Abstract
Bullous pemphigoid, mucous membrane pemphigoid, and pemphigus vulgaris are different cutaneous autoimmune blistering diseases, with complex pathogenic mechanisms. In all of them, a type‐2 response is thought to have a central role. Interleukin 4 and Interleukin 13 are crucial cytokines in type‐2 response. Treatment of these conditions is often challenging. Dupilumab, a recombinant fully human IgG4 monoclonal antibody with binding specificity to human interleukin‐4 receptor IL‐4Rα, has the potential to inhibit both IL‐4 and IL‐13. We propose IL‐4Rα as a theoretical drug target for cutaneous autoimmune bullous diseases.
Keywords: blistering diseases, bullous pemphigoid, dupilumab, mucous membrane pemphigoid, pemphigus vulgaris
1. INTRODUCTION
Interleukin‐4 (IL‐4) and Interleukin‐13 (IL‐13) share features that make them crucial cytokines in type‐2 model diseases (McCormick & Heller, 2015; Nakayama et al., 2017). Both cytokines are produced by Th2‐polarized T cells, granulocytes, and monocytes/macrophages, and have the potential to activate Th2 T cells differentiation, M2 macrophage polarization, MHCII expression, B cell and plasma cell differentiation (McCormick & Heller, 2015). Moreover, both IL‐4 and IL‐13 amplify IgE production from plasma cells (Moyle, Cevikbas, Harden, & Guttman‐Yassky, 2019). IL‐4 and IL‐13 share receptor subunits (IL4Rα) and signaling molecules. Cell membrane receptor heterodimers bind IL‐4 and IL‐13; three different subunits can signal through three different pathways. Normally the receptor is poorly expressed but its levels become higher when stimulated properly. The fact that the receptor IL4Rα is ubiquitous means that every cell in the human body has the potential to respond to IL‐4 and IL‐13 signal (McCormick & Heller, 2015).
Nowadays dupilumab, a recombinant fully human IgG4 monoclonal antibody with binding specificity to human interleukin‐4 receptor IL‐4Rα, is available. In 2017, it received its approval in United States and Europe for the treatment of adult patients with moderate‐to‐severe atopic dermatitis (European Medicine Agency, 2017).
Trials on this agent are ongoing for nasal polyposis, eosinophilic oesophagitis, chronic hands eczema, and cholinergic urticaria despite H1‐antihistamine treatment.
Hardly any clinical data are available, but theoretically this monoclonal antibody could find a further field of application in cutaneous autoimmune bullous diseases. In fact, although different pathogenic mechanisms are involved in different autoimmune blistering diseases, they have in common the role of type‐2 response in their pathogenic mechanisms, with IL‐4 and IL‐13 playing a central role, via IL‐4Rα.
2. BULLOUS PEMPHIGOID
Bullous pemphigoid (BP) is the most common autoimmune subepidermal blistering disease of the skin, usually affecting elderly people. Currently, the treatment relies on corticosteroids and immunosuppressant drugs. High doses of corticosteroids are often required, which can be damaging. Overall mortality is significantly increased in BP, due to either comorbidities or immunosuppressive therapy. Therefore, newer therapeutic agents which could be more selective would represent a new treatment horizon for BP (Bernard & Antonicelli, 2017). BP is characterized by a humoral and cellular response against two self‐antigens: BP antigen 180 (BPAg2, BP180 or Collagene XVII) and BP230 (BPAg1, BP230). Both are components of the hemidesmosomes. Patients with BP generally have circulating IgG autoantibodies binding to BP180, which are proved to have a pathogenic role (Bernard & Antonicelli, 2017). Also IgE autoantibodies targeting BP180 have been recognized. They probably have a pathogenic role, triggering eosinophil and mast cells degranulation, and their titre seems to correlate with disease activity (Cozzani, Gasparini, Di Zenzo, & Parodi, 2018; Maglie & Hertl, 2019). IgE subtype antibodies are known to be associated with Th2 cells regulation, via IL‐4 and IL‐13 stimulation (Moyle et al., 2019). In fact, BP patients show a predominant type‐2 response, suggesting that Th2 cells are primary involved in the loss of tolerance against BP180 (Cozzani et al., 2018; Maglie & Hertl, 2019). Th2‐related cytokines, including IL‐4 and IL‐5, and chemokines, including eotaxin and monocyte chemoattractant protein 4 (MCP‐4, also called CCL‐13), are overrepresented in lesional BP skin, especially in the early phase of the disease. Autoreactive Th2 cells are thought to exert a dual role in BP: they stimulate proliferation and autoantibody production by B‐cells via CD40–CD40L interaction and contribute to eosinophil recruitment and activation. Eosinophils may be involved in the maintenance of a Th2‐type response, via the production of IL‐4, IL‐5, and IL‐13 (Cozzani et al., 2018; Giomi, Caproni, Calzolari, Bianchi, & Fabbri, 2002; Gounni Abdelilah et al., 2006; Kaye, Gordon, Deverapalli, Her, & Rosmarin, 2018; Maglie & Hertl, 2019). Furthermore, Büdinger et al. (1998) demonstrated a type‐2 response to BP180 in BP patients, in opposition to MHCII restricted controls who mounted a type‐1 response without disease. Of note, IL‐4 as well as IL‐5 was found to be higher in blister fluid than in serum, suggesting a lesional gradient (Kowalski, Kneibner, Kridin, & Amber, 2019).
Teraki, Hotta, and Shiohara (2001) investigated the frequencies of IL‐4 and IL‐13 producing T‐cells and their correlation with the expression of skin‐homing receptors (cutaneous lymphocyte‐associated antigen) in peripheral blood and skin blisters of patients with BP. IL‐4 and IL‐13 producing T‐cells were found to be more represented in peripheral blood of BP patients, as compared with healthy controls; the majority of these cells was found among the cutaneous lymphocyte‐associated antigen‐positive ones. In the skin blister, the frequencies of IL‐4 and IL‐13 producing T‐cells were found to be higher than in peripheral blood of patients with BP. After systemic corticosteroid therapy, IL‐13 producing T‐cells were significantly decreased among the cutaneous lymphocyte‐associated antigen‐positive ones.
When used in patients with atopic dermatitis, a single agent playing a dual antagonism of IL‐4 and IL‐13 showed the potential to inhibit type‐2 responses, by revealing strong and significant modulation of type‐2‐associated chemokines, including Chemokine‐Ligand 18 (CCL18; Hamilton et al., 2014). Günther, Carballido‐Perrig, Kopp, Carballido, and Pfeiffer (2009) reported CCL18 levels in sera from patients with BP 84% higher than those in healthy individuals; in addition, they described blister fluid concentrations of CCL18 fivefold and sevenfold higher than those found in the sera of patients with BP and healthy individuals, respectively. Langerhans cells, antigen‐presenting cells of the dermis and eosinophils were identified as producers of CCL18 in BP skin. Nonetheless, CCL18 levels correlated with the disease course in most of the patients.
For these reasons, targeting IL‐4 and IL‐13 appears to be an interesting new horizon for treatment of BP, since it may reduce the type‐2 response. Actually, a successful treatment of a single patient with severe BP and treatment limitation using dupilumab has already been reported. After 3 months of therapy, the patient had clinical and serological resolution, and no lesions were detected at 10 months while on dupilumab monotherapy (Kaye et al., 2018).
3. MUCOUS MEMBRANE PEMPHIGOID
The pathogenesis of mucous membrane pemphigoid (MMP) is similar to that of BP. Subepithelial blistering of mucous membranes occurs due to autoantibodies targeting parts of the basement membrane zone (BMZ), including BPAg1, BPAg2, collagen VII, laminin‐332, and α6β4 integrin (Kamaguchi & Iwata, 2019; Stump, Messingham, & Fairley, 2019). Anyway, the exact mechanism of blistering is not completely known to date. A hypothetic mechanism, showing less inflammatory features than that of BP, has been purposed: depletion of collagen XVII (which is BPAg2) or inhibition of collagen IV‐collagen XVII binding could be the cause of blistering formation (Kamaguchi & Iwata, 2019). A recent study demonstrated the presence of tissue‐bond IgE deposits in lesions from patients with MMP; in one patient, the IgE positivity was the only one recorded. The presence of those deposits reportedly showed correlation with disease activity index. Circulating IgE anti‐BMZ were also detected (Corti et al., 2018). The production of IgE class antibodies is well known to be amplified by both IL‐4 and IL‐13 stimulation (Moyle et al., 2019). At least for ocular MMP, three distinct pathogenic phases are described: the injury phase, with autoantibodies binding to autoantigens, activating an immunological cascade; then the acute inflammation phase, with neutrophils and proinflammatory cytokines (IL‐1, TNFa, and IL‐17) playing a crucial role, while Th1 cells produce IL‐2 and interferon gamma, and Th2 cells release IL‐4, IL‐5, and IL‐13; the production of these Th2‐related cytokines leads to the fibrosis phase, with fibroblasts proliferating and producing extracellular matrix, connective tissue growth factor and TGFβ, causing fibrosis and mucosal scarring (Georgoudis et al., 2019). Likewise, various studies have well recognized the role by IL‐4 and IL‐13 in promoting fibrosis in many different cutaneous and extracutaneous diseases, via different pathways and mechanisms (Aoudjehane et al., 2008; Bailey et al., 2012; Bellini et al., 2012; Belperio et al., 2002; Doucet et al., 1998; Fallon, Richardson, McKenzie, & McKenzie, 2000; Firszt, Francisco, Church, & Thomas, 2014; Gasparini, Cozzani, & Parodi, 2019; Huaux, Liu, McGarry, Ullenbruch, & Phan, 2003; Kanellakis, Ditiatkovski, Kostolias, & Bobik, 2012; O'Reilly, 2013; Oh et al., 2011; Saito, Okazaki, Sugawara, & Yamamoto, 2003; Shimamura et al., 2008; Sugimoto, Enjoji, Nakamuta, & Ohta, 2005; Wynes, Franke, & Riches, 2004; Wynn, 2004).
Patients with end‐stage disease, with important scarring of mucosae, do not benefit from the common systemic immunosuppressants. Currently, in those patients treatment only aims to manage symptoms and sequelae (Georgoudis et al., 2019). Considering the above described role of IL‐4 and IL‐13 in fibrosis development, we hypothesize that inhibition of both cytokines could be helpful for patients going through the third pathogenic phase, no more responsive to systemic treatment, possibly causing massive fibrosis and scarring.
4. PEMPHIGUS VULGARIS
Pemphigus vulgaris is a chronic autoimmune disease characterized by intraepithelial blisters formation on skin and mucosae, due to the presence of autoantibodies directed against desmoglein 1 and 3 (Dsg 1 and Dsg3). These are desmosome‐forming proteins of keratinocytes, involved in cell‐to‐cell adhesion. The action of the autoantibodies leads to acantholysis, the separation of keratinocytes from one another (Buonavoglia et al., 2019). Pathogenic autoantibodies are mostly of IgG4 and IgE subtypes, suggesting a promoting role by type‐2 cells (Pollmann, Schmidt, Eming, & Hertl, 2018). High doses of corticosteroids and immunosuppressant drugs are needed to treat PV, with their respective complications (Buonavoglia et al., 2019). Therefore, new therapeutic horizons are always being explored.
The possible usefulness of targeting IL‐4 and IL‐13 in PV has already been presented (Tavakolpour & Tavakolpour, 2016). Th2 cells are reported to play a critical role in PV: Th2 activity has been postulated to be associated with the severity of PV, with relapsing phases, and also with anti‐Dsg IgG production (Rizzo et al., 2005; Veldman et al., 2004; Zhu et al., 2012). Moreover, Dsg3‐reactive Th1 CD4+ T‐cells can be identified in PV patients and in healthy individuals, while Dsg3‐reactive Th2‐cells are found only in PV patients (Buonavoglia et al., 2019; Veldman et al., 2004).
Serum and tissue‐bond IgE directed against Dsg3 were found in a series of patients with active PV, supporting the assumption of a Th2‐mediated disease. IL‐4 and IL‐13 are potent inducers of IgE, as well as IgG4, production. Moreover, both Dsg‐3 specific IgE and IgG4 levels reportedly correlated with active disease, not with chronic or remittent disease; this gives strength to the hypothesis of a crucial role of Th2 cells in PV (Nagel et al., 2010). Although IgG1 and IgG4 are widely considered the predominant anti‐Dsg1 and anti‐Dsg3 IgG subclasses in PV (Tavakolpour & Tavakolpour, 2016), IgG4 production is usually promoted by Th2 cytokines, including IL‐4 and IL‐13, whereas class switch to IgG1 is normally driven by Th1 cells (Nagel et al., 2010). In addition, IgG1 appeared to be increased in patients with less active disease, in contrast to IgG4, which were found to be higher in patients with acute, active PV (Nagel et al., 2010). Together with various experimental evidences supporting a nonpathogenic role of IgG1 in PV, compared to a central function attributed to IgG4, those findings strengthen the concept of PV as a Th2‐mediated disease (Bhol et al., 1995; Hacker, Janson, Fairley, & Lin, 2002; Nagel et al., 2010; Yeh et al., 2006).
Several studies reported IL‐4 elevation in patients with PV and pemphigus foliaceus; in a series of patients, the level of IL‐4 decreased after 4 weeks of azathioprine treatment (Caproni et al., 2001; Lin et al., 1997; Mortazavi et al., 2008; Satyam, Khandpur, Sharma, & Sharma, 2009). High level of IL‐4 in perilesional skin biopsies of patients with PV has been confirmed (Rico, Benning, Weingart, Streilein, & Hali, 1999).
IL‐4 could also determine mast cells and eosinophils activation, with a positive feedback loop which may cause T cells differentiation toward the Th2 cell; in fact, eosinophilia can be seen in patients with PV (Tavakolpour & Tavakolpour, 2016).
In summary, IL‐4 is linked to PV active phases, due to the properties of inducing Th2 cells differentiation and increase, promoting isotype switching to IgG4 and IgE, and stimulating proliferation and degranulation of eosinophil and mast cells; oppositely, the activity of Th1 cells may lead to remission phases by producing IgG1 rather than IgG4. When the balance of Th1:Th2 is changed into Th2 dominancy, then PV may flare or appear for the first time (Nagel et al., 2010; Tavakolpour & Tavakolpour, 2016).
The inhibition of IL‐4 and IL‐13 via a single agent playing a dual antagonism of both has not been experimented in PV. For the reasons explained above, we hypothesize that it could be a useful treatment against a potentially lethal disease, often occurring with episodic flares, which we could control by reducing the Th2 unbalance. On the other hand, a possible role of TH1/TH17 response in pemphigus was suggested, based on higher serum levels of Th1 cytokines found in 20 PV patients compared to controls (Timoteo et al., 2017). Actually, those patients had very different stages of skin involvement (some of them showed no lesions at all), and all of them were on therapy (some of them for years). It might be postulated that both Th1/Th17 type of response and Th2 type interact and cooperate to develop pemphigus vulgaris; or, alternatively, we may hypothesize that some patients develop the same disease because of a Th2 type response, whereas other patients because of a Th1/Th17 response. In all the cases above, it can be supposed that opposing the Th2 type response could be at least partially useful, so clinical studies might deserve to be carried out.
Of course our hypothesis has further limitations. First, no information is reported about consequences of blocking IL4 and IL13 on activated anti‐desmoglein clonal B‐cells. This represents a minus point in comparison to rituximab, which depletes these B‐cells. On the other hand, blocking IL4 and IL 13 would affect class switching and activation of naïve B cells. Therefore, we can state that using dupilumab would at least oppose part of the cells involved in the pathogenesis. Moreover, it would block the turnover of the activated B cells, once they are dead. Thus, we consider dupilumab a theoretical option to be studied, since it could have at least a partial efficacy. In addition, from a clinical point of view, if it was demonstrated to be efficient in treating PV, its best advantage against rituximab would be its safety.
Besides, some studies reported high levels of IL‐10 in PV; anyway, little information is available about their role in the pathogenesis (Cho, Ellebrecht, & Payne, 2015). However, we cannot totally exclude that high levels of IL‐10 would weaken the efficacy of an IL4Ra inhibitor in AIBDs. This is an interesting hypothesis to be studied in the context of a clinical trial, which we still think deserves to be carried out.
In conclusion, different pathogenic mechanisms are involved in different autoimmune blistering diseases; anyway BP, MMP, and PV seem to have in common an important role played by Th2 cells, which exert their role by means of cytokines including IL‐4 and IL‐13. Due to the challenging treatment of such diseases, with considerable morbidity and mortality, new therapeutic agents with few side effects would be of enormous value. In our opinion, a monoclonal antibody targeting IL‐4Rα could be of interest in the field of cutaneous autoimmune bullous diseases. In fact, by reducing IL‐4 and IL‐13 activity, it could reduce autoantibodies production, chemokines secretion and eosinophil activation in BP, decrease fibrosis development in MMP, oppose the Th2 unbalance in PV (Figure 1). Large, randomized trials are obviously required to prove its efficacy. Although we are conscious that some clinical experience would give more strength to our hypothesis, unfortunately we could not use dupilumab for patients with skin blistering diseases yet, due to the need for approval by ethical committees. Anyway, for all the reasons above, we believe that dupilumab would deserve to be studied in the setting of cutaneous autoimmune bullous diseases.
Figure 1.
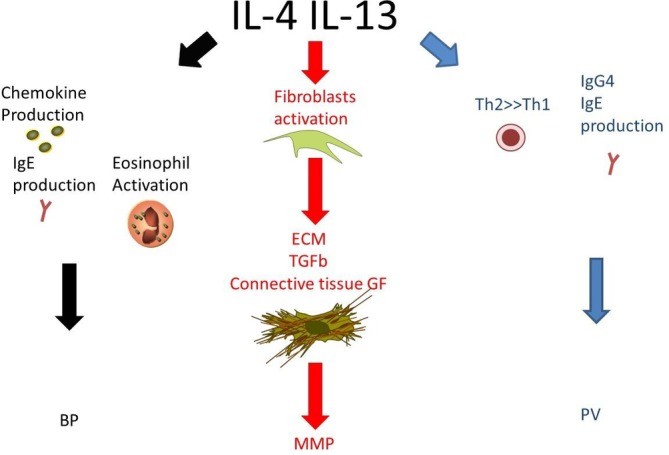
Role of Interleukin 4 (IL‐4) and Interleukin 13 (IL‐13) in pathogenesis of bullous pemphigoid (BP), mucous membrane pemphigoid (MMP), and pemphigus vulgaris (PV). Interleukin 4 and Interleukin 13 activities would be opposed by dupilumab
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Russo R, Cozzani E, Gasparini G, Parodi A. Targeting interleukin 4 receptor α: A new approach to the treatment of cutaneous autoimmune bullous diseases? Dermatologic Therapy. 2020;33:e13190 10.1111/dth.13190
REFERENCES
- Aoudjehane, L. , Pissaia, A., Jr. , Scatton, O. , Podevin, P. , Massault, P. P. , Chouzenoux, S. , … Conti, F. (2008). Interleukin‐4 induces the activation and collagen production of cultured human intrahepatic fibroblasts via the stat‐6 pathway. Laboratory Investigation, 88, 973–985. [DOI] [PubMed] [Google Scholar]
- Bailey, J. R. , Bland, P. W. , Tarlton, J. F. , Peters, I. , Moorghen, M. , Sylvester, P. A. , … Whiting, C. V. (2012). IL‐13 promotes collagen accumulation in Crohn's disease fibrosis by down‐regulation of fibroblast MMP synthesis: A role for innate lymphoid cells? PLoS One, 7, e52332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini, A. , Marini, M. A. , Bianchetti, L. , Barczyk, M. , Schmidt, M. , & Mattoli, S. (2012). Interleukin (IL)‐4, IL‐13, and IL‐17A differentially affect the profibrotic and proinflammatory functions of fibrocytes from asthmatic patients. Mucosal Immunology, 5, 140–149. [DOI] [PubMed] [Google Scholar]
- Belperio, J. A. , Dy, M. , Burdick, M. D. , Xue, Y. Y. , Li, K. , Elias, J. A. , & Keane, M. P. (2002). Interaction of IL‐13 and C10 in the pathogenesis of bleomycin‐induced pulmonary fibrosis. American Journal of Respiratory Cell and Molecular Biology, 27, 419–427. [DOI] [PubMed] [Google Scholar]
- Bernard, P. , & Antonicelli, F. (2017). Bullous pemphigoid: A review of its diagnosis, associations and treatment. American Journal of Clinical Dermatology, 18, 513–528. [DOI] [PubMed] [Google Scholar]
- Bhol, K. , Natarajan, K. , Nagarwalla, N. , Mohimen, A. , Aoki, V. , & Ahmed, A. R. (1995). Correlation of peptide specificity and IgG subclass with pathogenic and nonpathogenic autoantibodies in pemphigusvulgaris: A model for autoimmunity. Proceedings of the National Academy of Sciences of the United States of America, 92, 5239–5243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Büdinger, L. , Borradori, L. , Yee, C. , Eming, R. , Ferencik, S. , Grosse‐Wilde, H. , … Hertl, M. (1998). Identification and characterization of autoreactive T cell responses to bullous pemphigoid antigen 2 in patients and healthy controls. The Journal of Clinical Investigation, 102, 2082–2089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonavoglia, A. , Leone, P. , Dammacco, R. , di Lernia, G. , Petruzzi, M. , Bonamonte, D. , … Dammacco, F. (2019). Pemphigus and mucous membrane pemphigoid: An update from diagnosis to therapy. Autoimmunity Reviews, 18, 349–358. [DOI] [PubMed] [Google Scholar]
- Caproni, M. , Giomi, B. , Cardinali, C. , Salvatore, E. , Pestelli, E. , D'Agata, A. , … Fabbri, P. (2001). Further support for a role for Th2‐like cytokines in blister formation of pemphigus. Clinical Immunology, 98, 264–271. [DOI] [PubMed] [Google Scholar]
- Cho, M. J. , Ellebrecht, C. T. , & Payne, A. S. (2015). The dual nature of Interleukin‐10 in pemphigus vulgaris. Cytokine, 73, 335–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti, L. , Fanoni, D. , Venegoni, L. , Muratori, S. , Recalcati, S. , & Berti, E. (2018). Detection of IgE autoantibodies in mucous membrane pemphigoid and their association with disease severity. Giornale Italiano di Dermatologia e Venereologia. 10.23736/S0392-0488.18.06167-9. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Cozzani, E. , Gasparini, G. , Di Zenzo, G. , & Parodi, A. (2018). Immunoglobulin E and bullous pemphigoid. European Journal of Dermatology, 28, 440–448. [DOI] [PubMed] [Google Scholar]
- Doucet, C. , Brouty‐Boye, D. , Pottin‐Clemenceau, C. , Canonica, G. W. , Jasmin, C., & Azzarone, B. (1998). Interleukin (IL) 4 and IL‐13 act on human lung fibroblasts. Implication in asthma. Journal of Clinical Investigation, 101, 2129–2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Medicines Agency . (2017). Dupixent: Summary of Product Characteristics. Retrieved from https://www.ema.europa.eu/en/documents/product-information/dupixent-epar-product-information_en.pdf
- Fallon, P. G. , Richardson, E. J. , McKenzie, G. J. , & McKenzie, A. N. (2000). Schistosome infection of transgenic mice defines distinct and contrasting pathogenic roles for IL‐4 and IL‐13: IL‐13 is a profibrotic agent. Journal of Immunology, 164, 2585–2591. [DOI] [PubMed] [Google Scholar]
- Firszt, R. , Francisco, D. , Church, T. D. , & Thomas, J. M. (2014). Interleukin‐13 induces collagen type‐1 expression through matrix metalloproteinase‐2 and transforming growth factor‐b1 in airway fibroblasts in asthma. The European Respiratory Journal, 43, 464–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini, G. , Cozzani, E. , & Parodi, A. (2019). Interleukin‐4 and interleukin‐13 as possible therapeutic targets in systemic sclerosis. Cytokine, 125, 154799. [DOI] [PubMed] [Google Scholar]
- Georgoudis, P. , Sabatino, F. , Szentmary, N. , Palioura, S. , Fodor, E. , Hamada, S. , … Gatzioufas, Z. (2019). Ocular mucous membrane pemphigoid: Current state of pathophysiology, diagnostics and treatment. Ophthalmolology and Therapy, 8, 5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giomi, B. , Caproni, M. , Calzolari, A. , Bianchi, B. , & Fabbri, P. (2002). Th1, Th2 and Th3 cytokines in the pathogenesis of bullous pemphigoid. Journal of Dermatological Science, 30, 116–128. [DOI] [PubMed] [Google Scholar]
- Gounni Abdelilah, S. , Wellemans, V. , Agouli, M. , Guenounou, M. , Hamid, Q. , Beck, L. A. , & Lamkhioued, B. (2006). Increased expression of Th2‐associated chemokines in bullous pemphigoid disease. Role of eosinophils in the production and release of these chemokines. Clinical Immunology, 120, 220–231. [DOI] [PubMed] [Google Scholar]
- Günther, C. , Carballido‐Perrig, N. , Kopp, T. , Carballido, J. M. , & Pfeiffer, C. (2009). CCL18 is expressed in patients with bullous pemphigoid and parallels disease course. The British Journal of Dermatology, 160, 747–755. [DOI] [PubMed] [Google Scholar]
- Hacker, M. K. , Janson, M. , Fairley, J. A. , & Lin, M. S. (2002). Isotypes and antigenic profiles of pemphigus foliaceus and pemphigus vulgaris autoantibodies. Clinical Immunology, 105, 64–74. [DOI] [PubMed] [Google Scholar]
- Hamilton, J. D. , Suárez‐Fariñas, M. , Dhingra, N. , Cardinale, I. , Li, X. , Kostic, A. , … Guttman‐Yassky, E. (2014). Dupilumab improves the molecular signature in skin of patients with moderate‐to‐severe atopic dermatitis. The Journal of Allergy and Clinical Immunology, 134, 1293–1300. [DOI] [PubMed] [Google Scholar]
- Huaux, F. , Liu, T. , McGarry, B. , Ullenbruch, M. , & Phan, S. H. (2003). Dual roles of IL‐4 in lung injury and fibrosis. Journal of Immunology, 170, 2083–2092. [DOI] [PubMed] [Google Scholar]
- Kamaguchi, M. , & Iwata, H. (2019). The diagnosis and blistering mechanisms of mucous membrane pemphigoid. Frontiers in Immunology, 10, 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanellakis, P. , Ditiatkovski, M. , Kostolias, G. , & Bobik, A. (2012). A pro‐fibrotic role for interleukin‐4 in cardiac pressure overload. Cardiovascular Research, 95, 77–85. [DOI] [PubMed] [Google Scholar]
- Kaye, A. , Gordon, S. C. , Deverapalli, S. C. , Her, M. J. , & Rosmarin, D. (2018). Dupilumab for the treatment of recalcitrant bullous pemphigoid. JAMA Dermatology, 154, 1225–1226. [DOI] [PubMed] [Google Scholar]
- Kowalski, E. H. , Kneibner, D. , Kridin, K. , & Amber, K. T. (2019). Serum and blister fluid levels of cytokines and chemokines in pemphigus and bullous pemphigoid. Autoimmunity Reviews, 18, 526–534. [DOI] [PubMed] [Google Scholar]
- Lin, M. S. , Swartz, S. J. , Lopez, A. , Ding, X. , Fernandez‐Vina, M. A. , Stastny, P. , … Diaz, L. A. (1997). Development and characterization of desmoglein‐3 specific T cells from patients with pemphigus vulgaris. The Journal of Clinical Investigation, 99, 31–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maglie, R. , & Hertl, M. (2019). Pharmacological advances in pemphigoid. Current Opinion in Pharmacology, 46, 34–43. [DOI] [PubMed] [Google Scholar]
- McCormick, S. M. , & Heller, N. M. (2015). Commentary: IL‐4 and IL‐13 receptors and signaling. Cytokine, 75, 38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortazavi, H. , Amirzargar, A. , Valikhani, M. , Halaji, Z. , Maryam, D. P. , Tabrizi, M. , … Davatchi, S. H. S. (2008). The influence of treatment of pemphigus Vulgarison the serum level of cytokines. Iranian Journal of Dermatology, 43, 11–16. [Google Scholar]
- Moyle, M. , Cevikbas, F. , Harden, J. L. , & Guttman‐Yassky, E. (2019). Understanding the immune landscape in atopic dermatitis: The era of biologics and emerging therapeutic approaches. Experimental Dermatology, 28, 756–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel, A. , Lang, A. , Engel, D. , Podstawa, E. , Hunzelmann, N. , de Pita, O. , … Hertl, M. (2010). Clinical activity of pemphigus vulgaris relates to IgE autoantibodies against desmoglein 3. Clinical Immunology, 134, 320–330. [DOI] [PubMed] [Google Scholar]
- Nakayama, T. , Hirahara, K. , Onodera, A. , Endo, Y. , Hosokawa, H. , Shinoda, K. , … Okamoto, Y. (2017). Th2 cells in health and disease. Annual Review of Immunology, 35, 53–84. [DOI] [PubMed] [Google Scholar]
- O'Reilly, S. (2013). Role of interleukin‐13 in fibrosis, particularly systemic sclerosis. BioFactors, 39, 593–596. [DOI] [PubMed] [Google Scholar]
- Oh, M. H. , Oh, S. Y. , Yu, J. , Myers, A. C. , Leonard, W. J. , Liu, Y. J. , … Zheng, T. (2011). IL‐13 induces skin fibrosis in atopic dermatitis by thymic stromal lymphopoietin. Journal of Immunology, 186, 7232–7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollmann, R. , Schmidt, T. , Eming, R. , & Hertl, M. (2018). Pemphigus: A comprehensive review on pathogenesis, clinical presentation and novel therapeutic approaches. Clinical Reviews in Allergy and Immunology, 54, 1–25. [DOI] [PubMed] [Google Scholar]
- Rico, M. J. , Benning, C. , Weingart, E. S. , Streilein, R. D. , & Hali, R. P. (1999). Characterization of skin cytokines in bullous pemphigoid and pemphigus vulgaris. The British Journal of Dermatology, 140, 1079–1086. [DOI] [PubMed] [Google Scholar]
- Rizzo, C. , Fotino, M. , Zhang, Y. , Chow, S. , Spizuoco, A. , & Sinha, A. (2005). Direct characterization of human T cells in pemphigus vulgaris reveals elevated autoantigen‐specific Th2 activity in association with active disease. Clinical and Experimental Dermatology, 30, 535–540. [DOI] [PubMed] [Google Scholar]
- Saito, A. , Okazaki, H. , Sugawara, I. , & Yamamoto, K. (2003). Potential action of IL‐4 and IL‐13 as fibrogenic factors on lung fibroblasts in vitro. International Archives of Allergy and Immunology, 132, 168–176. [DOI] [PubMed] [Google Scholar]
- Satyam, A. , Khandpur, S. , Sharma, V. , & Sharma, A. (2009). Involvement of T(H)1/T(H)2 cytokines in the pathogenesis of autoimmune skin disease‐pemphigus vulgaris. Immunological Investigations, 38, 498–509. [DOI] [PubMed] [Google Scholar]
- Shimamura, T. , Fujisawa, T. , Husain, S. R. , Kioi, M. , Nakajima, A. , & Puri, R. K. (2008). Novel role of IL‐13 in fibrosis induced by nonalcoholic steatohepatitis and its amelioration by IL‐13R‐directed cytotoxin in a rat model. Journal of Immunology, 181, 4656–4665. [DOI] [PubMed] [Google Scholar]
- Stump, M. , Messingham, K. N. , & Fairley, J. A. (2019). Concurrent mucous membrane pemphigoid and membranous glomerulonephritis in a patient with autoantibodies targeting the 1080 region of collagen XVII. The British Journal of Dermatology, 181, 835–836. 10.1111/bjd.17923 [DOI] [PubMed] [Google Scholar]
- Sugimoto, R. , Enjoji, M. , Nakamuta, M. , & Ohta, S. (2005). Effect of IL‐4 and IL‐13 on collagen production in cultured LI90 human hepatic stellate cells. Liver International, 25, 420–428. [DOI] [PubMed] [Google Scholar]
- Tavakolpour, S. , & Tavakolpour, V. (2016). Interleukin 4 inhibition as a potential therapeutic in pemphigus. Cytokine, 77, 189–195. [DOI] [PubMed] [Google Scholar]
- Teraki, Y. , Hotta, T. , & Shiohara, T. (2001). Skin‐homing Interleukin‐4 and ‐13‐producing cells contribute to bullous pemphigoid: Remission of disease is associated with increased frequency of Interleukin‐10‐producing cells. The Journal of Investigative Dermatology, 117, 1097–1102. [DOI] [PubMed] [Google Scholar]
- Timoteo, R. P. , da Silva, M. V. , Miguel, C. B. , Silva, D. A. A. , Catarino, J. D. S. , Junior, V. R. , … Oliveira, C. J. F. (2017). Th1/Th17‐related cytokines and chemokines and their implications in the pathogenesis of pemphigus vulgaris. Mediators of Inflammation, 2017, 7151285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman, C. M. , Gebhard, K. L. , Uter, W. , Wassmuth, R. , Grötzinger, J. , Schultz, E. , & Hertl, M. (2004). T cell recognition of desmoglein 3 peptides in patients with pemphigus vulgaris and healthy individuals. Journal of Immunology, 172, 3883–3892. [DOI] [PubMed] [Google Scholar]
- Wynes, M. W. , Franke, S. K. , & Riches, D. W. (2004). IL‐4‐induced macrophage‐derived IGF‐I protects myofibroblasts from apoptosis following growth factor withdrawal. Journal of Leukocyte Biology, 76, 1019–1027. [DOI] [PubMed] [Google Scholar]
- Wynn, T. A. (2004). Fibrotic disease and the TH1/TH2 paradigm. Nature Reviews. Immunology, 4, 583–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh, S. W. , Cavacini, L. A. , Bhol, K. C. , Lin, M. S. , Kumar, M. , Duval, M. , … Ahmed, A. R. (2006). Pathogenic human monoclonalantibody against desmoglein 3. Clinical Immunology, 120, 68–75. [DOI] [PubMed] [Google Scholar]
- Zhu, H. , Chen, Y. , Zhou, Y. , Wang, Y. , Zheng, J. , & Pan, M. (2012). Cognate Th2‐B cell interaction is essential for the autoantibody production in pemphigus vulgaris. Journal of Clinical Immunology, 32, 114–123. [DOI] [PubMed] [Google Scholar]


