Abstract
A fundamental question of eukaryotic cell biology is how membrane organelles are organised and interact with each other. Cell biologists address these questions by characterising the structural features of membrane compartments and the mechanisms that coordinate their exchange. To do so, they must rely on variety of cargo molecules and treatments that enable targeted perturbation, localisation, and labelling of specific compartments. In this context, bacterial toxins emerged in cell biology as paradigm shifting molecules that enabled scientists to not only study them from the side of bacterial infection but also from the side of the mammalian host. Their selectivity, potency, and versatility made them exquisite tools for uncovering much of our current understanding of membrane trafficking mechanisms. Here, we will follow the steps that lead toxins until their intracellular targets, highlighting how specific events helped us comprehend membrane trafficking and establish the fundamentals of various cellular organelles and processes. Bacterial toxins will continue to guide us in answering crucial questions in cellular biology while also acting as probes for new technologies and applications.
Keywords: cell membrane, mechanism of action, toxins, trafficking
1. INTRODUCTION
Bacteria and their hosts have co‐evolved to produce a plethora of intricate host‐pathogen interactions that have shaped their respective biological diversities (Masri et al., 2015). From such long‐standing interactions, secreted bacterial exotoxins (hereby toxins) have emerged to be exquisitely precise and target very specific biological processes. For example, these toxins can target host functions to acquire nutrients, control host defences, and optimise their replicative niches.
The great advances that have been made in the characterisation of such toxins in the past 50 years have had a direct impact on infection biology but have also been instrumental in uncovering fundamental cellular mechanisms (Schiavo & van der Goot, 2001). These types of studies can be especially useful because toxins are generally extremely potent, that is, act at very low concentrations such that toxin action can be often monitored with a very clear and specific readout. Additionally, because toxins are secreted by bacteria, they can be purified and studied in isolation of the producing organism. Finally, their addition to the outside of cells allows for optimal temporal control.
As toxins are added outside but act inside cells, studying their modes of action has been particularly beneficial to understanding host cell membrane compartmentalization and vesicular trafficking. This review will follow the variety of routes undertaken by toxins to reach their cellular targets, highlighting the specific processes where toxins have enriched our view of eukaryotic vesicular trafficking—from the cell‐penetrating activities of toxins at the cell surface through to the endomembrane system and actions in the cytosol.
2. TOXINS HIJACK HOST‐CELL SURFACE MACHINERIES
Bacterial toxins can hijack diverse host‐cell receptors: proteins, carbohydrates, lipids, or glycolipids. For example, anthrax toxin binds to two protein receptors, capillary morphogenesis gene 2 and tumour endothelial marker 8 (Bradley, Mogridge, Mourez, Collier, & Young, 2001; Scobie, Rainey, Bradley, & Young, 2003), cholera toxin targets monosialotetrahexosylganglioside (Fishman, 1982; Holmgren, Lönnroth, & Svennerholm, 1973), and Shiga toxin binds globotriaosylceramide (Lindberg et al., 1987; Sandvig, Olsnes, Brown, Petersen, & van Deurs, 1989). Even pore‐forming toxins bind to diverse cell‐surface molecules: Aerolysin binds the sugar moieties of glycophosphatidylinositol‐anchored proteins (Abrami, Fivaz, Glauser, Parton, & van der Goot, 1998b) and cholesterol‐dependent cytolysins, such as listeriolysin O or streptolysin O, target surface cholesterol (Duncan & Schlegel, 1975; Vazquez‐Boland, Dominguez, Rodriguez‐Ferri, Fernandez‐Garayzabal, & Suarez, 1989). Because toxins are highly opportunistic molecules that make use of the cellular properties of their receptors, the study of the toxin‐receptor behaviour provides highly relevant cellular information. For example, studies on the binding and internalisation of Shiga toxin have paved the way to the discovery of the galectin 3‐mediated endocytic pathway (Lakshminarayan et al., 2014). Similarly, understanding how capillary morphogenesis gene 2 controls the level of collagen VI in the extracellular matrix was dictated by studies on anthrax toxin endocytosis (Bürgi et al., 2017).
Although bacterial toxins bind to extremely different receptors, a remarkable common feature is that these receptors tend to concentrate in specialised membrane domains. Because of this aspect, both cholera (Révész & Greaves, 1975) and tetanus (Montesano, Roth, Robert, & Orci, 1982) were instrumental in early observations that the cell surface is actually non‐uniform, in contrast to the then proposed fluid mosaic model. These plasma membrane domains, often referred to as microdomains or lipid rafts, were found to be enriched in cholesterol, glycosphingolipids, and lipid‐anchored proteins such as glycophosphatidylinositol‐anchored proteins (Brown & Rose, 1992; Simons & Ikonen, 1997). Many other toxins, including Shiga toxin (Falguières et al., 2001; Kovbasnjuk, Edidin, & Donowitz, 2001) and pore‐forming toxins (Abrami et al., 1998b; Waheed et al., 2001), were also shown to preferentially attach to microdomains, highlighting a shared mechanism for cellular activity/entry. Decades later, toxins continue to be exploited to probe for membrane compartmentalization (Dumitru et al., 2018; Maekawa, Yang, & Fairn, 2016; Russo et al., 2018). Their binding domains, conjugates, or derivatives have been used to not only monitor lipid distribution and trafficking but as probes that directly reorganise surface lipids, therefore driving the formation of membrane domains.
Compartmentalization in the two‐dimensional membrane environment enables the local concentration of molecules. For toxins, this has several important consequences. One is that receptor clustering affects ligand binding avidity, thus allowing binding at lower toxin concentrations and also potentially facilitating downstream signalling (Bray, Levin, & Morton‐Firth, 1998). This is particularly relevant for multivalent toxins, such as cholera and Shiga toxins whose receptor‐binding subunits are pentameric (Chinnapen, Chinnapen, Saslowsky, & Lencer, 2007; Šachl et al., 2015). Other toxins are monomeric upon binding, but require oligomerization for their activity, as is the case for many pore‐forming toxins and anthrax toxin (Abrami, Fivaz, & van der Goot, 2000; Abrami, Liu, Cosson, Leppla, & van der Goot, 2003). This oligomerization process will be favoured by receptor concentration, allowing the toxin to be active at lower overall concentrations (Abrami & van der Goot, 1999). In contrast, to satisfy the multivalency of the toxin or oligomerization process, toxins may also trigger the recruitment of additional receptors leading to increased, or even de novo, formation of membrane domains (Chinnapen et al., 2007; Šachl et al., 2015). This, in turn, may favour signalling events that are unrelated to the activity of the toxin itself, but merely triggered due to the toxin‐induced receptor clustering, as shown for listeriolysin O (Gekara, Jacobs, Chakraborty, & Weiss, 2005). Receptor clustering is not a particularity of toxins and is now well established for eukaryotic cellular signalling (Alonso & Millán, 2001; Varshney, Yadav, & Saini, 2016). For example, after epidermal growth factor binds to its receptor, epidermal growth factor receptor, clustering allows distinct signalling entities at the plasma membrane (Needham et al., 2016).
Besides clustering within lipid microdomains, several toxins are activated by a limited proteolysis step at the cell surface. Studies on this activation were crucial in the discovery that membrane‐anchored furin family members can be present and active at the cell surface of mammalian cells (Abrami et al., 1998a; Gordon, Klimpel, Arora, Henderson, & Leppla, 1995; Klimpel, Molloy, Thomas, & Leppla, 1992). These proprotein convertases were originally proposed to be located and active in the Golgi (Bresnahan et al., 1990), though the latest evidence using non‐toxin‐derived biosensors demonstrates that some proprotein convertases are active in non‐Golgi compartments (Ginefra, Filippi, Donovan, Bessonnard, & Constam, 2018). A recent study on anthrax and aerolysin toxins demonstrated that not only should furin be present at the cell surface to process these toxins but the toxin‐protease encounter needs to be forced through their colocalization in surface microdomains (Sergeeva & van der Goot, 2019). Beyond the intrinsic properties of most toxin receptors to be found in lipid microdomains, targeting furin to these locations was found to depend on palmitoylation of its cytosolic domain (Sergeeva & van der Goot, 2019). These toxin studies highlight how cells can ensure that interactions between low abundant molecules can occur in a highly efficient manner.
As demonstrated above, the general cell‐surface dynamics of toxins involve binding to specific receptors that reside in or subsequently associate with membrane domains leading to some form of clustering, wherein cleavage by proprotein convertases can either contribute to or result from clustering. These steps have helped cell biologists understand host‐cell plasma membrane compartmentalization, activation, and receptor signalling, and the study of toxins is likely to continue revealing lipid‐domain properties and receptor dynamics.
3. TOXINS EXPLOIT HOST‐CELL ENDOCYTIC AND RETROGRADE TRAFFICKING PATHWAYS
Apart from pore‐forming toxins, most toxins require access to the cytosol to reach their targets. Their translocation mechanisms, however, are quite diverse and can occur in different cellular compartments. Rapid translocation upon toxin binding at the plasma membrane occurs for the Bordetella adenylyl cyclase toxin (Ladant & Ullmann, 1999), which utilises its intrinsic phospholipase activity to form proteolipid membrane pores that allow crossing of its large adenylyl cyclase domain (González‐Bullón, Uribe, Martín, & Ostolaza, 2017). So far, all other toxins have been shown to enter the cell by endocytosis and only subsequently crossing some membrane of the endomembrane system.
Toxins such as botulinum and tetanus neurotoxins cross the endosomal membrane. They respond to endosome acidification by inserting a pore into the membrane of the early endosome, mediating the direct passage of active subunits (Pirazzini et al., 2016). pH‐dependent translocation can also occur later in the endocytic pathway within multivesicular bodies. This is the case for the receptor‐binding subunit of anthrax toxin, which translocates its enzymatic subunit, lethal factor, from the lumen of endosomes into the lumen of intraluminal vesicles (ILVs; Collier & Young, 2003; Friebe, van der Goot, & Bürgi, 2016). Access to the cytosol subsequently occurs by back fusion of ILVs with the limiting membrane of the endosome (Abrami, Lindsay, Parton, Leppla, & van der Goot, 2004). This trafficking route demonstrated that cargo within ILVs could remain in multivesicular endosomes without being delivered to lysosomes and could be stochastically released from ILVs to the cytosol (Luzio, Gray, & Bright, 2010; Stahl & Barbieri, 2002). Anthrax toxin was also shown to be released extracellularly through lethal factor‐loaded exosomes upon fusion of multivesicular bodies with the plasma membrane (Abrami et al., 2013). These exosomes can then be taken up by neighbouring cells, leading to their intoxication (Abrami et al., 2013). Due to its unique ability to translocate its enzymatic subunit into the lumen of ILVs, anthrax toxin provides a unique tool for investigating how certain cargo within ILVs may escape lysosomal degradation and for following exosome biogenesis and uptake. These mechanisms are particularly relevant to viral cell biology given that multiple viruses have been also shown to utilise ILVs to traffic throughout the cell and spread to uninfected neighbouring cells (Nour & Modis, 2014).
Although the aforementioned toxins carry a subunit or domain with membrane translocation properties, not all toxins do. They instead rely on cellular translocation mechanisms and must travel from endosomes through the Golgi to the ER, where they can exploit the retro‐translocation machinery. Early studies employing ricin (a plant toxin), cholera, and Shiga toxins, yielded the first clues of the existence of a retrograde pathway from the plasma membrane to the Golgi apparatus and then to the ER, which could be hijacked, but was not generated, by toxins (Moya, Dautry‐Varsat, Goud, Louvard, & Boquet, 1985; Sandvig et al., 1989). Subsequent work helped characterise both clathrin‐dependent and independent internalisation routes and demonstrated that toxin retrograde trafficking could occur through multiple pathways (Lauvrak, Torgersen, & Sandvig, 2004; Nichols et al., 2001; Sandvig & Brown, 1987). Trafficking occurs via early and recycling, Rab11‐dependent, endosomes for Shiga and cholera toxins (Fuchs et al., 2007; Mallard et al., 1998), whereas Pseudomonas exotoxin A travels deeper in to the endocytic pathway through Rab9‐dependent routes. At the trans‐Golgi network (TGN), toxins also follow multiple routes to reach the ER. The classical route travelled by KDEL receptors involves transport through the cis‐Golgi to the ER via COP‐1 dependent trafficking (Spang, 2013). This is the path followed by exotoxin A, which contains the C‐terminal KDEL ER‐retrieval signal and binds the KDEL‐receptor following exit from endocytic compartments and arrival to the TGN (Chaudhary, Jinno, FitzGerald, & Pastan, 1990). In contrast, Shiga and Shiga‐like toxins do not harbour a KDEL sequence, which enabled the identification of COP‐I‐independent Golgi‐to‐ER trafficking (Girod et al., 1999). In both cases, transport depends on Rab6‐mediated sorting and requires the integrity of the entire Golgi complex (Sandvig, Skotland, van Deurs, & Klokk, 2013). Cholera toxin, on the other hand, travels directly from the TGN to the ER, without crossing the entire Golgi apparatus. This helped uncover another unique retrograde pathway that does not require an intact Golgi apparatus (Feng et al., 2004) despite being dependent on its KDEL ER‐retrieval signal (Fujinaga et al., 2003).
At the ER, toxins exploit ER‐associated degradation pathways to translocate to the cytosol, which was actually suggested before the characterisation of the ERAD translocation machinery. Toxins were proposed to mimic misfolded proteins in the ER to reach the cytosol where they avoid subsequent ubiquitin‐mediated targeting to the proteasome due to the low abundance of lysines in their sequences (Deeks et al., 2002; Hazes & Read, 1997). Since then, the ER translocation of toxins has helped to confirm the role of numerous ER chaperones and translocators in ER quality control mechanisms and has revealed the complex and flexible nature of ERAD degradation pathways (Morito & Nagata, 2015; Nowakowska‐Gołacka, Sominka, Sowa‐Rogozińska, & Słomińska‐Wojewódzka, 2019).
Thanks to toxins, retrograde trafficking is now established as a combination of constitutive transport routes that deliver cargo (proteins and lipids) from early, recycling, or late endosomes back to the TGN, Golgi apparatus, or the ER. Physiological roles of this retrograde transport include the equilibration of membrane flow within the endo membrane system, retrieval of ER‐resident proteins from the Golgi apparatus, receptor recycling, transport of proteins to the ER for retro translocation, and delivery of soluble extracellular antigens to the cytosol for antigen cross presentation (Bonifacino & Rojas, 2006; Johannes & Popoff, 2008). Thus, retrograde transport helps maintain cell homeostasis, coordinates signalling pathways, and controls antigen presentation (Johannes & Popoff, 2008; Spang, 2013). Additionally, toxin studies enabled the identification of various small molecule inhibitors and contributed to the characterisation of the molecular determinants that regulate sorting from different cellular membrane compartments, including clathrin adaptors, the retromer complex, Rab GTPases, and molecular motors (Bonifacino & Rojas, 2006; Johannes & Popoff, 2008).
4. TOXINS HAMPER MEMBRANE TRAFFICKING MACHINERIES
Bacterial toxins have played an oft‐forgotten though essential role in the understanding of vesicular trafficking. Indeed, the metalloprotease activity of tetanus and botulinum toxins against the vesicle‐associated membrane protein (VAMP) synaptobravin‐2 provided the first evidence that soluble NSF‐attachment protein receptors (SNAREs) controlled vesicle fusion and neurotransmitters secretion from neuronal synapses (Schiavo et al., 1992). SNAREs are now recognised as key regulators of the sequential steps that lead to vesicle fusion during transport between different membrane compartments. The characterisation of key components of neurotransmitter release, such as VAMP/synaptobrevins, SNAP‐25, and syntaxin 1, further helped cell biologists dissect SNARE function in neuronal exocytosis, trafficking, and survival (Lalli, Bohnert, Deinhardt, Verastegui, & Schiavo, 2003; Peng et al., 2013).
Similarly, lysosome secretion, visualised by the presence of lysosomal markers at the cell surface or the release of lysosomal enzymes, was first demonstrated to be induced by a rise in cytosolic calcium using both ionophores and plasma permeabilization by the pore‐forming toxin streptolysin O (Rodríguez, Webster, Ortego, & Andrews, 1997). Subsequent studies using pore‐forming toxins as triggers identified central regulators of lysosome exocytosis, including cytosolic calcium sensors (synaptotagmin VII), SNARE proteins (SNAP‐23, syntaxin 4, VAMP7), and numerous effector and regulatory molecules (Andrews & Corrotte, 2018). Today, the exocytosis of lysosomal‐related organelles has a recognised role in the repair of plasma membrane wounds and broader impact in various cell processes, such as polarity, immune granule secretion, and melanosome secretion (Andrews & Corrotte, 2018).
The damage to the plasma membrane caused by bacterial pore‐forming toxins also enabled the understanding of the diversity of roles of the endosomal sorting complexes required for transport (ESCRT) machinery, highlighting their involvement in membrane repair. The ESCRT machinery has established roles in the formation of vesicles that bud “out of the cytosol” such as ILV formation, viral budding at the cell surface, and cytokinesis. More recent studies using the pore‐forming toxins, streptolysin O and listeriolysin O, and other sources of plasma membrane damage found that ESCRT complexes mediated budding of pore containing membrane patches and membrane remodelling of plasma membrane wounds (Jimenez et al., 2014). These findings paved the way for subsequent discoveries that the ESCRT machinery is a central mediator of nuclear envelope and organelle repair (Raab et al., 2016; Skowyra, Schlesinger, Naismith, & Hanson, 2018).
Membrane damage can also be caused by the translocon pore‐forming proteins at the tip of Type III secretion systems of gram‐negative bacteria. Groundwork on membrane damage caused by internalised bacteria was critical in identifying cytosolic membrane‐damaging sensors (p62, NDP52, and optineurin) that promote selective autophagy of bacteria released into the cytosol as well as damaged organelles (Birmingham, Smith, Bakowski, Yoshimori, & Brumell, 2006; Thurston, Ryzhakov, Bloor, von Muhlinen, & Randow, 2009). Overall, these processes have not only showed us the clever ways toxins can harm host cells but the intricacies of mammalian cell trafficking.
5. FUTURE PERSPECTIVES
Bacterial toxins have been truly exceptional tools for revealing the complexities of host cell mechanisms, and in this perspective, we have provided an overview of how they have improved our view of eukaryotic membrane trafficking. The selected examples underscore the detailed mechanistic insights and the broad conceptual changes that their study has made to cell biology (Figure 1). Toxins have also equally impacted other cell biology systems/processes, such as the eukaryotic cytoskeleton, cell cycle, and post‐translational modifications of proteins. The story is not over, however—it is clear that we still have much to learn from the finely tuned, highly efficient (unmatched!) manner by which toxins make their way into cells. Also, the choice of their targets and the way they modify them is amazingly optimised and, therefore, every new mode of action of a bacterial toxin has also brought novel findings about cellular function and homeostasis. For example, what will we learn when we understand how toxins without a pore‐forming domain make it from the endosome to cytosol (Alami, Taupiac, Reggio, Bienvenüe, & Beaumelle, 1998; Beaumelle, Alami, & Hopkins, 1993; Garcia‐Castillo et al., 2015)? What is the relevance of multiple phenotypes targeted by pore‐forming toxins, namely, how do pore‐forming toxins lead to the fission or vacuolation of the ER (Brito, Cabanes, Sarmento Mesquita, & Sousa, 2019; Gonzalez et al., 2018; Mesquita et al., 2017)? Or how further can we exploit toxins to screen for regulators of organelle function and biosynthetic pathways (Tian et al., 2018)? Combined with model organisms, tailored pharmacological treatments, and emerging fine‐tuned genetic manipulations, bacterial toxins will continue to reveal clever strategies that help us to dissect and identify fundamental, and exceedingly precise, properties of membrane trafficking.
Figure 1.
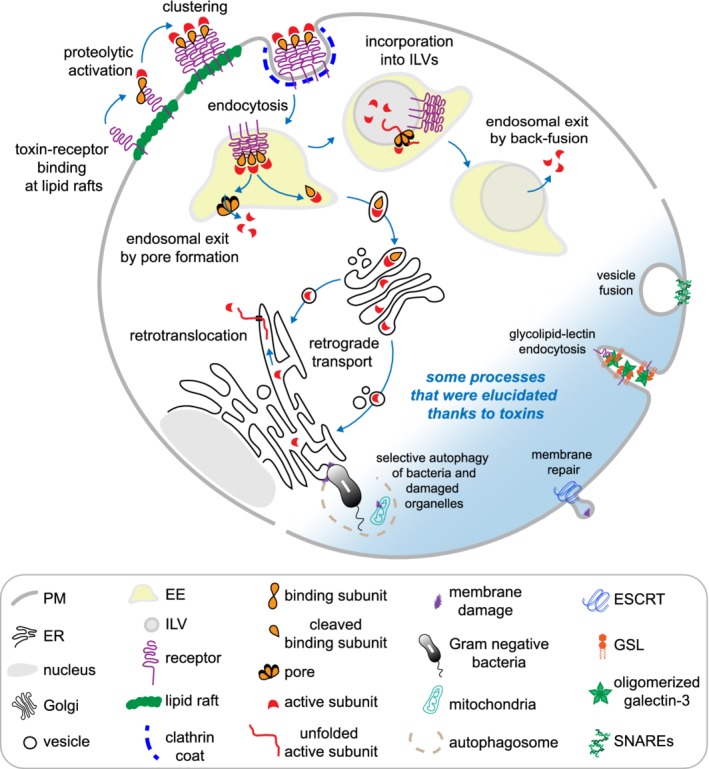
Trafficking routes influenced by bacterial toxins as shown for a hypothetical toxin with an active and binding subunit. The plasma membrane, endosome, and retrograde (Golgi to ER) pathways are depicted with their multiple mechanisms to access the cytosol. In addition, processes that were elucidated thanks to toxins are shown in the blue shading. EE, early endosome; ER, endoplasmic reticulum; ESCRT, endosomal sorting complexes required for transport; GSL, glycosphingolipids; ILV, intraluminal vesicle; PM, plasma membrane; SNAREs, soluble NSF‐attachment protein receptors (We thank Giorgia Brambilla Pisoni for her illustration)
CONFLICT OF INTEREST
The authors declare no conflicts of interest.
Mesquita FS, van der Goot FG, Sergeeva OA. Mammalian membrane trafficking as seen through the lens of bacterial toxins. Cellular Microbiology. 2020;22:e13167 10.1111/cmi.13167
Funding information The Swiss National Science Foundation grant 310030B_176393 to FGvdG is acknowledged.
Contributor Information
Francisco Sarmento Mesquita, Email: francisco.mesquita@epfl.ch.
Oksana A. Sergeeva, Email: oksana.sergeeva@epfl.ch.
REFERENCES
- Abrami, L. , Brandi, L. , Moayeri, M. , Brown, M. J. , Krantz, B. A. , Leppla, S. H. , & van der Goot, F. G. (2013). Hijacking multivesicular bodies enables long‐term and exosome‐mediated long‐distance action of anthrax toxin. Cell Reports, 5, 986–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami, L. , Fivaz, M. , Decroly, E. , Seidah, N. G. , Jean, F. , Thomas, G. , … van der Goot, F. G. (1998a). The pore‐forming toxin proaerolysin is activated by furin. The Journal of Biological Chemistry, 273, 32656–32661. [DOI] [PubMed] [Google Scholar]
- Abrami, L. , Fivaz, M. , Glauser, P.‐E. , Parton, R. G. , & van der Goot, F. (1998b). A pore‐forming toxin interacts with a GPI‐anchored protein and causes vacuolation of the endoplasmic reticulum. The Journal of Cell Biology, 140, 525–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami, L. , Fivaz, M. , & van der Goot, F. G. (2000). Surface dynamics of aerolysin on the plasma membrane of living cells. International Journal of Medical Microbiology, 290, 363–367. [DOI] [PubMed] [Google Scholar]
- Abrami, L. , Lindsay, M. , Parton, R. G. , Leppla, S. H. , & van der Goot, F. G. (2004). Membrane insertion of anthrax protective antigen and cytoplasmic delivery of lethal factor occur at different stages of the endocytic pathway. The Journal of Cell Biology, 166, 645–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami, L. , Liu, S. , Cosson, P. , Leppla, S. H. , & van der Goot, F. G. (2003). Anthrax toxin triggers endocytosis of its receptor via a lipid raft‐mediated clathrin‐dependent process. The Journal of Cell Biology, 160, 321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrami, L. , & van der Goot, F. G. (1999). Plasma membrane microdomains act as concentration platforms to facilitate intoxication by aerolysin. The Journal of Cell Biology, 147, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alami, M. , Taupiac, M.‐P. , Reggio, H. , Bienvenüe, A. , & Beaumelle, B. (1998). Involvement of ATP‐dependent pseudomonas exotoxin translocation from a late recycling compartment in lymphocyte intoxication procedure. Molecular Biology of the Cell, 9, 387–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso, M. A. , & Millán, J. (2001). The role of lipid rafts in signalling and membrane trafficking in T lymphocytes. Journal of Cell Science, 114, 3957–3965. [DOI] [PubMed] [Google Scholar]
- Andrews, N. W. , & Corrotte, M. (2018). Plasma membrane repair. Current Biology, 28, R392–R397. [DOI] [PubMed] [Google Scholar]
- Beaumelle, B. , Alami, M. , & Hopkins, C. R. (1993). ATP‐dependent translocation of ricin across the membrane of purified endosomes. The Journal of Biological Chemistry, 268, 23661–23669. [PubMed] [Google Scholar]
- Birmingham, C. L. , Smith, A. C. , Bakowski, M. A. , Yoshimori, T. , & Brumell, J. H. (2006). Autophagy controls salmonella infection in response to damage to the salmonella‐containing vacuole. The Journal of Biological Chemistry, 281, 11374–11383. [DOI] [PubMed] [Google Scholar]
- Bonifacino, J. S. , & Rojas, R. (2006). Retrograde transport from endosomes to the trans‐Golgi network. Nature Reviews. Molecular Cell Biology, 7, 568–579. [DOI] [PubMed] [Google Scholar]
- Bradley, K. A. , Mogridge, J. , Mourez, M. , Collier, R. J. , & Young, J. A. (2001). Identification of the cellular receptor for anthrax toxin. Nature, 414, 225–229. [DOI] [PubMed] [Google Scholar]
- Bray, D. , Levin, M. D. , & Morton‐Firth, C. J. (1998). Receptor clustering as a cellular mechanism to control sensitivity. Nature, 393, 85–88. [DOI] [PubMed] [Google Scholar]
- Bresnahan, P. A. , Leduc, R. , Thomas, L. , Thorner, J. , Gibson, H. L. , Brake, A. J. , … Thomas, G. (1990). Human fur gene encodes a yeast KEX2‐like endoprotease that cleaves pro‐beta‐NGF in vivo. The Journal of Cell Biology, 111, 2851–2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito, C. , Cabanes, D. , Sarmento Mesquita, F. , & Sousa, S. (2019). Mechanisms protecting host cells against bacterial pore‐forming toxins. Cellular and Molecular Life Sciences, 76, 1319–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown, D. A. , & Rose, J. K. (1992). Sorting of GPI‐anchored proteins to glycolipid‐enriched membrane subdomains during transport to the apical cell surface. Cell, 68, 533–544. [DOI] [PubMed] [Google Scholar]
- Bürgi, J. , Kunz, B. , Abrami, L. , Deuquet, J. , Piersigilli, A. , Scholl‐Bürgi, S. , … van der Goot, F. G. (2017). CMG2/ANTXR2 regulates extracellular collagen VI which accumulates in hyaline fibromatosis syndrome. Nature Communications, 8, 15861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary, V. K. , Jinno, Y. , FitzGerald, D. , & Pastan, I. (1990). Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proceedings of the National Academy of Sciences of the United States of America, 87, 308–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinnapen, D. J.‐F. , Chinnapen, H. , Saslowsky, D. , & Lencer, W. I. (2007). Rafting with cholera toxin: Endocytosis and trafficking from plasma membrane to ER. FEMS Microbiology Letters, 266, 129–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collier, R. J. , & Young, J. A. T. (2003). Anthrax toxin. Annual Review of Cell and Developmental Biology, 19, 45–70. [DOI] [PubMed] [Google Scholar]
- Deeks, E. D. , Cook, J. P. , Day, P. J. , Smith, D. C. , Roberts, L. M. , & Lord, J. M. (2002). The low lysine content of ricin a chain reduces the risk of proteolytic degradation after translocation from the endoplasmic reticulum to the cytosol. Biochemistry, 41, 3405–3413. [DOI] [PubMed] [Google Scholar]
- Dumitru, A. C. , Conrard, L. , Giudice, C. L. , Henriet, P. , Veiga‐da‐Cunha, M. , Derclaye, S. , … Alsteens, D. (2018). High‐resolution mapping and recognition of lipid domains using AFM with toxin‐derivatized probes. Chemical Communications, 54, 6903–6906. [DOI] [PubMed] [Google Scholar]
- Duncan, J. L. , & Schlegel, R. (1975). Effect of streptolysin O on erythrocyte membranes, liposomes, and lipid dispersions. A protein‐cholesterol interaction. The Journal of Cell Biology, 67, 160–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falguières, T. , Mallard, F. , Baron, C. , Hanau, D. , Lingwood, C. , Goud, B. , … Johannes, L. (2001). Targeting of Shiga toxin B‐subunit to retrograde transport route in association with detergent‐resistant membranes. Molecular Biology of the Cell, 12, 2453–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng, Y. , Jadhav, A. P. , Rodighiero, C. , Fujinaga, Y. , Kirchhausen, T. , & Lencer, W. I. (2004). Retrograde transport of cholera toxin from the plasma membrane to the endoplasmic reticulum requires the trans‐Golgi network but not the Golgi apparatus in Exo2‐treated cells. EMBO Reports, 5, 596–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fishman, P. H. (1982). Role of membrane gangliosides in the binding and action of bacterial toxins. The Journal of Membrane Biology, 69, 85–97. [DOI] [PubMed] [Google Scholar]
- Friebe, S. , van der Goot, F. G. , & Bürgi, J. (2016). The ins and outs of anthrax toxin. Toxins (Basel), 8, 69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs, E. , Haas, A. K. , Spooner, R. A. , Yoshimura, S. , Lord, J. M. , & Barr, F. A. (2007). Specific Rab GTPase‐activating proteins define the Shiga toxin and epidermal growth factor uptake pathways. The Journal of Cell Biology, 177, 1133–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujinaga, Y. , Wolf, A. A. , Rodighiero, C. , Wheeler, H. , Tsai, B. , Allen, L. , … Lencer, W. I. (2003). Gangliosides that associate with lipid rafts mediate transport of cholera and related toxins from the plasma membrane to endoplasmic reticulm. MBoC, 14, 4783–4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia‐Castillo, M. D. , Tran, T. , Bobard, A. , Renard, H.‐F. , Rathjen, S. J. , Dransart, E. , … Johannes, L. (2015). Retrograde transport is not required for cytosolic translocation of the B‐subunit of Shiga toxin. Journal of Cell Science, 128, 2373–2387. [DOI] [PubMed] [Google Scholar]
- Gekara, N. O. , Jacobs, T. , Chakraborty, T. , & Weiss, S. (2005). The cholesterol‐dependent cytolysin listeriolysin O aggregates rafts via oligomerization. Cellular Microbiology, 7, 1345–1356. [DOI] [PubMed] [Google Scholar]
- Ginefra, P. , Filippi, B. G. H. , Donovan, P. , Bessonnard, S. , & Constam, D. B. (2018). Compartment‐specific biosensors reveal a complementary subcellular distribution of bioactive furin and PC7. Cell Reports, 22, 2176–2189. [DOI] [PubMed] [Google Scholar]
- Girod, A. , Storrie, B. , Simpson, J. C. , Johannes, L. , Goud, B. , Roberts, L. M. , … Pepperkok, R. (1999). Evidence for a COP‐I‐independent transport route from the Golgi complex to the endoplasmic reticulum. Nature Cell Biology, 1, 423–430. [DOI] [PubMed] [Google Scholar]
- Gonzalez, M.R. , Bischofberger, M. , Frêche, B. , Ho, S. , Parton, R.G. , and Goot, F.G. van der (2018) Pore‐forming toxins induce multiple cellular responses promoting survival. Cellular Microbiology 13: 1026–1043. [DOI] [PubMed] [Google Scholar]
- González‐Bullón, D. , Uribe, K. B. , Martín, C. , & Ostolaza, H. (2017). Phospholipase A activity of adenylate cyclase toxin mediates translocation of its adenylate cyclase domain. Proceedings of the National Academy of Sciences of the United States of America, 114, E6784–E6793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon, V. M. , Klimpel, K. R. , Arora, N. , Henderson, M. A. , & Leppla, S. H. (1995). Proteolytic activation of bacterial toxins by eukaryotic cells is performed by furin and by additional cellular proteases. Infection and Immunity, 63, 82–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazes, B. , & Read, R. J. (1997). Accumulating evidence suggests that several AB‐toxins subvert the endoplasmic reticulum‐associated protein degradation pathway to enter target cells. Biochemistry, 36, 11051–11054. [DOI] [PubMed] [Google Scholar]
- Holmgren, J. , Lönnroth, I. , & Svennerholm, L. (1973). Tissue receptor for cholera exotoxin: Postulated structure from studies with GM1 ganglioside and related glycolipids. Infection and Immunity, 8, 208–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez, A. J. , Maiuri, P. , Lafaurie‐Janvore, J. , Divoux, S. , Piel, M. , & Perez, F. (2014). ESCRT machinery is required for plasma membrane repair. Science, 343, 1247136. [DOI] [PubMed] [Google Scholar]
- Johannes, L. , & Popoff, V. (2008). Tracing the retrograde route in protein trafficking. Cell, 135, 1175–1187. [DOI] [PubMed] [Google Scholar]
- Klimpel, K. R. , Molloy, S. S. , Thomas, G. , & Leppla, S. H. (1992). Anthrax toxin protective antigen is activated by a cell surface protease with the sequence specificity and catalytic properties of furin. Proceedings of the National Academy of Sciences of the United States of America, 89, 10277–10281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovbasnjuk, O. , Edidin, M. , & Donowitz, M. (2001). Role of lipid rafts in Shiga toxin 1 interaction with the apical surface of Caco‐2 cells. Journal of Cell Science, 114, 4025–4031. [DOI] [PubMed] [Google Scholar]
- Ladant, D. , & Ullmann, A. (1999). Bordetella pertussis adenylate cyclase: A toxin with multiple talents. Trends in Microbiology, 7, 172–176. [DOI] [PubMed] [Google Scholar]
- Lakshminarayan, R. , Wunder, C. , Becken, U. , Howes, M. T. , Benzing, C. , Arumugam, S. , … Johannes, L. (2014). Galectin‐3 drives glycosphingolipid‐dependent biogenesis of clathrin‐independent carriers. Nature Cell Biology, 16, 592–603. [DOI] [PubMed] [Google Scholar]
- Lalli, G. , Bohnert, S. , Deinhardt, K. , Verastegui, C. , & Schiavo, G. (2003). The journey of tetanus and botulinum neurotoxins in neurons. Trends in Microbiology, 11, 431–437. [DOI] [PubMed] [Google Scholar]
- Lauvrak, S. U. , Torgersen, M. L. , & Sandvig, K. (2004). Efficient endosome‐to‐Golgi transport of Shiga toxin is dependent on dynamin and clathrin. Journal of Cell Science, 117, 2321–2331. [DOI] [PubMed] [Google Scholar]
- Lindberg, A. A. , Brown, J. E. , Strömberg, N. , Westling‐Ryd, M. , Schultz, J. E. , & Karlsson, K. A. (1987). Identification of the carbohydrate receptor for Shiga toxin produced by Shigella dysenteriae type 1. The Journal of Biological Chemistry, 262, 1779–1785. [PubMed] [Google Scholar]
- Luzio, J. P. , Gray, S. R. , & Bright, N. A. (2010). Endosome‐lysosome fusion. Biochemical Society Transactions, 38, 1413–1416. [DOI] [PubMed] [Google Scholar]
- Maekawa, M. , Yang, Y. , & Fairn, G. D. (2016). Perfringolysin O theta toxin as a tool to monitor the distribution and inhomogeneity of cholesterol in cellular membranes. Toxins (Basel), 8, 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard, F. , Antony, C. , Tenza, D. , Salamero, J. , Goud, B. , & Johannes, L. (1998). Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of Shiga toxin B‐fragment transport. The Journal of Cell Biology, 143, 973–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masri, L. , Branca, A. , Sheppard, A. E. , Papkou, A. , Laehnemann, D. , Guenther, P. S. , … Schulenburg, H. (2015). Host–pathogen coevolution: The selective advantage of Bacillus thuringiensis virulence and its cry toxin genes. PLoS Biology, 13, e1002169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesquita, F. S. , Brito, C. , Mazon Moya, M. J. , Pinheiro, J. C. , Mostowy, S. , Cabanes, D. , & Sousa, S. (2017). Endoplasmic reticulum chaperone Gp96 controls actomyosin dynamics and protects against pore‐forming toxins. EMBO Reports, 18, 303–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montesano, R. , Roth, J. , Robert, A. , & Orci, L. (1982). Non‐coated membrane invaginations are involved in binding and internalization of cholera and tetanus toxins. Nature, 296, 651–653. [DOI] [PubMed] [Google Scholar]
- Morito, D. , & Nagata, K. (2015). Pathogenic hijacking of ER‐associated degradation: Is ERAD flexible? Molecular Cell, 59, 335–344. [DOI] [PubMed] [Google Scholar]
- Moya, M. , Dautry‐Varsat, A. , Goud, B. , Louvard, D. , & Boquet, P. (1985). Inhibition of coated pit formation in Hep2 cells blocks the cytotoxicity of diphtheria toxin but not that of ricin toxin. The Journal of Cell Biology, 101, 548–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Needham, S. R. , Roberts, S. K. , Arkhipov, A. , Mysore, V. P. , Tynan, C. J. , Zanetti‐Domingues, L. C. , … Martin‐Fernandez, M.L. (2016). EGFR oligomerization organizes kinase‐active dimers into competent signalling platforms. Nature Communications, 7, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichols, B. J. , Kenworthy, A. K. , Polishchuk, R. S. , Lodge, R. , Roberts, T. H. , Hirschberg, K. , … Lippincott‐Schwartz, J. (2001). Rapid cycling of lipid raft markers between the cell surface and Golgi complex. The Journal of Cell Biology, 153, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour, A. M. , & Modis, Y. (2014). Endosomal vesicles as vehicles for viral genomes. Trends in Cell Biology, 24, 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowakowska‐Gołacka, J. , Sominka, H. , Sowa‐Rogozińska, N. , & Słomińska‐Wojewódzka, M. (2019). Toxins utilize the endoplasmic reticulum‐associated protein degradation pathway in their intoxication process. International Journal of Molecular Sciences, 20, 1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng, L. , Liu, H. , Ruan, H. , Tepp, W. H. , Stoothoff, W. H. , Brown, R. H. , … Dong, M. (2013). Cytotoxicity of botulinum neurotoxins reveals a direct role of syntaxin 1 and SNAP‐25 in neuron survival. Nature Communications, 4, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirazzini, M. , Azarnia Tehran, D. , Leka, O. , Zanetti, G. , Rossetto, O. , & Montecucco, C. (2016). On the translocation of botulinum and tetanus neurotoxins across the membrane of acidic intracellular compartments. Biochimica et Biophysica Acta, 1858, 467–474. [DOI] [PubMed] [Google Scholar]
- Raab, M. , Gentili, M. , de Belly, H. , Thiam, H.‐R. , Vargas, P. , Jimenez, A. J. , … Piel, M. (2016). ESCRT III repairs nuclear envelope ruptures during cell migration to limit DNA damage and cell death. Science, 352, 359–362. [DOI] [PubMed] [Google Scholar]
- Révész, T. , & Greaves, M. (1975). Ligand‐induced redistribution of lymphocyte membrane ganglioside GM1. Nature, 257, 103–106. [DOI] [PubMed] [Google Scholar]
- Rodríguez, A. , Webster, P. , Ortego, J. , & Andrews, N. W. (1997). Lysosomes behave as Ca2+‐regulated exocytic vesicles in fibroblasts and epithelial cells. The Journal of Cell Biology, 137, 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo, D. , Della Ragione, F. , Rizzo, R. , Sugiyama, E. , Scalabrì, F. , Hori, K. , … D'Angelo, G. (2018). Glycosphingolipid metabolic reprogramming drives neural differentiation. The EMBO Journal, 37, e97674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Šachl, R. , Amaro, M. , Aydogan, G. , Koukalová, A. , Mikhalyov, I. I. , Boldyrev, I. A. , … Hof, M. (2015). On multivalent receptor activity of GM1 in cholesterol containing membranes. Biochimica et Biophysica Acta (BBA) ‐ Molecular Cell Research, 1853, 850–857. [DOI] [PubMed] [Google Scholar]
- Sandvig, K. , & Brown, J. E. (1987). Ionic requirements for entry of Shiga toxin from Shigella dysenteriae 1 into cells. Infection and Immunity, 55, 298–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig, K. , Olsnes, S. , Brown, J. E. , Petersen, O. W. , & van Deurs, B. (1989). Endocytosis from coated pits of Shiga toxin: A glycolipid‐binding protein from Shigella dysenteriae 1. The Journal of Cell Biology, 108, 1331–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig, K. , Skotland, T. , van Deurs, B. , & Klokk, T. I. (2013). Retrograde transport of protein toxins through the Golgi apparatus. Histochemistry and Cell Biology, 140, 317–326. [DOI] [PubMed] [Google Scholar]
- Schiavo, G. , Poulain, B. , Rossetto, O. , Benfenati, F. , Tauc, L. , & Montecucco, C. (1992). Tetanus toxin is a zinc protein and its inhibition of neurotransmitter release and protease activity depend on zinc. The EMBO Journal, 11, 3577–3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiavo, G. , & van der Goot, F. G. (2001). The bacterial toxin toolkit. Nature Reviews. Molecular Cell Biology, 2, 530–537. [DOI] [PubMed] [Google Scholar]
- Scobie, H. M. , Rainey, G. J. A. , Bradley, K. A. , & Young, J. A. T. (2003). Human capillary morphogenesis protein 2 functions as an anthrax toxin receptor. Proceedings of the National Academy of Sciences of the United States of America, 100, 5170–5174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sergeeva, O. A. , & van der Goot, F. G. (2019). Anthrax toxin requires ZDHHC5‐mediated palmitoylation of its surface‐processing host enzymes. Proceedings of the National Academy of Sciences of the United States of America, 116, 1279–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons, K. , & Ikonen, E. (1997). Functional rafts in cell membranes. Nature, 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Skowyra, M. L. , Schlesinger, P. H. , Naismith, T. V. , & Hanson, P. I. (2018). Triggered recruitment of ESCRT machinery promotes endolysosomal repair. Science, 360, eaar5078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang, A. (2013). Retrograde traffic from the Golgi to the endoplasmic reticulum. Cold Spring Harbor Perspectives in Biology, 5, a013391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl, P. D. , & Barbieri, M. A. (2002). Multivesicular bodies and multivesicular endosomes: The “ins and outs” of endosomal traffic. Science's STKE, 2002, pe32. [DOI] [PubMed] [Google Scholar]
- Thurston, T. L. M. , Ryzhakov, G. , Bloor, S. , von Muhlinen, N. , & Randow, F. (2009). The TBK1 adaptor and autophagy receptor NDP52 restricts the proliferation of ubiquitin‐coated bacteria. Nature Immunology, 10, 1215–1221. [DOI] [PubMed] [Google Scholar]
- Tian, S. , Muneeruddin, K. , Choi, M. Y. , Tao, L. , Bhuiyan, R. H. , Ohmi, Y. , … Dong, M. (2018). Genome‐wide CRISPR screens for Shiga toxins and ricin reveal Golgi proteins critical for glycosylation. PLoS Biology, 16, e2006951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varshney, P. , Yadav, V. , & Saini, N. (2016). Lipid rafts in immune signalling: Current progress and future perspective. Immunology, 149, 13–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez‐Boland, J. A. , Dominguez, L. , Rodriguez‐Ferri, E. F. , Fernandez‐Garayzabal, J. F. , & Suarez, G. (1989). Preliminary evidence that different domains are involved in cytolytic activity and receptor (cholesterol) binding in listeriolysin O, the Listeria monocytogenes thiol‐activated toxin. FEMS Microbiology Letters, 53, 95–99. [DOI] [PubMed] [Google Scholar]
- Waheed, A. A. , Shimada, Y. , Heijnen, H. F. , Nakamura, M. , Inomata, M. , Hayashi, M. , … Ohno‐Iwashita, Y. (2001). Selective binding of perfringolysin O derivative to cholesterol‐rich membrane microdomains (rafts). Proceedings of the National Academy of Sciences of the United States of America, 98, 4926–4931. [DOI] [PMC free article] [PubMed] [Google Scholar]


