing the data on FeNa and instead to provide the raw data.
Newer advances in determining acute renal injury have been reported. These include use of various biomarkers such as urinary N‐acetyl‐beta‐D‐glucosaminidase (U‐NAG) to creatinine ratio, urine retinol‐binding protein (RBP) to creatinine ratio and urinary neutrophil gelatinase‐associated lipocalin (NGAL) to creatinine ratio and blood plasma cystatin C levels. We have looked at these tests in this review update.
We believe it is important to emphasize that the lack of statistical significance described in this review could be due to many factors. We have already discussed heterogeneity as a significant factor; causes of heterogeneity include different types and durations of operations, gender differences, smoking status, state of nutrition, age, alcohol intake and co‐morbidities such as hypertension, diabetes or other unknown causes. The potential role of publication (or small sample) bias is also important. One of the common methods employed to facilitate recognition of publication bias is the funnel plot. The most common reasons for small sample bias are the reluctance of trialists or journal editors to publish because results are non-statistically significant, but other reasons include small numbers of cases investigated in trials; lack of allocation concealment; and inadequate blinding. All of these events may result in misleading positive outcomes, leading to publication bias. Apart from visual examination of funnel plots, various complicated statistical methods are available, but none are wholly satisfactory for recognizing and avoiding small sample bias. A high level of suspicion is always required when reviews consisting of poor quality studies and studies with small sample sizes are interpreted, as with this review. Of particular concern are the high I2 values seen in many analyses; these may be due to significant statistical and clinical heterogeneity.
Results obtained with the use of diuretics were disappointing and suggested no real advantage for participants who received the treatment. The same is true for the use of calcium channel blockers and ACE inhibitors, both of which apparently offer no advantages. The use of hydration fluids also showed no obvious advantage for clear fluids over specialized colloid solutions, although the methodology of the studies and the information provided were of poor quality. It is interesting to note that only four studies investigated the role of different types of intravenous fluids in the perioperative setting, although in most studies participants were well hydrated. Wahbah 2000 makes special mention of the fluid status of participants. Is it simply that kidneys are at their happiest when they have a good pre‐load to wash out toxic substances?
Other interventions such as N‐acetyl cysteine and EPO have failed to show any advantage. It is a matter of note that a meta‐analysis of studies using Atrial Natriuretic Peptides has shown some benefit of treatment in the form of improved creatinine clearance. However, caution is required in accepting these findings because two large studies conducted by one particular author reported an advantage of NAC over controls and considerably influenced the overall results (Sezai 2009; Sezai 2011).
Potential renal damage in participants with pre‐existing renal injury is widely recognized (Wijeysundera 2006). One area of interest for the authors of this review involved looking at the beneficial effects of treatment in participants with pre‐existing renal impairment. Fortunately, we were able to include in this updated review 14 studies that considered this topic. These studies used different interventions. Unfortunately, available data are somewhat limited for the purpose of analysis. No difference was noted in reported mortality, but some advantage seems to be associated with treatment intervention, thus avoiding acute renal injury. Note that we have previously discussed differing criteria between studies for the diagnosis of acute renal damage. Urine output seems to improve when interventions are provided for patients with pre‐existing renal damage, but the heterogeneity of the studies makes the results less valid.
We performed a sensitivity analysis of 24 studies of high and moderately high methodological quality. The results suggest no benefit for mortality or acute renal damage associated with treatment intervention. A marginal advantage seen in better urine output at 24 hours was offset by high heterogeneity, and no advantage was noted for creatinine clearance at 24 hours. These results substantiate the overall findings on questionable renal protection effects for various interventions given during the perioperative period.
It is important to remember that the effect of ADH is part of the usual stress response of surgery. Both urine output and free water clearance in the first 12 to 24 hours after surgery are reduced because of the influence of ADH (Brazel 1996). Fractional excretion of sodium is increased with the use of diuretics. Hence we would question the worth of measurements of urine output, free water clearance and fractional excretion of sodium as measures of renal function in the perioperative period. Glomerular filtration rate (and creatinine clearance) and renal plasma flow are good measures of renal function, but these must be measured accurately if meaningful conclusions are to be drawn.
Quality of the evidence
A major outcome of interest, mortality, was reported in a number of studies. Only a small number of deaths were reported in the trials, and this review shows that no advantage was conferred by individual interventions. Similarly, another outcome of major importance, acute renal injury after operation, was reported in only a few of the included studies. No evidence suggests that specific interventions offered any advantages for participants. However, it is important to recognize that the methodological quality of many of the included studies was poor and that the number of reported cases was small. Another point to consider is the inconsistency of the criteria used to diagnose renal injury across multiple studies. As a result, the statistical significance may not indicate a true advantage of interventions over no interventions.
In the subgroup of surgical procedures, cardiac surgery, aortic surgery and biliary surgery were considered for analysis. In cardiac surgery, interventions helped to increase urine output at 24 hours after surgery, but a high level of heterogeneity made the results unconvincing. Creatinine clearance also improved slightly at two to three days and five to seven days after surgery with treatment given to participants undergoing cardiac surgery, ,but the results are considerably swayed by the studies conducted by Sezai et al (Sezai 2009; Sezai 2011). None of the other tests showed any significant changes. Reported mortality and acute renal injury were no different after cardiac surgery and after abdominal aortic surgery.
No benefit was noted in other forms of interventions and surgery. However, the number and quality of studies in these areas are limited.
Potential biases in the review process
Even though we included 72 studies in this review, the overall methodological quality of the studies was poor. The methodological quality assessment identified twelve studies of good quality and another nine studies in which the methodological quality was considered moderately good. Most of the studies that we assessed (51 studies) were classified as showing poor methodological quality. This would reflect on any conclusions drawn from this review.
A note of caution: Many of the studies included in this review are old and were conducted before adaptations were made to the RIFLE (risk, injury, failure, loss of kidney function and end‐stage kidney disease) classification, which was introduced in 2004 (Bellomo 2007), and to acute kidney injury (AKI) diagnosis and classification criteria, introduced in 2007 (Mehta 2007). These classifications are generally accepted in modern clinical practice and research (Lopes 2013). So that the reader might find no uniformity or consistency for the diagnosis or classification of renal damage in many of the older studies included in this review; we have taken the criteria and diagnosis used by the authors. Any future update of this review should include a subgroup of studies conforming to such classification as RIFLE or AKI.
Another area of concern was that we were unable to standardize the administration or withholding of various medications, which may or may not have influenced individual study results.
Agreements and disagreements with other studies or reviews
Results of this review reflect previous versions of the review (Zacharias 2005; Zacharias 2008). A recent systematic review, undertaken to look at renal protection offered by perioperative haemodynamic manipulation, looks at the effects of haemodynamic stabilization and the effects of inotropes and fluids or a combination of these (Brienza 2009). Although we are reluctant to comment on the methodological rigor of the Brienza review, it is of note that this review agrees with the present review on the use of fluids as a measure to protect renal function, showing no clear advantage for fluid management alone. However, the focus of our review is exploration of the effects of pharmacological agents used in the perioperative period.
Authors' conclusions
Implications for practice.
No convincing evidence suggests that pharmacological or other interventions used to protect the kidneys during surgery are of benefit to patients, as seen from 72 included studies. Reasonable numbers of studies are currently available to substantiate this point. It is also of note that we were unable to find any significant adverse effects associated with the various interventions.
Implications for research.
Many studies are available on the use of various pharmaceutical agents to protect renal function during surgery. Further areas of possible research might include the use of newer methods of identifying acute renal damage, such as urinary biomarkers. Future studies should focus on evaluation of preventive measures or more precise and accurate methods of identifying the benefit or harm of interventions. Use of RIFLE or AKI classification to identify renal damage would result in better comparisons of the effects of interventions and procedures.
Feedback
Please forward any comments and feedback to Jane Cracknell (jane_cracknell@yahoo.com), Managing Editor, Cochrane Anaesthesia Review Group.
What's new
| Date | Event | Description |
|---|---|---|
| 9 September 2013 | New citation required but conclusions have not changed | We updated the search until August 2012; some of the authors have been changed from the previous version of this review (Zacharias 2008) We included 19 new studies We identified new interventions and incorporated them into the review (N‐acetyl cysteine; atrial natriuretic peptide; erythropoietin (EPO)) We identified new outcomes in the form of biomarkers of renal damage and added them to the review in keeping with current trends in the literature The conclusions of the new update have not changed from those provided in the previous published version of this review (Zacharias 2008) |
| 9 September 2013 | New search has been performed | Major update completed |
History
Protocol first published: Issue 2, 2002 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 7 August 2008 | New search has been performed | The following changes from the previous published review are made in this updated review. 1.We have modified the search strategy and updated it until June 2007. 2. A change in members of the review team from the first published review to the present update. Some of the original review authors were no longer available, and other authors have joined the review team. 3. Modifed search strategy in June 2007 identified further studies, which are incorporated in the updated review. 4. Some of the previous studies included in the review have been dropped following further detailed evaluation of the studies and new ones were added. 5. There are minor changes in the results following the above modifications, but the conclusions remain the same. |
| 7 August 2008 | New citation required but conclusions have not changed | New review team; review has undergone substantial work |
Notes
This review was published first in 2005 and was updated in 2008 (Zacharias 2005; Zacharias 2008).
Acknowledgements
We would like to thank Anna Lee, Nathan Pace, Mike Bennett, Giovanni Strippoli, Giuseppe Remuzzi, Amy Arkel, Janet Wale and Nete Villebro, as well as Suetonia Palmer, Marliers Ostermann and Giovanni Strippoli, for their valuable suggestions during the editorial process of preparing the previous review (Zacharias 2005) and this review. We are very grateful to Dr Ian Gilmore for his contribution as co‐author to the previous versions of this review. Our special thanks to Jane Cracknell, the Co‐ordinator for the Cochrane Anaesthesia Review Group, for her encouragement, support, help and great patience. We thank Dolores Matthews for the copy editing of this review.
We are also grateful to Toni Yalovitch, Mina Nishimori and Murat Genc for their assistance with manuscript translation, from Slovakian, Japanese and Turkish, respectively.
Appendices
Appendix 1. Search strategies employed
Search strategy for MEDLINE (Ovid SP) 1 exp Kidney Failure/ or exp Kidney Failure Acute/ or exp Kidney Failure Chronic/ or exp Kidney Function Tests/ or exp Glomerular Filtration Rate/ or exp Renal Circulation/ or exp Renal Plasma Flow/ or exp Renal Insufficiency/ or kidney.ti,ab. or (glomerul* adj3 filtration).mp. or (renal adj3 (failure or protect* or function*)).mp. or kidney function test*.mp. or renal function test*.mp. or free water clearance.mp. or fractional excretion of sodium.mp. or (urine adj3 (output or flow)).mp. 2 exp Angiotensin Converting Enzyme Inhibitors/ or exp Fluid Therapy/ or exp Infusions Intravenous/ or exp Angiotensin Converting Enzyme Inhibitors/ or exp diuretics/ or exp mannitol/ or exp Furosemide/ or exp Dopamine/ or exp Dopamine Agonists/ or (diuretic* or mannitol or frusemide or furosemide).mp. or (fluid* adj3 therap*).mp. or (intravenous adj3 fluid*).mp. or hydration.ti,ab. or angiotensin converting enzyme inhibitor*.mp. or ACE inhibitor*.mp. or dopamin*.ti,ab. 3 exp Perioperative Care/ or exp Intraoperative Period/ or exp Intraoperative Care/ or exp Intraoperative Complications/ or (peri?operativ* or intra?operativ*).ti,ab. 4 1 and 2 and 3 5 reno?protect*.af. 6 4 or 5 (2513) 7 ((randomized controlled trial or controlled clinical trial).pt. or randomized.ab. or placebo.ab. or clinical trials as topic.sh. or randomly.ab. or trial.ti.) not (animals not (humans and animals)).sh. 8 6 and 7 Search strategy for EMBASE (Ovid SP) 1 exp kidney failure/ or exp kidney failure/ or exp kidney function test/ or exp glomerulus filtration rate/ or exp kidney circulation/ or exp kidney clearance/ or exp kidney plasma flow/ or exp urine flow rate/ or exp urine volume/ or kidney.ti,ab. or (glomerul* adj3 filtration).mp. or (renal adj3 (failure or protect* or function*)).mp. or kidney function test*.mp. or renal function test*.mp. or free water clearance.mp. or fractional excretion of sodium.mp. or (urine adj3 (output or flow)).mp. 2 exp dipeptidyl carboxypeptidase inhibitor/ or exp fluid therapy/ or intravenous drug administration/ or exp diuretic agent/ or exp diuretic agent/ or exp mannitol/ or exp furosemide/ or exp dopamine/ or exp dopamine receptor stimulating agent/ or (diuretic* or mannitol or frusemide or furosemide).mp. or (fluid* adj3 therap*).mp. or (intravenous adj3 fluid*).mp. or hydration.ti,ab. or angiotensin converting enzyme inhibitor*.mp. or ACE inhibitor*.mp. or dopamin*.ti,ab. 3 exp perioperative period/ or exp intraoperative period/ or exp peroperative care/ or (peri?operativ* or intra?operativ*).ti,ab. 4 1 and 2 and 3 5 reno?protect*.ti,ab. 6 4 or 5 7 (placebo.sh. or controlled study.ab. or random*.ti,ab. or trial*.ti,ab. or ((singl* or doubl* or trebl* or tripl*) adj3 (blind* or mask*)).ti,ab.) not (animals not (humans and animals)).sh. 8 6 and 7 Search strategy for CENTRAL, The Cochrane Library #1 MeSH descriptor Acute Kidney Injury explode all trees #2 MeSH descriptor Kidney Failure, Chronic explode all trees #3 MeSH descriptor Kidney Function Tests explode all trees #4 MeSH descriptor Glomerular Filtration Rate explode all trees #5 MeSH descriptor Renal Circulation explode all trees #6 MeSH descriptor Renal Plasma Flow, Effective explode all trees #7 MeSH descriptor Renal Insufficiency explode all trees #8 kidney #9 glomerul* near filtration #10 renal near (failure or protect* or function*) #11 kidney function test* #12 renal function test* #13 free water clearance #14 (fractional excretion) of sodium #15 urine near (output or flow) #16 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15) #17 MeSH descriptor Angiotensin‐Converting Enzyme Inhibitors explode all trees #18 diuretic* or mannitol or frusemide or furosemide #19 MeSH descriptor Fluid Therapy explode all trees #20 fluid* near therap* #21 MeSH descriptor Infusions, Intravenous explode all trees #22 (intravenous near fluid*) or hydration #23 angiotensin converting enzyme inhibitor* #24 ACE inhibitor* #25 MeSH descriptor Diuretics explode all trees #26 MeSH descriptor Mannitol explode all trees #27 MeSH descriptor Furosemide explode all trees #28 MeSH descriptor Dopamine explode all trees #29 MeSH descriptor Dopamine Agonists explode all trees #30 dopamin* #31 (#16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29 OR #30) #32 MeSH descriptor Perioperative Care explode all trees #33 MeSH descriptor Intraoperative Care explode all trees #34 MeSH descriptor Intraoperative Complications explode all trees #35 MeSH descriptor Intraoperative Period explode all trees #36 perioperativ* or intraoperativ* #37 (#32 OR #33 OR #34 OR #35 OR #36) #38 (#16 AND #31 AND #37)
Appendix 2. data extraction form
Data extraction form
Study ID:
Language: English/
What was the surgical procedure?
What was the study intervention?
How many participants were studied?
How many in the intervention group?
How many in the control group?
Were the inclusion criteria clearly defined?
Were the exclusion criteria clearly defined?
Age group Intervention Control
Male:Female numbers:
Bias:
Was there randomization of allocation in the groups?
Was there adequate information about randomization?
Was there allocation concealment?
Was the allocation concealment adequate?
Were there any withdrawals from the study?
How many people withdrew from each group?
Was there blinding in the study?
Study details:
What was the actual nature of the intervention?
When did the intervention start and finish?
What was the actual nature of the control group?
When did the control group start and finish?
Were the two groups treated equally?
What were the outcomes studied?
Mortality
Acute renal failure
Urine output
Creatinine clearance
Free water clearance
Fractional excretion of sodium
Renal blood flow
Urinary microalbumin:creatinine ratio
Urinary NAG:creatinine ratio
Urinary RBOP:creatinine ratio
Urinary NGAL:creatinine ratio
Plasma cystatin C
When were the outcomes measured?
Preoperative Postoperative: 24 hours Postoperative: 48 hours Postoperative: 72 hours Postoperative: day (note)
Was the outcome assessment blinded?
Was there intention‐to‐treat analysis?
Are mean and standard deviation given?
Other measures of presentation of data
Graphic data?
Results:
Mean and SD Other measures: (please specify clearly)
Remarks:
Drug company sponsorship?
Other comments:
Data and analyses
Comparison 1. Dopamine and analogues versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 11 | 583 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.50 [0.48, 4.73] |
| 2 Acute renal injury | 10 | 541 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.44, 4.23] |
| 3 Urine output | 13 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 13 | 670 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.19, 0.54] |
| 3.2 2 to 4 days (mL/min) | 7 | 380 | Mean Difference (IV, Random, 95% CI) | 0.51 [0.04, 0.97] |
| 3.3 5 to 7 days (mL/min) | 4 | 103 | Mean Difference (IV, Random, 95% CI) | 0.23 [‐0.06, 0.51] |
| 4 Creatinine clearance | 15 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 14 | 616 | Mean Difference (IV, Random, 95% CI) | 7.17 [‐5.53, 19.86] |
| 4.2 2 to 4 days (mL/min) | 9 | 459 | Mean Difference (IV, Random, 95% CI) | 7.31 [‐6.19, 20.82] |
| 4.3 5 to 7 days (mL/min) | 5 | 115 | Mean Difference (IV, Random, 95% CI) | ‐3.33 [‐13.63, 6.98] |
| 5 Free water clearance | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 24 hours (mL/min) | 6 | 166 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.17, 0.22] |
| 6 Fractional excretion of sodium | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 24 hours (%) | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Renal plasma flow (24 hours) | 2 | 48 | Mean Difference (IV, Random, 95% CI) | 75.36 [‐63.27, 213.98] |
Comparison 2. Diuretics versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | 255 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.49 [0.80, 7.74] |
| 2 Acute renal injury | 5 | 305 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.39 [0.68, 8.47] |
| 3 Urine output | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 4 | 141 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.12, 0.33] |
| 3.2 2 to 4 days (mlL/min) | 2 | 89 | Mean Difference (IV, Random, 95% CI) | 0.15 [‐0.14, 0.45] |
| 4 Creatinine clearance | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 3 | 123 | Mean Difference (IV, Random, 95% CI) | ‐18.02 [‐41.78, 5.75] |
| 4.2 2 to 4 days (mL/min) | 3 | 120 | Mean Difference (IV, Random, 95% CI) | 2.33 [‐14.76, 19.42] |
Comparison 3. Calcium channel blockers versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 2 | 68 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Acute renal injury | 6 | 172 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.11 [0.01, 1.17] |
| 3 Urine output | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Urine output: 24 hours (mL/min) | 4 | 170 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [0.02, 0.45] |
| 4 Creatinine clearance | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 5 | 251 | Mean Difference (IV, Random, 95% CI) | 4.74 [‐3.30, 12.77] |
| 4.2 2 to 4 days (mL/min) | 2 | 130 | Mean Difference (IV, Random, 95% CI) | 13.92 [‐24.62, 52.46] |
| 5 Free water clearance | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 24 hours (mL/min) | 3 | 91 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.47, 0.29] |
Comparison 4. ACE inhibitors versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 14 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| 2 Acute renal injury | 3 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Renal plasma flow | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 RPF: end of operation (mL/min) | 3 | 62 | Mean Difference (IV, Random, 95% CI) | 46.37 [‐68.61, 161.34] |
Comparison 5. Atrial natriuretic peptide versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 3 | 825 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.19, 1.44] |
| 2 Acute renal injury | 4 | 865 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.08, 0.64] |
| 3 Urine output at 24 hours | 3 | 584 | Mean Difference (IV, Random, 95% CI) | 0.42 [0.18, 0.67] |
| 4 Creatinine clearance, 24 hours | 5 | 905 | Mean Difference (IV, Random, 95% CI) | 35.23 [‐0.48, 70.94] |
| 5 Creatinine clearance, 2 to 3 days | 5 | 905 | Mean Difference (IV, Random, 95% CI) | 27.30 [4.36, 50.23] |
Comparison 6. N‐Acetyl cysteine versus no intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 6 | 641 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.42, 2.42] |
| 2 Acute renal injury | 5 | 601 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.32, 2.62] |
| 3 Urine output, 24 hours | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 0.18 [‐0.24, 0.60] |
6.3. Analysis.
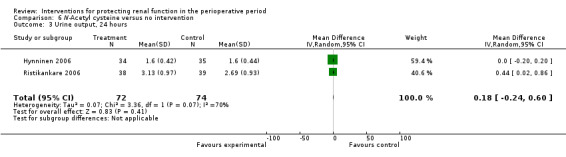
Comparison 6 N‐Acetyl cysteine versus no intervention, Outcome 3 Urine output, 24 hours.
Comparison 7. Erythropoietin (EPO) versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.63] |
| 2 Acute renal injury | 1 | 71 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Urine output: 24 hours | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.47, 0.21] |
| 4 Urine output: 2 to 3 days | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.56, 0.18] |
| 5 Urine output: 5 to 7 days | 1 | 71 | Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.50, 0.22] |
Comparison 8. Intravenous fluid versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 4 | 152 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.75 [0.16, 3.42] |
| 2 Acute renal injury | 3 | 123 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.05, 0.96] |
| 3 Creatinine clearance | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 2 | 77 | Mean Difference (IV, Random, 95% CI) | ‐10.34 [‐29.57, 8.88] |
Comparison 9. Cardiac surgery: subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 26 | 2390 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.56, 1.64] |
| 2 Acute renal injury | 31 | 2504 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.55 [0.32, 0.92] |
| 3 Urine output | 19 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 17 | 1475 | Mean Difference (IV, Random, 95% CI) | 0.26 [0.17, 0.36] |
| 3.2 2 to 3 days (mL/min) | 9 | 1058 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.13, 0.54] |
| 4 Creatinine clearance | 27 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 24 | 2136 | Mean Difference (IV, Random, 95% CI) | 9.38 [‐5.99, 24.74] |
| 4.2 2 to 3 days (mL/min) | 17 | 1844 | Mean Difference (IV, Random, 95% CI) | 14.21 [3.58, 24.85] |
| 4.3 5 to 7 days (mL/min) | 7 | 949 | Mean Difference (IV, Random, 95% CI) | 14.99 [0.84, 29.13] |
| 5 Free water clearance | 8 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 24 hours (mL/min) | 7 | 700 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.22, 0.19] |
| 5.2 2 to 3 days (mL/min) | 4 | 591 | Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.30, ‐0.28] |
| 6 Fractional excretion of sodium | 9 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 24 hours (%) | 8 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 2 to 4 days (%) | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 10. Aortic surgery: subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 8 | 236 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.76 [0.20, 2.89] |
| 2 Acute renal injury | 8 | 284 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.11, 3.70] |
| 3 Urine output | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 24 hours (mL/min) | 7 | 227 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.10, 0.19] |
| 3.2 2 to 3 days (mL/min) | 3 | 95 | Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.06, 0.58] |
| 3.3 5 to 7 days (mL/min) | 2 | 55 | Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.39, 0.21] |
| 4 Creatinine clearance | 9 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 24 hours (mL/min) | 9 | 323 | Mean Difference (IV, Random, 95% CI) | 7.99 [‐0.77, 16.74] |
| 4.2 2 to 3 days (mL/min) | 5 | 195 | Mean Difference (IV, Random, 95% CI) | 11.62 [‐6.13, 29.37] |
| 4.3 5 to 7 days (mL/min) | 4 | 116 | Mean Difference (IV, Random, 95% CI) | ‐12.85 [‐26.41, 0.72] |
| 5 Free water clearance | 5 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 5.1 24 hours (mL/min) | 5 | 154 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.51, 0.01] |
| 5.2 2 to 4 days (mL/min) | 2 | 85 | Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.12, 0.85] |
| 5.3 5 to 7 days (mL/min) | 2 | 85 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.13, 0.61] |
| 6 Fractional excretion of sodium | 5 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 6.1 24 hours (%) | 5 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 2 to 4 days (%) | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Renal plasma flow | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 End of operation (mL/min) | 2 | 44 | Mean Difference (IV, Random, 95% CI) | 50.29 [‐92.83, 193.40] |
| 7.2 24 hours (mL/min) | 2 | 47 | Mean Difference (IV, Random, 95% CI) | 45.86 [‐18.64, 110.36] |
Comparison 11. Biliary surgery: subgroup analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Urine output | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Urine output: 24 hours (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.99, ‐0.19] |
| 1.2 Urine output: 2 to 4 days (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.22, 0.69] |
| 1.3 Urine output: 5 to 7 days (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.23 [0.09, 0.37] |
| 2 Creatinine clearance | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 24 hours (mL/min) | 3 | 83 | Mean Difference (IV, Random, 95% CI) | ‐2.84 [‐14.07, 8.39] |
| 2.2 2 to 4 days (mL/min) | 3 | 74 | Mean Difference (IV, Random, 95% CI) | 0.42 [‐16.68, 17.52] |
| 2.3 5 to 7 days (mL/min) | 2 | 43 | Mean Difference (IV, Random, 95% CI) | 0.58 [‐16.43, 17.60] |
Comparison 12. Studies on participants with pre‐existing renal impairment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 10 | 959 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.74 [0.36, 1.52] |
| 2 Acute renal injury | 11 | 979 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.40 [0.22, 0.76] |
| 3 Urine output | 4 | 707 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.16, 0.85] |
| 3.1 Urine output, 24 hours | 4 | 416 | Mean Difference (IV, Random, 95% CI) | 0.35 [‐0.12, 0.81] |
| 3.2 Urine output, 2 to 3 days | 2 | 291 | Mean Difference (IV, Random, 95% CI) | 0.43 [‐0.78, 1.65] |
| 4 Creatinine clearance | 4 | 646 | Mean Difference (IV, Random, 95% CI) | 10.65 [0.04, 21.27] |
| 4.1 Creatinine clearance, 24 hours | 4 | 347 | Mean Difference (IV, Random, 95% CI) | 7.78 [‐10.39, 25.94] |
| 4.2 Creatinine clearance, 2 to 3 days | 3 | 299 | Mean Difference (IV, Random, 95% CI) | 14.16 [‐6.20, 34.52] |
Comparison 13. Studies with low risk of bias: sensitivity analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Reported mortality, low risk of bias studies only | 19 | 1604 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.52, 1.97] |
| 2 Acute renal injury, requiring dialysis, low risk of bias studies only | 16 | 1550 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.05 [0.55, 2.03] |
| 3 Urine output at 24 hours, low risk of bias studies only | 11 | 798 | Mean Difference (IV, Random, 95% CI) | 0.20 [‐0.04, 0.44] |
| 4 Creatinine clearance at 24 hours, low risk of bias studies only | 9 | 817 | Mean Difference (IV, Random, 95% CI) | 6.59 [‐3.53, 16.72] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Adabag 2008.
| Methods | Cardiac surgery patients, with pre‐existing chronic kidney disease were studied (GFR <60ml/min.1.73m2). Excluded patients in severe renal failure, emergency surgery and intravenous contrast within 4 days. | |
| Participants | Intervention group (N‐acetylcysteine group), n= 50, Age: mean=70, SD=9. Control group (matching placebo), n=52, Age: mean=72, SD=9. | |
| Interventions | Oral N‐acetyl cysteine. 14 doses, twice daily. Started 1 day before surgery, (3 doses before surgery and 11 doses after surgery). Matching placebo for same duration and time used. The 2 groups were comparable. | |
| Outcomes | 30 day mortality, acute kidney injury, acute renal injury needing haemodialysis | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by the investigational (?) pharmacist. Block randomization (blocks of 10). |
| Allocation concealment (selection bias) | Low risk | Participants, researchers and clinicians blinded to treatment assignment. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, researchers and clinicians (including data collecting nurse) were blinded. Drug packets matched in volume, colour, consistency and transparency and given mixed with fruit juice to mask taste. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
Amano 1994.
| Methods | Consecutive patients for coronary artery bypass graft (CABG); randomization done, but method not clear; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Glutathione group n = 10, age, mean = 58.2, SD = 2.5; control group n = 9, age, mean 56.8, SD 2.5 | |
| Interventions | Glutathione 200 mg/kg IV before bypass and same dose repeated during 1st and 2nd postoperative days. Control group had saline in the same manner | |
| Outcomes | Urine output, creatinine clearance, fractional excretion of sodium | |
| Notes | No response to letter for details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | ‘Randomly assigned’ into two groups. No details of randomization given |
| Allocation concealment (selection bias) | High risk | D ‐ Not used |
| Blinding (performance bias and detection bias) All outcomes | High risk | None described; control group had no treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
Amano 1995.
| Methods | Consecutive patients for CABG; randomization method not clear; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Diltiazem group n = 13, age, mean = 54.5, SD = 1.8; control group n = 10, age, mean = 54.2, SD = 1.6 | |
| Interventions | Diltiazem 0.1 mg/kg bolus, followed by infusion of 2 mcg/kg/min until end of aortic cross clamping, followed by nasogastric administration every 8 hours for 24 hrs; control group received 5% dextrose in the same manner | |
| Outcomes | Urine output, creatinine clearance, free water clearance, fractional excretion of sodium | |
| Notes | No response to letter for details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | ‘Patients were randomized into either diltiazem or no treatment groups'; no details of randomization method |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | None described; control group had no treatment |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
Ascione 1999.
| Methods | Patients having CABG; randomization by card allocation; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Off pump n = 25, age, mean = 59.4, SD = 10.5; control (on pump), n = 26, age, mean = 63.8, SD = 6.7 | |
| Interventions | On pump and off pump used for revascularization of coronary arteries | |
| Outcomes | Creatinine clearance | |
| Notes | Inadequate response to letter from the contact author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘Prospectively randomized by card allocation’ |
| Allocation concealment (selection bias) | Unclear risk | ‘Prospectively randomized by card allocation’; no further details given on allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not discussed in text |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not discussed |
Barr 2008.
| Methods | Cardiac surgery patients, over 18 yrs, elective or urgent surgery in patients with chronic renal impairment (preop CCl of under 40ml/min). Excluded renally crippled patients (on dialysis), pregnant or sensitivity to drug used. | |
| Participants | 3 intervention groups: Fenoldopam group – 19, NAC group – 20, NAC+ Fenoldopam group – 21, Placebo group ‐ 19. Ages: mean, SD G1 – 77.2, 1.2; G2 – 73.8, 2.2; G3 – 73.5, 2.0; G4 ‐ 72.4, 2.0 Sex (Male/ Female): G1 – 12 / 7; G2 – 15 / 5; G3 ‐ 12 / 9; G4 ‐ 13 / 6 |
|
| Interventions | Four groups of participants. Group 1: Fenoldopam 0.1 mcg/kg/min at induction and continued for 48 hrs Group 2: N‐acetyl cysteine orally 600mg per day 1 day preop and on the morning of surgery and the night of surgery. Group 3: Fenoldopam and N‐acetylcysteine together Group 4: Placebo: Normal saline instead of fenoldopam, for 48 hrs (‘double blinded’). Taste controlled placebo instead of NAC, same time |
|
| Outcomes | Mortality, acute renal injury (renal replacement therapy), creatinine clearance (Cockroft‐Gault) | |
| Notes | results reported as mean and SE | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization done by pharmacy dept; method of randomization uncertain |
| Allocation concealment (selection bias) | Unclear risk | No specific mention of allocation concealment except to say ‘double‐blinded’. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No specific mention of who all were blinded; reports as ‘Double‐blinded’, placebo controlled trial. Not sure if blinding was adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reports one withdrawal |
Berendes 1997.
| Methods | Patients having CABG; randomization done, method unclear; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Dopamine 0.5 mcg/kg/min, n = 10, age, mean = 60, SD = 7.1; dopamine 1 mcg/kg/min, n = 10, age, mean = 62, SD = 8.2; dopamine 2 mcg/kg/min, n = 10, age, mean = 62, SD = 10.2; placebo, n = 14, age, mean = 62, SD = 6.3 | |
| Interventions | Different doses of diltiazem, after induction of anaesthesia and for 24 hours post‐operation | |
| Outcomes | Creatinine clearance | |
| Notes | Did not contact authors; old study and no additional information required | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | ‘Placebo controlled prospective study’; no description of randomization |
| Allocation concealment (selection bias) | High risk | None described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
Bergman 2002.
| Methods | Patients undergoing cardiac surgery with cardiopulmonary bypass; randomization done using list from hospital pharmacy; allocation concealment method unclear; blinding of patients, researchers and care givers is unknown, but strong possibility; study of moderate methodological quality | |
| Participants | Cardiac surgery. diltiazem group, n = 12, age, mean = 72, range, 69‐76; placebo group, n = 12; age, mean = 73, range, 69‐74. All participants had high serum creatinine | |
| Interventions | Diltiazem 0. 25 mg/kg infusion for 15 min, followed by infusion of 1.7 mcg/kg/min for 24 hours | |
| Outcomes | Glomerular filtration rate (GFR) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were consented and randomized; method of randomization not described |
| Allocation concealment (selection bias) | High risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Accounted for drop outs |
Burns 2005.
| Methods | High risk patients undergoing cardiac surgery; randomization by pharmacy by permuted block strategy (alternate blocks of 4 or 6); Allocation concealed by central randomization and drug (or placebo) dispensed by pharmacy by colour and consistency matching; everyone blinded to the nature of drugs | |
| Participants | Cardiac surgery. N‐acetyl cysteine, 4 doses or 600mg or placebo 4 doses of 5% dextrose. Intervention group, n = 148, age, mean = 68.9, SD = 8.9; placebo group, n = 147; age = 69.2, SD = 9.7. Three patients from NAC group and 4 patients from placebo group withdrawn | |
| Interventions | N‐acetyl cysteine or placebo given. 1st dose after induction of anaesthesia, 2nd dose at end of bypass, 3rd dose at 12 hrs in ICU and 4th dose at 24hrs | |
| Outcomes | Data available only on mortality and acute renal injury | |
| Notes | Contacted the authors successfully for data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization done by pharmacy trial co‐ordinator using a permitted block strategy |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was using central randomization with drugs prepared by pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quadruple blinded (patients, clinicians, data collectors and data analyst) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Account for drop outs in the trial |
Carcoana 2003.
| Methods | Patients for cardiac surgery; randomization by computer generated random number tables by Pharmacy; allocation concealment clearly stated. Patients, researchers and care givers were blinded; study with good methodological quality | |
| Participants | Cardiac surgery. Mannitol group, n = 26, age, mean = 64.3, SD = 8.9; Dopamine group, n = 25, age, mean = 63.8, SD = 9.8; mannitol + dopamine group, n = 25, age, mean = 63.4, SD = 7.8 (this group excluded in review); Placebo group, n = 24, age, mean = 63.3, SD = 8.8; | |
| Interventions | Mannitol 1 g/kg into pump, dopamine 2 mcg/kg/min during surgery or both together as treatment; saline as placebo | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | No further information sought from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘Prospective randomized double‐blinded and placebo controlled study’. Computer generated random number tables were used |
| Allocation concealment (selection bias) | Unclear risk | Does not specifically describe it, but quite likely it was concealed allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded manner; drug or saline supplied by the dept investigational pharmacy in a blinded manner; additive for the CPB circuit prime (mannitol or saline, supplied similarly) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All allocated patients completed the trial (withdrawals before allocation) |
Chen 2007.
| Methods | Adults over 18yrs having cardiac surgery under cardiopulmonary bypass and having pre‐existing renal insufficiency (CCl <60ml/min) were studied. Exclcuded shocked patients and patients with aortic dissection. | |
| Participants | Intervention group: n=17; Age: mean=77, SD=10; Sex: M=12, F=5. Control group: n=19; Age: mean=78, SD=7; Sex: M=10, F=9. | |
| Interventions | Nesiritide infusion 0.005 mcg/kg/min, from start of induction of anaesthesia for 24 hrs. Control group received 'placebo', but not sure what it was. The 2 groups were not comparable. | |
| Outcomes | Mortality, acute renal injury (needing dialysis), and creatinine clearance (Cochroft‐Gault formula) | |
| Notes | Data available on plasma cystatin. plasma aldosterone, plasma cGMP and plasma BNP | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomized’; no details provided; described as ‘double‐blind, placebo‐controlled proof of concept trial’ |
| Allocation concealment (selection bias) | Unclear risk | No details provided apart from 'double‐blind, placebo‐controlled proof of concept trial’ |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details provided apart from 'double‐blind, placebo‐controlled proof of concept trial’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reports 4 withdrawals from trial |
Cho 2009.
| Methods | Patients undergoing robot‐assisted laparoscopic radical prostatectomy (RALRP). Excluded patients with chronic renal insufficiency preop | |
| Participants | Intervention group: n=50; Age: mean=67, SD=6. Control group: n=50; Age: mean=68, SD=4 | |
| Interventions | Intervention group: Nicardipine infusion ‐ after induction of anaesthesia infusion 0.5mcg/kg/min until end of surgery. Control group: Normal saline infusion at same rate. Both groups were comparable. |
|
| Outcomes | Mortality (mention only), calculated GFR, urine output | |
| Notes | Also reports 'renal insufficiency', based on eGFR values only. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization method used |
| Allocation concealment (selection bias) | Unclear risk | Computer allocation, no further details given (likely to be adequate) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not described except the statement ‘investigator blinded to the study group evaluated the postoperative data’; likely to be inadequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
Cogliati 2007.
| Methods | Elective cardiac surgery patients with high creatinine levels (> 1.5mg/dl) (patients with chronic renal failure), more than 70yrs, diabetes on insulin or previous cardiac surgery (at least one of these factors present = high risk pt). Excluded patients who were renal cripples (those on dialysis) and allergy to drug used | |
| Participants | Intervention group: n=95; Age: mean=70.3, SD=7.6; Sex: M=61; F=34 Control group: n=98; Age: mean=69.6, SD=10.4; Sex: M=63; F=35 |
|
| Interventions | Fenoldopam 0.1mcg/kg/min infusion immediately before incision and continued for 24 hrs. Control group: normal saline at the same rate for 24 hrs. Groups were comparable. | |
| Outcomes | Creatinine clearance (Cochroft‐Gault formula), urine output | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization from a computer list, in an envelope |
| Allocation concealment (selection bias) | Low risk | Sealed envelope used; ‘All personnel and patients were blinded to the assignment’ |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinded nurse, not involved with study, prepared the drug/placebo in identical 50ml filled syringes, ‘All personnel and patients were blinded to the assignment’ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant lost to follow‐up |
Colson 1990.
| Methods | Patients having CABG; randomization & double blinding stated, but method not specified; method of allocation concealment is unclear; blinding of patients, researchers and care givers is unknown, but possible it was adequate; poor methodological study | |
| Participants | Coronary artery bypass surgery. Captopril group n = 8, age, mean = 56, SD = 3; control group n = 8, age, mean = 60, SD =2 | |
| Interventions | Captopril 100 mg orally tid for 2 days preoperatively, last dose being 2 hrs before surgery; placebo tablets for control group | |
| Outcomes | Renal plasma flow | |
| Notes | Old study; no attempt made to contact authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Allocated in a randomized double‐blind fashion to two groups'; No details on randomization method |
| Allocation concealment (selection bias) | Unclear risk | 'Allocated in a randomized double‐blind fashion to 2 groups'; no description of method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details on blinding except ‘double‐blind fashion’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not given in text |
Colson 1992.
| Methods | Patients having abdominal aortic aneurysm (AAA) surgery; randomization and double blinding of treatments done, but method not specified; method of allocation concealment is unclear; blinding of patients, researchers and care givers is unknown, but possible it was adequate; poor methodological quality study | |
| Participants | Abdominal aortic surgery. Enalapril group n = 8, age, mean = 58, SD = 4; nicardipine group n = 8; age, mean = 63, SD = 3; control group n = 8, age, mean = 63, SD = 1 | |
| Interventions | Enalapril 10 mg orally BD for 2 days preoperation; placebo capsules for control group | |
| Outcomes | GFR, renal plasma flow; fractional excretion of sodium | |
| Notes | Old study; no attempt made to contact authors. The same study (with alternative treatment is quoted "Colson 1992A") | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Allocated in a randomized double‐blind fashion to 2 groups'; No details on randomization method |
| Allocation concealment (selection bias) | Unclear risk | 'Allocated in a randomized double‐blind fashion to 2 groups'; No description of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details on blinding except ‘double blind fashion’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not given in text |
Costa 1990.
| Methods | Patients with pre‐operative renal dysfunction (Creatinine clearance less than 50ml/min) having CABG; randomization method not specified; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Dopamine group n = 12, age, mean = 60.3, SD = 12.3; control group n = 12, age, mean = 61.3, SD = 8.9; dopamine and SNP group, n = 12, age, mean = 54.2, SD = 8.7 (this group excluded from review) | |
| Interventions | Dopamine infusion 2.5 mcg/kg/min during the operation (unsure for how long); control group had no treatment | |
| Outcomes | Creatinine clearance, free water clearance, fractional excretion of sodium | |
| Notes | Old study; no attempt made to contact authors. Excluded parallel treatment group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly divided into three groups’; no description of randomization |
| Allocation concealment (selection bias) | High risk | No description of allocation method |
| Blinding (performance bias and detection bias) All outcomes | High risk | No description of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details in text |
Cregg 1999.
| Methods | Idiopathic scoliosis surgery patients; randomization method not specified; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Corrective spinal surgery for scoliosis. Dopamine group, n = 15, age, mean = 14.6, SD = 3.6; control group, n = 15, age, mean = 12.1, SD = 2.8 | |
| Interventions | Dopamine infusion 3 mcg/kg/min after induction for 24 hrs; control group received 5% dextrose for 24 hrs | |
| Outcomes | Urine output, fractional excretion of sodium | |
| Notes | Unable to contact the author; the only study selected which is on a paediatric population | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly allocated’ into three groups; no description of randomization method |
| Allocation concealment (selection bias) | High risk | No description of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No details on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not given |
Dawidson 1991.
| Methods | Consecutive patients for abdominal aortic surgery; randomization by random card method; allocation concealment not used; blinding of patients, researchers and care givers is unknown, but possible; poor methodological quality study | |
| Participants | Corrective abdominal aortic surgery. Dextran 60 group, n = 10, age, mean = 62, SD = 10.4; Ringer's lactate group, n = 10, age, mean = 66.1, SD = 13.7 | |
| Interventions | Dextran 60 infusion during operation and Ringer's lactate solution during surgery, ratio being 1:3 for the solutions | |
| Outcomes | Urine output; mortality data | |
| Notes | Too old study to get any more information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘Randomized to either treatment group’ by pulling a card from a previously prepared deck |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | No details on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not given in text |
de Lasson 1995.
| Methods | Consecutive patients for abdominal aortic surgery; randomization method unclear; allocation concealment not used; blinding of patients and care givers is unknown, researcher blinded; poor methodological quality study | |
| Participants | Abdominal aortic surgery. Dopamine group n = 12, age, mean = 63; placebo n = 12, age, mean = 60 | |
| Interventions | Dopamine infusion 3 mcg/kg/min during operation and for 24 hrs | |
| Outcomes | Urine output, GFR, renal plasma flow, free water clearance | |
| Notes | No reply from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly allocated into infusion of dopamine or placebo’ by one of the authors who was unaware of the treatment allocation; method of randomization is not clear in text |
| Allocation concealment (selection bias) | Low risk | Not sure of any allocation concealment, but likely |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not described any blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not given in text |
de Lasson 1997.
| Methods | Patients for abdominal aortic surgery | |
| Participants | Abdominal aortic surgery. Felodipine group n = 11, age, mean = 65; control group n = 12, age, mean = 60 | |
| Interventions | Felodipine 5 mg slow release tab for 5 days preoperatively, last dose 1‐2 hrs before surgery; placebo tablets as control | |
| Outcomes | Urine output, GFR, renal plasma flow, free water clearance, fractional excretion of sodium | |
| Notes | No reply from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomiztion and drug or placebo preparation done by drug company; method not described was central and likely to be good |
| Allocation concealment (selection bias) | Unclear risk | Not sure of any allocation concealment, but likely possibility |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Possible, but does not describe blinded tables |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One patient had additional drugs, but not excluded |
Dehne 2001.
| Methods | CABG patients, randomized into four groups. Method of randomization unclear; allocation concealment or blinding not mentioned. Methodological quality poor | |
| Participants | Aortocoronary bypass surgery. Patients into four groups; Group (1), controls with normal renal function: n = 12; age: mean = 62.6, SD = 8.0; group (2), dopexamine infusion in patients with normal renal function: n = 12; age: mean = 64.0, SD = 7.5; group (3), controls with abnormal renal function; n = 12; age, mean = 62.4, SD = 7.5; group (4), dopexamine infusion in patients with abnormal renal function: n = 12; age, mean = 65.4, SD = 8.1 | |
| Interventions | Dopexamine 1 mcg/kg/min after induction of anaesthesia, until the end of surgery in groups 2 and 4; not sure how the controls were treated | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated into two groups, but does not describe the randomization method |
| Allocation concealment (selection bias) | High risk | Does not describe the allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | High risk | Does not mention blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients accounted for in calculations |
Donmez 1998.
| Methods | CABG patients. Patients were randomized, but method of randomization and allocation concealment not described | |
| Participants | CABG patients. All patients received dopamine 2mg/kg/min infusion. Group (1) verapamil 5mg added to prime solution: n = 25; age, mean = 58.3, SE = 1.9; group (2), nimodipine 1‐15mcg/kg/min during bypass; n = 25; age, mean = 56.1, SE = 2.6; group (3), control group; normal saline infusion only; n = 25; age, mean = 56.5, SE = 2.0 | |
| Interventions | Verapamil 5 mg in prime in group (1); group (2) received infusion of nimodipine 1‐15 mcg/kg/min during bypass; group (3), control group received normal saline only | |
| Outcomes | Creatinine clearance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly allocated into three groups’; method of randomization not described |
| Allocation concealment (selection bias) | Unclear risk | ‘Randomly allocated into three groups’; method of allocation not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | ‘Randomly allocated into three groups’; method of blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts described in text |
Dural 2000.
| Methods | CABG patients; randomized by opaque sealed envelopes; allocation concealment unclear; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery surgery. Dopamine group n = 12, age, mean = 53.2, SD = 10.9; mannitol group, n = 12, age, mean = 55.4, SD = 8.4; control group, n = 12, age, mean = 53.7, SD = 8.3 | |
| Interventions | Dopamine 3 mcg/kg/min started after induction, until end of operation; mannitol 1 mg/kg/hr from induction unto end of operation; no treatment for control group | |
| Outcomes | Urine output | |
| Notes | No reply so far from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly allocated into three groups’; method of randomization not described |
| Allocation concealment (selection bias) | Unclear risk | ‘Randomly allocated into 3 groups’; method of allocation concealment not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Randomly allocated into 3 groups’; method of blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Does not describe any dropouts |
Durmaz 2003.
| Methods | Patients for CABG, with renal dysfunction (preoperative creatinine more than 2.5mg/dl) | |
| Participants | CABG in patients with renal dysfunction. Preoperative dialysis group, n = 21; age, mean = 58.1, SD = 11.8; control group (no preoperative dialysis), n = 23; age, mean = 54.3, SD = 11.1 | |
| Interventions | Preoperative haemodialysis in the intervention group; control group had no preoperative haemodialysis. | |
| Outcomes | Mortality, acute renal injury | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomization done by the last digit of the medical record number of patient (Quasi‐randomization) |
| Allocation concealment (selection bias) | High risk | ‘Patients were prospectively allocated into 2 groups’ |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not given in text |
Fischer 2005.
| Methods | CABG. Used retrospective review of data from a randomized, double blinded, trial. No details are given about the method of randomization or allocation concealment, but confirms double‐blind status. Methodology poor | |
| Participants | CABG patients, age: mean = 66, SD = 9. Intervention group received N‐acetyl cysteine during bypass, n = 20; placebo group, n = 20, not sure what they received | |
| Interventions | N‐acetyl cysteine, 100 mg/kg in prime, followed by 20 mg/kg per hour until end of bypass. Unsure what placebo group received | |
| Outcomes | Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Retrospective chart review of a randomized trial in 2003, which used computer generated allocation list (randomly permuted blocks of random size) provided by dept of Medical Statistics |
| Allocation concealment (selection bias) | Unclear risk | Computer generated allocation list (randomly permuted blocks of random size) provided by dept of Medical Statistics |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Drugs were supplied in identical looking glass vials containing drug or placebo. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusions described in text |
Gubern 1988.
| Methods | Patients for biliary surgery (on patients with some renal impairment); randomization method not detailed; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Biliary tract surgery. Mannitol group n = 17, age, mean = 65.9, SD = 12; control group n = 14, age, mean = 68.5, SD = 9.9 | |
| Interventions | Mannitol 50 g intravenously 1 hour preoperation and for 2 days; Control treatment had no treatment | |
| Outcomes | Urine output, GFR | |
| Notes | Study too old to try to obtain details | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Prospectively randomized’; no details of method of randomization |
| Allocation concealment (selection bias) | Unclear risk | ’Prospectively randomized’; no details of method of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | ’Prospectively randomized’; no details of method of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Fate of participants discussed |
Haase 2007.
| Methods | Elective cardiac surgery patients with high creatinine (>150 mcmol/l), older patients, diabetes on insulin or previous cardiac surgery (at least one of these factors present = high risk patient, but none on dialysis) | |
| Participants | Intervention group: n=30; Age: mean= 68.9, SD=9.7; Sex: M=23, F=7 Control group: n=30; Age: mean=68.3; SD=9.3; Sex: M=21, F=9 |
|
| Interventions | Intervention group: N‐Acetyl cysteine infusion immediately after induction at dose of 150mg/kg over 15 min, followed by continuous infusion of 50mg/kg over 4 hours, then 100mg/kg over 20hrs. Control group: Normal saline at the same rate for 24 hrs |
|
| Outcomes | Mortality, urine output, acute renal injury (needing postop renal replacement therapy) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized using Microsoft Excel‐based random number generation to create a randomization list, in blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was ensured by quadruple‐blinding (patients, clinicians, data collectors and data analysers) were unaware of groups or treatment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quadruple‐blinding (patients, clinicians, data collectors and data analysers were blinded) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None missed |
Haase 2009.
| Methods | Elective cardiac surgery patients (CPB), with pre‐existing renal dysfunction (creatinine >120mmol/L), high risk patients (no patients on dialysis) | |
| Participants | Intervention group: n=50; Age: mean=71.5, SD=9.2; Sex: M=30; F=20 Control group: n=50; Age: mean=70.6; SD=9.5; Sex: M=33, F=17 |
|
| Interventions | Intervention group: Sodium bicarbonate 0.5mmol/kg in 250ml 5% dextrose bolus immediately after induction of anaesthesia, followed by continuous infusion of 0.15mmol/kg in 1000ml of 5% dextrose over 23 hrs: (Total of 4mmol/kg in 24 hrs) Control group: Sodium chloride infusion in a similar fashion for same period (same volume infused) |
|
| Outcomes | Mortality (hospital) and requirement for renal replacement therapy | |
| Notes | No missing data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Microsoft Excel based random number generation, with blocks of 10; central randomization by dept of Pharmacy |
| Allocation concealment (selection bias) | Low risk | Allocation concealment was achieved by central randomization, blinding to all researchers, patients and others. Allocation revealed only after data analysis. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Both infusions were in separate shrink‐wrapped black plastic bags that were identical in appearance (blinded to patients, anaesthetists, surgeons ICU personnel and nurses and others |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One in each group |
Halpenny 2002.
| Methods | Patients for AAA; randomization and blinding mentioned in text, but not detailed; allocation concealment not used; blinding of patients, researchers and care givers is unknown, but possible; overall poor methodological quality study | |
| Participants | Abdominal aortic surgery. Fenoldopam group n = 14, age, mean = 70, SD = 5; control group n = 13, age, mean = 69, SD = 6 | |
| Interventions | Fenoldopam infusion 0.1 mcg/kg/min (only during aortic cross clamping); placebo for control group | |
| Outcomes | Urine output, creatinine clearance, free water clearance, fractional excretion of sodium | |
| Notes | No response from authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Random allocation used’; method of randomization not given |
| Allocation concealment (selection bias) | Unclear risk | ‘Random allocation used’; method of allocation not given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Exclusion described |
Harten 2008.
| Methods | Emergency abdominal surgery, age >50yrs. Excluded night cases, patients on lithium and those having vascular surgery | |
| Participants | Intervention: n=14; Age: median=66, range=56‐75; Sex: M=11, F=3 Control: n=15; Age: median=64, range=51‐76; Sex: M=12, F=3 |
|
| Interventions | Intervention: Optimization of intraoperative fluids using arterial line and Lidco cardiovascular monitoring and gave fluid boluses of 250ml 6% hydroxyethyl starch over 15 min done as necessary Control: Standard care, fluids decided by clinicians in Operating Theatre. Groups not comparable |
|
| Outcomes | Mortality (30 days) and renal morbidity | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Randomized’, but no details given |
| Allocation concealment (selection bias) | Unclear risk | Allocated to control and intervention group using opaque envelopes immediately before surgery; not sure if allocation concealment was adequate |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 died before operation in the intervention group |
Hynninen 2006.
| Methods | Elective repair of AAA. Excluded patients with renal insufficiency (creatinine >130mmol/l) and those who had renal artery clamping done during surgery |
|
| Participants | Intervention group: n=34; Age: mean=66, SD=10; Sex: M=27; F=7 Control group: n=35; Age: mean=67, SD=10; Sex: M=27, F=8 |
|
| Interventions | Intervention: N‐Acetyl cysteine infusion, 150mg/kg NAC in 250ml 5% dextrose in 20min (bolus) after induction of anaesthesia, followed by 150mg/kg in 250ml 5% dextrose, infused over 24 hrs. Control: 250ml 5% dextrose in 20min, followed by 250ml 5% dextrose infusion for 24hrs |
|
| Outcomes | Mortality, patients needing renal replacement therapy (dialysis), urine output, urinary NAG/creatinine ratio, urinary albumin/ creatinine ratio, plasma Cystatin C | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized in blocks of 10, done by hospital pharmacy, no details given |
| Allocation concealment (selection bias) | Low risk | Allocation was done by hospital pharmacy. None of the clinical and study personnel was aware of study allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Likely that there was blinding, though not detailed in text |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One patient withdrew from study intraoperatively, does not mention which group, though most likely the intervention group (as seen from the numbers in each group) |
Kaya 2007.
| Methods | Cardiac surgery patients, with eGFR >30ml/min, LVEF <0.50 and a minimum of 2 lesions undergoing CABG. Excluded patients with CCF, cardiogenic shock, unstable angina, MI and renal cripples (on dialysis or creatinine >300mmol/l) Morbidly obese |
|
| Participants | Intervention group: n=124; Age: mean=60.8, SD=10.8. Sex: M=81, F=43 Control group: n=116; Age: mean=61.3, SD=9.7; Sex: M=72, F=44 |
|
| Interventions | Intervention: Sodium nitroprusside (SNP) infusion. SNP started with onset of rewarming 0.1mcg/kg/hr, until end of CPB. SNP in 50ml blinded syringe Control: Normal saline in 50ml blinded syringe |
|
| Outcomes | Mortality, renal injury, eGFR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomization done by statistician |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed, envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | SNP and saline in uniformly appearing 50ml syringes, blinded to surgeons, perfusionists and nurses; the investigators did not know the details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reported as none |
Kleinschmidt 1997.
| Methods | CABG patients. Three groups, randomization not described, no indication of concealment allocation, but indicated double‐blind status. Methodological quality poor | |
| Participants | CABG. Pentoxyfylline group, n = 14, age, mean = 61.7, SD = 8.0; gamma‐hydroxybutyrate group, n = 13, age, mean = 62.3, SD = 3.9; control group, n = 13, age, mean = 62.9, SD = 6.2 | |
| Interventions | Pentoxyphylline 1 mg/kg bolus, followed by 1 mg/kg/hour during operation. Gamma hydroxybutyrate bolus of 25 mg/kg, followed by 25 mg/kg/hour during operation. Control group received normal saline infusion | |
| Outcomes | Creatinine clearance, fractional excretion of sodium | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by computer |
| Allocation concealment (selection bias) | High risk | Not described in detail |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not described in detail |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not described |
Kramer 2002.
| Methods | Patients for CABG; randomized and double‐blinded study; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Theophylline group n = 28, age, mean = 60.4, SD = 10.1; control group n = 28, age, mean = 60.3, SD = 8.1 | |
| Interventions | Theophylline bolus of 4 mg/kg over 30 min, followed by infusion of 0.25 mg/kg/hr for 96 hrs; control group received saline | |
| Outcomes | GFR | |
| Notes | No response from author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized to receive one of two treatments |
| Allocation concealment (selection bias) | High risk | No details given |
| Blinding (performance bias and detection bias) All outcomes | High risk | No details given |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Early termination of study in 33 of 56 patients; Intention to treat (ITT) analysis used |
Kulka 1996.
| Methods | Patients for CABG; randomization method not described; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Clonidine group n = 23, age, mean = 58, SD = 7; control n = 25, age, mean = 57, SD = 2 | |
| Interventions | Preoperative infusion of clonidine 4 mcg/kg over 15 min, 1 hr before surgery; placebo in control group | |
| Outcomes | Urine volume, creatinine clearance | |
| Notes | No response from author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Allocated into two groups in a double‐blinded randomized fashion'; no details of randomization given |
| Allocation concealment (selection bias) | Unclear risk | 'Allocated into 2 groups in a double‐blinded randomized fashion'; no details of allocation given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | 'Allocated into 2 groups in a double‐blinded randomized fashion'; no details of blinding given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two patients were excluded |
Lassnigg 2000.
| Methods | Patients undergoing cardiac surgery; randomization by sealed envelopes; allocation concealment unclear, but strong possibility; blinding of patients, researchers and care givers done; good methodological quality study | |
| Participants | Cardiac surgery. Dopamine group n = 42, age, mean = 63, SD = 10; frusemide (furosemide) group n = 41, age, mean = 63, SD = 10; control group n = 40, age, mean = 65, SD = 10 | |
| Interventions | Dopamine infusion 2 mcg/kg/min for 48 hrs; 05 mcg/kg/min frusemide infusion for 48 hrs; saline in control group | |
| Outcomes | Urine volume, creatinine clearance, fractional excretion of sodium | |
| Notes | Same study (with alternative treatment) is quoted in "Lassnigg 2000A" | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Placebo controlled randomized double blind trial; block randomization done and used sealed envelopes; no further details of randomization |
| Allocation concealment (selection bias) | Low risk | Placebo controlled randomized double blind trial; block randomization done and used sealed envelopes; no further details of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Placebo controlled randomized double blind trial; block randomization done and used sealed envelopes; no details of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three patients were excluded from analysis |
Lau 2001.
| Methods | Abdominal aortic repair patients. Randomization done, but method not specified. Allocation concealment unclear or not done. Poor methodological quality | |
| Participants | AAA repair. Intervention is extraperitoneal approach; n = 10; age, mean = 69.8, SEM=3.1. Control group is intraperitoneal approach; n=10; age, mean=74.3, SEM=2.5 | |
| Interventions | Extraperitoneal approach for AAA repair compared with transperitoneal approach | |
| Outcomes | Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Recruited patients were allocated to one of two groups’; no details on randomization |
| Allocation concealment (selection bias) | Unclear risk | ‘Recruited patients were allocated to one of 2 groups’; no details on allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | ‘Recruited patients were allocated to one of 2 groups’; no details of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two patients accounted for |
Licker 1996.
| Methods | Infrarenal aortic surgery; randomization method not clear; allocation concealment not used; blinding of patients, researchers and care givers is unknown, but likely; overall poor methodological quality study | |
| Participants | Abdominal aortic surgery. Enalapril group n = 11, age, mean = 69; control group n = 9, age, mean = 68 | |
| Interventions | Enalapril bolus 50 mcg/kg injection 25 min before anaesthesia; saline injection in control group | |
| Outcomes | Urine output, creatinine clearance, renal plasma flow, free water clearance, fractional excretion of sodium | |
| Notes | Contacted authors to confirm or exclude duplicate reporting; no response; used only full publication in review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients were allocated in a randomized double‐blind manner’; no details of randomization given |
| Allocation concealment (selection bias) | Unclear risk | 'Patients were allocated in a randomized double‐blind manner’; no details of allocation given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | 'Patients were allocated in a randomized double‐blind manner’; no details of blinding given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two patients were excluded from trial |
Loef 2004.
| Methods | CABG surgery. Method of randomization unclear, allocation concealment not stated, double‐blind status stated. Methodological quality moderately poor | |
| Participants | CABG surgery. Dexamethasone group, n = 10, age, mean = 67.7, range = 58‐76; control group, n = 10, age, mean = 59.6, range = 47‐76 | |
| Interventions | Dexamethasone 1 mg/kg before induction of anaesthesia, followed by 0.5 mg/kg 8 hours later. Control group received a placebo, nature of which is unsure | |
| Outcomes | Urine output, creatinine clearance, free water clearance, fractional excretion of sodium | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Randomized in a double blind fashion’; no details of randomization given |
| Allocation concealment (selection bias) | High risk | 'Randomized in a double blind fashion’; no details of allocation given |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | 'Randomized in a double blind fashion’; no details of blinding given |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 'All patients completed the trial' |
Marathias 2006.
| Methods | Cardiac surgery (open heart) in patients with high creatinine. Randomization done, but method unclear. Allocation concealment not stated. Poor methodological quality | |
| Participants | Open heart surgery in patients with renal impairment (high creatinine). Intervention was fluid hydration preoperatively for 12 hours using half normal saline; n = 30; age, mean = 64, SEM = 1.7. Control group had fluid restriction for 12 hours preoperatively; n = 15; age, mean = 64.2, SEM = 2.8 | |
| Interventions | Preoperative fluid hydration using half isotonic saline, 1 ml/kg/hour for 12 hours; control group had no fluid hydration | |
| Outcomes | Mortality, acute renal injury | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Used a 2:1 ratio of randomization process, patients were randomized into groups; no other details of randomization given |
| Allocation concealment (selection bias) | High risk | Patients were randomized into groups; no other details of allocation given |
| Blinding (performance bias and detection bias) All outcomes | High risk | Used a 2:1 ratio of randomization process, patients were randomized into groups; no details of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not described |
Mitaka 2008.
| Methods | Repair of AAA (elective) in patients over 20yrs. Excluded patients on chronic dialysis and those with a preop creatinine >3.0mg/dl | |
| Participants | Intervention group: n=20; Age: mean=69.4, SD=7.7; Sex: M=18, F=2 Control group: n=20; Age: mean=73.3, SD=8.6; Sex: M=17, F=3 |
|
| Interventions | Intervention: hANP (Atrial natriuretic peptide) infusion, 0.01mcg/kg/min starting dose (to prevent hypotension), increasing by 0.01mcg/kg/min every 10min until dose of 0.05mcg/kg/min is reached. Start infusion of hANP just before cross clamping and continued for 48hrs Control group: Placebo infusion started at 2ml/hr, increased by 2ml every 10min, until 10ml/hr |
|
| Outcomes | Acute renal injury (needing dialysis), creatinine clearance, urinary NAG, urine volume | |
| Notes | No data in paper for creatinine clearance | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomized into two groups’; not sure what method of randomization was used |
| Allocation concealment (selection bias) | Unclear risk | Not sure how the allocation was done |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | ‘Blind infusion was performed’; not sure about blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described in paper |
Morariu 2005.
| Methods | CABG patients. Intervention dexamethasone IV; randomized, but method of randomization unclear. Allocation concealment not stated, but patients were double‐blinded. Methodological quality poor | |
| Participants | CABG patients. Intervention was dexamethasone and control group received a placebo, the nature of which is unclear. Dexamethasone group; n = 10; age, mean = 67.8, 95% CI = 63.4‐72.1. Control group, n = 10; age, mean = 59.5; 95% CI = 53.4‐65.5 | |
| Interventions | Dexamethasone 1 mg/kg at induction, followed by 0.5 mg/kg 8 hours later; placebo in the control group at the same time | |
| Outcomes | Mortality | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Designed as a prospective double‐blind placebo controlled randomized trial'; no other details of randomization provided |
| Allocation concealment (selection bias) | High risk | 'Designed as a prospective double‐blind placebo controlled randomized trial'; no description of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | 'Designed as a prospective double‐blind placebo controlled randomized trial'; but no other details of blinding provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 'All patients completed the trial' |
Morgera 2002.
| Methods | High risk patients for CABG; randomization not described; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Prostaglandin group n = 17, age, mean = 62, SD = 5.5; control group n = 17, age, mean = 61, SD = 7 | |
| Interventions | Prostaglandin infusion 2 ng/kg/min at start of anaesthesia and for 48 hrs; control group treatment not described | |
| Outcomes | Urine volume, creatinine clearance | |
| Notes | No response to letter | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomized’; no other details given |
| Allocation concealment (selection bias) | High risk | ‘Patients were randomized’; no mention of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | ‘Patients were randomized’; no mention of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two people were excluded from analysis |
Myles 1993.
| Methods | Patients for CABG; randomization by random number generated by pharmacy; allocation concealment adequate; blinding of patients, researchers and care givers are adequate; good methodological quality study | |
| Participants | Coronary artery bypass surgery. dopamine group n = 25, age, mean = 62.2, SD = 8; control group n = 24, age, mean = 61, SD = 10 | |
| Interventions | Dopamine infusion 3 mcg/kg/min for 24 hrs; 5% dextrose infusion for control group | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | No need for further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by use of a table of random numbers; ‘prospective double‐blind randomized trial’ |
| Allocation concealment (selection bias) | Low risk | Coded 50ml syringes from the pharmacy, with contents remaining unknown to investigators until the end of trial; allocation concealed |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Coded 50ml syringes from the pharmacy, with contents remaining unknown to investigators until the end of trial; blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3 withdrawals before start of trial |
Nicholson 1996.
| Methods | Consecutive patients for AAA surgery; randomization by sealed envelope (random number); allocation concealment adequate; blinding of patients done, but that of researchers and care givers unknown, but is possible; moderate methodological quality study | |
| Participants | Abdominal aortic surgery. Mannitol group n = 15, age, mean = 68; control group n = 13, age, mean = 71 | |
| Interventions | Mannitol 0.3 g/kg before cross‐clamp; normal saline for control group | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | Need for further information uncertain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Prospective randomized trial’; no further details on randomization |
| Allocation concealment (selection bias) | High risk | ‘Prospective randomized trial’; no details on allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | ‘Prospective randomized trial’; no details on blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | None reported |
Nouri‐Majalan 2009.
| Methods | Elective CABG surgery patients with renal impairment (GFR < 60ml/min), age >18yrs. Excluded emergency CABG surgery patients | |
| Participants | Intervention group: n=30; Age: mean=65, SD=9.5; Sex: M=17, F=13 Control group: n=30; Age: mean=61, SD=7.9; Sex: M=14, F=16 |
|
| Interventions | Intervention: Vitamin E 100 units QID and allopurinol 100mg BD, for 3‐5 days preop Control group: Had no treatment |
|
| Outcomes | Mortality, ARF (needing dialysis) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients were randomized’; no further details |
| Allocation concealment (selection bias) | Unclear risk | No indication of allocation concealment, but for statement ‘to prevent bias surgeons, nurses, and lab technicians were blinded to patient assignment’ |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Possible: states, ‘to prevent bias surgeons, nurses, and lab technicians were blinded to patient assignment’ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None indicated in text |
O'Hara 2002.
| Methods | Partial nephrectomy in patients with single kidney; Randomization mentioned in text, no details given; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Partial nephrectomy. Dopamine group n = 13, age, mean = 64.6, SD = 8; Control group n = 11, age, mean = 62.4, SD = 8.8 | |
| Interventions | Dopamine infusion 3 mcg/kg/min during surgery and for 1 hr afterwards; no intervention in control group | |
| Outcomes | Urine output, GFR, renal blood flow | |
| Notes | No response to letter | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Prospective randomized study’; no further details on randomization |
| Allocation concealment (selection bias) | High risk | 'Prospective randomized study’; no details on allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | 'Prospective randomized study’; no details on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 11 out of 35 excluded |
Parks 1994.
| Methods | Surgery for obstructive jaundice; randomized, but no details given. allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Elective surgery for obstructive jaundice. Control group, n = 10, age not given; dopamine group, n = 13, age not given | |
| Interventions | Control group had pre‐op IV fluids and frusemide on induction; dopamine group had the above + infusion of dopamine 3 mcg/kg/min for 48 hours | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | Old study, no real need for further information | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomly allocated into two groups’; no further details on randomization |
| Allocation concealment (selection bias) | High risk | ‘Patients were randomly allocated into 2 groups’; no details on allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | ‘Patients were randomly allocated into 2 groups’; no details on blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not disclosed |
Perez 2002.
| Methods | Laparoscopic colorectal surgery; randomization done by use of sealed envelopes; allocation concealment not used; blinding of patients, researchers done, but that of care givers is unknown; overall good methodological quality study (unfortunately no relevant observations useful for this review) | |
| Participants | Elective laparoscopic colorectal surgery patients. Dopamine group, n = 19, age, mean = 64.3, SD = 9.4; control group, n = 18, age, mean = 61.3, SD = 16.7 | |
| Interventions | Dopamine infusion, 2 mcg/kg/min during the operation; control group received saline in the same manner | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | Mailed authors for data because only the difference is given in the text. the data from authors contains details only during and for 2 hours after the operations. Continuous data not suitable for analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Randomization performed by aleatorized numbers prepared in closed envelopes'. |
| Allocation concealment (selection bias) | Unclear risk | No details on concealment of allocation except ‘Randomization performed by aleatorized numbers prepared in closed envelopes’; possible to have concealment of allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Drug or placebo given with an identical container in a double blind manner and the volume of drug or saline were same. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 patients were excluded |
Prasad 2010.
| Methods | Elective OP‐CABG surgery patients, with baseline creatinine >133mcmol/l. Inclusion and exclusion criteria detailed in text. | |
| Participants | Intervention group: n=35; Age: mean=55.6, SD=10.2. Sex: M=25; F=10 Control group: n=35; Age: mean=57.8, SD=9.4; Sex: M=28, F=7 |
|
| Interventions | Intervention: N‐actylcysteine (NAC). Oral NAC 600mg BD on preop day, followed by IV NAC 600mg prior to induction of anaesthesia and IV NAC 600mg BD for 2 postop days (total dose of NAC = 4.8g) Control: No treatment |
|
| Outcomes | Postop renal dysfunction (judged by rise of more than 44 mmol/l or 25% in creatinine from preop values), GFR (Cockroft‐Gault formula) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized, prospective, open label study; random number generated from a random number table |
| Allocation concealment (selection bias) | High risk | No concealment of assignment discussed |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding discussed |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four people excluded after randomization |
Prowle 2012.
| Methods | Elective high risk cardiac surgery patients (Patients with high creatinine >1.2mg/dl, older than 70yrs,lCHF,lLV EF<35%, diabetes on insulin or previous cardiac surgery; at least one of these factors present) | |
| Participants | Intervention group: n=50; mean age= 69.0; SD=11.1; Sex: M = 33; F=17 Control group: n=50; mean age= 67.3; SD=10.8; Sex: M = 37; F=13 |
|
| Interventions | Intervention group: Atorvastatin 40 mg orally a day before surgery and 3 further doses orally in the post op days 1,2 and 3 Control group: Matching placebo for same duration and time |
|
| Outcomes | Mortality; Acute kidney injury needing dialysis; Rise in Serum creatinine levels; Urinary NGAL, and urinary NGAL/urinary creatinine ratio; Rise in serum transminases and serum creatine kinase | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by the hospital pharmacy clinical trials coordinator. Microsoft excel –based random number generator‐permuted block strategy with blocks of 10 |
| Allocation concealment (selection bias) | Low risk | Allocation stratified into two groups based on preop use of statins. Allocation concealed to patients, anaesthetists, cardiac surgeons, intensive care specialists, bedside nurses and investigators |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double blind”. Atorvastatin or placebo medication was prepared in capsules of identical appearance and blinded to patients, anaesthetists, cardiac surgeons, intensive care specialists, bedside nurses and investigators |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eight in intervention group and seven in control |
Pull Ter Gunne 1990.
| Methods | Patients undergoing AAA surgery; randomized, method not described; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Aortic surgery for aneurysm. Preoperative hydration group, n = 11, age, mean = 65, SD, 9. Control group, n = 8, mean = 71, SD = 10 | |
| Interventions | Treatment group optimally hydrated preoperatively (guided by PCWP); control treatment no special treatment | |
| Outcomes | Creatinine clearance | |
| Notes | Unlikely to get authors to respond after this long after the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Random assignment into two groups'; no further details of randomization |
| Allocation concealment (selection bias) | Unclear risk | 'Random assignment into 2 groups'; the anaesthesiologist was aware of the allocation and treatment received; no further details on allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | 'Random assignment into 2 groups'; the anaesthesiologist was aware of the allocation and treatment received; no further details on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details provided |
Ristikankare 2006.
| Methods | Cardiac surgery patients with high creatinine levels (abnormal renal function). Randomization and allocation concealment methods not stated, but done by hospital pharmacy. Double‐blind status stated. Methodological quality moderately good | |
| Participants | Cardiac surgery (bypass) patients. N‐acetyl cysteine group, n = 38, age, mean = 72, range = 44‐87; control group, n = 42, age, mean = 69, range = 51‐81 | |
| Interventions | N‐acetyl cysteine group received loading dose of the drug 150 mg/kg in 15 min, followed by 50 mg/kg for next 4 hours, thereafter 100 mg/kg for next 16 hours. Placebo group received similar amount of saline (0.9%) over the same time | |
| Outcomes | Mortality, acute renal injury | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’ |
| Allocation concealment (selection bias) | Unclear risk | ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’; but no further details of allocation concealment provided in text |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | ‘Randomly allocated in a double‐blinded manner; the hospital pharmacy performed the randomization and prepared the study medications’; no details of blinding provided |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three patients were excluded |
Ryckwaert 2001.
| Methods | Cardiac surgery patients; randomized, but method not specified; allocation concealment unclear; blinding of patients, researchers and care givers is unknown, but is likely; overall poor methodological quality study | |
| Participants | Cardiac surgery (CABG). Enalapril group n = 7, mean = 60.1, SD = 3.6; control group n = 7, age, mean = 66.3, SD = 4.2 | |
| Interventions | Enalapril, 1 mg, 6 hourly for 2 days | |
| Outcomes | Urine output, GFR, renal plasma flow | |
| Notes | Unable to contact authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were allocated in a randomized double‐blind fashion to two groups’; no further details of randomization given |
| Allocation concealment (selection bias) | Unclear risk | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; No other details on allocation method |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; No other details on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No drop outs detailed in text |
Sezai 2000.
| Methods | CABG patients. Randomization done, but details unclear. Allocation concealment not detailed. Blinding is done. Methodological quality moderately good | |
| Participants | CABG patients. Atrial natriuretic peptide group, n=20, age, mean = 62.1, SD = 7.9; control group, n = 20, age, mean = 64.8, SD = 5.2 | |
| Interventions | Intervention group received atrial natriuretic peptide infusion, 0.03‐0.05 mcg/kg/min for 20 hours, starting during operation, then reduced to 0.02 mcg/kg/min for another 4 hours. Control group received placebo (nature not described). All patients received dopamine and dobutamine at the end of bypass | |
| Outcomes | Urine output, GFR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; No other details on randomization method |
| Allocation concealment (selection bias) | Unclear risk | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; No other details on allocation method |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‘Randomly allocated to two groups receiving blind infusion of drug or placebo’; No other details on blinding, but likely to be adequate |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described, but probably there were no dropouts |
Sezai 2009.
| Methods | CABG surgery. No patients with renal impairment (determined by Cr <1.3 mg/dl and CCr <80ml/min) were included | |
| Participants | Intervention group: n=251; Age: mean=65.6, SD=0.6; Sex: M=193, F=58 Control group: n=253; Age: mean=66.3, SD=0.6; Sex: M=205, F=48 |
|
| Interventions | Intervention: hANP (Human atrial natriuretic peptide) infusion of hANP 0.02mcg/kg/min from start of CPB, reduced to 0.01mcg/kg/min after start of oral medications and then stopped after 12hrs Control: Normal saline infusion in the same fashion |
|
| Outcomes | ARF needing dialysis, mortality, creatinine clearance, fractional excretion of sodium, free water clearance, urine output | |
| Notes | Too many confounders in the intervention and control groups such as use of dopamine infusion in some patients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomly allocated into 2 groups by drawing lots |
| Allocation concealment (selection bias) | Unclear risk | 'Randomly allocated by drawing lots’; no other details |
| Blinding (performance bias and detection bias) All outcomes | High risk | No evidence of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No mention of dropouts in the text |
Sezai 2011.
| Methods | Randomly allocated into 2 groups by lottery method | |
| Participants | Intervention group: n=141; age: 68.8, SD: 6.7; Males: 123/141 Control group: n=144; age: 68.8, SD: 7.8; Males 128/144 |
|
| Interventions | Intervention: Carperitide (hANP) infusion, 0.01mcg/kg/min for over 2 days Control: Saline infusion for similar period |
|
| Outcomes | Mortality, Acute renal injury needing dialysis, calculated GFR | |
| Notes | Poor quality study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Lottery method' |
| Allocation concealment (selection bias) | High risk | No evidence of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | None used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts discussed |
Shackford 1983.
| Methods | Patients for aortic reconstruction; randomized by random number method; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Elective aortic reconstruction surgery. Hypertonic saline group, n = 30, age, mean = 60.5, SD = 8.2; Ringer's lactate group, n = 28, age, mean= 61.7, SD = 8.5 | |
| Interventions | Hypertonic saline intraoperatively and Ringer's lactate solution intraoperatively | |
| Outcomes | Urine output, creatinine clearance, fractional excretion of sodium | |
| Notes | Very old study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were assigned by random number to one of 2 groups; no details on randomization |
| Allocation concealment (selection bias) | High risk | Patients were assigned by random number to one of 2 groups; no details on concealment of allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Patients were assigned by random number to one of 2 groups; no details on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts described |
Shim 2007.
| Methods | Cardiac surgery (off‐pump coronary artery surgery). Randomization using computer generated randomization table. Allocation concealment not stated, but blinding seems adequate. Methodological quality moderately good | |
| Participants | Off‐pump coronary artery surgery. Mannitol group, n = 25, age, mean = 63, SD = 8; control group, n = 25, age, mean = 63, SD = 8 | |
| Interventions | Mannitol 0.5 g/kg in 10 min during grafting; control group 2.5 ml/kg normal saline during the same time period | |
| Outcomes | Acute renal injury | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Patients were randomly allocated to one of 2 groups using a computer generated randomization table' |
| Allocation concealment (selection bias) | Low risk | Patients were randomly allocated to one of 2 groups using a computer generated randomization table; no further details on allocation concealment; likely to be adequate |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All medical personnel involved in the study were blinded to the contents of the infusion bottle. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts recorded |
Song 2009.
| Methods | Cardiac surgery patients, mostly off pump CABG. | |
| Participants | Adults undergoing CABG | |
| Interventions | EPO 300u/kg given immediately following induction of anaesthesia. Same volume of normal saline given as placebo. | |
| Outcomes | Acute kidney injury was primary outcome (serum creatinine rise of more than 50%); urine output and creatinine clearance (calculated) | |
| Notes | EPO study. All enrolled patients completed the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization done by research unit of hospital. Randomization was stratified by creatinine levels |
| Allocation concealment (selection bias) | Low risk | Allocation was via Internet |
| Blinding (performance bias and detection bias) All outcomes | Low risk | None of the clinicians, patients or researchers were aware of the nature of the drugs; matching syrings of EPO and normal saline used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients completed the trial |
Tang 1999.
| Methods | Consecutive patients for CABG; randomization method unclear; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Coronary artery bypass surgery. Dopamine group n = 20, age, mean = 61, SD = 10.3; control group n = 20, age, mean = 56.3, SD = 8.7 | |
| Interventions | Dopamine infusion, 2.5‐4 mcg/kg/min for 48 hrs; control group without any intervention | |
| Outcomes | Urine output | |
| Notes | No need to contact authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospectively randomized; no details given |
| Allocation concealment (selection bias) | High risk | No details of allocation provided in text |
| Blinding (performance bias and detection bias) All outcomes | High risk | No details of blinding provided in the text |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No dropouts recorded |
Tang 2002.
| Methods | Coronary artery surgery. Randomization done, but method not clear. No evidence of allocation concealment or blinding. Methodological quality poor | |
| Participants | Coronary artery surgery. Beating heart surgery group, n = 20, age, mean = 64.8, SD = 6.9; conventional bypass group (control), n = 20, age, mean = 62.1, SD = 9.3 | |
| Interventions | Intervention is off‐pump coronary artery surgery and control group is on pump coronary artery surgery | |
| Outcomes | Mortality, acute renal injury | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 'Patients were randomized'; no further details on randomization |
| Allocation concealment (selection bias) | High risk | 'Patients were randomized'; no further details on allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | 2 different types of procedures; no blinding possible |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 people were subsequently excluded from trial |
Thompson 1986.
| Methods | Surgery for obstructive jaundice. Randomized, but method of randomization, allocation concealment and blinding not stated. Methodological quality poor | |
| Participants | Surgery for obstructive jaundice. Oral ursodeoxycholic acid group, n = 20, age, mean = 57.0, range = 18‐72. Control group, n = 20, age, mean = 56.5, range = 45‐78 | |
| Interventions | Intervention oral ursodeoxycholic acid 900 mg, 8 hourly for 48 hours in the immediate preoperative period. Control group had no additional treatment | |
| Outcomes | Mortality, acute renal injury, creatinine clearance | |
| Notes | Too old study to contact authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomized’; no more details |
| Allocation concealment (selection bias) | High risk | ‘Patients were randomized’; no account of allocation method |
| Blinding (performance bias and detection bias) All outcomes | High risk | ‘Patients were randomized’; no details on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‘There were no withdrawals’ |
Turner 2008.
| Methods | Patients for elective AAA (infrarenal) repair. Excluded patients on steroids, diabetic patients and those with CRF (Creatinine >150mcmol/l) | |
| Participants | Intervention group: n=10; Age: mean=69.1, SD=5.4; Sex: not described Control group: n=10; Age: mean=71.9, SD=6.0; Sex: not described |
|
| Interventions | Intervention: Methyl prednisolone 10mg/kg in 500ml 5% dextrose, infusion over 30min, but mentions only that infusion was given ‘during the surgery’; not sure when Control group: Received 5% dextrose solution |
|
| Outcomes | Mortality, NAG/creatinine ratio | |
| Notes | Also did serum creatinine, cytokines, alpha‐1 microglobulin/ creatinine ratio and albumin/ creatinine ratio | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization done using computer generated randomization list |
| Allocation concealment (selection bias) | Low risk | Computer generated randomization list placed in sealed envelopes and opened in numerical order by a third party preparing the study infusion |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Third party prepared the infusion. The infusions were such that the volumes were equal in the bag and identical colour and the contents of bag were indistinguishable; the infusion was done over 30min to avoid any haemodynamic effects of treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Yes, none lost to follow‐up |
Urzua 1992.
| Methods | CABG surgery. Quasi randomization by using last digits of their notes, no allocation concealment or blinding | |
| Participants | CABG. Phenylephrine group, n = 7, age, mean = 55, SD = 7. Control group, n = 14, age, mean = 54, SD = 7 | |
| Interventions | Intervention is to maintain mean perfusion pressure above 70 mmHg during surgery. Control group had no intervention | |
| Outcomes | Acute renal injury, urine output, creatinine clearance, free water clearance, fractional excretion of sodium | |
| Notes | To old study to contact authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly assigned into one of 2 groups, according to the last digit of their clinical history number (Quasi‐randomization) |
| Allocation concealment (selection bias) | High risk | Allocation by last digit of clinical history number is impossible to conceal |
| Blinding (performance bias and detection bias) All outcomes | High risk | No report of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 'All patients completed the trial' |
Wahbah 2000.
| Methods | Patients for biliary surgery; randomization method unclear; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Biliary tract surgery. Dopamine group n = 10, age, median = 50, range = 37‐60; control group n = 10, age, median = 44.5, range = 36‐60; dopamine + mannitol group n = 10, age, median = 51, range = 44‐58; dopamine + frusemide (furosemide) group n = 10, age, median = 61, range = 55‐71 (excluded the last 2 groups from review) | |
| Interventions | Dopamine infusion 2.5 mcg/kg/min during surgery and for 2 days; control group had no treatment | |
| Outcomes | Urine output, creatinine clearance | |
| Notes | No need to contact authors. Excluded 2 parallel treatment groups in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomly allocated into 4 equal groups’; no further details on randomization |
| Allocation concealment (selection bias) | Unclear risk | 'Patients were randomly allocated into 4 equal groups’; no further details on allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | No description of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | None described |
Welch 1995.
| Methods | Infrarenal aortic surgery; Randomization method not described; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Abdominal aortic surgery. Dopexamine group n = 15, age, mean = 63.5; control group n = 17, age, mean = 62.1 | |
| Interventions | Dopexamine 2 mcg/kg/min infusion during surgery; saline in control group | |
| Outcomes | Creatinine clearance | |
| Notes | Need to contact authors uncertain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomly assigned’; no further details on randomization method used |
| Allocation concealment (selection bias) | High risk | ‘Patients were randomly assigned’; no further details on allocation method used |
| Blinding (performance bias and detection bias) All outcomes | High risk | No description of blinding |
| Incomplete outcome data (attrition bias) All outcomes | High risk | None described |
Wijnen 2002.
| Methods | AAA surgery (infrarenal). Randomized, but method of randomization, allocation concealment and blinding not stated in the text. Methodological quality poor | |
| Participants | Elective AAA repair. Antioxidant group, n = 20, age, mean = 67, range = 51‐75; control group, n = 22, age, mean = 70, range = 59‐82 | |
| Interventions | Intervention was multiple antioxidant therapy as follows: vitamin E 200 mg orally for 5 days before surgery + vitamin C 200 mg orally on morning of surgery + allopurinol 300 mg orally 1 day before surgery and 300 mg at induction + N‐acetyl cysteine 150 mg/kg bolus, followed by infusion of 200 mg/kg over 12 hours preoperatively + mannitol 10%, 500 ml over 12 hours from the time of surgery. Control group had no intervention | |
| Outcomes | Creatinine clearance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomized’; no further details on method of randomization used |
| Allocation concealment (selection bias) | High risk | ‘Patients were randomized’; no details on method of allocation used |
| Blinding (performance bias and detection bias) All outcomes | High risk | No details on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | One death described, but not of any dropouts |
Witczak 2008.
| Methods | Elective CPB surgery patients studied. Males with creatinine >150mcmol/L and females >130mcmol/l CABG or valve surgery were included. Excluded patients with unstable angina, EF <35%, renal cripple (on dialysis) or renal transplant patients. |
|
| Participants | Intervention: n=10; Age: mean=67.7, SD=9.0; Sex: M=8, F=2 Control: n=10; Age: mean=65.8, SD=10.6; Sex: M=8, F=2 |
|
| Interventions | Intervention: Infusion of nifedipine, from start of surgery and for 24hrs; Nifedipine infusion rate was 0.25 – 0.60 mcg/kg/min (adjusted according to BP) Control: No treatment |
|
| Outcomes | Creatinine clearance | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Patients were ‘randomized’. It appears that the anaesthesiologist ‘randomly drew an envelope with the assigned treatment’ |
| Allocation concealment (selection bias) | High risk | Allocation concealment was possible only for patients and statistician |
| Blinding (performance bias and detection bias) All outcomes | High risk | No; control had no treatment; only the patients and statistician were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | None described |
Woo 2002.
| Methods | Consecutive patients undergoing cardiac surgery; block randomized with sealed envelopes; allocation concealment described in follow‐up correspondence; blinding of patients done, but researchers and care givers not blinded; moderate methodological quality of study | |
| Participants | Elective cardiac surgery patients (with high risk of postoperative renal dysfunction). Dopamine group, n = 20, age, mean = 64.5, range 58‐82; control group, n = 22, age, mean = 66.5, range 48‐84) | |
| Interventions | Dopamine infusion 3 mcg/kg/min during operation and for 48 hrs post‐operation; saline infusion for the control group | |
| Outcomes | Data on urine output obtained following correspondence | |
| Notes | Contacted the author and obtained excellent response | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were randomized’; no further details on method of randomization used |
| Allocation concealment (selection bias) | High risk | ‘Patients were randomized’; no details on method of allocation used |
| Blinding (performance bias and detection bias) All outcomes | High risk | No details on blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Eight patients were excluded due to death or major complications |
Yavuz 2002A.
| Methods | Elective CABG patients; randomized, but method not described; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Elective CABG patients. Dopamine group, n = 11, age, mean = 55.7, SD = 5.2; control group, n = 11, age, mean = 56.4, SD = 9.5 | |
| Interventions | Dopamine 2 mcg/kg/min infusion, started 24 hrs before surgery and continued for 48 hrs after surgery; control group had no treatment | |
| Outcomes | Creatinine clearance, free water clearance | |
| Notes | Contacted the author (e‐mail), but no response yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients were prospectively randomized’; no details on method of randomization |
| Allocation concealment (selection bias) | High risk | ‘Patients were prospectively randomized’; no details on method of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | No description of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | States no deaths; no description of any dropouts |
Yavuz 2002B.
| Methods | CABG patients; randomized, but method of randomization unclear; allocation concealment not used; blinding of patients, researchers and care givers is unknown; poor methodological quality study | |
| Participants | Elective CABG patients. Control group, n = 15, age, mean = 61.3, SD = 8.3; dopamine group, n = 15, age, mean = 58.3, SD = 5.3; diltiazem group, n = 15, age, mean = 60.3, SD = 7.1; dopamine + diltiazem group, n = 15, age, mean = 58.7, SD = 7.1 (excluded this group from review) | |
| Interventions | Dopamine and diltiazem 2 mcg/kg/min, started 24 hrs pre‐operation and continued for 48 hrs post‐operation; no mention of how control group was treated | |
| Outcomes | Creatinine clearance, free water clearance | |
| Notes | Contacted (e‐mail) to author, no response yet | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Patients randomized into four groups’; no further details on randomization |
| Allocation concealment (selection bias) | High risk | ‘Patients randomized into four groups’; no description of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | No description of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 'No mortality' is described, but not any suggestion of dropouts |
Zanardo 1993.
| Methods | CABG surgery. Randomization done, but method of randomization, allocation concealment and blinding not described. Methodological quality poor | |
| Participants | Diltiazem infusion (2 doses): Group 1, diltiazem 1 mcg/kg/min, n = 11, age, mean = 58.1, SD = 10.7; Group 2, diltiazem 2 mcg/kg/min, n = 12, age, mean = 58.3, SD = 5.8; control group, n = 12, age, mean = 57.8, SD = 10.1 | |
| Interventions | Intervention, group 1, diltiazem 1 mcg/kg/min and group 2, diltiazem 2 mcg/kg/min, both started after chest opening, until 24 hours in ICU. Control group had no additional treatment | |
| Outcomes | Acute renal injury, urine output, creatinine clearance | |
| Notes | Old study, unlikely to get more data | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | ‘Randomly assigned’; no further details of randomization |
| Allocation concealment (selection bias) | High risk | ‘Randomly assigned’; no further details of allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts described |
CABG = coronary artery bypass graft; AAA = abdominal aortic aneurysm; GFR = glomerular filtration rate
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abe 1993 | Treatment only for very short duration during surgery |
| Aho 2004 | Not a randomized controlled trial |
| Amar 2001 | Treatment started only after operation |
| Antonucci 1996 | There is no control group |
| Baldwin 1994 | Treatment started only in the postoperative period |
| Boldt 2000 | A phase 2 trial, looking for side effects; no controls |
| Boldt 2006 | No control group without treatment in the trial |
| Boodhwani 2009 | Not relevant to the review |
| Boutros 1979 | This is not a randomized controlled trial |
| Bove 2005 | No control group without treatment in the trial |
| Caglikulekci 1998 | Not a randomized controlled trial |
| Cahill 1987 | Not very relevant to the review; no randomization |
| Caimmi 2003 | The control group received active treatment with dopamine, dobutamine or frusemide; some controls started in postoperative period only |
| Christakis 1992 | No data relevant to the review |
| Christenson 1995 | Not a randomized controlled trial |
| Dementi'eva 1996 | Data not relevant to the review |
| Feindt 1995 | Data not relevant to the review |
| Fischer 2002 | A retrospective study only |
| Fisher 1998 | Follow up only for 12 hours |
| Franklin 1997 | The intervention started in the postoperative period only |
| Frumento 2006 | Postoperative study in the intensive care unit |
| Garwood 2003 | This is an observational study, not a randomized controlled trial or controlled trial |
| Gatot 2004 | Started in the postoperative period in the intensive care unit |
| Gerola 2004 | Data not relevant to the review |
| Gilbert 2001 | Not a randomized controlled study; there is only treatment and no controls |
| Godet 2008 | No control group |
| Goto 1992 | No control group |
| Grundmann 1985 | This is a postoperative study |
| Halpenny 2001 | Started the intervention only towards the end of operation and continued only for 16 hours |
| Hayashida 1997 | Not a randomized controlled trial |
| Hayashida 2000 | Study lasted only 6‐14 hours |
| Hisatomi 2012 | Open study, with inadequate randomization; used multiple interventions (frusamide) in both groups |
| Izumi 2006 | Retrospective study |
| Izumi 2008 | Mostpatients received other interventions such as dopamine and frusamide |
| Junnarkar 2003 | Not a randomized controlled trial |
| Kulka 1993 | This is a conference abstract; data published as Kulka 1996 (included) |
| Kumle 1999 | No control groups |
| Kunt 2009 | There were no control group patients |
| Kuraoka 1995 | Not a randomized controlled trial |
| Lema 1995 | This is not a randomized controlled trial or controlled trial |
| Lema 1998 | Control group had received active treatment |
| Lemmer 1996 | No relevant data for the review |
| Levy 1995 | The intervention is not relevant for the review |
| Licker 1999 | We could not exclude if this study contained the same data as in Licker 1996; hence excluded this publication from analysis |
| Lim 2002 | Postoperative study |
| Loef 2002 | Not a randomized controlled trial |
| MacGregor 1994 | No control group; intervention mostly in the intensive care unit |
| Mahesh 2008 | The follow up was only for 12 hrs |
| Mahmood 2007 | No control group patients in the trial |
| Memmo 2011 | Only two treatment groups, no control group |
| Neimark 2005 | The data not relevant to the review |
| Nguyen 2001 | Not a randomized controlled trial |
| Nguyen 2002 | Not a randomized controlled trial |
| Niiya 2001 | Study started in the postoperative period only |
| Nuutinen 1976 | No randomization or controls |
| O'Hara 2002A | Conference abstract only; data published as O'Hara 2002 (included) |
| Oliver 2006 | No control group |
| Ovrum 2004 | Not a randomized controlled trial |
| Pain 1991 | The treatment using intravenous hydration is only during the preoperative period; no randomization |
| Paul 1986 | Randomization method inadequate; two treatments were given to the treatment groups (both dopamine and mannitol) |
| Pavoni 1998 | Postoperative study |
| Petry 1992 | No control group |
| Piper 2003 | Intervention started only in intensive care unit |
| Plusa 1991 | No control group; only 2 treatments |
| Priano 1993 | No relevant data for the review |
| Prifti 2001 | Study did not look at renal function |
| Regragui 1995 | No clear intervention in the study |
| Riess 2000 | Not a randomized controlled trial |
| Ryckwaert 1995 | Abstract publication only; data published as Ryckwaert 2001 (included) |
| Sanders 2001 | Study of intravenous fluid administration in the preoperative period only; no relevant data reported |
| Sezai 2006 | Not a randomized controlled trial |
| Sherry 1997 | Study started in intensive care unit |
| Skillman 1975 | There is no relevant data for the review |
| Stanitsh 2002 | Multiple measures used for the purpose of renal protection in the intervention group (mannitol + dopamine or frusemide) |
| Straka 2004 | There is no renal data in the study |
| Tataranni 1994 | Not a randomized controlled trial |
| Torsello 1993 | Used only retrospective controls |
| Tripathy 1996 | No control group |
| Ueki 1995 | Not a randomized controlled trial |
| Vogt 1996 | No control group |
| Vogt 1999 | No control group |
| Weisz 2009 | It is a group registry only and not a RCT |
| Welch 1993 | The renal function tests were done only for very short periods of time |
| Wool 2010 | Not relevant to the review |
Characteristics of studies awaiting assessment [ordered by study ID]
Fergany 2011.
| Methods | RCT |
| Participants | Patients undergoing partial nephrectomy of solitary kidney |
| Interventions | Fenoldopam and placebo |
| Outcomes | |
| Notes | Awaiting full publication; unable to contact authors |
Differences between protocol and review
We have included new interventions in this review (N‐acetyl cysteine, atrial natriuretic peptide, sodium bicarbonate, antioxidants and erythropoietin). We have looked at some of the newer biomarkers of kidney damage (urinary NAG/creatinine ratio; urinary RBP/creatinine ratio; plasma cystatin C; urinary NGAL/creatinine ratio) as surrogate markers for renal damage.
Contributions of authors
Dr Mathew Zacharias Contact reviewer. Involved in development of the protocol, the search strategy, retrieval of the papers, screening of the papers, data extraction and data input and writing of the protocol and review, including the updated review
Dr Mohan Mugawar Screening of the papers, extraction of data, checking of data input and writing of the updated review
Prof G Peter Herbison Statistical and general advice, checking of the review
Dr Palvannan Sivalingam Co‐reviewer. Involved with development of the protocol and screening of the papers, data extraction, checking of data input and checking of the review, including the update
Prof Robert J Walker Specialist advice on kidney and renal function tests
Dr Karen Hovhannisyan Development of new search strategy and performing the search
Dr Niamh P Conlon Co‐reviewer for the previous version of the review; special advisor on the review
Sources of support
Internal sources
Dunedin Hospital & Dunedin School of Medicine, Dunedin, New Zealand.
External sources
None, New Zealand.
Declarations of interest
Mathew Zacharias: none known
Mohan Mugawar: none known
G Peter Herbison: none known
Robert J Walker: none known
Karen Hovhannisyan: none known
Palvannan Sivalingam: none known
Niamh P Conlon: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Adabag 2008 {published data only}
- Adabag AS, Ishani A, Koneswaran S, Johnson DJ, Kelly RF, Ward HB, et al. Utility of N‐acetylcysteine to prevent acute kidney injury after cardiac surgery: a randomized controlled trial. American Heart Journal 2008;155:1143‐9. [PUBMED: PMID: 18513531] [DOI] [PubMed] [Google Scholar]
Amano 1994 {published data only}
- Amano J, Suzuki A, Sunamori M. Salutary effect of reduced glutathione on renal function in coronary artery bypass operation. Journal of the American College of Surgeons 1994;179:714‐20. [MEDLINE: ] [PubMed] [Google Scholar]
Amano 1995 {published data only}
- Amano J, Suzuki A, Sunamori M, Tofukuji M. Effect of calcium antagonist diltiazem on renal function in open heart surgery. Chest 1995;107:1260‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ascione 1999 {published data only}
- Ascione R, Lloyd CT, Underwood MJ, Gomes WJ, Angelini GD. On pump versus off pump coronary revascularization: evaluation of renal function. Annals of Thoracic Surgery 1999;68:493‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Barr 2008 {published data only}
- Barr LF, Kolodner K. N‐acetylcysteine and fenoldopam protect the renal function of patients with chronic renal insufficiency undergoing cardiac surgery. Critical Care Medicine 2008;36(5):1427‐35. [PUBMED: PMID: 18434903 ] [DOI] [PubMed] [Google Scholar]
Berendes 1997 {published data only}
- Berendes E, Mollhoff T, Aken H, Schmidt C, Erren M, Deng MC, et al. Effects of dopexamine on creatinine clearance, systemic inflammation, and splanchnic oxygenation in patients undergoing coronary artery bypass grafting. Anesthesia and Analgesia 1997;84:950‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bergman 2002 {published data only}
- Bergman AS, Odar‐Cederlof I, Westman L, Bjellerup P, Hoglund P, Ohqvist G. Diltiazem infusion for renal protection in cardiac surgical patients with preexisting renal dysfunction. Journal of Cardiothoracic and Vascular Anesthesia 2002;16(3):294‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Burns 2005 {published data only}
- Burns KEA, Chu MWA, Novick RJ, Fox SA, Gallo K, Martin CM, et al. Perooperative N‐acetylcysteine to prevent renal dysfunction in high‐risk patients undergoing CABG surgery. JAMA 2005;294(3):342‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Carcoana 2003 {published data only}
- Carcoana OV, Mathew JP, Davis E, Byrne DW, Hayslett JP, Hines RL, et al. Mannitol and dopamine in patients undergoing cardiopulmonary bypass: a randomized clinical trial. Anesthesia and Analgesia 2003;97:1222‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chen 2007 {published data only}
- Chen HH, Sundt TM, Cook DJ, Heublein DM, Burnett JC. Low dose nesiritide and the preservation of renal function in patients with renal dysfunction undergoing cardio‐pulmonary‐bypass surgery. Circulation 2007;116(11 Suppl):1134‐8. [PUBMED: PMID: 17846293] [DOI] [PubMed] [Google Scholar]
Cho 2009 {published data only}
- Cho JE, Shim JK, Chang JH, Oh YJ, Kil HK, Rha KH, et al. Effect of nicardipine on renal function after robot‐assisted laparoscopic radical prostatectomy. Urology 2009;73:1056‐60. [PUBMED: PMID: 19394503] [DOI] [PubMed] [Google Scholar]
Cogliati 2007 {published data only}
- Cogliati AA, Vellutini R, Nardini A, Urovi S, Hamdan M, Landoni G, et al. Fenoldopam infusion for renal protection in high‐risk cardiac surgery patients: a randomized clinical study. Journal of Cardiothoracic and Vascular Anesthesia 2007;21(6):847‐50. [PUBMED: PMID: 18068064] [DOI] [PubMed] [Google Scholar]
Colson 1990 {published data only}
- Colson P, Ribstein J, Mimran A, Grolleau D, Chaptal PA, Roquefeuil B. Effect of angiotensin converting enzyme inhibition on blood pressure and renal function during open heart surgery. Anesthesiology 1990;72:23‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Colson 1992 {published data only}
- Colson P, Ribstein J, Sequin JR, Marty‐Ane C, Roquefeuil B. Mechanisms of renal haemodynamic impairment during infrarenal aortic cross‐clamping. Anesthesia and Analgesia 1992;75:18‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Costa 1990 {published data only}
- Costa P, Ottino GM, Matani A, Pansini S, Canavese C, Passerini G, et al. Low‐dose dopamine during cardiopulmonary bypass in patients with renal dysfunction. Journal of Cardiothoracic Anesthesia 1990;4:469‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cregg 1999 {published data only}
- Cregg N, Mannion D, Casey W. Oliguria during corrective spinal surgery for idiopathic scoliosis: the role of antidiuretic hormone. Paediatric Anaesthesia 1999;9:505‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dawidson 1991 {published data only}
- Dawidson IJ, Willms CD, Sandor ZF, Coorpender LL, Reisch JS, Fry WJ. Ringer's lactate with or without 3% dextran‐60 as volume expanders during abdominal aortic surgery. Critical Care Medicine 1991;19:36‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dehne 2001 {published data only}
- Dehne MG, Klein TF, Muhling J, Sablotzki A, Osmer C, Hempelman G. Impairment of renal function after cardiopulmonary bypass is not influenced by dopamine. Renal Failure 2001;23(2):217‐30. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Lasson 1995 {published data only}
- Lasson L, Hansen HE, Juhl B, Paaske WP, Pedersen EB. A randomized, clinical study of the effect of low‐dose dopamine on cental and renal haemodynamics in infrarenal aortic surgery. European Journal of Endovascular Surgery 1995;10:82‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
de Lasson 1997 {published data only}
- Lasson L, Hansen HE, Juhl B, Paaske WP, Pedersen EB. Effect of felodipine on renal function and vasoactive hormones in infrarenal aortic surgery. British Journal of Anaesthesia 1997;79:719‐25. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Donmez 1998 {published data only}
- Donmez A, Ergun F, Kayhan Z, Tasdelen A, Dogan S. Verapamil and nimodipine do not improve renal function during cardiopulmonary bypass. Acta Anesthesiologica Italica 1998;49:173‐7. [Google Scholar]
Dural 2000 {published data only}
- Dural O, Ozkara A, Celebioglu B, Kanbak M, Ciliv G, Aypar U. Comparative study of dopamine and mannitol effects on renal function during cardiopulmonary bypass by using N‐acetyl‐beta‐D‐glucosaminidase assay. Turkish Journal of Medical Science 2000;30:453‐7. [Google Scholar]
Durmaz 2003 {published data only}
- Durmaz I, Yagdi T, Calkavur T, Mahmudov R, Apaydin AZ, Posacioglu H, et al. Prophylactic dialysis in patients with renal dysfunction undergoing on‐pump coronary artery bypass surgery. Annals of Thoracic Surgery 2003;75:859‐64. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fischer 2005 {published data only}
- Fischer UM, Tossios P, Mehlhorn U. Renal protection by scavenging in cardiac surgery patients. Current Medical Research and Opinion 2005;21(8):1161‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gubern 1988 {published data only}
- Gubern JM, Sancho JJ, Simo J, Sitges‐Serra A. A randomized trial on the effect of mannitol on postoperative renal function in patients with obstructive jaundice. Surgery 1988;103:39‐44. [MEDLINE: ] [PubMed] [Google Scholar]
Haase 2007 {published data only}
- Haase M, Haase‐Fielitz A, Bagshaw SM, Reade MC, Morgera S, Seevenayagam S, et al. Phase ll, randomized, controlled trial of high‐dose N‐acetylcysteine in high‐risk cardiac surgery patients. Critical Care Medicine 2007;35(5):1324‐31. [PUBMED: PMID: 17414730] [DOI] [PubMed] [Google Scholar]
Haase 2009 {published data only}
- Haase M, Haase‐Fielitz A, Bellomo R, Devarajan P, Story D, Matalanis G, et al. Sodium bicarbonate to prevent increases in serum creatinine after cardiac surgery: a pilot double‐blind, randomized controlled trial. Critical Care Medicine 2009;37(1):39‐47. [PUBMED: PMID: 19112278] [DOI] [PubMed] [Google Scholar]
Halpenny 2002 {published data only}
- Halpenny M, Rushe C, Breen P, Cunningham AJ, Boucher‐Hayes D, Shorten GD. The effect of fenoldopam on renal function in patients undergoing elective aortic surgery. European Journal of Anaesthesiology 2002;19(1):32‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harten 2008 {published data only}
- Harten J, Crozier JEM, McCreath B, Hay A, McMillan DC, McArdle CS, et al. Effect of intraoperative fluid optimization on renal function in patients undergoing emergency abdominal surgery: a randomized controlled pilot study. International Journal of Surgery 2008;6(3):197‐204. [PUBMED: PMID: 18424200] [DOI] [PubMed] [Google Scholar]
Hynninen 2006 {published data only}
- Hynninen MS, Niemi TT, Poyhia R, Raininko EI, Salmenpera MT, Lepantalo MJ, et al. N‐acetylcysteine for the prevention of kidney injury in abdominal aortic surgery: a randomized, double‐blind, placebo‐controlled trial. Anesthesia and Analgesia 2006;102:1638‐45. [PUBMED: PMID: 16717300] [DOI] [PubMed] [Google Scholar]
Kaya 2007 {published data only}
- Kaya K, Oguz M, Akar AR, Durdu S, Aslan A, Erturk S, et al. The effect of sodium nitroprusside infusion on renal function during reperfusion period in patients undergoing coronary artery bypass grafting: a prospective randomized clinical trial. European Journal of Cardiothoracic Surgery 2007;31:290‐7. [PUBMED: PMID: 17174559] [DOI] [PubMed] [Google Scholar]
Kleinschmidt 1997 {published data only}
- Kleinschmidt Von S, Bauer M, Grundmann U, Schneider A, Wagmer B, Graeter T. Influence of gamma‐hydroxybutyrate and pentoxifylline on renal function markers in coronary artery bypass graft surgery. Anaesthesiologie Reanimation (German) 1997;22(4):102‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Kramer 2002 {published data only}
- Kramer BK, Preuner J, Ebenburger A, Kaiser M, Bergner U, Eilles C, et al. Lack of renoprotective effect of theophylline during aortocoronary bypass surgery. Nephrology, Dialysis and Transplantation 2002;17:910‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kulka 1996 {published data only}
- Kulka PJ, Tryba M, Zenz M. Preoperative alpha2‐adrenergic receptor agonists prevent the deterioration of renal function after cardiac surgery: results of a randomized, controlled trial. Critical Care Medicine 1996;24:947‐52. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lassnigg 2000 {published data only}
- Lassnigg A, Donner E, Grubhofer G, Presterl E, Drubl W, Hiesmayr M. Lack of renoprotective effects of dopamine and furosemide during cardiac surgery. Journal of American Society of Nephrology 2000;11:97‐104. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lau 2001 {published data only}
- Lau LL, Halliday MI, Smye MG, Lee B, Hannon RJ, Gardiner KR, et al. Extraperitoneal approach reduces intestinal and renal dysfunction in elective abdominal aortic aneurysm repair. International Angiology 2001;20(4):282‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Licker 1996 {published data only}
- Licker M, Bednarkiewicz M, Neidhart P, Pretre R, Montessuit M, Favre H, et al. Preoperative inhibition of angiotensin‐converting enzyme improves systemic and renal haemodynamic changes during aortic abdominal surgery. British Journal of Anaesthesia 1996;76:632‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Loef 2004 {published data only}
- Loef BG, Henning RH, Epema AH, Rietman GW, Oeveren W, Navis GJ, et al. Effect of dexamethasone on perioperative renal function impairment during cardiac surgery with cardiopulmonary bypass. British Journal of Anaesthesia 2004;93(6):793‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Marathias 2006 {published data only}
- Marathias KP, Vassili M, Robola A, Alivizatos PA, Palatianos M, Geroulanos S, et al. Preoperative intravenous hydration confers renoprotection in patients with chronic kidney disease undergoing cardiac surgery. Artificial Organs 2006;30(8):615‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mitaka 2008 {published data only}
- Mitaka C, Kudo T, Jibiki M, Sugano N, Inoue Y, Makita K, et al. Effects of human atrial natriuretic peptide on renal function in patients undergoing abdominal aortic aneurysm repair. Critical Care Medicine 2008;36(3):745‐51. [PUBMED: PMID: 18431264] [DOI] [PubMed] [Google Scholar]
Morariu 2005 {published data only}
- Morariu AM, Loaf BG, Aarts LPHJ, Rietman GW, Bakhorst G, Oeveren W, et al. Dexamethasone: benefits and prejudice for patients undergoing on‐pump coronary artery bypass grafting. A study on myocardial, pulmonary, renal, intestinal and hepatic injury. Chest 2005;128(4):2677‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Morgera 2002 {published data only}
- Morgera S, Woydt R, Kern H, Schmutzler M, DeJonge K, Lun A, et al. Low‐dose prostacyclin preserves renal function in high risk patients after coronary bypass surgery. Critical Care Medicine 2002;30:107‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Myles 1993 {published data only}
- Myles PS, Buckland MR, Schenk NJ, Cannon GB, Langley M, Davis BB, et al. Effect of renal dose dopamine on renal function following cardiac surgery. Anaesthesia and Intensive Care 1993;21:56‐61. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nicholson 1996 {published data only}
- Nicholson ML, Baker DM, Hopkinson BR, Wenham PW. Randomized controlled trial of the effect of mannitol on renal reperfusion injury during aortic aneurysm surgery. British Journal of Surgery 1996;83:1230‐3. [MEDLINE: ] [PubMed] [Google Scholar]
Nouri‐Majalan 2009 {published data only}
- Nouri‐Majalan N, Ardakani EF, Forouzannia K, Moshtaghian H. Effect of allopurinol and vitamin E on renal function in patients with coronary artery bypass grafts. Vascular Health and Risk Management 2009;5:489‐94. [PUBMED: PMID: 19554089 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Hara 2002 {published data only}
- O'Hara JF, Thomas JR, Hsu THS, Sprung J, Cywinski JB, Rolin HA, et al. The effect of dopamine on renal function in solitary partial nephrectomy surgery. Journal of Urology 2002;167:24‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Parks 1994 {published data only}
- Parks RW, Diamond T, McCrory DC, Johnston GW, Rowlands BJ. Prospective study of postoperative renal function in obstructive jaundice and the effect of perioperative dopamine. British Journal of Surgery 1994;81:437‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Perez 2002 {published data only}
- Perez J, Taura P, Rueda J, Balust J, Anglada T, Beltran J. Role of dopamine in renal dysfunction during laparoscopic surgery. Surgical Endoscopy 2002;16(9):1297‐301. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prasad 2010 {published data only}
- Prasad A, Banakal S, Muralidhar K. N‐acetylcysteine does not prevent renal dysfunction after off‐pump coronary artery bypass surgery. European Journal of Anaesthesiology 2010;27:973‐7. [PUBMED: PMID: 20299984] [DOI] [PubMed] [Google Scholar]
Prowle 2012 {published data only}
- Prowle JR, Calzavacca P, Licari E, Ligabo EV, Echeverri JE, Haase M, et al. Pilot double‐blind, randomized controlled trial of short‐term atorvostatin for prevention of acute kidney injury after cardiac surgery. Nephrology 2012;17:215‐24. [PUBMED: PMID: 22117606] [DOI] [PubMed] [Google Scholar]
Pull Ter Gunne 1990 {published data only}
- Pull Ter Gunne AJ, Bruining HA, Obertop H. Haemodynamics and 'optimal' hydration in aortic cross clamping. The Netherlands Journal of Surgery 1990;42:113‐7. [PubMed] [Google Scholar]
Ristikankare 2006 {published data only}
- Ristikankare A, Kuitunen T, Kuitunen A, Uotila L, Vento A, Suojaranta‐Ylinen R, et al. Lack of renoprotective effect of i.v. N‐acetylcysteine in patients with chronic renal failure undergoing cardiac surgery. British Journal of Anaesthesia 2006;97(5):611‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ryckwaert 2001 {published data only}
- Ryckwaert F, Colson P, Ribstein J, Boccara G, Guillon G. Haemodynamic and renal effects of intravenous enalapril during coronary artery bypass graft surgery in patients with ischaemic heart dysfunction. British Journal of Anaesthesia 2001;86:169‐75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sezai 2000 {published data only}
- Sezai A, Shiono M, Orime Y, Hata H, Hata M, Negishi N, et al. Low‐dose continuous infusion of human atrial natriuretic peptide during and after cardiac surgery. Annals of Thoracic Surgery 2000;69:732‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sezai 2009 {published data only}
- Sezai A, Hata M, Niino T, Yoshitake I, Unosawa S, Wakui S, et al. Influence of continuous infusion of low‐dose human atrial natriuretic peptide on renal function during cardiac surgery. Journal of the American College of Cardiology 2009;54(12):1058‐64. [PUBMED: PMID: 19744614] [DOI] [PubMed] [Google Scholar]
Sezai 2011 {published data only}
- Sezai A, Hata M, Niino T, Yoshitake I, Unosawa S, Wakui S, et al. Results of low dose human atrial natriuretic peptide infusion in nondialysis patients with chronic kidney disease undergoing coronary artery bypass grafting. Journal of the American College of Cardiology 2011;58(9):897‐903. [PUBMED: PMID: 21851876] [DOI] [PubMed] [Google Scholar]
Shackford 1983 {published data only}
- Shackford SR, Sise MJ, Friedlund PH, Rowley WR, Peters RM, Virgilio RW, et al. Hypertonic sodium lactate versus lactated Ringer's solution for intravenous fluid therapy in operations on the abdominal aorta. Surgery 1983;94:41‐51. [MEDLINE: ] [PubMed] [Google Scholar]
Shim 2007 {published data only}
- Shim JK, Choi SH, Oh YJ, Kim CS, Yoo KJ, Kwak YL. The effect of mannitol on oxygenation and creatinine kinase MB release in patients undergoing multivessel off‐pump coronary artery bypass surgery. The Journal of Thoracic and Cardiovascular Surgery 2007;133(3):704‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Song 2009 {published data only}
- Song YR, Lee T, You SJ, Chin HJ, Chae D‐W, Lim C, et al. Prevention of acute kidney injury by erythropoietin in patients undergoing coronary artery bypass grafting: a pilot study. American Journal of Nephrology 2009;30:253‐60. [PUBMED: PMID: 19494484] [DOI] [PubMed] [Google Scholar]
Tang 1999 {published data only}
- Tang AT, El‐Gamel A, Keevil B, Yonan N, Deiraniya AK. The effect of renal dose dopamine on renal tubular function following cardiac surgery: assessed by measuring retinol binding protein (RBP). European Journal of Cardiothoracic Surgery 1999;15:717‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tang 2002 {published data only}
- Tang AT, Knott J, Nanson J, Hsu J, Haw MP, Ohri SK. A prospective randomized study to evaluate the renoprotective action of beating heart coronary surgery in low risk patients. European Journal of Cardiothoracic Surgery 2002;22:118‐23. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Thompson 1986 {published data only}
- Thompson JN, Cohen J, Blenkharn JI, McConnell JS, Barr J, Blumgart LH. A randomized clinical trial of oral ursodeoxycholic acid in obstructive jaundice. British Journal of Surgery 1986;73:634‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Turner 2008 {published data only}
- Turner S, Derham C, Orsi NM, Bosomworth M, Bellamy MC, Howell SJ. Randomized clinical trial of the effects of methylprednisolone on renal function after major vascular surgery. British Journal of Surgery 2008;95(1):50‐6. [PUBMED: PMID: 18027383] [DOI] [PubMed] [Google Scholar]
Urzua 1992 {published data only}
- Urzua J, Troncoso S, Bugedo G, Canessa R, Munoz H, Lema G, et al. Renal function and cardiopulmonary bypass: effect of perfusion pressure. Journal of Cardiothoracic and Vascular Anesthesia 1992;6(3):299‐303. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wahbah 2000 {published data only}
- Wahbah AM, el‐Hefny MO, Wafa EM, el‐Kharbotly W, el‐Enin AA, Zaglol A, et al. Perioperative renal protection in patients with obstructive jaundice using drug combinations. Hepatogastroenterology 2000;47:1691‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Welch 1995 {published data only}
- Welch M, Newstead CG, Smyth JV, Dodd PD, Walker MG. Evaluation of dopexamine hydrochloride as a renoprotective agent during aortic surgery. Annals of Vascular Surgery 1995;9:488‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wijnen 2002 {published data only}
- Wijnen MH, Vader HL, Wall Bake AW, Roumen RM. Can renal dysfunction after infra‐renal aortic aneurysm repair be modified by multi‐antioxidant supplementation?. Journal of Cardiovascular Surgery 2002;43:483‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Witczak 2008 {published data only}
- Witczak BJ, Hartmann A, Geiran OR, Bugge JF. Renal function after cardiopulmonary bypass surgery in patients with impaired renal function. A randomized study of the effect of nifedipine. European Journal of Anaesthesiology 2008;25:319‐25. [PUBMED: PMID: 18182121] [DOI] [PubMed] [Google Scholar]
Woo 2002 {published data only}
- Woo EB, Tang AT, el‐Gamel A, Keevil B, Greenhalgh D, Patrick M, et al. Dopamine therapy for patients at risk of renal dysfunction following cardiac surgery: science or fiction?. European Journal of Cardiothoracic Surgery 2002;22:106‐11. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yavuz 2002A {published data only}
- Yavuz S, Ayabaken N, Dilek K, Ozdemir A. Renal dose of dopamine in open heart surgery; does it protect renal tubular function?. Journal of Cardiovascular Surgery 2002;43:25‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Yavuz 2002B {published data only}
- Yavuz S, Ayabakan N, Goncu MT, Ozdemir A. Effect of combined dopamine and diltiazem on renal function after cardiac surgery. Medical Science Monitor 2002;8:145‐50. [MEDLINE: ] [PubMed] [Google Scholar]
Zanardo 1993 {published data only}
- Zanardo G, Michielon P, Rosi P, Teodori T, Antonucci F, Caenaro G, et al. Effect of a continuous diltiazem infusion on renal function during cardiac surgery. Journal of Cardiothoracic and Vascular Anesthesia 1993;7(6):711‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abe 1993 {published data only}
- Abe K, Fujino Y, Sakakibara T. The effect of prostaglandin E1 during cardiopulmonary bypass on renal function after cardiac surgery. European Journal of Clinical Pharmacology 1993;45:217‐20. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Aho 2004 {published data only}
- Aho PS, Niemi T, Lindgren L, Lepantalo M. Endovascular vs open AAA repair: similar effects on renal proximal tubular function. Scandinavian Journal of Surgery 2004;93:52‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Amar 2001 {published data only}
- Amar D, Fleisher M. Diltiazem treatment does not alter renal function after thoracic surgery. Chest 2001;119(5):1476‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Antonucci 1996 {published data only}
- Antonucci F, Calo L, Rizzolo M, Cantaro S, Bertoliswsi M, Travaglini M, et al. Nifedipine can preserve renal function in patients undergoing aortic surgery with infrarenal crossclamping. Nephron 1996;74(4):668‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Baldwin 1994 {published data only}
- Baldwin L, Henderson A, Hickman P. Effect of postoperative low‐dose dopamine on renal function after elective major vascular surgery. Annals of Internal Medicine 1994;120(9):744‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boldt 2000 {published data only}
- Boldt J, Lehmann A, Rompert R, Haisch G, Isgro F. Volume therapy with a new hydroxyethyl starch solution in cardiac surgical patients before cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia 2000;14:264‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boldt 2006 {published data only}
- Boldt J, Scholhorn T, Mayer J, Piper S, Suttner S. The value of albumin‐based intravascular volume replacement strategy in elderly patients undergoing major abdominal surgery. Anesthesia and Analgesia 2006;103(1):191‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Boodhwani 2009 {published data only}
- Boodhwani M, Rubens FD, Wozny D, Nathan HJ. Effects of mild hypothermia and rewarming on renal function after coronary artery bypass grafting. Annals of Thoracic Surgery 2009;87:489‐95. [PUBMED: PMID: 19161766] [DOI] [PubMed] [Google Scholar]
Boutros 1979 {published data only}
- Boutros AR, Ruess R, Olson L, Hoyt JL, Baker WH. Comparison of hemodynamic, pulmonary, and renal effects of use of three types of fluids after major surgical procedures on the abdominal aorta. Critical Care Medicine 1979;7(1):9‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bove 2005 {published data only}
- Bove T, Lanjdoni G, Calabro MG, Aletti G, Marino G, Cerchierini E, et al. Renoprotective action of fenoldopam in high‐risk patients undergoing cardiac surgery: a prospective, double‐blind, randomized clinical trial. Circulation 2005;111(24):3230‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Caglikulekci 1998 {published data only}
- Caglikulekciaz S, Yilmaz S, Kayaalp C, Demirbag A, Akoglu M. Postoperative renal function in obstructive jaundice and the effect of dopamine, mannitol and lactulose: a prospective clinical study. The Turkish Journal of Surgery 1998;14(4):230‐6. [Google Scholar]
Cahill 1987 {published data only}
- Cahill CJ, Pain JA, Bailey ME. Bile salts, endotoxin and renal function in obstructive jaundice. Surgery, Gynecology & Obstetrics 1987;165:519‐22. [MEDLINE: ] [PubMed] [Google Scholar]
Caimmi 2003 {published data only}
- Caimmi PP, Pagani L, Micalizzi E, Fiume C, Guani S, Bernardi M, et al. Fenoldopam for renal protection in patients undergoing cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia 2003;17(4):491‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Christakis 1992 {published data only}
- Christakis GT, Koch JP, Deemar KA, Fremes SE, Sinclair L, Chen E, et al. A randomized study of the systemic effects of warm heart surgery. Annals of Thoracic Surgery 1992;54:449‐57. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Christenson 1995 {published data only}
- Christenson JT, Maurice F, Simonet V, Velebit V, Schmuziger M. Normothermic versus hypothermic perfusion during primary coronary artery bypass grafting. Cardiovascular Surgery 1995;3(5):519‐24. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Dementi'eva 1996 {published data only}
- Dementi'eva II, Charnaia MA, Dzemeshkevich SL, Ziuliaeva TP. The preventive role of large doses of aprotinin in decreasing the degree of metabolic disorders during aortocoronary bypass operations. Anestheziologiia i Reanimatologica (Russian) 1996;1:55‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Feindt 1995 {published data only}
- Feindt PR, Walcher S, Volkmer I, Keller HE, Straub U, Hewer H, Seyfert UT, et al. Effect of high‐dose aprotinin on renal function in aortocoronary bypass grafting. Annals of Thoracic Surgery 1995;60:1076‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fischer 2002 {published data only}
- Fischer UM, Weissenberger WK, Warters RD, Geissler HJ, Allen SJ, Mehlhorn U. Impact of cardiopulmonary bypass management on postcardiac surgical renal function. Perfusion 2002;17:401‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fisher 1998 {published data only}
- Fisher AR, Jones P, Barlow P, Kennington S, Saville S, Farrimond J, et al. The influence of mannitol on renal function during and after open‐heart surgery. Perfusion 1998;13:181‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Franklin 1997 {published data only}
- Franklin SC, Moulton M, Sicard GA, Hammerman MR, Miller SB. Insulin‐like growth factor I preserves renal function postoperatively. American Journal of Physiology: Renal, Fluid and Electrolyte Physiology 1997;272(241‐2):F257‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Frumento 2006 {published data only}
- Frumento RJ, Logginidou HG, Wahlqander S, Wagener G, Playford HR, Sladen RN. Dexmedetomidine infusion is associated with enhanced renal function after thoracic surgery. Journal of Clinical Anesthesia 2006;18:422‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Garwood 2003 {published data only}
- Garwood S, Swamidoss CP, Davis EA, Samson L, Hines RL. A case series of low dose fenoldopam in seventy cardiac surgical patients at increased risk of renal dysfunction. Journal of Cardiothoracic and Vascular Anesthesia 2003;17(1):17‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gatot 2004 {published data only}
- Gatot I, Abramov D, Tsodikov V, Yeshaaiahu M, Orman S, Gavriel A, et al. Should we give prophylactic "renal‐dose" dopamine after coronary artery bypass surgery?. Journal of Cardiac Surgery 2004;19:128‐33. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gerola 2004 {published data only}
- Gerola LR, Buffolo E, Jasbik W, Botelho B, Bosco J, Brasil LA, et al. Off‐pump versus on‐pump myocardial revascularization in low‐risk patients with one or two vessel disease: perioperative results in a multicenter randomized controlled trial. Annals of Thoracic Surgery 2004;77:569‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gilbert 2001 {published data only}
- Gilbert TB, Hasnain JU, Flinn WR, Benjamin ME. Fenoldopam infusion associated with preserving renal function after aortic cross‐clamping for aneurysm repair. Journal of Cardiovascular Pharmacology and Therapeutics 2001;6:31‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Godet 2008 {published data only}
- Godet G, Lehot J‐J, Janvier G, Steib A, Castro V, Coriat P. Safety of HES 130/0.4 (Voluven) in patients with preoperative renal dysfunction undergoing abdominal aortic surgery: a prospective randomized controlled parallel‐group multicentre trial. European Journal of Anaesthesiology 2008;25:986‐94. [PUBMED: PMID: 18492315] [DOI] [PubMed] [Google Scholar]
Goto 1992 {published data only}
- Goto F, Kato S, Sudo I. Treatment of intraoperative hypertension with enflurane, nicardipine, or human atrial natriuretic peptide: haemodynamic and renal effects. Canadian Journal of Anaesthesia 1992;39(9):932‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Grundmann 1985 {published data only}
- Grundmann R, Heistermann S. Postoperative albumin infusion therapy based on colloid osmotic pressure. Archives of Surgery 1985;120:911‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Halpenny 2001 {published data only}
- Halpenny M, Lakshmi S, O'Donnell A, O'Callaghan‐Enright, Shorten GD. Fenoldopam: renal and splanchnic effects in patients undergoing coronary artery bypass grafting. Anaesthesia 2001;56:953‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hayashida 1997 {published data only}
- Hayashida M, Hanaoka K, Shimada Y, Namiki A, Amaha K. The effect of low‐dose prostglandin E1 on intra‐ and post‐operative renal function. The Japanese Journal of Anesthesiology 1997;46(4):464‐70. [MEDLINE: ] [PubMed] [Google Scholar]
Hayashida 2000 {published data only}
- Hayashida N, Chihara S, Kashikie H, Tayama E, Yokose S, Akasu K, et al. Effects of intraoperative administration of atrial natriuretic peptide. Annals of Annals of Thoracic Surgery 2000;70:1319‐26. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hisatomi 2012 {published data only}
- Hisatomi K, Eishi K. Multicenter trial of carperitide in patients with renal dysfunction undergoing cardiovascular surgery. General Thoracic Cardiovascular Surgery 2012;60:21‐30. [PUBMED: PMID: 22237735] [DOI] [PubMed] [Google Scholar]
Izumi 2006 {published data only}
- Izumi Y, Magishi K, Ishikawa N, Kimura F. On‐pump beating‐heart coronary artery bypass grafting for acute myocardial infarction. Annals of Thoracic Surgery 2006;81:573‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Izumi 2008 {published data only}
- Izumi K, Eishi K, Yamachika S, Hashizume K, Miura T, Nakaji S. The efficacy of human atrial natriuretic peptide in patients with renal dysfunction undergoing cardiac surgery. Annals of Thoracic and Cardiovascular Surgery 2008;14(5):294‐302. [PUBMED: PMID: 18989245 ] [PubMed] [Google Scholar]
Junnarkar 2003 {published data only}
- Junnerkar S, Lau LL, Edrees WK, Underwood D, Smye MG, Lee B, et al. Cytokine activation and intestinal mucosal and renal dysfunction are reduced in endovascular AAA repair compared to surgery. Journal of Endovascular Therapy 2003;10:195‐202. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kulka 1993 {published data only}
- Kulka PJ, Tryba M, Menzel C, Leurs F, Frankenberg C. Preoperative clonidine improves postoperative renal function in CABG patients. British Journal of Anaesthesia 1993;70:74. [Google Scholar]
Kumle 1999 {published data only}
- Kumle B, Boldt J, Piper S, Schmidt C, Suttner S, Salopek S. The influence of different intravascular volume replacement regimens on renal function in the elderly. Anesthesia and Analgesia 1999;89:1124‐30. [MEDLINE: ] [PubMed] [Google Scholar]
Kunt 2009 {published data only}
- Kunt AT, Akgun S, Atalan N, Bitir N, Arsan S. Frusamide infusion prevents the requirement of renal replacement therapy after cardiac surgery. The Anatolian Journal of Cardiology 2009;9(6):499‐504. [PUBMED: PMID: 19965324 ] [PubMed] [Google Scholar]
Kuraoka 1995 {published data only}
- Kuraoka S, Orita H, Watanabe T, Abe K, Abe H, Inui S, et al. Effect of combined aprotinin and prostaglandin E1 therapy on aortic arch replacement. The Japanese Journal of Thoracic Surgery 1995;48(3):198‐201. [MEDLINE: ] [PubMed] [Google Scholar]
Lema 1995 {published data only}
- Lema G, Meneses G, Urzua J, Jalil R, Canessa R, Moran S, et al. Effects of extracorporeal circulation on renal function in coronary surgical patients. Anesthesia and Analgesia 1995;81:446‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lema 1998 {published data only}
- Lema G, Urzua J, Jalil R, Canessa R, Moran S, Sacco C, et al. Renal protection in patients undergoing cardiopulmonary bypass with preoperative abnormal renal function. Anesthesia and Analgesia 1998;86(1):3‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lemmer 1996 {published data only}
- Lemmer JH Jr, Dilling EW, Morton JR, Rich JB, Robicsek F, Bricker DL, et al. Aprotinin for primary coronary artery bypass grafting: a multicenter trial of three dose regimens. Annals of Thoracic Surgery 1996;62:1659‐68. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Levy 1995 {published data only}
- Levy JH, Pifarre R, Schaff HV, Horrow JC, Albus R, Spiess B, et al. A multicenter, double‐blind, placebo‐controlled trial of aprotinin for reducing blood loss and the requirement for donor‐blood transfusion in patients undergoing repeat coronary artery bypass grafting. Circulation 1995;92(8):2236‐44. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Licker 1999 {published data only}
- Licker M, Schweizer A, Hohn L, Morel DR. Chronic angiotensin converting inhibition does not influence renal hemodynamic and function during cardiac surgery. Canadian Journal of Anaesthesia 1999;46(7):626‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lim 2002 {published data only}
- Lim E, Ali ZA, Attaran R, Cooper G. Evaluating routine diuretics after coronary surgery: a prospective randomized controlled study. Annals of Thoracic Surgery 2002;73:153‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Loef 2002 {published data only}
- Loef BG, Epema AH, Navis G, Ebels T, Oeveren W, Henning RH. Off‐pump coronary revascularization attenuates transient renal damage compared with on‐pump coronary revascularization. Chest 2002;121(4):1190‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
MacGregor 1994 {published data only}
- MacGregor DA, Butterworth JF 4th, Zaloga CP, Prielipp RC, James R, Royster RL. Hemodynamic and renal effects of dopexamine and dobutamine in patients with reduced cardiac output following coronary artery bypass grafting. Chest 1994;106:835‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mahesh 2008 {published data only}
- Mahesh B, Yim B, Robson D, Pillai R, Ratnatunga C, Pigott D. Does furosemide prevent renal dysfunction in high‐risk cardiac surgical patients? Results of a double‐blinded prospective randomised trial. European Journal of Cardiothoracic Surgery 2008;33:370‐6. [PUBMED: PMID: 18243724] [DOI] [PubMed] [Google Scholar]
Mahmood 2007 {published data only}
- Mahmood A, Gosling P, Vohra RK. Randomized clinical trial comparing the effects on renal function of hydroxyethyl starch or gelatine during aortic aneurysm surgery. British Journal of Surgery 2007;94:427‐33. [PUBMED: PMID: 17380548] [DOI] [PubMed] [Google Scholar]
Memmo 2011 {published data only}
- Memmo A, Carozzo A, Landoni G, Fano G, Sottocorna O, Bignami E, et al. Perioperative fenoldopam for the prevention of acute renal failure in non‐cardiac surgery, randomized clinical trial. Signa Vitae 2011;6(1):14‐9. [Google Scholar]
Neimark 2005 {published data only}
- Neimark MI, Merkulov IV, Flat MK. Renal protective function in surgical treatment for chronic infrarenal aortic aneurysms. Anesteziologiia i Reanimatologiia 2005;4:18‐22. [MEDLINE: ] [PubMed] [Google Scholar]
Nguyen 2001 {published data only}
- Nguyen NT, Lee SL, Anderson JT, Palmer LS, Canet F, Wolfe BM. Evaluation of intra‐abdominal pressure after laparoscopic and open gastric bypass. Obesity Surgery 2001;11:40‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nguyen 2002 {published data only}
- Nguyen NT, Perez RV, Fleming N, Rivers R, Wolfe BM. Effect of prolonged pneumoperitoneum on intraoperative urine output during laparoscopic gastric bypass. Journal of American College of Surgeons 2002;195:476‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Niiya 2001 {published data only}
- Niiya S, Fukusaki M, Nakamura T, Miyoshi H, Ogata K, Miyako M. Effects of dopamine and dobutamine on renal function and urinary excretion of prostaglandin E2 in elderly postoperative patients [In Japanese]. Japanese Journal of Anesthesiology 2001;50:122‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Nuutinen 1976 {published data only}
- Nuutinen L, Hollmen A. The effect of prophylactic use of furosemide on renal function during open heart surgery. Annales Chirurgiae et Gynaecologiae 1976;65:258‐66. [MEDLINE: ] [PubMed] [Google Scholar]
O'Hara 2002A {published data only}
- O'Hara JF, Sprung J. The effect of dopamine on renal function in solitary partial nephrectomy patients. Anesthesia and Analgesia 2002;94(Suppl):104. [Google Scholar]
Oliver 2006 {published data only}
- Oliver WC, Nuttall GA, Cherry KJ, Decker PA, Bower T, Ereth MH. A comparison of fenoldopam with dopamine and sodium nitroprusside in patients undergoing cross‐clamping of the abdominal aorta. Anesthesia and Analgesia 2006;103(4):833‐40. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ovrum 2004 {published data only}
- Ovrum E, Tangen G, Tollofsrud S, Oystese R, Ringdal MAL, Istad R. Cold blood cardioplegia versus cold crystalloid cardioplegia: a prospective randomized study of 1440 patients undergoing coronary artery bypass grafting. The Journal of Thoracic and Cardiovascular Surgery 2004;128(6):860‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pain 1991 {published data only}
- Pain JA, Cahill CJ, Gilbert JM, Johnson CD, Trapnell JE, Bailey ME. Prevention of postoperative renal dysfunction in patients with obstructive jaundice: a multi‐centre study of bile salts and lactulose. British Journal of Surgery 1991;78:467‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Paul 1986 {published data only}
- Paul MD, Mazer CD, Byrick RJ, Rose DK, Goldstein MB. Influence of mannitol and dopamine on renal function during elective infrarenal aortic clamping in man. American Journal of Nephrology 1986;6:427‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pavoni 1998 {published data only}
- Pavoni V, Verri M, Ferraro L, Volta CA, Paparella L, Capuzzo M, et al. Plasma dopamine concentration and effects of low dopamine doses on urinary output after major vascular surgery. Kidney International 1998;53(Suppl 66):S75‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Petry 1992 {published data only (unpublished sought but not used)}
- Petry A, Wulf H, Blomer U, Wawersik J. Nifedipine versus nitroglycerin in aortocoronary bypass surgery [Nifedipin versus nitrat bei aorto‐koronaren bypassoperationen [German]]. Anaesthesist 1992;41:39‐46. [1536439] [PubMed] [Google Scholar]
Piper 2003 {published data only}
- Piper SN, Kumle B, Maleck WH, Kiessling AH, Lehmann A, Rohm KD. Diltiazem may preserve renal tubular integrity after cardiac surgery. Canadian Journal of Anaesthesia 2003;50(3):285‐92. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Plusa 1991 {published data only}
- Plusa SM, Clark NW. Prevention of postoperative renal dysfunction in patients with obstructive jaundice: a comparison of mannitol‐induced diuresis and oral sodium taurocholate. Journal of the Royal College of Surgeons of Edinburgh 1991;36:303‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Priano 1993 {published data only}
- Priano LL, Smith JD, Cohen JI, Everts EE. Intravenous fluid administration and urine output during radical neck surgery. Head & Neck 1993;15:208‐15. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prifti 2001 {published data only}
- Prifti E, Boinacchi M, Frati G, Giunti G, Proietti P, Leacche M, et al. Beating heart myocardial revascularization on extracorporeal circulation in patients with end‐stage coronary artery disease. Cardiovascular Surgery 2001;9(6):608‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Regragui 1995 {published data only}
- Regragui IA, Izzat MB, Birdi I, Lapsley M, Bryan AJ, Angelini GD. Cardiopulmonary bypass perfusion temperature does not influence perioperative renal function. Annals of Thoracic Surgery 1995;60:160‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Riess 2000 {published data only}
- Riess FC, Moshar S, Bader R, Schofer J, Lower C, Kremer P, et al. Clinical outcome of patients with and without renal impairment undergoing a minimally invasive LIMA‐to‐LAD bypass operation. Heart Surgery Forum 2000;3(4):313‐8. [MEDLINE: ] [PubMed] [Google Scholar]
Ryckwaert 1995 {published data only}
- Ryckwaert F, Calvert B, Peckstaing M, Wintrebert P, Ribstein J, Colson P. Effects of enalaprilate on haemodynamics and renal function during cardiac surgery in patients with preoperative heart failure. British Journal of Anaesthesia 1995;74:A103. [Google Scholar]
Sanders 2001 {published data only}
- Sanders G, Mercer SJ, Saeb‐Parsey K, Akhavani MA, Hosie KB, Lambert AW. Randomized clinical trial of intravenous fluid replacement during bowel preparation for surgery. British Journal of Surgery 2001;88:1363‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sezai 2006 {published data only}
- Sezai A, Shiono M, Hata M, Iida M, Wakui S, Soeda M, et al. Efficacy of continuous low‐dose human atrial natriuretic peptide given from the beginning of cardiopulmonary bypass for thoracic aortic surgery. Surgery Today 2006;36:508‐14. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Sherry 1997 {published data only}
- Sherry E, Tooley MA, Bolsin SN, Monk CR, Wilcox J. Effect of dopexamine hydrochloride on renal vascular resistance index and haemodynamic responses following coronary artery bypass graft surgery. European Journal of Anaesthesiology 1997;14:184‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Skillman 1975 {published data only}
- Skillman JJ, Restall DS, Salzman EW. Randomized trial of albumin vs. electrolyte solutions during abdominal aortic operations. Surgery 1975;78(3):291‐303. [MEDLINE: ] [PubMed] [Google Scholar]
Stanitsh 2002 {published data only}
- Stanitsh MB, Sindjelitsh RB, Neshkovitsh V, Davidovitsh LB, Lotina SL. Renal protection during the operation of infra‐renal aorta [In Serbian]. Srpski Archv Za Celokupno Lekarstvo (Serbian) 2002;130(5‐6):168‐72. [Google Scholar]
Straka 2004 {published data only}
- Straka Z, Widimsky P, Jirasek K, Stros P, Votava J, Vanek T, et al. Off‐pump versus on‐pump coronary surgery: final results from a prospective randomized study PRAGUE‐4. Annals of Thoracic Surgery 2004;77:789‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tataranni 1994 {published data only}
- Tataranni G, Malacarne F, Farinelli R, Tarroni G, Gritti G, Guberti A, et al. Beneficial effects of verapamil in renal‐risk surgical patients. Renal Failure 1994;16(3):383‐90. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Torsello 1993 {published data only}
- Torsello G, Kutkuhn B, Kniemeyer H, Sandmann W. Prevention of acute renal failure after suprarenal aortic surgery: results of a pilot study. Zentralblatt fur Chirurgie 1993;118:390‐4. [MEDLINE: ] [PubMed] [Google Scholar]
Tripathy 1996 {published data only}
- Tripathy U, Dhiman RK, Attari A, Katariya RN, Ganguly NK, Chawla YK, et al. Preoperative bile salt administration versus bile salt refeeding in obstructive jaundice. The National Medical Journal of India 1996;9(2):66‐9. [MEDLINE: ] [PubMed] [Google Scholar]
Ueki 1995 {published data only}
- Ueki M, Yokono S, Nogaya J, Taei S, Komatsu H, Ogli K. Effect of ulinastatin on renal function after subrenal aortic cross‐clamping. The Japanese Journal of Anesthesiology 1995;44(3):357‐61. [MEDLINE: ] [PubMed] [Google Scholar]
Vogt 1996 {published data only}
- Vogt NH, Bothner U, Lerch G, Lindner KH, Georgieff M. Large‐dose administration of 6% hydroxyethyl starch 200/0.5 for total hip arthroplasty: plasma homeostasis, hemostasis, and renal function compared to use of 5% human albumin. Anesthesia and Analgesia 1996;83:262‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vogt 1999 {published data only}
- Vogt N, Bothner U, Brinkmann A, Petriconi R, Georgieff M. Peri‐operative tolerance to large‐dose 6% HES 200/0.5 in major urological procedures compared with 5% human albumin. Anaesthesia 1999;54:121‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Weisz 2009 {published data only}
- Weisz G, Filby SJ, Cohen MG, Allie DE, Weinstock BS, Kyriazis D, et al. Safety and performance of targeted renal therapy: the Be‐RITe! Registry. Journal of Endovascular Therapy 2009;16(1):1‐12. [PUBMED: PMID: 19281283] [DOI] [PubMed] [Google Scholar]
Welch 1993 {published data only}
- Welch M, Knight DG, Carr HM, Smyth JV, Walker MG. The preservation of renal function by isovolemic hemodilution during aortic operations. Journal of Vascular Surgery 1998;18:858‐66. [MEDLINE: ] [PubMed] [Google Scholar]
Wool 2010 {published data only}
- Wool DB, Lemmens HJM, Brodsky JB, Solomon H, Chong KP, Morton JM. Intraoperative fluid replacement and postoperative creatine phosphokinase levels in laparoscopic bariatric patients. Obesity Surgery 2010;20:698‐701. [PUBMED: PMID: 20198451] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Fergany 2011 {published and unpublished data}
- Fergany A, O'Hara J, Campbell S, Kaple K, Bonilla A, Mahboobi R. The effect of fenoldopam on renal function in solitary partial nephrectomy surgery. European Journal of Urology 2011;10:199. [Google Scholar]
Additional references
Anderson 1986
- Anderson SG, Rennke HG, Brenner BM. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in rat. The Journal of Clinical Investigation 1986;77:1993‐2000. [PUBMED: PMID: 3011863] [DOI] [PMC free article] [PubMed] [Google Scholar]
ANZICS CTG 2000
- Australian and New Zealand Intensive Care Society (ANZICS) Clinical Trials Group. Low‐dose dopamine in patients with early renal dysfunction: a placebo‐controlled randomized trial. Lancet 2000;356:2139‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Bellomo 2007
- Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Medicine 2007;33:409‐13. [PUBMED: PMID: 17165018 ] [DOI] [PubMed] [Google Scholar]
Brazel 1996
- Brazel PW, MacPhee IB. Inappropriate secretion of antidiuretic hormone in postoperative scoliosis patients: the role of fluid management. Spine 1996;21(6):724‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Brienza 2009
- Brienza N, Giglio MT, Marucci M, Riore T. Does perioperative hemodynamic optimization protect renal function in surgical patients? A meta‐analytic study. Critical Care Medicine 2009;37(6):2079‐90. [PUBMED: PMID: 19384211] [DOI] [PubMed] [Google Scholar]
Brown 1998
- Brown NJ, Vaughan DE. Angiotensin‐converting enzyme inhibitors. Circulation 1998;97:1411‐20. [PUBMED: PMID: 9577953] [DOI] [PubMed] [Google Scholar]
Chatterjee 2005
- Chatterjee PK. Pleiotropic renal actions of erythropoietin. Lancet 2005;365:1890‐2. [PUBMED: PMID: 15924987] [DOI] [PubMed] [Google Scholar]
Deeks 2008
- Deeks JJ, Higgins JPT, Altman DG (editors), on behalf of the Cochrane Statistical Methods Group. Chapter 9: Analysing data and undertaking meta‐analyses: In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. www.cochrane‐handbook.org.
Dillingham 1986
- Dillingham MA, Anderson RJ. Inhibition of vasopressin action by atrial natriuretic factor. Science 1986;231:1572‐3. [PUBMED: PMID: 3006248] [DOI] [PubMed] [Google Scholar]
Edelstein 1997
- Edelstein CL, Ling H, Wangsiripaisan A, Schrier RW. Emerging therapies for acute renal failure. American Journal of Kidney Diseases 1997;30(Suppl 4):89‐95. [PUBMED: PMID: 9372985] [DOI] [PubMed] [Google Scholar]
Endre 2011
- Endre ZH, Pickering JW, Walker RJ, Davarajan P, Edelstein CL, Bonventre JV, et al. Improved performance of urinary biomakers of acute kidney injury in the critically ill by stratification for injury duration and baseline renal function. Kidney International 2011;79:1119‐30. [PUBMED: 21307838] [DOI] [PMC free article] [PubMed] [Google Scholar]
Harris 1987
- Harris PJ, Thomas D, Morgan TO. Atrial natriuretic peptide inhibits angiotensin stimulated proximal tubular sodium and water reabsorption. Nature 1987;326:697‐8. [PUBMED: PMID: 2951600] [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.0.0 [updated February 2008]. The Cochrane Collaboration, 2008. www.cochrane‐handbook.org.
Johnson 2006
- Johnson DW, Forman C, Vesey DA. Novel renoprotective actions of erythropoietin; new uses for an old hormone. Nephrology 2006;11:306‐12. [PUBMED: PMID: 16889570] [DOI] [PubMed] [Google Scholar]
Kay 2003
- Kay J, Chow WH, Chan TM, Lo SK, Kwok OH, Yop A, et al. Acetylcysteine for prevention of acute deterioration of renal function following elective coronary angiography and intervention: a randomized controlled trial. JAMA 2003;289:553‐8. [PUBMED: PMID: 12578487] [DOI] [PubMed] [Google Scholar]
Kiefer 2000
- Kiefer P, Vogt J, Radermacher P. From mucolytic to antioxidant and liver protection: new aspects in the intensive care unit career of N‐acetylcysteine. Critical Care Medicine 2000;28:3935‐6. [PUBMED: PMID: 11153640 ] [DOI] [PubMed] [Google Scholar]
Levin 1998
- Levin ER, Gardiner DG, Samson WK. Natriuretic peptides. New England Journal of Medicine 1998;339:321‐28. [PUBMED: PMID: 9682046] [DOI] [PubMed] [Google Scholar]
Lopes 2013
- Lopes JA, Jorge S. The RIFLE and AKIN classifications for acute kidney injury: a critical and comprehensive review. Clinical Kidney Journal 2013;6:8‐14. [PUBMED: PMID: 20931312] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maiese 2005
- Maiese K, Li F, Chong ZZ. New avenues of exploration for erythropoietin. JAMA 2005;293:90‐5. [PUBMED: PMID: 15632341] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marin‐Grez 1986
- Marin‐Grez M, Fleming JT, Steinhausen M. Atrial natriuretic peptide causes pre‐glomerular vasodilation and post‐glomerular vasoconstriction in rat kidney. Nature 1986;324:473‐6. [PUBMED: PMID: 2946962] [DOI] [PubMed] [Google Scholar]
McDonald 1964
- McDonald RH, Goldberg LI, McNay JL, Tuttle EP. Effects of dopamine in man: augmentation of sodium excretion, glomerular filtration rate, and renal plasma flow. Journal of Clinical Investigation 1964;43:1116‐24. [PUBMED: PMID: 14171789 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehta 2007
- Mehta RL, Kellum JA, Shah SV, Molitoris BA, Ronco C, Warnock DG, et al. Acute Kidney Injury Network. Report of an initiative to improve outcomes in acute kidney injury. Critical Care 2007;11:R31. [PUBMED: PMID: 17331245 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moitra 2009
- Moitra V, Gaffney A, Playford H, Sladen RN. What is the best means of preventing perioperative renal injury?. Fleisher LA (editor). Evidence–Based Practice of Anesthesiology. 2nd Edition. Philadelphia: Saunders Elsevier, 2009. [ISBN: 978‐1‐4160‐5996‐7] [Google Scholar]
Moore 2011
- Moore E, Bellomo R. Erythropoetin in acute kidney injury. Annals of Intensive Care 2011;1(1):3. [PUBMED: PMID: 21906325] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morcos 2004
- Morcos SK. Prevention of contrast media nephrotoxicity ‐ the story so far. Clinical Radiology 2004;59:381‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Renton 2005
- Renton MC, Snowden CP. Dopexamine and its role in the protection of hepatosplanchnic and renal perfusion in high‐risk surgical and critically ill patients. British Journal of Anaesthesia 2005;94:459‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schrier 1984
- Schrier RW, Arnold PE, Gordon JA, Burke TJ. Protection of mitochondrial function by mannitol in ischemic acute renal failure. American Journal of Physiology ‐ Renal Physiology 1984;247:F365‐9. [PUBMED: PMID: 6431829] [DOI] [PubMed] [Google Scholar]
Schrier 1991
- Schrier RW, Burke TJ. Role of calcium–channel blockers in preventing acute and chronic renal injury. Journal of Cardiovascular Pharmacology 1991;18(Suppl 6):S38‐43. [PUBMED: PMID: 1725916] [PubMed] [Google Scholar]
Seri 1988
- Seri I, Kone BC, Gullans SR, Aperia A, Brenner BM, Ballerman BJ. Locally formed dopamine inhibits Na+K+‐ATPase activity in rat renal cortical tubule cells. American Journal of Physiology-Renal Physiology 1988;255:F666‐73. [PUBMED: PMID: 2845809] [DOI] [PubMed] [Google Scholar]
Sonnenberg 1986
- Sonnenberg H, Honrath U, Chong CK, Wilson DR. Atrial natriuretic factor inhibits sodium transport in medullary collecting duct. American Journal of Physiology-Renal Physiology 1986;250:F963‐6. [PUBMED: PMID: 2940876] [DOI] [PubMed] [Google Scholar]
Tepel 2000
- Tepel M, Giet M, Schwarzfeld C, Laufer U, Liermann D, Zidek W. Prevention of radiographic‐contrast‐agent‐induced reductions in renal function by acetylcysteine. New England Journal of Medicine 2000;343:180‐4. [PUBMED: PMID: 10900277] [DOI] [PubMed] [Google Scholar]
Wang 2003
- Wang F, Dupuis J‐Y, Nathan H, Williams K. An analysis of the association between preoperative renal dysfunction and outcome in cardiac surgery. Chest 2003;124:1852‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wijeysundera 2006
- Wijaysundera DN, Karoouti K, Beattie WS, Rao V, Ivanov J. Improving the identification of patients at risk of postoperative renal failure after cardiac surgery. Anesthesiology 2006;104:65‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zafarullah 2003
- Zafarullah M, Li WQ, Sylvester J, Ahmad M. Molecular mechanisms of N‐acetylcysteine actions. Cellular and Molecular Life Sciences 2003;60:6‐20. [PUBMED: PMID: 12613655 [PubMed] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Zacharias 2005
- Zacharias M, Gilmore ICS, Herbison GP, Sivalingam P, Walker RJ. Interventions for protecting renal function in the perioperative period. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003590.pub2] [DOI] [PubMed] [Google Scholar]
Zacharias 2008
- Zacharias M, Conlon NP, Herbison GP, Sivalingam P, Walker RJ, Hovhannisyan K. Interventions for protecting renal function in the perioperative period. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003590.pub3] [DOI] [PubMed] [Google Scholar]


