Summary
R‐CVP (cyclophosphamide, vincristine, prednisone) and R‐CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone + rituximab) are immunochemotherapy regimens frequently used for remission induction of indolent non‐Hodgkin lymphomas (iNHLs). Rituximab maintenance (RM) significantly improves progression‐free survival (PFS) in patients with complete/partial remission (CR/PR). Here we report the final results of a randomized study comparing R‐CVP to R‐CHOP both followed by RM. Untreated patients in need of systemic therapy with symptomatic and progressive iNHLs including follicular (FL) and marginal zone lymphoma (MZL), mucosa‐associated lymphoid tissue (MALT), small lymphocytic (SLL), and lymphoplasmacytic (LPL) lymphoma were eligible. Patients were randomized to receive R‐CVP or R‐CHOP for eight cycles or until complete response (CR). All patients with CR/PR (partial response) received RM 375 mg/m2 q 2 months for 12 cycles. Primary endpoint was event‐free survival (EFS). Two‐hundred and fifty patients [FL 42%, MZL/MALT 38%, LPL/ Waldenström Macroglobulinaemia (WM) 11%, SLL 9%] were enrolled and randomized (R‐CHOP: 127, R‐CVP: 123). Median age was 56 years (21–85), 44% were male, 90% were in stage III–IV, 43% of FL patients had a Follicular Lymphoma International Prognostic Index (FLIPI) score ≥3, and 33·4% of all patients had an IPI score ≥3. At the end of induction treatment, the CR/PR rate was 43·6/50·9% and 36·3/60·8% in the R‐CHOP and R‐CVP groups (P = 0·218) respectively. After a median follow‐up of 67, 66, and 70 months, five‐year EFS was 61% vs. 56% (not significant), progression‐free survival (PFS) was 71% vs. 69% (not significant) and overall survival (OS) was 84% vs. 89% in the R‐CHOP vs. the R‐CVP arm respectively. Grade III/IV adverse events (65 vs. 22) occurred in 40 (33·1%) and 18 (15·3%) patients, P = 0·001; neutropenia in 16 (11·6%) and 4 (3·4%) patients, P = 0·017; infection in 14 (10·7%) and 3 (2·5%) patients,; P = 0·011; and a second neoplasm in three versus seven patients., in the R‐CHOP and the R‐CVP groups respectively. This multicentre randomized study with >five‐year follow‐up shows similar outcome in patients with indolent lymphoma in need of systemic therapy treated with R‐CVP or R‐CHOP immunochemotherapy and rituximab maintenance in both arms. The minor toxicity of the R‐CVP regimen makes it a reasonable choice for induction treatment, leaving other active agents like doxorubicin or bendamustin for second‐line therapy.
Keywords: indolent lymphoma, first‐line induction immunochemotherapy, rituximab maintenance
Rituximab (R)‐based immunochemotherapy is a standard initial treatment of patients with advanced indolent non‐Hodgkin lymphomas (iNHLs) (Casulo et al., 2017). Adding R to either CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) or CVP (cyclophosphamide, vincristine, prednisone) regimens in randomized controlled trials (RCTs) improved event‐free (EFS), progression‐free (PFS), and overall survival (OS) (Hiddemann et al., 2005; Marcus et al., 2005; Marcus et al., 2008; Bachy et al., 2013). The PRIMA study demonstrated a significant PFS benefit to two‐year rituximab maintenance (RM) versus no maintenance in patients with follicular lymphoma (FL) responding to R plus alkylator‐based chemotherapy (Salles et al., 2010), and RM is generally accepted as the standard of care (Nastoupil et al., 2014; Madsen et al., 2018). Several RCTs were designed to identify an optimum induction therapy for iNHLs or FL. In the StiL study, bendamustine plus R (BR) demonstrated improved PFS and less toxicity as compared to R‐CHOP (Rummel et al., 2013). In the BRIGHT study, BR was non‐inferior to R‐CHOP or R‐CVP in terms of complete remission rate (CRR) in first‐line therapy with a safety profile distinct from standard regimens; however, direct comparison of BR to R‐CVP or R‐CHOP was not possible (Flinn et al., 2019). The FOLL05 trial compared R‐CVP versus R‐CHOP versus R‐FM (rituximab, fludarabine, mitoxantrone) for the initial treatment of patients with advanced symptomatic FL and improved PFS and time to failure (TTF) with R‐CHOP or R‐FM over R‐CVP with no OS difference, and R‐CVP was shown to have the lowest toxicity (Federico et al., 2013; Luminari et al., 2018). None of the prior studies directly compared R‐CHOP with R‐CVP induction regimens followed by RM. Therefore, the choice of induction chemotherapy is still debatable (Casulo et al., 2017).
We report here on the results of a prospective randomized trial directly comparing two induction immunochemotherapy regimens: R‐CVP and R‐CHOP with RM in both arms in patients with iNHLs. The PLRG4 study is registered with ClinicalTrials.gov (identifier: NCT00801281).
Patients and methods
Patient selection
Eligible patients were ≥18 years old with histologically confirmed FL grade 1, 2, 3a, marginal zone lymphoma (MZL), including mucosa‐associated lymphoid tissue (MALT) type, small lymphocytic lymphoma (SLL) (with bone marrow involvement <30%), and lymphoplasmacytic lymphoma/Waldenström Macroglobulinaemia (LPL/WM). Other main inclusion criteria included Ann Arbor stage II–IV; at least one measurable lesion; previously untreated, with indications for therapy including presence of B symptoms, complications or symptoms related to lymphoma, cytopenia related to bone marrow or spleen involvement, progression of lymphoma within the last 2 months; Eastern Cooperative Oncology Group (ECOG)/World Health Organization (WHO) performance status (PS) ≤2. Patients with grade 3b FL, transformed lymphoma and CNS involvement were excluded from the study. The full list of inclusion/exclusion criteria is presented in Table SI in the Appendix. Written informed consent was obtained for each patient. The study was approved by the independent central Ethics Committee at the Maria Sklodowska‐Curie Institute–Oncology Centre and conducted according to the principles of the Declaration of Helsinki 1964 (with further amendments) and Good Clinical Practice guidelines.
Randomization
Eligible patients were centrally randomized (1:1) with the use of a web response system to standard arm R‐CVP or experimental R‐CHOP. Randomization was stratified by centre and lymphoma subtype. A minimization technique was used to balance treatment over strata.
Study design
PLRG4 was a phase III, open‐label, randomized study, recruiting patients in 14 sites in Poland. Patients in the R‐CVP arm received R 375 mg/m2 iv on day 1, cyclophosphamide (CP) 750 mg/m2 iv on day 1, vincristine (VCR) 1·4 mg/m2 (max. 2 mg) iv on day 1, prednisone 40 mg/m2 orally on days 1–5 every 21 days (Q21D); and patients in the R‐CHOP arm received the same drugs plus doxorubicin 50 mg/m2 iv on day 1, with prednisone 100 mg orally on days 1–5 Q21D, until complete remission plus two cycles for a maximum of eight cycles. Restaging was performed after cycle 4 and after completion of induction therapy. Two months after the last induction chemotherapy, RM was initiated in responding patients (CR/PR) with R 375 mg/m2 IV every 2 months (Q2M) for 12 cycles or 24 months. Follow‐up visits were scheduled Q3M until an event or up to three years after therapy.
The primary endpoint was event‐free survival (EFS), defined as time from randomization to disease progression (PD), relapse, change of lymphoma therapy, serious adverse event (SAEs) resulting in patient withdrawal, patient refusal, or death from any cause. Secondary endpoints included: response rate (RR), time to best response (TBR), PFS, duration of response (RD), overall survival (OS), rate of febrile neutropenia, rate of infection (Table SII). Response was evaluated according to revised International Workshop Criteria (Cheson et al., 1999; Cheson et al., 2007). Response to treatment was evaluated with CT scans after four cycles, after end of induction (after six or eight cycles), and within 4 weeks of completion of maintenance. PET/CT was not required and was performed occasionally at the discretion of investigators. Data on adverse events (AEs) and SAEs were obtained for up to 110 days after last dose of study treatment. AEs were graded using the National Cancer Institute Common Terminology Criteria for Adverse Events version 3.0 (https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf; accessed 13 June 2018).
Histological diagnosis was done locally including 40% of patients diagnosed at two sites hosting the national hematopathology reference centres. Primary diagnostic samples were centrally reviewed (20% of patients) at the discretion of the treating physician. Response to treatment was evaluated with CT scans after four cycles, after end of induction (after six or eight cycles), and within 4 weeks of completion of maintenance. PET/CT was not required and was performed occasionally at the discretion of investigators.
Statistical analysis
We assumed a median EFS difference of 18 months as clinically meaningful in favour of the experimental arm R‐CHOP with 147 events needed to provide 80% power for the two‐sided log rank test to detect an expected difference at a 0·05 level of significance. Assuming a 10% drop‐out rate, a total number of 250 patients had to be recruited over 3 years and 4 months (125 per arm) and followed for 3 years and 6 months. Interim analyses were planned after 37, 74 and 111 events according to O'Brian and Fleming with levels of significance of 0·00009, 0·0055 and 0·022 respectively. The final analysis was performed after a median of 67 months of follow‐up with a 0·04 level of significance. Safety analysis was performed yearly. The Kaplan–Meier method was used to calculate survival curves and the two‐sided log rank test was used for comparing time‐to‐event distributions. The Cox model was applied for estimating appropriate hazard ratios. The chi‐square test or exact Fisher test was used to compare percentages. To compare the distributions of continuous variables, a two‐sided t‐test or the Mann–Whitney nonparametric test was used. All statistical analyses were performed using IBM SPSS Statistics version 23.0 (IBM Corp., Armonk, NY).
Results
Baseline characteristics
Between February 2007 and June 2011, 250 eligible patients were randomized to the R‐CVP arm (n = 123) and the R‐CHOP arm (n = 127) (Fig 1). Eleven patients did not start study treatment or discontinued it early due to change of diagnosis after pathology review (n = 7), spontaneous disease regression (n = 1), absence of active disease documented on PET/CT examination (n = 1), and consent withdrawal before study treatment was initiated (n = 2). Consequently, 239 patients were included in the final analysis (R‐CVP, n = 118; R‐CHOP, n = 121).
Figure 1.
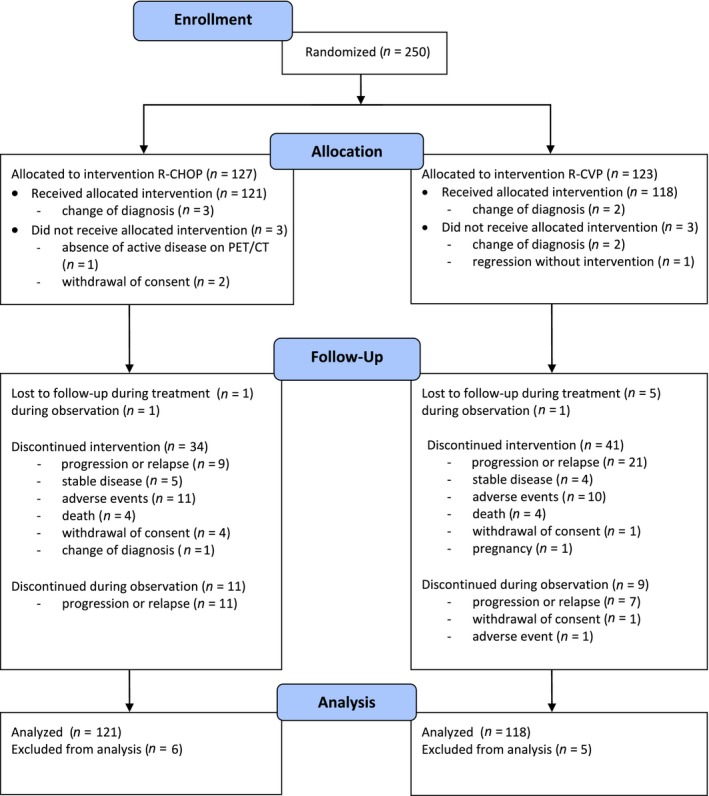
Treatment allocation and patients included in the analysis. [Colour figure can be viewed at wileyonlinelibrary.com].
Baseline characteristics of patients were well balanced in the two study arms except for Ann Arbor clinical stage 3–4, which was slightly more prevalent in R‐CHOP arm than in the R‐CVP arm (94·2% vs. 84·7%, P = 0·02) Selected variables are presented in Table 1, and a full list in Table SIII.
Table 1.
Selected demographic and clinical features of the patients (N = 250).
| R‐CVP | P‐CHOP | P‐value | |
|---|---|---|---|
| Patients randomized | 123 | 127 | |
| Patients evaluable | 118 | 121 | |
| Male/female | 55/63 | 52/69 | |
| Age, mean (range) | 55.9 (25–89) | 55.8 (21–80) | |
| ≥60 | 44 (37.3%) | 47 (38.8%) | |
| Histology | |||
| FL | 48 (40.6%) | 51 (42.2%) | >0.998* |
| MZL/MALT | 46 (39%) | 46 (38%) | |
| SLL | 11 (9%) | 11 (9%) | |
| LPL/WM | 13 (11%) | 13 (11%) | |
| Ann Arbor stage | |||
| 1 | 1 (0.8%) | 2 (1.7%) | 0.042 * |
| 2 | 17 (14.4%) | 5 (4.1%) | |
| 3 | 24 (20.3%) | 23 (19.0%) | |
| 4 | 76 (64.4%) | 91 (75.2%) | |
FL, follicular lymphoma; MZL, marginal zone lymphoma; MALT, mucosa associated lymphoid tissue lymphoma; SLL, small lymphocytic lymphoma; LPL, lymphoplasmacytic lymphoma; WM, Waldenström macroglobulinemia.
t‐student test.
Efficacy
The EFS was better than expected in both arms and a median was not reached. Consequently, estimation of the difference in median EFS was not possible. After medians at follow‐up (95% CI) of 67 (66, 68), 66 (65, 67) and 70 (68, 71) months respectively, no statistically significant difference was found between study arms regarding EFS, PFS and OS (P = 0·386, 0·849 and 0·434 respectively). The corresponding hazard ratios [HR (R‐CHOP)/(R‐CVP)] with 95% CIs were 0·840 (0·566, 1·247), 0·955 (0·594, 1·536) and 1·328 (0·651, 2·710) respectively.
The five‐year EFS rates for the R‐CHOP and R‐CVP groups were 0·61 (95% CI; 0·52, 0·70) and 0·56 (95% CI; 0·47, 0·65), five‐year PFS rate was 0·71 (95% CI; 0·62, 0·79) and 0·69 (95% CI; 0·60, 0·78), and five‐year OS was 0·84 (95% CI; 0·78, 0·92) and 0·89 (95% CI; 0·83, 0·95) respectively. EFS curves are presented in Fig 2A, and three‐, four‐, five‐year PFS and OS are presented in Fig 3 and Figure S1.
Figure 2.

Primary endpoint Event Free Survival in total study population (P = 0.386) (A) and by histology subtype (P < 0.001) (B). [Colour figure can be viewed at wileyonlinelibrary.com].
Figure 3.
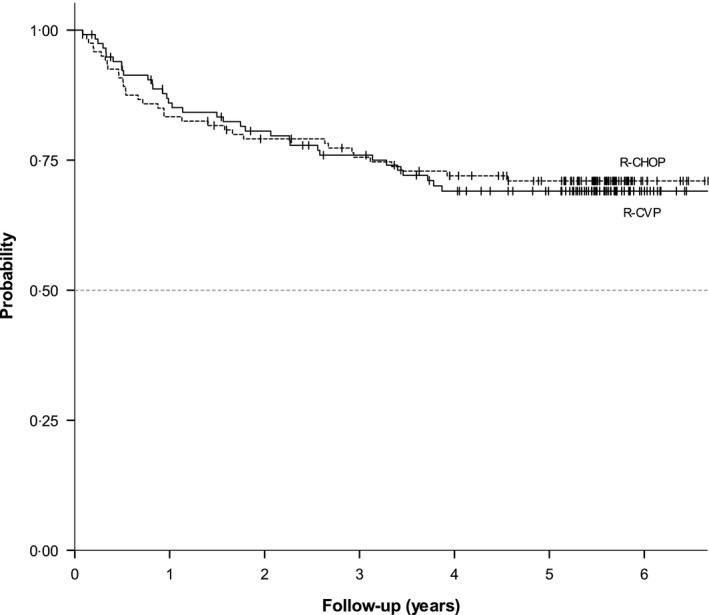
Progression Free Survival (P = 0.849).
Among 99 FL patients, early progression of disease (POD), i.e. progression within 2 years of the start of treatment, occurred in 8/48 (16·7%) patients in the R‐CVP arm and in 7/51 (13·7%) patients in the R‐CHOP arm; the difference in frequency of early POD between arms was not significant (P = 0·68). Early POD was highly significantly associated with reduced OS (HR 23·8, P < 0·001) (Figure S2A–C).
In post hoc evaluation of EFS by histology subtype (Fig 2B), the outcome in SLL patients was significantly (P < 0·001) worse than in patients with other subtypes (FL, MZL, LPL). The same was observed for PFS and OS, with corresponding P‐values of <0·001 and 0·039 (Figures S3 and S4). The forest plot of hazard ratios for EFS of patients with different histological subtypes by treatment arm is shown in Figure S5.
The FLIPI high‐risk group had a significant adverse effect on EFS (P = 0·015) and OS (0·001) and PFS (P = 0·039) of FL patients and IPI risk level had a significant influence on EFS (P < 0·001), PFS (P = 0·011), and OS (P < 0·001) of all patients. Corresponding Kaplan–Meier curves for EFS, PFS and OS according to FLIPI and IPI risk are shown in Figures [Link], [Link], [Link], [Link], [Link], [Link].
Overall response rate (CR + PR) in the R‐CVP and R‐CHOP arms was 97·1% and 94·5% respectively (CR = 36·3% and 43·6% respectively) in 212 evaluable patients (Table SIV). There was no difference in response rate (P = 0·218) including best response rate (P = 0·786) between treatment arms (Table SV). The Kaplan–Meier curves of the best response duration (BRD) in treatment arms are shown in Figure S11.
Safety
The number of AEs was higher in the R‐CHOP than in the R‐CVP arm. In total, there were 474 grade 1–4 AEs reported throughout the induction and maintenance period plus 110 days (296 in R‐CHOP and 178 in R‐CVP). The most frequent AE of any grade in the R‐CVP arm was neutropenia (n = 18, 10·3%), sensory neuropathy (n = 13, 7·4%), and elevated transaminase levels (n = 12, 6·9%), while in the R‐CHOP arm most frequent AEs were neutropenia (n = 43, 17·0%), leukopenia (n = 27, 10·0%) and elevated transaminase levels (n = 20, 7·4%). Grade 3–4 AEs were recorded in 65 vs. 22 patients in the R‐CHOP and R‐CVP arm respectively, including neutropenia (n = 16 [11·6%] vs. n = 4 3·4%]) and infections (n = 14 [10·7%] vs. n = 3 [2·5%]). Table SVI in presents asummary of all AEs.
There were four deaths attributed to cardiac arrest (n = 2) or heart failure (n = 2), one in each arm, and four cardiac events, all in the R‐CHOP arm including grade 1 arrhythmia, grade 1 atrial fibrillation, grade 2 cardiomyopathy and grade 3 circulatory insufficiency.
In total, 10 cases (3 vs. 7, in the R‐CHOP and R‐CVP group respectively) of second malignancies were recorded (lung cancer two, colorectal cancer two, and thyroid, urinary bladder, tongue, nose, uterine and prostate cancer). The Cumulative Incidence Function (CIF) with 95% CI for a second cancer was 1·69% (0·04%, 3·34%) after 1 year, and 4·52% (1·77%, 7·28%) after 6 years. Median time to onset of the second tumour was 12·33 months. Detailed information is presented in Figure S12.
There were four cases of uncomplicated pregnancy reported: one in the R‐CHOP and three cases in the R‐CVP arm, all in the follow‐up period.
Discussion
At the time when this study was designed in 2006, we hypothesized a better outcome of patients treated with R‐CHOP than R‐CVP based on the results of the German Low‐Grade Lymphoma Study Group (GLSG) published soon after the international study of R‐CVP versus CVP (Hiddemann et al., 2005; Marcus et al., 2005). However, with a median follow‐up of more than 5 years, medians of EFS, PFS, or OS in either arm were not reached, and the rates of survival including EFS, PFS, and OS at 5 years were comparable in the R‐CVP and R‐CHOP arms.
Event‐free survival was selected as a primary endpoint in our study based on the consideration of events dependent on therapy tolerance in addition to disease progression or death. EFS and similar endpoints like TTF (time to failure), FFS (failure‐free survival), and recently mPFS (modified PFS) are frequently used in studies where OS is unpractical due to the long natural course of disease or existence of effective salvage treatments (Marcus et al., 2005; Marcus et al., 2008; Bachy et al., 2013). In the pivotal M39021 study comparing R‐CVP with CVP, the median EFS (TTF) for the R‐CVP arm was 27 month at 30 month median follow‐up (Marcus et al., 2005; Marcus et al., 2008). Based on this outcome, we assumed a reference median EFS of 30 months in the standard arm R‐CVP and expected an improvement by 18 months in the experimental arm R‐CHOP.
However, the patient outcome in our study was better than in the Marcus study (Marcus et al., 2005; Marcus et al., 2008); median EFS was not reached in either arm, and 48‐month OS for R‐CHOP versus R‐CVP was 87% vs. 89% compared to 83% in the R‐CVP arm of the Marcus study. The difference between EFS/TTF in the Marcus and our study as well as the less than expected number of events were most likely due to the use of RM as the current standard of care. Indeed, in the PRIMA study (Salles et al., 2010) of FL patients with high tumour burden, two‐year RM reduced the risk of progression and improved EFS compared to no RM with HR 0·55 (95% CI 0·44, 0·68) and 0·59 (95% CI 0·48, 0·72) respectively, and the PFS difference reached 17·3% at 36 months with no difference in OS.
Early POD, a recently discovered powerful risk factor for OS in FL, was associated with markedly reduced OS of FL patients in both arms of our study but there was no statistically significant difference in the frequency of early POD between the treatment arms (Figure S2A–C).
In contrast to our study, the FOLL05 study (Federico et al., 2013) in patients with advanced‐stage FL comparing R‐CVP with R‐CHOP and R‐FM induction regimens, notably without RM, showed a statistically significant difference in three‐year TTF in favour of R‐CHOP (62%) compared to R‐CVP (46%) (P = 0·003) as well as a significant three‐year PFS difference of 68% vs. 52% (P = 0·011) respectively. However, the FOLL05 study expanded prespecified accrual from 252 to 531 patients due to faster than expected patient enrolment. A subsequent report of this study (Luminari et al., 2018) with the follow‐up extended to 7 years confirmed the superiority of R‐CHOP and R‐FM over R‐CVP with an eight‐year TTF of 45% and 49% vs. 38% respectively. Again, corresponding figures were higher for our study with five‐year EFS and PFS for R‐CHOP versus R‐CVP of 61% vs. 56% and 71% vs. 69% respectively.
An international phase III superiority trial (RELEVANCE) in patients with advanced untreated FL compared a chemo‐free combination of lenalidomide and rituximab with a standard rituximab plus chemotherapy followed by RM in both arms (Morschhauser et al., 2018). Chemotherapy included R‐CHOP (72%), R‐B (23%), and R‐CVP (5% of patients). Three‐year PFS in the lenalidomide and chemotherapy arm was 77% and 78% respectively, with a substantial rate of grade 3–4 neutropenia of 32% vs. 50% respectively. The expected 23% superiority in PFS of lenalidomide over the chemotherapy‐containing regimen was not observed. The three‐year PFS of 76% in both arms of our study is almost identical to PFS in the RELEVANCE trial, reflecting the current efficacy of induction immunochemotherapy regimens with the subsequent RM (Madsen et al., 2018). Interestingly, in the GALLIUM study (Hiddemann et al., 2018), where patient allocation to chemotherapy was not randomized but was stratified, three‐year PFS of patients who received R‐CHOP or R‐CVP assessed by the independent review committee was comparable and similar to the corresponding value in our study: 77% in both groups, and 76% in our study.
In the BRIGHT study, which was recently updated with around five‐year follow‐up, similarly to our study, the medians were not reached for any of the time‐to‐event endpoints for either treatment arm, and five‐year PFS for patients treated with BR and R‐CHOP or R‐CVP was 65·5% and 55·8% respectively. These figures were apparently lower than the corresponding five‐year PFS values in our study (71% and 69% respectively), which possibly resulted from less than half of the patients receiving rituximab maintenance (BR, 43%; R‐CHOP/R‐CVP, 45%) and a component of the mantle‐cell lymphoma (MCL) patients in the BRIGHT study. Even the five‐year PFS in the BR arm (65·5%), which was declared by the authors as a preferred first‐line treatment option for patients with indolent and MCL, was lower than the five‐year PFS of patients in our study treated with R‐CVP (69%), which makes the recommendation debatable.
In addition to FL patients (42%), our study included patients with other iNHL subtypes: MZL/MALT (38%), LPL/WM (11%), and SLL (9%), and randomization was stratified by histology given that principles of therapy were similar for FL and iNHL in 2006, and other studies like StiL (Rummel et al., 2013) or BRIGHT (Flinn et al., 2019) were addressing this patient population as well. Of note, even though the study was not powered to analyse outcome in histological subsets, it was significantly worse in the SLL subset, which strongly suggests that neither of the treatment arms is appropriate for this group of patients (Fig 2B, P < 0·001; Figures [Link], [Link], [Link]).
Response rates to induction immunochemotherapy were comparable between treatment arms and the median time to best response was 4 months in both arms. However, the magnitude of complete response rates, i.e. ORR/CR 97·1/36·3% and 94·5/43·6% in the R‐CVP and R‐CHOP arm respectively, was lower than in similar studies. In the FOLL05 study, ORR/CR rates in the R‐CVP and R‐CHOP arms were 88%/67% and 93%/73% respectively (Federico et al., 2013) and in the RELEVANCE study, ORR/CR (confirmed/ unconfirmed) rates for the R‐lenalidomide and R‐chemotherapy arms were 61/48% and 65/53% respectively (Morschhauser et al., 2018). We used revised response criteria as per Cheson et al. (2007) where the CRu category was abandoned and counted as PR unless the FDG‐PET scan was negative. However, a PET scan was not obligatory in our study as the primary endpoint was EFS, not response rate, and in fact PET was performed in only seven patients. This may explain why CR rates were lower in our study compared to FOLL05 and RELEVANCE where Cheson et al.'s (1999) criteria were used.
The most frequent AE of any grade in both arms was neutropenia. Other frequent AEs in R‐CVP and R‐CHOP were sensory neuropathy, elevated transaminase levels and leukopenia. Grade 3–4 AEs were recorded in 65 vs. 22 patients in the R‐CHOP and R‐CVP arm respectively, with neutropenia and infections being the most common. We have no data on the use of granulocyte‐colony stimulating factor. Summarizing, AEs were more prevalent with R‐CHOP therapy, which in general is in line with other trials; however, the data comparing toxicity profile of different treatment schemes with rituximab are variable (Hiddemann et al., 2005; Federico et al., 2013; Rummel et al., 2013; Flinn et al., 2019). All four cases of pregnancies were reported in the follow‐up period, and pregnancies and deliveries were uncomplicated with healthy newborns.
During the study, we noted 10 cases of second malignancies, with a CIF for second primary malignancy (SM) of 4·52% with 95% CIs of 1·77% and 7·28% after six years and median time to onset of the second malignancy of 12·33 months. This is about half the rate of SM observed in the FOLL05 study of 9·4% at eight years (Luminari et al., 2018).
Overall, this multicentre randomized study with >5 year follow‐up shows a similar outcome for patients with indolent lymphoma in need of systemic therapy treated with R‐CVP or R‐CHOP immunochemotherapy and RM in both arms. The minor toxicity of the R‐CVP regimen makes it a reasonable choice for induction treatment, keeping other active agents like doxorubicin or bendamustin for second‐line therapy.
Funding
The study was supported by research grant ML19931 to the Polish Lymphoma Research Group (PLRG) from Roche Polska Sp. z o.o. Rituximab was supplied free of charge by Roche Polska Sp. z o.o. PLRG is the sponsor of the study.
Conflict of interest
JW — Advisory role: Roche, Celgene, Takeda, Janssen‐Cilag, Servier, Amgen, BMS, Abbvie, Novartis, Gilead; research funding: Roche, GSK/Novartis, Takeda, Janssen‐Cilag; lecture honoraria: Roche, Celgene, Takeda, Janssen‐Cilag, Servier; Amgen; conference travel support: Roche. EPK — lecture honoraria: Roche, Takeda, Servier; conference travel support: Roche, Takeda, Celgene. GR — conference travel support: Roche, Takeda, Servier. TS — conference travel support: Roche. JMZ — lecture honoraria, conference travel support, research funding: Roche; advisory role: Takeda, Abbvie; lecture honoraria: Janssen‐Cilag. EKW — lecture honoraria: Roche; conference travel support: Roche. JRJ — lecture honoraria: Roche; conference travel support: Roche. ADI — conference travel support: Roche, Sanofi, Amgen. AD — advisory role: Roche, Celgene, Amgen; lecture honoraria: Roche, Celgene, Amgen, GSK/Novartis, Servier. WJ — research funding: Roche, Celtrion, Sandoz Novartis, Janssen, Acerta, Merck, Beigene, TG therapeutics, Gilead, Celgene; advisory role: Sandoz Novartis, Janssen, Acerta, Abbvie, Servier. WM, AB, AG, AW, DZC, WKP, AT, BG, MJJ, AB, WH, AP, AR, AH, BSH — none declared.
Author contributions
JW, EPK, WJ conceived the study; JW, WJ, EPK, GR, WM, AD, BSH designed the study; EPK, TS, ABu, AG, JMZ, EKW, AW, DZC, WKP, AT, JRJ, ADI, BG, MJJ, ABo, WH, AP, AR, WJ treated patients, acquired and analysed data; JW, WJ, EPK, GR, WM, JMZ, AD, BSH, EKW, JRJ, ADI, AH analysed and interpreted data; WM performed the statistical analysis; GR centrally reviewed primary biopsy material; JW wrote the manuscript; JW, EPK, ADI, WJ, JMZ edited the manuscript; all authors reviewed and approved the manuscript.
Supporting information
Figure S1. Overall survival by treatment arm (P = 0.434).
Figure S2. (A) Overall survival of 99 FL patients who had (n = 15) or had not (n = 84) early progression of disease (P < 0.001). (B) Overall survival of 48 FL patients in arm R‐CVP who had (n = 8) or had not (n = 40) early progression of disease (P < 0.001). (C) Overall survival of 51 FL patients in arm R‐CHOP who had (n = 7) or had not (n = 44) early progression of disease (P < 0.001).
Figure S3. Progression‐free survival by histology subtype (P < 0.001).
Figure S4. Overall survival by histology subtype (P = 0.039).
Figure S5. Forest plot of hazard ratios for event‐free survival of patients with different histological subtypes by treatment arm.
Figure S6. Event‐free survival by FLIPI index, FL group (P = 0.015).
Figure S8. Overall survival by FLIPI index, FL group, (P = 0.001).
Figure S9. EFS by IPI, whole group, P < 0.001.
Figure S10. PFS by IPI, whole group, P = 0.011.
Figure S11. OS by IPI, whole group, P < 0.001.
Figure S13. Median time to onset of the second malignancy.
Table SI . PLRG‐4 study inclusion and exclusion criteria.
Table SII . Definition of secondary endpoints.
Table SIII . Baseline demographic and clinical characteristics of the patients (N = 250).
Table SIV . Response to treatment after induction before maintenance (P = 0.218).
Table SV . Best response distribution (P = 0.786).
Table SVI . Adverse events reported during the study related to treatment group.
Acknowledgements
The authors acknowledge the other investigators participating in this study: K. Zawilska (Municipal Hospital, Poznan), L. Popławska, M. Świerkowska‐Czeneszew, M. Szymczyk, (all Maria Sklodowska‐Curie Institute–Oncology Center in Warsaw), M. Ciechańska (Regional Oncology Center Lublin), K. Domańska‐Czyż (Maria Sklodowska‐Curie Institute–Oncology Center in Warsaw), I. Ryniewicz‐Zander, S. Kwant (both Voivodeship Hospital, Elblag), R. Konecki, M. Osowiecki, A. Druzd‐Sitek, Ł. Targoński (all Maria Sklodowska‐Curie Institute–Oncology Center in Warsaw), A. Lange (Lower Silesia Cell Transplantation Center, Wroclaw), K. Kuliczkowski, M. Podolak‐Dawidziak (both Medical University Wroclaw), A.B. Skotnicki (Jagiellonian University Collegium Medicum, Krakow), K. Warzocha (Institute of Hematology and Transfusiology, Warsaw). The authors acknowledge Dr. Janusz Szczepanik et al., CRO Quantum Satis Sp. z o.o., Warsaw, for assistance with the regulatory procedures, study oversight and monitoring in accordance with the study protocol, GCP guidelines, and institutional guidelines. The authors acknowledge the writing assistance of Dariusz Stencel, MD, DaFonte, Poznan, during the development of this manuscript, which was funded by the Polish Lymphoma Research Group. We thank the data and safety monitoring committee including Prof. Dr. Anna Dmoszynska, Prof. Dr. Jadwiga Dwilewicz‐Trojaczek, Prof. Dr. Tadeusz Robak. We acknowledge Dr. Jarosław Stobiecki and Dr. Iwona Wyleżoł for recruiting and treating patients within the study.
Preliminary results of this study, based on the planned interim analysis after 74 events, were presented as oral presentation during 12 ICML in Lugano on 20th June 2013 and during the Polish Lymphoma Research Group meeting on 7th November 2017 in Warsaw.
Key description: In this prospective, multicentre, phase III randomized study by the Polish Lymphoma Research Group, the efficacy and toxicity of R‐CHOP regimen with rituximab maintenance (RM) were compared with standard R‐CVP regimen with RM in untreated patients with indolent lymphoma. After the median follow‐up of 70 months, no significant differences in EFS, PFS and OS were observed with higher toxicity of R‐CHOP.
References
- Bachy, E. , Houot, R. , Morschhauser, F. , Sonet, A. , Brice, P. , Belhadj, K. , Cartron, G. , Audhuy, B. , Ferme, C. , Feugier, P. , Sebban, C. , Delwail, V. , Maisonneuve, H. , Le Gouill, S. , Lefort, S. , Brousse, N. , Foussard, C. & Salles, G. (2013) Long‐term follow up of the FL2000 study comparing CHVP‐interferon to CHVP‐interferon plus rituximab in follicular lymphoma. Haematologica, 98, 1107–1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casulo, C. , Nastoupil, L. , Fowler, N.H , Friedberg, J.W & Flowers, C.R (2017) Unmet needs in the first‐line treatment of follicular lymphoma. Annals of Oncology, 28, 2094–2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheson, B.D. , Horning, S.J. , Coiffier, B. , Shipp, M.A. , Fisher, R.I. , Connors, J.M. , Lister, T.A. , Vose, J. , Grillo‐López, A. , Hagenbeek, A. & Cabanillas, F. (1999) Report of an international workshop to standardize response criteria for non‐Hodgkin's lymphomas. NCI Sponsored International Working Group. Journal of Clinical Oncology, 17, 1244. [DOI] [PubMed] [Google Scholar]
- Cheson, B.D. , Pfistner, B. , Juweid, M.E. , Gascoyne, R.D. , Specht, L. , Horning, S.J. , Coiffier, B. , Fisher, R.I. , Hagenbeek, A. , Zucca, E. & Rosen, S.T. (2007) Revised response criteria for malignant lymphoma. Journal of Clinical Oncology, 25, 579–586. [DOI] [PubMed] [Google Scholar]
- Federico, M. , Luminari, S. , Dondi, A. , Tucci, A. , Vitolo, U. , Rigacci, L. , Di Raimondo, F. , Carella, A.M. , Pulsoni, A. , Merli, F. & Arcaini, L. (2013) R‐CVP versus R‐CHOP versus R‐FM for the initial treatment of patients with advanced‐stage follicular lymphoma: results of the FOLL05 trial conducted by the Fondazione Italiana Linfomi. Journal of Clinical Oncology, 31, 1506–13. [DOI] [PubMed] [Google Scholar]
- Flinn, I.W. , van der Jagt, R. , Kahl, B. , Wood, P. , Hawkins, T. , MacDonald, D. , Simpson, D. , Kolibaba, K. , Issa, S. , Chang, J. & Trotman, J. (2019) First‐line treatment of patients with indolent non‐Hodgkin lymphoma or mantle‐cell lymphoma with bendamustine plus rituximab versus R‐CHOP or R‐CVP: results of the BRIGHT 5‐year follow‐up study. Journal of Clinical Oncology, 37, 984–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiddemann, W. , Kneba, M. , Dreyling, M. , Schmitz, N. , Lengfelder, E. , Schmits, R. , Reiser, M. , Metzner, B. , Harder, H. , Hegewisch‐Becker, S. & Fischer, T. (2005) Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) significantly improves the outcome for patients with advanced‐stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low‐Grade Lymphoma Study Group. Blood, 106, 3725–3732. [DOI] [PubMed] [Google Scholar]
- Hiddemann, W. , Barbui, A.M. , Canales, M.A. , Cannell, P.K. , Collins, G.P. , Dürig, J. , Forstpointner, R. , Herold, M. , Hertzberg, M. , Klanova, M. & Radford, J. (2018) Immunochemotherapy with obinutuzumab ot rituximab for previously untreated follicular lymphoma in the GALLIUM study: influence of chemotherapy on efficacy and safety. Journal of Clinical Oncology, 36, 2395–2404. [DOI] [PubMed] [Google Scholar]
- Luminari, S. , Ferrari, A. , Manni, M. , Dondi, A. , Chiarenza, A. , Merli, F. , Rusconi, C. , Tarantino, V. , Tucci, A. , Vitolo, U. & Kovalchuk, S. (2018) Long‐term of the FOLL05 trial comparing R‐CVP versus R‐CHOP versus R‐FM for the initial treatment of patients with advanced‐stage symptomatic follicular lymphoma. Journal of Clinical Oncology, 36, 689–696. [DOI] [PubMed] [Google Scholar]
- Madsen, C. , Clausen, M.R. , Plesner, T.L. , Pasanen, A. , Kuismanen, T. , Bentzen, H.H. , Jørgensen, J.M. , Sillesen, I.B. , Himmelstrup, B.M. , Rønnov‐Jessen, D. & Jensen, K.R. (2018) Up‐front rituximab maintenance improves outcome in patients with follicular lymphoma: a collaborative Nordic study. Blood Advance, 2, 1562–1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus, R. , Imrie, K. , Belch, A. , Cunningham, D. , Flores, E. , Catalano, J. , Solal‐Celigny, P. , Offner, F. , Walewski, J. , Raposo, J. & Jack, A. (2005) CVP chemotherapy plus rituximab compared with CVP as first‐line treatment for advanced follicular lymphoma. Blood, 105, 1417–1423. [DOI] [PubMed] [Google Scholar]
- Marcus, R. , Imrie, K. , Marcus, R. , Imrie, K. , Solal‐Celigny, P. , Catalano, J.V. , Dmoszynska, A. , Raposo, J.C. , Offner, F.C. , Gomez‐Codina, J. , Belch, A. , Cunningham, D. & Wassner‐Fritsch, E. (2008) Phase III Study of R‐CVP compared with cyclophosphamide, vincristine, and prednisone alone in patients with previously untreated advanced follicular lymphoma. Journal of Clinical Oncology, 26, 4579–4586. [DOI] [PubMed] [Google Scholar]
- Morschhauser, F. , Fowler, N.H. , Fowler, N.H. , Feugier, P. , Bouabdallah, R. , Tilly, H. , Palomba, M.L. , Fruchart, C. , Libby, E.N. , Casasnovas, R.O. , Flinn, I.W. & Haioun, C. (2018) Rituximab plus lenalidomide in advanced untreated follicular lymphoma. The New England Journal of Medicine, 379, 934–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nastoupil, L.J. , Sinha, R. , Byrtek, M. , Zhou, X. , Taylor, M.D. , Friedberg, J.W. , Link, B.K. , Cerhan, J.R. , Dawson, K. & Flowers, C.R. (2014) The use and effectiveness of rituximab maintenance in patients with follicular lymphoma diagnosed between 2004 and 2007 in the United States. Cancer, 120, 1830–1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rummel, M.J. , Niederle, N. , Maschmeyer, G. , Banat, G.A. , von Grünhagen, U. , Losem, C. , Kofahl‐Krause, D. , Heil, G. , Welslau, M. , Balser, C. & Kaiser, U. (2013) Bendamustine plus rituximab versus CHOP plus rituximab as first‐line treatment for patients with indolent and mantle‐cell lymphomas: an open‐label, multicentre, randomised, phase 3 non‐inferiority trial. Lancet, 381, 1203–10. [DOI] [PubMed] [Google Scholar]
- Salles, G. , Seymour, J.F. , Offner, F. , López‐Guillermo, A. , Belada, D. , Xerri, L. , Feugier, P. , Bouabdallah, R. , Catalano, J.V. , Brice, P. & Caballero, D. (2010) Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet, 377, 42–51. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Overall survival by treatment arm (P = 0.434).
Figure S2. (A) Overall survival of 99 FL patients who had (n = 15) or had not (n = 84) early progression of disease (P < 0.001). (B) Overall survival of 48 FL patients in arm R‐CVP who had (n = 8) or had not (n = 40) early progression of disease (P < 0.001). (C) Overall survival of 51 FL patients in arm R‐CHOP who had (n = 7) or had not (n = 44) early progression of disease (P < 0.001).
Figure S3. Progression‐free survival by histology subtype (P < 0.001).
Figure S4. Overall survival by histology subtype (P = 0.039).
Figure S5. Forest plot of hazard ratios for event‐free survival of patients with different histological subtypes by treatment arm.
Figure S6. Event‐free survival by FLIPI index, FL group (P = 0.015).
Figure S8. Overall survival by FLIPI index, FL group, (P = 0.001).
Figure S9. EFS by IPI, whole group, P < 0.001.
Figure S10. PFS by IPI, whole group, P = 0.011.
Figure S11. OS by IPI, whole group, P < 0.001.
Figure S13. Median time to onset of the second malignancy.
Table SI . PLRG‐4 study inclusion and exclusion criteria.
Table SII . Definition of secondary endpoints.
Table SIII . Baseline demographic and clinical characteristics of the patients (N = 250).
Table SIV . Response to treatment after induction before maintenance (P = 0.218).
Table SV . Best response distribution (P = 0.786).
Table SVI . Adverse events reported during the study related to treatment group.


