Abstract
Chronic and acute tendon injuries are frequent afflictions, for which treatment is often long and unsatisfactory. When facing extended injuries, matrices and scaffolds with sufficient biomechanical properties are required for surgical repair and could additionally serve as supports for cellular therapies to improve healing. In this study, protocols of either commonly used detergents only (SDS 1%, Triton 1%, TBP 1%, and Tween‐20 1%) or a combination of freeze/thaw (F/T) cycles with decellularization agents (NaCl 1M, ddH2O) were evaluated for the decellularization of horse equine superficial digital flexor tendon (SDFT) for hand flexor or extensor tendon reconstruction. Decellularization efficiency was assessed microscopically by histological staining (HE, DAPI) and DNA quantification. Macroscopical structure and biomechanical integrity of the tendon matrices were further assessed by gross observation, histological staining (SR), and mechanical testing (ultimate strain and stress, Young’s modulus, energy to failure) for select protocols. Decellularization with hypertonic NaCl 1M in association with F/T cycles produced the most robust tendon matrices, which were nontoxic after 10 days for subsequent recellularization with human fetal progenitor tendon cells (hFPTs). This standardized protocol uses a less aggressive decellularization agent than current practice, which allows subsequent reseeding with allogenic cells, therefore making them very suitable and bioengineered tendon matrices for human tendon reconstruction in the clinic.
Keywords: cell therapy, decellularization, extracellular matrix, hand flexor tendons, human fetal progenitor tenocytes, tendon healing
1. INTRODUCTION
Hand tendon injuries are a frequent challenge for the hand surgeon that often require surgical grafting for proper repair.1 While autograft remains the ideal choice, donor‐site morbidity and potential lack of material are limiting.2 Alternative matrices from biological or synthetic sources must then be considered for tendon grafts, providing that the resulting scaffolds are biocompatible, retain sufficient mechanical properties, and have potential for cellular reseeding.
Decellularized extracellular matrix (ECM) scaffolds prepared from tissues of human or animal sources are a potential option that have presented encouraging results for tendon allograft or xenograft.3 However, grafts directly obtained from allogenic tendon tissue have produced either lower biomechanical properties than native tendon or are associated with much higher costs than autografts.4, 5, 6, 7 Several studies of commercially available tendon grafts have reported their biological characteristics and clinical efficacy, but tendon matrices specifically designed for hand tendons are currently unavailable.6, 8, 9, 10 The use of animal tendon as a source material for this purpose could lead to an ideal matrix with better overall mechanical properties, and strategies have been tested to process tendon of various species, including rat,11 porcine,12, 13 canine, rabbit,14, 15 and equine.16, 17 The equine superficial digital flexor tendon (SDFT) presents a potential realistic source for human tendon grafting due to its large dimensions, availability in large quantities, and traceability through accredited food industries. Equine tissue is also known to be less immunogenic than other species, such as bovine.18 With an increase in security for disease transmission, this source could be valorized from an ecological point of view in the recycling of waste material for unmet biomedical needs.
All biological tendon sources must be efficiently decellularized prior to grafting with a treatment strong enough to eliminate cellular material while conserving the initial structure and mechanical properties of the tissue.19 Detergents, such as sodium dodecyl sulfate (SDS) and t‐octyl‐phenoxypolyethoxyethanol (Triton X‐100), tri‐n‐butyl phosphate (TBP), or Tween‐20, are often used to solubilize cell membranes.11, 20, 21 Enzymes, such as trypsin and collagenase, can also be used in processing, but may result in undesired ECM breakdown that would compromise tendon repair.22 Physical treatments can further aid in decellularization and the penetration of chemical agents into deeper tissue regions of the ECM. Indeed, freeze‐thaw (F/T) cycling coupled with detergents has been shown to increase the decellularization efficiency of large tendons.16 However, any chemical or enzymes used for decellularization must be thoroughly removed from the matrix, which can incur additional processing time compared to neutral solutions, such as NaCl or water, which have also been used for human skin and tissue processing.23
Correct matrix processing would ensure the conservation of proteins24 and growth factors,25 therefore promoting healing by cell adhesion and migration and the interaction with host tissue.24 During the last decade, biologics and cellular therapies have become of interest as potential alternative treatments for tendon injuries to aid in this healing process, and F/T treatment has been shown to be biocompatible with equine adipose‐derived stromal cells.16, 26 We have previously evaluated the characteristics of human fetal progenitor tenocytes (hFPTs) in vitro and have obtained promising results with this cell source.27 For chronic tendinopathies or simple lacerations, we have also investigated the use of hFTPs in an injectable, hyaluronic acid gel preparation to locally deliver these cells and potentially accelerate the healing process in small tendon defects.28
The aim of this study was to select an efficient decellularization method for equine tendon that retained the mechanical properties and functionality of the tendon, as well as that would support biocompatibility with subsequent cellular therapies. We show that harsh detergents are not necessary for effective decellularization and, for the first time, demonstrate the biocompatibility of SDFT with human cellular therapy using hFPTs. We present an optimized, simple, and cost‐effective processing using F/T cycles and NaCl that can be implemented for clinical preparation of tendon grafts for tendon reconstruction.
2. MATERIALS AND METHODS
2.1. Equine tendon source, processing, and conservation
Horse superficial digital flexor tendons (SDFT) were obtained from Profil Export (Chavrieu Chavagneux, France) with traceability for each source animal. Tendons were harvested, cleaned, and trimmed to standard dimensions, then put into hermetic bags, frozen immediately, and transferred to our unit, where they were stored at −80°C until further use. The tendons were later thawed at room temperature (RT) in order to facilitate the removal of surrounding tissue and epitenon with a scalpel and subsequently frozen at −20°C to obtain sufficient rigidity for processing into pieces of 150 mm × 10 mm. Thin slices of 1.2 mm thickness were then prepared with a dermatome (Aesculap AG, Tuttlingen, Germany) and conserved at −80°C.
2.2. Decellularization of standardized tendon tissue samples
The standardized tendon tissues were further cut to obtain pieces of 20 mm × 10 mm × 1.2 mm for decellularization. Two types of decellularization protocols were tested, using either common detergents alone or F/T cycles with a decellularizing agent. The first group tested commonly used detergent treatments: SDS 1% (Ambion, ThermoFischer Scientific, Waltham, MA, USA), Triton 1% (Applichem, Darmstadt, Germany), TBP 1% (Sigma‐Aldrich, Darmstadt, Germany), or Tween‐20 1% (Applichem). Chemical decellularization was performed by immersion of tendons in solutions with constant stirring at 300 RPM for 48 hours. The tendon tissues were then washed twice for 15 minutes in ddH2O.
The second decellularization protocol consisted of combined F/T with neutral solutions of hypertonic NaCl 1M (Sigma‐Aldrich), hypotonic ddH2O, or Triton 1%. A modified version of F/T decellularization described by Roth et al29 was used, where samples were submitted to five cycles of 2‐minute freezing in liquid nitrogen and 10 minutes at 37°C in 1X phosphate‐buffered saline (PBS; Interlaken, Switzerland). Samples were then incubated 48 hours at RT in ddH2O followed by 48 hours of incubation in NaCl 1M or ddH2O. Afterward, samples were washed twice for 15 minutes in ddH2O and 24 hours in DMEM (Dulbecco’s modified Eagle medium, ThermoFischer Scientific) supplemented with 10% fetal bovine serum (Gibco Qualified FBS, ThermoFischer Scientific).
All samples were kept in 1X PBS supplemented with 1% penicillin‐streptomycin (Pen Strep, ThermoFischer Scientific) until further analysis.
2.3. DNA quantification of decellularized tendon matrix
For all samples and controls, 25‐35 mg of tissue was minced into 1‐mm3 pieces and dehydrated at 60°C overnight. The tissues were then digested with proteinase K and purified, according to manufacturer instructions (NucleoSpin Tissue kit, Macherey‐Nagel, Düren, Germany). DNA quantification was measured by spectrophotometry (Nanodrop 1000, ThermoFischer Scientific). Each quantification was repeated in triplicate with samples from different tendons (n = 3) and treatments were compared using a 2‐tailed two‐sample t‐test compared to control, nondecellularized tendons.
2.4. Histology of standardized decellularized tendon matrix
A 5 mm × 5 mm × 1.2 mm piece of tendon matrix from each treatment was fixed in 4% (w/v) neutralized formalin solution (J.T. Baker, Deventer, The Netherlands) for 24 hours at RT, washed thrice in PBS, and subsequently dehydrated and embedded in paraffin (Merck Millipore, Darmstadt, Germany). Sections of 5 µm in length were excised and sections corresponding to the middle depth were taken for staining. Each tendon matrix was stained with hematoxylin and eosin (HE), 4ʹ,6‐diamidino‐2‐phenylindole (DAPI), or Sirius Red (SR). Histology images were taken with a Leica DM 5500 microscope equipped with a CMOS Camera DMC 2900 Color for HE and SR sections and with a CCD Camera DFC 3000 B/W for DAPI fluorescent staining. SR sections images were taken under polarized light to determine collagen fiber orientation.
At this point, the protocol producing the best resulting matrix samples in terms of decellularization and biological properties was selected for further testing. Select detergent protocols representing the most widely used methods (SDS 1% and Triton 1%) were retained for comparison.
2.5. Biomechanical testing of native tendon and decellularized tendon matrices
Biomechanical tests were performed on tendon slices (150 mm × 10 mm × 1.2 mm; Figure 1A) treated with selected protocols, and measures were taken with an Electropuls Dynamic Test System (Instron E3000; Instron, Norwood, MA, USA; Figure 1B). We developed a system for stable fixation to avoid slippage before rupture in collaboration with the Institute of Mechanical Engineering at EPFL in order to create an optimal system compared to published methods.30, 31, 32, 33, 34 Tendons were attached with the aid of two clamps specifically designed with a zigzag pattern to increase fixation while preventing weakening of the structure around clamps for tendon testing (Figure 1D). No slippage was observed during the measurements. The length of tendons was standardized at 150 mm, which was suitable for a distance of approximately 80 mm between clamps and more than 30 mm of grip on each side for good fixation. A sufficient length between the clamps was used in order to diminish the impact of end effects known to occur in the analysis of soft tissues.35, 36 A precharge of 5N was applied to the tissue, and the new position was defined as the origin; the gap was considered as the initial length and the elongation and force were set to zero. A rate of 25 mm/min was used to gradually put the tendon under tension until rupture (Figure 1C). The force and elongation were recorded every 20 milliseconds and normalized to stress and strain. Strain (%) was determined by dividing the deformation at each measure point by the initial length. Stress (MPa) was determined by dividing the force with the initial cross section of the sample. An example of the typical stress‐strain curve is shown in Figure 2E. Stress and strain allowed normalization of the results and determination of Young’s modulus by calculating the slope of the stress‐strain regression curve in its linear region, between 2% and 4% of strain prior to failure. The energy to failure was calculated by integration with the rectangle method for each measure of force and elongation. For each parameter, an F test was done to compare variances and the Student’s t‐test was used to compare mean results with control tendons. Biomechanical properties of tendon samples originating from the same (n = 7) and different (n = 10) tendon tissues were pooled to represent control values.
Figure 1.

Tensile testing of tendon slides. A, Example of tendon slices of 150 mm × 10 mm × 1.2 mm used for tensile testing. B, Instron tensil machine used for the mechanical measurements. The tendon band is fixed between the two clamps, one of which is attached to a force captor (visible at the bottom). C, Example of tendon being stretched until rupture. D, Detailed pattern of the clamps designed for the evaluation of tendon within this study. E, Typical curve obtained with stress versus strain plot for tendon biomechanics. Toe region corresponds to the strain where collagen becomes aligned to create tension. In the linear region, collagen fibers are aligned and further strain provokes higher stress until rupture. Physiological strain is usually situated between 2% and 6%
Figure 2.
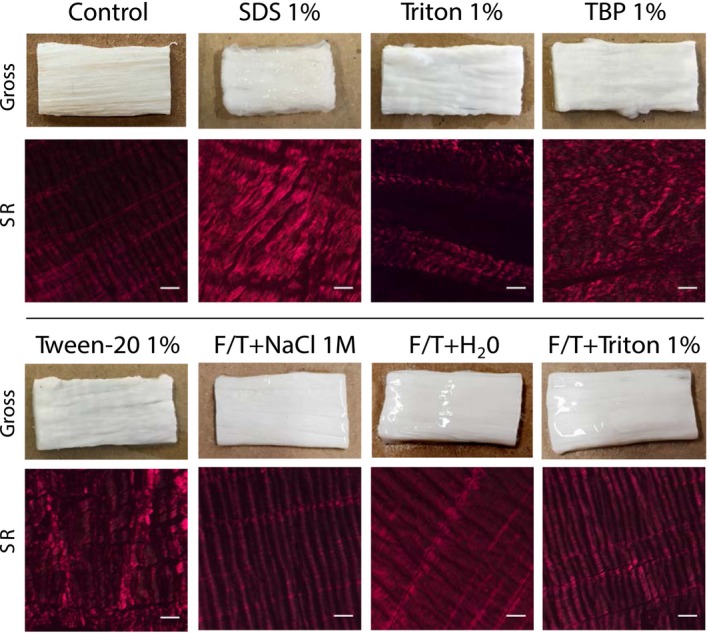
Macroscopic appearance and orientation of collagen fibers of decellularized matrices. Macroscopic aspects were observed after the different tendon tissue decellularization treatments, as indicated, for shape and coloration (Gross). SR staining shows the corresponding orientation of preserved collagen fibers. Scale bars: 100 μm. SR: Sirius Red
2.6. Tendon matrix recellularization
Reseeding of hFTPs and cytocompatibility were assessed on decellularized matrices, which included an additional sterilization step with ethanol 70% for 4 hours, subsequent rinsing with 1X PBS three times for 20 minutes, incubation at 37° with standard growth medium, and finally two rinses in PBS to allow for cell culture. The tendon matrices were further cut into pieces of 10 mm × 10 mm × 1.2 mm. Each sample was transferred to a 24‐well plate and seeded with hFPTs from an established cell bank (FE002‐Ten, passages 5‐6).27 Cell suspensions were prepared with DMEM supplemented to 5.97 mM L‐glutamine (L‐glut: Gibco, ThermoFischer Scientific) with 10% FBS. Suspensions of 50 µL were distributed on the decellularized matrices to seed 105 cells or 106 cells, and 50 µL of medium was used for controls. Five hundred microliters of media were added in each well around the tendon, avoiding the surface where cells were deposited, after 30 minutes. The media was changed after 24 hours, and then, every 2‐3 days. After 72 hours and 10 days, the samples were cut in half. Half of the samples were rinsed twice with PBS, fixed in formalin solution for 4 hours at 37°C, washed thrice with PBS, dehydrated, and embedded in paraffin with orientation to obtain sections of depth and stained with DAPI and HE for histological analysis. The other half of each sample was used for MTT staining (3‐(4,5‐dimethylthiazol‐2‐yl)‐2,5‐dephenyltetrazolium bromide) to detect metabolic activity of hFTP cells. The MTT assay was done accordingly to the manufacturer protocol (MTT, M6494, Thermo Fisher Scientific). In brief, after removing the culture medium the samples were covered with a 10% MTT solution in DMEM for 4 hours at 37°C in a humidified atmosphere containing 5% CO2. After 4 hours, the MTT solution was removed and samples were rinsed with 1X PBS. Images for the MTT assay were taken with an iPhone 7 plus (Apple). For Histology, images were taken with an inverted microscope equipped for fluorescence (IX81; Olympus, Tokyo, Japan) and a digital camera (iXon; Andor Technology Ltd., Belfast, United Kingdom). Each sample condition was tested thrice in duplicate.
3. RESULTS
3.1. Macroscopic structure
Macroscopic aspects of tendon matrices were observed after the different decellularization treatments (Figure 2). Treatment with SDS 1% resulted in a shrunken matrix that had a consistency similar to a hard gel with visible disorganization of collagen fibers within the tissue structure by SR staining. Tendon matrices treated with other detergent methods retained an approximately original shape, and no pink coloration due to eventual remaining vascularization was observed. Triton 1% appeared to preserve the structure of the tendon on SR, and, although some gaps appeared between fibers, the collagen remained well aligned and more intact than for other detergent treatments. For F/T treatments, the macroscopic appearance and collagen structure were well preserved and comparable to controls (Figure 2).
3.2. Decellularization
The effectiveness of decellularization protocols was assessed by the presence of nuclei (HE), nuclei remnants (DAPI), and DNA content (Figure 3). A large reduction in HE staining of cell nuclei was observed for both detergent only and F/T treatments compared to controls (Figure 3A). Nuclei remnants observed with HE staining for all detergent protocols were lower than for controls, but still detectable with DAPI. Triton 1% treatment of tendons was less effective on vessels, and some cellular material was clearly visible in these regions both with HE and DAPI. Regarding F/T protocols, HE stainings were devoid of visible nuclei, which was further supported by the absence of DAPI signal for F/T+NaCl 1M or F/T‐Triton and, to a lesser extent, for F/T+ddH2O.
Figure 3.
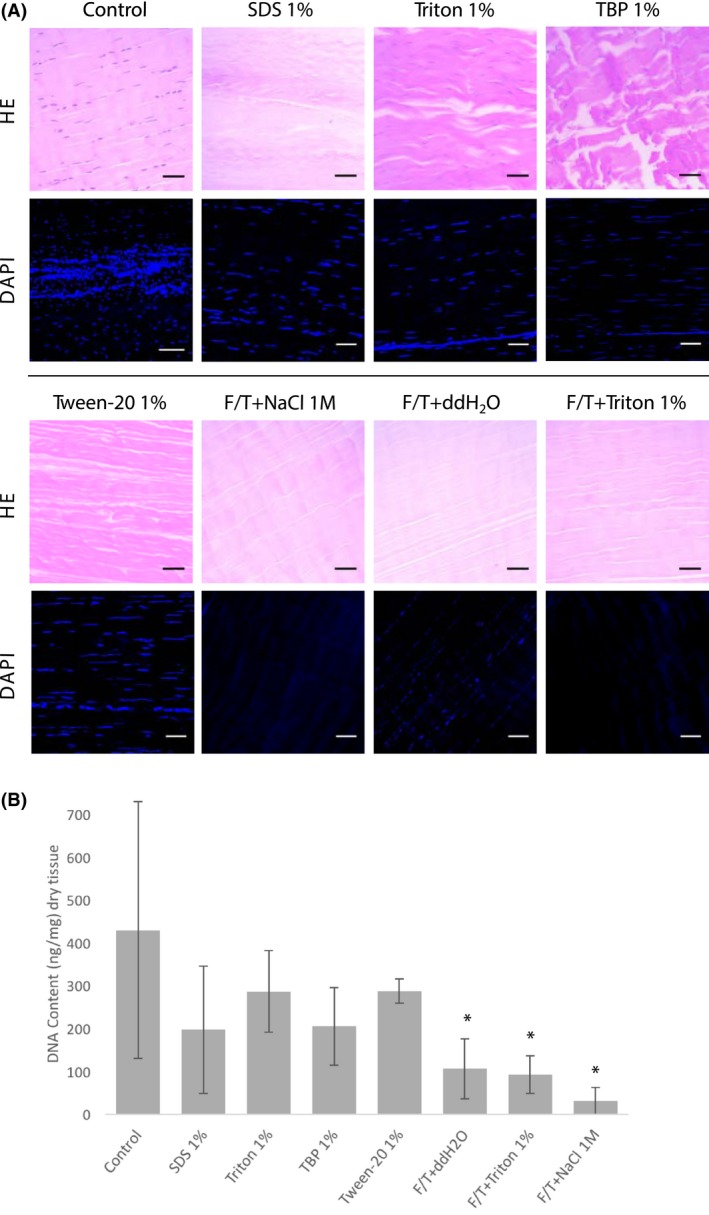
DNA content of tendons following decellularization. A, HE and DAPI staining of tendon sections after decellularization with indicated protocols. Nuclei are seen in purple with HE, and DAPI staining shows DNA content in blue. HE: hematoxylin and eosin, DAPI: 4ʹ,6‐diamidino‐2‐phenylindole. Scale bars: 100 μm. B, Amount of DNA recovered from tendon matrices with different decellularization protocols (ng/mg). *Significant differences from the mean of control experiments were determined by Student’s t‐tests (P < .005). Extractions were done in triplicate
The concentration of DNA extracted from the tissues treated with the different protocols are shown in Figure 3B. Control tendons contained an average DNA quantity of 430‐ng DNA/mg dry tissue with a high level of variation. Decellularization with detergents only was removed approximately 30%‐50% of DNA content, which was non‐significant. F/T treatments were superior to detergent treatments, removing 75%, 79%, and 93% of expected DNA content, for F/T plus ddH20, Triton 1%, and NaCl 1M, respectively.
At this point, F/T+NaCl was selected as the leading candidate protocol for further investigation due to its superior decellularization efficiency (least amount of DNA detected) and preservation of morphological tendon structure. The two most commonly used protocols were retained for comparative value (SDS 1% and Triton 1%).
3.3. Biomechanical assessment
Biomechanical results are presented in Table 1 and Figure 4 and show the ultimate strain (%), ultimate stress (MPa), Young’s modulus (MPa), and energy to failure (J) reported for native tendon controls and for each of the decellularization conditions. In terms of ultimate strain, the mean was significantly higher for SDS 1% (11%) and F/T+NaCl 1M (12%), while the Triton‐treated matrices were similar to controls. The mean ultimate stress for F/T+NaCl was the highest at 16.66 MPa, which was significantly higher than for controls (9.3 MPa) or detergent‐treated tendons. None of the decellularization treatments significantly affected the Young’s modulus compared to control tissues, which had an average of 122 MPa. Tendon matrices treated with SDS 1% obtained a slightly lower value of 119 MPa, whereas F/T+NaCl treatment reached an average value of 140 MPa. The total energy required to cause rupture of the native tissue and tendon matrices was again significantly higher for tendons decellularized with F/T+NaCl treatment. A mean of <1 J was required to break control tendons; higher values were found for F/T+NaCl (2 J).
Table 1.
Biomechanical tensil results
| Protocol | Strain (%) | Stress (MPa) | Young (Mpa) | E calc (J) |
|---|---|---|---|---|
| Control | 8.09% ± 1.54 | 7.95 ± 3.12 | 122.25 ± 50.78 | 0.86 ± 0.34 |
| SDS 1% | 11.11% ± 1.97a | 9.93 ± 3.18 | 118.79 ± 30.78 | 1.07 ± 0.35 |
| Triton 1% | 8.77% ± 2.72 | 8.77 ± 2.72 | 116.39 ± 45.83 | 0.94 ± 0.29 |
| F/T+NaCl 1M | 11.86% ± 5.69a | 16.66 ± 7.41a | 140.22 ± 51.90 | 1.83 ± 0.82a |
Value significantly different from controls.
Figure 4.
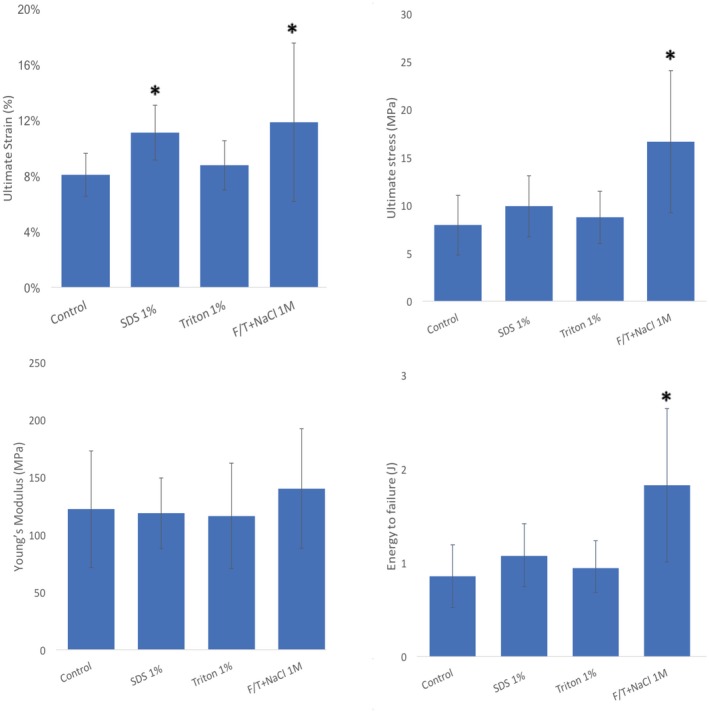
Biomechanical property testing with native control and decellularized tendon. Mean values for ultimate strain (%), ultimate stress (MPa), Young’s modulus (MPa), and energy to failure (J) of tissues after different decellularized protocols (Triton 1%, SDS 1%, F/T‐NaCl 1M). *Significant differences from controls were determined by Student’s t‐tests
3.4. Reseeding with hFTPs
Recellularization with hFPTs was visible with HE and MTT staining for Triton 1% and F/T+NaCl tendon matrices after 72 hours and 10 days (Figure 5A,B). No cells were visible for SDS‐treated or control matrices at either time point (Figure 5A,B). Reseeded cells remained rather superficial after 72 hours; but after 10 days, we have found that several cells infiltrate the matrices up to 300 microns for both conditions (Figure 5A). A higher inoculum of 106 cells produced a significantly more efficient recellularization of matrices than 105 cells after 72 hours, although after 10 days this difference was not observable, as indicated by the darker MTT staining, but had a lesser effect on Triton‐treated tendons (Figure 5B). Additionally, F/T+NaCl decellularized matrices appeared to have a higher MTT intensity than those treated with Triton, therefore suggesting that cells may more easily integrate the scaffold (Figure 5B).
Figure 5.
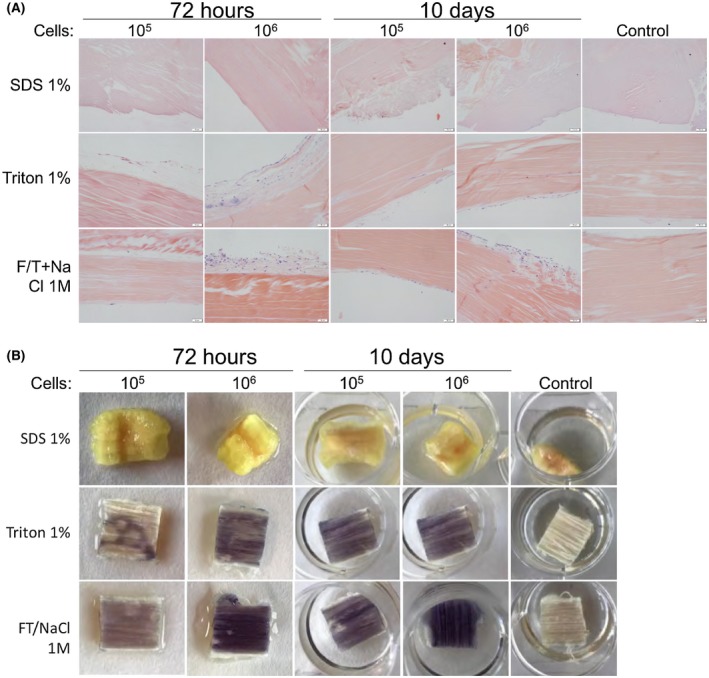
Decellularized tendon matrices after reseeding with 105 and 106 hFPTs. Tendon matrices previously decellularized with SDS 1%, Triton 1%, or F/T NaCl 1M were reseeded with hFPTs (0, 105 and 106 cells) and processed for (A) histology with HE stain and (B) MTT staining at 72 hours and 10 days. Control tendons where no hFPTs were added were imaged at 10 days
4. DISCUSSION
In this study, we identified a simplified, effective decellularization protocol for SDFT to be considered for hand tendon grafting with appropriate sizes of matrix. Processing should allow a proper decellularization of the tissue to diminish the risk of host immune reaction, as well as preserve biomechanical properties and support cell viability. F/T cycles combined with Triton 1% have previously been shown to be more effective than detergents alone for decellularization of SDFT, which was also supported by our results.16 This is the first report of using hypertonic NaCl 1M instead of detergent with F/T cycles to achieve a properly decellularized tendon matrix with good biomechanical properties and cytocompatibility. Other protocols tested in this study were included in order to compare results with commonly used methods in a standardized manner, as results are known to vary greatly between studies.
Decellularization was evaluated by the presence of cells (HE) or nuclei (DAPI) and the total amount of DNA remaining after treatment. While HE staining allowed the detection of cells and their distribution within the tissue, DAPI was more sensitive to detect remaining DNA. Detergents alone in this study were able to reduce detectable DNA by ~50%‐70%, which is slightly lower than previously reported for detergent decellularization.16, 37, 38 F/T were the only protocols where nuclei were not detectable, and the concentration of recovered DNA was dramatically reduced, particularly for F/T+NaCl‐treated matrices, to the point where almost no DNA was detected. F/T cycles combined with Triton have reported decreases of ~80%‐87% residual DNA,16 which is in line with the percentages reported here. The only protocol near the threshold of 20 ng/mg, which has been proposed as an acceptable definition of an efficient decellularization, was F/T+NaCl 1M at ~30 ng/mg representing a 93% removal of DNA content.20
Tendons generally have an off‐white, silver color with a dense and fibrous appearance. A conservative protocol should not impact macroscopic aspects, as alterations would possibly reduce the biomechanical properties of the tendon. The observed shrinkage and rigidity of tendons following SDS treatment indicated that this protocol is not well suited for the development a force‐resistant matrix, which was further confirmed by biomechanical testing. Other detergents allowed a better preservation of the macroscopic structure, both visibly and microscopically through SR staining of collagen fiber orientation. However, the best results were again obtained with F/T+NaCl treatment, where the tendon retained similar aspects to controls.
Biomechanical properties of tendon matrices are very important as these materials are destined to be grafted and will need to sustain and transmit forces in the hand while avoiding reruptures. We show here that decellularized tendon matrix obtained by F/T+NaCl 1M had comparable or higher values of strain, stress, modulus, and energy to rupture to native control tendons and was superior to other treatment protocols. The ultimate stress value is of high importance as to prevent premature tendon rupture, and F/T+NaCl matrices were able to bear higher tensile forces than control tendons. The higher strain value found with F/T+NaCl treatment would permit further deformation of the matrix before breakage. Modulus is also of high importance for hand tendon reconstruction, as a low modulus translates into low rigidity and higher elasticity. If a flexor or an extensor of the hand is grafted with a more elastic material than native tissue, it will be difficult to initiate fine, precise, and rapid movements with the affected finger, as muscle contraction force will be lost in tendon elastic elongation. The strong biomechanical properties observed for F/T+NaCl decellularized tendon matrices compared to native tendons indicates that they would be excellent candidates for further clinical development.
The F/T protocol produced resulting matrices with more robust features than with only detergent methods tested here, but also many commercial products available for tendon treatment. Indeed, Aurora et al4 reviewed mean Young’s modulus between 15.2 and 40.1 MPa for most tested products (Restore, CuffPatch, GraftJacket and TissueMend). Only AlloPatch presented a higher rigidity with a Young’s modulus of 304. Our scaffolds were also more rigid and resistant than those obtained by Youngstrom et al, which were also from equine SDFT. Their samples of 400 μm in depth presented a Young’s modulus of approximately 70‐76 MPa and maximum stress of 6 MPa for the treated and untreated samples, respectively.17 In comparison, our scaffolds were slightly more resistant at equally corrected cross sections.
The addition of hFPTs for tendon recellularization would be a potential way to improve tendon matrices for grafting, as hFTPs would be progressively replaced by host tissue after surgical placement, leading to an improved integration and better healing response.19 To date, studies of reseeding SDFT have used equine adipose‐derived mesenchymal stromal cells,16, 29, 39 which are a promising cellular therapy for veterinary medicine, but would be not suitable for human cellular therapy. Human fetal cells such as hFTPs are not only able to stimulate healing, but they are also highly proliferative and can be used to create cell banks for a consistent, safe clinical therapy.27 Additionally, they are already predefined cells, unlike stem cells, and do not de‐differentiate.
Surface reseeding with hFTPs was successful for both Triton 1% and F/T+NaCl treated matrices, visible at both short and prolonged time intervals. Cells were able to infiltrate both treated matrices after 10 days, but F/T NaCl matrices showed a stronger MTT staining indicating that presence of a higher quantity of cells after 10 days. Even if penetration of hFTPs was slightly deeper in regions where the ECM was more disorganized, we observed that cells were able to infiltrate the matrices up to 300 microns under both conditions. Injection within the mid‐substance of the scaffolds or the creation of small holes by needle puncture could also help for better distribution of cellular therapies within the matrix structure,40, 41 but the impact of such techniques on the mechanical properties remains to be verified. Alternatively, mechanical stimulation by stretching of reseeded tendons could further induce tenogenic differentiation and cellular alignment that may improve tendon healing.39 Overall, both HE and MTT staining indicated that the F/T+NaCl 1M protocol was biocompatible and allowed sterile cell culture, proving promising for future clinical development.
Decellularized tendon matrices would realistically need to be tailored for any size of injury. To replace hand flexor or extensor tendons, an adapted size would be a minimum of 3 mm in diameter and 15 cm in length.42 The decellularized tendon matrices tested here were smaller than what would be used in tendon reconstruction in order to favor reproducibility and be thin enough that the treatment could reach all the tissue in the same manner. However, vascular zones showed high cellularity, and it is possible that with the small quantities used here, high variability in DNA content occurred due to random selection of zones that were more vascularized than others. To optimize the protocols further, digestion should be done on larger pieces to avoid risks of small zone selection bias.
5. CONCLUSION
Overall, we were able to develop a simple and efficient protocol for the decellularization of equine SDFT using F/T+NaCl 1M. The use of NaCl has the advantage of easy leeching from tissues during the washing steps with less residual product remaining in the matrix that could be a cause for cellular toxicity, as potentiated with the use of strong detergents. The ability to automate F/T decellularization has recently been documented,29 which, combined with the use of biocompatible GMP‐grade human cellular therapies presented here, further supports the development of decellularized SDFT as a future clinical solution for human tendon surgery indications.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Concept/design: PAA, AG, NHB, WR, LAA
Data collection: PAA, AG, CP, JA
Data analysis/inter‐pretation: PAA, AG, SM, CP, JA, DDP, LAA
Drafting article: SM, PAA, AG, NHB, LAA
Critical revision of article: SM, DDP, WR, LAA
Funding secured by: WR, LAA
Approval of article: AG, PAA, CP, JA, DDP, WR, LAA
ACKNOWLEDGMENTS
This study was funded in part by the Lausanne Orthopedic Research Foundation (LORF) and the Foundation Leenaards. We thank the Sandoz Family Foundation and the S.A.N.T.E Foundation for their continued support in our Transplantation Program, Profil Export for Providing equine tendons, the Histology Core Facility at EPFL for the HE and SR staining, and the workshop of the Institute of Mechanical Engineering at EPFL for helping design and produce the traction clamps. This article is dedicated to Pierre‐Arnaud Aeberhard, M.D., who passed away on June 2, 2018 only several weeks before his mid‐thesis PhD examination, and, who without his motivation and work, this paper would not have been possible. His loss is felt in its entirety by members of the lab, who are proud of his early scientific achievements documented here.
Aeberhard P‐A, Grognuz A, Peneveyre C, et al. Efficient decellularization of equine tendon with preserved biomechanical properties and cytocompatibility for human tendon surgery indications. Artif Organs. 2020;44:E161–E171. 10.1111/aor.13581
Pierre‐Arnaud Aeberhard and Anthony Grognuz are considered as co‐first authors.
REFERENCES
- 1. de Jong JP, Nguyen JT, Sonnema AJ, Nguyen EC, Amadio PC, Moran SL. The incidence of acute traumatic tendon injuries in the hand and wrist: a 10‐year population‐based study. Clin Orthop Surg. 2014;6:196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Chang J. Studies in flexor tendon reconstruction: biomolecular modulation of tendon repair and tissue engineering. J Hand Surg Am. 2012;37:552–61. [DOI] [PubMed] [Google Scholar]
- 3. Lovati AB, Bottagisio M, Moretti M. Decellularized and engineered tendons as biological substitutes: a critical review. Stem Cells Int. 2016;2016:7276150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Aurora A, McCarron J, Iannotti JP, Derwin K. Commercially available extracellular matrix materials for rotator cuff repairs: state of the art and future trends. J Shoulder Elbow Surg. 2007;16:S171–8. [DOI] [PubMed] [Google Scholar]
- 5. Oro FB, Sikka RS, Wolters B, Graver R, Boyd JL, Nelson B, et al. Autograft versus allograft: an economic cost comparison of anterior cruciate ligament reconstruction. Arthroscopy. 2011;27:1219–25. [DOI] [PubMed] [Google Scholar]
- 6. Pridgen BC, Woon CYL, Kim M, Thorfinn J, Lindsey D, Pham H, et al. Flexor tendon tissue engineering: acellularization of human flexor tendons with preservation of biomechanical properties and biocompatibility. Tissue Eng Part C Meth. 2011;17:819–28. [DOI] [PubMed] [Google Scholar]
- 7. Raghavan SS, Woon CY, Kraus A, Megerle K, Choi MS, Pridgen BC, et al. Human flexor tendon tissue engineering: decellularization of human flexor tendons reduces immunogenicity in vivo. Tissue Eng Part A. 2012;18:796–805. [DOI] [PubMed] [Google Scholar]
- 8. Chong AK, Riboh J, Smith RL, Lindsey DP, Pham HM, Chang J. Flexor tendon tissue engineering: acellularized and reseeded tendon constructs. Plast Reconstr Surg 2009;123:1759–66. [DOI] [PubMed] [Google Scholar]
- 9. Longo UG, Lamberti A, Maffulli N, Denaro V. Tendon augmentation grafts: a systematic review. Br Med Bull. 2010;94:165–88. [DOI] [PubMed] [Google Scholar]
- 10. Valentin JE, Badylak JS, McCabe GP, Badylak SF. Extracellular matrix bioscaffolds for orthopaedic applications. A comparative histologic study. J Bone Joint Surg Am. 2006;88:2673–86. [DOI] [PubMed] [Google Scholar]
- 11. Cartmell JS, Dunn MG. Effect of chemical treatments on tendon cellularity and mechanical properties. J Biomed Mater Res. 2000;49:134–40. [DOI] [PubMed] [Google Scholar]
- 12. Deeken CR, White AK, Bachman SL, Ramshaw BJ, Cleveland DS, Loy TS, et al. Method of preparing a decellularized porcine tendon using tributyl phosphate. J Biomed Mater Res B Appl Biomater. 2011;96:199–206. [DOI] [PubMed] [Google Scholar]
- 13. Ingram JH, Korossis S, Howling G, Fisher J, Eileen I. The use of ultrasonication to aid recellularization of acellular natural tissue scaffolds for use in anterior cruciate ligament reconstruction. Tissue Eng. 2007;13:1561–72. [DOI] [PubMed] [Google Scholar]
- 14. Tischer T, Vogt S, Aryee S, Steinhauser E, Adamczyk C, Milz S, et al. Tissue engineering of the anterior cruciate ligament: a new method using acellularized tendon allografts and autologous fibroblasts. Arch Orthop Trauma Surg. 2007;127:735–41. [DOI] [PubMed] [Google Scholar]
- 15. Xing S, Liu C, Xu B, Chen J, Yin D, Zhang C. Effects of various decellularization methods on histological and biomechanical properties of rabbit tendons. Exp Ther Med. 2014;8:628–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Burk J, Erbe I, Berner D, Kacza J, Kasper C, Pfeiffer B, et al. Freeze‐thaw cycles enhance decellularization of large tendons. Tissue Eng Part C Meth. 2014;20:276–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Youngstrom DW, Barrett JG, Jose RR, Kaplan DL. Functional characterization of detergent‐decellularized equine tendon extracellular matrix for tissue engineering applications. PLoS ONE 2013;8:e64151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pastuszka R, Barlowska J, Litwinczuk Z. Allergenicity of milk of different animal species in relation to human milk. Postepy higieny i medycyny doswiadczalnej. 2016;70:1451–9. [DOI] [PubMed] [Google Scholar]
- 19. Schulze‐Tanzil G, Al‐Sadi O, Ertel W, Lohan A. Decellularized tendon extracellular matrix—a valuable approach for tendon reconstruction? Cells. 2012;1:1010–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Crapo PM, Gilbert TW, Badylak SF. An overview of tissue and whole organ decellularization processes. Biomaterials. 2011;32:3233–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gilbert TW, Sellaro TL, Badylak SF. Decellularization of tissues and organs. Biomaterials. 2006;27:3675–83. [DOI] [PubMed] [Google Scholar]
- 22. Grauss RW, Hazekamp MG, Oppenhuizen F, van Munsteren CJ, Gittenberger‐de Groot AC, DeRuiter MC. Histological evaluation of decellularised porcine aortic valves: matrix changes due to different decellularisation methods. Eur J Cardiothorac Surg. 2005;27:566–71. [DOI] [PubMed] [Google Scholar]
- 23. MacNeil S, Shepherd J, Smith L. Production of tissue‐engineered skin and oral mucosa for clinical and experimental use. Meth Mol Biol. 2011;695:129–53. [DOI] [PubMed] [Google Scholar]
- 24. Hodde J. Naturally occurring scaffolds for soft tissue repair and regeneration. Tissue Eng. 2002;8:295–308. [DOI] [PubMed] [Google Scholar]
- 25. Hoganson DM, O’Doherty EM, Owens GE, Harilal DO, Goldman SM, Bowley CM, et al. The retention of extracellular matrix proteins and angiogenic and mitogenic cytokines in a decellularized porcine dermis. Biomaterials. 2010;31:6730–7. [DOI] [PubMed] [Google Scholar]
- 26. Docheva D, Muller SA, Majewski M, Evans CH. Biologics for tendon repair. Adv Drug Deliv Rev. 2015;84:222–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Grognuz A, Scaletta C, Farron A, Raffoul W, Applegate LA. Human fetal progenitor tenocytes for regenerative medicine. Cell Transplant. 2016;25:463–79. [DOI] [PubMed] [Google Scholar]
- 28. Grognuz A, Scaletta C, Farron A, Pioletti DP, Raffoul W, Applegate LA. Stability enhancement using hyaluronic acid gels for delivery of human fetal progenitor tenocytes. Cell Med. 2016;8:87–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Roth SP, Erbe I, Burk J. Decellularization of large tendon specimens: combination of manually performed freeze‐thaw cycles and detergent treatment. Meth Mol Biol. 2018;1577:227–37. [DOI] [PubMed] [Google Scholar]
- 30. Giannini S, Buda R, Di Caprio F, Agati P, Bigi A, De Pasquale V, et al. Effects of freezing on the biomechanical and structural properties of human posterior tibial tendons. Int Orthop. 2008;32:145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Haraldsson BT, Aagaard P, Krogsgaard M, Alkjaer T, Kjaer M, Magnusson SP. Region‐specific mechanical properties of the human patella tendon. J Appl Physiol. 2005;98:1006–12. [DOI] [PubMed] [Google Scholar]
- 32. Kiss MO, Hagemeister N, Levasseur A, Fernandes J, Lussier B, Petit Y. A low‐cost thermoelectrically cooled tissue clamp for in vitro cyclic loading and load‐to‐failure testing of muscles and tendons. Med Eng Phys. 2009;31:1182–6. [DOI] [PubMed] [Google Scholar]
- 33. Schöttle P, Goudakos I, Rosenstiel N, Hoffmann J‐E, Taylor WR, Duda GN, et al. A comparison of techniques for fixation of the quadriceps muscle‐tendon complex for in vitro biomechanical testing of the knee joint in sheep. Med Eng Phys. 2009;31:69–75. [DOI] [PubMed] [Google Scholar]
- 34. Wren TA, Lindsey DP, Beaupre GS, Carter DR. Effects of creep and cyclic loading on the mechanical properties and failure of human Achilles tendons. Ann Biomed Eng. 2003;31:710–17. [DOI] [PubMed] [Google Scholar]
- 35. Horgan CO. End effects in mechanical testing of biomaterials. J Biomech. 2013;46:1040–1. [DOI] [PubMed] [Google Scholar]
- 36. Legerlotz K, Riley GP, Screen HR. Specimen dimensions influence the measurement of material properties in tendon fascicles. J Biomech. 2010;43:2274–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ning L‐J, Zhang YI, Chen X‐H, Luo J‐C, Li X‐Q, Yang Z‐M, et al. Preparation and characterization of decellularized tendon slices for tendon tissue engineering. J Biomed Mater Res, Part A. 2012;100:1448–56. [DOI] [PubMed] [Google Scholar]
- 38. Whitlock PW, Smith TL, Poehling GG, Shilt JS, Van Dyke M. A naturally derived, cytocompatible, and architecturally optimized scaffold for tendon and ligament regeneration. Biomaterials. 2007;28:4321–9. [DOI] [PubMed] [Google Scholar]
- 39. Burk J, Plenge A, Brehm W, Heller S, Pfeiffer B, Kasper C. Induction of tenogenic differentiation mediated by extracellular tendon matrix and short‐term cyclic stretching. Stem Cells Int. 2016;2016:7342379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Woon CYL, Farnebo S, Schmitt T, Kraus A, Megerle K, Pham H, et al. Human flexor tendon tissue engineering: revitalization of biostatic allograft scaffolds. Tissue Eng Part A. 2012;18:2406–17. [DOI] [PubMed] [Google Scholar]
- 41. Harvey FJ, Chu G, Harvey PM. Surgical availability of the plantaris tendon. J Hand Surg Am. 1983;8:243–7. [DOI] [PubMed] [Google Scholar]
- 42. Jakubietz MG, Jakubietz DF, Gruenert JG, Zahn R, Meffert RH, Jakubietz RG. Adequacy of palmaris longus and plantaris tendons for tendon grafting. J Hand Surg Am. 2011;36:695–8. [DOI] [PubMed] [Google Scholar]


