Summary
In daily haematological practice, predicting bleeding in thrombocytopenic patients is difficult, and clinicians adhere to transfusion triggers to guide patients through the aplastic phase of chemotherapy. Platelet count is not the only determinant of bleeding and additional mechanisms for impending haemostasis are likely. Beside clot formation, platelets are essential for the maintenance of integrity of vascular beds. We therefore prospectively investigated associations between biomarkers for endothelial damage (urine albumin excretion) and inflammation (C‐reactive protein) and bleeding (WHO grading) in 88 patients with 116 on‐protocol episodes. We found an increase in grade 2 bleeding with a higher urine albumin/creatinine ratio one day after the measurement [odds ratio (OR) 1·24 for every doubling of the ratio, 95% CI 1·05–1·46, P‐value 0·01] and a 29% increase in the odds of grade 2 bleeding for every doubling of serum C‐reactive protein (CRP) (95% CI 1·04–1·60, P‐value 0·02) after correction for morning platelet count. The 24 h post‐transfusion corrected count increment (CCI24) showed a significant association with these biomarkers: increasing urine albumin/creatinine ratio and CRP were associated with lower CCI24. We report two inexpensive and easy‐to‐apply biomarkers that could be useful in designing a prediction model for bleeding risk in thrombocytopenic patients.
Keywords: haemorrhage, platelet transfusion, thrombocytopenia, microalbuminuria, CRP
Over 40% of patients with thrombocytopenia due to haematological malignancy and intensive chemotherapy encounter one or more bleeding episodes in a broad range of platelet counts, despite trigger‐based preventive platelet transfusion practices (Heddle et al., 2009; Slichter et al., 2010; Ypma et al., 2012; Stanworth et al., 2013). As the current prophylactic trigger‐based platelet transfusion strategy is associated with adverse transfusion events, costs and resource issues (Stanworth et al., 2015a), better strategies to identify patients prone to bleeding are needed. Recently, the interest for on demand therapeutic platelet transfusions has revived. Some phenotypically “non‐bleeding” patient conditions were identified, one of which is being in the thrombocytopenic phase post‐autologous stem cell transplantation (Wandt et al., 2012; Stanworth et al., 2013). Despite the fact that some laboratory tests are significantly associated with bleeding, such as the absolute immature platelet count preluding the recovery of functional platelets (Estcourt et al., 2014), the alpha angle of thromboelastography (Opheim et al., 2017), a low haematocrit (≤25%) and abnormal global coagulation assays (Uhl et al., 2017), these tests are not commonly used in transfusion practice.
Along with their haemostatic function, platelets contribute to vascular integrity with a finite number of platelets (Hanson & Slichter, 1985; Nachman & Rafii, 2008). Spontaneous extravasation of red cells occurred in patients with prolonged deep thrombocytopenia. In agreement with this finding, a secondary analysis of the TOPPS study revealed that a greater number of days with a platelet count <10 × 109/l is associated with haemorrhage (Stanworth et al., 2015b). However, although a disturbed vascular lining is apparently an important risk for bleeding, surprisingly, laboratory tests do not take vascular integrity into account for transfusion management. In disorders with endothelial disruption, such as diabetes mellitus type 2, an important biomarker for endothelial damage is microalbuminuria, which is the loss of small amounts of albumin in the urine (Stehouwer & Smulders, 2006; Martens et al., 2018). Urine albumin excretion can be measured in a first morning urine specimen and is either expressed as albumin concentration (mg/l); daily albumin excretion in mg/24 h or as the albumin/creatinine ratio (de Jong & Curhan, 2006). Whether urine albuminuria reflecting endothelial damage enhances bleeding in the setting of a platelet deficit in haematological malignancies has not been studied before.
Fever is another risk factor for enhanced bleeding tendency, as can be concluded from several clinical studies (Webert et al., 2006; Stanworth et al., 2015b). Fever, when resulting from inflammation and/or infection often leads to increased C‐reactive protein (CRP), which is a predictor of bleeding severity in non‐thrombocytopenic populations (Koller et al., 2015; Tomizawa et al., 2016).
The aim of this study was (1) to investigate whether increased CRP levels and microalbuminuria, associated with inflammation and vascular damage respectively in individuals with normal platelet counts, also occur in thrombocytopenic patients and (2) to test the hypothesis that these parameters show an association with bleeding. The primary objective was to evaluate whether these biomarkers show an association with bleeding on the day of the measurement and the two days thereafter. The secondary objective was to investigate associations between the biomarkers and corrected count increments after platelet transfusion.
Methods and materials
The study was designed as a prospective observational substudy in patients that were included in the PREPAReS trial. This trial is registered at The Netherlands National Trial Registry as #NTR2106 and at ://www.clinicaltrials.gov as #NCT02783313. PREPAReS was a randomized controlled trial comparing pathogen‐inactivated platelet concentrates using riboflavin and ultraviolet (UV)‐B illumination technology (Mirasol, Terumo BCT, Lakewood, CO, USA) and untreated, plasma‐stored platelet concentrates in adult patients ≥18 years old with haematological malignancies requiring at least two platelet transfusions, and who had signed informed consent (van der Meer et al., 2018). The exclusion criteria were as follows: active bleeding at inclusion, immunological refractoriness to platelet transfusions, indications to use hyperconcentrated platelets, idiopathic thrombocytopenic purpura, pregnancy, microangiopathic thrombocytopenia, and known allergy to riboflavin or its photoactive products (Ypma et al., 2016). The current study, for which ethics approval was obtained, included patients in four Dutch participating centres between January 2014 and June 2016, irrespective of their treatment arm in the PREPAReS trial. The principles of the Declaration of Helsinki were adhered to. Included patients gave written informed consent for laboratory assessments collected for the MARBLE study (Microalbuminuria and bleeding complications in patients with hypoproliferative thrombocytopenia). The study observation period was from signing informed consent (usually one to several days before the first platelet transfusion) until recovery from thrombocytopenia, hospital discharge, or death. Patients could be included in the study more than once in case of subsequent intensive chemotherapeutical treatments, and inclusions will be referred to as “on‐protocol episodes”.
Analysis of urine and blood samples
All patients underwent measurement of urine and serum biomarkers twice a week. In total, 438 measurements of urine biomarkers and 462 measurements of CRP were carried out. This encompassed the entire observation period, including the period before the first transfusion. A variable number of daily measurements was carried out per inclusion depending on the duration of the study observation period. The urinary measurement was performed in an overnight first‐voided urine sample. Urine albumin excretion is expressed as urine albumin concentration (mg/l) and as albumin/creatinine ratio (mg/mmol). Microalbuminuria is defined as a urine albumin/creatinine ratio of 2·5–25 mg/mmol for men and 3·5–35 mg/mmol for women (de Jong & Curhan, 2006). Applying the ratio results in a somewhat more reliable rendition of albuminuric subjects as compared to that of the absolute urine albumin concentration (Bakker, 1999).
C‐reactive protein was measured on the same days as the urine albumin concentration and is expressed in mg/l. A CRP of <3 mg/l is considered to be normal, 3–10 mg/l as high‐normal, and >10 mg/l as increased (Pepys & Hirschfield, 2003; Markanday, 2015). CRP and urinary albumin were measured using a turbidimetric immunoassay at 340 nm (Cobas 6000, Roche Diagnostics, IN, USA). Urinary creatinine was measured by the enzymatic Jaffé method (Cobas 6000, Roche Diagnostics, IN, USA). A morning platelet count was performed 5–7 times a week according to standard procedures on a Sysmex XN1000 (Sysmex, Kobe, Japan).
Measurement of bleeding and body temperature
All patients underwent daily bleeding assessment (in total 1981 bleeding assessments) by independent research personnel. Bleeding symptoms were adjudicated afterwards by three adjudicators who were blinded to the patient’s randomization, and were categorized in grade 0–4 bleeding according to the WHO bleeding scale (Slichter et al., 2010; Ypma et al., 2016). For all patients, the morning temperature was measured daily (tympanic thermometry, Genius 2, Cardinal Health Netherlands, Amsterdam, the Netherlands). A temperature of ≥38·0°C is considered an increased body temperature.
Transfusion increments
Post‐transfusion corrected count increments were determined 1 h after platelet transfusion (CCI1) and after circa 24 h (18–30 h after transfusion) (CCI24), corrected for number of platelets transfused and body surface area (van der Meer et al., 2018).
Multiple transfusions could be recorded on one day. If both a pre‐ and a postcount were available for each transfusion, they entered the analysis as separate transfusions. If no separate precount for the second transfusion was available or no postcount for the first transfusion, then one overall CCI was calculated for both transfusions on that day, using the sum of the doses given as the denominator. CCIs at one hour generally have all pre‐ and postcounts, while for the CCI at 24 h the approach of averaging multiple transfusions was sometimes used.
Statistical analysis
Statistical analyses were performed with IBM SPSS Statistics for Windows, version 23 (IBM Corp., Armonk, NY, USA). All analyses were predetermined and were captured in a statistical analysis plan. The laboratory parameters (morning platelet count, urine albumin/creatinine ratio and CRP) are expressed as medians and interquartile range (IQR) or range. To control for the impact of very high laboratory values for urine albumin/creatinine ratio and serum CRP, the laboratory parameters were log2‐transformed for further analysis. The associations between laboratory parameters and bleeding were tested using generalized linear mixed models with logit link function taking into account dependent (within the same patients) repeated measurements. In particular, two random intercepts were included in the models, one accounting for multiple days (or multiple periods of two or three days) within the same on‐protocol episodes, and the other accounting for multiple on‐protocol episodes within the same patient. First, univariable unadjusted odds ratios for bleeding, together with 95% confidence intervals (CIs), were estimated and tested. Next, the association between urine albumin/creatinine ratio and CRP adjusted for platelet count was estimated in two multivariable models. The urine albumin/creatinine ratio and CRP were considered to be potentially predictive for bleeding if a statistically significant (α = 0·05) adjusted odds ratio would be found for bleeding occurring the day of the measurement or 24–48 h after the measurement using the multivariable analysis correcting for platelet count. Of secondary interest was the association with the measurement and bleeding outcome 48–72 h later. This was separately performed for bleeding grade ≥1 and bleeding grade 2. A sensitivity analysis was performed taking into account two additional adjustment factors: fever and cardiovascular risk profile (one or more of the following comorbidities: hypertension, diabetes mellitus, TIA/CVA, peripheral artery disease, ischaemic congestive heart failure, myocardial infarction).
Associations between the urine albumin/creatinine ratio and CCIs 1 h and 24 h after transfusions administered on the first and the second day after the urine analysis were tested using a general linear mixed model taking into account non‐independent (within same patients) repeated measurements. The same associations were tested for CRP and corrected count increments. Both the univariate unadjusted regression coefficients and the coefficients after adjustment for platelet count and randomisation arm in two multivariable models were estimated. Urine albumin/creatinine ratio and CRP were considered to be potentially associated with CCI if there was a statistically significant regression coefficient in the multivariable models.
Receiver operating characteristics curves were plotted to compare sensitivity and specificity at different thresholds of urine albumin/creatinine ratio and CRP with respect to bleeding grade 2 the day after the measurement.
Results
The 116 on‐protocol episodes analysed counted 88 patients of whom 28 were included twice. The patient characteristics, the number of laboratory measurements and the number of days with bleeding observations in total and per thrombocytopenic episode are described in Table 1. Platelet count rapidly decreased by the day; at inclusion, 18% of patients already had a low (<20 × 109/l) platelet count (Fig 1). The distribution of the laboratory measurements over time is shown in Fig 2 and Table SI. Both urine albumin excretion and CRP built up to their maximum value around the 15th day after chemotherapy (Fig 2, Table SI).
Table 1.
Patient characteristics. Patients could be included more than once and an inclusion is referred to as “on‐protocol episode”. A variable number of observation days per inclusion are incorporated. The cardiovascular risk profile is defined as one or more of the following comorbidities: hypertension, diabetes mellitus, TIA/CVA, peripheral artery disease, ischemic congestive heart failure, or myocardial infarction.
| n = 116 on‐protocol episodes | |
|---|---|
| Age [years; mean (±SD)] | 54 (±12) |
| Gender (male/female; n/n) | 75/41 |
| Diagnosis n (%) | |
| MDS/AML (myelodysplastic syndrome/acute myeloid leukemia) | 43 (37%) |
| MM (multiple myeloma) | 33 (28%) |
| NHL (non hodgkin lymphoma) | 27 (23%) |
| ALL (acute lymphoblastic leukemia) | 6 (6%) |
| Other (Hodgkin’s lymphoma, mantle cell lymphoma, chronic leukaemia) | 7 (6%) |
| Treatment n (%) | |
| Autologous transplantation | 66 (57%) |
| Remission induction chemotherapy | 32 (28%) |
| Consolidation chemotherapy | 12 (10%) |
| Allogeneic transplantation | 6 (5%) |
| Randomization arm in PREPAReS trial n (%) | |
| Control (plasma stored platelet concentrates) | 57 (49%) |
| Intervention (pathogen‐reduced platelet concentrates) | 59 (51%) |
| Number of observation days per on‐protocol episode median (IQR) with | |
| Daily bleeding assessment | 16 (12–20) |
| Measurement morning platelet count | 13 (10–17) |
| Measurement urine albumin/creatinine ratio | 4 (2–5) |
| Measurement of serum C‐reactive protein | 3 (2–5) |
| Cardiovascular risk profile present* n (%) | 30 (28·6%) |
| Fever present n (%) of days | 336 (29·5%) |
ALL, acute lymphoblastic leukemia; IQR, interquartile range; MDS/AML, myelodysplastic syndrome/acute myeloid leukemia; MM, multiple myeloma; NHL, non hodgkin lymphoma.
Measurement available for 105/116 on‐protocol episodes.
Figure 1.
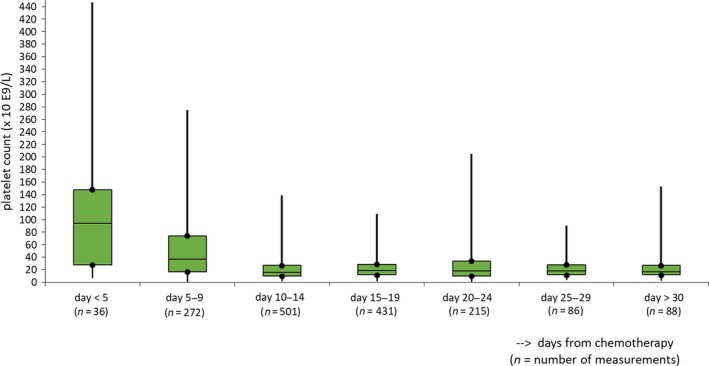
Morning platelet count: boxplots of median and interquartile range, maximum and minimum counts on days from chemotherapy.
Figure 2.
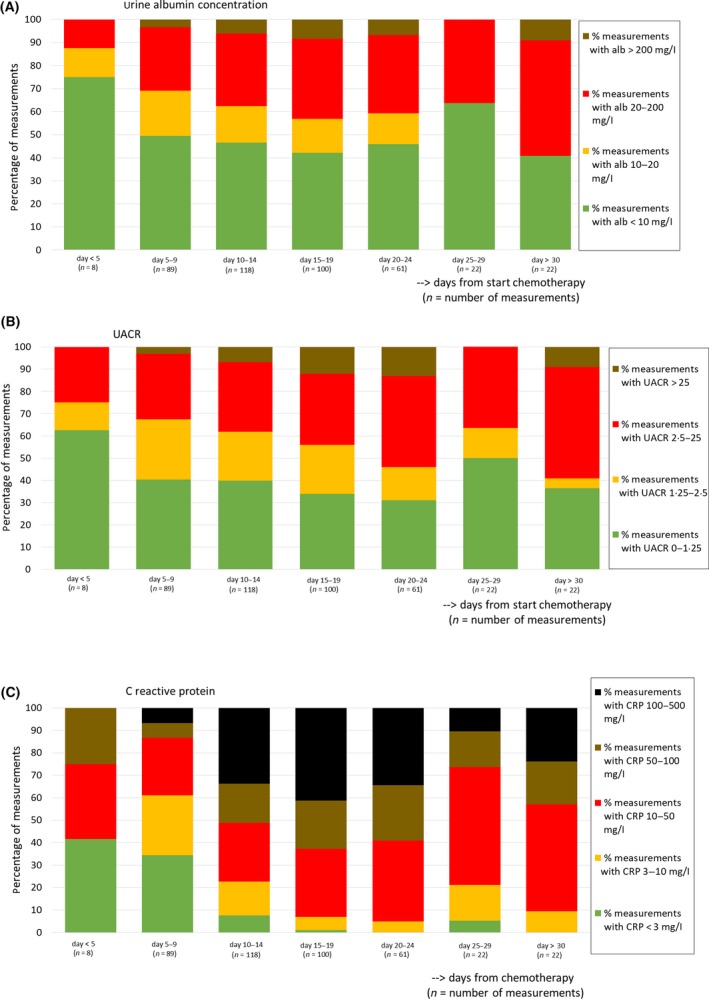
The distribution of laboratory measurements over time: urine albumin concentration (A); urine albumin/creatinine ratio (UACR) (B); C‐reactive protein (C). Only measurements of on‐protocol episodes for which the date of first chemotherapy was known are shown.
The patients received a total of 543 platelet transfusions. Most of the platelet transfusions (86%) were administered prophylactically at a trigger of 10 × 109/l, and 14% of all platelets were either transfused therapeutically or to prevent bleeding in case of an intervention at a trigger of 50 × 109/l.
There was no bleeding in 17% of all analysed on‐protocol episodes. In 40% of the episodes, the patient experienced a grade 1 bleeding and in 43% a grade 2 bleeding was observed. In none of the patients a grade 3 or grade 4 bleeding occurred. The percentage of bleeding days per total observed days was around 30% for bleeding grade ≥1 and about 9 % for grade 2 bleeding.
Laboratory parameters and bleeding on the day of the measurement and 1 and 2 days later
Platelet count. The univariable analyses for the association between bleeding of grade ≥1 and platelet count showed that a twofold lower platelet count was associated with a 32% increased odds for bleeding on the day of the measurement and a 29% increased odds for bleeding the day thereafter (Table IIA, 2). The platelet count was not significantly associated with bleeding two days later; the odds ratio for bleeding grade ≥1 was 0·92 (95% CI 0·81–1·04, P‐value 0·19). For bleeding grade 2, doubling of the platelet count was not significantly associated with the probability of bleeding (Table IIA, 2).
Table 2.
Associations between platelet count (PC), urine albumin/creatinine ratio (UACR) and C‐reactive protein (CRP) and WHO grade ≥1 and grade 2 bleeding on the same day the blood and urine samples were taken (A) and 24 h after sampling (B). The odds ratio for bleeding by platelet count, urine albumin/creatinine ratio and CRP is for every doubling of the respective measurement. For platelet count an odds ratio <1 means that the probability a bleeding occurs is higher with decreasing platelet count. For urine albumin/creatinine ratio and CRP an odds ratio >1 means the probability of a bleeding occurring is higher with increasing values for urine albumin/creatinine ratio and CRP.
| (A) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO grade ≥1 bleeding on the day of the measurement | WHO grade 2 bleeding on the day of the measurement | |||||||||||
| Univariate analysis | Analysis adjusted for PC | Univariate analysis | Analysis adjusted for PC | |||||||||
| Odds ratio per doubling of variable | 95% CI | P‐value | Odds ratio per doubling of variable | 95% CI | P‐value | Odds ratio per doubling of variable | 95% CI | P‐value | Odds ratio per doubling of variable | 95% CI | P‐value | |
| PC | 0·68 (n = 1708 total days of observation, n = 507 bleeding days) | (0·60–0·77) | <0·001 | 0·94* (n = 1708 total days of observation, n = 147 bleeding days) | (0·78–1·13) | 0·527 | ||||||
| UACR | 1·13* (n = 438 total days of observation, n = 135 bleeding days) | (1·01–1·26) | 0·027 | 1·12* (n = 429, n = 133 bleeding days) | (1·01–1·26) | 0·037 | 1·14 (n = 438 total days of observation, n = 42 bleeding days) | (0·95–1·36) | 0·157 | 1·14 (n = 429, n = 41 bleeding days) | (0·95–1·36) | 0·150 |
| CRP | 1·21 (n = 462 total days of observation, n = 137 bleeding days) | (1·07–1·37) | 0·002 | 1·18 (n = 447, n = 135 bleeding days) | (1·04–1·35) | 0·012 | 1·25 (n = 462 total days of observation, n = 50 bleeding days) | (1·03–1·52) | 0·027 | 1·31 (n = 447, n = 48 bleeding days) | (1·06–1·62) | 0·012 |
| (B) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WHO grade ≥1 bleeding 24 h after the measurement | WHO grade 2 bleeding 24 h after the measurement | |||||||||||
| Univariate analysis | Analysis adjusted for PC | Univariate analysis | Analysis adjusted for PC † | |||||||||
| Odds ratio per doubling of variable | 95% CI | P‐value | Odds ratio per doubling of variable | 95% CI | P‐value | Odds ratio per doubling of variable | 95% CI | P‐value | Odds ratio per doubling of variable | 95% CI | P‐value | |
| PC | 0·71 (n = 1598 total days of observation, n = 474 bleeding days) | (0·63–0·91) | <0·001 | 0·87 (n = 1598 total days of observation, n = 142 bleeding days) | (0·72–1·06) | 0·161 | ||||||
| UACR | 1·15* (n = 409 total days of observation, n = 125 bleeding days) | (1·04–1·29) | 0·009 | 1·16* (n = 402, n = 122 bleeding days) | (1·04–1·29) | 0·010 | 1·23* (409 total days of observation, n = 43 bleeding days) | (1·05–1·44) | 0·012 | 1·24* (n = 402, n = 41 bleeding days) | (1·05–1·46) | 0·010 |
| CRP | 1·12 (n = 435 total days of observation, n = 129 bleeding days) | (0·99–1·27) | 0·066 | 1·13 (n = 422, n = 124 bleeding days) | (1·04–1·60) | 0·076 | 1·20* (n = 435 total days of observation, n = 49 bleeding days) | (0·99–1·45) | 0·058 | 1·29* (n = 422, n = 45 bleeding days) | (1·04–1·60) | 0·020 |
Model run with only one random intercept due to low number of patients contributing more than one on‐protocol episode.
After correcting for PC, fever and vascular risk the odds ratio for UACR is 1·27, (n = 362, n = 39 bleeding days), 95% CI 1·06–1·52, P‐value 0·009 and the odds ratio for CRP is 1·39, (n = 372, n = 40 bleeding days), 95% CI 1·08–1·78, P‐value 0·012.
Urine albumin/creatinine ratio and CRP. Doubling of the urine albumin/creatinine ratio was associated with a 13% higher odds of bleeding grade ≥1 [odds ratio (OR) 1·13, 95% CI 1·01–1·26, P‐value 0·027]. The association remained statistically significant when adjusted for platelet count (OR 1·12, 95% CI 1·01–1·26, P = 0·037). The analysis for the association between bleeding grade ≥1 one day after measurement of the urine albumin/creatinine ratio showed an increase in the odds for bleeding of 15% (OR 1·15, 95% CI 1·04–1·29, P‐value 0·009), adjustment for platelet count hardly changed these results (OR 1·16, 95% CI 1·04–1·29, P = 0·010, Table IIA). Also, for bleeding grade 2, doubling of the urine albumin/creatinine ratio was associated with a higher probability for bleeding the day after the measurement (adjusted OR 1·24, 95% CI 1·05–1·46 and P = 0·010, Table IIB), but there was no association with bleeding two days later.
A higher value for CRP was significantly associated with a higher probability of bleeding measured on the days of the laboratory measurement, (both univariate and adjusted for platelet count) for grade ≥1 bleeding and grade 2 bleeding (18% and 31% increase respectively). A significant 29% higher probability for grade 2 bleeding remained one day after the CRP measurement, after adjusting for platelet count (Table IIB).
We performed sensitivity analyses and further corrected for fever and vascular risk besides the former adjustment for platelet count, since these factors could influence the results for CRP and urine albumin/creatinine ratio. The most important finding was a persistent association between grade 2 bleeding and doubling of urine albumin/creatinine ratio and CRP the day before the bleeding [OR for urine albumin/creatinine ratio (UACR) 1·27, 95% CI 1·06–1·52, P‐value 0·009 and OR for CRP 1·39, 95% CI 1·08–1·78, P‐value 0·012] (Table IIB).
With respect to the receiver operating characteristics curves that were plotted, the values for the AUC (area under the curve) of urine albumin/creatinine ratio were higher than for CRP (UACR AUC 0·665 and CRP AUC 0·642). The curves, however, did not show a specific threshold for both measurements.
Post‐platelet transfusion Corrected Count Increments
Table 3 shows the associations between corrected count increments and CRP and urine analysis. Corrected count increments at 1 h and 24 h after platelet transfusions administered on the day of the measurement and one day later were analysed. The associations after adjustment for randomization arm and platelet count are shown. The ΔCCI is a negative value, which means that the CCI decreases with increasing urine albumin/creatinine ratio or CRP. For the urine albumin/creatinine ratio, a ΔCCI24 of −0·52, 95% CI −0·93 to −0·11, P‐value 0·013 was observed, and for CRP the ΔCCI24 was −1·0, 95% CI −1·67 to −0·32, P‐value 0·004 respectively. Corrected count increments at 1 h were significantly associated with CRP measured on the day of or the day before the transfusion (ΔCCI −0·65, 95% CI −1·09 to −0·214, P‐value 0·004), whereas the urine albumin/creatinine ratio was not (Table 3). We found no association between CCIs for transfusion administered 48 h after the measurement of serum and urine biomarkers.
Table 3.
Associations between urinary albumin/creatinine ratio and C‐reactive protein (CRP) and corrected count increments (CCI1 h and CCI24 h) for platelet transfusions on the same day as the blood sampling and 24 h after the sampling, adjusted for platelet count and randomisation arm (pathogen‐reduced platelets versus plasma‐stored control platelets). The regression coefficient for urine albumin/creatinine ratio and CRP is for every doubling of the respective measurement. ΔCCI is a negative value, this means the count increment decreases with increasing urine albumin/creatinine ratio or CRP.
| CCIs for transfusion administered on the day of the measurement [of urine albumin/creatinine ratio (UACR) and CRP] |
CCIs for transfusion administered 24 h after the measurement (of UACR and CRP) |
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CCI 1 h | CCI 24 h | CCI 1 h | CCI 24 h | |||||||||||||
| Δ CCI | 95% CI | P‐value | Δ CCI | 95% CI | P‐value | Δ CCI | 95% CI | P‐value | Δ CCI | 95% CI | P‐value | |||||
| UACR | −0·12* (n = 98 transfusions, n = 68 episodes, n = 58 patients) | (−0·64 to 0·40) | 0·647 | −0·52 (n = 87 transfusions, n = 60 episodes, n = 50 patients) | (−0·93 to −0·11) | 0·013 | −0·32* (n = 99 transfusions, n = 64 episodes, n = 57 patients) | (−0·70 to 0·07) | 0·103 | −0·25 (n = 85 transfusions, n = 55 episodes, n = 50 patients) | (−0·69 to 0·19) | 0·262 | ||||
| CRP | −0·62 (n = 101 transfusions, n = 64 episodes, n = 56 patients) | (−1·26 to 0·02) | 0·056 | −1·00 (n = 86 transfusions, n = 58 episodes, n = 50 patients) | (−1·67 to −0·32) | 0·004 | −0·65* (n = 96 transfusions, n = 59 episodes, n = 54 patients) | (−1·09 to −0·214) | 0·004 | 0·057* (n = 85 transfusions, n = 51 episodes, n = 48 patients) | (−0·48 to 0·60) | 0·835 | ||||
Model run with only one random intercept due to low number of patients contributing more than one on‐protocol episode.
Discussion
This is the first study that prospectively collected and analysed (micro)albuminuria and CRP in patients with a chemotherapy‐induced platelet‐deficient state and related these biomarkers for endothelial dysfunction and inflammation to occurrence and severity of bleeding.
We studied 116 on‐protocol episodes comprising 1981 days of bleeding assessments with 737 days with a grade 1 (n = 401 days) or 2 bleeding (n = 168 days). A two times higher morning platelet count was associated with approximately 30% fewer grade 1 bleedings up to 24 h thereafter. However, grade 1 bleeding in the WHO grading scale consists of relatively insignificant mucosal and skin bleeds and poses no indication for platelet transfusion. In clinical practice, grade 2 bleedings represent the more clinically relevant bleedings elicited by minor trauma or mucosal ulceration. In agreement with previous large studies, the degree of thrombocytopenia had no effect on grade 2 bleedings, suggesting that concomitant factors play a role in the susceptibility for bleeding (Slichter et al., 2010).
We found an association between microalbuminuria and bleeding. Analyses with adjustment for the platelet count showed that the urine albumin/creatinine ratio is associated with grade 1 bleeding on the same day, and with grade 1 and 2 bleeding the day thereafter. For CRP, similar associations were found, which even remained present after correction for fever.
We assumed that albuminuria is a marker of generalised microvascular endothelial alterations. This assumption has been established indirectly in the literature concerning cardiovascular disease (Stehouwer & Smulders, 2006), and has only recently been proven more directly in the Maastricht Study, where the association between experimentally induced retinal arteriolar dilation and heat‐induced skin hyperaemia with 24‐h albuminuria in a large population was studied (Martens et al., 2018). In a pathological condition such as diabetes mellitus, microalbuminuria is defined in a spot urine sample or a first morning urine sample with a urine albumin concentration of 20–200 mg/l (equivalent to an albumin/creatinine ratio of 2·5–25 mg/mmol). An albumin concentration of 10–20 mg/l (urine albumin/creatinine ratio 1·25–2·5 mg/mmol) was considered to be “high normal”, while concentrations exceeding 200 mg/l (urine albumin/creatinine ratio >25 mg/mmol) were regarded as macroalbuminuria (de Jong & Curhan, 2006). In the current cohort of thrombocytopenic patients, up to 50% of all measurements showed micro‐ or macroalbuminuria between days 15 and 24 after chemotherapy. Endothelial damage in our patients is most likely multifactorial. A platelet‐deficient state may contribute to endothelial dysfunction because of a lack of repairing capacity of the endothelium. Endothelial thinning has been shown in an antibody‐induced thrombocytopenia model in rabbits; moreover earlier work in a limited patient group showed marked endothelial thinning and an increase in fenestrations in capillaries in skin and muscle biopsies of patients with thrombocytopenia (Kitchens & Weiss 1975; Kitchens & Pendergast, 1986). Also, an association with severe thrombocytopenia and leakage of 51Cr‐labelled red cells in the stool was shown in otherwise stable thrombocytopenic patients (Slichter & Harker, 1978). A fixed amount of platelets was shown to be necessary to preserve endothelial integrity. A requirement of 7·1 platelets/µl/day (18% of the normal rate of platelet turnover) was estimated from a model after measuring platelet life span and platelet clearance at different platelet counts (Hanson & Slichter, 1985). The presence of bleeding despite maintenance of a platelet count of >10 × 109/l is indicative of concomitant other factors. The global endothelial dysfunction in this population may reflect damage by chemotherapy (de Vos et al., 2004; Kvestad et al., 2014). Similarly, inflammation and infection can cause haemorrhage to become manifest in thrombocytopenia (Goerge et al., 2008; Ho‐Tin‐Noé et al., 2018). As a biomarker for local or generalised inflammation or infection, we found CRP to be associated with bleeding in thrombocytopenic patients, independent of the presence of fever. This is, to our knowledge, the first study to show this association in patients with thrombocytopenia. C‐reactive protein is an acute phase protein, produced by the liver in response to cytokines that are released from white blood cells. In several non‐thrombocytopenic patient groups, this was found to represent a factor contributing to the bleeding risk (Koller et al., 2015; Tomizawa et al., 2016). Both for CRP and urine albumin, a late peak is seen in a small group of patients with prolonged thrombocytopenia beyond 30 days. We suspect these to be patients who live through a very complicated phase with serious infection, but further confirmation is required.
Corrected count increments measured 24 h after the transfusion show an association with both urine albumin/creatinine ratio and CRP. In line with the former reasoning, in the condition of a deprived endothelium and a state of inflammation, the consumption of larger amounts of platelets could be a probable explanation; however, the effect of transfusion itself on the urine and serum biomarkers is not known. Associations between biomarkers of endothelial damage and repair and platelet transfusion requirements have been reported (McPherson et al., 2010), and these findings also suggested that vascular regeneration is associated with increased platelet consumption.
The strength of the current study is the carefully observed cohort with daily uniform measurement of minor and major bleeding complications as primary endpoint of the PREPAReS trial, as well as having a pre‐specified analysis plan. The laboratory tests, urine albumin and CRP, are rapidly and widely available and relatively inexpensive. A limitation of the study is the lack of more severe bleedings in the cohort. As has been mentioned by other authors, the WHO bleeding scale does not necessarily exclusively reflect increasing severity of bleedings from grade 1 to grade 4, but possibly designates bleeding types with differing pathogenesis (Heddle et al., 2010). Since WHO grade 3 and 4 bleeding was not observed in our cohort, we do not know whether the biomarkers tested also show an association with higher bleeding grades. Further, receiving chemotherapy may confound the study outcomes, and studies in non‐thrombocytopenic patients that receive chemotherapy may shed further light on this. The associations between biomarkers and bleeding that we found were moderately strong. The markers on their own will not be perfect predictors of bleeding. Also, the relative contribution of platelet transfusion to the associations between urine albumin/creatinine ratio, CRP and bleeding is unknown. Larger studies are needed to rule out transfusions as a confounder.
In conclusion, the occurrence and quantity of albuminuria and CRP in thrombocytopenic patients are associated with a bleeding phenotype as well as with increased platelet consumption as shown by lower CCIs after platelet transfusions. How these biomarkers can be incorporated into a model to predict which patient will develop a clinically significant bleeding requiring additional platelet transfusion support or alternative strategies such as antifibrinolytic agents, remains to be studied.
Author contributions
PY, JLK, AB and JE designed the study. PY, JLK, PB, JZ and EB contributed to the acquisition of data. PY, NG, JLK, AB and JE analysed and interpreted the data. PY, NG, JLK, JZ, AB, PM and JE contributed to the writing of the manuscript. All authors revised the manuscript and gave final approval.
Supporting information
Table SI. The distribution of laboratory measurements over time: urine albumin concentration (A); urine albumin/creatinine ratio (UACR) (B); C‐reactive protein (C). Only measurements of on‐protocol episodes for which the date of first chemotherapy was known are shown (n = number of observations).
Acknowledgements
Many research co‐workers in the Departments of Haematology of the HAGA Hospital, the Leiden University Medical Center, the Maastricht University Medical Center and the Erasmus Medical Center in Rotterdam worked on the various aspects of the study. The authors would in particular like to thank Rinie van Wordragen‐Vlaswinkel and Okke Eissen for data management and research coordination. The Research Bureau of the HAGA Hospital provided the opportunity for statistical support.
References
- Bakker, A.J. (1999) Detection of microalbuminuria. Receiver operating characteristic curve analysis favors albumin‐to‐creatinine ratio over albumin concentration. Diabetes Care, 22, 307–313. [DOI] [PubMed] [Google Scholar]
- CBO, Kwaliteitsinstituut voor de Gezondheidszorg, 2004CBO, Kwaliteitsinstituut voor de Gezondheidszorg . (2004) Richtlijn Bloedtransfusie. Available at: http://nvic.nl/sites/default/files/CBO%2520Richtlijn%2520Bloedtransfusie.pdf (Accessed April 4, 2019).
- Estcourt, L.J. , Stanworth, S.J. , Harrison, P. , Powter, G. , McClure, M. , Murphy, M.F. & Mumford, A.D. (2014) Prospective observational cohort study of the association between thromboelastometry, coagulation and platelet parameters and bleeding in patients with haematological malignancies‐ the ATHENA study. British Journal of Haematology, 166, 581–591. [DOI] [PubMed] [Google Scholar]
- Goerge, T. , Ho‐Tin‐Noe, B. , Carbo, C. , Benarafa, C. , Remold‐O'Donnell, E. , Zhao, B.Q. , Cifuni, S.M. & Wagner, D.D. (2008) Inflammation induces hemorrhage in thrombocytopenia. Blood, 111, 4958–4964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson, S.R. & Slichter, S.J. (1985) Platelet kinetics in patients with bone marrow hypoplasia: evidence for a fixed platelet requirement. Blood, 66, 1105–1109. [PubMed] [Google Scholar]
- Heddle, N.M. , Arnold, D.M. & Webert, K.E. (2010) Time to rethink clinically important outcomes in platelet transfusion trials. Transfusion, 51, 430–434. [DOI] [PubMed] [Google Scholar]
- Heddle, N.M. , Cook, R.J. , Tinmouth, A. , Kouroukis, C.T. , Hervig, T. , Klapper, E. , Brandwein, J.M. , Szczepiorkowski, Z.M. , AuBuchon, J.P. , Barty, R.L. & Lee, K.A. ; SToP Study Investigators of the BEST Collaborative . (2009) A randomized controlled trial comparing standard‐ and low‐dose strategies for transfusion of platelets (SToP) to patients with thrombocytopenia. Blood, 113, 1564–1573. [DOI] [PubMed] [Google Scholar]
- Ho‐Tin‐Noé, B. , Boulaftali, Y. & Camerer, E. (2018) Platelets and vascular integrity: how platelets prevent bleeding in inflammation. Blood, 131, 277–288. [DOI] [PubMed] [Google Scholar]
- de Jong, P.E. & Curhan, G.C. (2006) Screening, monitoring, and treatment of albuminuria: Public health perspectives. Journal of the American Society of Nephrology, 17, 2120–2126. [DOI] [PubMed] [Google Scholar]
- de Vos, F.Y. , Willemse, P.H. , de Vries, E.G. & Gietema, J.A. (2004) Endothelial cell effects of cytotoxics: balance between desired and unwanted effects. Cancer Treatment Reviews, 30, 495–513. [DOI] [PubMed] [Google Scholar]
- Kitchens, C.S. & Pendergast, J.F. (1986) Human Thrombocytopenia is associated with structural abnormalities of the endothelium that are ameliorated by glucocorticosteroid administration. Blood, 67, 203–206. [PubMed] [Google Scholar]
- Kitchens, C.S. & Weiss, L. (1975) Ultrastructural changes of endothelium associated with thrombocytopenia. Blood, 46, 567–578. [PubMed] [Google Scholar]
- Koller, L. , Rothgerber, D.J. , Sulzgruber, P. , El‐Hamid, F. , Förster, S. , Wojta, J. , Goliasch, G. , Maurer, G. & Niessner, A. (2015) History of previous bleeding and C‐reactive protein improve assessment of bleeding risk in elderly patients (≥ 80 years) with myocardial infarction. Thrombosis and Haemostasis, 114, 1085–1091. [DOI] [PubMed] [Google Scholar]
- Kvestad, H. , Evensen, L. , Lorens, J.B. , Bruserud, O. & Hatfield, K.J. (2014) In vitro characterization of valproic acid, ATRA, and cytarabine used for disease‐stabilization in human acute myeloid leukemia: antiproliferative effects of drugs on endothelial and osteoblastic cells and altered release of angioregulatory mediators by endothelial cells. Leukemia Research and Treatment, 2014, 143479 10.1155/2014/143479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markanday, A. (2015) Acute phase reactants in infections: evidence-based review and a guide for clinicians. Open Forum Infectious Diseases, 2, ofv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens, R.J.H. , Houben, A.J.H.M. , Kooman, J.P. , Berendschot, T.T.J.M. , Dagnelie, P.C. , van der Kallen, C.J.H. , Kroon, A.A. , Leunissen, K.M.L. , van der Sande, F.M. , Schaper, N.C. , Schouten, J.S.A.G. , Schram, M.T. , Sep, S.J.S. , Sörensen, B.M. , Henry, R.M.A. & Stehouwer, C.D.A. (2018) Microvascular endothelial dysfunction is associated with albuminuria: the Maastricht Study. Journal of Hypertension, 36, 1178–1187. [DOI] [PubMed] [Google Scholar]
- McPherson, M.E. , Owusu, B.Y. , August, K.J. , Qayed, M. , Lyles, R.H. , Chiang, K.Y. , Khoury, H.J. , Ofori‐Acquah, S.F. & Horan, J. (2010) Platelet transfusion requirements are associated with endothelial cell injury and angiogenesis during allogeneic hematopoietic stem cell transplantation. Blood (ASH Annual Meeting Abstracts), 116, 3487. [Google Scholar]
- van der Meer, P.F. , Ypma, P.F. , van Geloven, N. , van Hilten, J.A. , van Wordragen‐Vlaswinkel, R.J. , Eissen, O. , Zwaginga, J.J. , Trus, M. , Beckers, E.A.M. , Te Boekhorst, P. , Tinmouth, A. , Lin, Y. , Hsia, C. , Lee, D. , Norris, P.J. , Goodrich, R.P. , Brand, A. , Hervig, T. , Heddle, N.M. , van der Bom, J.G. & Kerkhoffs, J.H. (2018) Hemostatic efficacy of pathogen‐inactivated vs untreated platelets: a randomized controlled trial. Blood, 132, 223–231. [DOI] [PubMed] [Google Scholar]
- Nachman, R.L. & Rafii, S. (2008) Platelets, petechiae, and preservation of the vascular wall. New England Journal of Medicine, 359, 1261–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opheim, E.N. , Apelseth, T.O. , Stanworth, S.J. , Eide, G.E. & Hervig, T. (2017) Thromboelastography may predict risk of grade 2 bleeding in thrombocytopenic patients. Vox Sanguinis, 112, 578–585. [DOI] [PubMed] [Google Scholar]
- Pepys, M.B. & Hirschfield, G.M. (2003) C-reactive protein: a critical update. Journal of Clinical Investigation, 111, 1805–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slichter, S.J. & Harker, L.A. (1978) Thrombocytopenia: mechanisms and management of defects in platelet production. Clinics in Haematology, 7, 523–39. [PubMed] [Google Scholar]
- Slichter, S.J. , Kaufman, R.M. , Assmann, S.F. , McCullough, J. , Triulzi, D.J. , Strauss, R.G. , Gernsheimer, T.B. , Ness, P.M. , Brecher, M.E. , Josephson, C.D. , Konkle, B.A. , Woodson, R.D. , Ortel, T.L. , Hillyer, C.D. , Skerrett, D.L. , McCrae, K.R. , Sloan, S.R. , Uhl, L. , George, J.N. , Aquino, V.M. , Manno, C.S. , McFarland, J.G. , Hess, J.R. , Leissinger, C. & Granger, S. (2010) Dose of prophylactic platelet transfusions and prevention of hemorrhage. New England Journal of Medicine, 362, 600–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanworth, S.J. , Estcourt, L.J. , Powter, G. , Kahan, B.C. , Dyer, C. , Choo, L. , Bakrania, L. , Llewelyn, C. , Littlewood, T. , Soutar, R. , Norfolk, D. , Copplestone, A. , Smith, N. , Kerr, P. , Jones, G. , Raj, K. , Westerman, D.A. , Szer, J. , Jackson, N. , Bardy, P.G. , Plews, D. , Lyons, S. , Bielby, L. , Wood, E.M. , Murphy, M.F. ; TOPPS Investigators . (2013) A no‐prophylaxis platelet‐transfusion strategy for hematologic cancers. New England Journal of Medicine, 368, 1771–1780. [DOI] [PubMed] [Google Scholar]
- Stanworth, S.J. , Navarrete, C. , Estcourt, L. & Marsh, J. (2015a) Platelet refractoriness–practical approaches and ongoing dilemmas in patient management. British Journal of Haematology, 171, 297–305. [DOI] [PubMed] [Google Scholar]
- Stanworth, S.J. , Hudson, C.L. , Estcourt, L.J. , Johnson, R.J. , Wood, E.M. ; TOPPS Study Investigators . (2015b) Risk of bleeding and use of platelet transfusions in patients with hematologic malignancies: recurrent event analysis. Haematologica, 100, 740–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehouwer, C.D. & Smulders, Y.M. (2006) Microalbuminuria and risk for cardiovascular disease: analysis of potential mechanisms. Journal of the American Society of Nephrology, 17, 2106–2111. [DOI] [PubMed] [Google Scholar]
- Tomizawa, M. , Shinozaki, F. , Hasegawa, R. , Shirai, Y. , Motoyoshi, Y. , Sugiyama, T. , Yamamoto, S. & Ishige, N. (2016) Elevated C‐reactive protein level predicts lower gastrointestinal tract bleeding. Biomedical Reports, 4, 711–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhl, L. , Assmann, S.F. , Hamza, T.H. , Harrison, R.W. , Gernsheimer, T. & Slichter, S.J. (2017) Laboratory predictors of bleeding and the effect of platelet and RBC transfusions on bleeding outcomes in the PLADO trial. Blood, 130, 1247–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandt, H. , Schaefer‐Eckart, K. , Wendelin, K. , Pilz, B. , Wilhelm, M. , Thalheimer, M. , Mahlknecht, U. , Ho, A. , Schaich, M. , Kramer, M. , Kaufmann, M. , Leimer, L. , Schwerdtfeger, R. , Conradi, R. , Dölken, G. , Klenner, A. , Hänel, M. , Herbst, R. , Junghanss, C. & Ehninger, G. ; Study Alliance Leukemia . (2012) Therapeutic platelet transfusion versus routine prophylactic transfusion in patients with haematological malignancies: an open‐label, multicentre, randomised study. Lancet, 380, 1309–1316. [DOI] [PubMed] [Google Scholar]
- Webert, K. , Cook, R.J. , Sigouin, C.S. , Rebulla, P. & Heddle, N.M. (2006) The risk of bleeding in thrombocytopenic patients with acute myeloid leukemia. Haematologica, 91, 1530–1537. [PubMed] [Google Scholar]
- Ypma, P.F. , Kerkhoffs, J.L. , van Hilten, J.A. , Middelburg, R.A. , Coccoris, M. , Zwaginga, J.J. , Beckers, E.M. , Fijnheer, R. , van der Meer, P.F. & Brand, A. (2012) The observation of bleeding complications in haemato‐oncological patients: stringent watching, relevant reporting. Transfusion Medicine, 22, 426–431. [DOI] [PubMed] [Google Scholar]
- Ypma, P.F. , van der Meer, P.F. , Heddle, N.M. , van Hilten, J.A. , Stijnen, T. , Middelburg, R.A. , Hervig, T. , van der Bom, J.G. , Brand, A. & Kerkhoffs, J.L. ; PREPAReS Study Group . (2016) A study protocol for a randomised controlled trial evaluating clinical effects of platelet transfusion products: the Pathogen Reduction Evaluation and Predictive Analytical Rating Score (PREPAReS) trial. British Medical Journal Open, 6, e010156. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table SI. The distribution of laboratory measurements over time: urine albumin concentration (A); urine albumin/creatinine ratio (UACR) (B); C‐reactive protein (C). Only measurements of on‐protocol episodes for which the date of first chemotherapy was known are shown (n = number of observations).


