Abstract
Background
Cell‐free microRNAs (miRs) have emerged as early and sensitive biomarkers for tissue injury and function. This study aimed to investigate whether the release of hepatocyte‐derived microRNAs (HDmiRs) and cholangiocyte‐derived miRs (CDmiRs) correlates with hepato‐cholangiocellular injury and function during oxygenated, normothermic machine perfusion (NMP) of human liver grafts.
Methods
Donor livers (n = 12), declined for transplantation, were subjected to oxygenated NMP (6 hours) after a period of static cold storage (median 544 minutes (IQR 421‐674)). Perfusate and bile samples were analyzed by qRT‐PCR for HDmiR‐122 and CDmiR‐222. Spearman correlations were performed between miR levels and currently available indicators and classic markers.
Results
Both HDmiR‐122 and CDmiR‐222 levels in perfusate at 30 minutes of NMP strongly correlated with hepatocyte injury (peak perfusate AST) and cholangiocyte injury (peak biliary LDH). In bile, only CDmiR‐222 correlated with these injury markers. For hepato‐cholangiocellular function, both miRs in perfusate correlated with total bilirubin, while HDmiR‐122 (in perfusate) and CDmiR‐222 (in bile) correlated with bicarbonate secretion. Both the relative ratio of HDmiR‐122/CDmiR‐222 and AST in perfusate at 30 minutes significantly correlated with cumulative bile production, but only the relative ratio was predictive of histopathological injury after 6 hours NMP.
Conclusion
Early levels of HDmiR‐122 and CDmiR‐222, in perfusate and/or bile, are predictive of excretory functions and hepato‐cholangiocellular injury after 6 hours NMP. These miRs may represent new biomarkers for graft viability and function during machine perfusion.
Keywords: biomarker, cholangiocyte‐derived microRNA, hepatocyte‐derived microRNA, miR‐122, miR‐222, transplantation
1. INTRODUCTION
To address the global shortage of donor livers, the use of “extended criteria” donor livers, which include steatotic, elderly, and donation after circulatory death (DCD) livers, has substantially increased.1, 2 Organs from these donors typically are more susceptible to ischemic damage, resulting in an increased incidence of primary non‐function, early allograft dysfunction (EAD), and biliary complications such as non‐anastomotic strictures (NAS).3, 4, 5
Normothermic machine perfusion (NMP) is a technique whereby donor livers are perfused ex situ. At 37°C, NMP renders the organ metabolically active, offering a window for assessing the viability of the graft. Several research groups have proposed putative markers of organ viability in this context, but their utility in predicting outcome thus far is limited.6, 7, 8 The lack of reliable, objective measurements to determine organ quality prior to transplantation limits clinicians in predicting graft performance following transplantation and possibly improving graft function during machine perfusion.
In the field of biomarker discovery, small non‐coding RNAs, in particular microRNAs (miRs), have emerged as sensitive, specific and stable markers for cell function, cell stress and cell injury. MiRs are important regulators of post‐transcriptional gene expression and as such control many cellular processes. They can down‐regulate certain processes by preventing translation of messenger RNA (mRNA) into functional proteins whereas others upregulate other cellular functions.9 Several studies have recently investigated the release of miRs in relation to cellular injury resulting from liver transplantation.10, 11, 12, 13 It was shown that not only levels of released miRs differ between damaged and normal functioning hepatocytes and cholangiocytes but also that, during impaired excretory function and injury of hepatocytes, the liver shows polarized release of extracellular hepatocyte‐derived miRs (HDmiRs) and cholangiocyte‐derived miRs (CDmiRs) into both bile and serum, suggesting active rather than passive underlying release mechanisms.14
The aim of the current study was to assess whether miRs in the perfusate and bile of normothermically perfused liver grafts correlate with, and are predictive of, hepatocellular and cholangiocellular injury and liver function as measured by currently available indicators and classic markers. Furthermore, the levels of miRs on different time points during NMP were investigated and correlated with the aforementioned markers.
2. MATERIALS AND METHODS
2.1. Donor livers
Between August 2012 and August 2014, twelve consecutive human donor livers, declined for transplantation, were included in this study after informed consent had been obtained. Following a period of static cold storage (SCS) (544 minutes (421‐674)), livers underwent 6 hours of NMP for viability assessment. This cohort has been previously described in the study by Westerkamp et al 2016 (“SCS‐only” group).15
The study protocol was approved by the medical ethical committee of the University Medical Center Groningen (UMCG) (METC 2012.068) and the Dutch Transplantation Foundation (NTS).
2.2. Machine perfusion
Upon arrival at the UMCG, NMP was performed using the Liver Assist (Organ Assist, Groningen, the Netherlands) with a perfusion solution based on packed red blood cells and fresh frozen plasma as described previously.15 Bile and perfusion fluid samples were obtained at baseline prior to connecting the liver and at 30 minutes intervals, and essentially deprived of cells by centrifugation for 5 minutes at 2700 rpm. Supernatants were subsequently stored at −80°C until further use.
2.3. Assessment of hepatobiliary function and injury
Lactate, glucose, bicarbonate, and total bilirubin were measured using an ABL800 FLEX analyzer (Radiometer, Brønhøj, Denmark). Aspartate aminotransferase (AST) and lactate dehydrogenase (LDH) were measured using routine biochemical methods. Cumulative bile production, measured gravimetrically, was expressed as mL bile/kg liver.
2.4. RNA isolation
Fifty µl of essentially cell‐free bile or cell‐free perfusion fluid was supplemented with 150 µL PBS, and total RNA was extracted using the Qiagen miRNeasy kit (Qiagen, Venlo, the Netherlands) essentially as described previously.12, 14 Samples were spiked with 200 amol of artificial Caenorhabditis elegans miR‐39 (Cel‐miR‐39, Sigma Aldrich, Zwijndrecht, the Netherlands) during the lysis procedure to monitor loss during workup. RNA was eluted using 50 µL of nuclease‐free water and stored at −80°C until further use.
2.5. Reverse transcription and real‐time polymerase chain reaction (qRT‐PCR)
Complement DNA (cDNA) synthesis was performed using the Taqman microRNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA, USA) as suggested by the manufacturer with minor modifications as described previously.16 To eliminate qRT‐PCR inhibition by heparin, the RT reactions were co‐incubated with 6 IU heparinase (New England Biolabs).17 One hundred amol of synthetic Cel‐miR‐54 was added to monitor the presence of PCR inhibiting components that co‐eluted with total RNA, and/or incomplete heparin degradation. cDNA samples were diluted to 100 μL with nuclease‐free water and stored at −20°C until further use. PCR reactions were performed in duplicate essentially as described by the manufacturer with the following modifications: Six μL Taqman 2× Universal PCR Master Mix was mixed with 0.5 μL microRNA‐specific PCR primer (Applied Biosystems), 0.5 μL nuclease‐free water, and 5.0 μL of the diluted cDNA. The mature sequences of the miRNAs analyzed, both endogenous and synthetic, are summarized in Table 1.
Table 1.
Mature microRNA sequences and assays
| microRNA | Mature sequence | Assay ID |
|---|---|---|
| hsa‐miR‐122 | UGGAGUGUGACAAUGGUGUUUG | 002245 |
| hsa‐miR‐222 | AGCUACAUCUGGCUACUGGGU | 002276 |
| cel‐miR‐39 | UCACCGGGUGUAAAUCAGCUUG | 000200 |
| cel‐miR‐54 | UACCCGUAAUCUUCAUAAUCCGAG | 001361 |
2.6. Histological analysis
Liver biopsies were collected before and after 6 hours of NMP and fixed in formalin. Liver parenchymal injury (LPI) was determined on paraffin‐embedded and H&E stained sections by two independent investigators using the semi‐quantitative Suzuki injury score as described previously.18
2.7. Statistical analysis
Levels of endogenous miRs in perfusate and bile, represented as relative values (2‐Cq), were normalized using the relative values of the spiked‐in synthetic Cel‐miR‐39 as described by Vandesompele et al with the highest relative value set to 1.19 Continuous variables were presented as median with interquartile rage (IQR). In order to determine the predictive value of both miRs, relative miR levels in perfusate at 30 minutes and in bile at 2 hours were correlated with hepato‐cholangiocellular parameters using the Spearman correlations test. Suzuki scores for LPI before and after NMP were compared using a paired t test. The level of significance was set at P < .05. All statistical analyses were performed using SPSS software version 22.0 for Windows (SPSS, Inc) and GraphPad Prism 5.0 (GraphPad Software).
3. RESULTS
3.1. Hepato‐cholangiocellular injury and function parameters during NMP
An overview of donor characteristics has previously been published by Westerkamp et al (“SCS‐only” group) and is provided in Table 2.15 Supplemental figure S1 shows the levels of hepatocellular injury and function parameters during 6 hours of NMP. AST in perfusate and LDH in bile reflect hepatocyte and cholangiocyte injury, respectively. Lactate in perfusate, bilirubin in bile, and (cumulative) bile production reflect hepatocyte function during NMP. Bicarbonate in bile and the bile glucose/perfusate glucose ratio are indicators of cholangiocyte secretory function and cholangiocyte resorptive function, respectively.20
Table 2.
Donor characteristics
| n = 12 | |
|---|---|
| Age (y) | 61 (52‐64) |
| Gender | |
| Male | 8 (67%) |
| Female | 4 (33%) |
| Length (m) | 1.77 (1.67‐1.80) |
| Weight (kg) | 88 (76‐100) |
| Body mass index (kg/m2) | 27.3 (24.5‐36.0) |
| Type of donor liver | |
| DCD | 9 (75%) |
| DBD | 3 (25%) |
| Cause of death | |
| Cardiovascular | 2 (16%) |
| Post‐anoxia | 5 (42%) |
| Trauma | 5 (42%) |
| Reasons livers were declined for transplantation | |
| DCD + age >60 y | 5 (42%) |
| DCD + high BMI | 5 (42%) |
| DCD + high transaminases | 2 (16%) |
| Type of preservation solution | |
| UW solution | 9 (75%) |
| HTK solution | 3 (25%) |
| Time between withdrawal of life support treatment and circulatory arrest (min)a | 20 (4‐46) |
| Time between circulatory arrest and cold flush in situ (min)a | 18 (12‐22) |
| Cold ischemia time (min)b | 544 (421‐674) |
| Reasons livers were declined for transplantation | |
| DCD + age >60 y | 5 (42%) |
| DCD + high BMI | 5 (42%) |
| DCD + high transaminases | 2 (16%) |
| Histological findings before start NMP | |
| Suzuki injury score | 2 (1‐3) |
| Histological findings after 6 h of NMP | |
| Suzuki injury score | 3 (2‐4.25) |
| Donor risk index | 2.79 (2.24‐3.21) |
| Weight of liver (kg) | 2.11 (1.81‐2.30) |
Data represented as median (interquartile ranges) for continuous variables or as numbers (percentages) for categorical variables.
Abbreviations: BMI, body mass index; DCD, donation after circulatory death; DBD, donation after brain death; HTK, histidine‐tryptophan‐ketoglutarate; SCS, static cold storage; UW, University of Wisconsin.
The total of both periods can be defined as the total donor warm ischemia time during DCD donation.
Period between cold flush out of the liver in the donor and start of machine perfusion.
3.2. Hepatocyte‐ and cholangiocyte‐derived miRs in perfusate and bile during NMP
Figure 1A and 1B showed the relative values of HDmiR‐122 in perfusate and in bile during NMP, and Figure 1C and 1D showed the relative values of CDmiR‐222 in the same fluids. Overall, relative HDmiR‐122 values were higher than relative CDmiR‐222 values. Figure 1E and 1F illustrated the relative ratios between HDmiR‐122 and CDmiR‐222 in the respective fluids. Both HDmiR‐122 and CDmiR‐222 levels in perfusate initially increased during the first 30 minutes of perfusion, and the median values showed a slight, but not significant, decrease when relative values were determined in perfusate samples taken after 6 hours NMP (Figure 1A and 1). The HDmiR‐122/CDmiR‐222 ratio in perfusate decreased over time in most livers after an initial peak at 30 minutes of perfusion (Figure 1E). Relative levels of HDmiR‐122 in bile showed a different trend with a median continuous increase during perfusion (Figure 1B). The mean relative value of CDmiR‐222 in bile however, although also not significantly, decreased during the 6 hours of perfusion (Figure 1D). The HDmiR‐122/CDmiR‐222 ratio in bile therefore increased in most livers, indicating that there was relatively more HDmiR‐122 than CDmiR‐222 in bile after 5.5 hours of perfusion (P < .001; Figure 1F).
Figure 1.
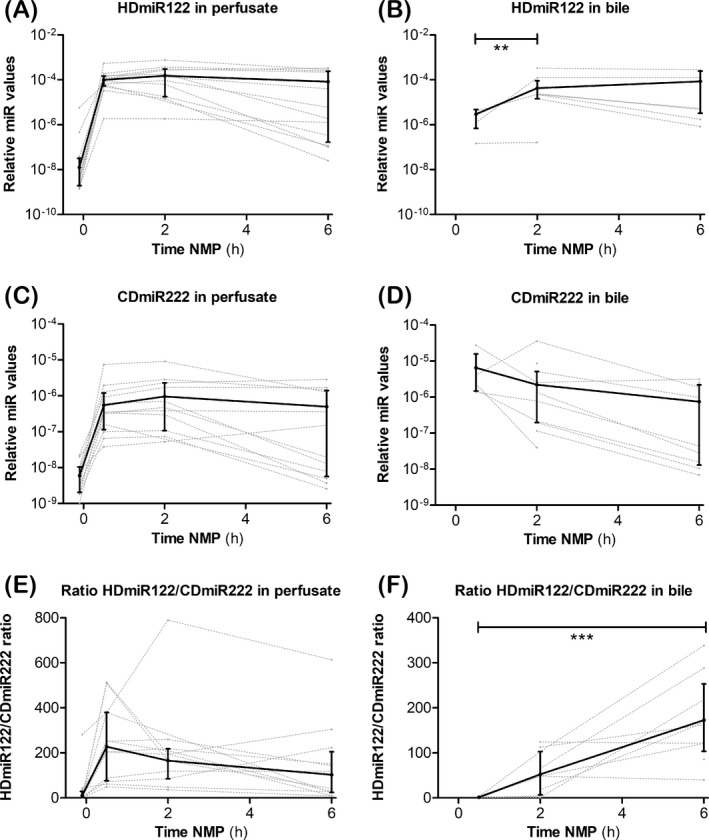
Dynamics of HDmiR‐122 and CDmiR‐222 levels in perfusate and bile during NMP. Relative values of HDmiR‐122 (A, B), CDmiR‐222 (C, D), and the HDmiR‐122/CDmiR‐222 ratio (E, F) in perfusate (A, C, E), and bile (B, D, F). In perfusate median values of both miRs peaked after 2 h of NMP and slowly declined thereafter (A, C). In bile, median relative values of HDmiR‐112 significantly increase between 0.5 and 2 h (B). The miR ratio in bile significantly increase over time during NMP. MiR levels are represented as relative values (2−Cq). Solid lines and bars: Median ± IQR, dotted lines: relative miR value profiles for each individual sample. **P < .01, ***P < .001
3.3. miR levels and microscopic assessment of liver parenchymal injury
Often livers, declined for transplantation on subjective assessment by the transplant surgeon, were shown to display minimal morphological changes after histological examination.21 We set out to evaluate the histological observations described earlier 22 and correlate these data with relative miRNA levels and the HDmiR122/CDmiR222 ratio. Suzuki injury scores at the start and end of NMP were compared with our early predictors HDmiR‐122, CDmiR‐222, and their ratio in perfusate at 30 minutes. Median (IQR) scores were 2 (1‐3) before NMP and 3 (2‐4.5) after 6 hours NMP and were significantly different (P = .001). This suggests progression of injury during NMP. A significant positive correlation with the Suzuki injury score and miRs at the start of NMP was observed (HDmiR‐122 r = .79, P < .05; CDmiR‐222 r = .68, P < .01; Figure 2A). The relative CDmiR‐222 levels, but not the relative HDmiR‐122 levels, at 30 minutes NMP were also significantly associated with the Suzuki injury score after 6 hours of NMP (P < .05) (data not shown). The HDmiR122/CDmiR222 ratio in perfusate after 30 minutes showed a good correlation with the Suzuki injury score after 6 hours NMP (Figure 2B).
Figure 2.
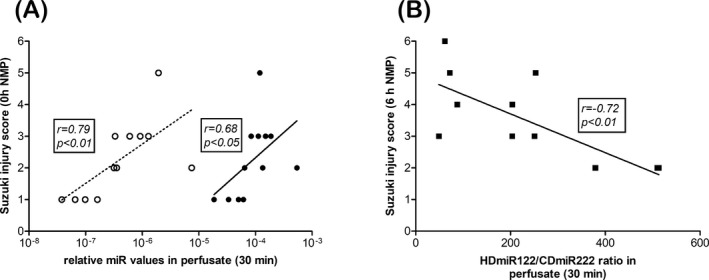
Correlation and prediction of relative miRNA levels and miR ratio with Suzuki injury score for liver injury. Relative level for HDmiR‐122 (closed circles) and CDmiR‐222 (open circles) in perfusate at 30 min correlated with Suzuki injury scores prior to NMP (0 h NMP, A). HDmiR122/CDmiR222 ratios at 30 min were predictive for liver parenchymal injury score after 6 h of NMP (black squares, B). Spearman's correlation coefficient (r) and P‐value are indicated
3.4. Early miR release into perfusate is predictive of late hepato‐cholangiocellular parameters
Peak HDmiR‐122 levels precede peak serum AST levels in predicting the onset of hepatic injury from acute rejection.16 To investigate this observation during NMP, the relative values of HDmiR‐122 and CDmiR‐222 in perfusate early during machine perfusion (30 minutes) were correlated to peak AST levels within 6 hours of NMP. A positive correlation was observed for both HDmiR‐122 and CDmiR‐222 with peak AST levels during the 6 hours reperfusion period (HDmiR‐122 r = .90, P < .001; CDmiR‐222 r = .78, P < .01; Figure 3A). A similar observation was made for peak LDH in bile, which also showed a positive and significant correlation with HDmiR‐122 (r = .76, P < .01) and CDmiR‐222 (r = .63, P = .04; Figure 3B). No significant correlation was observed between early miR levels and lactate at 6 hours of NMP and cumulative bile production (HDmiR‐122 r = .45, P = .15 and r = −0.39, P = .21, respectively; CDmiR‐222 r = .30, P = .34 and r = −.50, P = .10, respectively) (data not shown). There was, however, a significant negative correlation between early miR levels in perfusate and bilirubin levels in bile after 6 hours of NMP (HDmiR‐122 r = −.72, P = .02; CDmiR‐222 r = −.78, P = .01; Figure 3C). Bicarbonate concentration in bile at 6 hours, an indicator of cholangiocyte function, correlated significantly and negatively with early HDmiR‐122 levels (r = −.65, P = .02) in perfusate and nearly reached significance with early CDmiR‐222 (r = −.54, P = .07; Figure 3D). The bile glucose/perfusate glucose ratio did not show a correlation with early miR levels in perfusate (HDmiR‐122 r = .23, P = .55; CDmiR‐222 r = .20, P = .61; data not shown).
Figure 3.
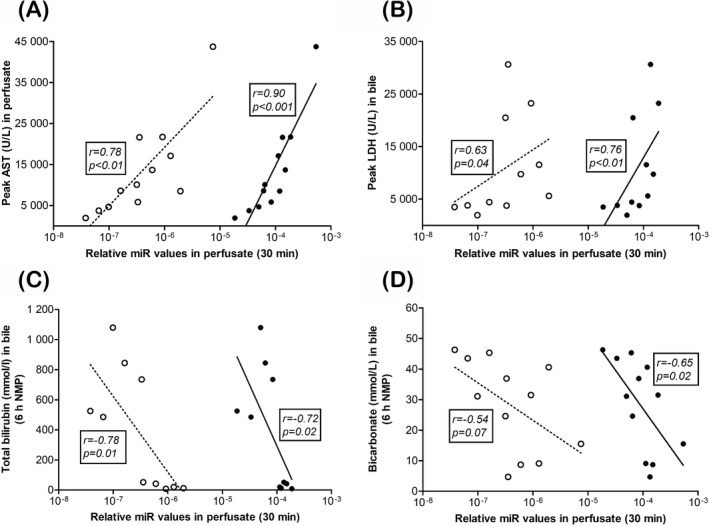
Early relative HDmiR‐122 and CDmiR‐222 levels in perfusate are predictive for hepato‐cholangiocellular injury at end of normothermic machine perfusion (NMP). Relative level for HDmiR‐122 (closed circles) and CDmiR‐222 (open circles) in perfusate at 30 min correlated with hepato‐cholangiocellular injury markers, peak aspartate aminotransferase in perfusate (A) and peak lactate dehydrogenase in bile (B) at 6 h of NMP. Total bilirubin in bile at 6 h NMP correlated with both HDmiR‐122 and CDmiR‐222 at 30 min (C). HDmiR‐122, but not CDmiR‐222, at 30 min was associated with bicarbonate level in bile at 6 h NMP (D). Spearman's correlation coefficient (r) and P‐value are indicated
3.5. CDmiR‐222 release into bile is predictive of later hepato‐cholangiocellular parameters, except for hepatocellular and cholangiocyte resorptive functions
The relative values of CDmiR‐222 in bile at 2 hours NMP correlated very strongly with peak AST in perfusate and peak LDH in bile (r = .88, P < .001 and r = .81, P = .003, respectively; Figure 4A‐B). HDmiR‐122 in bile correlated less strongly, and nearly significantly, with peak AST but not with peak LDH in bile (r = .61, P = .05 and r = .46, P = .16 respectively). None of the hepatocellular function parameters, lactate in perfusate, total bilirubin in bile, or total bile production correlated with either HDmiR‐122 (r = .21, P = .54; r = −.56, P = .09 and r = .03, P = .96, respectively) or CDmiR‐222 in bile (r = .47, P = .14; r = −.50, P = .14 and r = −.15, P = .20, respectively) (data not shown). Bicarbonate in bile, however, did show a strong and negative correlation with CDmiR‐222 in bile (r = −.89, P < .001; Figure 4C), while the glucose bile/perfusate ratio, which is higher in livers with poor cholangiocyte resorptive function, correlated strongly and positively with both HDmiR‐122 (r = −.70, P = .04) and CDmiR‐222 in bile (r = .72, P = .03; Figure 4D).
Figure 4.
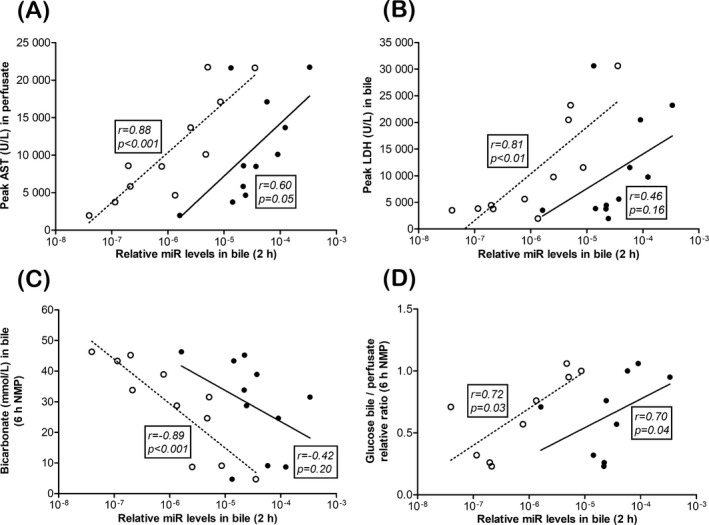
CDmiR‐222 levels in bile at 2 h of normothermic machine perfusion (NMP) are predictive of hepato‐cholangiocellular injury and function at end of NMP. Relative CDmiR‐222 (open circles) values, but not relative HDmiR‐122 values (closed circles), at 2 h of NMP correlated with peak aspartate aminotransferase (AST) in perfusate (A), and with lactate dehydrogenase (LDH) in bile (B) at 6 h of NMP (6 h NMP). In the case of bicarbonate in bile at 6 h NMP, also only CDmiR‐222, and not HDmiR‐122, at 2 h showed a strong, but negative, correlation (C), while both miRs were predictive of the bile/perfusate glucose ratio at 6 h (D). Spearman's correlation coefficient (r) and P‐value are indicated
3.6. The relative HDmiR‐122/CDmiR‐222 ratio in perfusate correlates with cumulative bile production
Cumulative bile production during NMP has been considered a good parameter of liver function in non‐transplanted research livers.15 Therefore, we correlated the previously mentioned clinical markers in perfusate and bile, as well as the HDmiR‐122/CDmiR‐222 ratio, to the cumulative bile production after 6 hours of perfusion (Figure 5). Early AST levels negatively correlated with cumulative bile production (r = −.76, P = .01) as is shown in Figure 5A. This was, however, not the case for LDH (r = −.58, P = .10), lactate (r = −.25, P = .46), total bilirubin in bile (r = .32, P = .41), bicarbonate in bile (r = −.21, P = .51), or HDmiR‐122 or CDmiR‐222 levels in perfusate (r = −.39, P = .21; r = −.5, P = .10, respectively) and bile (r = −.08, P = .99; r = −.42, P = .20, respectively) (data not shown). Interestingly, cumulative bile production at 30 minutes was not predictive for cumulative bile production 5.5 hours later (r = .28, P = .38) (not shown). Cumulative bile production did, however, correlate significantly with the ratio of the relative HDmiR‐122/CDmiR‐222 values in perfusate after 30 minutes (r = 0.67, P = .01; Figure 5B).
Figure 5.
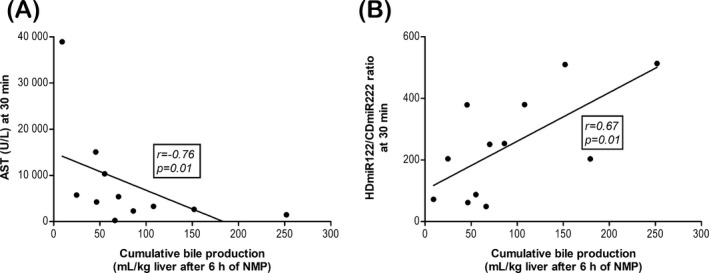
Aspartate aminotransferase (AST) and the miR ratio are predictive for good of liver function, as measured by cumulative bile production during normothermic machine perfusion (NMP). Cumulative bile production after 6 h plotted against clinical parameter AST (A) and relative HDmiR‐122/CDmiR‐222 ratio (B) measured after 30 min of NMP. Only these two parameters showed a good correlation with the cumulative bile production after 6 h. Spearman's correlation coefficient (r) and P‐value are indicated
4. DISCUSSION
The aim of this study was to assess whether hepatocyte‐ and cholangiocyte‐derived miR levels in perfusate and bile were predictive of function and injury after 6 hours of ex situ NMP of human liver grafts. This is the first study to report on miRs at different time points during NMP of human donor livers.
Overall, the current study showed that poor quality liver grafts with high hepato‐cholangiocellular injury (defined as high AST and LDH peaks in perfusate and bile, respectively) and low hepato‐cholangiocellular function (reflected by high lactate levels in perfusate, low levels of bilirubin in bile, low total bile production and a low glucose bile/perfusate ratio) had higher levels of HDmiR‐122 and CDmiR‐222 in both perfusate and bile. As expected, early relative values of HDmiR‐122 in perfusate showed a strong correlation with hepatocyte injury, but also with cholangiocyte injury after 6 hours of perfusion. A similar observation, although less pronounced, was made for CDmiR‐222 in perfusate. In bile, on the other hand, HDmiR‐122 did not correlate with either hepatocyte or cholangiocyte injury. CDmiR‐222, however, showed an even stronger correlation with both these injury markers than was observed for this miR in perfusate. In terms of hepatocyte function, both HDmiR‐122 and CDmiR‐222 in perfusate correlated significantly and negatively with bilirubin in bile, but lactate and cumulative bile production did not. The ratio of relative HDmiR‐122/CDmiR‐222 values after 30 minutes was an early predictor of cumulative bile production after 6 hours of perfusion and thereby a predictor of the function of non‐transplanted (research) livers.
Machine perfusion is a technique that is rapidly making its way into the clinic and can be applied for various purposes. NMP not only offers the possibility to perform viability testing of high‐risk livers in order to select transplantable organs,8, 13, 21, 22, 23 but also results in a lower rate of organ discard and lower levels of graft injury after NMP compared to SCS.24 In the early detection of hepatic injury during machine perfusion, miRs in perfusate offer several advantages. miRs can withstand harsh environmental conditions, and because the perfusate circulates through the entire liver during machine perfusion, miR levels in perfusate provide a better and non‐invasive representation of the entire liver compared to miR levels measured in total RNA samples from biopsies.11 Furthermore, miRs have been shown to be earlier and more sensitive markers of liver damage than more conventional markers such as AST or ALT, and machine perfusion potentially allows for an even more representative and dynamic evaluation of the liver graft.16, 25, 26 In addition, machine perfusion can be used for the resuscitation of grafts but fast and predictive biomarkers, such as miRs, are necessary to assess the effects of the treatment provided.27, 28, 29, 30 Especially HDmiR‐122, which is involved in several processes in the liver such as lipid metabolism, has the potential to serve as a biomarker to monitor the effect of interventions, or as a therapeutic target itself.31
Studies have shown that HDmiR‐122 levels in graft preservation fluid are higher in DCD grafts and in livers that went on to develop EAD and NAS.11, 12, 14 Higher HDmiR‐122 levels in perfusate were also measured in a porcine DCD NMP model with increasing warm ischemia times.11 Moreover, patients with high serum AST levels after transplantation, as well as patients with acute liver failure and chronic hepatitis C infection, showed higher serum HDmiR‐122 levels.32 Our finding that HDmiR‐122 levels in perfusate strongly and positively correlated with hepatocellular injury during NMP is entirely in line with the currently available literature.
In addition to being present in perfusate, HDmiR‐122 is also present in bile. HDmiR‐122 was present in bile of patients after liver transplantation and levels decrease upon episodes of liver injury.14 We confirm these findings in this study, with higher levels of HDmiR‐122 in bile from livers with higher AST levels.
In our study, both HDmiR‐122 and CDmiR‐222 levels in perfusate were highest at 2 hours of NMP and declined thereafter, a phenomenon that has not been described before. As the perfusion solution was contained in a closed circuit, it could be suggested that HDmiR‐122 was internalized or otherwise metabolized during NMP. This phenomenon, although less pronounced, was also observed for CDmiR‐222. This might be due to an active reuptake process and decreased passive release of miRs by better functioning and less injured grafts. However, to confirm this hypothesis further research is warranted.33, 34 Histological examination of the declined livers and comparing these data with HDmiR‐122 and CDmiR‐222 revealed a clear correlation with levels in perfusate prior, and, to a lesser extent, after NMP. This reduction in significance between scores prior to and after NMP also hints toward an active resorption of miRNA while, at the same time, injury that was initiated prior to NMP could progress.
Although not as extensively studied as HDmiR‐122, CDmiR‐222 was shown to be abundantly expressed in biliary epithelium.14, 35 Elevated levels have been reported in serum of patients with primary sclerosing cholangitis, in tissue of hepatocellular carcinoma and in primary colorectal cancer lesions with liver metastases.36, 37, 38 CDmiR‐222 was also found to be lower in the preservation solution of grafts that developed NAS after transplantation.12 In the present study, CDmiR‐222 levels in both perfusate and bile correlated strongly and positively with hepato‐cholangiocellular injury, and, in bile, with cholangiocyte function. These results are in concordance with a previous study showing that higher CDmiR‐222 levels in bile were associated with high hepatocyte injury and poor hepatocyte excretory function.14 The finding that both miRs were excreted into bile and strongly correlated with cellular excretory function, has been, as already mentioned, described previously.14
Subsequently, we observed that the HDmiR‐122/CDmiR‐222 ratio correlated with cumulative bile production during NMP, with significantly higher ratios in livers with better bile production. The relative ratio of hepatocyte‐derived and cholangiocyte‐derived miRs represents a relative degree of hepatocellular injury using an internal stabilization factor. Elevated HDmiR‐122/CDmiR‐222 ratios in the flushing solution of liver grafts, obtained prior to implantation, have previously been associated with NAS, EAD, graft loss, and DCD livers in humans,11, 12 and was, in a porcine DCD model, directly correlated with increasing WIT.11 Future research is necessary to determine whether miR levels in bile and perfusate during NMP (but also at other temperatures) can be used to predict clinical outcomes, such as NAS or EAD.
Several limitations should be considered in this study. The liver grafts used in this study were not transplanted after preservation; hence, post‐transplantation data are not available. Recent studies, however, showed that the use of NMP for viability assessment of otherwise discarded grafts was clinically feasible 13, 22, 39 and also helped to define optimal criteria for the final acceptance of these rejected grafts once their viability had been re‐assessed.22, 40 As a consequence, the clinical parameters for hepato‐cholangiocellular injury and function were used as mere viability markers to correlate with relative miR values.15, 22, 24 Mergental et al proposed composite viability criteria based on analysis of 12 discarded human livers. Multilevel statistical analysis showed that lactate clearance stratified his cohort in 2 distinct groups with pH and bile production as discriminative parameters.22 It should, however, be noted that in studies where livers were transplanted after NMP, these parameters tend to vary extensively and are not predictive for transplant outcome after NMP alone.8, 21 As lactate levels for the transplanted livers in the studies by Watson and by Mergental dropped below 2.5 mmol/L in 4 out of 6 grafts and 42 out of 47 grafts after 2 hours of NMP, respectively, in our cohort only one reached this level in this period. No correlation between this parameter and relative miRNA values in perfusate at 30 minutes and bile after 2 hours in our cohort was observed, nor with the other viability criteria components. It should be noted, however that, as far as lactate is concerned, 2 hours of NMP are sufficient to assess viability. For pH, to determine stability, longer periods of NMP are required as the first 2 hours are already needed to reach a plateau. At present, a 6‐hour period of NMP, either with or without additional perfusion treatments, is still considered the standard. Another limitation is the relatively small number of liver grafts included in this study due to a minimal availability of research livers. To avoid further stratification and reduced numbers, both DCD and DBD livers were included. Despite these small numbers and graft type heterogeneity, there were still significant differences observed in miR detection in perfusate and bile, which supports the utility of relative miR values to serve as potential, dynamic, biomarkers. Lastly, the measurement of miRs is currently a time‐consuming process, as it takes 4‐5 hours to complete the analysis, although techniques are under development to make clinical applications possible.41 Nonetheless, the predictive properties of these miRs might aid in the detection of transplantable livers or the effects of the efforts to improve the graft during machine perfusion. Therefore, the dynamics of miRs during perfusion in a closed circuit shown in this study can give new insights in the kinetics of miRs as biomarkers in general.
In conclusion, this study shows that HDmiR‐122 and CDmiR‐222 levels in bile and perfusate, already at early stages of NMP, are predictive of late classic markers of injury and function. Furthermore, the relative ratio of hepatocyte‐derived and cholangiocyte‐derived miRs is predictive of the cumulative bile production during perfusion. It will be of utmost importance to determine whether miR levels maintain a steady‐state profile or whether they will change during pre‐implant interventions and what the level profiles will be for (normothermic) machine perfused liver grafts once they are used for transplantation.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
Supporting information
Matton APM, Selten JW, Roest HP, et al. Cell‐free microRNAs as early predictors of graft viability during ex vivo normothermic machine perfusion of human donor livers. Clin Transplant. 2020;34:e13790 10.1111/ctr.13790
Matton and Selten are equally contributing authors.
Porte and van der Laan shared senior authorship.
REFERENCES
- 1. Jochmans I, van Rosmalen M, Pirenne J, Samuel U. Adult liver allocation in eurotransplant. Transplantation. 2017;101(7):1542. [DOI] [PubMed] [Google Scholar]
- 2. Barshes NR, Horwitz IB, Franzini L, Vierling JM, Goss JA. Waitlist mortality decreases with increased use of extended criteria donor liver grafts at adult liver transplant centers. Am J Transplant. 2007;7(5):1265. [DOI] [PubMed] [Google Scholar]
- 3. Blok JJ, Detry O, Putter H, et al. Longterm results of liver transplantation from donation after circulatory death. Liver Transpl. 2016;22(8):1107. [DOI] [PubMed] [Google Scholar]
- 4. de Vries Y, von Meijenfeldt FA, Porte RJ. Post‐transplant cholangiopathy: classification, pathogenesis, and preventive strategies. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4):1507‐1515. [DOI] [PubMed] [Google Scholar]
- 5. Meurisse N, Vanden Bussche S, Jochmans I, et al. Outcomes of liver transplantations using donations after circulatory death: a single‐center experience. Transplant Proc. 2012;44(9):2868. [DOI] [PubMed] [Google Scholar]
- 6. Verhoeven CJ, Farid WRR, De Jonge J, Metselaar HJ, Kazemier G, Van Der Laan LJW. Biomarkers to assess graft quality during conventional and machine preservation in liver transplantation. J Hepatol. 2014;61(3):672. [DOI] [PubMed] [Google Scholar]
- 7. Sutton ME, op den Dries S, Karimian N, et al. Criteria for viability assessment of discarded human donor livers during ex‐vivo normothermic machine perfusion. Liver Transpl. 2014;20:S240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Watson CJE, Kosmoliaptsis V, Pley C, et al. Observations on the ex situ perfusion of livers for transplantation. Am J Transplant. 2018;18(8):2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Mohr AM, Mott JL. Overview of microRNA biology. Semin Liver Dis. 2015;35(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Farid WR, Verhoeven CJ, de Jonge J, Metselaar HJ, Kazemier G, van der Laan LJ. The ins and outs of microRNAs as biomarkers in liver disease and transplantation. Transpl Int. 2014;27(12):1222. [DOI] [PubMed] [Google Scholar]
- 11. Selten JW, Verhoeven CJ, Heedfeld V, et al. The release of microRNA‐122 during liver preservation is associated with early allograft dysfunction and graft survival after transplantation. Liver Transpl. 2017;23(7):946. [DOI] [PubMed] [Google Scholar]
- 12. Verhoeven CJ, Farid WRR, de Ruiter PE, et al. MicroRNA profiles in graft preservation solution are predictive of ischemic‐type biliary lesions after liver transplantation. J Hepatol. 2013;59(6):1231. [DOI] [PubMed] [Google Scholar]
- 13. Watson CJE, Kosmoliaptsis V, Randle LV, et al. Preimplant normothermic liver perfusion of a suboptimal liver donated after circulatory death. Am J Transplant. 2016;16(1):353. [DOI] [PubMed] [Google Scholar]
- 14. Verhoeven CJ, Farid WRR, Roest HP, et al. Polarized release of hepatic microRNAs into bile and serum in response to cellular injury and impaired liver function. Liver Int. 2016;36(6):883. [DOI] [PubMed] [Google Scholar]
- 15. Westerkamp AC, Karimian N, Matton APM, et al. Oxygenated hypothermic machine perfusion after static cold storage improves hepatobiliary function of extended criteria donor livers. Transplantation. 2016;100(4):825. [DOI] [PubMed] [Google Scholar]
- 16. Farid WRR, Pan Q, van der Meer AJP, et al. Hepatocyte‐derived microRNAs as serum biomarkers of hepatic injury and rejection after liver transplantation. Liver Transpl. 2012;18(3):290. [DOI] [PubMed] [Google Scholar]
- 17. Roest HP, Verhoeven CJ, de Haan JE, de Jonge J, Ijzermans JNM, van der Laan LJW. Improving accuracy of urinary miRNA quantification in heparinized patients using heparinase I digestion. J Mol Diagn. 2016. [DOI] [PubMed] [Google Scholar]
- 18. Suzuki S, Toledo‐Pereyra LH, Rodriguez FJ, Cejalvo D. Neutrophil infiltration as an important factor in liver ischemia and reperfusion injury. Modulating effects of FK506 and cyclosporine. Transplantation. 1993;55(6):1265. [DOI] [PubMed] [Google Scholar]
- 19. Vandesompele J, De Preter K, Pattyn F, et al. Accurate normalization of real‐time quantitative RT‐PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3(7):RESEARCH0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Matton APM, de Vries Y, Burlage LC, et al. Biliary bicarbonate, pH, and glucose are suitable biomarkers of biliary viability during ex situ normothermic machine perfusion of human donor livers. Transplantation. 2019;103(7):1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mergental H, Perera MT, Laing RW, et al. Transplantation of declined liver allografts following normothermic ex‐situ evaluation. Am J Transplant. 2016;16(11):3235. [DOI] [PubMed] [Google Scholar]
- 22. Mergental H, Stephenson BTF, Laing RW, et al. Development of clinical criteria for functional assessment to predict primary nonfunction of high‐risk livers using normothermic machine perfusion. Liver Transpl. 2018;24(10):1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ravikumar R, Jassem W, Mergental H, et al. Liver transplantation after ex vivo normothermic machine preservation: a phase 1 (First‐in‐Man) clinical trial. Am J Transplant. 2016;16(6):1779. [DOI] [PubMed] [Google Scholar]
- 24. Nasralla D, Coussios CC, Mergental H, et al. A randomized trial of normothermic preservation in liver transplantation. Nature. 2018;557(7703):50. [DOI] [PubMed] [Google Scholar]
- 25. Shaked A, Chang B‐L, Barnes MR, et al. An ectopically expressed serum miRNA signature is prognostic, diagnostic, and biologically related to liver allograft rejection. Hepatology. 2017;65(1):269. [DOI] [PubMed] [Google Scholar]
- 26. van der Meer AJ, Farid WRR, Sonneveld MJ, et al. Sensitive detection of hepatocellular injury in chronic hepatitis C patients with circulating hepatocyte‐derived microRNA‐122. J Viral Hepat. 2013;20(3):158. [DOI] [PubMed] [Google Scholar]
- 27. Guarrera JV, Henry SD, Samstein B, et al. Hypothermic machine preservation in human liver transplantation: the first clinical series. Am J Transplant. 2010;10(2):372. [DOI] [PubMed] [Google Scholar]
- 28. Dutkowski P, Schlegel A, de Oliveira M, Mullhaupt B, Neff F, Clavien PA. HOPE for human liver grafts obtained from donors after cardiac death. J Hepatol. 2014;60(4):765. [DOI] [PubMed] [Google Scholar]
- 29. van Rijn R, Karimian N, Matton APM, et al. Dual hypothermic oxygenated machine perfusion in liver transplants donated after circulatory death. Br J Surg. 2017;104(7):907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Boteon YL, Boteon APCS, Attard J, et al. Ex situ machine perfusion as a tool to recondition steatotic donor livers: Troublesome features of fatty livers and the role of defatting therapies. A systematic review. Am J Transplant. 2018;18(10):2384. [DOI] [PubMed] [Google Scholar]
- 31. Bandiera S, Pfeffer S, Baumert TF, Zeisel MB. miR‐122–a key factor and therapeutic target in liver disease. J Hepatol. 2015;62(2):448. [DOI] [PubMed] [Google Scholar]
- 32. Dubin PH, Yuan H, Devine RK, et al. Micro‐RNA‐122 levels in acute liver failure and chronic hepatitis C. J Med Virol. 2014;86(9):1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Winter J, Jung S, Keller S, Gregory RI, Diederichs S. Many roads to maturity: microRNA biogenesis pathways and their regulation. Nat Cell Biol. 2009;11(3):228. [DOI] [PubMed] [Google Scholar]
- 34. Zhou M, Hara H, Dai Y, et al. Circulating organ‐specific microRNAs serve as biomarkers in organ‐specific diseases: implications for organ allo‐ and xeno‐transplantation. Int J Mol Sci. 2016; 17(8):1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Chen L, Yan H‐X, Yang W, et al. The role of microRNA expression pattern in human intrahepatic cholangiocarcinoma. J Hepatol. 2009;50(2):358. [DOI] [PubMed] [Google Scholar]
- 36. Bernuzzi F, Marabita F, Lleo A, et al. Serum microRNAs as novel biomarkers for primary sclerosing cholangitis and cholangiocarcinoma. Clin Exp Immunol. 2016;185(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karakatsanis A, Papaconstantinou I, Gazouli M, Lyberopoulou A, Polymeneas G, Voros D. Expression of microRNAs, miR‐21, miR‐31, miR‐122, miR‐145, miR‐146a, miR‐200c, miR‐221, miR‐222, and miR‐223 in patients with hepatocellular carcinoma or intrahepatic cholangiocarcinoma and its prognostic significance. Mol Carcinog. 2013;52(4):297. [DOI] [PubMed] [Google Scholar]
- 38. Iida M, Hazama S, Tsunedomi R, et al. Overexpression of miR221 and miR222 in the cancer stroma is associated with malignant potential in colorectal cancer. Oncol Rep. 2018;40(3):1621. [DOI] [PubMed] [Google Scholar]
- 39. van Leeuwen OB, de Vries Y, Fujiyoshi M, et al. Transplantation of high‐risk donor livers after ex situ resuscitation and assessment using combined hypo‐ and normothermic machine perfusion: a prospective clinical trial. Ann Surg. 2019;270(5):906. [DOI] [PubMed] [Google Scholar]
- 40. de Vries Y, Berendsen TA, Fujiyoshi M, et al. Transplantation of high‐risk donor livers after resuscitation and viability assessment using a combined protocol of oxygenated hypothermic, rewarming and normothermic machine perfusion: study protocol for a prospective, single‐arm study (DHOPE‐COR‐NMP trial). BMJ Open. 2019;9(8):e028596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cheng Y, Dong L, Zhang J, Zhao Y, Li Z. Recent advances in microRNA detection. Analyst. 2018;143(8):1758. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


