Abstract
This study tested the hypothesis that dietary activation of the master antioxidant and cell protective transcription factor nuclear factor, erythroid −2-like 2 (NRF2) protects against salt-induced vascular dysfunction by restoring redox homeostasis in the vasculature. Male Sprague-Dawley rats and Syrian hamsters were fed a HS (4.0% NaCl) diet containing ~60 mg/kg/day Protandim supplement for 2 weeks and compared to controls fed HS diet alone. Protandim supplementation prevented the loss of endothelium-dependent vasodilation in response to acetylcholine (ACh) in middle cerebral arteries (MCA) of HS-fed rats and hamster cheek pouch arterioles, and increased microvessel density in the cremaster muscle of HS-fed rats. The preserved dilation to ACh in MCA of Protandim-treated rats was prevented by inhibiting nitric oxide synthase (NOS) with L-NAME [100 μM] and was absent in MCA from Nrf2(−/−) knockout rats fed HS diet. Basilar arteries from HS-fed rats treated with Protandim exhibited significantly lower staining for mitochondrial oxidizing species than untreated animals fed HS diet alone; and Protandim treatment increased MnSOD (SOD2) protein expression in mesenteric arteries of HS-fed rats. These results suggest that dietary activation of NRF2 protects against salt-induced vascular dysfunction, vascular oxidative stress, and microvascular rarefaction by up-regulating antioxidant defenses and reducing mitochondrial ROS levels.
Keywords: Antioxidants, cerebral circulation, endothelial dysfunction, NRF2, oxidant stress, Protandim, reactive oxygen species, resistance arteries, salt, sodium, superoxide dismutase, vascular dysfunction, vasodilation
INTRODUCTION
Although oxidant stress has been implicated experimentally in a variety of human diseases including cardiovascular pathologies, clinical trials investigating oral antioxidant therapies in the prevention of morbidity and mortality have shown little-to-no benefit with antioxidant supplementation. For example, a recent meta-analysis of 50 randomized, controlled trials that included nearly 300,000 participants revealed no beneficial effect of vitamin or antioxidant supplementation in the prevention of cardiovascular outcomes, including cardiovascular death. [37]
While the pressure-independent effects of dietary salt on the cardiovascular system are still being investigated, work from our laboratory and others has revealed a critical link between redox homeostasis and salt-induced vascular dysfunction. In conduit arteries,[55] small resistance arteries [54], and arterioles [27,41], high salt (HS) diet attenuates or eliminates endothelium-dependent vasodilation by increasing superoxide levels and reducing nitric oxide (NO) bioavailability. In the skeletal muscle microcirculation, HS increases reactive oxygen species (ROS) production via increased NADPH and xanthine oxidase activity [25] and impairs antioxidant defenses by decreasing Cu/Zn superoxide dismutase (SOD) activity. [26] HS diet also leads to impaired angiogenic responses to muscle stimulation, endothelial cell apoptosis, and a reduction in microvessel density (microvascular rarefaction) in the skeletal muscle of HS fed rats. [6,7,16,19,40]
There is a growing body of evidence that salt-induced endothelial dysfunction also occurs in the human vasculature. Work by Tzemos and colleagues [48] showed an attenuation of endothelium-dependent flow-mediated vasodilation in the brachial arteries of healthy, normotensive, young adult volunteers following short-term (5 days) consumption of a high salt diet (200 mmol/day). In that study, ingestion of a high salt diet led to a significant reduction in the eNOS/NO contribution to flow-mediated vasodilation [48]. Impaired endothelium-dilation in response to short term high salt diet in humans is not restricted to males, as Cavka et al. [4] also showed that short term high salt diet led to impaired endothelium dependent dilation in healthy young females. These findings along with multiple reports of endothelial dysfunction and oxidative stress occurring in response to short term high salt diet in multiple animal models are alarming, as they indicate that HS-induced oxidative stress and vascular dysfunction occurs much more rapidly than previously believed. This has important implications, as endothelial dysfunction is a harbinger of multiple adverse cardiovascular effects, including cardiovascular death -- many of which are independent of an elevated blood pressure. [52]
In light of the disappointing results of clinical trials involving the administration of so-called “direct” antioxidants (e.g., vitamins C or E or beta carotene) [37], there is growing interest in strategies based on dietary or pharmacological upregulation of endogenous antioxidant defense genes, including those regulated by the master antioxidant and cell protective transcription factor nuclear factor (erythroid-derived 2)-like-2 (Nrf2), which regulates hundreds of antioxidant and cytoprotective proteins [20] by binding at antioxidant response element (ARE) consensus sequences in the promoter region of those genes. Previous studies in our laboratory [42] indicate that salt-induced angiotensin II (ANG II) suppression promotes endothelial dysfunction by suppressing antioxidant defenses normally maintained by NRF2.
A number of electrophilic compounds can activate NRF2, thereby increasing nuclear localization of the transcription factor. These include sulforaphane [46], resveratrol [49], and the nutritional supplement Protandim®, a mixture of phytochemical extracts that have a synergistic effect to activate NRF2. [20,38] Protandim induces NRF2 nuclear translocation and subsequent transcription of the prototypical NRF2-regulated gene heme oxygenase-1 (HO-1) in human endothelial cells in vitro, [51] as well as transcription of erythrocyte SOD and catalase in vivo [38].
In the present study, we tested the hypothesis that NRF2 activation via dietary supplementation with Protandim would prevent salt-induced oxidative stress and endothelial dysfunction and down-regulation of the expression of two key vascular antioxidant enzymes [Cu/Zn SOD (SOD1) and Mn SOD (SOD2)] in two established animal models of pressure-independent vascular dysfunction induced by high salt diet (Sprague-Dawley rats and Syrian hamsters). [11,12,32,33,41,54] We also tested the hypothesis that activation of NRF2 with Protandim would ameliorate salt-induced microvascular rarefaction in the skeletal muscle microcirculation, which has important implications for O2 supply to the tissues [17,28], muscle performance [39], and collateral vessel formation with ischemia [53].
MATERIALS AND METHODS
Animals:
Age-matched male Sprague-Dawley rats (Envigo, Indianapolis, IN) and Syrian hamsters (Charles River Laboratories, Wilmington, MA) were fed either high (HS) salt diet (4.0% NaCl), or a high salt diet fortified with Protandim (4.0% NaCl + ~60 mg/kg/day Protandim) for 10–14 days. In another series of experiments, Nrf2(−/−) knockout rats in the Sprague-Dawley genetic background [42] and wild type littermates were fed either high (HS) salt diet (4.0% NaCl), or a high salt diet fortified with Protandim (4.0% NaCl + ~60 mg/kg/day Protandim) for 10–14 days.
Protandim Treatment:
Protandim dosage calculations were based on the report of Qureshi et al., who fed mice Protandim-supplemented chow at a dose of 457 mg/m2 Protandim [43]. They reported that dosage to be “nearly equivalent to the manufacturer’s recommended human dose of 675 mg per day for a 60 kg adult, or 422 mg/m2” [43]. Using averaged metrics from our laboratory records where adult, male rats weighed 300 grams and consumed 22 grams of chow per day, we modified AIN-76A Purified Rodent Diet (Dyets, Inc., Bethelehem, PA) with 4.0% NaCl and 0.82 grams of Protandim per kilogram of chow, for a final dose of approximately 60.5 mg/kg/day or 450 mg/m2. Adult male hamsters weighing 100 grams and consuming 8 grams of food per day received the same food formulation and thus consumed approximately 59.6 mg/kg/day or 328 mg/m2. Water was available ad libitum. All protocols were reviewed and approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Experimental Approaches:
For the present studies, we employed three different experimental approaches that we had used in previous studies of salt-induced effects on vascular reactivity. These approaches included isolated cannulated middle cerebral arteries (MCA); direct measurement of active contractile force in small mesenteric resistance arteries using a wire myograph; and a single-layered hamster cheek pouch preparation, which allowed direct observation of changes in arteriolar diameter in the in situ microcirculation in an animal model previously shown to be especially vulnerable to oxidative stress and microvascular dysfunction when consuming a high salt diet [41]. Utilization of these diverse experimental approaches allowed us to evaluate the effects of NRF2 activation on different parameters in well-established experimental protocols previously used to demonstrate impaired vascular relaxation mechanisms in normotensive animals fed a high salt diet.
Cannulated Middle Cerebral Artery (MCA) Preparation:
Rats were euthanized by pentobarbital overdose (Beuthanasia-D, Merck Animal Health, Summit, NJ) and middle cerebral arteries (MCA) were isolated and cleaned of connective tissue in physiological salt solution (PSS) containing 119 mM NaCl, 24 mM NaHCO3, 5.5 mM dextrose, 4.7 mM KCl, 1.6 mM CaCl2•2H2O, and 1.17 mM MgSO4•7H2O in ddH2O and adjusted to pH 7.4. The arteries were cannulated with glass micropipettes and transmural pressure was set at 80 mmHg to approximate in vivo conditions. The vessels were perfused and superfused at 37° C with PSS equilibrated with a 21% O2, 5% CO2, 74% N2 gas mixture, as previously described [32].
Internal diameters of the arteries were measured via television microscopy, and concentration-response curves were obtained for the endothelium-dependent vasodilator acetylcholine (ACh; 10−10 to 10−5 M) and the NO-donor sodium nitroprusside (SNP; 10−10 to 10−4 M). ACh concentration-response curves were also obtained in the presence of the NOS inhibitor L-NG-nitroarginine methyl ester (L-NAME; 10−4 M). At the end of the experiment, the arteries were maximally relaxed by perfusing and superfusing them with Ca2+-free PSS having the following composition (mM): NaCl (119.0), KCl (4.7), NaH2PO4 (1.18), MgSO4 (1.17), NaHCO3 (24.0), D-glucose (5.5), and EDTA (0.03). Vessel diameters were measured at the 80 mmHg control pressure in Ca2+-free PSS to assess the level of active resting tone in the artery. Active tone (%) was calculated as [(Dmax-Drest)/Dmax] × 100, where Dmax is the maximum diameter in Ca2+-free PSS and Drest is the resting diameter at the equilibration pressure. Passive mechanical properties and distensibility of the maximally relaxed arteries in Ca2+-free solution were also evaluated by measuring vessel diameter as intraluminal pressure was increased in 20 mmHg increments from 20 mmHg to 160 mmHg.
Myograph Experiments:
In another series of experiments, second and third-order mesenteric resistance arteries were isolated from Sprague-Dawley rats, cleaned of fat and connective tissue, and mounted on tungsten wires in a wire myograph (Danish Myo Technology, Aarhus, Denmark) to measure active contractile force, as previously described [54]. Passive tension (2 mN) was applied and vessels were equilibrated for 30 minutes in PSS (see above) equilibrated with 95% O2 −5% CO2 at 37 °C. An initial challenge of 10 μM phenylephrine (PE), followed by 10 μM acetylcholine (ACh) was used to assess the condition of the artery. A contraction of less than 6.0 mN or a dilation of less than 25% in response to ACh warranted exclusion.
To assess vascular relaxation, 10 μM PE was added to the vessel chamber to pre-contract the artery. Endothelium-dependent vascular relaxation was assessed by measuring the amount of active contractile force that remained during exposure to increasing concentrations of ACh (10−10 M-10−6 M). Vessel responses to increasing concentrations of SNP (10−9 M-10−6 M) were also determined to assess vascular smooth muscle sensitivity to NO. In other experiments, active contractile force developed during exposure to increasing concentrations of phenylephrine (PE, 10−8 M–10−5 M) or serotonin (5-hydroxytryptamine (5-HT, 10−9 M – 10−6 M) was measured to assess the sensitivity of the arteries to vasoconstrictor agents in the various experimental groups,.
Arteriolar Reactivity in the Hamster Cheek Pouch:
For these experiments, Syrian hamsters were anesthetized with sodium pentobarbital (60 mg/kg, i.p.). The left femoral vein was cannulated to permit i.v. administration of supplemental anesthetic, a femoral artery was cannulated to permit arterial pressure measurements, and the trachea was cannulated to maintain a patent airway. A single-layered cheek pouch was prepared and continuously superfused with PSS warmed to 37°C and equilibrated with a 0% O2-5% CO2-95% N2 mixture to ensure that all O2 delivery occurred via the microcirculation. Diameters of third-order arterioles were measured with a video micrometer, as previously described [41]. Following a 30-minute equilibration period, active vessel tone was verified by assessing arteriolar dilation in response to 100 μM adenosine. Unresponsive vessels were excluded. Concentration-response curves for ACh (10−10 M - 10−5 M) and SNP (10−12 M - 10−5 M) were then determined by measuring arteriolar diameter during topical application of the respective agonists.
MnSOD and Cu/Zn SOD Expression in Rat Mesenteric Resistance Arteries:
The expression of two important antioxidant enzyme proteins [Cu/Zn SOD (SOD1) and MnSOD (SOD2)] that play a crucial role in determining cell redox status and vascular antioxidant defenses was evaluated via Western blotting in small intestinal mesenteric arteries of rats from the various groups. Earlier studies by our laboratory and others [26] have shown that downregulation or reduced activity of these enzymes contributes to vascular oxidative stress that occurs in response to salt-induced suppression of plasma ANG II levels in Sprague-Dawley rats [33] and in Nrf2(−/−) mutant rats [42].
For these studies, mesenteric resistance arteries supplying the small intestine were isolated and homogenized in a buffer containing 20 mM HEPES, 1 mM EDTA, 1 mM Triton × 20, 150 μl/20 ml of protease inhibitor (Sigma Aldrich P8340), and 150 μl/20 ml of phosphatase inhibitor (Sigma Aldrich P0044). Proteins were separated by electrophoresis under reducing conditions on a 4–20% Criterion Tris-Glycine protein gel (BioRad #3450033), transferred to a 0.2 μm nitrocellulose membrane (BioRad #162–0112), and then blocked for 90 min in 5% skim milk in TBS-Tween 20.
Primary antibodies included SOD1 (Cu/Zn SOD) (Enzo Life Sciences ADI-SOD-101, 1:1000), SOD2 (Mn/SOD) (Enzo Life Sciences ADI-SOD-111, 1:8000), and β-actin (Sigma-Aldrich A5441, 1:5,000) with secondary antibodies Goat-anti-rabbit HRP-conjugated IgG, (Santa-Cruz sc2004) (1:6,000 for SOD1, 1: 8,000 for SOD2) and Goat-anti-mouse HRP-conjugated IgG (Santa-Cruz sc 2005) (1:10,000 for β-actin). Primary antibodies were incubated at 4°C overnight and secondary antibodies were applied for 90 minutes at room temperature and then developed with Supersignal West Dura (Thermo Fisher #34075) or Supersignal West Femto (Thermo Fisher #34096). Chemiluminescent signal was read using a ChemiDoc system (BioRad #1708265), and the expression of bands of the target protein for each animal was expressed as % of β-actin loading control.
Mito-Sox Red Fluorescence:
To assess mitochondrial levels of reactive oxygen species (ROS), basilar arteries from individual animals were incubated in warm, aerated HEPES (5 mM)-buffered PSS for one hour. Whole vessels were then incubated in 5 μM MitoSOX- Red (Molecular Probes Invitrogen Detection Technologies) in PSS for 30 minutes, rinsed, and mounted on microscopy slides. Fluorescence (excitation 510 nm and emission 580 nm) was assessed along the length of vessel with a Nikon Eclipse E-80i microscope using MetaVue software (Molecular Devices Inc.). Generally, a vessel spanned 4–5 visual fields at 20× magnification. An average fluorescence value was calculated for each vessel by dividing the sum of fluorescence values for each visual field by the number of visual fields for that vessel.
Microvessel Density Measurements:
Microvessel density was assessed in the cremaster muscle of Sprague-Dawley rats fed HS diet for 2 weeks ± Protandim. Vessels were visualized using fluorescently labeled Griffonia simplicifolia-1 (GS-1) lectin as described previously [16,18,19]. Microvessel density in the tissue was quantified by counting the intersections of vessels with a superimposed 19×14 grid generated using Metamorph Imaging software in 60–80 photographs taken in the cremaster muscles of each animal [17]. In another series of experiments, microvessel density was evaluated via GS-1 lectin labeling in cremaster muscles from Nrf2(−/−) knockout rats fed HS diet with or without Protandim treatment.
Statistical Evaluation:
Data are summarized as mean ± SEM. Differences between treatment groups were assessed using ANOVA or repeated-measures ANOVA with a Newman-Keuls test post hoc, as appropriate. Homogeneity of variance was evaluated with a Cochran’s test and data failing the Cochran’s test were transformed and subsequent analysis performed on the transformed data. No transformation of data was required in the myograph studies, mito-SOX experiments, or SOD protein expression studies. A probability value of P<0.05 was considered to be statistically significant.
RESULTS
Vascular Reactivity:
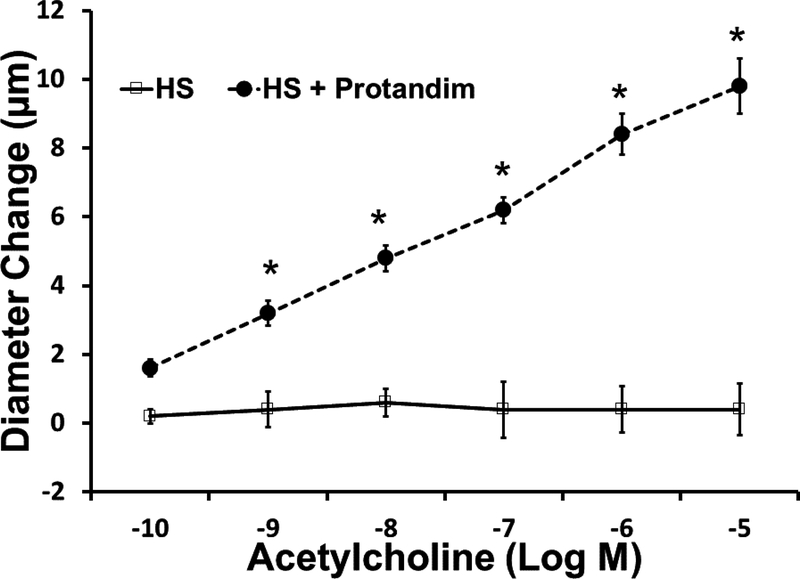
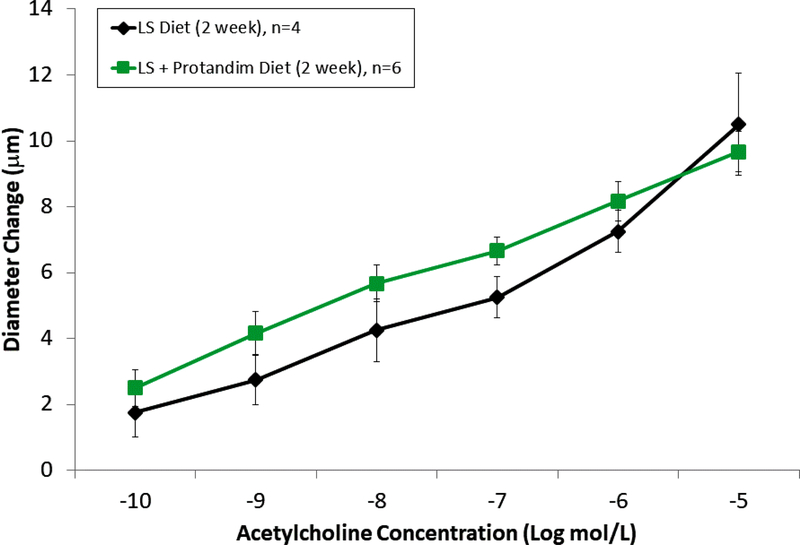
In the rat studies, there were no significant differences in baseline parameters, including weight, blood pressure, and resting vessel diameters between the treatment groups (Table 1). Similar to previous reports [32,42], HS diet eliminated acetylcholine-induced dilation of rat middle cerebral arteries. Supplementation of the HS diet with Protandim prevented the loss of endothelium-dependent relaxation of MCA in response to ACh (FIGURE 1). Incubation with the NOS inhibitor L-NAME (100 μM for 30 minutes) eliminated ACh-dependent relaxation in Protandim-treated rats fed HS diet (Supplement Figure 1), consistent with the hypothesis that NRF2 activation restores NO availability in cerebral arteries of HS-fed animals. However, Protandim treatment had no effect on the magnitude of ACh-induced dilation in MCA from S-D rats fed low salt diet, (Figure 2). MCA from all the experimental groups dilated in response to the endothelium-independent NO-donor SNP (not shown), verifying that vessels of salt-fed animals were still responsive to NO. The passive pressure-diameter relationship in middle cerebral arteries that were maximally dilated with Ca2+ free solution was also similar in S-D rats fed HS diet and LS Diet ± Protandim (Supplement Figure 2), demonstrating that HS diet and/or Protandim treatment had no effect on the passive mechanical properties of the arteries.
Table 1.
Baseline Statistics for Sprague-Dawley Rats ± Protandim Supplementation.
| High Salt (n) | High Salt + Protandim (n) | |
|---|---|---|
| Body Weight(g) | 324.7 ± 3.0 (22) | 319.3 ± 4.5 (27) |
| Mean Arterial Pressure (mmHg) | 105.0 ± 2.9 (9) | 106.5 ± 3.4 (11) |
| MCA Resting Diameter (μm) | 99.4 ± 4.5 (9) | 119.6 ± 8.8 (11) |
| MCA Maximum Diameter (μm) | 235.5 ± 4.9 (8) | 240.4 ± 3.4 (11) |
| MCA Tone (%) | 58.6 ± 1.8 (22) | 50.4 ± 3.3 (11) |
There were no differences in body weight, blood pressure, and the resting diameter, maximum diameter, and percent tone of middle cerebral arteries (MCA) in S-D rats fed HS diet with or without Protandim.
Figure 1:
Responses of middle cerebral arteries from Sprague-Dawley rats to the endothelium-dependent vasodilator acetylcholine in rats fed high salt (HS, 4.0% NaCl; n=5) or high salt with Protandim (60.5 mg/kg/day for 14 days; n=5). * -- P < 0.05 HS + Protandim versus untreated HS-fed controls.
Figure 2.
Effect of Protandim on ACh-induced dilation of middle cerebral arteries of from Sprague-Dawley rats maintained on low salt diet. There were no significant differences in the acetylcholine response in the treated and untreated animals. Data plotted as mean ± SEM for 4 rats fed LS diet and 6 rats fed LS diet + Protandim.
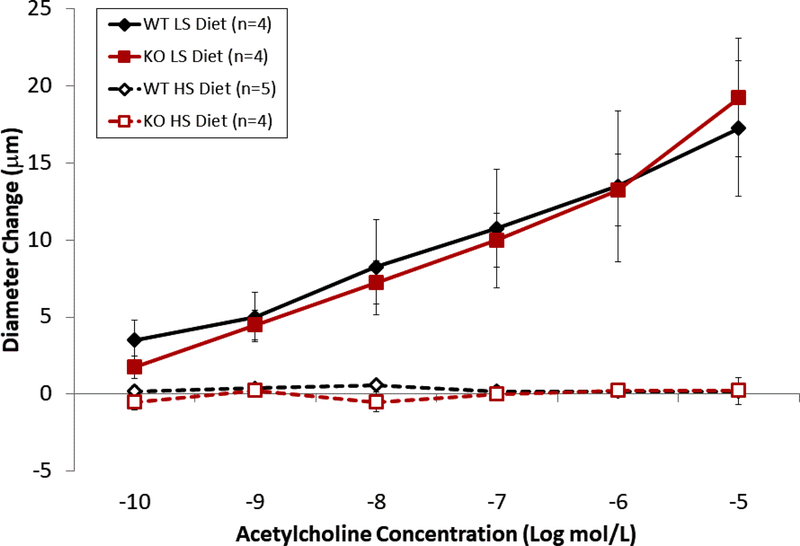
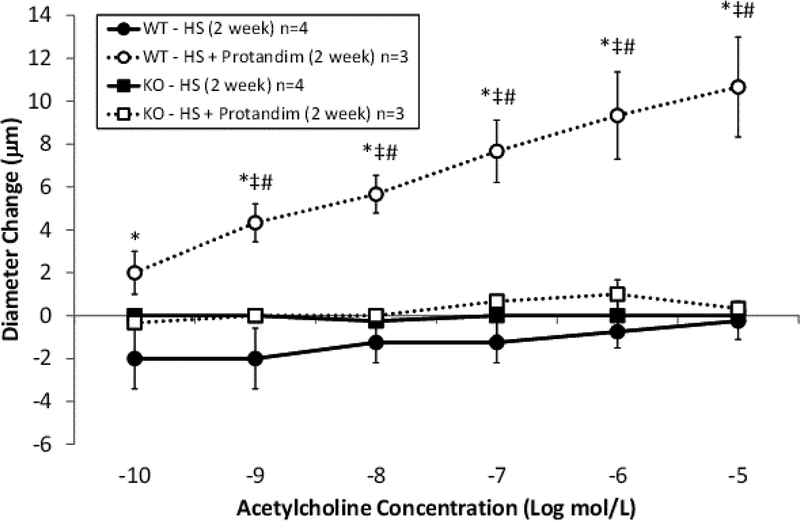
ACh-induced dilation was similar in wild type and Nrf2(−/−) mutant rats fed low salt diet, suggesting that NRF2 does not have a major role in regulating vascular oxidative stress and preserving endothelial function during normal, non-stressed resting conditions. However, high salt diet eliminated ACh-induced dilation in both groups (Figure 3), as previously reported for wild type and Nrf2(−/−) mutant rats [42] and Sprague-Dawley rats fed HS diet. [12,32,33]. Supplementation of the HS diet with Protandim prevented the loss of endothelium-dependent dilation to ACh in the wild type rats, but not in the Nrf2(−/−) knockout rats (Figure 4). The passive pressure-diameter relationships in middle cerebral arteries that were maximally dilated with Ca2+ free solution were similar in wild type and Nrf2(−/−) knockout rats fed LS diet or HS diet (± Protandim) (Supplement Figure 3), indicating that NRF2 activation with Protandim does not affect the passive mechanical properties or distensibility of cerebral arteries of HS-fed wild type or Nrf2(−/−) knockout rats (Supplement Figure 3).
Figure 3.
Effect of Protandim treatment on ACh-induced dilation of MCA from wild type (WT) and Nrf2(−/−) knockout (KO) rats fed low salt (LS) or high salt (HS) diet. Vasodilator responses to acetylcholine were similar in MCA from wild type and knockout rats fed low salt diet, and high salt diet eliminated acetylcholine -induced dilation of MCA in both wild type and Nrf2(−/−) knockout rats. Data plotted as mean ± SEM, n=number of animals.
Figure 4:
Responses of middle cerebral arteries to the endothelium-dependent vasodilator acetylcholine in Nrf2(−/−) knockout (KO) rats and wild type (WT) controls fed high salt (HS, 4.0% NaCl diet; n=4 KO and 4 WT) or high salt with Protandim (60.5 mg/kg/day; n=3 KO and 3 WT) for 14 days. * -- P < 0.05 WT + Protandim vs. WT-HS; ‡--P<0.05 WT + Protandim vs. KO-HS; # P<0.05 WT HS + Protandim vs. KO-HS + Protandim.
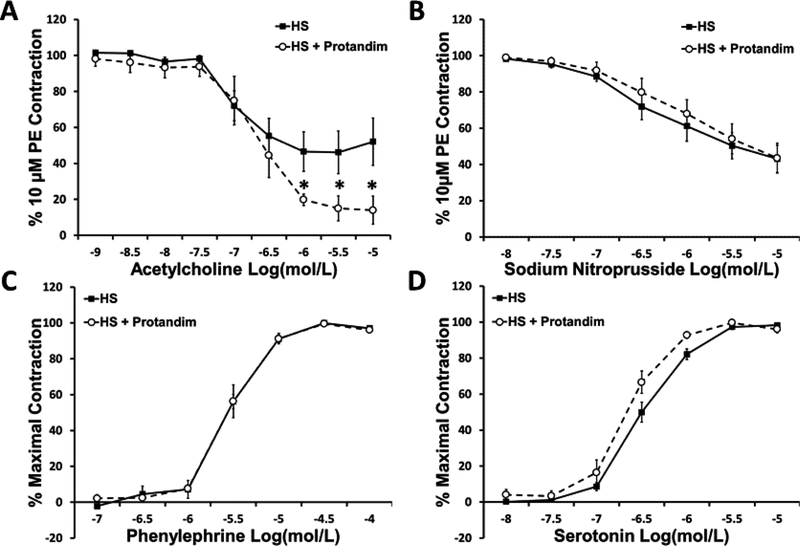
The effects of Protandim treatment on active contractile force in small mesenteric arteries from HS-fed Sprague-Dawley rats are shown in Figure 5. In those experiments, arteries from rats fed HS diet alone relaxed significantly less to acetylcholine than their HS + Protandim counterparts. However, concentration-dependent relaxation in response to SNP was similar at any dose tested in the two groups. Vessel responses to phenylephrine and serotonin were unaffected by supplementation of the HS diet with Protandim.
Figure 5:
Reponses to the endothelium-dependent vasodilator acetylcholine (Panel A), the NO-donor sodium nitroprusside (Panel B), the α1-adrenergic receptor agonist phenylephrine (Panel C), and the vasoconstrictor serotonin (Panel D) in mesenteric resistance arteries from rats fed high salt (HS, 4.0% NaCl) or high salt with Protandim (60.5 mg/kg/day for 14 days). N = 6–7 for all agonists in each treatment group. * -- P < 0.05 HS + Protandim vs. HS-fed controls.
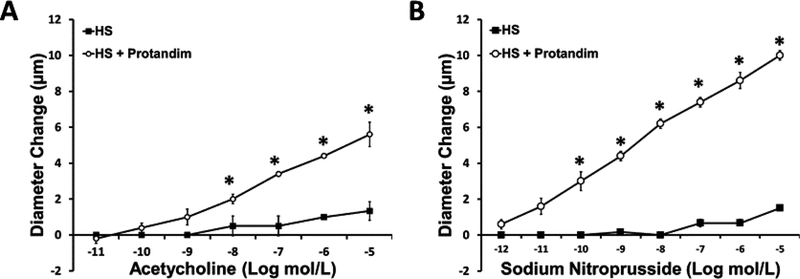
In the hamster studies, there were no significant differences in body weight in animals fed HS alone (129±2.3 g; n=4) vs. HS diet + Protandim (134 ±1.8 g; n=5). There was also no significant difference in resting diameter of arterioles in hamsters fed HS diet alone (20 ±2.5 μm; n=4) vs. animals fed HS diet + Protandim (15 ±1.4 μm; n=5). Similar to our previous report [41], HS diet eliminated arteriolar dilation in response to both ACh and SNP in the in situ hamster cheek pouch, and treatment of the HS-fed hamsters with Protandim prevented the loss of arteriolar dilation in response to ACh and SNP (FIGURE 6).
Figure 6:
Responses to acetylcholine (Panel A) and sodium nitroprusside (Panel B) in cheek pouch arterioles of Syrian hamsters fed high salt (HS, 4.0% NaCl) diet or HS diet with Protandim (60.5 mg/kg/day for 14 days). N = 5 hamsters in each treatment group. * -- P < 0.05 HS + Protandim vs. HS-fed controls.
Effect of High Salt Diet and Protandim on Mitochondrial Levels of Reactive Oxygen Species (ROS):
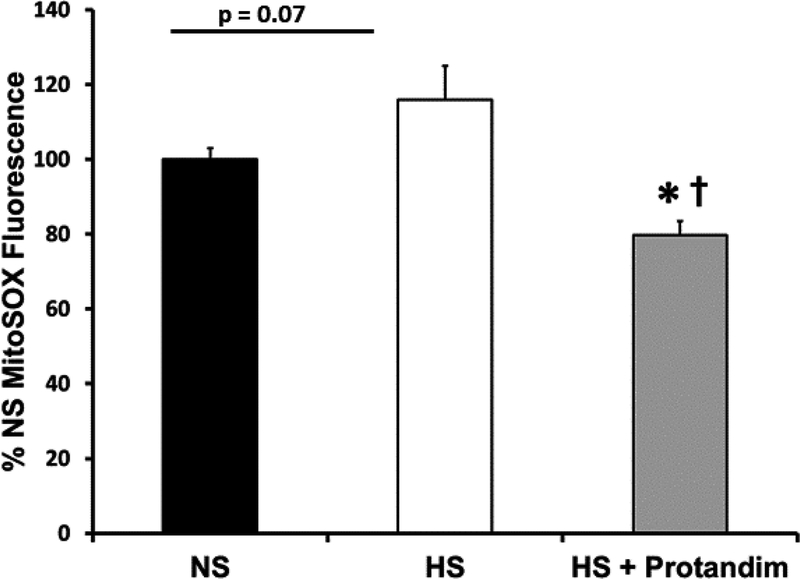
Mitochondria-specific levels of reactive oxygen species in rat basilar arteries, assessed with MitoSOX Red probe, are summarized in Figure 7. Protandim supplementation caused a significant reduction in mitochondrial ROS levels in basilar arteries of HS-fed rats compared to untreated rats fed HS diet.
Figure 7:
Fluorescence of the mitochondria-specific ROS indicator MitoSOX Red® in basilar arteries from Sprague-Dawley rats fed low salt (LS; 0.4% NaCl) diet, high salt (HS; 4.0% NaCl) diet, or HS diet + Protandim (60.5 mg/kg/day for 14 days). Average fluorescence value in arbitrary fluorescence units was calculated from 4–5 visual fields in each artery. Summarized data are normalized to average fluorescence value in arteries from rats fed low salt control diet. * -- P < 0.05 vs. LS vessels; †-- P< 0.05 vs. HS vessels; n= 5–8 animals per treatment group.
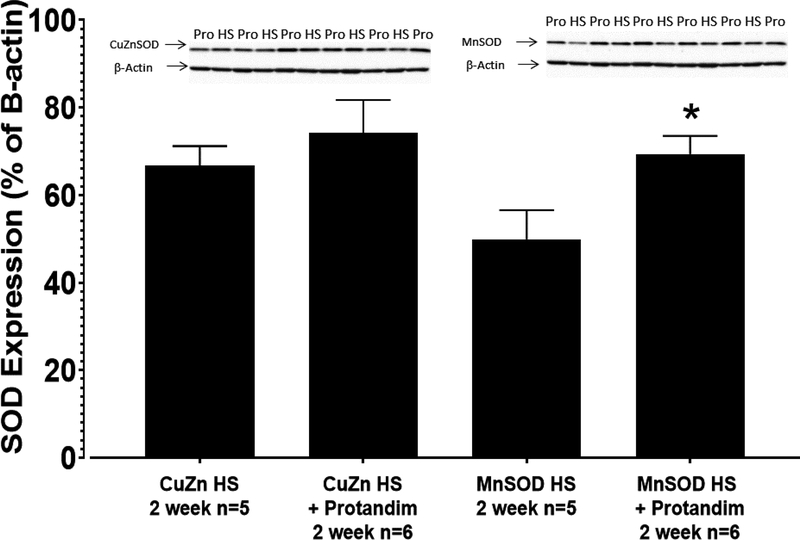
Expression of Mn SOD (SOD2) and Cu/Zn SOD (SOD1) Enzyme Proteins in Mesenteric Resistance Arteries:
Mn SOD protein expression (expressed as a percent of β-actin loading control) was significantly higher in mesenteric resistance arteries of rats fed HS + Protandim (69.4 ± 4.2%; n=5) relative to HS controls (49.9 ± 6.6%; n=5) (FIGURE 8), a finding consistent with the lower mitochondrial ROS levels detected with MitoSox Red (Figure 7). Protandim treatment also tended to upregulate Cu/Zn SOD expression in mesenteric arteries of HS-fed rats (FIGURE 8), although this difference was not significant (66.7 ± 4.5% β-actin in HS, n=5 vs. 74.2 ± 7.5% β-actin in HS + Protandim, n=5).
Figure 8:
Expression of Mn SOD and Cu/Zn SOD protein assessed by Western blot in mesenteric artery homogenates from rats fed HS diet of or HS diet + Protandim for 14 days. Densitometry values for each blot are normalized to β-actin loading control. N = 5 homogenates for each treatment group. * -- P < 0.05 HS + Protandim vs. HS-fed controls.
Effect of Protandim on Microvessel Density of HS-Fed Rats:
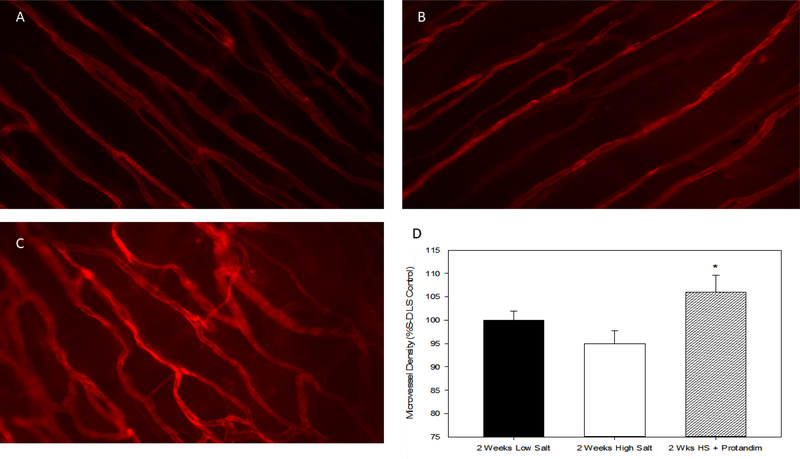
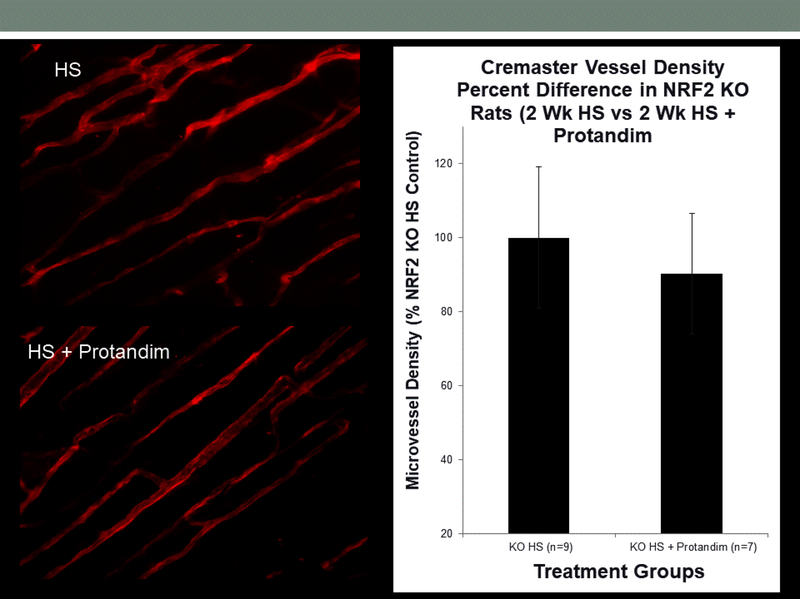
As noted above, previous studies have shown that HS diet leads to a reduced density of microvessels (rarefaction) [16,19] and impaired angiogenic responses to electrical stimulation of skeletal muscle [6,7,19,39]. In the present experiments, microvessel density in the cremaster muscle of HS-fed animals treated with Protandim was significantly higher than that in untreated fed HS diet (Figure 9). However, Protandim treatment failed to increase microvessel density in the cremaster muscle of Nrf2(−/−) knockout rats fed high salt diet. Figure 10.
Figure 9:
A. GS-1 labeled microvessels in cremaster muscles of Sprague-Dawley rats fed low salt (LS) diet (A), high salt diet (B), or HS diet +Protandim for 2 weeks (C). D. Microvessel density expressed as % Low Salt Control (Mean ± SEM) in lectin-labeled cremaster muscles from the different experimental groups. * -- P < 0.05 HS + Protandim vs. HS-fed controls, n=6 animals/group.
Figure 10.
Effect of Protandim on microvessel density (mean ± SEM) in lectin-labeled cremaster muscle of Nrf2(−/−) knockout rats fed high salt diet alone (n=9) or HS diet + Protandim (n=7).
DISCUSSION
The role of the NRF2/Keap1 system (expressed in endothelial cells [3] and in vascular smooth muscle cells [2,36]) in cardiovascular homeostasis is still emerging. A role for NRF2 in regulating endothelium-dependent vasodilation in the human vasculature is suggested by the results of a study by Marczak et al. who reported that the −653G polymorphism within the Nrf2 promoter region is associated with reduced forearm blood flow and increased forearm vascular resistance in healthy African American volunteers [31]. That polymorphism also significantly reduced Nrf2 transcription in vitro [31], suggesting that changes in Nrf2 transcriptional control can have profound effects on vascular function.
The present study shows that administration of the nutritional supplement Protandim® [20,43], a powerful activator of the NRF2 antioxidant defense system, prevents vascular dysfunction resulting from a high salt diet in a variety of vascular beds, including rat cerebral and mesenteric resistance arteries and hamster cheek pouch arterioles. Protandim treatment also increased microvessel density in the cremaster muscle of HS-fed Sprague-Dawley rats, a valuable experimental model of microvascular rarefaction [16,19,40]. Endothelium-dependent dilation to ACh in HS-fed rats treated with Protandim was NO-dependent, as it was eliminated when the vessels were acutely exposed to the eNOS inhibitor L-NAME. Consistent with our hypothesis, Protandim® prevented the loss of ACh-induced dilation in MCA of HS-fed wild type rats, but not in Nrf2(−/−) knockout rats lacking NRF2 in the Sprague-Dawley genetic background [42] (Figure 4).
In contrast to its effect on ACh-induced dilation in rats fed HS diet, Protandim treatment had no effect on endothelium-dependent dilation to ACh in MCA of animals fed LS diet (Figure 2). This is an important observation in view of the potential deleterious effects of NRF2 activation under some conditions. For example, exogenous activation of NRF2 during conditions of substantially elevated oxidative stress can activate the pro-oxidative and pro-apoptotic transcription Klf9 when the high affinity antioxidant response elements in promotor regions of beneficial antioxidant genes normally activated by NRF2 are saturated. [56]. Of particular importance, NRF2 activation in humans under some conditions can have disastrous consequences, as evidenced by the failed BEACON clinical trial of bardoxolone in human patients with type 2 diabetes and stage 4 chronic kidney disease [8]. In the present study, the similarity of ACh-induced dilation of MCA of Protandim-treated and nontreated rats fed low salt diet and the similar ACh-induced dilations of MCA from wild type and Nrf2(−/−) knockout rats fed low salt diet indicate that NRF2 mechanisms in the vasculature are not strongly active during normal physiological conditions, and that activation of NRF2 under those conditions has no deleterious effects.
In both vascular smooth muscle and endothelial cells, proteasome inhibition protects against oxidant stress via NRF2-mediated induction of Cu/Zn SOD and HO-1 [10,29,30,35]. Previous studies from our laboratory have shown that HS diet suppresses both Mn SOD and Cu/Zn SOD expression in cerebral arteries of S-D rats [11]. In the present study, protection from vascular dysfunction afforded by Protandim was accompanied by increased expression of the mitochondrial isoform of superoxide dismutase Mn SOD (SOD2) enzyme protein in small mesenteric arteries and a decrease in mitochondrial ROS levels, as measured with MitoSOX Red probe. Cu/Zn SOD expression also tended to be higher in arteries of HS-fed rats treated with Protandim vs. nontreated controls, but this difference was not significant. Taken together, these findings suggest that the protective effect of Protandim to reduce oxidative stress and preserve NO-mediated vasodilation in those vessels is mediated primarily via its ability to prevent salt-induced downregulation of Mn SOD expression.
The present studies demonstrate a critical role of the transcription factor NRF2 in the protection against salt-induced vascular dysfunction and microvascular rarefaction in HS-fed animals. This study also suggests a role for the mitochondria, a known source of reactive oxygen species, in the pathogenesis of salt-induced vascular dysfunction and the potential benefits of direct activation of NRF2-mediated antioxidant defenses in ameliorating mitochondrial oxidant stress in HS-fed animals. Together, these data support existing studies emphasizing the importance of redox balance in vascular function and the existence of a critical “redox window” necessary for angiogenic responses in the microcirculation. [53]
Nitric oxide bioavailability is critical in maintaining endothelium-dependent vasodilation and is compromised in a variety of cardiovascular pathologies [5,45,52]. In the present study, we found that Protandim-mediated protection against salt-induced endothelial dysfunction (presumably via NRF2 activation) was prevented by inhibiting NOS by L-NAME in isolated MCAs (Supplement Figure 1), indicating that NO bioavailability is improved in Protandim-supplemented animals.
After interrogating both smooth muscle cell and endothelial cell function, we found that the protective effects of the supplement on vascular function in the rat are most apparent in the endothelium, as the sensitivity of mesenteric resistance arteries to smooth muscle vasoconstrictor agonists (phenylephrine and serotonin) and the endothelium-independent NO-donor sodium nitroprusside (SNP) were unchanged by Protandim supplementation. In a similar fashion, MCA from Protandim-treated and untreated rats fed low salt or high salt diet dilated in response to SNP. Overall, these findings suggest that mechanisms of vasodilation downstream of endothelial NO generation are intact in salt-fed rats and are unaffected by Protandim treatment. However, because of potential differences in vascular bed responses to vasoactive stimuli, it will be important to conduct additional studies in the future, in order to verify the absence of any effect of Protandim (or other NRF2 activators) on NO sensitivity in cerebral arteries (versus mesenteric arteries or other vessels).
In contrast to the rat data, Protandim treatment also appears to exert beneficial effects on arteriolar smooth muscle in the hamster cheek pouch because Protandim supplementation prevented the loss of arteriolar sensitivity to SNP in HS-fed animals not receiving Protandim. Earlier studies by our laboratory [41] have shown that hamster arterioles are particularly sensitive to the deleterious effects of HS diet and that the loss of arteriolar sensitivity to SNP in salt-fed hamsters is due to the effects of oxidant stress [41]. In those experiments, SNP-induced dilation of cheek pouch arterioles that was eliminated by HS diet could be restored by superoxide scavenging with PEG-SOD or by low-dose ANG II infusion, which reduced oxidant stress via AT1 receptor-dependent mechanisms [41]. Based on reports in the literature [23,24], it is tempting to speculate that the loss of NO sensitivity in the HS-fed hamsters is due to down-regulation or impaired function of soluble guanylyl cyclase as a result of oxidant stress. Consistent with that hypothesis and based on the antioxidant effects of NRF2 activation in other systems [9,21,22], we believe that the most likely mechanism for the restoration of NO sensitivity by Protandim in the arterioles of HS-fed hamsters is maintenance of soluble guanylyl cyclase function via reduction of vascular oxidative stress.
Angiotensin II (ANG II) plays a critical role in the regulation of microvessel density and in exercise-induced angiogenesis; and previous studies have shown that HS diet leads to a reduction in microvessel density (microvascular rarefaction) in the rat cremaster muscle [16,19]. Restoration of physiological ANG II levels in salt-fed rats via chronic infusion of a low dose of ANG II prevents microvascular rarefaction and rescues angiogenesis in skeletal muscle following electrical stimulation of the muscle [1,6,39,40], and the protective effect of low dose ANG II infusion to prevent salt-induced microvascular rarefaction is lost in Nrf2(−/−) knockout rats [42].
Cultured human endothelial cells in which NRF2 is knocked down by siRNA or in which Keap1 is overexpressed show reduced cellular proliferation and reduced tube formation in vitro, indicating a role for NRF2 in maintaining angiogenesis [50]. Collectively, those findings suggest that the interaction between NRF2 and physiological levels of ANG II in the blood play an important role in maintaining microvessel density, although the mechanisms of this interaction remain unclear.
In the present study, we found that Protandim treatment maintained microvessel density in the cremaster muscle of Sprague-Dawley rats fed HS diet, supporting the hypothesis that oxidant stress plays an important role in salt-induced microvascular rarefaction. In contrast to its ability to prevent microvascular rarefaction in HS-fed S-D rats, Protandim treatment failed to increase microvessel density in the cremaster muscle of Nrf2(−/−) knockout rats fed HS diet. Taken together, these findings suggest that direct activation of NRF2-regulated antioxidant defenses, e.g., with compounds or supplements such as Protandim, resveratrol, bardoxolone, or sulforaphane may be beneficial in relieving microvascular rarefaction and possibly improving collateral function in ischemia [53]. However, it will be especially important to evaluate the effects of NRF2 activation during normal physiological conditions and normal (or elevated) plasma ANG II levels in view of a narrow “redox” window in which both abnormally low levels of ANG II and elevated levels of ANG II can lead to oxidative stress and impaired angiogenic responses and collateral vessel formation [53].
Recent studies indicate that NRF2 regulates hundreds of genes related to redox homeostasis and other physiological processes, and that dysregulation of NRF2-regulated genes provides a logical explanation for direct and indirect connections between oxidant stress and more than 200 human diseases [20]. In this regard, the connection between oxidant stress and nearly every cardiovascular disease suggests that pharmacological or dietary activation of the NRF2 antioxidant defense system may be a prime candidate for novel therapeutic approaches to disease [20].
Thirty years ago, Gey’s “antioxidant hypothesis” linked nutritionally derived plasma antioxidant levels with a reduced incidence of cardiovascular disease through the attenuation of oxidant stress [13–15]. Since then, tremendous resources have been allocated for randomized, double-blind trials investigating the potential for oral antioxidants to reduce cardiovascular disease risk in a variety of clinical populations. Ultimately, these efforts proved to be unsuccessful [37], even as case-control studies reported reduced cardiovascular event risk in populations consuming diets rich in both plant-based antioxidants and chemicals known to activate the NRF2 oxidant defense system, e.g., the Mediterranean diet [47].
Over the years, scores of NRF2-activating chemicals have been identified, many from natural sources, including sulforaphane from cruciferous vegetables [34,46] and resveratrol from grapes [49]. The nutritional supplement Protandim is especially promising as a NRF2 activator because it combines five known NRF2-inducing botanical extracts that act synergistically to induce NRF2 in vivo [38] and in vitro [9,44,51]. The results of the present study are consistent with the hypothesis that direct activation of NRF2-regulated enzymatic antioxidant defenses via dietary approaches may provide a more effective therapeutic approach to treat and prevent cardiovascular disease than supplementation with exogenous antioxidant compounds that act by stoichiometric scavenging mechanisms.
Supplementary Material
Supplement Figure 1. Effect of NOS inhibition with NOS inhibitor L-NAME (100 μM) on ACh-induced dilation of MCA from rats fed HS diet and treated with Protandim. HS and HS + Protandim data are replotted from Figure 1. Data plotted as mean ± SEM for n=4–6 rats.
Supplement Figure 2. Passive pressure-diameter curves for maximally dilated MCA from Sprague-Dawley rats fed low salt diet or high salt diet with or without Protandim treatment. Data plotted as mean diameter (μm) ± SEM for (n) animals.
Supplement Figure 3. Passive pressure-diameter curves for maximally dilated MCA from wild type and Nrf2(−/−) mutant rats fed low salt diet or high salt diet and Nrf2(−/−) mutant rats fed HS diet + Protandim. Data plotted as mean diameter (μm) ± SEM for (n) animals.
Acknowledgements:
The authors extend sincere thanks to Brian Weinberg, Kaleigh Kozak, Nadya Zheleznova Ph.D., and Lynn Dondlinger for their hard work and outstanding technical contributions to this study. Special thanks to Dr. Jacek Zielonka for his valuable advice regarding the conceptual aspects of the study and to Glenn Slocum in the Physiology Department microscopy core lab.
Source of Funding: NIH #R01-HL-65289–12; NIH #R56-HL-65289–13; NIH #R01-HL128242–01; NIH #R21-OD018309–01, and NIH #R21OD024781–01
Footnotes
DISCLOSURES:
JMM previously served as Chief Science Officer and as a member of the Science Advisory Board of LifeVantage Corp., the maker of Protandim®, but currently has no relationship or financial interest in the company.
REFERENCES
- 1.Amaral SL, Papanek PE, Greene AS. Angiotensin II and VEGF are involved in angiogenesis induced by short-term exercise training. Am.J.Physiol (Heart Circ.Physiol.) 281: H1163–H1169, 2001. [DOI] [PubMed] [Google Scholar]
- 2.Anwar AA, Li FY, Leake DS, Ishii T, Mann GE, Siow RC. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic Biol Med 39: 227–236, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Buckley BJ, Marshall ZM, Whorton AR. Nitric oxide stimulates Nrf2 nuclear translocation in vascular endothelium. Biochem Biophys Res Commun 307: 973–979, 2003. [DOI] [PubMed] [Google Scholar]
- 4.Cavka A, Rasic L, Cosic A, Jukic I, Drenjancevic I. Acute salt loading affects vascular function without significant change in body fluid status and body composition in young healthy women. J Hypertens 33 Suppl 1: e101, 2015. [Google Scholar]
- 5.Celermajer DS, Sorensen KE, Spiegelhalter DJ, Georgakopoulos D, Robinson R, Deanfield JE. Aging is associated with endotheial dysfunction in healthy men years before the age-related decline in women. J. Am. Coll. Cardiol 24: 471–476, 1994. [DOI] [PubMed] [Google Scholar]
- 6.de Resende MM, Amaral SL, Moreno C, Greene AS. Congenic strains reveal the effect of the renin gene on skeletal muscle angiogenesis induced by electrical stimulation. Physiol Genomics 33: 33–40, 2008. [DOI] [PubMed] [Google Scholar]
- 7.de Resende MM, Amaral SL, Munzenmaier DH, Greene AS. Role of endothelial cell apoptosis in regulation of skeletal muscle angiogenesis during high and low salt intake. Physiol Genomics 25: 325–335, 2006. [DOI] [PubMed] [Google Scholar]
- 8.deZeewu D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, and Chertow GM. Bardoxolone methyl in type 2 diabetes and stage 4 renal disease. N. Eng. J. Med 369:2492–2503, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Donovan EL, McCord JM, Reuland DJ, Miller BF, Hamilton KL. Phytochemical activation of Nrf2 protects human coronary artery endothelial cells against an oxidative challenge. Oxidative Medicine and Cellular Longevity 2012: 132931, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dreger H, Westphal K, Wilck N, Baumann G, Stangl V, Stangl K, Meiners S. Protection of vascular cells from oxidative stress by proteasome inhibition depends on Nrf2. Cardiovasc Res 85: 395–403, 2010. [DOI] [PubMed] [Google Scholar]
- 11.Durand MJ and Lombard JH. Low dose angiotensin II infusion restores vascular function in cerebral arteries of high salt-fed rats by increasing copper/zinc superoxide dismutase expression. Am. J. Hypertension 26:739–747, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Durand MJ, Raffai G, Weinberg BD, and Lombard JH. Angiotensin (1–7) and low dose angiotensin II infusion reverse salt-induced endothelial dysfunction via different mechanisms in rat middle cerebral arteries. Am. J. Physiol 299:H1024–H1033, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gey KF. On the antioxidant hypothesis with regard to arteriosclerosis. Bibl Nutr Dieta: 53–91, 1986. [DOI] [PubMed] [Google Scholar]
- 14.Gey KF. The antioxidant hypothesis of cardiovascular disease: epidemiology and mechanisms. Biochem Soc Trans 18: 1041–1045, 1990. [DOI] [PubMed] [Google Scholar]
- 15.Gey KF. Optimum plasma levels of antioxidant micronutrients. Ten years of antioxidant hypothesis on arteriosclerosis. Bibl Nutr Dieta: 84–99, 1994. [DOI] [PubMed] [Google Scholar]
- 16.Greene AS, Lombard JH, Cowley AW Jr., Hansen-Smith FM. Microvessel changes in hypertension measured by Griffonia simplicifolia I lectin. Hypertension 15: 779–783, 1990. [DOI] [PubMed] [Google Scholar]
- 17.Greene AS, Tonellato PJ, Zhang Z, Lombard JH, Cowley AW Jr. Effect of microvascular rarefaction on tissue oxygen delivery in hypertension. Am J Physiol (Heart Circ.Physiol.) 262: H1486–1493, 1992. [DOI] [PubMed] [Google Scholar]
- 18.Hansen-Smith FM, Watson L, Lu DY, Goldstein I. Griffonia simplicifolia I: Fluorescent tracer for microcirculatory vessels in nonperfused thin muscles and sectioned muscle. Microvascular Research 36: 199–215, 1988. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez I, Cowley AW Jr., Lombard JH, Greene AS. Salt intake and angiotensin II alter microvessel density in the cremaster muscle of normal rats. Am J Physiol (Heart Circ.Physiol.) 263: H664–667, 1992. [DOI] [PubMed] [Google Scholar]
- 20.Hybertson BM, Gao B, Bose SK, McCord JM. Oxidative stress in health and disease: the therapeutic potential of Nrf2 activation. Molecular Aspects of Medicine 32: 234–246, 2011. [DOI] [PubMed] [Google Scholar]
- 21.Ishii T, Itoh K, Takahashi S, Sato H, Yanagawa T, Katoh Y, Bannai S, Yamamoto M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J Biol Chem 275: 16023–16029, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M,Nabeshima Y. An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236: 313–322, 1997. [DOI] [PubMed] [Google Scholar]
- 23.Kagota S, Tamashiro A, Yamaguchi Y, Nakamura K, Kunitomo M. High salt intake impairs vascular nitric oxide/cyclic guanosine monophosphate system in spontaneously hypertensive rats. J Pharmacol Exp.Ther. 302: 344–351, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Kagota S, Tamashiro A, Yamaguchi Y, Sugiura R, Kuno T, Nakamura K, Kunitomo M. Downregulation of vascular soluble guanylate cyclase induced by high salt intake in spontaneously hypertensive rats. Br.J Pharmacol 134: 737–744, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenda DM, Boegehold MA. Effect of a high-salt diet on oxidant enzyme activity in skeletal muscle microcirculation. Am.J Physiol (Heart Circ.Physiol) 282: H395–H402, 2002. [DOI] [PubMed] [Google Scholar]
- 26.Lenda DM, Boegehold MA. Effect of a high salt diet on microvascular antioxidant enzymes. J Vasc.Res 39: 41–50, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Lenda DM, Sauls BA, and Boegehold MA. Reactive oxygen species may contribute to reduced endothelium-dependent dilation in rats fed high salt. Am. J. Physiol 279:H7–H14, 2000. [DOI] [PubMed] [Google Scholar]
- 28.Lombard JH, Frisbee JC, Greene AS, Hudetz AG, Roman RJ, Tonellato PJ. Microvascular flow and tissue PO2 in skeletal muscle of chronic reduced renal mass hypertensive rats. Am J Physiol (Heart Circ.Physiol) 279: H2295–H2302, 2000. [DOI] [PubMed] [Google Scholar]
- 29.Lorenz M, Wilck N, Meiners S, Ludwig A, Baumann G, Stangl K, Stangl V. Proteasome inhibition prevents experimentally-induced endothelial dysfunction. Life Sci 84: 929–934, 2009. [DOI] [PubMed] [Google Scholar]
- 30.Ludwig A, Fechner M, Wilck N, Meiners S, Grimbo N, Baumann G, Stangl V, Stangl K. Potent anti-inflammatory effects of low-dose proteasome inhibition in the vascular system. J Mol Med (Berl) 87: 793–802, 2009. [DOI] [PubMed] [Google Scholar]
- 31.Marczak ED, Marzec J, Zeldin DC, Kleeberger SR, Brown NJ, Pretorius M, Lee CR. Polymorphisms in the transcription factor NRF2 and forearm vasodilator responses in humans. Pharmacogenetics and Genomics 22: 620–628, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McEwen ST, Balus SF, Durand MJ, Lombard JH. Angiotensin II maintains cerebral vascular relaxation via EGF receptor transactivation and ERK1/2. Am.J Physiol (Heart Circ.Physiol) 297: H1296–H1303, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McEwen ST, Schmidt JR, Somberg L, de la Cruz L, Lombard JH. Time-course and mechanisms of restored vascular relaxation by reduced salt intake and angiotensin II infusion in rats fed a high-salt diet. Microcirculation 16: 220–234, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, Wolf CR, Cavin C,Hayes JD. The Cap’n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer Res 61: 3299–3307, 2001. [PubMed] [Google Scholar]
- 35.Meiners S, Ludwig A, Lorenz M, Dreger H, Baumann G, Stangl V, Stangl K. Nontoxic proteasome inhibition activates a protective antioxidant defense response in endothelial cells. Free Radic Biol Med 40: 2232–2241, 2006. [DOI] [PubMed] [Google Scholar]
- 36.Miller KP, Ramos KS. DNA sequence determinants of nuclear protein binding to the c-Ha-ras antioxidant/electrophile response element in vascular smooth muscle cells: identification of Nrf2 and heat shock protein 90 beta as heterocomplex components. Cell Stress Chaperones 10: 114–125, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, Park BJ. Korean Meta-Analysis Study. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta-analysis of randomised controlled trials. BMJ 346: f10, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nelson SK, Bose SK, Grunwald GK, Myhill P, McCord JM. The induction of human superoxide dismutase and catalase in vivo: a fundamentally new approach to antioxidant therapy. Free Radic Biol Med 40: 341–347, 2006. [DOI] [PubMed] [Google Scholar]
- 39.Petersen MC, Greene AS. Inhibition of angiogenesis by high salt diet is associated with impaired muscle performance following chronic muscle stimulation. Microcirculation 15: 405–416, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Petersen MC, Munzenmaier DH, Greene AS. Angiotensin II infusion restores stimulated angiogenesis in the skeletal muscle of rats on a high-salt diet. Am J Physiol (Heart Circ Physiol) 291: H114–120, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Priestley JR, Buelow MW, McEwen ST, Weinberg BD, Delaney M, Balus SF, Hoeppner C, Dondlinger L, Lombard JH. Reduced angiotensin II levels cause generalized vascular dysfunction via oxidant stress in hamster cheek pouch arterioles. Microvasc Res 89: 134–145, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Priestley JR, Kautenburg KE, Casati MC, Endres BT, Geurts AM, Lombard JH. The NRF2 knockout rat: a new animal model to study endothelial dysfunction, oxidant stress, and microvascular rarefaction. Am J Physiol (Heart Circ Physiol) 310: H478–487, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qureshi MM, McClure WC, Arevalo NL, Rabon RE, Mohr B, Bose SK, McCord JM,Tseng BS. The dietary supplement Protandim decreases plasma osteopontin and improves markers of oxidative stress in muscular dystrophy Mdx mice. Journal of Dietary Supplements 7: 159–178, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reuland DJ, Khademi S, Castle CJ, Irwin DC, McCord JM, Miller BF, Hamilton KL. Upregulation of phase II enzymes through phytochemical activation of Nrf2 protects cardiomyocytes against oxidant stress. Free Radic Biol Med 56: 102–111, 2013. [DOI] [PubMed] [Google Scholar]
- 45.Spieker LE, Hurlimann D, Ruschitzka F, Corti R, Enseleit F, Shaw S, Hayoz D, Deanfield JE, Luscher TF, Noll G. Mental stress induces prolonged endothelial dysfunction via endothelin-A receptors. Circulation 105: 2817–2820, 2002. [DOI] [PubMed] [Google Scholar]
- 46.Thimmulappa RK, Mai KH, Srisuma S, Kensler TW, Yamamoto M, Biswal S. Identification of Nrf2-regulated genes induced by the chemopreventive agent sulforaphane by oligonucleotide microarray. Cancer Res 62: 5196–5203, 2002. [PubMed] [Google Scholar]
- 47.Toledo E, Hu FB, Estruch R, Buil-Cosiales P, Corella D, Salas-Salvado J, Covas MI, Aros F, Gomez-Gracia E, Fiol M, Lapetra J, Serra-Majem L, Pinto X, Lamuela-Raventos RM, Saez G, Bullo M, Ruiz-Gutierrez V, Ros E, Sorli JV, Martinez-Gonzalez MA. Effect of the Mediterranean diet on blood pressure in the PREDIMED trial: results from a randomized controlled trial. BMC Med 11: 207, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tzemos N, Lim PO, Wong S, Struthers AD, MacDonald TM. Adverse cardiovascular effects of acute salt loading in young normotensive individuals. Hypertension 51: 1525–1530, 2008. [DOI] [PubMed] [Google Scholar]
- 49.Ungvari Z, Bagi Z, Feher A, Recchia FA, Sonntag WE, Pearson K, de Cabo R, Csiszar A. Resveratrol confers endothelial protection via activation of the antioxidant transcription factor Nrf2. Am J Physiol (Heart Circ Physiol) 299: H18–24, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valcarcel-Ares MN, Gautam T, Warrington JP, Bailey-Downs L, Sosnowska D, de Cabo R, Losonczy G, Sonntag WE, Ungvari Z, Csiszar A. Disruption of Nrf2 signaling impairs angiogenic capacity of endothelial cells: implications for microvascular aging. The Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 67: 821–829, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Velmurugan K, Alam J, McCord JM, Pugazhenthi. Synergistic induction of heme oxygenase-1 by the components of the antioxidant supplement Protandim. Free Radic Biol Med 46: 430–440, 2009. [DOI] [PubMed] [Google Scholar]
- 52.Widlansky ME, Gokce N, Keaney JF Jr., Vita JA. The clinical implications of endothelial dysfunction. J Am. Coll.Cardiol 42: 1149–1160, 2003. [DOI] [PubMed] [Google Scholar]
- 53.Yun J, Rocic P, Pung YF, Belmadani S, Carrao AC, Ohanyan V, Chilian WM. Redox-dependent mechanisms in coronary collateral growth: the “redox window” hypothesis. Antioxidants & Redox Signaling 11: 1961–1974, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhu J, Huang T, Lombard JH. Effect of high-salt diet on vascular relaxation and oxidative stress in mesenteric resistance arteries Journal of Vascular Research 44: 382–390, 2007. [DOI] [PubMed] [Google Scholar]
- 55.Zhu J, Mori T, Huang T, Lombard JH. Effect of high-salt diet on NO release and superoxide production in rat aorta. Am J Physiol (Heart Circ.Physiol) 286: H575–H583, 2004. [DOI] [PubMed] [Google Scholar]
- 56.Zucker SN, Fink EE, Bagati A, Mannava S, Bianchi-Smiraglia A, Bogner PN, Wawrzyniak JA, Foley C, Leonova KI, Grimm MJ, Moparthy K, Ionov Y, Wang J, Liu S, Sexton S, Kandel ES, Bakin AV, Zhang Y, Kaminski N, Segal BH, Nikiforov MA. Nrf2 amplifies oxidative stress via induction of Klf9. Mol. Cell 53:916–928 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplement Figure 1. Effect of NOS inhibition with NOS inhibitor L-NAME (100 μM) on ACh-induced dilation of MCA from rats fed HS diet and treated with Protandim. HS and HS + Protandim data are replotted from Figure 1. Data plotted as mean ± SEM for n=4–6 rats.
Supplement Figure 2. Passive pressure-diameter curves for maximally dilated MCA from Sprague-Dawley rats fed low salt diet or high salt diet with or without Protandim treatment. Data plotted as mean diameter (μm) ± SEM for (n) animals.
Supplement Figure 3. Passive pressure-diameter curves for maximally dilated MCA from wild type and Nrf2(−/−) mutant rats fed low salt diet or high salt diet and Nrf2(−/−) mutant rats fed HS diet + Protandim. Data plotted as mean diameter (μm) ± SEM for (n) animals.