Abstract
The deep sea plays a critical role in global climate regulation through uptake and storage of heat and carbon dioxide. However, this regulating service causes warming, acidification and deoxygenation of deep waters, leading to decreased food availability at the seafloor. These changes and their projections are likely to affect productivity, biodiversity and distributions of deep‐sea fauna, thereby compromising key ecosystem services. Understanding how climate change can lead to shifts in deep‐sea species distributions is critically important in developing management measures. We used environmental niche modelling along with the best available species occurrence data and environmental parameters to model habitat suitability for key cold‐water coral and commercially important deep‐sea fish species under present‐day (1951–2000) environmental conditions and to project changes under severe, high emissions future (2081–2100) climate projections (RCP8.5 scenario) for the North Atlantic Ocean. Our models projected a decrease of 28%–100% in suitable habitat for cold‐water corals and a shift in suitable habitat for deep‐sea fishes of 2.0°–9.9° towards higher latitudes. The largest reductions in suitable habitat were projected for the scleractinian coral Lophelia pertusa and the octocoral Paragorgia arborea, with declines of at least 79% and 99% respectively. We projected the expansion of suitable habitat by 2100 only for the fishes Helicolenus dactylopterus and Sebastes mentella (20%–30%), mostly through northern latitudinal range expansion. Our results projected limited climate refugia locations in the North Atlantic by 2100 for scleractinian corals (30%–42% of present‐day suitable habitat), even smaller refugia locations for the octocorals Acanella arbuscula and Acanthogorgia armata (6%–14%), and almost no refugia for P. arborea. Our results emphasize the need to understand how anticipated climate change will affect the distribution of deep‐sea species including commercially important fishes and foundation species, and highlight the importance of identifying and preserving climate refugia for a range of area‐based planning and management tools.
Keywords: climate change, cold‐water corals, deep‐sea, fisheries, fishes, habitat suitability modelling, octocorals, scleractinians, species distribution models, vulnerable marine ecosystems
We used environmental niche modelling to predict the habitat suitability for key cold‐water coral and commercially important deep‐sea fish species under present‐day environmental conditions and to forecast changes under future climate projections (RCP8.5) for the North Atlantic Ocean. Our models forecasted a significant decrease in suitable habitat for cold‐water corals and poleward expansion in suitable habitat for deep‐sea fishes in response to climate change. Our results emphasize the need to understand how climate change will affect the distribution of deep‐sea species and highlight the importance of identifying and preserving climate refugia for a range of area‐based planning and management tools.

1. INTRODUCTION
The deep sea represents at least 95% of the ocean and plays a critical role in climate regulation through uptake and storage of heat and carbon dioxide (Purkey & Johnson, 2010; Sabine et al., 2004). However, changes linked to these regulating services have consequences for the health of the ocean including warming, acidification, and deoxygenation of deep waters, leading to decrease in food availability at the seafloor (Bindoff et al., 2019; Chen et al., 2017; Gehlen et al., 2014; Mora et al., 2013; Perez et al., 2018; Sulpis et al., 2018; Sweetman et al., 2017). Recent projections of deep water mass properties suggested that portions of the seafloor will experience average temperature increases in excess of 1°C, pH decreases greater than 0.3 units, dissolved oxygen decreases up to 3.7%, and a 40%–55% decrease in particulate organic matter flux to the seafloor by 2100 (Gehlen et al., 2014; Sweetman et al., 2017). These projected changes may severely affect productivity, biodiversity, and distribution of deep‐sea fauna, including species that underpin vulnerable marine ecosystems (VMEs) as well as commercially important deep‐sea fishes, thereby compromising key ecosystem services (Johnson, Ferreira, & Kenchington, 2018; Jones et al., 2014; Levin & Le Bris, 2015; Pecl et al., 2017; Thurber et al., 2014).
Among deep‐sea VME indicators, cold‐water corals that form important biogenic habitats are known to be vulnerable to anthropogenic climate change, particularly to ocean acidification (FAO, 2019; Guinotte et al., 2006; Orr et al., 2005; Perez et al., 2018; Roberts et al., 2016; Tittensor, Baco, Hall‐Spencer, Orr, & Rogers, 2010). This vulnerability exists because most cold‐water corals with carbonate skeletons occur in waters supersaturated in carbonate that enable coral skeleton biocalcification. Although several experimental studies demonstrate high resilience of reef‐building scleractinian to ocean acidification (Büscher, Form, & Riebesell, 2017; Form & Riebesell, 2012; Hennige et al., 2014, 2015; Maier et al., 2013; Maier, Watremez, Taviani, Weinbauer, & Gattuso, 2012; Maier, Weinbauer, & Gattuso, 2019; Movilla et al., 2014), the projected shoaling of the calcite and aragonite saturation horizons along with warming is expected to lead to the loss of suitable habitat (Davies & Guinotte, 2011; Perez et al., 2018; Sulpis et al., 2018; Tittensor et al., 2010; Yesson et al., 2012), weakening of the reef frameworks that may result in structural collapse of slow‐growing scleractinian corals (Büscher et al., 2019; Gomez, Wickes, Deegan, Etnoyer, & Cordes, 2019; Hennige et al., 2015), and increased mortality of octocorals that form coral gardens (Cerrano et al., 2013; Gugliotti, DeLorenzo, & Etnoyer, 2019). Notwithstanding genotypic variability in cold‐water corals’ response to ocean acidification (Kurman, Gómez, Georgian, Lunden, & Cordes, 2017; Lunden, McNicholl, Sears, Morrison, & Cordes, 2014), these changes may result in the loss of biodiversity and provision of ecosystem services associated with these ecosystems (Cordes et al., 2016).
Along with climate regulation, provisioning of food from fish stocks is one of the most critical ecosystem services provided by the deep sea (Thurber et al., 2014); these stocks are increasingly important to global food security (Victorero, Watling, Palomares, & Nouvian, 2018; Watson & Morato, 2013). However, warming and deoxygenation will simultaneously affect fishes by increasing metabolic rates and oxygen demand while limiting supply of oxygen to their tissues to meet increased demand for oxygen in low‐oxygen environments (Holt & Jørgensen, 2015; Pörtner, Bock, & Mark, 2017; Pörtner & Knust, 2007). Decreasing food availability will indirectly exacerbate stress imposed by increased metabolism in warmer waters (Woodworth‐Jefcoats, Polovina, & Drazen, 2017). Although, many gaps remain in understanding the underlying physiological mechanisms that influence potential responses of fishes to climate change (Lefevre, McKenzie, & Nilsson, 2017), multiple lines of evidence project that climate change will reduce the fish size and growth, abundance and survival, and will shift the spatial distributions of bottom fishes and fisheries (Baudron, Needle, Rijnsdorp, & Marshall, 2014; Bryndum‐Buchholz et al., 2019; Cheung et al., 2010; Dulvy et al., 2008; Pecl et al., 2017; Perry, Low, Ellis, & Reynolds, 2005; Pörtner & Knust, 2007), potentially driving unforeseen and undocumented trophic cascade effects (Frank, Petrie, Choi, & Leggett, 2005). Previous studies demonstrate the importance of local stocks with adaptive diversity to long‐term sustainability of fish stocks, fisheries, and ecosystems (Bradbury et al., 2010).
Improved projections of how climate change can lead to shifts in the distribution of deep‐sea species is critically important in developing effective management measures that account for such changes, especially those spatial measures that aim to preserve refugia areas or local fish stocks, to aid conservation of VMEs, or secure food, income and livelihoods from fisheries (Bates et al., 2019; Cheung et al., 2017, 2010; Gaines et al., 2018; Thresher, Guinotte, Matear, & Hobday, 2015; Tittensor et al., 2010). Such improved projections can also inform the designation of ‘other effective area‐based conservation measures’ (OECMs; CBD, 2018a; IUCN WCPA, 2019). Environmental niche modelling, also known as species distribution modelling, habitat suitability modelling (HSM) or climate envelope modelling, represents a powerful tool for predicting the distribution of species over wide geographic regions and projecting changes under future climate scenarios (Hattab et al., 2014; Hijmans & Graham, 2006; Pearson & Dawson, 2003; Wiens, Stralberg, Jongsomjit, Howell, & Snyder, 2009). While acknowledging that species genetic variability, phenotypic plasticity, evolutionary changes and acclimation could limit the accuracy of such models (Austin & Van Niel, 2011; Elith & Leathwick, 2009; Fillinger & Richter, 2013; Kurman et al., 2017; Pearson & Dawson, 2003; Sandblom, Gräns, Axelsson, & Seth, 2014), researchers have widely applied these approaches to terrestrial (e.g. Fordham et al., 2012; Iverson & Prasad, 1998) and marine species and habitats (e.g. benthic macrofauna, Singer, Millat, Staneva, & Kroncke, 2017; seagrass, Chefaoui, Duarte, & Serrão, 2018; and fish, Morley et al., 2018). With few exceptions (Tittensor et al., 2010), the lack of reliable projections of future environmental conditions close to the seabed has constrained efforts to project shifts in distributions of deep‐sea bottom‐dwelling species.
The recent modelling of global‐scale scenarios for future deep ocean environmental conditions (e.g. Sweetman et al., 2017), in tandem with increased understanding of the ecology and distribution of key deep‐sea benthic species (e.g. Orejas & Jiménez, 2019; Priede, 2017; Rossi, Bramanti, Gori, & Orejas, 2017), enabled projections of distributional changes in deep‐sea species (FAO, 2019). Utilizing the best available curated species occurrence data obtained from multiple public and restricted sources, along with a set of static (depth, slope, among others) and near‐bottom dynamic environmental parameters (particulate organic carbon flux to the seabed, near seafloor pH, dissolved oxygen concentration and temperature, and near seafloor aragonite and calcite saturation state), we modelled habitat suitability for six cold‐water coral and six deep‐sea fish species under current conditions and projected changes under future projected high emission climate conditions for the whole North Atlantic Ocean. With this study, we asked how much suitable habitat we expect will be lost, gained or sustained as refugia areas under the business‐as‐usual emissions trajectory RCP8.5for indicators of VMEs and commercially important deep‐sea fishes at an ocean basin scale, to support climate change adaptive management.
2. MATERIALS AND METHODS
2.1. Study area
Habitat suitability models of VME indicator taxa and commercially important deep‐sea fish species were developed for the deep waters of the North Atlantic, from 18°N to 76°N and 36°E to 98°W. This region encompasses one of the best‐studied deep‐water regions in the world with respect to species distribution, environmental conditions and deep‐sea species responses to environmental variability. Additionally, the North Atlantic Ocean contains two well‐established Regional Fisheries Management Organisations, increasing the relevance of these analyses for fisheries management, for conserving and protecting VMEs, and for the designation of OECMs. This basin‐scale focus also enhances model performance because it accounts for a wide range of environmental variability and species’ ecological niches.
2.2. Species selection and presence data
Six VME indicator taxa and six commercially important deep‐sea fish species representative of both Eastern and Western North Atlantic deep‐sea habitats were selected based on their wide spatial distribution, ecological significance or catch relevance in deep‐sea fisheries, and on the availability and spatial coverage of existing occurrence records (Table 1). The VME indicator taxa included three scleractinian corals that form aragonite skeletons (Lophelia pertusa, Madrepora oculata and Desmophyllum dianthus) and three octocorals forming calcitic axial skeletons (Acanella arbuscula), and with sclerites in their axis or coenenchyme and polyps (Acanthogorgia armata, and Paragorgia arborea). Despite the widespread occurrence of these two groups of VME indicators in the North Atlantic (FAO, 2019), they are expected to respond differently to future water mass conditions properties. The six deep‐sea fish species selected were the commercially harvested round nose grenadier (Coryphaenoides rupestris), Atlantic cod (Gadus morhua), blackbelly rosefish (Helicolenus dactylopterus), American plaice (Hippoglossoides platessoides), Greenland halibut (Reinhardtius hippoglossoides) and beaked redfish (Sebastes mentella). Although many of these fishes are not strictly considered deep‐sea species, they all occur beyond 200 m depth (Table 1) and are relevant to deep‐sea fisheries including in areas beyond national jurisdictions.
Table 1.
Number of grid cells with occurrence data obtained from multiple sources for the six cold‐water corals and six deep‐sea fishes used to model the suitable habitat in the North Atlantic. Depth ranges of occurrence records, and mean and standard deviation (±) for slope, Bathymetric Position Index (BPI), temperature at seafloor (Temp), POC flux to seafloor, and Aragonite (Ωar) and Calcite (Ωcal) saturation state at seafloor are also shown. Depth ranges for scleractinian corals and octocorals are shown for reference purposes only since depth was not considered for these species
| Group | Species | No. cells | Depth range (m) | Slope (°) | BPI | Temp (°C) | POC flux (mg C m−2 day−1) | Ωar | Ωcal |
|---|---|---|---|---|---|---|---|---|---|
| Scleractinian corals | Lophelia pertusa | 1,311 | 20–2,840 | 1.55 ± 2.01 | 0.73 ± 1.06 | 8.24 ± 2.57 | 14.78 ± 8.31 | 1.99 ± 0.30 | – |
| Madrepora oculata | 418 | 100–2,120 | 2.23 ± 2.41 | 0.88 ± 1.79 | 9.41 ± 2.93 | 9.94 ± 6.48 | 1.96 ± 0.45 | – | |
| Desmophyllum dianthus | 312 | 50–3,250 | 3.67 ± 3.32 | 0.80 ± 1.79 | 8.28 ± 3.17 | 9.18 ± 5.84 | 1.81 ± 0.49 | – | |
| Octocorals | Acanthogorgia armata | 324 | 30–2,600 | 2.81 ± 2.18 | 0.78 ± 0.99 | 4.92 ± 2.21 | 15.24 ± 6.17 | – | 2.59 ± 0.30 |
| Acanella arbuscula | 852 | 50–4,810 | 2.52 ± 2.41 | 0.75 ± 1.44 | 4.71 ± 1.99 | 12.00 ± 5.54 | – | 2.54 ± 0.28 | |
| Paragorgia arborea | 434 | 40–2,170 | 1.26 ± 1.78 | 0.52 ± 0.71 | 3.87 ± 2.26 | 21.35 ± 8.36 | – | 2.69 ± 0.21 | |
| Deep‐water fish | Helicolenus dactylopterus | 4,508 | 20–1,790 | 1.49 ± 2.23 | 1.08 ± 1.19 | 8.59 ± 1.79 | 24.08 ± 16.35 | – | – |
| Sebastes mentella | 15,476 | 10–1,630 | 0.80 ± 1.15 | 0.44 ± 0.74 | 3.71 ± 1.70 | 21.08 ± 7.08 | – | – | |
| Gadus morhua | 52,463 | 10–990 | 0.29 ± 0.57 | 0.22 ± 0.52 | 5.64 ± 2.75 | 34.12 ± 14.97 | – | – | |
| Hippoglossoides platessoides | 56,734 | 10–1,490 | 0.34 ± 0.63 | 0.23 ± 0.54 | 5.21 ± 2.69 | 32.19 ± 14.66 | – | – | |
| Reinhardtius hippoglossoides | 23,491 | 10–1,690 | 0.74 ± 1.09 | 0.30 ± 0.67 | 3.58 ± 1.89 | 23.43 ± 9.84 | – | – | |
| Coryphaenoides rupestris | 3,009 | 70–1,800 | 2.23 ± 1.60 | 0.44 ± 0.88 | 4.55 ± 1.53 | 15.16 ± 5.73 | – | – |
Georeferenced presence‐only records were obtained from institutional databases of partners participating in this work (see Supporting Information Appendix S1, Table S1) as well as from public databases (Table 1) such as the Ocean Biogeographic Information System portal (OBIS), the NOAA Deep Sea Coral Data Portal, and the ICES Vulnerable Marine Ecosystems data portal. In order to reduce potential errors in the spatial position of the occurrence records, we compared the depth values given by OBIS and NOAA with depth values extracted from the depth raster layer, excluding any occurrence records with no depth information or with depths that differed more than 30% and more than 50 m in absolute depth. In the case of the ICES VMEs database, those records with a position accuracy lower than 5000 m of linear distance were excluded. Species occurrence provided directly by co‐authors was cross‐checked for accuracy of reported depth prior to submission of the data and were considered accurate, and took priority over OBIS data from the same institutional sources. Maps of the presence records used in the models are provided as supporting information (Figure S1).
Most HSM approaches require information on the location of both species presence and absence. However, existing biological datasets rarely include information on species absence, despite its importance for model performance and precision (Iturbide, Bedia, & Gutiérrez, 2018; Iturbide et al., 2015; Wisz & Guisan, 2009). To overcome this obstacle, we generated pseudo‐absence data (a.k.a. background points) by adapting the methodology in Iturbide et al. (2015) to our specific data. In the first step, we used the function OCSVMprofiling from the R package MOPA (Iturbide et al., 2018) to limit the geographic region for pseudo‐absence data generation using environmental profiling based on presence data. In the second step, pseudo‐absence data were randomly generated in the region defined above using the function pseudoAbsences from the MOPA package, but excluding a buffer distance to presence records of 6 km. The number of pseudo‐absence data points generated differed between cold‐water corals (10,000) and deep‐sea fishes (100,000) because of the different numbers of presence records. Finally, the pseudo‐absence data were randomly stratified subsampled by depth strata to match the proportion of the presence records distribution by depth of all cold‐water corals and fish species in public databases.
2.3. Environmental layers
A set of terrain (static in time; Wilson, O’Connell, Brown, Guinan, & Grehan, 2007) and environmental (dynamic in time) variables were used as candidate predictors of present‐day (1951–2000) distribution and to project future (2081–2100) changes. All predictor variables were projected with the Albers equal‐area conical projection centred in the middle of the study area, and were rescaled to a final grid cell resolution of 3x3 km; comprising about 3.8 million cells. The terrain variable depth was extracted from a bathymetry grid built from two data sources: the EMODnet Digital Terrain Model (EMODnet, 2018) and the General Bathymetric Chart of the Oceans (GEBCO 2014 described in Weatherall et al., 2015). The original resolution from EMODNET (0.002°) was rescaled to match the GEBCO resolution (0.008°) using a bilinear interpolation. The bathymetry layer consisted of EMODnet data, where available, merged with GEBCO 2014 and rescaled to the final resolution of 3x3 km using bilinear interpolation. Rescaled EMODnet is considered higher in accuracy than GEBCO 2014 at the same resolution because it contains the most recent multibeam coverage for the Northeast Atlantic (EMODnet, 2018; Schmitt, Schaap, Spoelstra, Loubrieu, & Poncelet, 2019). Slope (in degrees) was derived from the final bathymetry grid using the Raster package in R (Hijmans, 2016) and the Bathymetric Position Index (BPI) was computed using the Benthic Terrain Model 3.0 tool (Walbridge, Slocum, Pobuda, & Wright, 2018) in ArcGIS 10.1 with an inner radius of 3 and an outer radius of 25 grid cells. In order to avoid extreme values, BPI was standardized using the scale function from the Raster package.
Environmental variables of present‐day and future conditions, including particulate organic carbon (POC) flux at 100 m depth (epc100, mg C m−2 day−1), bottom water dissolved oxygen concentration (µmol/kg), pH and potential temperature (°C) were downloaded from the Earth System Grid Federation (ESGF) Peer‐to‐Peer (P2P) enterprise system. The epc100 was converted to export POC flux at the seafloor using the Martin curve (Martin, Knauer, Karl, & Broenkow, 1987) following the equation: epc = epc100 × (water depth/export depth)−0.858, and setting the export depth to 100 m. Near seafloor aragonite (Ωar) and calcite (Ωcal) saturation were also used as candidate predictors for habitat suitability of cold‐water coral species. These saturation states were computed by dividing the bottom water carbonate ion concentration (mol/m3) by the bottom water carbonate ion concentration (mol/m3) for seawater in equilibrium with pure aragonite and calcite. Yearly means of these parameters were calculated for the periods 1951–2000 (historical simulation) and 2081–2100 (RCP8.5 or business‐as‐usual scenario) using the average values obtained from the Geophysical Fluid Dynamics Laboratory's ESM 2G model (GFDL‐ESM‐2G; Dunne et al., 2012), the Institut Pierre Simon Laplace's CM6‐MR model (IPSL‐CM5A‐MR; Dufresne et al., 2013) and Max Planck Institute's ESM‐MR model (MPI‐ESM‐MR; Giorgetta et al., 2013) within the Coupled Models Intercomparison Project Phase 5 (CMIP5) for each grid cell of the present study area. CMIP5 environmental variables were available at a 0.5° resolution and rescaled to match the 3 × 3 km cell size using universal kriging and depth as a covariate. In order to evaluate the performance of the 1951–2000 seabed environmental layer, we compared modelled data against field observations. In general, modelled values correlated highly significantly with observed data, yielding adjusted R > .70 and low values of RMSE (Figure S2). While these results indicate appropriate environmental layers in this study, environmental model projections contain a degree of uncertainty likely associated with the spatiotemporal scales used. Environmental layers used in this study are available to download https://doi.org/10.1594/PANGAEA.911117 and provided as Supporting Information (Figure S3).
Collinearity between all candidate predictor variables was evaluated using Spearman's coefficient of correlation and the variation inflation factor (VIF, Zuur, Ieno, & Elphick, 2010). The final selection of predictor variables was based on the inferred ecological relevance of each variable for each group of species modelled (Fourcade, Besnard, & Secondi, 2018), and therefore differed among scleractinians, octocorals and fish species. Because scleractinian and octocoral species differ in environmental requirements based on how they incorporate calcium carbonate into their skeletons (Freiwald, 2002; Lewis, Barnowski, & Telesnicki, 1992), aragonite saturation was exclusively selected for scleractinians, whereas calcite saturation was selected for octocorals. In order to avoid collinearity, we retained the most ecologically relevant of those variables with Spearman coefficient of correlation >0.85 or with VIF values >10 (Dormann et al., 2013; Elith et al., 2006). After the collinearity analyses, depth (highly correlated with aragonite and calcite saturation), dissolved oxygen concentrations and pH were excluded from the cold‐water coral model development, while excluding only pH from the deep‐water fish model developments. Hence, the variables used for modelling the distribution of scleractinian and octocorals were slope, BPI, POC flux to the seafloor, and bottom water temperature and aragonite or calcite saturation state, whereas fish species distribution modelling included depth, slope, BPI, POC flux to the seafloor, and bottom water dissolved oxygen concentration and temperature.
2.4. Modelling approach
We used an ensemble modelling approach to predict habitat suitability under present‐day (1951–2000) conditions and to project changes under future (2081–2100) climate projections (RCP8.5 scenario). An ensemble projecting approach was selected because past studies considered it appropriate to assess uncertainties and enhance reliability in determining suitable habitats under projected climate change scenarios (Araújo & New, 2007; Buisson, Thuiller, Casajus, Lek, & Grenouillet, 2010). We employed three widely used modelling methods (González‐Irusta et al., 2015) capable of dealing with presence‐only data using pseudo‐absences: the maximum entropy model (Maxent, Phillips, Anderson, Dudík, Schapire, & Blair, 2017; Phillips, Anderson, & Schapire, 2006), generalized additive models (GAMs, Hastie & Tibshirani, 1986) and the random forest machine learning algorithms (RF, Breiman, 2001). Maxent models were developed using the function maxent from the R package Dismo (Hijmans, Phillips, Leathwick, & Elith, 2017), with prevalence set as the proportion of presences over the pseudo‐absences generated. GAMs were fitted with the function gam from the R package mgcv (Wood, 2018) using a binomial error distribution with logit link function, constraining the smooth curves to four knots to avoid overfitting; we used three knots for temperature and aragonite in cold‐water coral models. Finally, we computed RF models using the function randomForest from the R package of the same name (Liaw & Wiener, 2002).
Variable selection in GAMs used the Akaike information criterion and the function dredge from the R package MuMIn (Barton, 2018), whereas the other two models (Maxent and Random Forest) were fitted with the original set of variables. To assess the contribution of each variable to the final predictions, we used a randomization procedure adapted from Thuiller, Lafourcade, Engler, and Araújo (2009), which estimates the importance of each variable independently of the modelling technique, enabling direct comparisons among models. This methodology computes the Pearson correlation between the original predictions and predictions where the variable under evaluation was randomly permutated. This operation was repeated 10 times for each combination of model, variable and species. We report results as 1‐Pearson correlation in order to provide intuitive values of variable importance, where high values indicate high variable importance. The relationship between the environmental predictors and predicted habitat suitability was analysed using response curves, described by Elith et al. (2006).
Performance for the present‐day model was evaluated using a cross‐validation method based on a random ‘block’ selection of training and testing data (Guinotte & Davies, 2014). Past work suggests this method of partitioning a unique dataset provides the best spatial independence between training and testing datasets (Fourcade et al., 2018). We implemented this methodology with the get.block function from the EnMEVAL package in R (Muscarella et al., 2014), dividing the study area into four subareas containing similar numbers of occurrence data points. Selection of three of these four subareas provided training datasets, leaving the fourth area as the test dataset. This operation was repeated 10 times with a random selection of evaluation and training areas to compute the mean and the standard deviation of each metric. From each training and test dataset, we randomly selected 80% of the data in each iteration to avoid repeating the exact same selection in different iterations. Analysis of the ability of the training data to predict the test data in each iteration used the evaluation data and five different statistical metrics: area under the curve (AUC) of the receiver operating characteristic, kappa statistic, specificity (a.k.a. true negative rate), sensitivity (a.k.a. true positive rate, or probability of detection) and true skill statistic (TSS). From these analyses, we considered the overall accuracy of the model prediction good (AUC > 0.8; TSS > 0.6), moderate (0.7 ≤ AUC≤0.8; 0.2 ≤ TSS≤0.6) or poor (AUC < 0.7; TSS < 0.2; Mandrekar, 2010). Fielding and Bell (1997) provide a complete description of these statistics.
Each model was then used to predict a relative index of habitat suitability (HSI) across the study area under present‐day (1951–2000) conditions and to project the HSI for the period 2081–2100 by projecting the present‐day niche onto the environmental layers of projected future conditions. The modelled logistic outputs consisted of an HSI that ranked grid cells according to their predicted suitability for a particular species, rather than the probability of presence (Elith et al., 2011; Greathead et al., 2015). Applying threshold values of HSI into predicted/projected suitable and unsuitable areas for both current and future scenarios produced binary habitat suitability maps for each species, employing two thresholds: 10 percentile training presence logistic threshold and maximum sensitivity and specificity. We computed the uncertainty for each model prediction by bootstrapping the data with replacement (Anderson et al., 2016) using the function boot from the R package boot (Canty & Ripley, 2017), fitting new models using the bootstrapped data and predicting HSI values for the whole study area. Repeating this process 100 times for each model yielded 100 estimates of HSI for each cell. Finally, we computed the coefficient of variation (CV) of the bootstrap output for each species, modelling approach, and the present‐day predictions and future projections.
Finally, ensemble HSI and uncertainty were computed for all species and for the two study periods by calculating the average of these two indexes by cell after weighting the three families of model outputs with the evaluation metrics AUC and TSS, using the same approach as Rowden et al. (2017). We estimated the importance of each predictor variable to the ensemble habitat suitability model predictions as the average of the variable importance in the individual models weighted by the models' evaluation metrics. Ensemble model performance statistic calculations used the same methods as the individual models.
The binary maps were used to calculate the suitable habitat area, along with the median latitude and depth for all species in the North Atlantic, excluding the Mediterranean Sea. We inferred refugia areas (sensu Keppel & Wardell‐Johnson, 2012) for VMEs and local fish stocks of commercially important deep‐sea species in the North Atlantic Ocean from those suitable areas predicted under present‐day and projected under future conditions.
3. RESULTS
In general, the three families of modelling approaches (Table S2) and the ensemble model predictions (Table 2) achieved good accuracy for most species (AUC > 0.80 and TSS > 0.60), reasonably matching known species occurrences (sensitivity > 0.80). Ensemble models for all cold‐water coral species also achieved good accuracy, although models for scleractinian species performed slightly better than those for octocoral species. Of those corals examined, the predicted distribution of D. dianthus (AUC = 0.95; TSS > 0.74) and P. arborea (AUC = 0.95; TSS > 0.76) were most accurate, with lowest accuracy in A. arbuscula (AUC = 0.88; TSS < 0.67). Deep‐sea fish model prediction accuracies were slightly better compared to cold‐water corals but related inversely to sample size (Table 2). The predicted distribution of C. rupestris (AUC = 0.99; TSS > 0.88; n = 3,009) and H. dactylopterus (AUC = 0.97; TSS > 0.81; n = 4,508) were highest in accuracy among all fishes, in contrast to comparatively low accuracy in G. morhua (AUC = 0.94; TSS < 0.81; n = 52,463) and H. platessoides (AUC = 0.93; TSS < 0.81, n = 54,725), and the lowest accuracy in R. hippoglossoides (AUC = 0.87; TSS < 0.61, n = 23,491). The uncertainty associated with the habitat suitability ensemble model predictions under present‐day and projections under future environmental conditions were generally low for both cold‐water corals and deep‐sea fishes, but generally lower for fishes (Figure S4a,b). Supporting information provided the predicted habitat suitability indices and coefficients of variance for all three families of models and species (Figure S5). Model outputs are available for download from https://doi.org/10.1594/PANGAEA.910319.
Table 2.
Model performance statistics generated using an ensemble modelling approach. The ability of the training data to predict the probability of presence was tested with different statistical metrics: area under the curve (AUC) of the receiver operating characteristic, kappa statistic, sensitivity (% true positives), specificity (% true negatives) and true skill statistic (TSS). Statistics were calculated using two thresholds: 10 percentile training presence logistic threshold (10th) and maximum sensitivity and specificity (MSS)
| Group | Species | AUC | Kappa | Sensitivity | Specificity | TSS | Thresholds | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10th | MSS | 10th | MSS | 10th | MSS | 10th | MSS | 10th | MSS | |||
| Scleractinian corals | Lophelia pertusa | 0.91 ± 0.08 | 0.51 ± 0.31 | 0.57 ± 0.25 | 0.90 | 0.88 ± 0.06 | 0.78 ± 0.18 | 0.85 ± 0.11 | 0.68 ± 0.18 | 0.72 ± 0.17 | 0.34 | 0.24 |
| Madrepora oculata | 0.92 ± 0.06 | 0.31 ± 0.22 | 0.41 ± 0.19 | 0.90 | 0.88 ± 0.09 | 0.77 ± 0.16 | 0.87 ± 0.07 | 0.66 ± 0.16 | 0.75 ± 0.14 | 0.31 | 0.20 | |
| Desmophyllum dianthus | 0.95 ± 0.03 | 0.34 ± 0.14 | 0.39 ± 0.16 | 0.90 | 0.92 ± 0.07 | 0.85 ± 0.08 | 0.86 ± 0.09 | 0.74 ± 0.08 | 0.79 ± 0.08 | 0.33 | 0.22 | |
| Octocorals | Acanthogorgia armata | 0.92 ± 0.05 | 0.35 ± 0.29 | 0.43 ± 0.21 | 0.90 | 0.88 ± 0.06 | 0.77 ± 0.20 | 0.89 ± 0.07 | 0.66 ± 0.20 | 0.77 ± 0.12 | 0.26 | 0.18 |
| Acanella arbuscula | 0.88 ± 0.03 | 0.22 ± 0.20 | 0.49 ± 0.20 | 0.90 | 0.81 ± 0.07 | 0.60 ± 0.15 | 0.86 ± 0.10 | 0.50 ± 0.15 | 0.67 ± 0.10 | 0.32 | 0.19 | |
| Paragorgia arborea | 0.95 ± 0.05 | 0.44 ± 0.18 | 0.50 ± 0.17 | 0.90 | 0.90 ± 0.06 | 0.86 ± 0.13 | 0.90 ± 0.09 | 0.76 ± 0.13 | 0.79 ± 0.12 | 0.36 | 0.23 | |
| Deep‐water fish | Helicolenus dactylopterus | 0.97 ± 0.03 | 0.81 ± 0.08 | 0.84 ± 0.08 | 0.90 | 0.96 ± 0.02 | 0.91 ± 0.08 | 0.88 ± 0.08 | 0.81 ± 0.08 | 0.84 ± 0.08 | 0.54 | 0.33 |
| Sebastes mentella | 0.94 ± 0.06 | 0.68 ± 0.22 | 0.67 ± 0.21 | 0.90 | 0.95 ± 0.03 | 0.85 ± 0.15 | 0.82 ± 0.15 | 0.75 ± 0.15 | 0.78 ± 0.13 | 0.63 | 0.50 | |
| Gadus morhua | 0.94 ± 0.02 | 0.75 ± 0.01 | 0.79 ± 0.01 | 0.90 | 0.99 ± 0.01 | 0.86 ± 0.01 | 0.82 ± 0.02 | 0.76 ± 0.01 | 0.81 ± 0.01 | 0.74 | 0.60 | |
| Hippoglossoides platessoides | 0.93 ± 0.01 | 0.75 ± 0.00 | 0.80 ± 0.03 | 0.90 | 0.97 ± 0.04 | 0.86 ± 0.00 | 0.84 ± 0.01 | 0.76 ± 0.00 | 0.81 ± 0.04 | 0.73 | 0.59 | |
| Reinhardtius hippoglossoides | 0.87 ± 0.01 | 0.48 ± 0.05 | 0.52 ± 0.01 | 0.90 | 0.90 ± 0.08 | 0.68 ± 0.05 | 0.71 ± 0.06 | 0.58 ± 0.05 | 0.61 ± 0.02 | 0.61 | 0.54 | |
| Coryphaenoides rupestris | 0.99 ± 0.01 | 0.88 ± 0.01 | 0.93 ± 0.03 | 0.90 | 0.97 ± 0.02 | 0.98 ± 0.01 | 0.96 ± 0.01 | 0.88 ± 0.01 | 0.93 ± 0.03 | 0.63 | 0.26 | |
The habitat suitability models developed here included seven different predictors which contributed differently to the different modelled species (Table 3; Table S4). In general, POC flux to the seafloor, bottom water temperature, and aragonite or calcite saturation were the most important predictors for scleractinians and octocorals, in contrast to depth, POC flux, and temperature for deep‐sea fishes (Table 3). We also note, however, the importance of slope in predicting the suitable habitat for D. dianthus, A. armata, and M. oculata. Supporting information provides the response curves derived from all modelling approaches (Figures S6 and S7).
Table 3.
Importance of each predictor variable to the habitat suitability model predictions, measured as 1‐Pearson correlation, for six cold‐water corals and six deep‐sea fishes in the North Atlantic Ocean. Predictor variables were depth, slope, Bathymetric Position Index (BPI), temperature at seafloor (Temp), particulate organic carbon flux to seafloor (POC), aragonite (Ωar) and calcite (Ωcal) saturation state at seafloor, and dissolved oxygen (DO) concentration at seafloor
| Group | Species | Relative importance | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Depth | Slope | BPI | Temp | POC | Ωar | Ωcal | DO | ||
| Scleractinian corals | Lophelia pertusa | – | 0.06 ± 0.04 | 0.05 ± 0.06 | 0.41 ± 0.13 | 0.26 ± 0.10 | 0.36 ± 0.04 | – | |
| Madrepora oculata | – | 0.16 ± 0.09 | 0.07 ± 0.05 | 0.47 ± 0.14 | 0.40 ± 0.06 | 0.14 ± 0.09 | – | ||
| Desmophyllum dianthus | – | 0.27 ± 0.13 | 0.05 ± 0.04 | 0.44 ± 0.15 | 0.48 ± 0.20 | 0.11 ± 0.13 | – | ||
| Octocorals | Acanthogorgia armata | – | 0.19 ± 0.12 | 0.05 ± 0.05 | 0.15 ± 0.05 | 0.29 ± 0.20 | – | 0.35 ± 0.12 | |
| Acanella arbuscula | – | 0.09 ± 0.06 | 0.03 ± 0.04 | 0.17 ± 0.08 | 0.21 ± 0.15 | – | 0.41 ± 0.06 | ||
| Paragorgia arborea | – | 0.06 ± 0.05 | 0.08 ± 0.05 | 0.15 ± 0.09 | 0.35 ± 0.27 | – | 0.58 ± 0.05 | ||
| Deep‐water fish | Helicolenus dactylopterus | 0.64 ± 0.26 | 0.17 ± 0.22 | 0.19 ± 0.21 | 0.45 ± 0.03 | 0.24 ± 0.17 | – | – | 0.16 ± 0.16 |
| Sebastes mentella | 0.67 ± 0.22 | 0.17 ± 0.23 | 0.17 ± 0.22 | 0.38 ± 0.11 | 0.43 ± 0.17 | – | – | 0.13 ± 0.16 | |
| Gadus morhua | 0.70 ± 0.15 | 0.15 ± 0.17 | 0.14 ± 0.17 | 0.27 ± 0.13 | 0.40 ± 0.09 | – | – | 0.31 ± 0.25 | |
| Hippoglossoides platessoides | 0.64 ± 0.11 | 0.12 ± 0.14 | 0.11 ± 0.13 | 0.29 ± 0.12 | 0.41 ± 0.12 | – | – | 0.35 ± 0.13 | |
| Reinhardtius hippoglossoides | 0.64 ± 0.26 | 0.22 ± 0.30 | 0.23 ± 0.30 | 0.48 ± 0.14 | 0.50 ± 0.16 | – | – | 0.27 ± 0.17 | |
| Coryphaenoides rupestris | 0.67 ± 0.16 | 0.21 ± 0.19 | 0.16 ± 0.22 | 0.20 ± 0.20 | 0.41 ± 0.10 | – | – | 0.25 ± 0.17 | |
Patterns in suitable habitat under future climate conditions differed for the different VME indicator species (Figure 1) and deep‐sea fish species evaluated (Figure 2). In general, the model outputs for scleractinian corals and octocorals decreased markedly in the suitable habitat towards the year 2100, whereas deep‐sea fishes clearly shifted towards higher latitudes.
Figure 1.
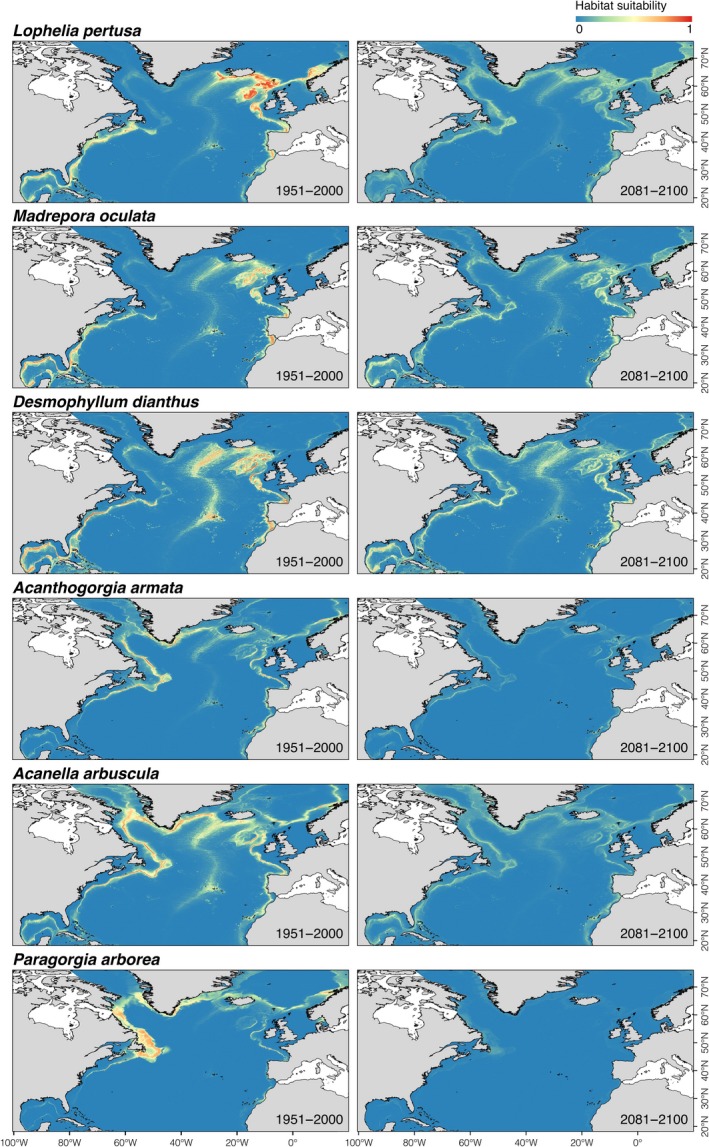
Habitat suitability index predicted under present‐day (1951–2000) and projected under future (2081–2100; RCP8.5 or business‐as‐usual scenario) environmental conditions for cold‐water corals in the North Atlantic Ocean using an ensemble modelling approach
Figure 2.
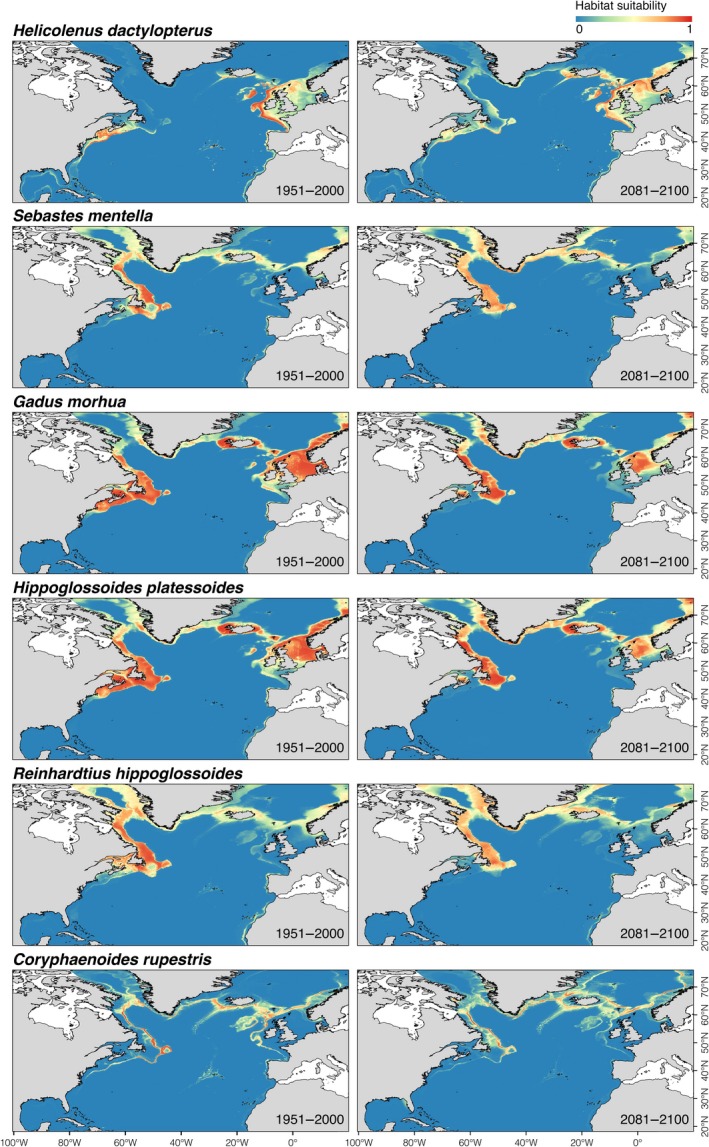
Habitat suitability index predicted under present‐day (1951–2000) and projected under future (2081–2100; RCP8.5 or business‐as‐usual scenario) environmental conditions for commercially important deep‐sea fishes in the North Atlantic Ocean, using an ensemble modelling approach
Potential habitat for scleractinian corals under present‐day conditions showed higher suitability indices in the eastern North Atlantic and the Mid‐Atlantic Ridge including the Azores, but also in the Gulf of Mexico, in contrast to higher suitability indices for octocorals in the western North Atlantic and south of Greenland (Figure 1). Most modelled deep‐sea fishes spanned a wide geographic range, with suitable habitats on the continental shelf and slope on both sides of the North Atlantic and along the coast of Iceland and Greenland (Figure 2). However, the predicted suitable habitat for H. dactylopterus was limited to areas south of Nova Scotia, south of Iceland, the Azores and around the British Isles, in contrast to deeper areas along the continental slope on both sides of the North Atlantic for C. rupestris.
Based on the ensemble model outputs, the scleractinian L. pertusa may experience a major reduction in suitable habitat of over 79% (Figure 3; Figure S8a,b). We observed no clear shift in the median latitudinal distribution of this coral species by 2100 (Figure 4) but we projected a shift in the median depth distribution towards deeper waters (Figure 5) resulting from loss of suitable habitat at shallower depths. The other two scleractinians (M. oculata and D. dianthus) may experience moderate reduction in suitable habitat of about 30%–55% (Figure 3; Figure S8a,b), with a northern shift in median latitudinal distributions (ranging from 1.9° to 4.6° in latitude; Figure 4) and a shift of M. oculata median suitable depths towards deeper waters (Figure 5). The models projected increased suitable habitat for all scleractinian corals not only in the Davis Strait and Labrador Sea, with corresponding decreases in most southern parts of the North Atlantic from the Gulf of Mexico to Nova Scotia, but also in the Mid‐Atlantic Ridge, Bay of Biscay, and in the Rockall and Faroe Shetland areas (Figure 6; Figure S8a,b). Projected climate refugia for scleractinian corals averaged 30%–42% of North Atlantic present‐day habitat on average, depending on the threshold considered (Table 4). However, refugia for L. pertusa estimated with the 10th percentile threshold was only about 1.5% of the North Atlantic present‐day habitat (Table 4). Projections indicated climate refugia for scleractinian corals on both sides of the North Atlantic (Figure 6).
Figure 3.
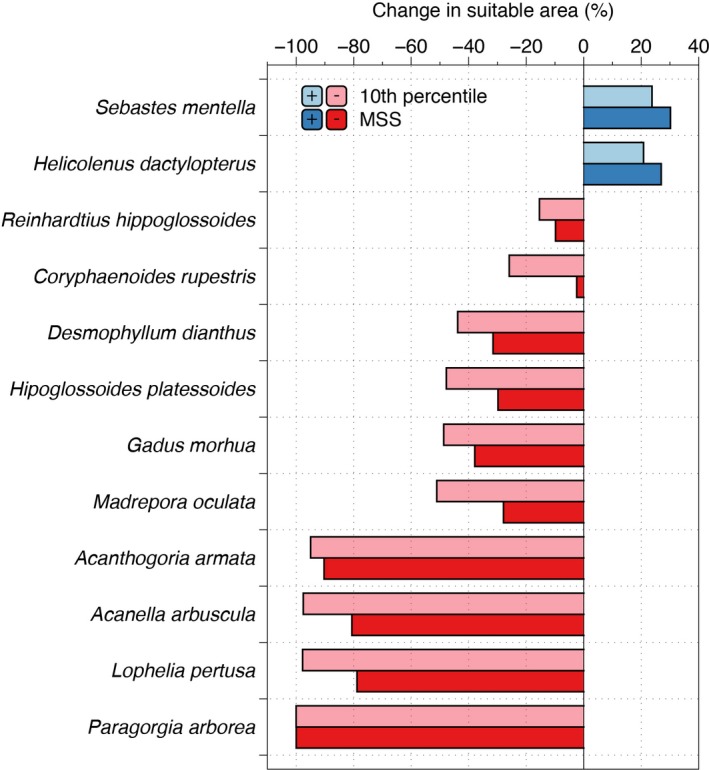
Climate‐induced projected changes (RCP8.5 or business‐as‐usual scenario) in the suitable habitat for cold water corals and deep‐sea fishes in the North Atlantic Ocean with an ensemble modelling approach. The extension of the habitat was calculated from binary maps built with two thresholds: 10 percentile training presence logistic (10th percentile) and maximum sensitivity and specificity (MSS)
Figure 4.
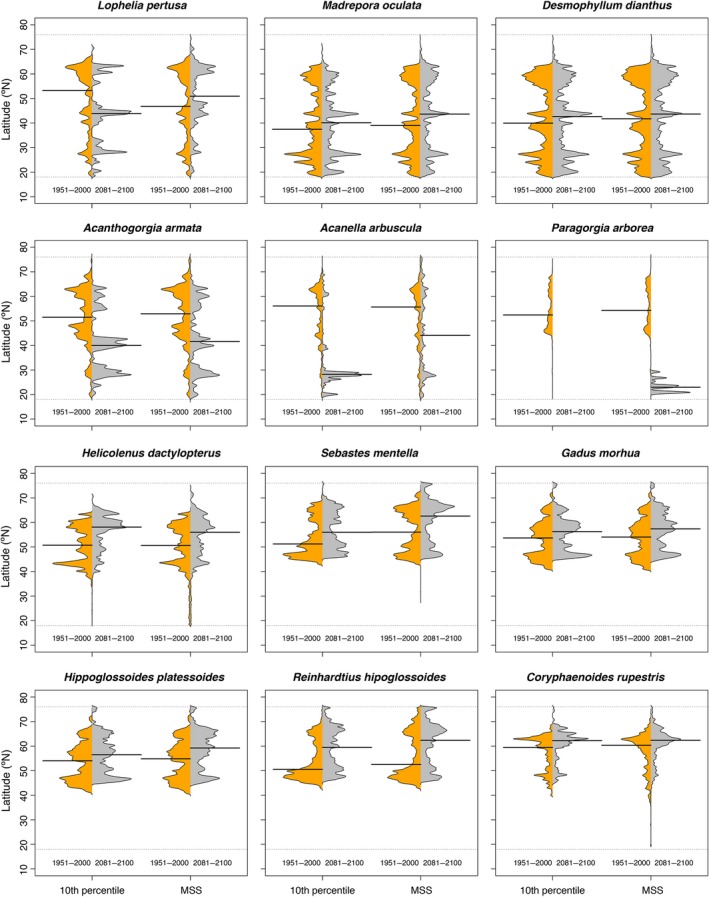
Climate‐induced projected changes (RCP8.5 or business‐as‐usual scenario) in the latitudinal distribution of cold water corals and deep‐sea fishes in the North Atlantic Ocean with an ensemble modelling approach. The extension of the habitat was calculated from binary maps built with two thresholds: 10 percentile training presence logistic (10th percentile) and maximum sensitivity and specificity (MSS). The black line indicates the median latitudinal
Figure 5.
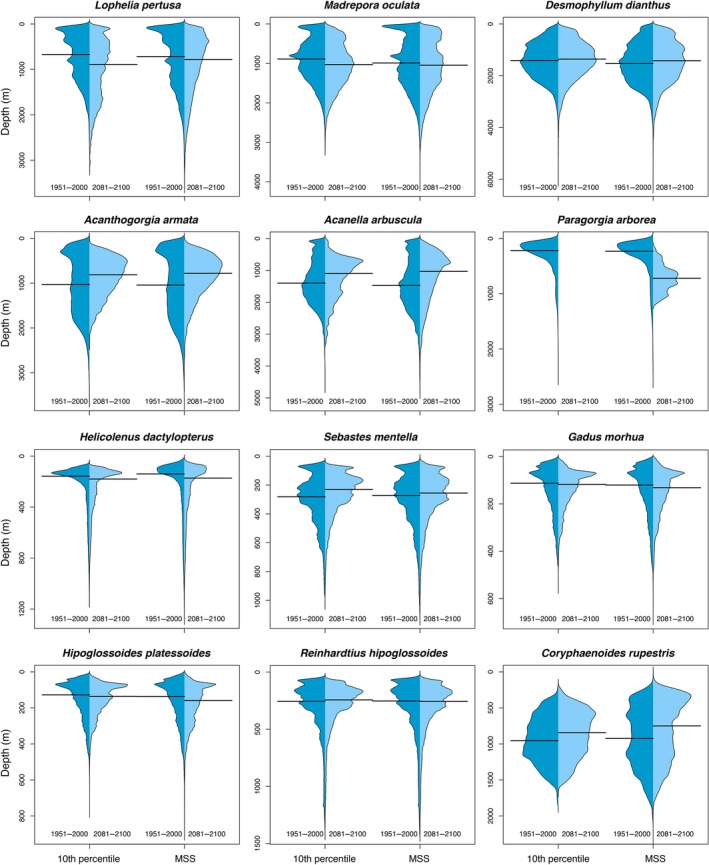
Climate‐induced projected changes (RCP8.5 or business‐as‐usual scenario) in the depth distribution of cold water corals and deep‐sea fishes in the North Atlantic Ocean with an ensemble modelling approach. The extension of the habitat was calculated from binary maps built with two thresholds: 10 percentile training presence logistic (10th percentile) and maximum sensitivity and specificity (MSS). The black line indicates the median depth
Figure 6.
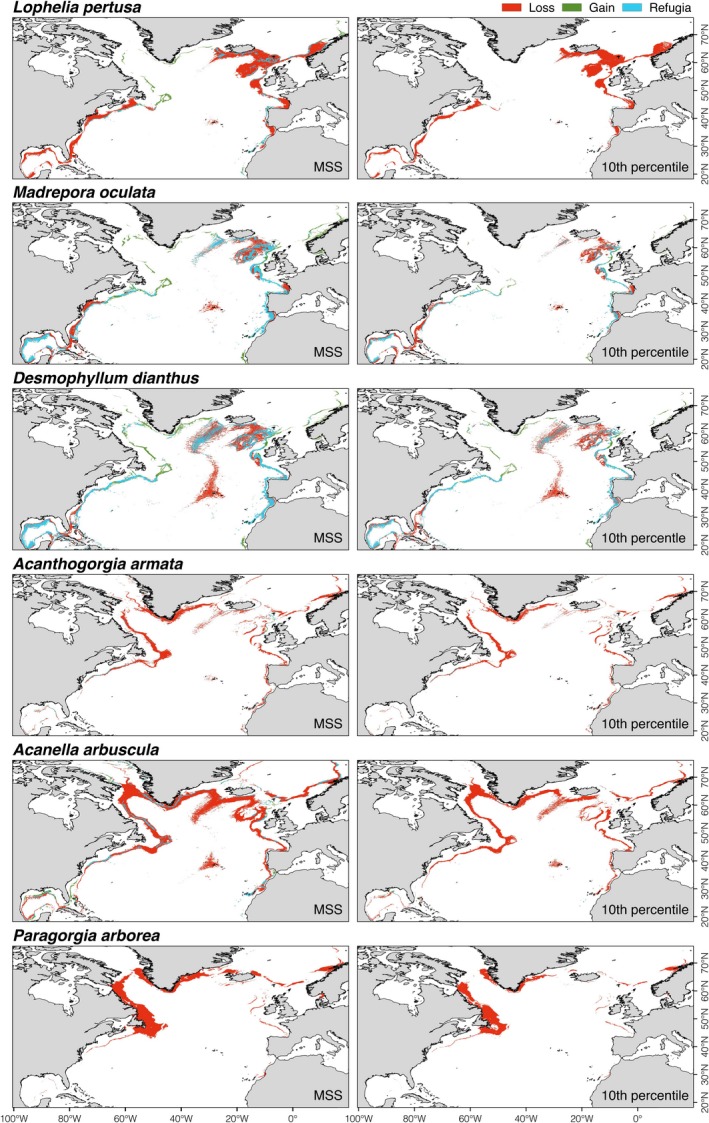
Projected present‐day suitable habitat loss, gain, and acting as climate refugia areas (sensu Keppel & Wardell‐Johnson, 2012) under future (2081–2100; RCP8.5 or business‐as‐usual scenario) environmental conditions for cold‐water corals in the North Atlantic Ocean. Areas were identified from binary maps built with an ensemble modelling approach and two thresholds: 10 percentile training presence logistic threshold (10th) and maximum sensitivity and specificity (MSS)
Table 4.
Proportion of the present‐day suitable habitat acting as refugia areas (Keppel & Wardell, 2012) under future (2081–2100; RCP8.5 or business‐as‐usual scenario) environmental conditions for cold‐water corals and commercially important deep‐sea fishes in the North Atlantic Ocean. Refugia areas were identified from binary maps built with an ensemble modelling approach and two thresholds: 10 percentile training presence logistic threshold (10th) and maximum sensitivity and specificity (MSS). Refugia areas estimated by the different models used to calculate the ensemble outputs are also shown: generalized additive model (GAM), maximum entropy model (Maxent) and random forest (RF)
| Group | Species | Refugia areas (% present‐day habitat) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ensemble | GAM | Maxent | RF | ||||||
| 10th | MSS | 10th | MSS | 10th | MSS | 10th | MSS | ||
| Scleractinian corals | Lophelia pertusa | 1.54 | 13.71 | 0.25 | 0.27 | 41.20 | 54.93 | 2.80 | 16.81 |
| Madrepora oculata | 38.98 | 53.97 | 27.95 | 42.30 | 61.50 | 73.52 | 12.81 | 45.95 | |
| Desmophyllum dianthus | 45.55 | 53.58 | 48.48 | 47.80 | 60.80 | 68.72 | 17.92 | 37.95 | |
| Octocorals | Acanthogorgia armata | 4.02 | 8.13 | 9.16 | 12.07 | 4.76 | 6.90 | 4.96 | 12.67 |
| Acanella arbuscula | 1.30 | 13.85 | 0.36 | 1.80 | 11.14 | 25.65 | 11.94 | 31.52 | |
| Paragorgia arborea | 0.00 | 0.00 | 0.00 | 0.00 | 0.11 | 0.23 | 0.00 | 9.99 | |
| Deep‐water fish | Helicolenus dactylopterus | 67.95 | 84.22 | 72.72 | 89.38 | 85.15 | 91.64 | 46.16 | 63.82 |
| Sebastes mentella | 55.92 | 64.78 | 51.05 | 61.59 | 53.05 | 70.24 | 48.04 | 64.22 | |
| Gadus morhua | 42.62 | 51.73 | 52.10 | 56.45 | 12.33 | 45.84 | 39.77 | 47.96 | |
| Hippoglossoides platessoides | 42.61 | 55.79 | 54.97 | 59.75 | 13.83 | 53.70 | 37.84 | 48.22 | |
| Reinhardtius hippoglossoides | 51.54 | 60.86 | 50.68 | 55.68 | 50.76 | 57.79 | 43.81 | 50.20 | |
| Coryphaenoides rupestris | 43.48 | 62.45 | 52.55 | 59.63 | 44.47 | 68.65 | 28.52 | 57.88 | |
Climate change impacts may threaten the persistence of all three octocorals (A. arbuscula, A. armata and P. arborea) in the North Atlantic. The ensemble model outputs suggest a reduction in suitable habitat of over 80% in all regions, irrespective of modelling approach or threshold (Figure 3; Figure S8a,b). The large reduction in suitable habitat resulted in a projected marked southern shift in median latitudinal distribution (−11.3° to −27.8°; Figure 4) and a shift towards shallower depths for A. arbuscula and A. armata (Figure 5). Our models projected new suitable habitat becoming available for these two species by 2100 in the shallower waters of the northernmost of the North Atlantic and Gulf of Mexico (Figure 6). Extremely small refugia may remain in the North Atlantic for octocorals A. arbuscula and A. armata (averaging 6%–14% of present‐day suitable habitat), whereas the ensemble model projects no climate refugia for P. arborea by 2100 (Table 4; Figure 6).
The ensemble model projected a 30%–50% reduction of suitable habitat for the fish species G. morhua and Hipoglossoides platessoides, between 10% and 15% for R. hippoglossoides, and between 2% and 25% for C. rupestris, mostly in their lower latitudinal limit (Figure 3; Figure S9a,b). The decrease in suitable habitat for G. morhua included important shallower water fishing grounds, such as Georges Bank, the Irish Sea, the Norwegian Sea and the southern North Sea (Figure S9a,b). Of those species examined, only H. dactylopterus and S. mentella increased in projected total suitable habitat by 2100 (by about 20%–30%), mostly by expanding their northern latitudinal limit (Figure 3; Figure S9a,b). Therefore, we observed a clear northern shift in the median latitude of suitable habitat for most fishes by 2100 (from 2.0° to 9.9°; Figure 4), but with no clear trend in depth distribution (Figure 5). Projected climate refugia for deep‐water fishes were comparatively large compared to corals, averaging between 51% and 63% of present‐day habitat, depending on the threshold used (Table 4). These projected climate refugia occur mostly on both sides of the northern part of the North Atlantic (Figure 7).
Figure 7.
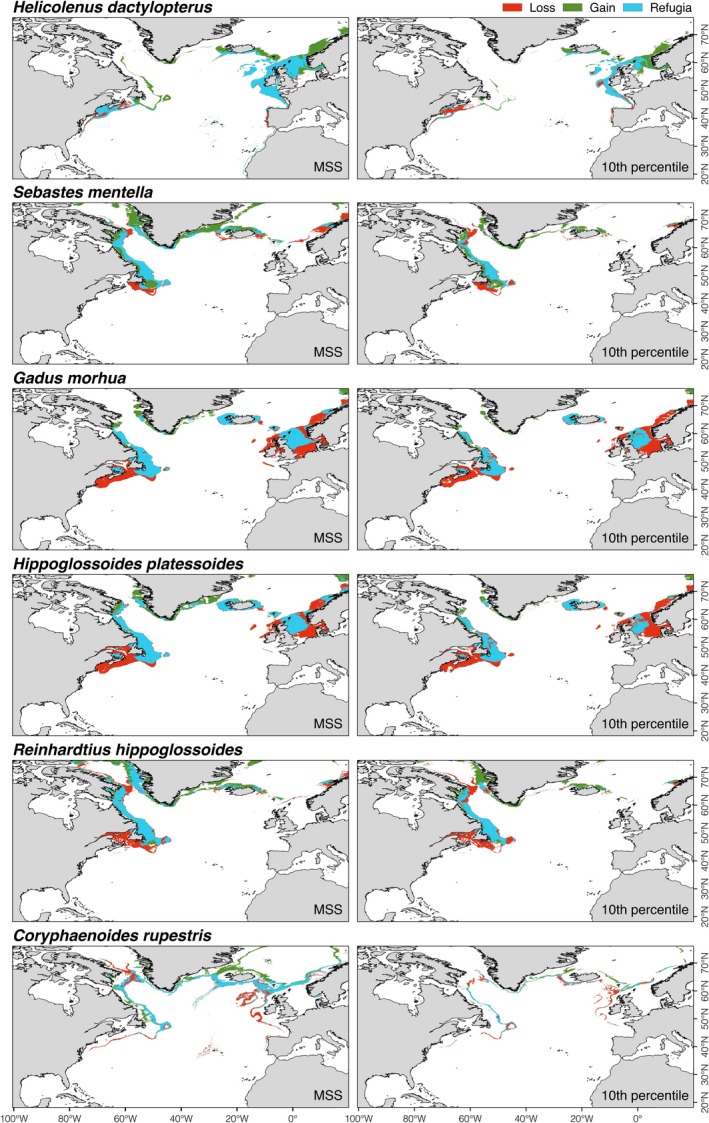
Projected present‐day suitable habitat loss, gain, and acting as climate refugia areas (sensu Keppel & Wardell‐Johnson, 2012) under future (2081–2100; RCP8.5 or business‐as‐usual scenario) environmental conditions for commercially important deep‐sea fishes in the North Atlantic Ocean. Areas were identified from binary maps built with an ensemble modelling approach and two thresholds: 10 percentile training presence logistic threshold (10th) and maximum sensitivity and specificity (MSS)
Our results strongly suggest that warming, acidification, and decreasing food availability (Figure S3) will act additively or synergistically to alter the availability of suitable habitat for deep‐sea species (Figures 6 and 7). Our analyses link marked loss of suitable habitat for cold‐water corals to the shoaling of aragonite and calcite saturation horizons as a consequence of ocean acidification in the NE (the Rockall and Faroe Shetland areas) and SE regions of the North Atlantic. This effect will act synergistically with a strong decrease in food availability on the Mid‐Atlantic Ridge. In the NW region of the North Atlantic (Davis Strait and Labrador Sea), reduced suitable habitat for octocorals in deeper waters was linked with the shoaling of the calcite saturation horizon as a consequence of ocean acidification. In the SW region from the Florida Strait to Georges Bank, loss of suitable habitat for scleractinian corals was linked to projected warming of deeper waters, whereas changes for octocorals were linked to shoaling of the calcite saturation horizon. In the Gulf of Mexico, the projected decreases in the suitable habitat of cold‐water corals were associated with anticipated warming of deeper waters. Increasing water temperature in most regions of the North Atlantic and decreasing food availability on the MAR contributed to the marked loss of suitable habitat for deep‐sea fishes. In contrast, warming of shallow waters in Davis Strait and the Labrador Sea and in the NE region resulted in projected suitable habitat gain for both cold‐water corals and fishes, along with suitable habitat gains for corals from the warming of deeper waters in North Atlantic lower latitudes.
4. DISCUSSION
Our model predictions and projections showed that North Atlantic deep‐sea species with the best‐studied distributions could experience a significant reduction in suitable habitat by 2100 as a result of climate change. Indeed, our results suggest that the suitable habitat of scleractinian corals in the North Atlantic may be reduced by more than 50%, potentially extirpating all three octocorals studied (A. arbuscula, A. armata and P. arborea). This reduction could be of particular concern for A. armata, a species limited in distribution to the North Atlantic Ocean. Our projection also suggested a northward shift of suitable habitat for many commercially important deep‐sea fishes, a finding consistent with the hypothesis of a poleward expansion in response to climate change (Jones et al., 2013; Perry et al., 2005; Poloczanska et al., 2013). Our study projected very limited, discrete climate‐change refugia for cold‐water corals, and especially octocorals, highlighting the need for accurate fine‐scale climate grids and methodologies to properly identify climate refugia (Ashcroft, 2010; Kavousi & Keppel, 2018; Keppel et al., 2012; Valdimarsson, Astthorsson, & Palsson, 2012). Occupancy of the future suitable habitats will depend on connectivity pathways and will differ greatly between deep‐sea fishes which have juvenile and adult mobility and the cold‐water corals which can only disperse as larvae (Andrello, Mouillot, Somot, Thuiller, & Manel, 2015; Baco et al., 2016; Hilário et al., 2015). The latter will be much more dependent on downstream connectivity paths that have not been evaluated in our projections but which are likely to further impose fragmentation of populations through loss of source populations (Fox, Henry, Corne, and Roberts (2016). Fox et al. (2016) have shown that past changes in the NAO significantly altered network connectivity and source–sink dynamics for L. pertusa in the northeast Atlantic, and there is every reason to anticipate similar impacts associated with the future climate scenarios shown here.
Previous studies suggested some of the general changes in distribution patterns identified here for both cold‐water corals and deep‐sea fishes, based on inferences about changes in distribution resulting from the likely effects of climate change on the marine environment. Specifically, several studies projected significant loss of suitable habitat for cold‐water coral reefs globally (Guinotte et al., 2006; Tittensor et al., 2010; Zheng & Cao, 2014) or in UK waters (Jackson, Davies, Howell, Kershaw, & Hall‐Spencer, 2014) as a result of ocean acidification. Other studies projected the loss of suitable habitat for Atlantic cod on Georges Bank, and in the Celtic and Irish Seas, a reduction in the southern North Sea and a northward expansion along Greenland and the Davis Strait and the Arctic Ocean (Drinkwater, 2005; Fogarty, Incze, Hayhoe, Mountain, & Manning, 2008; Fossheim et al., 2015).
Furthermore, field surveys already allude to pattern shifts, such as the collapse of Atlantic cod fisheries in the Gulf of Maine as a result of warming (Pershing et al., 2015). Other studies documented the northward movement of blackbelly rosefish and Atlantic cod in the North Sea (Beare et al., 2004; Engelhard, Righton, & Pinnegar, 2014; Perry et al., 2005), increased abundance of blackbelly rosefish in Icelandic waters (Sólmundsson et al., 2019), the northward movement of blackbelly rosefish and a reduction in suitable habitat for American plaice in the western North Atlantic (Nye, Link, Hare, & Overholtz, 2009) and reduced abundance of Greenland halibut in the Barents Sea (Fossheim et al., 2015). Such northward shifting of ranges is already being documented for some western North Atlantic shelf and slope fishes (Møller et al., 2010; Nye et al., 2009), and although Ross, Rhode, Viada, and Mather (2016) also reported northward range extensions, they cautioned that lack of historical baseline surveys limits interpretation of distributional data. Our study presents another example of the potential value of environmental niche modelling for projecting changes in suitable habitat for deep‐sea species under future climate scenarios, and extends the more localized or species‐specific studies already reported above, to much larger spatial scales and more consistent and rigorous analytical methods.
With climate change potentially affecting all species in the ecosystem its influence on community assembly processes remains a major knowledge gap. Field surveys suggest an association between some deep‐sea fishes (e.g. H. dactylopterus) and live cold‐water corals reefs (e.g. L. pertusa; D'Onghia et al., 2012; Pham et al., 2015). Fish species use such habitats as both spawning and nursery grounds (Corbera et al., 2019), calling into question whether the projected range expansion of the blackbelly rosefish is likely to occur given projected declines of a species forming one of its prime habitats, for example, L. pertusa. However, insufficient evidence exists to infer that blackbelly rosefish can occupy transitional and noncoral habitats in many regions (Biber et al., 2014; Milligan, Spence, Roberts, & Bailey, 2016; Ross & Quattrini, 2007), suggesting that declines in scleractinian suitable habitat may not translate into loss of habitat for this deep‐sea fish species.
Temperature and depth were important predictors of habitat suitability for cold‐water coral and deep‐sea fishes respectively. This result corresponds to similar studies on distributions of other deep‐sea corals (e.g. Buhl‐Mortensen, Olafsdottir, Buhl‐Mortensen, Burgos, & Ragnarsson, 2015; Chimienti, Taviani, & Mastrototaro, 2019; Davies & Guinotte, 2011; Georgian, Shedd, & Cordes, 2014; Guinotte & Davies, 2014; Lauria et al., 2017; Tittensor et al., 2010) and deep‐sea fishes (Gomez et al., 2015; Parra et al., 2017; Ross & Quattrini, 2007). However, the strong autocorrelation between depth and temperature and significant correlation with other environmental and biological factors complicates efforts to elucidate the environmental parameters primarily responsible for the observed patterns. Slope and other terrain attributes also help shape distributions of some cold‐water coral species (Rengstorf, Yesson, Brown, & Grehan, 2013) and are linked to the higher suitability of high geomorphological relief habitats that promote stronger near‐bed currents and enhanced food supply (Genin, Dayton, Lonsdale, & Spiess, 1986; Hebbeln, Van Rooij, & Wienberg, 2016; Soetaert Mohn, Rengstorf, Grehan, & Van Oevelen, 2016). Habitat slope and rugosity are also important elements influencing distributions of some deep‐sea fishes (Quattrini, Ross, Carlson, & Nizinski, 2012; Ross & Quattrini, 2009; Ross, Rhode, & Quattrini, 2015). For those fishes intimately tied to complex habitat, loss of corals may tend to disperse (as they search for remaining habitat) or concentrate (as they utilize shrinking habitats) fish communities.
Multiple studies document the importance of aragonite and calcite saturation state in determining cold‐water coral habitat suitability (Davies & Guinotte, 2011; Thresher et al., 2015; Tittensor et al., 2010; Yesson et al., 2012), because waters supersaturated in carbonate enable coral skeleton bio‐calcification. The chemical dissolution and biological erosion of the unprotected skeleton exposed to corrosive waters will impair the long‐term survival of cold‐water coral reefs (Hennige et al., 2015; Schönberg, Fang, Carreiro‐Silva, Tribollet, & Wisshak, 2017; Thresher, Tilbrook, Fallon, Wilson, & Adkins, 2011). However, cold‐water corals may occur in undersaturated waters of high productivity, leading to the hypothesis that increased food supply may compensate to some degree for undersaturation by providing the additional energy necessary to survive (Baco et al., 2017; Ross, unpublished data; Thresher et al., 2011). Elevated food supply may also compensate for low dissolved oxygen concentrations (Hanz et al., 2019). However, in an environment with consistently scarce food or low oxygen concentration, the metabolic costs of calcifying in extremely low carbonate conditions may become prohibitively expensive, thus compromising coral survival (Carreiro‐Silva et al., 2014; Hennige et al., 2015; Maier et al., 2016).
In fact, food availability measured as POC flux to the seafloor was also an important predictor of suitable habitat for most cold‐water corals and deep‐sea fishes in our study. This finding corroborates reports of abundant L. pertusa in regions of elevated POC flux, both in recent times (Davies & Guinotte, 2011; White, Mohn, Stigter, & Mottram, 2005) and since the last glacial events (Boavida et al., 2019; Henry et al., 2014; Matos et al., 2015; Wienberg et al., 2010). Indeed, multiple studies link reduced food availability to reduced physiological performance (e.g. calcification and respiratory metabolism) and condition of cold‐water corals (Büscher et al., 2017; Larsson, Lundälv, & van Oevelen, 2013; Naumann, Orejas, Wild, & Ferrier‐Pagès, 2011), as well as their ability to cope with ocean change (Büscher et al., 2017; Georgian et al., 2016; Gomez et al., 2019; Maier et al., 2016; Wood, Spicer, & Widdicombe, 2008). In contrast, the direct link between POC flux and deep‐sea fish abundances has proven difficult to demonstrate (Bailey, Ruhl, & Smith, 2006), despite some evidence that increased surface production may fuel key fish prey taxa such as benthic invertebrates (Bailey et al., 2006; Drazen, Bailey, Ruhl, & Smith, 2012; Ruhl & Smith, 2004). Therefore, the projected decrease in food availability by 2100 in the North Atlantic (Gehlen et al., 2014; Gomez et al., 2019; Sweetman et al., 2017) may exacerbate the likely negative effects of other environmental changes.
Inferring the capacity of deep‐sea species, and corals in particular, to adapt to changes in water chemistry projected by climatic models is challenging. For example, experimental (Keller & Os'kina, 2008) and palaeoecological studies (Wienberg et al., 2010) suggest that some M. oculata populations can tolerate elevated seawater temperature; this tolerance may explain their prevalence at shallower depths (180–360 m) in the Mediterranean Sea (Chimienti, Bo, & Mastrototaro, 2018; Chimienti et al., 2019; Freiwald et al., 2009; Gori et al., 2013). L. pertusa also may occur in regions (e.g. beneath the Florida Current, Gulf Stream) that experience periodic high temperatures (12–15°C) and rapid water property fluctuations (Brooke, Ross, Bane, Seim, & Young, 2013), but the impact of these conditions is unclear. However, D. dianthus, which may also tolerate high temperatures (Naumann, Orejas, & Ferrier‐Pagès, 2013) and survive in waters undersaturated in aragonite (Jantzen et al., 2013; Rodolfo‐Metalpa et al., 2015; Thresher et al., 2011), may experience reduced metabolism that compromise survival when exposed to the combined effects of increased temperature and reduced aragonite saturation (Gori et al., 2016). Additionally, although other scleractinians and octocorals may calcify and grow under low or undersaturated conditions (Büscher et al., 2017; Form & Riebesell, 2012; Hennige et al., 2014, 2015; Maier et al., 2012, 2013; Movilla et al., 2014; Thresher et al., 2011), their capacity to sustain calcification and other physiological processes under unfavourable conditions remains unclear, given studies that show effects of low carbonate concentrations on coral metabolism (Hennige et al., 2014) and increased energy demand required to maintain pH homeostasis at calcification sites (McCulloch et al., 2012; Raybaud et al., 2017). Despite the many uncertainties regarding potential acclimation and adaptation of cold‐water coral species to changes in climate, along with interspecific genetic variability (Kurman et al., 2017) and potential for local adaptation (Georgian et al., 2016), growing evidence points to limited long‐term capacity for adaptation to multiple stressors associated with climate change (Kurman et al., 2017).
Habitat suitability modelling approaches come with some caveats, and we, therefore, acknowledge multiple common and well‐known limitations that may be particularly pronounced when modelling deep‐sea taxa. For example, cold‐water coral and fish distributions respond to small‐scale variation in terrain, such as substrate type and seabed rugosity, as well as local oceanographic conditions such as food availability (Bennecke & Metaxas, 2017; De Clippele et al., 2017; Drazen et al., 2012; Rengstorf et al., 2013; Ross et al., 2015; White et al., 2005). We also recognize some limitations from the quantity, quality and spatial coverage of occurrence data, availability of absence records as well as some uncertainty in deep‐sea species identification (mostly for cold‐water corals). For example, two new species of Acanella were recently described from the Gulf of Mexico and Norfolk Canyon off the coast of eastern United States (Saucier, Sajjadi, & France, 2017). We, therefore, cannot state whether previous records from several databases include some of these new species. The deep sea remains one of the least studied and sampled areas on the planet, with many undescribed species and unresolved taxonomy that constrain determination of the full spatial distributions of many species. Extensive exploration of the deep‐sea environment may eventually reduce this uncertainty, but it will take time. Further integration of species‐level biogeochemical and physical data, as well as results of the ecophysiological performance of deep‐sea organisms from ex situ experimental work, will improve suitability and distribution mapping, but noting the need for additional mechanistic (experimentally derived) understanding of how climate drivers elicit ecological responses.
Finally, future climate scenarios always hinge on assumptions; indeed, the RCP8.5 or business‐as‐usual scenario projections for 2081–2100 assume specific future greenhouse gas emissions, world population growth and technology development (Riahi et al., 2011; Van Vuuren et al., 2011), and also encompass climate model errors shared by all scenarios. Consequently, our projected future models for cold‐water coral and fish species potentially represent worst‐case scenarios, with a high degree of uncertainty. Furthermore, future climate projections may not capture localized effects that may influence benthic organisms. Nevertheless, our projections of distribution changes in key species across the North Atlantic offer critical, best available information for decision makers to develop long‐term sustainable management plans (Rheuban, Doney, Cooley, & Hart, 2018), highlighting the utility of enhanced international dialogue on basin‐scale management.
Following sufficient ground‐truthing, habitat suitability models can become valuable tools to inform environmental management and conservation policy (Robinson, Nelson, Costello, Sutherland, & Lundquist, 2017) and provide a basis for taking climate change into consideration (Johnson et al., 2018; Johnson & Kenchington, 2019), as demonstrated here. In practice, these approaches can help put climate change aspects into area‐based management decisions such as those aimed to preserve VMEs (UNGA, 2006), Areas of Particular Environmental Interest designated by the International Seabed Authority, Ecologically and Biologically Significant Areas designated under the Convention on Biological Diversity (CBD), OECMs specified in CBD COP Decision 14/8 (CBD, 2018b; Garcia, Rice, Friedman, & Himes‐Cornell, in press; IUCN WCPA, 2019), or in the new legally binding international instrument for the conservation and sustainable use of biodiversity in areas beyond national jurisdiction (see Wright et al., 2019) which is currently under discussion. Knowledge of changing species distributions can also inform the United Nations Framework Convention on Climate Change.
Habitat suitability projections can help in developing research agendas that confirm and advance the model outputs and clarify the roles of predictor variables in determining species distributions. Incorporating these needs into the Deep Ocean Observing Strategy can help fill data gaps, and prioritize spatial locations for the collection of key physical and biogeochemical data (Canonico et al., 2019; Levin et al., 2019). As forward‐looking international entities, the United Nations’ Decade for Ocean Science, the Global Ocean Observing System, and the Regular Process for Global Reporting and Assessment of the State of the Marine Environment (a.k.a. World Ocean Assessment) can help set such science agendas.
In summary, we have shown that despite all the caveats, habitat suitability models can produce potentially useful projections of future changes in the distribution of deep‐water fish and invertebrate species, and areas where foundation species could be impacted by climate change and may be used to inform management decisions. This application is especially relevant for dramatic changes such as those projected here. Although ocean‐basin scale models provide useful coarse, directional information regarding climate change impacts on deep‐sea fauna, regional models could help to resolve changes of distribution and identification of refugia for monitoring purposes. We hope our study offers a suitable template for, and will stimulate, similar analyses on other taxa or regions.
Supporting information
ACKNOWLEDGEMENTS
This work contributes to the European Union's Horizon 2020 research and innovation programme under grant agreement No 678760 (ATLAS) and No 679849 (SponGES). This output reflects only the authors' views and the European Union cannot be held responsible for any use that may be made of the information contained therein. TM was supported by Program Investigador FCT (IF/01194/2013), IFCT Exploratory Project (IF/01194/2013/CP1199/CT0002) from the Fundação para a Ciência e Tecnologia (POPH and QREN), PO2020 MapGes (Acores‐01‐0145‐FEDER‐000056). TM, CDC, JMGI, GHT and MCS also acknowledge funds provided by the Fundação para a Ciência e a Tecnologia (FCT) through the strategic project (FCT/UID/MAR/04292/2013) granted to MARE. LAL is supported by NSF OCE OCE‐1829623 and a DOOS subcontract from the Consortium for Ocean Leadership. HE and SR acknowledge funds by the Icelandic Research Fund (174552‐052) and SR for grant from Fundación Séneca (20632/IV/18). DJ acknowledges the Global Ocean Biodiversity Initiative, part of the International Climate Initiative (grant no. 16‐IV‐049‐Global‐A‐Global Ocean Biodiversity Initiative GOBI). T Molodtsova acknowledges RF State Assignment 0149‐2019‐0009. We acknowledge all of those involved in data collection and modelling, namely Anna Rengstorf, Inge van den Beld and Tim Siferd, among many others. Finally, we thank the Food and Agriculture Organization ABNJ Deep‐Seas Program, the Deep Ocean Stewardship Initiative, Tony Thompson and Maria Baker for their contributions towards this work.
Morato T, González‐Irusta J‐M, Dominguez‐Carrió C, et al. Climate‐induced changes in the suitable habitat of cold‐water corals and commercially important deep‐sea fishes in the North Atlantic. Glob Change Biol. 2020;26:2181–2202. 10.1111/gcb.14996
Footnotes
Recently synonymized to Desmophyllum pertusum (Addamo et al., 2016).
DATA AVAILABILITY STATEMENT
The bathymetry data supporting the analyses are publicly available from the EMODnet Digital Terrain Model and the General Bathymetric Chart of the Oceans portals (https://portal.emodnet-bathymetry.eu/ and https://www.gebco.net/data_and_products/gridded_bathymetry_data/, respectively). The environmental data used in this study are publicly available from the Earth System Grid Federation (ESGF) Peer‐to‐Peer (P2P) enterprise system (https://esgf.llnl.gov/). All analyses were conducted using publicly available packages from the Comprehensive R Archive Network (https://cran.r-project.org/), namely the R packages MOPA, Raster, Dismo, mgcv, randomForest, MuMIn, EnMEVAL, and boot. The Benthic Terrain Model 3.0 tool is available from the ESRI Oceans GitHub repository (https://github.com/EsriOceans/btm). The processed environmental data layers used in this study are publicly available through the PANGAEA data publisher portal (https://doi.org/10.1594/PANGAEA.911117). The model outputs are also available for download from PANGAEA (https://doi.org/10.1594/PANGAEA.910319).
REFERENCES
- Addamo, A. M. , Vertino, A. , Stolarski, J. , García‐Jiménez, R. , Taviani, M. , & Machordom, A. (2016). Merging scleractinian genera: The overwhelming genetic similarity between solitary Desmophyllum and colonial Lophelia . BMC Evolutionary Biology, 16(1), 108 10.1186/s12862-016-0654-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson, O. F. , Guinotte, J. M. , Rowden, A. A. , Tracey, D. M. , Mackay, K. A. , & Clark, M. R. (2016). Habitat suitability models for predicting the occurrence of vulnerable marine ecosystems in the seas around New Zealand. Deep Sea Research Part I: Oceanographic Research Papers, 115, 265–292. 10.1016/j.dsr.2016.07.006 [DOI] [Google Scholar]
- Andrello, M. , Mouillot, D. , Somot, S. , Thuiller, W. , & Manel, S. (2015). Additive effects of climate change on connectivity between marine protected areas and larval supply to fished areas. Diversity and Distributions, 21(2), 139–150. 10.1111/ddi.12250 [DOI] [Google Scholar]
- Araújo, M. B. , & New, M. (2007). Ensemble forecasting of species distributions. Trends in Ecology & Evolution, 22(1), 42–47. 10.1016/j.tree.2006.09.010 [DOI] [PubMed] [Google Scholar]
- Ashcroft, M. B. (2010). Identifying refugia from climate change. Journal of Biogeography, 37(8), 1407–1413. 10.1111/j.1365-2699.2010.02300.x [DOI] [Google Scholar]
- Austin, M. P. , & Van Niel, K. P. (2011). Improving species distribution models for climate change studies: Variable selection and scale. Journal of Biogeography, 38(1), 1–8. 10.1111/j.1365-2699.2010.02416.x [DOI] [Google Scholar]
- Baco, A. R. , Etter, R. J. , Ribeiro, P. A. , von der Heyden, S. , Beerli, P. , & Kinlan, B. P. (2016). A synthesis of genetic connectivity in deep‐sea fauna and implications for marine reserve design. Molecular Ecology, 25(14), 3276–3298. 10.1111/mec.13689 [DOI] [PubMed] [Google Scholar]
- Baco, A. R. , Morgan, N. , Roark, E. B. , Silva, M. , Shamberger, K. E. , & Miller, K. (2017). Defying dissolution: Discovery of deep‐sea scleractinian coral reefs in the North Pacific. Scientific Reports, 7(1), 5436 10.1038/s41598-017-05492-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, D. M. , Ruhl, H. A. , & Smith, K. L. Jr. (2006). Long‐term change in benthopelagic fish abundance in the abyssal northeast Pacific Ocean. Ecology, 87(3), 549–555. 10.1890/04-1832 [DOI] [PubMed] [Google Scholar]
- Barton, K. (2018). MuMIn: Multi‐Model Inference. R package version 1.40.4. Retrieved from https://CRAN.R-project.org/package=MuMIn [Google Scholar]
- Bates, A. E. , Cooke, R. S. C. , Duncan, M. I. , Edgar, G. J. , Bruno, J. F. , Benedetti‐Cecchi, L. , … Stuart‐Smith, R. D. (2019). Climate resilience in marine protected areas and the ‘Protection Paradox’. Biological Conservation, 236, 305–314. 10.1016/j.biocon.2019.05.005 [DOI] [Google Scholar]
- Baudron, A. R. , Needle, C. L. , Rijnsdorp, A. D. , & Tara Marshall, C. (2014). Warming temperatures and smaller body sizes: Synchronous changes in growth of North Sea fishes. Global Change Biology, 20(4), 1023–1031. 10.1111/gcb.12514 [DOI] [PubMed] [Google Scholar]
- Beare, D. J. , Burns, F. , Greig, A. , Jones, E. G. , Peach, K. , Kienzle, M. , … Reid, D. G. (2004). Long‐term increases in prevalence of North Sea fishes having southern biogeographic affinities. Marine Ecology Progress Series, 284, 269–278. 10.3354/meps284269 [DOI] [Google Scholar]
- Bennecke, S. , & Metaxas, A. (2017). Is substrate composition a suitable predictor for deep‐water coral occurrence on fine scales? Deep Sea Research Part I: Oceanographic Research Papers, 124, 55–65. 10.1016/j.dsr.2017.04.011 [DOI] [Google Scholar]
- Biber, M. F. , Duineveld, G. C. , Lavaleye, M. S. , Davies, A. J. , Bergman, M. J. , & van den Beld, I. M. (2014). Investigating the association of fish abundance and biomass with cold‐water corals in the deep Northeast Atlantic Ocean using a generalised linear modelling approach. Deep Sea Research Part II: Topical Studies in Oceanography, 99, 134–145. 10.1016/j.dsr2.2013.05.022 [DOI] [Google Scholar]
- Bindoff, N. L. , Cheung, W. W. L. , Arístegui, J. G. K. J. , Guinder, V. A. , Hallberg, R. , Hilmi, N. , … Williamson, P. (2019). Chapter 5: Changing ocean, marine ecosystems, and dependent communities IPCC special report oceans and cryospheres in changing climate. Cambridge: University Press. [Google Scholar]
- Boavida, J. , Becheler, R. , Choquet, M. , Frank, N. , Taviani, M. , Bourillet, J.‐F. , … Arnaud‐Haond, S. (2019). Out of the Mediterranean? Post‐glacial colonization pathways varied among cold‐water coral species. Journal of Biogeography, 46(5), 915–931. 10.1111/jbi.13570 [DOI] [Google Scholar]
- Bradbury, I. R. , Hubert, S. , Higgins, B. , Borza, T. , Bowman, S. , Paterson, I. G. , … Bentzen, P. (2010). Parallel adaptive evolution of Atlantic cod on both sides of the Atlantic Ocean in response to temperature. Proceedings of the Royal Society B: Biological Sciences, 277(1701), 3725–3734. 10.1098/rspb.2010.0985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiman, L. (2001). Random forests. Machine Learning, 45, 5–32. 10.1023/A:1010933404324 [DOI] [Google Scholar]
- Brooke, S. , Ross, S. W. , Bane, J. M. , Seim, H. E. , & Young, C. M. (2013). Temperature tolerance of the deep‐sea coral Lophelia pertusa from the southeastern United States. Deep Sea Research Part II: Topical Studies in Oceanography, 92, 240–248. 10.1016/j.dsr2.2012.12.001 [DOI] [Google Scholar]
- Bryndum‐Buchholz, A. , Tittensor, D. P. , Blanchard, J. L. , Cheung, W. W. L. , Coll, M. , Galbraith, E. D. , … Lotze, H. K. (2019). Twenty‐first‐century climate change impacts on marine animal biomass and ecosystem structure across ocean basins. Global Change Biology, 25(2), 459–472. 10.1111/gcb.14512 [DOI] [PubMed] [Google Scholar]
- Buhl‐Mortensen, L. , Olafsdottir, S. H. , Buhl‐Mortensen, P. , Burgos, J. M. , & Ragnarsson, S. A. (2015). Distribution of nine cold‐water coral species (Scleractinia and Gorgonacea) in the cold temperate North Atlantic: Effects of bathymetry and hydrography. Hydrobiologia, 759(1), 39–61. 10.1007/s10750-014-2116-x [DOI] [Google Scholar]
- Buisson, L. , Thuiller, W. , Casajus, N. , Lek, S. , & Grenouillet, G. (2010). Uncertainty in ensemble forecasting of species distribution. Global Change Biology, 16(4), 1145–1157. 10.1111/j.1365-2486.2009.02000.x [DOI] [Google Scholar]
- Büscher, J. V. , Form, A. U. , & Riebesell, U. (2017). Interactive effects of ocean acidification and warming on growth, fitness and survival of the cold‐water coral Lophelia pertusa under different food availabilities. Frontiers in Marine Science, 4, 101 10.3389/fmars.2017.00101 [DOI] [Google Scholar]
- Büscher, J. V. , Wisshak, M. , Form, A. U. , Titschack, J. , Nachtigall, K. , & Riebesell, U. (2019). In situ growth and bioerosion rates of Lophelia pertusa in a Norwegian fjord and open shelf cold‐water coral habitat. PeerJ, 7, e7586 10.7717/peerj.7586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canonico, G. , Buttigieg, P. L. , Montes, E. , Muller‐Karger, F. E. , Stepien, C. , Wright, D. , … Murton, B. (2019). Global observational needs and resources for marine biodiversity. Frontiers in Marine Science, 6, 367 10.3389/fmars.2019.00367 [DOI] [Google Scholar]
- Canty, A. , & Ripley, B. (2017). boot: Bootstrap R (S‐Plus) functions. R package version 1.3‐20. Retrieved from https://cran.r-project.org/web/packages/boot/boot.pdf [Google Scholar]
- Carreiro‐Silva, M. , Cerqueira, T. , Godinho, A. , Caetano, M. , Santos, R. S. , & Bettencourt, R. (2014). Molecular mechanisms underlying the physiological responses of the cold‐water coral Desmophyllum dianthus to ocean acidification. Coral Reefs, 33(2), 465–476. 10.1007/s00338-014-1129-2 [DOI] [Google Scholar]
- CBD . (2018a). Protected areas and other effective area‐based conservation measures In Convention on biological diversity, 22nd meeting, Montreal, Canada 2–7 July 2018, Agenda item 7, CBD/SBSTTA/22/L.2. Retrieved from https://www.cbd.int/doc/c/9b1f/759a/dfcee171bd46b06cc91f6a0d/sbstta-22-l-02-en.pdf [Google Scholar]
- CBD . (2018b). Decisions adopted by the conference of the parties to the convention on biological diversity In Decision 14/8 on protected areas and other effective area‐based conservation measures. Cop 14. Convention on Biological Diversity, 14th meeting, Sharm El-Sheik, Egypt, 17–29 November 2018, Agenda item 24, CBD/COP/DEC/14/8. Retrieved from https://www.cbd.int/doc/decisions/cop-14/cop-14-dec-08-en.pdf [Google Scholar]
- Cerrano, C. , Cardini, U. , Bianchelli, S. , Corinaldesi, C. , Pusceddu, A. , & Danovaro, R. (2013). Red coral extinction risk enhanced by ocean acidification. Scientific Reports, 3, 1457 10.1038/srep01457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chefaoui, R. M. , Duarte, C. M. , & Serrão, E. A. (2018). Dramatic loss of seagrass habitat under projected climate change in the Mediterranean Sea. Global Change Biology, 24(10), 4919–4928. 10.1111/gcb.14401 [DOI] [PubMed] [Google Scholar]
- Chen, C. T. A. , Lui, H. K. , Hsieh, C. H. , Yanagi, T. , Kosugi, N. , Ishii, M. , & Gong, G. C. (2017). Deep oceans may acidify faster than anticipated due to global warming. Nature Climate Change, 7(12), 890–894. 10.1038/s41558-017-0003-y [DOI] [Google Scholar]
- Cheung, W. W. L. , Jones, M. C. , Lam, V. W. Y. , Miller, D. D. , Ota, Y. , Teh, L. , & Sumaila, U. R. (2017). Transform high seas management to build climate resilience in marine seafood supply. Fish and Fisheries, 18(2), 254–263. 10.1111/faf.12177 [DOI] [Google Scholar]
- Cheung, W. W. , Lam, V. W. , Sarmiento, J. L. , Kearney, K. , Watson, R. E. G. , Zeller, D. , & Pauly, D. (2010). Large‐scale redistribution of maximum fisheries catch potential in the global ocean under climate change. Global Change Biology, 16(1), 24–35. 10.1111/j.1365-2486.2009.01995.x [DOI] [Google Scholar]
- Chimienti, G. , Bo, M. , & Mastrototaro, F. (2018). Know the distribution to assess the changes: Mediterranean cold‐water coral bioconstructions. Rendiconti Lincei. Scienze Fisiche e Naturali, 29(3), 583–588. 10.1007/s12210-018-0718-3 [DOI] [Google Scholar]
- Chimienti, G. , Bo, M. , Taviani, M. , & Mastrototaro, F. (2019). Occurrence and biogeography of Mediterranean cold‐water corals In Orejas C. & Jiménez C. (Eds.), Mediterranean cold‐water corals: Past, present and future (pp. 213–243). Chapter 19. Berlin: Springer International Publishing; 10.1007/978-3-319-91608-8_19 [DOI] [Google Scholar]
- Corbera, G. , Lo Iacono, C. , Gràcia, E. , Grinyó, J. , Pierdomenico, M. , Huvenne, V. A. I. , … Gili, J. M. (2019). Ecological characterization of a Mediterranean cold‐water coral reef: Cabliers Coral Mound Province (Alboran Sea, western Mediterranean). Progress in Oceanography, 175, 245–262. 10.1016/j.pocean.2019.04.010 [DOI] [Google Scholar]
- Cordes, E. E. , Arnaud‐Haond, S. , Bergstad, O.‐A. , da Costa Falçao, A. P. , Freiwald, A. , & Roberts, J. M. (2016). Chapter 42: Cold‐water corals In Inniss L., Simcock A., Ajawin A. Y., Alcala A. C., Bernal P., Calumpong H. P., … Kamara O. K. (Eds.), The first global integrated marine assessment: World ocean assessment (pp. 803–816). New York: United Nations General Assembly. [Google Scholar]
- Davies, A. J. , & Guinotte, J. M. (2011). Global habitat suitability for framework‐forming cold‐water corals. PLoS ONE, 6(4), e18483 10.1371/journal.pone.0018483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Clippele, L. H. , Gafeira, J. , Robert, K. , Hennige, S. , Lavaleye, M. S. , Duineveld, G. C. A. , … Roberts, J. M. (2017). Using novel acoustic and visual mapping tools to predict the small‐scale spatial distribution of live biogenic reef framework in cold‐water coral habitats. Coral Reefs, 36(1), 255–268. 10.1007/s00338-016-1519-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Onghia, G. , Maiorano, P. , Carlucci, R. , Capezzuto, F. , Carluccio, A. , Tursi, A. , & Sion, L. (2012). Comparing deep‐sea fish fauna between coral and non‐coral “megahabitats” in the Santa Maria di Leuca cold‐water coral province (Mediterranean Sea). PLoS ONE, 7(9), e44509 10.1371/journal.pone.0044509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dormann, C. F. , Elith, J. , Bacher, S. , Buchmann, C. , Carl, G. , Carré, G. , … Lautenbach, S. (2013). Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography, 36(1), 27–46. 10.1111/j.1600-0587.2012.07348.x [DOI] [Google Scholar]
- Drazen, J. C. , Bailey, D. M. , Ruhl, H. A. , & Smith, K. L. Jr. (2012). The role of carrion supply in the abundance of deep‐water fish off California. PLoS ONE, 7(11), e49332 10.1371/journal.pone.0049332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drinkwater, K. F. (2005). The response of Atlantic cod (Gadus morhua) to future climate change. ICES Journal of Marine Science, 62(7), 1327–1337. 10.1016/j.icesjms.2005.05.015 [DOI] [Google Scholar]
- Dufresne, J.‐L. , Foujols, M.‐A. , Denvil, S. , Caubel, A. , Marti, O. , Aumont, O. , … Vuichard, N. (2013). Climate change projections using the IPSL‐CM5 Earth System Model: From CMIP3 to CMIP5. Climate Dynamics, 40(9–10), 2123–2165. 10.1007/s00382-012-1636-1 [DOI] [Google Scholar]
- Dulvy, N. K. , Rogers, S. I. , Jennings, S. , Stelzenmüller, V. , Dye, S. R. , & Skjoldal, H. R. (2008). Climate change and deepening of the North Sea fish assemblage: A biotic indicator of warming seas. Journal of Applied Ecology, 45(4), 1029–1039. 10.1111/j.1365-2664.2008.01488.x [DOI] [Google Scholar]
- Dunne, J. P. , John, J. G. , Adcroft, A. J. , Griffies, S. M. , Hallberg, R. W. , Shevliakova, E. , … Zadeh, N. (2012). GFDL’s ESM2 global coupled climate–carbon earth system models. Part I: Physical formulation and baseline simulation characteristics. Journal of Climate, 25(19), 6646–6665. 10.1175/JCLI-D-11-00560.1 [DOI] [Google Scholar]
- Elith, J. , Graham, C. H. , Anderson, R. P. , Dudík, M. , Ferrier, S. , Guisan, A. , … Zimmermann, N. E. (2006). Novel methods improve prediction of species’ distributions from occurrence data. Ecography, 29(2), 129–151. 10.1111/j.2006.0906-7590.04596.x [DOI] [Google Scholar]
- Elith, J. , & Leathwick, J. R. (2009). Species distribution models: Ecological explanation and prediction across space and time. Annual Review of Ecology, Evolution, and Systematics, 40(1), 677–697. 10.1146/annurev.ecolsys.110308.120159 [DOI] [Google Scholar]
- Elith, J. , Phillips, S. J. , Hastie, T. , Dudík, M. , Chee, Y. E. , & Yates, C. J. (2011). A statistical explanation of MaxEnt for ecologists. Diversity and Distributions, 17(1), 43–57. 10.1111/j.1472-4642.2010.00725.x [DOI] [Google Scholar]
- EMODnet Bathymetry Consortium . (2018). EMODnet Digital Bathymetry (DTM). Marine Information Service. 10.12770/18ff0d48-b203-4a65-94a9-5fd8b0ec35f6 [DOI]
- Engelhard, G. H. , Righton, D. A. , & Pinnegar, J. K. (2014). Climate change and fishing: A century of shifting distribution in North Sea cod. Global Change Biology, 20(8), 2473–2483. 10.1111/gcb.12513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- FAO . (2019). Deep‐ocean climate change impacts on habitat, fish and fisheries. FAO Fisheries and Aquaculture Technical Paper 638. Rome: Food and Agriculture Organization of the UN; Retrieved from http://www.fao.org/3/ca2528en/ca2528en.pdf [Google Scholar]
- Fielding, A. H. , & Bell, J. F. (1997). A review of methods for the assessment of prediction errors in conservation presence/absence models. Environmental Conservation, 24(1), 38–49. 10.1017/S0376892997000088 [DOI] [Google Scholar]
- Fillinger, L. , & Richter, C. (2013). Vertical and horizontal distribution of Desmophyllum dianthus in Comau Fjord, Chile: A cold‐water coral thriving at low pH. PeerJ, 1, e194 10.7717/peerj.194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty, M. , Incze, L. , Hayhoe, K. , Mountain, D. , & Manning, J. (2008). Potential climate change impacts on Atlantic cod (Gadus morhua) off the northeastern USA. Mitigation and Adaptation Strategies for Global Change, 13(5–6), 453–466. 10.1007/s11027-007-9131-4 [DOI] [Google Scholar]
- Fordham, D. A. , Resit Akçakaya, H. , Araujo, M. B. , Elith, J. , Keith, D. A. , Pearson, R. , … Brook, B. W. (2012). Plant extinction risk under climate change: Are forecast range shifts alone a good indicator of species vulnerability to global warming? Global Change Biology, 18(4), 1357–1371. 10.1111/j.1365-2486.2011.02614.x [DOI] [Google Scholar]
- Form, A. U. , & Riebesell, U. (2012). Acclimation to ocean acidification during long‐term CO2 exposure in the cold‐water coral Lophelia pertusa . Global Change Biology, 18(3), 843–853. 10.1111/j.1365-2486.2011.02583.x [DOI] [Google Scholar]
- Fossheim, M. , Primicerio, R. , Johannesen, E. , Ingvaldsen, R. B. , Aschan, M. M. , & Dolgov, A. V. (2015). Recent warming leads to a rapid borealization of fish communities in the Arctic. Nature Climate Change, 5(7), 673–677. 10.1038/nclimate2647 [DOI] [Google Scholar]
- Fourcade, Y. , Besnard, A. G. , & Secondi, J. (2018). Paintings predict the distribution of species, or the challenge of selecting environmental predictors and evaluation statistics. Global Ecology and Biogeography, 27(2), 245–256. 10.1111/geb.12684 [DOI] [Google Scholar]
- Fox, A. D. , Henry, L.‐A. , Corne, D. W. , & Roberts, J. M. (2016). Sensitivity of marine protected area network connectivity to atmospheric variability. Royal Society Open Science, 3(11), 160494 10.1098/rsos.160494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank, K. T. , Petrie, B. , Choi, J. S. , & Leggett, W. C. (2005). Trophic cascades in a formerly cod‐dominated ecosystem. Science, 308(5728), 1621–1623. 10.1126/science.1113075 [DOI] [PubMed] [Google Scholar]
- Freiwald, A. (2002). Reef‐forming cold‐water corals In Wefer G., Billett D., Hebbeln D., Jørgensen B. B., Schlüter M., & van Weering T. C. E. (Eds.), Ocean margin systems (pp. 365–385). Berlin: Springer; 10.1007/978-3-662-05127-6_23 [DOI] [Google Scholar]
- Freiwald, A. , Beuck, L. , Rüggeberg, A. , Taviani, M. , Hebbeln, D. , & R/V Meteor Cruise M70–1 Participants . (2009). The white coral community in the central Mediterranean Sea revealed by ROV surveys. Oceanography, 22(1), 58–74. 10.5670/oceanog.2009.06 [DOI] [Google Scholar]
- Gaines, S. D. , Costello, C. , Owashi, B. , Mangin, T. , Bone, J. , Molinos, J. G. , … Ovando, D. (2018). Improved fisheries management could offset many negative effects of climate change. Science Advances, 4(8), eaao1378 10.1126/sciadv.aao1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia, S. M. , Rice, J. , Friedman, K. , & Himes‐Cornell, A. (in press). Identification, assessment and governance of Other Effective Area‐Based Conservation Measures in the marine fishery sector: A background document. Retrieved from https://www.openchannels.org/literature/24881 [Google Scholar]
- Gehlen, M. , Séférian, R. , Jones, D. O. B. , Roy, T. , Roth, R. , Barry, J. , … Tjiputra, J. (2014). Projected pH reductions by 2100 might put deep North Atlantic biodiversity at risk. Biogeosciences, 11(23), 6955–6967. 10.5194/bg-11-6955-2014 [DOI] [Google Scholar]
- Genin, A. , Dayton, P. K. , Lonsdale, P. F. , & Spiess, F. N. (1986). Corals on seamount peaks provide evidence of current acceleration over deep‐sea topography. Nature, 322(6074), 59–61. 10.1038/322059a0 [DOI] [Google Scholar]
- Georgian, S. E. , Dupont, S. , Kurman, M. , Butler, A. , Strömberg, S. M. , Larsson, A. I. , & Cordes, E. E. (2016). Biogeographic variability in the physiological response of the cold‐water coral Lophelia pertusa to ocean acidification. Marine Ecology, 37(6), 1345–1359. 10.1111/maec.12373 [DOI] [Google Scholar]
- Georgian, S. E. , Shedd, W. , & Cordes, E. E. (2014). High‐resolution ecological niche modelling of the cold‐water coral Lophelia pertusa in the Gulf of Mexico. Marine Ecology Progress Series, 506, 145–161. 10.3354/meps10816 [DOI] [Google Scholar]
- Giorgetta, M. A. , Jungclaus, J. , Reick, C. H. , Legutke, S. , Bader, J. , Böttinger, M. , … Stevens, B. (2013). Climate and carbon cycle changes from 1850 to 2100 in MPI‐ESM simulations for the Coupled Model Intercomparison Project phase 5. Journal of Advances in Modeling Earth Systems, 5(3), 572–597. 10.1002/jame.20038 [DOI] [Google Scholar]
- Gomez, C. E. , Wickes, L. , Deegan, D. , Etnoyer, P. J. , & Cordes, E. E. (2019). Growth and feeding of deep‐sea coral Lophelia pertusa from the California margin under simulated ocean acidification conditions. PeerJ, 6, e5671 10.7717/peerj.5671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez, C. , Williams, A. J. , Nicol, S. J. , Mellin, C. , Loeun, K. L. , & Bradshaw, C. J. (2015). Species distribution models of tropical deep‐sea snappers. PLoS ONE, 10(6), e0127395 10.1371/journal.pone.0127395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- González‐Irusta, J. M. , González‐Porto, M. , Sarralde, R. , Arrese, B. , Almón, B. , & Martín‐Sosa, P. (2015). Comparing species distribution models: A case study of four deep sea urchin species. Hydrobiologia, 745(1), 43–57. 10.1007/s10750-014-2090-3 [DOI] [Google Scholar]
- Gori, A. , Ferrier‐Pagès, C. , Hennige, S. J. , Murray, F. , Rottier, C. , Wicks, L. C. , & Roberts, J. M. (2016). Physiological response of the cold‐water coral Desmophyllum dianthus to thermal stress and ocean acidification. PeerJ, 4, e1606 10.7717/peerj.1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gori, A. , Orejas, C. , Madurell, T. , Bramanti, L. , Martins, M. , Quintanilla, E. , … Gili, J. M. (2013). Bathymetrical distribution and size structure of cold‐water coral populations in the Cap de Creus and Lacaze‐Duthiers canyons (northwestern Mediterranean). Biogeosciences, 10, 2049–2060. 10.5194/bg-10-2049-2013 [DOI] [Google Scholar]
- Greathead, C. , González‐Irusta, J. M. , Clarke, J. , Boulcott, P. , Blackadder, L. , Weetman, A. , & Wright, P. J. (2015). Environmental requirements for three sea pen species: Relevance to distribution and conservation. ICES Journal of Marine Science, 72(2), 576–586. 10.1093/icesjms/fsu129 [DOI] [Google Scholar]
- Gugliotti, E. F. , DeLorenzo, M. E. , & Etnoyer, P. J. (2019). Depth‐dependent temperature variability in the Southern California bight with implications for the cold‐water gorgonian octocoral Adelogorgia phyllosclera. Journal of Experimental Marine Biology and Ecology, 514, 118–126. [Google Scholar]
- Guinotte, J. M. , & Davies, A. J. (2014). Predicted deep‐sea coral habitat suitability for the US West Coast. PLoS ONE, 9(4), e93918 10.1371/journal.pone.0093918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinotte, J. M. , Orr, J. , Cairns, S. , Freiwald, A. , Morgan, L. , & George, R. (2006). Will human‐induced changes in seawater chemistry alter the distribution of deep‐sea scleractinian corals? Frontiers in Ecology and the Environment, 4(3), 141–146. 10.1890/1540-9295(2006)004[0141:WHCISC]2.0.CO;2 [DOI] [Google Scholar]
- Hanz, U. , Wienberg, C. , Hebbeln, D. , Duineveld, G. , Lavaleye, M. , Juva, K. ,… Flögel, S. (2019). Environmental factors influencing cold‐water coral ecosystems in the oxygen minimum zones on the Angolan and Namibian margins. Biogeosciences, 16, 4337–4356. 10.5194/bg-16-4337-2019 [DOI] [Google Scholar]
- Hastie, T. J. , & Tibshirani, R. J. (1986). Generalized additive models. Statistical Science, 1, 297–318. 10.1214/ss/1177013604 [DOI] [PubMed] [Google Scholar]
- Hattab, T. , Albouy, C. , Lasram, F. B. R. , Somot, S. , Le Loc'h, F. , & Leprieur, F. (2014). Towards a better understanding of potential impacts of climate change on marine species distribution: A multiscale modelling approach. Global Ecology and Biogeography, 23(12), 1417–1429. 10.1111/geb.12217 [DOI] [Google Scholar]
- Hebbeln, D. , Van Rooij, D. , & Wienberg, C. (2016). Good neighbours shaped by vigorous currents: Cold‐water coral mounds and contourites in the North Atlantic. Marine Geology, 378, 171–185. 10.1016/j.margeo.2016.01.014 [DOI] [Google Scholar]
- Hennige, S. J. , Wicks, L. C. , Kamenos, N. A. , Bakker, D. C. , Findlay, H. S. , Dumousseaud, C. , & Roberts, J. M. (2014). Short‐term metabolic and growth responses of the cold‐water coral Lophelia pertusa to ocean acidification. Deep Sea Research Part II: Topical Studies in Oceanography, 99, 27–35. 10.1016/j.dsr2.2013.07.005 [DOI] [Google Scholar]
- Hennige, S. J. , Wicks, L. C. , Kamenos, N. A. , Perna, G. , Findlay, H. S. , & Roberts, J. M. (2015). Hidden impacts of ocean acidification to live and dead coral framework. Proceedings of the Royal Society B: Biological Sciences, 282(1813), 20150990 10.1098/rspb.2015.0990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry, L.‐A. , Frank, N. , Hebbeln, D. , Wienberg, C. , Robinson, L. , de Flierdt, T. V. , … Roberts, J. M. (2014). Global ocean conveyor lowers extinction risk in the deep sea. Deep Sea Research Part I: Oceanographic Research Papers, 88, 8–16. 10.1016/j.dsr.2014.03.004 [DOI] [Google Scholar]
- Hijmans, R. J. (2016). Introduction to the ’raster’ package (version 2.5‐8). 27 pp. Retrieved from https://cran.r-project.org/web/packages/raster/vignettes/Raster.pd [Google Scholar]
- Hijmans, R. J. , & Graham, C. H. (2006). The ability of climate envelope models to predict the effect of climate change on species distributions. Global Change Biology, 12(12), 2272–2281. 10.1111/j.1365-2486.2006.01256.x [DOI] [Google Scholar]
- Hijmans, R. J. , Phillips, S. , Leathwick, J. , & Elith, J. (2017). dismo: Species distribution modeling. R package version 1.1‐4. Retrieved from https://CRAN.R-project.org/package=dismo [Google Scholar]
- Hilário, A. , Metaxas, A. , Gaudron, S. M. , Howell, K. L. , Mercier, A. , Mestre, N. Ã. C. , … Young, C. (2015). Estimating dispersal distance in the deep sea: Challenges and applications to marine reserves. Frontiers in Marine Science, 2, 6 10.3389/fmars.2015.00006 [DOI] [Google Scholar]
- Holt, R. E. , & Jørgensen, C. (2015). Climate change in fish: Effects of respiratory constraints on optimal life history and behaviour. Biology Letters, 11(2), 20141032 10.1098/rsbl.2014.1032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturbide, M. , Bedia, J. , & Gutiérrez, J. M. (2018). Tackling uncertainties of species distribution model projections with package mopa. The R Journal, 10(1), 122 10.32614/RJ-2018-019 [DOI] [Google Scholar]
- Iturbide, M. , Bedia, J. , Herrera, S. , del Hierro, O. , Pinto, M. , & Gutiérrez, J. M. (2015). A framework for species distribution modelling with improved pseudo‐absence generation. Ecological Modelling, 312, 166–174. 10.1016/j.ecolmodel.2015.05.018 [DOI] [Google Scholar]
- IUCN WCPA . (2019). Guidelines for recognising and reporting other effective area‐based conservation measures. Switzerland: International Union for Conservation of Nature. [Google Scholar]
- Iverson, L. R. , & Prasad, A. M. (1998). Predicting abundance of 80 tree species following climate change in the eastern United States. Ecological Monographs, 68(4), 465–485. 10.1890/0012-9615(1998)068[0465:PAOTSF]2.0.CO;2 [DOI] [Google Scholar]
- Jackson, E. L. , Davies, A. J. , Howell, K. L. , Kershaw, P. J. , & Hall‐Spencer, J. M. (2014). Future‐proofing marine protected area networks for cold water coral reefs. ICES Journal of Marine Science, 71(9), 2621–2629. 10.1093/icesjms/fsu099 [DOI] [Google Scholar]
- Jantzen, C. , Häussermann, V. , Försterra, G. , Laudien, J. , Ardelan, M. , Maier, S. , & Richter, C. (2013). Occurrence of a cold‐water coral along natural pH gradients (Patagonia, Chile). Marine Biology, 160(10), 2597–2607. 10.1007/s00227-013-2254-0 [DOI] [Google Scholar]
- Johnson, D. , Ferreira, M. A. , & Kenchington, E. (2018). Climate change is likely to severely limit the effectiveness of deep‐sea ABMTs in the North Atlantic. Marine Policy, 87, 111–122. 10.1016/j.marpol.2017.09.034 [DOI] [Google Scholar]
- Johnson, D. E. , & Kenchington, E. L. (2019). Should potential for climate change refugia be mainstreamed into the criteria for describing EBSAs? Conservation Letters, 12(4), e12634 10.1111/conl.12634 [DOI] [Google Scholar]
- Jones, D. O. , Yool, A. , Wei, C. L. , Henson, S. A. , Ruhl, H. A. , Watson, R. A. , & Gehlen, M. (2014). Global reductions in seafloor biomass in response to climate change. Global Change Biology, 20(6), 1861–1872. 10.1111/gcb.12480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones, M. C. , Dye, S. R. , Fernandes, J. A. , Frölicher, T. L. , Pinnegar, J. K. , Warren, R. , & Cheung, W. W. (2013). Predicting the impact of climate change on threatened species in UK waters. PLoS ONE, 8(1), e54216 10.1371/journal.pone.0054216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavousi, J. , & Keppel, G. (2018). Clarifying the concept of climate change refugia for coral reefs. ICES Journal of Marine Science, 75(1), 43–49. 10.1093/icesjms/fsx124 [DOI] [Google Scholar]
- Keller, N. B. , & Os’kina, N. S. (2008). Habitat temperature ranges of azooxantellate scleractinian corals in the world ocean. Oceanology, 48(1), 77–84. 10.1134/S0001437008010098 [DOI] [Google Scholar]
- Keppel, G. , Van Niel, K. P. , Wardell‐Johnson, G. W. , Yates, C. J. , Byrne, M. , Mucina, L. , … Franklin, S. E. (2012). Refugia: Identifying and understanding safe havens for biodiversity under climate change. Global Ecology and Biogeography, 21(4), 393–404. 10.1111/j.1466-8238.2011.00686.x [DOI] [Google Scholar]
- Keppel, G. , & Wardell‐Johnson, G. W. (2012). Refugia: Keys to climate change management. Global Change Biology, 18(8), 2389–2391. 10.1111/j.1365-2486.2012.02729.x [DOI] [Google Scholar]
- Kurman, M. D. , Gómez, C. E. , Georgian, S. E. , Lunden, J. J. , & Cordes, E. E. (2017). Intra‐specific variation reveals potential for adaptation to ocean acidification in a cold‐water coral from the Gulf of Mexico. Frontiers in Marine Science, 4, 111 10.3389/fmars.2017.00111 [DOI] [Google Scholar]
- Larsson, A. I. , Lundälv, T. , & van Oevelen, D. (2013). Skeletal growth, respiration rate and fatty acid composition in the cold‐water coral Lophelia pertusa under varying food conditions. Marine Ecology Progress Series, 483, 169–184. 10.3354/meps10284 [DOI] [Google Scholar]
- Lauria, V. , Garofalo, G. , Fiorentino, F. , Massi, D. , Milisenda, G. , Piraino, S. , … Gristina, M. (2017). Species distribution models of two critically endangered deep‐sea octocorals reveal fishing impacts on vulnerable marine ecosystems in central Mediterranean Sea. Scientific Reports, 7(1), 8049 10.1038/s41598-017-08386-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefevre, S. , McKenzie, D. J. , & Nilsson, G. E. (2017). Models projecting the fate of fish populations under climate change need to be based on valid physiological mechanisms. Global Change Biology, 23(9), 3449–3459. 10.1111/gcb.13652 [DOI] [PubMed] [Google Scholar]
- Levin, L. A. , Bett, B. J. , Gates, A. R. , Heimbach, P. , Howe, B. M. , Janssen, F. , … Weller, R. A. (2019). Global observing needs in the deep ocean. Frontiers in Marine Science, 6, 241 10.3389/fmars.2019.00241 [DOI] [Google Scholar]
- Levin, L. A. , & Le Bris, N. (2015). The deep ocean under climate change. Science, 350, 766–768. 10.1126/science.aad0126 [DOI] [PubMed] [Google Scholar]
- Lewis, J. C. , Barnowski, T. F. , & Telesnicki, G. J. (1992). Characteristics of carbonates of gorgonian axes (Coelenterata, Octocorallia). The Biological Bulletin, 183(2), 278–296. 10.2307/1542215 [DOI] [PubMed] [Google Scholar]
- Liaw, A. , & Wiener, M. (2002). Classification and regression by randomForest. R News, 2(3), 18–22. http://cran.r-project.org/doc/Rnews/Rnews_2002-3.pdf [Google Scholar]
- Lunden, J. J. , McNicholl, C. G. , Sears, C. R. , Morrison, C. L. , & Cordes, E. E. (2014). Acute survivorship of the deep‐sea coral Lophelia pertusa from the Gulf of Mexico under acidification, warming, and deoxygenation. Frontiers in Marine Science, 1, 78 10.3389/fmars.2014.00078 [DOI] [Google Scholar]
- Maier, C. , Popp, P. , Sollfrank, N. , Weinbauer, M. G. , Wild, C. , & Gattuso, J. P. (2016). Effects of elevated pCO2 and feeding on net calcification and energy budget of the Mediterranean cold‐water coral Madrepora oculata . Journal of Experimental Biology, 219, 3208–3217. 10.1242/jeb.127159 [DOI] [PubMed] [Google Scholar]
- Maier, C. , Schubert, A. , Sànchez, M. M. B. , Weinbauer, M. G. , Watremez, P. , & Gattuso, J. P. (2013). End of the century pCO2 levels do not impact calcification in Mediterranean cold‐water corals. PLoS ONE, 8(4), e62655 10.1371/journal.pone.0062655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier, C. , Watremez, P. , Taviani, M. , Weinbauer, M. G. , & Gattuso, J. P. (2012). Calcification rates and the effect of ocean acidification on Mediterranean cold‐water corals. Proceedings of the Royal Society B: Biological Sciences, 279(1734), 1716–1723. 10.1098/rspb.2011.1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier, C. , Weinbauer, M. G. , & Gattuso, J.‐P. (2019). Fate of Mediterranean scleractinian cold‐water corals as a result of global climate change In Orejas C. & Jiménez C. (Eds.), Mediterranean cold‐water corals: Past, present and future (pp. 517–529). Chapter 44. Berlin: Springer International Publishing; 10.1007/978-3-319-91608-8_44 [DOI] [Google Scholar]
- Mandrekar, J. N. (2010). Receiver operating characteristic curve in diagnostic test assessment. Journal of Thoracic Oncology, 5(9), 1315–1316. 10.1097/JTO.0b013e3181ec173d [DOI] [PubMed] [Google Scholar]
- Martin, J. H. , Knauer, G. A. , Karl, D. M. , & Broenkow, W. W. (1987). VERTEX: Carbon cycling in the northeast Pacific. Deep Sea Research Part A. Oceanographic Research Papers, 34(2), 267–285. 10.1016/0198-0149(87)90086-0 [DOI] [Google Scholar]
- Matos, L. , Mienis, F. , Wienberg, C. , Frank, N. , Kwiatkowski, C. , Groeneveld, J. , … Hebbeln, D. (2015). Interglacial occurrence of cold‐water corals off Cape Lookout (NW Atlantic): First evidence of the Gulf Stream influence. Deep Sea Research Part I: Oceanographic Research Papers, 105, 158–170. 10.1016/j.dsr.2015.09.003 [DOI] [Google Scholar]
- McCulloch, M. , Trotter, J. , Montagna, P. , Falter, J. , Dunbar, R. , Freiwald, A. , … Taviani, M. (2012). Resilience of cold‐water scleractinian corals to ocean acidification: Boron isotopic systematics of pH and saturation state up‐regulation. Geochimica et Cosmochimica Acta, 87, 21–34. 10.1016/j.gca.2012.03.027 [DOI] [Google Scholar]
- Milligan, R. J. , Spence, G. , Roberts, J. M. , & Bailey, D. M. (2016). Fish communities associated with cold‐water corals vary with depth and substratum type. Deep Sea Research Part I: Oceanographic Research Papers, 114, 43–54. 10.1016/j.dsr.2016.04.011 [DOI] [Google Scholar]
- Møller, P. R. , Nielsen, J. G. , Knudsen, S. W. , Poulsen, J. Y. , Sünksen, K. , & Jørgensen, O. A. (2010). A checklist of the fish fauna of Greenland waters. Zootaxa, 2378, 1–84. 10.11646/zootaxa.2378.1.1 [DOI] [Google Scholar]
- Mora, C. , Wei, C.‐L. , Rollo, A. , Amaro, T. , Baco, A. R. , Billett, D. , … Yasuhara, M. (2013). Biotic and human vulnerability to projected changes in ocean biogeochemistry over the 21st century. PLoS Biology, 11(10), e1001682 10.1371/journal.pbio.1001682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley, J. W. , Selden, R. L. , Latour, R. J. , Frölicher, T. L. , Seagraves, R. J. , & Pinsky, M. L. (2018). Projecting shifts in thermal habitat for 686 species on the North American continental shelf. PLoS ONE, 13, e0196127 10.1371/journal.pone.0196127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Movilla, J. , Gori, A. , Calvo, E. , Orejas, C. , López‐Sanz, À. , Domínguez‐Carrió, C. , … Pelejero, C. (2014). Resistance of two Mediterranean cold‐water coral species to low‐pH conditions. Water, 6(1), 59–67. 10.3390/w6010059 [DOI] [Google Scholar]
- Muscarella, R. , Galante, P. J. , Soley‐Guardia, M. , Boria, R. A. , Kass, J. M. , Uriarte, M. , & Anderson, R. P. (2014). ENM eval: An R package for conducting spatially independent evaluations and estimating optimal model complexity for Maxent ecological niche models. Methods in Ecology and Evolution, 5(11), 1198–1205. 10.1111/2041-210X.12261 [DOI] [Google Scholar]
- Naumann, M. S. , Orejas, C. , & Ferrier‐Pagès, C. (2013). High thermal tolerance of two Mediterranean cold‐water coral species maintained in aquaria. Coral Reefs, 32(3), 749–754. 10.1007/s00338-013-1011-7 [DOI] [Google Scholar]
- Naumann, M. S. , Orejas, C. , Wild, C. , & Ferrier‐Pagès, C. (2011). First evidence for zooplankton feeding sustaining key physiological processes in a scleractinian cold‐water coral. Journal of Experimental Biology, 214(21), 3570–3576. 10.1242/jeb.061390 [DOI] [PubMed] [Google Scholar]
- Nye, J. A. , Link, J. S. , Hare, J. A. , & Overholtz, W. J. (2009). Changing spatial distribution of fish stocks in relation to climate and population size on the Northeast United States continental shelf. Marine Ecology Progress Series, 393, 111–129. 10.3354/meps08220 [DOI] [Google Scholar]
- Orejas, C. , & Jiménez, C. (2019). Mediterranean cold‐water corals: Past, present and future. Understanding the deep‐sea realms of coral (pp. 1–862). Berlin: Springer International Publishing; 10.1007/978-3-319-91608-8 [DOI] [Google Scholar]
- Orr, J. C. , Fabry, V. J. , Aumont, O. , Bopp, L. , Doney, S. C. , Feely, R. A. , … Yool, A. (2005). Anthropogenic ocean acidification over the twenty‐first century and its impact on calcifying organisms. Nature, 437(7059), 681 10.1038/nature04095 [DOI] [PubMed] [Google Scholar]
- Parra, H. E. , Pham, C. K. , Menezes, G. M. , Rosa, A. , Tempera, F. , & Morato, T. (2017). Predictive modeling of deep‐sea fish distribution in the Azores. Deep Sea Research Part II: Topical Studies in Oceanography, 145, 49–60. 10.1016/j.dsr2.2016.01.004 [DOI] [Google Scholar]
- Pearson, R. G. , & Dawson, T. P. (2003). Predicting the impacts of climate change on the distribution of species: Are bioclimate envelope models useful? Global Ecology and Biogeography, 12(5), 361–371. 10.1046/j.1466-822X.2003.00042.x [DOI] [Google Scholar]
- Pecl, G. T. , Araújo, M. B. , Bell, J. D. , Blanchard, J. , Bonebrake, T. C. , Chen, I.‐C. , … Williams, S. E. (2017). Biodiversity redistribution under climate change: Impacts on ecosystems and human well‐being. Science, 355(6332), eaai9214 10.1126/science.aai9214 [DOI] [PubMed] [Google Scholar]
- Perez, F. F. , Fontela, M. , García‐Ibáñez, M. I. , Mercier, H. , Velo, A. , Lherminier, P. , … Padin, X. A. (2018). Meridional overturning circulation conveys fast acidification to the deep Atlantic Ocean. Nature, 554(7693), 515 10.1038/nature25493 [DOI] [PubMed] [Google Scholar]
- Perry, A. L. , Low, P. J. , Ellis, J. R. , & Reynolds, J. D. (2005). Climate change and distribution shifts in marine fishes. Science, 308(5730), 1912–1915. 10.1126/science.1111322 [DOI] [PubMed] [Google Scholar]
- Pershing, A. J. , Alexander, M. A. , Hernandez, C. M. , Kerr, L. A. , Le Bris, A. , Mills, K. E. , … Thomas, A. C. (2015). Slow adaptation in the face of rapid warming leads to collapse of the Gulf of Maine cod fishery. Science, 350(6262), 809–812. 10.1126/science.aac9819 [DOI] [PubMed] [Google Scholar]
- Pham, C. K. , Vandeperre, F. , Menezes, G. , Porteiro, F. , Isidro, E. , & Morato, T. (2015). The importance of deep‐sea vulnerable marine ecosystems for demersal fish in the Azores. Deep Sea Research Part I: Oceanographic Research Papers, 96, 80–88. 10.1016/j.dsr.2014.11.004 [DOI] [Google Scholar]
- Phillips, S. J. , Anderson, R. P. , Dudík, M. , Schapire, R. E. , & Blair, M. E. (2017). Opening the black box: An open‐source release of Maxent. Ecography, 40(7), 887–893. 10.1111/ecog.03049 [DOI] [Google Scholar]
- Phillips, S. J. , Anderson, R. P. , & Schapire, R. E. (2006). Maximum entropy modeling of species geographic distributions. Ecological Modelling, 190(3–4), 231–259. 10.1016/j.ecolmodel.2005.03.026 [DOI] [Google Scholar]
- Poloczanska, E. S. , Brown, C. J. , Sydeman, W. J. , Kiessling, W. , Schoeman, D. S. , Moore, P. J. , … Richardson, A. J. (2013). Global imprint of climate change on marine life. Nature Climate Change, 3(10), 919–925. 10.1038/nclimate1958 [DOI] [Google Scholar]
- Pörtner, H. O. , Bock, C. , & Mark, F. C. (2017). Oxygen‐and capacity‐limited thermal tolerance: Bridging ecology and physiology. Journal of Experimental Biology, 220(15), 2685–2696. 10.1242/jeb.134585 [DOI] [PubMed] [Google Scholar]
- Pörtner, H. O. , & Knust, R. (2007). Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science, 315(5808), 95–97. 10.1126/science.1135471 [DOI] [PubMed] [Google Scholar]
- Priede, I. (2017). Deep‐sea fishes: Biology, diversity, ecology and fisheries. Cambridge: Cambridge University Press; 10.1017/9781316018330 [DOI] [Google Scholar]
- Purkey, S. G. , & Johnson, G. C. (2010). Warming of global abyssal and deep Southern Ocean waters between the 1990s and 2000s: Contributions to global heat and sea level rise budgets. Journal of Climate, 23(23), 6336–6351. 10.1175/2010JCLI3682.1 [DOI] [Google Scholar]
- Quattrini, A. M. , Ross, S. W. , Carlson, M. C. , & Nizinski, M. S. (2012). Megafaunal‐habitat associations at a deep‐sea coral mound off North Carolina, USA. Marine Biology, 159(5), 1079–1094. 10.1007/s00227-012-1888-7 [DOI] [Google Scholar]
- Raybaud, V. , Tambutté, S. , Ferrier‐Pagès, C. , Reynaud, S. , Venn, A. A. , Tambutté, É. , … Allemand, D. (2017). Computing the carbonate chemistry of the coral calcifying medium and its response to ocean acidification. Journal of Theoretical Biology, 424, 26–36. 10.1016/j.jtbi.2017.04.028 [DOI] [PubMed] [Google Scholar]
- Rengstorf, A. M. , Yesson, C. , Brown, C. , & Grehan, A. J. (2013). High‐resolution habitat suitability modelling can improve conservation of vulnerable marine ecosystems in the deep sea. Journal of Biogeography, 40(9), 1702–1714. 10.1111/jbi.12123 [DOI] [Google Scholar]
- Rheuban, J. E. , Doney, S. C. , Cooley, S. R. , & Hart, D. R. (2018). Projected impacts of future climate change, ocean acidification, and management on the US Atlantic sea scallop (Placopecten magellanicus) fishery. PLoS ONE, 13(9), e0203536 10.1371/journal.pone.0203536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riahi, K. , Rao, S. , Krey, V. , Cho, C. , Chirkov, V. , Fischer, G. , … Rafaj, P. (2011). RCP 8.5—A scenario of comparatively high greenhouse gas emissions. Climatic Change, 109(1–2), 33 10.1007/s10584-011-0149-y [DOI] [Google Scholar]
- Roberts, J. M. , Murray, F. , Anagnostou, E. , Hennige, S. , Gori, A. , Henry, L. A. , … Foster, G. L. (2016). Cold‐water corals in an era of rapid global change: Are these the deep ocean’s most vulnerable ecosystems? In Goffredo S. & Dubinsky Z. (Eds.), The Cnidaria, past, present and future (pp. 593–606). Cham: Springer; 10.1007/978-3-319-31305-4_36 [DOI] [Google Scholar]
- Robinson, N. M. , Nelson, W. A. , Costello, M. J. , Sutherland, J. E. , & Lundquist, C. J. (2017). A systematic review of marine‐based species distribution models (SDMs) with recommendations for best practice. Frontiers in Marine Science, 4, 421 10.3389/fmars.2017.00421 [DOI] [Google Scholar]
- Rodolfo‐Metalpa, R. , Montagna, P. , Aliani, S. , Borghini, M. , Canese, S. , Hall‐Spencer, J. M. , … Houlbrèque, F. (2015). Calcification is not the Achilles’ heel of cold‐water corals in an acidifying ocean. Global Change Biology, 21(6), 2238–2248. 10.1111/gcb.12867 [DOI] [PubMed] [Google Scholar]
- Ross, S. W. , & Quattrini, A. M. (2007). The fish fauna associated with deep coral banks off the southeastern United States. Deep Sea Research Part I: Oceanographic Research Papers, 54(6), 975–1007. 10.1016/j.dsr.2007.03.010 [DOI] [Google Scholar]
- Ross, S. W. , & Quattrini, A. M. (2009). Deep‐sea reef fish assemblage patterns on the Blake Plateau (Western North Atlantic Ocean). Marine Ecology, 30(1), 74–92. 10.1111/j.1439-0485.2008.00260.x [DOI] [Google Scholar]
- Ross, S. W. , Rhode, M. , & Quattrini, A. M. (2015). Demersal fish distribution and habitat use within and near Baltimore and Norfolk Canyons, US middle Atlantic slope. Deep Sea Research Part I: Oceanographic Research Papers, 103, 137–154. 10.1016/j.dsr.2015.06.004 [DOI] [Google Scholar]
- Ross, S. W. , Rhode, M. , Viada, S. T. , & Mather, I. R. (2016). Fish species associated with shipwreck and natural hard‐bottom habitats from the middle to outer continental shelf of the Middle Atlantic Bight near Norfolk Canyon. Fishery Bulletin, 114, 45–57. 10.7755/FB.114.1.4 [DOI] [Google Scholar]
- Rossi, S. , Bramanti, L. , Gori, A. , & Orejas, C. (2017). Marine animal forests. The ecology of benthic biodiversity hotspots (pp. 1–1366). Berlin: Springer International Publishing; 10.1007/978-3-319-17001-5 [DOI] [Google Scholar]
- Rowden, A. A. , Anderson, O. F. , Georgian, S. E. , Bowden, D. A. , Clark, M. R. , Pallentin, A. , & Miller, A. (2017). High‐resolution habitat suitability models for the conservation and management of vulnerable marine ecosystems on the Louisville Seamount Chain, South Pacific Ocean. Frontiers in Marine Science, 4, 335 10.3389/fmars.2017.00335 [DOI] [Google Scholar]
- Ruhl, H. A. , & Smith, K. L. (2004). Shifts in deep‐sea community structure linked to climate and food supply. Science, 305(5683), 513–515. 10.1126/science.1099759 [DOI] [PubMed] [Google Scholar]
- Sabine, C. L. , Feely, R. A. , Gruber, N. , Key, R. M. , Lee, K. , Bullister, J. L. , … Millero, F. J. (2004). The oceanic sink for anthropogenic CO2 . Science, 305(5682), 367–371. 10.1126/science.1097403 [DOI] [PubMed] [Google Scholar]
- Sandblom, E. , Gräns, A. , Axelsson, M. , & Seth, H. (2014). Temperature acclimation rate of aerobic scope and feeding metabolism in fishes: Implications in a thermally extreme future. Proceedings of the Royal Society B: Biological Sciences, 281(1794), 20141490 10.1098/rspb.2014.1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucier, E. H. , Sajjadi, A. U. Z. I. T. A. , & France, S. C. (2017). A taxonomic review of the genus Acanella (Cnidaria: Octocorallia: Isididae) in the North Atlantic Ocean, with descriptions of two new species. Zootaxa, 4323(3), 359–390. 10.11646/zootaxa.4323.3.2 [DOI] [Google Scholar]
- Schmitt, T. , Schaap, D. , Spoelstra, G. , Loubrieu, B. , & Poncelet, C. (2019). EMODnet Bathymetry a compilation of bathymetric data in the European waters In OCEANS 2019‐Marseille (pp. 1–7). IEEE; 10.1109/OCEANSE.2019.8867250 [DOI] [Google Scholar]
- Schönberg, C. H. , Fang, J. K. , Carreiro‐Silva, M. , Tribollet, A. , & Wisshak, M. (2017). Bioerosion: The other ocean acidification problem. ICES Journal of Marine Science, 74(4), 895–925. 10.1093/icesjms/fsw254 [DOI] [Google Scholar]
- Singer, A. , Millat, G. , Staneva, J. , & Kroncke, I. (2017). Modelling benthic macrofauna and seagrass distribution patterns in a North Sea tidal basin in response to 2050 climatic and environmental scenarios. Estuarine, Coastal and Shelf Science, 188, 99–108. 10.1016/j.ecss.2017.02.003 [DOI] [Google Scholar]
- Soetaert, K. , Mohn, C. , Rengstorf, A. , Grehan, A. , & van Oevelen, D. (2016). Ecosystem engineering creates a direct nutritional link between 600‐m deep cold‐water coral mounds and surface productivity. Scientific Reports, 6, 35057 10.1038/srep35057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sólmundsson, J. , Karlsson, H. , Pétursson, H. , Björnsson, H. , Jónsdóttir, I. G. , Pálsson, J. , … Bogason, V. (2019). Icelandic groundfish survey 2019—implementation and main results. Iceland: Marine and Freshwater Research in Iceland, HV‐2019–26. [Google Scholar]
- Sulpis, O. , Boudreau, B. P. , Mucci, A. , Jenkins, C. , Trossman, D. S. , Arbic, B. K. , & Key, R. M. (2018). Current CaCO3 dissolution at the seafloor caused by anthropogenic CO2 . Proceedings of the National Academy of Sciences of the United States of America, 115(46), 11700–11705. 10.1073/pnas.1804250115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweetman, A. K. , Thurber, A. R. , Smith, C. R. , Levin, L. A. , Mora, C. , Wei, C.‐L. , … Roberts, J. M. (2017). Major impacts of climate change on deep‐sea benthic ecosystems. Elementa: Science of the Anthropocene, 5, 4 10.1525/elementa.203 [DOI] [Google Scholar]
- Thresher, R. E. , Guinotte, J. M. , Matear, R. J. , & Hobday, A. J. (2015). Options for managing impacts of climate change on a deep‐sea community. Nature Climate Change, 5(7), 635–639. 10.1038/nclimate2611 [DOI] [Google Scholar]
- Thresher, R. E. , Tilbrook, B. , Fallon, S. , Wilson, N. C. , & Adkins, J. (2011). Effects of chronic low carbonate saturation levels on the distribution, growth and skeletal chemistry of deep‐sea corals and other seamount megabenthos. Marine Ecology Progress Series, 442, 87–99. 10.3354/meps09400 [DOI] [Google Scholar]
- Thuiller, W. , Lafourcade, B. , Engler, R. , & Araújo, M. B. (2009). BIOMOD–a platform for ensemble forecasting of species distributions. Ecography, 32(3), 369–373. 10.1111/j.1600-0587.2008.05742.x [DOI] [Google Scholar]
- Thurber, A. R. , Sweetman, A. K. , Narayanaswamy, B. E. , Jones, D. O. B. , Ingels, J. , & Hansman, R. L. (2014). Ecosystem function and services provided by the deep sea. Biogeosciences, 11(14), 3941–3963. 10.5194/bg-11-3941-2014 [DOI] [Google Scholar]
- Tittensor, D. P. , Baco, A. R. , Hall‐Spencer, J. M. , Orr, J. C. , & Rogers, A. D. (2010). Seamounts as refugia from ocean acidification for cold‐water stony corals. Marine Ecology, 31, 212–225. [Google Scholar]
- UNGA . (2006). Resolution 61/105. Sustainable fisheries: Agreement for the implementation of the provision of the United Nations Convention on the Law of the Sea of 10 December 1982 relating to the Conservation and Management of Straddling Fish Stocks and Highly Migratory Fish Stocks, and related instruments. A/RES/61/105. 10.1163/221160007X00263. [DOI]
- Valdimarsson, H. , Astthorsson, O. S. , & Palsson, J. (2012). Hydrographic variability in Icelandic waters during recent decades and related changes in distribution of some fish species. ICES Journal of Marine Science, 69(5), 816–825. 10.1093/icesjms/fss027 [DOI] [Google Scholar]
- van Vuuren, D. P. , Edmonds, J. , Kainuma, M. , Riahi, K. , Thomson, A. , Hibbard, K. , … Rose, S. K. (2011). The representative concentration pathways: An overview. Climatic Change, 109(1–2), 5 10.1007/s10584-011-0148-z [DOI] [Google Scholar]
- Victorero, L. , Watling, L. , Deng Palomares, M. L. , & Nouvian, C. (2018). Out of sight, but within reach: A global history of bottom‐trawled deep‐sea fisheries from > 400 m depth. Frontiers in Marine Science, 5, 98 10.3389/fmars.2018.00098 [DOI] [Google Scholar]
- Walbridge, S. , Slocum, N. , Pobuda, M. , & Wright, D. (2018). Unified geomorphological analysis workflows with Benthic Terrain Modeler. Geosciences, 8(3), 94 10.3390/geosciences8030094 [DOI] [Google Scholar]
- Watson, R. A. , & Morato, T. (2013). Fishing down the deep: Accounting for within‐species changes in depth of fishing. Fisheries Research, 140, 63–65. 10.1016/j.fishres.2012.12.004 [DOI] [Google Scholar]
- Weatherall, P. , Marks, K. M. , Jakobsson, M. , Schmitt, T. , Tani, S. , Arndt, J. E. , … Wigley, R. (2015). A new digital bathymetric model of the world's oceans. Earth and Space Science, 2(8), 331–345. 10.1002/2015EA000107 [DOI] [Google Scholar]
- White, M. , Mohn, C. , de Stigter, H. , & Mottram, G. (2005). Deep‐water coral development as a function of hydrodynamics and surface productivity around the submarine banks of the Rockall Trough, NE Atlantic In Freiwald A. & Roberts J. M. (Eds.), Cold‐water corals and ecosystems (pp. 503–514). Berlin: Springer; 10.1007/3-540-27673-4_25 [DOI] [Google Scholar]
- Wienberg, C. , Frank, N. , Mertens, K. N. , Stuut, J.‐B. , Marchant, M. , Fietzke, J. , … Hebbeln, D. (2010). Glacial cold‐water coral growth in the Gulf of Cádiz: Implications of increased palaeo‐productivity. Earth and Planetary Science Letters, 298(3–4), 405–416. 10.1016/j.epsl.2010.08.017 [DOI] [Google Scholar]
- Wiens, J. A. , Stralberg, D. , Jongsomjit, D. , Howell, C. A. , & Snyder, M. A. (2009). Niches, models, and climate change: Assessing the assumptions and uncertainties. Proceedings of the National Academy of Sciences of the United States of America, 106(Supplement_2), 19729–19736. 10.1073/pnas.0901639106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, M. F. , O’Connell, B. , Brown, C. , Guinan, J. C. , & Grehan, A. J. (2007). Multiscale terrain analysis of multibeam bathymetry data for habitat mapping on the continental slope. Marine Geodesy, 30(1–2), 3–35. 10.1080/01490410701295962 [DOI] [Google Scholar]
- Wisz, M. S. , & Guisan, A. (2009). Do pseudo‐absence selection strategies influence species distribution models and their predictions? An information‐theoretic approach based on simulated data. BMC Ecology, 9(1), 8 10.1186/1472-6785-9-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, H. L. , Spicer, J. I. , & Widdicombe, S. (2008). Ocean acidification may increase calcification rates, but at a cost. Proceedings of the Royal Society B: Biological Sciences, 275(1644), 1767–1773. 10.1098/rspb.2008.0343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, S. (2018) Mixed GAM computation vehicle with automatic smoothness estimation (version 1.8‐28), 302 pp. Retrieved from https://r.meteo.uni.wroc.pl/web/packages/mgcv/mgcv.pdf [Google Scholar]
- Woodworth‐Jefcoats, P. A. , Polovina, J. J. , & Drazen, J. C. (2017). Climate change is projected to reduce carrying capacity and redistribute species richness in North Pacific pelagic marine ecosystems. Global Change Biology, 23(3), 1000–1008. 10.1111/gcb.13471 [DOI] [PubMed] [Google Scholar]
- Wright, G. , Gjerde, K. , Johnson, D. E. , Finkelstein, A. , Ferreira, M. A. , Dunn, D. C. , … Grehan, A. (2019). Marine spatial planning in areas beyond national jurisdiction. Marine Policy, in press. 10.1016/j.marpol.2018.12.003 [DOI] [Google Scholar]
- Yesson, C. , Taylor, M. L. , Tittensor, D. P. , Davies, A. J. , Guinotte, J. , Baco, A. , … Rogers, A. D. (2012). Global habitat suitability of cold‐water octocorals. Journal of Biogeography, 39(7), 1278–1292. 10.1111/j.1365-2699.2011.02681.x [DOI] [Google Scholar]
- Zheng, M. D. , & Cao, L. (2014). Simulation of global ocean acidification and chemical habitats of shallow‐ and cold‐water coral reefs. Advances in Climate Change Research, 5(4), 189–196. 10.1016/j.accre.2015.05.002 [DOI] [Google Scholar]
- Zuur, A. F. , Ieno, E. N. , & Elphick, C. S. (2010). A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution, 1(1), 3–14. 10.1111/j.2041-210X.2009.00001.x [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The bathymetry data supporting the analyses are publicly available from the EMODnet Digital Terrain Model and the General Bathymetric Chart of the Oceans portals (https://portal.emodnet-bathymetry.eu/ and https://www.gebco.net/data_and_products/gridded_bathymetry_data/, respectively). The environmental data used in this study are publicly available from the Earth System Grid Federation (ESGF) Peer‐to‐Peer (P2P) enterprise system (https://esgf.llnl.gov/). All analyses were conducted using publicly available packages from the Comprehensive R Archive Network (https://cran.r-project.org/), namely the R packages MOPA, Raster, Dismo, mgcv, randomForest, MuMIn, EnMEVAL, and boot. The Benthic Terrain Model 3.0 tool is available from the ESRI Oceans GitHub repository (https://github.com/EsriOceans/btm). The processed environmental data layers used in this study are publicly available through the PANGAEA data publisher portal (https://doi.org/10.1594/PANGAEA.911117). The model outputs are also available for download from PANGAEA (https://doi.org/10.1594/PANGAEA.910319).


