Abstract
Introduction
We studied the association of carotid intima‐media thickness (CIMT) with hippocampal volume (HV) in community dwelling individuals, testing the hypothesis that persons with carotid atherosclerosis progression would have lower HV.
Methods
We studied 1376 Framingham Offspring participants with two carotid ultrasounds and brain magnetic resonance imaging (MRIs). We used multivariable linear regression analyses to relate CIMT progression and HV and total brain volume. Regression models were adjusted for demographics and vascular risk factors, time interval between imaging examinations, and baseline CIMT. We assessed effect modification by hypertension treatment (HRx).
Results
Participants with higher ICA IMT progression had significantly lower HV after adjustment for vascular risk factors and baseline IMT (standardized beta ± standard error: −0.067 ± 0.027, P = .01). We observed weaker association between ICA IMT change and HV among subjects treated for hypertension (β = −0.047, P = .19 vs β = −0.096, P = .026).
Discussion
Cumulative vascular risk factor exposure, reflected by CIMT progression, may increase the risk of neurodegeneration.
Keywords: atherosclerosis, carotid artery, carotid ultrasound, hippocampus, magnetic resonance imaging
1. INTRODUCTION
In the face of increasing prevalence of dementia and lack of effective prevention or treatment strategies, the search for identifying modifiable risk factors and possible treatment strategies has amplified. 1 Traditionally thought to only contribute to vascular dementia, carotid artery disease has increasingly been associated with all‐cause dementia and Alzheimer's disease. 2 , 3 Mechanisms underlying the association between carotid atherosclerosis and dementia include increased arterial stiffness and cerebral hypoperfusion, which may interfere with amyloid β (aβ) clearance from the brain leading to increased deposition. 4 , 5 , 6 , 7 Despite these potential pathophysiologic associations, there has been limited community‐based investigation into the relation of carotid artery disease progression and dementia.
Carotid intima‐media thickness (CIMT), measured on ultrasound (US), is a surrogate marker for subclinical atherosclerosis and a potentially modifiable vascular risk factor. Baseline elevations in CIMT are associated with poor cognitive function, 7 stroke, and dementia. 8 , 9 CIMT progression may be a particularly good marker for cerebrovascular risk as it can identify those who have vascular instability, increased arterial stiffness, 10 progressive atherosclerotic disease, and ongoing poor control of cardiovascular risk factors. 11 Magnetic resonance imaging (MRI) markers of brain aging, including hippocampal volume (HV) loss, are also associated with dementia and cognitive decline, but their relation to CIMT progression is unknown. 12 Characterizing the relation of CIMT progression and HV loss would further elucidate the contribution of carotid vascular disease progression to ongoing neurodegeneration. Based on the pathophysiologic associations described, we hypothesize that individuals with higher CIMT progression will demonstrate lower HV, indicating a link between carotid atherosclerosis and neurodegeneration.
We evaluated the association of the progression of carotid artery disease as measured by changes in CIMT and HV on MRI in the community‐based Framingham Heart Study (FHS) Offspring Cohort. Beyond that, we explored any modifications of the observed associations by cardiovascular risk factor treatments.
RESEARCH IN CONTEXT
Systematic review: The authors reviewed the literature using traditional (eg, PubMed, Google Scholar) sources and meeting presentations. While there were many studies evaluating the link between baseline carotid intima‐media thickness (CIMT) and imaging findings of hippocampal volume (HV), there were not any relevant publications regarding CIMT progression and HV.
Interpretation: Our findings identify an association between CIMT progression and lower HV in a large population‐based cohort, building upon the existing knowledge of an association between baseline CIMT and lower HV.
Future directions: The findings require further validation in additional independent cohorts and in prospective studies to confirm that progression of atherosclerosis may lead to HV loss and that anti‐hypertensive medications may blunt that association.
2. METHODS
2.1. Sample
The FHS Offspring Cohort consists of 5124 participants (2483 men; mean age 37 years) who are the offspring of the original cohort and their spouses and began enrollment in 1971. We included FHS Offspring Cohort participants with available carotid duplex US on two occasions and brain MRI allowing for HV quantification. Carotid duplex US was performed during exams cycle 6 (1991 to 1998) and 8 (2005 to 2008), and brain MRI was obtained close to exam cycle 8. Of the participants who had the two carotid duplex examinations, 1459 participants also had volumetric brain MRI data with hippocampal volumetric measurements. We excluded participants with prevalent stroke, prevalent dementia, or other neurological diagnoses that affected MRI interpretation (n = 83). The final sample included 1376 participants; Figure 1 shows a flow diagram for sample selection. The institutional review board of Boston University Medical Center approved the study protocol, and informed consent was obtained from all participants.
FIGURE 1.
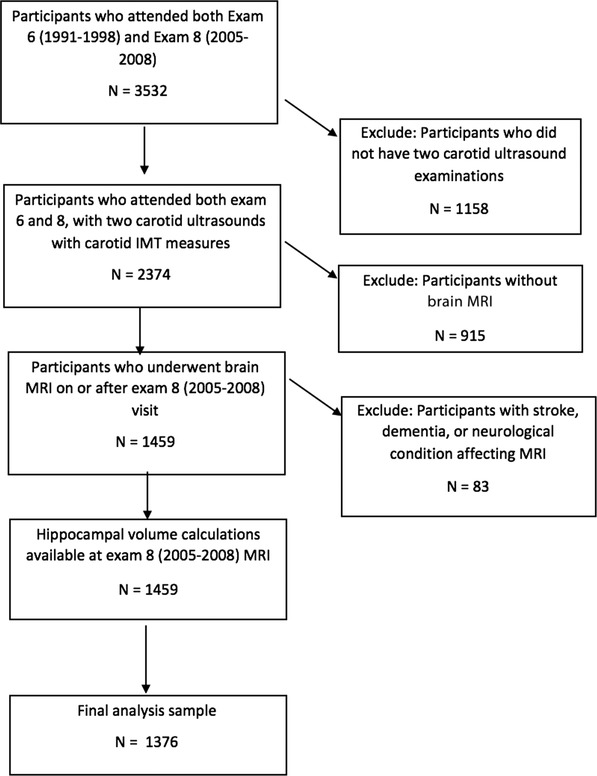
Flow chart of selection of the study sample. IMT, intima‐media thickness; MRI, magnetic resonance imaging
2.2. Carotid ultrasound
Carotid US was acquired by a certified sonographer, following a standard protocol. 13 A high‐resolution linear‐array transducer with color Doppler and Doppler spectral analysis was used (model SSH‐140A; Toshiba America Medical Systems). The common carotid arteries (CCAs) were imaged using a 7.5‐MHz transducer and both the carotid bulb and internal carotid arteries were imaged using a 5‐MHz transducer (‐3‐dB point; 6.2 MHz). Images were gated to an electrocardiogram taken at end‐diastole.
2.3. Carotid intima‐media thickness change
CIMT was measured at three locations: the CCA, the carotid bulb, and the internal carotid artery (ICA) bilaterally. The mean of the maximal IMT measurements of the near and far walls was used. The ICA/bulb IMT was defined as the mean of the four maximal IMT measurements made in the carotid artery bulb and the ICA on both sides for up to 16 wall segments. The carotid US examinations were interpreted by a single lab, side‐by‐side to align the fiduciary markers and ensure that the same site, both longitudinally and circumferentially, was being measured. The examinations were interpreted by two readers blinded to all participant demographic and clinical data. Reproducibility of IMT measurements has been previously reported: Pearson correlation coefficient for repeat readings in 37 participants was 0.94 for the mean IMT of the CCA and 0.76 for the maximum IMT of the ICA. 14 We separately evaluated CCA and ICA IMT progression, because there is evidence that ICA IMT progression is a better correlate to vascular risk factors. 15 , 16 , 17 , 18 Carotid site IMT rate of change (mm/y) was defined as the difference between site‐specific (ie, CCA or ICA) IMT measured in the second US minus same site specific IMT measured in the first carotid US, divided by the time interval between the two studies.
2.4. Brain MRI
The majority of participants were imaged on a 1‐T or 1.5‐T MRI machine (Siemens Magnetom) with the following parameters: T2‐weighted double spin‐echo coronal imaging sequence of 4 mm contiguous slices from nasion to occiput with a repetition time of 2420 ms, echo time (TE) of TE1 20/TE2 90 ms; echo train length 8 ms; field of view 22 cm; and an acquisition matrix of 182 × 256 interpolated to a 256 × 256 with one excitation. MRI data were analyzed using a custom‐designed in‐house image analysis package, QUANTA 2, written for the Linux operating system. All analyses were performed blinded to each participant's demographic and clinical information. 19
2.5. MRI volume measurements
Detailed description of the acquisition and data processing of the MRI examinations has been previously published. 20 , 21 Briefly, the volume analyses were performed using semiautomated measurements of pixel distributions based on mathematical modeling of MRI pixel intensity histograms. This was performed for cerebrospinal fluid (CSF) and brain (both white and gray matter) to determine the optimal pixel intensity threshold to most accurately distinguish cerebral spinal fluid from brain matter.
Brain volume determination was performed manually using coronal MRI sections by outlining the supratentorial intracranial cavity above the tentorium to determine total cranial volume. Then, the calvarium and other non‐brain tissue were excluded from the image, and mathematical modeling was used to determine total brain volume. Total brain volume includes all supratentorial gray and white matter but excludes CSF.
HV was determined manually using coronal MRI sections, resliced to alignment perpendicular to the long axis of the left hippocampal formation. For this study, the hippocampus was defined as fields CA1 to CA4, the dentate gyrus, and the subicular complex. Using 1.5 mm thick reformatted coronal slices, the left hippocampal formation was manually outlined in the anterior to posterior direction using corresponding sagittal and axial reformations for verification. The HV was analyzed as a percent of total cranial volume. Intrarater and interrater reliability for this method of measuring HVs was good, with coefficient of variation of 0.96. 19 , 20 , 21 , 22
2.6. Vascular risk factors
Vascular risk factors were assessed at exam cycle 8, which was the closest exam to the brain MRI. Systolic and diastolic blood pressures were each taken as the average of the Framingham clinic physician's two measurements. Hypertension was defined by the JNC‐7 classification (systolic blood pressure ≥ 140 mmHg and/or diastolic blood pressure ≥ 90 mmHg, or use of antihypertensive medications). Current cigarette smoking was defined as self‐reported use in the year prior to the examination. Diabetes was defined as a fasting glucose ≥126 mg/dL (≥7 mmol/L) or use of insulin or oral hypoglycemic medications. Prevalent cardiovascular disease included coronary heart disease, heart failure, and peripheral arterial disease.
Medication use was assessed by self‐report during interview at the corresponding examination cycle closest to baseline carotid duplex, including antiplatelet agents, anticoagulant therapies, and statin use.
2.7. Statistical analysis
Descriptive statistics are provided for sample characteristics (Table 1). Multivariate linear regression analyses were used to assess the associations between measures of carotid atherosclerosis and site‐specific CIMT change to hippocampal MRI volume. Primary analysis was adjusted for age, sex, and the time interval between CIMT measure and MRI. A second model was performed to additionally adjust for levels of systolic blood pressure, diabetes, current smoking, hypertension, prevalent cardiovascular disease, and total cholesterol levels. Finally, a third model additionally adjusted for baseline CIMT.
TABLE 1.
Characteristics of study partipants
| Clinical characteristics | n = 1376 |
| Women | 732 (53.2) |
| Age at exam 6 (years) | 56.9 (8.8) |
| Age at exam 8 (years) | 66.4 (8.8) |
| Age at MRI examination (years) | 67.0 (8.7) |
| Time between CIMT follow‐up US and MRI (years) | 0.61 (0.88) |
| Exam 8 covariates | |
| Systolic blood pressure (mmHg) | 127.7 (16.9) |
| Diabetes mellitus | 179 (13.1) |
| Current smokers | 108 (7.8) |
| Prevalent cardiovascular disease | 165 (12.0) |
| Hypertension JNC‐7 Stage I or greater | 800 (58.1) |
| Total cholesterol level (mg/dL) | 187.0 (36.4) |
| Hypertension treatment | 683 (49.7) |
| Statin use | 529 (38.4) |
| Carotid ultrasound measures (in mm) | |
| ICA IMT duplex 1 | 1.64 (0.85) |
| ICA IMT duplex 2 (in mm) | 2.25 (1.1) |
| ICA IMT change per year | 0.064 (0.0083) |
| CCA IMT duplex 1 | 0.62 (0.12) |
| CCA IMT duplex 2 | 0.70 (0.16) |
| CCA IMT change per year | 0.008 (0.008) |
| MRI measures (in cm3) | |
| Hippocampal volume | 0.55 (0.049) |
Values are mean (standard deviation) for continuous variables and n (%) for categorical variables.
Prevalent cardiovascular disease includes coronary heart disease, heart failure, and intermittent claudication. Excluded: Participants attending baseline exam (exam 6) with prevalent stroke or dementia.
Abbreviations: CCA, common carotid artery; ICA, internal carotid artery; IMT, intima‐media thickness; JNC‐7, Seventh Report of the Joint National Committee on Prevention, Detection Evaluation, and Treatment of High Blood Pressure.
We, then, explored effect modification in the association between CIMT change and HV by both hypertension treatment use (yes vs no) and statin use (yes vs no). All analyses were done using SAS version 9.4. Statistical significance was set at P < .1 for interaction analyses and P < .05 for remaining analyses.
3. RESULTS
We included a total of 1376 Framingham Offspring participants with mean age of 67 ± 8.7 years at the time of the MRI examination. Females comprised 53% of cohort, and vascular risk factors are described in Table 1. In the ICA, the mean CIMT change per year was 0.064 ± 0.082 mm and in the CCA, the mean CIMT change per year was 0.008 ± 0.008 mm. The mean (standard deviation [SD]) HV (analyzed as a percent of total cranial volume) in our sample was 0.55 cm3 (0.05). After adjustment for age, vascular risk factors, and baseline CIMT, we found that participants with higher ICA CIMT progression had significantly lower HV (standardized beta ± standard error [β ± SE]: −0.067 ± 0.027, P = .01) Table 2. We did not see the same association with higher CCA IMT progression (β ± SE: 0.01 ± 0.0276 P = .72). In addition, after adjustment for age, vascular risk factors, and baseline CIMT, we did not see any association between ICA or CCA CIMT progression and total brain volume (β ± SE: 0.0203 ± 0.029, P = .47 and −0.0244 ± 0.029, P = .39, respectively).
TABLE 2.
Association between progression of carotid atherosclerosis and hippocampal volume
| Combined sample (n = 1345) | No hypertension treatment (N = 679) | Hypertension treatment (N = 665) | P‐value for interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Exposure | Model | B (SE) | P‐value | B (SE) | P‐value | B (SE) | P‐value | P‐value for *interaction | |
| Change in IMT (per SD increment) | CCA | 1 | 0.010 (0.027) | .713 | −0.045 (0.052) | .386 | 0.030 (0.031) | .334 | .259 |
| 2 | 0.010 (0.027) | .695 | −0.051 (0.053) | .336 | 0.030 (0.032) | .348 | .196 | ||
| 3 | 0.010 (0.028) | .719 | −0.040 (0.054) | .458 | 0.024 (0.033) | .466 | .195 | ||
| ICA | 1 | −0.055 (0.026) | .039 | −0.087 (0.042) | .039 | −0.029 (0.034) | .403 | .364 | |
| 2 | −0.057 (0.027) | .033 | −0.091 (0.043) | .033 | −0.035 (0.035) | .322 | .340 | ||
| 3 | −0.067 (0.027) | .014 | −0.096 (0.043) | .026 | −0.047 (0.036) | .187 | .390 | ||
Abbreviations: ICA, internal carotid artery; IMT, intima‐media thickness; CCA, common carotid artery; SD, standard deviation; SE, standard error.
Interaction between change in IMT and hypertension treatment. Bold indicates statistical significance.
In subgroup analyses, we found that there was a stronger association between ICA CIMT change and HV among participants who were not treated for hypertension (β = −0.096, P = .026 vs β = −0.047, P = .19). However, the difference was not statistically significant between hypertension treatment and CCA IMT change (P‐value = .39 for interaction). This association between ICA CIMT change and HV was similar in statin users and non‐users.
4. DISCUSSION
We have found an association of ICA CIMT progression with lower HV. This association was independent of vascular risk factors and baseline CIMT measurements, and was stronger among patients not treated with anti‐hypertensive medications. These findings suggest that the progression of carotid atherosclerosis is associated with lower HVs, indicating that the impact of progressing vascular disease is associated with changes in brain morphology, in particular a measure that indicates ongoing neurodegeneration. Notably, however, the association with lower HVs was attenuated in those being treated with anti‐hypertensive medications.
To our knowledge, this study is the first to relate the progression of CIMT to HVs. Other studies, including studies with the FHS cohorts, have shown that baseline CIMT is associated with lower total brain volumes; 17 , 23 however, other smaller studies found no significant association of baseline CIMT with brain volume and cortical thickness. 24 Others have found no association of CIMT progression to the risk of subsequent cardiovascular events. 25 Additionally, midlife vascular risk factor exposure is associated with lower HVs. 26 Our findings, however, suggest that the progression of carotid atherosclerosis is related to lower HVs. Because lower HVs generally predate the onset of clinical dementia, carotid IMT may represent a potential target for preventative efforts: atherosclerosis. Although we saw a robust association with ICA CIMT progression and lower HV, we did not find a significant association of CCA IMT progression to HVs. This finding is not surprising given the evidence that ICA IMT progression is a better correlate to vascular risk factors, 15 , 16 , 17 , 18 and suggests that measurements at the ICA may be more relevant, partially because the degree of progression is also greater than when measured at the CCA.
Many epidemiologic studies have suggested a link between atherosclerosis and cognitive impairment. CIMT is established as a surrogate marker for subclinical atherosclerosis and has been used in many randomized clinical trials as an endpoint for treatment. 2 , 3 , 4 , 11 Brain atrophy, particularly lower HV, is also associated with cognitive impairment. 27 , 28 , 29 The exact mechanism underlying the association between CIMT progression and lower HV is unclear; however, cerebrovascular disease may mediate the association between atherosclerosis and lower brain volume, possibly because of a combination of relative hypoperfusion secondary to arterial stiffness leading to microvascular injury and early brain capillary damage with blood brain barrier breakdown. 23 , 30 , 31 , 32 , 33 , 34 Further examination of how the progression of atherosclerosis leads to changes in HV is warranted.
Additionally, our study found that anti‐hypertensive use may blunt the association between increasing CIMT change and lower HVs. Hypertension is a known risk factor for poor cognitive function and dementia. 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 Furthermore, randomized controlled trials have shown that treatment with anti‐hypertensives may be protective against dementia. 46 , 47 The findings of our study also support the notion that use of anti‐hypertensive treatment may influence neurodegeneration.
The strengths of our study are the ability to follow a cohort of participants longitudinally to assess for changes in CIMT, the large number of participants investigated with volumetric measures of HV, which allowed for sufficient power to detect associations. An additional strength of our study is that the included participants are relatively younger than other studies, with most in mid‐life, are free of dementia at baseline, and are of generally lower vascular risk than in prior studies. Despite this, there was a robust association between increasing CIMT and lower HV. In addition, a number of vascular risk factors were evaluated that allowed us to determine whether the association between CIMT progression and HV was independent of these possible confounders. Our study has several limitations. Because we evaluated the HV at a single point in time, we are unable to draw definite conclusions on the causal relationship between carotid atherosclerotic progression and changes in HV. An additional limitation is the homogeneity of the sample. The FHS participants are predominantly of white, European descent, so it is unclear to what extent we can generalize these findings to other ethnic and racial groups.
Our results suggest that cumulative vascular risk factor exposure, as reflected by ICA IMT progression, may increase the risk of neurodegeneration represented by hippocampal atrophy on MRI. Such effects occur prior to onset of clinical dementia, suggesting that atherosclerosis, as measured by carotid IMT, may be a target for preventive efforts. Furthermore, anti‐hypertensive medications may blunt the association, supporting more stringent medical management of hypertension. 48 If our results are confirmed in further studies confirming the link between carotid atherosclerosis and lower HVs, our findings could have potential implications for the treatment and prevention of cognitive impairment, including more aggressive medical control of CIMT progression.
CONFLICT OF INTEREST
The authors report no conflicts of interest.
Supporting information
Supporting Information
FUNDING INFORMATION
This work was supported by the Foundation of the American Society of Neuroradiology Scholar Award following NIH grants: AG054076 and NS017950.
Baradaran H, Demissie S, Himali JJ, et al. The progression of carotid atherosclerosis and imaging markers of dementia. Alzheimer's Dement. 2020;6:1–7. 10.1002/trc2.12015
REFERENCES
- 1. International AsD . World Alzheimer Report 2010: The Global Economic Impact of Dementia. London: Alzheimer's Disease International; 2010. [Google Scholar]
- 2. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia. Stroke. 2011;42:2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. van Oijen M, Jan de Jong F, Witteman J, Hofman A, Koudstaal PJ, Breteler M. Atherosclerosis and risk for dementia. Ann Neurol. 2007;61:403‐410. [DOI] [PubMed] [Google Scholar]
- 4. de Groot E, Hovingh GK, Wiegman A, et al. Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation. 2004;109:III33‐III38. [DOI] [PubMed] [Google Scholar]
- 5. Ruitenberg A, den Heijer T, Bakker SL, et al. Cerebral hypoperfusion and clinical onset of dementia: the Rotterdam Study. Ann Neurol. 2005;57:789‐794. [DOI] [PubMed] [Google Scholar]
- 6. Mawuenyega KG, Sigurdson W, Ovod V, et al. Decreased clearance of cns β‐amyloid in Alzheimer's disease. Science. 2010;330:1774‐1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Mathiesen E, Waterloo K, Joakimsen O, Bakke S, Jacobsen E, Bønaa K. Reduced neuropsychological test performance in asymptomatic carotid stenosis: the Tromsø Study. Neurology. 2004;62:695‐701. [DOI] [PubMed] [Google Scholar]
- 8. O'leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK, Jr . Carotid‐artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14‐22. [DOI] [PubMed] [Google Scholar]
- 9. Hofman A, Ott A, Breteler MM, et al. Atherosclerosis, apolipoprotein E, and prevalence of dementia and Alzheimer's disease in the Rotterdam Study. Lancet. 1997;349:151‐154. [DOI] [PubMed] [Google Scholar]
- 10. van Popele NM, Grobbee DE, Bots ML, et al. Association between arterial stiffness and atherosclerosis: the Rotterdam Study. Stroke. 2001;32:454‐460. [DOI] [PubMed] [Google Scholar]
- 11. Crouse JR, Raichlen JS, Riley WA, et al. Effect of rosuvastatin on progression of carotid intima‐media thickness in low‐risk individuals with subclinical atherosclerosis: the METEOR Trial. JAMA. 2007;297:1344‐1353. [DOI] [PubMed] [Google Scholar]
- 12. Erten‐Lyons D, Dodge HH, Woltjer R, et al. Neuropathological basis of age‐associated brain atrophy. JAMA Neurol. 2013;70:616‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Polak JF, O'Leary DH, Kronmal RA, et al. Sonographic evaluation of carotid artery atherosclerosis in the elderly: relationship of disease severity to stroke and transient ischemic attack. Radiology. 1993;188:363‐370. [DOI] [PubMed] [Google Scholar]
- 14. Polak JF, Pencina MJ, Pencina KM, O'donnell CJ, Wolf PA, D'Agostino RB. Carotid‐wall intima–media thickness and cardiovascular events. N Engl J Med. 2011;365:213‐221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heiss G, Sharrett AR, Barnes R, et al. Carotid atherosclerosis measured by B‐mode ultrasound in populations: associations with cardiovascular risk factors in the ARIC study. Am J Epidemiol. 1991;134:250‐256. [DOI] [PubMed] [Google Scholar]
- 16. Mackinnon AD, Jerrard‐Dunne P, Sitzer M, Buehler A, von Kegler S, Markus HS. Rates and determinants of site‐specific progression of carotid artery intima‐media thickness. Stroke. 2004;35:2150‐2154. [DOI] [PubMed] [Google Scholar]
- 17. Romero JR, Beiser A, Seshadri S, et al. Carotid artery atherosclerosis, MRI indices of brain ischemia, aging, and cognitive impairment. Stroke. 2009;40:1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rubba P, Panico S, Bond MG, et al. Site‐specific atherosclerotic plaques in the carotid arteries of middle‐aged women from southern Italy: associations with traditional risk factors and oxidation markers. Stroke. 2001;32:1953‐1959. [DOI] [PubMed] [Google Scholar]
- 19. DeCarli C, Massaro J, Harvey D, et al. Measures of brain morphology and infarction in the Framingham Heart Study: establishing what is normal. Neurobiol Aging. 2005;26:491‐510. [DOI] [PubMed] [Google Scholar]
- 20. DeCarli C, Maisog J, Murphy DG, Teichberg D, Rapoport SI, Horwitz B. Method for quantification of brain, ventricular, and subarachnoid CSF volumes from MR images. J Comput Assist Tomogr. 1992;16:274‐284. [DOI] [PubMed] [Google Scholar]
- 21. Seshadri S, Wolf P, Beiser A, et al. Stroke risk profile, brain volume, and cognitive function the framingham offspring study. Neurology. 2004;63:1591‐1599. [DOI] [PubMed] [Google Scholar]
- 22. Jeerakathil T, Wolf PA, Beiser A, et al. Stroke risk profile predicts white matter hyperintensity volume. Stroke. 2004;35:1857. [DOI] [PubMed] [Google Scholar]
- 23. Muller M, van der Graaf Y, Algra A, Hendrikse J, Mali WP, Geerlings MI. Carotid atherosclerosis and progression of brain atrophy: the SMART‐MR study. Ann Neurol. 2011;70:237‐244. [DOI] [PubMed] [Google Scholar]
- 24. Cardenas VA, Reed B, Chao LL, et al. Associations among vascular risk factors, carotid atherosclerosis, and cortical volume and thickness in older adults. Stroke. 2012;43:2865‐2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lorenz MW, Polak JF, Kavousi M, et al. Carotid intima‐media thickness progression to predict cardiovascular events in the general population (the PROG‐IMT collaborative project): a meta‐analysis of individual participant data. Lancet. 2012;379:2053‐2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Debette S, Seshadri S, Beiser A, et al. Midlife vascular risk factor exposure accelerates structural brain aging and cognitive decline. Neurology. 2011;77:461‐468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chao L, Mueller S, Buckley S, et al. Evidence of neurodegeneration in brains of older adults who do not yet fulfill MCI criteria. Neurobiol Aging. 2010;31:368‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jack CR, Petersen R, Xu Y, et al. Rates of hippocampal atrophy correlate with change in clinical status in aging and AD. Neurology. 2000;55:484‐490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Apostolova LG, Mosconi L, Thompson PM, et al. Subregional hippocampal atrophy predicts Alzheimer's dementia in the cognitively normal. Neurobiol Aging. 2010;31:1077‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. de la Torre JC. Is Alzheimer's disease a neurodegenerative or a vascular disorder? Data, dogma, and dialectics. Lancet Neurol. 2004;3:184‐190. [DOI] [PubMed] [Google Scholar]
- 31. Scuteri A, Brancati AM, Gianni W, Assisi A, Volpe M. Arterial stiffness is an independent risk factor for cognitive impairment in the elderly: a pilot study. J Hypertens. 2005;23:1211‐1216. [DOI] [PubMed] [Google Scholar]
- 32. Nation DA, Sweeney MD, Montagne A, et al. Blood–brain barrier breakdown is an early biomarker of human cognitive dysfunction. Nat Med. 2019;25:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ivanidze J, Mackay M, Hoang A, et al. Dynamic contrast‐enhanced MRI reveals unique blood‐brain barrier permeability characteristics in the hippocampus in the normal brain. AJNR Am J Neuroradiol. 2019;40:408‐411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. De la Torre JC. Critically attained threshold of cerebral hypoperfusion: the catch hypothesis of Alzheimer's pathogenesis. Neurobiol Aging. 2000;21:331‐342. [DOI] [PubMed] [Google Scholar]
- 35. Lande MB, Kaczorowski JM, Auinger P, Schwartz GJ, Weitzman M. Elevated blood pressure and decreased cognitive function among school‐age children and adolescents in the United States. J Pediatr. 2003;143:720‐724. [DOI] [PubMed] [Google Scholar]
- 36. Power MC, Tchetgen EJT, Sparrow D, Schwartz J, Weisskopf MG. Blood pressure and cognition: factors that may account for their inconsistent association. Epidemiology. 2013;24:886‐893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Swan GE, DeCarli C, Miller B, et al. Association of midlife blood pressure to late‐life cognitive decline and brain morphology. Neurology. 1998;51:986‐993. [DOI] [PubMed] [Google Scholar]
- 38. Singh‐Manoux A, Marmot M. High blood pressure was associated with cognitive function in middle‐age in the Whitehall II study. J Clin Epidemiol. 2005;58:1308‐1315. [DOI] [PubMed] [Google Scholar]
- 39. Launer LJ, Masaki K, Petrovitch H, Foley D, Havlik RJ. The association between midlife blood pressure levels and late‐life cognitive function. The Honolulu‐Asia Aging Study. JAMA. 1995;274:1846‐1851. [PubMed] [Google Scholar]
- 40. Wolf PA, Beiser A, Elias MF, Au R, Vasan RS, Seshadri S. Relation of obesity to cognitive function: importance of central obesity and synergistic influence of concomitant hypertension. The Framingham Heart Study. Curr Alzheimer Res. 2007;4:111‐116. [DOI] [PubMed] [Google Scholar]
- 41. Tzourio C, Dufouil C, Ducimetière P, Alpérovitch A. Cognitive decline in individuals with high blood pressure: a longitudinal study in the elderly. EVA Study Group. Epidemiology of Vascular Aging. Neurology. 1999;53:1948‐1948. [DOI] [PubMed] [Google Scholar]
- 42. Knopman D, Boland L, Mosley T, et al. Cardiovascular risk factors and cognitive decline in middle‐aged adults. Neurology. 2001;56:42‐48. [DOI] [PubMed] [Google Scholar]
- 43. Knopman DS, Mosley TH, Catellier DJ, Coker LH; Atherosclerosis Risk in Communities Study Brain MRI Study . Fourteen‐year longitudinal study of vascular risk factors, APOE genotype, and cognition: the ARIC MRI study. Alzheimers Dement. 2009;5:207‐214. [DOI] [PubMed] [Google Scholar]
- 44. Gottesman RF, Schneider AL, Albert M, et al. Midlife hypertension and 20‐year cognitive change: the atherosclerosis risk in communities neurocognitive study. JAMA Neurol. 2014;71:1218‐1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kilander L, Nyman H, Boberg M, Hansson L, Lithell H. Hypertension is related to cognitive impairment: a 20‐year follow‐up of 999 men. Hypertension. 1998;31:780‐786. [DOI] [PubMed] [Google Scholar]
- 46. Forette F, Seux M‐L, Staessen JA, et al. The prevention of dementia with antihypertensive treatment: new evidence from the systolic hypertension in Europe (Syst‐Eur) study. JAMA Intern Med. 2002;162:2046‐2052. [DOI] [PubMed] [Google Scholar]
- 47. Forette F, Seux M‐L, Staessen JA, et al. Prevention of dementia in randomised double‐blind placebo‐controlled systolic hypertension in Europe (Syst‐Eur) trial. Lancet. 1998;352:1347‐1351. [DOI] [PubMed] [Google Scholar]
- 48. SPRINT Research Group , Wright JT, Jr , Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood‐pressure control. N Engl J Med. 2015;373:2103‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


