Abstract
This editorial refers to ‘Association between physical activity and risk of incident arrhythmias in 402 406 individuals: evidence from the UK Biobank cohort’†, by A.D. Elliott et al., on page 1479.
Atrial fibrillation (AF) is a significant and growing contributor to morbidity, mortality, and preventable stroke.1 A variety of approaches are being applied to develop better ways to combat this expanding epidemic. One strategy that has received great interest is the control of cardiac risk factors, increasingly recognized to be an important and targetable contributor to the likelihood of AF.1,2 An important weapon in the fight against uncontrolled risk factors is exercise, known to have beneficial effects against a range of adverse risk predictors such as obesity, diabetes, hypertension, and dyslipidaemia. However, there is evidence that high levels of exercise, especially endurance training, can promote AF.3 These contradictory effects have been difficult to sort out in epidemiological studies, most of which support a simple protective effect; however, the available studies have been limited by relatively small sample sizes and few patients in any given study that achieve regular high-level physical activity.
Results from the UK Biobank
In this issue of the European Heart Journal, Elliott et al. report the results of a study exploiting the very extensive and well-structured UK Biobank to provide a detailed and elegant analysis of the relationship between levels of physical activity and AF risk.4 Data were obtained from 402 406 individuals who completed self-reported physical activity screening questionnaires at baseline. Analysis of the information in the questionnaire allowed for the estimation of the total weekly physical activity at baseline (in MET-min/week), as well as the proportion attributable to vigorous physical activity. Participants were followed (median follow-up of 7 years) for arrhythmia endpoints with the use of national electronic health record databases. In addition to the occurrence of AF and atrial flutter (which are the focus here), they examined the occurrence of ventricular arrhythmias and bradyarrhythmic events.
As previously reported in the literature,2 the authors noted a predominantly protective effect of regular physical activity against AF/flutter events. However, their large database and detailed quantitative information on activity levels allowed the authors to identify sex-related complexity within the data. Women showed a more pronounced risk reduction with activity than men, as well as a protective effect over the entire range of physical activity levels examined, with statistically significant reductions in AF risk up to 2500 MET-min/week. For men, however, increasing physical activity was only protective against AF to a level of 1500 MET-min/week; beyond that, the protective effect was lost. Furthermore, there was a statistically significant interaction between sex and vigorous activity effects. In women, a beneficial anti-AF effect was observed for vigorous exercise, whereas for men, there was a progressive increase in AF risk with greater amounts of vigorous physical activity, with a statistically significant 12% risk enhancement at 5000 MET-min/week.
Physical activity has a complex relationship to AF risk
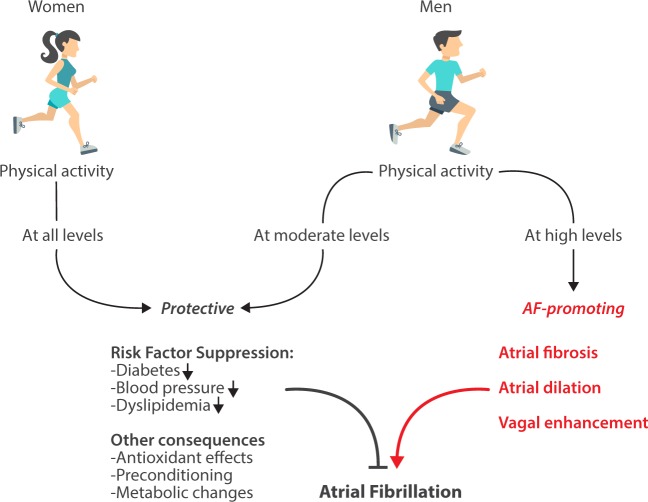
It is clear from the data of Elliott et al. that exercise has complex effects on the AF-promoting atrial substrate, with some actions being protective and others potentially harmful. This complicated relationship is illustrated in the Take home figure. Physical activity presents a duality of effects on AF susceptibility, with the net outcome depending on which set of actions is predominant. By controlling such prominent AF risk factors as obesity, diabetes, and hypertension,2 regular exercise reduces AF risk. In addition, exercise has other protective effects such as metabolic adaptations,5 cardioprotective pre-conditioning,6 and antioxidant properties.7 These and possibly other (unknown) actions underlie the ability of regular physical activity to reduce the AF likelihood.
Take home figure.
Schematic representing the opposing effects of physical activity on AF likelihood. Physical activity can either reduce or increase the risk of AF, with the balance of effects at high exercise levels being quite sex-dependent.
On the other hand, intense physical activity can produce adverse remodelling consequences that increase AF risk. Intense endurance training in animal models produces atrial fibrosis and dilatation, along with autonomic changes such as enhanced vagal tone that, in addition to causing the well-known slow heart rates of trained athletes, also promote the initiation of reentrant AF.8,9 Stopping training allows for some of the adverse effects (like the autonomic changes) to reverse, whereas others (like fibrosis and dilatation) recover much more slowly, if at all.9 Prominent among the underlying mechanisms is the engagement of inflammatory pathways like that associated with tumour necrosis factor-a (TNF-α).10,11 These systems activate profibrotic signaling that is responsible for atrial structural remodeling with intense exercise; the fibrosis-promotion and AF-substrate enhancement can be prevented by interventions that block TNF-α action.10,11.
A predominance of the detrimental remodelling consequences of exercise accounts for the well-described occurrence of AF in high-level athletes with no known heart disease;12 whereas the beneficial effects predominate in population studies involving few individuals that regularly engage in high-level physical training.13
Sex, physical activity, and AF
There is an increasing awareness of the important role of sex in controlling cardiac outcomes and the need for better data to guide clinical practice.14 It has long been suspected that the AF-promoting effects of exercise are predominantly expressed in males.9,12 The study of Elliott et al. is the clearest and most definitive demonstration to date of the sex-related dichotomy, represented in Take home figure, in the effects of physical activity level on AF likelihood. Among women, only a protective effect was noted, and was expressed over the entire range of activity levels. However, men showed clear protection with moderate physical activity. With regular vigorous exercise, men became more AF prone.
The mechanistic basis of this phenomenon is unknown and merits investigation. Are sex-related differences in hormonal background and their cardiovascular actions responsible? What about male–female differences in body habitus, a known determinant of AF risk?15 Sex-dependent differences in the cardiovascular or autonomic response to training? Discrepancies in the type or intensity of maximum exercise attained? Varying reprogramming of cardiovascular gene-expression in response to high-level physical activity, and/or the post-transcriptional control of critical proteins and signaling pathways, in men versus women? At this point, we do not know how to explain the sex-based differences in the response of AF risk to vigorous physical activity, but the answers might provide intriguing new insights into sex-dependent cardiovascular mechanisms and the determinants of AF occurrence.
Clinical implications
What are the clinical implications of the study of Elliott et al.? The first and foremost implication is that regular physical activity is protective against most forms of cardiac arrhythmias, including AF, and is to be encouraged. Secondly, regular involvement in very vigorous physical activity can promote AF, predominantly in men, and this may need to be considered in managing highly active patients with the arrhythmia. Finally, there is a lot we do not know about how sex can determine important biological responses, and much more research is needed in this area.
Funding
The Canadian Institutes of Health Research (Foundation Grant 148401) and the Heart and Stroke Foundation of Canada (G-16-00012708).
Conflict of interest: none declared.
Footnotes
doi: 10.1093/eurheartj/ehz897.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1. Andrade J, Khairy P, Dobrev D, Nattel S.. The clinical profile and pathophysiology of atrial fibrillation: relationships among clinical features, epidemiology, and mechanisms. Circ Res 2014;114:1453–1468. [DOI] [PubMed] [Google Scholar]
- 2. Lau DH, Nattel S, Kalman JM, Sanders P.. Modifiable risk factors and atrial fibrillation. Circulation 2017;136:583–596. [DOI] [PubMed] [Google Scholar]
- 3. Elliott AD, Linz D, Verdicchio CV, Sanders P.. Exercise and atrial fibrillation: prevention or causation? Heart Lung Circ 2018;27:1078–1085. [DOI] [PubMed] [Google Scholar]
- 4. Elliott AD, Linz D, Mishima R, Kadhim K, Gallagher C, Middledorp ME, Verdicchio CV, Hendriks JML, Lau DH, Gerche LA, Sanders P.. Association between physical activity and risk of incident arrhythmias in 402 406 individuals: evidence from the UK Biobank cohort. Eur Heart J 2020;41:1479–1486. [DOI] [PubMed] [Google Scholar]
- 5. Vega RB, Konhilas JP, Kelly DP, Leinwand LA.. Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab 2017;25:1012–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Borges JP, da Silva Verdoorn K.. Cardiac ischemia/reperfusion injury: the beneficial effects of exercise. Adv Exp Med Biol 2017;999:155–179. [DOI] [PubMed] [Google Scholar]
- 7. de Sousa CV, Sales MM, Rosa TS, Lewis JE, de Andrade RV, Simões HG.. The antioxidant effect of exercise: a systematic review and meta-analysis. Sports Med 2017;47:277–293. [DOI] [PubMed] [Google Scholar]
- 8. Benito B, Gay-Jordi G, Serrano-Mollar A, Guasch E, Shi Y, Tardif JC, Brugada J, Nattel S, Mont L.. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation 2011;123:13–22. [DOI] [PubMed] [Google Scholar]
- 9. Guasch E, Benito B, Qi X, Cifelli C, Naud P, Shi Y, Mighiu A, Tardif JC, Tadevosyan A, Chen Y, Gillis MA, Iwasaki YK, Dobrev D, Mont L, Heximer S, Nattel S.. Atrial fibrillation promotion by endurance exercise: demonstration and mechanistic exploration in an animal model. J Am Coll Cardiol 2013;62:68–77. [DOI] [PubMed] [Google Scholar]
- 10. Aschar-Sobbi R, Izaddoustdar F, Korogyi AS, Wang Q, Farman GP, Yang F, Yang W, Dorian D, Simpson JA, Tuomi JM, Jones DL, Nanthakumar K, Cox B, Wehrens XH, Dorian P, Backx PH.. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNFα. Nat Commun 2015;6:6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lakin R, Polidovitch N, Yang S, Guzman C, Gao X, Wauchop M, Burns J, Izaddoustdar F, Backx PH.. Inhibition of soluble TNFα prevents adverse atrial remodeling and atrial arrhythmia susceptibility induced in mice by endurance exercise. J Mol Cell Cardiol 2019;129:165–173. [DOI] [PubMed] [Google Scholar]
- 12. Flannery MD, Kalman JM, Sanders P, La Gerche A.. State of the art review: atrial fibrillation in athletes. Heart Lung Circ 2017;26:983–989. [DOI] [PubMed] [Google Scholar]
- 13. Tikkanen E, Gustafsson S, Ingelsson e. associations of fitness, physical activity, strength, and genetic risk with cardiovascular disease: longitudinal analyses in the UK Biobank Study. Circulation 2018;137:2583–2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tannenbaum C, Norris CM, McMurtry MS.. Sex-specific considerations in guidelines generation and application. Can J Cardiol 2019;35:598–605. [DOI] [PubMed] [Google Scholar]
- 15. Nattel S. Atrial fibrillation and body composition: is it fat or lean that ultimately determines the risk? J Am Coll Cardiol 2017;69:2498–2501. [DOI] [PubMed] [Google Scholar]