Abstract
Background
High-intensity focused electromagnetic (HIFEM) field technology has been reported to increase muscle thickness and hypertrophy. However, this process has not yet been confirmed on a histologic level.
Objectives
The aim of this study was to evaluate in-vivo structural changes in striated porcine muscle tissue following HIFEM treatment.
Methods
Three Yorkshire pigs received four 30-minute HIFEM treatments applied to the biceps femoris muscle on 1 side only. The fourth pig served as a control subject. At baseline and 2 weeks after the last treatment, biopsy specimens of the muscle tissue were collected from the treatment site. The control pig underwent muscle biopsy from a similar but untreated site. Twenty-five histology slides were evaluated from each pig. A certified histopathologist analyzed sliced biopsy samples for structural changes in the tissue.
Results
Histologic analysis showed hypertrophic changes 2 weeks posttreatment. The muscle mass density increased by 20.56% (to a mean of 17,053.4 [5617.9] µm2) compared with baseline. Similarly, muscle fiber density (hyperplasia) increased: the average change in the number of fibers in a slice area of 136,533.3 µm2 was +8.0%. The mean size of an individual muscle fiber increased by 12.15% (to 332.23 [280.2] µm2) 2 weeks posttreatment. Control samples did not show any significant change in fiber density or hyperplasia.
Conclusions
Histopathologic quantification showed significant structural muscle changes through a combination of fiber hypertrophy and hyperplasia. Control biopsies showed a lack of similar changes. The data correlate with findings of other HIFEM research and suggest that HIFEM could be used for noninvasive induction of muscle growth.
Muscles have long been neglected in the body-shaping industry, which predominantly deals with subcutaneous fat deposits. However, strong and firm muscles significantly contribute to the overall aesthetic appearance. High-intensity focused electromagnetic (HIFEM) field technology has recently been introduced in the field of aesthetic medicine to provide physicians with a tool for muscle toning and strengthening beyond the capability of normal exercise.
Current noninvasive body-shaping devices are based on heating or cooling of subcutaneous fat tissue to levels that fat cells can no longer tolerate, consequently triggering programmed cell death—apoptosis.1 The heating modalities of these radiofrequency devices are based on emitting electromagnetic waves of high frequencies (0.5-50 MHz)2 which are predominantly absorbed in the subcutaneous fat tissue, where the energy of the waves is transformed into heat. HIFEM technology, on the other hand, does not deliver any heating through electromagnetic radiation, as it utilizes magnetic waves of very low frequencies (3-5 kHz) which propagate through the tissue without being absorbed. In this case, an interaction between the wave and human tissue occurs according to the principles of electromagnetic induction, first described by Michael Faraday in 1831. The law of electromagnetic induction says that any change in a magnetic field induces an electric current and vice versa. The HIFEM device comprises a circular coil located in the applicator, which is placed over the treatment area. During the treatment, an alternating electric current is sent into the circular coil. The alternations in the electric current induce rapidly changing magnetic waves which propagate into the underlying tissue, where they induce a secondary electric current. These electric currents within the tissue depolarize the muscle-innervating motor neurons and induce muscle contractions.3
Several studies have shown that humans are unable to fully activate muscles voluntarily as the power of muscle contraction is limited by the firing rates and conductivity of neural pathways.4-7 Application of HIFEM bypasses the central and peripheral nervous system and directly stimulates the muscle-innervating motor neurons, allowing full muscle contraction. In addition, the frequency of delivered pulses does not allow the muscle to relax between 2 consecutive stimuli, which results in supramaximal tension within the muscle and thus supramaximal muscle contraction.
Multiple studies have investigated the effects of rapidly changing magnetic fields delivered through HIFEM technology.8-13 The studies by Kent et al,11 Katz et al,12 and Kinney et al8 employed computed tomography (CT), ultrasound, and magnetic resonance imaging (MRI), respectively, to investigate changes in abdominal composition post-HIFEM treatments. The thickness of abdominal muscles measured in CT and MRI images increased on average by 14.8% to 15.4%, indicating muscle hypertrophy. Although HIFEM technology directly affects muscles, the studies also found that the thickness of abdominal fat was reduced on average by 17.5% to 19%. The effect of the HIFEM procedure on adipose tissue was confirmed by a veterinary study,13 which reported increased apoptotic index and apoptotic markers in the fat tissue post-HIFEM treatments.
Results from human trials suggest that HIFEM technology is a feasible modality for the aesthetic industry and could be widely used in body contouring for simultaneous fat reduction and muscle toning. Clinical trials are currently underway to assess the use of this technology to improve strength and tone in biceps, triceps, and gastrocnemius muscles. HIFEM has also been successfully used for strengthening the pelvic floor.14
Unlike fat apoptosis, which was confirmed on a histologic level, there is no histologic evidence for muscle hypertrophy. Because muscle thickness was found to be increased posttreatment, it might not necessarily indicate muscle fiber hypertrophy, but could be linked to swelling,15 overall hydration, or increased water content in the muscle,16 which may change with time. Therefore, histologic evaluation is necessary to confirm the observations on a cellular level.
The present study aimed to investigate the effect of an HIFEM-based procedure on muscle cells in a porcine model. The goal was to determine whether muscle hypertrophy is present on a cellular level.
METHODS
Four Yorkshire pigs served as subjects. Inclusion in the study required the animals to be in full physical health, which was assessed via blood samples collected 2 days before the treatment began. Three pigs received active treatment applied to the unilateral thigh, and the fourth untreated animal served as a control. The treatment procedure consisted of 4 sessions (30 minutes each) with a device that utilizes HIFEM technology (EMSCULPT; BTL Industries Inc., Boston, MA). The treatment sessions were scheduled twice a week for 2 weeks. The study was approved by the Institutional Animal Care and Use Committee (Bulgarian Food Safety Agency—BFSA committee, ID 195/2018). Animal care complied with the convention for the protection of vertebrate animals used for experimental and other scientific purposes. The experiment was conducted in July and August 2018.
During each treatment session, the animals were placed under general anesthesia to minimize their discomfort; this process was supervised by a veterinarian who chose the type and dosing of the anesthetic. A single applicator of the device was placed over the back thigh of the pig and secured by a fixation belt. All parameters used were identical to those commonly used in humans. Device settings were controlled by the operator. The intensity was gradually increased to 100% of the maximum device output, at which level it was maintained for the rest of the treatment time. For the 30 minutes of the treatment, the applicator was continuously delivering electromagnetic pulses with a magnetic field intensity of up to 1.8 T. The applicator position was adjusted during the treatment to ensure maximum contraction in the entire treatment area.
Punch biopsy specimens of muscle tissue were collected with a disposable biopsy punch (diameter, 5 mm) before the first treatment and 2 weeks after the last treatment . The samples were fixed in 10% neutral buffered formalin and stained with hematoxylin and eosin. For microscopic evaluation, the samples were cut into 5-µm thick slices.
The slices were screened under a microscope (DFC295; Leica Microsystems Ltd, Germany) and an image of the entire slice was obtained for further analysis with Leica Application Suite (version 4.9.0) software. Each slice area was 136,533.3 µm2. The analysis comprised the calculation of muscle fiber density, muscle mass density, and muscle fiber volume. Muscle fiber density was obtained as an average number of muscle fibers calculated individually in each slice. Muscle mass density was defined as the slice area occupied by muscle tissue. Muscle volume represents the area per single muscle fiber. ImageJ 1.52a software (National Institutes of Health, Bethesda, MD)17 was used to calculate the muscle mass density and muscle fiber volume. Based on individual pixel color the software automatically segments the muscle tissue within the slices and calculates the area occupied by the muscle tissue.
In addition, the animals were monitored for any possible external manifestations of adverse events or side effects related to the procedure. The test animals were examined after every procedure to ascertain whether they exhibited any change in their condition compared with the baseline examination.
The sliced biopsy samples collected at baseline and 2 weeks posttreatment were compared for histologic changes. The statistical significance of possible changes was assessed by t test with a significance level set to 5%.
RESULTS
The 4 recruited Yorkshire sows (females) were between 1.5 and 2 years old (mean, 1.7 [0.2] years) and their mean weight was 74.6 [1.5] kg. All animals recovered from anesthesia without any complications or adverse events. The skin of test animals did not show any signs of adverse events such as erythema, scarring, ruptures, or skin texture change. The weights of all animals (treated and control) did not change after the treatments. In total 104 slices were obtained by slicing the punch biopsy samples (26 slices per subject). The statistical analysis showed a significant increase (P < 0.01) of muscle mass in the samples from treated animals.
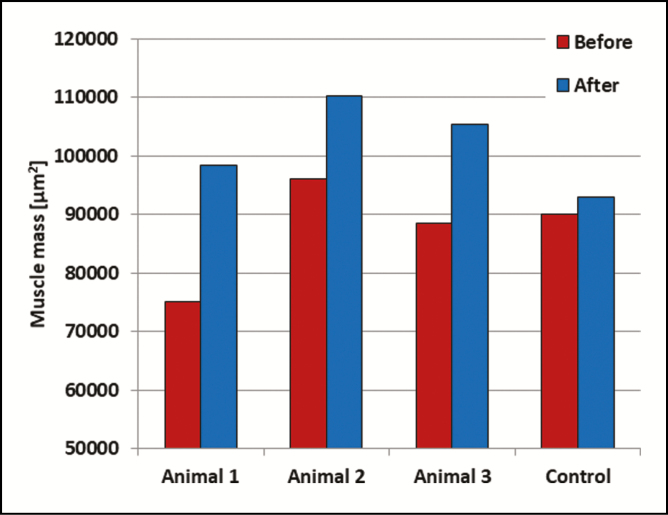
In the treated animals, the muscle mass density increased on average by 20.56% (to a mean of 17,053.4 [5617.9] µm2). An increase was observed in each of the treated animals, although the density remained constant in the control animal, with the change being within the standard deviation. The results for each animal are shown in Figure 1.
Figure 1.
The average muscle mass per slice for each of the animals. All treated animals showed a significant growth in muscle mass. The muscle mass in the control animal did not change significantly.
The change in the number of muscle fibers per slice was not statistically significant (P > 0.05), although a increasing trend was present in the treated animals as the average fiber density increased by 8.0% from 35.0 [6.8] to 38.2 [10.5]. The average muscle fiber density per slice in the control animal was 36.0 [9.1] at baseline and 37.0 [10.2] 2 weeks posttreatment. The difference was not statistically significant (P > 0.05).
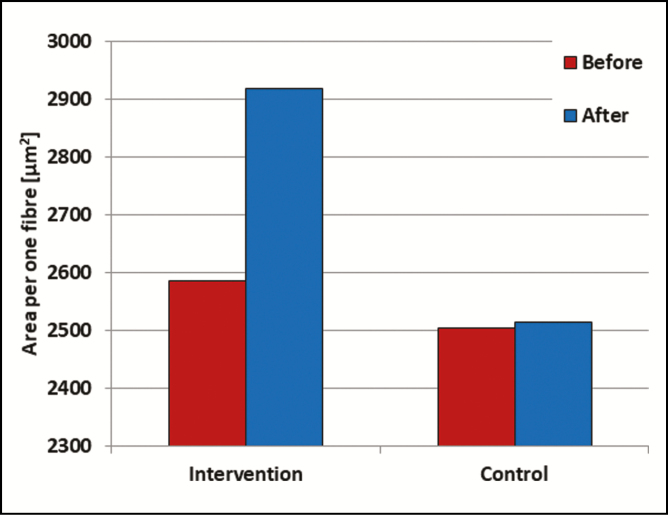
Posttreatment, the average area per single muscle fiber increased significantly (P < 0.05) by 12.15% (to 332.23 [280.16] µm2) in the intervention group. In the control animal the fiber area remained constant. See Figure 2 for the average results.
Figure 2.
The average area per single muscle fiber in the treated (left) and control (right) animals at baseline (red) and posttreatment (blue).
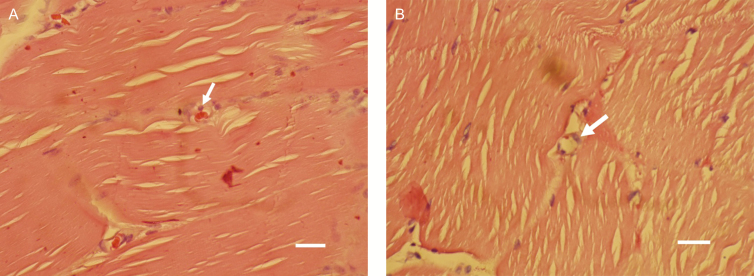
A further observation was neovascularization of the muscle tissue, which was widely seen in the 2-week follow-up histologic samples of the intervention group. Figure 3 shows samples exhibiting new capillary build-up.
Figure 3.
(A, B) The white arrows point to areas with the appearance of the endothelial cells with the onset of new capillary build-up in 2-week follow-up samples from the treated animals. White bar, 35 µm.
DISCUSSION
Hypertrophy is normally seen in humans. There is, however, little evidence as to whether the overall muscle increase is simply due to increased thickness of individual muscle fibers (fiber hypertrophy) or due to a combination of fiber hypertrophy and multiplication of existing fibers/creation of new fibers (hyperplasia). Hyperplasia in humans is controversial among the scientific community, but existing studies have assessed this phenomenon after sets of ordinary exercises.18 HIFEM, on the other hand, induces approximately 20,000 strong, supramaximal contractions within a time frame of 30 minutes, which cannot be achieved voluntarily, and the effects thus could be significantly higher, even leading to hyperplasia. Previous research on HIFEM technology showed an increase in muscle thickness in MRI, CT, and ultrasound images, providing evidence of muscle hypertrophy.8,11 However, no study to date has looked at what happens to the muscle on a histologic level. The current study extends the scope of the existing literature by evaluating the effect of HIFEM treatments on individual muscle fibers, which has not been studied before.
This study aimed to determine whether HIFEM treatments can induce muscle hypertrophy on a cellular level. The histologic examination demonstrated that 4 HIFEM treatments induced prominent growth in the muscle tissue. The observed increase in total muscle mass by 20.56% appears to be mainly caused by a volumetric growth in individual muscle fibers, ie, muscle fiber hypertrophy (contributing 12.15%), and partially by an increase in the number of muscle fibers, ie, hyperplasia (contributing 8.0%), although the latter was not statistically significant.
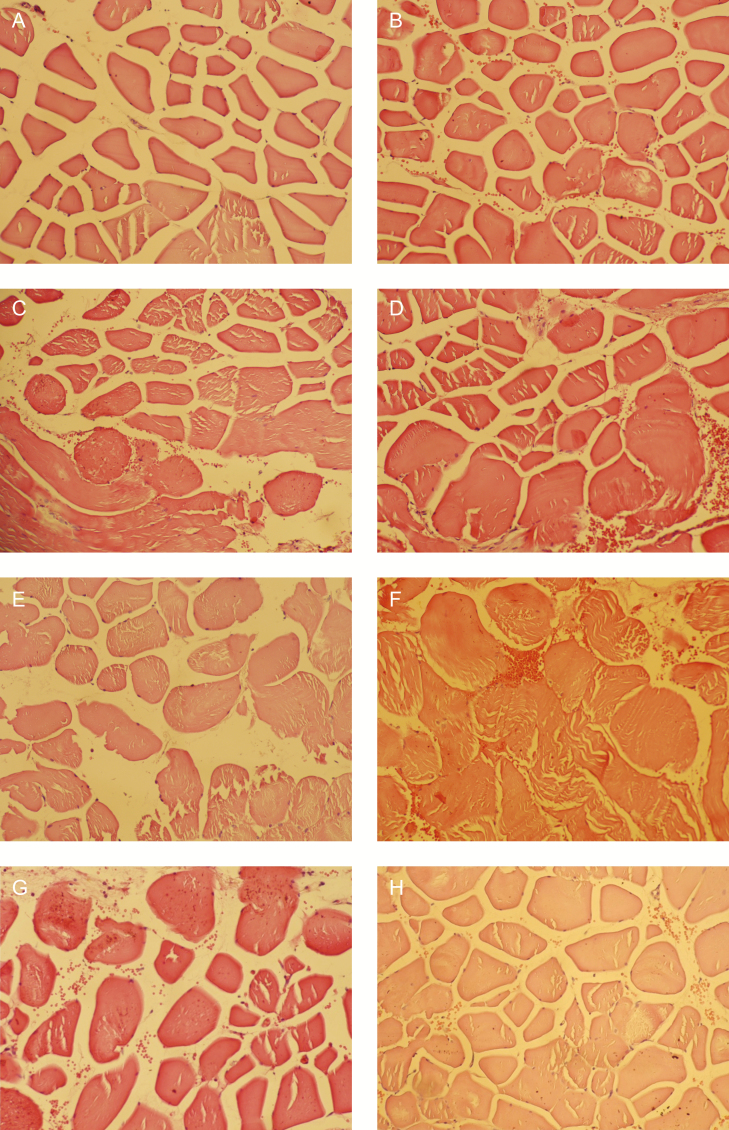
The muscle growth observed in the current study correlates with previous research investigating the effect of HIFEM treatments on muscles. Kent et al11 and Kinney et al8 reported an increase in the muscle thickness by 14.8% and 15.4%, respectively. In comparison with these studies, the 20.56% increase on a cellular level seen in this study is larger, possibly due to densifying of the muscle tissue, as the connective tissue surrounding muscle fibers (endomysium) is compressed by increased muscle mass. This has indeed been observed in the histologic slices, and examples are shown in Figure 4. This is the first study investigating the hypertrophic effects of HIFEM technology on a histologic level, and hence there is no other histologic research with which the present results can be compared.
Figure 4.
Example of histologic images of slices taken at (A, C, E, G) baseline and (B, D, F, H) 2 weeks posttreatment. The baseline images (A, C, E, G) show normal structure of muscle fibers, whereas the posttreatment images (B, D, F, H) show hypertrophy of muscle fibers with the muscle cell diameter being noticeably larger. The same magnification is used in all the images.
The lack of significant hyperplasia could be attributed to the short duration of the follow-up period. The posttreatment samples were collected 2 weeks after the last treatment and this period might not have been enough to fully manifest the hyperplastic changes as they might require more time to occur than fiber hypertrophy. A study by Crameri et al19 found that it took 4 to 8 days to capture increased levels of myosatellite cells after a single bout of exercise. Therefore the terminal differentiation of these cells into clearly recognizable new muscle fibers might require more than 2 weeks.
The role of muscle fiber hyperplasia and muscle hypertrophy in humans is controversial because no evidence conclusively documents hyperplasia in human muscle.20,21 Although the indications of hyperplasia observed in our study are not necessarily transferable to humans, it would be convenient to investigate whether the same pattern can be seen in human studies. Previous studies investigated hyperplasia only postexercise, but HIFEM induced contractions of significantly higher strength and power than “exercise contractions” and could eventually trigger the terminal differentiation of myosatellite cells into new muscle fibers.
Besides HIFEM technology, which is based on magnetic stimulation, modalities based on electrical stimulation, such as electrical muscle stimulation (EMS) or transcutaneous electrical nerve stimulation (TENS), have been used in the past for muscle training.22-24 Although TENS and EMS are predominantly used in rehabilitation and physiotherapy, HIFEM is the first muscle-affecting technology intended for body contouring. However, electromagnetic stimulation appears to offer a number of advantages over electrical stimulation: it induces 2 times higher peak torque25 and, unlike with electrical stimulation, there is no pain25 or risk of burns26,27 with high stimulation intensities. Electromagnetic stimulation was further found to penetrate deeper into the tissue,28 which is linked with the larger peak torques observed. The absence of adverse events in our study correlates with previous studies on humans. Due to the nonthermal nature of HIFEM technology, any risk of thermal tissue damage is eliminated. It might be assumed that rhabdomyolysis could occur following supramaximal contractions, but this has not been observed. Other expected complications or adverse events could be prolonged muscle soreness, swelling, bruising, cramping, or erythema of the overlying skin, but none of these were noted.
Observed neovascularization appears to be an adaptation response to the high load induced by HIFEM treatments when the growth of new capillaries is initiated to supply nutrition to the increased muscle mass.29,30 Nevertheless, the level of neovascularization was not quantified and should thus not be considered as a definite conclusion. As such, this observation will be subjected to additional research in the future to provide objective evidence.
One of the limitations of the present study is the sample size; the study included only 4 animals (3 treated animals and 1 control) to minimize the number of animals in order to conform to the convention for the protection of vertebrate animals. However, to increase the statistical power of the study, over 104 histologic slices were examined and evaluated. Another limitation of the study is the short time period between the treatment and the muscle biopsy, because with longer terms larger hypertrophy and higher levels of hyperplasia may be noted, as discussed above. The use of animal subjects in the study may also be considered as a limitation because the observed results may not be fully transferable to humans. On the other hand, the porcine model is widely used as a suitable substitute due to its high biological similarity with humans.
The results suggest that HIFEM induces intense muscle contractions, causing a response of the muscle tissue in the form of muscle fiber hypertrophy, which correlates with previous studies reporting increased muscle thickness in CT11 and MRI8 images posttreatment. Future studies should focus on further verification of the observed hyperplastic effects via additional evaluation methods such as monitoring the levels of myosatellite cells.31-33 In addition, longer follow-ups are required to capture potential terminal differentiation of the satellite cells.
To the best of our knowledge, no previous studies investigating changes in strength after HIFEM have been reported, although several studies have reported increased muscle mass posttreatment.8,11,12 Anecdotally, patients often report increased strength during exercise after the treatment procedure, and one may infer that an increase in muscle mass is also linked with increased strength. Further studies should include strength assessment prior to and following HIFEM to document this hypothesized benefit.
CONCLUSIONS
Histopathologic evaluation found a hypertrophic effect of HIFEM application on a cellular level, which correlates to the muscle growth observed in previous studies. The results indicate that intense muscle activity is induced during the HIFEM treatments and suggests this technology could serve as a convenient tool for muscle toning.
Disclosures
The authors declared no potential conflicts of interest with respect to the research, authorship, and publication of this article.
Funding
The authors received no financial support for the research, authorship, and publication of this article.
REFERENCES
- 1. Kennedy J, Verne S, Griffith R, Falto-Aizpurua L, Nouri K. Non-invasive subcutaneous fat reduction: a review. J Eur Acad Dermatol Venereol. 2015;29(9):1679-1688. [DOI] [PubMed] [Google Scholar]
- 2. Beasley KL, Weiss RA. Radiofrequency in cosmetic dermatology. Dermatol Clin. 2014;32(1):79-90. [DOI] [PubMed] [Google Scholar]
- 3. Roth BJ, Basser PJ. A model of the stimulation of a nerve fiber by electromagnetic induction. IEEE Trans Biomed Eng. 1990;37(6):588-597. [DOI] [PubMed] [Google Scholar]
- 4. Gabriel DA, Kamen G, Frost G. Neural adaptations to resistive exercise: mechanisms and recommendations for training practices. Sports Med. 2006;36(2):133-149. [DOI] [PubMed] [Google Scholar]
- 5. Dowling JJ, Konert E, Ljucovic P, Andrews DM. Are humans able to voluntarily elicit maximum muscle force? Neurosci Lett. 1994;179(1-2):25-28. [DOI] [PubMed] [Google Scholar]
- 6. Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81(4):1725-1789. [DOI] [PubMed] [Google Scholar]
- 7. Knight CA, Kamen G. Adaptations in muscular activation of the knee extensor muscles with strength training in young and older adults. J Electromyogr Kinesiol. 2001;11(6):405-412. [DOI] [PubMed] [Google Scholar]
- 8. Kinney BM, Lozanova P. High intensity focused electromagnetic therapy evaluated by magnetic resonance imaging: safety and efficacy study of a dual tissue effect based non-invasive abdominal body shaping. Lasers Surg Med. 2019;51(1):40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jacob CI, Paskova K. Safety and efficacy of a novel high-intensity focused electromagnetic technology device for noninvasive abdominal body shaping. J Cosmet Dermatol. 2018;17(5):783-787. [DOI] [PubMed] [Google Scholar]
- 10. Jacob C, Kinney B, Busso M, et al. High intensity focused electro-magnetic technology (HIFEM) for non-invasive buttock lifting and toning of gluteal muscles: a multi-center efficacy and safety study. J Drugs Dermatol. 2018;17(11):1229-1232. [PubMed] [Google Scholar]
- 11. Jacob C. Novel non-invasive technology based on simultaneous induction of changes in adipose and muscle tissues: safety and efficacy of a high intensity focused electro-magnetic (HIFEM) field device used for abdominal body shaping. Presented at the 38th Annual ASLMS Conference in Dallas, TX; April 2018.
- 12. Katz B, Bard R, Goldfarb R, Shiloh A, Kenolova D. Ultrasound assessment of subcutaneous abdominal fat thickness after treatments with a high-intensity focused electromagnetic field device: a multicenter study. Dermatol Surg. 2019. doi: 10.1097/DSS.0000000000001902. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 13. Weiss RA, Bernardy J. Induction of fat apoptosis by a non-thermal device: mechanism of action of non-invasive high-intensity electromagnetic technology in a porcine model. Lasers Surg Med. 2019;51(1):47-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Samuels JB, Pezzella A, Berenholz J, Alinsod R. Safety and efficacy of a non-invasive high-intensity focused electromagnetic field (HIFEM) device for treatment of urinary incontinence and enhancement of quality of life. Lasers Surg Med. 2019. doi: 10.1002/lsm.23106. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cleak MJ, Eston RG. Muscle soreness, swelling, stiffness and strength loss after intense eccentric exercise. Br J Sports Med. 1992;26(4):267-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Francaux M, Poortmans JR. Effects of training and creatine supplement on muscle strength and body mass. Eur J Appl Physiol Occup Physiol. 1999;80(2):165-168. [DOI] [PubMed] [Google Scholar]
- 17. Schneider CA, Rasband WS, Eliceiri KW. NIH image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9(7):671-675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Reggiani C, Kronnie T. Hyperplasia in exercise-induced muscle growth? Basic Appl Myol. 1999;9(6):289-292. [Google Scholar]
- 19. Crameri RM, Langberg H, Magnusson P, et al. Changes in satellite cells in human skeletal muscle after a single bout of high intensity exercise. J Physiol. 2004;558(Pt 1):333-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Spiering BA, Kraemer WJ, Anderson JM, et al. Resistance exercise biology: manipulation of resistance exercise programme variables determines the responses of cellular and molecular signalling pathways. Sports Med. 2008;38(7):527-540. [DOI] [PubMed] [Google Scholar]
- 21. McCall GE, Byrnes WC, Dickinson A, Pattany PM, Fleck SJ. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol (1985). 1996;81(5):2004-2012. [DOI] [PubMed] [Google Scholar]
- 22. Langeard A, Bigot L, Chastan N, Gauthier A. Does neuromuscular electrical stimulation training of the lower limb have functional effects on the elderly? A systematic review. Exp Gerontol. 2017;91:88-98. [DOI] [PubMed] [Google Scholar]
- 23. Matsuse H, Hashida R, Takano Y, et al. Walking exercise simultaneously combined with neuromuscular electrical stimulation of antagonists resistance improved muscle strength, physical function, and knee pain in symptomatic knee osteoarthritis: a single-arm study. J Strength Cond Res. 2017;31(1):171-180. [DOI] [PubMed] [Google Scholar]
- 24. Lin VW, Hsieh C, Hsiao IN, Canfield J. Functional magnetic stimulation of expiratory muscles: a noninvasive and new method for restoring cough. J Appl Physiol (1985). 1998;84(4):1144-1150. [DOI] [PubMed] [Google Scholar]
- 25. Han TR, Shin HI, Kim IS. Magnetic stimulation of the quadriceps femoris muscle: comparison of pain with electrical stimulation. Am J Phys Med Rehabil. 2006;85(7):593-599. [DOI] [PubMed] [Google Scholar]
- 26. Balmaseda MT Jr, Fatehi MT, Koozekanani SH, Sheppard JS. Burns in functional electric stimulation: two case reports. Arch Phys Med Rehabil. 1987;68(7):452-453. [PubMed] [Google Scholar]
- 27. Lambert H, Baetselier ED, Vanalme G, Mey GD. Skin burn risks using transcutaneous direct current. In: Proceedings of 17th International Conference of the Engineering in Medicine and Biology Society. Vol 1; 1995:647-648. [Google Scholar]
- 28. Zborowski M, Androjna C, Waldorff EI, Midura RJ. Comparison of therapeutic magnetic stimulation with electric stimulation of spinal column vertebrae. IEEE Trans Magn. 2015;51(12):1-9.26203196 [Google Scholar]
- 29. Deschenes MR, Ogilvie RW. Exercise stimulates neovascularization in occluded muscle without affecting bFGF content. Med Sci Sports Exerc. 1999;31(11):1599-1604. [DOI] [PubMed] [Google Scholar]
- 30. Cheng XW, Kuzuya M, Kim W, et al. Exercise training stimulates ischemia-induced neovascularization via phosphatidylinositol 3-kinase/Akt-dependent hypoxia-induced factor-1 alpha reactivation in mice of advanced age. Circulation. 2010;122(7):707-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kadi F, Eriksson A, Holmner S, Butler-Browne GS, Thornell LE. Cellular adaptation of the trapezius muscle in strength-trained athletes. Histochem Cell Biol. 1999;111(3):189-195. [DOI] [PubMed] [Google Scholar]
- 32. Kadi F, Eriksson A, Holmner S, Thornell LE. Effects of anabolic steroids on the muscle cells of strength-trained athletes. Med Sci Sports Exerc. 1999;31(11):1528-1534. [DOI] [PubMed] [Google Scholar]
- 33. Kadi F, Schjerling P, Andersen LL, et al. The effects of heavy resistance training and detraining on satellite cells in human skeletal muscles. J Physiol. 2004;558(Pt 3):1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]