Abstract
Background
Antibodies against hepatitis B surface antigen (HBsAg) wane over time following hepatitis B immunisation; hence, it is unclear whether people vaccinated in three‐dose or four‐dose schedules of the hepatitis B vaccine are still immune when the hepatitis B surface antibody (anti‐HBs) level in their body is undetectable, or lower than the level usually considered protective. This question may potentially be answered indirectly by measuring the anamnestic immune response to a booster dose of vaccine. The term 'booster' (or revaccination) refers to an additional dose of hepatitis B vaccine (HBV) given some time post‐primary vaccination to induce immune memory and improve protection against hepatitis B virus (HBV) infection.
Objectives
To assess the benefits and harms of booster dose hepatitis B vaccination, more than five years after the primary vaccination, for preventing HBV infection in healthy individuals previously vaccinated with the hepatitis B vaccine, and with hepatitis B surface antibody (anti‐HBs) levels below 10 mIU/mL.
Search methods
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register, the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, Science Citation Index Expanded, conference databases, and reference lists of articles to January 2016. We also contacted authors of articles. In addition, we searched ClinicalTrials.gov and the World Health Organization (WHO) International Clinical Trials Registry Platform for ongoing trials (May 2016).
Selection criteria
Randomised clinical trials addressing anamnestic immune response to a booster dose of hepatitis B vaccine, more than five years after the primary vaccination, in apparently healthy participants, vaccinated in a three‐dose or four‐dose schedule of the hepatitis B vaccine during the primary vaccination, without receiving an additional dose or immunoglobulin.
Data collection and analysis
Both review authors decided if the identified studies met the inclusion criteria or not. Primary outcomes included the proportion of participants with anamnestic immune response in non‐protected participants and signs of HBV infection. Secondary outcomes were the proportion of participants that developed local and systemic adverse events following a booster dose injection. We planned to report the weighted proportion with 95% confidence intervals (CIs).
Main results
There were no eligible randomised clinical trials fulfilling the inclusion criteria of this review.
Authors' conclusions
We were unable to include any randomised clinical trials on the topic; only randomised clinical trials will be able to provide an answer as to whether a booster dose vaccination is able to protect against hepatitis B infection.
Plain language summary
Booster dose for preventing hepatitis B infection
Background Antibodies against hepatitis B surface antigen (HBsAg) wane over time following hepatitis B immunisation; hence, it is unclear whether people vaccinated in 3‐dose or 4‐dose schedules of the hepatitis B vaccine during their primary vaccination are still immune when the hepatitis B surface antibody (anti‐HBs) level in their body is undetectable, or lower than the level usually considered protective. This question may potentially be answered indirectly by measuring the anamnestic immune response to a booster dose of vaccine given to people previously immunised with the hepatitis B vaccine.
Aim The authors selected to assess the benefits and harms of a booster dose of hepatitis B vaccine, more than five years after the primary vaccination.
Searches Electronic searches were performed up until January 2016.
Selection criteria Randomised clinical trials addressing immune response (i.e., the way your body recognises and defends itself against bacteria, viruses, and substances that appear foreign and harmful to the body) to a booster dose of hepatitis B vaccine, more than five years after the primary vaccination in apparently healthy participants, vaccinated in a three‐dose or four‐dose schedule of hepatitis B vaccine during their primary vaccination, without receiving an additional dose of the hepatitis B vaccine or immunoglobulin.
Main results and conclusions We were unable to find any eligible randomised clinical trials to include in this review. There is no scientific evidence, based on randomised clinical trials, to support or reject the need for booster doses of hepatitis B vaccine in healthy individuals with normal immune status. We need evidence, based on randomised clinical trials, to formulate future booster vaccination policies.
Background
Description of the condition
The protection provided by the hepatitis B vaccine has been well documented (Chen 2005; McMahon 2005; Mast 2006; Poorolajal 2009a). Hepatitis B surface antibody (anti‐HBs) concentrations equal to or greater than 10 mIU/mL are generally considered protective against hepatitis B virus (HBV) infection (WHO 2002; Mast 2006). However, the protective antibodies induced by the hepatitis B vaccine wane gradually over time and may reach very low or even undetectable levels (Wainwright 1997; Dentinger 2005). It is not known if anti‐HBs concentrations below 10 mIU/mL offer protection against HBV infection. Furthermore, we do not know the exact benefits and harms of a booster dose vaccination in people previously vaccinated against the HBV. The term 'booster' (or revaccination) refers to an additional dose of hepatitis B vaccine given some time post‐primary vaccination to induce immune memory and improve protection against HBV infection.
Description of the intervention
The evidence based on several long‐term follow‐up studies has indicated that the protection provided by three or four doses of monovalent hepatitis B vaccine during the primary vaccination persists for at least two decades (Poorolajal 2009b; Poorolajal 2010a). In addition, immunologic studies have revealed that hepatitis B vaccine induces immunologic memory, so that memory B cells can proliferate, differentiate, and retain the capacity to generate a rapid and vigorous anamnestic immune response upon re‐exposure to hepatitis B surface antigen (HBsAg), even if the anti‐HBs titre falls below 10 mIU/mL (Watson 2001; van der Sande 2007). Hence, disappearance of the antibody may not necessarily imply loss of protection against hepatitis B infection. Nonetheless, a HBV breakthrough infection, detected by the presence of the hepatitis B core antibody (anti‐HBc) in the blood, and chronic HBV carriage, detected by the presence of HBsAg in the blood, are reported in some vaccinees, especially in endemic regions (Hadler 1986; Liao 1999; McMahon 2005). Moreover, adults are less likely than infants to demonstrate an anamnestic response of their immune reaction to the HBV or hepatitis B vaccine as they grow older (Samandari 2007), and the risk of HBV infection increases by sexual and occupational exposures during adulthood (Whittle 2002). In the context of these relatively limited results, the duration of immunity provided by a complete course of primary vaccine is unknown because vaccine protection may not be parallel to the anti‐HBs titre. Indeed, it is not clear whether a decline in serum anti‐HBs level implies the need for a booster dose of the vaccine or not.
How the intervention might work
When anti‐HBs levels fall to low or undetectable levels, a HBV vaccine booster dose may raise antibody levels, leading to increased protection against subclinical and clinical HBV infection. Subclinical infection can be detected by measuring the occurrence of anti‐HBc. Clinical infection can be measured by detecting clinical symptoms and verifying the diagnosis of acute hepatitis B infection using hepatitis B serology.
A practical approach in determining the duration of protection provided by hepatitis B vaccine could be if we assume that the response to a booster dose of hepatitis B vaccine mimics the response to hepatitis B wild virus infection. Accordingly, the serologic response to a booster dose may be considered as a surrogate marker for assessing the presence of protection against the wild virus. Therefore, through measuring the immune response to a booster dose of vaccine in definite post‐primary vaccination periods, we can assess the presence of anamnestic immune response, and potentially assess the long‐term immunity induced by hepatitis B vaccine against HBV infection.
Why it is important to do this review
As unnecessary hepatitis B revaccination is wasteful, none of the international guidelines recommend booster doses to be applied universally (WHO 2003; John 2005; Puro 2005; Mast 2006). Furthermore, duration of protection provided by the hepatitis B vaccine is important for public health authorities who have to plan immunisation programmes and formulate future booster vaccination policies. Hence, protective immunity of the vaccine still requires further investigation (European Consensus Group 2000; FitzSimons 2005; John 2005; Poorolajal 2010b). We found some review articles (European Consensus Group 2000; Banatvala 2003; Chen 2005; FitzSimons 2005; Lee 2006; Mast 2006), and two meta‐analyses that address the anamnestic immune response to a booster dose of hepatitis B vaccine (Poorolajal 2009b; Poorolajal 2010a). However, these meta‐analyses were based on the results of observational studies rather than randomised clinical trials. This raises the risks of confounding and bias. In this systematic review, we aim to determine the long‐term protection of hepatitis B vaccine and the need for a hepatitis B vaccine booster dose, using the results of randomised clinical trials.
Objectives
To assess the benefits and harms of booster dose hepatitis B vaccination, more than five years after the primary vaccination, for preventing hepatitis B virus (HBV) infection in healthy individuals previously vaccinated with the hepatitis B vaccine, and with hepatitis B surface antibody levels (anti‐HBs) below 10 mIU/mL.
Methods
Criteria for considering studies for this review
Types of studies
We planned to include randomised clinical trials addressing response to a hepatitis B vaccine booster dose in non‐protected vaccinees, i.e., vaccinees with hepatitis B surface antibody (anti‐HBs) level below 10 mIU/mL (Figure 1). We planned to include randomised clinical trials, irrespective of blinding, publication status, or language.
1.
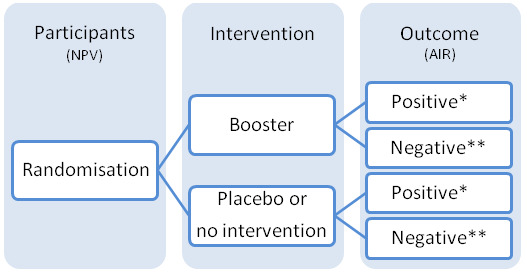
NPV: non‐protected vaccinees (with anti‐HBs less than 10 mIU/mL) AIR: anamnestic immune response * Positive: number with anti‐HBs at or above 10 mIU/mL ** Negative: number with anti‐HBs below 10 mIU/mL
For our review, we planned to consider randomised clinical trials only with more than five years follow‐up after the primary vaccination because several observational follow‐up studies indicated that none of the vaccinated participants became hepatitis B surface antigen (HBsAg) positive during the first five years following their primary hepatitis B vaccination (Wainwright 1989; Lai 1993; Mintai 1993; Zhang 1993; Goh 1995; Joshi 1995; Yuen 1999; But 2008; Gilca 2009). In addition, the World Health Organization (WHO) stated that the duration of vaccine‐induced immunity was uncertain, but it was definitely long‐term, i.e., more than 15 years (WHO 2002). Accordingly, we planned to exclude short‐term randomised clinical trials, i.e., trials with equal to or less than five years interval between the initial vaccination and the booster dose (Appendix 1).
Types of participants
We planned to include those apparently healthy, non‐protected participants with intact immune status, without previous serological signs of hepatitis B virus (HBV) infection (i.e., positive regarding HBsAg and/or hepatitis B core antibody (anti‐HBc)), and who have already received vaccination against hepatitis B in a three‐dose or four‐dose schedule more than five years earlier during their primary vaccination. Non‐protected participants were those vaccinees whose anti‐HBs concentrations in the blood fell to below 10 mIU/mL (WHO 2002; Mast 2006).
We planned to exclude randomised clinical trials with participants who: a) were not screened for serologic markers of HBV infection (HBsAg and anti‐HBc) before admission into the trial; b) have no clear vaccination history; c) were immunised in a less than three‐dose vaccination schedule during their primary vaccination; d) received hepatitis B vaccine plus immunoglobulin; and e) had predisposing factors for immunodeficiency, such as HIV‐positive or haemodialysis (Appendix 1).
Types of interventions
The planned intervention of interest was administration of a booster dose of hepatitis B vaccine versus placebo or no intervention to already immunised participants to assess long‐term (more than five years) presence of anamnestic immune response to booster dose versus placebo (Figure 1). The term 'booster' refers to an additional dose of hepatitis B vaccine given some time post‐primary vaccination to induce immune memory and improve protection against HBV infection. We planned to assess the booster effect, irrespective of type of hepatitis B vaccine, dosage, route, or site of injection (Appendix 1).
Types of outcome measures
Primary outcomes
Any sign or symptom of hepatitis B virus (HBV) infection, either acute or chronic hepatitis B infection, or the development of hepatitis B core antibody (anti‐HBc) in serum or plasma.
Cirrhosis or hepatocellular carcinoma caused or associated with chronic hepatitis B infection, and mortality due to hepatitis B infection.
Proportion of participants that developed serious adverse events after the booster dose injection, including fever, headache, malaise, irritability, rash, nausea, myalgia, arthralgia, or any other systemic adverse events (WHO 2001). Serious adverse events were defined as any outward medical occurrence that was life‐threatening, resulted in death, or persistent or significant disability, or any medical event, which may have jeopardised the patient, or required intervention to prevent it (ICH‐GCP 1997).
Secondary outcomes
Quality of life.
Non‐serious adverse events.
Proportion of participants that developed local adverse events at the booster dose injection site, including pain, redness, swellings, or any other local adverse events (WHO 2001).
The dichotomous outcome of interest was the proportion with anamnestic immune response in non‐protected participants and signs of HBV infection. The continuous outcome of interest was the intensity of anamnestic immune response in non‐protected participants. The intensity of immune response is the amount of fold rise in geometric mean titre post‐booster compared to pre‐booster administration. Anamnestic immune response to booster doses is defined in the following two ways (Watson 2001; Williams 2003; Yuen 2004; van der Sande 2007):
Proportion with a four‐fold or greater rise in the post‐booster anti‐HBs titre within two to four weeks of the booster dose administration in participants having any measurable antibody in the pre‐booster blood sample.
Proportion with development of post‐booster anti‐HBs level equal to or greater than 10 mIU/mL within two to four weeks of the booster dose administration in participants with no detectable antibody in the pre‐booster blood sample.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Hepato‐Biliary Group Controlled Trials Register (Gluud 2016), the Cochrane Central Register of Controlled Trials (Wiley) (CENTRAL; 2015, Issue 12), MEDLINE (Ovid SP), EMBASE (Ovid SP), and Science Citation Index Expanded (Web of Science) (Royle 2003), until January 2016. The search strategies with the time spans of the searches are described in Appendix 2.
We searched ClinicalTrials.gov (clinicaltrials.gov/) and the WHO International Clinical Trial Registry Platform (www.who.int/ictrp) for ongoing trials (May 2016).
Searching other resources
We scanned the reference lists of all retrieved studies and pertinent reviews for additional references. We contacted authors of retrieved studies as well as vaccine manufacturers for additional unpublished randomised trials. We searched the following conference databases for unpublished data until December 2014.
Annual Meeting of the Infectious Diseases Society of America (IDSA); available from www.idsociety.org.
European Congress of Clinical Microbiology and Infectious Diseases (ECCMID); available from www.escmid.org.
Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC); available from www.icaac.org.
Data collection and analysis
Selection of studies
We (JP and EH) read the retrieved publications separately in order to identify the trials that would meet the inclusion criteria of this review (Appendix 1). We were not blinded to the authors' names, journals, or results. We resolved any disagreements through discussion. We had to exclude all identified publications, and we have provided the reasons for exclusion in the Characteristics of excluded studies tables.
Data extraction and management
We entered the extracted data regarding the 'Data collection and abstraction form' in electronic data sheets (Appendix 3). In case of missing data or need for clarification, we contacted study authors.
Assessment of risk of bias in included studies
We intended to assess the risk of bias of the included studies using the ‘Risk of bias’ tool recommended by Cochrane (Higgins 2011) (Appendix 4). It was to be done independently by the review authors (JP and EH) and any disagreements were to be resolved through discussion among the review authors until consensus was reached. If information was not available in the published trial, we planned to contact any of the authors of the trial in order to assess the trials correctly.
The trials judged at 'low' risk of bias in the domains of sequence generation, allocation concealment, blinding of participants and caregivers, blinding of outcome assessors, handling of incomplete outcome data, selective outcome reporting, vested interests, and without other bias risks were to be considered trials at low risk of bias.
The trials judged to be at 'high' or 'unclear' risk of bias regarding any of the domains above were to be considered trials with high risk of bias. Any disagreements were to be resolved through discussion among the review authors, until consensus was reached.
Measures of treatment effect
The effect measure of choice for dichotomous outcomes was the risk ratio (RR), and the effect measure of choice for continuous outcomes was the mean difference (MD). We planned to report all estimates with 95% confidence intervals (CIs).
Dealing with missing data
To handle withdrawals and dropouts in the analysis, we planned to use the 'available data approach' (Higgins 2011), as well as include data on only those participants whose results were known, using as a denominator the total number of people who had data recorded for anamnestic immune response (Higgins 2011).
Assessment of heterogeneity
We planned to consider the Chi2 test at the 10% significance level (P < 0.10) to explore statistical heterogeneity. We also planned to quantify inconsistency across results of the trials using the I2 statistic (Higgins 2003), and to estimate the between‐studies variance by using the Tau2 statistic (Higgins 2011).
Assessment of reporting biases
We planned to create a funnel plot to assess publication bias and other bias risks.
Data synthesis
We planned to use Review Manager 5 for data analysis (RevMan 2014). We planned to analyse data using both a random‐effects model (DerSimonian 1986) and a fixed‐effect model (DeMets 1987) with 95% CI. We planned to report both analyses in case there were discrepancies regarding the significance of the intervention effects; otherwise, the results of the fixed‐effect model only. We planned to put most weight on the most conservative finding in our interpretation (Jakobsen 2014).
Trial Sequential Analysis
We intended to conduct Trial Sequential Analysis (Thorlund 2011; TSA 2011) to control the risk of random error and prevent premature statements of superiority of the experimental or control intervention (Wetterslev 2008). We intended to conduct the Trial Sequential Analysis for primary and secondary outcomes with a type I error of 2.5%, type II error of 20% (80% power), and adjusted for diversity among the included trials (Brok 2008; Wetterslev 2008; Brok 2009; Thorlund 2009; Wetterslev 2009; Thorlund 2010). We assumed an event proportion as observed in the control group and an anticipated intervention effect of 20% relative risk reduction.
Subgroup analysis and investigation of heterogeneity
We planned to assess anamnestic immune response to booster dose for the following subgroups.
Various periods: every five years from initial vaccination.
Various methodological quality: trials with low risk of bias compared to trials with high risk of bias.
Various endemic regions: low endemicity (prevalence of HBV infection less than 2%) compared to intermediate endemicity (prevalence of HBV infection 2% to 7%) and high endemicity (prevalence of HBV infection more than 7%).
Various age groups: every 10 years.
Various participants: apparently healthy participants compared to healthcare workers, or intravenous drug abusers, or sex partners.
Various vaccination schedules of the primary vaccination: three‐dose compared to four‐dose.
Various vaccine or booster types: recombinant vaccine compared to plasma derived vaccine.
Various booster dosages: 5 μg compared to 10 μg.
Various injection sites: deltoid or thigh compared to gluteus.
Various injection routes: intramuscular compared to intradermal.
Sensitivity analysis
We planned to conduct a sensitivity analysis to assess the impact of dropouts and withdrawals for whom no outcome data were obtained, based on the following two scenarios (Gamble 2005).
'Best‐case scenario': assuming all missing participants responded to the booster dose in the booster arm and failed to respond in the control arm, using the total number of participants as the denominator.
'Worst‐case scenario': assuming all missing participants failed to respond to the booster dose in the booster arm and responded in the control arm, using the total number of participants as the denominator.
A true worst‐case scenario (from the perspective of the use of a booster) would be to consider all lost cases in the booster arm to be failures and all lost cases in the control arm to be successes. A best‐case scenario would be the opposite. Any estimate that remains significant in both of these scenarios is robust.
'Summary of findings' tables
We planned to summarise the evidence in 'Summary of findings' tables using the GRADE (Grading of Recommendations, Assessment, Development, and Evaluation) criteria (GRADEpro). We planned to assess five factors referring to limitations in the study design and implementation of included studies that suggest the quality of the evidence; risk of bias ‐ indirectness of evidence (population, intervention, control, outcomes); unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses); imprecision of results (wide CIs and as evaluated with our Trial Sequential Analyses) (Jakobsen 2014); and a high probability of publication bias. If we include studies in future updates, we will define the levels of evidence as 'high', 'moderate', 'low', or 'very low'. These grades are defined as follows.
High certainty: this research provides a very good indication of the likely effect; the likelihood that the effect will be substantially different is low.
Moderate certainty: this research provides a good indication of the likely effect; the likelihood that the effect will be substantially different is moderate.
Low certainty: this research provides some indication of the likely effect; however, the likelihood that it will be substantially different is high.
Very low certainty: this research does not provide a reliable indication of the likely effect; the likelihood that the effect will be substantially different is very high.
Results
Description of studies
Results of the search
We developed a search strategy to include randomised clinical trials exploring anamnestic immune response to booster doses of hepatitis B vaccine. Up to January 2016, we retrieved 660 references through searching electronic databases, 118 references through checking reference lists, and 296 references through checking relevant clinical trial registries. Of 12 references we considered potentially eligible after screening, we did not consider any eligible to be included in the review, and we excluded these references from the review (Figure 2) (Characteristics of excluded studies).
2.
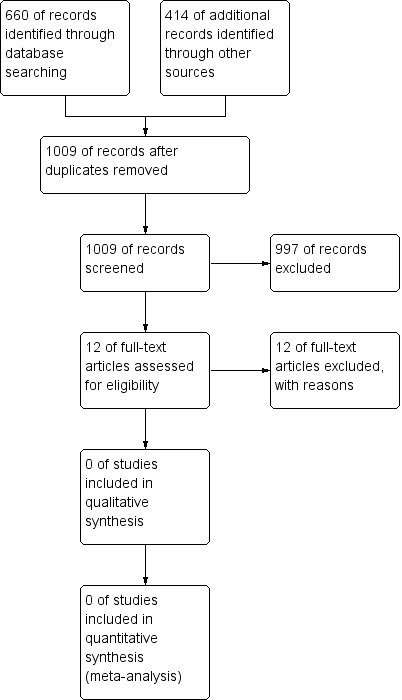
Study flow diagram.
Included studies
We did not find any randomised clinical trials to meet the objectives and the inclusion criteria of the review.
Excluded studies
We excluded 12 studies from the review because: four had no control (placebo or no intervention) group; four assessed immune response to booster dose administered before five years from initial vaccination; three did not exclude protected vaccinees from non‐protected vaccinees; and one compared modified regimens of hepatitis B vaccine with the recommended regimen (Characteristics of excluded studies).
Risk of bias in included studies
There were no eligible trials to be included in the review and hence to be assessed for risk of bias.
Effects of interventions
There were no eligible trials to be included in the review and hence to be assessed for the effects of intervention.
Discussion
Summary of main results
According to the objectives of this review, we intended to assess anamnestic immune response to the hepatitis B vaccine booster dose in vaccinees after five years from the initial hepatitis B vaccination with hepatitis B surface antibody (anti‐HBs) levels below 10 mIU/mL. We restricted our systematic review to randomised clinical trials only. We did not identify any randomised trials to fulfil the inclusion criteria of this review, and hence, we could not meet the objectives of our review. However, in our search process for identification of randomised trials, as well as through previous research done by us (Poorolajal 2009b; Poorolajal 2010a), we identified several non‐randomised studies which addressed anamnestic immune response to a booster dose of hepatitis B vaccine in non‐protected vaccinees, without considering any control group.
Quality of the evidence
We developed a wide search strategy to encompass as many studies as possible. Although we retrieved 660 references, we could not find any eligible randomised trials to include in the review and therefore we could not assess any quality of evidence.
Potential biases in the review process
For this review, we found no randomised clinical trials or controlled clinical studies.
Agreements and disagreements with other studies or reviews
Non‐randomised studies
All studies, found through our previous non‐Cochrane research, included a total of 3551 participants (Poorolajal 2009b; Poorolajal 2010a). These studies assessed anamnestic immune response to a booster dose five to 20 years post‐initial vaccination.
We divided the participants into four strata based on duration from the last primary vaccination (Poorolajal 2009b; Poorolajal 2010a). Stratum 1 included studies that investigated anamnestic immune response to a booster dose five years post‐initial vaccination; stratum 2 included studies that assessed anamnestic immune response to a booster dose six to 10 years post‐initial vaccination; stratum 3 included studies that assessed anamnestic immune response to a booster dose 11 to 15 years post‐initial vaccination; and stratum 4 included studies that assessed anamnestic immune response to a booster dose 16 to 20 years post‐initial vaccination. Stratum 1 included 12 studies with 480 participants; stratum 2 included 27 studies with 1405 participants; stratum 3 included 12 studies with 1883 participants; and stratum 4 included two studies with 711 participants.
We conducted a meta‐analysis on non‐randomised studies to estimate the overall anamnestic immune response to a booster dose five to 20 years after initial vaccination. Based on the results of this meta‐analysis, the response proportion to a booster dose was 92% (95% CI 88% to 96%) after five years; 92% (95% CI 89% to 95%) after six to 10 years; 80% (95% CI 72% to 88%) after 11 to 15 years; and 76% (95% CI 73% to 80%) after 16 to 20 years (Poorolajal 2009b). However, we should remember that considering the response to a booster dose of hepatitis B vaccine for assessing the presence of protection against the wild virus is only an unvalidated surrogate marker (Gluud 2007). Therefore, no response to booster dose does not necessarily mean susceptibility to live virus infection. And, on the other hand, one does not know if response to a booster dose means effective prevention against infection, although this is likely.
The results of previously published meta‐analyses revealed the fact that although anti‐HBs concentrations equal to or greater than 10 mIU/mL are generally considered protective against hepatitis B virus (HBV) infection (WHO 2002; Mast 2006), the opposite of this does not seem correct. In other words, anti‐HBs concentrations less than 10 mIU/mL or absence of anamnestic immune response cannot be considered absence of immunity.
Another meta‐analysis was conducted on non‐randomised studies to estimate the duration of protection provided by the hepatitis B vaccine (Poorolajal 2010a). The results indicated that the overall incidence rate of HBV breakthrough infection five to 20 years after initial vaccination was 0.007 (95% CI 0.005 to 0.010) with a variation among studies from 0 to 0.094. Available data do not allow us to exclude an increased risk for infection with time since vaccination. We concluded that the protection provided by three or four doses of hepatitis B vaccine could persist for at least two decades (Poorolajal 2010a).
Randomised clinical trials
We found three randomised trials addressing the effect of various types of hepatitis B booster dose. However, these trials did not meet our inclusion criteria because they did not have a placebo control group or assessed the effect of booster dose before five years from the initial vaccination. The results of these trials are described below.
A randomised multicentre, open‐label clinical trial was conducted in Spain to assess the anamnestic immune response to a hepatitis B booster dose among four to eight year‐old children after initial hepatitis B vaccination. A total of 1478 children were enrolled in this multicentre trial and stratified into three cohorts (A, B, and C) from 77 primary care centres in Spain and one site in Canada. Participants in cohort A included 751 participants who initially were vaccinated with Recombivax. Participants in cohort B included 707 participants who were initially vaccinated with Engerix‐B. And cohort C included 20 participants who received no primary hepatitis B vaccine series. The participants were randomised to receive the hepatitis B booster dose as follows. In cohort A, 374 participants received a booster dose of minipool HBV (mpHBV) (group 1) and 375 participants received Engerix‐B (group 2). In cohort B, 349 participants received mpHBV (group 3) and 352 participants received Engerix‐B (group 4). All 20 participants in cohort C received a dose of mpHBV (group 5). Some participants were lost during the follow‐up period. Before the booster dose vaccination, 15.9% to 51.2% of participants had hepatitis B antibody concentrations equal to or greater than 10 mIU/mL. One month after the booster dose vaccination, 91.6% to 97.3% of the participants had antibody concentrations equal to or greater than 10 mIU/mL. The authors concluded that measuring anti‐HBs post‐booster dose may be an indicator of long‐term post‐vaccination protection against hepatitis B infection even if the pre‐booster anti‐HBs level is undetectable (Diez‐Domingo 2010).
A randomised open‐label trial was conducted in Italy to investigate the response to a booster dose of monovalent hepatitis B vaccine in 410 children immunised with three doses of either Hexavac (n = 201) or Infanrix‐Hexa (n = 209) during infancy. Children were randomised into two groups to receive a single booster dose of either HBVaxPro (n = 62) or Engerix‐B (n = 348). Anti‐HBs concentrations were measured before and one month after the booster dose. One month post‐booster: 91% (86% to 95%) of children in the Hexavac group and 98% (95% to 99%) in the Infanrix‐Hexa group had anti‐HBs concentrations equal to or greater than 10 mIU/mL (Zanettia 2012).
A randomised double‐blind, placebo‐controlled field trial was conducted to assess the efficacy of a booster dose of hepatitis B vaccine in 104 primary school children with a good response to initial vaccination three years after the primary vaccination. The participants were randomised to receive either hepatitis B booster dose (53 participants) or placebo (51 participants). At the end of the six‐year follow‐up (three years after the revaccination), the proportion of anti‐HBs positive in the revaccinated group was 88% versus 69% in the control group (P < 0.01) (Zhuang 1998).
Authors' conclusions
Implications for practice.
There were no eligible randomised clinical trials to include in the review. There is no scientific evidence based on randomised clinical trials to support or refute the need for a booster dose of hepatitis B vaccine in healthy individuals, with normal immune status, who had fully responded to a complete course of the vaccine.
Implications for research.
The clinical consequences of offering a booster dose to healthy people with hepatitis B surface antibody (anti‐HBs) levels below 10 mIU/mL more than five years after initial hepatitis B vaccination are not known. In principle, therefore, we need to conduct such randomised clinical trials. However, such trials will need to be very large in order to be meaningful, and accordingly expensive. Such costs have to be weighed against a policy where one offers a booster vaccination without knowing the clinical consequences. This review did not aim to include immunocompromised persons such as HIV‐infected individuals, haemodialysis patients, and persons receiving chemotherapy, so we cannot make any implications for research for these groups. Hence, the need for a booster dose in these groups also has to be investigated. These trials ought to be conducted according to the SPIRIT statement and reported according to the CONSORT statement.
What's new
| Date | Event | Description |
|---|---|---|
| 4 April 2016 | New citation required but conclusions have not changed | We could identify no randomised clinical trials for inclusion in the review until the very end of its resubmission for publication. This is why, in the future, the review will be updated only if such trials are identified. |
| 4 April 2016 | New search has been performed | The review has been updated following the latest Cochrane requirements for review preparation. There is also a change in the author team. |
Acknowledgements
We thank previous authors of the first version of this review ‐ Mahmoodi M, Haghdoost A, Majdzadeh R, Nasseri‐Moghaddam S, Ghalichi L, Fotouhi A ‐ for their dedicated work (Poorolajal 2010b).
We thank Dimitrinka Nikolova who was involved in the formulation, supervision, and improvement of the Cochrane protocol and review as well as Sarah Louise Klingenberg for designing the search strategies. We also thank Kate Whitfield for sending us papers on trials.
Peer Reviewer: Ronald L Koretz, USA. Contact Editors: Gennaro D'Amico, Italy; Christian Gluud, Denmark.
Cochrane Review Group funding acknowledgement: The Danish State is the largest single funder of the Cochrane Hepato‐Biliary Group through its investment in the Copenhagen Trial Unit, Centre for Clinical Intervention Research, Rigshospitalet, Copenhagen University Hospital, Denmark.
Disclaimer: The views and opinions expressed in this review are those of the authors and do not necessarily reflect those of the Danish State or the Copenhagen Trial Unit.
Appendices
Appendix 1. Inclusion‐exclusion criteria
| Criteria | Included | Excluded |
| Types of studies | ||
| Has the trial assessed anamnestic immune response to booster dose? | Yes | No |
| Have the participants been randomised to booster hepatitis B vaccination versus placebo or no vaccination? | Yes | No |
| Types of participants | ||
| Were they apparently healthy participants, with intact immune status, without previous hepatitis B virus infection? | Yes | No |
| Were they free of predisposing factors for immunodeficiency? | Yes | No |
| Were they screened for serologic markers of hepatitis B virus infection before admission into the trial? | Yes | No |
| Have the participants already received either a 3‐dose or a 4‐dose schedule of hepatitis B vaccine? | Yes | No |
| Was their vaccination history clear and reliable? | Yes | No |
| Did they receive a monovalent hepatitis B vaccine not in fixed combination with other vaccines? | Yes | No |
| Did they receive hepatitis B vaccine without immunoglobulin? | Yes | No |
| Types of interventions | ||
| Was the administered booster dose a monovalent vaccine of either recombinant vaccine (RV) or plasma derived vaccine (PDV)? | Yes | No |
| Primary outcomes | ||
| Was the anamnestic immune response to booster dose of hepatitis B vaccine versus placebo investigated? | Yes | No |
Appendix 2. Search strategies
| Database | Time of searches | Search terms |
| The Cochrane Hepato‐Biliary Group Controlled Trials Register | January 2016 | boost* AND (vaccin* OR immuni* OR engerix‐B OR euvax‐B OR recombivax OR twinrix) AND hepatitis B |
| The Cochrane Central Register of Controlled Trials (CENTRAL) (Wiley) | Issue 12 of 12, 2015 | #1 MeSH descriptor: [Immunization, Secondary] explode all trees #2 boost* #3 #1 or #2 #4 MeSH descriptor: [Hepatitis B Vaccines] explode all trees #5 vaccin* or immuni* or engerix‐B or euvax‐B or recombivax or twinrix #6 #4 or #5 #7 MeSH descriptor: [Hepatitis B] explode all trees #8 hepatitis B #9 #7 or #8 #10 #3 and #6 and #9 |
| MEDLINE (Ovid SP) | 1946 to January 2016 | 1. exp Immunization, Secondary/ 2. boost*.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 3. 1 or 2 4. exp Hepatitis B Vaccines/ 5. (vaccin* or immuni* or engerix‐B or euvax‐B or recombivax or twinrix).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 6. 4 or 5 7. exp Hepatitis B/ 8. hepatitis B.mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 9. 8 or 7 10. 6 and 3 and 9 11. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, original title, abstract, name of substance word, subject heading word, unique identifier] 12. 11 and 10 |
| EMBASE (Ovid SP) | 1974 to January 2016 | 1. boost*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 2. exp Hepatitis B Vaccine/ 3. (vaccin* or immuni* or engerix‐B or euvax‐B or recombivax or twinrix).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 4. 3 or 2 5. exp Hepatitis B/ 6. hepatitis B.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 7. 6 or 5 8. 4 and 1 and 7 9. (random* or blind* or placebo* or meta‐analysis).mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name] 10. 8 and 9 |
| Science Citation Index Expanded (Web of Science) | 1900 to January 2016 | #6 #5 AND #4 #5 TS=(random* or blind* or placebo* or meta‐analysis) #4 #3 AND #2 AND #1 #3 TS=(hepatitis B) #2 TS=(vaccin* OR immuni* OR engerix‐B OR euvax‐B OR recombivax OR twinrix) #1 TS=(boost*) |
Appendix 3. Data collection and abstraction form
| Row | Data | Results | |||
| Booster | Placebo | ||||
| 1 | 1st author | ||||
| 2 | Date of publication | ||||
| 3 | Design of clinical trial | Randomised clinical trial | |||
| Quasi‐randomised study | |||||
| 4 | Follow‐up time from last vaccination (year) | ||||
| 5 | Endemicity | High | |||
| Intermediate | |||||
| Low | |||||
| 6 | Participants | General population | |||
| Healthcare workers | |||||
| Intravenous (IV) drug abusers | |||||
| Sex partners | |||||
| Others | |||||
| 7 | Mean age (year) | ||||
| 8 | Vaccine schedule | 3‐dose | |||
| 4‐dose | |||||
| 9 | Initial vaccine type | Recombinant vaccine (RV) | |||
| Plasma derived vaccine (PDV)? | |||||
| 10 | Proportion with response to initial vaccination (%) | ||||
| 11 | Booster type | Recombinant vaccine (RV) | |||
| Plasma derived vaccine (PDV)? | |||||
| 12 | Booster dosage (mcg) | ||||
| 13 | Injection site | Deltoid | |||
| Thigh | |||||
| Gluteus | |||||
| 14 | Injection route | IM | |||
| ID | |||||
| SD | |||||
| 15 | Sample size | ||||
| 16 | Dropouts | ||||
| 17 | Anamnestic immune response (AIR) | ||||
| 18 | Proportion of anamnestic immune response (PAIR) | ||||
| 19 | Before intervention (booster) | GMT (mIU/mL) | |||
| 95% CI of GMT | |||||
| 20 | 1 week after intervention (booster) | GMT (mIU/mLl) | |||
| 95% CI of GMT | |||||
| 21 | 2 weeks after booster dose (booster) | GMT (mIU/mL) | |||
| 95% CI of GMT | |||||
| 22 | 3 weeks after intervention (booster) | GMT (mIU/mL) | |||
| 95% CI of GMT | |||||
| 23 | 4 weeks after intervention (booster) | GMT (mIU/mL) | |||
| 95% CI of GMT | |||||
| 24 | 2 months after intervention (booster) | GMT (mIU/mL) | |||
| 95% CI of GMT | |||||
| 25 | 1 year after intervention (booster) | GMT (mIU/mL) | |||
| 95% CI of GMT | |||||
| 26 | Adverse events of booster | Local | Pain | ||
| Tenderness | |||||
| Redness | |||||
| Swelling | |||||
| Other | |||||
| Systemic | Fever | ||||
| Headache | |||||
| Malaise | |||||
| Irritability | |||||
| Rash | |||||
| Nausea | |||||
| Myalgia | |||||
| Arthralgia | |||||
| Other | |||||
Appendix 4. Assessment of risk of bias of the included studies
| Random sequence generation |
|
Criteria for a judgement of ‘low risk’ of bias The investigators describe a random component in the sequence generation process from the following.
Criteria for the judgement of ‘high risk’ of bias The investigators describe a non‐random component in the sequence generation process. Usually, the description would involve some systematic, non‐random approach, such as the following.
Criteria for the judgement of ‘unclear risk’ of bias Insufficient information about the sequence generation process to permit judgement of ‘low risk’ or ‘high risk’ |
| Allocation concealment |
|
Criteria for a judgement of ‘low risk’ of bias Participants and investigators enrolling participants could not foresee assignment because one of the following, or an equivalent method, was used to conceal allocation.
Criteria for the judgement of ‘high risk’ of bias Participants or investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on the following.
Criteria for the judgement of ‘unclear risk’ of bias Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. This is usually the case if the method of concealment is not described or not described in sufficient detail to allow a definite judgement – for example, if the use of assignment envelopes is described, but it remains unclear whether envelopes were sequentially numbered, opaque, and sealed |
| Blinding of participants and personnel |
|
Criteria for a judgement of ‘low risk’ of bias Any one of the following.
Criteria for the judgement of ‘high risk’ of bias Any one of the following.
Criteria for the judgement of ‘unclear risk’ of bias Any one of the following.
|
| Blinding of outcome assessment |
|
Criteria for a judgement of ‘low risk’ of bias Any one of the following.
Criteria for the judgement of ‘high risk’ of bias Any one of the following.
Criteria for the judgement of ‘unclear risk’ of bias Any one of the following.
|
| Incomplete outcome data |
|
Criteria for a judgement of ‘low risk’ of bias Any one of the following.
Criteria for the judgement of ‘high risk’ of bias Any one of the following.
Criteria for the judgement of ‘unclear risk’ of bias Any one of the following.
|
| Selective reporting |
|
Criteria for a judgement of ‘low risk’ of bias Any of the following.
Criteria for the judgement of ‘high risk’ of bias Any one of the following.
Criteria for the judgement of ‘unclear risk’ of bias Insufficient information to permit judgement of ‘low risk’ or ‘high risk’. It is likely that the majority of studies will fall into this category |
| For a trial to be classified as a trial with low risk of bias, it must be judged at low risk of bias in all domains. If this is not the case, the trial will be classified as a trial at high risk of bias |
Characteristics of studies
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Cassidy 2001 | Comparing modified regimens of hepatitis B vaccine with the recommended regimen |
| Chan 1991 | Did not exclude protected vaccinees from non‐protected vaccinees |
| Diez‐Domingo 2010 | Assessing immune response to booster dose before five years from initial vaccination |
| Gilca 2013 | No control (placebo or no intervention) group |
| Teoharov 2013 | No control (placebo or no intervention) group |
| Trivello 1995 | Did not exclude protected vaccinees from non‐protected vaccinees |
| van der Sande 2007 | Did not exclude protected vaccinees from non‐protected vaccinees |
| Williams 2001 | No control (placebo or no intervention) group |
| Wu 2010 | Assessing immune response to booster dose before five years from initial vaccination |
| Yao 2011 | No control (placebo or no intervention) group |
| Zanettia 2012 | Assessing immune response to booster dose before five years from initial vaccination |
| Zhuang 1998 | Assessing immune response to booster dose before five years from initial vaccination |
Differences between protocol and review
We expanded the review protocol outcomes before we started on the review update. In addition, we expanded the methods of our review protocol with Trial Sequential Analysis and Summary of Findings. We updated the bias risk domains.
Contributions of authors
Jalal Poorolajal (JP): developed and wrote the protocol, and was responsible for the reference searching, article retrieval, study inclusion and exclusion, data extraction, assessment of risk of bias in included studies, data analysis, interpretation of results, and writing of the review.
Elham Hooshmand (EH) was responsible for the reference searching, article retrieval, and study inclusion and exclusion.
Sources of support
Internal sources
-
UMSHA, Hamadan, Iran.
Department of Epidemiology and Biostatistics, School of Public Health, Hamadan Univesity of Medical Science (UMSHA)
External sources
No sources of support supplied
Declarations of interest
Jalal Poorolajal declares no conflicts of interest. Elham Hooshmand declares no conflicts of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies excluded from this review
Cassidy 2001 {published data only}
- Cassidy WM, Watson B, Ioli VA, Williams K, Bird S, West DJ. A randomized trial of alternative two‐ and three‐dose hepatitis B vaccination regimens in adolescents: antibody responses, safety, and immunologic memory. Pediatrics 2001;107(4):626‐31. [DOI] [PubMed] [Google Scholar]
Chan 1991 {published data only}
- Chan C‐Y, Lee S‐D, Tsai Y‐T, Lo K‐J. Booster response to recombinant yeast‐derived hepatitis‐B vaccine in vaccinees whose anti‐HBs responses were initially elicited by a plasma‐derived vaccine. Vaccine 1991;9(10):765‐7. [DOI] [PubMed] [Google Scholar]
Diez‐Domingo 2010 {published data only}
- Diez‐Domingo J, Flores SA, Martin JC, Klopfer SO, Schodel FP, Bhuyan PK. A randomized, multicenter, open‐label clinical trial to assess the anamnestic immune response 4 to 8 years after a primary hepatitis B vaccination series. Pediatric Infectious Disease Journal 2010;29(10):972‐4. [DOI] [PubMed] [Google Scholar]
Gilca 2013 {published data only}
- Gilca V, Serres G, Boulianne N, Murphy D, Wals P, Ouakki M, et al. Antibody persistence and the effect of a booster dose given 5, 10 or 15 years after vaccinating preadolescents with a recombinant hepatitis B vaccine. Vaccine 2013;31(3):448‐51. [DOI] [PubMed] [Google Scholar]
Teoharov 2013 {published data only}
- Teoharov P, Kevorkyan A, Petrova N, Baltadzhiev I, Damme P. Immune memory and immune response in children from Bulgaria 5‐15 years after primary hepatitis B vaccination. Pediatric Infectious Disease Journal 2013;32(1):51‐3. [DOI] [PubMed] [Google Scholar]
Trivello 1995 {published data only}
- Trivello R, Chiaramonte M, Ngatchu T, Baldo V, Majori S, Moschen ME, et al. Persistence of anti‐HBs antibodies in health care personnel vaccinated with plasma‐derived hepatitis B vaccine and response to recombinant DNA HB booster vaccine. Vaccine 1995;13(2):139‐41. [DOI] [PubMed] [Google Scholar]
van der Sande 2007 {published data only}
- Sande MA, Waight PA, Mendy M, Zaman S, Kaye S, Sam O, et al. Long‐term protection against HBV chronic carriage of Gambian adolescents vaccinated in infancy and immune response in HBV booster trial in adolescence. Plos One 2007;2(8):e753. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 2001 {published data only}
- Williams JL, Christensen CJ, McMahon BJ, Bulkow LR, Cagle HH, Mayers JS, et al. Evaluation of the response to a booster dose of hepatitis B vaccine in previously immunized healthcare workers. Vaccine 2001;19(28‐29):4081‐5. [DOI] [PubMed] [Google Scholar]
Wu 2010 {published data only}
- Wu ZH, Cui FQ, Gong XH. Effect analysis on non‐and‐low response infants after revaccinated hepatitis B vaccine. Zhongguo Yi Miao He Mian Yi 2010;16(3):207‐10. [PubMed] [Google Scholar]
Yao 2011 {published data only}
- Yao J, Ren J, Shen L, Chen Y, Liang X, Cui F, et al. The effects of booster vaccination of hepatitis B vaccine on anti‐HBV surface antigen negative children 11‐15 years after primary vaccination. Human Vaccines 2011;7(10):1055‐9. [DOI] [PubMed] [Google Scholar]
Zanettia 2012 {published data only}
- Zanettia A, Parlatob A, Romanòa L, Desolec MG, Ferrerad G, Giurdanellae F, et al. Challenge with a hepatitis B vaccine in two cohorts of 4‐7‐year‐old children primed with hexavalent vaccines: an open‐label, randomised trial in Italy. Vaccine 2012;30(39):5770‐5. [DOI] [PubMed] [Google Scholar]
Zhuang 1998 {published data only}
- Zhuang G, Xu H, Zuo H, Wang X, Liu P, Kong L. Six‐year efficacy of hepatitis B revaccination: a double‐blind, placebo‐controlled and randomized field trial. Journal of Xi'an Medical University 1998;10(1):44‐8. [Google Scholar]
Additional references
Banatvala 2003
- Banatvala JE, Damme P. Hepatitis B vaccine – do we need boosters?. Journal of Viral Hepatitis 2003;10:1‐6. [DOI] [PubMed] [Google Scholar]
Brok 2008
- Brok J, Thorlund K, Gluud C, Wetterslev J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta‐analyses. Journal of Clinical Epidemiology 2008;61:763‐9. [DOI] [PubMed] [Google Scholar]
Brok 2009
- Brok J, Thorlund K, Wetterslev J, Gluud C. Apparently conclusive meta‐analyses may be inconclusive ‐ Trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta‐analyses. International Journal of Epidemiology 2009;38(1):287‐98. [DOI] [PubMed] [Google Scholar]
But 2008
- But DYK, Lai CL, Lim WL, Fung J, Wong DKH, Yuen MF. Twenty‐two years follow‐up of a prospective randomized trial of hepatitis B vaccines without booster dose in children: Final report. Vaccine 2008;26:6587‐91. [DOI] [PubMed] [Google Scholar]
Chen 2005
- Chen W, Gluud C. Vaccines for preventing hepatitis B in health‐care workers. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD000100.pub3] [DOI] [PubMed] [Google Scholar]
DeMets 1987
- DeMets DL. Methods for combining randomized clinical trials: strengths and limitations. Statistics in Medicine 1987;6(3):341‐50. [DOI] [PubMed] [Google Scholar]
Dentinger 2005
- Dentinger CM, McMahon BJ, Butler JC, Dunaway CE, Zanis CL, Bulkow LR, et al. Persistence of antibody to hepatitis B and protection from disease among Alaska natives immunized at birth. Pediatric Infectious Disease Journal 2005;24(9):786‐92. [DOI] [PubMed] [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
European Consensus Group 2000
- European Consensus Group on Hepatitis B Immunity. Are booster immunizations needed for lifelong hepatitis B immunity?. Lancet 2000;355:561–5. [PubMed] [Google Scholar]
FitzSimons 2005
- FitzSimons D, Francois G, Hall A, McMahon B, Meheus A, Zanetti A, et al. Long‐term efficacy of hepatitis B vaccine, booster policy and impact of hepatitis B virus mutants. Vaccine 2005;23:4158‐66. [DOI] [PubMed] [Google Scholar]
Gamble 2005
- Gamble C, Hollis S. Uncertainty method improved on best‐worst case analysis in a binary meta‐analysis. Journal of Clinical Epidemiology 2005;58:579‐88. [DOI] [PubMed] [Google Scholar]
Gilca 2009
Gluud 2007
- Gluud C, Brok J, Gong Y, Koretz RL. Hepatology may have problems with putative surrogate outcome measures. Journal of Hepatology 2007;46(4):734‐42. [DOI] [PubMed] [Google Scholar]
Gluud 2016
- Gluud C, Nikolova D, Klingenberg SL. Cochrane Hepato‐Biliary Group. About Cochrane (Cochrane Review Groups (CRGs)) 2016, Issue 4. Art. No.: LIVER.
Goh 1995
- Goh KT, Oon CJ, Heng BH, Lim GK. Long‐term immunogenicity and efficacy of a reduced dose of plasma‐based hepatitis B vaccine in young adults. Bulletin of the World Health Organization 1995;73(4):523‐7. [PMC free article] [PubMed] [Google Scholar]
GRADEpro [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro. Version 3.2 for Windows. Grade Working Group 2004‐2007, 2008.
Hadler 1986
- Hadler SC, Francis DP, Maynard JE, Thompson SE, Judson FN, Echenberg DF, et al. Long‐term immunogenicity and efficacy of hepatitis B vaccine in homosexual men. New England Journal of Medicine 1986;315(4):209‐14. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical Research Ed.) 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
ICH‐GCP 1997
- International Conference on Harmonisation Expert Working Group. International conference on harmonisation of technical requirements for registration of pharmaceuticals for human use. ICH harmonised tripartite guideline. Guideline for good clinical practice CFR & ICH Guidelines. Vol. 1, Philadelphia: Barnett International/PAREXEL, 1997. [Google Scholar]
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14(120):1‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
John 2005
- John TJ, Cooksley G. Hepatitis B vaccine boosters: Is there a clinical need in high endemicity populations?. Journal of Gastroenterology and Hepatology 2005;20:5‐10. [DOI] [PubMed] [Google Scholar]
Joshi 1995
- Joshi N, Kumar YRN, Srinivas DV, Kumar A, Rao PN. Recombinant hepatitis‐B vaccine long‐term efficacy in hospital personnel. Gastroenterology 1995;108(4):A1093. [Google Scholar]
Lai 1993
- Lai CL, Wong BC, Yeoh EK, Lim WL, Chang WK, Lin HJ. Five‐year follow‐up of a prospective randomized trial of hepatitis B recombinant DNA yeast vaccine vs. plasma‐derived vaccine in children: immunogenicity and anamnestic responses. Hepatology 1993;18(4):763‐7. [DOI] [PubMed] [Google Scholar]
Lee 2006
- Lee C, Gong Y, Brok J, Boxall EH, Gluud C. Hepatitis B immunisation for newborn infants of hepatitis B surface antigen‐positive mothers. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004790.pub2] [DOI] [PubMed] [Google Scholar]
Liao 1999
- Liao SS, Li RC, Li H, Yang JY, Zeng XJ, Gong J, et al. Long‐term efficacy of plasma‐derived hepatitis B vaccine: a 15‐year follow‐up study among Chinese children. Vaccine 1999;17:2661‐6. [DOI] [PubMed] [Google Scholar]
Mast 2006
- Mast EE, Weinbaum CM, Fiore AE, Alter MJ, Bell BP, Finelli L, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) Part II: immunization of adults. MMWR. Morbidity and Mortality Weekly Report 2006;55(RR‐16):1‐33. [PubMed] [Google Scholar]
McMahon 2005
- McMahon BJ, Bruden DL, Petersen KM, Bulkow LR, Parkinson AJ, Nainan O, et al. Antibody levels and protection after hepatitis B vaccination: results of a 15‐year follow‐up. Annals of Internal Medicine 2005;142(5):333‐41. [DOI] [PubMed] [Google Scholar]
Mintai 1993
- Mintai Z, Kezhou L, Lieming D, Raymond S. Duration and efficacy of immune response to hepatitis‐B vaccine in high‐risk Chinese adolescents. Clinical Infectious Diseases 1993;16(1):165‐7. [DOI] [PubMed] [Google Scholar]
Poorolajal 2009a
- Poorolajal J, Majdzadeh R. Prevalence of chronic hepatitis B infection in Iran: a review article. Journal of Research in Medical Sciences 2009;14(4):249‐58. [PMC free article] [PubMed] [Google Scholar]
Poorolajal 2009b
- Poorolajal J, Mahmoodi M, Majdzadeh R, Haghdoost A, Nasseri‐Moghaddam S, Fotouhi A, et al. Seroprotection of Hepatitis B vaccine and need for booster dose: a meta‐analysis. Hepatitis Monthly 2009;9(4):293‐304. [DOI] [PubMed] [Google Scholar]
Poorolajal 2010a
- Poorolajal J, Mahmoodi M, Haghdoost A, Majdzadeh R, Nasseri‐Moghaddam S, Fotouhi A. Long‐term protection provided by hepatitis B vaccine and need for booster dose: A meta‐analysis. Vaccine 2010;28(3):623‐31. [DOI] [PubMed] [Google Scholar]
Puro 2005
- Puro V, Carli GD, Cicalini S, Soldani F, Balslev U, Begovac J, et al. European recommendations for the management of healthcare workers occupationally exposed to hepatitis B virus and hepatitis C virus. Euro Surveillance 2005;10(10):260‐4. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Royle 2003
- Royle P, Milne R. Literature searching for randomized controlled trials used in Cochrane reviews: rapid versus exhaustive searches. International Journal of Technology Assessment in Health Care 2003;19(4):591‐603. [DOI] [PubMed] [Google Scholar]
Samandari 2007
- Samandari T, Fiore AE, Negus S, Williams JL, Kuhnert W, McMahon BJ, et al. Differences in response to a hepatitis B vaccine booster dose amongAlaskan children and adolescents vaccinated during infancy. Pediatrics 2007;120(2):e373‐81. [DOI] [PubMed] [Google Scholar]
Thorlund 2009
- Thorlund K, Devereaux PJ, Wetterslev J, Guyatt G, Ioannidis JP, Thabane L, et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta‐analyses. International Journal of Epidemiology 2009;38(1):276‐86. [DOI] [PubMed] [Google Scholar]
Thorlund 2010
- Thorlund K, Anema A, Mills E. Interpreting meta‐analysis according to the adequacy of sample size. An example using isoniazid chemoprophylaxis for tuberculosis in purified protein derivative negative HIV‐infected individuals. Clinical Epidemiology 2010;2:57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thorlund 2011
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). ctu.dk/tsa/files/tsa_manual.pdf 2011 (accessed 12 May 2016).
TSA 2011 [Computer program]
- Copenhagen Trial Unit. TSA ‐ Trial Sequential Analysis. Version 0.9 Beta. Copenhagen: Copenhagen Trial Unit, 2011.
Wainwright 1989
- Wainwright RB, McMahon BJ, Bulkow LR, Hall DB, Fitzgerald MA, Harpster AP, et al. Duration of immunogenicity and efficacy of hepatitis B vaccine in a Yupik Eskimo population. JAMA 1989;261(16):2362‐6. [PubMed] [Google Scholar]
Wainwright 1997
- Wainwright RB, Bulkow LR, Parkinson AJ, Zanis C, McMahon BJ. Protection provided by hepatitis B vaccine in a Yupik Eskimo population ‐ Results of a 10‐year study. Journal of Infectious Diseases 1997;175:674‐7. [DOI] [PubMed] [Google Scholar]
Watson 2001
- Watson B, West DJ, Chilkatowsky A, Piercy S, Ioli VA. Persistence of immunologic memory for 13 years in recipients of a recombinant hepatitis B vaccine. Vaccine 2001;19:3164‐8. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61(1):64‐75. [DOI] [PubMed] [Google Scholar]
Wetterslev 2009
- Wetterslev J, Thorlund K, Brok J, Gluud C. Estimating required information size by quantifying diversity in a random‐effects meta‐analysis. BMC Medical Research Methodology 2009;9:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
Whittle 2002
- Whittle H, Jaffar S, Wansbrough M, Mendy M, Dumpis U, Collinson A, et al. Observational study of vaccine efficacy 14 years after trial of hepatitis B vaccination in Gambian children. BMJ (Clinical Research Ed.) 2002;325:569‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
WHO 2001
- World Health Organization. Introduction of hepatitis B vaccine into childhood immunization services. www.who.int/immunization/documents/WHO_VB_01.31/en/ 2001 (accessed 11 May 2015).
WHO 2002
- World Health Organization. Hepatitis B. www.who.int/csr/disease/hepatitis/whocdscsrlyo20022/en/ 2002 (accessed 11 May 2015).
WHO 2003
- World Health Organization. Expanded program on immunization: measles and hepatitis B. www2.wpro.who.int/rcm/en/archives/rc54/rc_resolutions/wpr_rc54_r03.htm 2003 (accessed 11 May 2015).
Williams 2003
- Williams IT, Goldstein ST, Tufa J, Tauillii S, Margolis HS, Mahoney FJ. Long term antibody response to hepatitis B vaccination beginning at birth and to subsequent booster vaccination. Pediatric Infectious Disease Journal 2003;22(2):157‐63. [DOI] [PubMed] [Google Scholar]
Yuen 1999
- Yuen MF, Lim WL, Cheng CC, Lam SK, Lai CL. Twelve‐year follow‐up of a prospective randomized trial of hepatitis B recombinant DNA yeast vaccine versus plasma‐derived vaccine without booster doses in children. Hepatology 1999;29(3):924‐7. [DOI] [PubMed] [Google Scholar]
Yuen 2004
- Yuen MF, Lim WL, Chan AOO, Wong DKH, Sum SSM, Lai CL. 18‐year follow‐up study of a prospective randomized trial of hepatitis B vaccinations without booster doses in children. Clinical Gastroenterology and Hepatology 2004;2:941‐5. [DOI] [PubMed] [Google Scholar]
Zhang 1993
- Zhang M, Liu K, Jin J, Ding L, Zhou S, Yu Q, et al. Five‐year follow‐up of immune response to hepatitis B vaccine in juveniles. Chinese Medical Journal 1993;106(2):97‐9. [PubMed] [Google Scholar]
References to other published versions of this review
Poorolajal 2010b
- Poorolajal J, Mahmoodi M, Haghdoost A, Majdzadeh R, Nasseri‐Moghaddam S, Ghalichi L, et al. Booster dose vaccination for preventing hepatitis B. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD008256.pub2] [DOI] [PubMed] [Google Scholar]


