Summary
The treatment landscape in relapsed/refractory chronic lymphocytic leukaemia (CLL) has rapidly evolved over the past five years, with one such emergent treatment being the BCL2 inhibitor, venetoclax. This oral treatment has demonstrated significant clinical advantages in indicated patients, but rapid tumour debulking can lead to a treatment‐related risk of the acute condition known as tumour lysis syndrome (TLS). Here, I present real patient cases to show how I have used the recommended predose monitoring and prophylactic procedures to mitigate the risk of TLS. I also used the ramp‐up dose escalation schedule of venetoclax therapy initiation to safely take patients through the treatment, successfully providing them with sustained clinical benefits.
Keywords: chronic lymphocytic leukaemia, tumour lysis syndrome, venetoclax, risk management, case studies
Chronic lymphocytic leukaemia (CLL), a neoplastic growth of lymphocytes in the bone marrow, is the most common leukaemia in adults (Seer Hematopoietic & Lymphoid Neoplasm Database, 2019). Treatment options for patients with CLL include chemoimmunotherapy and B‐cell‐targeted therapies (Eichhorst et al., 2015; ESMO, 2017). Venetoclax is one such targeted therapy which inhibits the antiapoptotic protein, B‐cell lymphoma‐2 (BCL2), and is approved as a monotherapy treatment of CLL in: (i) the presence of 17p deletion or TP53 mutation in adult patients who are unsuitable for, or have failed, a B‐cell receptor (BCR) pathway inhibitor; or (ii) in the absence of 17p deletion or TP53 mutation in adult patients who have failed both chemoimmunotherapy and BCR pathway inhibitor (Venetoclax SmPC, 2018). Venetoclax in combination with rituximab is indicated for the treatment of adult patients with CLL who have received at least one prior therapy (Venetoclax SmPC, 2018). Venetoclax has demonstrated substantial clinical benefits in the above patient groups in late‐phase clinical trials (Stilgenbauer et al., 2016; Coutre et al., 2018; Jones et al., 2018; Kater et al., 2018; Seymour et al., 2018).
A clinically researched titration pattern and monitoring schedule has been designed to support effective and tolerable treatment with venetoclax, which optimises clinical outcomes for patients with relapsed/refractory (R/R) CLL (Roberts et al., 2017; Venetoclax SmPC, 2018). This article will discuss the factors for consideration when initiating and maintaining venetoclax treatment as a monotherapy or in combination with rituximab, using real patient cases as examples.
Optimising CLL treatment to mitigate the risk of TLS
In asymptomatic CLL patients who do not present with the disease at an advanced stage, a ‘watch‐and‐wait’ approach to treatment on diagnosis is initially adopted, as there is no evidence that treating stable patients leads to a better long‐term outcome (CLL Trialists’ Collaborative Group, 1999). However, possible increases in tumour load during the watch‐and‐wait period can lead to a risk of tumour lysis syndrome (TLS). TLS is the uncontrolled release of phosphorus, nucleic acids, potassium and inflammatory cytokines, which cause clinically relevant electrolyte and metabolic disturbances, leading to renal insufficiency, seizures and death due to cardiac arrythmias and multiorgan failure. Although it can occur spontaneously, it more commonly occurs after anti‐cancer treatment and is characterised by the rapid lysis of malignant cells and release of their cellular contents into the bloodstream (Howard, 2011).
Risk‐stratification for TLS
Patients with cancer, including haematological malignancy, can be stratified into low‐, intermediate‐ and high‐risk categories for treatment‐related TLS, based on specific risk factors (Jones et al., 2015): (i) tumour burden, (ii) tumour grade and cell turnover rate, (iii) pre‐existing renal impairment or renal involvement by tumour, (iv) age, (v) treatment with highly active cell‐cycle specific agents, (vi) concomitant use of drugs that increase uric acid levels. When risk‐stratifying CLL patients for treatment with venetoclax, it is recommended that their TLS‐risk category is primarily based upon assessment of their tumour burden (measured by lymph node size and blood count) and reduced renal function (measured by creatinine clearance) (Table 1) (Seymour et al., 2018).
Table 1.
TLS‐risk‐stratification based on CLL tumour burden Seymour et al. (2018).
| TLS‐risk | Tumour burden assessment |
|---|---|
| Low |
AND
|
| Medium* |
OR
|
| High |
AND
|
ALC, absolute lymphocyte count; CLL, chronic lymphocytic leukaemia; LN, lymph node; TLS, tumour lysis syndrome.
Patients with medium TLS‐risk who have creatinine clearance of <80 mg/ml are to be managed as high‐risk.
Mitigating for TLS
TLS can be categorised depending on whether it is detectable by changes in metabolic measurements alone ‒ either laboratory‐TLS or, if both metabolic and clinical observations are made, clinical‐TLS (Table II) (Jones et al., 2015). Patients at risk of TLS should be monitored for the development of laboratory‐TLS as a precursor to more severe clinical‐TLS, so they can be managed prophylactically to avoid progression.
Table 2.
Laboratory‐ and clinical‐TLS definitions Jones et al. (2015).
| Criterion | Metabolic/clinical abnormalities | |
|---|---|---|
| Laboratory‐TLS | The presence of two or more metabolic abnormalities in a patient with cancer, or undergoing treatment for cancer within three days prior to, and up to seven days after, initiation of treatment | Uric acid ≥476 μmol/l or 25% increase from baseline |
| Potassium ≥6·0 mmol/l or 25% increase from baseline | ||
| Phosphate ≥1·45 mmol/l or 25% increase from baseline (adults) | ||
| Calcium ≤1·75 mmol/l or 25% decrease from baseline | ||
| Clinical‐TLS | A patient with laboratory‐TLS and at least one clinical abnormality | Creatinine ≥1·5 × ULN (age >12 years or age‐adjusted) |
| Cardiac arrhythmia | ||
| Sudden death | ||
| Seizure |
TLS, tumour lysis syndrome; ULN, upper limit of normal.
To mitigate the risk of laboratory‐TLS, there are several key steps and appropriate interventions that should be used to prevent and manage TLS before venetoclax treatment is started (Coiffier et al., 2008; Cairo et al., 2010; Venetoclax SmPC, 2018). Firstly, patients should undergo evaluation for TLS‐risk factors and, if they are deemed to be at risk, they should be closely monitored in an inpatient or outpatient hospital setting, depending upon the level of risk (Fig 1A). TLS‐risk management and prophylaxis include intensive oral and IV (intravenous) hydration, as well as administration of anti‐hyperuricaemic agents to manage high uric acid levels (Fig 1B) (Venetoclax SmPC, 2018).
Figure 1.
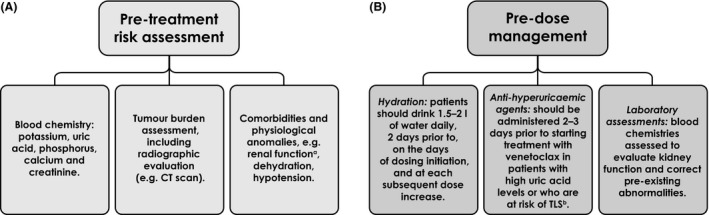
Predose initiation for venetoclax. (A) Pretreatment risk assessments are carried out before initiating venetoclax therapy to evaluate a patient’s TLS‐risk. (B) Predose prophylactic measures are implemented based on a patient’s TLS‐risk assessment. Venetoclax SmPC, 2018. aCreatine clearance <80 ml/min. bMay be continued through dose titration phase. CT, computed tomography; TLS, tumour lysis syndrome.
Clinical trial evidence has demonstrated that when the dose escalation and risk management protocol for TLS is used, there is a low incidence of TLS in patients treated with venetoclax, and that these patients can be robustly managed and their TLS‐risk mitigated. In an open‐label Phase 1b trial of venetoclax in 49 patients with R/R CLL, five patients developed laboratory‐TLS during dose escalation; two of these patients advanced to clinical‐TLS, of which one case was fatal. These findings led to the institution of a venetoclax dose escalation modification (a reduced starting dose of 20 mg) and TLS‐risk management procedures, following which there were no subsequent clinical‐TLS events (Seymour et al., 2017). In a Phase 2 study of venetoclax in 107 R/R CLL patients, laboratory‐TLS was reported in five patients during the dose escalation period. These events were mitigated by robust TLS prophylactic and monitoring procedures ‒ none of these five patients progressed to having clinical‐TLS, and there were no TLS fatalities (Stilgenbauer et al., 2016). Similar rates of TLS were observed in a larger, open‐label Phase 3 trial of venetoclax in combination with rituximab in 194 CLL patients: five patients developed grade 3 or 4 laboratory‐TLS, and one patient developed non‐fatal clinical‐TLS (Seymour et al., 2018).
The five‐week dose escalation schedule of venetoclax (and the phased introduction of rituximab in combination therapy) is designed to gradually debulk tumour burden and decrease the risk of TLS (Fig 2A) (Venetoclax SmPC, 2018). By initiating venetoclax at a low dose and incrementally increasing it weekly where appropriate, patients can undergo regular blood chemistry monitoring and be proactively dose‐adjusted, if necessary (Fig 2B) (Venetoclax SmPC, 2018). In addition to clinical evidence, real‐world experience demonstrates that patients can be individually managed on venetoclax, depending on their risks of TLS (illustrated by patients A and B; Boxes 1 and 2). These case studies are based on real patients who are suitable for either venetoclax monotherapy or venetoclax and rituximab combination therapy, to practically demonstrate that similar concepts around treatment initiation apply in both situations.
Figure 2.

(A) Venetoclax five‐week dose titration schedule. (B) Management strategies during venetoclax ramp‐up dosing. Venetoclax SmPC, 2018. *In monotherapy treatment, venetoclax 400 mg should be administered until disease progression or until it is no longer tolerated. TLS, tumour lysis syndrome.
Box 1. Patient A: Venetoclax monotherapy treatment initiation.
Diagnosis and first‐line treatment
Patient A is a 76‐year‐old male who was diagnosed with CLL [with the prognostically poor del(11q) chromosomal deletion] (Döhner et al., 2000), after presenting in 2011 with colon cancer, and a lymph node biopsy revealed a small lymphocytic lymphoma. A watch‐and‐wait monitoring approach was taken until his CLL progressed to the point of requiring treatment: he was treated with fludarabine, cyclophosphamide and rituximab (FCR) chemotherapy, which was well tolerated and led to complete remission (CR).
Relapse
This patient relapsed after five years: his haemoglobin dropped to 85 g/l (no evidence of haemolysis) and in addition to his persistent del(11q) mutation, he had acquired a del(17p) in 30% of his CLL cells. He was treated with ibrutinib 420 mg once daily, which was initially well tolerated; however, eight months following ibrutinib initiation, he developed a severe, therapy‐related rash. Despite a short treatment break of two weeks and a reduced ibrutinib dose to 140 mg, his rash immediately worsened, and he was taken off treatment for four months, with his disease subsequently progressing*.
Venetoclax treatment initiation and TLS‐risk management
At disease progression, the decision was made to start him on venetoclax monotherapy. He had an absolute lymphocyte count (ALC) of 30 × 109/l and his largest lymph node (LN) was 6 cm; his creatinine clearance was 50 mg/ml. Due to his high risk of TLS (Table 1), I started him on the anti‐hyperuricaemic, allopurinol, two to three days before treatment; instructed him to drink 1·5–2 l of fluid daily for two days before starting venetoclax; and he was hospitalised for hydration and observation. This patient’s blood chemistry was analysed before and after he received the first 20 mg dose titration of venetoclax; 8 h after his first dose, his potassium had increased from 3·5 mmol/l at baseline to 4·5 mmol/l, and although this level remained within the normal range, the rise was sufficient for us to start aggressive IV fluid replacement. Twenty‐four hours after venetoclax dose initiation, the patient’s biochemistry remained normal and he was discharged from inpatient care to continue with 20 mg venetoclax, once daily, until weekly dose escalation (50 mg, 100 mg, 200 mg, 400 mg; Weeks 2–5, respectively). His blood chemistry was analysed at baseline and at eight and 24 h post‐treatment after every dose escalation. For three years, this patient has been maintained successfully on well‐tolerated 400 mg venetoclax daily and remains in CR.
*Patient A’s intolerance to ibrutinib (as evidenced by a rash that did not resolve with dose reduction) meant that his ibrutinib therapy was completely stopped. In the special case of a patient developing resistance to ibrutinib, the therapy should however not be stopped immediately, due to the potential risk of very rapid disease progression. Instead, it is recommended that ibrutinib should be continued during ramp‐up dosing of venetoclax until the patient is established on the 400 mg dose, at which point ibrutinib is stopped. This patient was monitored until his disease progressed; it is important that the disease should not progress to the point where the patient is high‐risk before starting venetoclax.
Box 2. Patient B: Treatment initiation of venetoclax in combination with rituximab.
Diagnosis and first‐line treatment
Patient B is a female, currently aged 45, who first presented in 2016 with increased swelling in the neck. She was diagnosed with CLL when her complete blood count revealed lymphocytosis with a classical CLL immunophenotype and an ALC of 15 × 109/l. As her disease progressed rapidly, she started a six‐cycle course of FCR chemotherapy in 2018. She required growth factor support during FCR therapy due to profound neutropenia, and she eventually achieved only a partial remission (PR).
Venetoclax and rituximab treatment initiation with TLS‐risk management
I discussed treatment options with the patient when her disease progressed following FCR therapy and, as she was keen to be on a fixed duration of treatment, venetoclax in combination with rituximab was initiated, allowing for a fixed duration treatment for 24 months. Her pretreatment computed tomography (CT) scan showed moderately enlarged LNs (≤4 cm at largest diameter), an ALC of 45 × 109/l, and normal renal function, so she was therefore stratified as medium‐risk for TLS (Table II). She was advised to drink plenty of fluids for two days before starting venetoclax.
This patient did not require hospitalisation, but was managed as an outpatient, with blood chemistry performed during the five‐week ramp‐up schedule, both before, and eight and 24 h after each weekly venetoclax dose escalation (20 mg, 50 mg, 100 mg, 200 mg, 400 mg daily; Weeks 1–5, respectively).
In practice, I arrange the blood test on the day before starting treatment in order to check the biochemistry and risk stratification. If treatment as an outpatient is appropriate, I administer the first dose of 20 mg of venetoclax early in the morning to allow the 6–8 h blood test to be performed when staff are available to check the biochemistry and ensure there is no need for biochemical correction. I then bring the patient back the next morning to re‐test bloods and administer the second dose of venetoclax, once it is confirmed that there is no need for any biochemical correction. This process is repeated for each dose escalation until the patient is on the recommended dose of 400 mg.
When this patient had been established on 400 mg venetoclax daily for one week, rituximab was introduced via IV infusion at 375 mg/m2 on Day 1 of Cycle 1 and then increased to 500 mg/m2 every 28 days for Cycles 2–6.
Postvenetoclax remission and monitoring
Patient B has now completed her rituximab cycles and is currently successfully maintained on 400 mg once‐daily venetoclax monotherapy which she is tolerating well, having achieved CR at nine months of therapy. She is minimal residual disease (MRD)‐negative in her peripheral blood (PB) and continues to have her MRD monitored by flow cytometry, with a bone marrow (BM) MRD‐test scheduled at one year of treatment.
Case studies of treatment initiation with venetoclax
Optimising venetoclax treatment in routine clinical practice
Step 1: Patient–doctor discussions to determine treatment choice
When discussing treatment options with the patient and deciding on optimal treatment selection, there are a number of factors that I believe are important to consider:
(i) informed consent; (ii) rationale for selecting venetoclax; (iii) discussion of continuous treatment strategy vs. fixed duration strategy (Venetoclax SmPC, 2018); (iv) potential side effects; (v) safety aspects, e.g. relative safety of 400 mg dose of venetoclax but requirement for a ramp‐up period to avoid TLS; (6) explaining TLS and its associated risk factors and mitigation protocols. For example, in the case of Patient A (Box 1), I would explain to him that he is at high risk for TLS but that there are specific management strategies for someone in his risk category.
Another factor for consideration is in the treatment of elderly patients, where the ramp‐up dose may be viewed as a burden in terms of the need for frequent and potentially prolonged hospital visits. As with patients A and B, once the patient and I have agreed to initiate venetoclax therapy, there are several steps to take, both before and after therapy is started (Boxes 1 and 2). The following sections constitute an example of a week‐by‐week strategy in venetoclax treatment initiation; importantly, this needs to be individualised for each patient, as not all patients will react in the same way. For instance, just because there is no laboratory‐TLS with lower doses does not mean that vigilance should be decreased, as TLS can occur for the first time on subsequent dose escalation.
Step 2: Actions to take before initiating venetoclax therapy
Pretreatment assessments and prophylactic strategies
Before initiating venetoclax, I carry out specific pretreatment assessments to evaluate patient TLS‐risk and necessary predose prophylactic measures to be implemented, based on the patient’s level of risk (Fig 1).
Prophylactic measures can be maintained during the venetoclax dose titration phase, as needed (Fig 2B) (Venetoclax SmPC, 2018). For example, Patient A is high‐risk for TLS and was admitted for IV fluids, whereas based upon the results of the CT scan for patient B, showing moderately enlarged lymph nodes and ALC of 45 × 109/l, and therefore a medium risk for TLS, she was therefore advised to drink plenty of fluids for two days before starting venetoclax (Box 1).
The importance of multidisciplinary management in the practical considerations during dose‐planning
There is a need to involve and educate the whole clinical team – especially clinical nurse specialists, treating nurses, junior doctors and pharmacists, because they are involved in biochemical monitoring and administering venetoclax – on individual responsibilities for the management of high‐risk TLS patients. The whole team needs to be made aware of the clinical signs of TLS (e.g. increased creatinine levels, risks for seizures, cardiac dysrhythmia) and other side effects, e.g. neutropoenia. Early team‐planning and communication processes need to be in place before venetoclax dose initiation to ensure monitoring of patients during the dose titration phase is managed effectively (especially, but not limited to, results and out‐of‐hours events) and that appropriate staff and beds are available. Ideally, patients should have a baseline blood test the evening before receiving the first dose so that venetoclax can be administered early in the morning. I accept results from laboratories based outside the hospital, provided the laboratory participates in an accredited quality assurance program. Laboratory results mean that we do not have to wait for the results of early‐morning blood work before administering venetoclax, and the eight‐hour blood test can be performed and then reviewed within working hours, as discussed for Patient B (Box 2).
Management approaches for patients at risk of TLS
Tumour lysis syndrome prophylaxis measures within the venetoclax protocol should be followed throughout the dose titration phase (with more intensive measures used to moderate any increase in overall risk) for patients who are considered to be at risk of developing TLS (Fig 2B) (Venetoclax SmPC, 2018).
Step 3: Venetoclax dose titration schedule
When starting the initial venetoclax dose titration of 20 mg daily, patient blood chemistry should be monitored closely, as electrolyte changes indicating TLS can occur as early as 6–8 h following the first dose. Blood chemistry should be monitored following each stepwise dose increase until a dose of 400 mg per day is reached at Week 5, as patients can still be at risk of TLS with subsequent doses (not just the initial dose) (Venetoclax SmPC, 2018). As illustrated in Patient A, even when blood test results remain within the normal range, changes in biochemistry should still be reviewed and may require action, such as extra fluids (Box 1).
Venetoclax dose modifications for TLS
If, following a venetoclax dose, a patient’s blood chemistry meets the criteria for TLS (Table II), the following day’s dose should be withheld. If their blood biochemistry returns to normal within 24–48 h after their last dose, venetoclax treatment can be resumed at the same dose received prior to TLS occurrence. If the blood chemistry change does not resolve within 48 h, or there is development of clinical‐TLS, venetoclax should be resumed at a lower dose before that week’s dose escalation. If dose interruption was necessary for more than one week during the five‐week titration period or for more than two weeks after the end of the titration period, then a given patient’s TLS‐risk should be reassessed to determine whether restarting at a reduced venetoclax dose is necessary (Venetoclax SmPC, 2018) (Table III).
Table 3.
Dose modification for TLS during venetoclax treatment Venetoclax SmPC (2018.
| Dose at interruption, mg | Restart dose, mg* |
|---|---|
| 400 | 300 |
| 300 | 200 |
| 200 | 100 |
| 100 | 50 |
| 50 | 20 |
| 20 | 10 |
TLS, tumour lysis syndrome.
The modified dose should be continued for one week before increasing it.
Step 4: Postdose titration: ongoing treatment and management
When venetoclax dose titration is successfully completed in monotherapy treatment, patients should continue to be maintained on 400 mg daily until disease progression or until it is no longer tolerated by the patient. When venetoclax is given in combination with rituximab, patients should take 400 mg venetoclax for 24 months from Day 1 Cycle 1 of rituximab therapy (Venetoclax SmPC, 2018). All patients should be regularly monitored for side effects, including: (1) TLS: continue to monitor blood chemistry and adjust dose if required (Table III), although it should be noted that TLS is rare after the 400 mg dose is established; (2) neutropoenia: the most common grade 3–4 adverse event in a Phase 2 trial of venetoclax monotherapy in 107 CLL patients (40%) (Stilgenbauer et al., 2016), and in a Phase 3 trial of venetoclax in combination with rituximab in 194 patients (60·8%) (Seymour et al., 2018). Patients with neutropoenia may require omission of a venetoclax dose but can often be managed with growth factor support. There needs to be specific monitoring of patients who have discontinued venetoclax and those who are restarting venetoclax.
Potential future direction for venetoclax management
There are several potential combination treatments with venetoclax, in addition to rituximab, that are currently being explored. With these emerging combinations, protocol evolution is necessary to optimise their safety and improve clinical benefits. For instance, during combination therapy in a Phase 1b study combining venetoclax with an antibody, treatment‐naive and relapsed CLL patients receiving venetoclax first were compared to patients receiving obinutuzumab first ‒ this showed no difference in the incidence of clinical‐TLS (Flinn et al., 2019), demonstrating the need to standardise the knowledge of combination therapy protocol safety.
The Phase 2 CLARITY study of 50 patients demonstrated that the combination of ibrutinib and venetoclax in R/R CLL was generally tolerated by patients. Patients were started on ibrutinib monotherapy before venetoclax was started, using the dose escalation schedule. With this approach, there was only one case of laboratory‐TLS, which later resolved with treatment (Hillmen et al., 2018). This combination is being further explored in a Phase 2 study of patients who have developed treatment‐resistant mutations following ibrutinib treatment (ClinicalTrials.gov: NCT03513562, 2019), and in front‐line treatment in the CAPTIVATE study (ClinicalTrials.gov: NCT02910583, 2019) (Wierda et al., 2018). This is currently also a treatment regime being explored in the front‐line CLL FLAIR clinical trial in the UK, a study investigating alternative treatment schedules with venetoclax in the first‐line setting, specifically the approach of administering ibrutinib before starting venetoclax. The four treatment arms of this trial consist of FCR, ibrutinib and rituximab, ibrutinib monotherapy, and ibrutinib and venetoclax (Cancer Research UK, 2019).
Conclusion
Venetoclax has demonstrated improved clinical outcomes in defined groups of patients with CLL, although there is the potential for a slightly increased risk of an acute comorbidity, namely TLS. In this report, clinical examples have been shared on how to successfully use venetoclax to take on and treat patients with CLL in the real world, outlining the steps which are taken to mitigate the risk of TLS. In the therapeutic context of both monotherapy, and in combination with rituximab, venetoclax treatment can be optimised through collaborative, appropriate and proactive management of the risk of TLS by the multidisciplinary team to successfully deliver clinical benefit to our patients.
Disclosures
Grant funding: Janssen, AstraZeneca, Celgene; Honoraria: AbbVie, AstraZeneca, Celgene, Gilead, Janssen, Roche.
Author contributions
J.G.G. conceived and wrote this article and provided the patient case studies.
Acknowledgements
Medical writing support was provided by Kiran Nandra, PhD, and Omoremi Williams of Leading Edge Medical Communications Ltd., Loudwater, UK, funded by AbbVie Inc.
References
- Cairo, M.S. , Coiffier, B. , Reiter, A. & Younes, A. & TLS Expert Panel (2010) Recommendations for the evaluation of risk and prophylaxis of tumour lysis syndrome (TLS) in adults and children with malignant diseases: an expert TLS panel consensus. British Journal of Haematology, 149, 578–586. [DOI] [PubMed] [Google Scholar]
- Cancer Research UK (2019) A trial of ibrutinib with rituximab for chronic lymphocytic leukaemia (FLAIR). Available at https://www.cancerresearchuk.org/about-cancer/find-a-clinical-trial/a-trial-ibrutinib-rituximab-chronic-lymphocytic-leukaemia-flair. Last accessed March 2019.
- ClinicalTrials.gov: NCT02910583 (2019) Available at https://clinicaltrials.gov/ct2/show/NCT02910583. Last accessed March 2019.
- ClinicalTrials.gov: NCT03513562 (2019) Available at https://clinicaltrials.gov/ct2/show/NCT03513562. Last accessed March 2019.
- CLL Trialists’ Collaborative Group (1999) Chemotherapeutic options in chronic lymphocytic leukemia: a meta‐analysis of the randomized trials. Journal of the National Cancer Institue, 91, 861–868. [DOI] [PubMed] [Google Scholar]
- Coiffier, B. , Altman, A. , Pui, C.H. , Younes, A. & Cairo, M.S. (2008) Guidelines for the management of pediatric and adult tumor lysis syndrome: an evidence‐based review. Journal of Clinical Oncology, 26, 2767–2778. [DOI] [PubMed] [Google Scholar]
- Coutre, S. , Choi, M. , Furman, R.R. , Eradat, H. , Heffner, L. , Jones, J.A. , Chyla, B. , Zhou, L. , Agarwal, S. , Waskiewicz, T. , Verdugo, M. , Humerickhouse, R.A. , Potluri, J. , Wierda, W.G. & Davids, M.S. (2018) Venetoclax for patients with chronic lymphocytic leukemia who progressed during or after idelalisib therapy. Blood, 131, 1704–1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Döhner, H. , Stilgenbauer, S. , Benner, A. , Leupolt, E. , Kröber, A. , Bullinger, L. , Döhner, K. , Bentz, M. & Lichter, P. (2000) Genomic aberrations and survival in chronic lymphocytic leukemia. New England Journal of Medicine, 343, 1910–1916. [DOI] [PubMed] [Google Scholar]
- Eichhorst, B. , Robak, T. , Montserrat, E. , Ghia, P. , Hillmen, P. , Hallek, M. , Buske, C. , & ESMO Guidelines Committee (2015) Chronic lymphocytic leukaemia: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Annals of Oncology, 26, v78–v84. [DOI] [PubMed] [Google Scholar]
- ESMO (2017) Clinical Guidelines. eUpdate – Chronic Lymphocytic Leukaemia Treatment Recommendations, UK. Available at https://www.esmo.org/Guidelines/Haematological-Malignancies/Chronic-Lymphocytic-Leukaemia/eUpdate-Treatment-Recommendations. Last accessed March 2019.
- Flinn, I.W. , Gribben, J.G. , Dyer, M.J.S. , Wierda, W. , Maris, M.B. , Furman, R.R. , Hillmen, P. , Rogers, K.A. , Padmanabhan Iyer, S. , Quillet‐Mary, A. , Ysebaert, L. , Walter, H.S. , Verdugo, M. , Klein, C. , Huang, H. , Jiang, Y. , Lozanski, G. , Pignataro, D.S. , Humphrey, K. , Mobasher, M. & Kipps, T.J. (2019) Phase 1b study of venetoclax‐obinutuzumab in previously untreated and relapsed/refractory chronic lymphocytic leukemia. Blood, 133, 2765–2775. [E‐publ ahead of print]. doi: 10.1182/blood-2019-01-896290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillmen, P. , Rawstron, A. , Brock, K. , Munoz Vicente, S. , Yates, F. , Bishop, R. , Macdonald, D. , Fegan, C. , McCaig, A. , Schuh, A. , Pettitt, A. , Gribben, J.G. , Patten, P.E.M. , Devereux, S. , Bloor, A. , Fox, C.P. , Forconi, F. & Munir, T. (2018) Ibrutinib plus venetoclax in relapsed/refractory CLL: results of the bloodwise TAP CLARITY study. Blood, 132, 182. [Google Scholar]
- Howard, S.C. (2011) The tumor lysis syndrome. New England Journal of Medicine, 364, 1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, G.L. , Will, A. , Jackson, G.H. , Webb, N.J. , Rule, S. & British Committee for Standards in Haematology (2015) Guidelines for the management of tumour lysis syndrome in adults and children with haematological malignancies on behalf of the British Committee for Standards in Haematology. British Journal of Haematology, 169, 661–671. [DOI] [PubMed] [Google Scholar]
- Jones, J.A. , Mato, A.R. , Wierda, W.G. , Davids, M.S. , Choi, M. , Cheson, B.D. , Furman, R.R. , Lamanna, N. , Barr, P.M. , Zhou, L. , Chyla, B. , Salem, A.H. , Verdugo, M. , Humerickhouse, R.A. , Potluri, J. , Coutre, S. , Woyach, J. & Byrd, J.C. (2018) Venetoclax for chronic lymphocytic leukaemia progressing after ibrutinib: an interim analysis of a multicentre, open‐label, phase 2 trial. Lancet Oncology, 19, 65–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kater, A.P. , Seymour, J.F. , Hillmen, P. , Eichhorst, B. , Langerak, A.W. , Owen, C. , Verdugo, M. , Wu, J. , Punnoose, E.A. , Jiang, Y. , Wang, J. , Boyer, M. , Humphrey, K. , Mobasher, M. & Kipps, T.J. (2018) Fixed duration of venetoclax‐rituximab in relapsed/refractory chronic lymphocytic leukemia eradicates minimal residual disease and prolongs survival: post‐treatment follow‐up of the MURANO phase III study. Journal of Clinical Oncology, 37, 269–277. [DOI] [PubMed] [Google Scholar]
- Roberts, R.W. , Stilgenbauer, S. , Seymour, J.F. & Huang, D.C.S. (2017) Venetoclax in patients with previously treated chronic lymphocytic leukemia. Clinical Cancer Research, 23, 4527–4533. [DOI] [PubMed] [Google Scholar]
- SEER (2019) Hematopoietic and Lymphoid Neoplasm Database: Chronic Lymphocytic Leukemia/small lymphocytic lymphoma. National Cancer Institute, USA: Available at https://seer.cancer.gov/seertools/hemelymph/51f6cf59e3e27c3994bd5447/. Last accessed March 2019. [Google Scholar]
- Seymour, J.F. , Ma, S. , Brander, D.M. , Choi, M.Y. , Barrientos, J. , Davids, M.S. , Anderson, M.A. , Beaven, A.W. , Rosen, S.T. , Tam, C.S. , Prine, B. , Agarwal, S.K. , Munasinghe, W. , Zhu, M. , Lash, L.L. , Desai, M. , Cerri, E. , Verdugo, M. , Kim, S.Y. , Humerickhouse, R.A. , Gordon, G.B. , Kipps, T.J. & Roberts, A.W. (2017) Venetoclax plus rituximab in relapsed or refractory chronic lymphocytic leukaemia: a phase 1b study. Lancet Oncology, 18, 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seymour, J.F. , Kipps, T.J. , Eichhorst, B. , Hillmen, P. , D'Rozario, J. , Assouline, S. , Owen, C. , Gerecitano, J. , Robak, T. , De la Serna, J. , Jaeger, U. , Cartron, G. , Montillo, M. , Humerickhouse, R. , Punnoose, E.A. , Li, Y. , Boyer, M. , Humphrey, K. , Mobasher, M. & Kater, A.P. (2018) Venetoclax‐rituximab in relapsed or refractory chronic lymphocytic leukemia. New England Journal of Medicine, 378, 1107–1120. [DOI] [PubMed] [Google Scholar]
- Stilgenbauer, S. , Eichhorst, B. , Schetelig, J. , Coutre, S. , Seymour, J.F. , Munir, T. , Puvvada, S.D. , Wendtner, C.M. , Roberts, A.W. , Jurczak, W. , Mulligan, S.P. , Böttcher, S. , Mobasher, M. , Zhu, M. , Desai, M. , Chyla, B. , Verdugo, M. , Enschede, S.H. , Cerri, E. , Humerickhouse, R. , Gordon, G. , Hallek, M. & Wierda, W.G. (2016) Venetoclax in relapsed or refractory chronic lymphocytic leukaemia with 17p deletion: a multicentre, open‐label, phase 2 study. Lancet Oncology, 17, 768–778. [DOI] [PubMed] [Google Scholar]
- Venetoclax [Summary of Product Characteristics] (2018) AbbVie Ltd, December 2018.
- Wierda, W.G. , Siddiqi, T. , Flinn, I. , Badoux, X.C. , Kipps, T.J. , Allan, J.N. , Tedeschi, A. , Pagel, J.M. , Kuss, B.J. , Barca, E.G. , Ghia, P. , Eckert, K. , Zhou, C. , Ninimoto, J. , Dean, J.P. , James, D.F. & Tam, C. (2018) Phase 2 CAPTIVATE results of ibrutinib (ibr) plus venetoclax (ven) in first‐line chronic lymphocytic leukemia (CLL). Journal of Clinical Oncology, 36, 7502–7502. [Google Scholar]


