Abstract
This present study examined excess copper (Cu) effects on seedling growth, leaf Cu concentration, gas exchange, and protein profiles identified by a two-dimensional electrophoresis (2-DE) based mass spectrometry (MS) approach after Citrus sinensis and Citrus grandis seedlings were treated for six months with 0.5 (control), 200, 300, or 400 μM CuCl2. Forty-one and 37 differentially abundant protein (DAP) spots were identified in Cu-treated C. grandis and C. sinensis leaves, respectively, including some novel DAPs that were not reported in leaves and/or roots. Most of these DAPs were identified only in C. grandis or C. sinensis leaves. More DAPs increased in abundances than DAPs decreased in abundances were observed in Cu-treated C. grandis leaves, but the opposite was true in Cu-treated C. sinensis leaves. Over 50% of DAPs were associated with photosynthesis, carbohydrate, and energy metabolism. Cu-toxicity-induced reduction in leaf CO2 assimilation might be caused by decreased abundances of proteins related to photosynthetic electron transport chain (PETC) and CO2 assimilation. Cu-effects on PETC were more pronounced in C. sinensis leaves than in C. grandis leaves. DAPs related to antioxidation and detoxification, protein folding and assembly (viz., chaperones and folding catalysts), and signal transduction might be involved in Citrus Cu-toxicity and Cu-tolerance.
Keywords: Citrus grandis, Citrus sinensis, CO2 assimilation, copper-toxicity, 2-DE, leaves
1. Introduction
Microelement copper (Cu) is highly toxic to plants when in excess. Cu-containing fungicides and bactericides are widely used in agriculture to control fungal and bacterial diseases in crops including Citrus in order to improve crop production and quality. Cu contamination in agriculture soils is on the rise all over the world [1,2]. Cu accumulation in soils can cause Cu-toxicity and related nutritional disorders, resulting in a series of adverse effects on plants ranging from morphological and physiological to molecular levels [1,3]. In old Citrus orchards, the excess accumulation of Cu in soils is a common phenomenon because of the extensive and continued use of Cu-containing agricultural chemicals against fruit and foliar diseases such as anthracnose and canker [3,4]. Cu concentration and availability in soils under continuous Citrus production orchards increase with increasing production period [2]. In Citrus, the common Cu-toxic symptoms include leaf iron (Fe) chlorosis, poor growth, and stunted, and discolored root systems [3,5].
Cu, which can act as a cofactor for over 100 proteins including plastocyanin, laccase, cytochrome c oxidase, Cu/zinc (Zn) superoxide dismutase (SOD), ethylene receptors, amino oxidase, polyphenol oxidases, ascorbate (ASC) oxidase, diamine oxidases, and phytocyanin, is involved in photosynthesis, respiration, ATP biosynthesis, ethylene reception, reactive oxygen species (ROS) metabolism, cell wall formation, and carbon, lipid, and nitrogen metabolisms [6]. Accordingly, a lot of researchers have examined the toxic effects of Cu on the uptake of nutrients and water [1,5], growth [1,3], photosynthetic pigment production [7], photosynthetic electron transport [5,8], CO2 assimilation [8], carbohydrate and nitrogen (N) metabolism [7,9], respiration [10], hormonal status [11], cell wall metabolism [12], phenolic metabolism [13], as well as ROS generation and detoxification [8].
Although Cu-toxic effects on plant growth and physiology have been investigated in some details [2,14], little is known about Cu-toxicity-induced alteration of protein profiles in plants. Proteomics is a powerful approach to elucidate the complicated responses of plants to unfavorable environments [15,16]. Recently, there have been several reports investigating Cu toxicity responsive proteins. Most reports, however, have focused on herbaceous plants, including rice [17,18,19], Allium cepa [20], Oenothera glazioviana [21], Arabidopsis [22], Cannabis sativa [23], Agrostis capillaris [24], Elsholtzia splendens [25,26], sorghum [27,28] and wheat [29], while only one study investigated Cu-toxic effects on protein profiles in leaves of woody plant Eucalyptus camaldulensis [30]. Also, most of the above studies mainly focused on Cu-toxicity-responsive proteins occurring in roots because Cu is preferentially accumulated in Cu-stressed roots, while only few studies investigated Cu-toxic effects on protein profiles in leaves [25,27,29,30]. Evidence shows that the toxic effects of Cu on plant proteomics vary with Cu concentration, plant species, populations and/or cultivars, and plant tissues [17,18,24,25,27,28,29]. Therefore, more extensive proteomic research on the leaves of woody plants is needed to elucidate the molecular mechanisms of plants under Cu-toxicity.
Here, a two-dimensional electrophoresis (2-DE) based mass spectrometry (MS) approach was used to examine Cu-toxicity-responsive proteins in Citrus grandis and Citrus sinensis leaves. Meanwhile, we examined excess Cu effects on seedling growth, and leaf Cu concentration and gas exchange. The objectives were (a) to identify Cu toxicity responsive proteins in Citrus leaves and (b) to screen the candidate proteins possibly responsible for Cu tolerance in Citrus.
2. Results
2.1. Excess Cu-Effects on Seedling Growth, Leaf Cu and Gas Exchange
As shown in Supplementary Figures S1 and S2, C. sinensis (C. grandis) biomass remained little changed as Cu concentration in the nutrient solution elevated from 0.5 to 300 (200) μM, then declined at 400 (300–400) μM Cu. Biomass was lower in C. sinensis seedlings than that in C. grandis seedlings at each given Cu supply.
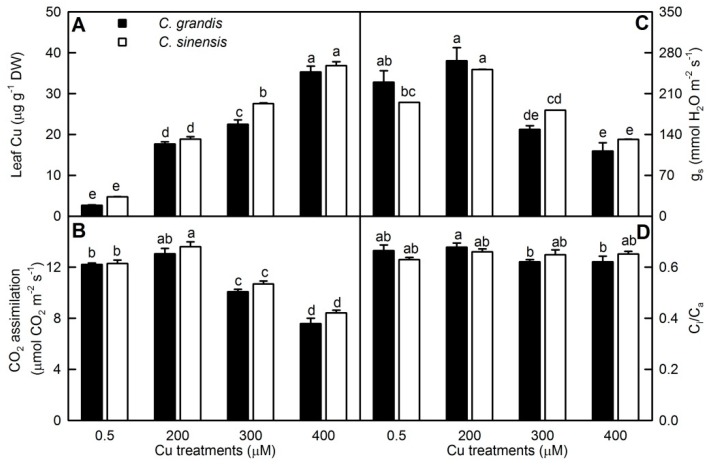
Leaf Cu concentration increased with Cu supply and did not differ between the two Citrus species with the exception that its concentration in leaves was higher in C. sinensis than that in C. grandis at 300 μM (Figure 1A).
Figure 1.
Cu-effects on Cu concentration (A), CO2 assimilation (B), stomatal conductance (gs, C) and ratio of intercellular to ambient CO2 concentration (Ci/Ca, D) in Citrus grandis and Citrus sinensis leaves. Bars represent means ± SE (n = 8 except for 4 for leaf Cu). Different letters above the bars indicate significant differences at p < 0.05.
Leaf CO2 concentration and stomatal conductance (gs) kept unchanged or increased as Cu concentration in the nutrient solution rose from 0.5 to 200 μM, then declined with further rise in Cu concentration. Cu supply had little influence on the ratio of intercellular to ambient CO2 concentration (Ci/Ca) except for that Ci/Ca in C. grandis leaves was slightly higher at 200 μM Cu than that at 300–400 μM Cu. No significant differences were observed in the three parameters between the two Citrus species over the range of Cu supply (Figure 1B–D).
Based on these results, seedlings that received 300–400 μM Cu were regarded as Cu excess.
2.2. Protein Yield and Cu-responsive Proteins in Leaves
Three biological replicates were performed in order to obtain reliable data. No significant differences were observed in protein yields and the number of protein spots per gel among eight means (Table 1, Figure 2, Supplementary Figures S3 and S4).
Table 1.
Protein yield, number of differentially abundant protein (DAP) spots and number of identified DAP spots in 0.5 (control), 200 (Cu200), 300 (Cu300) or 400 (Cu400) Cu-treated Citrus grandis and C. sinensis leaves.
| Citrus grandis | Citrus sinensis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control | Cu200 | Cu300 | Cu400 | Total | Control | Cu200 | Cu300 | Cu400 | Total | |
| Protein yield (mg g−1DW) | 49.4 ± 5.1a | 45.3 ± 0.7a | 47.6 ± 1.2a | 44.7 ± 8.3a | 44.4 ± 5.0a | 43.8 ± 5.4a | 40.9 ± 5.4a | 35.6 ± 3.1a | ||
| Number of spots per gel | 613 ± 4a | 627 ± 8a | 621 ± 12a | 621 ± 22a | 614 ± 7a | 625 ± 15a | 617 ± 12a | 618 ± 9a | ||
| Number of DAP spots | ||||||||||
| Increased in abundances | 15 | 12 | 13 | 2 | 4 | 5 | ||||
| Decreased in abundances | 4 | 2 | 7 | 8 | 12 | 18 | ||||
| Disappeared | 2 | 2 | 1 | 13 | ||||||
| Total | 19 | 14 | 22 | 42 | 12 | 17 | 36 | 45 | ||
| Number of identified DAP spots | ||||||||||
| Increased in abundances | 15 | 12 | 12 | 2 | 4 | 5 | ||||
| Decreased in abundances | 4 | 2 | 7 | 7 | 11 | 12 | ||||
| Disappeared | 2 | 2 | 1 | 11 | ||||||
| Total | 19 | 14 | 21 | 41 | 11 | 16 | 28 | 37 | ||
Note: Means (±SE, n = 3) with a row followed by the same letter are not significant different at p < 0.05.
Figure 2.
Representative 2-DE images of proteins extracted from 0.5 (A,E), 200 (B,F) 300 (C,G) and 400 (D,H) Cu-treated Citrus grandis (A–D) and Citrus sinensis (E–H) leaves.
As shown in Table 1 and Table 2, Supplementary Table S1 and Figure S5, a total of 42 and 45 differentially abundant protein (DAP) spots were obtained from Cu-treated C. grandis and C. sinensis leaves, respectively. All of these DAP spots were submitted to matrix-assisted laser desorption/ionization tandem time-of-flight mass spectrometry (MALDI-TOF/TOF-MS) based identification. In total, 41 and 37 DAP spots were identified in 200, 300, and/or 400 μM Cu-treated C. sinensis and C. grandis leaves, responsively. Most of these DAP spots only presented in Cu-treated C. sinensis or C. grandis leaves. Only seven DAPs with the same accession number [viz., Orange1.1t05091.1, S-adenosyl-L-homocysteine (AdoHcy) hydrolase (Orange1.1t01892.1), chaperonin CPN60-1, (Orange1.1t01459.2), major allergen Pru ar 1 (Cs9g03630.1), ribulose bisphosphate carboxylase/oxygenase (Rubisco) activase 1 (Cs7g31800.3), sedoheptulose-1,7-bisphosphatase (Cs7g31640.4), and 29 kDa ribonucleoprotein A (CP29A; Cs6g11900.1)] presented in the two Citrus species. Fifteen, 12 and 12 (2, 4, and 5) spots increased in abundances and 4, 2, and 9 (9, 12, and 23) spots decreased (including disappeared) in abundances were identified in 200, 300, or 400 μM Cu-treated C. grandis (C. sinensis) leaves, responsively. Obviously, more (less) DAPs increased in abundances than DAPs decreased in abundances were obtained in 200, 300, or 400 μM Cu-treated C. grandis (C. sinensis) leaves. For C. grandis, 10, 6, or 14 DAP spots were identified only in 200, 300 or 400 μM Cu-treated leaves, respectively, only 2 DAP spots with the same accession number (viz., malate dehydrogenase (MDH, Cs9g10470.1) and glutathione S-transferase (GST, Cs5g32800.1)) were shared by the three. For C. sinensis, 3, 3, or 15 DAP spots were identified only in 200, 300, or 400 μM Cu-treated leaves, respectively, only 2 DAP spots with the same accession number (viz., ferritin-3 (Cs6g09150.2) and enolase (Cs6g15540.1)) were shared by the three.
Table 2.
Differentially abundant protein (DAP) spots and their identification by MALDI-TOF/TOF-MS in 0.5 (control), 200 (Cu200), 300 (Cu300) or 400 (Cu400) Cu-treated Citrus grandis and C. sinensis leaves.
| Spot No. | Protein Identity | Accession No | Mr(kDa)/PI Exp. | Mr(kDa)/PI Theor. | Protein Score | Peptide Ions | NMP | Ratio | CS (%) | Charge | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cu0.5 | Cu200 | Cu300 | Cu400 | ||||||||||
| Citrus Grandis | |||||||||||||
| Photosynthesis, Carbohydrate and Energy Metabolism | |||||||||||||
| G1 | 29 kDa ribonucleoprotein A, chloroplastic; Ribonucleoprotein At2g37220, chloroplastic | Cs6g11900.1 | 30.37/5.17 | 36.20/4.33 | 358 | 186 | 9 | 1.00 ± 0.11c | 1.68 ± 0.19ab | 1.20 ± 0.14bc | 2.24 ± 0.26a | 8 | 1 |
| G30 | Photosystem II stability/assembly factor HCF136, chloroplast, putative | Cs7g13970.1 | 45.06/8.46 | 48.11/5.91 | 902 | 42 | 22 | 1.00 ± 0.11b | 1.19 ± 0.20b | 1.17 ± 0.13b | 1.91 ± 0.12a | 19 | 1 |
| G31 | Photosystem II stability/assembly factor HCF136, chloroplast, putative | Cs7g13970.1 | 53.11/5.75 | 59.08/5.94 | 684 | 172 | 28 | 1.00 ± 0.23b | 1.23 ± 0.25b | 2.40 ± 0.19a | 1.58 ± 0.21b | 24 | 1 |
| G10 | Ferredoxin—NADP reductase, leaf-type isozyme, chloroplastic | Cs1g25510.4 | 55.49/5.09 | 62.32/4.92 | 74 | 47 | 14 | 1.00 ± 0.11a | 0.53 ± 0.08b | 0.69 ± 0.10ab | 0.36 ± 0.16b | 12 | 1 |
| G42 | Ferredoxin—NADP reductase, leaf-type isozyme, chloroplastic | Cs1g25510.4 | 40.48/8.68 | 35.76/6.62 | 458 | 122 | 26 | 1.00 ± 0.23b | 3.66 ± 0.24a | 1.64 ± 0.10b | 1.54 ± 0.32b | 23 | 1 |
| G8 | RuBisCO subunit binding-protein alpha subunit, chloroplast, putative, expressed; Chaperonin 60 subunit alpha 1, chloroplastic | Cs8g16040.3 | 61.50/5.23 | 68.61/4.72 | 1350 | 169 | 47 | 1.00 ± 0.22b | 0.31 ± 0.09c | 1.86 ± 0.24a | 1.06 ± 0.13b | 41 | 1 |
| G9 | Ribulose bisphosphate carboxylase/oxygenase activase 1, chloroplastic | Cs7g31800.3 | 47.86/6.29 | 53.65/5.10 | 617 | 119 | 17 | 1.00 ± 0.20ab | 1.38 ± 0.05a | 0.80 ± 0.08bc | 0.48 ± 0.08c | 24 | 1 |
| G6 | Sedoheptulose-1,7-bisphosphatase, chloroplastic | Cs7g31640.4 | 42.40/5.82 | 48.51/4.64 | 489 | 84 | 29 | 1.00 ± 0.22b | 1.66 ± 0.20a | 1.09 ± 0.18ab | 1.32 ± 0.10ab | 25 | 1 |
| G38 | Glyceraldehyde-3-phosphate dehydrogenase B, chloroplastic | Cs3g27520.2 | 48.00/7.10 | 52.45/6.38 | 515 | 104 | 30 | 1.00 ± 0.27b | 2.43 ± 0.31a | 1.42 ± 0.08b | 1.34 ± 0.14b | 26 | 1 |
| G29 | Triosephosphate isomerase, cytosolic (Fragment) | Cs5g16495.1 | 26.96/5.73 | 32.66/6.15 | 326 | 129 | 7 | 1.00 ± 0.04ab | 1.58 ± 0.36a | 0.63 ± 0.12b | 0.18 ± 0.10c | 6 | 1 |
| G35 | Triosephosphate isomerase, cytosolic | Cs7g32500.1 | 26.96/5.73 | 31.99/6.16 | 236 | 125 | 14 | 1.00 ± 0.34a | 0.69 ± 0.14a | 0.51 ± 0.24a | 0 | 11 | 1 |
| G41 | Triosephosphate isomerase, cytosolic | Cs8g18560.2 | 27.24/5.75 | 33.66/6.67 | 428 | 102 | 17 | 1.00 ± 0.14b | 1.97 ± 0.27a | 1.49 ± 0.05ab | 1.16 ± 0.19b | 15 | 1 |
| G4 | Probable 6-phosphogluconolactonase 4, chloroplastic | Orange1.1t02542.1 | 35.38/6.24 | 34.54/4.72 | 1050 | 179 | 25 | 1.00 ± 0.10b | 2.05 ± 0.35a | 2.04 ± 0.28a | 1.30 ± 0.20ab | 20 | 1 |
| G36 | Fructose-1,6-bisphosphatase, cytosolic | Cs3g21280.1 | 37.65/5.95 | 47.35/6.37 | 408 | 80 | 22 | 1.00 ± 0.42b | 4.05 ± 1.70a | 2.31 ± 0.11ab | 1.63 ± 0.18ab | 19 | 1 |
| G17 | Malate dehydrogenase [NADP], chloroplastic | Cs7g21820.2 | 47.97/6.37 | 52.65/5.59 | 654 | 104 | 29 | 1.00 ± 0.28a | 0.71 ± 0.05ab | 1.01 ± 0.02a | 0.32 ± 0.15b | 25 | 1 |
| G37 | Malate dehydrogenase, cytoplasmic | Cs9g10470.1 | 35.54/6.10 | 48.01/6.44 | 212 | 63 | 15 | 1.00 ± 0.28b | 1.66 ± 0.13a | 1.01 ± 0.01b | 0.82 ± 0.15b | 13 | 1 |
| G39 | Malate dehydrogenase, cytoplasmic | Cs9g10470.1 | 42.35/5.94 | 56.49/6.33 | 193 | 144 | 11 | 1.00 ± 0.21b | 2.76 ± 0.46a | 2.11 ± 0.20a | 2.35 ± 0.48a | 10 | 1 |
| G18 | ATP synthase subunit beta, mitochondrial | Cs2g13550.1 | 59.85/6.06 | 61.56/5.45 | 1240 | 203 | 21 | 1.00 ± 0.11bc | 0.73 ± 0.20c | 1.66 ± 0.12a | 1.40 ± 0.11ab | 18 | 1 |
| G23 | ATP synthase gamma chain, chloroplastic | Cs2g03080.1 | 40.62/6.08 | 45.19/5.67 | 594 | 115 | 25 | 1.00 ± 0.08bc | 0.64 ± 0.20c | 1.65 ± 0.09a | 1.38 ± 0.04ab | 22 | 1 |
| G26 | Bis(5’-adenosy l)-triphosphatase | Cs9g13060.1 | 17.37/5.94 | 18.09/5.99 | 216 | 105 | 7 | 1.00 ± 0.07b | 1.03 ± 0.37b | 1.47 ± 0.09ab | 1.86 ± 0.14a | 14 | 1 |
| G24 | Glucose-1-phosphate adenylyltransferase small subunit 2, chloroplastic | Cs2g18800.1 | 57.08/6.74 | 58.03/5.66 | 1120 | 121 | 45 | 1.00 ± 0.12b | 1.97 ± 0.42a | 2.24 ± 0.27a | 1.64 ± 0.31ab | 39 | 1 |
| G25 | Glucose-1-phosphate adenylyltransferase small subunit 2, chloroplastic | Cs2g18800.1 | 65.86/8.50 | 68.69/5.67 | 751 | 139 | 37 | 1.00 ± 0.18b | 2.11 ± 0.43a | 1.09 ± 0.08b | 1.36 ± 0.19ab | 32 | 1 |
| Antioxidation and Detoxification | |||||||||||||
| G40 | Glutathione S-transferase | Cs5g32800.1 | 23.83/6.17 | 30.07/6.75 | 389 | 100 | 20 | 1.00 ± 0.13b | 78.9 ± 28.10a | 93.20 ± 15.40a | 47.10 ± 8.39a | 17 | 1 |
| G34 | Glutathione S-transferase DHAR1, mitochondrial | Cs7g28340.4 | 23.85/6.18 | 30.34/6.46 | 544 | 127 | 21 | 1.00 ± 0.41a | 0.57 ± 0.33a | 0.26 ± 0.06a | 0 | 18 | 1 |
| G21 | Copper/zinc superoxide dismutase (Fragment) | Cs3g12000.1 | 15.09/5.47 | 19.86/5.80 | 83 | 46 | 7 | 1.00 ± 0.07b | 1.03 ± 0.41b | 1.18 ± 0.16b | 2.43 ± 0.51a | 12 | 1 |
| G33 | Manganese superoxide dismutase (Fragment) | Cs7g29850.1 | 25.29/6.79 | 28.64/6.34 | 520 | 107 | 19 | 1.00 ± 0.08b | 1.51 ± 0.15ab | 1.47 ± 0.16ab | 2.02 ± 0.47a | 17 | 1 |
| G16 | Quinone oxidoreductase-like protein At1g23740, chloroplastic | Cs7g08640.2 | 41.88/8.77 | 48.90/5.38 | 728 | 189 | 27 | 1.00 ± 0.12a | 1.17 ± 0.08a | 1.01 ± 0.04a | 0.66 ± 0.03b | 23 | 1 |
| Chaperones and Folding Catalysts | |||||||||||||
| G15 | Probable protein disulfide-isomerase A6 | Cs5g33860.2 | 41.75/6.91 | 44.67/5.58 | 522 | 130 | 28 | 1.00 ± 0.11b | 1.45 ± 0.18ab | 1.49 ± 0.03ab | 1.60 ± 0.21a | 24 | 1 |
| G12 | 20 kDa chaperonin, chloroplastic | Cs4g07030.2 | 26.59/8.89 | 30.93/5.32 | 874 | 186 | 27 | 1.00 ± 0.18b | 0.69 ± 0.13b | 1.66 ± 0.08a | 0.73 ± 0.19b | 23 | 1 |
| G11 | Heat shock cognate 70 kDa protein 2 | Cs7g29010.1 | 70.99/5.09 | 74.6/4.89 | 794 | 113 | 5 | 1.00 ± 0.18a | 0.81 ± 0.12ab | 0.52 ± 0.12b | 0.56 ± 0.04b | 4 | 1 |
| G19 | Chaperonin CPN60-1, mitochondrial, putative, expressed | Orange1.1t01459.2 | 61.73/5.85 | 68.57/5.40 | 632 | 60 | 46 | 1.00 ± 0.23b | 0.46 ± 0.04c | 1.76 ± 0.06a | 0.81 ± 0.22bc | 40 | 1 |
| Signal Transduction | |||||||||||||
| G3 | Calreticulin-1 | Cs3g15060.3 | 52.52/6.29 | 62.84/4.12 | 122 | 99 | 11 | 1.00 ± 0.33a | 0.72 ± 0.17ab | 0.54 ± 0.08ab | 0.19 ± 0.02b | 15 | 1 |
| G27 | Major allergen Pru ar 1 (Major pollen allergen Bet v 1-D/H; Major pollen allergen Bet v 1-A) | Cs9g03630.1 | 17.60/5.67 | 21.51/6.05 | 253 | 104 | 19 | 1.00 ± 0.09c | 3.02 ± 0.42a | 1.66 ± 0.28bc | 2.17 ± 0.49ab | 17 | 1 |
| G5 | 14-3-3 protein 7 (14-3-3-like protein GF14 epsilon) | Cs3g18200.2 | 28.86/4.92 | 37.48/4.61 | 670 | 174 | 25 | 1.00 ± 0.20b | 1.36 ± 0.08ab | 1.39 ± 0.13ab | 1.83 ± 0.16a | 21 | 1 |
| G22 | Annexin D1 | Cs3g18360.1 | 35.88/5.17 | 46.63/5.66 | 784 | 138 | 31 | 1.00 ± 0.72b | 3.18 ± 1.21ab | 4.77 ± 0.99a | 2.03 ± 0.43ab | 27 | 1 |
| Cellular Transport | |||||||||||||
| G28 | Ferritin-2, chloroplastic | Cs7g30630.1 | 29.47/5.41 | 32.63/5.90 | 499 | 155 | 27 | 1.00 ± 0.12a | 0.55 ± 0.12b | 0.88 ± 0.05ab | 1.21 ± 0.16a | 23 | 1 |
| Cell Wall and Cytoskeleton | |||||||||||||
| G7 | Tubulin beta-6 chain | Cs3g26180.1 | 50.38/4.75 | 62.28/4.68 | 775 | 162 | 30 | 1.00 ± 0.10b | 3.48 ± 0.55a | 1.59 ± 0.08b | 1.23 ± 0.39b | 26 | 1 |
| G2 | Endochitinase 1 | Cs8g01850.1 | 35.39/4.85 | 52.52/4.16 | 75 | 33 | 9 | 1.00 ± 0.16a | 0.55 ± 0.27ab | 0.44 ± 0.05b | 0.75 ± 0.09ab | 8 | 1 |
| Stress Response | |||||||||||||
| G14 | Abscisic stress-ripening protein 1-like | Cs3g21500.1 | 17.88/6.00 | 30.30/5.56 | 323 | 123 | 8 | 1.00 ± 0.35b | 1.75 ± 0.48b | 1.97 ± 0.10b | 5.75 ± 0.59a | 29 | 1 |
| Others | |||||||||||||
| G20 | Orange1.1t05091.1 | 157.30/6.83 | 19.29/5.70 | 161 | 12 | 31 | 1.00 ± 0.16c | 1.38 ± 0.32bc | 1.80 ± 0.26ab | 2.31 ± 0.19a | 30 | 1 | |
| G32 | S-adenosyl-L-homocysteine hydrolase (adenosylhomocysteinase) | Orange1.1t01892.1 | 80.71/6.26 | 77.69/6.12 | 577 | 97 | 35 | 1.00 ± 0.18b | 2.72 ± 0.72a | 0.87 ± 0.09b | 0.79 ± 0.10b | 3 | 1 |
| Unidentified Protein Spots | |||||||||||||
| G13 | Receptor serine-threonine protein kinase, putative | Cs9g04750.2 | 25.57/8.87 | 23.24/5.56 | 45 | 109 | 13 | 1.00 ± 0.15b | 1.06 ± 0.28b | 1.13 ± 0.25b | 2.12 ± 0.35a | 22 | 1 |
| Citrus Sinensis | |||||||||||||
| Photosynthesis, Carbohydrate and Energy Metabolism | |||||||||||||
| S19 | Chlorophyll a-b binding protein 8,chloroplastic | Cs3g06180.2 | 29.52/6.84 | 32.89/5.42 | 222 | 95 | 12 | 1.00 ± 0.25b | 1.80 ± 0.27a | 1.28 ± 0.05ab | 0.44 ± 0.09c | 10 | 1 |
| S41 | Protease Do-like 1, chloroplastic | Cs2g28080.1 | 53.11/5.75 | 95.23/4.75 | 553 | 139 | 17 | 1.00 ± 0.15a | 0.12 ± 0.05c | 0.68 ± 0.04ab | 0.51 ± 0.10bc | 15 | 1 |
| S13 | PsbP domain-containing protein 3, chloroplastic | Cs3g27720.1 | 27.63/8.28 | 21.66/5.68 | 366 | 126 | 9 | 1.00 ± 0.17a | 0.81 ± 0.11ab | 0.55 ± 0.10bc | 0.36 ± 0.11c | 12 | 1 |
| S2 | 29 kDa ribonucleoprotein A, chloroplastic; Ribonucleoprotein At2g37220, chloroplastic | Cs6g11900.1 | 30.37/5.17 | 45.1/6.21 | 418 | 195 | 13 | 1.00 ± 0.08a | 0.82 ± 0.16ab | 0.43 ± 0.09c | 0.53 ± 0.06bc | 11 | 1 |
| S32 | 29 kDa ribonucleoprotein A, chloroplastic; Ribonucleoprotein At2g37220, chloroplastic | Cs7g01430.1 | 28.53/7.78 | 33.75/5.11 | 392 | 114 | 19 | 1.00 ± 0.20a | 0.76 ± 0.02ab | 0.49 ± 0.14b | 0 | 23 | 1 |
| S17 | Oxygen-evolving enhancer protein 1, chloroplastic | Cs1g23450.1 | 35.38/5.83 | 24.74/5.56 | 261 | 116 | 7 | 1.00 ± 0.05c | 1.06 ± 0.01bc | 1.34 ± 0.10ab | 1.54 ± 0.14a | 19 | 1 |
| S3 | Carbonic anhydrase, chloroplastic | Cs2g28060.4 | 36.77/6.66 | 53.09/6.25 | 171 | 162 | 5 | 1.00 ± 0.26c | 3.02 ± 0.38ab | 3.14 ± 0.23a | 1.18 ± 0.35bc | 5 | 1 |
| S11 | Rubisco subunit binding-protein alpha subunit, chloroplast, putative, expressed; Chaperonin 60 subunit alpha 1, chloroplastic | Cs8g16040.1 | 61.50/5.23 | 99.02/5.94 | 1250 | 182 | 39 | 1.00 ± 0.1a | 0.70 ± 0.16a | 0.80 ± 0.17a | 0.27 ± 0.04b | 34 | 1 |
| S9 | Ribulose bisphosphate carboxylase/oxygenase activase 1, chloroplastic | Cs7g31800.3 | 50.90/5.33 | 81.78/6.10 | 505 | 107 | 21 | 1.00 ± 0.23a | 0.75 ± 0.10ab | 0.66 ± 0.10ab | 0.51 ± 0.04b | 28 | 1 |
| S14 | Ribulose bisphosphate carboxylase/oxygenase activase 1, chloroplastic | Cs7g31800.3 | 46.96/5.94 | 75.7/5.65 | 505 | 107 | 21 | 1.00 ± 0.11a | 0.65 ± 0.02b | 0.48 ± 0.05b | 0.45 ± 0.05b | 28 | 1 |
| S4 | Ribulose bisphosphate carboxylase/oxygenase activase 1, chloroplastic | Cs7g31800.3 | 46.96/5.94 | 83.26/6.21 | 579 | 139 | 19 | 1.00 ± 0.46a | 0 | 0.38 ± 0.03a | 0.77 ± 0.06a | 17 | 1 |
| S10 | Ribulose bisphosphate carboxylase/oxygenase activase 1, chloroplastic | Cs7g31800.3 | 46.96/5.94 | 79.78/6.02 | 641 | 175 | 24 | 1.00 ± 0.16b | 1.38 ± 0.30b | 2.16 ± 0.23a | 1.05 ± 0.04b | 21 | 1 |
| S21 | Phosphoribulokinase, chloroplastic | Cs3g08480.1 | 45.19/5.97 | 67.41/5.58 | 686 | 137 | 31 | 1.00 ± 0.05a | 1.01 ± 0.04a | 1.02 ± 0.15a | 0 | 27 | 1 |
| S33 | Sedoheptulose-1,7-bisphosphatase, chloroplastic | Cs7g31640.4 | 36.77/6.66 | 43.56/4.96 | 576 | 101 | 27 | 1.00 ± 0.06a | 1.02 ± 0.06a | 0.75 ± 0.24a | 0 | 23 | 1 |
| S44 | Malate dehydrogenase, mitochondrial | Cs7g25390.1 | 35.48/8.52 | 66.81/4.32 | 613 | 144 | 24 | 1.00 ± 0.02b | 1.18 ± 0.21ab | 0.95 ± 0.15b | 1.64 ± 0.26a | 21 | 1 |
| S45 | Malate dehydrogenase, mitochondrial | Cs7g25390.3 | 37.65/5.95 | 63.69/4.53 | 287 | 171 | 9 | 1.00 ± 0.16bc | 1.15 ± 0.2b | 0.46 ± 0.21c | 1.85 ± 0.22a | 8 | 1 |
| S30 | Enolase | Cs6g15540.1 | 15.09/5.47 | 18.14/4.99 | 928 | 154 | 30 | 1.00 ± 0.12a | 0.53 ± 0.07b | 0.63 ± 0.09b | 0.22 ± 0.08c | 26 | 1 |
| S36 | Enolase | Cs6g15540.1 | 47.79/5.54 | 89.59/5.04 | 531 | 136 | 15 | 1.00 ± 0.25b | 1.04 ± 0.22b | 1.78 ± 0.13a | 1.92 ± 0.11a | 47 | 1 |
| S43 | Dihydrolipoyllysine-residue succinyltransferase component of 2-oxoglutarate dehydrogenase complex 2, mitochondrial | Cs2g21190.3 | 40.39/6.95 | 87.84/4.79 | 268 | 144 | 8 | 1.00 ± 0.26a | 0.56 ± 0.20ab | 0.30 ± 0.06b | 0 | 25 | 1 |
| Antioxidation and Detoxification | |||||||||||||
| S1 | 2-Cys peroxiredoxin BAS1, chloroplastic | Cs6g13880.1 | 29.49/7.65 | 30.56/6.56 | 465 | 161 | 17 | 1.00 ± 0.10a | 0.89 ± 0.11a | 0 | 0.81 ± 0.19a | 15 | 1 |
| S20 | Cysteine synthase, chloroplastic/chromoplastic | Orange1.1t02144.1 | 41.35/8.29 | 56.87/5.55 | 880 | 197 | 28 | 1.00 ± 0.14a | 0 | 0.91 ± 0.09a | 1.17 ± 0.06a | 21 | 1 |
| S39 | Cysteine synthase | Cs9g06970.1 | 29.29/6.78 | 60.56/4.69 | 114 | 49 | 6 | 1.00 ± 0.28a | 1.04 ± 0.12a | 0.78 ± 0.15a | 0 | 17 | 1 |
| S34 | L-ascorbate peroxidase 1, cytosolic | Cs8g17370.1 | 28.68/5.42 | 46.45/5.05 | 392 | 114 | 19 | 1.00 ± 0.05a | 0.82 ± 0.18a | 0.77 ± 0.14a | 0.35 ± 0.01b | 23 | 1 |
| S24 | Glutathione peroxidase (Fragment) | Cs5g03830.1 | 18.58/5.72 | 25.08/5.15 | 646 | 135 | 21 | 1.00 ± 0.14a | 1.02 ± 0.07a | 0.88 ± 0.16ab | 0.55 ± 0.12b | 30 | 1 |
| Chaperones and Folding Catalysts | |||||||||||||
| S16 | Luminal-binding protein 5 | Cs5g01840.2 | 73.56/5.09 | 108.09/5.72 | 578 | 112 | 27 | 1.00 ± 0.18b | 1.07 ± 0.21b | 2.57 ± 0.19a | 2.39 ± 0.23a | 23 | 1 |
| S8 | Peptidyl-prolylcis-transisomerase CYP37, chloroplastic | Cs1g06710.1 | 50.39/6.42 | 58.95/5.92 | 109 | 92 | 5 | 1.00 ± 0.13a | 1.03 ± 0.07a | 0.73 ± 0.05ab | 0.66 ± 0.12b | 2 | 1 |
| S26 | Chaperonin CPN60-1, mitochondrial, putative, expressed | Orange1.1t01459.2 | 46.12/8.24 | 52.38/5.28 | 727 | 152 | 39 | 1.00 ± 0.13a | 0.90 ± 0.14a | 1.16 ± 0.12a | 0 | 34 | 1 |
| Signal Transduction | |||||||||||||
| S35 | Major allergen Pru ar 1 (Major pollen allergen Bet v 1-D/H; Major pollen allergen Bet v 1-A) | Cs9g03630.1 | 48.33/6.19 | 89.59/5.09 | 230 | 94 | 15 | 1.00 ± 0.07a | 0.57 ± 0.15b | 1.20 ± 0.13a | 0.91 ± 0.05ab | 13 | 1 |
| S7 | 14-3-3 protein 6 | Orange1.1t01991.1 | 29.44/4.84 | 45.11/6.09 | 439 | 137 | 18 | 1.00 ± 0.18a | 0.28 ± 0.02b | 0.51 ± 0.13ab | 0.54 ± 0.04ab | 16 | 1 |
| Cellular Transport | |||||||||||||
| S5 | Ferritin-3, chloroplastic | Cs6g09150.2 | 28.97/5.46 | 41.03/5.97 | 406 | 129 | 13 | 1.00 ± 0.03a | 0.65 ± 0.03b | 0.61 ± 0.12b | 0.46 ± 0.03b | 33 | 1 |
| Nucleic acid Metabolism | |||||||||||||
| S42 | RuvB-like helicase 1 | Cs6g16920.1 | 38.08/6.90 | 90.61/4.73 | 268 | 99 | 16 | 1.00 ± 0.17a | 0.82 ± 0.33a | 0.56 ± 0.08a | 0 | 25 | 1 |
| Others | |||||||||||||
| S27 | Orange1.1t05091.1 | 53.64/5.26 | 85.72/5.32 | 246 | 73 | 19 | 1.00 ± 0.38a | 0.15 ± 0.01b | 0.23 ± 0.04b | 0.44 ± 0.18ab | 17 | 1 | |
| S28 | Orange1.1t05091.1 | 61.73/5.85 | 95.17/5.28 | 212 | 70 | 16 | 1.00 ± 0.24a | 0.91 ± 0.15a | 0.32 ± 0.05b | 0.60 ± 0.07ab | 14 | 1 | |
| S31 | Orange1.1t05091.1 | 177.77/7.11 | 24.86/4.86 | 170 | 45 | 15 | 1.00 ± 0.07a | 0.75 ± 0.15ab | 0.53 ± 0.12b | 0 | 14 | 1 | |
| S23 | Anthranilate N-methyltransferase | Cs5g24940.1 | 39.48/5.20 | 23.56/5.23 | 300 | 146 | 17 | 1.00 ± 0.03a | 0.93 ± 0.02a | 0.27 ± 0.10b | 0 | 15 | 1 |
| S37 | S-adenosyl-L-homocysteine hydrolase (adenosylhomocysteinase) | Orange1.1t01892.1 | 17.60/5.67 | 26.91/4.83 | 776 | 172 | 24 | 1.00 ± 0.12a | 1.18 ± 0.07a | 0.73 ± 0.24a | 0 | 21 | 1 |
| S38 | Dihydroflavonol-4-reductase | Cs3g01140.1 | 15.15/4.94 | 43.76/4.84 | 396 | 152 | 14 | 1.00 ± 0.07a | 1.28 ± 0.35a | 2.04 ± 0.45a | 0 | 12 | 1 |
| Unidentified Protein Spots | |||||||||||||
| S6 | Light-harvesting chlorophyll-a/b binding protein Lhca6 (Fragment) | Cs7g27290.1 | 26.56/5.43 | 35.60/5.91 | 105 | 50 | 4 | 1.00 ± 0.24a | 0.87 ± 0.06ab | 0.71 ± 0.09ab | 0.49 ± 0.02b | 31 | 1 |
| S12 | Thioredoxin M-type, chloroplastic | Cs3g20630.1 | 19.91/8.83 | 16.79/5.85 | 60 | 43 | 4 | 1.00 ± 0.18a | 0.95 ± 0.13a | 0.80 ± 0.08ab | 0.40 ± 0.17b | 7 | 1 |
| S15 | Nicotinate-nucleotide pyrophosphorylase [carboxylating], putative | Orange1.1t04780.1 | 55.49/5.09 | 91.64/5.82 | 50 | 131 | 26 | 1.00 ± 0.26a | 0.79 ± 0.18ab | 0.59 ± 0.04ab | 0.30 ± 0.02b | 58 | 1 |
| S18 | Disease resistance protein RFL1, putative | Cs3g08210.1 | 49.77/9.44 | 31.35/5.42 | 50 | 18 | 10 | 1.00 ± 0.20a | 0.39 ± 0.13b | 0.33 ± 0.04b | 0.19 ± 0.1b | 17 | 1 |
| S22 | Dehydration-responsive family protein, putative, expressed | Orange1.1t00308.3 | 49.93/5.04 | 88.43/5.57 | 57 | 117 | 24 | 1.00 ± 0.19a | 0.94 ± 0.06ab | 0.87 ± 0.02ab | 0.65 ± 0.07b | 57 | 1 |
| S25 | Transducin/WD40 domain-containing protein-like protein | Cs9g09840.1 | 29.76/6.18 | 31.98/5.16 | 67 | 41 | 9 | 1.00 ± 0.14a | 0.83 ± 0.15a | 0.51 ± 0.12a | 0 | 8 | 1 |
| S29 | ATPase 8, plasma membrane-type | Cs4g01370.1 | 14.72/5.41 | 22.10/5.07 | 68 | 98 | 11 | 1.00 ± 0.09a | 0.90 ± 0.07ab | 0.72 ± 0.05bc | 0.50 ± 0.09c | 21 | 1 |
| S40 | 4-hydroxy-3-methylbut-2-enyl diphosphate reductase | Cs5g28200.1 | 52.22/6.60 | 81.69/4.72 | 63 | 49 | 5 | 1.00 ± 0.16a | 0.68 ± 0.06a | 0.66 ± 0.14a | 0 | 17 | 1 |
Note: Spot number corresponds to the 2-DE imagines in Figure 2. Ratio means the ratio of 0.5, 200, 300 or 400 μM Cu-treated leaves to 0.5 μM Cu-treated leaves. NMP: the number of matched peptides; CS: covered sequence. Means (±SE, n = 3) with a row followed by different letters are significant different at p < 0.05.
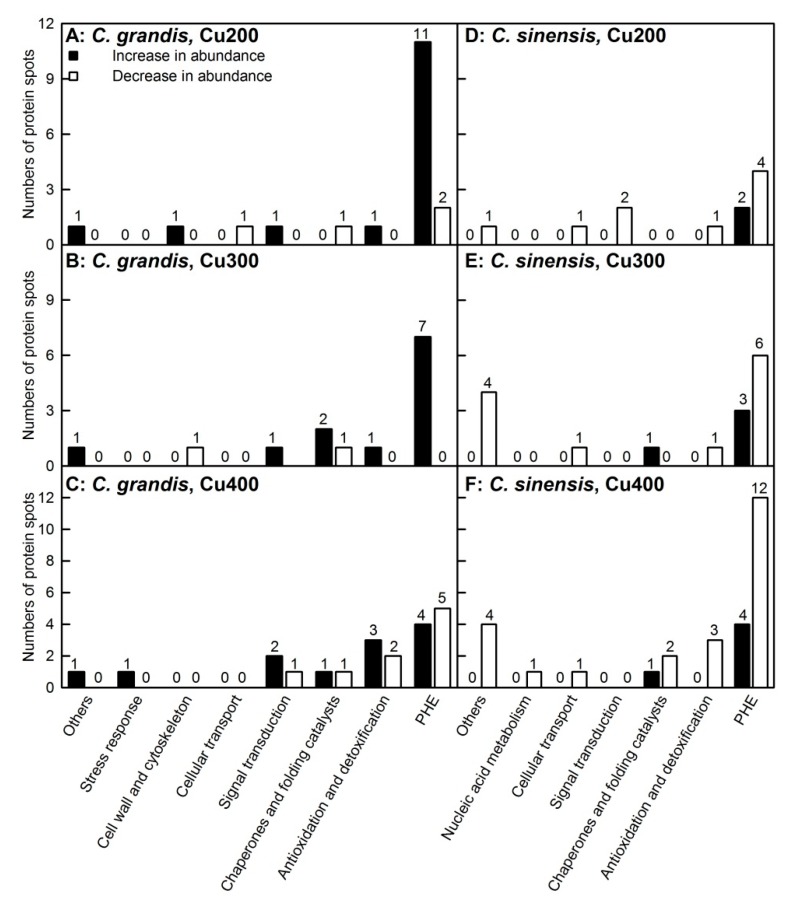
DAPs were mainly involved in photosynthesis, carbohydrate and energy metabolism, antioxidation and detoxification, protein folding and assembly (viz., chaperones and folding catalysts), and others. Cell wall, cytoskeleton (G7 and G2), and stress response (G14) related DAPs were obtained only in Cu-treated C. grandis leaves, but nucleic acid metabolism related DAP (S42) was identified only in Cu-treated C. sinensis leaves (Table 2 and Figure 3).
Figure 3.
Differentially abundant proteins (DAPs) in 200, 300 and 400 μM Cu-treated Citrus grandis (A–C) and Citrus sinensis (D–F) leaves. PHE: photosynthesis, carbohydrate and energy metabolism.
2.3. KEGG Pathway Analysis of DAPs
For total DAPs in C. grandis leaves, there were eight significantly enriched KEGG pathways-namely carbon fixation in photosynthetic organisms (ko00710), exosome (ko04147), glycolysis/gluconeogenesis (ko00010), fructose and mannose metabolism (ko00051), photosynthesis (ko00195), chaperones and folding catalysts (ko03110), photosynthesis proteins (ko00194) and inositol phosphate metabolism (ko00562). Four, six, and ten KEGG pathways were significantly enriched by DAPs in 200, 300, and 400 μM Cu-treated C. grandis leaves, respectively. For total DAPs in C. sinensis leaves, carbon fixation in photosynthetic organisms, photosynthesis proteins, exosome, tricarboxylic acid (TCA) cycle (ko00020) and photosynthesis were the significantly enriched KEGG pathways. One [photosynthesis-antenna proteins (ko00196)], one (exosome) and five KEGG pathways were significantly enriched by DAPs in 200, 300 and 400 μM Cu-treated C. sinensis leaves, respectively (Supplementary Figure S6).
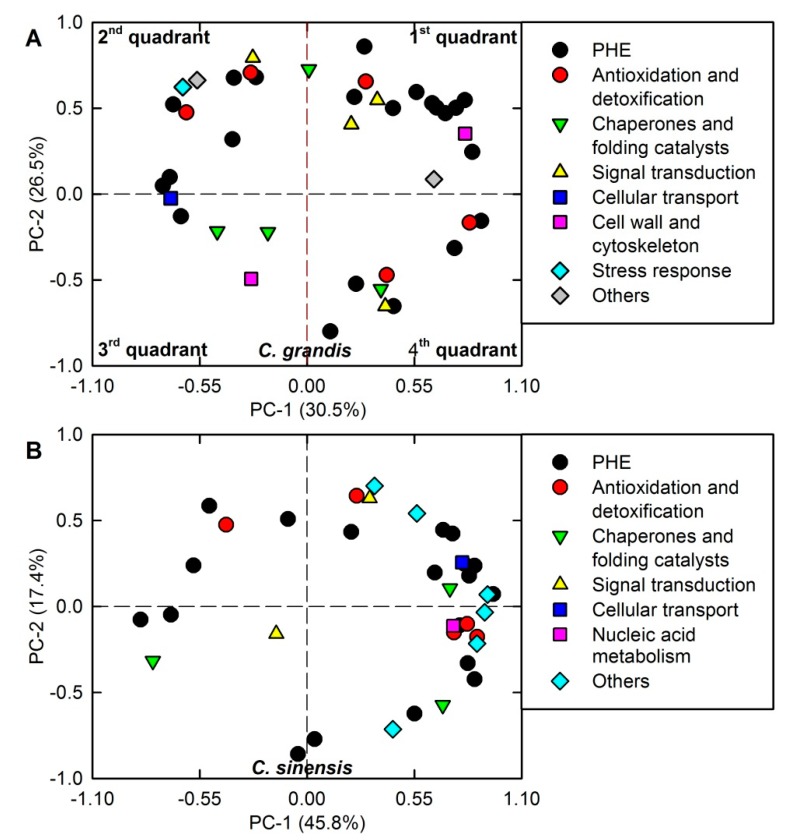
2.4. PCA Loading Plots and Correlation Matrices of DAPs
As shown in Figure 4 and Supplementary Tables S2 and S3, PC1 and PC2 accounted for 30.5% and 26.5%, and 45.8% and 17.4% of the total variation in C. grandis and C. sinensis leaves, respectively. The association patterns of DAPs were more obvious in C. sinensis leaves than those in C. grandis leaves. Similarly, more positive and negative relationships between DAP spots existed in C. sinensis leaves than those in C. grandis leaves (Supplementary Figure S7).
Figure 4.
Principal component analysis (PCA) of differentially abundant proteins (DAPs) in Cu-treated Citrus grandis (A) and Citrus sinensis (B) leaves. PHE: photosynthesis, carbohydrate and energy metabolism.
2.5. qRT-PCR Analysis of Genes for DAPs
The expression levels of genes for 22 DAPs from 400 μM Cu-treated C. grandis (viz., G3, G9, G10, G11, G14, G26, G29, G33, G34, and G35) and C. sinensis (viz., S2, S5, S9, S16, S17, S23, S24, S30, S32, S33, S37, and S43) leaves were analyzed by qRT-PCR. With the exceptions of G26, G33, S9, S16, S23, S32, and S33, the abundances of the other 16 DAPs matched well with the expression levels of the corresponding genes regardless of whether PRPF31 or actin served as an internal standard (Table 2 and Supplementary Figure S8).
3. Discussion
3.1. DAPs Related to Photosynthesis, Carbohydrate and Energy Metabolism
Excess Cu-treated C. grandis and C. sinensis leaves had lower CO2 assimilation (Figure 1) and higher concentrations of nonstructural carbohydrates relative to controls [5]. Accordingly, many Cu-toxicity-responsive proteins related to photosynthesis, carbohydrate and energy were identified in these leaves (Table 2 and Figure 3). Damkjær et al. reported that Arabidopsis mutants lacking light-harvesting chlorophyll (Chl) a/b binding protein Lhcb3 had a lower maximum photosystem (PSII) efficiency of dark-adapted leaves (Fv/Fm) than wild type under high light condition and still displayed a lower Fv/Fm after 7 d of recovery under normal light, implying that PSII in these plants suffered from photoinhibition under high light [31]. The abundance of Chl a-b binding protein 8 (Lhca3; S19) was increased and decreased in 200 and 400 μM Cu-treated C. sinensis leaves, respectively. Thus, the decreased abundance of Lhca3 in 400 μM Cu-treated C. sinensis leaves might contribute to the Cu-induced photoinhibition. This could explain why photoinhibition was slightly greater in 400 μM Cu-treated C. sinensis leaves than that in 400 μM Cu-treated C. grandis leaves [5]. Also, the abundance of protease Do-like 1 (DEGP1; S41) was decreased in 200 and 400 μM Cu-treated C. sinensis leaves. DEGP1, an enzyme responsible for the degradation of damaged proteins, plays a role in photoinhibition repair of PSII in Arabidopsis [32]. Also, the abundance of PsbP domain-containing protein 3 (PPD3, S13) involved in PSII light reaction was decreased in 300 and 400 μM Cu-treated C. sinensis leaves.
Phosphorylation of PSII antenna protein RNA-binding protein CP29, localized in chloroplasts, was induced under conditions of decreased photosynthetic capacity and excess light. Maize plants lacking the ability to perform the phosphorylation of CP29 were more sensitive to cold-induced photoinhibition [33]. CP29 phosphorylation has been indicated to play a role in lowering 1O2 generation and improving excess energy dissipation [34]. The abundance of CP29A (G1) was increased in 200 and 400 μM Cu-treated C. grandis leaves, while the abundances of CP29A (S2 and S32) were decreased in 300 and 400 μM Cu-treated C. sinensis leaves. The different response of CP20A to excess Cu between the two agreed with the report that excess Cu had less influence on Chl a fluorescence (OJIP) transients in C. grandis leaves than those in C. sinensis leaves [5]. Similarly, the abundance of PSII stability/assembly factor HCF136, an essential protein for the stability/assembly of PSII, was increased in 300 (G31) and 400 (G30) μM Cu-treated C. grandis leaves, but not in Cu-treated C. sinensis leaves. Increased abundance of HCF136 has been obtained in cadmium (Cd) treated Arabidopsis shoots [35]. However, the abundances of oxygen-evolving enhancer protein 1 (PSBO2, S17) were enhanced significantly in 400 μM Cu-treated C. sinensis leaves. PSBO2 is required for the stability of the photosynthetic water-splitting complex [36]. Interestingly, the damage of the oxygen evolving complexes (OEC) was greater in C. sinensis leaves than that in C. grandis leaves under 400 μM Cu [5]. Evidently, other factors play a role in stabilizing the water-splitting complex.
The abundance of G10 (ferredoxin-NADP reductase, leaf-type isozyme (LFNR2)) was decreased in 200 and 400 μM Cu-treated C. grandis leaves, and of G42 (LFNR2) was increased in 200 μM Cu-treated C. grandis leaves. LFNR oxidizes ferredoxin (Fd) to yield NADPH, which is utilized in various reactions such as lipid and Chl biosynthesis, CO2 fixation and stromal redox regulation. Arabidopsis fnr2 RNAi mutants had decreased concentrations of photosynthetic thylakoid proteins and Chls, and rate of carbon fixation relative to the wild type plants [37]. The abundances of Rubisco subunit binding-protein α subunit [chaperonin 60 subunit α 1 (Cpn60α1); S11] involved in protein folding and Rubisco activase 1 (G4, S4, and S14) involved in the activation of Rubisco were decreased in 400 μM Cu-treated C. sinensis and/or C. grandis leaves. The abundance of Rubisco activase 1 (S14) was also decreased in 200 and 300 μM Cu-treated C. sinensis leaves. Cpn60α1 is necessary for the folding of Rubisco large subunit (rbcL) and proper chloroplast development [38]. Rubisco activase-deficient transgenic tobacco plants had decreased Rubisco carbamylation and CO2 assimilation [39]. Also, the abundances of sedoheptulose-1,7-bisphosphatas (SBPase; S33) and phosphoribulokinase (PRK; S21) involved in Calvin cycle were decreased in 400 μM Cu-treated C. granids leaves. The lower abundances of LFNR2 (G10), Cpn60α1 (S11), Rubisco activase 1 (G4, S4, S9 and S14), PRK (S21) and SBPase (S33) agreed with our finding and previous report that excess Cu-treated Citrus leaves had reduced CO2 assimilation and Chl concentrations (Figure 1) [5]. However, the abundances of LFNR2 (G42), SBPase (G6) and glyceraldehyde-3-phosphate dehydrogenase B (GAPB, G38) were increased in 200 μM Cu-treated C. grandis leaves. This was agreement with the finding that CO2 assimilation displayed an upward trend in 200 μM Cu-treated C. grandis leaves relative to controls (Figure 1). Similarly, the abundance of fructose-1,6-bisphosphatase, cytosolic (cyFBPase; G36), a major site for controlling sucrose synthesis, was increased in 200 μM Cu-treated C. grandis. Strand et al. reported that photosynthesis was inhibited in antisense cyFBPase Arabidopsis mutants [40]. Also, the abundance of Cpn60α1 (G8) in C. grandis leaves was decreased and increased at 200 and 300 μM Cu, respectively, and the abundance of Rubisco activase 1 (S10) was increased in 300 μM Cu-treated C. sinensis leaves.
Carbonic anhydrase (CA, a Zn-metalloenzyme) is required for CO2 assimilation in cotyledons. The abundance of CA (S3) was increased or unaffected by Cu supply in C. sinensis leaves. However, CA activity was reduced in Cu excess Brassica juncea [41]. The difference between CA abundance and activity could be explained by the Cu-induced decrease in Zn level in C. sinensis [5], because its activity is regulated by Zn availability.
Mitochondrial MDH (mMDH) is necessary for CO2 and energy partitioning in leaves. Antisense mMDH tomato plants displayed increased photosynthetic electron transport rate, CO2 assimilation, gs and growth rate, but decreased respiration rate [42]. The increased abundances of mMDH (S44 and S45) in 400 μM Cu-treated C. sinensis leaves agreed with the report that 400 μM Cu-treated C. sinensis seedlings had decreased growth, leaf CO2 assimilation and gs, and impaired photosynthetic electron transport chain (PETC) [5]. Chloroplastic NADP-MDH, which catalyzes the excess NADPH produced through PETC and oxaloacetate to malate and NADP+, plays a key role in counteracting PETC over-reduction and in H2O2 signaling by exporting chloroplast NADPH to other cell compartments. Arabidopsis nadp-mdh mutants lacked the reversible inactivation of catalase activity and the concomitant accumulation of H2O2, but had a higher reduction state of the plastoquinone (PQ) pool when exposed to high light [43]. The decreased abundance of NADP-MDH (G17) in 400 μM Cu-treated C. grandis leaves might contribute to the Cu-induced inhibition of photosynthesis and the increased reduction of the PSII acceptor side, as indicated by the positive ΔJ- and ΔI-bands in 400 μM Cu-treated C. grandis leaves [5]. However, Cu treatments increased or did not alter the abundance of cytosolic MDH (cyMDH; G37 and G39) in C. grandis leaves. cyMDH plays a key role in the transport of chloroplast or mitochondria NADPH to other cell compartments. Transgenic apple plants overexpressing an apple cyMDH gene displayed a higher stress-tolerance accompanied by increased reducing power, as indicated by increased concentrations of ASC and reduced glutathione (GSH) and ratios of ASC/dehydroascorbate (DHA), GSH/GSSG and NAD(P)H/NAD(P)+ [44]. Thus, the Cu-toxicity-induced increases of cyMDH abundances in C. grandis leaves might be an adaptive strategy.
Pentose phosphate pathway (PPP) provides NADPH for biosynthesis of GSH and maintenance of cellular redox state necessary to deal with oxidative stress. Arabidopsis PGL3 T-DNA insertion mutants with decreased flux through the plastidial PPP displayed a decrease in plant size and a lower cellular redox potential [45]. The increased abundance of probable 6-phosphogluconolactonase 4 (PGL4, an enzyme involved in PPP; G4) in 200 and 300 μM Cu-treated C. grandis leaves agreed with the increased needs for ROS scavenging [5].
Triosephosphate isomerase (TPI), which catalyzing the reversible interconversion of glyceraldehydes 3-phosphate (GAP) and dihydroxyacetone phosphate (DHAP), may prevent the spontaneous degradation of DHAP into methylglyoxal (MG, a cytotoxic metabolite). TPI-deficiency led to increased generation of MG in red blood cells [46]. The decreased abundances of TPI (G29) in 400 μM Cu-treated C. grandis leaves implied that MG formation was increased in these leaves, thus increasing ROS generation and lipid peroxidation [5].
The increased abundances of glucose-1-phosphate adenylyltransferase (APS, G24, and G25) in 200 and 300 μM Cu-treated C. grandis leaves implied that starch biosynthesis was enhanced in these leaves. However, this way could not explain starch accumulation in 400 μM Cu-treated C. grandis leaves, because APS abundance was not increased in these leaves. A weaker sink for the photosynthetic requirement due to Cu toxicity-induced inhibition of growth has been suggested to be responsible for the accumulation of nonstructural carbohydrates including starch in Cu-toxic Citrus leaves [5].
There is a close relation between energy availability and stress-tolerance [47]. An extra energy supply is necessary for stressed plants to fortify their tolerance. The increased abundances of ATP synthase subunit β (G12) and ATP synthase γ chain (G23) 300 μM Cu-treated C. grandis leaves and bis(5’-adenosyl)-triphosphatase (Ap3A, G26) in 400 μM Cu-treated C. grandis leaves suggested that ATP biosynthesis was enhanced in these leaves to meet the increased energy needs. Similar result has been obtained in Cu-stressed Elsholtzia splendens leaves [25].
To conclude, Cu-toxicity might affect the abundances of proteins involved in PETC and CO2 assimilation, thus decreasing electron transport rate and CO2 assimilation. Cu-toxic effects on PETC were more pronounced in C. sinensis leaves than those in C. grandis leaves.
3.2. DAPs Related to Antioxidation and Detoxification
Five (five) DAP spots involved in antioxidation and detoxification were identified in Cu-treated C. sinensis (C. grandis) leaves (Table 2). The striking Cu-mediated alteration was the big increase in GST (G40) abundance in Cu-treated C. grandis leaves. Dianthus superbus plants overexpressing GST were observed to biosynthesize phytochelatins (PCs), thus sequestering and detoxifying excess Cu [48]. Lambda class of GSTs could be used to enhance plant tolerance against various stresses including heavy metals [49]. However, the abundance of GST DHAR1 (G34), an enzyme having glutathione-dependent thiol transferase and DHA reductase (DHAR) activities, was decreased in 400 μM Cu-treated C. grandis leaves. SOD can rapidly dismutate O2− to H2O2 and protect organisms against oxidative damage. The increased abundances of Cu/Zn SOD (G21) and manganese (Mn) SOD (G33) in 400 μM Cu-treated C. grandis leaves agreed with the report that Cu stress increased Cu/Zn SOD and Mn SOD activity in Arabidopsis leaves [50]. Cu/Zn SOD abundance increased and Fe SOD abundance decreased in Cu-sufficient Arabidopsis leaves, but the reverse was true in Cu-limited leaves, which could save Cu for the biosynthesis of plastocyanin necessary for photosynthesis [51]. Thus, excess Cu increased the biosynthesis of Cu/Zn SOD by a direct effect of Cu on the gene for SOD, hence preventing a Cu-toxic effect on photosynthesis. Methyl viologen (mainly to enhance PSI-originated ROS formation) induced decrease of Fv/Fm was more severe in aor [a chloroplastic NADPH-dependent alkenal/one oxidoreductase (AOR, At1g23740)] Arabidopsis mutants than in Col-0 plants, concluding that AOR played a role in the scavenging of stromal reactive carbonyls (RCs) generated under oxidative stress [52]. Therefore, the decreased abundance of quinone oxidoreductase-like protein At1g23740 (G16) in 400 μM Cu-treated C. grandis might contribute to the Cu-induced inhibition of photosynthesis by lowering the photosynthetic electron transport rate.
The abundances of all the five DAP spots were decreased in Cu-treated C. sinensis leaves. The decreased abundances of three H2O2 detoxifying enzymes in 300 (S1) and 400 (S24 and S34) μM Cu-treated C. sinensis leaves agreed with the report that H2O2 production was increased in these leaves [5]. Cysteine (Cys) synthase (CS) catalyzes the final step for Cys biosynthesis in plants. The overexpression of CS conferred tolerance to Cd and selenium (Se) by over-production of Cys, GSH and presumably PCs, but not to Cu in transgenic tobacco plants [53]. PCs have been proven not to be the major factor responsible for plant Cu-tolerance [54]. Thus, the Cu-induced decrease of CS abundance (S20 and S39) might not lower the tolerance of C. sinensis seedlings to Cu.
To conclude, the antioxidation and detoxification system as a whole could not effectively protect Citrus leaves from Cu-toxicity-induced oxidative stress, as indicated by the increased H2O2 production and electrolyte leakage [5].
3.3. Chaperones and Folding Catalysts
Luminal binding protein (BiP) functions in both protein folding and endoplasmic reticulum (ER) quality control mechanism. Heterologous expression of an ER BiP gene alleviated Cd-induced ER stress and programmed cell death in transgenic tobacco BY-2 cells [55]. Transgenic tobacco plants overexpressing an ER chaperone BiP gene had enhanced Cd-tolerance accompanied by decreased level of ROS and increased level of GSH [56]. Thus, the increased abundance of luminal-binding protein 5 (BiP5, S16) might contribute to Cu-tolerance of C. sinensis. Protein disulfide isomerase (PDI), which catalyzes thiol-disulfide interchange, is the most abundant oxidative protein folding catalyst and a multifunctional protein chaperone. PDI could serve as a Cu chelator or Cu delivering protein to protect cells against Cu-toxicity [57]. The increased abundance of probable PDI A6 (G15) in 400 μM Cu-treated C. grandis leaves might play a role in preventing these leaves from Cu-toxicity by binding Cu and/or decreasing oxidative damage. Like Cpn60α1 (G8), the abundance of 20 kDa chaperonin (Cpn20, a co-chaperonin of CPN60; G12) was increased in 300 μM Cu-treated C. grandis leaves. Cpn20 played a role in oxidative stress protection and chloroplast development via positively regulating the activation of Fe SOD [58]. Interestingly, the abundance of chaperonin CPN60-1 (G19 and S26) involved in the correct folding of imported proteins was decreased and increased in 200 and 300 μM Cu-treated C. grandis leaves, respectively, but was decreased in 400 μM Cu-treated C. sinensis leaves. Also, the abundance of heat shock cognate 70 kDa protein 2 (HSP70-2, G11) involved in the folding of de novo translocation of precursor proteins into organelles, and degradation of damaged protein under disadvantaged conditions was decreased in 300 and 400 μM Cu-treated C. grandis leaves. These results demonstrate the involvement of chaperones and folding catalysts in the Cu tolerance and Cu toxicity of Citrus.
3.4. DAPs Related to Signal Transduction
Plant plasma membrane (PM) H+-ATPase activity can be regulated by 14-3-3 proteins involved in brassinosteroid (BR)-mediated signaling pathway [59]. The increased abundance of 14-3-3 protein 7 (G5) in 400 μM Cu-treated C. grandis leaves agreed with the increased expression of a 14-3-3 gene in Fucus vesiculosus in response to moderately elevated level of Cu [60], and the increased activity of PM H+-ATPase in Cu-treated cucumber roots [61]. However, the abundance of 14-3-3 protein 6 (S7) was reduced in 200 μM Cu-treated C. sinensis leaves.
Major pollen allergen, which is involved in abscisic acid (ABA)-activated signaling pathway, have high sequence homology to pathogenesis related (PR) proteins. The increased or unaltered abundance of major allergen Pru ar 1 (G27) in 200–400 μM C. grandis leaves agreed with the elevated abundances of Bet v 1-Sc3 (PR-10c) and PvPR1 in Cu-stressed Betula pendula and bean leaves, respectively [62,63]. Annexins, a key element of Ca2+-signaling pathways, are involved in counteracting oxidative stress. Transgenic tobacco plants overexpressing an annexin displayed elevated total peroxidase activity, improved tolerance/resistance to Cd, oxidative stress and diseases, and increased message levels for several PR proteins [64]. The increased or unchanged abundance of annexin D1 (G22) in Cu-treated C. grandis leaves agreed with the increased abundance of annexin D1 in Cu-stressed Allium cepa roots [20]. Thus, Cu supply might enhance the resistance of C. grandis to diseases [65]. However, the abundance of major allergen Pru ar 1 (S35) was decreased in 200 μM Cu-treated C. sinensis leaves.
Calreticulin (CRT), a crucial Ca2+-binding protein mainly in the ER, functions in Ca2+ signaling in response to stress in plants. The decreased abundance of CRT-1 (G3) agreed the decreased abundance of CRT in excess Cu-treated Ectocarpus siliculosus [66] and the decreased expression level of CRT in Mg-deficient Citrus reticulata leaves [67], because Mg concentration was decreased in Cu-stressed C. grandis leaves [5].
To conclude, hormone (ABA and BR)- and Ca2+-mediated signaling pathways might function in Citrus Cu-tolerance and Cu-toxicity. This was also supported by data suggesting that 28-homobrassinolide [41] and Ca [68] could alleviate plant Cu-toxicity, and that a reciprocal cross-talk existed between Cu status and ABA metabolism and signaling in Arabidopsis [69].
3.5. DAPs Related to Cellular Transport, Nucleic Acid and Cell Wall Metabolisms, and Cytoskeleton
Ferritin can protect plant cells from Fe-toxicity by storing excess Fe in a non-toxic form in plant cells [70]. A characteristic of Cu-toxicity in Citrus leaves is Fe chlorosis [5,71]. The decreased abundance of ferritin-3 (S5) in 200–400 μM Cu-treated C. sinensis leaves agreed with the report that ferritin accumulation in plant cells increased under high Fe concentrations [72]. The decreased abundance of ferritin-3 might contribute to Fe homeostasis by lowering the chelation of Fe to ferritin.
Both α- and β-tubulins are the primary constituents of microtubules (MTs), one of the cytoskeletal components. MTs have been proposed to function in plant Cu-toxicity and Cu-tolerance. Song et al. found that the abundances of three protein spots-namely tubulin α-1 chain, putative tubulin α-1 chain and tubulin α-2 chain, were decreased in excess Cu-treated rice roots, concluding that the decreased accumulation of α-tubulin might impair MT polymerization and alignment, thus influencing MT functions [18]. However, the abundance of tubulin β-6 chain (G7) in C. grandis leaves increased or did not alter in response to Cu supply, implying that MTs might be not impaired in these leaves. This might be related to the preferential accumulation of most Cu in the roots under Cu-stress [5].
DNA helicases, which are ATP-dependent DNA unwinding enzymes, are involved in DNA repair, replication and recombination. Ectopic expression of a Medicago sativa helicase 1 (a homolog of the pea DNA helicase 4) gene conferred Arabidopsis tolerance to drought, salt and oxidative stress [73]. The decreased abundance of RuvB-like helicase 1 (S42) in 400 μM Cu-treated C. sinensis leaves implied that DNA repair was impaired in these leaves.
The decreased or unaltered abundance of endochitinase 1 (G2) related to cell wall polysaccharide (macromolecule) catabolic process in Cu-treated C. grandis leaves implied that the level of cell wall polysaccharides might be increased in these leaves because of decreased degradation. This agreed with the increased concentration of total polysaccharide in the cell walls of Cu-treated Elsholtzia splendens roots [26]. However, the abundance of chitinase was enhanced in rice leaves treated with 100 μM Cu for 72 h [74]. Chitinase activity was not altered in pepper roots, stems, and cotyledons after 28 days of treatment with 50 μM Cu [65]. Thus, it seems that the effects of Cu on chitinase vary with plant species, Cu concentration, and time of exposure to Cu.
3.6. Other DAPs
AdoHcy hydrolase, which catalyzes the reversible hydrolysis of AdoHcy to L-homocysteine and adenosine, plays a crucial role in maintaining methyl cycling via the removal of AdoHcy. Taddei et al. observed that AdoHcy hydrolase was induced by Cu stress in in vitro-cultured pith explants of Nicotiana glauca, suggesting that AdoHcy hydrolase played a crucial role in regulating Cu level and intracellular distribution [75]. B-induced alleviation of C. grandis Al-toxicity was accompanied by increased root expression of adenosylhomocysteinase-like [76]. The increased abundance of AdoHcy hydrolase (G32) in 200 μM Cu-treated C. grandis leaves might contribute to their Cu-tolerance. However, its (S37) abundance was decreased in 400 μM Cu-treated C. sinensis leaves.
Flavonoids can act as ROS scavengers, and inhibit ROS production by chelating metals. The decreased abundance of dihydroflavonol-4-reductase (DFR; S38) in 400 μM Cu-treated C. sinensis leaves suggested that anthocyanin biosynthesis might be decreased in these leaves. This disagreed with the increased expression level of DFR in Cu-stressed rice leaves [77].
4. Materials and Methods
4.1. Plant Materials
Seedling culture and Cu treatments were made according to Li et al. [5]. Briefly, 6-week-old uniform seedlings of ‘Xuegan’ (Citrus sinensis) and ‘Shatian pummelo’ (Citrus grandis) were transported to 6 L pots (two plants per pot) filled with sand thoroughly washed with tap water, then grown in a greenhouse under natural conditions at Fujian Agriculture and Forestry University. Six weeks after transporting, seedlings were watered daily with freshly papered nutrient solution at a Cu concentration of 0.5 (Cu0.5, control), 200 (Cu200), 300 (Cu300), or 400 (Cu400) μM from CuCl2 until nutrients begin to flow out of the bottom hole of the pot (~500 mL per pot). Nutrient solution pH was adjusted to 4.8 with 1 M HCl before supply. Six months after Cu treatments, the fully expanded (about 7-week-old) leaves were used for all measurements. Firstly, leaf gas exchange was measured. Then, leaves (winged leaves, petioles and midribs removed) were taken at a sunny noon and immediately frozen in liquid N2. All samples were stored at −80 °C until extraction of proteins and total RNA. These seedlings unused for the collection of leaves were used for the measurements of plant dry weight (DW) and leaf Cu.
4.2. Measurements of Plant DW, and Leaf Gas Exchange and Cu Concentration
Root, stem, and leaf DW were weighted after being washed with tap water and dried to a constant weight at 70 °C (~48 h) [78].
Gas exchange was measured with a CIARS-2 portable photosynthesis system (PP systems, Herts, UK) at a controlled light intensity of ~1000 μmol m−2 s−1 and a controlled CO2 concentration of ~380 μmol mol−1 between 9:30 and 12:30 a.m. on a sunny day [79].
Leaf Cu was determined with a NexION 300X Inductively Coupled Plasma Mass Spectrometer (ICP-MS, PerkinElmer, Shelton, CT, USA).
4.3. Leaf Protein Extraction, 2-DE and Image Analysis
About 1 g of frozen leaves harvested equally from four seedlings (one seedling per pot) was mixed as one biological replicate. There were three biological replicates per treatment (a total of 12 seedlings from 12 pots). Proteins were extracted using a phenol extraction procedure [80] and their concentration was measured as described by Bradford [81]. 2-DE was performed according to Sang et al. [82]. Stained gels were scanned with an Epson Scanner (Seiko Epson Corporation, Japan) at a resolution of 300 dpi. Images were analyzed with PDQuest version 8.0.1 (BioRad, Hercules, CA, USA), including background subtraction, normalization, spot detection, matching, Gaussian fitting and gel alignment [83]. A fold change of >1.5 or <0.67 was set to determine DAP spots in addition to a p-value < 0.05. After being visually checked and manually excised from gels, all DAP spots were submitted to MALDI-TOF/TOF-MS-based identification.
4.4. MALDI-TOF/TOF-MS-Based Protein Identification and Bioinformatic Analysis
Peptide identification was carried out on an AB SCIEX 5800 TOF/TOF plus MS (AB SCIEX, Shanghai, China) as described by Peng et al. [83]. After being processed with TOF/TOF Explorer™ Software (AB SCIEX, Shanghai, China) in a default mode, all acquired spectra were submitted to MASCOT (Version 2.3, Matrix Science Inc., Boston, MA) by GPS Explorer (Version 3.6) for the search of C. sinensis databases (http://citrus.hzau.edu.cn/orange/index.php) using following search parameters: trypsin cleavage with one missed, MS tolerance of 100 ppm and MS/MS tolerance of 0.6 Da. At least two of matched peptides were necessary for each protein. Protein identifications were accepted if MASCOT score was ≥ 70, and the sequence coverage was ≥ 20% or the number of matched peptides (NMP) was ≥ five [84,85]. DAPs were classified according to KEGG (http://www.kegg.jp/), GO (http://www.geneontology.org/) and Uniprot (http://www.uniprot.org/) databases [86,87].
4.5. KEGG Pathway Analysis of DAPs
KEGG pathway was analyzed using KOBAS 3.0 (Peking University, Beijing, China). Pathways were considered as significantly enriched if the corrected p-value was less than 0.05
4.6. qRT-PCR Analysis
Total RNA were extracted from ~300 mg frozen of leaves (mixed sample from four seedlings, one seedling per pot) using Recalcirtant Plant Total RNA Extraction Kit (Bioteke Corporation, Beijing, China). There were three biological replicates per treatment (a total of 12 seedlings from 12 pots). The sequences of specific primers designed using Primer Primier Version 5.0 (PREMIER Biosoft International, CA, USA), were listed in Table S4. qRT-PCR was performed with three biological and two technical replicates [86]. Two Citrus genes: U4/U6 small nuclear ribonucleoprotein PRP31 (PRP31, Cs7g08440.1) and actin (Cs1g05000.1) were used as internal standards and 0.5 μM Cu-treated leaves were used as reference (set as 1).
4.7. Data Analysis
There were 15 pots (30 seedlings) per treatment in a completely randomized design. Results were presented as the mean ± SE for n = 3–10. Eight means [two (species) × four (Cu levels)] were tested by two ANOVA followed by the least significant difference at p < 0.05 level.
Pearson correlation analysis and principal component analysis (PCA) for all identified DAP spots were made using SPSS (version 17.0, IBM, NY, USA) [88].
4.8. Data Deposit
The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium via the PRIDE partner repository with the dataset identifier PXD017049.
5. Conclusions
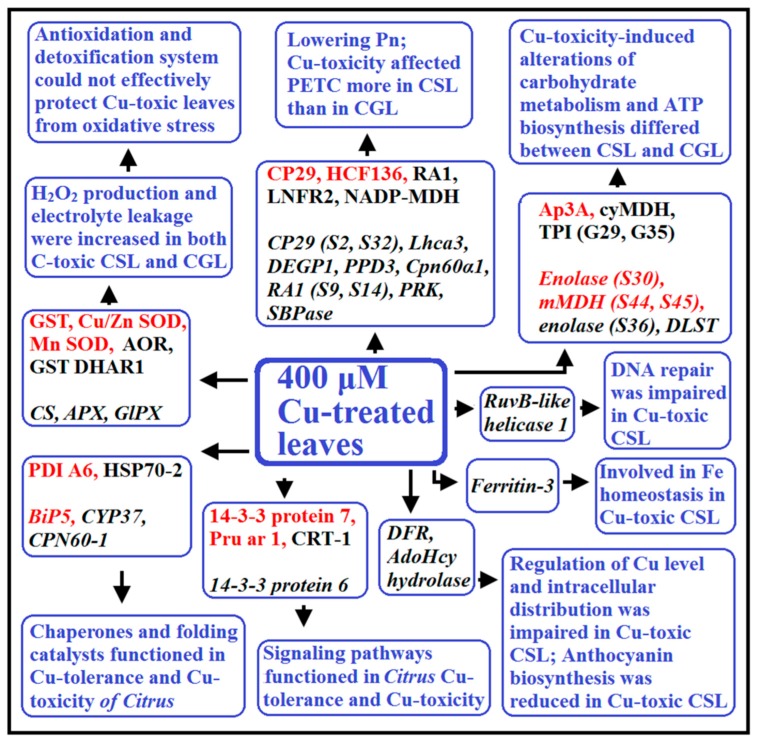
In this study, a 2-DE based MS approach was used to investigate Cu-toxicity-responsive proteins in Citrus leaves. Forty-one and 37 DAP spots were identified in 200, 300 and/or 400 μM Cu-treated C. grandis and C. sinensis leaves, respectively. Over 50% of these DAPs were involved in photosynthesis, carbohydrate, and energy metabolism, followed by antioxidation and detoxification, protein folding and assembly (viz., chaperones and folding catalysts), and signal transduction. More than 80% of these DAPs were identified only in C. grandis or C. sinensis leaves. More (Less) DAPs increased in abundances than DAPs decreased in abundances were identified in Cu-treated C. grandis (C. sinensis) leaves. Impaired PETC and decreased abundances of proteins involved in CO2 assimilation might be responsible for the Cu-induced inhibition of photosynthesis. Cu-toxicity affected the PETC more in C. sinensis leaves than in C. grandis leaves. DAPs related to antioxidation and detoxification, protein folding and assembly (viz., chaperones and folding catalysts), and signal transduction might be involved in Citrus Cu-toxicity and Cu-tolerance. Also, we identified some new DAPs (viz., LFNR2, SBPase, probable PGL4, ferritin, AdoHcy hydrolase and abscisic stress-ripening protein 1-like) that were not reported in leaves and/or roots (Figure 5). In conclusion, this study revealed some novel mechanisms on Cu-toxicity and Cu-tolerance in plants.
Figure 5.
A possible model displaying the differentially abundant proteins (DAPs) in 400 μM Cu-treated Citrus grandis leaves (CGL) and Citrus sinensis leaves (CSL). In this Figure, plain format and italics were used for Cu-toxic CGL and CSL, respectively. Red: DAPs increased in abundance; Black: DAPs decreased in abundances; APX, L-ascorbate peroxidase; DLST: dihydrolipoyllysine-residue succinyltransferase component of 2-oxoglutarate dehydrogenase complex 2; GlPX: glutathione peroxidase; Pn: photosynthesis; RA1: Rubisco activase A1.
Supplementary Materials
The following are available online at https://www.mdpi.com/2223-7747/9/3/291/s1, Figure S1: Excess Cu effects on growth of Citrus grandis (A) and Citrus sinensis (B), Figure S2: Excess Cu effects on root (A), stem (B), leaf (C) and whole plant (D) dry weight (DW) in Citrus grandis and Citrus sinensis seedlings, Figure S3: 2-DE images of proteins extracted from 0.5 (A, E, I, M), 200 (B, F, J, N) 300 (C, G, K, O) and 400 (D, H, L, P) Cu-treated Citrus grandis (A-D and I-L) and Citrus sinensis (E-H and M-P) leaves for the other two replicates, Figure S4: Close-up views of 24 DAP spots in 200, 300 and 400 μM Cu-treated Citrus sinensis and Citrus grandis leaves, Figure S5: Venn diagram analysis of Cu-responsive proteins in Citrus grandis and Citrus sinensis leaves, Figure S6: Significantly enriched (A-E and H) and the most enriched (F-G) KEGG pathways for annotated differentially abundant proteins (DAPs) in Cu-treated Citrus grandis (A-D) and Citrus sinensis (E-H) leaves, Figure S7: Matrices of Pearson correlation coefficients for differentially abundant proteins (DAPs) in Citrus grandis (A) and Citrus sinensis (B) leaves, Figure S8: Relative expression levels of genes encoding 22 differentially abundant proteins (DAPs) identified in 400 μM Cu-treated Citrus grandis (G3, G9, G10, G11, G14, G26, G29, G33, G34 and G35) and Citrus sinensis (S2, S5, S9, S16, S17, S23, S24, S30, S32, S33, S37 and S43) leaves using PRPF31 (A) and actin (B) as internal standards, Table S1: Master list of proteins identified in MALDI TOF/TOF MS from 200, 300 and or 400 μM Cu-treated Citrus sinensis and Citrus grandis leaves using 2DE and DIGE experiments, Table S2: Principal component analysis (PCA) for copper-responsive proteins in Citrus sinensis leaves, Table S3: Principal component analysis (PCA) for copper-responsive proteins in Citrus grandis leaves, Table S4: Specific primer pairs used for qRT-PCR analysis.
Author Contributions
Conceptualization, W.-L.H. and L.-S.C.; methodology, W.-L.H., L.-T.Y. and L.-S.C.; software, W.-L.H. and Z.-R.H.; validation, W.-L.H.; formal analysis, W.-L.H. and C.-L.D.; investigation, W.-L.H., F.-L.W., H.-Y.H. and W.-T.H.; resources, W.-L.H. and L.-S.C.; data curation, W.-L.H. and L.-T.Y.; writing—original draft preparation, W.-L.H.; writing—review and editing, L.-S.C.; supervision, L.-T.Y. and L.-S.C.; project administration, L.-S.C.; funding acquisition, L.-S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Key Research and Development Program of China (2018YFD1000305), and the earmarked fund for China Agriculture Research System (CARS27). The APC was funded by the National Key Research and Development Program of China (2018YFD1000305).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.Adrees M., Ali S., Rizwan M., Ibrahim M., Abbas F., Farid M., Zia-ur-Rehman M., Irshad M.K., Bharwana S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015;22:8148–8162. doi: 10.1007/s11356-015-4496-5. [DOI] [PubMed] [Google Scholar]
- 2.Fan J., He Z., Ma L.Q., Stoffella P.J. Accumulation and availability of copper in Citrus grove soils as affected by fungicide application. J. Soils Sediments. 2011;11:639–648. doi: 10.1007/s11368-011-0349-0. [DOI] [Google Scholar]
- 3.Mozaffari M., Alva A.K., Chen E.Q. Relation of copper extractable from soil and pH to copper content and growth of two Citrus rootstocks. Soil Sci. 1996;161:786–792. doi: 10.1097/00010694-199611000-00007. [DOI] [Google Scholar]
- 4.Yuan M., Li Y., Zhang C., Wang J., Li S., Fu X., Ling L., Cao L., Peng L. Review of research on copper stress in Citrus. J. Fruit Sci. 2018;35:347–357. [Google Scholar]
- 5.Li Q., Chen H.-H., Qi Y.P., Ye X., Yang L.T., Huang Z.R., Chen L.S. Excess copper effects on growth, uptake of water and nutrients, carbohydrates, and PSII photochemistry revealed by OJIP transients in Citrus seedlings. Environ. Sci. Pollut. Res. 2019;26:30188–30205. doi: 10.1007/s11356-019-06170-2. [DOI] [PubMed] [Google Scholar]
- 6.Burkhead J.L., Reynolds K.A.G., Abdel-Ghany S.E., Cohu C.M., Pilon M. Copper homeostasis. New Phytol. 2009;182:799–816. doi: 10.1111/j.1469-8137.2009.02846.x. [DOI] [PubMed] [Google Scholar]
- 7.Ambrosini V.G., Rosa D.J., Basso A., Borghezan M., Pescador R., Miotto A., George de Melo W.B., de Sousa Soares C.R.F., Comin J.J., Brunetto G. Liming as an ameliorator of copper toxicity in black oat (Avena strigosa Schreb.) J. Plant Nutr. 2017;40:404–416. doi: 10.1080/01904167.2016.1240203. [DOI] [Google Scholar]
- 8.Hippler F.W.R., Boaretto R.M., Dovis V.L., Quaggio J.A., Azevedo R.A., Mattos D., Jr. Oxidative stress induced by Cu nutritional disorders in Citrus depends on nitrogen and calcium availability. Sci. Rep. 2018;8:1641. doi: 10.1038/s41598-018-19735-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang L.L., He X.J., Chen M., An R.D., An X.L., Li J. Responses of nitrogen metabolism to copper stress in Luffa cylindrica roots. J. Soil Sci. Plant Nutr. 2014;14:616–624. doi: 10.4067/S0718-95162014005000049. [DOI] [Google Scholar]
- 10.Fürtig K., Pavelic D., Brunold C., Brändle R. Copper-and iron-induced injuries in roots and rhizomes of reed (Phragmites australis) Limnologica. 1999;29:60–63. doi: 10.1016/S0075-9511(99)80039-5. [DOI] [Google Scholar]
- 11.Lequeux H., Hermans C., Lutts S., Verbruggen N. Response to copper excess in Arabidopsis thaliana: Impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol. Biochem. 2010;48:673–682. doi: 10.1016/j.plaphy.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 12.Colzi I., Doumett S., Del Bubba M., Fornaini J., Arnetoli M., Gabbrielli R., Gonnelli C. On the role of the cell wall in the phenomenon of copper tolerance in Silene paradoxa L. Environ. Exp. Bot. 2011;72:77–83. doi: 10.1016/j.envexpbot.2010.02.006. [DOI] [Google Scholar]
- 13.Kováčik J., Klejdus B., Hedbavny J., Štork F., Bačkor M. Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. Plant Soil. 2009;320:231–242. doi: 10.1007/s11104-009-9889-0. [DOI] [Google Scholar]
- 14.Yruela I. Copper in plants: Acquisition, transport and interactions. Funct. Plant. Biol. 2009;36:409–430. doi: 10.1071/FP08288. [DOI] [PubMed] [Google Scholar]
- 15.Greenbaum D., Colangelo C., Williams K., Gerstein M. Comparing protein abundance and mRNA expression levels on a genomic scale. Genome Biol. 2003;4:117–124. doi: 10.1186/gb-2003-4-9-117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rose J.K., Bashir S., Giovannoni J.J., Jahn M.M., Saravanan R.S. Tackling the plant proteome: Practical approaches, hurdles and experimental tools. Plant J. 2004;39:715–733. doi: 10.1111/j.1365-313X.2004.02182.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen C., Song Y., Zhuang K., Li L., Xia Y., Shen Z. Proteomic analysis of copper-binding proteins in excess copper-stressed roots of two rice (Oryza sativa L.) varieties with different Cu tolerances. PLoS ONE. 2015;10:e0125367. doi: 10.1371/journal.pone.0125367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song Y., Cui J., Zhang H., Wang G., Zhao F.J., Shen Z. Proteomic analysis of copper stress responses in the roots of two rice (Oryza sativa L.) varieties differing in Cu tolerance. Plant Soil. 2013;366:647–658. doi: 10.1007/s11104-012-1458-2. [DOI] [Google Scholar]
- 19.Zhang H., Lian C., Shen Z. Proteomic identification of small, copper-responsive proteins in germinating embryos of Oryza sativa. Ann. Bot. 2009;103:923–930. doi: 10.1093/aob/mcp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qin R., Ning C., Björn L.O., Li S. Proteomic analysis of Allium cepa var. agrogarum L. roots under copper stress. Plant Soil. 2016;401:197–212. [Google Scholar]
- 21.Wang C., Wang J., Wang X., Xia Y., Chen C., Shen Z., Chen Y. Proteomic analysis on roots of Oenothera glazioviana under copper-stress conditions. Sci. Rep. 2017;7:10589. doi: 10.1038/s41598-017-10370-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gill T., Dogra V., Kumar S., Ahuja P.S., Sreenivasulu Y. Protein dynamics during seed germination under copper stress in Arabidopsis over-expressing Potentilla superoxide dismutase. J. Plant Res. 2012;125:165–172. doi: 10.1007/s10265-011-0421-2. [DOI] [PubMed] [Google Scholar]
- 23.Bona E., Marsano F., Cavaletto M., Berta G. Proteomic characterization of copper stress response in Cannabis sativa roots. Proteomics. 2007;7:1121–1130. doi: 10.1002/pmic.200600712. [DOI] [PubMed] [Google Scholar]
- 24.Hego E., Bes C.M., Bedon F., Palagi P.M., Chaumeil P., Barré A., Claverol S., Dupuy J.W., Bonneu M., Lalanne C., et al. Differential accumulation of soluble proteins in roots of metallicolous and nonmetallicolous populations of Agrostis capillaris L. exposed to Cu. Proteomics. 2014;14:1746–1758. doi: 10.1002/pmic.201300168. [DOI] [PubMed] [Google Scholar]
- 25.Li F., Shi J., Shen C., Chen G., Hu S., Chen Y. Proteomic characterization of copper stress response in Elsholtzia splendens roots and leaves. Plant Mol. Biol. 2009;71:251–263. doi: 10.1007/s11103-009-9521-y. [DOI] [PubMed] [Google Scholar]
- 26.Liu T., Shen C., Wang Y., Huang C., Shi J. New insights into regulation of proteome and polysaccharide in cell wall of Elsholtzia splendens in response to copper stress. PLoS ONE. 2014;9:e109573. doi: 10.1371/journal.pone.0109573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roy S.K., Kwon S.J., Cho S.W., Kamal A.H.M., Kim S.W., Sarker K., Oh M.W., Lee M.S., Chung K.Y., Xin Z., et al. Leaf proteome characterization in the context of physiological and morphological changes in response to copper stress in sorghum. Biometals. 2016;29:495–513. doi: 10.1007/s10534-016-9932-6. [DOI] [PubMed] [Google Scholar]
- 28.Roy S.K., Cho S.W., Kwon S.J., Kamal A.H.M., Lee D.G., Sarker K., Moon-Soon Lee M.S., Xin Z., Woo S.H. Proteome characterization of copper stress responses in the roots of sorghum. Biometals. 2017;30:765–785. doi: 10.1007/s10534-017-0045-7. [DOI] [PubMed] [Google Scholar]
- 29.Li G., Peng X., Xuan H., Wei L., Yang Y., Guo T., Kang G. Proteomic analysis of leaves and roots of common wheat (Triticum aestivum L.) under copper-stress conditions. J. Proteome Res. 2013;12:4846–4861. doi: 10.1021/pr4008283. [DOI] [PubMed] [Google Scholar]
- 30.Alotaibi M.O., Mohammed A.E., Almutairi T.A., Elobeid M.M. Morpho-physiological and proteomic analyses of Eucalyptus camaldulensis as a bioremediator in copper-polluted soil in Saudi Arabia. Plants. 2019;8:43. doi: 10.3390/plants8020043. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 31.Damkjær J.T., Kereiche S., Johnson M.P., Kovacs L., Kiss A.Z., Boekema E.J., Ruban A.V., Horton P., Jansson S. The photosystem II light-harvesting protein Lhcb3 affects the macrostructure of photosystem II and the rate of state transitions in Arabidopsis. Plant Cell. 2009;21:3245–3256. doi: 10.1105/tpc.108.064006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapri-Pardes E., Naveh L., Adam Z. The thylakoid lumen protease Deg1 is involved in the repair of photosystem II from photoinhibition in Arabidopsis. Plant Cell. 2007;19:1039–1047. doi: 10.1105/tpc.106.046573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bergantino E., Dainese P., Cerovic Z., Sechi S., Bassi R. A post-translational modification of the PSII subunit CP29 protects maize from cold stress. J. Biol. Chem. 1995;270:8474–8481. doi: 10.1074/jbc.270.15.8474. [DOI] [PubMed] [Google Scholar]
- 34.Betterle N., Ballottari M., Baginsky S., Bassi R. High light-dependent phosphorylation of photosystem II inner antenna CP29 in monocots is STN7 independent and enhances nonphotochemical quenching. Plant Physiol. 2015;167:457–471. doi: 10.1104/pp.114.252379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Farinati S., DalCorso G., Bona E., Corbella M., Lampis S., Cecconi D., Polati R., Berta G., Vallini G., Furini A. Proteomic analysis of Arabidopsis halleri shoots in response to the heavy metals cadmium and zinc and rhizosphere microorganisms. Proteomics. 2009;9:4837–4850. doi: 10.1002/pmic.200900036. [DOI] [PubMed] [Google Scholar]
- 36.Lundin B., Hansson M., Schoefs B., Vener A.V., Spetea C. The Arabidopsis PsbO2 protein regulates dephosphorylation and turnover of the photosystem II reaction centre D1 protein. Plant J. 2007;49:528–539. doi: 10.1111/j.1365-313X.2006.02976.x. [DOI] [PubMed] [Google Scholar]
- 37.Lintala M., Allahverdiyeva Y., Kangasjärvi S., Lehtimäki N., Keränen M., Rintamäki E., Aro E.M., Mulo P. Comparative analysis of leaf-type ferredoxin-NADP+ oxidoreductase isoforms in Arabidopsis thaliana. Plant J. 2009;57:1103–1115. doi: 10.1111/j.1365-313X.2008.03753.x. [DOI] [PubMed] [Google Scholar]
- 38.Kim S.R., Yang J.I., An G. OsCpn60α1, encoding the plastid chaperonin 60α subunit, is essential for folding of rbcL. Mol. Cells. 2013;35:402–409. doi: 10.1007/s10059-013-2337-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mate C.J., von Caemmerer S., Evans J.R., Hudson G.S., Andrews T.J. The relationship between CO2-assimilation rate, Rubisco carbamylation and Rubisco activase content in activase-deficient transgenic tobacco suggests a simple model of activase action. Planta. 1996;198:604–613. doi: 10.1007/BF00262648. [DOI] [PubMed] [Google Scholar]
- 40.Strand Å., Zrenner R., Trevanion S., Stitt M., Gustafsson P., Gardeström P. Decreased expression of two key enzymes in the sucrose biosynthesis pathway, cytosolic fructose-1, 6-bisphosphatase and sucrose phosphate synthase, has remarkably different consequences for photosynthetic carbon metabolism in transgenic Arabidopsis thaliana. Plant J. 2000;23:759–770. doi: 10.1046/j.1365-313x.2000.00847.x. [DOI] [PubMed] [Google Scholar]
- 41.Fariduddin Q., Yusuf M., Hayat S., Ahmad A. Effect of 28-homobrassinolide on antioxidant capacity and photosynthesis in Brassica juncea plants exposed to different levels of copper. Environ. Exp. Bot. 2009;66:418–424. doi: 10.1016/j.envexpbot.2009.05.001. [DOI] [Google Scholar]
- 42.Nunes-Nesi A., Carrari F., Lytovchenko A., Smith A.M., Loureiro M.E., Ratcliffe R.G., Sweetlove L.J., Fernie A.R. Enhanced photosynthetic performance and growth as a consequence of decreasing mitochondrial malate dehydrogenase activity in transgenic tomato plants. Plant Physiol. 2005;137:611–622. doi: 10.1104/pp.104.055566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Heyno E., Innocenti G., Lemaire S.D., Issakidis-Bourguet E., Krieger-Liszkay A. Putative role of the malate valve enzyme NADP-malate dehydrogenase in H2O2 signalling in Arabidopsis. Philos. Trans. R. Soc. B. 2014;369:20130228. doi: 10.1098/rstb.2013.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang Q.J., Sun H., Dong Q.L., Sun T.Y., Jin Z.X., Hao Y.J., Yao Y.X. The enhancement of tolerance to salt and cold stresses by modifying the redox state and salicylic acid content via the cytosolic malate dehydrogenase gene in transgenic apple plants. Plant Biotech. J. 2016;14:1986–1997. doi: 10.1111/pbi.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Xiong Y., DeFraia C., Williams D., Zhang X., Mou Z. Characterization of Arabidopsis 6-phosphogluconolactonase T-DNA insertion mutants reveals an essential role for the oxidative section of the plastidic pentose phosphate pathway in plant growth and development. Plant Cell Physiol. 2009;50:1277–1291. doi: 10.1093/pcp/pcp070. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed N., Battah S., Karachalias N., Babaei-Jadidi R., Horányi M., Baróti K., Hollan S., Thornalley P.J. Increased formation of methylglyoxal and protein glycation, oxidation and nitrosation in triosephosphate isomerase deficiency. Biochim. Biophys. Acta-Mol. Basis Dis. 2003;1639:121–132. doi: 10.1016/j.bbadis.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 47.Baena-González E., Sheen J. Convergent energy and stress signaling. Trends Plant Sci. 2008;13:474–482. doi: 10.1016/j.tplants.2008.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lim J.D., Hahn S.J., Yu C.Y., Chung I.M. Expression of the glutathione S-transferase gene (NT107) in transgenic Dianthus superbus. Plant Cell Tiss. Org. Cult. 2005;80:277–286. doi: 10.1007/s11240-004-1032-6. [DOI] [Google Scholar]
- 49.Kumar S., Asif M.H., Chakrabarty D., Tripathi R.D., Dubey R.S., Trivedi P.K. Expression of a rice Lambda class of glutathione S-transferase, OsGSTL2, in Arabidopsis provides tolerance to heavy metal and other abiotic stresses. J. Hazard. Mater. 2013;248:228–237. doi: 10.1016/j.jhazmat.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Drążkiewicz M., Skórzyńska-Polit E., Krupa Z. The redox state and activity of superoxide dismutase classes in Arabidopsis thaliana under cadmium or copper stress. Chemosphere. 2007;67:188–193. doi: 10.1016/j.chemosphere.2006.08.032. [DOI] [PubMed] [Google Scholar]
- 51.Cohu C.M., Pilon M. Regulation of superoxide dismutase expression by copper availability. Physiol. Plant. 2007;129:747–755. doi: 10.1111/j.1399-3054.2007.00879.x. [DOI] [Google Scholar]
- 52.Yamauchi Y., Hasegawa A., Mizutani M., Sugimoto Y. Chloroplastic NADPH-dependent alkenal/one oxidoreductase contributes to the detoxification of reactive carbonyls produced under oxidative stress. FEBS Lett. 2012;586:1208–1213. doi: 10.1016/j.febslet.2012.03.013. [DOI] [PubMed] [Google Scholar]
- 53.Kawashima C.G., Noji M., Nakamura M., Ogra Y., Suzuki K.T., Saito K. Heavy metal tolerance of transgenic tobacco plants over-expressing cysteine synthase. Biotechnol. Let. 2004;26:153–157. doi: 10.1023/B:BILE.0000012895.60773.ff. [DOI] [PubMed] [Google Scholar]
- 54.Lee S., Kang B.S. Phytochelatin is not a primary factor in determining copper tolerance. J. Plant Biol. 2005;48:32–38. doi: 10.1007/BF03030562. [DOI] [Google Scholar]
- 55.Xu H., Xu W., Xi H., Ma W., He Z., Ma M. The ER luminal binding protein (BiP) alleviates Cd2+-induced programmed cell death through endoplasmic reticulum stress–cell death signaling pathway in tobacco cells. J. Plant Physiol. 2013;170:1434–1441. doi: 10.1016/j.jplph.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 56.Guan C., Jin C., Ji J., Wang G., Li X. LcBiP, a endoplasmic reticulum chaperone binding protein gene from Lycium chinense, confers cadmium tolerance in transgenic tobacco. Biotechnol. Prog. 2015;31:358–368. doi: 10.1002/btpr.2046. [DOI] [PubMed] [Google Scholar]
- 57.Narindrasorasak S., Yao P., Sarkar B. Protein disulfide isomerase, a multifunctional protein chaperone, shows copper-binding activity. Biochem. Biophys. Res. Commun. 2003;311:405–414. doi: 10.1016/j.bbrc.2003.09.226. [DOI] [PubMed] [Google Scholar]
- 58.Kuo W.Y., Huang C.H., Liu A.C., Cheng C.P., Li S.H., Chang W.C., Weiss C., Azem A., Jinn T.L. CHAPERONIN 20 mediates iron superoxide dismutase (Fe SOD) activity independent of its co-chaperonin role in Arabidopsis chloroplasts. New Phytol. 2013;197:99–110. doi: 10.1111/j.1469-8137.2012.04369.x. [DOI] [PubMed] [Google Scholar]
- 59.Palmgren M.G. Plant plasma membrane H+-ATPases: Powerhouses for nutrient uptake. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001;52:817–845. doi: 10.1146/annurev.arplant.52.1.817. [DOI] [PubMed] [Google Scholar]
- 60.Owen J.R., Morris C.A., Nicolaus B., Harwood J.L., Kille P. Induction of expression of a 14-3-3 gene in response to copper exposure in the marine alga, Fucus vesiculosus. Ecotoxicology. 2012;21:124–138. doi: 10.1007/s10646-011-0772-4. [DOI] [PubMed] [Google Scholar]
- 61.Janicka-Russak M., Kabała K., Burzyński M. Different effect of cadmium and copper on H+-ATPase activity in plasma membrane vesicles from Cucumis sativus roots. J. Exp. Bot. 2012;63:4133–4142. doi: 10.1093/jxb/ers097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cuypers A., Koistinen K.M., Kokko H., Kärenlampi S., Auriola S., Vangronsveld J. Analysis of bean (Phaseolus vulgaris L.) proteins affected by copper stress. J. Plant Physiol. 2005;162:383–392. doi: 10.1016/j.jplph.2004.07.018. [DOI] [PubMed] [Google Scholar]
- 63.Utriainen M., Kokko H., Auriola S., Sarrazin O., Kärenlampi S. PR-10 protein is induced by copper stress in roots and leaves of a Cu/Zn tolerant clone of birch, Betula pendula. Plant Cell Environ. 1998;21:821–828. doi: 10.1046/j.1365-3040.1998.00326.x. [DOI] [Google Scholar]
- 64.Jami S.K., Clark G.B., Turlapati S.A., Handley C., Roux S.J., Kirti P.B. Ectopic expression of an annexin from Brassica juncea confers tolerance to abiotic and biotic stress treatments in transgenic tobacco. Plant Physiol. Biochem. 2008;46:1019–1030. doi: 10.1016/j.plaphy.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 65.Chmielowska J., Veloso J., Gutierrez J., Silvar C., Díaz J. Cross-protection of pepper plants stressed by copper against a vascular pathogen is accompanied by the induction of a defence response. Plant Sci. 2010;178:176–182. doi: 10.1016/j.plantsci.2009.11.007. [DOI] [Google Scholar]
- 66.Ritter A., Ubertini M., Romac S., Gaillard F., Delage L., Mann A., Cock J.M., Tonon T., Correa J.A., Potin P. Copper stress proteomics highlights local adaptation of two strains of the model brown alga Ectocarpus siliculosus. Proteomics. 2010;10:2074–2088. doi: 10.1002/pmic.200900004. [DOI] [PubMed] [Google Scholar]
- 67.Jin X.L., Ma C.L., Yang L.T., Chen L.S. Alterations of physiology and gene expression due to long-term magnesium-deficiency differ between leaves and roots of Citrus reticulata. J. Plant Physiol. 2016;198:103–115. doi: 10.1016/j.jplph.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 68.Zhai F.Q., Wang X.L., Hua J.M., Si J.Y., Feng K. Copper toxicity on seedlings of wheat and the detoxification of calcium. J. Agro-Environ. Sci. 2006;26:694–698. [Google Scholar]
- 69.Carrió-Seguí À., Romero P., Sanz A., Peñarrubia L. Interaction between ABA signaling and copper homeostasis in Arabidopsis thaliana. Plant Cell Physiol. 2016;57:1568–1582. doi: 10.1093/pcp/pcw087. [DOI] [PubMed] [Google Scholar]
- 70.Briat J.F. Roles of ferritin in plants. J. Plant Nutr. 1996;19:1331–1342. doi: 10.1080/01904169609365202. [DOI] [Google Scholar]
- 71.Alva A.K., Chen E.Q. Effects of external copper concentrations on uptake of trace elements by Citrus seedlings. Soil Sci. 1995;159:59–64. doi: 10.1097/00010694-199501000-00007. [DOI] [Google Scholar]
- 72.Ravet K., Pilon M. Copper and iron homeostasis in plants: The challenges of oxidative stress. Antioxid. Redox Sign. 2013;19:919–932. doi: 10.1089/ars.2012.5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Luo Y., Liu Y.B., Dong Y.X., Gao X.Q., Zhang X.S. Expression of a putative alfalfa helicase increases tolerance to abiotic stress in Arabidopsis by enhancing the capacities for ROS scavenging and osmotic adjustment. J. Plant Physiol. 2009;166:385–394. doi: 10.1016/j.jplph.2008.06.018. [DOI] [PubMed] [Google Scholar]
- 74.Rakwal R., Yang G., Komatsu S. Chitinase induced by jasmonic acid, methyl jasmonate, ethylene and protein phosphatase inhibitors in rice. Mol. Biol. Rep. 2004;31:113–119. doi: 10.1023/B:MOLE.0000031407.18708.95. [DOI] [PubMed] [Google Scholar]
- 75.Taddei S., Bernardi R., Salvini M., Pugliesi C., Durante M. Effect of copper on callus growth and gene expression of in vitro-cultured pith explants of Nicotiana glauca. Plant Biosyst. 2007;141:194–203. doi: 10.1080/11263500701401521. [DOI] [Google Scholar]
- 76.Zhou X.X., Yang L.T., Qi Y.P., Guo P., Chen L.S. Mechanisms on boron-induced alleviation of aluminum-toxicity in Citrus grandis seedlings at a transcriptional level revealed by cDNA-AFLP analysis. PLoS ONE. 2015;10:e0115485. doi: 10.1371/journal.pone.0115485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sudo E., Itouga M., Yoshida-Hatanaka K., Ono Y., Sakakibara H. Gene expression and sensitivity in response to copper stress in rice leaves. J. Exp. Bot. 2008;59:3465–3474. doi: 10.1093/jxb/ern196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Huang J.H., Xu J., Ye X., Luo T.Y., Ren L.H., Fan G.C., Yi P.Q., Li Q., Ferarezi R.H., Chen L.S. Magnesium deficiency affects secondary lignification of the vascular system in Citrus sinensis seedlings. Trees Struct. Funct. 2019;33:171–182. doi: 10.1007/s00468-018-1766-0. [DOI] [Google Scholar]
- 79.Ye X., Chen X.F., Deng C.L., Yang L.T., Lai L.W., Guo J.X., Chen L.S. Magnesium-deficiency effects on pigments, photosynthesis and photosynthetic electron transport of leaves, and nutrients of leaf blades and veins in Citrus sinensis seedlings. Plants. 2019;8:389. doi: 10.3390/plants8100389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.You X., Yang L.T., Lu Y.B., Li H., Zhang S.Q., Chen L.S. Proteomic changes of Citrus roots in response to long-term manganese toxicity. Trees Struct. Funct. 2014;28:1383–1399. doi: 10.1007/s00468-014-1042-x. [DOI] [Google Scholar]
- 81.Bradford M.M. A rapid and sensitive method for quantitation of microgramand quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 82.Sang W., Huang Z.R., Yang L.T., Guo P., Ye X., Chen L.S. Effects of high toxic boron concentration on protein profiles in roots of two Citrus species differing in boron tolerance revealed by a 2-DE based MS approach. Front. Plant Sci. 2017;8:180. doi: 10.3389/fpls.2017.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Peng H.Y., Qi Y.P., Lee J., Yang L.T., Guo P., Jiang H.X., Chen L.S. Proteomic analysis of Citrus sinensis roots and leaves in response to long-term magnesium-deficiency. BMC Genom. 2015;16:253. doi: 10.1186/s12864-015-1462-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Figueiredo A., Martins J., Sebastiana M., Guerreiro A., Silva A., Matos A.R., Monteiro F., Pais M.S., Roepstorff P., Coelho A.V. Specific adjustments in grapevine leaf proteome discriminating resistant and susceptible grapevine genotypes to Plasmopara viticola. J. Proteome. 2017;152:48–57. doi: 10.1016/j.jprot.2016.10.012. [DOI] [PubMed] [Google Scholar]
- 85.Zhang J., Li Q., Qi Y.P., Huang W.L., Yang L.T., Lai N.W., Ye X., Chen L.S. Low pH-responsive proteins revealed by a 2-DE based MS approach and related physiological responses in Citrus leaves. BMC Plant Biol. 2019;18:188. doi: 10.1186/s12870-018-1413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Guo P., Qi Y.P., Huang W.L., Yang L.T., Huang Z.R., Lai N.W., Chen L.S. Aluminum-responsive genes revealed by RNA-Seq and related physiological responses in leaves of two Citrus species with contrasting aluminum-tolerance. Ecotoxicol. Environ. Saf. 2018;158:213–222. doi: 10.1016/j.ecoenv.2018.04.038. [DOI] [PubMed] [Google Scholar]
- 87.Yang L.T., Qi Y.P., Lu Y.B., Guo P., Sang W., Feng H., Zhang H.X., Chen L.S. iTRAQ protein profile analysis of Citrus sinensis roots in response to long-term boron-deficiency. J. Proteome. 2013;93:179–206. doi: 10.1016/j.jprot.2013.04.025. [DOI] [PubMed] [Google Scholar]
- 88.Guo P., Qi Y.P., Cai Y.T., Yang T.Y., Yang L.T., Huang Z.R., Chen L.S. Aluminum effects on photosynthesis, reactive oxygen species and methylglyoxal detoxification in two Citrus species differing in aluminum tolerance. Tree Physiol. 2018;38:1548–1565. doi: 10.1093/treephys/tpy035. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.