Abstract
Since their discovery about 10 years ago, Th9 cells have been increasingly linked to allergic pathologies. Within this review, we summarize the current knowledge on associations between Th9 cells and allergic diseases and acknowledge Th9 cells as important targets in future treatment of allergic diseases. However, until today, it is not fully understood how these Th9 cell responses are modulated. We describe current literature suggesting that these Th9 cell responses might be stimulated by microbial species such as Staphylococcus aureus and Candida albicans, while on the other hand, microbial and dietary compounds such as retinoic acid (RA), butyrate and vitamin D show suppressive capacity on allergy‐related Th9 responses. By reviewing this recent research, we provide new insights into the modulating capacity of the microbiota on Th9 cell responses. Consequently, microbial and dietary factors may be used as innovative tools to target Th9 cells in the treatment of allergic diseases. However, further research is needed to elucidate the mechanisms behind these interactions in order to translate this knowledge into clinical allergy settings.
Keywords: allergy, microbiota, T helper 9 cells
1. INTRODUCTION
With their discovery about 10 years ago, Th9 cells are the most recently emerged subtype of CD4 T cells and they are characterized by a potent secretion of interleukin‐9 (IL‐9), a pleiotropic cytokine with both protective and disease‐promoting effects. Historically, IL‐9 was considered a Th2 cytokine, but two independent studies discovered the differential development of IL‐9 producing cells, distinguishing Th9 cells as a distinct cell type. These studies observed that the Th9 promoting conditions during naïve T cell differentiation, which consisted of a combination of IL‐4 and TGF‐β, actually inhibited the development of Th2 cells.1, 2 Nevertheless, the hypothesis that Th9 cells are an activated subpopulation of Th2 cells was debated again by recent research again.3 Until now, Th9 cells have shown to be involved in protection against parasitic infections4 and anti‐tumour immunity,5 but they are also linked to pathologies as described for allergic conditions, such as asthma, allergic rhinitis, atopic dermatitis (AD) and food allergies.6, 7
Today, there is substantial evidence to support the idea that the microbiota and microbiota‐derived metabolites influence the development of T cell responses.8 Within this review, we aim to provide an overview of the recent knowledge on Th9 cells, their relation to allergic diseases as well as of the effects of the microbiota and associated factors on Th9 cell development.
2. Th9 CELL CHARACTERIZATION
IL‐9 is not only made by CD4 T cells, but can be produced by a broad range of immune cells, such as mucosal mast cells,9 type 2 innate lymphoid cells (ILC2),10 γδ T cells 11 and CD8 T cells.12 The downstream functionality of IL‐9 seems to depend on the cellular source as well as the microenvironment of the secreting cells. Consequently, IL‐9 production on its own is not sufficient to uniquely define Th9 cells.
In vitro studies show that naïve CD4 T cells develop into Th9 cells following polarization with IL‐4 and TGF‐β.1, 2 The levels of IL‐9 produced by these Th9 cells fluctuate over time, but usually peak after 72 hours.13 It has additionally been suggested that costimulatory molecules, such as OX40, also known as tumour necrosis factor receptor superfamily 4, selectively and potently promote Th9 cell development.14
The transcription factor network regulating this characteristic Th9 response is not completely understood, and there is no single master transcription factor that uniquely characterizes Th9 cells determined as yet. Notwithstanding, several candidate transcription factors have been proposed.15
It was demonstrated that Th9 cell development failed in interferon regulatory factor 4 (IRF4)–deficient CD4 T cells and that IL‐9 production is strongly reduced in established Th9 cells where IRF4 was silenced with a small interfering RNA (siRNA). Moreover, it was shown that IRF4 regulates the development of Th9 cells by directly binding to the IL‐9 promoter. Nevertheless, the role of IRF4 is not Th9 cell specific, since this transcription factor is also clearly involved in the regulation of Th2 and Th17 cells.16, 17
STAT6, downstream of the IL‐4 signalling pathway, was observed to be required for the induction of IRF4 and was subsequently considered important in the early stages of Th9 cell development.18 In addition, upregulation of the transcription factors PU.1, FOXO1, and BATF were found in Th9 cells, and inhibition of these transcription factors significantly reduced IL‐9 expression in the CD4 T cells.19, 20, 21 FOXO1 seemed to perform a dual action by binding both to the IL‐9 and IRF4 promoter and thereby modulating the stimulating effect of IRF4.20 Th9 cell development might also be stimulated indirectly, by intracellular binding of the two transcription factors FOXP3 and GATA‐3 to each other.2 This binding leads to the formation of a transcription factor complex which consequently inhibits the generation of Treg and Th2 cells, favouring the formation of Th9 cells. Very recently, the expression of peroxisome proliferator‐activated receptor γ (PPARγ) was found to be essential for IL‐9 production by CD4 T cells,3 and a novel role for the amino acid sensor general control non‐derepressible 2 (GCN2) was discovered in Th9 cell differentiation.22 Finally, also Smad proteins and Notch signalling were found to influence IL‐9 production by binding directly to the IL‐9 promoter. In particular, Smad 2 and Smad 4 which are activated by TGF‐β signalling, seem to regulate IL‐9 gene expression by inducing epigenetic changes at the IL‐9 locus, while Notch receptors cooperate with Smad 3 to promote Th9 cell differentiation.23, 24
In conclusion, a broad spectrum of transcription factors and signalling pathways are involved in Th9 cell development and further investigation is necessary to determine the master transcription factor that may uniquely characterize these cells.
3. Th9 CELLS IN ALLERGIC DISEASES
Following the discovery of Th9 cells as a separate T helper subtype, these cells have emerged as important mediators of allergic inflammation. This potential role of Th9 cells in allergic diseases relates to the functional capacity of IL‐9 to stimulate IgE production by B cells,25, 26 accumulate and activate mast cells,27 enhance eosinophil chemotaxis 28 and stimulate mucin production in lung epithelial cells.29 Moreover, Th9 cells were recently discovered to express histamine H4 receptors, which might enhance the allergy promoting and inflammatory potential of these cells during allergic responses.30 In addition, IL‐9 negatively influenced epithelial barrier integrity during intestinal inflammation, for example during inflammatory bowel disease (IBD). In IBD, IL‐9 secretion by Th9 cells recruits and stimulates mast cells, which in turn secrete pro‐inflammatory cytokines and proteases affecting the gut barrier permeability. This disruption of the intestinal barrier results in increased entry of antigens, which might over‐activate the mucosal immune cells and therefore also enhance the risk for allergic responses.31
Numerous studies, conducted both in humans and mice, recognized the role of IL‐9 and Th9 cells in airway allergic diseases, due to their contribution to airway inflammation. Research on human cohort material revealed an increased IL‐9 expression in bronchoalveolar lavage (BAL) from atopic asthma patients,32 which is in line with findings of increased IL‐9 production by CD4 T cells in peripheral blood mononuclear cells (PBMCs) from adult atopic asthma patients,33 as well as from atopic infants after allergen‐specific and polyclonal challenge.26, 34 Importantly, a correlation between the frequencies of these IL‐9 producing cells and serum IgE levels was also observed in these asthma patients.33 Interestingly, using a mouse asthma model, it was proven that an anti‐IL‐9 antibody treatment inhibits airway inflammation by reducing the numbers of eosinophils and lymphocytes as well as the levels of type 2 cytokines IL‐4 and IL‐13 in the lungs.35 However, in human trials, anti‐IL9 treatment was not yet found to ameliorate asthma symptoms,36 while blocking Th2 responses has shown promising results.37
Next to these associations with allergic asthma, single‐nucleotide polymorphisms in both IL‐9 and IL‐9 receptor genes were found associated with an increased susceptibility to AD.36 This relation between AD and Th9 cells was strengthened by the finding that large part of the Th9 cells are skin‐tropic and skin‐resident, expressing cutaneous homing receptors and being highly present in skin lesions, for example in psoriasis.38, 39 Moreover, enhanced IL‐9 expression levels have been described in atopic skin lesions of AD patients compared to healthy controls.40, 41
Besides this convincing evidence in asthma and AD, associations between food allergies and Th9 cells have also emerged from studies on peanut allergy. Peanut‐specific IL‐9 responses revealed high discriminatory capacity between PBMCs from peanut‐allergic and peanut‐tolerant individuals.42 These results were complemented by a study showing that IL‐9 is an important component of the peanut‐specific memory T cells in peanut‐allergic children. Moreover, this study clearly characterized these IL‐9 secreting cells as Th9 cells and showed even a discriminatory Th9 response between peanut‐allergic and peanut‐sensitized children.43 Apart from the association with peanut allergy, a role for IL‐9 secreting cells is also suggested in cow's milk allergy, since a study in cow's milk allergic children showed a correlation between IL‐9 plasma levels and eosinophilia, which is known to contribute to allergy development.44
Combined, these results stress the importance of Th9 cells in allergic pathologies and underline their potential as target for future allergy treatment, although further investigation is clearly needed.
4. ASSOCIATION BETWEEN THE MICROBIOTA, DIETARY COMPOUNDS AND Th9 CELLS
While the microbiota is referred to as the microbes lining the mucosa and epithelial surfaces of the entire body, the microbial community of the gut is the most abundant. The composition of the gut microbiota is developing already in early life but it is dynamic and shaped by both genetic and non‐genetic factors.45, 46 Previous research indicated strong associations between host immunity and the composition of the microbiota, not only in the gut, but also in organs such as the lung and skin.46, 47 This connection between microbiota and immunity was already suggested by the ‘hygiene hypothesis’, which described early life infections and microbe encounters as important to balance the Th1 and Th2 cell responses and consequently to prevent allergy development.46, 48 Nowadays, CD4 T cells are considered to have high plasticity, being able to switch between subtypes and functional capacity depending on received environmental triggers. A large part of these environmental stimuli comes from the microbiota and microbiota‐derived factors, although the exact mechanisms and modulating compounds are still largely unknown.8, 49
Specialized organ‐resident T cells are present in every organ and are influenced by the organ‐specific microbiota. However, evidence is accumulating that effects of gut‐derived microbial factors are not limited to the gut microenvironment, but also signals to immune cells in other organs such as the lung and brain, via the so‐called gut‐lung and gut‐brain axes. These broad effects might be mediated by the release of gut‐derived microbial and dietary factors, such as short‐chain fatty acids (SCFAs), into the circulation, or due to traffic of gut‐derived activated T cells towards other compartments of the body.45, 47 Consequently, the gut microbiota might influence the immune system in the entire organism.
With Th9 cells being a new player in the field and being strongly related to allergy development, it is of importance to investigate whether Th9 cells are modulated, either induced or inhibited, by microbial species or microbial and dietary metabolites.
Although research on associations between microbial species and Th9 cell responses is scarce, some interesting observations have been made. First of all, Staphylococcus aureus, a commensal but potential pathogenic Gram‐positive bacterium commonly found in the nasopharynx and on the skin, was suggested to enhance Th9 cell responses. This effect might be dependent on the staphylococcal enterotoxins, since Staphylococcal enterotoxin B enhanced Th9 cell properties among CD4 T cells in a cancer murine model.51 Moreover, S aureus has been frequently linked to AD and asthma in humans, clinical manifestations that are also often related to Th9 cell responses and could potentially be mediated by staphylococcal toxin‐specific IgE.51, 52 Noteworthy, S aureus is also often found in the infant gut,53 but whether this could influence Th9 development in the gut during infancy is not known.
In addition, the opportunistic fungus Candida albicans, inhabiting most of the body's epithelial barriers, was observed to stimulate Th9 cells both in the human skin and gut.39, 54 Specifically, it was shown that a high proportion of skin‐tropic Th9 cells possessed specificity for C albicans, since stimulation of these Th9 cells with a heat‐killed preparation of the fungus lead to enhancement of IL‐9 secretion.39 Moreover, C albicans enhanced Th9 cell development in CD4 T cells residing in Peyer's patches and mesenteric lymph nodes.54 Importantly, it was also hypothesized that C albicans may exacerbate asthma symptoms.55 However, mechanisms or possible mediators behind these effects remain to be unravelled.
On the other hand, several reports determined inhibiting effects of gut‐derived compounds on Th9 cell responses. First of all, recent murine studies showed that butyrate, one of the microbiota‐derived SCFAs, suppressed Th9 cell responses in a lung inflammation setting. Specifically, butyrate reduced the frequency of Th9 cells in the lung and subsequently reduced eosinophil infiltration and lung inflammation.56
In addition, antagonizing effects of retinoic acid (RA), a dietary metabolite of vitamin A which is synthesized by mucosal dendritic cells (DCs), were described.57 RA showed to impact the transcriptome of Th9 cells while not affecting other T helper subtypes to the same extent. Th9 cells development was inhibited by direct binding of RA to its receptor RARα and subsequent repression of the extended IL9 locus. In addition, it was determined that allergic inflammation in human asthma is associated with a decreased expression of RA target genes.57 These results complement a previous finding showing an association between vitamin A deficiency and a higher prevalence and severity of allergic asthma.58
Finally, the metabolic active form of vitamin D, 1α,25‐dihydroxyvitamin D3, was shown to inhibit the Th9 cell development in PBMCs from asthma patients in vitro. Vitamin D is either taken up by the skin or gut and transformed to the active form by the liver and kidneys. However, the mechanisms by which it does so, as well as the clinical applications, are to date not fully understood.59
These findings suggest that dietary and microbial compounds have broad modulatory effects on Th9 cell immune responses, and their influence is not limited to their organ of origin but can be extended throughout the body. The above‐mentioned potential modulatory effects of microbial species and microbial and diet‐derived factors are summarized in Table 1. and graphically depicted in Figure 1.
Table 1.
Summary of the current research on associations between microbial species, dietary metabolites and Th9 cells
| Microbial species and dietary metabolites | Effect on Th9 cells | Model organism | Ref. |
|---|---|---|---|
|
Staphylococcal enterotoxin B (SEB) |
|
Murine cancer model | 50 |
| Candida albicans |
|
Human peripheral blood and skin tissue | 39 |
| C albicans |
|
Murine model and human duodenum biopsies | 54 |
| Butyrate |
|
Murine lung inflammation model | 56 |
| Retinoic acid |
|
Human peripheral blood and murine asthma model | 57 |
| 1α,25‐dihydroxyvitamin D3 |
|
Peripheral blood mononuclear cells from human asthma patients | 59 |
Figure 1.
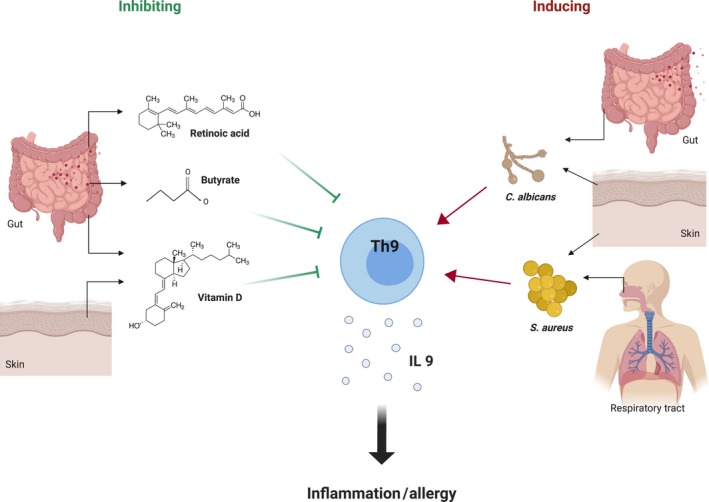
Schematic representation of the current knowledge on associations between microbial species as well as microbial and dietary metabolites and Th9 cells. Inhibiting effects on Th9 cells are represented by green lines, while stimulating effects are indicated with red arrows. Origin site and/or localization of the microbes and microbial and dietary metabolites is shown. (Figure made with BioRender)
Further investigation on the associations between the microbiota, dietary factors and Th9 cells is clearly needed, while at the same time relating these associations to clinical outcomes such as allergy development.
5. CONCLUSION
In the 10 years since the discovery of Th9 cells, these cells have been associated with a broad range of allergic diseases. Consequently, Th9 cells are increasingly acknowledged as potential targets to prevent and treat allergic pathologies. Since the microbiota and microbial derived factors as well as dietary compounds have been recognized as important immunomodulators, also in relation to allergic disease, it is of interest to investigate whether they can have an impact on Th9 cells and their responses.
This review of current literature suggests that S aureus and C albicans enhance Th9 cell responses in different organs. Future research should further explore these associations as well as the underlying mechanisms and clinical consequences. Moreover, we highlight retinoic acid, butyrate and vitamin D as important modulating and antagonistic factors for Th9 cells that could eventually be used in allergic disease management. Further investigation is needed to understand how and to what extent these metabolites influence the immune responses in different organs. Lastly, it is of interest to investigate the possible applications of these compounds in clinical allergy settings.
CONFLICT OF INTEREST
The authors have no conflict of interest.
AUTHOR CONTRIBUTIONS
MvdH and ESE conceptualized the manuscript; IB and MvdH wrote the manuscript; and all authors critically revised the manuscript.
ACKNOWLEDGMENT
This review was financially supported by The Swedish Asthma and Allergy Association's Research Foundation, The Swedish Research Council 2016‐01715_3, Hjärt‐ och Lungfonden, and Stiftelsen Olle Engkvist Byggmästare.
Badolati I, Sverremark‐Ekström E, van der Heiden M. Th9 cells in allergic diseases: A role for the microbiota?. Scand J Immunol. 2020;91:e12857 10.1111/sji.12857
REFERENCES
- 1. Veldhoen M, Uyttenhove C, van Snick J, et al. Transforming growth factor‐β “reprograms” the differentiation of T helper 2 cells and promotes an interleukin 9‐producing subset. Nat Immunol. 2008;9:1341‐1346. [DOI] [PubMed] [Google Scholar]
- 2. Dardalhon V, Awasthi A, Kwon H, et al. IL‐4 inhibits TGF‐β‐induced Foxp3+ T cells and together with TGF‐β, generates IL‐9+ IL‐10+ Foxp3‐ effector T cells. Nat Immunol. 2008;9:1347‐1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Micossé C, von Meyenn L, Steck O, et al. Human “TH9” cells are a subpopulation of PPAR‐+ TH2 cells. Sci Immunol. 2019;4:1‐14. [DOI] [PubMed] [Google Scholar]
- 4. Licona‐Limón P, Henao‐Mejia J, Temann AU, et al. Th9 cells drive host immunity against gastrointestinal worm infection. Immunity. 2013;39:744‐757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rivera Vargas T, Humblin E, Végran F, et al. TH9 cells in anti‐tumor immunity. Semin Immunopathol. 2017;39:39‐46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kaplan MH. Th9 cells in allergic disease. Curr Allergy Asthma Rep. 2019;19:1‐9. [DOI] [PubMed] [Google Scholar]
- 7. Garn H. Is 9 more than 2 also in allergic airway inflammation? J Allergy Clin Immunol. 2018;141:2024‐2026. [DOI] [PubMed] [Google Scholar]
- 8. Pezoldt J, Yang J, Zou M, Huehn J. Microbiome and gut immunity: T cells. Gut Microbiome Heal Dis. 2018:119‐140. [Google Scholar]
- 9. Chen CY, Lee JB, Liu B, et al. Induction of Interleukin‐9‐producing mucosal mast cells promotes susceptibility to IgE‐mediated experimental food allergy. Immunity. 2015;43:788‐802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rauber S, Luber M, Weber S, et al. Resolution of inflammation by interleukin‐9‐producing type 2 innate lymphoid cells. Nat Med. 2017;23:938‐944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peters C, Häsler R, Wesch D, Kabelitz D. Human Vδ2 T cells are a major source of interleukin‐9. Proc Natl Acad Sci. 2016;113:12520‐12525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Visekruna A, Ritter J, Scholz T, et al. Tc9 cells, a new subset of CD8+ T cells, support Th2‐mediated airway inflammation. Eur J Immunol. 2013;43:606‐618. [DOI] [PubMed] [Google Scholar]
- 13. Tan C, Aziz MK, Lovaas JD, et al. Antigen‐specific Th9 cells exhibit uniqueness in their kinetics of cytokine production and short retention at the inflammatory site. J Immunol. 2010;185:6795‐6801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Xiao X, Balasubramanian S, Liu W, et al. OX40 signaling favors the induction of TH9 cells and airway inflammation. Nat Immunol. 2012;13:981‐990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kaplan MH. The transcription factor network in Th9 cells. Semin Immunopathol. 2017;39:11‐20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Staudt V, Bothur E, Klein M, et al. Interferon‐regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity. 2010;33:192‐202. [DOI] [PubMed] [Google Scholar]
- 17. Veldhoen M. Interferon regulatory factor 4: combinational control of lymphocyte differentiation. Immunity. 2010;33:141‐143. [DOI] [PubMed] [Google Scholar]
- 18. Goswami R, Jabeen R, Yagi R, et al. STAT6‐dependent regulation of Th9 development. J Immunol. 2012;188:968‐975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chang HC, Sehra S, Goswami R, et al. The transcription factor PU.1 is required for the development of IL‐9‐producing T cells and allergic inflammation. Nat Immunol. 2010;11:527‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buttrick TS, Wang W, Yung C, et al. Foxo1 promotes Th9 cell differentiation and airway allergy. Sci Rep. 2018;8:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jabeen R, Goswami R, Awe O, et al. Th9 cell development requires a BATF‐regulated transcriptional network. J Clin Invest. 2013;123:4641‐4653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang P, Xu Y, Zhang J, et al. The amino acid sensor general control nonderepressible 2 (GCN2) controls Th9 cells and allergic airway inflammation. Mech Allergy Immunol. 2019;144:1091‐1105. [DOI] [PubMed] [Google Scholar]
- 23. Wang A, Pan D, Lee Y‐H, et al. Cutting Edge: Smad2 and Smad4 regulate TGF‐ ‐mediated Il9 gene expression via EZH2 displacement. J Immunol. 2013;191:4908‐4912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elyaman W, Bassil R, Bradshaw EM, et al. Notch receptors and Smad3 signaling cooperate in the induction of interleukin‐9‐producing T cells. Immunity. 2012;36:623‐634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Petit‐Frere C, Dugas B, Braquet P, Mencia‐Huerta JM. Interleukin‐9 potentiates the interleukin‐4‐induced IgE and IgG1 release from murine B lymphocytes. Immunology. 1993;79:146‐151. [PMC free article] [PubMed] [Google Scholar]
- 26. Jia L, Wang Y, Li J, et al. Detection of IL‐9 producing T cells in the PBMCs of allergic asthmatic patients. BMC Immunol. 2017;18:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sehra S, Yao W, Nguyen ET, et al. TH9 cells are required for tissue mast cell accumulation during allergic inflammation. J Allergy Clin Immunol. 2015;136:433‐440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dong Q, Louahed J, Vink A, et al. IL‐9 induces chemokine expression in lung epithelial cells and baseline airway eosinophilia in transgenic mice. Eur J Immunol. 1999;29:2130‐2139. [DOI] [PubMed] [Google Scholar]
- 29. Longphre M, Li D, Gallup M, et al. Allergen‐induced IL‐9 directly stimulates mucin transcription in respiratory epithelial cells. J Clin Invest. 1999;104:1375‐1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schaper‐Gerhardt K, Wohlert M, Mommert S, et al. Stimulation of histamine H 4 receptors increases the production of IL‐9 in Th9 polarized cells. Br J Pharmacol. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vyas SP, Goswami R. A decade of Th9 cells: role of Th9 cells in inflammatory bowel disease. Front Immunol. 2018;9:7‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Erpenbeck VJ, Hohlfeld JM, Volkmann B, et al. Segmental allergen challenge in patients with atopic asthma leads to increased IL‐9 expression in bronchoalveolar lavage fluid lymphocytes. J Allergy Clin Immunol. 2003;111:1319‐1327. [DOI] [PubMed] [Google Scholar]
- 33. Jones CP, Gregory LG, Causton B, Campbell GA, Lloyd CM. Activin A and TGF‐β promote TH9 cell‐mediated pulmonary allergic pathology. J Allergy Clin Immunol. 2012;129:1000‐1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yao W, Tepper RS, Kaplan MH. Predisposition to the development of IL‐9–secreting T cells in atopic infants. J Allergy Clin Immunol. 2011;128:1357‐1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cheng G, Arima M, Honda K, et al. Anti‐Interleukin‐9 antibody treatment inhibits airway inflammation and hyperreactivity in mouse asthma model. Am J Respir Crit Care Med. 2002;166:409‐416. [DOI] [PubMed] [Google Scholar]
- 36. Oh CK, Leigh R, McLaurin KK, et al. A randomized, controlled trial to evaluate the effect of an anti‐interleukin‐9 monoclonal antibody in adults with uncontrolled asthma. Respir Res. 2013;14:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. McGregor MC, Krings JG, Nair P, Castro M. Role of biologics in asthma. Am J Respir Crit Care Med. 2019;199:433‐445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Namkung JH, Lee JE, Kim E, et al. An association between IL‐9 and IL‐9 receptor gene polymorphisms and atopic dermatitis in a Korean population. J Dermatol Sci. 2011;62:16‐21. [DOI] [PubMed] [Google Scholar]
- 39. Schlapbach C, Gehad A, Yang C, et al. Human T H 9 cells are skin‐tropic and have autocrine and paracrine proinflammatory capacity. Sci Transl Med. 2014;6:1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Hamza AM, Omar SS, Abo El‐Wafa RAH, Elatrash MJ. Expression levels of transcription factor PU.1 and interleukin‐9 in atopic dermatitis and their relation to disease severity and eruption types. Int J Dermatol. 2017;56:534‐539. [DOI] [PubMed] [Google Scholar]
- 41. Sismanopoulos N, Delivanis DA, Alysandratos KD, et al. IL‐9 induces VEGF secretion from human mast cells and IL‐9/IL‐9 receptor genes are overexpressed in atopic dermatitis. PLoS ONE. 2012;7:e33271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Xie J, Lotoski LC, Chooniedass R, et al. Elevated antigen‐driven IL‐9 responses are prominent in peanut allergic humans. PLoS ONE. 2012;7:e45377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Brough HA, Cousins DJ, Munteanu A, et al. IL‐9 is a key component of memory TH cell peanut‐specific responses from children with peanut allergy. J Allergy Clin Immunol. 2014;134:1329‐1338. [DOI] [PubMed] [Google Scholar]
- 44. Barros KV, Silveira VLF, Laranjeira MS, et al. Evidence for involvement of IL‐9 and IL‐22 in cows’ milk allergy in infants. Nutrients. 2017;9:1‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schroeder BO, Bäckhed F. Signals from the gut microbiota to distant organs in physiology and disease. Nat Med. 2016;22:1079‐1089. [DOI] [PubMed] [Google Scholar]
- 46. Pascal M, Perez‐Gordo M, Caballero T, et al. Microbiome and allergic diseases. Front Immunol. 2018;9:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Anand S, Mande SS. Diet, microbiota and gut‐lung connection. Front Microbiol. 2018;9:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Strachan DP. Hay fever, hygiene, and household size. BMJ. 1989;299:1259‐1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Brucklacher‐Waldert V, Carr EJ, Linterman MA, Veldhoen M. Cellular plasticity of CD4+ T cells in the intestine. Front Immunol. 2014;5:1‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Miao BP, Zhang RS, Sun HJ, et al. Inhibition of squamous cancer growth in a mouse model by Staphylococcal enterotoxin B‐triggered Th9 cell expansion. Cell Mol Immunol. 2017;14:371‐379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hon KL, Tsang YC, Pong NH, et al. Clinical features and Staphylococcus aureus colonization/infection in childhood atopic dermatitis. J Dermatolog Treat. 2016;27:235‐240. [DOI] [PubMed] [Google Scholar]
- 52. Bachert C, Van Steen K, Zhang N, et al. Specific IgE against Staphylococcus aureus enterotoxins: an independent risk factor for asthma. J Allergy Clin Immunol. 2012;130:376‐381. [DOI] [PubMed] [Google Scholar]
- 53. Lindberg E, Adlerberth I, Hesselmar B, et al. High rate of transfer of staphylococcus aureus from parental skin to infant gut flora. J Clin Microbiol. 2004;42:530‐534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Renga G, Moretti S, Oikonomou V, et al. IL‐9 and mast cells are key players of candida albicans commensalism and pathogenesis in the gut. Cell Rep. 2018;23:1767‐1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Goldman DL, Huffnagle GB. Potential contribution of fungal infection and colonization to the development of allergy. Med Mycol. 2009;47:445‐456. [DOI] [PubMed] [Google Scholar]
- 56. Vieira RS, Castoldi A, Basso PJ, et al. Butyrate attenuates lung inflammation by negatively modulating Th9 cells. Front Immunol. 2019;10:1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Schwartz DM, Farley TK, Richoz N, et al. Retinoic acid receptor alpha represses a Th9 transcriptional and epigenomic program to reduce allergic pathology. Immunity. 2019;50:106‐120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Marquez HA, Cardoso WV. Vitamin A‐retinoid signaling in pulmonary development and disease. Mol Cell Pediatr. 2016;3:3‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Keating P, Munim A, Hartmann JX. Effect of vitamin D on T‐helper type 9 polarized human memory cells in chronic persistent asthma. Ann Allergy Asthma Immunol. 2014;112:154‐162. [DOI] [PubMed] [Google Scholar]


