Abstract
Peri‐implantitis is an inflammatory disease of hard and soft tissues around osseointegrated implants, followed by a progressive damage of alveolar bone. Oral microorganisms can adhere to all types of surfaces by the production of multiple adhesive factors. Inherent properties of materials will influence not only the number of microorganisms, but also their profile and adhesion force onto the material surface. In this perspective, strategies to reduce the adhesion of pathogenic microorganisms on dental implants and their components should be investigated in modern rehabilitation concepts in implant dentistry. To date, several metallic nanoparticle films have been developed to reduce the growth of pathogenic bacteria. However, the main drawback in these approaches is the potential toxicity and accumulative effect of the metals over time. In view of biological issues and in attempt to prevent and/or treat peri‐implantitis, biomaterials as carriers of antimicrobial substances have attracted special attention for application as coatings on dental implant devices. This review will focus on biomaterial‐based possibilities to prevent and/or treat peri‐implantitis by describing concepts and dental implant components suitable for engagement in preventing and treating this disease. Additionally, we raise important criteria referring to the geometric parameters of dental implants and their components, which can directly affect peri‐implant tissue conditions. Finally, we overview currently available biomaterial systems that can be used in the field of oral implantology.
Keywords: biomaterials, dental implants, peri‐implantitis
1. INTRODUCTION
Routinely, missing teeth are being replaced by dental implants. According to the American Academy of Implant Dentistry (AAID), 3 million US citizens have dental implants and this number is rapidly growing by 500 000 annually (https://www.aaid-implant.org/dental-implants/what-are-dental-implants/). As a consequence of the increased number of dental implants, biological complications surrounding these medical devices, prior to, during, or after the osseointegration process, also increase.1 The complications affecting peri‐implant tissue are categorized as peri‐implant mucositis and peri‐implantitis. Peri‐implant mucositis is considered the preceding step of the infection process that evokes peri‐implantitis, during which a biofilm‐induced inflammatory process is initiated clinically diagnosed by bleeding on probing and visual signs of inflammation limited to the soft tissues.2 The continued accumulation of inflammatory infiltrate around the implants promotes disease progression of the hard tissues and concomitantly peri‐implant bone loss.2, 3 Understanding the pathogenesis causing peri‐implantitis is required prior to considering any methods to prevent this disease and/or establishing a therapeutic strategy.
Comparable to periodontal infection, peri‐implantitis is a polymicrobial disease, which can also lead to bone resorption and eventually implant loss.2, 4 Studies have reported infection‐related implant loss in 20% of patients during 5‐10 years after implant placement.5 Etiological factors for this multifactorial disease have been discussed in recent literature in order to manage and control this peri‐implant inflammatory process. Despite the high rate of dental implant success (ie, up to 98%),6 clinicians are increasingly confronted with a new challenge: How to prevent and/or treat peri‐implantitis? In this sense, an important and antecedent event that precedes and directly affects bacterial adhesion is the irreversible interaction between material and bacteria, which is mandatory for direct biofilm development. Since bacteria interact with the substrate surface and with other species by specific and/or non‐specific interactions, the design and physicochemical properties of material surfaces used for dental implants can determine the magnitude of attractive or repulsive forces.7 Therefore, all physical and chemical dental implant factors capable of interfering with the bacterial adhesion process might be imperative for future management possibilities.
Controlling peri‐implant disease first requires the right diagnosis. Limited bone loss from the first thread of the implant is not necessarily considered as a diseased state.8 A recent concept defines peri‐implant health as the absence of bone loss beyond crestal bone level changes resulting from initial bone remodeling. The workgroup 4 of the 2017 World Workshop stated that peri‐implant tissue health can exist around implants with variable levels of bone support.9 The commonly exposed dental implant surfaces to the oral environment have an important role to adaptive behavior of bacterial species, becoming the base for pathogen colonization. In fact, subgingival implant components have gained a steadily important role in conserving implant health due to their intimate contact with the peri‐implant soft and hard tissues.10, 11 Although inconclusive definitions about the threshold levels of bone loss and/or attachment loss remain being discussed, peri‐implantitis has been defined as “a change in the level of the crestal bone in combination with bleeding upon probing with deepening of peri‐implant pockets in the light of previous examinations.”12, 13, 14
The issues involved in progressive peri‐implantitis opened a window of new possibilities in managing this clinical complication. It has instigated the development for new biomaterials, their modification, design, and function. Here, we review the literature on emerging options to prevent and manage peri‐implant infections from a biomaterial perspective.
2. CURRENT BIOMATERIAL‐BASED SOLUTIONS TO PREVENT PERI‐IMPLANTITIS
2.1. Titanium as a biomaterial: Design and engineering concepts for dental implants
Biomaterials, including dental implants and associated components, can be defined as “any material, natural or synthetic, that can be used for any period of time that interacts with biological systems, in order to maintain or improve the quality of life of the individual.” To date, titanium (Ti) is still contemplated as the most useful biomaterial in dental implant therapy for implant screw material and abutment connection. However, even though Ti has appropriate mechanical and biological properties,15 its antimicrobial assets16 are insufficient to avoid colonization by microorganisms. Consequently, microbial infection remains the major cause of implant loss.17
Although peri‐implantitis is modulated and mediated by the host, microorganisms are always responsible to initiate the inflammatory response.9 Consequently, even though other factors have been identified as potential risk factors including clinical history of the patient and poor plaque control,18, 19 any complication, which can favor microbial accumulation, can increase the risk of peri‐implantitis. Regardless the type of material, the design of a dental implant has a considerable role in peri‐implant tissue health. The macroscopic geometric parameters of a dental implant and the implant position within the bone influence the load transfer and stress distribution to the connective tissue. In the absence of infection, occlusal stress can stimulate an undesirable response of the mineralized tissue as a consequence of the natural bone metabolism reaction.20 Here, it is important to clarify that occlusal overload as a risk indicator for peri‐implantitis remains to be determined. So far, scientific evidence correlates the biological complication of occlusal overload with the potential risk of peri‐implantitis development. Although evidence is lacking that occlusal overload itself induces pocket formation in a healthy oral environment, the excessive and continuous load on dental implant components can disrupt the beneficial interaction between the abutment surface and barrier epithelial, which increases the risk of microbial leakage.21, 22 Further, excessive loading forces on dental implant abutments and crowns can cause screw fracture and also disturb the bone‐implant interface, facilitating the bacterial colonization in the inner part of implants.23 The pathogenic microorganism accumulation on dental implants and their components might stimulate inflammatory reactions in the peri‐implant tissues and induce peri‐implantitis development.24 Consequently, prosthetic platforms in implant dentistry must be correctly employed for each particular clinical case.
In the absence of periodontal ligament, abutments act as a critical component that provides vertical and lateral support for the dental implant structure. Commonly, it has hexagonal retaining walls to resist lateral movement during the mastication process. Initially, the external hexagon was developed to facilitate the insertion of dental implants into bone.25 However, this abutment system had limited resistant to rotation under lateral movements and concentrated forces along the axis of the implant create possible gaps on the implant‐abutment interface and increase the possibility of abutment fracture.26 Prospective studies revealed a high incidence (ie, up to 40%) of screw loosening for this type of abutment system in the molar region of the mandible.27 These complications combined with the risk of fracture of the external hexagon in situations of overload induced the development of anti‐rotational internal connections, by internal hexagons and the combination of screws and frictional systems. Although cracks and fractures have been observed independent of the connection type, multiple studies indicated that deep joints increase stability favoring stress distribution around dental implants.28, 29 In order to avoid microleakage at the implant‐abutment interface, morse taper abutments have been introduced as an efficient system to reduce microgap occurrence (1‐3 μm) at the implant‐abutment interface.30 Sealing capability of the implant screw–healing connection has been investigated, and recent findings have confirmed that Morse taper healing screws seem to better resist bacterial penetration compared with the internal hexagon.31 This is possible because the abutment is linked to the implant through an internal tapered connection and the high mechanical stability conferred by this system provides an adequate biological seal.32, 33
Conversely, even considering all benefits of the dental implant design evolution, interface microgap values under dynamic‐loading conditions, below bacteria dimensions, are still a challenge. Studies have shown microgaps for internal and external connection around 0.97 and 1.22 μm, respectively,34 that is, 5 to 6 times larger than overall bacterial size. With bacteria generally having a size of 0.2 μm in diameter and 1‐8 μm in length, this means that microbial penetration through this space is inevitable. Overall geometry of the fixture‐abutment interface affects the risk of bacterial invasion into the internal part of the implant, even when using Morse taper.35, 36, 37, 38 Consequently, other methods to prevent pathogenic microorganism colonization and posterior biofilm formation are required in an attempt to avoid peri‐implantitis initiation.
2.2. Physicochemical surface properties of dental implants on peri‐implantitis
Similar to the effect of geometric parameters of dental implant components on disease initiation, physicochemical properties of different substrates are suggested to play a major role in peri‐implant tissue conditions. Two surface characteristics, which interfere directly with prokaryotic and eukaryotic cell behavior, are roughness and hydrophilicity.39 Depending on the dental implant component function, different roughness values are required. In case of an implant surface, it has been known that relatively rough surfaces, average roughness (Ra) values around 2 μm,40, 41 directly promote the secretion of proteins required for osteoblast differentiation and function, forming a stable mechanical bone‐implant interface.42 On the other hand, roughness values around 2 μm may facilitate the interaction between surface and bacterial cells, affecting their morphology and microbial profile.43, 44, 45 Indeed rough surfaces display irregular topography, which protect bacteria against shear forces during their initial reversible binding. Many previous works have reported a high positive correlation between surface roughness and bacterial adhesion.46, 47, 48 Quantitative observation by CFU/mL showed a clear and direct proportional growth of Streptococcus mutans according to the increased roughness values.49 Similar finding was recorded by another genus of bacteria. In a recent study, the authors assessed the role of surface finish on biofilm formation and found a positive relationship between roughness and methicillin‐resistant Staphylococcus pseudintermedius biofilm growth.50 Previous studies investigated the minimum roughness incapable to interfere with bacteria adhesion and values lower than 0.2 μm were defined as a too limited roughness below, which no further significant changes occur regarding biofilm accumulation.51
In an attempt to counteract bacterial colonization, implants have been confectioned with different topographies, such as smoother coronal threads of the implant (Ra ~ 0.2 μm) and rougher apical threads (Ra ~ 2 μm). Especially for abutment surfaces, physical properties can be of clinical importance not only for interfering with microbial adhesion, but also for improving the connection to soft peri‐implant tissue.52
The quality of water‐surface interactions displays the capacity of the materials to influence microbial flora adhesion and promote osteogenic and non‐osteogenic soft tissue responses. As a non‐pharmacological treatment, recent studies have shown that physicochemical modification of titanium implant surfaces by UV irradiation leads to a significant reduction in the attachment and biofilm formation by human oral bacteria in the first 24 hours.53 In addition, previous analyses have confirmed a favorable effect from hydrophilic surfaces on osteoblastic differentiation and maturation.54, 55, 56, 57 The successful of primary stability of implants is dependent upon the magnitude of bone directly deposited onto the titanium surface without soft tissue intervention. Overall, bone cells are not entirely attracted by hydrophobic property of titanium implant. Nevertheless, superhydrophilic surfaces can increase adsorption of protein, osteoblast migration, and proliferation and promote osteoblastic differentiation.56, 58 In vitro and in vivo studies have proved the rapid and complete establishment of bone‐titanium integration in superhydrophilic surfaces, regardless surface topography.58, 59, 60 This technology has already been applied in dental implant therapies with a new chemically modified hydrophilic sand‐blasted large‐grit acid‐etched surface (SLActive), for example.61 However, with regard to the physicochemical modification of titanium implant surfaces by UV irradiation, the disadvantage of this treatment approach against peri‐implantitis is the limited effect of the surface modification, on late biological complications. Furthermore, the premise of the hydrophilic surface as a desired feature for soft tissue behavior is not totally clear.
Epithelial connective tissue provides a biological seal to resist external mechanical forces. Additionally, soft tissue surrounding the implant neck acts as a defensive wall protecting the internal environment against foreign invaders.39, 62 Interestingly, epithelial cells have shown a positive response when in contact with hydrophobic materials, in terms of quality of spreading and cell morphology.63, 64 And the importance goes further cell viability. It is already known that alterations in cell morphology can affect gene expression modulation and consequently the molecular connections between integrins, cytoskeletal filaments and surface.65, 66 This indicates that the knowledge about the cell‐surface interaction ability is the key in the development and application of new implant materials. Therefore, physicochemical optimizations of dental implant components represent a straightforward modality to minimize bacterial accumulation and maximize tissue integration.
3. BIOMATERIAL‐BASED SOLUTIONS AS FUTURE PERSPECTIVES TO PREVENT/TREAT PERI‐IMPLANTITIS
In an attempt to prevent/treat bacterial colonization on dental implant system components,67, 68, 69 metallic nanoparticles have been investigated as a potential coating material on titanium substrates.70 However, the wide broad‐spectrum antimicrobial properties depend on the metallic nanoparticles concentration. Potential concerns have been raised about the effect of metallic nanoparticles on human cells, even at low concentrations. The unanswered questions about cytotoxicity of these compounds upon long‐term exposure have triggered great efforts in setting up new possibilities to fight peri‐implantitis.71
Nowadays, an interesting paradigm shift brings up new possibilities to antimicrobial drug applications. Considering that antibiotics still remain as important protagonists to treat infection diseases, the idea in reducing microbial resistance can be achieved by controlled and directed delivery of specific drugs. The overall short‐term benefit of local antibiotic administration has been proven in controlling inflammation from affected periodontal sites.72, 73 The efficacy of the locally delivered antibiotic in managing peri‐implantitis has shown improvements in probing depths that were significantly reduced compared with (non‐treated) controls.74 From this principle, biomaterials emerge as a valuable tool to improve drug specificity to the desired site of action. Among drugs profile, doxycycline, tetracycline, metronidazole, and minocycline have been selected as antibiotics to be incorporated in the delivery system.75, 76, 77 Besides antibiotics, antimicrobial peptides have been investigated as drug possibilities to fight oral infections.78 Further, depending on the target, biomaterial modifications can be employed on different parts of implants to either prevent infection or treat peri‐implantitis. In this section, we outline some biomaterials as a straightforward prospect to prevent peri‐implant mucositis, which means that the disease has not established yet and the reversible inflammatory process is limited to the soft tissue.79 Furthermore, we discuss the possibility of using the same biomaterial systems to an advanced stage of the disease.
Hydrogels are hydrated polymers that exhibit meaningful therapeutic versatility, designed for human application.80 These biomaterials form a strong cross‐linked network of natural or synthetic molecules capable of storing biological drugs on their internal spaces. Among biomaterials used for hydrogel fabrication, polysaccharides (eg, dextran and chitosan81) and proteins (eg, gelatin and fibrin) are well‐studied standards of natural polymers.82, 83 Regarding synthetic biomaterials, polyvinyl alcohol (PVA),84, 85 polyethylene glycol (PEG),86 and poly(acrylic acid) (PAA) are widely used examples of hydrogel‐forming polymers. Overall action mechanisms involve either hydrogel ability to encompass different antimicrobial substances into the multilayered polysaccharide for eventual release or by covalently attaching therapeutics to the network.87 This hydrogel engine was shown effective as a coating on titanium implants without inducing an inflammatory reaction. The idea was to develop an antibiotic‐loaded hydrogel loaded to offer a local protection for implants against bacteria without interfering with bone apposition or inducing a local or systemic inflammatory reaction.88, 89 The versatility of hydrogels and the succeeded in vitro and in vivo outcomes88, 89 makes it an interesting candidate to counteract implant‐related infections.
Another recent approach is layer‐by‐layer (LbL) coating deposition, which could lead to higher efficacy and fewer adverse effects regarding controlled release of antimicrobial substances.90 The multilayer coating buildup via LbL is based solely on electrostatic attractions between polyelectrolytes with opposite charges. For this, three important characteristics must be taken into account: properties of the substrate, polyelectrolytes, and antimicrobial agents. For LbL construction, different polyelectrolytes have been used to develop the multilayered coating, including synthetic polymers poly‐L‐lysine (PLL), polyethyleneimine (PEI), polyacrylic acid (PAA), natural polymers (chitosan, hyaluronic acid, DNA, and proteins), and lipids.91, 92, 93, 94, 95, 96 As a key concept in LbL technology, self‐assembly is primitively based on noncovalent interactions between molecules. This means that these interactions can suffer from external factors related to environmental conditions.94, 97 The dynamic essence of those noncovalent interactions maintains the three‐dimensional structures of large molecules, which can exhibit stimuli responsiveness to various physical, chemical, or biological stimuli, including temperature, light, pH, ionic strength, redox agent, and enzymes.98 This allows for controlling the desired substance delivery to a specific target site. Based upon current knowledge of peri‐implantitis pathology, a wide pH variation within peri‐implant tissue is generally observed at different stages of disease. For instance, an acidic pH is present during acute inflammation, which shifts toward alkaline values during chronic inflammation.99 As a consequence of acid‐base conditions, each inflammation phase will directly affect the microbial profile. The alkaline pH may play a role in peri‐implant infections since Gram‐negative anaerobic bacteria species often flourish in relatively higher pH levels.100, 101 Hence, LbL systems could deliver antimicrobial substances under varying pH conditions and initiate drug release against peri‐implantitis once required.
Since hydrogels easily respond to certain stimuli, these polymeric structures could also be used to control antimicrobial agents release behavior and ultimately therapeutic outcomes. A number of different mechanisms, including those based on pH, redox potential, or enzymatic changes, produced during the disease initiation, could destitute the drug and affect the multilayers morphology.102, 103 As a consequence of this action, the structural and chemical LbL modifications would allow slow release of antimicrobial agents overtime (Figure 1). For example, to achieve slower release kinetics, a pH‐responsive polymeric capsules were assembled combining poly(2‐diisopropylaminoethyl methacrylate PDPA and poly(methacrylic acid) (PMA) via LbL assembly and cross‐linked by click chemistry.103 Effectively, PDPA undergoes a charge‐shifting transition from hydrophobic to hydrophilic when in an acidic environment. With this strategy, the capsules swell, allowing degradation of the instable disulfide bond moieties, and release the drug.104, 105 In order to create effective multilayer systems on implant devices to reduce implant failures, it is important to understand how each material responds to biological conditions and how it interacts ion with the antimicrobial agents.90
Figure 1.
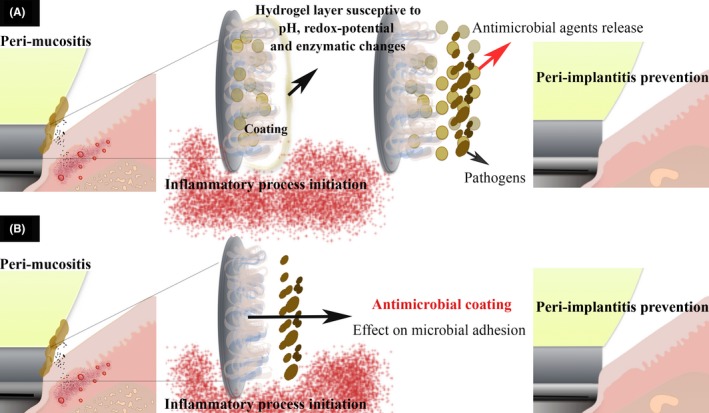
Proposed antimicrobial mechanism addressed to peri‐implantitis prevention. Biomaterial coating onto Ti substrate to release antimicrobial substances after inflammatory process initiation
Besides antimicrobial coating properties required to prevent bacterial colonization on subgingival dental implant surfaces, it is also a necessary functional peri‐implant epithelial sealing at the interface of the implant, especially at the coronal margin.106 The natural ability of epithelial cells in attaching to the tooth surfaces and maintaining the integrity of internal environment should be preserved to dental implant structures. In a recent investigation, it was shown that the potential of platelets to improve peri‐implant epithelial sealing with basal lamina attachment at the interface of the implant. The idea in modifying the titanium surface with protease‐activated receptor 4‐activating peptide and platelet‐rich plasma was successful in demonstrating epithelial attachment with no bacterial invasion into the interface of the epithelial sheet.107 The antimicrobial peptides naturally released from epithelial cells were competent in avoiding bacterial colonization at the epithelial sheet.107
In an advanced stage of the disease, with the constant and increased presence of dangerous bacterial products and consequent immune inflammatory biological response, elimination of the biofilm from the implant surface is the prime process to interrupt disease progression.108 However, depending on the bone loss extension, complete eradication of biofilm attached on dental implants is not easy, mainly in the apical region109, 110 because peri‐implant pocket becomes deeper as the bone resorbs. Nowadays, focus on peri‐implantitis treatment requires primarily mechanical nonsurgical/surgical therapy for removal of the biofilm and calcified deposits from the implant surface.109, 110, 111 Although, those current therapies have been found to reduce probe depth and bleeding, from a clinical perspective, the evidences on their efficacy to treat peri‐implantitis are still limited.
In order to improve the efficiency of the mechanical debridement of peri‐implant pockets, biomolecules/drugs have been incorporated into an LbL system to be applied to the affected implant sites.112 To achieve this goal, LbL deposition process on Ti substrates should be capable of releasing a sustained high dose of antibiotic specifically into affected sites and facilitating new bone formation to ensure intimate contact between the bone and implant surface (Figure 2).113 Concomitantly, coating should combine advancements in surface chemistry to disclose the capacity of stimulating an effective epithelial tissue barrier surrounded implant‐abutment components against bacterial penetration.114 Although most of the conclusions regarding the biomedical applications of LbL self‐assembly has been derived from in vitro studies,113, 115, 116 the ability of this system in incorporating drugs and the versatile chemical properties of polyelectrolytes for coating any surface render the method attractive.
Figure 2.
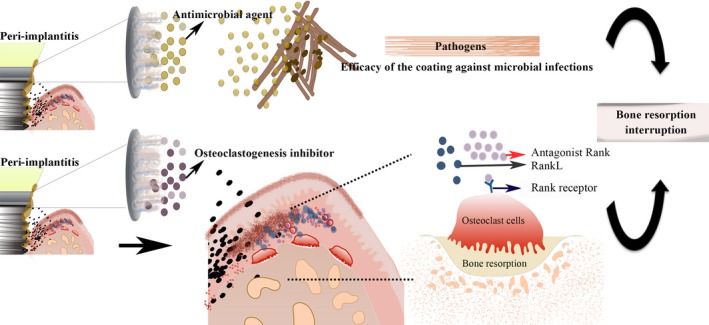
Proposed antimicrobial mechanism addressed to peri‐implantitis treatment. Biomaterial coating onto Ti substrate to release a sustained high dose of antimicrobial substances into affected sites
As mentioned earlier, antibiotics are still considered important protagonists to fight disease and the drastic issue provoked by the microorganism resistance can be relieved by its punctual and local application. However, the undesired collateral effects provoked by indiscriminate antibiotic prescription has pushed alternative antimicrobial agents to take this approach to the next level.117 Another special interest in the field of biomaterial‐based solutions for peri‐implantitis is mimicking the structural and functional complexity of the biological system by developing synthetic biomaterials. Since peri‐implantitis is modulated and regulated by the host, a mechanism to interrupt the inflammatory pathway could be convenient to block bone resorption. Virulence factors, such as lipopolysaccharides (LPS) of the outer cell wall of pathogenic bacteria, can bind to Toll‐like receptors (TLR) from epithelial cells and trigger the innate inflammatory response. The subsequent release of the complex pro‐inflammatory agents, like cytokines and other mediators, evoke bone resorption. These molecules can overall activate the immune response; however, the pathway involved in this process will depend on the bacterial species. For example, it has been demonstrated that B‐cell proliferation is differently regulated by P gingivalis and Escherichia coli (E coli) LPS.118, 119 The advance in understanding the function of LPSs from different species in triggering the immune system represents the possibility of recognizing the role of pathogenic bacterial species in the disease beginning and its progression.
4. CONCLUSION
In this review, we presented a wide range of biomaterial‐based possibilities for the treatment of peri‐implantitis. Physicochemical modifications of dental implants play a role in the reduction in microorganism adhesion but do not avoid peri‐implantitis. Biomaterials can also be used as carrier coating for antimicrobial agents to support the prevention and/or treatment of peri‐implant mucositis and peri‐implantitis. Remaining challenges in this field involve optimization of coating assembly to improve long‐term storage of multilayer systems and to control new triggered and responsive release mechanisms. Further research in the advancement in materials capable of coordinated and sustained multidrug release is needed to provide new avenues for prevention and/ or treatment of peri‐implantitis.
ACKNOWLEDGEMENTS
Erica Dorigatti de Avila was supported by the São Paulo Research Foundation (FAPESP) grant #2016/19650‐3 and 2015/03567‐7.
de Avila ED, van Oirschot BAJA, van den Beucken JJJP. Biomaterial‐based possibilities for managing peri‐implantitis. J Periodont Res. 2020;55:165–173. 10.1111/jre.12707
REFERENCES
- 1. Lindhe J, Meyle J, Group D of European Workshop on Periodontology . Peri‐implant diseases: Consensus Report of the Sixth European Workshop on Periodontology. J Clin Periodontol. 2008;35(8 suppl):282‐285. [DOI] [PubMed] [Google Scholar]
- 2. Caton J, Berglundh T, Chapple I, et al. A new classification scheme for periodontal and peri‐implant diseases and conditions – Introduction and key changes from the 1999 classification. J Clin Periodontol. 2018;45:S1‐S8. [DOI] [PubMed] [Google Scholar]
- 3. Carcuac O, Berglundh T. Composition of human peri‐implantitis and periodontitis lesions. J Dent Res. 2014;93:1083‐1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Charalampakis G, Leonhardt A, Rabe P, Dahlen G. Clinical and microbiological characteristics of peri‐implantitis cases: a retrospective multicentre study. Clin Oral Implants Res. 2012;23:1045‐1054. [DOI] [PubMed] [Google Scholar]
- 5. Mombelli A, Muller N, Cionca N. The epidemiology of peri‐implantitis. Clin Oral Implants Res. 2012;23(suppl 6):67‐76. [DOI] [PubMed] [Google Scholar]
- 6. Pjetursson BE, Asgeirsson AG, Zwahlen M, Sailer I. Improvements in implant dentistry over the last decade: comparison of survival and complication rates in older and newer publications. Int J Oral Maxillofac Implants. 2014;29:308‐324. [DOI] [PubMed] [Google Scholar]
- 7. Chen Y, Harapanahalli AK, Busscher HJ, Norde W, van der Mei HC. Nanoscale cell wall deformation impacts long‐range bacterial adhesion forces on surfaces. Appl Environ Microbiol. 2014;80:637‐643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abrahamsson I, Berglundh T, Lindhe J. Soft tissue response to plaque formation at different implant systems. A comparative study in the dog. Clin Oral Implants Res. 1998;9:73‐79. [DOI] [PubMed] [Google Scholar]
- 9. Berglundh T, Armitage G, Araujo MG, et al. Peri‐implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri‐implant diseases and conditions. J Periodontol. 2018;89(suppl 1):S313‐S318. [DOI] [PubMed] [Google Scholar]
- 10. Hahnel S, Wieser A, Lang R, Rosentritt M. Biofilm formation on the surface of modern implant abutment materials. Clin Oral Implants Res. 2015;26(11):1297‐1301. [DOI] [PubMed] [Google Scholar]
- 11. Zheng H, Xu L, Wang Z, et al. Subgingival microbiome in patients with healthy and ailing dental implants. Sci Rep. 2015;5:10948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lang NP, Berglundh T. Working Group 4 of Seventh European Workshop on P. Periimplant diseases: where are we now?–Consensus of the Seventh European Workshop on Periodontology. J Clin Periodontol. 2011;38(suppl 11):178‐181. [DOI] [PubMed] [Google Scholar]
- 13. Coli P, Christiaens V, Sennerby L, Bruyn H. Reliability of periodontal diagnostic tools for monitoring peri‐implant health and disease. Periodontol 2000. 2017;73(1):203‐217. [DOI] [PubMed] [Google Scholar]
- 14. Berglundh T, Armitage G, Araujo MG, et al. Peri‐implant diseases and conditions: consensus report of workgroup 4 of the 2017 world workshop on the classification of periodontal and peri‐implant diseases and conditions. J Clin Periodontol. 2018;45(suppl 20):S286‐S291. [DOI] [PubMed] [Google Scholar]
- 15. Niinomi M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J Mech Behav Biomed Mater. 2008;1:30‐42. [DOI] [PubMed] [Google Scholar]
- 16. Rupp F, Haupt M, Klostermann H, et al. Multifunctional nature of UV‐irradiated nanocrystalline anatase thin films for biomedical applications. Acta Biomater. 2010;6:4566‐4577. [DOI] [PubMed] [Google Scholar]
- 17. Mei S, Wang H, Wang W, et al. Antibacterial effects and biocompatibility of titanium surfaces with graded silver incorporation in titania nanotubes. Biomaterials. 2014;35:4255‐4265. [DOI] [PubMed] [Google Scholar]
- 18. Monje A, Aranda L, Diaz KT, et al. Impact of maintenance therapy for the prevention of peri‐implant diseases: A systematic review and meta‐analysis. J Dent Res. 2016;95:372‐379. [DOI] [PubMed] [Google Scholar]
- 19. Renvert S, Persson GR. Periodontitis as a potential risk factor for peri‐implantitis. J Clin Periodontol. 2009;36(suppl 10):9‐14. [DOI] [PubMed] [Google Scholar]
- 20. Kozlovsky A, Tal H, Laufer BZ, et al. Impact of implant overloading on the peri‐implant bone in inflamed and non‐inflamed peri‐implant mucosa. Clin Oral Implants Res. 2007;18:601‐610. [DOI] [PubMed] [Google Scholar]
- 21. Miyata T, Kobayashi Y, Araki H, Ohto T, Shin K. The influence of controlled occlusal overload on peri‐implant tissue. Part 4: a histologic study in monkeys. Int J Oral Maxillofac Implants. 2002;17:384‐390. [PubMed] [Google Scholar]
- 22. Schou S, Holmstrup P, Stoltze K, Hjorting‐Hansen E, Fiehn NE, Skovgaard LT. Probing around implants and teeth with healthy or inflamed peri‐implant mucosa/gingiva. A histologic comparison in cynomolgus monkeys (Macaca fascicularis). Clin Oral Implants Res. 2002;13:113‐126. [DOI] [PubMed] [Google Scholar]
- 23. Steinebrunner L, Wolfart S, Bossmann K, Kern M. In vitro evaluation of bacterial leakage along the implant‐abutment interface of different implant systems. Int J Oral Maxillofac Implants. 2005;20(6):875‐881. [PubMed] [Google Scholar]
- 24. Jansen VK, Conrads G, Richter EJ. Microbial leakage and marginal fit of the implant‐abutment interface. Int J Oral Maxillofac Implants. 1997;12:527‐540. [PubMed] [Google Scholar]
- 25. Finger IM, Castellon P, Block M, Elian N. The evolution of external and internal implant/abutment connections. Pract Proced Aesthet Dent. 2003;15:625‐632; quiz 634. [PubMed] [Google Scholar]
- 26. Maeda Y, Satoh T, Sogo M. In vitro differences of stress concentrations for internal and external hex implant‐abutment connections: a short communication. J Oral Rehabil. 2006;33:75‐78. [DOI] [PubMed] [Google Scholar]
- 27. Becker W, Becker BE. Replacement of maxillary and mandibular molars with single endosseous implant restorations: a retrospective study. J Prosthet Dent. 1995;74:51‐55. [DOI] [PubMed] [Google Scholar]
- 28. Freitas‐Junior AC, Rocha EP, Bonfante EA, et al. Biomechanical evaluation of internal and external hexagon platform switched implant‐abutment connections: an in vitro laboratory and three‐dimensional finite element analysis. Dent Mater. 2012;28:e218‐228. [DOI] [PubMed] [Google Scholar]
- 29. Steinebrunner L, Wolfart S, Ludwig K, Kern M. Implant‐abutment interface design affects fatigue and fracture strength of implants. Clin Oral Implants Res. 2008;19:1276‐1284. [DOI] [PubMed] [Google Scholar]
- 30. Dibart S, Warbington M, Su MF, Skobe Z. In vitro evaluation of the implant‐abutment bacterial seal: the locking taper system. Int J Oral Maxillofac Implants. 2005;20:732‐737. [PubMed] [Google Scholar]
- 31. Scarano A, Lorusso C, Di Giulio C, Mazzatenta A. Evaluation of the sealing capability of the implant healing screw by using real time volatile organic compounds analysis: internal hexagon versus cone morse. J Periodontol. 2016;87:1492‐1498. [DOI] [PubMed] [Google Scholar]
- 32. Mangano C, Mangano F, Piattelli A, Iezzi G, Mangano A, La Colla L. Prospective clinical evaluation of 1920 Morse taper connection implants: results after 4 years of functional loading. Clin Oral Implants Res. 2009;20:254‐261. [DOI] [PubMed] [Google Scholar]
- 33. Pereira J, Morsch CS, Henriques B, et al. Removal torque and biofilm accumulation at two dental implant‐abutment joints after fatigue. Int J Oral Maxillofac Implants. 2016;31:813‐819. [DOI] [PubMed] [Google Scholar]
- 34. Gil FJ, Herrero‐Climent M, Lazaro P, Rios JV. Implant‐abutment connections: influence of the design on the microgap and their fatigue and fracture behavior of dental implants. J Mater Sci Mater Med. 2014;25:1825‐1830. [DOI] [PubMed] [Google Scholar]
- 35. Koutouzis T, Wallet S, Calderon N, Lundgren T. Bacterial colonization of the implant‐abutment interface using an in vitro dynamic loading model. J Periodontol. 2011;82:613‐618. [DOI] [PubMed] [Google Scholar]
- 36. Bressan E, Stocchero M, Jimbo R, et al. Microbial leakage at morse taper conometric prosthetic connection: an in vitro investigation. Implant Dent. 2017;26:756‐761. [DOI] [PubMed] [Google Scholar]
- 37. Koutouzis T, Gadalla H, Lundgren T. Bacterial colonization of the implant‐abutment interface (IAI) of dental implants with a sloped marginal design: an in‐vitro study. Clin Implant Dent Relat Res. 2016;18:161‐167. [DOI] [PubMed] [Google Scholar]
- 38. Ranieri R, Ferreira A, Souza E, et al. The bacterial sealing capacity of morse taper implant‐abutment systems in vitro. J Periodontol. 2015;86:696‐702. [DOI] [PubMed] [Google Scholar]
- 39. Zhao B, van der Mei HC, Subbiahdoss G, et al. Soft tissue integration versus early biofilm formation on different dental implant materials. Dent Mater. 2014;30:716‐727. [DOI] [PubMed] [Google Scholar]
- 40. Gittens RA, McLachlan T, Olivares‐Navarrete R, et al. The effects of combined micron‐/submicron‐scale surface roughness and nanoscale features on cell proliferation and differentiation. Biomaterials. 2011;32:3395‐3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Silva‐Bermudez P, Almaguer‐Flores A, Garcia VI, Olivares‐Navarrete R, Rodil SE. Enhancing the osteoblastic differentiation through nanoscale surface modifications. J Biomed Mater Res A. 2017;105:498‐509. [DOI] [PubMed] [Google Scholar]
- 42. Anselme K, Bigerelle M. Statistical demonstration of the relative effect of surface chemistry and roughness on human osteoblast short‐term adhesion. J Mater Sci Mater Med. 2006;17:471‐479. [DOI] [PubMed] [Google Scholar]
- 43. de Avila ED, Avila‐Campos MJ, Vergani CE, Spolidorio DM, Mollo Fde A Jr. Structural and quantitative analysis of a mature anaerobic biofilm on different implant abutment surfaces. J Prosthet Dent. 2016;115:428‐436. [DOI] [PubMed] [Google Scholar]
- 44. de Avila ED, de Molon RS, Lima BP, et al. Impact of physical chemical characteristics of abutment implant surfaces on bacteria adhesion. J Oral Implantol. 2016;42:153‐158. [DOI] [PubMed] [Google Scholar]
- 45. de Avila ED, Vergani CE, Mollo Junior FA, Junior MJ, Shi W, Lux R. Effect of titanium and zirconia dental implant abutments on a cultivable polymicrobial saliva community. J Prosthet Dent. 2017;118:481‐487. [DOI] [PubMed] [Google Scholar]
- 46. Bevilacqua L, Milan A, Del Lupo V, Maglione M, Dolzani L. Biofilms developed on dental implant titanium surfaces with different roughness: comparison between in vitro and in vivo studies. Curr Microbiol. 2018;75:766‐772. [DOI] [PubMed] [Google Scholar]
- 47. Cury MS, Silva CB, Nogueira RD, Campos M, Palma‐Dibb RG, Geraldo‐Martins VR. Surface roughness and bacterial adhesion on root dentin treated with diode laser and conventional desensitizing agents. Lasers Med Sci. 2018;33:257‐262. [DOI] [PubMed] [Google Scholar]
- 48. Yuan C, Wang X, Gao X, Chen F, Liang X, Li D. Effects of surface properties of polymer‐based restorative materials on early adhesion of Streptococcus mutans in vitro. J Dent. 2016;54:33‐40. [DOI] [PubMed] [Google Scholar]
- 49. Nogueira RD, Silva CB, Lepri CP, Palma‐Dibb RG, Geraldo‐Martins VR. Evaluation of surface roughness and bacterial adhesion on tooth enamel irradiated with high intensity lasers. Braz Dent J. 2017;28:24‐29. [DOI] [PubMed] [Google Scholar]
- 50. McGaffey M, Zur Linden A, Bachynski N, Oblak M, James F, Weese JS. Manual polishing of 3D printed metals produced by laser powder bed fusion reduces biofilm formation. PLoS ONE. 2019;14:e0212995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Bollen CM, Papaioanno W, Van Eldere J, Schepers E, Quirynen M, van Steenberghe D. The influence of abutment surface roughness on plaque accumulation and peri‐implant mucositis. Clin Oral Implants Res. 1996;7:201‐211. [DOI] [PubMed] [Google Scholar]
- 52. Lauer G, Wiedmann‐Al‐Ahmad M, Otten JE, Hubner U, Schmelzeisen R, Schilli W. The titanium surface texture effects adherence and growth of human gingival keratinocytes and human maxillar osteoblast‐like cells in vitro. Biomaterials. 2001;22:2799‐2809. [DOI] [PubMed] [Google Scholar]
- 53. de Avila ED, Lima BP, Sekiya T, et al. Effect of UV‐photofunctionalization on oral bacterial attachment and biofilm formation to titanium implant material. Biomaterials. 2015;67:84‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Park JW, Kim YJ, Jang JH. Enhanced osteoblast response to hydrophilic strontium and/or phosphate ions‐incorporated titanium oxide surfaces. Clin Oral Implants Res. 2010;21:398‐408. [DOI] [PubMed] [Google Scholar]
- 55. Lotz EM, Olivares‐Navarrete R, Berner S, Boyan BD, Schwartz Z. Osteogenic response of human MSCs and osteoblasts to hydrophilic and hydrophobic nanostructured titanium implant surfaces. J Biomed Mater Res A. 2016;104:3137‐3148. [DOI] [PubMed] [Google Scholar]
- 56. Miyauchi T, Yamada M, Yamamoto A, et al. The enhanced characteristics of osteoblast adhesion to photofunctionalized nanoscale TiO2 layers on biomaterials surfaces. Biomaterials. 2010;31:3827‐3839. [DOI] [PubMed] [Google Scholar]
- 57. Tsukimura N, Yamada M, Iwasa F, et al. Synergistic effects of UV photofunctionalization and micro‐nano hybrid topography on the biological properties of titanium. Biomaterials. 2011;32:4358‐4368. [DOI] [PubMed] [Google Scholar]
- 58. Aita H, Hori N, Takeuchi M, et al. The effect of ultraviolet functionalization of titanium on integration with bone. Biomaterials. 2009;30:1015‐1025. [DOI] [PubMed] [Google Scholar]
- 59. Funato A, Ogawa T. Photofunctionalized dental implants: a case series in compromised bone. Int J Oral Maxillofac Implants. 2013;28:1589‐1601. [DOI] [PubMed] [Google Scholar]
- 60. Hirota M, Ikeda T, Tabuchi M, Ozawa T, Tohnai I, Ogawa T. Effects of ultraviolet photofunctionalization on bone augmentation and integration capabilities of titanium mesh and implants. Int J Oral Maxillofac Implants. 2017;32:52‐62. [DOI] [PubMed] [Google Scholar]
- 61. Qian SJ, Mo JJ, Shi JY, Gu YX, Si MS, Lai HC. Endo‐sinus bone formation after transalveolar sinus floor elevation without grafting with simultaneous implant placement: histological and histomorphometric assessment in a dog model. J Clin Periodontol. 2018;45:1118‐1127. [DOI] [PubMed] [Google Scholar]
- 62. Zinger O, Zhao G, Schwartz Z, et al. Differential regulation of osteoblasts by substrate microstructural features. Biomaterials. 2005;26:1837‐1847. [DOI] [PubMed] [Google Scholar]
- 63. Allen LT, Fox EJ, Blute I, et al. Interaction of soft condensed materials with living cells: phenotype/transcriptome correlations for the hydrophobic effect. Proc Natl Acad Sci U S A. 2003;100:6331‐6336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yumoto H, Hirota K, Hirao K, et al. Anti‐inflammatory and protective effects of 2‐methacryloyloxyethyl phosphorylcholine polymer on oral epithelial cells. J Biomed Mater Res A. 2015;103:555‐563. [DOI] [PubMed] [Google Scholar]
- 65. Maniotis AJ, Chen CS, Ingber DE. Demonstration of mechanical connections between integrins, cytoskeletal filaments, and nucleoplasm that stabilize nuclear structure. Proc Natl Acad Sci U S A. 1997;94:849‐854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Jain N, Iyer KV, Kumar A, Shivashankar GV. Cell geometric constraints induce modular gene‐expression patterns via redistribution of HDAC3 regulated by actomyosin contractility. Proc Natl Acad Sci U S A. 2013;110:11349‐11354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Hickok NJ, Shapiro IM. Immobilized antibiotics to prevent orthopaedic implant infections. Adv Drug Deliv Rev. 2012;64:1165‐1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rasmussen K, Nikrad J, Reilly C, Li Y, Jones RS. N‐Acetyl‐l‐cysteine effects on multi‐species oral biofilm formation and bacterial ecology. Lett Appl Microbiol. 2016;62:30‐38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Tsuchiya H, Shirai T, Nishida H, et al. Innovative antimicrobial coating of titanium implants with iodine. J Orthop Sci. 2012;17:595‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Haro Chavez NL, de Avila ED, Barbugli PA, et al. Promising effects of silver tungstate microcrystals on fibroblast human cells and three dimensional collagen matrix models: a novel non‐cytotoxic material to fight oral disease. Colloids Surf B Biointerfaces. 2018;170:505‐513. [DOI] [PubMed] [Google Scholar]
- 71. Ahamed M, Karns M, Goodson M, et al. DNA damage response to different surface chemistry of silver nanoparticles in mammalian cells. Toxicol Appl Pharmacol. 2008;233:404‐410. [DOI] [PubMed] [Google Scholar]
- 72. Jepsen K, Jepsen S. Antibiotics/antimicrobials: systemic and local administration in the therapy of mild to moderately advanced periodontitis. Periodontol 2000. 2016;71(1):82‐112. [DOI] [PubMed] [Google Scholar]
- 73. Finkelman RD, Polson AM. Evidence‐based considerations for the clinical use of locally delivered, controlled‐release antimicrobials in periodontal therapy. J Dent Hyg. 2013;87:249‐264. [PubMed] [Google Scholar]
- 74. Renvert S, Lessem J, Dahlen G, Renvert H, Lindahl C. Mechanical and repeated antimicrobial therapy using a local drug delivery system in the treatment of peri‐implantitis: a randomized clinical trial. J Periodontol. 2008;79:836‐844. [DOI] [PubMed] [Google Scholar]
- 75. Alecio A, Ferreira CF, Babu J, et al. Doxycycline release of dental implants with nanotube surface, coated with poly lactic‐co‐glycolic acid (PLGA) for extended pH‐controlled drug delivery. J Oral Implantol. 2019;45:267‐273. [DOI] [PubMed] [Google Scholar]
- 76. Bassetti M, Schar D, Wicki B, et al. Anti‐infective therapy of peri‐implantitis with adjunctive local drug delivery or photodynamic therapy: 12‐month outcomes of a randomized controlled clinical trial. Clin Oral Implants Res. 2014;25:279‐287. [DOI] [PubMed] [Google Scholar]
- 77. Lee JB, Kweon HH, Cho HJ, Kim CS, Kim YT. Characteristics of local delivery agents for treating peri‐implantitis on dental implant surfaces: a preclinical study. J Oral Implantol. 2019;45:116‐126. [DOI] [PubMed] [Google Scholar]
- 78. Guo J, Sun H, Lei W, et al. MMP‐8‐responsive polyethylene glycol hydrogel for intraoral drug delivery. J Dent Res. 2019;98:564‐571. [DOI] [PubMed] [Google Scholar]
- 79. Jovanovic SA. The management of peri‐implant breakdown around functioning osseointegrated dental implants. J Periodontol. 1993;64(11 suppl):1176‐1183. [DOI] [PubMed] [Google Scholar]
- 80. Wichterle O, Lim D. Hydrophilic gels for biological use. Nature. 1960;185:117‐118. [Google Scholar]
- 81. Pichayakorn W, Boonme P. Evaluation of cross‐linked chitosan microparticles containing metronidazole for periodontitis treatment. Mater Sci Eng C Mater Biol Appl. 2013;33:1197‐1202. [DOI] [PubMed] [Google Scholar]
- 82. Yue K, Li X, Schrobback K, et al. Structural analysis of photocrosslinkable methacryloyl‐modified protein derivatives. Biomaterials. 2017;139:163‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Yue K, Trujillo‐de Santiago G, Alvarez MM, Tamayol A, Annabi N, Khademhosseini A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials. 2015;73:254‐271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Liu Y, Geever LM, Kennedy JE, Higginbotham CL, Cahill PA, McGuinness GB. Thermal behavior and mechanical properties of physically crosslinked PVA/Gelatin hydrogels. J Mech Behav Biomed Mater. 2010;3:203‐209. [DOI] [PubMed] [Google Scholar]
- 85. Choi J, Bodugoz‐Senturk H, Kung HJ, Malhi AS, Muratoglu OK. Effects of solvent dehydration on creep resistance of poly(vinyl alcohol) hydrogel. Biomaterials. 2007;28:772‐780. [DOI] [PubMed] [Google Scholar]
- 86. Zhu J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials. 2010;31:4639‐4656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Veiga AS, Schneider JP. Antimicrobial hydrogels for the treatment of infection. Biopolymers. 2013;100:637‐644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Boot W, Gawlitta D, Nikkels P, et al. Hyaluronic acid‐based hydrogel coating does not affect bone apposition at the implant surface in a rabbit model. Clin Orthop Relat Res. 2017;475:1911‐1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Drago L, Boot W, Dimas K, et al. Does implant coating with antibacterial‐loaded hydrogel reduce bacterial colonization and biofilm formation in vitro? Clin Orthop Relat Res. 2014;472:3311‐3323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Boudou T, Crouzier T, Ren K, Blin G, Picart C. Multiple functionalities of polyelectrolyte multilayer films: new biomedical applications. Adv Mater. 2010;22:441‐467. [DOI] [PubMed] [Google Scholar]
- 91. Caruso F, Caruso RA, Mohwald H. Nanoengineering of inorganic and hybrid hollow spheres by colloidal templating. Science. 1998;282:1111‐1114. [DOI] [PubMed] [Google Scholar]
- 92. Cavalieri F, Postma A, Lee L, Caruso F. Assembly and functionalization of DNA‐polymer microcapsules. ACS Nano. 2009;3:234‐240. [DOI] [PubMed] [Google Scholar]
- 93. Stadler B, Chandrawati R, Goldie K, Caruso F. Capsosomes: subcompartmentalizing polyelectrolyte capsules using liposomes. Langmuir. 2009;25:6725‐6732. [DOI] [PubMed] [Google Scholar]
- 94. van den Beucken JJ, Vos MR, Thune PC, et al. Fabrication, characterization, and biological assessment of multilayered DNA‐coatings for biomaterial purposes. Biomaterials. 2006;27:691‐701. [DOI] [PubMed] [Google Scholar]
- 95. Guillot R, Pignot‐Paintrand I, Lavaud J, et al. Assessment of a polyelectrolyte multilayer film coating loaded with BMP‐2 on titanium and PEEK implants in the rabbit femoral condyle. Acta Biomater. 2016;36:310‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Zhong X, Song Y, Yang P, et al. Titanium surface priming with phase‐transited lysozyme to establish a silver nanoparticle‐loaded chitosan/hyaluronic acid antibacterial multilayer via layer‐by‐layer self‐assembly. PLoS ONE. 2016;11:e0146957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. van den Beucken JJ, Walboomers XF, Boerman OC, et al. Functionalization of multilayered DNA‐coatings with bone morphogenetic protein 2. J Control Release. 2006;113(1):63‐72. [DOI] [PubMed] [Google Scholar]
- 98. Min J, Braatz RD, Hammond PT. Tunable staged release of therapeutics from layer‐by‐layer coatings with clay interlayer barrier. Biomaterials. 2014;35:2507‐2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Nyako EA, Watson CJ, Preston AJ. Determination of the pH of peri‐implant crevicular fluid in successful and failing dental implant sites: a pilot study. Arch Oral Biol. 2005;50:1055‐1059. [DOI] [PubMed] [Google Scholar]
- 100. Aino K, Hirota K, Okamoto T, Tu Z, Matsuyama H, Yumoto I. Microbial communities associated with indigo fermentation that thrive in anaerobic alkaline environments. Front Microbiol. 2018;9:2196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Mombelli A. Microbiology of the dental implant. Adv Dent Res. 1993;7:202‐206. [DOI] [PubMed] [Google Scholar]
- 102. Gao H, Goriacheva OA, Tarakina NV, Sukhorukov GB. Intracellularly biodegradable polyelectrolyte/silica composite microcapsules as carriers for small molecules. ACS Appl Mater Interfaces. 2016;8:9651‐9661. [DOI] [PubMed] [Google Scholar]
- 103. Liang K, Such GK, Zhu Z, Yan Y, Lomas H, Caruso F. Charge‐shifting click capsules with dual‐responsive cargo release mechanisms. Adv Mater. 2011;23:H273‐277. [DOI] [PubMed] [Google Scholar]
- 104. Du J, Tang Y, Lewis AL, Armes SP. pH‐sensitive vesicles based on a biocompatible zwitterionic diblock copolymer. J Am Chem Soc. 2005;127(51):17982‐17983. [DOI] [PubMed] [Google Scholar]
- 105. Lomas H, Johnston AP, Such GK, et al. Polymersome‐loaded capsules for controlled release of DNA. Small. 2011;7:2109‐2119. [DOI] [PubMed] [Google Scholar]
- 106. Pollanen MT, Salonen JI, Uitto VJ. Structure and function of the tooth‐epithelial interface in health and disease. Periodontol 2000. 2003;31(1):12‐31. [DOI] [PubMed] [Google Scholar]
- 107. Maeno M, Lee C, Kim DM, et al. Function of platelet‐induced epithelial attachment at titanium surfaces inhibits microbial colonization. J Dent Res. 2017;96:633‐639. [DOI] [PubMed] [Google Scholar]
- 108. Peri‐implant mucositis and peri‐implantitis: a current understanding of their diagnoses and clinical implications. J Periodontol. 2013;84:436‐443. [DOI] [PubMed] [Google Scholar]
- 109. Renvert S, Polyzois I. Treatment of pathologic peri‐implant pockets. Periodontol 2000. 2018;76(1):180‐190. [DOI] [PubMed] [Google Scholar]
- 110. Renvert S, Samuelsson E, Lindahl C, Persson GR. Mechanical non‐surgical treatment of peri‐implantitis: a double‐blind randomized longitudinal clinical study. I: clinical results. J Clin Periodontol. 2009;36:604‐609. [DOI] [PubMed] [Google Scholar]
- 111. La Monaca G, Pranno N, Annibali S, Cristalli MP, Polimeni A. Clinical and radiographic outcomes of a surgical reconstructive approach in the treatment of peri‐implantitis lesions: a 5‐year prospective case series. Clin Oral Implants Res. 2018;29:1025‐1037. [DOI] [PubMed] [Google Scholar]
- 112. Mombelli A, Feloutzis A, Bragger U, Lang NP. Treatment of peri‐implantitis by local delivery of tetracycline. Clinical, microbiological and radiological results. Clin Oral Implants Res. 2001;12:287‐294. [DOI] [PubMed] [Google Scholar]
- 113. Mattioli‐Belmonte M, Cometa S, Ferretti C, et al. Characterization and cytocompatibility of an antibiotic/chitosan/cyclodextrins nanocoating on titanium implants. Carbohydr Polym. 2014;110:173‐182. [DOI] [PubMed] [Google Scholar]
- 114. Ivanovski S, Lee R. Comparison of peri‐implant and periodontal marginal soft tissues in health and disease. Periodontol 2000. 2018;76(1):116‐130. [DOI] [PubMed] [Google Scholar]
- 115. de Avila ED, Castro AGB Tagit O, et al. Anti‐bacterial efficacy via drug‐delivery system from layer‐by‐layer coating for percutaneous dental implant components. Appl Surf Sci. 2019;488:194‐204. [Google Scholar]
- 116. Jin S, Gu H, Chen X, et al. A facile method to prepare a versatile surface coating with fibrinolytic activity, vascular cell selectivity and antibacterial properties. Colloids Surf B Biointerfaces. 2018;167:28‐35. [DOI] [PubMed] [Google Scholar]
- 117. Kaye KS, Pogue JM. Infections caused by resistant gram‐negative bacteria: epidemiology and management. Pharmacotherapy. 2015;35:949‐962. [DOI] [PubMed] [Google Scholar]
- 118. Nebel D, Arvidsson J, Lillqvist J, Holm A, Nilsson BO. Differential effects of LPS from Escherichia coli and Porphyromonas gingivalis on IL‐6 production in human periodontal ligament cells. Acta Odontol Scand. 2013;71:892‐898. [DOI] [PubMed] [Google Scholar]
- 119. Yu X, Wang Y, Lin J, et al. Lipopolysaccharides‐induced suppression of innate‐like B cell apoptosis is enhanced by CpG oligodeoxynucleotide and requires toll‐like receptors 2 and 4. PLoS ONE. 2016;11:e0165862. [DOI] [PMC free article] [PubMed] [Google Scholar]


