Key Points
Question
Is birth by cesarean delivery associated with obesity and type 2 diabetes in adulthood?
Findings
In this cohort study of 33 226 women, those born by cesarean delivery were 11% more likely to be obese as adults and had a 46% higher risk of developing type 2 diabetes than women born by vaginal delivery.
Meaning
This study found an association between being born by cesarean delivery and increased risks of obesity and type 2 diabetes in adulthood.
Abstract
Importance
Cesarean delivery is associated with an increased risk of childhood obesity in offspring. However, whether this increased risk also includes obesity-associated conditions remains unclear.
Objective
To evaluate the association of birth by cesarean delivery with offspring’s risks of obesity and type 2 diabetes in adulthood.
Design, Setting, and Participants
This prospective cohort study compared the incidence of obesity and type 2 diabetes between birth by cesarean delivery and vaginal delivery among 33 226 women participating in the Nurses’ Health Study II who were born between 1946 and 1964, with follow-up through the end of the 2013-2015 follow-up cycle. Participants’ mothers provided information on mode of delivery and pregnancy characteristics. Participants provided information every 2 years on weight and diagnosis of type 2 diabetes. Relative risks of obesity and type 2 diabetes were estimated using log-binomial and proportional hazards regression accounting for maternal body mass index and other confounding factors. Statistical analysis was performed from June 2017 to December 2019.
Exposure
Birth by cesarean delivery compared with birth by vaginal delivery.
Main Outcomes and Measures
Risk of obesity and incidence of type 2 diabetes.
Results
At baseline, the participants’ mean (SD) age was 33.8 (4.6) years (range, 24.0-44.0 years). A total of 1089 of the 33 226 participants (3.3%) were born by cesarean delivery. After 1 913 978 person-years of follow-up, 12 156 (36.6%) women were obese and 2014 (6.1%) had received a diagnosis of type 2 diabetes. Women born by cesarean delivery were more likely to be classified as obese and to have received a diagnosis of type 2 diabetes during follow-up. The multivariable-adjusted relative risk of obesity among women born by cesarean vs vaginal delivery was 1.11 (95% CI, 1.03-1.19). The multivariable-adjusted hazard ratio for type 2 diabetes among women born by cesarean vs vaginal delivery was 1.46 (95% CI, 1.18-1.81); this association remained significant after additional adjustment for participant’s own body mass index (relative risk, 1.34 [95% CI, 1.08-1.67]). These associations persisted when analyses were restricted to women at low risk of cesarean delivery based on maternal characteristics.
Conclusions and Relevance
This study suggests that women born by cesarean delivery may have a higher risk than women born by vaginal delivery of being obese and developing type 2 diabetes during adult life.
This cohort study evaluates the association of birth by cesarean delivery with offspring’s risks of obesity and type 2 diabetes in adulthood.
Introduction
More than 1.2 million cesarean deliveries are performed yearly in the United States, making it the most common inpatient surgical procedure and accounting for nearly one-third of births nationwide.1,2 Starting at approximately 2.6% of all births in the 1930s (prior to the widespread availability of penicillin) and remaining stable around 5% between the 1950s3 and 1970s,4 the cesarean delivery rate in the United States rose to 24% in 1986,4 reaching a peak of 33% in 2009 and stabilizing around 30% thereafter,1 with primary cesarean delivery accounting for 50% of the increasing rate.5 When indicated, cesarean delivery is a lifesaving intervention to mother and fetus.5,6,7,8 Like all surgical procedures, however, cesarean deliveries are not without risks. Women without medical or obstetric risk factors for obstetric complications undergoing a planned cesarean delivery at term experience a 3-fold greater risk of major morbidity—including greater risks of cardiac arrest, hysterectomy, puerperal infection, and thromboembolism—relative to comparable women undergoing vaginal deliveries.9 For newborns, the most common immediate risk with cesarean delivery is a higher frequency of respiratory complications.10,11 Moreover, many cesarean deliveries performed in the United States do not have a clear indication,12,13 raising concerns that the excess maternal and newborn morbidity and mortality may be largely preventable. With these concerns in mind, leading professional organizations have advocated for the prevention of primary cesarean delivery as a strategy to reduce their overall frequency.13,14
Increasing evidence also suggests that being born by cesarean delivery may have long-term consequences on the health of offspring.15,16,17,18 One of the most consistent findings to date is that birth by cesarean delivery is associated with a higher risk of childhood obesity. Two meta-analyses summarizing data from 24 studies have reported an increased risk of obesity for individuals born by cesarean delivery (pooled odds ratio, 1.33 [95% CI, 1.19-1.48]19 and 1.22 [95% CI, 1.05-1.42]20), with little difference for risk of obesity during childhood vs adolescence and suggestive evidence that the elevated risk persists in adult life (odds ratio, 1.50 [95% CI, 1.02-2.20]).19 Subsequent studies, including studies based on long-term follow-up of large populations with stringent control for maternal body mass index (BMI) and other confounders, replicated these results.21,22 It is not clear whether the increased risk of obesity may also be accompanied by an increased risk of metabolic syndrome or type 2 diabetes during adult life. In 1 study, birth by cesarean delivery was associated with higher BMI, total cholesterol, low-density lipoprotein cholesterol, and leptin levels at age 20 years,23 but other studies have not identified associations between cesarean delivery and markers of metabolic risk.24 To further investigate the long-term association of birth by cesarean delivery with obesity and metabolic risk, we evaluated the association of cesarean delivery with obesity and incidence of type 2 diabetes in an ongoing prospective cohort study followed up for nearly 3 decades.
Methods
Study Population
The Nurses’ Health Study II (NHS-II) is an ongoing prospective cohort study established in 1989 when 116 671 female nurses aged 24 to 44 years enrolled in the study. Participants completed a self-administered questionnaire regarding lifestyle factors, anthropometric variables, and disease prevalence at recruitment. Updated information is collected through follow-up questionnaires every 2 years. In 2001, 39 904 mothers of NHS-II participants completed a questionnaire regarding their pregnancy with their NHS-II participant daughter and additional information regarding their daughter’s infancy, forming the Nurses’ Mothers Cohort Study.25 After excluding participants who did not provide information on height or weight, were not born of a singleton pregnancy, and whose mothers did not provide information on delivery mode, the final study sample included 33 226 NHS-II participants born between 1946 and 1964, with follow-up through the end of the 2013-2015 follow-up cycle. The study was approved by the Harvard School of Public Health and Brigham and Women’s Hospital Institutional Review Boards. In follow-up questionnaires, participants are informed in writing of the risks and benefits of participating in the study, and of their rights as participants; returning a completed questionnaire is considered evidence of informed consent. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline.
Exposure Assessment
Mode of delivery (cesarean vs vaginal delivery) was reported by the participants’ mothers in 2001. A validation study conducted among 154 women enrolled in the Collaborative Perinatal Project found perfect maternal recall of cesarean delivery at a mean of 32 years after delivery.26
Ascertainment of Outcomes
At baseline, participants reported their height and weight, which are validly reported by adults,27 and updated this information every 2 years. Body mass index was calculated from these data as weight in kilograms divided by height in meters squared. We defined obesity (BMI ≥30) using the World Health Organization cutoffs.28
Participants reporting physician-diagnosed type 2 diabetes on follow-up questionnaires were mailed a supplemental questionnaire to confirm diagnoses. Cases of type 2 diabetes were confirmed based on the following American Diabetes Association criteria29: (1) 1 or more classic symptoms (excessive thirst, polyuria, weight loss, hunger, pruritus, or coma) plus elevated glucose levels (fasting plasma glucose concentration 126 mg/dL or more or random plasma glucose 200 mg/dL or more [to convert glucose to millimoles per liter, multiply by 0.0555]), or (2) no symptoms reported but 2 or more elevated plasma glucose concentrations on more than 1 occasion (fasting, 126 mg/dL or more; random, 200 mg/dL or more; or 2-hour oral glucose tolerance test, 200 mg/dL or more), or (3) treatment with insulin or an oral hypoglycemic agent. Before 1998, a fasting plasma glucose concentration of 140 mg/dL or more was used instead of 126 mg/dL or more for the diagnosis of diabetes according to the criteria of National Diabetes Data Group.30 In a validation study, a high consistency (98%) was observed comparing questionnaire-confirmed cases of type 2 diabetes against confirmation by medical record review.31 Participants were followed up from enrollment until the diagnosis of type 2 diabetes, death, or completion of the 2013-2015 follow-up cycle, whichever came first.
Assessment of Covariates
Information on race/ethnicity, maternal educational level, maternal prepregnancy BMI, gestational weight gain, diagnosis of gestational diabetes, preeclampsia or gestational hypertension, gestational age, birth weight, and smoking during pregnancy was reported by participants’ mothers in 2001. A validation study showed that long-term maternal recall of many pregnancy-related events, including diagnosis of pregnancy complications (hypertensive disorders of pregnancy, gestational diabetes, placental abruption, and placenta previa), offspring birth weight, gestational age at delivery, smoking status, prepregnancy anthropometry, and gestational weight gain, were very accurately reported.26 Region of residence at birth was reported by NHS-II participants. Maternal age at delivery was calculated as the difference, in years, between participants’ date of birth and their mother’s date of birth. Missing indicators were used whenever data were missing for a covariate.
Statistical Analysis
Statistical analysis was performed from June 2017 to December 2019. Maternal and offspring characteristics were presented according to delivery mode for which we used a Kruskal-Wallis test for continuous variables and a χ2 test for categorical variables when estimating differences between delivery modes. All P values were from 2-sided tests and results were deemed statistically significant at P < .05. To evaluate the association between cesarean delivery and offspring’s risk of obesity, we calculated relative risks (RRs) and 95% CIs using log-binomial regression models, or log-Poisson models when log-binomial models did not converge.2 To assess the association between cesarean delivery and offspring’s risk of type 2 diabetes, we calculated hazard ratios (HRs) using Cox proportional hazards regression models. We obtained crude and multivariable-adjusted estimates of these associations. The multivariable-adjusted models included terms for the following maternal variables: age at delivery (continuous, in years), race/ethnicity (white or other), educational level (≤high school or ≥college), prepregnancy BMI group (<18.5, 18.5-24.99, 25-29.99, or ≥30), gestational weight gain (continuous, in pounds), height (continuous, in inches), gestational diabetes (yes or no), preeclampsia (yes or no), pregnancy-induced hypertension (yes or no), year of birth (1946-1951, 1952-1961, or 1961-1963), gestational age at delivery (<37, 37-39, 40-42, or ≥43 weeks), birth weight group (<2.3, 2.3-3.1, 3.2-3.8, 3.9-4.4, ≥4.5 kg), smoking during pregnancy (no, first trimester, or second and third trimesters), and region of residence at birth (Northeast, Midwest, West, or South).
We conducted sensitivity analyses to address the possibility of residual confounding and evaluate the robustness of the findings. We fitted marginal structural models where the probability of cesarean delivery was assessed for each woman based on baseline characteristics and subsequently used to weight each observation using stabilized weights.32,33 In addition, we fitted models where we adjusted for maternal BMI with a linear and a quadratic term (instead of categories), restricted the obesity case definition to individuals who remained obese during follow-up, and allowed obesity status to vary over time. When assessing the association of cesarean delivery with the risk of type 2 diabetes, we additionally adjusted for offspring breastfeeding and fit log-binomial models for risk. To evaluate whether BMI explained any association between cesarean delivery and type 2 diabetes, the multivariable-adjusted model was additionally adjusted for offspring BMI during follow-up. We also conducted analyses restricted to participants in low-risk categories for cesarean delivery based on maternal characteristics (ie, maternal prepregnancy BMI <25, no gestational diabetes, no hypertensive disorders of pregnancy, no smoking during pregnancy, maternal age <30 years, gestational age at delivery between 37 and 42 weeks, and birth weight between 2.3 and 4.4 kg). All analyses were conducted using SAS software, version 9.2 (SAS Institute Inc).
Results
At baseline, the participants’ mean (SD) age was 33.8 (4.6) years (range, 24.0-44.0 years). Of the 33 226 participants in the study, 1089 (3.3%) were born by cesarean delivery. Participants’ mothers who delivered by cesarean method had a higher mean (SD) prepregnancy BMI than those who delivered vaginally (21.7 [3.0] vs 21.2 [2.5]), were older (mean [SD] age at delivery, 28.2 [5.6] vs 26.2 [4.9] years), and were more likely to have preeclampsia (54 of 1089 [5.0%] vs 1018 of 32 137 [3.2%]), pregnancy-induced hypertension (55 of 1089 [5.1%] vs 1089 of 32 137 [3.4%]), preterm birth (64 [9.2%] vs 757 [4.1%]), and low birth weight (94 of 1089 [8.6%] vs 1903 of 32 137 [5.9%]) (Table 1). They were also less likely than those who delivered vaginally to smoke during pregnancy (249 of 1089 [22.9%] vs 8394 of 32 137 [26.1%]) and to breastfeed their participant daughters (350 of 1089 [32.1%] vs 14 820 of 32 137 [46.1%]). We documented 12 156 cases of obesity and 2014 new cases of type 2 diabetes during 1 913 978 person-years of follow-up. The cumulative risk of obesity through the end of follow-up was 36.5% (11 722 of 32 137) among women born by vaginal delivery and 39.9% (434 of 1089) among women born by cesarean delivery. The incidence of type 2 diabetes per 10 000 person-years was 10.4 among participants born by vaginal delivery and 14.1 among participants born by cesarean delivery.
Table 1. Maternal and Offspring Characteristics According to Mode of Delivery.
| Characteristic | All participants (N = 33 226) | Mode of delivery | P valuea | |
|---|---|---|---|---|
| Vaginal (n = 32 137) | Cesarean (n = 1089) | |||
| Maternal characteristics | ||||
| Age at delivery, mean (SD), y | 26.3 (4.9) | 26.2 (4.9) | 28.2 (5.6) | <.001 |
| Prepregnancy BMI, mean (SD) | 21.2 (2.5) | 21.2 (2.5) | 21.7 (3.0) | <.001 |
| Height, mean (SD), cm | 163.3 (6.1) | 163.6 (6.1) | 160.8 (6.4) | .06 |
| White race, No. (%) | 32 321 (97.3) | 31 265 (97.3) | 1056 (97.0) | .53 |
| Geographic region of birth, No. (%) | ||||
| Northeast | 10 932 (32.9) | 10 568 (32.9) | 364 (33.4) | .32 |
| Midwest | 12 081 (36.4) | 11 705 (36.4) | 376 (34.5) | |
| West | 3120 (9.4) | 3009 (9.4) | 121 (11.1) | |
| South | 4061 (12.2) | 3932 (12.2) | 129 (11.9) | |
| Missing | 3022 (9.1) | 2923 (9.1) | 99 (9.1) | |
| Gestational diabetes, No. (%) | 140 (0.4) | 133 (0.4) | 7 (0.6) | .14 |
| Preeclampsia, No. (%) | 1072 (3.2) | 1018 (3.2) | 54 (5.0) | <.001 |
| Gestational hypertension, No. (%) | 1144 (3.4) | 1089 (3.4) | 55 (5.1) | <.001 |
| Smoking during pregnancy, No. (%) | ||||
| No | 24 583 (74.0) | 23 743 (73.9) | 840 (77.1) | .05 |
| Yes, first trimester | 1233 (3.7) | 1197 (3.7) | 36 (3.3) | |
| Yes, second and third trimesters | 7410 (22.3) | 7197 (22.4) | 213 (19.6) | |
| Educational level, No. (%) | ||||
| ≤High school degree | 21 036 (63.3) | 20 371 (63.4) | 665 (61.1) | .10 |
| Some college or college degree | 12 071 (36.3) | 11 654 (36.3) | 417 (38.3) | |
| Missing | 119 (0.4) | 112 (0.4) | 7 (0.6) | |
| Gestational weight gain, No. (%) | ||||
| <9.1 kg | 10 920 (32.9) | 10 526 (32.8) | 394 (36.2) | .06 |
| ≥9.1 kg | 19 699 (59.3) | 19 088 (59.4) | 611 (56.1) | |
| Missing | 2607 (7.9) | 2523 (7.9) | 84 (7.7) | |
| Offspring characteristics | ||||
| Year of birth, No. (%) | ||||
| 1946-1951 | 10 382 (31.3) | 10 096 (31.4) | 286 (26.3) | <.001 |
| 1952-1961 | 19 634 (59.1) | 18 969 (59.0) | 665 (61.1) | |
| 1962-1964 | 3210 (9.7) | 3072 (9.6) | 138 (12.7) | |
| Gestational age at delivery, No. (%)b | ||||
| <37 wk | 821 (4.3) | 757 (4.1) | 64 (9.2) | <.001 |
| 37-39 wk | 8954 (46.8) | 8498 (46.1) | 456 (65.7) | |
| 40-42 wk | 7220 (37.7) | 7111 (38.6) | 109 (15.7) | |
| ≥43 wk | 2136 (11.2) | 2071 (11.3) | 65 (9.4) | |
| Birth weight group, No. % | ||||
| <2.3 kg | 1997 (6.0) | 1903 (5.9) | 94 (8.6) | <.001 |
| 2.3-3.1 kg | 10 731 (32.3) | 10 313 (32.1) | 418 (38.4) | |
| 3.2-3.8 kg | 18 657 (56.2) | 18 135 (56.4) | 522 (47.9) | |
| 3.9-4.4 kg | 1544 (4.7) | 1495 (4.7) | 49 (4.5) | |
| ≥4.5 kg | 297 (0.9) | 291 (0.9) | 6 (0.6) | |
| Breastfeeding duration, No. (%) | ||||
| Never | 17 837 (53.7) | 17 103 (53.2) | 734 (67.4) | <.001 |
| ≤6 mo | 11 419 (34.4) | 11 159 (34.7) | 269 (24.7) | |
| >6 mo | 3751 (11.3) | 3661 (11.4) | 81 (7.4) | |
| Missing | 219 (0.7) | 214 (0.7) | 5 (0.5) | |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
The 2-sample t test was used to test the difference of continuous variables and the χ2 test was used to test the difference of categorical variables.
For 19 131 participants.
Being born by cesarean delivery was associated with a higher risk of obesity (RR, 1.09 [95% CI, 1.01-1.18]) (Table 2). This association persisted in multivariable-adjusted analyses (adjusted RR, 1.11 [95% CI, 1.03-1.19]) and was similar across strata of age. Results were also similar when analyzing data using marginal structural models (RR, 1.11 [95% CI, 1.03-1.19]), when maternal prepregnancy BMI was modeled as a continuous variable (RR, 1.11 [95% CI, 1.03-1.19]), when case definition was restricted to women whose BMI remained ≥30 in all follow-up cycles after obesity was first documented (RR, 1.13 [95% CI, 1.05-1.22]), and when obesity status was allowed to change in each follow-up cycle (RR, 1.18 [95% CI, 1.08-1.29]).
Table 2. Association Between Mode of Delivery With Obesity in Offspring Among 33 226 Women.
| Mode of delivery | Obese participants, No./total No. (%)a | Offspring obesity | |
|---|---|---|---|
| RR (95% CI) | P value | ||
| Overall | 12 156/33 226 (36.6) | NA | NA |
| Vaginal | 11 722/32 137 (36.5) | 1 [Reference] | NA |
| Cesarean | 434/1089 (39.9) | ||
| Crude | 1.09 (1.01-1.18) | .02 | |
| Adjustedb | 1.11 (1.03-1.19) | .005 | |
| Additional analysis | |||
| Marginal structural model estimate | 434/1089 (39.9) | 1.11 (1.03-1.19) | <.001 |
| Maternal BMI as continuous variablec | 434/1089 (39.9) | 1.11 (1.03-1.19) | .006 |
| Allowing obesity status to change over time | 434/1089 (39.9) | 1.18 (1.08-1.29) | <.001 |
| Women who remained obese during follow-up (n = 31 867) | 391/1046 (37.4) | 1.13 (1.05-1.22) | .002 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); NA, not applicable; RR, relative risk.
Body mass index of 30 or higher.
Adjusted models included terms for maternal age at delivery, race/ethnicity (white or other), maternal educational level (≥high school or ≥college), maternal prepregnancy BMI group (<18.5, 18.5-24.99, 25-29.99, or ≥30), gestational weight gain (<9.1 kg or ≥9.1 kg), maternal height, gestational diabetes (yes or no), preeclampsia (yes or no), pregnancy-induced hypertension (yes or no), year of birth (1946-1951, 1952-1961, or 1962-1964), gestational age at delivery (<37, 37-39, 40-42, or ≥43 weeks), birth weight group (<2.3, 2.3-3.1, 3.2-3.8, 3.9-4.4, or ≥4.5 kg), smoking during pregnancy (no, first trimester, second and third trimesters, or current), and region of residence at birth (Northeast, Midwest, West, or South).
Adjusted model modeling prepregnancy BMI as a continuous variable with a linear term and a quadratic term.
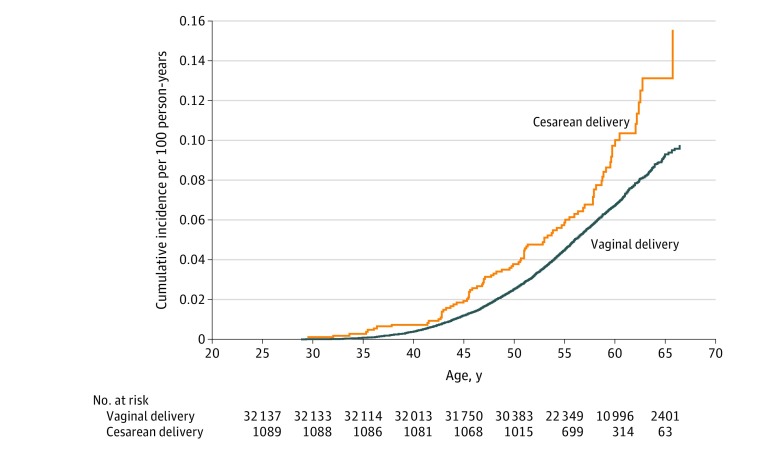
The incidence of type 2 diabetes was also higher among women born by cesarean delivery than among women born by vaginal delivery (Figure 1). The HR for type 2 diabetes in women born by cesarean vs vaginal delivery was 1.42 (95% CI, 1.14-1.76) (Table 3). This association persisted after multivariable adjustment (HR, 1.46 [95% CI, 1.18-1.81]), when analyzing data using marginal structural models (HR, 1.19 [95% CI, 0.96-1.50]), when maternal prepregnancy BMI was modeled as a continuous variable (HR, 1.47 [95% CI, 1.18-1.82]), and when risk of obesity was modeled using log-binomial models (HR, 1.49 [95% CI, 1.19-1.87]). Adjustment for breastfeeding did not change the association (HR, 1.45 [95% CI, 1.17-1.80]). Adjustment for updated offspring BMI status attenuated the association by 12% but the association remained statistically significant (HR, 1.34 [95% CI, 1.08-1.67]).
Figure 1. Cumulative Incidence of Type 2 Diabetes Among Women Born by Vaginal vs Cesarean Delivery.
Table 3. Association Between Mode of Delivery With the Risk of Type 2 Diabetes.
| Characteristic | HR (95%CI) | P value | |
|---|---|---|---|
| Vaginal delivery | Cesarean delivery | ||
| Cases of type 2 diabetes, No. | 1927 | 87 | NA |
| Person-time, Person-years | 1 852 102 | 61 876 | NA |
| Incidence rate of type 2 diabetes, per 10 000 person-years | 10.4 | 14.1 | NA |
| Crude | 1 [Reference] | 1.42 (1.14-1.76) | .002 |
| Adjusteda | 1 [Reference] | 1.46 (1.18-1.81) | .001 |
| Additional analyses | |||
| Marginal structural model estimate | 1 [Reference] | 1.19 (0.96-1.50) | .12 |
| Treating maternal BMI as continuous variableb | 1 [Reference] | 1.47 (1.18-1.82) | .001 |
| Log-binomial model for risk ratio | 1 [Reference] | 1.49 (1.19-1.87) | <.001 |
| Additional adjustment for offspring characteristics | |||
| Breastfeeding | 1 [Reference] | 1.45 (1.17-1.80) | .001 |
| Updated BMI | 1 [Reference] | 1.34 (1.08-1.67) | .008 |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); HR, hazard ratio; NA, not applicable.
Adjusted models included terms for maternal age at delivery, race/ethnicity (white or other), maternal educational level (≥high school or ≥college), maternal prepregnancy BMI group (<18.5, 18.5-24.99, 25-29.99, or ≥30), gestational weight gain (<9.1 kg or ≥9.1 kg), maternal height, gestational diabetes (yes or no), preeclampsia (yes or no), pregnancy-induced hypertension (yes or no), year of birth (1946-1951, 1952-1961, or 1962-1964), gestational age at delivery (<37, 37-39, 40-42, or ≥43 weeks), birth weight group (<2.3, 2.3-3.1, 3.2-3.8, 3.9-4.4, or ≥4.5 kg), smoking during pregnancy (no, first trimester, second and third trimesters, or current), and region of residence at birth (Northeast, Midwest, West, or South).
Adjusted model modeling prepregnancy BMI as a continuous variable with a linear term and a quadratic term.
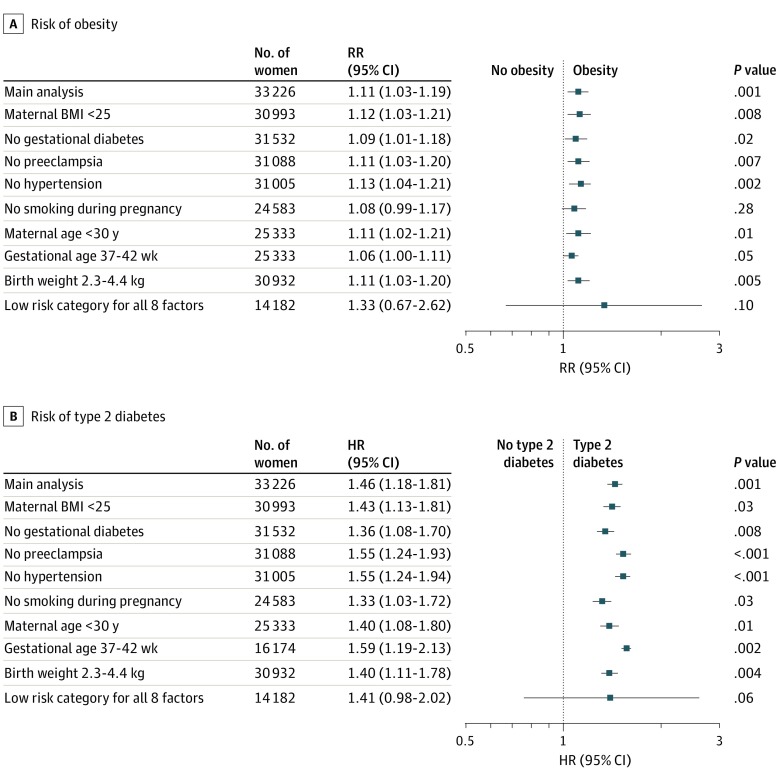
The associations of cesarean delivery with risks of obesity and type 2 diabetes were of comparable magnitude across each of the low-risk categories for cesarean delivery based on maternal characteristics, separately and when all were simultaneously considered (Figure 2). However, estimates were no longer statistically significant when analyses were restricted to women in all 8 low-risk groups.
Figure 2. Associations of Birth by Cesarean Delivery With Risks of Offspring Obesity and Type 2 Diabetes Among Women in Low-Risk Categories for Cesarean Delivery Based on Maternal Characteristics.
A, Risk of obesity among offspring in adulthood. B, Risk of type 2 diabetes among offspring in adulthood. BMI indicates body mass index (calculated as weight in kilograms divided by height in meters squared); HR, hazard ratio; and RR, relative risk.
Discussion
In this prospective cohort of women aged of 24 to 66 years during the entire follow-up period, we found that being born by cesarean delivery was associated with an 11% higher risk of obesity and a 46% higher risk of type 2 diabetes. The association of cesarean delivery with type 2 diabetes persisted after adjustment for BMI during follow-up, suggesting that the association with type 2 diabetes is only partly mediated by elevated BMI. Findings were consistent across multiple strategies to account for confounding, suggesting that these associations are consistent with a true biological association of birth by cesarean delivery. Whether these findings are applicable to men or to individuals born today, when cesarean delivery rates are substantially higher, is uncertain.
Although the mechanisms for the development of obesity and type 2 diabetes in adulthood among individuals born by cesarean delivery remains unclear, growing evidence points to the hygiene theory and changes in the offspring’s gut microbiota.34,35 Gut microbiota can modulate host energy harvest from the diet and bacterial lipopolysaccharide-induced chronic inflammation. Thus, changes in gut microbiota can be associated with host adiposity and glucose metabolism.36 Mode of delivery is associated with the diversity in gut microbiota of the offspring.37,38,39,40 Vaginally delivered neonates are rapidly colonized by microbes from their mother’s birth canal and feces, while neonates delivered by cesarean delivery are colonized by environmental microbes.36 As a result, neonates born by cesarean delivery harbor a less diverse gut microbiota, particularly less Bifidobacteria and less Bacteroides spp, which have shown to be protective against obesity.36,40 Differences in gut microbiota composition by mode of delivery have been described in infants40,41 and children up to 7 years of age.42 Whether these differences are sustained long-term is unknown. Differences in DNA methylation patterns between children born by cesarean delivery and those born by vaginal delivery have also been proposed as a biological explanation underlying long-term health outcomes of cesarean delivery, but data are scarce.43,44,45,46 Higher global DNA methylation has been reported in infants born by cesarean delivery,43,44 including a study in which a genome-wide analysis identified 343 loci that were nominally (P < .01) differentially methylated between infants born by cesarean delivery and those born by vaginal delivery.44 Other studies, however, have found no difference in DNA methylation between children born by vaginal delivery or cesarean delivery.45,46
We found that, compared with those born by vaginal delivery, offspring born by cesarean delivery had an 11% higher risk of obesity and a 46% higher risk of type 2 diabetes in adulthood. Our finding of an association with obesity in adulthood is in agreement with the results of 2 meta-analyses, which reported an increased risk in offspring obesity of 22%20 and 50%,19 and with more recent prospective cohorts that included young adults and reported associations of similar magnitude to that reported here.21 However, to our knowledge, an association of birth by cesarean delivery with risk of type 2 diabetes has not been previously described. Two prospective cohort studies24,47 previously reported that cesarean delivery was not associated with metabolic risk factors in offspring after 20 to 23 years of follow-up. On the other hand, a recent prospective cohort study23 found that, compared with those born by vaginal delivery, young adults born by cesarean delivery showed a more adverse cardiometabolic risk profile. More important, participants in these 3 studies were all in their early 20s and the prevalence of type 2 diabetes may not have been sufficiently high to identify differences in prevalence by mode of delivery. In fact, the incidence data from our study (Figure 1) suggest that differences in risk of type 2 diabetes may not become evident until the fourth decade of life. Although, to our knowledge, our study has the largest sample size and longest duration of follow-up of all studies addressing the association of cesarean delivery with type 2 diabetes to date, these findings should be interpreted with caution given that it is the first time that an association with type 2 diabetes is reported and very few studies have examined the association with obesity-related metabolic abnormalities. Further research is needed to replicate the association with type 2 diabetes and address the biological mechanisms underlying the association of cesarean delivery with the increased risk of offspring obesity and type 2 diabetes in adulthood.
Limitations and Strengths
Our study has some limitations. The most important limitation is the lack of data on indications for cesarean delivery. Historical data3 suggest that the most common indications of cesarean delivery during the time period in which participants in this study were born were cephalopelvic disproportion, previous cesarean delivery, and placenta previa, jointly accounting for approximately 80% of cesarean deliveries.3 These data further suggest that no less than 30% and as much as half of all cesarean deliveries during this period were planned and presumably performed before the onset of labor. Although we cannot determine whether these figures apply to our study participants, they do highlight the importance of our analyses restricted to low-risk groups, as these analyses may have eliminated many cesarean deliveries performed owing to cephalopelvic disproportion. Moreover, other common indications of cesarean delivery during this period, namely labor arrest and breech presentation, are not known risk factors for offspring obesity and are therefore unlikely to be important confounders of the association of cesarean delivery with offspring obesity or type 2 diabetes. The individuals in this study are nurses participating in a long-term health study; while this facilitated long-term follow-up and the prospective collection of high-quality detailed data, it may hamper the generalizability of the findings to the general population. The fact that maternal report of mode of birth and other pregnancy-related information was retrospective and thus subject to recall bias could be reasonably identified as a major potential limitation. However, cesarean delivery rates in this cohort are comparable to cesarean delivery rates in the general population at the time participants were born4 and, as previously mentioned, past studies have shown perfect recall of cesarean delivery and highly accurate reporting of other pregnancy events and important potential confounders (including smoking status during pregnancy, prepregnancy weight, and gestational weight gain) decades later.26 An additional limitation is the underrepresentation of minorities in our cohort. However, there are no a priori reasons to believe these associations would differ across race or ethnicity. We also acknowledge the possibility of residual confounding owing to the lack of information about potentially relevant covariates such as the use of antibiotics during pregnancy or intrapartum and birth order. We also lacked information on offspring gut microbiota, DNA methylation patterns, or other potential biological mediators to further explore the underlying mechanisms.
It is also important to consider the generalizability of the findings to current practice in light of the differences in cesarean delivery rates at the time of the study and today. As this is an observational study, further research is needed before causality can be assumed. Nevertheless, the current study has multiple strengths and was able to address the most salient limitations of previous studies. The prospective study design, large sample size, and long-term follow-up allowed us to examine the associations of cesarean delivery and the risk of obesity and type 2 diabetes of the offspring in adulthood, and to provide precise estimates of these associations. The availability of key prepregnancy and pregnancy information allowed for multiple sensitivity analyses aimed at addressing residual confounding.
Conclusions
We observed associations of cesarean delivery with increased risks of obesity and type 2 diabetes of the offspring in adulthood. Most important, the association remained significant in most of the analyses restricted to participants in low-risk categories for cesarean delivery based on maternal characteristics. Greater evidence from large, prospective studies with high-quality data on prepregnancy, pregnancy, and delivery information (particularly information regarding the timing of cesarean delivery relative to the onset of labor or rupture of membranes), as well as data from studies with sibling pairs discordant in delivery mode, is needed to address if these findings are generalizable and to investigate whether offspring born by cesarean delivery are at a higher risk of developing other adverse metabolic and cardiovascular outcomes.
References
- 1.Martin JA, Hamilton BE, Osterman MJK, Driscoll AK. Births: final data for 2018. Natl Vital Stat Rep. 2019;68(13):-. [PubMed] [Google Scholar]
- 2.Healthcare Cost and Utilization Project McDermott KW, Freeman WJ, Elixhauser A. Statistical brief #233: overview of operating room procedures during inpatient stays in U.S. hospitals, 2014. Accessed August 22, 2018. https://www.hcup-us.ahrq.gov/reports/statbriefs/sb233-Operating-Room-Procedures-United-States-2014.pdf [PubMed]
- 3.Woodman M. Mid-century trends in cesarean section. N Engl J Med. 1950;243(14):528-530. doi: 10.1056/NEJM195010052431404 [DOI] [PubMed] [Google Scholar]
- 4.Pokras R, Kozak LJ, McCarthy E, Graves EJ. Trends in hospital utilization, 1965-86. Am J Public Health. 1990;80(4):488-490. doi: 10.2105/AJPH.80.4.488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tita AT. When is primary cesarean appropriate: maternal and obstetrical indications. Semin Perinatol. 2012;36(5):324-327. doi: 10.1053/j.semperi.2012.04.014 [DOI] [PubMed] [Google Scholar]
- 6.Hannah ME, Hannah WJ, Hewson SA, Hodnett ED, Saigal S, Willan AR; Term Breech Trial Collaborative Group . Planned caesarean section versus planned vaginal birth for breech presentation at term: a randomised multicentre trial. Lancet. 2000;356(9239):1375-1383. doi: 10.1016/S0140-6736(00)02840-3 [DOI] [PubMed] [Google Scholar]
- 7.Humberg A, Härtel C, Paul P, et al. ; German Neonatal Network (GNN) . Delivery mode and intraventricular hemorrhage risk in very-low-birth-weight infants: observational data of the German Neonatal Network. Eur J Obstet Gynecol Reprod Biol. 2017;212:144-149. doi: 10.1016/j.ejogrb.2017.03.032 [DOI] [PubMed] [Google Scholar]
- 8.Simpson LL. When is primary cesarean appropriate: fetal indications. Semin Perinatol. 2012;36(5):328-335. doi: 10.1053/j.semperi.2012.04.015 [DOI] [PubMed] [Google Scholar]
- 9.Liu S, Liston RM, Joseph KS, Heaman M, Sauve R, Kramer MS; Maternal Health Study Group of the Canadian Perinatal Surveillance System . Maternal mortality and severe morbidity associated with low-risk planned cesarean delivery versus planned vaginal delivery at term. CMAJ. 2007;176(4):455-460. doi: 10.1503/cmaj.060870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hansen AK, Wisborg K, Uldbjerg N, Henriksen TB. Risk of respiratory morbidity in term infants delivered by elective caesarean section: cohort study. BMJ. 2008;336(7635):85-87. doi: 10.1136/bmj.39405.539282.BE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loebel G, Zelop CM, Egan JF, Wax J. Maternal and neonatal morbidity after elective repeat Cesarean delivery versus a trial of labor after previous Cesarean delivery in a community teaching hospital. J Matern Fetal Neonatal Med. 2004;15(4):243-246. doi: 10.1080/14767050410001668653 [DOI] [PubMed] [Google Scholar]
- 12.Barber EL, Lundsberg LS, Belanger K, Pettker CM, Funai EF, Illuzzi JL. Indications contributing to the increasing cesarean delivery rate. Obstet Gynecol. 2011;118(1):29-38. doi: 10.1097/AOG.0b013e31821e5f65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Boyle A, Reddy UM, Landy HJ, Huang CC, Driggers RW, Laughon SK. Primary cesarean delivery in the United States. Obstet Gynecol. 2013;122(1):33-40. doi: 10.1097/AOG.0b013e3182952242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American College of Obstetricians and Gynecologists; Society for Maternal-Fetal Medicine . Obstetric care consensus no. 1: safe prevention of the primary cesarean delivery. Obstet Gynecol. 2014;123(3):693-711. doi: 10.1097/01.AOG.0000444441.04111.1d [DOI] [PubMed] [Google Scholar]
- 15.Black M, Bhattacharya S, Philip S, Norman JE, McLernon DJ. Planned cesarean delivery at term and adverse outcomes in childhood health. JAMA. 2015;314(21):2271-2279. doi: 10.1001/jama.2015.16176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Blustein J, Liu J. Time to consider the risks of caesarean delivery for long term child health. BMJ. 2015;350:h2410. doi: 10.1136/bmj.h2410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cho CE, Norman M. Cesarean section and development of the immune system in the offspring. Am J Obstet Gynecol. 2013;208(4):249-254. doi: 10.1016/j.ajog.2012.08.009 [DOI] [PubMed] [Google Scholar]
- 18.Nielsen NM, Bager P, Stenager E, et al. Cesarean section and offspring’s risk of multiple sclerosis: a Danish nationwide cohort study. Mult Scler. 2013;19(11):1473-1477. doi: 10.1177/1352458513480010 [DOI] [PubMed] [Google Scholar]
- 19.Li HT, Zhou YB, Liu JM. The impact of cesarean section on offspring overweight and obesity: a systematic review and meta-analysis. Int J Obes (Lond). 2013;37(7):893-899. doi: 10.1038/ijo.2012.195 [DOI] [PubMed] [Google Scholar]
- 20.Darmasseelane K, Hyde MJ, Santhakumaran S, Gale C, Modi N. Mode of delivery and offspring body mass index, overweight and obesity in adult life: a systematic review and meta-analysis. PLoS One. 2014;9(2):e87896. doi: 10.1371/journal.pone.0087896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yuan C, Gaskins AJ, Blaine AI, et al. Association between cesarean birth and risk of obesity in offspring in childhood, adolescence, and early adulthood. JAMA Pediatr. 2016;170(11):e162385. doi: 10.1001/jamapediatrics.2016.2385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pei Z, Heinrich J, Fuertes E, et al. ; Influences of Lifestyle-Related Factors on the Immune System and the Development of Allergies in Childhood plus Air Pollution and Genetics (LISAplus) Study Group . Cesarean delivery and risk of childhood obesity. J Pediatr. 2014;164(5):1068-1073.e2. doi: 10.1016/j.jpeds.2013.12.044 [DOI] [PubMed] [Google Scholar]
- 23.Hansen S, Halldorsson TI, Olsen SF, et al. Birth by cesarean section in relation to adult offspring overweight and biomarkers of cardiometabolic risk. Int J Obes (Lond). 2018;42(1):15-19. doi: 10.1038/ijo.2017.175 [DOI] [PubMed] [Google Scholar]
- 24.Bernardi JR, Pinheiro TV, Mueller NT, et al. Cesarean delivery and metabolic risk factors in young adults: a Brazilian birth cohort study. Am J Clin Nutr. 2015;102(2):295-301. doi: 10.3945/ajcn.114.105205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michels KB, Willett WC, Graubard BI, et al. A longitudinal study of infant feeding and obesity throughout life course. Int J Obes (Lond). 2007;31(7):1078-1085. doi: 10.1038/sj.ijo.0803622 [DOI] [PubMed] [Google Scholar]
- 26.Tomeo CA, Rich-Edwards JW, Michels KB, et al. Reproducibility and validity of maternal recall of pregnancy-related events. Epidemiology. 1999;10(6):774-777. doi: 10.1097/00001648-199911000-00022 [DOI] [PubMed] [Google Scholar]
- 27.McAdams MA, Van Dam RM, Hu FB. Comparison of self-reported and measured BMI as correlates of disease markers in US adults. Obesity (Silver Spring). 2007;15(1):188-196. doi: 10.1038/oby.2007.504 [DOI] [PubMed] [Google Scholar]
- 28.World Health Organization Physical Status: The Use and Interpretation of Anthropometry: Report of a WHO Expert Committee, WHO Technical Report Series No. 854. World Health Organization; 1995. [PubMed] [Google Scholar]
- 29.Expert Committee on the Diagnosis and Classification of Diabetes Mellitus Report of the Expert Committee on the Diagnosis and Classification of Diabetes Mellitus. Diabetes Care. 1997;20(7):1183-1197. doi: 10.2337/diacare.20.7.1183 [DOI] [PubMed] [Google Scholar]
- 30.National Diabetes Data Group Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. Diabetes. 1979;28(12):1039-1057. doi: 10.2337/diab.28.12.1039 [DOI] [PubMed] [Google Scholar]
- 31.Manson JE, Rimm EB, Stampfer MJ, et al. Physical activity and incidence of non–insulin-dependent diabetes mellitus in women. Lancet. 1991;338(8770):774-778. doi: 10.1016/0140-6736(91)90664-B [DOI] [PubMed] [Google Scholar]
- 32.Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656-664. doi: 10.1093/aje/kwn164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu S, Ross C, Raebel MA, Shetterly S, Blanchette C, Smith D. Use of stabilized inverse propensity scores as weights to directly estimate relative risk and its confidence intervals. Value Health. 2010;13(2):273-277. doi: 10.1111/j.1524-4733.2009.00671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greiner T, Bäckhed F. Effects of the gut microbiota on obesity and glucose homeostasis. Trends Endocrinol Metab. 2011;22(4):117-123. doi: 10.1016/j.tem.2011.01.002 [DOI] [PubMed] [Google Scholar]
- 35.Neu J, Rushing J. Cesarean versus vaginal delivery: long-term infant outcomes and the hygiene hypothesis. Clin Perinatol. 2011;38(2):321-331. doi: 10.1016/j.clp.2011.03.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Musso G, Gambino R, Cassader M. Obesity, diabetes, and gut microbiota: the hygiene hypothesis expanded? Diabetes Care. 2010;33(10):2277-2284. doi: 10.2337/dc10-0556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biasucci G, Benenati B, Morelli L, Bessi E, Boehm G. Cesarean delivery may affect the early biodiversity of intestinal bacteria. J Nutr. 2008;138(9):1796S-1800S. doi: 10.1093/jn/138.9.1796S [DOI] [PubMed] [Google Scholar]
- 38.Dominguez-Bello MG, Costello EK, Contreras M, et al. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci U S A. 2010;107(26):11971-11975. doi: 10.1073/pnas.1002601107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Huurre A, Kalliomäki M, Rautava S, Rinne M, Salminen S, Isolauri E. Mode of delivery—effects on gut microbiota and humoral immunity. Neonatology. 2008;93(4):236-240. doi: 10.1159/000111102 [DOI] [PubMed] [Google Scholar]
- 40.Sordillo JE, Zhou Y, McGeachie MJ, et al. Factors influencing the infant gut microbiome at age 3-6 months: findings from the ethnically diverse Vitamin D Antenatal Asthma Reduction Trial (VDAART). J Allergy Clin Immunol. 2017;139(2):482-491. doi: 10.1016/j.jaci.2016.08.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Grönlund MM, Lehtonen OP, Eerola E, Kero P. Fecal microflora in healthy infants born by different methods of delivery: permanent changes in intestinal flora after cesarean delivery. J Pediatr Gastroenterol Nutr. 1999;28(1):19-25. doi: 10.1097/00005176-199901000-00007 [DOI] [PubMed] [Google Scholar]
- 42.Salminen S, Gibson GR, McCartney AL, Isolauri E. Influence of mode of delivery on gut microbiota composition in seven year old children. Gut. 2004;53(9):1388-1389. doi: 10.1136/gut.2004.041640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schlinzig T, Johansson S, Gunnar A, Ekström TJ, Norman M. Epigenetic modulation at birth—altered DNA-methylation in white blood cells after Caesarean section. Acta Paediatr. 2009;98(7):1096-1099. doi: 10.1111/j.1651-2227.2009.01371.x [DOI] [PubMed] [Google Scholar]
- 44.Almgren M, Schlinzig T, Gomez-Cabrero D, et al. Cesarean delivery and hematopoietic stem cell epigenetics in the newborn infant: implications for future health? Am J Obstet Gynecol. 2014;211(5):502.e1-502.e8. doi: 10.1016/j.ajog.2014.05.014 [DOI] [PubMed] [Google Scholar]
- 45.Franz MB, Poterauer M, Elhenicky M, et al. Global and single gene DNA methylation in umbilical cord blood cells after elective caesarean: a pilot study. Eur J Obstet Gynecol Reprod Biol. 2014;179:121-124. doi: 10.1016/j.ejogrb.2014.05.038 [DOI] [PubMed] [Google Scholar]
- 46.Virani S, Dolinoy DC, Halubai S, et al. Delivery type not associated with global methylation at birth. Clin Epigenetics. 2012;4(1):8. doi: 10.1186/1868-7083-4-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horta BL, Gigante DP, Lima RC, Barros FC, Victora CG. Birth by caesarean section and prevalence of risk factors for non-communicable diseases in young adults: a birth cohort study. PLoS One. 2013;8(9):e74301. doi: 10.1371/journal.pone.0074301 [DOI] [PMC free article] [PubMed] [Google Scholar]