Abstract
Background
In 1998 secretin, a gastrointestinal hormone, was suggested as an effective treatment for autism spectrum disorders (ASD) based on anecdotal evidence.
Objectives
To assess whether intravenous secretin improves the core features of ASD, other aspects of behaviour or function such as self‐injurious behaviour, and the quality of life of affected individuals and their carers. We also assessed whether secretin causes harm. This is an updated version of our review of this topic originally published in 2005.
Search methods
We searched CENTRAL (2010 Issue 1), MEDLINE (1950 to January 2010) , EMBASE (1980 to 2010 Week 2), PsycINFO (1806 to 2010 Week 2), CINAHL (1938 to January 2010), ERIC (1966 to January 2010), Sociological Abstracts (1952 to January 2010). Sociofile and HealthStar were searched in March 2005 when this review was first published, but were not available for this update. Records were limited to studies published since 1998 as this is when secretin was first proposed as a possible treatment for ASD. We searched reference lists of trials and reviews; we also contacted experts and trialists to find unpublished studies.
Selection criteria
Randomised controlled trials of intravenous secretin compared to a placebo treatment in children or adults diagnosed with ASD, where at least one standardised outcome measure was reported.
Data collection and analysis
Sixteen studies met the inclusion criteria but for two of these, conducted by Repligen, the only available multisite data were reported in press releases. All outcome data from the other 14 trials were continuous. Where trials used cross‐over designs, we conducted analysis on results from the first treatment phase. Where mean change from baseline was reported, we used this in preference to post‐treatment scores for meta‐analyses or forest plots. Meta‐analysis was able to be attempted for only one outcome (Childhood Autism Rating Scale). Insufficient data were available to conduct sensitivity or subgroup analyses to assess the impact of study quality, clinical differences in the intervention or clinically relevant differences between groups, such as age or presence of gastrointestinal symptoms.
Main results
Over 900 children were recruited for the secretin trials. Twenty‐five established standardised outcome measures were reported to assess core features of ASD, communication, behaviour, visuospatial skills, affect and adverse events. One standardised measure of global impression was also used. No more than four studies used any one outcome measure similarly. When duration from the start of the intervention to outcome assessment was known, outcomes were reported at between three and six weeks. Meta‐analysis of data was not possible but there is now consistency of findings, with RCTs of the efficacy of secretin in autism not showing improvements for core features of ASD.
Authors' conclusions
There is no evidence that single or multiple dose intravenous secretin is effective and as such currently it should not be recommended or administered as a treatment for ASD. Further experimental assessment of secretin's effectiveness for ASD can only be justified if there is new high‐quality and replicated scientific evidence that either finds that secretin has a role in neurotransmission in a way that could benefit all children with ASD or identifies important subgroups of children with ASD who could benefit from secretin because of a proven link between the action of secretin and the known cause of their ASD, or the type of problems they are experiencing.
Keywords: Humans; Autistic Disorder; Autistic Disorder/drug therapy; Autistic Disorder/psychology; Behavior; Behavior/drug effects; Communication; Gastrointestinal Agents; Gastrointestinal Agents/administration & dosage; Hormones; Hormones/administration & dosage; Injections, Intravenous; Randomized Controlled Trials as Topic; Secretin; Secretin/administration & dosage; Treatment Outcome
Plain language summary
Intravenous secretin for autism spectrum disorders (ASD)
Secretin is a gastrointestinal hormone that was first presented as an effective treatment for ASD in 1998, based on anecdotal evidence. On the basis of these first reports many families sought treatment with intravenous secretin for their children with ASD even though secrein was not a proven, effective treatment and there was inadequate information about side effects when used in this group of children. This review included 16 randomised trials with a placebo control group, with over 900 children involved. The review found no evidence that single or multiple dose intravenous secretin is effective in improving the main problems seen in ASD, namely a lack of social interaction and communication and restrictive, repetitive behaviours and routines. As such, currently it should not be recommended or administered as a treatment for ASD. Further experimental assessment of secretin's effectiveness for ASD can only be justified if there is convincing new evidence that finds that secretin can influence brain function in a way that could benefit children with ASD or a link is proven between secretin and the known cause of ASD for some or all children.
Background
Description of the condition
What are autism spectrum disorders?
Although autism was first described in the 1940s (Kanner 1943), the classification of autism and its aetiologies are still disputed. More recently the term 'autism spectrum disorders', or 'ASD', has been developed because children with abnormalities of communication, behaviour and social interaction of the type seen in autism have been identified who do not fulfil all the diagnostic criteria for autism. However, there is no diagnostic classification for autism spectrum disorders. As such, ASD are still diagnosed using either DSM‐IV (APA 2000) or ICD‐10 (WHO 1993) classification systems (Table 1). These versions have superseded all previous DSM and ICD classifications. Along with childhood autism or autistic disorder, diagnoses like Pervasive Developmental Disorder‐Not Otherwise Specified (PDD‐NOS), Asperger syndrome and atypical autism are now considered by many to be part of the autism spectrum. In addition, ICD‐10 categories of other childhood disorders of social functioning (F94.8) and childhood disorders of social functioning, unspecified (F94.9), may also be relevant; as well as other codes for behavioural and language disturbances. As well as the diagnostic features of autism, many affected children also have intellectual impairment and some have other physical, behavioural and emotional problems. As such, ASD are heterogeneous conditions. In an attempt to standardise diagnostic practice and research, several diagnostic instruments have also been developed for ASD. Only four instruments are available for research use at present that relate scores to current diagnostic criteria. One of these tools is a behavioural assessment, the Autism Diagnostic Observation Scale (ADOS) (Lord 1997); the other three are interviews: Autism Diagnostic Interview‐Revised (ADI‐R) (Lord 1994), The Diagnostic Interview for Social and Communication Disorders (DISCO) (Wing 1999), and the third is The Developmental, Dimensional and Diagnostic Interview (Skuse 2004). The Childhood Autism Rating Scale (CARS) (Schopler 1980) is another frequently used standardised assessment tool. Unfortunately, there is not complete agreement between these diagnostic methods.
1. DSM‐IV and ICD‐10 diagnoses and codes of Autism Spectrum Disorders.
| DSM‐IV | ICD‐10 |
| Autistic disorder (299.00) | Childhood autism (F84.0) |
| Pervasive developmental disorder, not otherwise specified (299.80) | Atypical autism (F84.1) |
| Asperger's disorder (299.80) | Asperger's syndrome (F84.5) |
| Rett's disorder (299.80) | Other pervasive developmental disorders (F84.8) |
| Childhood disintegrative disorder (299.10) | Pervasive developmental disorder, unspecified (F84.9) |
A review and an update of epidemiological studies published between 1966 and 2003 show reports of the estimated prevalence for autism varying between 0.7 and 40 children per 10,000 (Fombonne 1999; Fombonne 2003). Estimates of the prevalence of ASD using the DSM‐III, DSM‐IIIR, DSM‐IV or ICD‐10 diagnostic classification systems from published synthesised literature up to April 2004 varied between 3 and 82 in 10,000 (Williams 2006), and from 2000 to 2007 between 16 and 181 in 10,000 (Fombonne 2009). The problems seen in ASD usually present in childhood. Boys are affected about four times more frequently than girls. Although there is considerable agreement that the aetiology of ASD is multifactorial and probably involves genetic, neurodevelopmental and environmental pathways, the relative contribution of these aspects is still uncertain (Gillberg 2000).
Issues in therapy for autism spectrum disorders
There are several generic issues that apply to therapies for ASD. The heterogeneity of symptoms and associated problems seen with this spectrum of disorders, and even within one diagnostic group such as classical autism, mean it is often difficult to be sure which individuals will benefit from therapies. As in other developmental abnormalities, there are likely to be changes over time for individuals with autism spectrum disorders. It is also likely that outcomes of therapy will be different depending on timing of therapy in relation to age and onset of problems. To date, pharmacological treatments have been, at best, useful adjuncts to behavioural intervention. They have been associated with improvements in problems such as sleep disturbance, mood disorder, poor attention and concentration, and self‐harm or aggression to others. Many therapies suggested for individuals with ASD are invasive, time consuming and expensive, and there is little known about their potential to do harm. However, individuals with ASD and their families are seeking ways to improve their lives and futures. The many needs of these individuals trigger hope for cure. As a result, therapies like secretin have become widely used after limited reports of success.
Description of the intervention
Secretin
Secretin is a 27 amino‐acid polypeptide produced in the intestine. Its role in gastrointestinal function is well described. There is a postulated role in decreasing immune responses in the gut lumen. Secretin receptors have also been demonstrated in the brains of rats and pigs (Freier 1981) but the exact role of secretin and its mechanism of action in the central nervous system have not been determined. The behaviour of rats has been studied after secretin infusion (Charlton 1983). When injected intracerebrally, secretin decreased the locomotor activity of rats. However, there is uncertainty about the role of secretin in the human brain.
How the intervention might work
Autism spectrum disorders and secretin
Clinicians investigating children with a combination of gastrointestinal disorders and developmental symptoms of the type seen in ASD noticed improvements in ASD symptoms during their investigations. In a series of three cases, the administration of porcine secretin was seen to positively affect the behaviour of children with ASD (Horvath 1998). Improvements in eye contact, alertness and language were noted. The children had incidentally been given the secretin during an endoscopic procedure. The study included some laboratory data suggesting physiological changes in some of these children. Following this report, the use of secretin became widespread. Subsequently, following reviews of published trials, it was stated that secretin is not an effective or recommended treatment for ASD (Perry 2003; Roberts 2006; Myers 2007; SIGN 2007).
Why it is important to do this review
Controlled studies of the effectiveness of intravenous secretin have been completed. It is important to update this systematic review to ensure that there is no new evidence that contradicts previous findings and to assess whether there is sufficient evidence to declare that this review should no longer be updated. It is also important to present the strengths and weaknesses of methodologies used to date, relevant to secretin specifically and to ASD trials in general.
Objectives
Aim
The aim of this systematic review was to determine if intravenous secretin is effective in improving the lives of individuals affected by autism spectrum disorders (ASD) and their families.
Objectives
To determine if intravenous secretin improves the core features of ASD (social interaction, communication and behaviour problems)
To determine if intravenous secretin improves non‐core aspects of behaviour or function such as self‐injurious behaviour
To determine if intravenous secretin improves the quality of life of affected individuals and their carers
To determine if intravenous secretin has short‐term and long‐term effects on outcome
To determine if intravenous secretin causes harm
Methods
Criteria for considering studies for this review
Types of studies
Trials were eligible for inclusion in the review if:
the assignment of study participants to the intervention or control group was random;
the study intervention had its major focus on intravenous secretin;
there was at least one standardised measure such as a behaviour checklist used for the intervention and control groups, pre‐ and post‐intervention.
Types of participants
Inclusion was limited to individuals with Pervasive Developmental Disorders, excluding Rett syndrome and Childhood Disintegrative Disorder. The diagnosis must have been made using a standardised diagnostic instrument or by using established diagnostic criteria. We intended to include participants with dual diagnosis. No such participants were found.
Types of interventions
Intravenous administration of secretin regardless of dosage used or frequency of administration. The control group must have been a placebo group.
Types of outcome measures
Outcomes
Improvement of core features of ASD, that is social interaction, communication and behavioural problems including stereotypy or obsessive behaviours
Improvement in non‐core behaviours such as sleep disturbance, self‐mutilation, aggression, attention and concentration problems, gastrointestinal function
Global impression of health
Improvement in quality of life for the individual or their family
Adverse events
Short‐, medium‐ and long‐term outcomes were sought (six weeks, six months and 12 months after therapy).
Measures
Standardised diagnostic assessment instruments (for example, Autism Diagnostic Interview, Revised (ADI‐R) (Lord 1994), Autism Diagnostic Observation Schedule (ADOS) (Lord 1997), Diagnostic Interview for Social and Communication Disorders (DISCO) (Wing 1999))
Standardised communication assessments
Quality of life questionnaires
Behaviour scales (for example, the Child Behavior Checklist (Achenbach 1991))
Global Impression Rating Scales (for example, the Clinical Global Impression Scale (CGIS) (Guy 1976))
Other health outcome rating scales
Search methods for identification of studies
Because of the recent nature of work with secretin in ASD, we only sought trials conducted after 1998 and prioritised contact with experts in the field to identify unpublished studies.
We performed searches for the original review in March 2005. Records were limited by date to studies published since 1998. During the period between the original review and this update, Sociological Abstracts replaced Sociofile and HealthStar became unavailable. We updated the searches in June 2007 and in January 2010. We searched the following databases.
Cochrane Central Register of Controlled Trials (CENTRAL) ( 2010, Issue 1), last searched 20 January 2010 MEDLINE (1950 to 15 January 2010), last searched 20 January 2010 EMBASE (1980 to Week 2, 2010), last searched 20 January 2010 PsycINFO (1806 to Week 2, 2010), last searched 20 January 2010 CINAHL (1938 to current), last searched 20 January 2010 Sociofile (searched March 2005), unavailable for update Sociological Abstracts (1952 to current), last searched 20 January 2010 ERIC (1966 to current), last searched 20 January 2010 HealthStar (searched March 2005), unavailable for update
We based searches for this update on the strategy used in the original review to search the Cochrane Central Register of Controlled Trials (Appendix 1). Subsequent search strategies are in Appendix 2 (2007) and Appendix 3 (2010). The aim of the search strategy was for high precision and recall. There were no language restrictions. We also reviewed reference lists of articles identified through the search strategy to identify trials.
In addition to the core searches for this update, we ran supplementary searches to locate data from the studies conducted by Repligen. On 17 October 2011 we searched MEDLINE (1948 to week 1 October 2011), EMBASE (1948 to week 1 October 2011) and ABI/Inform using the terms (RG‐1068 or repligen or secreflo). In November 2011 we also searched the websites of ClinicalTrials.gov, CenterWatch, US Food and Drug Administration, Autistic Society, Autism Research Institute, Autism Society, National Autistic Society, National Institute of Child Health & Human Development and the Repligen Corporation. We made further contact by email with CenterWatch, the Autism Society, Autism Research Institute and the Repligen Corporation.
Data collection and analysis
Selection of studies
Two review authors (KW, DW) screened titles and abstracts from the searches for the first published review and one author (KW) for the 2010 search. We resolved disagreement by consensus and discarded articles that did not fulfil inclusion criteria. We retrieved potentially relevant articles for full‐text assessment and data extraction. In 2005 we also conducted web searches, searched conference proceedings and contacted clinicians to determine if any unpublished or ongoing trials existed.
Data extraction and management
We organised data using Review Manager 4.2.6 (Review Manager 4.2). As no new data were added in this update, the organisation as established for the prior review was not changed. We developed data extraction forms a priori and included information regarding study location, methods, participant details, dose and frequency of secretin administration and outcome. Two review authors (KW, DW) independently completed data extraction forms for each included study. No disagreements arose.
Assessment of risk of bias in included studies
In 2005 two review authors (KW and DW) independently assessed each included study for risk of bias, without blinding to authorship or source. The assessments were compared to identify inconsistencies. We resolved any differences in interpretation by discussion and consensus was reached. One author (KW) used data extracted during 2005 to reclassify studies according to risk of bias criteria outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008). Risk of bias was assessed according to the following four domains, with ratings of low risk of bias, high risk of bias and unclear (uncertain) risk of bias.
1. Sequence generation
Was the allocation sequence adequately generated?
Low risk of bias: computer‐generated random numbers, table of random numbers, coin tossing or similar. High risk of bias: day of week, even and odd clinic record numbers, clinician judgment, participant preference, laboratory test result such as haemoglobin level or similar. Unclear risk of bias: insufficient information about the sequence generation process to permit judgment.
2. Allocation concealment
Was allocation adequately concealed?
Low risk of bias: central independent unit, sequentially numbered drug containers, sealed envelopes of identical appearance or similar. High risk of bias: alternation or rotation, date of birth, non‐opaque envelopes, open table of random numbers or similar. Unclear risk of bias: randomisation stated but no information on method used was available.
3. Blinding
Was knowledge of the allocated intervention adequately prevented during the study?
Low risk of bias: identical placebo medication or similar. High risk of bias: intravenous secretin or placebo easily identified. Unclear: blinding stated but no information on method used was available.
4. Incomplete outcome data
Were incomplete data dealt with adequately by the researchers?
Low risk of bias: no missing outcome data, missing outcome data balanced in numbers across intervention groups, reasons for dropouts and withdrawals described or similar. High risk of bias: reason for missing outcome data likely to be related to true outcome or similar. Unclear risk of bias: number or reasons for dropouts and withdrawals not described.
Selective outcome reporting was not assessed.
Measures of treatment effect
All outcome data reported in included papers were continuous.
Unit of analysis issues
Where trials used a cross‐over design, we conducted analysis on results from the first treatment phase, that is, before cross‐over. This approach to analysis was used in the first review and not modified for this review in order to avoid the possible bias towards underestimation of treatment effectiveness if the chosen washout period was inadequate.
Dealing with missing data
Where insufficient data were reported, we contacted trials authors. Where no reply was forthcoming and missing data could not be imputed using standard methods, we included the study in the text but not in any statistical synthesis.
Assessment of heterogeneity
We assessed consistency of results visually and by examining the I2 statistic, a quantity which approximately describes the proportion of variation in point estimates that is due to heterogeneity rather than sampling error (Higgins 2002). We supplemented this with a test of homogeneity to determine the strength of evidence that the heterogeneity was genuine. We performed meta‐analysis using a random‐effects model in order to account for the possibility of heterogeneity.
Assessment of reporting biases
We intended to use funnel plots to investigate any relationship between effect size and study precision (closely related to sample size) (Egger 1997). However, the number of studies was too small and the outcome measures too inconsistent for this method to be viable.
Data synthesis
Where standardised assessment tools generated a score as the outcome measure, we made comparisons between the means of these scores. Where baseline means were reported, we determined differences between treatment and control groups to assess possible bias. Where mean change from baseline was reported, we used this in preference to post‐treatment scores for meta‐analyses or forest plots. Meta‐analysis and forest plots included either post‐treatment means or mean change, but these were not entered as subgroups because of small study numbers. One author (Kern 2002) provided raw data and we calculated mean change where change = post‐treatment score ‐ baseline score. We checked data from all included studies to ensure consistency. One study (Sandler 1999) did not use this convention and data were multiplied by ‐1 as a correction.
We calculated standard deviations from standard errors or confidence intervals, where necessary, using the calculation for small sample size. In one instance where standard deviation for the control was zero (Kern 2002), the standard deviation of the treatment group was used to produce a forest plot. One study (Unis 2002) used two secretin treatment groups and a single placebo group. We formed a single treatment group using an Excel spreadsheet to calculate combined means and standard deviations as directed at the time and now published in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2008).
Sensitivity analysis
Sensitivity and subgroup analysis
As meta‐analysis was possible for only one outcome (the Childhood Autism Rating Scale (CARS) (Schopler 1980), neither sensitivity analysis or subgroup analysis were viable options to assess the impact of study quality, clinical differences in the intervention, or clinically relevant differences between participant groups, such as age or presence of gastrointestinal symptoms.
Results
Description of studies
Results of the search
Selection procedure
The first search for this review was completed at the end of February 2003 and yielded 84 titles (Figure 1). Seventy‐three papers were excluded because they were not randomised trials, did not have a placebo group or were not primarily about ASD. Full paper reviews were conducted for 11 studies and from these seven RCTs were identified. The search was repeated in February 2004 and six further published trials were identified. One unpublished study of 22 children, conducted by one of the authors of this review, was also provided (Wray, personal communication). An author of one of the included trials informed us of phase II and III Repligen Corporation pharmaceutical trials (personal communication with T Owley (Owley 2001)).
1.
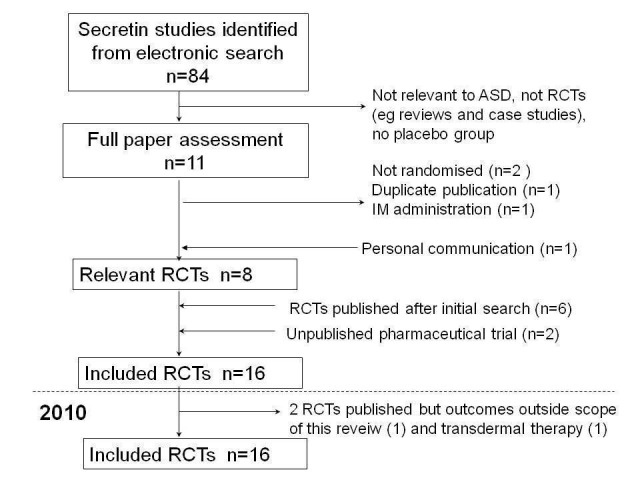
Study inclusion pathway
We excluded four of the 11 studies that received full‐paper review. One study presented data that were also presented in an included study (Owley 2001) and as such were not presented separately, one study used intramuscular rather than intravenous administration of secretin (Shin‐Siung 2000) and two studies were not randomised (Lonsdale 2000; Lightdale 2001).
Repligen published a series of press releases reporting results of its phase II trials (Repligen 2002) and one phase III (NCT00036244) trial. No further information about trial methods or data were forthcoming for either the phase II or phase III trials despite requests. A dissertation completed in 2004 was also identified and was unclassifiable at the time of first publication. Subsequently a dissertation abstract for that PhD has been found that reported results for 37 children who were part of a multicentre trial in which children aged three to six years were recruited and given three doses of intravenous secretin. This is the same method used for the phase II Repligen trial, which is the only multisite trial of this kind that has been reported. As such this study was listed as part of the Repligen 2002 study (Repligen 2002) and data was not be presented separately. Another phase III Repligen trial, with participants who were children with autism and gastrointestinal dysfunction, was terminated and no information published (NCT00036231), so this study was excluded.
A search in March 2005 prior to publication of the initial review found no new trials. An updated search in 2007 yielded no new trials. From the 2010 search, two studies were identified as trials but one used transdermal, not intravenous, administration of secretin (Ratliff‐Schaub 2005) and the other was a trial of healthy males that reported only neurological imagining outcomes (Yurgelun‐Todd 2008). The remaining studies were either reviews (4) or not related to the topic of secretin and autism (2). No new information about the Repligen trials was forthcoming from the supplementary searches conducted in October 2011.
Included studies
Design of included studies
Sixteen randomised controlled trials including 911 children under 18 years were available at study completion. Nine studies used a cross‐over treatment design (Wray; Chez 2000; Corbett 2001; Owley 2001; Carey 2002; Kern 2002; Molloy 2002; Sponheim 2002; Levy 2003). That is, participants were given either secretin or placebo, outcomes were measured, a washout period followed (to allow any effects of the drug to wear off), then all participants were given the alternative treatment and outcomes measured again. The remaining seven trials were of parallel design where participants were given only one treatment, secretin or placebo (Sandler 1999; Dunn‐Geier 2000; Coniglio 2001; Roberts 2001; Repligen 2002; Unis 2002; NCT00036244).
Diagnostic criteria
Twelve studies required participants to have been diagnosed using DSM‐IV (APA 1994) or ICD‐10 (WHO 1993) criteria (Wray; Sandler 1999; Chez 2000; Dunn‐Geier 2000; Coniglio 2001; Corbett 2001; Owley 2001; Carey 2002; Kern 2002; Molloy 2002; Sponheim 2002; Unis 2002). Two studies (Roberts 2001; Levy 2003) confirmed diagnosis using the Autism Diagnostic Interview‐R (ADI‐R) (Lord 1994) or ADOS‐G (Lord 2000), or both. In six studies, the children had either autism or another pervasive developmental disorder (Sandler 1999; Chez 2000; Roberts 2001; Carey 2002; Kern 2002; Unis 2002). In one study, the participants had either autism, pervasive developmental disorder not otherwise specified or childhood disintegrative disorder (Wray). In the remaining seven studies, participants were required to have autism according to DSM‐IV, ICD‐10 or a standardised assessment instrument (Dunn‐Geier 2000; Coniglio 2001; Corbett 2001; Owley 2001; Molloy 2002; Sponheim 2002; Levy 2003). Diagnostic inclusion criteria for the two Repligen studies were not available in published information (Repligen 2002; NCT00036244) but the children recruited to both studies were said to have "moderate to severe symptoms of autism" and children recruited to the phase II trial also had "gastrointestinal disorders".
Age of participants
The age groups of study participants varied between studies. Twelve studies recruited children from the age of two or three years (Wray; Sandler 1999; Dunn‐Geier 2000; Coniglio 2001; Owley 2001; Roberts 2001; Carey 2002; Kern 2002; Molloy 2002; Sponheim 2002; Unis 2002; Levy 2003). In one study, only a mean age was given (Chez 2000) and one study recruited children from the age of four years (Corbett 2001). In the published studies, the age range within a study varied between five years (Dunn‐Geier 2000; Roberts 2001; Carey 2002; Levy 2003) and 13 years (Molloy 2002). The age range for one unpublished study was 15 years (from three to 18) (Wray), but was narrower for the Repligen studies, 2 years 8 months to 4 years 11 months (NCT00036244) and three years to six years 11 months (Repligen 2002).
Dosage of secretin
Six published studies (Chez 2000; Dunn‐Geier 2000; Coniglio 2001; Corbett 2001; Owley 2001; Kern 2002) and one unpublished study (Wray) used single dose porcine secretin in a dose of 2 CU/kg or 2 IU/kg administered intravenously. Three studies used synthetic secretin in a single 2 CU/kg dose (Carey 2002; Molloy 2002; Levy 2003). One study used synthetic human secretin 0.4 µg/kg intravenously as a single dose (Sandler 1999). Four studies used repeated secretin doses; one administered two doses of 2 ml/kg six weeks apart (Roberts 2001), one study used three doses of synthetic secretin in a dose of 4 CU/kg administered intravenously four weeks apart (Sponheim 2002), one used three administrations of RG1068 (Repligen 2002) and one used six injections of RG1068 over 18 weeks (NCT00036244). One study used both synthetic and 'biological' or extracted porcine secretin in a dose of 2 CU/kg (Unis 2002). All studies used intravenously administered normal saline as the control.
Excluded studies
Risk of bias in included studies
Risk of bias for the Repligen studies was unclear for all criteria as neither detailed published methods nor information from the company were available at the time of writing.
We assessed allocation concealment as adequate in six studies (Dunn‐Geier 2000; Corbett 2001; Owley 2001; Kern 2002; Molloy 2002; Levy 2003) and inadequate in one (Chez 2000). We contacted the authors of the trials in which allocation concealment or randomisation was unclear and asked them to provide further information. Information was forthcoming for three studies (Sandler 1999; Owley 2001; Sponheim 2002). Two studies provided details to indicate that randomisation or allocation concealment, or both, were adequate (Owley 2001; Sponheim 2002). One study provided details of pharmacy‐based randomisation and allocation, but syringes were colour‐tagged according to treatment allocation (Sandler 1999). The unpublished study was randomised with adequate allocation concealment (Wray).
For the nine cross‐over trials, the 'washout' or treatment‐free time ranged from three to eight weeks. It has been suggested that secretin treatment effects 'taper off' at about five weeks, but may continue for eight or more weeks (Rimland 2000). If the treatment is effective, and if this is the case, participants who had secretin as their first treatment may still exhibit the beneficial effects in the placebo phase. While cross‐over trials are a useful methodological tool for boosting sample power, this systematic review has been limited to the first phase of cross‐over trials to prevent biased underestimation of treatment effectiveness due to potentially inadequate washout periods.
In one study, there was an initial open‐label trial of secretin followed by a second phase RCT for a subsample of 'responders' from the open‐label study (Chez 2000). This study design would tend to overestimate treatment effectiveness. Because the RCT followed an open‐label trial, this study assessed the effectiveness of a second dose of secretin, six weeks following an initial dose, rather than primary secretin treatment (Chez 2000).
Five studies reported no loss to follow‐up (Wray; Dunn‐Geier 2000; Owley 2001; Kern 2002; Sponheim 2002). For the remainder, loss to follow‐up was 1.5% (Roberts 2001), 1.6% (Levy 2003), 4% (Chez 2000), 5% (Coniglio 2001), 6% (Unis 2002), 7% (Sandler 1999), 12.5% (Corbett 2001) and 62% (Carey 2002). Reasons for dropouts were not given by the authors. One study was terminated after 70% of randomised participants had completed treatment and follow‐up because interim analysis indicated that the treatment difference was not large enough to justify continuation of the trial (Molloy 2002). No study reported that intention‐to‐treat analysis was used. As an example of the use of analyses other than intention‐to‐treat analysis, in one paper the overall baseline data presented differs from the baseline presented graphically to illustrate treatment effect (Sandler 1999).
The risk of bias assessments for all included studies are summarised in Figure 2.
2.
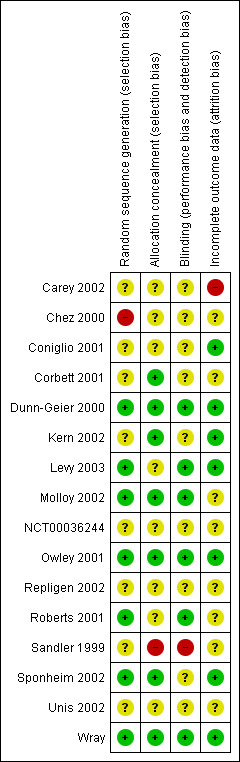
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
Outcome measures
Twenty‐one established standardised outcome measures were reported to assess core features of autism, communication, behaviour, visuospatial skills, affect and adverse events (see Appendix 4). Few studies used any one outcome measure in the same way. Thus possible meta‐analyses were limited. Outcomes were reported at between three and six weeks for those where timing of outcome was available. Timing of outcome assessment for the Repligen studies (Repligen 2002; NCT00036244) was unclear. The Repligen phase III study reported that dual primary outcomes were used: improvements in social interaction as measured by the ADOS and the parental Clinical global Impressions of change (CGI) (NCT00036244). Outcome measures for the phase II study included the ADOS (Repligen 2002), but how it was used and whether other outcome measures were used is unclear from the reports available. Additional outcome assessments included a global assessment score (Kern 2002), a parent global behavior rating scale (Levy 2003; Wray), visual analogue scale (VAS) for multiple domains (Sponheim 2002), a modified version of the secretin outcome survey (Unis 2002), an autism treatment evaluation checklist (Wray) and gastrointestinal symptoms (Dunn‐Geier 2000; Roberts 2001). One study also collected blood for biochemical and metabolic outcomes (Levy 2003).
Analysis methods
Data were analysed and presented in varying ways. Four studies presented secretin and control group means at baseline and post‐treatment (Chez 2000; Coniglio 2001; Owley 2001; Molloy 2002). One of these studies also presented in the text some additional data about mean change (Owley 2001). Four studies presented mean change post‐treatment (Sandler 1999; Dunn‐Geier 2000; Unis 2002; Levy 2003). One study presented information graphically only (Kern 2002). Individual patient data, provided on request, were re‐analysed for the purpose of this review (Kern 2002). Individual patient data were also provided for the unpublished study (Wray). Two studies presented the results of an analysis of variance (Corbett 2001; Roberts 2001). Two small studies presented individual participant changes, one (N = 8) used the Reliable Change Index (Carey 2002) and the other (N = 6) used the means of summary repeated measures for secretin and placebo (Sponheim 2002). One of these studies (Carey 2002) also presented post‐test means by group for the Autism Behavior Checklist (ABC) (Aman 1986).
One study reported Reliable Change Index and an analysis of variance, with graphical representation of treatment effects and baseline measures for outcomes (Coniglio 2001). No additional data were available following correspondence with the authors.
For outcome measures used by more than one study, analysis was often performed using different methods. In addition, although two studies reported results of the Preschool Language Scale (PLS)‐3 (Zimmerman 1992), one did so as a standard score (Dunn‐Geier 2000) and the other as total language age equivalents (Coniglio 2001). Appendix 5 shows the baseline scores for secretin and placebo groups in nine studies where the outcome measures were used by more than one study, when baseline information was available and data were available in a suitable form for meta‐analysis (Sandler 1999; Chez 2000; Dunn‐Geier 2000; Coniglio 2001; Owley 2001; Carey 2002; Kern 2002; Molloy 2002; Unis 2002).
Scores were different at baseline for the secretin and placebo groups for the three studies (Owley 2001; Kern 2002; Unis 2002) reporting the same outcome for the subscales of the Aberrant Behaviour Checklist (Aman 1986) and the Gilliam Autism Rating Scale (GARS (Gilliam 1995)) (Coniglio 2001; Owley 2001; Molloy 2002). Of the two studies (Owley 2001; Unis 2002) that reported social and communication subscales of the ADOS (Lord 1997), one had large baseline differences that were significant for the social subscale (Owley 2001). The baseline communication component of the Vineland Adaptive Behaviour Scale (VABS) (Sparrow 1984) was different for the secretin and placebo groups for one of the two studies reporting data in a form useable for meta‐analysis (Owley 2001) and was not available for the other (Sandler 1999). The PLS baseline scores were different for both studies reporting this outcome, albeit data were presented in different units (Dunn‐Geier 2000; Coniglio 2001). Baseline measures of the CARS score (Schopler 1980) for the four studies in which it was used as an outcome measure were similar (Chez 2000; Dunn‐Geier 2000; Coniglio 2001; Molloy 2002).
When differences between baseline measures exist between groups, there is potential for biased estimation of treatment effect size. The direction of the bias (favouring treatment or control) will vary depending on the correlation between baseline score and change score and the type of analysis used (change score or post‐treatment) (Vickers 2001). As such, data suitable for analysis were presented for different outcomes but a combination of data using different methods was only performed for the CARS (Schopler 1980). All cross‐over trials that presented data suitable for meta‐analysis reported sufficient data to enable assessment of outcomes prior to treatment cross‐over.
Details about baseline and outcome data and analysis methods were not available for the Repligen studies (Repligen 2002; NCT00036244).
There were insufficient data available in a format suitable for meta‐analysis to explore the impact of different age groups, diagnostic classification inclusion or methodological rigor on treatment effectiveness.
Outcomes
Core features of ASD
Twelve studies presented information about core features of autism as an outcome measure (see also Appendix 4). Figure 3 presents the overall scores for the CARS, GARS and Autism Behaviour Checklist, as standardised mean differences, for the six studies with suitable data available. As shown, no statistically significant result for treatment effectiveness was seen for the CARS, GARS or Autism Behaviour Checklist in these studies. One further study (Roberts 2001) also reported that there was no significant effect for secretin versus placebo for the Autism Behaviour Checklist but the data available were not suitable for inclusion in a meta‐analysis. Figure 4 presents outcomes for the subgroups of the Autism Diagnostic Observation Scale (Lord 1997) for the two studies with data suitable for this presentation. No significant differences for subgroup scores for these two tools, between secretin and placebo treatments, were reported. A further three studies reported no significant treatment effect for secretin as measured by the ADOS, but insufficient data were available for further analysis (Corbett 2001; Roberts 2001, NCT00036244).
3.
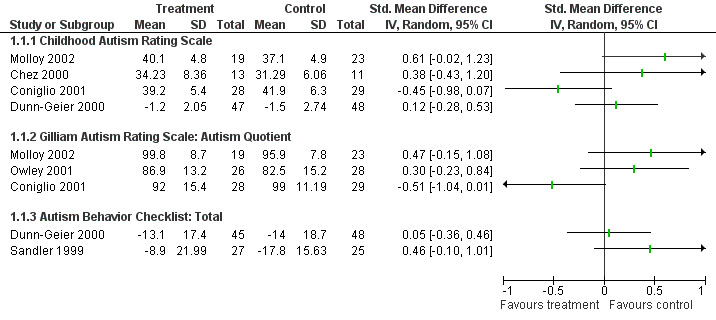
Forest plot of comparison: 1 Core features of autism, outcome: 1.1 Core features of autism, total scores.
4.
Forest plot of comparison: 4 ADOS, outcome: 4.1 ADOS.
Communication
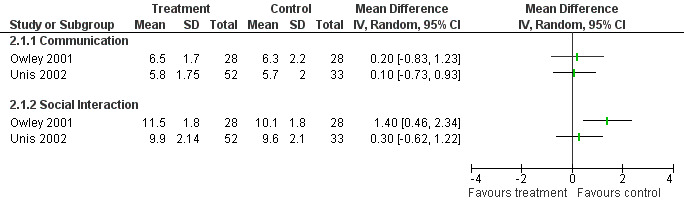
Ten structured instruments were used to assess communication outcomes in eight studies, including the communication subscale of the ADOS‐G and the VABS (see Appendix 4). Only the VABS communication score and the PLS‐3 were used by two or more studies as an outcome measure. For the VABS only two studies had data available in a format suitable for meta‐analysis and this is displayed in Figure 5. For the PLS‐3, data were only available in one study in a form suitable for meta‐analysis (Dunn‐Geier 2000). That study and two others (Coniglio 2001; Roberts 2001) reported no significant treatment effect over time as measured by the PLS‐3. No significant improvements were found for other tools used by individual studies.
5.
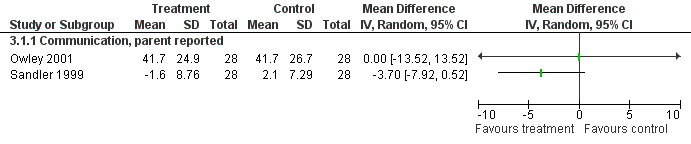
Forest plot of comparison: 5 Vineland (communication), outcome: 5.1 Vineland four weeks post treatment.
Non‐core behaviours
Behaviour
Seven studies presented outcome information for changes to behaviours that were not core features of autism (see Appendix 4). Five used the Aberrant Behaviour Checklist (ABC) (Aman 1986). The subgroup scores for the subscales of the ABC are presented in Figure 6 for the three studies in which data were available in a suitable form. Data for parent and teacher report for the ABC was also available from one study (Carey 2002) and there was no improvement for secretin compared to placebo. However the data could not be included in the meta‐analysis because there was considerable baseline variation between groups and the standard deviation for the change between baseline and post‐treatment was not available. Another study reported that "ABC ratings did not differ throughout the study and were inconsistently completed by both parents and teachers", and no data were provided (Sponheim 2002). For the ABC, a high score is an indicator of a greater incidence of problem behaviours. Scores for the inappropriate speech subcategory are low across all studies. This may indicate that the children in these studies were generally pre‐verbal due to the severity of their condition. In addition, one study analysed treatment response for the ABC subgroup scores for a subgroup of five children with current chronic diarrhoea and found a greater response to secretin compared to placebo in this group, which was significantly different for social withdrawal (P < 0.002) (Kern 2002). This subgroup analysis was a post hoc analysis inclusion when the research team noticed a greater response in participants with chronic diarrhoea.
6.
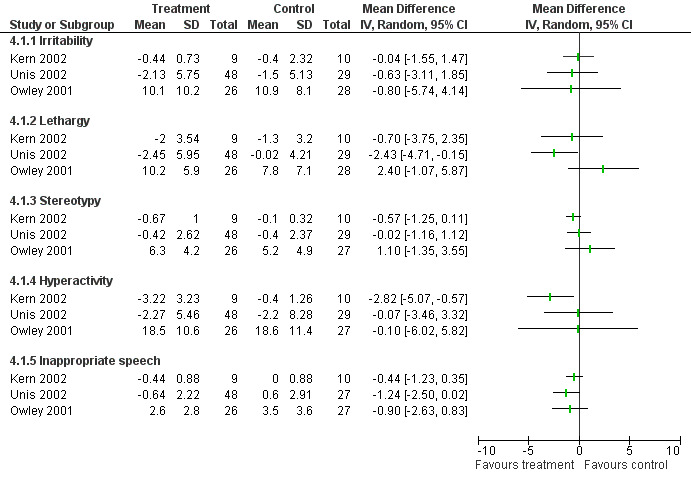
Forest plot of comparison: 6 Aberrant Behavior Checklist, outcome: 6.1 Aberrant Behavior Checklist 3‐4 weeks post treatment.
An additional two tools were used by three studies. Two studies (Sandler 1999; Owley 2001) reported no significant differences between secretin and placebo groups when using the VABS (Vineland Adaptive Behaviour Scale) (Sparrow 1984). One study (Levy 2003) used the change score for the Ritvo Real‐Life Rating Scale (Freeman 1986) and found no statistically significant difference between secretin and placebo groups.
Affect
The Minnesota Preschool Affect Rating Scale (MN‐PARS) (Shapiro 1994) was used by one study to assess the effectiveness of secretin for improving affect (Corbett 2001). A significant difference was found for two variables, positive affect (P = 0.01) and activity (P = 0.05). The remaining variables (negative affect and self‐regulation) were reported as not statistically significant. When the results of the parent completed MN‐PARS subscales were compared with other standardised subscales measuring similar attributes, the results were not consistent. For example, the shared positive affect measure of the Communication and Symbolic Behavior Scale showed no difference between treatment and placebo groups (Corbett 2001). Because the Activity Level scale records the child's level of motor activity regardless of their concentration, it was not thought to be a useful outcome measure when interpreted in isolation.
Visuospatial skills
Three tools (see Appendix 4) were used by three studies (Owley 2001; Roberts 2001; Molloy 2002) to assess visuospatial or fine motor skills, or both. No study reported significant differences between secretin and placebo groups.
Global impression of health
Parent impression
No difference in the CGI was reported for the overall group or the high functioning subgroup in one study (NCT00036244). Another study also used the Clinical Global Impression (CGI) Scale (NIMH 1985) and reported no statistically significant difference in behaviours (Sandler 1999). Anecdotal parent reports were reported in six studies (Chez 2000; Coniglio 2001; Corbett 2001; Roberts 2001; Kern 2002; Molloy 2002). Parent reports focused on sleep improvements (Roberts 2001), toilet training (Roberts 2001), gastrointestinal changes (Chez 2000; Coniglio 2001; Roberts 2001; Kern 2002; Molloy 2002), aggression (Corbett 2001) and eye contact (Chez 2000; Corbett 2001; Roberts 2001). One study found three children with marked gastrointestinal improvements following secretin injection (Kern 2002). None of the other parent reported improvements were consistent with measurable gains and there was no significant improvement in the treatment group compared with placebo. In one study, parents were asked about their continuing interest in secretin to treat their child. Despite knowing that the study found no significant improvement, 63% of parents in the treatment group and 76% of parents in the control remained interested in secretin (Sandler 1999).
Quality of life
No standardised quality of life measures were used for the children or the families.
Adverse events and harm
No serious adverse events, such as anaphylaxis, were reported. One study used a standardised measure, the Treatment Emergent Symptoms Scale (NIMH 1985), to collect information about adverse events (Sandler 1999). Results showed a non‐significant (P = 0.15) increase in severity scores for the secretin group. One study reported worsening of symptoms in one child following secretin administration, including tantrums, hyperactivity and aggression (Kern 2002). One study reported nine adverse events, including increased liver function tests, hyperactivity, emotional lability, fractures and stomach ache. No differences were reported between treatment and control groups (Levy 2003). One study reported two seizures in one child, vomiting in two children, including one child who developed flu‐like symptoms three weeks after secretin injection, and brief hyperactivity in one child flowing placebo. No side effects could be directly attributed to secretin (Owley 2001). One study reported that 13% of parents observed side effects including irritability, hyperactivity and vomiting in both treatment and placebo groups (Coniglio 2001). One study reported adverse events that may have been attributed to secretin, including single cases of rash, fever, tachycardia and vomiting, and photosensivity; three children with increased irritability; and 21% of the secretin injections resulting in generalised flushing (Roberts 2001). This study also reported increased aggression and hyperactivity in children from both the treatment and placebo groups, with one child withdrawn from the study by parents as a result of this increased hyperactivity. One other study reported flushing, which occurred equally in both groups (Dunn‐Geier 2000). Six studies reported no serious adverse events (Sandler 1999; Chez 2000; Dunn‐Geier 2000; Repligen 2002; Sponheim 2002; NCT00036244).
Subgroup analyses of treatment effectiveness
Seven studies reported analysis of subgroups to test the hypothesis that secretin was more effective in children with specific conditions (Roberts 2001; Unis 2002; Kern 2002; Sandler 1999; Levy 2003; NCT00036244; Repligen 2002). For five of these the study was not designed with adequate sample size to determine a statistically significant difference between groups (Roberts 2001; Unis 2002; Kern 2002; Sandler 1999; Levy 2003) and for two this information was not available but the analyses were planned prospectively (NCT00036244; Repligen 2002). Three studies reported no significant improvements regardless of existing gastrointestinal problems (Roberts 2001; Unis 2002; Kern 2002). One study (Unis 2002) reported no differences when grouped for age of participants or when grouped for scores on the Vineland communication scale (Sparrow 1984). One study reported a significant improvement in the ADOS social interaction scale for one age subgroup (three to four year olds) (Repligen 2002). One study (Roberts 2001) reported no difference when grouped for IQ or history of 'regression' of developmental skills. Three studies reported no difference in outcomes for different severities of ASD (Sandler 1999; Roberts 2001; Levy 2003). In one study a significant improvement was reported for a prospectively defined subset analysis of the higher functioning participants (NCT00036244). Only one study supplied, on request, information for analysis by age of child in years and autism severity (Dunn‐Geier 2000).
Discussion
The possible effectiveness of secretin as a treatment for ASD was first suggested by a report of improvement of the core features of autism following secretin administration during pancreatic stimulation testing of three children who had ASD with a history of regression in their development in association with bowel dysfunction problematic enough to warrant investigation (Horvath 1998). The product development and dissemination that followed this report reflects the high level of unmet needs of children with ASD and their families, the power of market forces and the way we now function as a global community. The report was also followed by research studies attempting to provide evidence to aid the decision making of families, clinicians, pharmaceutical companies and health services.
RCTs of the efficacy of secretin in ASD have not shown improvements in the core features of ASD as initially reported (Horvath 1998). A wide range of different potentially positive outcomes have been explored. However, the study methodology of many RCTs has meant that both over‐ and under‐identification of true differences has been possible. Although limited data are available from the multisite studies conducted by Repligen, reported data indicate that secretin was not an effective treatment and further development of secretin for use in ASD has not continued.
Firstly, large numbers of outcomes and subscale scores have often been analysed, which has increased the likelihood of finding significant positive effects by chance (Type I error). Reporting results using adjusted statistical significance levels would address this problem. It is noteworthy that, even in the studies using multiple outcome measures, few positive effects of secretin have been shown.
Secondly, although some studies used sample size based on reported power calculation, many studies had a low sample size, which creates the possibility of not finding significant differences when they do exist (Type II error). The two largest studies (N = 124 and N = 132) failed to report detailed methods or results (Repligen 2002; NCT00036244). Meta‐analytic combination of data would have clarified if Type II errors were creating a lack of significant results, but this was not possible for reasons discussed previously and summarised below.
Thirdly, the outcome measures used have not always been suitable to assess change. Initial reports were of improvements of the core features of ASD, but there are few, if any, standardised tools that assess core features of ASD that have been designed to monitor change. Rather, these tools were designed to provide systematic diagnostic classification. Because the initial report of improvements did not include standardised assessments, it is unclear whether these tools would be adequate for monitoring the substantial changes expected. It is also unclear what degree of change would be considered of sufficient importance to the child and the family to warrant therapy.
Finally, the small sample size of studies, uncertain adequacy of allocation concealment and the play of chance have led to variations in outcome measures at baseline between treatment and placebo groups in a way that could bias outcomes and over‐ or under‐estimate treatment effects. The largest study, by Repligen, has still not been fully reported. The variation in baseline scores has made synthesis of change scores and post‐treatment scores inappropriate.
Clinical and methodological differences have also prevented meta‐analyses of existing data. The 14 included studies used a diverse range of outcome measures, with no more than four studies using any one measure. There is also a great deal of variation between studies in regard to their diagnostic inclusion criteria, age range of participants, types of secretin used, dosage regime and length of follow‐up. These variations mean that included studies are clinically diverse such that statistical heterogeneity is likely. In addition, the information presented to assess study methods was often inadequate.
Attempts to clarify methodological variations and gather missing data were frequently not successful, even though all studies were published during the period CONSORT guidelines were available (Moher 2001) and data would usually be stored.
Additionally, published reports rarely contained subgroup analyses for important clinical groups, such as children who had early developmental regression or gastrointestinal problems, as in the first publication that suggested secretin was an effective treatment for autism. Structured assessment of these clinical features is not straightforward and the proportion of children with ASD with these problems is small, leading to sample size problems.
The research response to the theory about the effectiveness of secretin has shown that RCTs can be conducted for children with ASD and that research groups can organise themselves quickly when necessary. It has also highlighted the potential problems of different methodological approaches and contributed to the increasing awareness of the need for agreed diagnostic classification systems and responsive outcomes to monitor change over time (Kasari 2002).
One dose of secretin has not been shown to be effective in improving the core features of autism for all children with autism or other ASDs. There is no evidence to suggest that a single dose of intravenous secretin should be made available to all children with ASD. It also seems unlikely that multiple dose therapy is effective (NCT00036244). Although no serious side effects have been reported, the risk to a child of serious side effects is likely to increase with repeated doses and more adverse events are likely to be reported if secretin is made widely available. A similar conclusion that "...studies have demonstrated that secretin is not effective for improving language, cognition, behavior, communication, autism symptom severity, or socialization skills" was reached by a recent systematic review of secretin, which included eight studies (Krishnaswami 2011).
Whether secretin is effective for some subgroups of children with ASD is uncertain but guidelines have reported that "...no subgroup of children who benefit has been consistently identified" (SIGN 2007). It is still true that information about effectiveness in subgroups of children has not yet been measured in an adequate sample size. Further explorations of effectiveness of secretin for subgroups of children with autism should only be undertaken if there is biological plausibility for effectiveness in the subgroup and the findings can be translated into practice. Any such research would also need to provide valid evidence about improvements in outcomes that are of value to children with autism and their families. For example, effectiveness of therapy for a subgroup of children that are only reliably identified using procedures only available in a research setting would not be able to be translated to improved outcomes for children and families in a clinical setting.
Authors' conclusions
Implications for practice.
There is no evidence that single or multiple dose intravenous secretin is effective and as such currently it should not be recommended or administered as a treatment for ASD.
Implications for research.
Further experimental assessment of secretin's effectiveness for autism can only be justified if there is new high‐quality and replicated scientific evidence that either:
1. finds that secretin has a role in neurotransmission in a way that could benefit all children with autism; or
2. identifies important subgroups of children with autism who could benefit from secretin because of a proven link between the action of secretin and the known cause of their autism or type of problems they are experiencing.
If further studies are planned they must overcome the methodological problems of existing research, so that precise, valid, applicable and clinically useful information is gathered. As such they must:
follow CONSORT guidelines for study design and reporting;
allow an adequate wash‐out period where a cross‐over trial is planned;
carefully review appropriate outcome measures for their suitability for assessing change, their clinical importance, and equivalence to existing evidence, and consult consumers about outcome selection.
What's new
| Date | Event | Description |
|---|---|---|
| 1 December 2011 | New citation required but conclusions have not changed | No new studies to include. Conclusions unchanged. |
| 20 July 2010 | New search has been performed | New search run. |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 9 November 2008 | Amended | Converted to new review format. |
| 24 May 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to thank those trialists who provided us with information and debate about secretin. We thank Drs Julian Higgins, Alex Sutton, Jon Deeks, Jenny Peat and Andrew Hayen for their advice and assistance with methods and meta‐analysis. We commend the tireless efforts of the Cochrane Developmental, Psychosocial and Learning Problems Group for their help with literature searching, review and editing, in particular the support of Dr Jane Dennis. Danielle Wheeler was funded by the Small Grants Scheme of The Children's Hospital at Westmead and the Financial Markets Foundation for Children.
Appendices
Appendix 1. Search strategy for Cochrane Central Register of Controlled Trials (CENTRAL)
#1 MeSH descriptor Child Development Disorders, Pervasive explode all trees
#2 MeSH descriptor Communication, this term only
#3 (autis*)
#4 (pdd)
#5 (pervasive developmental disorder*)
#6 (language near/3 delay*)
#7 (communicat*)
#8 (speech near/3 disorder*)
#9 (childhood schizophrenia)
#10 (kanner*)
#11 (asperg*)
#12 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11)
#13 MeSH descriptor Secretin explode all trees
#14 (secretin)
#15 (#13 OR #14)
#16 (#12 AND #15)
Appendix 2. Search strategies used to update records in 2007
CENTRAL (2007 Issue 2)
#1 MeSH descriptor Child Development Disorders, Pervasive explode all trees
#2 MeSH descriptor Communication, this term only
#3 (autis*)
#4 (pdd)
#5 (pervasive developmental disorder*)
#6 (language near/3 delay*)
#7 (communicat*)
#8 (speech near/3 disorder*)
#9 (childhood schizophrenia)
#10 (kanner*)
#11 (asperg*)
#12 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11)
#13 MeSH descriptor Secretin explode all trees
#14 (secretin)
#15 (#13 OR #14)
#16 (#12 AND #15)
CINAHL via Ovid (2005 to June Week 3 2007)
1 autis$.tw.
2 pdd.tw.
3 pervasive developmental disorder$.tw.
4 (language adj3 delay$).tw.
5 communicat$.tw.
6 (speech adj3 disorder$).tw.
7 childhood schizophrenia.tw.
8 kanner$.tw.
9 asperg$.tw.
10 Autistic Disorder/
11 COMMUNICATION/
12 or/1‐11
13 secretin.tw.
14 12 and 13
15 limit 14 to yr="2005 ‐ 2007"
16 randomi$.mp.
17 clin$.mp.
18 trial$.mp.
19 (clin$ adj3 trial$).mp.
20 singl$.mp.
21 doubl$.mp.
22 tripl$.mp.
23 trebl$.mp.
24 mask$.mp.
25 blind$.mp.
26 (20 or 21 or 22 or 23) and (24 or 25)
27 crossover.mp.
28 random$.mp.
29 allocate$.mp.
30 assign$.mp.
31 (random$ adj3 (allocate$ or assign$)).mp.
32 Random Assignment/
33 exp Clinical Trials/
34 exp Meta Analysis/
35 31 or 27 or 26 or 19 or 16 or 32 or 33 or 34
36 15 and 35
EMBASE (2005 to Week 24 2007)
1 autis$.tw.
2 pdd.tw.
3 pervasive developmental disorder$.tw.
4 (language adj3 delay$).tw.
5 communicat$.tw.
6 (speech adj3 disorder$).tw.
7 childhood schizophrenia.tw.
8 kanner$.tw.
9 asperg$.tw.
10 Secretin/
11 secretin.tw.
12 or/10‐11
13 Autism/
14 Interpersonal Communication/
15 (or/1‐9) or 13 or 14
16 15 and 12
17 clin$.tw.
18 trial$.tw.
19 (clin$ adj3 trial$).tw.
20 singl$.tw.
21 doubl$.tw.
22 trebl$.tw.
23 tripl$.tw.
24 blind$.tw.
25 mask$.tw.
26 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
27 randomi$.tw.
28 random$.tw.
29 allocat$.tw.
30 assign$.tw.
31 (random$ adj3 (allocat$ or assign$)).tw.
32 crossover.tw.
33 32 or 31 or 27 or 26 or 19
34 exp Randomized Controlled Trial/
35 exp Double Blind Procedure/
36 exp Crossover Procedure/
37 exp Single Blind Procedure/
38 exp RANDOMIZATION/
39 34 or 35 or 36 or 37 or 38 or 33
40 16 and 39
41 limit 40 to yr="2005 ‐ 2007"
MEDLINE via Ovid (2005 to June 2007)
1 autis$.tw.
2 pdd.tw.
3 pervasive developmental disorder$.tw.
4 (language adj3 delay$).tw.
5 communicat$.tw.
6 (speech adj3 disorder$).tw.
7 childhood schizophrenia.tw.
8 kanner$.tw.
9 asperg$.tw.
10 Secretin/
11 secretin.tw.
12 or/10‐11
13 Autism/
14 Interpersonal Communication/
15 (or/1‐9) or 13 or 14
16 15 and 12
17 clin$.tw.
18 trial$.tw.
19 (clin$ adj3 trial$).tw.
20 singl$.tw.
21 doubl$.tw.
22 trebl$.tw.
23 tripl$.tw.
24 blind$.tw.
25 mask$.tw.
26 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw.
27 randomi$.tw.
28 random$.tw.
29 allocat$.tw.
30 assign$.tw.
31 (random$ adj3 (allocat$ or assign$)).tw.
32 crossover.tw.
33 32 or 31 or 27 or 26 or 19
34 exp Randomized Controlled Trial/
35 exp Double Blind Procedure/
36 exp Crossover Procedure/
37 exp Single Blind Procedure/
38 exp RANDOMIZATION/
39 34 or 35 or 36 or 37 or 38 or 33
40 16 and 39
41 limit 40 to yr="2005 ‐ 2007"
PsycINFO (2005 to June 2007, Week 5)
(random* or trial* or blind* or crossover) and ((secretin) and ((( language near3 delay* ) or
( communicat* ) or ( speech near3 disorder* )) or (( autis* )or( pdd )or( pervasive developmental disorder* )) or (explode "Interpersonal‐Communication‐+" in MJ,MN) or (explode "Pervasive‐Developmental‐Disorders" in MJ,MN) or (( childhood schizophrenia )or( kanner* )or( asperg* ))) and (PY:PSYI = 2005‐2007))
Appendix 3. Search strategies used to update records in 2010
CENTRAL (2010 issue 1)
#1 MeSH descriptor Child Development Disorders, Pervasive explode all trees
#2 MeSH descriptor Communication, this term only
#3 (autis*)
#4 (pdd)
#5 (pervasive developmental disorder*)
#6 (language near/3 delay*)
#7 (communicat*)
#8 (speech near/3 disorder*)
#9 (childhood schizophrenia)
#10 (kanner*)
#11 (asperg*)
#12 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11)
#13 MeSH descriptor Secretin explode all trees
#14 (secretin)
#15 (#13 OR #14)
#16 (#12 AND #15)
CINAHL via EBSCO (2007 to 20 January 2010)
S32 S15 and S31
S31 S16 or S17 or S18 or S19 or S20 or S21 or S22 or S23 or S24 or S25 or
S26 or S27 or S28 or S29 or S30
S30 allocat* random*
S29 (MH "Quantitative Studies")
S28 (MH "Placebos")
S27 placebo*
S26 random* allocat*
S25 (MH "Random Assignment")
S24 (Randomi?ed control* trial*)
S23 (singl* mask* )
S22 (doubl* mask* )
S21 (tripl* mask* )
S20 (trebl* mask* )
S19 (trebl* blind* )
S18 (tripl* blind* )
S17 (doubl* blind* )
S16 (singl* blind* )
S15 S12 and S13
S14 (secretin) and (S12 and S13)
S13 secretin
S12 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11
S11 asperg*
S10 kanner*
S9 childhood schizophrenia
S8 speech N3 disorder*
S7 communicat*
S6 language N3 delay*
S5 pervasive developmental disorder*
S4 pdd
S3 autis*
S2 (MH "Communication")
S1 (MH "Child Development Disorders, Pervasive+")
EMBASE via Ovid (2007 ‐ 2010 Week 2)
1 autis$.tw.
2 pdd.tw.
3 pervasive developmental disorder$.tw.
4 (language adj3 delay$).tw.
5 communicat$.tw.
6 (speech adj3 disorder$).tw.
7 childhood schizophrenia.tw.
8 kanner$.tw.
9 asperg$.tw.
10 Secretin/
11 secretin.tw.
12 or/10‐11
13 Autism/
14 Interpersonal Communication/
15 (or/1‐9) or 13 or 14
16 15 and 12
17 random$.tw.
18 factorial$.tw.
19 crossover$.tw.
20 cross over$.tw.
21 cross‐over$.tw.
22 placebo$.tw.
23 (doubl$ adj blind$).tw.
24 (singl$ adj blind$).tw.
25 assign$.tw.
26 allocat$.tw.
27 volunteer$.tw.
28 Crossover Procedure/
29 double‐blind procedure.tw.
30 Randomized Controlled Trial/
31 Single Blind Procedure/
32 or/17‐31
33 16 and 32
34 limit 33 to yr="2007 ‐Current"
MEDLINE via Ovid (2007 to January 15 2010)
1 exp Child Development Disorders, Pervasive/
2 Communication/
3 autis$.tw.
4 pdd.tw.
5 pervasive developmental disorder$.tw.
6 (language adj3 delay$).tw.
7 communicat$.tw.
8 (speech adj3 disorder$).tw.
9 childhood schizophrenia.tw.
10 kanner$.tw.
11 asperg$.tw.
12 or/1‐11
13 Secretin/
14 secretin.tw.
15 or/13‐14
16 12 and 15
17 limit 16 to yr="2007 ‐Current"
18 randomized controlled trial.pt.
19 controlled clinical trial.pt.
20 randomized.ab.
21 placebo.ab.
22 drug therapy.fs.
23 randomly.ab.
24 trial.ab.
25 groups.ab.
26 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25
27 humans.sh.
28 26 and 27
29 17 and 28
PsycINFO (2007 to January Week 2 2010)
1 exp Child Development Disorders, Pervasive/
2 Communication/
3 autis$.tw.
4 pdd.tw.
5 pervasive developmental disorder$.tw.
6 (language adj3 delay$).tw.
7 communicat$.tw.
8 (speech adj3 disorder$).tw.
9 childhood schizophrenia.tw.
10 kanner$.tw.
11 asperg$.tw.
12 or/1‐11 (
13 Secretin/
14 secretin.tw.
15 or/13‐14
16 12 and 15
17 limit 16 to yr="2007 ‐Current"
18 randomized controlled trial.pt.
19 controlled clinical trial.pt.
20 randomized.ab.
21 placebo.ab.
22 drug therapy.fs.
23 randomly.ab.
24 trial.ab.
25 groups.ab.
26 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25
27 humans.sh.
28 26 and 27
29 17 and 28
Appendix 4. Outcomes by measure and trial
| Outcome | Tool(s) | Trial | Between group difference |
| Core features of ASD | Autism Behaviour Checklist (Krug 1980) | Dunn‐Geier 2000 | NS |
| Sandler 1999 | NS | ||
| Roberts 2001 | NS | ||
| GARS (Gilliam 1995) | Owley 2001 | NS | |
| Molloy 2002 | NS | ||
| Coniglio 2001 | NS | ||
| ADOS‐G (Lord 2000) | Owley 2001 | Communication NS Social interaction favours control |
|
| Unis 2002 | Communication and social interaction NS | ||
| Roberts 2001 | NS | ||
| Corbett 2001 | NS | ||
| Wray | Data available only for 10 of 29 participants | ||
| Repligen 2002 | Favoured treatment (uncertain if a priori specified analysis) for social interaction scale for 3‐4 year olds | ||
| NCT00036244 | NS for social interaction Favoured treatment for higher functioning participants (a priori specified analysis) |
||
| CARS (Schopler 1980) | Dunn‐Geier 2000 | NS | |
| Chez 2000 | NS | ||
| Molloy 2002 | NS | ||
| Coniglio 2001 | NS | ||
| Communication | Mullen (Mullen 1997) | Molloy 2002 | NS |
| PPVT‐III (Lloyd 1997) | Molloy 2002 | NS | |
| CDI (Fenson 1993) | Levy 2003 | NS | |
| MUL/TTR (Shipley 1992) | Molloy 2002 | NS | |
| PLS‐3 (Zimmerman 1992) | Dunn‐Geier 2000 | NS | |
| Roberts 2001 | NS | ||
| Conglio 2001 | NS | ||
| CSBS (Wetherby 1993) | Levy 2003 | NS | |
| VABS (Sparrow 1984) | Owley 2001 | NS | |
| Sandler 1999 | NS | ||
| EOPVT (Gardener 1990) | Unis 2002 | NS | |
| Behaviour problems | Autism Behaviour Checklist (Aman 1986) | Owley 2001 | All subscales NS |
| Carey 2002 | NS (parent and teacher report) | ||
| Kern 2002 | Four subscales NS Hyperactivity favours treatment Post hoc sub‐group analysis of 5 children with chronic diarrhoea favoured secretin for lethargy |
||
| Unis 2002 | Four subscales NS Lethary favours treatment |
||
| Sponheim 2002 | Significance not reported | ||
| RLBLS (Freeman 1986) | Levy 2003 | NS | |
| VABS (Sparrow 1984) | Owley 2001 | NS | |
| Sandler 1999 | NS | ||
| Visiospatial skills | DTVP‐2 (Hammill 1993) | Owley 2001 | NS |
| Molloy 2002 | NS | ||
| M‐PSMT (Stutsman 1931) | Molloy 2002 | NS | |
| LIPS (Leiter 1980) | Roberts 2001 | NS | |
| Mullen (Mullen 1997) | Owley 2001 | NS | |
| Affect | MN‐PARS (Shapiro 1994) | Corbett 2001 | NS for two subscales Favours treatment for two subscales |
| Global impression of health | CGIS (Guy 1976) | Sandler 1999 | NS |
| NCT00036244 | NS | ||
| Adverse events | TESS (NIMH 1985) | Sandler 1999 | NS |
NS: No statistically significant between group difference
Appendix 5. Baseline data for secretin and control groups for data used in forest plots, by outcome measure and trial
| Mean (SD) (N) | ||||||||||
| Name of measure | Treatment Group | Owley 2001 | Kern 2002 | Unis 2002 | Carey 2002 | Chez 2000 | Molloy 2002 | Sandler 1999 | Dunn‐Geier 2000 | Coniglio 2001 |
| CARS ‐ Total (Schopler 1980) | Secretin | 34.7 (8.7) (N=13) | 40.2 (5.0) (N=19) | 38.5 (4.5) (N=47) | 42.4 (5.4) (N=28) | |||||
| Control | 33.3 (5.0) (N =12) | 39.2 (5.6) (N=23) | 37.9 (4.4) (N=47) | 41.3 (6.3) (N=29) | ||||||
| GARS ‐ Autism Quotient (Gilliam 1995) | Secretin | 93.6 (12.0) (N=26) | 102.0 (7.9) (N=19) | 104.1 (15.4) (N=28) | ||||||
| Control | 85.5 (11.7) (N=27) | 98.6 (9.2) (N=23) | 108.1 (11.0) (N=29) | |||||||
| ADOS ‐ Social interaction Lord 1997) | Secretin | 11.9 (1.5) (N=28) | 9.9 (2.1) (N=52) | |||||||
| Control | 10.8 (1.9) (N=28) | 9.6 (2.1) (N=33) | ||||||||
| ADOS ‐ Communication (Lord 1997) | Secretin | 6.8 (1.8) (N=28) | 5.8 (1.8) (N=52) | |||||||
| Control | 6.5 (2.1) (N=28) | 5.7 (2.0) (N=33) | ||||||||
| AuBC ‐ Total (Krug 1980) | Secretin | 62.7 (22.2) (N=30) | 79.0 (31.6) (N=45) | |||||||
| Control | 66.2 (23.4) (N=30) | 79.9 (26.0) (N=48) | ||||||||
| AuBC ‐ Sensory | Secretin | Not available | 13.3 (7.4) (N=43) | |||||||
| Control | 13.9 (6.5) (N=45) | |||||||||
| AuBC ‐ Social relatedness | Secretin | Not available | 19.1 (8.3) (N=44) | |||||||
| Control | 19.1 (8.6) (N=47) | |||||||||
| AuBC ‐ Body and object use | Secretin | Not available | 17.5 (9.4) (N=45) | |||||||
| Control | 17.0 (8.7) (N=48) | |||||||||
| AuBC ‐ Language | Secretin | Not available | 13.3 (7.6) (N=42) | |||||||
| Control | 13.5 (6.7) (N=45) | |||||||||
| AuBC ‐ Socialisation | Secretin | Not available | 14.7 (5.9) (N=42) | |||||||
| Control | 15.3 (5.4) (N=45) | |||||||||
| VABS ‐ Communicatio (Sparrow 1984) | Secretin | 35.9 (22.2) (N=28) | Not available | |||||||
| Control | 41.7 (26.7) (N=28) | |||||||||
| Preschool language scale (PLS‐3) (Zimmerman 1992) | Secretin | 25.2 (11.8) (N=41) | 15.4 (9.5) (N=28) | |||||||
| Control | 31.0 (15.6) (N=44) | 22.0 (12.8) (N=22) | ||||||||
| ABC ‐ Irritability (Aman 1986) | Secretin | 11.6 (7.5) (N=27) | 11.3 (8.4) (N= 9) | Not available | 22.5 (5.0) (N=4) | |||||
| Control | 10.1 (7.1) (N=27) | 16.0 (8.5) (N=10) | 15.5 (5.0) (N=4) | |||||||
| ABC ‐ Lethargy | Secretin | 13.7 (7.1) (N=27) | 15.9 (7.4) (N= 9) | Not available | 12.5 (6.4) (N=4) | |||||
| Control | 8.3 (6.7) (N=27) | 22.5 (6.3) (N=10) | 14.7 (5.0) (N=4) | |||||||
| ABC ‐ Stereotypy | Secretin | 7.3 (4.0) (N=27) | 9.1 (5.3) (N= 9) | Not available | 8.5 (0.7) (N=4) | |||||
| Control | 5.1 (3.5) (N=27) | 6.5 (4.5) (N=10) | 7.0 (5.1) (N=4) | |||||||
| ABC ‐ Hyperactivity | Secretin | 21.3 (10.3) (N=27) | 22.8 (8.8) (N= 9) | Not available | 29.0 (12.7) (N=4) | |
||||
| Control | 18.6 (9.6) (N=27) | 26.0 (14.1) (N=10) | 27.0 (8.0) (N=4) | |||||||
| ABC ‐ Inappropriate speech | Secretin | 2.6 (2.3) (N=27) | 2.8 (2.8) (N= 9) | Not available | 3.0 (2.8) (N=4) | |||||
| Control | 2.9 (2.3) (N=27) | 2.2 (2.0) (N=10) | 1.8 (1.9) (N=4) | |||||||
| TYPE OF DATA AVAILABLE FOR EACH TRIAL | Predominantly post‐treatment data, but some mean change in text | Both post‐treatment and mean change presented | Mean change scores presented | Post‐treatment scores | Post‐treatment scores | Post‐treatment scores | Mean change scores presented | Mean change scores presented | Post‐treatment scores | |
GARS Gilliam Autism Rating Scale CARS The Childhood Autism Rating Scale ADOS Autism Diagnostic Observation Scale
AuBC Autism Behaviour Checklist VABS Vineland Adaptive Behaviour Scale ABC Aberrant Behaviour Checklist
Data and analyses
Comparison 1. Core features of autism.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Core features of autism, total scores | 6 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Childhood Autism Rating Scale | 4 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Gilliam Autism Rating Scale: Autism Quotient | 3 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Autism Behavior Checklist: Total | 2 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
1.1. Analysis.

Comparison 1 Core features of autism, Outcome 1 Core features of autism, total scores.
Comparison 2. ADOS.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ADOS | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Communication | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Social Interaction | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.

Comparison 2 ADOS, Outcome 1 ADOS.
Comparison 3. Vineland (communication).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vineland four weeks post treatment | 2 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Communication, parent reported | 2 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.

Comparison 3 Vineland (communication), Outcome 1 Vineland four weeks post treatment.
Comparison 4. Aberrant Behavior Checklist.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Aberrant Behavior Checklist 3‐4 weeks post treatment | 3 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Irritability | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 Lethargy | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 Stereotypy | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Hyperactivity | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.5 Inappropriate speech | 3 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
4.1. Analysis.

Comparison 4 Aberrant Behavior Checklist, Outcome 1 Aberrant Behavior Checklist 3‐4 weeks post treatment.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Carey 2002.
| Methods | Cross‐over, "double‐blind" ‐ no details 13/21 LTFU | |
| Participants | Autism or PDD‐NOS (DSM‐IV) all male, 3‐8 yrs | |
| Interventions | Treatment: synthetic secretin 2CU/kg baseline or week 4 | |
| Outcomes | Aberrant Behaviour Checklist | |
| Notes | Reliable Change Index to assess treatment effect, MANOVA. High loss to follow‐up (62%). Low sample size | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" but no details |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 62% loss to follow‐up |
Chez 2000.
| Methods | Cross‐over Double blind Blinding of outcome assessor unclear 1/25 LTFU | |
| Participants | Autism or PDD‐NOS (DSM‐IV) 6yrs ± 2.4 22 males, 3 females | |
| Interventions | Treatment: porcine secretin 2CU/kg IV single dose at baseline or wk 4 Control: IV saline, single dose at baseline or wk 4 | |
| Outcomes | CARS Parent reported outcomes | |
| Notes | Biased sample ("responders") from non‐controlled trial. Secretin in this trial each child's 2nd dose | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Alternating schedule at entry |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double blind" but blinding of outcome assessors unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4% loss to follow‐up |
Coniglio 2001.
| Methods | Parallel, "double‐blind" Method of randomisation unclear 3/60 LTFU | |
| Participants | Autism (DSM‐IV) 45 males, 15 females age 3‐10 yrs | |
| Interventions | Treatment: porcine secretin 2 CU/kg or placebo | |
| Outcomes | CARS GARS Preschool Language Scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not speified |
| Allocation concealment (selection bias) | Unclear risk | Not specified |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5% loss to follow‐up. Reported that "Study completers were comparable to the original subjects assigned to the treatment and placebo control groups for all variables at study entry" |
Corbett 2001.
| Methods | Cross‐over Participants, treating physician blind. Blinding of outcome assessors unclear 2/16 LTFU No ITT reported | |
| Participants | Autism (DSM‐IV, ADOS, ADI). IQ<70 4‐12 yrs (6.5) Gender not stated | |
| Interventions | Treatment: porcine secretin 2CU/kg IV single dose baseline or wk 8 Control: IV saline, single dose baseline or wk 8 | |
| Outcomes | ADOS, MN PARS | |
| Notes | Multivariate analysis. P values only. Data not suitable for meta‐analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not stated |
| Allocation concealment (selection bias) | Low risk | Stated as "randomized treatment" by University of Minnesota Investigational Pharmacy |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants and treating physicians were blinded but the blinding of outcome assessors was unclear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 12.5% loss to follow‐up and intention‐to‐treat analysis not conducted |
Dunn‐Geier 2000.
| Methods | Parallel Triple blind, no LTFU | |
| Participants | DSM‐IV>6, age stratified 2‐4, 5‐7yrs (mean 5.1yrs), age stratified randomisation (2‐4, 5‐7yrs). No previous secretin administration or changes to current therapy CARS >30 88 males, 7 females | |
| Interventions | Treatment: porcine secretin 2CU/kg IV single dose Control: IV saline, single dose | |
| Outcomes | Preschool Language Scale (primary outcome measure), CARS, Autism Behavior Checklist, GI symptoms, Preschool Language Scale | |
| Notes | SD calculated from SEM. Power analysis: 80% power to detect change of 6 points on PLS‐3 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisation sequence stratified by site and age (2 to 4 or 5 to 7 years of age) was generated by the study biostatistician (BP) and kept at the pharmacy at each of the participating sites throughout the trial |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All personnel involved in clinical assessments, as well as the physician performing the infusion, were blinded to patients’ allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
Kern 2002.
| Methods | Cross‐over, outcome assessors blind, no LTFU | |
| Participants | Autism or PDD‐NOS (DSM‐IV). Age 3‐10 yrs. 15 males, 4 females | |
| Interventions | Treatment: secretin 2 CU/kg at week 3 or week 6 | |
| Outcomes | Aberrant Behavior Checklist, MacArther Communicative Development Inventory | |
| Notes | Data presented graphically. Individual patient data provided on request. Post hoc subgroup analysis for chronic diarrhoea | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not stated |
| Allocation concealment (selection bias) | Low risk | The pharmacists at Children’s Medical Center of Dallas prepared both the treatment and placebo and were responsible for random group assignments |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Only outcome assessors reported to be blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
Levy 2003.
| Methods | Cross‐over, triple blind, 1/62 LTFU | |
| Participants | ASD (ADI‐R) 3‐8 yrs, 50 males, 12 females | |
| Interventions | Treatment: human synthetic secretin 2CU/kg at baseline or week 6 | |
| Outcomes | Global Behavior Rating Scale, Communication and Symbolic Behavior Scale, Ritvo Real‐Life Rating Scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised sequence |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Pharmacist and research nurse not involved in assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1.6% loss to follow‐up only |
Molloy 2002.
| Methods | Cross‐over, participants, parents and researchers blinded. 18/60 planned for trial were not included because trial terminated | |
| Participants | Autism (DSM‐IV), age: 2‐15yrs, 37 males, 5 females | |
| Interventions | Treatment: 2IU/kg synthetic human secretin at baseline or week 6 | |
| Outcomes | CARS, GARS, Aberrant Behavior Checklist, GARS, Mullen Scales of Early Learning, PPVT‐III, mean length of utterance, Type‐token ratio, DTVP‐2, Merrill‐Palmer Scale of Mental Tests | |
| Notes | Power analysis: 6 pt change on GARS 81% power. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to the randomisation table in the research pharmacy |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Researchers, participants and caregivers were blind to the treatment condition |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 18/60 (30%) of participants planned for trial were not included because trial terminated |
NCT00036244.
| Methods | Double‐blind placebo‐controlled | |
| Participants | 132 children aged 2 years 8 months to 4 years 11 months with moderate to severe symptoms of autism | |
| Interventions | Six injection of synthetic human secretin (RG1068) or a placebo over 18 weeks | |
| Outcomes | Improvements of social interaction as measured by the Autism Diagnostic Observation Scale (ADOS) and parental Clinical Global Impression of Change (CGI) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Press release information only |
| Allocation concealment (selection bias) | Unclear risk | Press release information only |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Press release information only |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Press release information only |
Owley 2001.
| Methods | Cross‐over, multisite, parents and assessors blind Triple blind 0 LTFU no ITT reported | |
| Participants | Autism (DSM‐IV) 3‐12 yrs (80.5±22.9mo) 48 males, 8 females | |
| Interventions | Treatment: porcine secretin 2CU/kg IV single dose, baseline or week 4 baseline or wk 4 Control: IV saline, single dose baseline or wk 4 | |
| Outcomes | ADOS, GARS, Aberrant Behavior Checklist, Vineland, see table VABS Clinical Global Impression Scale | |
| Notes | Significant baseline differences for ADOS: social interaction (P=0.18), stereotypy (P=0.007) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Information provided |
| Allocation concealment (selection bias) | Low risk | Randomisation was done by the investigational pharmacy at each site |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All patients, their parents, and all members of the assessment team were blind to drug assignments until all subjects at that site had completed the trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
Repligen 2002.
| Methods | Double‐blind placebo‐controlled | |
| Participants | 124 children age 3 to 6 years 11 months | |
| Interventions | Three doses of intravenous secretin or placebo | |
| Outcomes | ||
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Press release information only |
| Allocation concealment (selection bias) | Unclear risk | Press release information only |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Press release information only |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Press release information only |
Roberts 2001.
| Methods | Triple blind 1/68 LTFU | |
| Participants | Autism/ASD, (ADI‐R, ADOS‐G) and previous diagnosis autism or PDD‐NOS. Age 2‐7 yrs 55 males, 9 female | |
| Interventions | Treatment: porcine secretin 2CU/kg IV 2 doses 6 wks apart Control: IV saline, 2 doses 6 wks apart | |
| Outcomes | ADOS, Preschool language scale, Autism Behavior Checklist, Leiter International Performance Scale, GI symptoms ADIR Preschool Language Scale | |
| Notes | Repeated measures ANOVA. Analysis of subgroups for GI, IQ, history of regression Two doses of secretin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, parents, and examiners were all blind to group membership |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | But loss to follow‐up only 1.5% |
Sandler 1999.
| Methods | Triple blind 4/60 LTFU | |
| Participants | Autism, PDD‐NOS (DSM‐IV), CARS, Autism Behavior Checklist, age 3‐14 yrs Gender not available | |
| Interventions | Treatment: synthetic human secretin 0.4 micrograms/kg IV single dose, week 2 Control: saline single dose IV | |
| Outcomes | Clinical Global Impression Scale, Autism Behavior Checklist, Vineland, Treatment emergent symptoms scale ABC VABS | |
| Notes | Baseline data in text does not match baseline data in figures due to loss to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information about type of randomisation |
| Allocation concealment (selection bias) | High risk | Colour tagged syringes acording to treatment allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | As syringes colour tagged and clinicians and parents performing outcome measurements |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Intention to treat analysis not reported, 7% loss to follow‐up |
Sponheim 2002.
| Methods | Cross‐over, "double‐blind" no further details, No LTFU | |
| Participants | Childhood autism (ICD‐10), ADI‐R, age 3‐12 yrs, 5 males, 1 female | |
| Interventions | Three doses each of human synthetic secretin or placebo in random order | |
| Outcomes | Visual Analogue Scale, Aberrant Behavior Checklist | |
| Notes | small sample size (n=6), VAS scale graphical data only, AbBC no change, so no data presented | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Details provided by authors |
| Allocation concealment (selection bias) | Low risk | Details provided by authors |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | "Double‐blind" with no further details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
Unis 2002.
| Methods | Parallel, "double blind" no further specific details 1/90 LTFU | |
| Participants | Autism or PDD‐NOS (DSM‐IV, ADOS), age 3‐12 yrs, age, communication level matched, gender not available | |
| Interventions | Two treatment groups: extracted porcine secretin (2 CU/KG) or synthetic porcine secretin (0.4 micrograms/kg) or placebo | |
| Outcomes | ADOS communication, social. Aberrant behavior checklist total | |
| Notes | Synthetic and extracted ("biologic") secretin results combined mean and SD used in meta‐analysis. Aberrant Behavior Checklist total score only provided ‐ not valid measure | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Only stated "double‐blind" with no specific details |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 6% loss to follow‐up |
Wray.
| Methods | Cross‐over, triple‐blind, 5/27 LTFU | |
| Participants | Autism, PDD‐NOS or Childhood disintegrative disorder (DSM‐III‐R or DSM‐IV), age 3‐18 yrs, 20 males, 2 females | |
| Interventions | Single dose, porcine secretin 2IU/kg or placebo | |
| Outcomes | ADOS | |
| Notes | Raw data available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Details provided by Wray |
| Allocation concealment (selection bias) | Low risk | Details provided by Wray |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Details provided by Wray |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up |
LTFU: lost to follow‐up
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Honomichl 2002 | Outcomes not relevant |
| Lightdale 2001 | Not RCT, no placebo |
| Lonsdale 2000 | Not RCT, no placebo |
| NCT00036231 | Terminated trial and no data reported |
| Ratliff‐Schaub 2005 | Study of healthy males not children with ASD and only neurological imaging outcome |
| Shin‐Siung 2000 | Not intravenous administration of secretin. Administration by intramuscular injection |
| Yurgelun‐Todd 2008 | Secretin administered transdermally not intravenously |
Differences between protocol and review
'Adverse events' was inadvertently omitted from the list of primary outcomes in the protocol. 'Improvement in' was omitted from the outcome subheadings in the review to avoid confusion.
Contributions of authors
KW, DW and JW developed the protocol. DW ran searches. KW and DW independently reviewed citations and full texts and selected trials for inclusion within the review. KW and DW independently extracted data, conducted the analysis and wrote the review, with consultation from JW.
Sources of support
Internal sources
Small Grants Scheme of the Children's Hospital at Westmead, Australia.
External sources
Financial Markets Foundation for Children, Australia.
Declarations of interest
Katrina Williams ‐ I gave a talk about treatments for autism at a symposium organised by Janssen‐Cilag Pty Ltd. Janssen‐Cilag had no control over the content of the talk and the speaker's fee was paid to the University that employs me. I have no ongoing relationship with Janssen‐Cilag.
John A Wray ‐ was involved in two trials included within this review (Wray; Levy 2003). Dr Wray has declared he delivered a previous testimony for inclusion of risperidone for children with autism on Australia's Public Medicines Prescription Benefit Scheme. Airfares and accommodation were paid for by Janssen Pharmaceutical.
Danielle M Wheeler ‐ none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Carey 2002 {published data only}
- Carey T, Ratcliff‐Schaub K, Funk J, Weinle C, Myers M, Jenks J. Double‐blind placebo‐controlled trial of secretin: effects on aberrant behavior in children with autism. Journal of Autism and Developmental Disorders 2002;32(3):161‐7. [DOI] [PubMed] [Google Scholar]
Chez 2000 {published data only}
- Chez M, Buchanan C, Bagan B, Hammer M, Carthy K, Ovrutskaya I, et al. Secretin and autism: a two‐part clinical investigation. Journal of Autism and Developmental Disorders 2000;30(2):87‐94. [DOI] [PubMed] [Google Scholar]
Coniglio 2001 {published data only}
- Coniglio S, Lewis J, Lang C, Burns T, Subhani‐Siddique R, Weintraub A, et al. A randomized, double‐blind, placebo‐controlled trial of single‐dose intravenous secretin as treatment for children with autism. Journal of Pediatrics 2001;138(5):649‐55. [DOI] [PubMed] [Google Scholar]
Corbett 2001 {published data only}
- Corbett B, Khan K, Czapansky‐Beilman, Brady N, Dropik P, Zelinsky Goldman D, et al. A double‐blind, placebo‐controlled cross‐over study investigating the effect of porcine secretin in children with autism. Clinical Pediatrics 2001;40(6):327‐31. [DOI] [PubMed] [Google Scholar]
Dunn‐Geier 2000 {published data only}
- Dunn‐Geier J, Ho H, Auersperg E, Doyle D, Eaves L, Matsuba C, et al. Effect of secretin on children with autism: a randomised controlled trial. Developmental Medicine and Child Neurology 2000;42(12):796‐802. [DOI] [PubMed] [Google Scholar]
Kern 2002 {published and unpublished data}
- Kern J, Miller V, Evans P, Trivedi M. Efficacy of porcine secretin in children with autism and pervasive developmental disorder. Journal of Autism and Developmental Disorders 2002;32(3):153‐60. [DOI] [PubMed] [Google Scholar]
Levy 2003 {published data only}
- Levy S, Souders M, Wray J, Jawad A, Gallagher P, Coplan J, et al. Children with autistic spectrum disorders. I:comparison of placebo and single dose of human synthetic secretin. Archives of Disease in Childhood 2003;88(8):731‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Molloy 2002 {published data only}
- Molloy C, Manning‐Courtney P, Swayne S, Bean J, Brown J, Murray D, et al. Lack of benefit of intravenous synthetic human secretin in the treatment of autism. Journal of Autism and Developmental Disorders 2002;32(6):545‐51. [DOI] [PubMed] [Google Scholar]
NCT00036244 {unpublished data only}
- NCT00036244. A phase III, randomised, double blind, placebo‐controlled, multiple dose study to assess the efficacy, safety and tolerability of RG1068 (synthetic human secretin) in children with autism. ClinicalTrials.gov: www.clinicaltrials.gov/ct2/show/NCT00036244 Study completed January 2004 (accessed 17 Nov 2011).
Owley 2001 {published data only}
- Owley T, McMahon W, Cook E, Laulhere T, South M, Mays L, et al. Multisite, double‐blind placebo‐controlled trial of porcine secretin in autism. Journal of the American Academy of Child and Adolescent Psychiatry 2001;40(11):1293‐9. [DOI] [PubMed] [Google Scholar]
- Owley T, Steele E, Corsello C, Risi S, McKaig K, Lord C, et al. A double‐blind, placebo‐controlled trial of secretin for the treatment of autistic disorder. Medscape General Medicine 1999;1(3):e1006. [PubMed] [Google Scholar]
Repligen 2002 {unpublished data only}
- Krusch DA. Effects of repeated secretin administration on a subset of children with pervasive developmental disorder [PhD dissertation]. Rochester, NY: University of Rochester, 2004. [Google Scholar]
- Repligen Corporation. Repligen Announces Initiation of Phase 3 Clinical Trials for Secretin in Autism. Press release 14 February 2002.
Roberts 2001 {published data only}
- Roberts W, Weaver L, Brian J, Bryson S, Emelianova S, Griffiths A, et al. Repeated doses of porcine secretin in the treatment of autism: a randomized, placebo‐controlled trial. Pediatrics 2001;107(5):e71. [DOI] [PubMed] [Google Scholar]
Sandler 1999 {published data only}
- Sandler A, Sutton K, DeWeese J, Girardi M, Sheppard V, Bodfish J. Lack of benefit of a single dose of synthetic human secretin in the treatment of autism and pervasive developmental disorder. New England Journal of Medicine 1999;341(24):1801‐6. [DOI] [PubMed] [Google Scholar]
Sponheim 2002 {published data only}
- Sponheim E, Oftedal G, Helverschou S. Multiple doses of secretin in the treatment of autism: a controlled study. Acta Paediatrica 2002;91(5):540‐5. [DOI] [PubMed] [Google Scholar]
Unis 2002 {published data only}
- Unis A, Munson J, Rogers S, Goldson E, Osterling J, Gabriels R, et al. A randomized, double‐blind, placebo‐controlled trial of porcine versus synthetic secretin for reducing symptoms of autism. Journal of the American Academy of Child and Adolescent Psychiatry 2002;41(11):1315‐21. [DOI] [PubMed] [Google Scholar]
Wray {unpublished data only}
- Wray J, Wilkins‐Shurmer A, O'Connor G, Knott H, Bint L, Gilroy A. Lack of communication and behavioural response of children with autism from single dose of intravenous porcine secretin. Unpublished.
References to studies excluded from this review
Honomichl 2002 {published data only}
- Honomichl R, Goodlin‐Jones B, Burnham M, Hansen R, Anders T. Secretin and sleep in children with autism. Child Psychiatry and Human Development 2002;33(2):107‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lightdale 2001 {published data only}
- Lightdale J, Hayer C, Duer A, Lind‐White C, Jenkins S, Siegal B, et al. Effects of intravenous secretin on language and behavior of children with autism and gastrointestinal symptoms: a single‐blinded, open‐label pilot study. Pediatrics 2001;108(5):e90. [DOI] [PubMed] [Google Scholar]
Lonsdale 2000 {published data only}
- Lonsdale D, Shamberger R. A clinical study of secretin in autism and pervasive developmental delay. Journal of Nutritional and Environmental Medicine 2000;10(4):271‐80. [Google Scholar]
NCT00036231 {unpublished data only}
- NCT00036231. A phase III, randomized, double blind, placebo‐controlled, multiple dose study to assess the efficacy, safety and tolerability of RG1068 (synthetic human secretin) in children with autism and gastrointestinal dysfunction [Study terminated]. ClinicalTrials.gov: www.clinicaltrials.gov/ct2/show/NCT00036231 (accessed 17 Nov 2011).
- Repligen Corporation. Phase 3 study for secretin for autism fails to meet dual primary endpoints. Development of secretin for schizophrenia to continue. Press release January 5 2004.
Ratliff‐Schaub 2005 {published data only}
- Ratliff‐Schaub K, Carey T, Reeves G, Rogers M. Randomized controlled trial of transdermal secretin on behavior of children with autism. Autism 2005;9(3):256‐65. [DOI] [PubMed] [Google Scholar]
Shin‐Siung 2000 {published data only}
- Shin‐Siung J, Kao P, Lee YC. Double blind crossover study of secretin/secrepan treatment for children with autistic symptoms. Tzu Chi Medical Journal 2000;12(3):173‐81. [Google Scholar]
Yurgelun‐Todd 2008 {published data only}
- Yurgelun‐Todd DA, Rogowska J, Gruber SA, Bogorodzki P, Simpson NS, Irvin RW, et al. Increased amygdala fMRI activation after secretin administration. Experimental and Clinical Psychopharmacology. 2008;16(3):191‐8. [DOI] [PubMed] [Google Scholar]
Additional references
Achenbach 1991
- Achenbach TM. Manual for the Child Behavior Checklist /4‐18, 1991 Profile. Burlington, VT: The University of Vermont, 1991. [Google Scholar]
Aman 1986
- Aman M, Singh N. Aberrant Behaviour Checklist (ABC). New York: Slosson Educational Publications, 1986. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV). 4th Edition. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM‐IV‐TR). 4th Edition. Washington, DC: American Psychiatric Press, 2000. [0890420246] [Google Scholar]
Charlton 1983
- Charlton CG, Miller RI, Crawley JN, Handelmann GE, O'Donohue TL. Secretin modulation of behavioural and physiological functions in the rat. Peptides 1983;4(5):739‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test [see comments]. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fenson 1993
- Fenson L, Dale P, Resnick J, Thal D, Bates E, Hartung J, et al. Words and sentences. MacArthur Communicative Development lnventories (CDI). San Diego, CA: Singular Publishing, 1993. [Google Scholar]
Fombonne 1999
- Fombonne E. The epidemiology of autism: a review. Psychological Medicine 1999;29(4):769‐86. [DOI] [PubMed] [Google Scholar]
Fombonne 2003
- Fombonne E. The prevalence of autism. JAMA 2003;289(1):87‐9. [DOI] [PubMed] [Google Scholar]
Fombonne 2009
- Fombonne E. Epidemiology of pervasive developmental disorders. Pediatric Research 2009;65(6):591‐8. [DOI] [PubMed] [Google Scholar]
Freeman 1986
- Freeman BJ, Ritvo ER, Yokota A, Ritvo A. A scale for rating symptoms of patients with the syndrome of autism in real life settings. Journal of the American Academy of Child Psychiatry 1986;25(1):130‐6. [DOI] [PubMed] [Google Scholar]
Freier 1981
- Charlton CG, O'Donohue TL, Miller RI, Jacobowitz DM. Secretin immunoreactivity in rat and pig brain. Peptides 1981;2(1):45‐9. [DOI] [PubMed] [Google Scholar]
Gardener 1990
- Gardener MF. Expressive One‐Word Picture Vocabulary Test (Revised). Novato, CA: Academic Therapy Publications, 1990. [Google Scholar]
Gillberg 2000
- Gillberg C, Coleman M. The neurology of autism. The Biology of Autistic Syndromes. 3rd Edition. London: MacKeith Press, 2000:291‐309. [Google Scholar]
Gilliam 1995
- Gilliam JE. The Gilliam Autism Rating Scale (GARS). Austin, TX: PRO‐ED, Inc, 1995. [Google Scholar]
Guy 1976
- Guy W. Early Clinical Drug Evaluation Unit (ECDEU) Assessment Manual for Psychopharmacology. Revised. Bethesda, MD: National Institute of Mental Health, 1976:217‐222. [Google Scholar]
Hammill 1993
- Hammill D, Pearson N, Voress J. Developmental Test of Visual Perception (DTVP‐2). Austin, TX: PRO‐ED, 1993. [Google Scholar]
Higgins 2002
- Higgins J, Thompson S. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21(11):1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2008
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions. Chichester: John Wiley & Sons, 2008. [Google Scholar]
Horvath 1998
- Horvath K, Stefanotos G, Sokolski KN, Wachtel R, Nabros L, Tildon JT. Improved social and language skills after secretin administration in patients with autistic spectrum disorders. Journal of the Association for Academic Minority Physicians 1998;9(1):9‐15. [PubMed] [Google Scholar]
Kanner 1943
- Kanner L. Autistic disturbances of affective contact. Nervous Child 1943;2:217‐50. [PubMed] [Google Scholar]
Kasari 2002
- Kasari C. Assessing change in early intervention problems for children with autism. Journal of Autism and Developmental Disorders 2002;32(5):447‐78. [DOI] [PubMed] [Google Scholar]
Krishnaswami 2011
- Krishnaswami S, McPheeters ML, Veenstra‐VanderWeele J. A systematic review of secretin for children with autism spectrum disorders. Pediatrics 2011;127:e1322‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krug 1980
- Krug DA, Arick JR, Almond PJ. Autism Screening Instrument for Educational Planning. 1st Edition. Portland, OR: ASIEP Educational Co, 1980. [Google Scholar]
Leiter 1980
- Leiter RG. Leiter International Performance Scale Instruction Manual (revised edition). Wood Dale, IL: Stoelting, 1980. [Google Scholar]
Lloyd 1997
- Lloyd M, Dunn LM. Peabody Picture Vocabulary Test ‐ Third Edition (PPVT‐III). Circle Pines, MN: American Guidance Service, 1997. [Google Scholar]
Lord 1994
- Lord C, Rutter M, Couteur A. Autism Diagnostic Interview‐Revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. Journal of Autism and Developmental Disorders 1994;24(5):659‐85. [DOI] [PubMed] [Google Scholar]
Lord 1997
- Lord C, Rutter M, DiLavore PC. Autism Diagnostic Observation Scale ‐ Generic. Chicago, IL: University of Chicago Press, 1997. [Google Scholar]
Lord 2000
- Lord C, Risi S, Lambrecht L, Cook EH Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule‐generic: a standard measure of social and communication deficits associated with the spectrum of autism. Journal of Autism and Developmental Disorders. 2000;30(3):205‐23. [PubMed] [Google Scholar]
Moher 2001
- Moher D, Schulz KF, Altman D, for the CONSORT Group. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomized trials. JAMA 2001;285(15):1987‐91. [DOI] [PubMed] [Google Scholar]
Mullen 1997
- Mullen E. Mullen Scales of Early Learning. Los Angeles: Western Psychological Services, 1997. [Google Scholar]
Myers 2007
- Myers SM, Plauche´ Johnson C, the Council on Children With Disabilities. Management of Children with Autism Spectrum Disorders. Pediatrics 2007;120(5):1162‐82. [DOI] [PubMed] [Google Scholar]
NIMH 1985
- National Institute of Mental Health. Treatment Emergent Symptoms Scale ‐‐ write‐in (TESS). Psychopharmacology Bulletin 1985;21:1069‐73. [Google Scholar]
Perry 2003
- Perry A, Condillac R. Evidence‐Based Practices for Children and Adolescents with Autism Spectrum Disorders: Review of the Literature and Practice Guide. Ontario, Canada: Children's Mental Health, 2003. [Google Scholar]
Review Manager 4.2 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 4.2.6. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2005.
Rimland 2000
- Rimland B. Comments on "Secretin and autism: a two‐part clinical investigation" by M.G. Chez et al. Journal of Autism and Developmental Disorders 2000;30(2):95. [DOI] [PubMed] [Google Scholar]
Roberts 2006
- Roberts JMA, Prior M. A review of the Research to Identify the most Effective Models of Practice in Early Intervention of Children with Autism Spectrum Disorders. Australia: Australian Government, Department of Health and Ageing, 2006. [Google Scholar]
Schopler 1980
- Schopler E, Reichler RJ, Vellis RF, Daly K. Toward objective classification of childhood autism: Childhood Autism Rating Scale (CARS). Journal of Autism and Developmental Disorder 1980;10(1):91‐103. [DOI] [PubMed] [Google Scholar]
Shapiro 1994
- Shapiro E, McPhee J, Abbot A, Sulzbacher S. Minnesota Preschool Affect Rating Scales: development, reliability, and validity. Journal of Pediatric Psychology 1994;19(3):325‐45. [DOI] [PubMed] [Google Scholar]
Shipley 1992
- Shipley K, McAfee J. Assessment in Speech‐Language Pathology. San Diego: Singular Publishing, 1992. [Google Scholar]
SIGN 2007
- Scottish Intercollegiate Guidelines Network. Assessment, Diagnosis and Clinical Interventions for Children and Young People with Autism Spectrum Disorders. A National Clinical Guideline. Edinburgh: Scottish Intercollegiate Guidelines Network, 2007. [Google Scholar]
Skuse 2004
- Skuse D, Warrington R, Bishop D, Chowdhury U, Lau J, Mandy W, et al. The developmental, dimensional and diagnostic interview (the 3di): a novel computerised assessment for autism spectrum disorders. Journal of the American Academy of Child and Adolescent Psychiatry 2004;43(5):548‐58. [DOI] [PubMed] [Google Scholar]
Sparrow 1984
- Sparrow S, Balla D, Cicchetti D. Vineland Scales of Adaptive Behavior, Survey Form Manual. Circle Pines, MN: American Guidance Service, 1984. [Google Scholar]
Stutsman 1931
- Stutsman R. Mental Measurement of Preschool Children with a Guide for the Administration of the Merrill‐Palmer Scale of Mental Tests. Yonkers on Hudson, NY: Worldbook, 1931. [Google Scholar]
Vickers 2001
- Vicker AJ, Altman DG. Analysing controlled trials with baseline and follow up measurements. BMJ 2001;323(7321):1123‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wetherby 1993
- Wetherby A, Prizant B. Communication and Symbolic Behaviour Scales (CSBS). 1st Edition. Chicago, IL: Riverside Publishing, 1993. [Google Scholar]
WHO 1993
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders: Diagnostic Criteria for Research. Geneva: World Health Organization, 1993. [Google Scholar]
Williams 2006
- Williams JG, Higgins JPT, Brayne CEG. Systematic review of prevalence studies of autism spectrum disorders. Archives of Disease in Childhood 2006;91(1):8‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wing 1999
- Wing L. Diagnostic Interview for Social and Communication Disorders (DISCO). 10th Edition. Kent: The National Autistic Society, 1999. [Google Scholar]
Zimmerman 1992
- Zimmerman IL, Steiner VG, Pond RE. Pre‐school Language Test (PLS‐3). San Antonio, TX: The Psychological Corporation, 1992. [Google Scholar]


