Abstract
Objective
To describe seizure outcomes in patients with medically refractory epilepsy who had evidence of bilateral mesial temporal lobe (MTL) seizure onsets and underwent MTL resection based on chronic ambulatory intracranial EEG (ICEEG) data from a direct brain‐responsive neurostimulator (RNS) system.
Methods
We retrospectively identified all patients at 17 epilepsy centers with MTL epilepsy who were treated with the RNS System using bilateral MTL leads, and in whom an MTL resection was subsequently performed. Presumed lateralization based on routine presurgical approaches was compared to lateralization determined by RNS System chronic ambulatory ICEEG recordings. The primary outcome was frequency of disabling seizures at last 3‐month follow‐up after MTL resection compared to seizure frequency 3 months before MTL resection.
Results
We identified 157 patients treated with the RNS System with bilateral MTL leads due to presumed bitemporal epilepsy. Twenty‐five patients (16%) subsequently had an MTL resection informed by chronic ambulatory ICEEG (mean = 42 months ICEEG); follow‐up was available for 24 patients. After MTL resection, the median reduction in disabling seizures at last follow‐up was 100% (mean: 94%; range: 50%‐100%). Nine patients (38%) had exclusively unilateral electrographic seizures recorded by chronic ambulatory ICEEG and all were seizure‐free at last follow‐up after MTL resection; eight of nine continued RNS System treatment. Fifteen patients (62%) had bilateral MTL electrographic seizures, had an MTL resection on the more active side, continued RNS System treatment, and achieved a median clinical seizure reduction of 100% (mean: 90%; range: 50%‐100%) at last follow‐up, with eight of fifteen seizure‐free. For those with more than 1 year of follow‐up (N = 21), 15 patients (71%) were seizure‐free during the most recent year, including all eight patients with unilateral onsets and 7 of 13 patients (54%) with bilateral onsets.
Significance
Chronic ambulatory ICEEG data provide information about lateralization of MTL seizures and can identify additional patients who may benefit from MTL resection.
Keywords: brain‐responsive neurostimulation, electrocorticography, epilepsy surgery, intractable temporal lobe epilepsy, mesial temporal lobe resection, temporal lobectomy
Key Points.
Chronic intracranial EEG (ICEEG) data obtained by the RNS System may identify candidates for curative or palliative mesial temporal lobe (MTL) resections
Twenty‐five of 157 patients (16%) treated with the RNS System with bilateral MTL leads had a resection based on chronic ambulatory ICEEG data
All nine patients with only unilateral electrographic seizures were seizure‐free after MTL resection; eight continued RNS System treatment
All 15 patients with bilateral‐onset seizures treated with MTL resection and RNS System had ≥50% seizure reduction; 8 of 15 were seizure‐free
In carefully selected patients with presumed bitemporal epilepsy, MTL resection informed by chronic ICEEG may yield excellent outcomes
1. INTRODUCTION
Mesial temporal lobe (MTL) resection or ablation (from herein referred to as “MTL resection”) can eliminate seizures in many patients with unilateral MTL seizures.1, 2, 3 However, if seizures arise bilaterally, achieving seizure remission is unlikely because bilateral MTL resection is not an option due to high risk of severe anterograde memory loss.4 Unilateral MTL resection in a patient with bilateral independent MTL onset can provide substantial palliation in carefully selected patients. However, it may not be possible to establish a confident estimate of MTL seizure lateralization within 1‐2 weeks of inpatient video–electroencephalography (EEG) monitoring. In a group of 82 patients treated with the RNS System with bilateral MTL leads and confirmed bilateral MTL seizures, the average time to record the first contralateral MTL electrographic seizure was 41.6 days,5 considerably longer than the average length of typical epilepsy monitoring unit (EMU) inpatient stays.6 In addition, some patients cluster from alternate sides, with most or all seizures arising from one side for weeks to months, only to switch to the other side at other times.7 In addition to the short time window permissible with typical scalp or intracranial recordings in the EMU, withdrawal of antiseizure medications, sleep deprivation, anesthesia, stress, and the postoperative setting could alter normal patterns of seizures and even trigger seizures that otherwise would not occur.8, 9, 10, 11 Thus, limitations in the data provided by the typical presurgical evaluation may lead to a faulty conclusion about a patient's laterality, especially in patients with possible bitemporal epilepsy.
The study's intent was threefold: (a) to evaluate how information provided by chronic ambulatory intracranial EEG (ICEEG) monitoring data captured by the RNS System contributed to the decision to proceed with an MTL resection; (b) to determine clinical outcome after MTL resection as assessed by reduction in frequency of patient‐reported disabling seizures; and (c) to assess how many of these patients continued to be treated with the RNS System after MTL resection.
2. METHODS
A retrospective chart review was conducted at 17 comprehensive epilepsy centers of all patients with medically intractable focal‐onset seizures who were treated with the RNS System between 2006 and 2016 using bilateral hippocampal depth or subtemporal cortical strip leads, and in whom an MTL resection was subsequently performed.
The RNS System (NeuroPace, Inc) is approved by the US Food and Drug Administration (FDA) as an adjunctive treatment for adults with refractory focal‐onset epilepsy having one or two seizure foci. Safety and efficacy were demonstrated in a randomized controlled trial and in a long‐term open‐label follow‐up study.12, 13 The RNS System includes a cranially implanted neurostimulator that is connected to one or two depth or cortical strip leads, each containing four electrode contacts. The leads are placed at that patient's seizure focus/foci (Figure 1). The neurostimulator continuously monitors ICEEG activity, and when patient‐specific abnormal patterns are detected, stimulation is automatically delivered. In addition, the neurostimulator provides continuous counts of pattern detections, data regarding the time and location of detections, recordings during pattern detections, as well as scheduled brief recordings of ICEEG activity.
Figure 1.
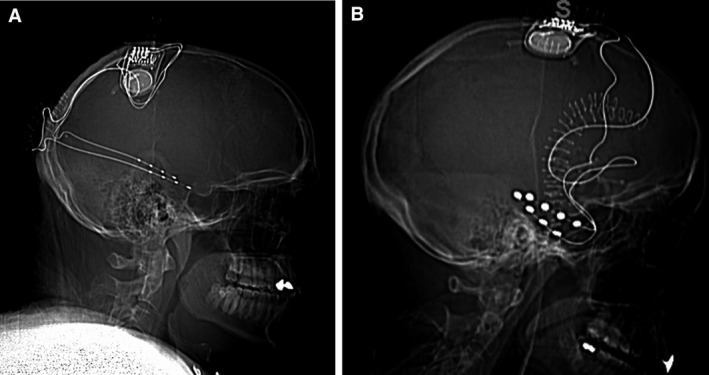
A, Neurostimulator connected to longitudinal bilateral hippocampal depth leads (most common mesial temporal lobe [MTL] treatment configuration). B, Neurostimulator connected to bilateral subtemporal strip leads
2.1. Clinical outcomes
Data obtained from patients’ medical records included demographics (age, age at epilepsy onset, and etiology), results of presurgical localization studies (scalp and intracranial EEG, and neuroimaging), EEG lateralization of seizures before RNS System treatment, type and location of leads connected to the neurostimulator, electrographic seizure lateralization with RNS System chronic ambulatory ICEEG, and type of MTL resection performed. Patient‐reported frequency of disabling seizures (focal aware motor, focal with impaired awareness, focal to bilateral tonic‐clonic), number of antiseizure medications, and driving status were determined for the 3 months before RNS System treatment, the 3 months before MTL resection, and at the most recent follow‐up. The primary outcome measure was seizure frequency at the most recent follow‐up compared to the 3 months before MTL resection. Seizure frequency was determined by medical record review, including any information available in the record as part of clinical care (clinician notes, patient diaries, patient questionnaires, and so on). In addition, all treating physicians completed a Clinical Global Impression Scale (CGIS) to assess the physician's subjective opinion of patient outcome after MTL resection. Adverse events attributed to the neurostimulator or MTL resection were identified based on chart review.
2.2. Chronic intracranial EEG review
RNS System chronic ambulatory ICEEG recordings were reviewed on the NeuroPace Patient Data Management System (PDMS), a secure online interface, in order to determine lateralization of MTL electrographic seizures. An electrographic seizure was defined as “a sustained rhythmic discharge, including repetitive spiking or spike‐and‐wave pattern faster than or equal to 2 Hz, with definite evolution in frequency, location, or morphology, and clearly distinguishable from background, lasting at least 10 seconds in duration.”14 The chronic ambulatory ICEEG review was performed pre‐MTL resection and post‐MTL resection through most recent follow‐up for patients who continued to be monitored with the RNS System. The final lateralization of electrographic seizures was determined by the patient's treating neurologist.
3. RESULTS
Twenty‐five of 157 patients (16%) treated with direct brain‐responsive neurostimulation using bilateral MTL leads underwent a subsequent MTL resection. One patient transferred institutions and is not included in the analyses. Demographic characteristics for the 24 patients with follow‐up are presented in Table 1.
Table 1.
Patient characteristics based on lateralization with long‐term ICEEG monitoring
|
Unilateral electrographic MTL seizures on chronic ambulatory ICEEG (N = 9) |
Bilateral electrographic MTL seizures on chronic ambulatory ICEEG (N = 15) |
|
|---|---|---|
| Age (y) |
Mean: 46 (median: 42, range: 31‐67) |
Mean: 40 (median: 36, range: 19‐61) |
| Duration of epilepsy (y) |
Mean: 18 (median: 13, range: 8‐50) |
Mean: 20 (median: 13, range: 2‐46) |
| Etiology | ||
| Unknown | 6 (67%) | 7 (47%) |
| Autoimmune epilepsy | 1 (11%) | 2 (13%) |
| Head trauma | 1 (11%) | 1 (7%) |
| Encephalitis | 0 | 3 (20%) |
| Hypoxic injury | 1 (11%) | 0 |
| Other | 0 | 2 (13%) |
| MTL abnormality detected on MRI | ||
| None | 1 (11%) | 3 (20%) |
| Unilateral | 2 (22%) | 7 (47%) |
| Bilateral | 6 (67%) | 5 (33%) |
| EEG monitoring with intracranial electrodes prior to RNS System | ||
| Yes | 4 (44%) | 8 (53%) |
| No | 5 (56%) | 7 (47%) |
| MTL seizure lateralization prior to RNS System | ||
| Bilateral | 4 (44%) | 15 (100%) |
| Unilateral | 4 (44%) | 0 |
| Unclear | 1 (11%) | 0 |
|
Duration of treatment with RNS System prior to MTL resection (mo) |
Mean: 48 (median: 24, range: 8‐115) |
Mean: 38 (median: 22, range: 10‐117) |
| Surgical procedure | ||
| Anteromesial temporal lobectomy (AMTL) | 8 (89%) | 14 (93%) |
| Selective Amygdalohippocampectomy (SAH) | 1 (11%) | 1 (7%) |
|
Follow‐up post‐MTL resection (mo) |
Mean: 29 (median: 18, range: 11‐67) |
Mean: 38 (median: 21, range: 6‐111) |
Abbreviations: ICEEG, intracranial EEG; MTL, mesial temporal lobe.
3.1. Presurgical localization and RNS System lead placement
Nineteen of the 24 patients had bilateral MTL electrographic seizures recorded during presurgical localization (inpatient scalp EEG, intracranial EEG, or both). The remaining five patients had data suggesting bilateral MTL seizures: one had unclear lateralization of electrographic seizures, one had bilateral mesial temporal sclerosis (MTS), one had good memory and language on the side of seizure onset and some seizures that were not well lateralized, one had bilateral interictal spikes and fast seizure spread contralaterally, and one had bilateral spikes and declined a right MTL resection in favor of neurostimulation. As a result, all patients underwent bilateral MTL lead placement for RNS System treatment. Twenty‐two patients had bilateral longitudinal hippocampal depth leads (inserted along the long axis of the hippocampus with a posterior approach), one patient had bilateral orthogonal hippocampal depth leads (implanted perpendicular to the long axis of the hippocampus from a lateral approach), and one patient had bilateral subtemporal strip leads.
3.2. Seizure reductions with direct brain‐responsive neurostimulation
Prior to RNS System treatment, the average number of disabling seizures per month was 21.2 (range: 2‐150). Patients were treated with the RNS System for an average of 42 months (median: 24 months, range: 8‐117 months) before MTL resection. The median percent reduction in frequency of disabling clinical seizures during the last 3 months of follow‐up prior to MTL resection was 37%, with a responder rate (≥50% reduction) of 54%.
3.3. Lateralization after chronic ambulatory ICEEG
Based on RNS System chronic ambulatory ICEEG, 9 of the 24 patients (38%) had exclusively unilateral electrographic MTL seizures recorded, 4 exclusively from the left and 5 from the right. The remaining 15 patients (62%) had electrographic seizures recorded bilaterally. However, 13 of 15 had more than 90% of their electrographic seizures coming from one side. Five patients had electrographic seizures arising predominantly from the left (mean: 92.4%, range: 90%‐97%) and 10 patients predominantly from the right (mean: 87.6%, range: 60%‐96%). In all cases, surgery was performed on the MTL with the majority of electrographic seizures.
3.4. MTL surgeries and outcomes
Twenty‐two of the 24 patients had an anteromesial temporal lobectomy (AMTL) and two had a selective amygdalohippocampectomy (SAH). No patient underwent laser ablation. Fifteen surgeries (62%) were right and nine surgeries (38%) were left.
Twenty‐three of the 24 patients continued to be treated with the RNS System post‐MTL resection (monitored and receiving brain‐responsive neurostimulation). Seventeen of these 23 patients were monitored with the RNS System bilaterally by either adding or newly connecting an existing cortical strip lead on the resected side (N = 8) or, as shown in Figure 2, by pulling back the depth lead on the resected side (N = 9). Six of the 23 patients continued to be monitored with the RNS System only in the temporal lobe contralateral to the MTL resection. One patient had the neurostimulator and leads explanted at the time of resection (Table 2).
Figure 2.

Illustration of bilateral hippocampal depth leads connected to the neurostimulator. The right hippocampal depth lead was pulled back into the posterior hippocampus and medial temporooccipital lobe at the time of a right anteromedial temporal lobectomy
Table 2.
Pre‐RNS System seizure recording and imaging data, and RNS System long‐term chronic ICEEG results pre‐ and post‐MTL surgery
| Patient number | Prior to RNS system | Post RNS system implant, Prior to MTL surgery | MTL surgery | Post‐MTL surgery | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Scalp seizures | Inpatient short‐term ICEEG | MRI findings | Reason for RNS system with bilateral leads | Follow‐up (mo) | Long‐term ICEEG seizures | Long‐term ICEEG seizures (% from each hemisphere) | Side resected | Follow‐up (mo) | RNS system leads | Long‐term ICEEG seizures | Seizure reduction at last follow‐up | |
| 4 |
0 left 5 right |
Bilateral atrophy & increased signal | Bilateral MTS | 62 | Unilateral | 100% right | Right | 48 | Bilateral | Contralaterala | Seizure free | |
| 6 |
0 left 20 right |
Right atrophy & increased signal | Bilateral spiking & fast contralateral seizure spread | 21 | Unilateral | 100% right | Right | 18 | Bilateral | Contralateral a | Seizure free | |
| 11 |
1 left 2 unclear |
13 left 2 unclear |
Left atrophy | Bilateral spiking; patient declined resection | 115 | Unilateral | 100% left | Left | 19 | None | Not applicable | Seizure free |
| 24 | 6 unclear | Left atrophy & increased signalb | Unclear lateralization | 74 | Unilateral | 100% right | Right | 57 | Unilateral | None | Seizure free | |
| 27 |
5 right 2 unclear |
Normal | Bilat spiking; fast ictal spread | 8 | Unilateral | 100% right | Right | 11 | Unilateral | None | Seizure free | |
| 14 |
3 left 2 right |
4 leftc 2 right |
Right atrophy | Bilateral seizures | 19 | Unilateral | 100% right | Right | 14 | Bilateral | None | Seizure free |
| 17 | 12 left |
11 leftc 13 right |
Normal | Bilateral seizures | 78 | Unilateral | 100% left | Left | 17 | Bilateral | None | Seizure free |
| 18 |
1 left 2 right |
Left atrophy & increased signal | Bilateral seizures | 42 | Unilateral | 100% left | Left | 67 | Unilateral | None | Seizure free | |
| 19 |
2 left 5 right |
4 leftc 9 right |
Bilateral atrophy & increased signal | Bilateral seizures | 11 | Unilateral | 100% right | Right | 18 | Bilateral | Contralateral a | Seizure free |
| 1 |
2 left 1 right |
Bilateral atrophy & increased signal | Bilateral seizures | 15 | Bilateral |
97% left 3% right |
Left | 7 | Unilateral | Contralateral a | Seizure free | |
| 2 |
16 left 1 right 1 unclear |
9 leftc | Left atrophy & increased signal | Bilateral seizures | 17 | Bilateral |
92% left 8% right |
Left | 19 | Bilateral | Contralateral a | Seizure free |
| 7 |
6 left 1 right |
4 left 6 right |
Right increased signal | Bilateral seizures | 11 | Bilateral |
25% left 75% right |
Right | 40 | Unilateral | Contralateral a | Seizure free |
| 8 |
3 left 7 right |
Bilateral atrophy & increased signal | Bilateral seizures | 15 | Bilateral |
93% left 7% right |
Left | 13 | Bilateral | Contralateral a | Seizure free | |
| 13 | 3 right |
1 left 5 right |
Normal | Bilateral seizures | 10 | Bilateral |
10% left 90% right |
Right | 13 | Bilateral | Ipsilateral a | Seizure free |
| 20 |
4 left 5 right |
Bilateral increased signal | Bilateral seizures | 22 | Bilateral |
4% left 96% right |
Right | 101 | Bilateral | Ipsilateral a | Seizure free | |
| 21 |
1 left 4 right |
Bilateral atrophy & increased signal | Bilateral seizures | 117 | Bilateral |
5% left 95% right |
Right | 31 | Bilateral | Contralateral a | Seizure free | |
| 25 |
9 left 5 right 1 unclear |
26 left 8 right |
Right atrophy & increased signal | Bilateral seizures | 14 | Bilateral |
90% left 10% right |
Left | 13 | Bilateral | Contralaterala | Seizure free |
| 5 |
1 left 1 right 1 unclear |
Normal | Bilateral seizures | 107 | Bilateral |
5% left 95% right |
Right | 21 | Bilateral | Contralateral | 96% | |
| 10 |
3 left 3 right 3 unclear |
6 left 3 right 2 unclear |
Bilateral atrophy & increased signal | Bilateral seizures | 94 | Bilateral |
90% left 10% right |
Left | 26 | Unilateral | Contralateral | 65% |
| 15 |
11 left 8 right |
Bilateral atrophy & increased signal | Bilateral seizures | 13 | Bilateral |
40% left 60% right |
Right | 111 | Bilateral | Contralateral | 94% | |
| 16 |
8 left 1 right |
Bilateral atrophy & increased signal | Bilateral seizures | 42 | Bilateral |
10% left 90% right |
Right | 78 | Bilateral | Contralateral | 89% | |
| 23 |
5 left 5 right 1 unclear |
6 leftc 3 right |
Right increased signal | Bilateral seizures | 38 | Bilateral |
10% left 90% right |
Right | 72 | Bilateral | Contralateral | 88% |
| 26 |
7 left 8 right |
2 rightc | Normal | Bilateral seizures | 30 | Bilateral |
5% left 95% right |
Right | 14 | Bilateral | Bilateral | 75% |
| 28 |
5 left 8 right |
6 leftc 4 right |
Right atrophy & increased signal | Bilateral seizures | 27 | Bilateral |
8% left 92% right |
Right | 6 | Bilateral | Contralateral | 50% |
Abbreviations: ICEEG, intracranial EEG; MTL, mesial temporal lobe.
Continued to have some electrographic seizures postsurgery but did not report clinical seizures at the patients' last follow‐up. This occurred in 11/17 clinically seizure‐free patients.
Note that all seizures arose from the side opposite the hippocampal atrophy and increased signal in this case.
Cases for which inpatient short‐term ICEEG monitoring was misleading, either for bilateral or unilateral onsets (five cases: patients 2, 14, 17, 19, and 26), or for the predominant side of seizure onset (two cases: patients 25 and 28). This occurred in 7 of 12 cases with inpatient short‐term ICEEG monitoring.
The mean follow‐up after MTL resection was 35 months (median: 19 months, range: 6‐111 months). The median reduction in patient‐reported seizures at last follow‐up was 100% (mean: 92%; range: 50%‐100%) and the responder rate (≥50% reduction in disabling seizures) was 100%. Seventy‐one percent of patients (17/24) were free of disabling clinical seizures for at least 3 months at the most recent follow‐up (Table 3); the mean duration of seizure freedom was 14 months (median: 12 months, range: 3‐57 months; Figure 3). Of the patients with more than 1 year of follow‐up (N = 21), 15 patients (71%) were seizure‐free during the most recent year, including all 8 patients with unilateral onsets and 7 of 13 patients (54%) with bilateral onsets. At last follow‐up, 71% (17/24) were seizure‐free for at least 3 months.
Table 3.
Seizure outcomes and Clinician Global Impression Scale scores at last follow‐up
|
Unilateral MTL seizures by chronic ambulatory ICEEG [N = 9] |
Bilateral MTL seizures by chronic ambulatory ICEEG [N = 15] |
Total [N = 24] | |
|---|---|---|---|
| Seizure outcomes | |||
| Seizure‐free ≥3 mo | 9/9 (100%) | 8/15 (53%) | 71% (17/24) |
| Seizure‐free ≥12 mo | 8/8 (100%) | 7/13 (54%) | 71% (15/21) |
| Respondersa (≥50% reduction) | 9/9 (100%) | 15/15 (100%) | 24/24 (100%) |
| Nonrespondersa (<50% reduction) | 0 | 0 | 0 |
| Clinician Global Impression Scale Scores | |||
| Very much improved | 5 | 9 | 14 |
| Much Improved | 2 | 4 | 6 |
| Minimally Improved | 1 | 2 | 3 |
| No change | 1 | 0 | 1 |
Abbreviations: ICEEG, intracranial EEG; MTL, mesial temporal lobe.
Based on the final 3 mo period of follow‐up compared to the 3 mo prior to MTL resection.
Figure 3.

Percent changes in clinical seizure frequency at most recent follow‐up in patients with unilateral (gray) and bilateral (blue) mesial temporal lobe (MTL) electrographic seizures recorded on RNS System chronic ambulatory intracranial EEG (ICEEG)
Using the CGIS Scores (Table 3), physicians indicated that 20 of 24 patients (83%) were “very much improved” (14; 58%) or “much improved” (6; 25%), three (12.5%) were minimally improved, and one (4%) had no change (details in subsections below). Five patients (21%) obtained their driver's license.
3.5. Patients with only unilateral MTL seizure onsets recorded with chronic ambulatory ICEEG
Nine of the 24 patients (38%) had only unilateral electrographic seizure onsets captured by chronic ambulatory ICEEG over an average of 48 months prior to MTL resection. The median seizure‐frequency reduction with brain‐responsive neurostimulation prior to MTL resection was 75% (range: 0%‐87%). Follow‐up after MTL resection ranged from 11‐67 months. At last follow‐up, all nine patients were free of disabling clinical seizures, (Figure 3, gray bars). Seven of the nine patients (78%) were continuously free of disabling clinical seizures after MTL resection, and two of the nine (22%) had rare disabling clinical seizures in the first year and later became free of disabling clinical seizures (for >1 year).
Eight of the nine patients continued to be treated with the RNS System after MTL resection. Three of the eight were monitored using only leads contralateral to the resection. Five of the eight were monitored bilaterally (strip added at the time of resection or lead remained in/next to resection); three of these patients had an electrographic seizure recorded by the RNS System from the temporal lobe contralateral to the resection, but none from the side of the resection. Details of the lateralization before and after RNS System treatment can be found in Table 2.
On the CGIS at most recent follow‐up, five of the nine patients (56%) were classified by their physician as “very much improved” and two of the nine (22%) as “much improved.” One patient was considered to be “minimally improved” because of a worsening in preexisting depression and complaints of chronic pain at the craniotomy site (postsurgery). One patient was considered to have “no change” after MTL resection because of worsened short‐term memory, worsening of baseline depression, and recurrent psychogenic nonepileptic seizures requiring emergency department or hospital visits. Four of the nine patients (44%) were able to stop at least one antiseizure medication. No patient stopped all antiseizure medications. Three of the nine patients (33%) obtained a driver's license.
3.6. Patients with bilateral MTL electrographic seizure onsets with chronic ambulatory ICEEG monitoring
Fifteen of the 24 patients (62%) had bilateral electrographic seizures on chronic ambulatory ICEEG monitoring over 6‐111 months of follow‐up. Nonetheless, 8 of the 15 patients (53%) were free of disabling clinical seizures (Figure 3, blue bars). In these eight seizure‐free patients, the percentage of seizures arising from the resected side on chronic ambulatory ICEEG ranged from 75%‐97% (median 91% [Table 2]). Three of the eight seizure‐free patients (38%) were free of disabling clinical seizures continuously after MTL resection; five seizure‐free patients (62%) had rare disabling clinical seizures in the first year and then became free of clinical seizures (four of five were seizure‐free for the final year or longer, and the fifth patient was seizure‐free for the final 11 months).
All 15 patients in this group continued to be treated with the RNS System after MTL resection. Thirteen of the 15 patients (87%) continued to be monitored bilaterally (strip added at time of resection or lead remained in/next to resection). All 13 patients had electrographic seizures recorded by the RNS System after MTL resection (which may or may not have manifested clinically); 10 patients had electrographic seizure onsets recorded contralateral to the MTL resection (6 of 10 were clinically seizure‐free), two patients had electrographic seizure onsets ipsilateral to MTL resection (both were clinically seizure‐free), and one patient had bilateral independent electrographic seizure onsets recorded (75% clinical seizure reduction). Two patients were monitored only contralateral to MTL resection; both had post‐MTL resection electrographic seizures recorded. One of these patients was free of disabling clinical seizures and the other had a 65% reduction in disabling clinical seizures. Details of lateralization before and after RNS System treatment can be found in Table 2.
On the CGIS after MTL resection, 9 of these 15 patients (60%) were classified as “Very Much Improved,” four (27%) were “Much Improved,” and two (13%) were “Minimally Improved” (Table 3). One of the “minimally improved” patients was completely seizure‐free since MTL resection but had a worsening of baseline depression, requiring multiple hospitalizations for suicidal ideation. The second patient with minimal improvement had a 50% reduction in clinical seizures. Four patients were able to reduce at least one antiseizure medication. No patients discontinued antiseizure medication completely. Two patients obtained a driver's license.
3.7. Safety
RNS System related adverse events were captured for all patients prior to the MTL resection. Three patients (12.5%) had adverse events. Two patients developed an implant‐site infection (8.3%) and one patient had a scalp dehiscence (4.2%). All three events resolved, and none of the neurostimulators or leads were explanted permanently. No other adverse events were reported.
Adverse events related to MTL resection occurred in nine patients (37.5%). These included worsening in baseline depression (three patients; 12.5%), worsening of preexisting memory complaints (two patients; 8.3%), infection (two patients; 8.3%), recurrence of psychogenic seizures (one patient; 4.2%), and chronic pain at the craniotomy site (one patient; 4.2%). Of the three patients with worsening of depression, two had right MTL surgeries and one had left MTL resection; all depressive episodes were ongoing at data cutoff. The infections in two patients resolved. One of the patients delayed reporting a scalp‐incision site infection due to their distance from the hospital and the infection eventually required removal of the neurostimulator and leads. The other patient had a scalp‐ and craniotomy‐site infection which did not require explant of the neurostimulator and leads. Of the two patients with subjectively reported worsening of memory, one had a right MTL resection, bilateral MTS, left hemisphere language dominance, and intact left hemisphere memory on Wada testing. The memory decline was only present postictally after clusters of seizures arising from the left. The other patient with subjective memory decline had a left MTL resection, left hemisphere language dominance, and bilateral intact memory on Wada testing. Both memory complaints were ongoing, although the postictal deficit was infrequent due to markedly improved seizure frequency. The adverse events related to psychogenic seizures and craniotomy site pain were reported to be ongoing at last follow‐up.
4. DISCUSSION
Chronic ambulatory bilateral MTL ICEEG monitoring with a direct brain‐responsive neurostimulator identified patients who benefitted from a unilateral MTL resection. Based on acute intracranial monitoring and other presurgical data, these patients were not initially considered to be appropriate for resective surgery. All nine patients whose seizures were determined to be unilateral with RNS System chronic ambulatory ICEEG became seizure‐free after MTL resection. Even patients with independent bilateral onsets with a unilateral preponderance had substantial benefit, with a median percent seizure reduction of 100% (range: 50%‐100%) and just over half (8/15) becoming seizure‐free. In all but one patient, surgery was combined with continued RNS System treatment. Clinicians rated patients as much improved or very much improved in 20 of 24 cases. Five patients (21%) obtained their driver's licenses.
Lateralization of an MTL seizure focus relies on a multimodal evaluation that incorporates data from history, neuroimaging, neuropsychological testing, and inpatient video and scalp or ICEEG recordings over days to weeks. Patients whose data are entirely concordant for a unilateral MTL onset have a high probability of seizure remission after an MTL resection or ablation. However, electrographic, neurocognitive, or imaging abnormalities suggestive of abnormalities in both temporal lobes reduce the probability of seizure freedom.15
Failed MTL surgeries may be due to bilateral MTL epilepsy that was not identified during preoperative localization studies.16 Overall, 27%‐42% of patients undergoing MTL surgeries do not remit over the first postoperative year,3, 15 and the percentage increases to 48% at 5 years and 53% at 10 years.17, 18 If localization data are not entirely concordant for unilateral MTL onsets, then the chance of seizure remission is reduced. In a series of 27 MTL resection failures, Abosch et al17 reported that bilateral temporal lobe onsets, bilateral abnormal MRI findings, or both, were a predictor of seizure relapse. Jeha, et al19 demonstrated that in 371 patients treated with unilateral temporal lobe surgery, seizure freedom rate was 73% at 6 months and 58% at 2 years if there were bilateral MRI abnormalities, compared to 88% at 6 months and 78% at 2 years for those without bilateral MRI abnormalities.
Chronic ambulatory ICEEG monitoring provides electrographic data over months and years that could importantly supplement the spatially rich but temporally limited data from the EMU. Data from chronic ambulatory ICEEG indicate that typical in‐patient EEG evaluations, whether scalp or intracranial, could lead to inaccurate conclusions about whether a patient has unilateral or bilateral MTL seizures. In a retrospective study of 82 patients with bilateral MTL seizures treated with brain‐responsive neurostimulation, about half had only unilateral MTL seizures in the first 2 weeks of chronic ambulatory ICEEG. Without prolonged monitoring, these patients with MTL seizures could have been incorrectly identified as having only unilateral onsets. Conversely, some patients who have bilateral MTL seizures during in‐patient scalp or ICEEG may have only unilateral seizures in the real world when on their antiseizure medications. This was the case in one study of 71 patients with presumed bilateral MTL onset by in‐patient video‐EEG monitoring with scalp (52%) or scalp and intracranial electrodes (48%). Thirteen percent had only unilateral electrographic seizures over a mean 42 months of RNS System chronic ambulatory ICEEG monitoring.5
Lateralization of MTL seizures by inpatient ICEEG was misleading in some of the patients in this study. Twelve of the 24 patients were monitored with inpatient ICEEG and the inpatient lateralization was misleading in 7 of 12 (58%, Table 2). Three patients had bilateral MTL seizures during short‐term inpatient ICEEG but only unilateral seizures by chronic ambulatory ICEEG, two patients had only unilateral MTL seizures with short‐term inpatient ICEEG but had bilateral seizures by chronic ambulatory ICEEG, and the preponderance of left compared to right MTL seizures based on short‐term inpatient ICEEG was incorrect in two patients. These data confirm the danger of relying on lateralizing information based on short‐term inpatient intracranial EEG.
There are several reasons for possible discrepancies between inpatient EEG monitoring results (scalp and intracranial) and chronic ambulatory ICEEG. These include undersampling due to the short time window for inpatient recordings, as already discussed, and likely the most important factor. Other reasons could be that atypical seizures are triggered by the stress of the inpatient environment (including sleep disruption, medication changes, and sometimes anesthesia, analgesics, and steroids), that atypical seizures arise because of inpatient medication withdrawal, or because either medications or brain‐responsive neurostimulation suppresses seizures in one MTL, but not the other.
There are other reports of patients treated with MTL surgeries based on information from chronic ambulatory ICEEG monitoring. In some cases, MTL resection was intended to result in seizure remission and in others, significant palliation.20, 21 DiLorenzo, et al described four patients who were not considered surgery candidates because seizures arose from more than one focus. Based on chronic ambulatory ICEEG monitoring data from the brain‐responsive neurostimulator, these patients underwent a resective procedure and were seizure‐free.20 Enatsu et al described a patient who was not considered a surgical candidate due to bilateral MTL onsets. Chronic ambulatory ICEEG recordings from bilateral hippocampal depth leads revealed a preponderance of right‐sided electrographic seizure onsets. The patient subsequently underwent a right MTL resection, continued to be treated with brain‐responsive neurostimulation, and became seizure free.21
The majority of patients with seizures of MTL onsets benefit substantially from brain‐responsive neurostimulation, whether treated unilaterally or bilaterally.22, 23 Patients in the RNS System clinical trials who had MTL onsets (N = 111) were selected for treatment with brain‐responsive neurostimulation because seizures were bilateral (72%) or unilateral (28%) and there was concern that a resection would cause memory or language deficits, because the patient had already had a MTL resection, or because the patient chose not to have a MTL resection. Over 9 years of prospective follow‐up, the clinical response for patients with unilateral or bilateral MTL onsets improved over time, from 50% median reduction in seizure frequency at 2 years to 73% at 9 years.22, 23
In some patients, combining a resective or ablative procedure with brain‐responsive neurostimulation can be effective. The majority of patients (71%) in this cohort became free of disabling clinical seizures after MTL resection; 23 of 24 (96%) continued to be monitored and treated with the brain‐responsive neurostimulator, either contralateral to the resection (5/24; 21%), or bilaterally (18/24; 75%). All seven patients who did not achieve seizure freedom after MTL resection had bilateral electrographic seizures recorded by chronic ambulatory ICEEG, and surgery was intended to provide significant palliation. These seven patients continued to be treated with brain‐responsive neurostimulation. As hoped, all of these patients had subjective and objective improvements in seizure burden, assessed by the CGIS and change in seizure frequency (all >50% seizure reduction). The infrequent adverse memory and mood events seen in this cohort are similar to those seen in MTL surgeries in general, reminding us that all patients should be carefully screened and counseled about these possibilities.
The efficacy of brain‐responsive neurostimulation increases over time. Based on the experience in the RNS System clinical trials and in this series, 24 months seems an appropriate period of time to assess the response to brain‐responsive neurostimulation and to draw a confident conclusion about lateralization of stimulation‐resistant and medication‐resistant seizures. At that point, a decision can be made regarding continued treatment with brain‐responsive neurostimulation alone or in combination with a resective or ablative procedure.
Data from the RNS System complement but cannot replace inpatient EEG monitoring. The RNS System chronic ambulatory ICEEG data cannot determine whether an electrographic seizure is also a clinically evident seizure. In addition, there are limitations for RNS System data sampling and storage. First, chronic ambulatory ICEEG sampling is limited to eight electrodes. Second, the chronic ambulatory ICEEG storage capacity of the RNS System is limited. Memory is freed up when patients upload daily neurostimulator data to their home‐use remote monitor. Although the RNS System is programmed to prioritize storage of electrographic seizures, it is possible that records of electrographic seizures are overwritten if there are more events than can be stored between patient uploads. Another potential data storage confounder is that the RNS System detection settings are modified throughout the years of treatment and this will influence the types of events that are detected and stored. However, in typical practice, detection settings are stable after the first year of treatment.
In conclusion, direct brain‐responsive neurostimulation is demonstrated to be safe and effective in patients with medically intractable epilepsy, including those with unilateral and bilateral MTL epilepsy.22, 23 For many patients, direct brain‐responsive neurostimulation will achieve the therapeutic goal. However, for some MTL patients, resective or ablative procedures can provide additional benefit. In patients with MTL epilepsy, if a confident lateralization cannot be achieved with standard localization techniques alone (as is often the case if there is any evidence of bitemporal epilepsy), chronic ambulatory ICEEG monitoring can establish whether a patient has unilateral or bilateral onsets, the preponderance of seizures in those with bilateral onsets, and who is likely, or unlikely, to benefit from a MTL resection. In carefully selected patients with MTL epilepsy, combining resective or ablative MTL procedures with direct brain‐responsive neurostimulation may achieve excellent seizure outcomes.
CONFLICT OF INTEREST
Author Lawrence J Hirsch has received: (a) research support to Yale University for investigator‐initiated studies from Eisai, Proximagen, Sunovion, and The Daniel Raymond Wong Neurology Research Fund at Yale; (b) consultation fees for advising from Adamas, Aquestive, Ceribell, Eisai, Marinus, Medtronic, Monteris, Neuropace, and UCB; (c) royalties for authoring chapters for UpToDate Neurology, and from Wiley for co‐authoring the book “Atlas of EEG in Critical Care,” by Hirsch and Brenner; and (d) honoraria for speaking from NeuroPace. Author Emily A Mirro has equity ownership/stock options with NeuroPace and is an employee of NeuroPace. Author Paul Rutecki has received support from the VA Research & Development unrelated to this study. Author Vikram R Rao has received support from and/or has served as a paid consultant for NeuroPace. Author Deepak S Madhavan has received support from and/or has served as a paid consultant for NeuroPace, LivaNova, and Greenwich Biosciences. Author Anli A Liu has received support from and/or has served as a paid consultant for NeuroPace (speaker fee). Author Christianne N Heck has received support from NeuroPace for research programs only. Author Janet E Greenwood has equity ownership/stock options with NeuroPace and is a former employee of NeuroPace. Author Dileep R Nair has received support from and/or has served as a paid consultant for NeuroPace. Author Ryder P Gwinn has received support from and/or has served as a paid consultant for NeuroPace. Author Eric B Geller has received support from and/or has served as a paid consultant for NeuroPace. Author Martha J Morrell has equity ownership/stock options with NeuroPace and is an employee of NeuroPace. The remaining authors have no conflict of interest. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGEMENTS
We would like to thank the patients and families for participating in this investigator‐initiated study.
Hirsch LJ, Mirro EA, Salanova V, et al. Mesial temporal resection following long‐term ambulatory intracranial EEG monitoring with a direct brain‐responsive neurostimulation system. Epilepsia. 2020;61:408–420. 10.1111/epi.16442
REFERENCES
- 1. Wiebe S, Blume WT, Girvin JP, Eliasziw M, Effectiveness and Efficiency of Surgery for Temporal Lobe Epilepsy Study Group . A randomized, controlled trial of surgery for temporal‐lobe epilepsy. N Engl J Med. 2001;345(5):311–8. [DOI] [PubMed] [Google Scholar]
- 2. Engel J Jr. Finally, a randomized, controlled trial of epilepsy surgery. N Engl J Med. 2001;345(5):365–7. [DOI] [PubMed] [Google Scholar]
- 3. Engel J Jr, McDermott MP, Wiebe S, Langfitt JT, Stern JM, Dewar S, et al. Early surgical therapy for drug‐resistant temporal lobe epilepsy: a randomized trial. JAMA. 2012;307(9):922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957;20(1):11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. King‐Stephens D, Mirro E, Weber PB, Laxer KD, Van Ness PC, Salanova V, et al. Lateralization of mesial temporal lobe epilepsy with chronic ambulatory electrocorticography. Epilepsia. 2015;56(6):959–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gazzola DM, Thawani S, Agbe‐Davies O, Carlson C. Epilepsy monitoring unit length of stay. Epilepsy Behav. 2016;58:102–5. [DOI] [PubMed] [Google Scholar]
- 7. Smart O, Rolston JD, Epstein CM, Gross RE. Hippocampal seizure‐onset laterality can change over long timescales: a same‐patient observation over 500 days. Epilepsy Behav Case Rep. 2013;13(1):56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobulashvili T, Höfler J, Dobesberger J, Ernst F, Ryvlin P, Cross JH, et al. Current practices in long‐term video‐EEG monitoring services: a survey among partners of the E‐PILEPSY pilot network of reference for refractory epilepsy and epilepsy surgery. Seizure. 2016;38:38–45. [DOI] [PubMed] [Google Scholar]
- 9. Rose AB, McCabe PH, Gilliam FG, Smith BJ, Boggs JG, Ficker DM, et al. Occurrence of seizure clusters and status epilepticus during inpatient video‐EEG monitoring. Neurology. 2003;60(6):975–8. [DOI] [PubMed] [Google Scholar]
- 10. Engel J Jr, Crandall PH. Falsely localizing ictal onsets with depth EEG telemetry during anticonvulsant withdrawal. Epilepsia. 1983;24(3):344–55. [DOI] [PubMed] [Google Scholar]
- 11. Spencer SS, Spencer DD, Williamson PD, Mattson RH. Ictal effects of anticonvulsant medication withdrawal in epileptic patients. Epilepsia. 1981;22(3):297–307. [DOI] [PubMed] [Google Scholar]
- 12. Morrell MJ, RNS System in Epilepsy Study Group . Responsible cortical stimulation for the treatment of medically intractable partial epilepsy. Neurology. 2011;77(13):1295–304. [DOI] [PubMed] [Google Scholar]
- 13. Bergey GK, Morrell MJ, Mizrahi EM, Goldman A, King‐Stephens D, Nair D, et al. Long‐term treatment with responsive brain stimulation in adults with refractory partial seizures. Neurology. 2015;84(8):810–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Durazzo TS, Spencer SS, Duckrow RB, Novotny EJ, Spencer DD, Zaveri HP. Temporal distribution of seizure occurrence from various epileptogenic regions. Neurology. 2008;70(15):1265–71. [DOI] [PubMed] [Google Scholar]
- 15. de Tisi J, Bell GS, Peacock JL, McEvoy AW, Harckness WF, Sander JW, et al. The long‐term outcome of adult epilepsy surgery, patterns of seizure remission, and relapse: a cohort study. Lancet. 2011;378(9800):1388–95. [DOI] [PubMed] [Google Scholar]
- 16. Hennessy MJ, Elwes RD, Binnie CD, Polkey CE. Failed surgery for epilepsy: a study of persistence and recurrence of seizures following temporal resection. Brain. 2000;123(12):2445–66. [DOI] [PubMed] [Google Scholar]
- 17. Abosch A, Bernasconi N, Boling W, Jones‐Gotman M, Pulin N, Dubeau F, et al. Factors predictive of suboptimal seizure control following selective amygdalohippocampectomy. J Neurosurg. 2002;97(5):1142–51. [DOI] [PubMed] [Google Scholar]
- 18. Spencer S, Huh L. Outcomes of epilepsy surgery in adults and children. Lancet Neurol. 2008;7(6):525–37. [DOI] [PubMed] [Google Scholar]
- 19. Jeha LE, Najm IM, Bingaman WE, Khandwala F, Widdess‐Walsh P, Morris HH, et al. Predictors of outcome after temporal lobectomy for the treatment of intractable epilepsy. Neurology. 2006;66(12):1938–40. [DOI] [PubMed] [Google Scholar]
- 20. DiLorenzo DJ, Mangubat EZ, Rossi MA, Bryne RW. Chronic unlimited recording electrocorticography‐guided respective surgery: technology‐enabled enhanced fidelity in seizure focus localization with improved surgical efficiency. J Neurosurg. 2014;120(6):1402–14. [DOI] [PubMed] [Google Scholar]
- 21. Enatsu R, Alexopoulos A, Bingaman W, Nair D. Complementary effect of surgical resection and responsive brain stimulation in the treatment of bitemporal lobe epilepsy: a case report. Epilepsy Behav. 2012;24(4):513–6. [DOI] [PubMed] [Google Scholar]
- 22. Geller EB, Skarpaas TL, Gross RE, Goodman RR, Barkley GL, Bazil CW, et al. Brain‐responsive neurostimulation in patients with medically intractable mesial temporal lobe epilepsy. Epilepsia. 2017;58(6):994–1004. [DOI] [PubMed] [Google Scholar]
- 23. Nair DR, Morrell M.Nine‐year prospective safety and effectiveness outcomes from the long‐term treatment trial of the RNS® System. Presented at the American Epilepsy Society 2018 Annual Meeting. 2018 Dec. Poster.


