Abstract
Cardiopulmonary bypass (CPB) is often necessary for congenital cardiac surgery, but CPB can alter drug pharmacokinetic parameters resulting in underdosing. Inadequate plasma levels of antibiotics could lead to postoperative infections with increased morbidity. The influence of pediatric CPB systems on cefazolin and clindamycin plasma levels is not known. We have measured plasma levels of cefazolin and clindamycin in in vitro pediatric CPB systems. We have tested three types of CPB systems. All systems were primed and spiked with clindamycin and cefazolin. Samples were taken at different time points to measure the recovery of cefazolin and clindamycin. Linear mixed model analyses were performed to assess if drug recovery was different between the type of CPB system and sampling time point. The experiments were conducted at a tertiary university hospital. 81 samples were analyzed. There was a significant difference in the recovery over time between CPB systems for cefazolin and clindamycin (P < .001). Cefazolin recovery after 180 minutes was 106% (95% CI: 91‐123) for neonatal, 99% (95% CI: 85‐115) for infant, and 77% (95% CI: 67‐89) for pediatric systems. Clindamycin recovery after 180 minutes was 143% (95% CI: 116‐177) for neonatal, 111% (95% CI: 89‐137) for infant, and 120% (95% CI: 97‐149) for pediatric systems. Clindamycin recovery after 180 minutes compared to the theoretical concentration was 0.4% for neonatal, 1.2% for infants, and 0.6% for pediatric systems. The recovery of cefazolin was high in the neonatal and infant CPB systems and moderate in the pediatric system. We found a large discrepancy between the theoretical and measured concentrations of clindamycin in all tested CPB systems.
Keywords: antibiotics, cardiopulmonary bypass, cefazolin, clindamycin, in vitro, infant, neonatal, pediatric, sequestration
1. INTRODUCTION
During pediatric cardiac surgery, the use of the cardiopulmonary bypass (CPB) is often necessary. However, CPB has several effects that can alter drug pharmacokinetic parameters leading to potentially increased or decreased plasma drug concentration in the patient. This may be a problem when dosing antibiotics to prevent surgical site infections (SSI), since inadequate antibiotic plasma concentration may lead to an increased risk to develop SSI and subsequent morbidity and mortality.1
The most profound changes CPB systems induce on drug concentrations are due to hemodilution, causing changes in drug distribution and clearance, changed hemodynamic status and protein binding, hypothermia and occasionally hemofiltration. The influence of these changes on plasma drug concentration is known for some routinely used drugs, such as midazolam and propofol.2
The CPB system itself has an effect on drugs administered during surgery. Mainly lipophilic drugs are known to sequester in the CPB system.3, 4 Factors that can contribute are type and coating of tubing,5, 6 and the type of priming fluid.2 Studies on the effects of CPB on plasma levels of drugs have not been performed recently and may no longer be comparable to current practice, since CPB systems and perioperative management may have changed. Data on pediatric CPB systems are lacking, since the literature published on in vitro assessment of the CPB systems investigates only adult CPB systems. The size of the system, added volume, and composition of priming fluid are different in adult CPB systems compared to pediatric CPB systems and data from adult CPB systems cannot simply be extrapolated to pediatric systems.
We have designed the CPB‐PHARM study to investigate the influence of CPB on routinely used drugs during and after pediatric cardiac surgery (MEC2011‐400). We have added an in vitro substudy to investigate the effects of the CPB without the influence of surgery or patients. This in vitro study describes the influence of different pediatric CPB systems on plasma levels of cefazolin and clindamycin, our first and second choice antibiotic prophylaxis.
2. MATERIALS AND METHODS
We have investigated three types of pediatric CPB systems used at the Department of Cardio‐Thoracic Surgery of the Erasmus MC, Rotterdam, The Netherlands. Since all experiments were in vitro and no patients were involved, there is no issue of informed consent in accordance with Dutch law. Circuits that would soon expire were made available free of charge by Terumo Europe NV, Leuven, Belgium and Sorin Group, Mirandola, Italy. Terumo Europe NV and Sorin Group had no influence on study design and protocol, data collection and analyses, or publication.
2.1. CPB systems
We have tested three CPB systems used for congenital cardiac surgery in our institution. The neonatal, infant, and pediatric CPB systems are used for patients with bodyweights of under 10, 10‐20, and 20‐40 kg, respectively. An overview of the components of these CPB systems is given in Table 1. In summary, all systems consist of a hollow‐fiber oxygenator and an open hard‐shell reservoir. The oxygenator of the neonatal and pediatric systems is coated with X‐coating (poly(2‐methoxyethyl acrylate) (PMEA)), a nonheparin biocompatible polymer with hydrophilic and hydrophobic properties. The neonatal and pediatric systems have an integrated arterial filter, whereas the infant system has a stand‐alone arterial filter. The arterial filter is a polyester screen‐type filter in the neonatal and pediatric systems and a phosporylcholine screen‐type filter in the infant system, with varying surface areas. The venous reservoirs are made of polycarbonate with capacities of 1000, 1500, and 3000‐4000 mL, respectively, for the neonatal, infant, and pediatric CPB systems. A roller pump is used in the neonatal and infant systems. In the pediatric system, a centrifugal pump is used, with a pump casing of polycarbonate. A combination of silicone and polyvinylchloride (PVC) tubing is used in the neonatal and infant systems. In the pediatric system, only PVC tubing is used. The tubing in all systems is coated with Phisio coating: a nonheparin, biomimetic layer consisting of a phosphorylcholine polymer.
Table 1.
CPB systems' characteristics
| Oxygenator | Reservoir | Arterial filter | Venous filter cardiotomy | Defoaming sponge | Silicone tubing | PVC tubing | Priming volume | |
|---|---|---|---|---|---|---|---|---|
| Neonatal Roller | Capiox FX05, Terumo Europe NV, Leuven, Belgium | Open hard‐shell polycarbonate, minimum capacity 15 mL, maximum capacity 1000 mL | Integrated polyester screen type | Polyester screen type, pore size 47 μm | Polyurethane | Sorin Kids neonate set, custom made, Sorin Group, Mirandola, Italy | Sorin Kids neonate set, custom made, Sorin Group, Mirandola, Italy | 260 mL |
| Hollow fiber | Surface area 130 cm2, pore size 32 μm | Diameter ¼ inch, length 1.10 m, 0.02 m2 contact surface area, Phisio coating | Diameter ¼ inch, length 2.95 m, 0.069 m2 contact surface area, Phisio coating | |||||
| Polycarbonate housing, polypropylene membrane 0.5 m2, priming volume 43 mL, X‐coating | ||||||||
| Infant Roller | Sorin Kids D101, Sorin Group, Mirandola, Italy | Open hard‐shell, polycarbonate, minimum capacity 30 mL, maximum capacity 1500 mL | Sorin Kids D131 stand‐alone arterial filter, Sorin Group, Mirandola, Italy | Polyester, pore size 51 μm | Polyurethane | Sorin Kids, custom made, Sorin Group, Mirandola, Italy | Sorin Kids neonate set, custom made, Sorin Group, Mirandola, Italy | 430 mL |
| Hollow fiber | Polycarbonate housing, phosphoryl chloride screen type membrane | Diameter ¼ inch, length 1.05 m, 0.02 m2 contact surface area, Phisio coating | Arterial part diameter ¼ inch, length 1.88 m, Venous part diameter ⅜ inch, length 1.51 m, Total 0.08 m2 contact surface area, Phisio coating | |||||
| Polycarbonate housing, polypropylene membrane 0.61 m2, priming volume 87 mL, Phisio coating | Surface area 27 cm2, pore size 40 μm, priming volume 28 mL | |||||||
| Pediatric Centrifugal | Capiox FX15, Terumo Europe Europe NV, Leuven, Belgium | Open hard‐shell polycarbonate, minimum capacity 70 or 200 mL, maximum capacity 3000 or 4000 mL | Integrated polyester screen type | Polyester screen type, pore size 47 μm | Polyurethane | (Sorin Kids Pediatric set, custom made, Sorin Group, Mirandola, Italy) | 68 mL | |
| Revolution, Sorin Group, Mirandola, Italy | Hollow fiber | Surface area 360 cm2, pore size 32 μm | Diameter ⅜ inch, length 4.87 m, 0.15 m2 contact surface area, Phisio coating | |||||
| Pump casing polycarbonate priming volume 57 mL | Polycarbonate housing, polypropylene membrane 1.5 m2, priming volume 144 mL, X‐coating |
Three systems were assembled for each category and placed on a mast‐mounted, remote pump head console (Stöcker S5 Perfusion System, Sorin Group, Mirandola, Italy), with a pediatric configuration. A ¼‐¼ or a ¼‐⅜ connection piece was used to make the continuous CPB systems.
All systems were primed according to hospital protocol (Table 2). Priming fluid in all systems consists of fresh frozen plasma (FFP), Gelofusine (B. Braun, Melsungen, Germany), red blood cells (RBCs), human albumin (Sanquin Plasma Products BV, Amsterdam, The Netherlands), and sodium bicarbonate 8.4% (Fresenius Kabi Nederland BV, Zeist, NL). Heparin was added to avoid the clotting of blood in the system. Hematocrit was aimed to be 28% during CPB. FFP’s and RBC’s those were just expired were obtained from the local blood bank.
Table 2.
Prime fluid
| Neonatal | Infant | Pediatric | |
|---|---|---|---|
| Priming volume (mL) | 263.4 | 430 | 683 |
| RBC (mL) | 135 | 235 | 365 |
| FFP (mL) | 30 | 40 | 50 |
| Gelofusine (mL) | 30 | 40 | 50 |
| Albumin 20 % (mL) | 40 | 50 | 100 |
| Mannitol 15 % (mL | 20 | 50 | 100 |
| NaHCO3 8.4 % (mL) | 3 | 15 | 18 |
| Heparin (mL) | 0.4 | 0.5 | 1 |
| Flow (L/min) | 0.5 | 1.5 | 3 |
| Temperature (°C) | 36 | 36 | 36 |
| Line pressure (mm Hg) | 100 | 100 | 100 |
CPB systems were kept running at a temperature of 36°C for 6 hours, since this is the maximum runtime for which the quality is guaranteed by the manufacturers. Before the start of the experiments the pCO2, pO2, and pH were measured with iStat (Abbot BV, Hoofddorp, The Netherlands). These parameters were kept within physiologic ranges during the experiments using sweep gas flow, gas composition and if needed, additional of sodium bicarbonate 8.4%.
Flow rates were kept at 0.5, 1.5, and 3 L/min for the neonatal, infant, and pediatric CPB systems. Using the venous clamp, postmembrane pressures were kept constant at 100 mm Hg.
2.2. Drug administration
Drug doses were standardized based on the average weight for a patient for the neonatal, infant, and pediatric CPB system. Drugs for the neonatal system were based on a 5 kg patient, for the infant system on a 15 kg patient, and for the pediatric system a 30 kg patient. Drugs were dosed according to our institutional guidelines for the induction for general anesthesia, that is, cefazolin at 30 mg/kg and clindamycin at 12 mg/kg for neonates and 6.7 mg/kg for children with a body weight of above 10 kg (see Table 3).
Table 3.
Drug concentration administered to the CPB systems
| Neonate (mg) | Infant (mg) | Pediatric (mg) | |
|---|---|---|---|
| Cefazolin (Kefzol, Eurocept BV, Ankeveen, The Netherlands, 100 mg/mL) | 250 | 750 | 1500 |
| Clindamycin (Dalacin, Pfizer BV, Capelle aan den IJssel, The Netherlands) | 60 | 100 | 200 |
Drugs were injected into the CPB systems at the venous side of the system via a manifold sample port. After each drug, 2 mL of physiological saline solution (0.9%) flush was injected to prevent pooling or crystallization of drugs in the sample port. Drugs were administered in the same order for all systems and all experiments.
2.3. Samples
From each CPB system, 4 mL of blood samples was taken at different time points. The first samples were immediately after injection of the drugs (T1), thereafter samples were taken at 2, 5, 7, 10, 30, 60, 180, and 300 minutes. Samples were taken from the arterial (postoxygenator) side of the CPB system via a manifold sample port. Polypropylene EDTA tubes were used (7.2 mg EDTA, BD Vacutainer, BD Life Sciences, Plymouth, UK).
Samples were stored at 4°C until centrifugation (10 minutes at 3600 rpm). The supernatant serum was transferred to polypropylene cryogenic vials with polypropylene screw caps (Sarstedt Aktiengesellschaft & Co, Nümbrecht, Germany) and stored at −80°C until further analysis.
2.4. Assay methods
Liquid chromatography‐mass spectrometry (LC‐MS/MS) was used to measure the total plasma drug concentrations of cefazolin and clindamycin.
Drug concentrations for clindamycin were measured using LC‐MS/MS in a Thermo TSQ Quantiva triple‐stage quadrupole mass spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) at the pharmacy laboratory of the University Medical Center Groningen, The Netherlands.
Drug concentrations for cefazolin were measured using LC‐MS/MS in an AB SCIEX QTRAP 5500 triple quadrupole mass spectrometer (AB SCIEX, Concord, Ontario, Canada) and Analyst software version 1.6.2 (AB SCIEX, Concord, Ontario, Canada) at the IBMP Institute for Biomedical and Pharmaceutical Research in Nürnberg‐Heroldsberg, Germany.
Limits of quantification are specified in Table 4. A certified research technician from the ISO‐certified laboratories performed the FDA validated drug analyses. In all analyses, quality control samples are included, as is obliged in FDA analyses and ISO and GCP certified laboratory.
Table 4.
Limits of quantification
| Drug | Lower limit of quantification (mg/L) | Upper limit of quantification (mg/L) |
|---|---|---|
| Cefazolin | 209.3 | 2009.23 |
| Clindamycin | 0.6 | 57.00 |
2.5. Statistical analysis
We performed linear mixed model analyses with drug concentration as the dependent variable and type of CPB system and sample time point as independent variables. We used mixed models analysis because a correlation can be expected between multiple measurements of the same variable (drug concentration) in the same subject (CPB systems). Given the nonnormal distribution of the drug concentrations, the dependent variable in the linear mixed model was defined as the log‐transformed concentration of cefazolin and clindamycin. The independent variables were treated as categorical variables and an interaction effect between the independent variables was included in the model. We used a random intercept and assumed a first‐order autoregressive error covariance matrix to correct for within‐system correlations between time points. This model specification was chosen by comparing the values of the Akaike information criterion between different structures for the random effects and the error covariance matrix.
The predicted values for the log‐transformed concentration for each CPB system and at each time point were provided by the linear mixed models. We calculated the differences between the predicted values for each time point and each CPB system and T1, including the 95% Confidence Interval (95% CI) of this difference. We exponentiated this difference and the 95% CI to obtain drug recovery (percentage of drugs still present in the system) for T2 to T300. We calculated the maximum expected concentration (MEC) of the drug in the CPB systems by dividing the amount of drug added to the CPB systems by the total priming volume in the systems. We used the MEC since it was unclear whether the drug was mixed completely with the prime fluid at T1.
Statistical analyses were performed using IBM SPSS Statistics 24 and all statistical tests used a two‐sided significance level of 0.05.
3. RESULTS
A total of 81 samples, 27 for each CPB system category, was analyzed. We encountered no technical problems during the execution of the experiments.
Figure 1 shows predicted drug recovery (based on the estimated marginal means of the linear mixed models) versus time for cefazolin and clindamycin, for each CPB‐system category. Based on the interaction effects in the mixed models, there was a significant difference in the pattern of recovery over time between systems for both cefazolin and clindamycin (P < .001). For cefazolin, the lowest drug recovery was in the pediatric systems, while the highest drug recovery was in the neonatal systems. In the pediatric system, cefazolin concentration seems to show an initial decline, but then increased again to the starting drug concentration. This maximum in recovered concentration was found after two hours of CPB runtime. After these first two hours cefazolin concentration decreased to approximately 50% recovery at the end of the experiment.
Figure 1.
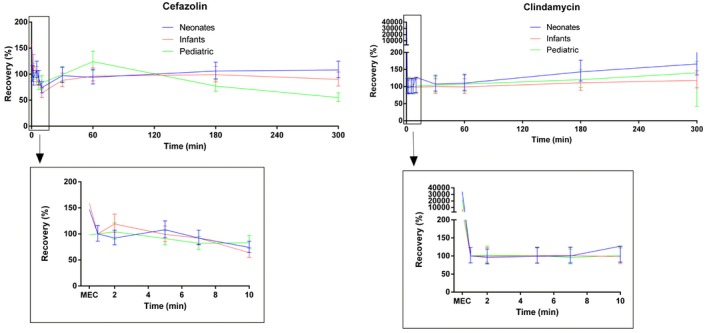
Predicted recovery of cefazolin and clindamycin (in % of theoretical concentration) versus time [Color figure can be viewed at https://www.wileyonlinelibrary.com]
For clindamycin, the highest recovery was observed in the neonatal systems, while the lowest recovery was in the infant systems. The clindamycin concentration was relatively stable during the experiment, with a mild increase in drug recovery in all systems starting at 60 minutes to the end of the experiment.
Mean recovery for cefazolin after 60 and 180 minutes, compared to T1, was 94% (95% CI: 81‐109) and 106% (95% CI: 91‐123) for the neonatal systems, 96% (95% CI: 83‐112) and 99% (95% CI: 85‐115) for the infant systems, and 124% (95% CI: 107‐144) and 77% (95% CI: 67‐89) for the pediatric systems.
Mean recovery for clindamycin after 60 and 180 minutes, compared to T1, was 110% (95% CI: 89‐136) and 143% (95% CI: 116‐177) for the neonatal CPB systems, 99% (95% CI: 80‐123) and 111% (95% CI: 89‐137) for the infant systems, and 107% (95% CI: 86‐132) and 120% (95% CI: 97‐149) for the pediatric systems.
For cefazolin the MEC was in the range of the measurements of T1 and T2, indicating complete mixing of cefazolin after injection in the CB system. For clindamycin MEC was much higher than all measurements, indicating a steep decline after injection. Mean clindamycin recovery after 60 and 180 minutes compared to the MEC was 0.3% and 0.4% for neonatal systems, 1.1% and 1.2% for infants’ systems, and 0.5% and 0.6% for pediatric systems.
4. DISCUSSION
The influence of pediatric CPB systems on plasma drug concentration is largely unknown. However, the use of CPB is inevitable in most congenital cardiac surgery procedures. For adult patients, in vivo and in vitro studies have been published.4, 7, 8, 9 We have investigated cefazolin and clindamycin plasma concentrations in three pediatric CPB systems that are used in our institution.
For cefazolin, there are no previous in vitro publications for adult or pediatric CPB systems. However, cefazolin was investigated in vitro in pediatric extracorporeal membrane oxygenation systems (ECMO).10 In these experiments, the recovery of cefazolin was high for both neonatal and pediatric ECMO systems, with an average cefazolin recovery at 2 minutes of 87%, and recovery at 180 minutes of 84.3%, with almost no difference between systems. After approximately 10 minutes plasma cefazolin concentration reached a steady state. These results are much in line with our findings. Our findings indicate that cefazolin mixes within minutes in the CPB systems and concentration moderately decreases throughout the experiments. Only in our pediatric systems the cefazolin concentration decreased by 50% by the end of the experiment. We cannot fully explain this decrease in only the pediatric systems. The components of the neonatal and pediatric CPB systems are similar and cefazolin plasma concentration is stable in the neonatal systems. The type and length of tubing could make a difference, with a larger contact surface area in the pediatric CPB systems. Absorption of drugs to PVC tubing with various coatings has been shown for fentanyl and morphine. This may also affect the cefazolin plasma concentration and account for the more profound decrease in the larger CPB system. Recently, De Cock et al published in vivo data on cefazolin plasma concentration during and after pediatric cardiac surgery.11 The authors collected blood samples from children undergoing cardiac surgery and concentration‐time profiles were analyzed using population pharmacokinetic modeling.12 The authors estimated that the volume of distribution of the CPB compartment in the pharmacokinetic model was equal to the priming volume of the CPB system. This means there should be no sequestration of cefazolin in the CPB system. However, the duration of surgical procedure and subsequent CPB use was the primary factor for not achieving the optimal cefazolin plasma levels. Considering the apparent lack of sequestration in the CPB systems, other factors may be responsible for the moderate decrease in cefazolin plasma levels that are not yet understood.
For clindamycin, the measured plasma concentrations remained stable over time, but the difference between the MEC and measured plasma concentration was large. These results were comparable for all tested CPB systems.
There are no previous studies published on the clindamycin use during cardiac surgery in adults or children. Our results, therefore, are the first to present these plasma concentrations. Measured clindamycin concentration remains stable during the CPB run, with an increase in the concentration toward the end of the experiment. This mild increase may be due to the recirculation of clindamycin from tubing or other CPB components. The most striking result is the large difference between the MEC and the first measured plasma concentration. This large difference may have several reasons. The first reason may be incomplete mixing of clindamycin with the prime fluid after injection in the CPB systems. This may lead to a preferential flow in the reservoir causing clindamycin to precipitate without circulating in the entire CPB system. An open hard‐shell reservoir is used in all CPB systems and this may be the compartment to cause clindamycin precipitation. However, we cannot explain the cause of precipitation other than incomplete mixing. Another potential cause for the discrepancy between MEC and measured concentration is sequestration of clindamycin to the plastic components or the defoaming area in the CPB system. Hydrophilic drugs such as clindamycin are not known to sequester in CPB systems. Sequestration in these systems is expected for lipophilic drugs, such as sufentanil or midazolam.13 As shown by Shekar et al14 in ECMO circuits, drugs with a LogP greater than 2.3 have a significantly higher decrease in the concentration compared to less lipophilic or hydrophilic drugs. As both cefazolin and clindamycin have a lower LogP they are not expected to sequester in the CPB systems (see Table 5).
Table 5.
Drug characteristics
| Blood/plasma ratio | LogP | Protein binding (%) | Vd (L/kg) | pKa | |
|---|---|---|---|---|---|
| Cefazolin | 1.21 | −0.4 | 74‐86 | 12 L | 3.03 (strongest acidic) |
| Clindamycin | Unavailable | 1.59 | 92‐94 | 70 L | 6.74 (strongest basic) |
Abbreviations: Vd, volume of distribution; pKa, acid dissociation constant at logarithmic scale; L, liter; Kg, kilogram; LogP, logarithm of partition coefficient.
Highly protein‐bound drugs (>80% protein bound) also sequester significantly more in ECMO systems and this could be a possible cause for the sequestration of clindamycin, since clindamycin is strongly protein bound. The Vd of clindamycin is mainly influenced by albumin and alpha‐1 acid glycoprotein.15 Albumin, but not alpha‐1 acid glycoprotein was added to the prime fluid and may have caused a shift in clindamycin protein binding resulting in a higher unbound fraction. However, we have measured the total serum concentration and we would not expect this concentration to change so dramatically due to changes in protein binding. The acid dissociation constant (pKa) could make a difference in mixing and recovery, with cefazolin being a strong acid compared to clindamycin. To our knowledge, no influence of pKa on sequestration of drugs in CPB or ECMO circuits has been published before. During the experiments, clindamycin concentration seems to gradually increase to above 100% of the T1 concentration, but not to the MEC values. No previous results have been published on a potentially reversible binding of hydrophilic drugs to CPB compartments. However, considering our results this may be a potential explanation for the increasing clindamycin concentration or we may have an incomplete mixing of clindamycin that gradually evolves. We cannot completely exclude an error in medication administration or analysis. However, the CPB systems were run on several days and medication was always double‐checked.
Our experiments show the effects of pediatric CPB systems on plasma concentration of cefazolin and clindamycin. Adequate concentration of cefazolin and clindamycin is imperative to prevent SSI. In children after cardiac surgery, SSI reportedly occurs in 1.9%‐8% of patients.16 Correct plasma concentrations of antibiotics are a relatively easily modifiable factor to prevent SSI and are therefore important to investigate in vitro and in vivo. As shown by Shah et al incorrect dosing of antibiotics gives a 1.7‐fold increased risk of developing an SSI in pediatric patients.17 Suboptimal dosing due to the influence of the CPB system may affect the occurrence of SSI. To date, no uniform guidelines for the dosing of antibiotic prophylaxis in children are available. Also, potentially increased risks of SSI after cardiac surgery are not incorporated in current dosing advice.11 Several procedural risk factors, such as the use of a bladder catheter, longer duration of CPB or prolonged surgery time, may increase the risk of SSI in pediatric cardiac surgery patients.1 Our results show that the plasma cefazolin concentration only moderately decreases during CPB in the pediatric system. Cefazolin concentration remains stable over time in the neonatal and infant systems. The increased risk of SSI with longer CPB runtime may be associated with other surgery and patient‐related factors, such as systemic inflammatory response syndrome (SIRS) and increased volume of distribution with subsequent lower plasma concentration. The most important moment to obtain adequate plasma concentrations is at the time of wound closure. This should be investigated in vivo, with the added patient‐related influence on plasma drug concentration. The loss of clindamycin in the CPB systems, with the recovery of only 0.3%‐1.2% compared to the MEC, should be further investigated. If this indeed holds true, antibiotic prophylaxis with clindamycin is not effective for children undergoing cardiac surgery.
Our study has several limitations. First, we have not corrected for the blood/plasma ratio of the measured drugs. For clindamycin, the blood/plasma ratio is not known. The blood/plasma ratio for cefazolin could potentially slightly change the measured plasma concentration. However, the measured concentrations are relatively high compared to clinically relevant values and therefore the blood/plasma ratio was not considered a large influence. Also, the blood/plasma ratio remains constant over time and therefore does not influence the increase or decrease in the drug concentration. Second, we did not correct for spontaneous degradation. Spontaneous degradation may introduce a potential bias in the measured plasma concentration, since this is a cause for drug loss. However, a study on the spontaneous degradation of cefazolin by Donnelly showed that cefazolin remains stable over 30 days.18 To our knowledge, no data have been published on clindamycin stability. From personal communication with our laboratory we know clindamycin to be stable for two years when kept at −20°C between processing and analysis, as is in line with good laboratory practice. Finally, during cardiac surgery, antibiotic administration is often repeated at the start of the CPB system. In contrast, we spiked the CPB systems at the beginning of the experiments and did not administer a redose during the experiment. This timing of administration only resembles short cardiac surgical procedures, without redosing at the onset of CPB. However, this method is often used in in vitro studies and most accurately reflects the influence of CPB runtime on drugs.
5. CONCLUSION
Our study shows a high recovery of cefazolin in the neonatal and infant CPB system, and the moderate recovery of cefazolin in the pediatric CPB system. We found a large discrepancy between the MEC and measured concentrations of clindamycin in all tested CPB systems. Our results do not explain this discrepancy and this should be further investigated to ensure adequate antibiotic prophylaxis in children undergoing cardiac surgery.
6. DATA SHARING AND DATA ACCESSIBILITY
Data published in this article can be provided by the authors upon request to support future research.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest with the contents of this article.
Zeilmaker‐Roest GA, van Saet A, van Hoeven MPJ, et al. Recovery of cefazolin and clindamycin in in vitro pediatric CPB systems. Artif Organs. 2020;44:394–401. 10.1111/aor.13595
Funding information
We did not receive funding for these studies.
REFERENCES
- 1. Bucher BT, Guth RM, Elward AM, Hamilton NA, Dillon PA, Warner BW, et al. Risk factors and outcomes of surgical site infection in children. J Am Coll Surg. 2011;212:1033‐1038.e1. [DOI] [PubMed] [Google Scholar]
- 2. van Saet A, de Wildt SN, Knibbe CA, Bogers AJ, Stolker RJ, Tibboel D. The effect of adult and pediatric cardiopulmonary bypass on pharmacokinetic and pharmacodynamic parameters. Curr Clin Pharmacol. 2013;8:297‐318. [DOI] [PubMed] [Google Scholar]
- 3. Hynynen M, Hammaren E, Rosenberg PH. Propofol sequestration within the extracorporeal circuit. Can J Anaesth. 1994;41:583‐588. [DOI] [PubMed] [Google Scholar]
- 4. Hudson RJ, Thomson IR, Jassal R. Effects of cardiopulmonary bypass on sufentanil pharmacokinetics in patients undergoing coronary artery bypass surgery. Anesthesiology. 2004;101:862‐871. [DOI] [PubMed] [Google Scholar]
- 5. Preston TJ, Ratliff TM, Gomez D, Olshove VE Jr, Nicol KK, Sargel CL, et al. Modified surface coatings and their effect on drug adsorption within the extracorporeal life support circuit. J Extra Corpor Technol. 2010;42:199‐202. [PMC free article] [PubMed] [Google Scholar]
- 6. Baksaas ST, Videm V, Fosse E, Karlsen H, Pedersen T, Mollnes TE, et al. In vitro evaluation of new surface coatings for extracorporeal circulation. Perfusion. 1999;14:11‐19. [DOI] [PubMed] [Google Scholar]
- 7. Dawson PJ, Bjorksten AR, Blake DW, Goldblatt JC. The effects of cardiopulmonary bypass on total and unbound plasma concentrations of propofol and midazolam. J Cardiothorac Vasc Anesth. 1997;11:556‐561. [DOI] [PubMed] [Google Scholar]
- 8. Hammaren E, Rosenberg PH, Hynynen M. Coating of extracorporeal circuit with heparin does not prevent sequestration of propofol in vitro. Br J Anaesth. 1999;82:38‐40. [DOI] [PubMed] [Google Scholar]
- 9. Su HB, Tseng CC, Jenn CT, Chang CL, Huang JD. Changes of propofol levels in isolated cardiopulmonary bypass circuit. Acta Anaesthesiol Sin. 1996;34:17‐20. [PubMed] [Google Scholar]
- 10. Wildschut ED, Ahsman MJ, Allegaert K, Mathot RAA, Tibboel D. Determinants of drug absorption in different ECMO circuits. Intensive Care Med. 2010;36:2109‐2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. De Cock PA, Mulla H, Desmet S, De Somer F, McWhinney BC, Ungerer JP, et al. Population pharmacokinetics of cefazolin before, during and after cardiopulmonary bypass to optimize dosing regimens for children undergoing cardiac surgery. J Antimicrob Chemother. 2017;72:791‐800. [DOI] [PubMed] [Google Scholar]
- 12. Benet LZ, Zia‐Amirhosseini P. Basic principles of pharmacokinetics. Toxicol Pathol. 1995;23:115‐123. [DOI] [PubMed] [Google Scholar]
- 13. Raffaeli G, Allegaert K, Koch B, Cavallaro G, Mosca F, Tibboel D, et al. In vitro adsorption of analgosedative drugs in new extracorporeal membrane oxygenation circuits. Pediatr Crit Care Med. 2018;19:e251‐e258. [DOI] [PubMed] [Google Scholar]
- 14. Shekar K, Roberts JA, Mcdonald CI, Ghassabian S, Anstey C, Wallis SC, et al. Protein‐bound drugs are prone to sequestration in the extracorporeal membrane oxygenation circuit: results from an ex vivo study. Crit Care. 2015;19:164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Smith MJ, Gonzalez D, Goldman JL, Yogev R, Sullivan JE, Reed MD, et al. Pharmacokinetics of clindamycin in obese and nonobese children. Antimicrob Agents Chemother. 2017;61:e02014‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Costello JM, Graham DA, Morrow DF, Morrow J, Potter‐Bynoe G, Sandora TJ, et al. Risk factors for surgical site infection after cardiac surgery in children. Ann Thorac Surg. 2010;89:1833‐1842; discussion 41‐2. [DOI] [PubMed] [Google Scholar]
- 17. Shah GS, Christensen RE, Wagner DS, Pearce BK, Sweeney J, Tait AR. Retrospective evaluation of antimicrobial prophylaxis in prevention of surgical site infection in the pediatric population. Paediatr Anaesth. 2014;24:994‐998. [DOI] [PubMed] [Google Scholar]
- 18. Donnelly RF. Stability of cefazolin sodium in polypropylene syringes and polyvinylchloride minibags. Can J Hosp Pharm. 2011;64:241‐245. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data published in this article can be provided by the authors upon request to support future research.


