Summary
Lenalidomide maintenance therapy prolonged progression‐free survival (PFS) versus placebo in elderly patients with diffuse large B‐cell lymphoma (DLBCL) responding to induction chemotherapy in the phase 3 REMARC study. This subpopulation analysis assessed the impact of lenalidomide maintenance and treatment‐emergent adverse events (TEAEs) on health‐related quality of life (HRQOL). Global health status (GHS), and physical functioning and fatigue subscales were evaluated in patients who completed the European Organisation for Research and Treatment of Cancer quality‐of‐life questionnaire‐C30 v3.0. The impact of TEAEs classified post hoc as subjective (patients can feel) or observable (only measurable by physicians) on dose reductions and discontinuations was assessed. Among 457 patients (lenalidomide, n = 229; placebo, n = 228), mean (standard deviation) GHS was similar between treatment arms [68·2 (20·7) Versus 72·0 (17·8)] at randomisation and remained similar during maintenance. Patients receiving lenalidomide experienced no meaningful changes in GHS, physical functioning, or fatigue. Observable TEAEs were more common (81·1% Versus 66·3%) and more likely to lead to dose reductions, than subjective TEAEs in both arms. PFS was superior in the lenalidomide arm regardless of dose reduction. Lenalidomide maintenance prolonged PFS and did not negatively impact HRQOL in patients with DLBCL despite TEAEs being more common, when compared with placebo.
Keywords: non‐Hodgkin lymphoma, quality of life, therapy
Diffuse large B‐cell lymphoma (DLBCL) is the most frequent type of aggressive non‐Hodgkin lymphoma (NHL) (Friedberg, 2011; Chiappella et al., 2017a) with incidence peaking in the sixth decade of life (Chiappella et al., 2017b). The combination regimen of rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone (R‐CHOP) is considered standard first‐line therapy for patients with DLBCL and is given with curative intent (Chiappella et al., 2017a,b; Davies, 2017). Patients with DLBCL who are event‐free at 24 months have a subsequent overall survival (OS) equivalent to that of the age‐ and sex‐matched general population (Maurer et al., 2014). However, around one‐third of patients relapse or require retreatment within the first 24 months post first‐line induction (Maurer et al., 2014). This is associated with poor outcome and a median survival after relapse or retreatment of 10 months (McMillan et al., 2016). Effective salvage options after failure of R‐CHOP are limited, particularly for elderly patients ineligible for autologous stem cell transplantation (ASCT) (Nowakowski et al., 2016; Gisselbrecht & Van Den Neste, 2018). Therefore, innovative strategies are needed to optimise induction and to prevent or delay relapse after R‐CHOP without compromising the patient's health‐related quality of life (HRQOL).
In the phase 3 REMARC study, patients were assigned to a starting dose of 25 mg/day lenalidomide or placebo on days 1–21 of 28‐day cycles for 24 months. Lenalidomide maintenance significantly prolonged progression‐free survival (PFS) compared with placebo in patients aged ≥60 years with DLBCL who had achieved a response [complete response (CR) or partial response (PR)] to R‐CHOP induction therapy [median PFS not reached Versus 58·9 months; hazard ratio (HR) 0·708; 95% confidence interval (CI) 0·54–0·93; P = 0·01] (Thieblemont et al., 2017). There was no difference in OS between the treatment arms at a median follow‐up of 52 months. In maintenance therapy, it is important to consider not just efficacy measures but also HRQOL, toxicities and tolerability of this treatment and manageability of treatment‐emergent adverse events (TEAEs). Previous studies have shown limited impact of maintenance therapy for DLBCL on HRQOL. Rituximab maintenance therapy significantly reduced pain and symptom severity following ASCT, but was not associated with the rapid post‐transplant improvements observed across all domains of HRQOL (Heutte et al., 2011). The SIMONAL study also found no difference in HRQOL between patients receiving rituximab‐containing regimens and those who did not (Mounier et al., 2019).
In the REMARC study, the most common grade 3–4 TEAEs associated with lenalidomide maintenance were neutropaenia (56% Versus 22% with placebo) and cutaneous reactions (5% Versus 1%) (Thieblemont et al., 2017). These TEAEs are often manageable with dose reductions; however, some patients may need to discontinue treatment. In the REMARC study, 61% of patients receiving lenalidomide and 41% of patients receiving placebo discontinued treatment prematurely, although reasons for discontinuation were not assessed in the primary analysis (Thieblemont et al., 2017). Therefore, a subpopulation analysis was performed to assess the impact of maintenance therapy and TEAEs on HRQOL in patients who completed the European Organisation for Research and Treatment of Cancer (EORTC) quality‐of‐life questionnaire (QLQ)‐C30. As the decision to adjust the dose or discontinue treatment must balance the severity of the TEAE and patient HRQOL against the need to continue maintenance therapy for as long as possible to maximise survival outcomes, we also describe analyses from the REMARC study that were conducted to understand the possible relationship between treatment dosing, reasons for dose reductions, and TEAEs. Furthermore, the impact of dose reductions on PFS was studied.
Materials and methods
HRQOL analyses in the REMARC study
Details on the REMARC study design have been reported elsewhere (Thieblemont et al., 2017). Briefly, REMARC (NCT01122472) was a double‐blinded, randomised, placebo‐controlled phase 3 trial conducted in nine countries (Australia, Austria, Belgium, France, Israel, Poland, Portugal, Spain and Switzerland) and was sponsored by the Lymphoma Academic Research Organization (LYSARC).
Health‐related quality of life was a prespecified exploratory endpoint of the REMARC study only evaluated in patients in France or Belgium. Changes in HRQOL during maintenance were assessed using the French‐language or Dutch‐language paper version of the EORTC QLQ‐C30 version 3.0, a validated and reliable instrument for cancer patients, including patients with haematological malignancies (Aaronson et al., 1993; Osborne et al., 2012). The HRQOL intention‐to‐treat (ITT) population included all randomised patients from French or Belgian study sites. The HRQOL‐evaluable population was defined as HRQOL ITT patients with an evaluable QLQ‐C30 questionnaire at randomisation and at least one evaluable QLQ‐C30 questionnaire at a postrandomisation visit. A QLQ‐C30 questionnaire was considered as evaluable if at least the global health status (GHS) or quality‐of‐life scale of the QLQ‐C30 was completed.
Assessments were performed at randomisation (i.e. at the start of maintenance treatment), at cycles 6, 12 and 21 of maintenance treatment, at the end of the maintenance treatment period (EOM, cycle 26), and at 1 year after the last study treatment dose. QLQ‐C30 questionnaires were posted to patients after each study visit. The subscale of primary interest was the GHS scale of the QLQ‐C30 questionnaire. The physical functioning and fatigue subscales were also evaluated. Post hoc analyses were conducted to determine the impact of TEAEs on GHS.
Analysis of adverse events
Treatment‐emergent adverse events were collected and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) version 4.0. For the analysis of ‘subjective’ TEAEs [i.e. AEs that a patient can feel (for example headache)] and ‘observable’ TEAEs [i.e. those that a patient was less likely to be aware of (for example laboratory values)], AEs were categorised post hoc based on the principles described to identify patient‐reported TEAEs that were included in the patient‐reported outcomes version of the CTCAE (PRO‐CTCAE) (Basch et al., 2014). TEAEs that a patient can feel were considered to be subjective; TEAEs that a patient could not feel and would only be measurable by a physician were considered to be observable. The first author (CT) conducted a blind review of subjective and observable TEAEs and identified additional subjective TEAEs for inclusion. The complete list of subjective and observable TEAEs is presented in Table SI.
Dose reductions
To evaluate whether GHS had an impact on dose reductions, GHS was compared between patients with and without dose reductions and treatment discontinuations. Subjective versus observable TEAEs were evaluated to understand if these factors had an impact on dose reductions and treatment discontinuations. Furthermore, the impact of dose reductions on PFS was studied.
Statistical analyses
Health‐related quality of life mean changes from randomisation were assessed at each postrandomisation visit and analysed using two‐sided Wilcoxon signed‐rank tests. Minimally important difference (MID) is defined as the smallest change in a treatment outcome that an individual patient would identify as important or noticeable. This threshold for change has been previously determined for the EORTC QLQ‐30 as 10 points (Osoba et al., 1998; Cocks et al., 2011). Therefore, the prespecified threshold for a clinically meaningful difference in HRQOL scores was defined as a MID of 10 points, in accordance with these published thresholds (Osoba et al., 1998; Cocks et al., 2011). Clinically meaningful changes from baseline were determined by calculating the proportion of patients reaching or exceeding the MID in each subscale at each postbaseline visit. A linear mixed‐effects model with random intercept/slope was used to estimate the effect of treatment on each of the QLQ‐C30 subscales over time, as well as differences in treatment effect between groups. We compared QLQ‐C30 compliance between the treatment groups at each assessment visit using the two‐sided Fisher’s exact test. No imputation was performed for missing HRQOL data; missing data were assumed to be missing at random for both incomplete and missing forms. No multiplicity adjustments for inflation in Type I errors were made to the exploratory statistical analyses. The impact of TEAEs on rates of dose modifications and treatment discontinuation was analysed using descriptive statistics unless otherwise specified. The impact of dose modification on PFS was assessed using a Cox model including treatment group and dose reduction as time‐dependent variables. First‐degree interaction between these two variables was tested; in the final model, the interaction term was only kept if it was statistically significant. In addition, a subgroup analysis was run using the Kaplan–Meier method. All statistical analyses were performed using SAS® version 9.2 or higher (SAS Institute, Cary, NC, USA).
Results
Participants
In the REMARC study, 650 patients were randomised to receive lenalidomide maintenance (n = 323) or placebo (n = 327) (Thieblemont et al., 2017). Median age was 69 years (range 58–80) in the lenalidomide arm and 68 years (range 59–80) in the placebo arm, and 57% vs. 55% of patients were male respectively. In the lenalidomide arm, 78% of patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of 2, versus 72% in the placebo arm (Table 1).
Table 1.
Baseline characteristics of patients enrolled in the REMARC study.
| Characteristic | Lenalidomide (n = 323) | Placebo (n = 327) | P value |
|---|---|---|---|
| Median age (range), years | 69 (58–80) | 68 (59–80) | 0·25 |
| Sex, n (%) | |||
| Male | 183 (57) | 180 (55) | 0·68 |
| Female | 140 (43) | 147 (45) | |
| Histology, n (%) | |||
| DLBCL NOS | 225 (70) | 233 (71) | 0·04 |
| FL grade 3B | 2 (1) | 3 (1) | |
| De novo transformed | 31 (10) | 16 (5) | |
| Other | 32 (10) | 38 (12) | |
| Central review missing | 33 (10) | 37 (11) | |
| ECOG PS score, n (%) | |||
| 0–1 | 252 (78) | 237 (72) | 0·16 |
| ≥2 | 65 (20) | 80 (24) | |
| Missing aaIPI score, n (%) | 6 (2) | 10 (3) | |
| 0–1 | 125 (39) | 124 (38) | 0·86 |
| 2–3 | 185 (57) | 189 (58) | |
| Missing | 13 (4) | 14 (4) | |
| Extranodal sites | |||
| <1 | 160 (50) | 167 (51) | 0·70 |
| 1 | 163 (51) | 160 (49) | |
| Elevated LDH (>ULN), n (%) | |||
| No | 118 (37) | 116 (35) | 0·77 |
| Yes | 193 (60) | 199 (61) | |
| Missing | 12 (4) | 12 (4) | |
| Albumin, n (%) | |||
| ≤35 g/l | 91 (28) | 91 (28) | 0·73 |
| 35 g/l | 172 (53) | 183 (56) | |
| Missing | 60 (19) | 53 (16) | |
| CIRS score, n (%) | |||
| 0–6 | 223 (69) | 251 (77) | — |
| ≥7 | 100 (31) | 76 (33) | |
| R‐CHOP induction cycles, n (%) | |||
| 6 | 119 (37) | 118 (36) | — |
| 8 | 204 (63) | 208 (64) | |
| Response to R‐CHOP induction, n (%) | |||
| CR | 251 (78) | 244 (75) | 0·25 |
| PR | 69 (21) | 83 (25) | |
| ORR | 320 (99) | 327 (100) | |
| If PR, n (%) | |||
| Positive PET scan | 41 (59) | 43 (52) | — |
| BM missing | 26 (38) | 36 (43) | |
aaIPI, age‐adjusted International Prognostic Index; BM, bone marrow; CIRS, cumulative illness rating scale; CR, complete response; DLBCL, diffuse large B‐cell lymphoma; ECOG PS, Eastern Cooperative Oncology Group performance status; FL, follicular lymphoma; LDH, lactate dehydrogenase; NOS, not otherwise specified; ORR, overall response rate; PET, positron emission tomography; PR, partial response; R‐CHOP, rituximab, cyclophosphamide, doxorubicin, vincristine and prednisone; ULN, upper limit of normal.
A total of 457 patients from France and Belgium (70·3% of all patients) enrolled and were defined as the HRQOL ITT population (lenalidomide, n = 229; placebo, n = 228) (Figure S1). Of these patients, 263 (57·5%) provided HRQOL questionnaires and met the QLQ‐C30 compliance criterion (lenalidomide, n = 136; placebo, n = 127) at randomisation (defined as HRQOL‐evaluable population). While there was no evidence of differences in questionnaire compliance rates between the treatment arms at all time points, the number of patients in the HRQOL‐evaluable population decreased with successive time points. At cycle 6, 191 out of 338 patients still on the study remained in the HRQOL‐evaluable population, 151 out of 272 patients at cycle 12, 113 out of 227 patients at cycle 21, 103 out of 166 patients at cycle 26 and 39 out of 73 patients at one year after EOM (Figure S1). As patient numbers at one‐year follow‐up were low, one‐year follow‐up was excluded from further analyses. In post hoc analyses of the generalisability of the HRQOL findings, key demographic and clinical characteristics at randomisation and treatment outcomes did not differ significantly between the compliant HRQOL group and the non‐HRQOL group (i.e. those from countries outside France and Belgium who never participated in the HRQOL assessment). For example, there was no significant difference in response between compliant HRQOL patients and the non‐HRQOL population (CR or PR 89·8% Versus 86·5% respectively; P = 0·248), suggesting that the HRQOL results can be extrapolated to the wider REMARC population. Within the HRQOL ITT population, compliant HRQOL patients (i.e. patients from France and Belgium who had ≥1 valid HRQOL assessment) were younger and more frequently male than non‐compliant HRQOL patients (i.e. patients from France and Belgium who did not complete HRQOL assessments). Duration of maintenance therapy was significantly shorter for non‐compliant patients (60% of compliant HRQOL patients received ≥18 cycles, versus 28% of non‐compliant HRQOL patients; P < 0·0001). The remaining disease characteristics did not differ significantly between the two groups. Significant differences between the two groups were found across all treatment outcomes. Non‐compliant HRQOL patients did worse than compliant HRQOL patients in terms of PFS (median PFS 59·7 months Versus 72·9 months respectively; P = 0·0018) and had poorer response (CR or PR 79·1% Versus 89·8% respectively; P = 0·018).
Patient HRQOL during lenalidomide maintenance therapy
Within the HRQOL ITT population, GHS at randomisation was similar between patients receiving lenalidomide [median GHS score (Q1, Q3), 66·7 (58·3, 83·3)] or placebo [75·0 (58·3, 83·3)] and remained similar during maintenance therapy (Fig 1A). Patients receiving lenalidomide had no clinically meaningful changes in GHS (Figure S2), physical functioning, or fatigue (Figure S3A, B). Mean changes from randomisation for all QLQ‐C30 subscales over time are shown in Table SII. Most of these did not exceed the MID of ±10 when compared with scores at randomisation, with the exception of diarrhoea in the lenalidomide arm where the subscale scores were +12·1 and +17·6 in cycles 12 and 21 respectively, and social functioning in the placebo arm where the subscale score was +16·5 at EOM. While there was a worsening in fatigue scores at cycle 21, this did not meet the threshold for MID. The percentage of patients with worsening fatigue was also not significantly different between treatment arms.
Figure 1.

(A) Overall GHS. (B) GHS in patients with grade 3–4 TEAEs. (C) GHS in patients without grade 3–4 TEAEs. (D) GHS in patients with grade 3–4 treatment‐emergent neutropaenia. (E) GHS in patients without grade 3–4 treatment‐emergent neutropaenia. (F) GHS in patients undergoing dose reductions. Patients were enrolled in the HRQOL‐evaluable population. Median and interquartile ranges are shown. A change in MID of ±10 points was considered clinically meaningful. EOM, end of maintenance; GHS, global health status; HRQOL, health‐related quality of life; ITT, intention‐to‐treat; LEN, lenalidomide; MID, minimal important difference; PBO, placebo; TEAE, treatment‐emergent adverse event.
Impact of TEAEs and dose reductions on GHS
As previously reported (Thieblemont et al., 2017), among 645 patients in the REMARC trial safety population, 564 (87%) experienced ≥1 TEAE: 296 of 322 patients (92%) in the lenalidomide arm and 268 of 323 patients (83%) in the placebo arm. Grade 3–4 TEAEs occurred in 181 patients (56·2%) in the lenalidomide arm and 72 patients (22·3%) in the placebo arm. To assess the possible relationship between TEAEs or dose reductions and patient‐reported HRQOL, changes in GHS scores in the HRQOL‐evaluable population were analysed according to occurrence of grade 3–4 TEAEs, grade 3–4 neutropaenia and dose reductions. No significant differences were observed in GHS changes from randomisation between patients who experienced any grade 3–4 TEAE (n = 95) compared with those who did not (n = 41). Changes in GHS score from baseline remained within the MID threshold for patients with or without grade 3–4 TEAEs (Fig 1B, C) and for those with or without grade 3–4 neutropaenia in both treatment groups throughout the study period (Fig 1D, E). Among patients receiving lenalidomide who underwent dose reductions, there was no clinically meaningful change in GHS during the maintenance treatment period. Patients receiving lenalidomide without dose reductions experienced a clinically meaningful reduction in GHS at cycle 21 only (Fig 1F). Patients receiving placebo who underwent dose reductions achieved a clinically meaningful increase in GHS at cycle 12 only, while there was no clinically meaningful change in GHS for patients receiving placebo without dose reductions (Fig 1F).
TEAEs leading to dose reductions
Among 645 patients in the REMARC safety population, 212 of 322 patients (65·8%) in the lenalidomide arm had ≥1 TEAE leading to a dose reduction versus 103 of 323 patients (31·9%) in the placebo arm (Table 2). The median time to first onset of a TEAE leading to dose reduction did not differ between the lenalidomide and placebo arms {1·2 months [interquartile ratio (IQR) 0·4–2·8] Versus 1·2 months [IQR 0·5–2·3]}. The median time to first onset of grade 3–4 TEAEs leading to dose reduction was also similar [1·6 months (IQR 0·7–2·8) Versus 1·4 months (IQR 0·6–2·3)].
Table 2.
TEAEs leading to lenalidomide dose reductions among patients in the REMARC study.
| Lenalidomide (n = 322) | Placebo (n = 323) | |
|---|---|---|
| Patients with ≥1 TEAE leading to dose reduction, n (%) | 212 (65·8) | 103 (31·9) |
| Median time to first onset of TEAE leading to dose reduction, days (IQR) | 36·5 (15·0–85·0) | 35·0 (14·0–71·0) |
| Median time to first onset of grade 3–4 TEAE leading to dose reductions, days (IQR) | 50·0 (20·0–85·0) | 43·0 (19·0–71·0) |
| Number of TEAEs leading to dose reductions | 512 | 168 |
| Grade 1, n (%) | 46 (9·0) | 33 (19·6) |
| Grade 2, n (%) | 109 (21·3) | 45 (26·8) |
| Grade 3, n (%) | 275 (53·7) | 60 (35·7) |
| Grade 4, n (%) | 82 (16·0) | 30 (17·9) |
| Most frequent causes of dose reductions,* n (%) | ||
| Neutropaenia | 146 (45·3) | 49 (15·2) |
| Leucopaenia | 15 (4·7) | 6 (1·9) |
| Rash | 14 (4·3) | 2 (0·6) |
| Decreased neutrophil count | 13 (4·0) | 5 (1·5) |
| Thrombocytopaenia | 12 (3·7) | 5 (1·5) |
| Asthenia | 8 (2·5) | 2 (0·6) |
| Peripheral neuropathy | 8 (2·5) | 8 (2·5) |
| Pruritus | 8 (2·5) | 2 (0·6) |
| Lymphopaenia | 7 (2·2) | 2 (0·6) |
| Diarrhoea | 6 (1·9) | 4 (1·2) |
| Decreased white blood cell count | 6 (1·9) | 0 (0) |
IQR, interquartile range; TEAE, treatment‐emergent adverse event.
Top ten any grade TEAEs for the lenalidomide arm are shown.
Events of grade 3–4 severity accounted for 69·7% and 53·6% of TEAEs leading to dose reduction in the lenalidomide and placebo arms respectively. The most common TEAE leading to dose reductions was neutropaenia in the lenalidomide (45·3%) and placebo (15·2%) arms (Table 2).
Treatment‐emergent adverse events continued to occur even after dose reduction. Of 231 patients in the lenalidomide arm with a dose reduction, 88·7% experienced a TEAE after dose reduction, while 68·9% of 135 patients in the placebo arm undergoing dose reduction experienced a TEAE after dose reduction. The most frequent TEAEs occurring after dose reductions in the lenalidomide and placebo arms were neutropaenia (55·0% and 14·8% respectively) and infection (38·5% and 27·4% respectively) (Table SIII).
Subjective and observable TEAEs
Observable TEAEs were reported in 261 of 322 (81·1%) lenalidomide‐treated patients in the REMARC study versus 214 of 323 (66·3%) patients in the placebo arm (Table 3). Neutropaenia was the most frequent observable TEAE in both the lenalidomide (56·2%) and placebo (21·4%) arms. Subjective TEAEs were reported in 203 (63·0%) patients in the lenalidomide‐treated arm (63·0%) versus 151 patients (46·7%) in the placebo arm (Table 4). The most frequent subjective TEAE in both arms was bronchitis (11·5% Versus 6·8% respectively).
Table 3.
Overall observable TEAEs and observable TEAEs leading to dose reductions in patients receiving lenalidomide maintenance or placebo in the REMARC study.
| Observable TEAEs occurring in ≥2% of patients, n (%) | Lenalidomide (n = 322) | Placebo (n = 323) | ||
|---|---|---|---|---|
| Total | Leading to dose reduction | Total | Leading to dose reduction | |
| Patients with ≥1 TEAE | 261 (81·1) | 175 (54·3) | 214 (66·3) | 73 (22·6) |
| Neutropaenia | 181 (56·2) | 146 (45·3) | 69 (21·4) | 49 (15·2) |
| Leucopaenia | 68 (21·1) | 15 (4·7) | 29 (9·0) | 6 (1·9) |
| Lymphopaenia | 49 (15·2) | 7 (2·2) | 32 (9·9) | 2 (0·6) |
| Drug administration error | 34 (10·6) | 0 (0) | 27 (8·4) | 0 (0) |
| Respiratory tract infection | 20 (6·2) | 2 (0·6) | 8 (2·5) | 0 (0) |
| Thrombocytopaenia | 17 (5·3) | 12 (3·7) | 9 (2·8) | 5 (1·5) |
| Decreased neutrophil count | 16 (5·0) | 13 (4·0) | 5 (1·5) | 5 (1·5) |
| Anaemia | 10 (3·1) | 3 (0·9) | 5 (1·5) | 0 (0) |
| Febrile neutropaenia | 9 (2·8) | 3 (0·9) | 3 (0·9) | 2 (0·6) |
| Atrial fibrillation | 8 (2·5) | 1 (0·3) | 2 (0·6) | 1 (0·3) |
| Decreased white blood cell count | 7 (2·2) | 6 (1·9) | 1 (0·3) | 0 (0) |
TEAE, treatment‐emergent adverse event.
Table 4.
Overall subjective TEAEs and subjective TEAEs leading to dose reductions in patients receiving lenalidomide maintenance or placebo in the REMARC study.
| Subjective TEAEs occurring in ≥1% of patients, n (%) | Lenalidomide (n = 322) | Placebo (n = 323) | ||
|---|---|---|---|---|
| Total | Leading to dose reduction | Total | Leading to dose reduction | |
| Patients with ≥1 TEAE | 203 (63·0) | 85 (26·4) | 151 (46·7) | 38 (11·8) |
| Bronchitis | 37 (11·5) | 3 (0·9) | 22 (6·8) | 0 (0) |
| Rash | 22 (6·8) | 14 (4·3) | 6 (1·9) | 2 (0·6) |
| Respiratory tract infection | 20 (6·2) | 2 (0·6) | 8 (2·5) | 0 (0) |
| Pruritus | 17 (5·3) | 8 (2·5) | 4 (1·2) | 2 (0·6) |
| Peripheral neuropathy | 15 (4·7) | 8 (2·5) | 15 (4·6) | 8 (2·5) |
| Diarrhoea | 13 (4·0) | 6 (1·9) | 5 (1·5) | 4 (1·2) |
| Upper respiratory tract infection | 13 (4·0) | 2 (0·6) | 10 (3·1) | 0 (0) |
| Asthenia | 12 (3·7) | 8 (2·5) | 5 (1·5) | 2 (0·6) |
| Urinary tract infection | 12 (3·7) | 4 (1·2) | 12 (3·7) | 1 (0·3) |
| Nasopharyngitis | 11 (3·4) | 0 (0) | 7 (2·2) | 0 (0) |
| Rhinitis | 9 (2·8) | 0 (0) | 6 (1·9) | 0 (0) |
| Paraesthesia | 7 (2·2) | 2 (0·6) | 5 (1·5) | 2 (0·6) |
| Influenza | 7 (2·2) | 2 (0·6) | 5 (1·5) | 0 (0) |
| Fatigue | 7 (2·2) | 4 (1·2) | 4 (1·2) | 0 (0) |
| Pharyngitis | 7 (2·2) | 1 (0·3) | 3 (0·9) | 0 (0) |
| Pneumonia | 7 (2·2) | 3 (0·9) | 3 (0·9) | 2 (0·6) |
| Exfoliative rash | 7 (2·2) | 2 (0·6) | 2 (0·6) | 1 (0·3) |
| Herpes zoster | 6 (1·9) | 1 (0·3) | 11 (3·4) | 2 (0·6) |
| Cystitis | 6 (1·9) | 0 (0) | 6 (1·9) | 1 (0·3) |
| Sinusitis | 6 (1·9) | 0 (0) | 6 (1·9) | 0 (0) |
| Peripheral oedema | 5 (1·6) | 1 (0·3) | 5 (1·5) | 1 (0·3) |
| Dizziness | 5 (1·6) | 1 (0·3) | 2 (0·6) | 0 (0) |
| Dyspnoea | 5 (1·6) | 1 (0·3) | 2 (0·6) | 0 (0) |
| Muscle spasms | 5 (1·6) | 3 (0·9) | 1 (0·3) | 1 (0·3) |
| Cough | 5 (1·6) | 0 (0) | 1 (0·3) | 0 (0) |
| Tooth abscess | 5 (1·6) | 0 (0) | 0 (0) | 0 (0) |
| Back pain | 4 (1·2) | 3 (0·9) | 5 (1·5) | 1 (0·3) |
| Constipation | 4 (1·2) | 3 (0·9) | 3 (0·9) | 2 (0·6) |
| Vomiting | 4 (1·2) | 1 (0·3) | 4 (1·2) | 1 (0·3) |
| Nausea | 4 (1·2) | 2 (0·6) | 4 (1·2) | 3 (0·9) |
| Dyspepsia | 4 (1·2) | 3 (0·9) | 1 (0·3) | 1 (0·3) |
| Laryngitis | 4 (1·2) | 0 (0) | 0 (0) | 0 (0) |
| Headache | 3 (0·9) | 1 (0·3) | 5 (1·5) | 1 (0·3) |
| Depression | 2 (0·6) | 0 (0) | 9 (2·8) | 1 (0·3) |
| Vertigo | 2 (0·6) | 0 (0) | 6 (1·9) | 2 (0·6) |
| Cataract operation | 0 (0) | 0 (0) | 7 (2·2) | 0 (0) |
TEAE, treatment‐emergent adverse event.
Observable TEAEs were more likely to lead to dose reductions than subjective TEAEs in both the lenalidomide arm (54·3% Versus 26·4% respectively) and the placebo arm (22·6% Versus 11·8% respectively) (Tables 3 and 4). The most frequent observable TEAE leading to dose reductions was neutropaenia in both the lenalidomide and placebo arms (45·3% Versus 15·2% respectively) (Table 3). The most frequent subjective TEAE leading to dose reduction was rash in the lenalidomide arm (4·3%) and peripheral neuropathy in the placebo arm (2·5%) (Table 4).
Thrombotic events were analysed in more detail as a TEAE of interest. In the REMARC study, nine patients in the lenalidomide arm and four patients in the placebo arm had a venous thromboembolism (VTE) (P = 0·17). Of these, six patients (66·7%) in the lenalidomide arm and all four patients in the placebo arm had received prophylaxis for VTE. In the lenalidomide arm, the prophylaxis agent used was aspirin in five patients, heparin or a related agent in four patients and a vitamin‐K antagonist in one patient (patients could receive more than one prophylactic agent). In the placebo arm, three patients had used aspirin and two had used heparin or a related agent; no patients used vitamin‐K antagonists. One patient in the lenalidomide arm had an arterial thromboembolism (ATE) and had received prophylaxis with aspirin. No patients in the placebo arm had an ATE.
Impact of TEAEs on treatment exposure
Most patients in the REMARC study safety population received a starting dose of 25 mg; however, only 24·5% in the lenalidomide arm received 25 mg as the final prescribed dose compared with 51·1% in the placebo arm. Median dose intensity and median relative dose intensity during maintenance were similar between treatment arms. However, the median cumulative dose was lower in the lenalidomide arm than in the placebo arm (3885 mg Versus 8190 mg), reflecting the difference in the median duration of maintenance treatment of 14·9 months (range 0·03–25·6) Versus 23·7 months (range 0·03–25·2) respectively, for lenalidomide or placebo. Due to the potential impact of active treatment on TEAEs and patient‐reported outcomes, adherence was evaluated. Most patients took 90–100% of the treatment capsules in the lenalidomide and placebo arms (74·5% Versus 89·8% respectively).
Impact of dose reductions on PFS
In the REMARC study, PFS in the lenalidomide arm was superior to PFS in the placebo arm, regardless of whether the patient had a dose reduction (Fig 2). The HR for PFS for lenalidomide versus placebo was 0·795 (95% CI 0·531–1·190; P = 0·2632) in the 366 patients with a dose reduction and 0·788 (95% CI 0·515–1·205; P = 0·2694) in the 279 patients without a dose reduction. The Cox model, which included treatment and dose reduction as time‐dependent variables as well as the first‐degree interaction term, showed that the treatment–dose interaction was not statistically significant (P = 0·9912). The final model was therefore run with treatment and dose reduction as time‐dependent variables but without the interaction term. No statistically significant differences were seen (HR 1·353, P = 0·2978), suggesting that there was a treatment benefit regardless of dose reduction.
Figure 2.
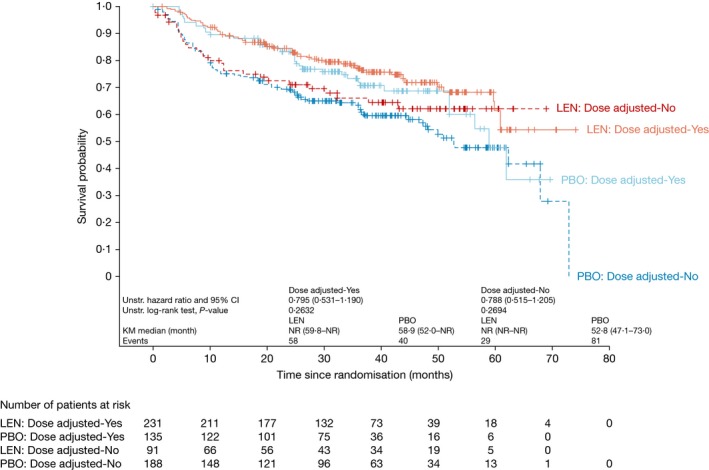
PFS (central review) in patients with and without dose reductions receiving lenalidomide or placebo. Patients in the overall REMARC study population. KM, Kaplan–Meier; LEN, lenalidomide; NR, not reached; PBO, placebo; PFS, progression‐free survival.
Impact of patient characteristics at randomisation on PFS
To assess the potential impact of patient baseline characteristics on PFS, the International Prognostic Index (IPI) score at baseline and number of cycles of induction R‐CHOP received were examined in post hoc analyses. Among patients with an IPI score of 1–2 (n = 174), PFS was significantly longer in patients receiving lenalidomide than in patients receiving placebo (HR 0·307, 95% CI 0·141–0·668; P = 0·0016). Among patients with an IPI score ≥3, there was a trend towards improved PFS in patients receiving lenalidomide; however, this was not significant (HR 0·774, 95% CI 0·565–1·061; P = 0·1106) (Figure S4A). Among patients receiving six versus eight cycles of R‐CHOP induction therapy, the difference in PFS between lenalidomide and placebo was greater in patients receiving six cycles of R‐CHOP (HR 0·615, 95% CI 0·369–1·023; P = 0·0587) than in patients receiving eight cycles of R‐CHOP (HR 0·791, 95% CI 0·566–1·106; P = 0·1691) (Figure S4B), although these differences were not statistically significant.
Discussion
In this subpopulation analysis from the REMARC study, there were no indications that lenalidomide maintenance had a negative impact on HRQOL in patients with DLBCL. GHS was similar between the lenalidomide and placebo groups at baseline and during maintenance. There was also no significant change in GHS score from baseline for patients who experienced any grade 3–4 TEAE, including grade 3–4 neutropaenia. In the lenalidomide‐treated patients, there were no clinically meaningful changes from baseline in most other QLQ‐C30 HRQOL subscales, including physical functioning and fatigue. Fatigue can have a profound effect on patients' HRQOL. In the SIMONAL study of long‐term survivors of NHL, persistent fatigue was found to be a serious long‐term complication, reported by 62% of survivors (Mounier et al., 2019). In contrast, only 7% of patients reported fatigue in the REMARC study. The SIMONAL study showed that increased fatigue was significantly correlated with increased age, obesity and comorbidities, but was unrelated to the initial therapy (CHOP‐like, high‐dose CHOP, or ASCT) that the survivors had received (Mounier et al., 2019). The REMARC study is one of the few longitudinal studies of fatigue in NHL and importantly shows that maintenance therapy with lenalidomide does not increase the burden of fatigue in patients, nor does it have an impact on other key measures of HRQOL such as GHS and physical functioning.
In the analysis of patients from the wider REMARC study, observable TEAEs, especially neutropaenia, were more likely to lead to dose reductions than subjective TEAEs, suggesting that the decision to implement dose reductions was primarily physician‐led and that most TEAEs were not detrimental to the patients' overall HRQOL. The most frequent TEAE in this analysis was neutropaenia, similar to previous studies of lenalidomide maintenance therapy in multiple myeloma (Attal et al., 2012; McCarthy et al., 2012; Palumbo et al., 2012, 2014).
Rates of compliance to the HRQOL questionnaires of 58% were achieved. This rate of compliance is within the range of 50–82% observed in other studies of HRQOL in lymphoma (Holzner et al., 2004; Mols et al., 2007; Holland et al., 2016; Oerlemans et al., 2017), albeit at the lower end of the scale. In view of these low compliance rates to the HRQOL questionnaires, subjective and observable TEAEs were also assessed by analysing the TEAE data collected using CTCAE criteria to provide supportive patient‐reported data. This additional data analysis is in support of the preliminary data from HRQOL analyses suggesting that lenalidomide maintenance treatment has no or little effect on HRQOL. The detailed collection of these subjective and observable TEAEs included more frequent data collection than the HRQOL questionnaire, which was collected only every six cycles.
As reported previously, PFS was higher for patients receiving lenalidomide maintenance therapy compared with patients receiving placebo (Thieblemont et al., 2017). Interestingly in the current analysis, there was a trend towards improved PFS in patients undergoing dose reductions, although the sample size was too small to draw definite conclusions. One speculation to explain this observation is that tolerance to lenalidomide or placebo reflects bone marrow reserve, possibly due to the effects of the R‐CHOP chemotherapy previously received. The observed trend towards increased PFS in patients receiving six cycles of R‐CHOP followed by lenalidomide, compared with patients receiving eight cycles of R‐CHOP and lenalidomide, also suggests that the extent of bone marrow exposure to chemotherapy may contribute to patient outcomes. There was also a trend towards increased PFS in patients with an IPI score of 1–2 compared with patients with an IPI score of ≥3, suggesting that prognostic factors at diagnosis also contribute to treatment outcomes. However, it is difficult to discern associations versus cause–effect observations.
The dose of lenalidomide used in the REMARC study (25 mg/day) was higher than the maintenance dose used in myeloma trials (typically 10–15 mg/day) (Cocks et al., 2011; Attal et al., 2012; Palumbo et al., 2014). However, it is the same as the dose used in a study of patients with multiple myeloma randomised to maintenance treatment with 25 mg or 5 mg of lenalidomide (Fenk et al., 2018). In that study, the higher dose of lenalidomide was associated with superior PFS but also with higher toxicity. The optimal maintenance dose has not been established for patients with DLBCL, but the higher starting dose used in REMARC may have contributed to the need for dose reductions and possibly treatment discontinuations, similar to that observed by Fenk et al. (2018).
There are several limitations of this analysis that should be considered. First, this was a post hoc analysis and therefore hypothesis‐generating only. Second, the results from this study should be viewed in the context of patient numbers. The number of patients completing all HRQOL assessments varied between 50% and 63%, and the data may be subject to bias because of the decreasing numbers of patients completing the HRQOL questionnaires at later time points in the trial. Importantly, while there was a trend towards an improvement in fatigue when patients discontinued treatment, patient numbers were too small to draw a conclusion. Third, these data may not be generalisable to the entire patient population because of the localised collection of HRQOL data in France and Belgium. Fourth, although this study suggests that lenalidomide maintenance therapy and TEAEs did not negatively impact patient HRQOL, the correlation between timing of the TEAEs and HRQOL measurements was not assessed. The correlation between HRQOL and observable TEAEs is also unknown. To our knowledge, this is the first analysis that has proposed a correlation between HRQOL and observable or subjective TEAEs. As such, this correlation should be further validated in other studies. Further, while growth factor use was permitted for the treatment of neutropaenia, the potential effect of growth factor therapy on neutropaenia and HRQOL was not assessed. Finally, it is also important to note that as non‐compliant HRQOL patients had worse outcomes than compliant HRQOL patients, potentially due to higher toxicity in this group driving non‐compliance, this informative censoring may mean results are not generalisable to the whole REMARC population. Non‐compliance of patients to HRQOL assessments has been recognised as a problem in cancer clinical trials, and the difficulty of generalising findings to a wider patient population has been identified as a limitation of HRQOL analyses (Bernhard et al., 1998). Previous studies of patients with rectal cancer, asthma and depression have also suggested poorer outcomes are more likely for non‐compliant HRQOL patients (Kopp et al., 2003; Mäkela et al., 2013; Novick et al., 2014).
The REMARC study demonstrated that lenalidomide maintenance therapy following chemotherapy in patients with DLBCL prolongs PFS. The secondary analysis reported in this article further demonstrated a trend towards higher PFS in patients undergoing dose reductions than in those who did not undergo dose reductions. While TEAEs were the leading cause of early discontinuation of lenalidomide maintenance therapy following chemotherapy, subjective TEAEs occurred less frequently than observable TEAEs and less frequently led to dose reductions. Lenalidomide maintenance did not negatively impact patient HRQOL in three key measures (GHS, fatigue and physical functioning). Taken together, these data show that lenalidomide maintenance has no appreciable effect on quality of life and that maintenance therapy is tolerable and manageable. These data provide risk‐benefit information for clinical decision‐making purposes.
Conflicts of interest
CT has been on an advisory board for Celgene Corporation, Gilead, Kyte, Novartis, and Roche. SH is an employee of Celgene Corporation. R‐OC has received grants, personal fees, and non‐financial support from Roche, Takeda, and Gilead; and personal fees from Bristol‐Myers Squibb, Merck, and Janssen. NM has no conflict of interest to disclose. AP has no conflict of interest to disclose. FM has been on an advisory board and provided scientific lectures to Celgene Corporation and Roche; has been on an advisory board for Gilead and Bristol‐Myers Squibb; provided scientific lectures for Janssen; and provided consultancy to Epizyme. CF has no conflict of interest to disclose. ND has no conflict of interest to disclose. KVE has no conflict of interest to disclose. LO has no conflict of interest to disclose. RB has no conflict of interest to disclose. GMP has no conflict of interest to disclose. EN‐V has no conflict of interest to disclose. JA has no conflict of interest to disclose. OF has no conflict of interest to disclose. SS has no conflict of interest to disclose. J‐CE has no conflict of interest to disclose. PL‐H has no conflict of interest to disclose. DB has received research funding. ST has no conflict of interest to disclose. DD has no conflict of interest to disclose. HG has no conflict of interest to disclose. RC has no conflict of interest to disclose. KLD has no conflict of interest to disclose. MGdS has received a research grant from, and provided consultancy to, Gilead Sciences; been on an advisory board for AbbVie; received institutional payments and travel reimbursement from Roche; provided consultancy to, and received travel reimbursement from, Janssen Cilag; and received travel reimbursement from Celgene Corporation. SG has no conflict of interest to disclose. JT has received non‐financial support from Celgene Corporation; and received clinical trials funding from BeiGene, Celgene, Janssen, PCYC, and Roche. JC has received travel reimbursement from Amgen and Celgene Corporation. DC has no conflict of interest to disclose. RG has received honoraria, research funding, travel and accommodation expenses, and provided consultancy or been on an advisory board for, Celgene Corporation, Roche, Merck, Takeda, AstraZeneca, Novartis, Amgen, Bristol‐Myers Squibb, MSD, Sandoz, AbbVie, and Janssen. AMC has no conflict of interest to disclose. PG has received a grant and personal fees from Takeda. LR has no conflict of interest to disclose. KT is a former employee of, and has stock ownership in, Celgene Corporation. MLC is an employee of Celgene Corporation. HT has received personal fees and non‐financial support from Roche; and received personal fees from AstraZeneca, Bristol‐Myers Squibb, and Karyopharm.
Author contributions
All authors had full access to all of the data in the study and are fully responsible for the content and editorial decisions for this manuscript. CT, R‐OC, NM, AP, FM, CF, ND, KVE, LO, RB, GMP, EN‐V, JA, OF, SS, J‐CE, PL‐H, DB, ST, DD, HG, RC, KLD, MGdS, SG, JT, JC, DC, RG, AMC, PG, LR, HT, and BC enrolled patients to the study, collected data, and provided critical review of the manuscript. CT, KT, and BC were responsible for the study design. SH, KT, and MLC provided statistical data analysis and interpretation, and provided critical review of the manuscript.
Supporting information
Fig S1. CONSORT diagram of patients included in the REMARC study HRQOL analyses.
Fig S2. Mean change and SD from baseline in QLQ‐C30 scores for GHS over time.
Fig S3. Mean change from baseline in QLQ‐C30 scores for physical functioning and fatigue over time.
Fig S4. PFS (central review) in patients with baseline IPI score of 1–2 or ≥3 receiving lenalidomide or placebo, and patients receiving six or eight cycles of induction R‐CHOP therapy and either lenalidomide or placebo.
Table SI. Subjective TEAEs and observable TEAEs.
Table SII. Mean change from baseline in QLQ‐C30 scores for all subscales over time.
Table SIII. TEAEs occurring after dose reduction (TEAEs occurring in ≥2% of patients).
Acknowledgements
The authors wish to thank all the patients and their families who participated in this study. The authors received medical writing support in the preparation of this manuscript from Victoria Edwards, PhD, and Nicky Dekker, MD, PhD, of Excerpta Medica BV, funded by Celgene Corporation. This study was sponsored by the Lymphoma Academic Research Organisation of France, funded by Celgene Corporation.
References
- Aaronson, N.K. , Ahmedzai, S. , Bergman, B. , Bullinger, M. , Cull, A. , Duez, N.J. , Filiberti, A. , Flechtner, H. , Fleishman, S.B. , de Haes, J.C. , Kaasa, S. , Klee, M. , Osoba, D. , Razavi, D. , Rofe, P.B. , Schraub, S. , Sneeuw, K. , Sullivan, M. & Takeda, F. (1993) The European Organization for Research and Treatment of Cancer QLQ‐C30: a quality‐of‐life instrument for use in international clinical trials in oncology. Journal of the National Cancer Institute, 85, 365–376. [DOI] [PubMed] [Google Scholar]
- Attal, M. , Lauwers‐Cances, V. , Marit, G. , Caillot, D. , Moreau, P. , Facon, T. , Stoppa, A.M. , Hulin, C. , Benboubker, L. , Garderet, L. , Decaux, O. , Leyvraz, O. , Vekemans, M.C. , Voillat, L. , Michallet, M. , Pegourie, B. , Dumontet, C. , Roussel, M. , Leleu, X. , Mathiot, C. , Payen, C. , Avet‐Loiseau, H. , Harousseau, J.L. & for the IFM Investigators . (2012) Lenalidomide maintenance after stem‐cell transplantation for multiple myeloma. New England Journal of Medicine, 366, 1782–1791. [DOI] [PubMed] [Google Scholar]
- Basch, E. , Reeve, B.B. , Mitchell, S.A. , Clauser, S.B. , Minasian, L.M. , Dueck, A.C. , Mendoza, T.R. , Hay, J. , Atkinson, T.M. , Abernethy, A.P. , Bruner, D.W. , Cleeland, C.S. , Sloan, J.A. , Chilukuri, R. , Baumgartner, P. , Denicoff, A. , St Germain, D. , O'Mara, A.M. , Chen, A. , Kelaghan, J. , Bennett, A.V. , Sit, L. , Rogak, L. , Barz, A. , Paul, D.B. & Schrag, D. (2014) Development of the National Cancer Institute's patient‐reported outcomes version of the common terminology criteria for adverse events (PRO‐CTCAE). Journal of the National Cancer Institute, 106, dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhard, J. , Cella, D.F. , Coates, A.S. , Fallowfield, L. , Ganz, P.A. , Moinpour, C.M. , Mosconi, P. , Osoba, D. , Simes, J. & Hürny, C. (1998) Missing quality of life data in cancer clinical trials: serious problems and challenges. Statistics in Medicine, 17, 517–532. [DOI] [PubMed] [Google Scholar]
- Chiappella, A. , Castellino, A. , Nicolosi, M. , Santambrogio, E. & Vitolo, U. (2017a) Diffuse large B‐cell lymphoma in the elderly: standard treatment and new perspectives. Expert Review of Hematology, 10, 289–297. [DOI] [PubMed] [Google Scholar]
- Chiappella, A. , Santambrogio, E. , Castellino, A. , Nicolosi, M. & Vitolo, U. (2017b) Integrating novel drugs to chemoimmunotherapy in diffuse large B‐cell lymphoma. Expert Review of Hematology, 10, 697–705. [DOI] [PubMed] [Google Scholar]
- Cocks, K. , King, M.T. , Velikova, G. , Martyn St‐James, M. , Fayers, P.M. & Brown, J.M. (2011) Evidence‐based guidelines for determination of sample size and interpretation of the European Organisation for the Research and Treatment of Cancer Quality of Life Questionnaire Core 30. Journal of Clinical Oncology, 29, 89–96. [DOI] [PubMed] [Google Scholar]
- Davies, A. (2017) Tailoring front‐line therapy in diffuse large B‐cell lymphoma: who should we treat differently? Hematology American Society of Hematology Education Program, 2017, 284–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenk, R. , Giagounidis, A. , Goldschmidt, H. , Heinsch, M. , Rummel, M.J. , Kröger, N. , Boquoi, A. , Lopez, D. , Hauck, K. , Gerrlich, C. , Baier, J. , Liesenjohann, S. , Boelke, E. , Mai, E. , Aul, C. , Strapatsas, J. , Dienst, A. , Kondakci, M. , Haas, R. & Kobbe, G. (2018) Maintenance therapy (MT) with 25 mg versus 5 mg lenalidomide (Len) after prolonged Len consolidation therapy (CT) in newly‐diagnosed, transplant‐eligible patients (pts) with multiple myeloma (MM). Journal of Clinical Oncology, 36(Suppl. 5), 8016. [Google Scholar]
- Friedberg, J.W. (2011) Relapsed/refractory diffuse large B‐cell lymphoma. Hematology American Society of Hematology Education Program, 2011, 498–505. [DOI] [PubMed] [Google Scholar]
- Gisselbrecht, C. & Van Den Neste, E. (2018) How I manage patients with relapsed/refractory diffuse large B cell lymphoma. British Journal of Haematology, 182, 633–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heutte, N. , Haioun, C. , Feugier, P. , Coiffier, B. , Tilly, H. , Ferme, C. , Gabarre, J. , Morschhauser, F. , Gisselbrecht, C. , Mounier, N. & Groupe d'Etude des Lymphomes de l'Adulte . (2011) Quality of life in 269 patients with poor‐risk diffuse large B‐cell lymphoma treated with rituximab versus observation after autologous stem cell transplant. Leukemia & Lymphoma, 52, 1239–1248. [DOI] [PubMed] [Google Scholar]
- Holland, R.A. , Bird, B.R. , Cahill, M. , Murphy, C.G. , O'Reilly, S. , O'Connor, E. , Boyce, M. & Crotty, R. (2016) Assessing the quality of life of non–Hodgkin lymphoma survivors: a population‐based study. Journal of Clinical Oncology, 34(Suppl. 3), 227.26573078 [Google Scholar]
- Holzner, B. , Kemmler, G. , Cella, D. , De Paoli, C. , Meraner, V. , Kopp, M. , Greil, R. , Fleischhacker, W.W. & Sperner‐Unterweger, B. (2004) Normative data for functional assessment of cancer therapy – general scale and its use for the interpretation of quality of life scores in cancer survivors. Acta Oncologica, 43, 153–160. [DOI] [PubMed] [Google Scholar]
- Kopp, I. , Lorenz, W. , Rothmund, M. & Koller, M. (2003) Relation between severe illness and non‐completion of quality‐of‐life questionnaires by patients with rectal cancer. Journal of the Royal Society of Medicine, 96, 442–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mäkela, M.J. , Backer, V. , Hedegaard, M. & Larsson, K. (2013) Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respiratory Medicine, 107, 1481–1490. [DOI] [PubMed] [Google Scholar]
- Maurer, M.J. , Ghesquières, H. , Jais, J.P. , Witzig, T.E. , Haioun, C. , Thompson, C.A. , Delarue, R. , Micallef, I.N. , Peyrade, F. , Macon, W.R. , Jo Molina, T. , Ketterer, N. , Syrbu, S.I. , Fitoussi, O. , Kurtin, P.J. , Allmer, C. , Nicolas‐Virelizier, E. , Slager, S.L. , Habermann, T.M. , Link, B.K. , Salles, G. , Tilly, H. & Cerhan, J.R. (2014) Event‐free survival at 24 months is a robust end point for disease‐related outcome in diffuse large B‐cell lymphoma treated with immunochemotherapy. Journal of Clinical Oncology, 32, 1066–1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy, P.L. , Owzar, K. , Hofmeister, C.C. , Hurd, D.D. , Hassoun, H. , Richardson, P.G. , Giralt, S. , Stadtmauer, E.A. , Weisdorf, D.J. , Vij, R. , Moreb, J.S. , Callander, N.S. , Van Besien, K. , Gentile, T. , Isola, L. , Maziarz, R.T. , Gabriel, D.A. , Bashey, A. , Landau, H. , Martin, T. , Qazilbash, M.H. , Levitan, D. , McClune, B. , Schlossman, R. , Hars, V. , Postiglione, J. , Jiang, C. , Bennett, E. , Barry, S. , Bressler, L. , Kelly, M. , Seiler, M. , Rosenbaum, C. , Hari, P. , Pasquini, M.C. , Horowitz, M.M. , Shea, T.C. , Devine, S.M. , Anderson, K.C. & Linker, C. (2012) Lenalidomide after stem‐cell transplantation for multiple myeloma. New England Journal of Medicine, 366, 1770–1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillan, A. , Martin, A. , Haioun, C. , Chiappella, A. , Di Rocco, A. , Rueda, A. , Palaska, C. & Davies, A.J. (2016) Post relapse survival rates in diffuse large B‐cell lymphoma. Blood, 128, 4204. [Google Scholar]
- Mols, F. , Aaronson, N.K. , Vingerhoets, A.J. , Coebergh, J.W. , Vreugdenhil, G. , Lybeert, M.L. & van de Poll‐Franse, L.V. (2007) Quality of life among long‐term non‐Hodgkin lymphoma survivors: a population‐based study. Cancer, 109, 1659–1667. [DOI] [PubMed] [Google Scholar]
- Mounier, N. , Anthony, S. , Busson, R. , Thieblemont, C. , Nerich, V. , Ribrag, V. , Castera, M. , Tilly, H. , Haioun, C. , Casasnovas, R.O. , Morschhauser, F. , Feugier, P. , Delarue, R. , Ysebaert, L. , Sebban, C. , Broussais‐Guillaumot, F. , Damaj, G. , Nerich, V. , Jais, J.P. , Laborde, L. , Salles, G. & Henry‐Amar, M. (2019) Long‐term fatigue in survivors of non‐Hodgkin lymphoma: the Lymphoma Study Association SIMONAL cross‐sectional study. Cancer, 125, 2291–2299. [DOI] [PubMed] [Google Scholar]
- Novick, D. , Montgomery, W. , Moneta, V. , Peng, X. , Brugnoli, R. & Haro, J.M. (2014) Impact of medication non‐compliance on outcomes of Asian patients with depression. European Psychiatry, 29(Suppl. 1), EPA1096. [Google Scholar]
- Nowakowski, G.S. , Blum, K.A. , Kahl, B.S. , Friedberg, J.W. , Baizer, L. , Little, R.F. , Maloney, D.G. , Sehn, L.H. , Williams, M.E. , Wilson, W.H. , Leonard, J.P. & Smith, S.M. (2016) Beyond RCHOP: a blueprint for diffuse large B cell lymphoma research. Journal of the National Cancer Institute, 108, djw257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oerlemans, S. , Arts, L.P. , Horevoorts, N.J. & van de Poll‐Franse, L.V. (2017) “Am I normal?” The wishes of patients with lymphoma to compare their patient‐reported outcomes with those of their peers. Journal of Medical Internet Research, 19, e288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne, T.R. , Ramsenthaler, C. , Siegert, R.J. , Edmonds, P.M. , Schey, S.A. & Higginson, I.J. (2012) What issues matter most to people with multiple myeloma and how well are we measuring them? A systematic review of quality of life tools. European Journal of Haematology, 89, 437–457. [DOI] [PubMed] [Google Scholar]
- Osoba, D. , Rodrigues, G. , Myles, J. , Zee, B. & Pater, J. (1998) Interpreting the significance of changes in health‐related quality‐of‐life scores. Journal of Clinical Oncology, 16, 139–144. [DOI] [PubMed] [Google Scholar]
- Palumbo, A. , Hajek, R. , Delforge, M. , Kropff, M. , Petrucci, M.T. , Catalano, J. , Gisslinger, H. , Wiktor‐Jędrzejczak, W. , Zodelava, M. , Weisel, K. , Cascavilla, N. , Iosava, G. , Cavo, M. , Kloczko, J. , Bladé, J. , Beksac, M. , Spicka, I. , Plesner, T. , Radke, J. , Langer, C. , Ben Yehuda, D. , Corso, A. , Herbein, L. , Yu, Z. , Mei, J. , Jacques, C. , Dimopoulos, M.A. & MM‐015 Investigators . (2012) Continuous lenalidomide treatment for newly diagnosed multiple myeloma. New England Journal of Medicine, 366, 1759–1769. [DOI] [PubMed] [Google Scholar]
- Palumbo, A. , Cavallo, F. , Gay, F. , Di Raimondo, F. , Ben Yehuda, D. , Petrucci, M.T. , Pezzatti, S. , Caravita, T. , Cerrato, C. , Ribakovsky, E. , Genuardi, M. , Cafro, A. , Marcatti, M. , Catalano, L. , Offidani, M. , Carella, A.M. , Zamagni, E. , Patriarca, F. , Musto, P. , Evangelista, A. , Ciccone, G. , Omedé, P. , Crippa, C. , Corradini, P. , Nagler, A. , Boccadoro, M. & Cavo, M. (2014) Autologous transplantation and maintenance therapy in multiple myeloma. New England Journal of Medicine, 371, 895–905. [DOI] [PubMed] [Google Scholar]
- Thieblemont, C. , Tilly, H. , Gomes da Silva, M. , Casasnovas, R.O. , Fruchart, C. , Morschhauser, F. , Haioun, C. , Lazarovici, J. , Grosicka, A. , Perrot, A. , Trotman, J. , Sebban, C. , Caballero, D. , Greil, R. , van Eygen, K. , Cohen, A.M. , Gonzalez, H. , Bouabdallah, R. , Oberic, L. , Corront, B. , Choufi, B. , Lopez‐Guillermo, A. , Catalano, J. , Van Hoof, A. , Briere, J. , Cabeçadas, J. , Salles, G. , Gaulard, P. , Bosly, A. & Coiffier, B. (2017) Lenalidomide maintenance compared with placebo in responding elderly patients with diffuse large B‐cell lymphoma treated with first‐line rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. Journal of Clinical Oncology, 35, 2473–2481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. CONSORT diagram of patients included in the REMARC study HRQOL analyses.
Fig S2. Mean change and SD from baseline in QLQ‐C30 scores for GHS over time.
Fig S3. Mean change from baseline in QLQ‐C30 scores for physical functioning and fatigue over time.
Fig S4. PFS (central review) in patients with baseline IPI score of 1–2 or ≥3 receiving lenalidomide or placebo, and patients receiving six or eight cycles of induction R‐CHOP therapy and either lenalidomide or placebo.
Table SI. Subjective TEAEs and observable TEAEs.
Table SII. Mean change from baseline in QLQ‐C30 scores for all subscales over time.
Table SIII. TEAEs occurring after dose reduction (TEAEs occurring in ≥2% of patients).


