Abstract
The temperature of the nest influences fitness in cavity-nesting bees. Females may choose nest cavities that mitigate their offspring’s exposure to stressful temperatures. This study aims to understand how cavity temperature impacts the nesting preference of the solitary bee Megachile rotundata (Fabricius) under field conditions. We designed and 3D printed nest boxes that measured the temperatures of 432 cavities. Nest boxes were four-sided with cavity entrances facing northeast, northwest, southeast, and southwest. Nest boxes were placed along an alfalfa field in Fargo, ND and were observed daily for completed nests. Our study found that cavity temperature varied by direction the cavity faced and by the position of the cavity within the nest box. The southwest sides recorded the highest maximum temperatures while the northeast sides recorded the lowest maximum temperatures. Nesting females filled cavities on the north-facing sides faster than cavities on the south-facing sides. The bees preferred to nest in cavities with lower average temperatures during foraging hours, and cavities that faced to the north. The direction the cavity faced was associated with the number of offspring per nest. The southwest-facing cavities had fewer offspring than nests on the northeast side. Our study indicates that the nesting box acts as a microclimate, with temperature varying by position and direction of the cavity. Variation in cavity temperature affected where females chose to nest, but not their reproductive investment.
Keywords: microclimate, heat stress, nesting behavior, solitary bee
Insects are sensitive to changes in temperature due to their ability to derive heat from their environment and their close relationship between external environmental temperature and internal body temperature (Martin and Huey 2008). The relationship between insect performance and temperature is nonlinear (Potter et al. 2011, Colinet et al. 2015, Sinclair et al. 2016). Insects experiencing temperatures past the optimum peak will have a steep decline in performance, while small decreases in temperature before the optimal peak do not drastically change performance measures. If temperatures continue to rise past the optimal temperature, insect performance rapidly declines and can eventually result in death (Colinet et al. 2015).
While atmospheric temperatures are often used to describe the temperature of an entire environment, microclimates—representing the specific conditions of a small area—often differ from the ambient temperature, or macroclimate. Microclimate temperatures better predict insect performance than atmospheric temperature, because they are a more accurate measurement of the specific environment that an insect experiences (Richards 1996). For example, the apple maggot Rhagoletis pomonella (Walsh) (Diptera: Tephridae) can experience internal apple temperatures of 45°C, well above the ambient temperature of 33°C and hot enough to cause expression of heat shock proteins (Lopez-Martinez and Denlinger 2008). Evapotranspiration at leaf surfaces buffers temperatures experienced by insect eggs, producing a microclimate that increases hatching success (Potter et al. 2009). Higher temperature nesting boxes of solitary bees have also been shown to increase mortality (CaraDonna et al. 2018). Microclimates have the potential to both protect from stressful temperatures or expose organisms to heat stress, suggesting that an insect’s ability to choose a more preferable microclimate may have a significant impact on fitness.
Many organisms, from a variety of taxa, have been shown to avoid exposure to high temperatures by moving to cooler microclimates. Bird communities associated with shepherd trees, Boscia albitrunca (Burch.) (Brassicales: Capparaceae), spend more time in densely shaded trees on days above 35°C (Martin et al. 2015). Atlantic salmon, Salmo salar (Linnaeus) (Salmoniformes: Salmonidae), swim to cooler waters to avoid warmer temperatures (Breau et al. 2011). Insects can also reduce exposure to high temperatures through behavior. Several insect species move to decrease exposure to stressful temperatures (Huey et al. 2002, Kearney et al. 2009), including choosing cooler microclimates (Woods et al. 2015).
However, not all insect developmental stages are able to move. Many insects that go through complete metamorphosis have a larval stage with limited ability to disperse from stressful microclimates and must complete development in the microclimate chosen by the parent. Females can mitigate the temperature stress of offspring by choosing favorable microclimates for juvenile development. For example, females of the silver-spotted skipper, Hesperia comma (Linnaeus) (Lepidoptera: Hesperiidae), will lay eggs on warmer host plants during low ambient temperatures and on cooler hosts plants during high ambient temperatures, resulting in increased fitness of offspring (Davies et al. 2006). The majority of larval hymenopterans are immobile and remain in the nest until fully grown, relying on the provisions and space provided by the mother. Thus, mothers can mitigate offspring exposure to hot environments by choosing microclimates with favorable temperatures.
The alfalfa leafcutting bee, Megachile rotundata, offers a model for exploring how insects respond to microclimates, and specifically, how the parent may avoid exposing offspring to suboptimal temperatures. Megachile rotundata is a solitary, cavity-nesting bee that builds linear nests out of leaves, creating an individual brood cell for each offspring (Pitts-Singer and Cane 2011). The agricultural industry uses them to pollinate alfalfa and provides man-made nest boxes consisting of thousands of linear cavities (Stephen 1981). Juveniles grow and develop in the brood cell from egg to adult, feeding off the provisions left by the nesting female (Pitts-Singer and Cane 2011). The offspring is entirely dependent on the mother’s choice of cavity and provision. The agricultural nest box allows an opportunity to test how females determine cavity choice and to explore whether variation in temperature influences nesting decisions.
Previous work has suggested that the nesting box is a microclimate. Nesting boxes have been found to have different temperatures due to nest box material (Richards 1996) and color (CaraDonna et al. 2018). High temperatures increase larval mortality (Pitts-Singer and James 2008), larval developmental rate (Kemp and Bosch 2000, Fischman et al. 2017), and susceptibility to pathogens (Xu and James 2012). These studies suggest that female M. rotundata have the potential to influence offspring fitness by choosing nesting cavities with favorable microclimate temperatures. However, previous studies were either laboratory studies or field studies that measured the temperature of the entire nest box, not the temperature of individual cavities.
The goal of this study was to determine how nest cavity microclimate corresponds to nest cavity choice and nest investment in M. rotundata by tracking individual cavity temperature and nesting behavior. In order to expose M. rotundata to naturally occurring temperature fluctuations and microclimates, we established nesting boxes facing four directions and placed temperature loggers throughout the nest box. We aimed to test the following: First, does cavity temperature vary by direction the cavity is facing and position of the cavity in the nest box? Second, does variation in cavity temperature influence M. rotundata nesting choice? And third, does cavity temperature influence the number of offspring?
Materials and Methods
Nest Box Design and Description of Field Site
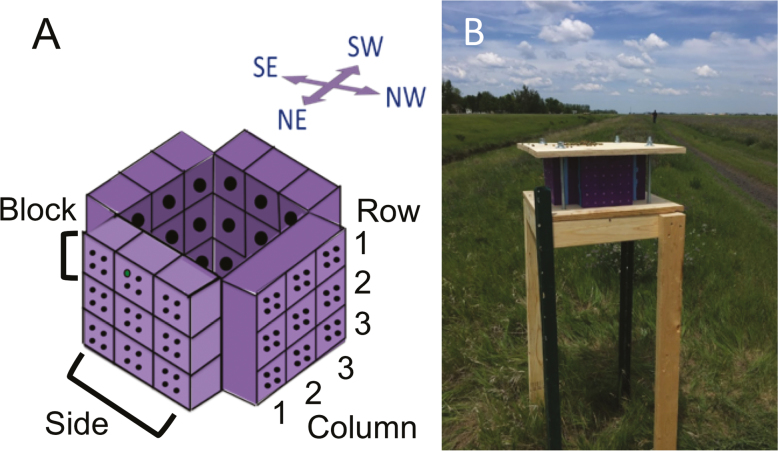
Three replicate nest boxes were designed and fabricated for use in a field site in Fargo, ND (details on field site below). Each nest box consisted of 36 blocks with four nest cavities each, which were 3D printed using purple polylactic acid (PLA) plastic (Lulzbot, Inc., Loveland, CO). Blocks were printed on a Taz 5 and Taz 6 3D printer (LulzBot, Loveland, CO) with a 20% infill. The dimensions of each block were 60 mm × 60 mm × 82 mm (Fig. 1A). Each block contained four nest cavities spaced equal distance apart, with a diameter of 7 mm and length of 78 mm. Nest boxes were made by stacking blocks in a three by three pattern, resulting in 36 nesting cavities per side, and a total of 144 cavities per nest box (Fig. 1A). A 10.25 mm × 41 mm hole was added to the back of each block to accommodate a Thermochron 5 iButton (Digi-Key, Inc., Thief River Falls, MN). To ensure that one iButton was able to accurately measure the temperatures of each of the four cavities within one block, an incubator pretrial was run. In the trial, four blocks were placed in an incubator with a HOBO temperature probe in each cavity (Onset Computer Corporation, Borne, MA) and an iButton placed in the back. The incubator was set to ramp from 10°C to 30°C and then back down to 10°C over the course of 4 d. This trial showed significant correlations between the four HOBO probes and the iButtons in the four trials, with Pearson’s correlation r-values ranging from 0.978 to 0.997. This pretrial confirmed that one iButton placed in the back of the block would be able to accurately measure the temperature of the four surrounding cavities.
Fig. 1.
Field design. (A) Diagram of 3D printed design. The iButtons were placed in holes in the back of each block. Each block had four nest cavities, and blocks were arranged in a 3 × 3 design with cavities facing four directions (northeast, northwest, southeast, and southwest). (B) Nest box (replicate 1) at alfalfa field site.
The three replicate nest boxes were placed along the side of an alfalfa field in Fargo, ND (46°55′15″N, 96°51′17″W). A drainage ditch containing multiple forb species ran along the side of the field. The three nest box replicates were placed 200 m apart, a distance that minimizes adult migration between replicates (Bradner et al. 1965). Each nest box was oriented in the field so that the entrances of the nest cavities faced northwest (NW), northeast (NE), southwest (SW), and southeast (SE) (Fig. 1). Nest boxes were placed on a wooden base 1.2 m above the ground and topped with a wooden board with 7.62 cm of overhang on all sides to provide shade (Fig. 1B). Paper straws (Jonesville Paper Tube Corp., Jonesville, MI) measuring 76.2 mm long with an internal diameter of 5.54 mm and a wall thickness of 0.305 mm were placed in each cavity to allow for nest removal and analysis. iButtons in each block recorded the block temperature to the nearest 0.5°C every 15 min. iButtons were downloaded and reprogrammed approximately every 20 d. iButtons were initially deployed on 21 June 2018 and the final temperature reading was 22 September 2018. Of the 108 iButtons and five time periods, three time intervals had data loss due to iButton failure.
Monitoring Nesting Behavior and Number of Offspring
Megachile rotundata were purchased from JWM Leafcutters (Nampa, ID) as post-diapause quiescent prepupae. They were incubated at 29°C until the first males emerged and then placed on top of the nest block and allowed to nest in any of the available cavities. Five hundred bees were released at each nest box replicate on 20 June 2018 and 1,068 bees were released at each replicate on 26 June 2018 to guarantee a large nesting population. Starting on 25 June 2018 boxes were checked every day for capped nests.
Number of brood cells per nest was determined by X-ray. Newly capped nests were removed from their cavities for X-ray analysis and were placed back into their cavities on the same day, usually within a few hours. Nests were X-rayed (Faxitron Bioptics LLC, Tucson, AZ) for 4 s, at 28 KVM. Megachile rotundata build about one brood cell per day (Maeta and Kitamura 2005). Assuming this rate, the start date of each nest was calculated by using the total number of brood cells in a nest to count backwards from the day the nest was completed. This analysis gave the start date for each nest.
Data Processing
iButton data were combined using RStudio (RStudio Team 2019, version 1.1.419) and R (R Core Team 2019, version 3.5.2) with packages lubridate (Grolemund and Wickham 2011), tidyr (Wickham and Henry 2019), and stringr (Wickham 2019). These packages were used to facilitate the analysis of dates in R (lubridate), subset data and create graphs (tidyr), and to manipulate string functions (stringr). Megachile rotundata are only actively foraging during the daylight hours (Szabo and Smith 1972, Lerer et al. 1982); thus, average foraging temperature was determined by temperatures collected between 9:00 a.m. and 9:00 p.m. using the package dplyr (Wickham et al. 2019). We hypothesized that temperature on the first day of nesting would be the most important temperature in determining nest choice. We calculated the average temperature in each cavity by each day of nesting. We used the first 30 d of capped nests for the preference analysis because this is when 50% of the cavities across all replicates had filled. To calculate choice, nests were either labeled with (0) for nest not chosen by calendar date or (1) for chosen on that date. Cavities that were already chosen from a previous day were not included in the analysis. Thus, each female’s preference was only calculated based on the number of cavities available on that day of nesting. We hypothesized the average cavity temperature during the nesting period would influence the number of offspring a female provisioned in each nest. Average cavity temperature was calculated by averaging the daily foraging temperature from the date each nest was started to the date it was capped. To determine whether cavity temperatures differed from ambient, ambient temperatures were downloaded from the National Climatic Data Center using Hector International Airport’s weather station, which was 2.7 km from the field site.
Statistical Analyses
In order to test the influence of cavity position and direction on average nest cavity temperature, we tested a linear mixed-effects model that incorporated direction as a categorical variable and row and column as continuous variables using the lme4 package (Bates et al. 2015). Row and column of the nest box refers to the row (1–3) or column (1–3) of the block in that nest, not the row or column of each nest cavity (see Fig. 1A). Replicate was included as a random effect. To test rate of cavity fill by direction, a Kaplan–Meier analysis was conducted using JMP Pro (version 14, SAS Institute, Cary, NC) with replicate as a random effect. A log-rank ANOVA with a Tukey’s post hoc determined whether the number of days until half the cavities were occupied differed by direction. To determine if the difference in nesting patterns was dependent on temperature, a binomial generalized linear mixed-effect model was run using the lme4 package (Bates et al. 2015) and the results were visualized using the effects package (Fox and Weisberg 2019). We restricted the model to the first 30 d of nesting because after that point half of all the nesting cavities were already filled, limiting the available choices for nesting females. The response variable for our model was chosen (1) or not chosen (0) for a cavity for every date of nesting. Cavities could be ‘not chosen’ for multiple days, because a female did not start a nest, but could only be ‘chosen’ once. After a cavity was chosen, it was no longer included in the data set because it was no longer a possible choice. Because individual cavities were represented multiple times in the data set, we included cavity ID as a random effect. iButton was also included as a random effect because each iButton temperature was used for four cavities adjacent to the iButton. Replicate nest box was also a random effect. The fixed effects were average foraging temperature of each day, direction, row, and column of nest box. To test for collinearity between temperature and direction, we calculated the variance inflation factors for direction, row, and temperature. To determine whether cavity temperature influenced the number of offspring a female laid in a nest, we tested a linear mixed-effects model that used average temperature of the cavity over the days the nest was being built, direction, row, and column of the nest block, and the date the nest was completed as fixed effects and replicate and iButton as random effects using the lme4 package (Bates et al. 2015).
Results
Nest Cavity Temperature Variation
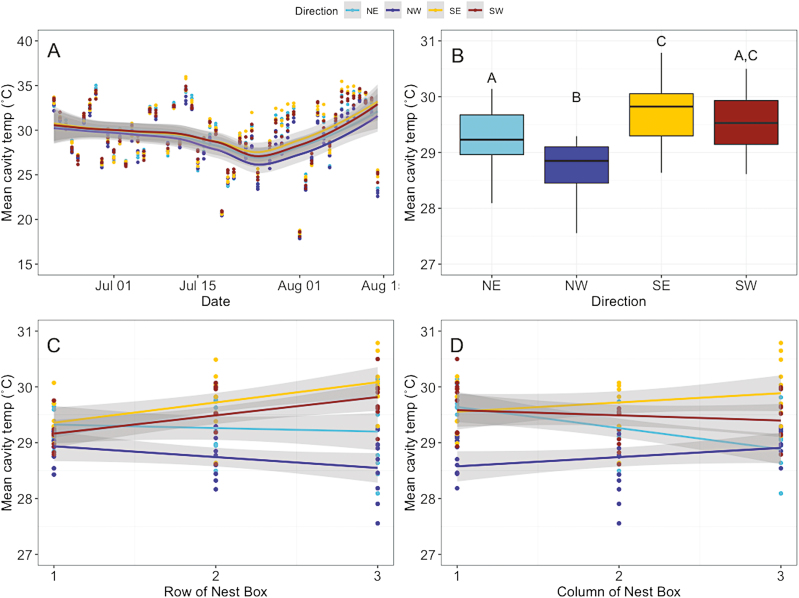
We wanted to understand how cavity temperature varied across the nesting box during foraging hours, which occurs only during daylight for M. rotundata (Szabo and Smith 1972, Lerer et al. 1982). The minimum cavity temperature recorded during foraging hours was 9.5°C, and the maximum cavity temperatures recorded was 46.5°C. All directions experienced similar minimum temperatures, but maximum temperatures ranged from 42°C on the NE to 46.5°C on the SW side. Minimum temperature for each side of the nest box were reached on 2 August, but maximum temperatures were reached on different days (Fig. 2A). The NW side reached the maximum temperature on 21 June, the NE on 28 June, SE on 8 August, and the SW on 13 July. Ambient air did not reach temperatures this high, with an average of 12–28°C during the nesting period and a maximum temperature of 33°C (National Climatic Data Center 2018).
Fig. 2.
Average cavity temperature during foraging hours was influenced by multiple factors. (A) Cavity temperature averaged by day and direction for each replicate. Lines represent a loess fit. (B) Cavity temperature averaged by block and grouped by direction the cavity faced. (C) Average cavity temperature by row, with 1 being the top row of the box and 3 being the bottom row. Lines represent linear fits from the single predictor of temperature, grouped by direction. (D) Average foraging temperature by column, with 1 denoting the left most column and 3 denoting the right most column. Lines represent linear fits from the single predictor of temperature, grouped by direction. In (B–D), temperatures were averaged across the field season by block of the nest box. Gray shading in all panels indicates the 95% confidence intervals.
Direction was a significant predictor of cavity temperature (Fig. 2B; F(3,100) = 27.35, P < 0.0001), and row was also significant (Fig. 2C; F(1,100) = 4.857, P = 0.0299). Column of the nest box was not a significant predictor of temperature (Fig. 2D; F(1,100) = 0.5058, P = 0.4787), but there was a significant interaction between column and direction (F(3,100) = 6.983, P = 0.0003) in average cavity temperature. There was also a significant interaction between row and direction (F(3,100) = 8.072, P < 0.0001). The combination of row, column, and direction explained 53.19% of the variation in average cavity temperature. The first row of all boxes had similar temperatures regardless of direction, which may be explained by the shade overhang. The SE- and SW-facing cavities increased in temperature in rows 2 and 3 (Fig. 2C), while the NE and NW directions decreased in average temperature in rows 2 and 3.
Rate of Nest Completion by Direction
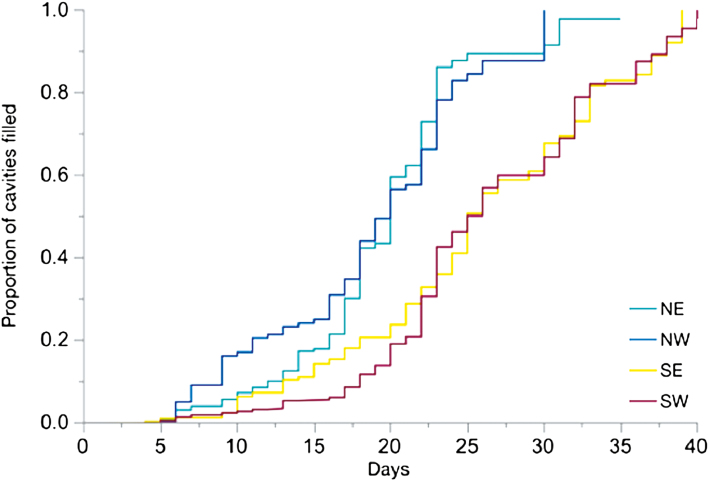
Over the course of the nesting season all boxes filled to capacity. However, rate of fill varied significantly by direction (Fig. 3; χ 2 = 1627, df = 3, P < 0.0001). The NW- and NE-facing cavities were preferred by nesting females and capped first, followed by the SE- and SW-facing cavities. The NW- and NE-facing cavities reached 50% capacity on day 20, which was 5 d before the SW and SE cavities. A log-rank ANOVA with a Tukey’s post hoc test on the number of days until a cavity was filled showed that the NE cavities were significantly different from the SW (P ≤ 0.0001) and SE (P = 0.0004). The NW cavities were also significantly different from the SW (P ≤ 0.0001) and SE (P ≤ 0.0001). There was not a significant difference between the two northern sides (P = 0.8106) or the two southern sides (P = 0.9206).
Fig. 3.
Females filled NE- and NW-facing cavities faster than SW- and SE-facing cavities. Kaplan–Meier analysis of box fill over nesting season (P < 0.0001). NE- and NW-facing cavities reached 50% filled 5 d earlier, on average, than SE- and SW-facing cavities.
Nest Cavity Preference by Temperature
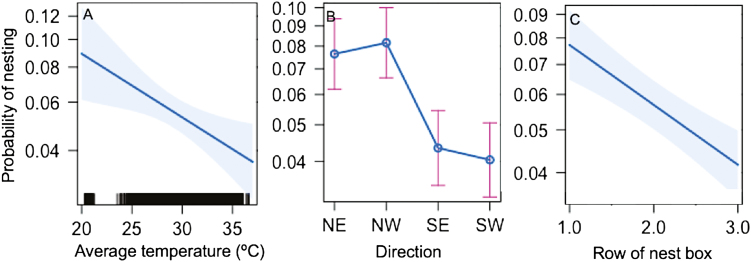
Average temperature during foraging hours influenced nest cavity preference. The final model included the average temperature during foraging hours (F(1,6015) = 7.940, P = 0.0049), direction (F(3,6015) = 12.80, P = 0.0003), and row (F(1,6015) = 22.80, P < 0.0001). Column was not significant (F(1,6007) = 0.0004, P = 0.9840). None of the interaction terms were significant. The probability that a female would nest in a cavity decreased with increasing temperatures (Fig. 4A). As was found in the Kaplan–Meier analysis, females favored the NE- and NW-facing cavities (Fig. 4B) and preferred the top rows of the nest box (Fig. 4C). Because the initial analysis on nest box temperature had determined temperatures were not evenly distributed across the four directions, we tested whether the preference for direction was a result of a collinearity between temperature and direction. The variance inflation factors were all less than 3, suggesting that preference for north-facing cavities was independent of those cavities having cooler temperatures. Our model explained 6.18% of the variation in nest choice, and the predictive power of the factors was low. The average probability that a female would choose a 20°C cavity was 8.91%, whereas the average probability of choosing a 37°C cavity was 3.63%. In summary, females showed a preference for cooler, north-facing cavities at the top of the nest box.
Fig. 4.
The probability of nesting in a cavity decreases as the average temperature of the cavity increases. (A) Cavity temperature significantly predicted nesting (P = 0.0049). (B) Direction the cavity faced significantly influenced the probability of nesting (P = 0.0003), as did the row of the nest box (C, P < 0.0001). Lines represent fitted values from the multivariate model. Shading on (A) and (C) is a confidence band based on standard errors, as are the whiskers in (B). Black bars on the x-axis of (A) show the range of values in the data set.
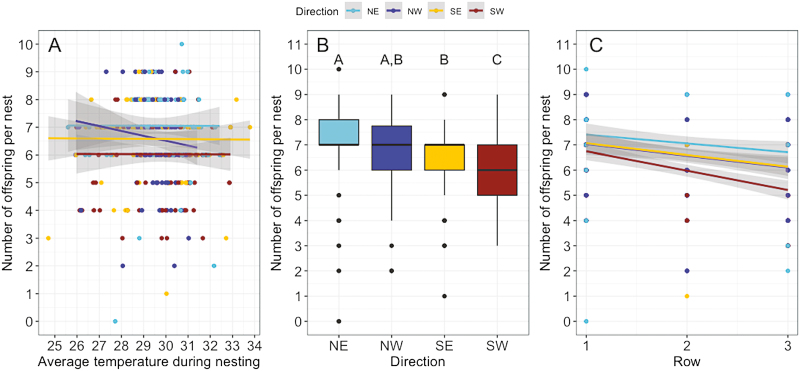
Number of Offspring
Females can choose how many brood cells to build per nest and we hypothesized that they would lower their reproductive investment in nest cavities with poor microclimates. However, the average temperature of the cavity during the days the female was building a nest did not significantly affect the number of brood cells (Fig. 5A; F(1,399) = 0.0246, P = 0.8755). Direction the cavity faced (Fig. 5B; F(3,399) = 8.731, P < 0.0001), and row of the nest box (Fig. 5C; F(1,399) = 35.01, P < 0.0001) significantly influenced the number of brood cells. Neither column (F(1,399) = 0.5599, P = 0.4560) nor the date the nest was completed (F(1,114) = 1.478, P = 0.2261) were significant. Females built more brood cells in NE-facing cavities, and fewer in SW-facing cavities (Fig. 5B). Nesting females built more brood cells in rows at the top of the nest box (Fig. 5C). The model explained 24.8% of the variation in the number of brood cells in each nest.
Fig. 5.
Nest size differs by direction the cavity faces and the row of the nest box. (A) Average cavity temperature during the nesting period did not affect nest size (P = 0.9967). Lines in (A) represent linear fits from the single predictor of temperature, grouped by direction. (B) Direction the cavity faced (P < 0.0001), and (C) row of the nest box (P < 0.0001) significantly influenced nest size. Lines in (C) represent linear fits from the single predictor of row, grouped by direction. Points correspond to individual nests. However, points overlap due to identical values. Gray shading in (A) and (C) indicates the 95% confidence intervals.
Discussion
Insects can behaviorally regulate their exposure to harmful temperatures by choosing favorable microclimates (Huey et al. 2002, Kearney et al. 2009, Woods et al. 2015). Hymenopterans tend to have immobile larvae, whose mothers choose the location for juvenile development. For cavity-nesting bees like the alfalfa leafcutting bee, M. rotundata, females have the potential to limit the exposure of their offspring to harmful temperatures by choosing favorable cavities to lay eggs. The nesting cavity has been demonstrated to have multiple impacts on the physiology and reproduction of M. rotundata, including sex ratio (Stephen and Osgood 1965) and development (Yocum et al. 2014), making the nesting cavity a significant influence in the life history of M. rotundata. However, the impact of individual cavity temperature has not been studied in this species. Our goal was to test whether female bees make reproductive decisions based on cavity temperature.
We found that nesting cavities are highly variable in temperature, not only due to the direction the nesting cavity is facing, but the cavity position within a single box (Fig. 2). Nesting box cavities increased in temperature by row from top to bottom within the nesting box on the south-facing sides (Fig. 2A–D), but cavities on the northern sides had a narrower range of temperatures (Fig. 2C). We did find that the interaction between direction and block position was significant in determining average temperature. These data on cavity temperatures are important because they provide fine-scale measurement of within-nest box variability.
The nest cavity temperatures exceeded ambient temperatures and sometimes reached stressfully high levels. The SW side reached 46.5°C, which is above the 45°C threshold that increases mortality in developing pupae (Undurraga and Stephen 1980, Barthell et al. 2002). The NW and SE sides reached 45.5°C and 44°C, respectively. However, the cavities facing NE remained below 45°C and did not exceed the threshold found to affect developing pupae. Ambient temperatures from the closest weather station reached a maximum temperature of 33°C (National Climatic Data Center 2018). Thus, nest cavity temperature sometimes exceeds ambient temperature by over 10°C. Our maximum nest temperatures were similar to those found in other studies. In Utah, nesting boxes reached temperatures of 44°C during a tent experiment (Rossie et al. 2010). Nesting boxes placed in Arizona reached temperatures exceeding 45°C (CaraDonna et al. 2018). Maximum temperatures are especially important in the relationship between performance and temperature, because increases could potentially exceed the thermal optimum causing a disproportionate decrease in performance (Sinclair et al. 2016).
We find that M. rotundata females preferred to nest in cooler cavities on the northern sides of the nest box (Fig. 4B). As cavity temperatures increased, the probability of nesting in that cavity decreased (Fig. 4A). We found that row of the nest box also influenced nesting preference with females favoring the top row. The top row was shaded by the wooden lid of the box (Fig. 1B), which could reduce temperature variation and possibly added protection from wind. Nest choice based on temperature suggests that M. rotundata could compensate for stressful temperatures by selecting the cooler nest cavities. These choices could have significant consequences for offspring since the less-preferred nesting cavities reached stressfully high temperatures. However, we did not directly measure whether these maternal choices impacted offspring survival. Female skippers also choose egg laying sites based on temperature cues (Davies et al. 2006), suggesting that these behavioral choices should be investigated in more species.
Direction the nest box faced also predicted cavity choice because females preferred north-facing sides of the nest boxes even in a model that accounted for temperature. This result suggests that an environmental factor besides temperature is causing females to prefer north-facing cavities. Although not explicitly tested in this study, we suspect that wind direction may partially explain the preference for north-facing cavities. Using data from the NDAWN (North Dakota Agricultural Weather Network) Fargo NW station we analyzed the wind direction from 21 June through 23 July, which was the nesting period used in the preference analyses. The average wind direction was from the SSE at 157.9 ± 1.7° (Raleigh test, test statistic = 0.2287, P < 0.0001), suggesting that the NE- and NW-facing cavities were sheltered from wind by the nest block. Wind preference has been demonstrated in a few Hymenoptera species. Trap-nesting for bees and wasps is most successful when nest entrances are turned away from prevailing winds (Martins et al. 2012). However, nesting bumble bees showed no preference for direction in the presence of wind, but without wind preferred north-facing nesting boxes (Hemple de Ibarra et al. 2009). Future studies should consider wind direction when investigating nesting choice in solitary bees.
Considering we found a difference in preference between nesting cavities, we wanted to determine if choice in cavity influenced the number of eggs laid. We hypothesized that females would invest less reproductive effort, as measured by the number of brood cells constructed, in warmer cavities. However, our hypothesis was not supported because there was no relationship between the average cavity temperature during the nesting period and nest size. Our analysis has a limitation in that the length of the nesting period is estimated using the date the nest was completed, which we recorded, and the assumption that a female builds her nest at the rate of one brood cell per day (Maeta and Kitamura 2005). If a female built her nest faster or slower than this estimate, the temperature the female experienced could have been slightly higher or lower than the averages used in the analysis. Previous studies have found that number of brood cells (Pitts-Singer and James 2008) and offspring survival (Barthell et al. 2002) decrease with warmer ambient temperatures, suggesting that temperature does impact nest size under other conditions. We did find that nests in cavities facing the SW were significantly smaller than those facing other directions. Females that nested on the SW side provisioned, on average, one offspring fewer than females on the NE side (Fig. 5B). Nest size decreased by half a brood cell, on average, from the top row to the bottom row of the nest box (Fig. 5C). Wind direction may have contributed to this pattern of nest size because females make multiple flights to and from the cavity while carrying nesting materials.
Nest box microclimates may influence agricultural production of M. rotundata. Megachile rotundata does not reproduce well in the United States and has a 50% return rate, which is less than Canadian agricultural populations (James and Pitts-Singer 2013, Pitts-Singer and Bosch 2010). The nest boxes in this study differed from agricultural management practices in three important ways: the use of materials, the amount of shelter, and the orientation. Commercial nest boxes are made of either wood or polystyrene (Pitts-Singer and James 2005), which have different heat retention from each other and from the plastic used in our study. Cavity temperatures in commercial wood and straw nests can exceed 42°C (Stephen 1981), and M. rotundata is used in the Central Valley of California, where ambient temperatures consistently rise above 40°C (Barthell et al. 2002). Polystyrene has been recorded reaching temperatures comparable to those found in this study (M. Bennett, unpublished data). The cavity temperatures in this study span the range that a female might experience during commercial management. We found that females prefer cooler nest cavities (20–30°C), and that these cavity temperatures were exceeded at our field site. Commercial nest boxes are placed in shed-like shelters (Pitts-Singer and Cane 2011) that provide more shade than our box design. We found that females preferred to nest in the top row of the nest box, which had the most shade and most limited temperature range, and also may have been protected from the wind. Management practice in the agricultural industry is to face the nest box shelters to the SE (Stephen 1981). We found a 15% increase in the number of offspring on the NE side compared to the SE orientation. Our study demonstrates that growers may be able to manipulate the microclimate experienced by M. rotundata, and lead to increased nesting rates and offspring laid.
In conclusion, ambient temperature is not a reliable predictor of cavity temperature, indicating that the nesting cavity provides a unique microclimate that can provide both beneficial and potentially harmful exposure to temperatures. We found the cavities in M. rotundata nesting box heat up disproportionately throughout the day, suggesting a microclimate that amplifies thermal extremes instead of buffering (Woods et al. 2015). The tendency to amplify ambient heat is dependent on the thermal properties of the material that contains the cavity but is likely to be true of many natural nest materials like straw and wood (Stephen 1981). Although this study focuses on stressfully high temperatures, relatively warmer cavities might be beneficial in cooler climates or earlier in the nesting season. The ability to choose microclimates, as demonstrated by nesting females in this study, needs to be integrated into species-specific predictions of response to thermal stress (Woods et al. 2015, Kingsolver and Woods 2016, Pincebourde and Casas 2019). The precise measurements of nest cavity temperature and the behavioral response of females in choosing cavities improves the ability to predict how climate change may impact cavity-nesting insects.
Data Accessibility
The raw data are available on Dryad (data will be made available upon acceptance of publication).
Acknowledgments
Thank you to Nyle Jonason for his help in building materials and field site setup. Thank you to Courtney Grula and Korie Debardlabon for assisting in the field. Thank you to Giancarlo Lopez-Martinez and two anonymous reviewers who provided thoughtful comments on this manuscript. This project was funded by National Science Foundation RII Track-2 FEC 1826835 and United States Department of Agriculture-Agricultural Research Service. E.S.W., C.E.M., J.P.R., and G.Y. conceived the ideas and designed the methodology; E.S.W. and C.E.M. collected the data; E.S.W., C.E.M., G.Y., and J.H.B. analyzed the data; E.S.W. and J.H.B. led the writing of the manuscript.
References Cited
- Barthell J. F., Hranitz J. M., Thorp R. W., and Shue M. K.. . 2002. High temperatures responses in two exotic leafcutting bee species: Megachile apicalis and Megachile rotundata (Hymenoptera: Megachilidae). Pan-Pac. Entomol. 78: 235–246. [Google Scholar]
- Bates D., Maechler M., Bolker B., and Walker S.. . 2015. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 67: 1–48. [Google Scholar]
- Bradner N. R., Frakes R. V., and Stephen W. P.. . 1965. Effects of bee species and isolation distance on possible varietal contamination in alfalfa. Agron. J. 57: 247–248. [Google Scholar]
- Breau C., Cunjak R. A., and Peake S. J.. . 2011. Behavior during elevated water temperatures: can physiology explain movement of juvenile Atlantic salmon to cool water? J. Anim. Ecol. 80: 844–853. [DOI] [PubMed] [Google Scholar]
- Caradonna P. J., Cunningham J. L., and Iler A. M.. . 2018. Experimental warming in the field delays phenology and reduces body mass, fat content, and survival: implications for the persistence of a pollinator under climate change. Funct. Ecol. 32: 2345–2356. [Google Scholar]
- Colinet H., Sinclair B. J., Vernon P., and Renault D.. . 2015. Insects in fluctuating thermal environments. Annu. Rev. Entomol. 60: 123–140. [DOI] [PubMed] [Google Scholar]
- Davies Z. G., Wilson R. J., Coles S., and Thomas C. D.. . 2006. Changing habitat associations of a thermally constrained species, the silver-spotted skipper butterfly, in response to climate warming. J. Anim. Ecol. 75: 247–256. [DOI] [PubMed] [Google Scholar]
- Fischman B. J., Pitts-Singer T. L., and Robinson G. E.. . 2017. Nutritional regulation of phenotypic plasticity in a solitary bee (Hymenoptera: Megachilidae). Environ. Entomol. 46: 1070–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox J., and Weisberg S.. . 2019. An R companion to applied regression, 3rd ed. Thousand Oaks, CA: http://tinyurl.com/carbook. [Google Scholar]
- Grolemund G., and Wickham H.. . 2011. Dates and times made easy with lubridate. J. Stat. Softw. 40: 1–25. http://www.jstatsoft.org/v40/i03/. [Google Scholar]
- Hemple de Ibarra N., Philippides A., Riabinina O., and Collett T. S.. . 2009. Preferred viewing directions of bumblebees (Bombus terrestris L.) when learning and approaching their nest site. J. Exp. Biol. 212: 3193–3204. [DOI] [PubMed] [Google Scholar]
- Huey R. B., Carlson M., Crozier L., Frazier M., Hamilton H., Harley C., Hoang A., and Kingsolver J. G.. . 2002. Plants versus animals: do they deal with stress in different ways? Integr. Comp. Biol. 42: 415–423. [DOI] [PubMed] [Google Scholar]
- James R. R., and Pitts-Singer T. L.. . 2013. Health status of alfalfa leafcutting bee larvae (Hymenoptera: Megachilidae) in United States alfalfa seed fields. Environ. Entomol. 42: 1166–1173. [DOI] [PubMed] [Google Scholar]
- Kearney M., Shine R., and Porter W. P.. . 2009. The potential for behavioral thermoregulation to buffer “cold-blooded” animals against climate warming. Proc. Natl. Acad. Sci. USA. 106: 3835–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemp W. P., and Bosch J.. . 2000. Development and emergence of the alfalfa pollinator Megachile rotundata. (Hymenoptera: Megachilidae). Ann. Entomol. Soc. Am. 93: 904–911. [Google Scholar]
- Kingsolver J. G., and Woods H. A.. . 2016. Beyond thermal performance curves: modeling time-dependent effects of thermal stress on ectotherm growth rates. Am. Nat. 187: 283–294. [DOI] [PubMed] [Google Scholar]
- Lerer H. l., Bailey W. G., Mills P. F., and Pankiw P.. . 1982. Pollination activity of Megachile rotundata (Hymenoptera: Apoidea). Environ. Entomol. 11: 997–1000. [Google Scholar]
- Lopez-Martinez G., and Denlinger D. L.. . 2008. Regulation of heat shock proteins in the apple maggot Rhagoletis pomonella during hot summer days and overwintering diapause. Physiol. Entomol. 33: 346–352. [Google Scholar]
- Maeta Y., and Kitamura T.. . 2005. On the number of eggs laid by one individual of females in the alfalfa leaf-cutting bee, Megachile (Eutricharaea) rotundata (Fabricius) (Hymenoptera, Megachilidae). Chugoku Kontyu. 19: 39–43. [Google Scholar]
- Martin T. L., and Huey R. B.. . 2008. Why “suboptimal” is optimal: Jensen’s inequality and ectotherm thermal preferences. Am. Nat. 17: 102–118. [DOI] [PubMed] [Google Scholar]
- Martin R. O., Cunningham S. J., and Hockey P. A. R.. . 2015. Elevated temperatures drive fine-scale patterns of habitat use in a savanna bird community. J. Afr. Ornithol. 86: 127–135. [Google Scholar]
- Martins C. F., Ferreira R. P., and Carneiro L. T.. . 2012. Influence of the orientation of nest entrance, shading, and substrate on sampling trap-nesting bees and wasps. Neotrop. Entomol. 41: 105–111. [DOI] [PubMed] [Google Scholar]
- National Climatic Data Center 2018. WFO monthly/daily climate data [CXUS53 KFGF 011602] http://www.ncdc.noaa.gov.
- Pincebourde S., and Casas J.. . 2019. Narrow safety margin in the phyllosphere during thermal extremes. Proc. Natl. Acad. Sci. USA. 116: 5588–5596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts-Singer T. L., and Bosch J.. . 2010. Nest establishment, pollination efficiency, and reproductive success of Megachile rotundata (Hymenoptera: Megachilidae) in relation to resource availability in field enclosures. Environ. Entomol. 39: 149–158. [DOI] [PubMed] [Google Scholar]
- Pitts-Singer T. L., and Cane J. H.. . 2011. The alfalfa leafcutting bee, Megachile rotundata: the world’s most intensively managed solitary bee. Annu. Rev. Entomol. 56: 221–237. [DOI] [PubMed] [Google Scholar]
- Pitts-Singer T. L., and James R. R.. . 2005. Emergence success and sex ratio of commercial alfalfa leafcutting bees from the United States and Canada. J. Econ. Entomol. 98: 1785–1790. [DOI] [PubMed] [Google Scholar]
- Pitts-Singer T. L., and James R. R.. . 2008. Do weather conditions correlate with findings in failed, provision-filled nest cells of Megachile rotundata (Hymenoptera: Megachilidae) in western North America? J. Econ. Entomol. 101: 674–685. [DOI] [PubMed] [Google Scholar]
- Potter K., Davidowitz G., and Woods H. A.. . 2009. Insect eggs protected from high temperatures by limited homeothermy of plant leaves. J. Exp. Biol. 212: 3448–3454. [DOI] [PubMed] [Google Scholar]
- Potter K. A., Davidowitz G., and Woods H. A.. . 2011. Cross-stage consequences of egg temperature in the insect Manduca sexta. Funct. Ecol. 25: 548–556. [Google Scholar]
- R Core Team 2019. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria: https://www.R-project.org/. [Google Scholar]
- Richards K. W. 1996. Effect of environment and equipment on productivity of alfalfa leafcutter bees (Hymenoptera: Megachilidae) in Southern Alberta, Canada. Can. Entomol. 128: 47–56. [Google Scholar]
- Rossi B. H., Nonacs P., and Pitts-Singer T. L.. . 2010. Sexual harassment by males reduces female fecundity in the alfalfa leafcutting bee, Megachile rotundata. Anim. Behav. 79: 165–171. [Google Scholar]
- RStudio Team 2019. RStudio: integrated development for R. RStudio, Inc., Boston, MA: http://www.rstudio.com/. [Google Scholar]
- Sinclair B. J., Marshall K. E., Sewell M. A., Levesque D. L., Willett C. S., Slotsbo S., Dong Y., Harley C. D., Marshall D. J., Helmuth B. S., . et al. 2016. Can we predict ectotherm responses to climate change using thermal performance curves and body temperatures? Ecol. Lett. 19: 1372–1385. [DOI] [PubMed] [Google Scholar]
- Stephen W. P. 1981. The design and function of field domiciles and incubators for leafcutting bee management (Megachile rotundata (Fabricius)). Oregon State College Agric. Exp. Stat. Bull. 654: 1–13. [Google Scholar]
- Stephen W. P., and Osgood C. E.. . 1965. Influence of tunnel size and nesting medium on sex ratios in a leaf-cutter bee, Megachile rotundata. J. Econ. Entomol. 58: 965–968. [Google Scholar]
- Szabo T. I., and Smith M. V.. . 1972. The influence of light intensity and temperature on the activity of the alfalfa leaf-cutter bee Megachile rotundata under field conditions. J. Apic. Res. 11: 157–165. [Google Scholar]
- Undurraga J. M., and Stephen W. P.. . 1980. Effect of temperature on development and survival in post-diapausing alfalfa leafcutting bee prepupae and pupae (Megachile rotundata (F.): Hymenoptera: Megachilidae). I. High temperatures. J. Kansas Entomol. Soc. 53: 669–676. [Google Scholar]
- Wickham H. 2019. stringr: simple, consistent wrappers for common string operations. R package version 1.4.0 https://CRAN. R-project.org/package=stringr.
- Wickham H., and Henry L.. . 2019. tidyr: easily tidy data with ‘spread()’ and ‘gather()’ functions. R package version 0.8.3 https://CRAN.R-project.org/package=tidyr.
- Wickham H., François R., Henry L., and Müller K.. . 2019. dplyr: a grammar of data manipulation. R package version 0.8.0.1 https://CRAN.R-project.org/package=dplyr.
- Woods H. A., Dillon M. E., and Pincebourde S.. . 2015. The roles of microclimatic diversity and of behavior in mediating the responses of ectotherms to climate change. J. Therm. Biol. 54: 86–97. [DOI] [PubMed] [Google Scholar]
- Xu J., and James R. R.. . 2012. Temperature stress affects the expression of immune response genes in the alfalfa leafcutting bee, Megachile rotundata. Insect Mol. Biol. 21: 269–280. [DOI] [PubMed] [Google Scholar]
- Yocum G. D., Rinehart J. P., and Kemp W. P.. . 2014. Cell position during larval development affects postdiapause development in Megachile rotundata (Hymenoptera: Megachilidae). Environ. Entomol. 43: 1045–1052. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data are available on Dryad (data will be made available upon acceptance of publication).