Abstract
Catalytic approaches to pharmaceutically important bioactive skeletons through gold carbene intermediates have experienced a dramatic development in the last decade. Although various carbene precursors continue to play an important role in heterocyclic syntheses, these reagents are associated with some drawbacks in terms of functional group tolerance, synthetic methods and safety limitations. A new generation of nitrene transfer reagents was established in 2019: the sulfilimines. These are safe, inexpensive and readily available. They can conveniently be stored and handled, and thus represent ideal reagents for the fast and modular modification of scaffolds and the preparation of libraries by intermolecular reactions of two components. Both the practical methods for synthesizing sulfilimines and the versatility of these ylidic species in gold‐catalyzed preparation of structural diversity, for both heterocycles and carbocycles, will be outlined in this Concept article.
Keywords: heterocycles, nitrene transfer, sulfilimines, sulfur ylides, α-imino gold carbenes
Sulfilimines, a new generation nitrene transfer reagent: The concise and scalable methods for preparing differently substituted sulfilimines are discussed. The gold‐catalyzed syntheses of diverse nitrogen‐containing compounds from these aza‐sulfur ylides through α‐imino gold carbene intermediates are summarized.
Introduction
Sulfur ylides,1 comprised of sulfonium ylides, sulfoxonium ylides, sulfilimines, sulfoximines, sulfoxides and sulfones (Figure 1), are highly reactive and possess important applications in organic synthesis. Sulfilimines,2 also named sulfimides or imino sulfuranes, have a long history. The sulfilimine bond naturally exists in a series of biomolecules, which has recently been confirmed.3 The electronic properties of the substituents on the nitrogen atom carrying a partial negative charge, are important for modulating the stability of the sulfilimines. Despite some useful applications in cycloadditions with other unsaturated systems,2b electrochemical oxidative cross‐coupling reactions4 and thioaminations of arynes,5 this versatile ylide is still in its infancy in modern transition metal catalysis.
Figure 1.
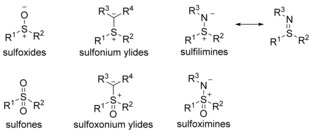
Sulfur ylides in six categories.
The eminence of gold catalysis in organic synthesis has been demonstrated in the last two decades by various gold‐initialed transformations for constructing complex molecular architectures.6 Functionalized gold carbenes have been frequently proposed as highly reactive intermediates in such reactions. Generally, gold carbene intermediates are generated by decomposition of diazo compounds,7 1,2‐acyloxy migration of propargylic esters,8 cycloisomerization of 1,n‐enynes or9 enynones,10 ring‐opening reaction of cyclopropenes,11 dual activation of 1,5‐diynes,12 retro‐Buchner reaction of cycloheptatrienes,13 and gold‐catalyzed oxygen,14 nitrene15 as well as carbene16 transfer reactions. Among these modes, the generation of highly reactive gold carbene intermediates through gold‐catalyzed group‐transfer to C≡C triple bonds and subsequent diverse evolutions has experienced significant attention in the last decade. The π‐acidity of gold catalysts is crucial to this reaction pattern. By π‐interactions with gold catalysts, the C≡C triple bonds become more electrophilic because of the dramatic decrease of electron density and thus undergo nucleophilic attack to form highly electrophilic gold carbene intermediates. These carbene electrophiles enable the facile cyclopropanation of alkenes, functionalization of C−H, N−H or O−H bonds, affording a broad range of useful building blocks.
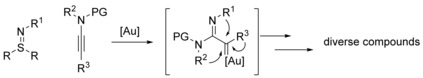
Among these gold carbene species, α‐imino gold carbenes have drawn considerable attention in the last five years. The development of synthetic methods involving imino gold carbene intermediates to facilitate the synthesis of challenging heterocycles is an ongoing endeavor.15 Chemists can benefit from exploiting such methodologies for rapid synthesis or late‐stage modification of biologically important compounds. Under this principle, a series of cyclic nitrenoid precursors, including 2H‐azirines,17 isoxazoles,18 1,2,4‐oxadiazoles,19 1,4,2‐dioxazoles,20 4,5‐dihydro‐1,2,4‐oxadiazoles,21 2,1‐benzisoxazoles,22 1,2‐benzisoxazoles,23 pyrido[1,2‐b]indazoles,24a and triazapentalenes24b, 24c have been developed for the direct introduction of nitrogen‐containing heterocyclic frameworks, whereas the corresponding gold carbenes generally underwent a limited number of transformations (Scheme 1 A). By contrast, azides25 and pyridium aza‐ylides26 potentially provide more opportunities to obtain reaction and product diversity (Scheme 1 B). These two reagents, however, are associated with drawbacks in terms of functional group tolerance. For example, the attempt to employ phenyl azide as an intermolecular nitrene transfer reagent was unsuccessful.24a The use of pyridium aza‐ylides in transition metal catalysis is restricted to substrates with strong acceptors (acyl, sulfonyl, amidinyl, pyridinyl) on the nitrogen anions.26 Furthermore, azides are potentially toxic and explosive. Pyridium ylides are comparatively difficult to synthesize. Thus, an elaborately designed nitrene transfer reagent that can overcome the above‐mentioned drawbacks regarding functional group tolerance, synthetic routes and safety limitations will be of paramount importance. In this Concept article, sulfilimines, a new generation of nitrene precursors, will be introduced (Scheme 1 C). The concise synthetic routes to diverse sulfilimines will be summarized, and their ability to give rise to high levels of complexity by means of gold catalysis will be highlighted.
Scheme 1.
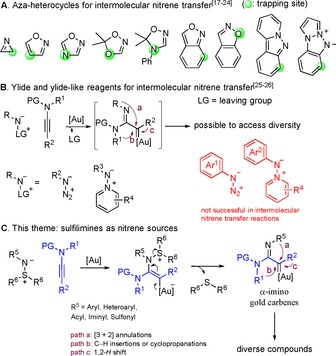
Gold‐catalyzed nitrene transfer reactions.
Synthesis of Sulfilimines
Compared to other nitrene equivalents, the cheap and concise synthesis of sulfilimines by scalable one‐pot reactions using readily available reactants is one of the major advantages and expands the synthetic applicability. The reagents can be stored for a long time without loss of activity, which was tested in the group for a period of two years. The five most general and practical methods will be described.
N‐Halogeno‐N‐metallo reagents as nitrene sources
The commercially available chloramine‐T and related N‐halo compounds can act as nitrene sources.27 The reaction between chloramine‐T and sulfides represents the first reported method to prepare sulfilimines as demonstrated by two research groups in 192128 and 1922.29 Since then this reaction has been developed as one of the most straightforward synthetic routes to a wide range of sulfilimines by employing amide, sulfonamide and amidine derived N‐halogeno‐N‐metallo reagents.30 The reaction between sulfide 1 and chloramine T (2) can be conducted on 40 gram scale (Scheme 2).31 Notably, these N‐halogeno‐N‐metallo reagents can be in situ generated by treating amino‐containing compounds with tert‐butyl hypochlorite followed by treatment with a strong base (NaOH or KOH).
Scheme 2.
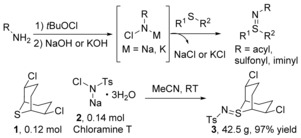
Synthesis of sulfilimines from sulfides and N‐halogeno‐N‐metallo reagents.
Oxidative addition reactions of sulfides with amines or amides
Initial oxidations of sulfides with N‐chlorosuccinimide form sulfonium salts, the nucleophilic attacks of which by NH2 groups can afford azasulfonium salts. Sulfilimines are formed from such salts in the presence of a base (NaOH, NaOCH3 or Et3N).32 This reaction is scalable and can be conducted on a 100 millimol scale (Scheme 3).33
Scheme 3.
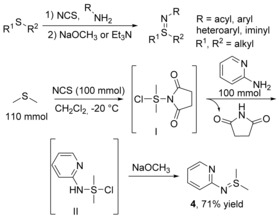
One‐pot synthesis of sulfilimine 4 from 2‐aminopyridine, DMS and NCS.
Sulfoxides as starting materials
Sulfoxides are also suitable starting materials for preparing sulfilimines. As shown in Scheme 4, an electrophilic addition of an activating reagent, such as trifluoroacetic anhydrides,34 to the S=O bond of a sulfoxide generates an oxosulfonium salt 5, the nucleophilic substitution of which by an arylamine, amide or sulfonamide forms aminosulfurane 6. Sulfilimine 7 can be easily obtained by a subsequent alkalization.
Scheme 4.
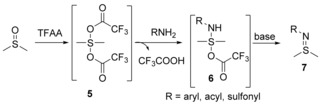
One‐pot synthesis of sulfilimines from sulfoxides.
Reaction of diaryldialkoxysulfuranes with amines or amides
The most frequently used diaryldialkoxysulfuranes is the isolable Martin's sulfurane 8.35 This reagent is commercially available and can be prepared from diaryl sulfides, bromine, and potassium hexafluoro‐2‐phenyl‐2‐propoxide in mole scale.36 Martin and Franz have developed a general method for preparing S,S‐diarylsulfilimines 9 in good yields by the mild reactions of these sulfuranes with amines, amides, and sulfonamides (Scheme 5).37
Scheme 5.
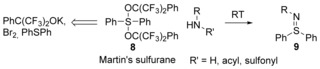
One‐pot synthesis of sulfilimines 9 by using Martin's sulfurane.
Direct N−H functionalization of free NH‐sulfilimines
The sulfilimine with a hydrogen on the nitrogen anion is named NH‐sulfilimine, which can be prepared in large‐scale by a deprotection reaction of N‐sulfonyl sulfilimine 10 (Scheme 6).38 This ylide core can deliver a diverse set of sulfilimines with different N‐substitutions, including N‐acyl, sulfonyl, aryl,39 vinyl,40 alkyl41 and iminyl,42 through simple N−H functionalizations.
Scheme 6.
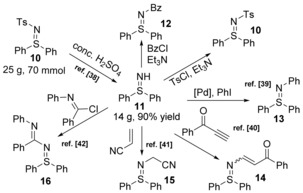
Preparation of free sulfilimine 11 and further N−H functionalizations.
Sulfilimines as Nitrene Transfer Reagents in Gold Catalysis
Ynamides, alkynes bearing an amido group, are broadly useful building blocks in organic reactions.43 The highly electron‐donating ability of the nitrogen atom strongly activates the triple bond, which enables facile electrophilic attacks. Gold‐catalyzed regioselective nitrene transfer from sulfilimines to ynamides efficiently generates α‐imino gold carbenes. By trapping such gold carbenes with functionalities originating from either the ynamides or the sulfilimines, diverse nitrogen‐containing molecules can be obtained.
1,2‐H insertion of the gold carbenes
Prior to our research, Zhang and co‐workers26b developed an intermolecular gold nitrene transfer reaction from N‐sulfonyl sulfilimines to alkyl ynamides through gold(I) carbene intermediates 21, which inserted into the α‐alkyl C−H bond affording α,β‐unsaturated amidines 19 and 20 in low yield with poor E/Z ratio (Scheme 7). This reactivity was not well explored until our group chose 2‐acylphenyl sulfilimines 22 as a nitrene source in our recent report (Scheme 8).39 By reacting with propargylic silyl ether derivatives 23 under gold(III) catalysis, a nitrene transfer, 1,2‐H‐shift and Mukaiyama aldol condensation cascade reaction afforded 3‐acyl quinolines 26–28 in good yield, offering a new alternative for constructing this building block.
Scheme 7.
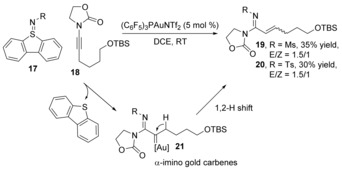
Synthesis of α,β‐unsaturated amidines from N‐sulfonyl sulfilimines.
Scheme 8.
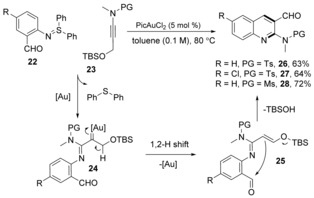
Synthesis of quinolines from N‐aryl sulfilimines.
Nucleophilic attack of nitrogen centers at the gold carbenes
Conjugated ylides that can offer 1,3‐dipoles are prone to undergo [3+2] annulations with C≡C bonds (Scheme 1 B, path a). In this context, the reaction between ynamides and sulfilimines with pyridinyl groups on the nitrogen atoms can deliver imidazopyridines by trapping the α‐imino gold carbenes with another nitrogen atom.44 Gold(III) catalysts are more efficient than gold(I) catalysts in this transformation. PicAuCl2 afforded product 31 in 95 % yield (Scheme 9).45 A further application by the in situ modification of adenine 32 demonstrated the great synthetic potential of this protocol. This reaction tolerated sulfilimines bearing OBn, CF3, dibromo, or trisubstituents on the pyridine rings. Other heteroaryl substituted sulfilimines were also suitable substrates, giving imidazo[1,2‐a]pyrazine 38, imidazo[2,1‐b]thiazole 39, benzo[d]imidazo[2,1‐b]oxazole 40 and benzo[d]imidazo[2,1‐b]thiazole 41 in typically good yields. A variety of ynamides were also tested, yielding imidazopyridines with different 3‐substituents, including pyridinyl, thienyl, alkyl, cyclohexenyl, alkynyl and bulkyl quaternary carbon, in moderate to good yields. Bidirectional reactions for the synthesis of 48–49 were also efficiently conducted. In an analogous [3+2] transformation, N‐iminyl sulfilimines led to 4‐aminoimidazoles 50–52 in high yields (Scheme 10).
Scheme 9.
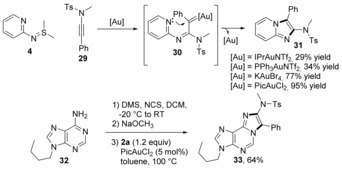
Synthesis of imidazopyridines from N‐pyridinyl sulfilimines.
Scheme 10.
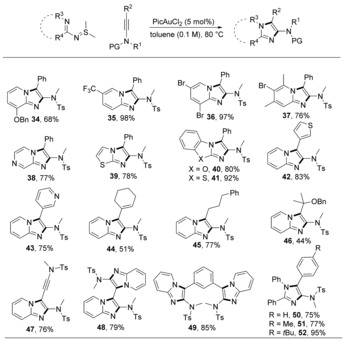
Synthesis of imidazole derivatives from N‐pyridinyl or iminyl sulfilimines.
Nucleophilic attack of oxygen centers at the gold carbenes
The successful use of N‐iminyl sulfilimines motivated us to explore N‐acyl sulfilimines. The oxygen atom of the acyl group can trap the gold carbene to form an oxazole. As illustrated in Scheme 11, under the same reaction conditions, N‐benzoyl sulfilimine reacted efficiently with a broad range of differently N‐substituted ynamides, providing oxazoles 53–63 in 58–95 % yield.46 An alkyl ynamide successfully delivered the target product 64 and no 1,2‐H‐shift product was observed. In addition to various benzoyl sulfilimines, heteroaromatic and alkyl acyl sulfilimines all afforded high yields in this transformation.
Scheme 11.
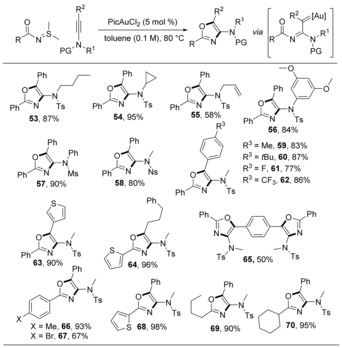
Synthesis of oxazole derivatives from N‐acyl sulfilimines.
1,5‐C−H insertion of the gold carbenes
In further studies our group focused on sulfilimines without any strong acceptors on the anions. The already described generation of gold carbenes was further extended to N‐aryl sulfilimines.39 The gold carbenes inserted into the ortho‐C−H bonds of the introduced aryl groups, which can easily form C−C bonds. This C−H annulation tolerated diverse ynamides, affording 2‐aminoindoles 71–73 with high efficiency (Scheme 12). A wide range of sulfilimines were also tested. The electron density on the aromatic ring is contrary with the product yield. More complex structures were afforded by this operationally simple method.
Scheme 12.
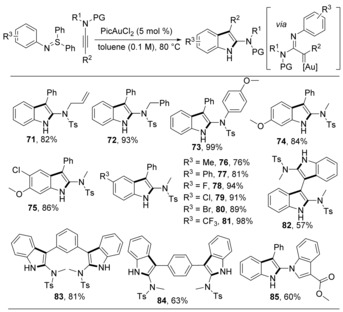
[3+2] Annulations for the synthesis of indole derivatives.
The use of N‐phenyl ynamides creates another possibility for C−H insertions, yielding 1‐protected 2‐aminoindoles.47 When N‐phenyl sulfilimines were used (Scheme 13, products 86–89), this reaction benefited from the electron‐deficient aryl groups on the anions of sulfilimines and the electron‐rich aryl groups on the nitrogen atoms of ynamides. Employing N‐sulfonyl sulfilimines can avoid the [3+2] annulations, and the corresponding gold carbenes can only be attacked by the aryl group within the ynamide to give a single product. In this manner, the product yield is typically high (90–97).
Scheme 13.
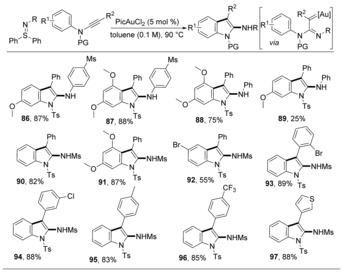
Synthesis of 1‐protected 2‐aminoindoles.
Cyclopropanation of the gold carbenes
The cyclopropanation of α‐oxo gold carbenes by alkenyl groups have been previously demonstrated.48 We speculated that α‐imino gold carbenes could also undergo a similar cyclopropanation in the presence of an allyl group, which would lead to azabicyclo[3.1.0]hexan‐2‐imines. Gratifyingly, by employing sulfilimines bearing electron‐poor aryl groups, the chemoselective cyclopropanation reaction proceeded smoothly with diverse N‐allylynamides, providing an array of azabicyclo[3.1.0]hexan‐2‐imines 98–108 in excellent yields (Scheme 14).39
Scheme 14.
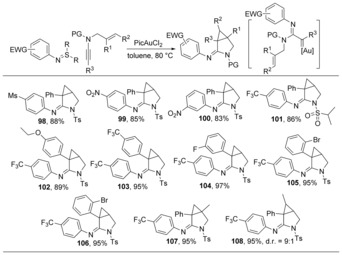
Synthesis of azabicyclo[3.1.0]hexan‐2‐imines.
Summary and Outlook
The straightforward, efficient and scalable methods for the preparation of sulfilimines show that these represent a class of reagents with high potential, which can be used in organic synthesis, in late stage functionalization and even in modular syntheses aiming at libraries of products. The recent advances of these ylides in gold‐catalyzed transformations allow to access diverse compounds, and show the high potential of the sulfilimine reagents in the synthesis of nitrogen‐containing heterocycles and carbocycles bearing amino substituents. The ability to generate α‐imino gold carbenes from these readily available starting materials is key to the efficient construction of biologically important nitrogen‐containing frameworks. In comparison to previous explored reagents, the use of sulfilimines overcomes some challenging problems with respect to functional group tolerance, synthetic methods and safety problems, and thus should be of high interest for reactions involving other metal catalysts, for example ruthenium, rhodium or platinum. However, the lack of reactivity with non‐polarized alkynes represents a major drawback of this reagent, overcoming which by using other catalytic systems can open a completely new field in heterocyclic chemistry.
Conflict of interest
The authors declare no conflict of interest.
Acknowledgements
X.T. and L.S. are grateful to the CSC (China Scholarship Council) for a PhD fellowship.
X. Tian, L. Song, A. S. K. Hashmi, Chem. Eur. J. 2020, 26, 3197.
References
- 1.
- 1a. Ando W., Acc. Chem. Res. 1977, 10, 179; [Google Scholar]
- 1b. Lu L.-Q., Chen J.-R., Xiao W.-J., Acc. Chem. Res. 2012, 45, 1278; [DOI] [PubMed] [Google Scholar]
- 1c. Trost B. M., Melvin L. S., in Sulfur ylides, Emerging Synthetic Intermediates, Vol. 2, Academic Press, New York, 1975, pp. 13–156; [Google Scholar]
- 1d. Oae S., in Organic Chemistry of Sulfur, Springer, 1977, pp. 383–471; [Google Scholar]
- 1e. Neuhaus J. D., Oost R., Merad J., Maulide N., Top. Curr. Chem. 2018, 376, 15; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1f. Mondal M., Chen S., Kerrigan N. J., Molecules 2018, 23, 738; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 1g. Gessner V. H., in Modern Ylide Chemistry: Applications in Ligand Design, Organic and Catalytic Transformations, Vol. 177, Springer, 2018. [Google Scholar]
- 2.
- 2a. Tsujihara K., Furukawa N., Oae K., Oae S., Bull. Chem. Soc. Jpn. 1969, 42, 2631; [Google Scholar]
- 2b. Gilchrist T. L., Moody C. J., Chem. Rev. 1977, 77, 409; [Google Scholar]
- 2c. Koval I. V., Sulfur Rep. 1993, 14, 149; [Google Scholar]
- 2d. Taylor P. C., Sulfur Rep. 1999, 21, 241; [Google Scholar]
- 2e. Bizet V., Hendriks C. M. M., Bolm C., Chem. Soc. Rev. 2015, 44, 3378. [DOI] [PubMed] [Google Scholar]
- 3. Vanacore R., Ham A.-J. L., Voehler M., Sanders C. R., Conrads T. P., Veenstra T. D., Sharpless K. B., Dawson P. E., Hudson B. G., Science 2009, 325, 1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hayashi R., Shimizu A., Yoshida J.-i., J. Am. Chem. Soc. 2016, 138, 8400. [DOI] [PubMed] [Google Scholar]
- 5. Yoshida S., Yano T., Misawa Y., Sugimura Y., Igawa K., Shimizu S., Tomooka K., Hosoya T., J. Am. Chem. Soc. 2015, 137, 14071. [DOI] [PubMed] [Google Scholar]
- 6.Selected Reviews on gold catalysis:
- 6a. Hashmi A. S. K., Hutchings G. J., Angew. Chem. Int. Ed. 2006, 45, 7896; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 8064; [Google Scholar]
- 6b. Fürstner A., Davies P. W., Angew. Chem. Int. Ed. 2007, 46, 3410; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 3478; [Google Scholar]
- 6c. Gorin D. J., Toste F. D., Nature 2007, 446, 395; [DOI] [PubMed] [Google Scholar]
- 6d. Hashmi A. S. K., Chem. Rev. 2007, 107, 3180; [DOI] [PubMed] [Google Scholar]
- 6e. Corma A., Leyva-Pérez A., Sabater M. J., Chem. Rev. 2011, 111, 1657; [DOI] [PubMed] [Google Scholar]
- 6f. Rudolph M., Hashmi A. S. K., Chem. Soc. Rev. 2012, 41, 2448; [DOI] [PubMed] [Google Scholar]
- 6g. Xie J., Pan C., Abdukader A., Zhu C., Chem. Soc. Rev. 2014, 43, 5245; [DOI] [PubMed] [Google Scholar]
- 6h. Qian D., Zhang J., Chem. Soc. Rev. 2015, 44, 677; [DOI] [PubMed] [Google Scholar]
- 6i. Dorel R., Echavarren A. M., Chem. Rev. 2015, 115, 9028; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6j. Wang Y., Muratore M. E., Echavarren A. M., Chem. Eur. J. 2015, 21, 7332; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6k. Pflästerer D., Hashmi A. S. K., Chem. Soc. Rev. 2016, 45, 1331; [DOI] [PubMed] [Google Scholar]
- 6l. Harris R. J., Widenhoefer R. A., Chem. Soc. Rev. 2016, 45, 4533. [DOI] [PubMed] [Google Scholar]
- 6m.For early results on gold catalysis: Hashmi A. S. K., Schwarz L., Choi J.-H., Frost T. M., Angew. Chem. Int. Ed. 2000, 39, 2285; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2000, 112, 2382; [Google Scholar]
- 6n. Hashmi A. S. K., Frost T. M., Bats J. W., J. Am. Chem. Soc. 2000, 122, 11553. [Google Scholar]
- 7.Reviews on gold carbenes from diazo compounds:
- 7a. Wei F., Song C., Ma Y., Zhou L., Tung C.-H., Xu Z., Sci. Bull. 2015, 60, 1479 ; [Google Scholar]
- 7b. Liu L., Zhang J., Chem. Soc. Rev. 2016, 45, 506; [DOI] [PubMed] [Google Scholar]
- 7c. Fructos M. R., Díaz-Requejo M. M., Pérez P. J., Chem. Commun. 2016, 52, 7326. [DOI] [PubMed] [Google Scholar]
- 8.Reviews on gold carbenes from propargylic esters:
- 8a. Marion N., Nolan S. P., Angew. Chem. Int. Ed. 2007, 46, 2750; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 2806; [Google Scholar]
- 8b. Wang S., Zhang G., Zhang L., Synlett 2010, 692; [Google Scholar]
- 8c. Shiroodi R. K., Gevorgyan V., Chem. Soc. Rev. 2013, 42, 4991; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8d. Day D. P., Chan P. W. H., Adv. Synth. Catal. 2016, 358, 1368; [Google Scholar]
- 8e. Mouriès-Mansuy V., Fensterbank L., Isr. J. Chem. 2018, 58, 586; [Google Scholar]
- 8f. Lauterbach T., Gatzweiler S., Nösel P., Rudolph M., Rominger F., Hashmi A. S. K., Adv. Synth. Catal. 2013, 355, 2481. [Google Scholar]
- 9.Reviews on 1,n-enynes:
- 9a. Michelet V., Toullec P. Y., Genêt J.-P., Angew. Chem. Int. Ed. 2008, 47, 4268; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2008, 120, 4338; [Google Scholar]
- 9b. Ebner C., Carreira E. M., Chem. Rev. 2017, 117, 11651; [DOI] [PubMed] [Google Scholar]
- 9c. Hashmi A. S. K., Rudolph M., Siehl H.-U., Tanaka M., Bats J. W., Frey W., Chem. Eur. J. 2008, 14, 3703. [DOI] [PubMed] [Google Scholar]
- 10.Examples on gold carbenes from enynones:
- 10a. Ma J., Jiang H., Zhu S., Org. Lett. 2014, 16, 4472; [DOI] [PubMed] [Google Scholar]
- 10b. Liu P., Sun J., Org. Lett. 2017, 19, 3482; [DOI] [PubMed] [Google Scholar]
- 10c. Miao M., Xu H., Jin M., Chen Z., Xu J., Ren H., Org. Lett. 2018, 20, 3096. [DOI] [PubMed] [Google Scholar]
- 11.For selected examples:
- 11a. Seidel G., Mynott R., Fürstner A., Angew. Chem. Int. Ed. 2009, 48, 2510; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2009, 121, 2548; [Google Scholar]
- 11b. Li C., Zeng Y., Zhang H., Feng J., Zhang Y., Wang J., Angew. Chem. Int. Ed. 2010, 49, 6413–6417; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 6557–6561. [Google Scholar]
- 12.Reviews:
- 12a. Braun I., Asiri A. M., Hashmi A. S. K., ACS Catal. 2013, 3, 1902; [Google Scholar]
- 12b. Hashmi A. S. K., Acc. Chem. Res. 2014, 47, 864; [DOI] [PubMed] [Google Scholar]
- 12c. Asiri A. M., Hashmi A. S. K., Chem. Soc. Rev. 2016, 45, 4471; [DOI] [PubMed] [Google Scholar]
- 12d. Gómez-Suárez A., Nolan S. P., Angew. Chem. Int. Ed. 2012, 51, 8156; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 8278; [Google Scholar]
- 12e. Gagosz F., Synthesis 2019, 1087; [Google Scholar]
- 12f. Zhao X., Rudolph M., Hashmi A. S. K., Chem. Commun. 2019, 55, 12127; [DOI] [PubMed] [Google Scholar]
- 12g.initial publication: Hashmi A. S. K., Braun I., Rudolph M., Rominger F., Organometallics 2012, 31, 644. [Google Scholar]
- 13. Mato M., García-Morales C., Echavarren A. M., ChemCatChem 2019, 11, 53, and the references therein. [Google Scholar]
- 14.For Reviews on α-oxo gold carbenes see:
- 14a. Yeom H.-S., Shin S., Acc. Chem. Res. 2014, 47, 966; [DOI] [PubMed] [Google Scholar]
- 14b. Zhang L., Acc. Chem. Res. 2014, 47, 877; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14c. Wang Y., Zhang L., Synthesis 2015, 47, 289; [Google Scholar]
- 14d. Zheng Z., Wang Z., Wang Y., Zhang L., Chem. Soc. Rev. 2016, 45, 4448. [DOI] [PubMed] [Google Scholar]
- 15.Reviews on gold nitrene transfer reactions, see:
- 15a. Davies P. W., Garzon M., Asian J. Org. Chem. 2015, 4, 694; [Google Scholar]
- 15b. Li L., Tan T.-D., Zhang Y.-Q., Liu X., Ye L.-W., Org. Biomol. Chem. 2017, 15, 8483; [DOI] [PubMed] [Google Scholar]
- 15c. Aguilar E., Santamaría J., Org. Chem. Front. 2019, 6, 1513. [Google Scholar]
- 16.
- 16a. Kramer S., Skrydstrup T., Angew. Chem. Int. Ed. 2012, 51, 4681; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 4759; [Google Scholar]
- 16b. Huang X., Peng B., Luparia M., Gomes L. F., Veiros L. F., Maulide N., Angew. Chem. Int. Ed. 2012, 51, 8886; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 9016. [Google Scholar]
- 17.
- 17a. Prechter A., Henrion G., dit Bel P. F., Gagosz F., Angew. Chem. Int. Ed. 2014, 53, 4959; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2014, 126, 5059; [Google Scholar]
- 17b. Zhu L., Yu Y., Mao Z., Huang X., Org. Lett. 2015, 17, 30; [DOI] [PubMed] [Google Scholar]
- 17c. Pawar S. K., Sahani R. L., Liu R. S., Chem. Eur. J. 2015, 21, 10843. [DOI] [PubMed] [Google Scholar]
- 18.Leading examples:
- 18a. Zhou A.-H., He Q., Shu C., Yu Y.-F., Liu S., Zhao T., Zhang W., Lu X., Ye L.-W., Chem. Sci. 2015, 6, 1265; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18b. Sahani R. L., Liu R.-S., Angew. Chem. Int. Ed. 2017, 56, 1026; [Google Scholar]; Angew. Chem. 2017, 129, 1046. [Google Scholar]
- 19. Zeng Z., Jin H., Xie J., Tian B., Rudolph M., Rominger F., Hashmi A. S. K., Org. Lett. 2017, 19, 1020. [DOI] [PubMed] [Google Scholar]
- 20. Xu W., Wang G., Sun N., Liu Y., Org. Lett. 2017, 19, 3307. [DOI] [PubMed] [Google Scholar]
- 21. Chen M., Sun N., Chen H., Liu Y., Chem. Commun. 2016, 52, 6324. [DOI] [PubMed] [Google Scholar]
- 22.Selected examples:
- 22a. Jin H., Huang L., Xie J., Rudolph M., Rominger F., Hashmi A. S. K., Angew. Chem. Int. Ed. 2016, 55, 794; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 804; [Google Scholar]
- 22b. Jin H., Tian B., Song X., Xie J., Rudolph M., Rominger F., Hashmi A. S. K., Angew. Chem. Int. Ed. 2016, 55, 12688; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2016, 128, 12880; [Google Scholar]
- 22c. Zeng Z., Jin H., Sekine K., Rudolph M., Rominger F., Hashmi A. S. K., Angew. Chem. Int. Ed. 2018, 57, 6935; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 7051; [Google Scholar]
- 22d. Tsai M.-H., Wang C.-Y., Raja A. S. K., Liu R.-S., Chem. Commun. 2018, 54, 10866; [DOI] [PubMed] [Google Scholar]
- 22e. Zeng Z., Jin H., Rudolph M., Rominger F., Hashmi A. S. K., Angew. Chem. Int. Ed. 2018, 57, 16549; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2018, 130, 16787; [Google Scholar]
- 22f. Hsu Y.-C., Hsieh S.-A., Liu R.-S., Chem. Eur. J. 2019, 25, 5288; [DOI] [PubMed] [Google Scholar]
- 22g. Song L., Tian X., Rudolph M., Hashmi A. S. K., Chem. Commun. 2019, 55, 9007; [DOI] [PubMed] [Google Scholar]
- 22h. Tian X., Song L., Farshadfar K., Rudolph M., Rominger F., Oeser T., Ariafard A., Hashmi A. S. K., Angew. Chem. Int. Ed. 2020, 59, 471; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2020, 132, 479. [Google Scholar]
- 23.
- 23a. Jadhav P. D., Lu X., Liu R.-S., ACS Catal. 2018, 8, 9697; [Google Scholar]
- 23b. Xu W., Zhao J., Li X., Liu Y., J. Org. Chem. 2018, 83, 15470. [DOI] [PubMed] [Google Scholar]
- 24.
- 24a. Yu Y., Chen G., Zhu L., Liao Y., Wu Y., Huang X., J. Org. Chem. 2016, 81, 8142; [DOI] [PubMed] [Google Scholar]
- 24b. González J., Santamaría J., Suárez-Sobrino Á. L., Ballesteros A., Adv. Synth. Catal. 2016, 358, 1398; [Google Scholar]
- 24c. Allegue D., González J., Fernández S., Santamaría J., Ballesteros A., Adv. Synth. Catal. 2019, 361, 758. [Google Scholar]
- 25.Selected examples on α-imino gold carbenes from azides, see:
- 25a. Gorin D. J., Davis N. R., Toste F. D., J. Am. Chem. Soc. 2005, 127, 11260; [DOI] [PubMed] [Google Scholar]
- 25b. Lu B., Luo Y., Liu L., Ye L., Wang Y., Zhang L., Angew. Chem. Int. Ed. 2011, 50, 8358; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 8508; [Google Scholar]
- 25c. Wetzel A., Gagosz F., Angew. Chem. Int. Ed. 2011, 50, 7354; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 7492; [Google Scholar]
- 25d. Yan Z. Y., Xiao Y., Zhang L., Angew. Chem. Int. Ed. 2012, 51, 8624; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 8752; [Google Scholar]
- 25e. Shu C., Wang Y.-H., Zhou B., Li X.-L., Ping Y.-F., Lu X., Ye L.-W., J. Am. Chem. Soc. 2015, 137, 9567. [DOI] [PubMed] [Google Scholar]
- 26.Selective examples, see:
- 26a. Davies P. W., Cremonesi A., Dumitrescu L., Angew. Chem. Int. Ed. 2011, 50, 8931; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 9093; [Google Scholar]
- 26b. Li C., Zhang L., Org. Lett. 2011, 13, 1738; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26c. Garzón M., Davies P. W., Org. Lett. 2014, 16, 4850; [DOI] [PubMed] [Google Scholar]
- 26d. Hung H.-H., Liao Y.-C., Liu R.-S., Adv. Synth. Catal. 2013, 355, 1545. [Google Scholar]
- 27. Campbell M. M., Johnson G., Chem. Rev. 1978, 78, 165. [Google Scholar]
- 28. Nicolet B. H., Willard I. D., Science 1921, 53, 217.17831200 [Google Scholar]
- 29. Mann F. G., Pope W. J., J. Chem. Soc. 1922, 121, 1052. [Google Scholar]
- 30.
- 30a. Yoshino H., Kawazoe Y., Taguchi T., Synthesis 1974, 713; [Google Scholar]
- 30b. Heesing A., Imsieke G., Chem. Ber. 1974, 107, 1536. [Google Scholar]
- 31. Marzinzik A. L., Sharpless K. B., J. Org. Chem. 2001, 66, 594. [DOI] [PubMed] [Google Scholar]
- 32.
- 32a. Vilsmaier E., Sprugel W., Tetrahedron Lett. 1972, 13, 625; [Google Scholar]
- 32b. Corey E. J., Kim C. U., J. Am. Chem. Soc. 1972, 94, 7586; [Google Scholar]
- 32c. Claus P. K., Rieder W., Hofbauer P., Vilsmaier E., Tetrahedron 1975, 31, 505. [Google Scholar]
- 33. Taylor E. C., Tseng C. P., Rampal J. B., J. Org. Chem. 1982, 47, 552. [Google Scholar]
- 34. Sharma A. K., Ku T., Dawson A. D., Swern D., J. Org. Chem. 1975, 40, 2758. [Google Scholar]
- 35. Arhart R. J., Martin J. C., J. Am. Chem. Soc. 1972, 94, 5003. [Google Scholar]
- 36. Martin J. C., Arhart R. J., Franz J. A., Perozzi E. F., Kaplan L. J., Org. Synth. 1977, 57, 22. [Google Scholar]
- 37. Martin J. C., Franz J. A., J. Am. Chem. Soc. 1975, 97, 6137. [Google Scholar]
- 38. Yoshimura T., Omata T., J. Org. Chem. 1976, 41, 1728. [Google Scholar]
- 39. Tian X., Song L., Rudolph M., Rominger F., Oeser T., Hashmi A. S. K., Angew. Chem. Int. Ed. 2019, 58, 3589; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2019, 131, 3627. [Google Scholar]
- 40. Tamura Y., Sumoto K., Matsushima H., Taniguchi H., Ikeda M., J. Org. Chem. 1973, 38, 4324. [DOI] [PubMed] [Google Scholar]
- 41. Furukawa N., Oae S., Yoshimura T., Synthesis 1976, 30. [Google Scholar]
- 42. Gilchrist T. L., Moody C. J., Rees C. W., J. Chem. Soc. Perkin Trans. 1 1975, 19, 1964. [Google Scholar]
- 43.
- 43a. Evano G., Coste A., Jouvin K., Angew. Chem. Int. Ed. 2010, 49, 2840; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2010, 122, 2902; [Google Scholar]
- 43b. DeKorver K. A., Li H., Lohse A. G., Hayashi R., Lu Z., Zhang Y., Hsung R. P., Chem. Rev. 2010, 110, 5064; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43c. Evano G., Jouvin K., Coste A., Synthesis 2013, 45, 17; [Google Scholar]
- 43d. Wang X.-N., Yeom H.-S., Fang L.-C., He S., Ma Z.-X., Kedrowski B. L., Hsung R. P., Acc. Chem. Res. 2014, 47, 560; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43e. Pan F., Shu C., Ye L.-W., Org. Biomol. Chem. 2016, 14, 9456. [DOI] [PubMed] [Google Scholar]
- 44. Tian X., Song L., Rudolph M., Wang Q., Song X., Rominger F., Hashmi A. S. K., Org. Lett. 2019, 21, 1598. [DOI] [PubMed] [Google Scholar]
- 45.For the benefits of N,O-ligands, see: Hashmi A. S. K., Weyrauch J. P., Rudolph M., Kurpejović E., Angew. Chem. Int. Ed. 2004, 43, 6545; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2004, 116, 6707. [Google Scholar]
- 46. Tian X., Song L., Han C., Zhang C., Wu Y., Rudolph M., Rominger F., Hashmi A. S. K., Org. Lett. 2019, 21, 2937. [DOI] [PubMed] [Google Scholar]
- 47. Tian X., Song L., Rudolph M., Rominger F., Hashmi A. S. K., Org. Lett. 2019, 21, 4327. [DOI] [PubMed] [Google Scholar]
- 48.
- 48a. Vasu D., Hung H.-H., Bhunia S., Gawade S. A., Das A., Liu R.-S., Angew. Chem. Int. Ed. 2011, 50, 6911; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 7043; [Google Scholar]
- 48b. Wang K.-B., Ran R.-Q., Xiu S.-D., Li C.-Y., Org. Lett. 2013, 15, 2374. [DOI] [PubMed] [Google Scholar]


