Summary
While rejection prevention with innovator tacrolimus (Tac) is one of the key factors for long‐lasting graft function, the use of generic Tac is still under debate. Thus, we performed a systematic review and meta‐analysis to provide an overview on the current body of evidence for the effect of generic Tac in adult liver (LT) and kidney transplantation (KT) with focus on both biopsy‐proven acute rejection (BPAR) and bioequivalence. A systematic literature search for trials comparing generic versus innovator Tac was conducted accordingly. Seventeen studies (5 LT, 11 KT, 1 LT/KT) including 1412 patients were identified. About 92.9% (13/14; 5/5 LT, 8/9 KT) of studies reported the same or lower BPAR with generics (pooled RR: 0.84, 95% CI: 0.65–1.09); however, de novo studies showed a significantly lower risk with generic Tac (RR: 0.75, 95% CI: 0.63–0.90), whereas conversion studies showed increased risk (RR: 1.93, 95% CI: 1.00–3.70). Bioequivalence was demonstrated primarily in studies on conversion. The current evidence is mostly based on observational data and studies showing some risk of bias. In conclusion, whereas overall there was no significant difference in terms of BPAR, there is some evidence suggesting lower BPAR risk with generic Tac for de novo use.
Keywords: generic immunosuppression, kidney, liver, transplantation
Introduction
With the expiry of patents for commonly prescribed immunosuppressive drugs, generic products become commonly available. Generics contain the same effective ingredient dosed in the same way as the innovator drug. Generics seem to be more cost‐efficient since their manufacturers do not need to provide safety and efficacy data; however, for their approval, a proof of bioequivalence and pharmaceutical equivalence to its innovator is mandatory 1, 2, 3, 4, 5, 6, 7, 8, 9, 10. Generics promote competition and crumbling of prizes, which is of special importance for countries with limited healthcare resources.
Generally, generics are safe alternatives for the treatment of various diseases that are already well‐accepted standard; however, in the past, there were safety concerns in the field of immunosuppression, that is, potential drug interactions and trough level variability 11.
Tacrolimus (Tac) is the main constituent of most immunosuppressive regimens worldwide. There is little evidence supporting therapeutic equivalence of generic formulations of immunosuppressive medications, including Tac, in solid organ transplantation 12. There is not only a lack of high‐quality data supporting the equivalence of generic and innovator immunosuppressive drugs but also a lack of data to suggest that they are not equivalent 11.
Tac lost its patent in 2008. Since then, a number of generic preparations have come to the market. To summarize the available evidence, a systematic review and meta‐analysis of studies on generic versus innovator Tac in adult solid liver (LT) and kidney transplantation (KT) with a special focus on both biopsy‐proven acute rejection (BPAR) and bioequivalence was performed.
Patients and methods
Literature search strategy
A comprehensive systematic search of published articles on generic Tac from database inception to August 31, 2018, was performed using PubMed, CENTRAL and Embase (OvidSP). The search was carried out with the assistance of a librarian experienced in systematic reviews. A structured search strategy (Appendix S1) was conducted with controlled vocabulary and relevant key terms to enhance sensitivity. The search strategy combined the following search terms: “immunosuppressive OR immunosuppress*,” “generic OR generic tacrolimus OR generic*,” “tacrolimus OR FK506* OR FK506,” and “transplantation OR transplant*.” In addition, reference lists of included papers and previous reviews were reviewed to identify potentially eligible studies.
Study selection
First, all abstracts identified by the search strategy after removal of duplicates were independently screened by two investigators (JK and PS). If no abstract was available, the full text was obtained unless the article could be confidently excluded by title alone. Studies reporting on BPAR, which was the primary clinical efficacy outcome, or bioequivalence criteria, specifically area under the curve (AUC) and concentration maximum (C max), in adult patients after LT and KT taking generic Tac for immunosuppression were considered. Randomized and non‐randomized studies comparing the generic version of Tac with innovator Tac in parallel groups or with a crossover design were eligible. Case reports, case series, studies including children or animals, and in vitro studies were excluded, as were studies with a before‐after design without a control group.
Conference abstracts collected by hand search (published proceedings) from international transplant congresses (American Transplant Congress (ATC), European Socitey of organ transplantation (ESOT) Congress, Congress of the British transplantation society (BTS), the German Transplant Society (DTG), The Transplantation Society, and the International Liver Transplantation Society (ILTS)) covering the same time period as the literature search were also considered and are presented separately. Any disagreements during the screening process were resolved through discussion among the authors. We obtained the full texts of potentially eligible studies and again determined their suitability based on the selection criteria. Only full‐text papers published in English were assessed.
Data extraction
The following information was extracted from all studies: study design, characteristics of the population studied, organ transplanted, number of study participants per group, duration of follow‐up, type of generic Tac formulation used, clinical safety and efficacy parameters as well as BPAR and bioequivalence parameters.
Quality assessment
The methodological quality of included randomized trials was evaluated with the Cochrane risk of bias assessment tool 13. The methodological quality of the non‐randomized included studies was assessed using the Newcastle–Ottawa Quality Assessment Scale for Cohort Studies 14.
Data synthesis
We performed a random‐effects meta‐analysis with inverse variance weighting for each of the three outcomes, BPAR, AUC0–12, and C max, and the data are presented in forest plots. For BPAR, the risk ratio and the respective 95% confidence interval (CI) for generic versus innovator Tac in each study were estimated from the reported events. If a study observed no event in one of the two groups, 0.5 was added to each count to allow for an estimate 15. If a study observed no event in either group, no risk ratio was calculable.
For bioequivalence studies, the geometric mean ratios (GMR) of the AUC0–12 and C max with the respective 90% CI were extracted and standard errors estimated therefrom. Due to initial heterogeneity, subgroup analyses were conducted for organ transplanted (liver versus kidney) and use (de novo versus conversion).
The analyses were performed using R version 3.5.3, in particular package “meta.”
Results
Literature search
The initial search identified 574 hits, 453 of which remained after the elimination of duplicates. A total of 390 publications were excluded during abstract screening. After the elimination of preliminary reports (2), case reports (3), reviews (25), and studies without a control group (16), 17 studies met the inclusion criteria 16, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32 (Fig. 1).
Figure 1.
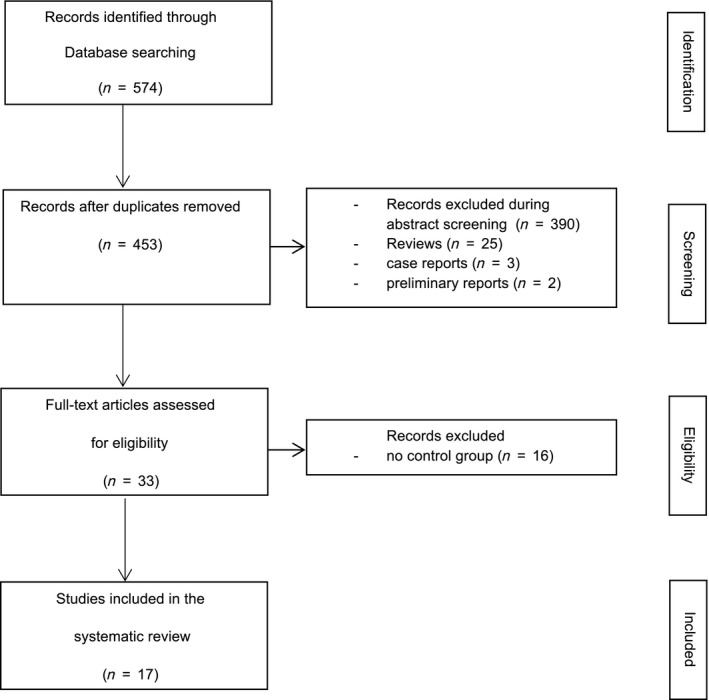
Flow chart depicting the screening and selection process for the systematic review of immunosuppression with generic vs. innovator tacrolimus in liver and kidney transplantation on biopsy‐proven acute rejection or bioequivalence.
Study characteristics
Fourteen of the 17 studies that were included in the systematic review used a parallel design and three used a crossover design; however, only five were randomized trials (Table 1). They were published between 2012 and 2017. Five different Tac generic formulations were used in these 17 studies: Tac Sandoz (Tac Hexal, Adoport, Hercoria) in eight studies, Tac Chong Kun Dang (Tacrobell) in five studies, Tac Teva (Tacni) in two studies, and Tac Dr. Reddy in one study; in one study, the generic formulation was not specified. The studies were conducted in Germany, Italy, US, Norway, UK, Sweden, and South Korea. To have a more complete insight, congress reports that did not proceed to publication in a peer‐reviewed journal have also been carefully reviewed. The 20 identified abstracts are listed in Table S1 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51.
Table 1.
17 studies comparing generic and innovator tacrolimus and presenting biopsy proven acute rejection or bioequivalence in patients after liver and kidney transplantation
| Study (Ref.) | Generic product | Study population | Control group | Results | AE | Follow‐up | Study design |
|---|---|---|---|---|---|---|---|
| LT de novo | n = 191 | ||||||
| Dannhorn et al. 16 | Tacrolimus Sandoz | 48 LT de novo | 46 de novo PRG |
Significantly lower Adoport starting dose Greater variability of dose/level ratio during the first 2 weeks No significant difference of AR, infection, acute kidney injury Similar patient and graft survival Significant cost saving within first 14 days |
2 graft losses in PRG group 1 death due to chronic rejection in Adoport group |
1 year |
Prosp., non‐rand. NOS: 8 |
| Yu et al. 17 |
Tac Chong Kun Dang |
57 LDLT de novo | 57 PRG LDLT | 8.3% AR PRG group |
Endocrine, nutritional, gastrointestinal, hepatobiliary disorders no graft loss, no death |
26 weeks |
Retrosp. NOS: 7 |
| Choi et al. 18 |
Tac Chong Kun Dang |
86 LT de novo | 81 de novo PRG |
No difference trough levels and dose 1 week after LT No difference of variation coefficient for trough levels at 1 and 5 years |
AE: no difference between groups 68.6%: drug side effects GEN 76.5% drug side effects PRG; itching: most frequent AE generic group (24.4%), skin rash: most frequent AE in PRG group (35.8%) |
53.0 ± 25.52 months |
Retrosp. NOS: 8 |
| No sign. difference in BPAR rates (17.4% GEN vs. 29.6% PRG, n.s.) | No difference rehospitalizations, infections, GFR | ||||||
| Significantly > patients discontinued/switched immunosuppression in generic group (29.1% vs. 14.8%, P = 0.027), nephrotoxicity: most common cause for switch in both groups‐difference n.s. | |||||||
| KT de novo | n = 760 | ||||||
| Connor et al. 19 | Tacrolimus Sandoz | 51 KT de novo | 48 KT de novo PRG |
AR (generic): 18%, AR (brand): 17% similar patient and graft survival, DGF, CNI toxicity, CMV infection |
Graft loss (generic): 16%, graft loss (brand): 13% | 6 months |
Retrosp. NOS: 7 |
| Min et al. 20 |
Tac Chong Kun Dang |
54 KT de novo | 63 KT de novo PRG |
Higher C max and higher AUC with Tacrobell demanded dose reductions day 10: comparable C0, but significantly higher C max, and higher AUC0–12 6 months: early and high C max, but equivalent dose‐normalized AUC0–12 BPAR 4.8% (generic) vs. 3.7% (brand, P < 0.85) no death, similar renal function (eGFR) |
NR | 9 months |
Prosp. rand. |
| Robertsen et al. 21 | Tacrolimus Teva | 25 KT de novo | 2‐sequence crossover | Bioequivalence criteria in elderly RT recipients not met (not reflected by C0) | No SAE, similar rates of AE | 2 weeks | Prosp. rand. |
| Arns et al. 22 | Tacrolimus Sandoz | 35 (37) KT de novo | 44 KT de novo PRG | No relevant differences of pharmacokinetic parameters at month 1, 3 and 6 | 6 months |
Prosp. rand. |
|
| Similar eGFR and BPAR (5.7% generic vs. 7.9% brand) | Similar AE and SAE (37.1% generic vs. 42.1% brand) | ||||||
| Mellili et al. 23 | Tacrolimus Sandoz | 60 KT de novo | 60 KT de novo PRG |
AR: 3 of 60 (generic) vs. 8 of 60 (brand) no difference in AR, DGF, renal function, de novo DSA, proteinuria |
NR | 6 months |
Retrosp. NOS: 8 |
| Son et al. 24 |
Tac Chong Kun Dang |
444 KT de novo | 245 KT de novo PRG |
5 year‐AR‐free graft survival 67% (generic) vs. 68.8% (brand) similar 5‐year patient and graft survival, 5‐year efficacy and safety |
similar AE rates (69% vs. 48%) predom. cardiovascular, cerebrovascular, maligancy, NODAT, infectious |
5 years |
Retrosp. NOS: 8 |
| Lindner et al. 25 | Tacrolimus Teva | 91 KT de novo | 95 KT de novo PRG | BPAR: 12% (generic) vs. 14% (PRG) | SAE: 47.3% (generic) vs. 43.2% (PRG) | 1 year |
Retrosp. NOS: 7 |
| Similar GFR, tacrolimus levels, similar patient and graft survival | 1 death in Tacni group (not related to study drug) | ||||||
| LT conversion | n = 210 | ||||||
| Vollmar et al. 26 | Tacrolimus Panacea | 25 LT c | 25 LT PRG |
Similar concentration‐dose ratio, no AR cost‐effective |
No graft loss 17 mild side effects (i.e. gastrointestinal) |
6 months |
Retrosp. NOS: 7 |
| Kim et al. 27 | Tacrolimus Chong Kun Dang | 149 LT c | LDLT database | No significant differences in trough levels, doses, laboratory parameters | 65 AE in both groups | 17.3 months |
Prosp., non‐rand. NOS: 6 |
| 3 AR (generic) vs. 2 AR (brand) | |||||||
| Alloway et al. 28, a | generic Hi (Tacrolimus Sandoz), generic Lo (Dr. Reddy) | 36 LT c | 6‐period crossover |
Bioequivalence of generic Hi and generic Lo with innovator tacrolimus and with each other, no AR within subject variability for AUC and C max was similar for all 3 products FDA and EMA bioequivalence criteria met (only exception: innovator versus generic Lo ‐ EMA bioequivalence criteria not met) |
NR | 8 weeks | Prosp. rand. |
| KT conversion | n = 251 | ||||||
| Alloway et al. 29 | Tacrolimus Sandoz | 68 KT c | 2‐sequence crossover | No AR. Correlations between 12 h trough levels and AUC were: | 9 AE (generic) vs. 21 AE (PRG) | 28 days | Prosp. rand. |
| r = 0.917 for generic tacrolimus and r = 0.887 for reference drug at day 28 | No graft loss | ||||||
| Marfo et al. 30 | Generic Tacrolimus | 73 KT c | 33 KT PRG |
Mean tacrolimus trough levels were similar pre‐ and postconversion no significant changes in mean serum creatinine values pre‐ and postconversion > infections, 1 Ab mediated rejection in generic group |
No AE, no toxicity | 1 year |
Retrosp. NOS: 6 |
| Heavner et al. 31 | Tacrolimus Sandoz | 36 KT c | 52 KT PRG |
Similar mean trough concentrations. 1 AR brand group no significant difference in dosage adjustments required or trough tacrolimus levels |
NR | 6 months |
Retrosp. NOS: 7 |
| Hauch et al. 32 | Tacrolimus Sandoz | 39 KT c | 159 KT PRG (historic) |
20% +/− change in trough levels, needing more dose adjustments (P < 0.038) in the first year after transplantation > AR: 23.1% (generic) vs. 10.2% (brand) at 1 yr., no difference AR at 6 months, no difference chron.rejection, more costs in generic group |
1 year |
Retrosp. NOS: 6 |
|
| Alloway et al. 28, a | generic Hi (Tacrolimus Sandoz), generic Lo (Dr. Reddy) | 35 KT c | 6‐period crossover |
Bioequivalence of generic Hi and generic Lo with innovator tacrolimus and with each other, no AR within subject variability for AUC and C max was similar for all 3 products FDA and EMA bioequivalence criteria met (only exception: innovator versus generic Lo ‐ EMA bioequivalence criteria not met) |
NR | 8 weeks | Prosp. rand. |
LT liver transplantation, KT kidney transplantation, LDLT living donor liver transplantation, c conversion, Tac tacrolimus, PRG Prograf, GEN generic tacrolimus, AR acute rejection, AE adverse events, SAE serious adverse events, DSA donor‐specific antibodies, DGF delayed graft function, CNI calcineurin inhibitor, CMV cytomegalovirus, NR not reported, and NOS Newcastle–Ottawa Scale.
Studies including LT and KT recipients.
Study quality
Study quality of the 12 non‐randomized studies according to the Newcastle–Ottawa scale was 8 (out of a maximum of 9) in four of the studies, 7 in 5 of them, and 6 in 3 (Table 1). There were 2 prospective non‐randomized interventional studies and 10 retrospective observational studies. Potential confounders like dose adjustments were often not outlined.
The methodological quality of the five included randomized studies is presented in a risk of bias summary (Fig. 2). As summarized there, the methodological quality was generally poor; performance bias was detected in 80% and attrition bias in 40% of the analyzed RCTs, whereas detection bias was mostly unclear.
Figure 2.
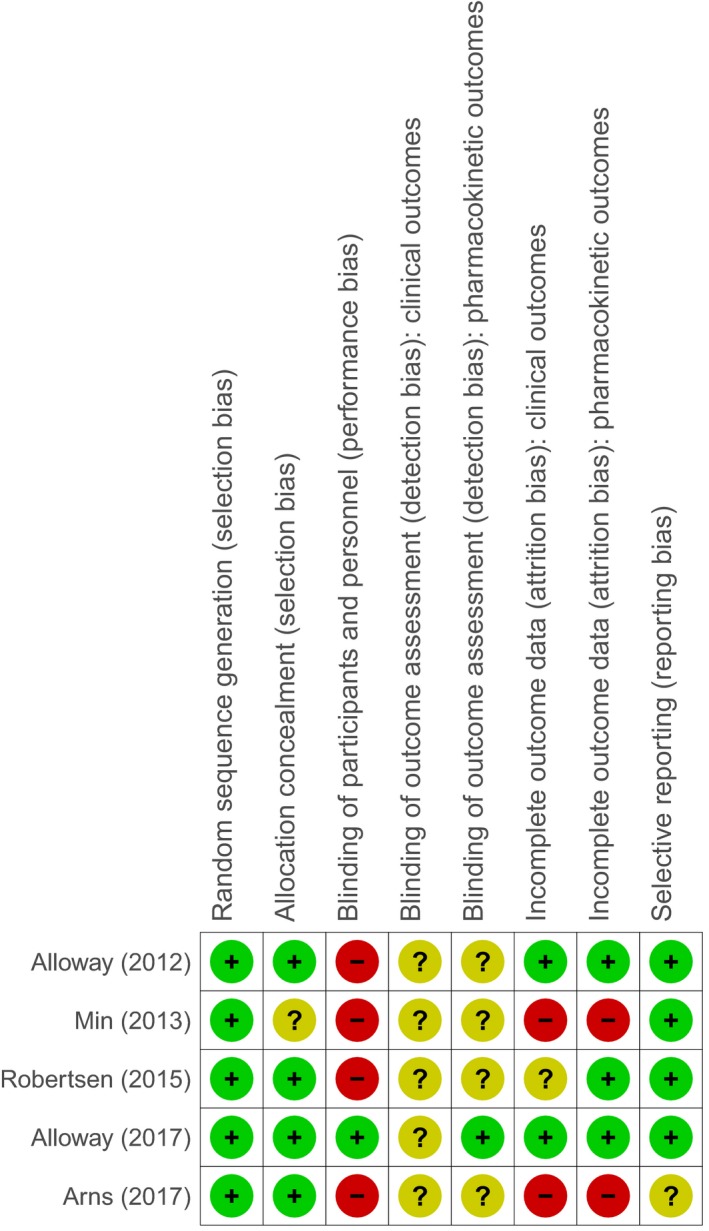
Risk of bias summary: review authors’ judgement about the risk of bias for each included randomized controlled trial.
The follow‐up times ranged from 2 weeks to 53 ± 25.52 months.
Biopsy‐proven acute rejection
Five studies including 365 patients after LT (191 de novo, 174 conversion) and nine studies including 883 patients after KT (735 de novo, and 148 conversion) compared BPAR of patients on generic Tac with those on innovator Tac after LT or KT, all of them in a parallel design (Table 1). However, only two of those studies were randomized controlled trials 20, 22 and a further two were non‐randomized prospective studies 16, 27. Risk ratios for BPAR rates of patients on generic Tac versus innovator Tac were compared in a forest plot stratified visually by organ and study design (Fig. 3). Only one study after KT conversion found a significant difference between generic and innovator Tac favoring innovator Tac 32, while one in KT de novo found a significant benefit of generic Tac 24. Two further crossover studies including 103 patients after KT and 36 patients after LT 28, 29 conversion did not report any BPAR event. Pooling the results in a meta‐analysis, the estimate for the risk ratio was 0.84 (n = 2369, 95% CI: 0.65–1.09) and there was low heterogeneity (I 2 = 16%, P = 0.28). When stratifying by organ, the pooled estimate for LT studies was 0.65 (n = 703, 95% CI: 0.41–1.03) and that of KT studies 0.93 (n = 1666, 95% CI: 0.67–1.31), showing no significant differences between generic and innovator Tac in either subgroup. Performing a subgroup analysis on de novo use versus conversion no residual heterogeneity remained (I 2 = 0%, P = 0.81). The pooled estimate for de novo use was 0.75 (n = 1659, 95% CI: 0.63–0.90), significantly favoring generic Tac, whereas the pooled estimate for conversion studies was 1.93 (n = 710, 95% CI: 1.00–3.70), favoring innovator Tac (Figs 3 and 4).
Figure 3.
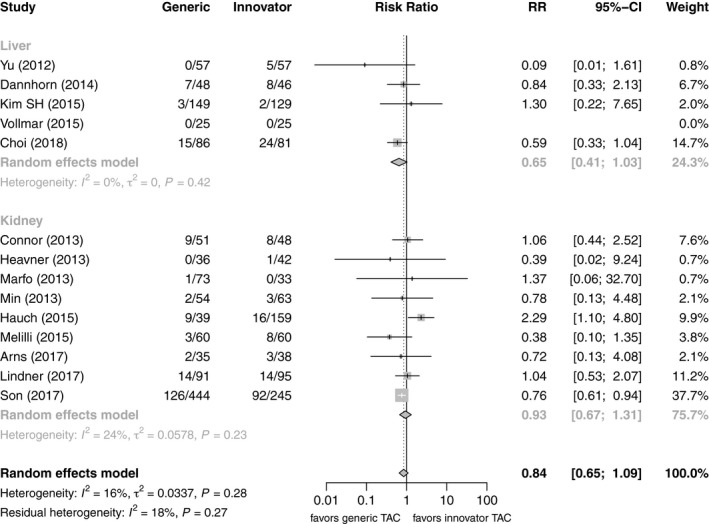
Forest plot of studies comparing risk of biopsy‐proven acute rejection between generic and innovator tacrolimus – stratified by organ transplanted.
Figure 4.
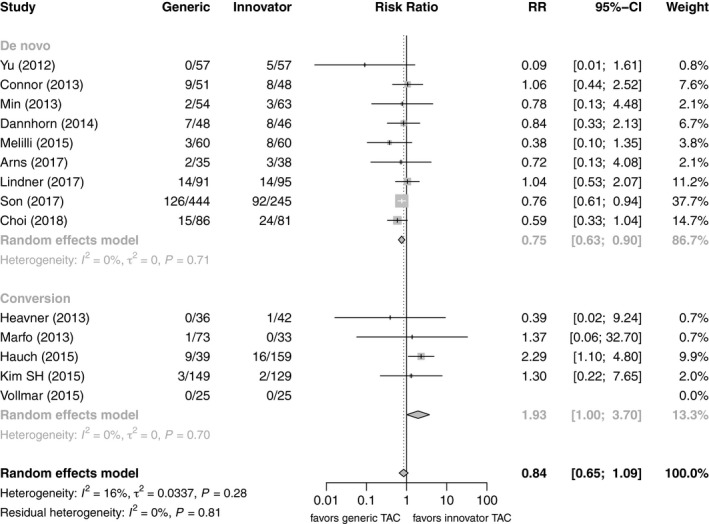
Forest plot of studies comparing risk of biopsy‐proven acute rejection between generic and innovator tacrolimus – stratified by use.
Bioequivalence
There were three crossover (25 patients de novo KT, 139 patients conversion LT and KT) and two parallel design studies (79 patients de novo KT), all RCTs, reporting the primary pharmacokinetic outcome of C max and AUC0–12 (Fig. 5) 20, 21, 22, 28, 29. Three prospective randomized pharmacokinetic studies in KT were conducted with de novo generic Tac 20, 21, 22 (one study including a crossover substudy), and one after conversion 29. Arns et al. 22 reported similar pharmacokinetics for Tac Sandoz as compared with innovator Tac after KT, but EMA bioequivalence criteria were not met. Also, Tac Teva did not meet the bioequivalence criteria in elderly KT recipients with a shorter time to C max 21, and Tac Chong Kun Dang showed higher dose‐normalized C max and AUC0–12 than the innovator 20. In 1 KT conversion study with a crossover design, the 90% CIs of the ratio generic/innovator for AUC0–12 were within the EMA bioequivalence acceptance criteria and the 90% CIs of the ratio generic/innovator for C max were within the FDA bioequivalence acceptance criteria 29. One prospective randomized 3‐treatment 6‐period crossover pharmacokinetic study on the switch of innovator to two generic Tac formulations (Tac Sandoz and Tac Dr. Reddy) showed bioequivalence in both KT and LT recipients, except for the conversion from innovator Tac to Tac Dr. Reddy according to EMA bioequivalence criteria 28. No BPAR event has been reported after conversion to generic Tac in both KT and LT patients (Table 1). In summary, 33.3% (1/2 LT, 2/7 KT) of (sub)studies show bioequivalence of generics for AUC0–12, and 55.6% according to the C max (2/2 LT, 3/7 KT). Two further studies (one each LT and KT) were almost entirely within range regarding AUC0–12. Pooling GMR and 90% CI of the AUC0–12, the combined estimate of all studies was 106.3 (n = 461, 95% CI: 103.8–108.7), which is within the bioequivalence acceptance interval of 90.00–111.11, and there was low heterogeneity (I 2 = 9%, P = 0.27). The subgroup analysis by organ yielded similar estimates for LT (105.8, n = 72, 95% CI: 102.4–109.2) and KT studies (106.9, n = 389, 95% CI: 102.9–110.8). The subgroup analysis on de novo use versus conversion revealed substantial differences between the subgroups. Whereas studies on de novo use did not fulfill EMA requirements for bioequivalence (115.2, n = 185, 95% CI: 107.6–122.8), conversion studies were well within the required limits (105.3, n = 276, 95% CI: 103.0–107.7) (Figs 5 and 6).
Figure 5.
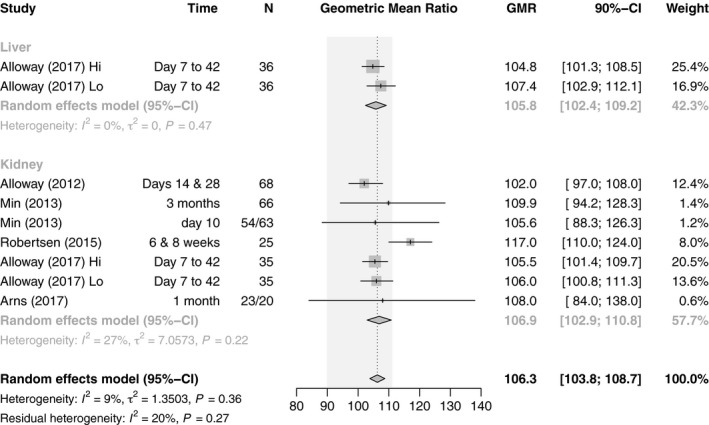
Forest plot of studies comparing geometric mean ratio of AUC0–12h between generic and innovator tacrolimus – stratified by organ transplanted. The EMA bioequivalence acceptance interval of 90.00–111.11 is displayed shaded in gray.
Figure 6.
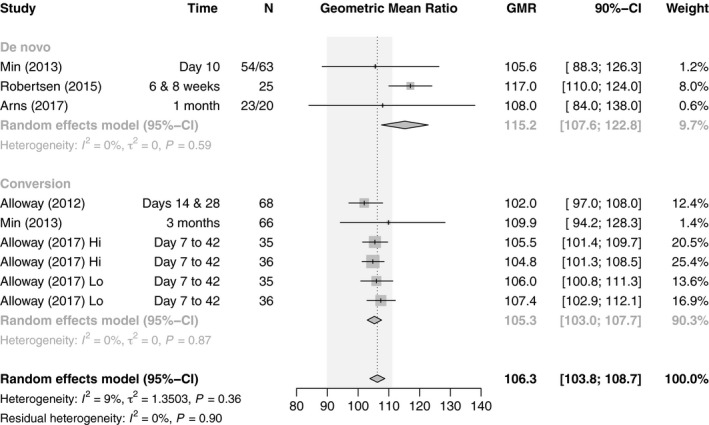
Forest plot of studies comparing geometric mean ratio of AUC0–12h between generic and innovator tacrolimus – stratified by use. The EMA bioequivalence acceptance interval of 90.00–111.11 is displayed shaded in gray.
For C max, the combined estimate of all studies was 114.9 (n = 461, 95% CI: 108.0–121.8), which is within the bioequivalence acceptance interval of 80.00–125.00. However, there was substantial heterogeneity (I 2 = 69%, P < 0.01). When stratifying by organ, there were differences between LT studies, which albeit few in number were within the required limits (108.6, n = 72, 95% CI: 103.3–113.8), and KT studies, which exceded the upper limit (119.7, n = 389, 95% CI: 108.7–130.7). However, there was substantial heterogeneity in both subgroups and thus the residual heterogeneity was unchanged (I 2 = 70%, P <0.01). The initial heterogeneity could be explained performing a subgroup analysis on de novo use versus conversion which resulted in no residual heterogeneity (I 2 = 0%, P = 0.62). Whereas de novo studies with a GMR of 142.4 (n = 185, 95% CI: 126.4–158.3) did not fulfill the requirements for bioequivalence, conversion studies with a GMR of 108.9 (n = 276, 95% CI: 105.6–112.2) did (Figs 7 and 8).
Figure 7.
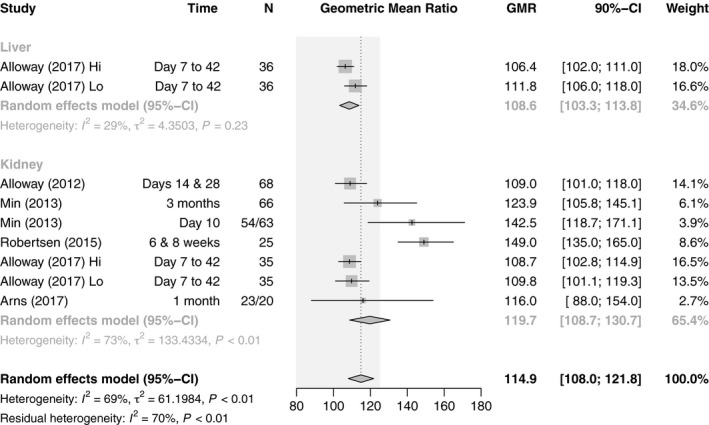
Forest plot of studies comparing geometric mean ratio of C max between generic and innovator tacrolimus – stratified by organ transplanted. The EMA bioequivalence acceptance interval of 80.00–125.00 is displayed shaded in gray.
Figure 8.
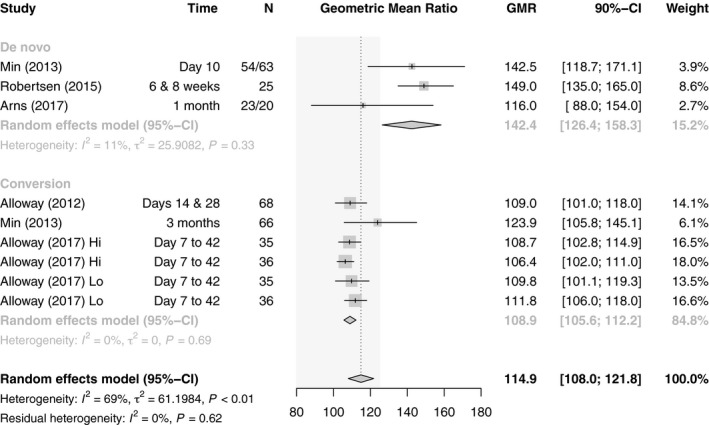
Forest plot of studies comparing geometric mean ratio of C max between generic and innovator tacrolimus – stratified by use. The EMA bioequivalence acceptance interval of 80.00–125.00 is displayed shaded in gray.
Further study outcomes
Patient and graft survival
Similar patient and graft survival was reported in the selected studies. Graft loss was reported in two of the de novo studies after KT: in eight patients (15.7%) versus six patients (12.5 %) after generic and innovator Tac, respectively 19, and the Spartacus trial revealed 0% graft loss versus 2.6% (one patient) with generic and innovator Tac after KT, respectively 22. Furthermore, there were 2 (4.3%) versus no graft losses reported with de novo innovator Tac after LT 16. More drug level variability and dose adjustments necessary during the first weeks after transplantation in de novo use and also after conversion was reported 16, 20, 32, 40, 41, 42, 46.
Congress reports that did not proceed to publication in a peer‐reviewed journal have been carefully reviewed with similar results, that is, similar BPAR rates and safety profiles as well as higher variability of dose/level ratio in the early phase after transplantation.
Costs
One de novo study with generic Tac evaluated cost‐effectiveness and reported cost savings within the first 14 days 16. One conversion study indicated higher costs with generic Tac due to monitoring and hospitalization 32, whereas one conference abstract 49 confirmed cost‐effectiveness.
Discussion
The narrow therapeutic index of Tac 52 and the potential severe adverse consequences of subtherapeutic or toxic concentrations necessitate close monitoring of patients’ exposure to the drug. Great experience with the innovator Tac has been achieved with excellent clinical outcomes. Both Tac pivotal trials 53 and trials with the innovator Tac in combination with other immunosuppressive agents to further develop immunosuppressive regimen for best possible clinical outcome after LT and KT have led to a very familiar and confident use of the innovator Tac 54. When generic Tac became available there was no need to shift from the long‐lasting standard with innovator Tac to a protocol with generic Tac for most centers or patients. This was especially important since both the grafts’ and patients’ survival highly depend on reliable immunosuppression. Moreover, there was limited experience with generic Tac in these days.
In contrast to the innovator drug, no pivotal trials that enable physicians to gain experience with new formulas are mandatory. Approval for generics is given after demonstration of their bioequivalence to its innovator. None of the regulatory agencies require the demonstration of bioequivalence with any other approved generic formulation, which seems to be a potential shortcoming of the current generic approval process. Following approval, generic manufacturers generally do not fund clinical trials to test their generic products against the reference or other generic products 55. All of this did not help to introduce generic Tac to a large patient cohort.
Despite these facts academically driven studies have been performed with generic Tac over the past years. Many of these datasets have been carefully put together by many scientific societies and transplant organisations to provide opinion statements on the use of generic immunosuppression. The most important statements of the American Society of Transplantation 56, International Society for Heart & Lung Transplantation 57, The Kidney Disease Improving Global Outcomes Society (KDIGO) 58, European Society for Organ Transplantation 9, and the Canadian Society of Transplantation 12 can be summarized as follows: (i) there is strong recommendation on the patients’ education to avoid an unintended switch between generic and innovator formulations, (ii) conversion between generic and innovator immunosuppressants should be avoided or at least limited to specialized transplant physicians, and (iii) any conversion should be accompanied by strict follow‐up and monitoring 9, 12, 56, 57, 58.
Since publication of the most recent position statement by the Canadian Society of Transplantation in 2012, the use of generic immunosuppressants has become routine in many centers all over the world 59, and several high‐quality RCTs have been published 20, 21, 22, 28, 29 together with both a considerable number of observational studies (Table 1) and congress reports (Table S1). Therefore, guidelines could be updated based on the current knowledge on the safety and efficacy of generic immunosuppression after transplantation.
Most of the recent studies have focused on generic Tac with special focus on BPAR rates and both patients’ and grafts’ survival, which are most important. Furthermore, there is outcome associated data available on drug levels (C max, c0, AUC, dose/level ratio, dose adjustments necessary), laboratory values for graft function, safety/efficacy, bioequivalence and on cost savings. However, there is still a lack of grade 1b level prospective RCTs in target populations according to EBM. The available evidence is mainly retrospective, from case reports, or from studies that are either underpowered, have no appropriate control group, analyze trough concentrations only, lack an analysis of confounders (comedications, comorbidities), or use nonspecific immunoassays to assess Tac concentrations.
Thus, this study was designed to provide an overview on the current body of evidence by performing both a systematic review on BPAR, reflecting the clinical safety and efficacy of generic Tac use, on the one hand and on bioequivalence in target populations, to address the concerns reflected by the current position statements of the Transplant Societies as mentioned above, on the other.
BPAR rates
A few studies on generic Tac have been performed on BPAR rates 16, 17, 18, 19, 20, 22, 23, 24, 25, 26, 27, 30, 31, 32, whereas five studies found risk ratios favoring innovator Tac and eight studies favoring generic Tac, CIs were often wide due to the low sample sizes. Only one study each found a significant benefit of innovator and generic Tac. However, none of these studies was designed to show equivalence or non‐inferiority of the generic Tac, whereas pooling the results of all studies showed no significant differences between innovator and generic Tac, after stratifying by use studies on de novo use significantly favored the generic and studies on conversion favored the innovator Tac.
How likely is it to see more RCTs on BPAR with generic Tac in the future? Protocol biopsies for exact monitoring of the immune situation after KT are done in some centers. This is in contrast to the practice after LT. Thus, these parameters are more commonly available after KT and more studies are available for the switch or de novo use of generic Tac after KT in general. Since protocol biopsies are not commonly performed after LT it is more sophisticated to specify AR episodes after LT with less evidence for BPAR episodes after LT. Biopsies are only performed in exceptional cases since clinicians mostly rely on standard liver values and clinical performance 16. Based on these facts, it is unlikely to see more RCTs on generic Tac with focus on BPAR.
Apart from that, large sample sizes would be needed, and additional costs would arise.
Bioequivalence
Among the five RCTs 20, 21, 22, 28, 29 demonstrating comparable safety and efficacy of generic Tac and its innovator, there are two parallel design studies 20, 22 in KT patients and three crossover studies on bioequivalence, two after KT 21, 29 and one study combining a cohort of patients after LT and KT 28, the two latter clearly showing bioequivalence between two generic Tac themselves and their innovator. The other studies did not demonstrate bioequivalence according to EMA criteria. Interestingly, in one trial, the bioequivalence criteria have not been met in elderly patients after KT 21. One parallel design study 22 was underpowered, and so unable to demonstrate bioequivalence. The combined estimate for the GMR was within the EMA bioequivalence acceptance range for both AUC0–12 and C max. However, when stratifying for de novo use and conversion, which resulted in homogeneous subgroups, conversion studies showed bioequivalence, with only one study being clearly outside the limits, whereas all three de novo studies did not meet bioequivalence criteria for the AUC as well as for the C max. Of the three de novo studies, one was a crossover study with a relatively small sample size 21. Although the CIs for this study were not too wide, that for C max was totally outside the acceptance interval and that of the AUC barely touched it. The remaining two de novo studies had a parallel design 20, 22; they had small sample sizes and thus wide CIs but were not actually designed to show bioequivalence.
Further study outcomes
Patient and graft survival
Similar patient and graft survival was reported in most of the studies. Graft performance was reported in most of the studies indicated by laboratory findings. Markers of graft function like serum creatinine and liver enzymes were measured at different time points in each study; however, there were no differences in serum creatinine or liver enzymes between generic and innovator arms in any of the studies. Since methods of measurement and timepoints samples were taken from patients were different in every study, data are not comparable.
Graft loss was reported in two of the de novo studies after KT: in eight patients (15.7%) versus six patients (12.5 %) after generic and innovator Tac, respectively 19, and the Spartacus trial revealed 0% graft loss versus 2.6% (one patient) with generic and innovator Tac after KT, respectively 22. Furthermore, there were 2 (4.3%) versus no graft losses reported with de novo innovator Tac after LT 16.
Many studies reported dose/level ratio and dose titrations after the introduction of generic immunosuppressive medications. It was remarkable that quite a few studies reported more drug level variability and dose adjustments necessary during the first weeks after transplantation in de novo use and also after conversion 16, 20, 32, 40, 41, 42, 46. Most importantly, these findings were not associated with any negative effects on clinical outcome.
Congress reports that did not proceed to publication in a peer‐reviewed journal have also been carefully reviewed with similar results, that is, similar BPAR rates and safety profiles as well as higher variability of dose/level ratio in the early phase after transplantation (Table S1).
Costs
According to patient surveys, cost is a considerable barrier to immunosuppressant adherence in healthcare systems not covering these costs in an adequate manner, which would be crucial in order to preserve graft function. One de novo study with generic Tac evaluated cost‐effectiveness and reported cost savings within the first 14 days 16, whereas one conversion study indicated higher costs with generic Tac due to monitoring and hospitalization 32, whereas one conference abstract 49 confirmed cost‐effectiveness.
Study quality
The majority of the included studies were observational and prospective non‐randomized, and five studies were randomized. The methodological quality of these studies was generally poor, with mostly small sample sizes, holding a serious risk of bias and its consequences on the validity of the results, which have to be interpreted with caution.
Limitations of this work are (i) combining interventional and observations studies holding potentially more risk of bias and confounding, and (ii) insufficient data for long‐term outcomes (follow‐up > 1 year). Therefore, this study was unable to address the effect of generic tacrolimus on long‐term BPAR sufficiently. Only few of the studies reported the 90% confidence intervals for AUC0–12 and C max geometric mean ratios as standard criteria for bioequivalence, and those were partially underpowered for the demonstration of bioequivalence. Only one study included in the systematic review investigated the null‐hypothesis that the generic was inferior to the innovator tacrolimus 24, the other studies did not.
Conclusion
The systematic review of immunosuppression with generic and innovator Tac in the prevention of BPAR in adult LT and KT did not reveal a difference. There is some evidence suggesting lower BPAR risk with generic Tac for de novo use. However, the current evidence is mostly based on observational data and the remaining studies showed some risk of bias. Bioequivalence regarding AUC0–12 and C max was demonstrated primarily in studies on conversion. Generic products have the potential to reduce costs for payers, patients, and healthcare systems 58 and potentially account for greater adherence in heathcare systems where patients are required to cover costs for immunosuppression to a relevant amount themselves. High‐quality studies with adequate study cohorts and follow‐up times are warranted to facilitate updates of transplant society position statements on generic immunosuppressant use 60.
Authorship
JK: wrote manuscript and performed the study. GP: analyzed data and wrote the manuscript. PS: designed and performed the study, and wrote the manuscript.
Funding
The authors have declared no funding.
Conflicts of Interest
The authors have declared no conflicts of interest.
Supporting information
Table S1. 20 Conference abstracts comparing generic and innovator tacrolimus after liver and kidney transplantation.
Appendix S1. Search strategies.
Acknowledgments
We are grateful to Konstanze Kral, MD who helped us finding literature of all sources used here for analysis.
References
- 1. Health Canada .Guidance document‐comparative bioavailability standards: formulations used for systemic effects; 2012. [cited 2017 Oct 26]. http://www.hc-sc.gc.ca/dhp-mps/prodpharma/applic-demande/guide-ld/bio/gd_standards_ld_normes-eng.php-a2.1.
- 2. WC500070039.pdf [Internet]. emea.europa.eu; 2010 [cited 2018 Aug 5]. Available from: http://www.emea.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf], European Medicines Agency. Guidelines on the investigation of bioequivalence. http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/01/WC500070039.pdf.
- 3. FDA Bioequivalence guidelines: Food and Drug Administration Therapeutic Equivalence of Generic Drugs‐Response to National Association of Boards of Pharmacy; 1997 [cited 2017 Oct 26]. Available from: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/HowDrugsareDevelopedandApproved/ApprovalApplications/AbbreviatedNewDrugApplicationANDAGenerics/ucm073224.htm.
- 4. Food and Drug Administration Centre for Drug Evaluation and Research Approved Drug Products CellCept; 1997 [cited 2017 Oct 26]. Available from: http://www.accessdata.fda.gov/scripts/cder/drugsatfda/index.cfm?fuseaction1%20444Search.Label_ApprovalHistory#labelinfo.
- 5. Food and Drug Administration . Guidance for industry: bioavailability and bioequivalence studies submitted in NDAs or INDs ‐ general considerations; 2014 [cited 2017 Oct 26]. http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm389370.pdf.
- 6. Food and Drug Administration . Guidance for Industry: bioavailability and bioequivalence studies for orally administered drug products ‐ general considerations; 2003 [cited 2017 Oct 26]. Available from: http://www.fda.gov/ohrms/dockets/ac/03/briefing/3995B1_07_GFI-BioAvail-BioEquiv.pdf.
- 7. Directive 2001/83/EC of the European Parliament and of the Council of 6 April 2001 on the Community Code Relating to Medicinal Products for Human Use; 2001 [cited 2017 Oct 26]. Official Journal of the European Communities L 311, 67–128; 2004. Available at: http://www.emea.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2009/10/WC500004481.pdf.
- 8. European Commission Enterprise Directorate‐General: Marketing Authorisations; VOLUME 2A Procedures for marketing authorisation. Brussels; 2005. [cited 2017 Oct 26]. Available at: http://ec.europa.eu/health/files/eudralex/vol-2/a/vol2a_chap1_2005-11_en.pdf
- 9. van Gelder T, ESOT Advisory Committee on Generic Substitution . European Society for Organ Transplantation Advisory Committee recommendations on generic substitution of immunosuppressive drugs. Transplant Int. 2011; 24: 1135. [DOI] [PubMed] [Google Scholar]
- 10. Phillips K, Reddy P, Gabardi S. Is there evidence to support brand to generic interchange of the mycophenolic acid products? J Pharm Pract 2017; 30: 9. [DOI] [PubMed] [Google Scholar]
- 11. Molnar AO, Fergusson D, Tsampalieros AK, et al Generic immunosuppression in solid organ transplantation: systematic review and meta‐analysis. BMJ 2015; 350: h3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Harrison JJ, Schiff JR, Coursol CJ, et al Generic immunosuppression in solid organ transplantation: a Canadian perspective. Transplantation 2012; 93: 657. [DOI] [PubMed] [Google Scholar]
- 13. Higgins JP, Altman DG, Gotzsche PC, et al The Cochrane Collaboration`s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wells GA, Shea B, O'Connell D, et al The Newcastle‐Ottawa Scale (NOS) for assessing the quality of non‐randomised studies in meta‐analyses; 2014. [cited 2017 Oct 27]. Available from: http://www.ahri.a/programs/clinical_epidemiology/oxford.asp.
- 15. Higgins JPT, Green S. (eds). Studies with zero-cell counts. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from: www.handbook.cochrane.org.
- 16. Dannhorn E, Cheung M, Rodrigues S, et al De novo use of generic tacrolimus in liver transplantation ‐ a single center experience with one‐yr follow‐up. Clin Transplant 2014; 28: 1349. [DOI] [PubMed] [Google Scholar]
- 17. Yu YD, Lee SG, Joh JW, et al Results of a phase 4 trial of Tacrobell in liver transplantation patients: a multicenter study in South Korea. Hepatogastroenterology 2012; 59: 357. [DOI] [PubMed] [Google Scholar]
- 18. Choi HJ, Kim DG, Kwak BJ, Han JH, Hong TH, You YK. Comparison of the long‐term efficacy and safety of generic tacrolimus, Tacrobell, with Prograf in liver transplant recipients. Drug Des Devel Ther 2018; 12: 295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Connor A, Prowse A, Newell P, Rowe PA. A single‐centre comparison of the clinical outcomes at 6 months of renal transplant recipients administered Adoport® or Prograf® preparations of tacrolimus. Kidney J 2013; 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Min SI, Ha J, Kim YS, et al Therapeutic equivalence and pharmacokinetics of generic tacrolimus formulation in de novo kidney transplant patients. Nephrol Dialysis Transplant 2013; 28: 3110. [DOI] [PubMed] [Google Scholar]
- 21. Robertsen I, Asberg A, Ingero AO, et al Use of generic tacrolimus in elderly renal transplant recipients: precaution is needed. Transplantation 2015; 99: 528. [DOI] [PubMed] [Google Scholar]
- 22. Arns W, Huppertz A, Rath T, et al Pharmacokinetics and clinical outcomes of generic tacrolimus (Hexal) versus branded tacrolimus in de novo kidney transplant patients: a multicenter randomized trial. Transplantation 2017; 101: 2780. [DOI] [PubMed] [Google Scholar]
- 23. Melilli E, Crespo E, Sandoval D, et al De novo use of a generic formulation of tacrolimus versus reference tacrolimus in kidney transplantation: evaluation of the clinical results, histology in protocol biopsies, and immunological monitoring. Transpl Int 2015; 28: 1283. [DOI] [PubMed] [Google Scholar]
- 24. Son SY, Jang HR, Iee JE, et al Comparison of the long‐term efficacy and safety of generic tacrobell with original tacrolimus (Prograf) in kidney transplant recipients. Drug Des Devel Ther 2017; 11: 203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lindner C, Lindner P. A 1‐year comparison of generic tacrolimus (Tacni®) and Prograf® in renal transplant patients: a retrospective matched group analysis. J Clin Exp Transplant 2017; 2: 1. [Google Scholar]
- 26. Vollmar J, Bellmann MC, Darstein F, et al Efficacy and safety of a conversion from the original tacrolimus and mycophenolate mofetil to the generics Tacpan and Mowel after liver transplantation. Drug Des Devel Ther 2015; 9: 6139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kim SH, Lee SD, Kim YK, Park SJ. Outcomes of early conversion from Prograf to generic tacrolimus in adult living donor liver transplant recipients. Transplant Proc 2015; 47: 1915. [DOI] [PubMed] [Google Scholar]
- 28. Alloway RR, Vinks AA, Fukuda T, et al Bioequivalence between innovator and generic tacrolimus in liver and kidney transplant recipients: a randomized, crossover clinical trial. PLoS Medicine 2017; 14: e1002428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Alloway RR, Sadaka B, Trofe‐Clark J, Wiland A, Bloom RD. Randomized pharmacokinetic study of generic tacrolimus versus reference tacrolimus in kidney transplant recipients. Am J Transplant 2012; 12: 2825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Marfo K, Aitken S, Akalin E. clinical outcomes after conversion from brand‐name tacrolimus (Prograf) to a generic formulation in renal transplant recipients. P&T 2013; 38: 484. [PMC free article] [PubMed] [Google Scholar]
- 31. Heavner MS, Tichy EM, Yazdi M, Formica RN, Kulkarni S, Emre S. Clinical outcomes associated with conversion from brand‐name to generic tacrolimus in hospitalized kidney transplant recipients. Am J Health Syst Pharm 2013; 70: 1507. [DOI] [PubMed] [Google Scholar]
- 32. Hauch A, John M, Smith A, et al. Generics: Are all immunosuppression agents created equally? Surgery 2015; 158: 1049. [DOI] [PubMed] [Google Scholar]
- 33. Heldenbrand S, Jones GD, Bornhorst J, Payakachat N. Extended comparison of therapeutic treatment outcomes of de novo liver and kidney transplant recipients with generic tacrolimus (SandozTM) or brand name (PrografR). Am J Transplant. 2012; 12(S3): Abstract 713. [Google Scholar]
- 34. Pasangulapati S, Featherstone B, Allison MED. Retrospective study comparing safety and efficacy of generic preparation of tacrolimus with prograf as primary immunosuppression after liver transplantation. J Hepatol 2014; 60: 382. [Google Scholar]
- 35. Siddiqui N, Lu A, Jones T, Akalin E, Marfo K. Clinical and economical outcomes: de novo use of FDA‐approved bioequivalent formulation of generic tacrolimus versus brand tacrolimus (Prograf). Am J Transplant. 2011; 11(S2): Abstract706. [Google Scholar]
- 36. Mojdeh S, Tichy E, Yazdi M, et al Clinical impact of an in‐patient tacrolimus therapeutic substitution program. Am J Transplant 2011; 11(S2): Abstract982. [Google Scholar]
- 37. Dick T, Raines A, Van der Werf W, et al Comparison of dose requirements of Sandoz generic tacrolimus with brand Prograf in kidney transplant recipients. Am J Transplant 2011; 11(S2): Abstract1102. [Google Scholar]
- 38. Qazi Y, Bolonesi R, Monis T, et al Effect of generic tacrolimus on the incidence of acute rejection in kidney transplant recipients‐a single center experience. Am J Transplant 2012; 12(S3): 205. [Google Scholar]
- 39. Babu, et al Adoport versus Prograf in the nove renal transplants: single center experience 2013; BTS meeting: AbstractP76.
- 40. Sharma, et al Assessment of posttransplantation results of Adoport compared to Prograf. A short sample study 2013; BTS Meeting: AbstractP68.
- 41. Storey, et al Tac trough levels in week 1 similar but more patients had higher levels above target with Adoport Tacrolimus dosing in renal transplant recipients following introduction of generic preparation. ESOT Vienna 2013; Abstract BO149.
- 42. Ariaudo, et al Tacrolimus brand and generic drug: comparison in de novo kidney transplant (KT) in a monocentric analysis. ESOT Vienna 2013; AbstractP536.
- 43. Cagna, et al Generic tacrolimus and branded tacrolimus have similar bioavailability in genetically matched patients. ESOT Vienna 2013; Abstract.p318.
- 44. Goldfarb D, Poggio E, Flechner S, Fatica R, Schold J. No significant outcome benefit for brand‐name versus generic tacrolimus in kidney transplant recipients: SRTR analysis. Available from: http://www.atcmeeetingabstracts.com/abstract/
- 45. Rickman H, Hull R, Popola J, et al Intrapatient variability in trough drug concentrations in renal transplant patients receiving branded (Prograf) and generic (Adoport) tacrolimus: a retrospective cohort analysis. Transplantation 2014; 98: 476. [Google Scholar]
- 46. Del Gaudio M, Cescon M, Ravaioli M, et al The impact of conversion from Prograf to generic tacrolimus in liver transplant recipients with stable graft function. Transplantation 2017; 101: S11. [DOI] [PubMed] [Google Scholar]
- 47. Sanhueza EB, Rius MA, Zapata RL, et al Initial experience with a generic tacrolimus formulation in a liver transplant center in Chile. Liver Transplant 2009; 15: 191. [Google Scholar]
- 48. De Zottel L, Blunck K, Guy J, et al Evaluation of the conversion from brand name to generic immunosuppression in hospitalized organ transplant recipients. AJT 2017; 17: 747. [Google Scholar]
- 49. Lee E, Bunniran S, Kamble P, et al Treatment patterns, healthcare resource utilization, and costs in kidney transplant recipients using a fixed source regimen of tacrolimus vs. a variable source regimen. Am J Transplant 2015; 15(S3): Abstract 231. [Google Scholar]
- 50. Merlotti G, Quaglia M, Musetti C, Terrazzino S, Genazzani A, Cantaluppi V. Genetically matched renal transplant recipients show similar bioavailability of generic and brand name tacrolimus. Nephrol Dial Transplant 2018; 33(S1) i598. [Google Scholar]
- 51. Mizuno T, Fukuda T, Emoto C, et al Population pharmacokinetic‐pharmacogenetic analysis of tacrolimus in renal transplant patients participating in a prospective bioequivalence study. Clin Pharm Ther 2016; 99: 40. [Google Scholar]
- 52. Staatz CE, Tett SE. Clinical pharmacokinetics and pharmacodynamics of tacrolimus in solid organ transplantation. Clin Pharmacokinet 2004; 43: 623. [DOI] [PubMed] [Google Scholar]
- 53. Modern Medicine Network . First‐time generic drug approval, Tacrolimus capsules, 2009. [cited 2018 Aug 11]. Available from: http://http:/formularyjournal.modernmedicine.com/formulary-journal/news/clinical/clinicalpharmacology/first-time-generic-drugapproval-september2009page=full.
- 54. Ekberg H, Tedesco‐Silva H, Demirbas A, et al ELITE‐Symphony Study. Reduced exposure to calcineurin inhibitors in renal transplantation. N Engl J Med 2007; 357: 2562. [DOI] [PubMed] [Google Scholar]
- 55. Hajj SE, Kim M, Phillips K, Gebardi S. Generic immunosuppression in transplantation: current evidence and controversial issues. Expert Rev Clin Immunol 2015; 11: 659. [DOI] [PubMed] [Google Scholar]
- 56. Alloway RR, Isaacs R, Lake K, et al Report of the American Society of Transplantation conference on immunosuppressive drugs and the use of generic immunosuppressants. Am J Transplant 2003; 3: 1211. [DOI] [PubMed] [Google Scholar]
- 57. Uber PA, Ross HJ, Zuckermann AO, et al Generic drug immunosuppression in thoracic transplantation: an ISHLT educational advisory. J Heart Lung Transplant 2009; 28: 655. [DOI] [PubMed] [Google Scholar]
- 58. Kasiske L, Zeier MG, Chapman JR, et al KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int 2010; 77: 299. [DOI] [PubMed] [Google Scholar]
- 59. Liu Q, Smith AR, Park JM, et al The adoption of generic immunosuppressant medications in kidney, liver and heart transplantation among recipients in Colorado or nationally with Medicare part D. Am J Transplant 2018; 18: 1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Phillips K, Reddy P, Gabardi S. There evidence to support brand to generic interchange of the mycophenolic acid products? J Pharm Pract 2017; 30: 9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. 20 Conference abstracts comparing generic and innovator tacrolimus after liver and kidney transplantation.
Appendix S1. Search strategies.


