Abstract
Objective
In this study, in order to investigate the usefulness of intratracheal instillation in assessing the pulmonary toxicity of nanomaterials, intratracheal instillation of nickel oxide‐nanoparticles (NiO‐NP) was performed.
Methods
In this study, rats were administered test materials by intratracheal instillation at five different research institutions in order to assess the validity of using intratracheal instillation for hazard identification of nanomaterials. Eight‐week‐old male SD rats were administered NiO‐NP dispersed in deionized water by a single intratracheal instillation at doses of 0 (vehicle control), 0.2, 0.67, and 2 mg/kg BW. Three days after instillation, histopathological examination of the lungs was performed.
Results
NiO‐NP was distributed in the vicinity of hilus of the lung and in the alveoli around the bronchioles. Histopathological changes such as degeneration/necrosis of macrophages, inflammation, and proliferation of type II pneumocyte in the lung were observed, and their severity corresponded with increasing dose. The histopathological observations of pulmonary toxicity were almost similar at each institution.
Conclusion
The similarity of the histopathological changes observed by five independent groups indicates that intratracheal instillation can be a useful screening method to detect the pulmonary toxicity of nanomaterials.
Keywords: inter‐laboratory comparison, intratracheal instillation, nanomaterial, NiO, pulmonary toxicity
1. INTRODUCTION
Many types of manufactured nanomaterials exist, such as carbon nanotubes and metal oxide nanoparticles, and there is tremendous variation in their physical characteristics. In addition, nanomaterials of the same composition that vary in size, length, and surface structure are being produced. These nanomaterials, even nanomaterials of the same composition but different structure, display different levels of toxicity. 1 Therefore, it is necessary to evaluate the toxicity of each respirable nanomaterial in inhalation exposure studies when there is a risk of human exposure in the workplace or in the environment. However, considering the cost, quantity of test materials, and specialized techniques required for inhalation studies and the low number of available facilities where inhalation studies can be performed, it is not practical to conduct inhalation exposure studies for each nanomaterial. Therefore, we propose using intratracheal instillation to identify nanomaterials that require further testing using inhalation studies. Intratracheal instillation studies with rodents is a simple and inexpensive method of administering test materials to the lung to identify nanomaterials with potential pulmonary toxicity. 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 , 11 , 12 , 13 However, if different research institutions employ different procedures when performing instillation studies, there could be considerable variation in the data obtained from different studies, which would make it difficult to analyze and evaluate the pulmonary toxicity of the tested nanomaterial.
In this study, in order to investigate the usefulness of intratracheal instillation in assessing the pulmonary toxicity of nanomaterials, intratracheal instillation of nickel oxide‐nanoparticles (NiO‐NP) was performed at five different institutions using a standardized procedure.
The histopathological findings of each institution were similar, indicating that when using a standardized protocol, the results of intratracheal instillation is comparable between independent groups, allowing analysis and evaluation of the pulmonary toxicity of the tested nanomaterial.
2. MATERIALS AND METHODS
The test substance used was NiO‐NP (US. 3352, purity 99.98%, Super fine 18 nm; US Research Nanomaterials, Inc). The NiO‐NP was dispersed in deionized water at the National Institute of Advanced Industrial Science and Technology (AIST) as described previously, 4 and the suspension was sent to each participating institution (A, B, C, D, and E).
Seven‐week‐old male Crl:CD (SD) rats were purchased from Charles liver Japan Inc. After a one‐week quarantine and acclimatization period, the rats were divided into four groups of five rats each, and instilled with the test material. Feed (CRF‐1: Oriental East Co. Ltd.) and water were available ad libitum.
Intratracheal instillation was performed at each of the five institutions by a standardized procedure. 9 The test substance suspension was re‐dispersed using a sonicator immediately prior to instillation. Each animal received a single intratracheal instillation of the test substance at a 45°‐90° angle in a supine position under isoflurane anesthesia, using an oral gavage needle (18G) (Institutions A, B, and C) or an aerosolizer (Institutions D and E). Each device was orally inserted into the trachea to a depth of approximately 6 cm from the corner of the mouth. The dosage was 0 (pure water: vehicle control), 0.2, 0.67, or 2 mg/kg BW, and the dose volume was 1 mL/kg BW. The high dose of NiO‐NP was determined from the results of a previous experiment, 4 which clearly induced pulmonary toxicity when administered to rats at a dose of 2 mg/kg BW. The ratio of administered NiO‐NP was set to approximately 3 (0.2, 0.67, and 2) to delineate its dose‐response toxicity.
On the third day after instillation, each animal was euthanized by exsanguination from abdominal aorta under deep isoflurane anesthesia. The left lungs were then injected with 10% neutral buffered formalin at a constant pressure of 20 cm of water for fixation, and H&E stained slides were prepared for histological examination. Severity of histopathological changes were classified into five grades with intensity and extent of the lesion such as —, normal; ±, very slight; +, slight; 2+, moderate; 3+, severe.
The study was conducted with the approval of the Animal Experimentation Committee of each institution in accordance with the 3Rs based on the principle of animal welfare.
3. RESULTS AND DISCUSSION
The histopathological findings in the lungs from the five research institutions are summarized in Table 1. The test NiO‐NP displayed little agglomeration, indicating that it was well dispersed. An unevenness was seen in the distribution of the NiO‐NP, mostly observed in the vicinity of the hilus of the lung and in the alveoli around the bronchiole, and its distribution was somewhat focal. Little test material was observed in the subpleural alveoli. Moreover NiO‐NP was seldom observed in the cranial area of the lung at which was the upper position when NiO‐NP was instilled. NiO‐NP existed intact in the alveolar space or phagocytosed by alveolar macrophages. The number of alveolar macrophages increased with dose. Degeneration and necrosis were occasionally seen in the macrophages that phagocytosed the NiO‐NP (Figures 1, 2), and the occurrence and degree of degeneration and necrosis also increased with dose. These trends were observed in all institutions. Alveolar protein retention was occasionally associated in areas of the lungs where severe necrosis of macrophages was seen. Inflammation induced by the intratracheal instillation of NiO‐NP was observed (Figure 2A, B), and mostly involved neutrophil and eosinophil infiltration (in the alveolar space and the alveolar wall). In addition, proliferation of type II pneumocyte was noted in the alveolar wall around the degenerating and/or necrotic alveolar macrophages (Figure 2B). The intensities of these inflammatory changes also depended on the dose, and were more marked in the alveoli closer to the hilus of the lung, where most of the NiO‐NP was deposited. Some animals showed alveolar hemorrhage and/or alveolar edema in institution D. Thus, the extent of inflammation caused by NiO‐NP is rather limited, and somewhat poorly distributed in the cranial area of the lung. The inflammation caused by NiO‐NP in the present study was the same as reported in the literature, 2 , 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10 and the spread of lesions was similar. Also, the tendency for particles to enter to the peribronchial alveoli of the lung was seen in all five participating institutions in this study, as previously reported by Hasegawa‐Baba et al. 3
TABLE 1.
Histopathological findings of the lung in rats
| Institution (instillation device) | A (oral gavage needle) | B (oral gavage needle) | C (oral gavage needle) | D (aerosolizer) | E (aerosolizer) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Cont | Lo | Mid | Hi | Cont | Lo | Mid | Hi | Cont | Lo | Mid | Hi | Cont | Lo | Mid | Hi | Cont | Lo | Mid | Hi |
| Deposit of particle | ||||||||||||||||||||
| Alveolar macrophage | — | ±:5 | ±:5 | ±:5 | — | — | ±:5 | ±:5 | — | ±:2 | ±:5 | +:5 | — | ±:5 | ±:5 | ±:5 | — | ±:5 | +:5 | +:5 |
| Alveolar space | — | ±:5 | ±:5 | ±:5 | — | — | ±:5 | ±:5 | — | ±:4 | ±:5 | +:5 | — | — | +:4 | +:5 | — | ±:5 | +:5 | +:5 |
| Alveolar macrophage | ||||||||||||||||||||
| Appearance in alveolar space | ±:5 |
±:1 +:4 |
+:4 2+:1 |
2+:5 | ±:4 | ±:5 |
±:1 +:2 2+:2 |
+:3 2+:2 |
±:5 |
±:2 +:3 |
+:4 2+:1 |
+:2 2+:3 |
±:2 +:1 |
+:5 | +:5 |
+:1 2+:4 |
±:4 +:1 |
+:5 | 2+:5 | 2+:5 |
| Degeneration/necrosis | — |
±:4 +:1 |
±:1 +:4 |
+:5 | — | — |
±:2 +:2 |
±:4 +:1 |
— | ±:5 | ±:5 |
+:2 2+:3 |
— |
±:4 +:1 |
±:1 +:3 2+:1 |
+:2 2+:3 |
— | ±:1 | +:5 |
+:4 2+:1 |
| Inflammatory cell infiltration | ||||||||||||||||||||
| Alveolar space/alveolar wall | — | ±:5 |
±:1 +:4 |
+:1 2+:3 3+:1 |
— | — |
+:3 2+:1 |
+:4 2+:1 |
— | — |
±:3 +:1 |
+:1 2+:1 3+:3 |
— |
±:3 +:2 |
+:3 2+:2 |
2+:1 3+:4 |
±:4 |
±:4 +:1 |
±:1 +:2 2+:2 |
3+:5 |
| Peribronchus/perivascular | — | — | ±:3 |
+:3 2+:2 |
— | — |
±:1 +:2 2+:1 |
+:5 | ±:2 | +:1 |
±:1 +:1 |
2+:5 | — |
±:2 +:2 2+:1 |
+:2 2+:3 |
2+:1 3+:4 |
±:3 +:1 |
±:5 |
±:2 +:3 |
+:3 2+:2 |
| Eosinophil: peribronchus/perivascular | ±:3 | ±:4 |
±:4 +:1 |
+:5 | ±:5 | ±:4 |
±:3 +:1 |
±:1 +:3 2+:1 |
±:4 |
±:4 +:1 |
±:1 +:3 |
+:5 | ±:5 | ±:5 |
±:4 +:1 |
±:3 +:2 |
±:2 +:1 |
±:5 |
±:4 +:1 |
±:2 +:2 2+:1 |
| Proliferation: type II pneumocyte | — | — |
±:2 +:1 |
+:3 2+:2 |
— | — | ±:2 |
±:2 +:1 |
— | — | — |
±:2 +:1 2+:2 |
— | ±:1 | +:4 |
+:1 2+:4 |
±:2 | ±:1 |
±:3 +:2 |
+:1 2+:4 |
| Hemorrhage | — | — | — | — | — | — | — | — | — | — | — | — | — | +:1 | — |
+:1 2+:1 |
— | — | — | — |
| Edema | — | — | — | — | — | — | — | — | — | — | — | — | — | — | — | +:1 | — | — | — | — |
Cont: Vehicle control, Lo: 0.2 mg/kg bw, Mid: 0.67 mg/kg bw, Hi: 2 mg/kg bw.
Severity of the histopathological changes: —, normal; ±, very slight; +, slight; 2+, moderate; 3+, severe.
FIGURE 1.
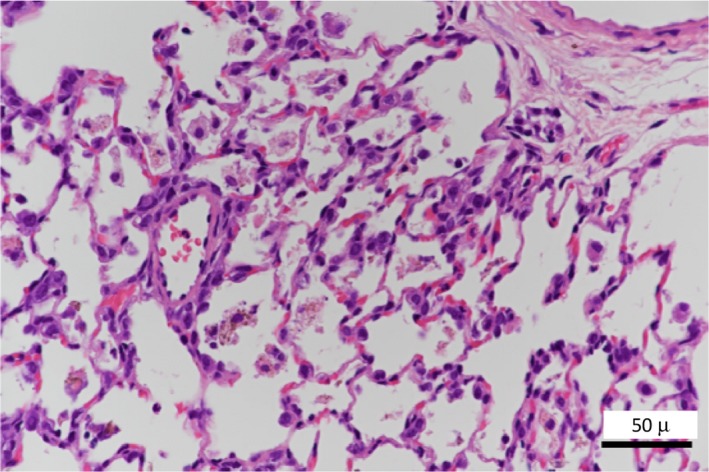
Histopathological findings of the lung. Degeneration/necrosis of increased alveolar macrophages phagocytosed NiO
FIGURE 2.
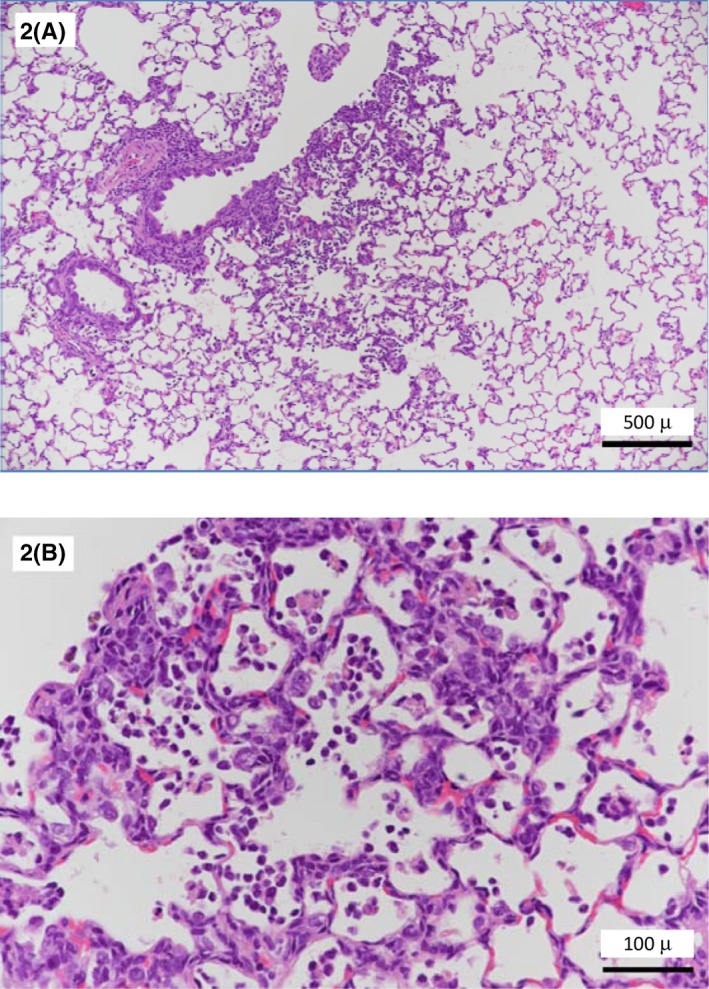
Infiltration of inflammatory cells and alveolar macrophages, and proliferation of typeII pneumocytes in the alveoli around the bronchiole. (2A: Low magnification. 2B: High magnification)
A comparison of the devices used for intratracheal instillation (A‐C: oral gavage needles and D, E: aerosolizer) revealed that the pulmonary lesions tended to be spread over a wider area when using an aerosolizer (Figure 3A, B). However, the lesions were histopathologically the same when administered by these two devices. In previous comparative experiments, there was no difference between aerosolizers and oral gavage needles, 13 and the procedure described in Koybayashi et al, 9 which we followed, indicates that either device can be used. From the results of this experiment, intratracheal instillation using an aerosolizer is slightly better for dispersion in the lungs, but aerosolizers are expensive and can be difficult to obtain. Although there was a slight difference in the severity of lesions between institutions, degeneration/necrosis of alveolar macrophages, inflammatory cell infiltration and proliferation of type II pneumocyte were observed at each institution, and the lesions showed an increase in the area or severity in response to increased dose. The doses at which these toxic effects were observed were ≥0.2 mg/kg in four institutions (A, C, D, and E) and ≥0.67 mg/kg in one (B). In institution B, NiO‐NP was not observed histopathologically in rats administered 0.2 mg/kg.
FIGURE 3.
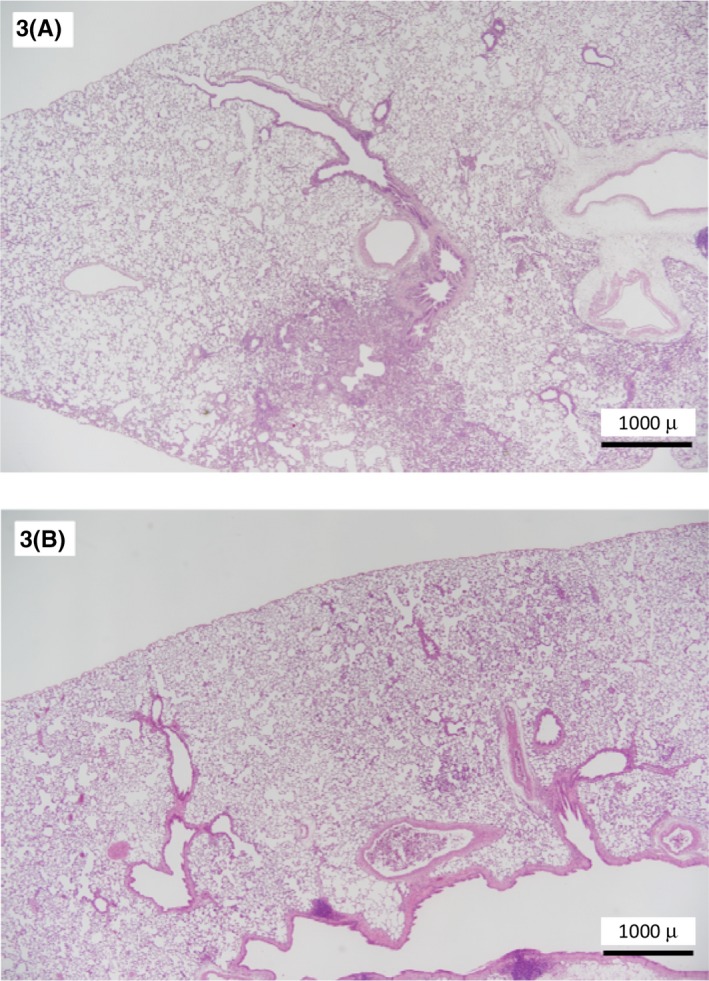
Comparison of the devices used for intratracheal instillation (3A: Oral gavage needles, 3B: Aerosolizer). The pulmonary lesions tend to be spread over a wider area when using aerosolizer
Previously, Morimoto et al 10 reported that the intratracheal instillation method can be useful for screening nanomaterials for hazard identification. In the current study, intratracheal instillation of NiO‐NP was conducted at five institutions in accordance with a standard procedure, and this method was able to detect similar toxic responses. Therefore, to prevent health effects from occupational exposure, intratracheal instillation is a useful and inexpensive method for screening for rat lung toxicity of nanomaterials.
DISCLOSURE
Approval of the research protocol: The study was conducted with the approval of the Animal Experimentation Committee of each institution in accordance with the 3Rs based on the principle of animal welfare. Informed consent: N/A Registry and the registration no. of the study/trial: N/A Animal studies: Rats Studies. Conflict of interest: The authors have no conflict of interest.
ACKNOWLEDGMENTS
This work was supported by the grant “Development of Innovative methodology for Safety Assessment of Industrial Nanomaterials” from the Ministry of Economy, Trade and Industry (METI) of Japan. The authors thank Kenji Kawaguchi (AIST) for the preparation of samples and Dr David Alexander for the valuable editorial advice.
Senoh H, Kano H, Suzuki M, et al. Inter‐laboratory comparison of pulmonary lesions induced by intratracheal instillation of NiO nanoparticle in rats: Histopathological examination results. J Occup Health. 2020;62:e12117 10.1002/1348-9585.12117
REFERENCES
- 1. Sager TM, Kommineni C, Castranova V. Pulmonary response to intratracheal instillation of ultrafine versus fine titanium dioxide: role of particle surface area. Part Fibre Toxicol. 2008;5(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Driscoll KE, Costa DL, Hatch G, et al. Intratracheal instillation as an exposure technique for the evaluation of respiratory tract toxicity: uses and limitations. Toxicol Sci. 2000;55(1):24‐35. [DOI] [PubMed] [Google Scholar]
- 3. Hasegawa‐Baba Y, Kubota H, Takata A, Miyagawa M. Intratracheal instillation methods and the distribution of administered material in the lung of the rat. J Toxicol Pathol. 2014;27:197‐204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Senoh H, Kano H, Suzuki M, et al. Comparison of single or multiple intratracheal administration for pulmonary toxic responses of nickel oxide nanoparticles in rats. J Occup Health. 2017;59(2):112‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Cho WS, Duffin R, Poland CA, et al. Metal oxide nanoparticles induce unique inflammatory footprints in the lung: important implications for nanoparticle testing. Environ Health Perspect. 2010;118:1699‐1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morimoto Y, Ogami A, Todoroki M, et al. Expression of inflammation‐related cytokines following intratracheal instillation of nickel oxide nanoparticles. Nanotoxicol. 2010;4(2):161‐176. [DOI] [PubMed] [Google Scholar]
- 7. Ogami A, Morimoto Y, Myojo T, Oyabu T, Murakami M, Todoroki M. Pathological features of different sizes of nickel oxide following intratracheal instillation in rats. Inhal Toxicol. 2009;21(10):812‐818. [DOI] [PubMed] [Google Scholar]
- 8. Bonner JC, Silva RM, Taylor AJ, et al. Interlaboratory evaluation of rodent pulmonary responses to engineered nanomaterials: the NIEHS Nano GO Consortium. Environ Health Perspect. 2013;121(6):676‐682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kobayashi T, Oshima Y, Tsubokura Y, et al. Standardization of intratracheal instillation study of manufactured nanomaterials In: Takebayashi T, Landsiedel R, Gamo M, eds. In Vivo Inhalation Toxicity Screening Methods for Manufactured Nanomaterials. Singapore: Springer Nature Singapore Pte Ltd.; 2019:107‐122. [Google Scholar]
- 10. Morimoto Y, Izumi H, Yoshimura Y, Fujisawa Y, Fujita K. Significance of intratracheal instillation test for the screening of pulmonary toxicity of nanomaterials. J UOEH. 2017;39(2):123‐132. [DOI] [PubMed] [Google Scholar]
- 11. Cao Z, Fang Y, Lu Y, et al. Exposure to nickel oxide nanoparticles induces pulmonary inflammation through NLRP3 inflammasome activation in rats. Int J Nanomed. 2016;11:3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Morimoto Y, Izumi H, Yoshiura Y, et al. Comparison of pulmonary inflammatory responses following intratracheal instillation and inhalation of nanoparticles. Nanotoxicol. 2016;10(5):607‐661. [DOI] [PubMed] [Google Scholar]
- 13. Kobayashi T, Oshima Y, Tsubokura Y, et al. Effects of dose volume and delivery device on bronchoalveolar lavage parameters of intratracheally administered nano‐sized TiO2 in rats. Regul Toxicol Pharmacol. 2016;81:233‐241. [DOI] [PubMed] [Google Scholar]


