Abstract
Background
Visuo‐spatial neglect (VSN) is generally assessed with neuropsychological paper‐and‐pencil tasks, which are often not sensitive enough to detect mild and/or well‐compensated VSN. It is of utmost importance to develop dynamic tasks, resembling the dynamics of daily living.
Objective
A simulated driving task was used to assess (1) differences in performance (i.e., position on the road and magnitude of sway) between patients with left‐ and right‐sided VSN, recovered VSN, without VSN, and healthy participants; (2) the relation between average position and VSN severity; and (3) its diagnostic accuracy in relation to traditional tasks.
Methods
Stroke inpatients were tested with a cancellation task, the Catherine Bergego Scale and the simulated driving task.
Results
Patients with left‐sided VSN and recovered VSN deviated more regarding position on the road compared to patients without VSN. The deviation was larger in patients with more severe VSN. Regarding diagnostic accuracy, 29% of recovered VSN patients and 6% of patients without VSN did show abnormal performance on the simulated driving task. The sensitivity was 52% for left‐sided VSN. Right‐sided VSN was not well detected, probably due to the asymmetric layout.
Conclusions
Based on these results, the simulated driving task should not be the only task to assess VSN, especially in its current form. Given the heterogenic nature of VSN, the assessment should always consist of several tasks varying in nature and complexity and include a dynamic task to detect mild and/or recovered VSN. A symmetric design should be used when designing novel tasks to assess right‐sided VSN.
Keywords: computerized simulations, dynamic testing, neuropsychological tests, rehabilitation, stroke, virtual reality, visuo‐spatial neglect
Background
Visuo‐spatial neglect (VSN) is a common cognitive disorder after stroke. VSN is defined as the inability to attend to, respond to, or orient towards novel stimuli presented in the contralesional space (Heilman, Valenstein, & Watson, 2000). This deficiency in lateralized attention is the core deficit of VSN (Buxbaum et al., 2004) and is usually measured with neuropsychological paper‐and‐pencil tasks. Left‐sided VSN is more common (16–50%) than right‐sided VSN (9–30%) and is more severe when measured with neuropsychological tasks (Chen, Hreha, Kong, & Barrett, 2015; Ten Brink, Verwer, Biesbroek, Visser‐Meily, & Nijboer, 2016). The consequences in daily life activities are, however, largely comparable between left and right‐sided VSN (Ten Brink et al., 2016). VSN is associated with a slower and decreased functional and motor recovery (Chen et al., 2015; Nijboer, Kollen, & Kwakkel, 2014; Nijboer, van de Port, Schepers, Post, & Visser‐Meily, 2013), resulting in prolonged hospitalization, safety risks, and a decreased chance of successful reintegration. For this reason, adequate assessment of VSN is important.
As mentioned above, VSN is generally assessed with neuropsychological paper‐and‐pencil tasks, such as cancellation, line bisection, and copying tasks. Although these tasks are convenient and easy to administer, research has often reported a lack of ecological validity and limitations in sensitivity (Pedroli, Serino, Cipresso, Pallavicini, & Riva, 2015; Ten Brink, Visser‐Meily, & Nijboer, 2017; Tsirlin, Dupierrix, Chokron, Coquillart, & Ohlmann, 2009). During VSN treatment, patients are explicitly taught compensatory attentional strategies and consequently perform quite well on these static tasks with no time limit (Pedroli et al., 2015; Ten Brink et al., 2017). These tasks, therefore, do not capture mild deficits in lateralized attention that might only occur in dynamic daily life situations (e.g., walking on a busy sidewalk).
Complementary tasks have been developed, such as observational scales for clinicians. The Catherine Bergego Scale (CBS) is an example of a structured scale to observe VSN behaviour during daily activities, such as walking and eating (Azouvi et al., 2003; Ten Brink et al., 2013). The assignment of the scores, however, might vary significantly among clinicians due to differences in interpretation. In addition, the daily activities cannot always be observed in one time period, by one therapist or in the same observational context (Chen, Hreha, Fortis, Goedert, & Barrett, 2012). Next to observational scales, investigators have developed ecologically valid multitasks (i.e., performing multiple operations simultaneously) conducted in the real world. As a real‐world environment continuously changes, the required responses also change (Pedroli et al., 2015; Rizzo, Schultheis, Kerns, & Mateer, 2004). This makes a task more demanding – or even in competition – for attentional processes (Ten Brink et al., 2017). An example of such a task is the Mobility Assessment Course, where participants have to perform a wayfinding task in a corridor while detecting targets (Ten Brink et al., 2017). An important limitation is the lack of a standardized and controlled setting, which results in an inconsistent degree of distraction within or between assessments.
In recent years, promising new techniques like virtual reality (VR) have been used to simulate daily life situations in a safe and controlled manner (Rose, Brooks, & Rizzo, 2005; Tsirlin et al., 2009). By using VR simulations in neuropsychological assessment, new possibilities exist that go beyond paper‐and‐pencil tests. Researchers and clinicians can assess a patient's performance in a controlled and dynamic environment and predict the functional outcome based on those results.
Patients with VSN tend to deviate towards one side while walking. Previous research suggests that attention towards the ipsilesional side of space generally leads to contralesional deviations while navigating in real life (Huitema et al., 2006; Turton et al., 2009). For example, patients with left‐sided VSN tend to position themselves too close to walls on their left side, which often results in collisions into doorframes and objects (Turton et al., 2009). A recent study showed that patients with left‐sided VSN allowed obstacles to be closer on their left side while walking down a virtual path, compared to obstacles on their right side (Houston et al., 2015). In the current study, a simulated driving task was used to detect this lateral deviation and to investigate whether a dynamic task can detect VSN behaviour in patients who show well‐compensated or even ‘recovered’ VSN on traditional tasks (i.e., shape cancellation task (SC) and/or CBS). This simulated driving task has already been used to investigate reaction time asymmetries in patients with VSN admitted for inpatient rehabilitation (Van Kessel, Van Nes, Brouwer, Geurts, & Fasotti, 2010). However, this study included a small group of VSN patients (n = 12), and they did not investigate the navigational deviations. In our study, we investigated the differences in performance (i.e., position on the road, magnitude of sway) between patients with left‐ versus right‐sided VSN, patients without VSN, and healthy control participants. The performance of patients with ‘recovered’ VSN was compared with the performance of patients with and without VSN. Our second aim was to investigate the relation between the average position on the road, as a measure of lateralized attention, and VSN severity (measured with the SC and CBS). As a third aim, the diagnostic accuracy of the simulated driving task was assessed in relation to traditional VSN tasks. The sensitivity, specificity, positive, and negative predictive values were designated, in addition to the task's added value to the existing assessment of VSN.
Materials and methods
Participants
We included stroke patients admitted to inpatient rehabilitation in De Hoogstraat Rehabilitation Centre, from August 2013 to February 2017. All stroke patients were screened for VSN within the first 2 weeks of admission. We recruited patients based on this screening. Some of the patients additionally participated in a randomized clinical trial (PAiR: #NTR3278; approved by the Medical Ethical Committee of the University Medical Centre Utrecht, #12‐183/O) (Ten Brink, Visser‐Meily, & Nijboer, 2015). In this RCT, only patients with VSN, indicated with the SC or CBS administered during the screening, were included. Inclusion criteria for the current study were as follows: (1) clinical diagnosed stroke (confirmed by a MRI or CT scan); (2) age between 18 and 80 years old; and (3) sufficient comprehension and communication (evaluated by a neuropsychologist). Exclusion criteria were as follows: (1) physically or mentally unable to participate; (2) no (complete) data on the simulated driving task; or (2) no data on the SC and CBS.
Finally, a healthy control group with a comparable age distribution was recruited. We excluded healthy controls with neurological or psychiatric disorder(s) in their previous medical history. All participants gave written informed consent. The experiment was performed in accordance with the Declaration of Helsinki. The research protocol was approved by the Medical Ethics Committee of De Hoogstraat Rehabilitation Centre.
Procedure
The VSN screening included, among other tasks, the SC and the CBS. The CBS was administered by the nursing staff separately (see ‘Traditional VSN tasks’ for task descriptions). The screening was part of usual care and took about 45 min in total. Approximately 2 weeks later, a second measurement containing the SC and the simulated driving task was conducted and took about 30 min. The CBS was only re‐administered for patients with VSN who were also included in the randomized clinical trial (PAiR).
Simulated driving task
The simulated driving task (Van Kessel, Geurts, Brouwer, & Fasotti, 2013; Van Kessel et al., 2010) consisted of a driving scene projected on a large screen (2.13 m × 3.18 m; Figure 1). A straight road without intersections or oncoming traffic was projected on the screen. Participants were seated in front of the screen, which was placed at approximately 90 cm from their eyes. No car interior was projected, only a steering wheel was fixed on a table in front of the participant. A white plain board was placed on top of the table, to prevent the participant from using the table as visuo‐spatial reference. The simulated driving speed was approximately 50 km/hr at a fixed pace. Participants were instructed to use the steering wheel to maintain the starting position at the centre of the right lane, which is in line with Dutch road traffic regulations. Participants needed to adjust their position continuously, which was manipulated by simulated ‘side wind’ from both directions. When participants drove off the road into the left or right verge, the projection of the driving scene vibrated as a warning sign. No other feedback was given, to minimize interference with the task at hand. Prior to the task, the participant received a 1‐min practice trial. The simulated driving task took 2 min.
Figure 1.
A schematic and achromatic overview of the driving scene (the display used in the current study was in colour).
Outcome measures consisted of the average position on the road and the average standard deviation of the position, as an indication of the magnitude of sway. Outcome measures were averaged every 15 s (i.e., 8 values in total). The total range of positions on the road (i.e., limited by the left and right verge) ranged between −600 (as virtual world distances) up to 200, with the position of 0 indicating the centre of the right lane.
Traditional VSN tasks
Shape cancellation task
A digitized SC consisting of 54 targets (i.e., small shapes) and 75 distractors in different sizes (i.e., shapes, letters, and words) was used. Patients were instructed to cancel all targets. No time limit was given. After each designation, a small circle appeared around the target and remained on screen. The asymmetry score (i.e., the difference in a number of missed targets between the contralesional and ipsilesional sides) was computed. An asymmetry score of two or more was considered as indicative for VSN (Van der Stoep et al., 2013). The asymmetry score was used to determine VSN severity (range between 0 and 27).
Catherine Bergego Scale
The CBS is an observation scale to assess VSN behaviour during basic activities of daily living. The nursing staff observed and rated behaviour during 10 activities (e.g., dressing or eating) with a score of 0 (no VSN) to 3 (severe VSN). For computing the total score, we corrected for missing items (e.g., because patients were unable to independently perform the activity or the situation was not observed). The total score was the sum of the item scores, divided by the number of valid items, multiplied by 10 (resulting in a total score ranging from 0 [no VSN] to 30 [severe VSN]). A total score of ≥6 was considered as indicative for VSN (Azouvi et al., 2003; Ten Brink et al., 2013).
Demographic and clinical characteristics
We collected data on sex, age, and level of education from the medical files. Level of education was assessed using a Dutch classification system (Verhage, 1965), that consists of seven levels, with 1 being the lowest (less than primary school) and 7 being the highest (academic degree). These levels were converted into three categories for analysis: low (Verhage 1–4), average (Verhage 5), and high (Verhage 6–7). Additionally, we extracted the following characteristics from the medical files: days post‐stroke onset, stroke type (i.e., ischaemic, haemorrhage, or subarachnoid haemorrhage), the presence of language or communication deficits measured with the Stichting Afasie Nederland (SAN) score (Deelman, Koning‐Haanstra, Liebrand, & Van den Burg, 1981), the level of independence during daily life activities measured with the Barthel Index (Collin, Wade, Davies, & Horne, 1988), and the level of motor strength of upper and lower extremities measured with the Motricity Index (Collin & Wade, 1990). Global cognitive functioning was assessed with the Montreal Cognitive Assessment (MoCA) (Nasreddine et al., 2005), or the Mini Mental State Examination (MMSE) (Folstein, Folstein, & McHugh, 1975). In order to create one score for global cognitive functioning, the MMSE scores were converted into a MoCA score by using the following formula: MoCA = (1.124 × MMSE) − 8.165 (Solomon et al., 2014).
Statistical analyses
Categorization of patients
We categorized patients based on their performance on the SC and the CBS during the VSN screening and the second measurement. If there was a discrepancy concerning VSN side between the CBS and the SC, the patient was excluded. Patients who showed left‐sided VSN on the SC and/or the CBS during the screening and second measurement were assigned to the stroke group with left‐sided VSN (left‐sided VSN+). Patients who showed right‐sided VSN on the SC and/or the CBS during the screening and second measurement were assigned to the stroke group with right‐sided VSN (right‐sided VSN+). Patients who showed left‐sided VSN during the screening, but not on the second measurement, were referred to as the recovered group (left‐sided R‐VSN). Patients who showed right‐sided VSN during the screening, but not on the second measurement, were referred to as the recovered group (right‐sided R‐VSN). However, right‐sided R‐VSN patients were excluded because of a small sample size (n = 2). Patients who did not show VSN on both measurements were assigned to the group without VSN (VSN−).
Demographic and clinical characteristics
Non‐parametric tests (Kruskal–Wallis non‐parametric ANOVA and post‐hoc Mann–Whitney U test for continuous variables, and Chi‐square test for categorical variables) were used to compare demographic and clinical characteristics between the five groups (i.e., [1] left‐sided VSN+, [2] right‐sided VSN+, [3] left‐sided R‐VSN, [4] VSN−, and [5] healthy control participants). For the post‐hoc tests, a Bonferroni correction was applied to counteract the problem of multiple comparisons.
Differences between groups on the simulated driving task
Mann–Whitney U tests were used to compare the average position on the road and the magnitude of sway between left‐sided VSN+, right‐sided VSN+, and VSN− patients. VSN− patients were compared to healthy control participants (adjusted p for four tests = .013). To investigate the performance of left‐sided R‐VSN patients, we compared their performance with left‐sided VSN+ and VSN− patients (adjusted p for two tests = .025).
Relation with VSN severity
Spearman correlations between the average position on the road (absolute) and VSN severity (SC asymmetry score [absolute] and CBS total score) were computed. We used the absolute values in order to be able to analyse combined data of patients with left‐ and right‐sided VSN. An r of .1 was considered a small, .3 a moderate, and .5 a large relation (Field, 2009). The level of significance was set at p = .05.
Diagnostic accuracy
The normal range was computed based on the performance of 21 healthy control participants. An SD of 2 above and below the average position was used to define the normal range. The average position on the road of the healthy control participants was −16.73 (SD = 42.39), resulting in a normal range of −101.51 to 68.05.
Furthermore, we calculated the sensitivity, specificity, positive predictive value (i.e., the probability that a patient with an average position outside normal range did have VSN, based on the SC and/or CBS), and the negative predictive value (i.e., the probability that a patient with an average position within normal range did not have VSN, based on the SC and/or CBS). To determine the added value, we provided the percentages of left‐sided R‐VSN and VSN− patients, who performed outside normal range on the simulated driving task.
Finally, Receiver Operating Characteristic (ROC) curves were constructed by computing the sensitivity and specificity of the average position in predicting VSN for the following groups: left‐sided VSN+ and right‐sided VSN+.
Results
A total of 138 stroke patients were recruited. For the current study, 38 patients were excluded due to the following reasons: (1) no data on the simulated driving task (n = 13), (2) no data on the SC and CBS (n = 10), (3) right‐sided R‐VSN patients because of the small sample size (n = 2), and (4) discrepancy between affected side (left/right) based on the SC and CBS (n = 13). In addition, 36 healthy control participants were recruited, but 15 participants were excluded due to the following reasons: (1) no data on simulated driving task (n = 4) and (2) <30 years old (n = 11). In total, 33 patients with left‐sided VSN+, 7 patients with right‐sided VSN+, 7 patients with left‐sided R‐VSN, and 53 patients without VSN and 21 healthy control participants were included.
Demographic and clinical characteristics
Demographic and clinical characteristics are reported in Table 1. Most patients showed contralesional VSN (left‐sided VSN due to right hemispheric damage and right‐sided VSN due to left hemispheric damage). One patient with recovered left‐sided VSN had left hemispheric damage (ipsilesional VSN). Three patients had bilateral lesions.
Table 1.
Demographic and clinical characteristics, means (SD), or percentages split per group
| Left‐sided VSN+ (n = 33) | n | Right‐sided VSN+ (n = 7) | n | Left‐sided R‐VSN (n = 7) | n | VSN− (n = 53) | n | Healthy (n = 21) | n | Statistics | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex (% male) | 66.7 | 33 | 85.7 | 7 | 57.1 | 7 | 75.5 | 53 | 52.4 | 21 | χ2(4) = 5.16, p = .271 |
| Age (years) | 58.83 (9.18) | 33 | 54.75 (11.48) | 7 | 54.47 (14.69) | 7 | 58.86 (12.14) | 53 | 58.77 (9.86) | 21 | H(4) = 1.21, p = .877a |
| Level of education (%) | 33 | 6 | 7 | 51 | 21 | χ2(8) = 12.99, p = .112 | |||||
| Low | 27.3 | 50 | 14.3 | 23.5 | 0 | ||||||
| Moderate | 36.4 | 33.3 | 42.9 | 29.4 | 28.6 | ||||||
| High | 36.4 | 16.7 | 42.9 | 47.1 | 71.4 | ||||||
| Time post‐stroke (days) | 60.36 (31.83) | 33 | 43.00 (26.54 | 7 | 50.00 (37.76) | 7 | 41.89 (39.77) | 53 | H(3) = 11.49, p = .009 | ||
| Stroke type (%) | 26 | 6 | 6 | 39 | χ2(6) = 4.47, p = .613 | ||||||
| Ischaemic | 76.9 | 66.7 | 66.7 | 84.6 | |||||||
| Haemorrhage | 15.4 | 33.3 | 33.3 | 10.3 | |||||||
| SAH | 7.7 | 0 | 0 | 5.1 | |||||||
| Lesion side (%)b | 32 | 7 | 7 | 28 | |||||||
| Left | 0 | 71.4 | 14.3 | 59.6 | |||||||
| Right | 96.9 | 0 | 85.7 | 36.2 | |||||||
| Bilateral | 3.1 | 28.6 | 0 | 4.3 | |||||||
| SAN (1–7) | 6.23 (1.06) | 26 | 2.93 (2.09) | 7 | 5.86 (1.86) | 7 | 5.40 (1.68) | 40 | H(3) = 13.36, p = .004 | ||
| BI (0–20) | 7.92 (4.83) | 33 | 7.86 (3.89) | 7 | 11.14 (3.24) | 7 | 14.90 (4.51) | 40 | H(3) = 33.29, p < .001 | ||
| MI arm (0–100) | 41.13 (41.29) | 24 | 28.67 (45.28) | 6 | 57.40 (37.16) | 5 | 81.00 (28.21) | 41 | H(3) = 19.14, p < .001 | ||
| MI leg (0–100) | 51.71 (38.57) | 24 | 40.43 (41.77) | 7 | 67.83 (35.29) | 6 | 77.38 (27.96) | 42 | H(3) = 11.04, p = .012 | ||
| MoCA (0–30) | 19.59 (4.50) | 28 | 16.73 (7.63) | 4 | 23.46 (1.98) | 6 | 22.24 (5.10) | 34 | H(3) = 10.25, p = .017 | ||
| CBS (0–30) | 18.94 (7.32) | 33 | 14.49 (4.13) | 7 | 13.99 (6.22) | 7 | .66 (1.22) | 53 | |||
| SC (0–27) | 9.31 (7.61) | 32 | 1.83 (3.13) | 6 | 1.29 (1.38) | 7 | .25 (.43) | 53 |
BI = Barthel Index; MI = Motricity Index; MoCA = Montreal Cognitive Assessment; SAH = subarachnoid haemorrhage; SAN = Stichting Afasie Nederland.
We tested the relation between the average position on the road (absolute) and age. We did not found a significant relation within the left‐sided VSN+ patients (rs = .10 p = .585), right‐sided VSN+ patients (rs = −.11, p = .819), left‐sided R‐VSN patients (rs = .29, p = .535), VSN‐patients (rs = .16, p = .265), and healthy control participants (rs = .15, p = .507).
Most patients showed contralesional VSN, except for one patient showing ipsilesional VSN. Three patients had bilateral lesions.
Statistical comparisons were conducted between the five groups (i.e., left‐sided VSN+, right‐sided VSN+, left‐sided R‐VSN, VSN−, and healthy control participants). There was a significant difference in time post‐stroke, the presence of language or communication deficits, independence in daily life, and motor strength of upper and lower extremities between the patient groups. The post‐hoc tests (adjusted p = .008) showed that the time after stroke was higher for left‐sided VSN+ patients compared to VSN− patients (U = 497.00, z = −3.35, p = .001). Compared to VSN− patients, left‐sided VSN+ patients had a lower motor strength of upper and lower extremities (arm: U = 214.00, z = −3.89, p < .0011; leg: U = 298.00, z = −2.81, p = .005). Compared to left‐sided VSN+ patients, right‐sided VSN+ patients had more language and communication deficits (U = 20.50, z = −3.27, p = .001). Also, VSN− patients were more independent in basic daily activities compared to left‐sided VSN+ patients (U = 190.50, z = −5.21, p < .001) and right‐sided VSN+ patients (U = 32.50, z = −3.22, p = .001).
Differences between groups on the simulated driving task
Left‐sided VSN+, right‐sided VSN+, VSN−, and healthy control participants
The average position of left‐sided VSN+ patients deviated more (M = −125.75) compared to VSN− patients (M = −11.75; U = 315.00, z = −4.97, p < .001, r = −.54) and compared to right‐sided VSN+ patients (M = 0.57; U = 41.00, z = −2.65, p = .008, r = −.42). The average position on the road did not differ significantly between right‐sided VSN+ and VSN− patients (U = 142.00, z = −1.00, p = .316, r = −.13). The average position of VSN− patients and healthy controls (M = −16.73) did not differ significantly (U = 512.50, z = −.53, p = .598, r = −.07). See Figure 2a.
Figure 2.
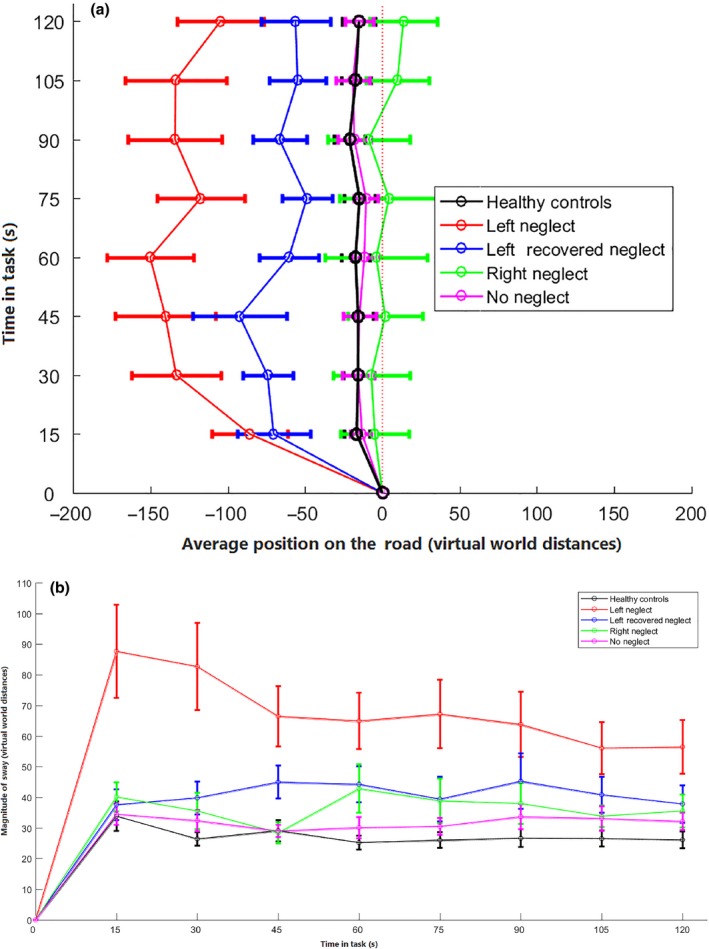
Overview of the (a) average position on the road and (b) average magnitude of sway for left‐sided VSN+, right‐sided VSN+, left‐sided R‐VSN, VSN− patients, and healthy control participants. The horizontal error bars represent the variability (SD) in the average position or sway. With regard to Figure 2a, the dashed line represents the starting position at the centre of the right lane. [Color figure can be viewed at http://wileyonlinelibrary.com]
Likewise, left‐sided VSN+ patients showed a larger magnitude of sway (M = 68.45) compared to VSN− patients (M = 32.48; U = 276.00, z = −5.32, p < .001, r = −.57) and right‐sided VSN+ patients (M = 36.66; U = 45.00, z = −2.51, p = .012, r = −.40). The magnitude of sway did not differ significantly between right‐sided VSN+ patients and VSN− patients (U = 130.50, z = −1.27, p = .205, r = −.16). The magnitude of sway did not differ significantly between VSN− patients and healthy controls (M = 27.51; U = 389.50, z = −2.00, p = .045, r = −.23). See Figure 2b.
Left‐sided VSN+, left‐sided recovered VSN, and VSN−
The average position on the road of left‐sided R‐VSN patients (M = −65.46) did not significantly differ from the position of left‐sided VSN+ patients (U = 76.50, z = −1.39, p = .165, r = −.22). However, left‐sided R‐VSN deviated more to the left compared to VSN− patients (U = 79.00, z = −2.45, p = .014, r = −.32).
Likewise, the magnitude of sway in left‐sided R‐VSN patients (M = 41.27) did not significantly differ from the sway in left‐sided VSN+ patients (U = 63.00, z = −1.87, p = .062, r = −.30) nor from the sway of VSN− patients (U = 115.50, z = −1.61, p = .107, r = −.21).
Relation with VSN severity
There was a moderate positive relation between the average position and VSN severity as measured with the SC (rs = .47, p < .001; Figure 3a). A high positive correlation was found between the average position and VSN severity as measured with the CBS (rs = .53, p < .001; Figure 3b).
Figure 3.
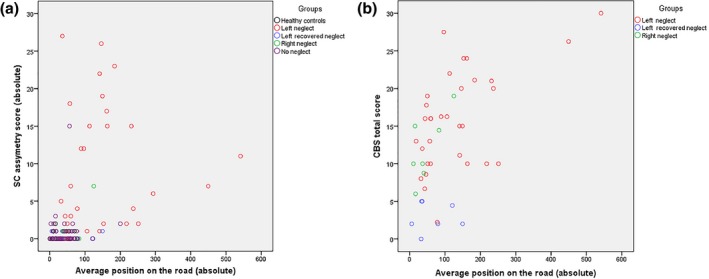
The average position on the road and its relation with VSN severity measured with (a) SC asymmetry score; and (b) CBS total score. [Color figure can be viewed at http://wileyonlinelibrary.com]
Diagnostic accuracy
With respect to sensitivity, 51.5% of left‐sided VSN+ patients and 28.6% of right‐sided VSN+ patients performed outside the normal range regarding position on the road. With respect to specificity, 94.3% of VSN− patients performed within normal range. Regarding left‐sided VSN, the positive predictive value was 85%, and the negative predictive value was 75.8%. Regarding right‐sided VSN, the positive predictive value was 40%, and the negative predictive value was 90.9%. Of the left‐sided R‐VSN patients, 28.6% performed outside normal range on the simulated driving task. Regarding VSN− patients, 5.7% performed outside normal range.
A ROC curve was computed for left‐sided VSN+ and right‐sided VSN+ patients. We found that the simulating driving task was more accurately as an assessment tool for left‐sided VSN+ patients (area under the curve = 0.844) compared to right‐sided VSN+ patients (area under the curve = 0.429; Figure 4).
Figure 4.
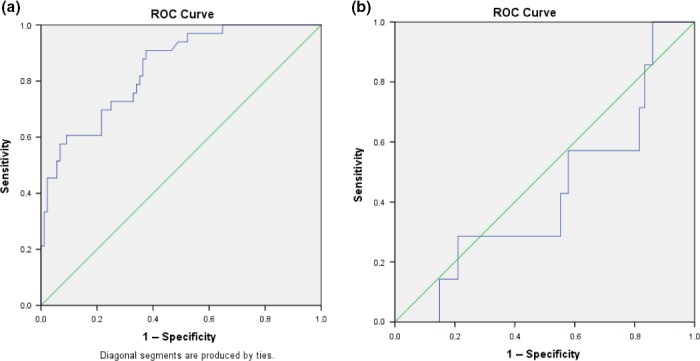
ROC curve for average position on the road for (a) left‐sided VSN+ patients and (b) right‐sided VSN+ patients. [Color figure can be viewed at http://wileyonlinelibrary.com]
Discussion
The aims of the current study were threefold: (1) to assess differences in performance (i.e., position on the road, magnitude of sway) on a simulated driving task between patients with left‐sided VSN, right‐sided VSN, ‘recovered’ left‐sided VSN, without VSN, and healthy control participants; (2) to investigate the relation between the average position and VSN severity; and (3) to assess the diagnostic accuracy of the simulated driving task in relation to traditional VSN tasks.
With respect to the first aim, left‐sided VSN+ patients showed a larger magnitude of sway compared to VSN− patients and tended to deviate more to the left side of the right lane, even up to the left verge. This leftward deviation is in line with previous findings that attention towards the ipsilesional side may lead to contralesional deviations (Houston et al., 2015; Huitema et al., 2006; Turton et al., 2009). The position of right‐sided VSN+ patients was comparable with the position of VSN− patients. This is likely the result of the asymmetric layout of the simulated driving task. There were only two lanes demarcated by two verges. Patients started in the centre of the right lane. As a result, there was less room for the expected rightward deviation. Also, a relatively small deviation towards the right, in the right verge, was directly interrupted with the warning sign. The ROC curve analyses supported this finding, as we found that the simulating driving task is a more accurate assessment tool for left‐sided VSN compared to right‐sided VSN. In future research, a symmetric design should be used to enhance the probability to detect right‐sided VSN. The average position on the road of left‐sided recovered VSN patients was of intermediary level between the positions on the road of left‐sided VSN+ and VSN− patients. The ‘recovered’ patients deviated significantly more from the centre compared to VSN− patients. Other studies also reported persistent VSN behaviour in patients showing ‘recovered’ VSN on paper‐and‐pencil tasks (Buxbaum et al., 2004; Houston et al., 2015; Ten Brink et al., 2017). These results fit the clinical observations that neuropsychological paper‐and‐pencil tasks are not always sensitive enough to assess mild or well‐compensated VSN. This is probably due to the lack of multitasking, attentional engagement, distractions, and/or time limit (Azouvi, 2017; Bonato, 2012, 2015; Ten Brink et al., 2017). Furthermore, the ability to reorient attention contralesionally may recover rather quickly, but the ipsilesional attention bias may be relatively persistent (Mattingley, Bradshaw, Bradshaw, & Nettleton, 1994). Regaining the ability to reorient contralesionally may explain why ‘recovered’ VSN patients do well on static paper‐and‐pencil tasks. In our study, it is possible that the remaining ipsilesional attention bias in ‘recovered’ VSN patients might cause the deviation to the contralesional side as the simulated driving task might be more demanding for attentional processes. Also, attention towards the ipsilesional side leads to contralesional deviations while navigating in dynamic, real‐life situations (Houston et al., 2015; Huitema et al., 2006; Turton et al., 2009). A note of caution is due here since we cannot state which underlying process causes the effect of the ‘recovered’ VSN patients in our study. This is an important issue for future research, as well as determining which factors (e.g., clinical severity of stroke (Nijboer, Winters, Kollen, & Kwakkel, 2018), specific white matter disconnections (Lunven et al., 2015), but also demographic factors and comorbid conditions (Kwakkel et al., 2017)) predict the recovery of VSN measured with different measures (static versus dynamic).
As for the second objective, a moderate positive relation was found between VSN severity as measured with a paper‐and‐pencil task (SC) and the average position on the road, indicating that more severe VSN was related to a more deviant position. A strong positive relation was found between VSN severity as measured with an observational scale (CBS) and the average position. As both the CBS and simulated driving task are more dynamic in nature than the SC, this finding suggests that dynamic tasks, like the simulated driving task, demand more natural behaviour and consequently relate more to daily activities (Tsirlin et al., 2009). Also, by using dynamic tasks in neuropsychological assessment, the results have a greater clinical relevance because of the enhanced ecological validity and could subsequently be a first step for the development of effective functional rehabilitation approaches (Schultheis, Himelstein, & Rizzo, 2002).
With respect to the third aim, the sensitivity was 52% for left‐sided VSN and 29% for right‐sided VSN, and the specificity was 94%. The positive predictive value for left‐sided VSN was 85% and right‐sided VSN 40%. The negative predictive value for left‐sided VSN was 76% and right‐sided VSN 91%. Based on these findings, the simulated driving task cannot be used in isolation to detect VSN. For example, a percentage of patients do show VSN on the SC and/or CBS, but not on the simulated driving task (49% of left‐sided VSN and 71% of right‐sided VSN patients). Similar percentages are found when patients are categorized based on the SC and CBS separately, indicating that approximately 50% of patients show VSN on a dynamic task irrespectively of the test you use to categorize them. For this reason, the assessment of VSN should always consist of more than a single task and, ideally, of several tasks varying in nature and complexity (Azouvi et al., 2006) and include dynamic tasks with an improved ecological validity. When developing such a test battery, it is important to investigate whether a new test improves the diagnostic accuracy by going beyond the available diagnostic information from traditional tests (Moons, De Groot, Linnet, Reitsma, & Bossuyt, 2012). Therefore, the most important clinically relevant finding of the current study was the added value of the simulated driving task. In a sequence of steps, diagnostic information has been documented: first using a widely used cancellation task (SC) and observational scale (CBS), and second using a simulated driving task as the dynamic counterpart. In total, 29% of patients, who showed left‐sided recovered VSN on a paper‐and‐pencil task and during observations through daily activities still showed abnormal performance on the simulated driving task. This finding shows the ‘clinical utility’ (Bossuyt, Reitsma, Linnet, & Moons, 2012) of dynamic testing, as the use of the simulated driving task can identify more patients who will benefit from the necessary treatment. Likewise, an additional 5.7% of patients not showing VSN on the SC and CBS did show abnormal performance on the simulated driving task. Although the sample sizes are rather small, this study shows that the addition of a dynamic task, such as a 2‐min simulated driving task, might improve the diagnostic accuracy of the existing clinical pathway for detecting VSN.
Previous research emphasized the need for divers dynamic tasks, resembling real life, because paper‐and‐pencil tasks are often not sensitive enough to detect mild and/or well‐compensated VSN (Appelros, Nydevik, Karlsson, Thorwalls, & Seiger, 2003). In dynamic tasks, there is (moving) interference of stimuli or time pressure, in which stimuli are presented for a short period of time. An example is the Mobility Assessment Course (MAC). Such tasks can be more sensitive for the lateralized attention deficit compared to paper‐and‐pencil tasks (Ten Brink et al., 2017). Regarding the MAC, 10–19% of patients without VSN on paper‐and‐pencil tasks showed VSN behaviour on the MAC. This task, however, lacks standardization and experimental control. For the assessment of VSN, we need tasks that are dynamic and ecological valid but also consist a controlled setting to purely measure lateralized inattention. The simulated driving task is such an example, because of the high level of control that enables a consistent presentation of stimuli and increases standardization of the task (Rizzo et al., 2004). Hence, fluctuations in performance can additionally be measured during the task, but also in the course of rehabilitation, because of the consistency across assessments. Previous studies with VSN patients have reported inconsistency in performance throughout the day (Corbetta, 2014) and during the period of recovery (Jehkonen, Laihosalo, Koivisto, Dastidar, & Ahonen, 2007). The simulated driving task could serve as dynamic task to assess mild VSN and to further explore fluctuations in performance among VSN patients.
Another reason to extend the traditional assessment of VSN with dynamic tasks is the heterogeneity of the VSN syndrome and its divers manifestation (Appelros et al., 2003; Corbetta, 2014). Some patients may perform within normal range with respect to the primary outcome measures on paper‐and‐pencil tasks, but show VSN when measured with dynamic tasks, and vice versa. The latter finding (i.e., showing VSN on static, paper‐and‐pencil tasks while performing normally on a dynamic, driving task) seems counterintuitive, as a more dynamic situation likely demands more attention. It could be explained by stochastic resonance, which describes the phenomena where ‘noise’ (e.g., additional visual, auditory, tactile stimuli) can enhance or decrease sensory information processing and perception (Moss, Ward, & Sannita, 2004). In other words, some patients benefit from additional stimuli, and subsequently perform better in a dynamic environment, whereas others do not. Thus, the nature of the task (static versus dynamic) can cause differences in performance. Furthermore, the heterogeneity of VSN also extends to modality (i.e., visual, auditory or tactile), frame of reference (i.e., egocentric or allocentric), or region of space (i.e., peripersonal or extrapersonal) (Corbetta, 2014; Rode, Pagliari, Huchon, Rossetti, & Pisella, 2016; Van der Stoep et al., 2013). With the simulated driving task, we only measured lateralized visuo‐spatial inattention. It can be concluded that the assessment of VSN should not consist of one single task, but should always consist of several tasks to detect all VSN patients.
A strength of this study is the use of a dynamic task in a stroke population in the sub‐acute phase of rehabilitation. Accurate assessment in an early phase is of utmost importance to provide the necessary information to determine the appropriate approach for rehabilitation. Administration of the simulated driving task, as part of neuropsychological assessment, was feasible, as all patients were able to perform this task. Even patients with lower motor strength were able to perform this task with one hand without negatively affecting the position on the road (i.e., the main outcome measure for VSN). Also, the inclusion of the different subgroups (left‐sided VSN+, right‐sided VSN+, and left‐sided R‐recovered) can be considered as a strength, as it allows an in‐depth exploration of VSN.
An important limitation of the task was the asymmetric layout that should be adjusted before it can be used to detect right‐sided VSN patients. Previous research has emphasized the necessity of accurate assessment tools to detect right‐sided VSN, as right‐sided VSN is often not detected when measured with paper‐and‐pencil tasks (Ten Brink et al., 2016). Consequences in daily life, however, are similar to left‐sided VSN patients, and accurate diagnosis is, therefore, of great importance. Hence, a symmetric design should be used when designing novel tasks to assess VSN. In addition, other visual field deficits, such as hemianopia, might also result in a deviated position on a driving task (Bowers, Mandel, Goldstein, & Peli, 2010; Wood et al., 2011). No systematic screening for hemianopia was done in the rehabilitation centre nor in the VSN screening, so it remains unclear whether hemianopia was present in a subset of stroke patients. Based on the scores of the tests, the observations during activities of daily living, and the inspection of the MRI scans in a subset of patients, however, we are convinced that it is highly unlikely that hemianopia has had a major influence on the current results. The SC measures inattention, and patients with hemianopia usually use compensatory strategies and find all targets in this phase post‐stroke onset. Furthermore, the nurses who filled in the CBS were instructed to score VSN behaviour only and no behaviours due to other sensory deficits (including visual field defects). Regarding the simulated driving task, it is highly unlikely that that hemianopia might be a potential cofounder. Hemianopic patients tend to deviate towards their seeing field, thus, in the opposite direction to that of VSN patients (Bowers et al., 2010; Wood et al., 2011). If anything, therefore, hemianopic patients in the VSN sample would have weaken the results.
Finally, we would like to stress that the current test is not intended for assessing fitness to drive after stroke. This simulated driving task did not represent the complexity of real life, because of its relatively ‘simple’ design (e.g., the lack of intersections and oncoming traffic, and the limited driving operations the user had to encounter). Nevertheless, even though this task was not intended to assess traffic participation, VR simulations can play an important role for such an assessment after stroke. A recent study used a driving simulator involving various traffic‐based events to assess fitness to drive in stroke patients (Blane, Falkmer, Lee, & Dukic Willstrand, 2017). In future research, substantial adaptations need to be made with regard to the current simulated driving task in order to design a suitable VR simulation to these aims. Also, in the simulated driving task, the outcome measures were averaged every 15 s (resulting in eight values in total). In future research, a continuous data acquisition would give more detailed and precise information and could subsequently give insight into the exact timing of onset of deviations, stabilization of lane position, and time‐dependent changes.
Conclusions
This study proposes a dynamic task as supplement to improve the diagnostic accuracy of the existing clinical pathway and consequently detect more VSN patients who can benefit from VSN treatment during rehabilitation. An extra 6–29% of patients who did not show VSN on a paper‐and‐pencil task nor on an observational scale did show VSN behaviour on a simple 2‐min simulated driving task. It is important to note that this conclusion is based on a rather small sample. The sensitivity was 52% for left‐sided VSN. Right‐sided VSN was not well detected, probably due to the asymmetric layout. Based on these results, the simulated driving task should not be used in isolation to assess VSN, especially in its current form. Given the heterogenic nature of VSN, the assessment should always consist of several tasks varying in nature and complexity and include a dynamic task to detect mild and/or recovered VSN.
Acknowledgements
This work was supported by the Revalidatiefonds (R2012134) and the Netherlands Organization for Scientific Research (NWO; 451‐10‐013). We would like to thank Merel Pieters, Marit Dorresteijn, Roemi Wikarta, Sanne Loosschilder, Inge Meeuwissen, and Irene Bonthond for their help in collecting the data.
Note
We tested the relation between the average position on the road (absolute) and motor strength in the upper extremities. We did not found a significant relation within the left‐sided VSN+ patients (rs = −.29, p = .168), right‐sided VSN+ patients (rs = −.68, p = .140), left‐sided R‐VSN patients (rs = .30, p = .624), and VSN− patients (rs = .03, p = .842).
References
- Appelros, P. , Nydevik, I. , Karlsson, G. M. , Thorwalls, A. , & Seiger, Å. (2003). Assessing unilateral neglect: Shortcomings of standard test methods. Disability and Rehabilitation, 25, 473–479. 10.1080/0963828031000071714 [DOI] [PubMed] [Google Scholar]
- Azouvi, P. (2017). The ecological assessment of unilateral neglect. Annals of Physical and Rehabilitation Medicine, 60, 186–190. 10.1016/j.rehab.2015.12.005 [DOI] [PubMed] [Google Scholar]
- Azouvi, P. , Bartolomeo, P. , Beis, J.‐M. , Perennou, D. , Pradat‐Diehl, P. , & Rousseaux, M. (2006). A battery of tests for the quantitative assessment of unilateral neglect. Restorative Neurology and Neuroscience, 24, 273–285. [PubMed] [Google Scholar]
- Azouvi, P. , Olivier, S. , De Montety, G. , Samuel, C. , Louis‐Dreyfus, A. , & Tesio, L. (2003). Behavioral assessment of unilateral neglect: Study of the psychometric properties of the Catherine Bergego Scale. Archives of Physical Medicine and Rehabilitation, 84(1), 51–57. 10.1053/apmr.2003.50062 [DOI] [PubMed] [Google Scholar]
- Blane, A. , Falkmer, T. , Lee, H. C. , & Dukic Willstrand, T. (2017). Investigating cognitive ability and self‐reported driving performance of post‐stroke adults in a driving simulator. Topics in Stroke Rehabilitation, 9357, 1–10. 10.1080/10749357.2017.1373929 [DOI] [PubMed] [Google Scholar]
- Bonato, M. (2012). Neglect and extinction depend greatly on task demands: A review. Frontiers in Human Neuroscience, 6, 1–13. 10.3389/fnhum.2012.00195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonato, M. (2015). Unveiling residual, spontaneous recovery from subtle hemispatial neglect three years after stroke. Frontiers in Human Neuroscience, 9, 1–9. 10.3389/fnhum.2015.00413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bossuyt, P. M. M. , Reitsma, J. B. , Linnet, K. , & Moons, K. G. M. (2012). Beyond diagnostic accuracy: The clinical utility of diagnostic tests. Clinical Chemistry, 58, 1636–1643. 10.1373/clinchem.2012.182576 [DOI] [PubMed] [Google Scholar]
- Bowers, A. R. , Mandel, A. J. , Goldstein, R. B. , & Peli, E. (2010). Driving with hemianopia, II: Lane position and steering in a driving simulator. Investigative Ophthalmology and Visual Science, 51(12), 6605–6613. 10.1167/iovs.10-5310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum, L. J. , Ferraro, M. K. , Veramonti, T. , Farne, A. , Whyte, J. , Ladavas, E. , … Coslett, H. B. (2004). Hemispatial neglect: Subtypes, neuroanatomy, and disability. Neurology, 62, 749–756. 10.1212/01.WNL.0000113730.73031.F4 [DOI] [PubMed] [Google Scholar]
- Chen, P. , Hreha, K. , Fortis, P. , Goedert, K. M. , & Barrett, A. M. (2012). Functional assessment of spatial neglect: A review of the Catherine Bergego Scale and an introduction of the Kessler Foundation Neglect Assessment Process. Top Stroke Rehabilitation, 19, 423–435. 10.1310/tsr1905-423.Functional [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, P. , Hreha, K. , Kong, Y. , & Barrett, A. M. (2015). Impact of spatial neglect in stroke rehabilitation: Evidence from the setting of an inpatient rehabilitation facility. Archives of Physical Medicine and Rehabilitation, 96, 1458–1466. 10.1002/aur.1474.Replication [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin, C. , & Wade, D. (1990). Assessing motor impairment after stroke: A pilot reliability study. Journal of Neurology, Neurosurgery & Psychiatry, 53, 576–579. 10.1136/jnnp.53.7.576Collin [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collin, C. , Wade, D. T. , Davies, S. , & Horne, V. (1988). The Barthel ADL Index: A reliability study. International Disability Studies, 10, 61–63. 10.3109/09638288809164103 [DOI] [PubMed] [Google Scholar]
- Corbetta, M. (2014). Hemispatial neglect: Clinic, pathogenesis, and treatment. Seminars in Neurology, 34, 514–523. 10.1055/s-0034-1396005 [DOI] [PubMed] [Google Scholar]
- Deelman, B. , Koning‐Haanstra, M. , Liebrand, W. , & van den Burg, W. (1981). Stichting Afasie Nederland ‐ de SAN‐test [Dutch Organisation of Aphasia – the SAN‐test]. Lisse: Swets & Zeitlinger. [Google Scholar]
- Field, A. P. (2009). Discovering statistics using SPSS: (and sex and drugs and rock ‘n’ roll). Los Angeles, CA: SAGE. [Google Scholar]
- Folstein, M. F. , Folstein, S. E. , & McHugh, P. R. (1975). “Mini‐mental state”. A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research, 12, 189–198. 10.1016/0022-3956(75)90026-6 [DOI] [PubMed] [Google Scholar]
- Heilman, K. M. , Valenstein, E. , & Watson, R. T. (2000). Neglect and related disorders. Seminars in Neurology, 20, 463–470. 10.1055/s-2000-13179 [DOI] [PubMed] [Google Scholar]
- Houston, K. E. , Woods, R. L. , Goldstein, R. B. , Peli, E. , Luo, G. , & Bowers, A. R. (2015). Asymmetry in the collision judgments of people with homonymous field defects and left hemispatial neglect. Investigative Ophthalmology and Visual Science, 56(6), 4135–4142. 10.1167/iovs.14-15492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitema, R. B. , Brouwer, W. H. , Hof, A. L. , Dekker, R. , Mulder, T. , & Postema, K. (2006). Walking trajectory in neglect patients. Gait & Posture, 23, 200–205. 10.1016/j.gaitpost.2005.02.003 [DOI] [PubMed] [Google Scholar]
- Jehkonen, M. , Laihosalo, M. , Koivisto, A. M. , Dastidar, P. , & Ahonen, J. P. (2007). Fluctuation in spontaneous recovery of left visual neglect: A 1‐year follow‐up. European Neurology, 58, 210–214. 10.1159/000107941 [DOI] [PubMed] [Google Scholar]
- Kwakkel, G. , Lannin, N. , Karen, B. , English, C. , Ali, M. , Churilov, L. , … Bernhardt, J. (2017). Standardized measurement of sensorimotor recovery in stroke trials: Consensus‐based core recommendations from the Stroke Recovery and Rehabilitation Roundtable. Neurorehabilitation and Neural Repair, 31(10–11), 864–876. 10.1177/1545968317732680 [DOI] [PubMed] [Google Scholar]
- Lunven, M. , De Schotten, M. T. , Bourlon, C. , Duret, C. , Migliaccio, R. , Rode, G. , & Bartolomeo, P. (2015). White matter lesional predictors of chronic visual neglect: A longitudinal study. Brain, 138(3), 746–760. 10.1093/brain/awu389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattingley, J. B. , Bradshaw, J. L. , Bradshaw, J. A. , & Nettleton, N. C. (1994). Residual rightward attentional bias after apparent recovery from right hemisphere damage: Implications for a multicomponent model of neglect. Journal of Neurology Neurosurgery and Psychiatry, 57, 597–604. 10.1136/jnnp.57.5.597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moons, K. G. M. , De Groot, J. A. H. , Linnet, K. , Reitsma, J. B. R. , & Bossuyt, P. M. M. (2012). Quantifying the added value of a diagnostic test or marker. Clinical Chemistry, 58, 1408–1417. 10.1373/clinchem.2012.182550 [DOI] [PubMed] [Google Scholar]
- Moss, F. , Ward, L. M. , & Sannita, W. G. (2004). Stochastic resonance and sensory information processing: A tutorial and review of application. Clinical Neurophysiology, 115, 267–281. 10.1016/j.clinph.2003.09.014 [DOI] [PubMed] [Google Scholar]
- Nasreddine, Z. S. , Philips, N. A. , Bedirian, V. , Charbonneau, S. , Whitehead, V. , Collin, I. , … Chertkow, H. (2005). The Montreal Cognitive Assessment, MoCA : A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society, 53, 695–699. 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- Nijboer, T. C. W. , Kollen, B. , & Kwakkel, G. (2014). The impact of recovery of visuo‐spatial neglect on motor recovery of the upper paretic limb after stroke. PLoS ONE, 9(6), 1–8. 10.1371/journal.pone.0100584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijboer, T. C. W. , van de Port, I. , Schepers, V. , Post, M. , & Visser‐Meily, A. (2013). Predicting functional outcome after stroke: The influence of neglect on basic activities in daily living. Frontiers in Human Neuroscience, 7, 1–6. 10.3389/fnhum.2013.00182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijboer, T. C. W. , Winters, C. , Kollen, B. , & Kwakkel, G. (2018). Impact of clinical severity of stroke on the severity and recovery of visuospatial neglect. PLoS ONE, 13(7), e0198755 10.1371/journal.pone.0198755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroli, E. , Serino, S. , Cipresso, P. , Pallavicini, F. , & Riva, G. (2015). Assessment and rehabilitation of neglect using virtual reality: A systematic review. Frontiers in Behavioral Neuroscience, 9, 226 10.3389/fnbeh.2015.00226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo, A. A. , Schultheis, M. , Kerns, K. A. , & Mateer, C. (2004). Analysis of assets for virtual reality applications in neuropsychology. Neuropsychological Rehabilitation, 14(1–2), 207–239. 10.1080/09602010343000183 [DOI] [Google Scholar]
- Rode, G. , Pagliari, C. , Huchon, L. , Rossetti, Y. , & Pisella, L. (2016). Semiology of neglect: An update. Annals of Physical and Rehabilitation Medicine, 60, 177–185. 10.1016/j.rehab.2016.03.003 [DOI] [PubMed] [Google Scholar]
- Rose, F. D. , Brooks, B. M. , & Rizzo, A. A. (2005). Virtual reality in brain damage rehabilitation: Review. CyberPsychology & Behavior, 8, 241–262. 10.1089/cpb.2005.8.241 [DOI] [PubMed] [Google Scholar]
- Schultheis, M. T. , Himelstein, J. , & Rizzo, A. A. (2002). Virtual reality and neuropsychology: Upgrading the current tools. The Journal of Head Trauma Rehabilitation, 17, 378–394. 10.1097/00001199-200210000-00002 [DOI] [PubMed] [Google Scholar]
- Solomon, T. M. , DeBros, G. B. , Budson, A. E. , Mirokovic, N. , Murphy, C. A. , & Solomon, P. R. (2014). Correlational analysis of 5 commonly used measures of cognitive functioning and mental status: An update. American Journal of Alzheimer's Disease and Other Dementias, 29, 718–722. 10.1177/1533317514534761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Brink, A. F. , Nijboer, T. C. W. , Van Beekum, L. , Van Dijk, J. , Peeters, R. , Post, M. , & Visser‐Meily, J. M. A. (2013). De Nederlandse Catherine Bergego schaal: een bruikbaar en valide instrument in de CVA‐zorg [The Dutch version of the Catherine Bergego Scale: a valid instrument in stroke care]. Wetenschappelijk Tijdschrift Voor Ergotherapie, 6, 27–36. [Google Scholar]
- Ten Brink, A. F. , Verwer, J. H. , Biesbroek, J. M. , Visser‐Meily, J. M. A. , & Nijboer, T. C. W. (2016). Differences between left and right sided neglect revisited: A large cohort study across multiple domains. Journal of Clinical and Experimental Neuropsychology, 39, 707–723. 10.1080/13803395.2016.1262333 [DOI] [PubMed] [Google Scholar]
- Ten Brink, A. F. , Visser‐Meily, J. M. A. , & Nijboer, T. C. W. (2015). Study protocol of ‘Prism Adaptation in Rehabilitation’: A randomized controlled trial in stroke patients with neglect. BMC Neurology, 15(5), 1–5. 10.1186/s12883-015-0263-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ten Brink, A. F. , Visser‐Meily, J. M. A. , & Nijboer, T. C. W. (2017). Dynamic assessment of visual neglect: The Mobility Assessment Course as a qualitative diagnostic tool. Journal of Clinical and Experimental Neuropsychology, 40, 161–172. 10.1080/13803395.2017.1324562 [DOI] [PubMed] [Google Scholar]
- Tsirlin, I. , Dupierrix, E. , Chokron, S. , Coquillart, S. , & Ohlmann, T. (2009). Uses of virtual reality for diagnosis, rehabilitation and study of unilateral spatial neglect: Review and analysis. Cyberpsychology and Behavior, 12, 175–181. 10.1089/cpb.2008.0208 [DOI] [PubMed] [Google Scholar]
- Turton, A. J. , Dewar, S. J. , Lievesley, A. , O'Leary, K. , Gabb, J. , & Gilchrist, I. D. (2009). Walking and wheelchair navigation in patients with left visual neglect. Neuropsychological Rehabilitation, 19, 274–290. 10.1080/09602010802106478 [DOI] [PubMed] [Google Scholar]
- Van der Stoep, N. , Visser‐Meily, J. M. A. , Kappelle, L. J. , de Kort, P. L. M. , Huisman, K. D. , Eijsackers, A. L. H. , … Nijboer, T. C. W. (2013). Exploring near and far regions of space: Distance‐specific visuospatial neglect after stroke. Journal of Clinical and Experimental Neuropsychology, 35, 799–811. 10.1080/13803395.2013.824555 [DOI] [PubMed] [Google Scholar]
- Van Kessel, M. E. , Geurts, A. C. H. , Brouwer, W. H. , & Fasotti, L. (2013). Visual scanning training for neglect after stroke with and without a computerized lane tracking dual task. Frontiers in Human Neuroscience, 7, 1–11. 10.3389/fnhum.2013.00358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Kessel, M. E. , Van Nes, I. J. W. , Brouwer, W. H. , Geurts, A. C. H. , & Fasotti, L. (2010). Visuospatial asymmetry and non‐spatial attention in subacute stroke patients with and without neglect. Cortex, 46, 602–612. 10.1016/j.cortex.2009.06.004 [DOI] [PubMed] [Google Scholar]
- Verhage, F. (1965). Intelligence and age in a Dutch sample. Human Development, 8, 238–245. 10.1159/000270308 [DOI] [Google Scholar]
- Wood, J. M. , McGwin, G. , Elgin, J. , Vaphiades, M. S. , Braswell, R. A. , de Carlo, D. K. , … Owsley, C. (2011). Hemianopic and quadrantanopic field loss, eye and head movements, and driving. Investigative Ophthalmology and Visual Science, 52(3), 1220–1225. 10.1167/iovs.10-6296 [DOI] [PMC free article] [PubMed] [Google Scholar]


