Abstract
Endemic plant species are usually more vulnerable to anthropogenic threats and natural changes and, therefore, hold a higher extinction risk. The preservation of these species is a major concern on a worldwide context and in situ protection alone will not guarantee their conservation. Ex situ conservation measures must be undertaken to support the conservation of these species, and seed banking is the more efficient and cost-effective method. However, when seed banking is not an option, alternative approaches should be considered. Biotechnological tools provide new and complementary options for plant conservation including short-, medium-, and long-term strategies, and their application for plant species conservation has increased considerably in the last years. This review provides information about the status of the use biotechnology-based techniques for the conservation of endemic plant species. Particular attention is given to cryopreservation, since is the only long-term ex situ conservation strategy that can complement and support the other conservation measures. The cryopreservation of plant genetic resources is, however, more focused on crop or economically important species and few studies are available for endemic plant species. The plant material used, the cryopreservation methods employed, and the assessment of cryogenic effects are reviewed. The reasons to explain the difficulties in cryopreserving these species are discussed and new strategies are proposed to facilitate and increase the interest on this matter. We expect that further studies on the conservation of endemic plant species will increase in a near future, thus contributing to maintain these valuable genetic resources.
Keywords: cryopreservation techniques, explant, ex situ conservation, genetic stability, micropropagation, shoot tips, seeds, vitrification
1. The Conservation of Endemic Plant Species
Plants are vital for life on earth and a crucial element in all ecosystems. Despite their importance, all over the world, plant biodiversity is at risk and every year the number of threatened species increases dramatically [1]. The loss of natural populations or even entire species is usually related to the destruction and alteration of their habitats, as a consequence of human overexploitation, and more recently, pollution, and climate changes, leading to the loss of genetic diversity [2,3,4]. Many of these species are endemic, therefore unique, and often only a few and small wild populations resist [4,5].
An endemic species can be defined as a species that occurs naturally and exclusively, and it is highly adapted to a specific geographic area [6,7,8,9]. According to the size and limits of that area, these species can be classified as “local endemic” (restricted to a small area), “provincial endemic” (restricted to the limits of a province), “national endemic” (restricted to the limits of a nation), “regional endemic” (restricted to a geographical region) and “continental endemic” (restricted to a continent) [9,10]. A set of characteristics, alone or together, can be found in most endemic species, which make them more vulnerable than others to anthropogenic threats and/or natural changes: restricted distribution, one or few populations, small population size, declining population size, excessive collection by humans, short reproduction capacity, specific habitat conditions, and necessity of stable and constant environments. The more of these characteristics these species display, the more vulnerable they are to extinction [9]. Hence, endemic species should be carefully monitored and managed, and their conservation considered a global priority [8,9].
The most important source of information concerning the global conservation status of species is the IUCN (International Union for Conservation of Nature) Red List of Threatened Species [9,11], and according to the latest numbers, there are 15,774 threatened plant species, out of the 38,630 species evaluated so far, of the 422,683 described species [11]. Many plant species (more than 75%) are endemic to the 35 Global Biodiversity Hotspots and conservation efforts pointed to these regions could greatly contribute to reduce the loss of these unique species [12,13].
In situ conservation, the conservation of ecosystems and biodiversity in their natural habitats, is the most appropriate conservation approach for the preservation of species, including endemic species, because it preserves the original genetic and geographical centers of biodiversity [14,15]. However, for a more complete and effective conservation program, different strategies and methods should be implemented to complement and support in situ protection [15]. In fact, ex situ conservation, the conservation of biodiversity outside its natural habitats, is sometimes the only option for the preservation of rare and endemic species [4,5,14]. Conventional seed bank storage (dry storage at −20 °C) is the simplest and most efficient method for ex situ conservation of plant germplasm, and it is also the best choice because it preserves genetically diverse material [16]. However, not all plant species can be preserved by this technique. Species with recalcitrant (desiccation-sensitive) and intermediate (relatively desiccation-tolerant) seeds cannot stand the desiccation conditions and cold storage without losing viability; only orthodox seeds (desiccation-tolerant) are able to do so [14]. Besides, there are plant species that do not produce seeds and are propagated vegetatively. Furthermore, there are others that, even though they can produce seeds, are highly heterozygous. For these plant species, the traditional ex situ conservation method is in the form of field collections [14,17]. But this method also has its limitations: it usually requires large areas of land, is labor intensive, and collections are unprotected from plagues and natural disasters [17]. In addition, field collections are mainly used to preserve crop species. Thus, it is important to implement alternative approaches for the preservation of these species, such as biotechnological methods. Even so, the use of alternative conservation methods should not only be considered for vegetatively propagated and non-orthodox seeded species. In some cases, in addition to traditional conservation methods, it is necessary to implement complementary strategies to augment the chances of survival even for species with orthodox seeds that are easily preserved in seed banks.
2. Biotechnological Approaches
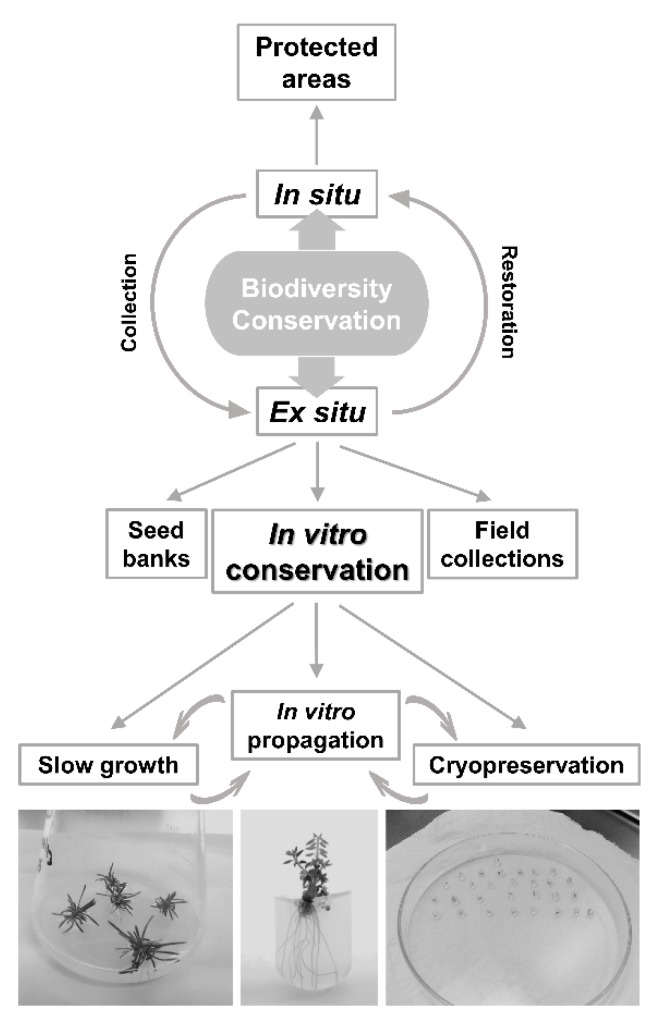
Biotechnology has given a major contribution to plant conservation and the purpose is not to replace the traditional conservation methods but to complement and improve the methods available (Figure 1) [14,18]. Plant conservation biotechnology comprises not only the conservation of plant genetic resources but also its management, characterization, and application (sustainable use) [18]. In particular, in vitro conservation, which is the maintenance of plant germplasm in culture collections using tissue culture technologies, can provide easy access for the evaluation, utilization, and safe exchange of plant material [4,14,15]. According to the storage duration, plant germplasm is maintained in culture collections for short-term periods, using standard tissue culture, or medium-term periods (slow growth), achieved by reducing growth. The long-term storage refers to the cryopreservation of plant material in liquid nitrogen (LN) [14]. There are a few reviews that discuss the use of biotechnological tools for the conservation of rare and endangered species, either presenting a synopsis on the subject [4,5,14] or focusing on a certain region/country [19,20] or groups of plants [21].
Figure 1.
Schematic representation of different conservation strategies with focus on biotechnological-based techniques (adapted from [18]).
Nonetheless, and considering the significant progress on the use of biotechnological tools and their importance to complement other ex situ methods, the purpose of this review is to make a global overview of the conservation of endemic plant species with small populations and limited distribution based on in vitro culture techniques. Cryopreservation will be discussed in more detail, since it is the only technique that allows long-term conservation and, as far as our literature survey can ascertain, there is no comprehensive review on its application for the conservation of narrow endemic plant species, hereafter referred to just as endemic plant species.
3. Short- (In Vitro Propagation) and Medium-Term Preservation (Slow Growth)
In vitro propagation is considered an effective alternative to the conventional propagation methods, since it allows the recovery of numerous plants in a short period of time and limited space and facilitates the transportation of propagation material [14]. This is particularly useful in the case of rare and endangered species and plants that are difficult to propagate by conventional techniques or with slow propagation rates. Other advantage of this technique is the necessity of small amounts of starting plant material from the mother plant, which allows the mass propagation with scarce impact on wild habitats [14]. Reinforcement of wild plant populations using individuals raised ex situ is considered a valid means of reducing the risk of extinction of threatened species or populations [22]. Thus, the development of effective propagation protocols is fundamental in conservation programs. In vitro culture in normal growth conditions allows the short-term (active state growth) conservation of genetic material of rare and endangered plants being a fundamental step for the propagation of plants directly but also for the application of medium- (slow growth) and long-term conservation techniques (cryopreservation).
In vitro propagation is described for the propagation and conservation of many endemic plant species and some selected examples are presented in Table 1. Meristem culture and the differentiation of adventitious buds (organogenesis) are the methods usually used, although the first has more examples. Meristem culture is more adequate from the conservation point of view because meristematic cells are less differentiated and genetically more stable, originating genetic integral plants [23]. Many factors affecting the process, such as the basal medium composition, the plant growth regulators, the environmental parameters, as well as the explant type, have been investigated in order to optimize it for each species. A recent example of the development and optimization of an in vitro propagation protocol using seedlings as the initial explant was performed for Eryngium viviparum Gay, a small, biennial, and aquatic plant endemic to the Southwest Europe (namely France, Spain, and Portugal) [24]. The effect of two types of cytokinins (6- benzylaminopurine and kinetin) and different concentrations were tested to assess shoot multiplication. For shoot elongation and rooting, the authors tested different concentrations of sucrose and MS (Murashige and Skoog) [25] macronutrients. With the optimized protocol high rates for shoot multiplication, 5.1-5.8 new shoots, 100% rooting, and 96% acclimatization were obtained [24].
Table 1.
Selected scientific publications on micropropagation of endemic plant species, including the name of the species, the region of distribution, and the explant type.
| Species | Region | Explant | Reference |
|---|---|---|---|
| Aechmea ramosa Mart. ex Schult. f. | Atlantic Forest (Brazil) | Leaf | [26] |
| Asparagus macrorrhizus (Pedrol, Regalado & López-Encina) | “Mar Menor” lagoon, Murcia (Spain) | Rhizome buds | [27] |
| Brachystelma glabrum Hook.f. | Eastern Ghats (India) | Shoot tips and nodal explants | [28] |
| Calophyllum apetalum Willd. | Western Ghats of southern India | Shoot tips and nodal explants | [29] |
| Ceropegia noorjahaniae Ans. | Western Ghats (India) | Shoot segments (node explant) | [30] |
| Coelogyne nervosa A. Rich. | Western Ghats (South India) | Seedlings | [31] |
| Cryptanthus sinuosus (L.B. Smith) | Rio de Janeiro State (Brazil) | Stem–root axes with intact shoot apices and approximately 6–10 nodes | [32] |
| Cycladenia humilis Benth. var. jonesii (Eastw.) S.L. Welsh &N.D. Atwood | Canyonlands region of Utah and Arizona (Western U.S.A.) | Seedlings and nodal segments | [33] |
| Dianthus giganteus D’Urv subsp. banaticus (Heuff.) Tutin | Southwest Carpathians (Romenia) | Nodal explants | [34] |
| Dianthus pinifolius Sibth. et Sm. | South-eastern Balkans | Seedlings | [35] |
| Eryngium viviparum Gay | Southwest Europe (France, Spain and Portugal) | Seedlings | [24] |
| Henckelia incana (Vahl) Spreng. | Peninsular hills of India | Leaves | [36] |
| Hladnikia pastinacifolia Rchb | Plateau of Trnovski Gozd, Alps (Slovenia) | Seedlings and shoots | [37] |
| Hypericum richeri ssp. transsilvanicum (Čelak) Ciocârlan | Southeast Carpathians (Romania) | Seedlings (nodes, leaves, internodal stems and and root segments) | [38] |
| Juniperus navicularis Gand | Southwest Portugal | Shoot tips and nodal segments | [39] |
| Juniperus thurifera L. | Aure‘s Mountains (Northeastern Algeria) | Shoots | [40] |
| Magnolia dealbata Zucc. | Mexico (south–central) | Zygotic embryos | [41] |
| Laelia anceps Lindl | Mexico | Seedlings | [42] |
| Leucocroton havanensis Borhidi | Cuba | Seedlings | [43] |
| Picconia azorica (Tutin) Knobl. | Azores (Portugal) | Nodal segments | [44] |
| Plantago algarbiensis Samp. | Algarve Region (Portugal) | Seedlings | [45] |
| Plantago almogravensis Franco | Portuguese Southwest coast | Seedlings | [45] |
| Quercus euboica Pap. | Northeastern Euboia island (middle eastern Greece) | Seedlings | [46] |
| Quercus lusitanica Lam. | Iberian Peninsula and North Africa | Shoots | [47] |
| Reseda pentagyna Abdallah & A.G. Miller | Saudi Arabia | Axillary and apical buds | [48] |
| Salvia valentina Vahl and Salvia blancoana Webb & Heldr subsp. mariolensis Figuerola | Valencia Community (Spain) | Apical and nodal segments | [49] |
| Thymus lotocephalus G. López & R. Morales | Algarve Region (Portugal) | Seedlings | [50] |
| Tuberaria major (Willk.) P. Silva and Rozeira | Algarve Region (Portugal) | Seedlings | [51] |
| Zelkova sicula Di Pasquale, Garfì and Quézel | South-eastern Sicily (Italy) | Shoot segments | [52] |
The maintenance of in vitro cultures in active growth (short-term conservation), involving the transference of cultures to fresh medium at regular and short periods (some weeks) is a laborious and expensive task. Slow growth storage is a strategy for the medium-term conservation of in vitro plant germplasm with the objective of reducing plant growth and, therefore, the number of sub-cultures required without affecting the viability of the explants. Using this strategy, cultures can be stored in the same medium for several months without subculture. Growth reduction is obtained by inducing osmotic stress, using growth retardants, reducing partial pressure of oxygen, or by changing the media composition (e.g., growth regulators, minerals) and/or the environmental conditions (e.g., reduction of temperature and/or light) [53]. Though the use of this technique in plant germplasm conservation significantly reduces costs and labor time, by reducing the number of subcultures, when compared to standard in vitro propagation, it still requires high storage space and some level of maintenance and the risk of somaclonal variation persists. There are several reports on the application of slow growth as a medium-term conservation strategy, but those are mainly focused on economic important crops. Regarding endemic plant species the studies are scarce [54,55], probably because efforts and resources have been concentrated in other strategies, like cryopreservation, that will be addressed in detail in the next section of this review.
4. Cryopreservation
Cryopreservation is a type of long-term ex situ conservation in which viable biological resources are stored at ultra-low temperatures, such as those of LN (–196 °C) and/or its vapor phase (–150 °C) [14,56,57]. Different types of plant cells, tissues, and organs can be cryopreserved, theoretically, without loss of their viability, because at these extreme low temperatures, metabolic processes cease, avoiding alterations and/or degeneration of plant material [14,57]. In addition, the material is stored in a small space, protected from contamination, and requires limited maintenance at a low cost. The cryopreservation techniques can be applied to different genotypes and the need for regular subcultures is eliminated, keeping the genetic stability of plant material [14,58,59].
To accomplish an efficient cryopreservation protocol, it is essential that plant cells can be cooled and recovered from ultra-low temperatures without injuries. The main cause of cellular damage is the transition of water into ice, producing ice crystals, during the cooling process [60]. Most plant cells/structures are extremely vulnerable to intracellular freezing, because of their cellular constitution and high-water content [14,57]. To prevent the formation of ice crystals, cells must be adequately dehydrated and exposed to cryoprotectant solutions, before cooling in LN. The exceptions are, for instance, orthodox seeds and dormant buds, that withstand desiccation better, and therefore can be directly cryopreserved without pretreatment [14].
The capacity to survive cryopreservation differs from species to species and even between different clonal lines of the same species. Protocols must be optimized individually according to the species/clone and explant typeto be cryopreserved to obtain the best recovery rates after cryostorage [20,61]. There are many cryopreservation studies concerning crops or economically relevant plant species, due to their value and the large knowledge of their growing conditions [14,62]. In the case of most endemic and wild species, they are highly variable and the information about their biology is scarce, which makes the process of developing a cryopreservation protocol more complex. Several factors/parameters that highly influence cryopreservation success are addressed below.
4.1. Explant Type
Different types of plant material, such as seeds, pollen, spores, cell suspensions, calli, shoot tips, somatic and zygotic embryos and dormant buds, can be cryopreserved. As shown in Table 2 and Table 3, most cryopreservation approaches developed for endemic plant species use seeds or shoot tips as the explant.
Table 2.
Scientific publications on the cryopreservation of seeds of endemic plant species (after 2010). Data refers to maximum germination rate obtained after cryopreservation. The IUCN Status 2020 is also included (NE—Not Evaluated; DD—Data Deficient; LC—Least Concern; NT—Near Threatened; VU—Vulnerable; EN—Endangered; CR—Critically Endangered).
| Species | Germination Rate (%) | Reference | IUCN |
|---|---|---|---|
| Encholirium spectabile Martius ex Schultes f. | 97 | [67] | LC |
| Ermania parryoides Cham. Ex Botsch. | 70 | [66] | - |
| Ferula iliensis Krasn. ex Korov | 90 | [68] | - |
| Hedysarum austrokurilense (N.S. Pavlova) N.S. Pavlova | 85 | [66] | - |
| Hedysarum sachalinense B. Fedtch. (Fabaceae) | 75 | [66] | - |
| Melocactus conoideus Buining and Brederoo | 10 | [69] | CR |
| Micranthocereus flaviflorus subsp. densiflorus (Buining and Brederoo) P. J. Braun and Esteves | 59 | [69] | NT |
| Micranthocereus polyanthus subsp. alvinii M. Machado and Hofacker | 23 | [69] | EN |
| Myosotis sachalinensis M. Pop. | 30 | [66] | - |
| Oxytropis chankaensis Jurtz. | 75 | [66] | - |
| Oxytropis kamtschatica Hult. | 90 | [66] | - |
| Oxytropis retusa Matsum. | 40 | [66] | - |
| Pitcairnia encholirioides L.B.Sm. | 85 | [65] | - |
| Plantago algarbiensis Samp. | 100 | [70] | EN |
| Saxifraga purpurascens Kom. | 20 | [66] | - |
| Stellaria eschscholziana Fenzl | 35 | [66] | - |
| Tanacetum ulutavicum Tzvel. | 72 | [71] | - |
| Thymus lotocephalus G. López & R. Morales | 90 | [63] | NT |
| Tuberaria major (Willk.) P. Silva and Rozeira | 65 | [64] | EN |
| Vaccinium vulcanorum Kom. | 90 | [66] | - |
| Vicia subrotunda (Maxim.) Czefr. | 50 | [66] | - |
Table 3.
Scientific publications on cryopreservation of endemic plant species, by method (D-V—Droplet-vitrification; E-D—Encapsulation-dehydration; E-V—Encapsulation-vitrification; Direct LN—Direct immersion in liquid nitrogen; Vit—Vitrification) and explant type. Data refers to maximum survival (S)/regrowth (R)/germination (G) rate obtained after cryopreservation. The IUCN Status 2020 is also included (NE—Not Evaluated; DD—Data Deficient; LC—Least Concern; NT—Near Threatened; VU—Vulnerable; EN—Endangered; CR—Critically Endangered).
| Method(s) | Species | Region | Explant | Rate (%) | Reference | IUCN |
|---|---|---|---|---|---|---|
| Vit | Anigozanthos viridis ssp terraspectans Hopper | Western Australia | Shoot tips | 89 (S) | [83,84] | - |
| Hypericum rumeliacum Boiss. | Balkan Peninsula | Shoot tips | 2 (S) | [85] | - | |
| Luisia macrantha Blatt. & McCann | Western Ghats (India) | Pollinia | 56 (G) | [75] | - | |
| Macropidia fuliginosa (Hook.) Druce | Western Australia | Shoot tips Somatic embryos |
84 (S) 91 (S) |
[86] | - | |
| Thymus moroderi Pau ex Martínez | South-eastern Spain | Shoot tips | 70 (S) | [81] | - | |
| Tuberaria major (Willk.) P. Silva and Rozeira | Algarve Region (Portugal) | Shoo tips | 60 (R) | [82] | EN | |
| D-V | Aster altaicus var. uchiyamae Kitam | Korea | Shoot tips | 65 (R) | [87] | - |
| Cycladenia humilis Benth. var. jonesii (Eastw.) S.L. Welsh & N.D. Atwood | Canyonlands region of Utah and Arizona (USA) | Shoot tips | 54 (R) | [33] | - | |
| Dianthus giganteus D’Urv subsp. banaticus (Heuff.) Tutin | Southwest Carpathians (Romenia) | Shoot tips | 43 (R) | [34] | - | |
| Hypericum richeri ssp. transsilvanicum (Čelak) Ciocârlan | Southeast Carpathians, Transilvania (Romania) | Axillary buds | 68 (S) | [38] | - | |
| Lithodora rosmarinifolia (Ten.) I. M. Johnst. | Sicily (Italy) | Nodal segments | 33 (R) | [88] | - | |
| Lomandra sonderi (F.Muell.) Ewart | Western Australia | Shoot tips | 32 (S) | [89] | - | |
| Loxocarya cinerea R. Br. | Western Australia | Callus | 90 (S) | [78] | - | |
| Paraisometrum mileense W. T. Wang | Yunnan, China | Shoot tips | 86 (R) | [79] | - | |
| Plantago algarbiensis Samp. | Algarve Region (Portugal) | Nodal segments | 60 (R) | [70] | EN | |
| Thymus cariensis Hub-Mor. & Jalas | Turkey | Shoot tips | 25 (R) | [90] | - | |
| Thymus lotocephalus G. López & R. Morales | Algarve Region (Portugal) | Shoot tips | 67 (R) | [91] | NT | |
| Thymus moroderi Pau ex Martínez | South-eastern Spain | Shoot tips | 79 (S) | [80] | - | |
| E-D | Antirrhinum microphyllum Rothm. | Spain | Nodal segments | 70 (S) | [92] | - |
| Hladnikia pastinacifolia Rchb | Plateau of Trnovski Gozd, Alps (Slovenia) | Shoot tips | 53 (R) | [62] | DD | |
| Centaurium rigualii Esteve | Iberian Peninsula | Nodal segments | 70 (S) | [93] | - | |
| Plantago algarbiensis Samp. | Algarve Region (Portugal) | Nodal segments | 63 (R) | [70] | EN | |
| Pteris adscensionis Swartz | Ascension Island | Gametophytes | 48 (S) | [21] | CR | |
| Thymus moroderi Pau ex Martínez | South-eastern Spain | Shoot tips | 50 (S) | [81] | - | |
| Thymus lotocephalus G. López & R. Morales | Algarve Region (Portugal) | Shoot tips | 44 (R) | [91] | NT | |
| Tuberaria major (Willk.) P. Silva and Rozeira | Algarve Region (Portugal) | Shoot tips | 67 (R) | [82] | EN | |
| E-V | Hladnikia pastinacifolia Rchb | Plateau of Trnovski Gozd, Alps (Slovenia) | Shoot tips | 64 (R) | [62] | DD |
| Cryo-mesh | Anigozanthos viridis Endl. | Western Australia | Shoot tips | 83 (R) | [94] | - |
| VIV | Anigozanthos viridis ssp. terraspectans Hopper | Western Australia | Shoo tips | 31 (R) | [95] | - |
| Lomandra sonderi (F.Muell.) Ewart | Western Australia | Shoo tips | 42 (R) | [95] | - | |
| Loxocarya cinerea R. Br. | Western Australia | Shoo tips | 10 (R) | [95] | - | |
| Desiccation | Nothapodytes nimmoniana (Graham) Melbbery | Western Ghats (India) | Embryonic axes | 60 (G) | [74] | - |
| Direct LN | Pleopeltis lepidopteris Langsd. & Fisch. | South Brazil | Spores | 89 (G) | [96] | - |
Though conventional seed storage is widely used for the conservation of orthodox seeds, cryopreservation presents as an alternative for the long-term conservation of seeds, which can prolong their longevity when compared to other storage temperatures. Several seeds from endemic plant species have been successfully cryopreserved by direct immersion into LN (Table 2). Considering the cryopreservation process used (dehydration followed by direct plunging into LN) these seeds can be described as orthodox. In most studies, there was no loss in seed viability and no significant differences were observed on the germination rate between cryopreserved and non-cryopreserved seeds [63,64,65,66].
On the contrary, the cryopreservation of recalcitrant and intermediate seeds, is a more complex procedure dependent on the seeds’ capacity to survive desiccation and cryogenic conditions. Usually these seeds are larger in size and instead of the complete seed, desiccated embryos or embryonic axes excised from the seed are often used for cryopreservation [72,73]. This approach was used to cryopreserve the germplasm of Nothapodytes nimmoniana (Graham) Melbbery, an endangered forest tree endemic to Western Ghats, India, with large and intermediate type seeds [74]. The embryonic axes of this species were dehydrated for 120 min and then immersed in LN, achieving a germination percentage of 60%. Pollen is another alternative for the conservation of species with recalcitrant or intermediate seeds. Pollinia of Luisia macrantha Blatt. & McCann, an endemic and endangered orchid of Western Ghats, India, was effectively cryopreserved with germination percentages around 55% [75].
For clonally propagated species, cryopreservation in seed form is not a valid option to preserve their germplasm. Besides, many endemic endangered species have small populations and/or scarce seed production, and the collection of seeds may compromise the species’ survival [76]. To cryopreserve these species, different explant types, other than seeds, have to be used, and shoot tips excised from in vitro-grown plants are a frequent option [17]. In fact, shoot tips are also the explant of choice for many other species. As previously mentioned, among the cryopreservation protocols developed for endemic species, the majority (not considering seed cryopreservation) uses shoot tips as plant material (Table 3). Shoot tips, containing the apical meristem, from where growth emerges, are organized and genetically stable structures. Cells within the apical meristem are slight differentiated, small and unvacuolated, and can resist desiccation and freezing better than highly vacuolated and differentiated cells [77,78]. Therefore, after cryostorage and rewarming, these characteristics enable direct shoot formation from cryopreserved shoot tips and maintenance of the genetic integrity [14,77,78]. Several endemic plant species, such as Paraisometrum mileense W. T. Wang (endemic to Yunan, China) [79], Thymus moroderi Pau ex Martínez (endemic to South-eastern Spain) [80,81], and Tuberaria major (Willk.) P. Silva and Rozeira (endemic to the south of Portugal) [82], were successfully cryopreserved using shoot tips, with survival (swollen green shoots or callus formation) or regrowth (normal shoot development) percentages above 50%.
Callus is another structure that can be used in cryopreservation. Despite being an unorganized tissue and more prone to genetic abnormalities, when compared to shoot tips, calli are easier and faster to handle, and the final amount of regenerated material can be higher [77,78]. Cryopreservation of shoot tips of Loxocarya cinerea R.Br., an endemic species to the southwest Western Australia, allowed a very low regeneration rate post-cryopreservation (below 5%) [78]. On the other hand, applying the same protocol to callus tissues, authors obtained survival percentages above 90%. There are other studies using diverse plant material for the cryopreservation of germplasm from endemic plant species. For instance, axillary buds and shoot tips were used as explants for the cryopreservation of Hypericum richeri ssp. transsilvanicum (Čelak) Ciocârlan, a plant species endemic to Transylvania, Romania, and the highest recovery percentage, 68%, was obtained using axillary buds [38]. Nodal segment was the explant chosen for the cryopreservation of Lithodora rosmarinifolia (Ten.) I. M. Johnst., a shrub endemic to Sicily, Italy [88]; Plantago algarbiensis Samp., endemic to the south of Portugal [70]; Centaurium rigualii Esteve, endemic to the southeast of the Iberian Peninsula [93]; and Antirrhinum microphyllum Rothm., endemic to Spain [92].
The cryopreservation of spores is recurrently a valid option for the long-term preservation of ferns. As with seeds, the conservation of spores allows the storage of a wider variety of genetic material. Besides, at sub-freezing temperatures, spores can be effectively stored as seeds [21]. Spores of Pleopeltis lepidopteris Langsd. & Fisch., a fern endemic to the south of Brazil, were successfully stored in LN, achieving germination percentages above 89% [96]. Pteris adscensionis Swartz, an endemic fern from Ascension Island, was chosen as a model species for the development of a standard cryopreservation procedure to be applied to other endemic and rare fern species from biodiversity hotspots and small islands over the world [21]. After germination of P. adscensionis spores, the obtained gametophytes were multiplied in vitro and further used for the cryopreservation trials. A survival of 48% was obtained for P. adscensionis after cryopreservation. When applying the same protocol to other fern species, the results were variable: 90, 86% and 47% of gametophytes survived for Ctenitis pauciflora (Kaulf.) Holttum. (not endemic), Lepisorus longifolius (Blume) Holttum. (not endemic) and Macroglossum smithii (Racib.) Campbell (endemic to Borneo), respectively, while no growth was achieved for Marattia purpurescens de Vriese (endemic to St. Helena). The explant type chosen to cryostorage germplasm from endemic plant species is highly dependent on the species and can have a major influence on the success of the cryopreservation process.
4.2. Cryopreservation Techniques
The classical cryopreservation techniques (controlled rate cooling, slow cooling, or two step cooling) are based on the use of cryoprotectants, to regulate cell water content, combined with slow cooling using a programmable freezer, to induce cell dehydration, followed by immersion in LN [14,61,97]. However, these techniques have been supplanted along the years for simpler and faster procedures and the most used techniques nowadays are vitrification and encapsulation-dehydration, and their derivates [14,56,57]. These techniques are based in the physical process named “vitrification” in which liquids solidify without crystallizing. There are several pre- and post-cryopreservation conditions/factors that can be considered to improve the efficiency of the cryopreservation process, namely the use of preconditioning strategies, standardization of the culture conditions of the in vitro-grown material (e.g., age, morphogenetic status, subculture period, light and nutritional requirements), and size of the explants, composition of the recovery medium, among others [97,98].
4.2.1. Vitrification, Encapsulation-Dehydration, and Encapsulation-Vitrification
Vitrification and encapsulation-dehydration are conventional cryopreservation techniques successfully used in the cryopreservation of a wide range of plant species. In the vitrification technique plant material is exposed to highly concentrated cryoprotectant solutions for short periods. The most commonly used vitrification solutions are the glycerol-based ones developed by Sakai and co-workers [99] in a series known as plant vitrification solution (PVS) [100,101]. A complete vitrification-based procedure comprises several steps: pretreatment, preconditioning, preculture, osmoprotection, dehydration, cooling, warming, dilution, and regrowth [100,101,102]. All these steps must be optimized, so that cells are able to vitrify, after dehydration, upon rapid cooling in LN without any damage resulting from freezing and/or the high toxicity of the vitrification solutions [101]. Several preculture media were investigated, prior to cryopreservation by vitrification, for shoot tips of Anigozanthos viridis ssp terraspectans Hopper [84], and somatic embryos of Macropidia fuliginosa (Hook.) Druce [86], plant species endemic to the southwest of Western Australia. The survival of the explants after rewarming was improved up to 84% and 90.6%, respectively, proving that the composition of the preculture medium has a significant impact on the regeneration of cryopreserved germplasm. Another vital step for an effective cryopreservation protocol by any vitrification-based procedure is the exposure to the vitrification solution. Shoot tips of T. moroderi [80] and T. major [82], were exposed to PVS2 between a time range from 0 to 120 min. The highest survival rates, 71.4% and 60%, respectively, were achieved after 60 min of exposure for both species. Pollinia of L. macranta, also subjected to several PVS2 exposure times, showed 56% germination after 10 min [75]. Overall, the vitrification technique is fast, because it reduces the time needed to dehydrate samples, and reaches high recovery levels. The drawbacks are the difficulty to handle simultaneously numerous and small explants and that it requires careful timing and cryoprotectant solutions that can be toxic to certain plants [56,99].
The encapsulation-dehydration technique consists of the encapsulation of plant material in calcium alginate beads which are partially dehydrated, before rapid exposure to LN [14,17,103]. The rewarming step is simplified because the beads containing the plant material, once properly dehydrated, form a stable structure which prevents devitrification and therefore the formation of ice crystals [56]. An example of an endemic species cryopreserved using this method is C. rigualii, that achieved a survival of 70%, after 4 h desiccation [93]. Despite the advantages, this technique has not been largely used for the cryopreservation of endemic plant species. The complete technique comprises several steps and is very time demanding [56], which may limit its application.
The encapsulation-vitrification technique is a combination of encapsulation-dehydration and vitrification procedures. Plant material is encapsulated in calcium alginate beads and then subjected to dehydration using vitrification solutions [100]. This method was compared with encapsulation-dehydration in the cryopreservation of Hladnikia pastinacifolia Rchb., an endemic species of Slovenia [62]. The best regrowth percentages were obtained with 85 min exposure to PVS2, for encapsulation-vitrification, and 150 min desiccation with silica gel for encapsulation-dehydration, 64% and 53%, respectively. The authors considered that both methods can be applied for the cryopreservation of H. pastinacifolia. The encapsulation-vitrification merges advantages of the conventional vitrification and encapsulation-dehydration techniques: fast procedure and easy to manipulate encapsulated plant material [100,101]. However only the previous publication mentioned was found using this method for the cryopreservation of endemic plant species. As demonstrated by the example above, different techniques can be effectively applied to the same plant species and obtain similar results [14,104]. Close regrowth percentages were also obtained in the cryopreservation of T. major using two different methods [82]. The techniques investigated were vitrification and encapsulation-dehydration with regrowth percentages of 60% and 67%, respectively.
4.2.2. Droplet-Vitrification
One of the most successful cryopreservation techniques is the droplet-vitrification technique, which is a combination of conventional vitrification and the droplet-freezing technique developed by Kartha et al. [105,106]. In this procedure, plant material is placed in droplets of cryoprotectant solution, on small aluminum foil strips, before rapid immersion in LN [14,101]. The use of a minimum volume of cryoprotective solution allows ultra-rapid cooling and warming rates [56,101]. However, there is a contamination risk since the set droplet/plant material is directly exposed to LN [56]. As with the conventional vitrification technique, the various steps of the process must be optimized. There are some reports that describe the optimization of the several steps of the cryopreservation protocol for endemic plant species. For Aster altaicus var. uchiyamae Kitam, an endemic species to Korea, the conditions that gave the best regeneration results, 65.3%, were loading solution (17.5% glucose + 17.5% sucrose) at 0 °C for 60 min, followed by exposure to an altered PVS2 solution (33.3% glucose + 13.3% DMSO + 13.3% ethylene glycol + 20.1% sucrose) at 0 °C for another 60 min [87]. These authors also proved that preculture had no effect on the regeneration of A. altaicus cryopreserved shoot tips, but the composition of the recovery medium was relevant to improve shoot tips regrowth. A regeneration of 86% was achieved for P. mileense when shoot tips were precultured with 0.3 M sucrose for 24 h and then exposed to PVS2 for 90 min at 0 °C, before immersion in LN [79]. Regeneration of P. mileense shoot tips was not improved by cold acclimation. Thymus lotocephalus G. López & R. Morales, endemic to the south of Portugal, attained 67% recovery after subculture of in vitro-donor plants at 25 °C for four weeks, preculture of shoot tips for one day on MS medium containing 0.3 M sucrose and 60 min exposure to PVS2 [91]. Cycladenia humilis Benth. var. jonesii (Eastw.) S.L. Welsh & N.D. Atwood, endemic to Utah and Arizona, USA, was cryopreserved by droplet-vitrification and the success in the recovery of the shoot tips after exposure to LN was determined by the recovery medium composition [33]. Testing different exposure times to the vitrification solution is essential during the optimization of cryopreservation protocols by droplet-vitrification. Other steps of the procedure appear to have a minor impact on the regeneration of cryopreserved germplasm from endemic plant species using this technique.
4.2.3. Cryo-Plate Methods
Some of the most recent and innovative cryopreservation procedures use cryo-plates: V cryo-plate and D cryo-plate methods. Yamamoto et al. [107] created an aluminum plate with small wells (cryo-plate) where explants can be placed. This was the basis for the development of a new cryogenic procedure based on vitrification dehydration, the V cryo-plate method [57,102]. The method is similar to the droplet-vitrification technique, but instead of an aluminum foil stripe, a cryo-plate is used. Before placing the plant material, droplets of calcium alginate are added to the cryo-plate wells to better adhere it to the plate. Attaching the explants to the cryo-plate enables an easier manipulation throughout the process of loading, exposure to the vitrification solution, immersion in LN, and thawing [20,102]. This method is highly advantageous because by attaching the explants to the cryo-plate, there is no need to move them directly, avoiding their damage and/or loss. The very rapid cooling and warming rates protect the explants from cryogenic injuries allowing high regrowth rates. Besides, the implementation and training for this technique is simple and fast [20,57,102,107]. The D cryo-plate method is a combination of V cryo-plate and encapsulation-dehydration. Explants placed in the cryo-plate wells are air-dehydrated in a laminar flow cabinet, instead of being exposed to a vitrification solution, before immersion in LN [57,102,108]. In addition to the advantages of the V cryo-plate method, D cryo-plate can be implemented with larger samples and avoids the toxicity of cryoprotectant solutions [102,108]. The specific construction requirements of the cryo-plates can be limitative to its use, especially at a large scale [20,94]. A stainless-steel mesh strip (cryo-mesh) was created by Funnekotter et al. to overcome this issue [94]. The cryo-mesh is fabricated using wire mesh strips which are simpler to obtain. Its structure enables a faster infiltration of the cryo-solutions and the use of plant material of different dimensions and shapes [94]. The cryo-mesh was tested in the cryopreservation of A. viridis shoot tips and compared to the droplet-vitrification technique. A regeneration of 83% was obtained after 30 min exposure to PVS2 using the cryo-mesh. There were no significant differences when compared to the droplet-vitrification technique, which resulted in 78% regeneration. The use of cryo-mesh greatly simplifies the cryopreservation process by reducing the handling of the plant material, in comparison with standard droplet-vitrification [20,94].
4.2.4. Vacuum-Infiltration Vitrification (VIV)
The vacuum infiltration vitrification (VIV) technique [109] is another recent and innovative cryopreservation method, also based on the conventional vitrification technique. In this technique, vacuum is used to speed the infiltration of the cryoprotectant solution into plant material, therefore reducing the total incubation time required before vitrification, and allowing higher regrowth rates [109]. The vacuum allows a more uniform penetration of the cryoprotectant solutions because it increases the contact between the cryoprotectant solution and cell membranes by reducing intracellular air on the surface of plant material to be cryopreserved [20,102,109]. This technique was tested on shoot tips of several species endemic to southwest Western Australia, that already had cryopreservation protocols developed, such as A. viridis ssp terraspectans and L. cinerea, above-mentioned in this manuscript, and Lomandra sonderi (F.Muell.) Ewart. Overall, comparing to droplet-vitrification, the VIV technique significantly reduced optimal PVS2 incubation time for cryoprotection and improved the survival rates in the regeneration of cryopreserved shoot tips of all the species studied. In the case of L. cinerea, no regeneration was achieved after shoot tip cryopreservation with conventional droplet-vitrification [95]. This had also been proved earlier by Kaczmarczyk et al. [78], which replaced shoot tips for callus. However, with the VIV technique, up to 10% regeneration of L. cinerea shoot tips was obtained after 10 min exposure to PVS2 under vacuum conditions [95].
4.3. Studies to Evaluate Cryogenic Effects
The quality and survival of plant material after storage is extremely important for any conservation protocol to succeed. During the entire cryopreservation process, which includes different stages, from the in vitro culture until the regeneration of complete plants from the cryopreserved plant material, plant cells are subjected to a variety of stresses which may induce morphological and cytological variations and affect their genome, resulting in low regeneration after thawing [14,58,110]. This is particularly risky for endemic plant species because the number of populations and individuals is frequently limited in these species, and any additional loss of genetic diversity may be unmanageable. Therefore, learning and investigating how the different factors of the cryogenic procedure can interfere in the final result is fundamental for the development of effective cryopreservation protocols.
As previously described in the beginning of the current paper, preventing ice formation is pivotal for the success of any cryopreservation procedure and in recent years, new methods have been used to study its effects. There are a few techniques that allow to observe and measure ice formation in cells and tissues and therefore are important tools to assist in the development of cryopreservation protocols. Low temperature scanning electron microscopy is useful in the monitorization of biological material which can be used to observe intra- and extra-cellular ice in conditions similar to those of cryopreservation procedures [111]. Differential scanning calorimetry (DSC) is a thermophysical analysis method that can measure ice formation in plant material during cryopreservation [78,89]. The optimization of PVS2 exposure time of L. sonderi shoots tips, an endemic plant of southwest Western Australia, was assisted by thermal analysis using DSC to detect ice formation in their cells [89]. Other parameters, such as preconditioning of in vitro-donor plants and preculture medium composition and duration, were also assessed, but the thermal analysis proved that only PVS2 exposure time influenced the reduction of ice formation. Thermal analysis using DSC also confirmed that PVS2 treatment prior to cryopreservation prevents ice formation in shoot tips of L. cinerea [78].
Another factor that can cause damages to cells is oxidative stress, that results from the accumulation of reactive oxygen species, and can appear at any step of the cryopreservation procedure [20,110]. Oxidative stress can be determined, for instance, by measuring the activity of antioxidant enzymes. The antioxidant activity of various enzymes, exposed to different preconditioning conditions, was analyzed along the cryopreservation process of L. sonderi [112]. The activity of glutathione reductase decreased in the recovery stage, after cryopreservation, while the activity of glutathione peroxidase and catalase remained equal. In addition, superoxide dismutase presented a positive correlation between post-cryopreservation survival and antioxidant activity, but other enzymes, glutathione reductase, glutathione peroxidase and catalase, presented no correlation [112]. The antioxidant defense against oxidative stress was evaluated for recovered plants from cryopreserved shoot tips of Hypericum rumeliacum Boiss., an endemic species to the Balkans [85]. The results showed that high oxidative stress, provoked by the preculture treatment, decreased the enzymatic antioxidant defense of regenerated cryopreserved material after long culture periods. However, cryopreservation did not affect the capacity of in vitro cultured plantlets to produce phenolics and flavonoids [85].
The assessment of the genetic integrity of cryopreserved plant material is also important to understand the viability of the procedures applied. Several techniques can be employed to assess the genetic stability of cryopreserved plant material, such as phenotypic, cytological, biochemical and molecular [58]. The number of studies concerning plant germplasm integrity after cryopreservation storage is very limited for endemic plant species. Nevertheless, the studies performed so far demonstrated that there are no differences or very few variations between cryopreserved and non-cryopreserved plant material [62,81,85,91]. Random amplified polymorphic DNA (RAPD) is one of the most used molecular techniques for the assessment of genetic stability, including for endemic species. [62,91]. The genetic stability of T. lotocephalus cryopreserved shoot tips was assessed using RAPD markers [91]. Variations at a low frequency (0.06%) were observed, although these had no influence on the morphological characteristics of the plants recovered from the cryopreserved shoot tips. T. moroderi plants, derived from cryopreserved shoot tips, one month after acclimatization to ex vitro conditions were assessed for their genetic and phytochemical stability [113]. A variation of 0.34% was detected using RAPD markers and no morphological differences were detected between plants from cryopreserved and non-cryopreserved plant material. The phytochemical analysis was performed by GS-MS and the results demonstrated that the major components found were the same as those usually found in T. moroderi wild plants. The variations observed during the genetic stability studies might not be phenotypically perceptible because they only affect non-coding regions. On the other hand, RAPD is a molecular technique that screens a low fraction of the genome and some genetic changes are not detected [58].
5. Synopsis and Future Perspectives
By combining in vitro propagation with cryopreservation, a powerful strategy is created. The development and optimization of in vitro propagation protocols is fundamental to ensure the propagation of plants after the recovery of the stored plant material independently of the in vitro strategy followed. Plants raised ex situ can be then used to restore or reinforce wild depauperated populations and for other applications including breeding programs. Cryopreservation secures the plant material in cryobanks, in theory for an unlimited time, for further use in plant production for many applications including the re-introduction in the wild [56]. However, the publications available in international journals concerning the cryopreservation of endemic plant species is still limited, considering their global distribution and number. The overall conclusion is that much more can be done in the future to benefit from cryopreservation in the conservation of these unique plant species. Several causes can be pointed out to why the number of scientific publications is not high in this matter:
-
-
Unpublished data: there could be a reasonable number of protocols that were developed at cryobanks or botanic gardens for specific endemic species that are been used but have not been published;
-
-
Low funding: the investment in the conservation of wild plants is not retrieved as the investment on crop, medicinal and ornamental species. Their economic value makes these last species more engaging for research and therefore more accessible to funds, not only from funding agencies at an academic level but also from private investors, leaving few resources for the conservation of endemic and rare species. In addition, there are considerably more funding and resources for the conservation of animals rather than plants [114,115];
-
-
Scarce plant material: endemic plant species frequently occur in small populations in the wild, occasionally with difficult access, and thus the plant material available is very limited. In addition, a considerable number of endemic species are legally protected and, therefore, subjected to restrictions for their use and collection [115];
-
-
No standard protocols: protocol development and optimization is the most labor-intensive phase of cryopreservation [56]. In the case of endemic species, their unique nature and often unknown biology and physiology makes it even harder to determine the best conditions for cryopreservation and almost impossible to establish standard protocols.
-
-
Cryobank facilities: although to develop a cryopreservation protocol, with the vitrification-based techniques, only a standard tissue culture lab is necessary, to effectively store germplasm under cryogenic conditions more complex and costly facilities are required. Not always laboratories that develop the cryopreservation protocols are prepared to maintain germplasm for long periods of time. The scale-up of the germplasm and cryopreservation procedures requires the establishment of specific measures for their management [14]. Priority is given once again to species economically more desirable.
-
-
High number: presently it is not possible to extend conservation efforts to all endemic species. There are just too many and too less resources to cover all species.
The limitations in the preservation of endemic plant species are variable because of their high diversity, geographic range, and/or accessibility. Research on this matter is urgent and the potential for future scientific studies on the cryopreservation of endemic plant species is enormous. There are considerably more studies on in vitro propagation than cryopreservation for the conservation of endemic plant species. Despite all the advantages of cryopreservation, this technique is still more costly and difficult to implement when compared to in vitro plant propagation. Nonetheless, there have been efforts made in different parts of the world to create cryopreservation protocols for these unique species. The scientific publications found (information obtained using a combination of the keywords “cryopreservation+endemic+plant+species” via Science Direct, Google Scholar, and standard Google search, in the English language) related to the development of cryopreservation protocols for endemic plant species were less than ten until 2009. Between 2010–2014, there was a substantial increase, approximately twenty publications; however, that number decreased after 2015 until the present and most publications are related to improvements in protocols and not the report of new protocols for other species. It is interesting to notice that many of the publications regarding the cryopreservation of endemic plants belong to species that occur in Biodiversity Hotspots. These are identified areas all over the planet with high concentration of endemic species and high habitat loss. There are 35 Biodiversity Hotspots that cover only 17.3% of the land surface of the Earth but maintain 77% of all endemic plant species [12]. Cryopreservation protocols were established, for instance, for species from the Biodiversity Hotspots Mediterranean basin (T. lotocephalus [91], T. moroderi [80,81], T. major [82], P. algarbiensis [70], C. rigualii [93], among others); Southwest Australia (L. sonderi [89,95], L. cinerea [78,95], M. fuliginosa [86] and A. viridis spp terraspectans [83,84,95]); and Western Ghats and Sri Lanka (L. macranta [75] and N. nimmoniana [74]).
The resources available are not enough to preserve all biodiversity and a recognized conservation strategy is to direct efforts and prioritize the preservation of species in areas rich in endemic species, such as the Biodiversity Hotspots [13]. However, within these areas, the number of plant species is still very high, considering the existing means. Since not all endemic species are rare, the identification of the species more vulnerable, i.e., with restricted ranges and low-density populations, is therefore very important in the selection of biodiversity to be preserved [7]. Once identified, the conservation planning for these species should encompass combined conservation strategies, including seed banking and in vitro techniques, and the setting of conservation objectives [4,7].
Other essential aspects to establish an efficient conservation program are the understanding of the natural populations’ structure and the assessment of the species’ genetic diversity, particularly when it includes ex situ conservation measures, such as cryopreservation [7,19,116]. Storage methods should comprise extensive collections with representative genetic diversity. Genetic variation is the basis for evolution and the plant material to be preserved ex situ should be demonstrative of the wild population diversity it represents, so that in the future, if need be, the material can be used for restoration of natural populations [19]. Classic molecular markers, such as RAPD, RFLP (restriction fragment length polymorphism), AFLP (amplified fragment length polymorphism), and ISSR (inter simple sequence repeats) were very useful in the study of genetic diversity and in the identification of species [19,117], although nowadays there are more updated methods. The number of publications reporting the genetic diversity of wild populations from endemic plant species is considerably higher than the publications regarding cryopreservation. As for the species mentioned in this review, a few were assessed for the genetic diversity of their wild populations: C. humilis [118], A. microphyllum [119], Oxytropis chankaensis Jurtz. [120], H. pastinacifolia [121], T. major [122], P. mileense [123], P. algarbiensis [116,124], Pitcairnia encholirioides L.B.Sm. [65], N. nimmoniana [125] and Encholirium spectabile Martius ex Schultes f. [126]. Recommendations for their conservation were given according to the results and the characteristics of the populations from each species. Though in some cases the genetic diversity studies were performed after the development of in vitro conservation techniques, the information obtained about the structure and genetic diversity of those populations will be very useful for further collections and storage of plant germplasm from these species. The next-generation sequencing methods, which are faster and less expensive than the classical ones, can facilitate the connection between molecular markers and conservation management. These new molecular techniques can identify thousands of markers in a single step and evaluate metabolisms even for unknown genomes, which is the case for most endemic plant species [2,117]. Examples of these methods are DNA microarray and their derived methods, diversity array technology (DArT) and subtracted diversity array (SDA), which are more appropriate for species with no previous knowledge of the genome [117].
As for cryopreservation itself, a deeper knowledge about the overall extent of the cryopreservation process could assist in a faster development of new protocols or even in the creation of easy-to-use procedures. Several factors and their interactions influence the success of any cryopreservation protocol. Understanding these factors, such as plant physiology and stress tolerance, will contribute to a faster and easier optimization of new cryopreservation procedures [109,110]. The advances in metabolomic, genomic, transcriptomic, and proteomic technologies are very promising for the acquisition of more detailed information about the physiological, biochemical, molecular, and ultrastructural changes that plant material undergoes during cryogenic storage. These techniques can help finding where (in the plant tissues) and/or what (cryoprotectant, ice crystals, etc.) exactly is damaging the cells, increase the knowledge on cryoinjury, and, consequently, facilitate the development of cryopreservation protocols by solving specific problems [102,110]. This information coupled with data from protocols already developed for closely related species or species from similar habitats can be very beneficial in the successful regeneration of cryopreserved plant germplasm [102]. Ultimately, time and resources spent on the development of cryopreservation protocols for endemic plant species can be significantly reduced.
The innovative cryopreservation techniques recently developed, cryo-plate methods and VIV, are important improvements for the cryopreservation of plant gemplasm. The application of these methods may ease and accelerate the development of new protocols due to their novel approaches to the whole cryopreservation process. Cryo-plate methods are simple and easy to use and could be applied for large scale storage in cryobanks [107]. As for VIV, this method reduces the exposure time to cryoprotectants, thus reducing their toxicity, and enables high regrowth percentages [109]. These novel methods should be tested for the cryopreservation of germplasm of more endemic plant species or even in the improvement of protocols already established.
Overall, a deeper knowledge on the cryopreservation process allied to new technologies can greatly aid and facilitate the development of simpler and standardized procedures that can ultimately be used by a wider range of institutions and laboratories and therefore augment the efforts on the preservation of endemic plant species worldwide.
Concerns about the environment, climate changes, earth’s genetic heritage, mass extinction, and weather alterations are increasing worldwide. The general public is now more aware of the importance of endemic plant species, their unique genetic material, and the need to preserve them. Hopefully, this increasing interest will lead to the expansion of the resources available to support the preservation of endemic plant species. Besides, the new technologies available can greatly facilitate and haste not only the development of new cryopreservation protocols but also in the establishment of a bridge between in situ and ex situ conservation strategies. There is an urgent need to expand conservation research and particularly transfer the academic knowledge acquired to the actual implementation of conservation strategies in practice. The cryopreservation of endemic plant species is a challenge and there is a considerable amount of work that needs to be done in a near future to complement the preservation of these unique plant species and prevent their extinction in the wild. Furthermore, it is important to promote studies that can confirm the economic value of many unstudied and unexplored endemic plant species, namely their potential for the extraction of valuable bioactive compounds, for biofuels production, for bioremediation, among others. This may increase the interest in these plants and encourage the implementation of conservation strategies. As above-mentioned in this manuscript the in vitro techniques allow the mass propagation of plants and therefore the sustainable use of plant biomass for many applications.
Author Contributions
N.C. and A.R. provided the key ideas of the review; N.C. and S.G. wrote the manuscript. All authors reviewed, edited, and discussed this review. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the project INTERREG–MD.NET: When Brand Meets People and by National Funds through FCT—Foundation for Science and Technology under the Project UIDB/05183/2020. Sandra Gonçalves is funded by national funds through the FCT, I.P., under the Norma Transitória–DL 57/2016/CP1361/CT0022.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.FAO . The State of the World’s Biodiversity for Food and Agriculture. In: Bélanger J., Pilling D., editors. FAO Commission on Genetic Resources for Food and Agriculture Assessments. FAO; Rome, Italy: 2019. [(accessed on 18 January 2020)]. Available online: http://www.fao.org/3/CA3129EN/CA3129EN.pdf. [Google Scholar]
- 2.Corlett R.T. A Bigger Toolbox: Biotechnology in Biodiversity Conservation. Trends Biotechnol. 2017;35:55–65. doi: 10.1016/j.tibtech.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Cuttelod A., García N., Abdul Malak D., Temple H., Katariya V. In: The 2008 Review of the IUCN Red List of Threatened Species. Vié J.-C., Hilton-Taylor C., Stuart S.N., editors. IUCN; Gland, Switzerland: 2008. p. 1. [Google Scholar]
- 4.Reed B.M., Sarasan V., Kane M., Bunn E., Pence V.C. Biodiversity conservation and conservation biotechnology tools. In Vitro Cell. Dev. Biol.-Plant. 2011;47:1–4. doi: 10.1007/s11627-010-9337-0. [DOI] [Google Scholar]
- 5.Sarasan V., Cripps R., Ramsay M.M., Atherton C., Mcmichen M., Prendergast G., Rowntree J.K. Conservation in vitro of threatened plants—Progress in the past decade. In Vitro Cell. Dev. Biol.-Plant. 2006;42:206–214. doi: 10.1079/IVP2006769. [DOI] [Google Scholar]
- 6.Anderson S. Area and endemism. Q. Rev. Biol. 1994;69:451–471. doi: 10.1086/418743. [DOI] [Google Scholar]
- 7.Burlakova L.E., Karatayev A.Y., Karatayev V.A., May M.E., Bennett D.L., Cook M.J. Endemic species: Contribution to community uniqueness, effect of habitat alteration, and conservation priorities. Biol. Conserv. 2011;144:155–165. doi: 10.1016/j.biocon.2010.08.010. [DOI] [Google Scholar]
- 8.Foggi B., Viciani D., Baldini R.M., Carta A., Guidi T. Conservation assessment of the endemic plants of the Tuscan Archipelago, Italy. Orix. 2014;49:118–126. doi: 10.1017/S0030605313000288. [DOI] [Google Scholar]
- 9.Işik K. Rare and endemic species: Why are they prone to extinction? Turk. J. Bot. 2011;35:411–417. doi: 10.3906/bot-1012-90. [DOI] [Google Scholar]
- 10.Ladle R.J., Whittaker R.J. Conservation Biogeography. Wiley-Blackwell; Oxford, UK: 2011. [Google Scholar]
- 11.IUCN The IUCN Red List of Threatened Species. [(accessed on 20 January 2020)];Version 2019-2. Available online: http://www.iucnredlist.org.
- 12.Marchese C. Biodiversity hotspots: A shortcut for a more complicated concept. Glob. Ecol. Conserv. 2015;3:297–309. doi: 10.1016/j.gecco.2014.12.008. [DOI] [Google Scholar]
- 13.Myers N., Mittermeier R.A., Mittermeier C.G., Fonseca G.A.B., Kent J. Biodiversity hotspots for conservation priorities. Nature. 2000;403:853–858. doi: 10.1038/35002501. [DOI] [PubMed] [Google Scholar]
- 14.Engelmann F. Use of biotechnologies for the conservation of plant biodiversity. In Vitro Cell. Dev. Biol.-Plant. 2011;47:5–16. doi: 10.1007/s11627-010-9327-2. [DOI] [Google Scholar]
- 15.Maxted N., Ford-Lloyd B.V., Hawkes J.G. Complementary conservation strategies. In: Maxted N., Ford-Lloyd B.V., Hawkes J.G., editors. Plant Genetic Conservation: The In Situ Approach. Chapman and Hall; London, UK: 1997. p. 15. Chapter 2. [Google Scholar]
- 16.Pritchard H.W. Cryopreservation of desiccation tolerant seeds. In: Day J.G., Stacey G.N., editors. Cryopreservation and Freeze-Drying Protocols. Humana Press Inc; Totowa, NJ, USA: 2007. p. 183. Chapter 13. [Google Scholar]
- 17.González-Benito M.E., Clavero-Ramírez I., López-Aranda J.M. Review. The use of cryopreservation for germplasm conservation of vegetatively propagated crops. Span. J. Agric. Res. 2004;2:341–351. doi: 10.5424/sjar/2004023-88. [DOI] [Google Scholar]
- 18.Benson E.E. An Introduction to Plant Conservation Biotechnology. In: Benson E.E., editor. Plant Conservation Biotechnology. Taylor & Francis; London, UK: 1999. p. 3. Part 1. Chapter 1. [Google Scholar]
- 19.González-Benito M.E., Martín C. In Vitro Preservation of Spanish Biodiversity. In Vitro Cell. Dev. Biol.-Plant. 2011;417:46–54. doi: 10.1007/s11627-010-9333-4. [DOI] [Google Scholar]
- 20.Streczynski R., Clark H., Whelehan L., Ang S.-T., Hardstaff L., Funnekotter B., Bunn E., Offord C.A., Sommerville K., Mancera R. Current issues in plant cryopreservation and importance for ex situ conservation of threatened Australian native species. Aust. J. Bot. 2019;67:1–15. doi: 10.1071/BT18147. [DOI] [Google Scholar]
- 21.Barnicoat H., Cripps R., Kendon J., Sarasan V. Conservation in vitro of rare and threatened ferns—Case studies of biodiversity hotspot and island species. In Vitro Cell. Dev. Biol.-Plant. 2011;47:37–45. doi: 10.1007/s11627-010-9303-x. [DOI] [Google Scholar]
- 22.Bowes B.G. A Colour Atlas of Plant Propagation and Conservation. Manson Publishing Ltd.; London, UK: 1999. [Google Scholar]
- 23.Kartha K.K. Cryopreservation of Plant Cells and Organs. CRC Press; Boca Raton, FL, USA: 1985. [Google Scholar]
- 24.Ayuso M., García-Pérez P., Ramil-Rego P., Gallego P.P., Barreal M.E. In vitro culture of the endangered plant Eryngium viviparum as dual strategy for its ex situ conservation and source of bioactive compounds. Plant Cell Tissue Organ Cult. 2019;138:427–435. doi: 10.1007/s11240-019-01638-y. [DOI] [Google Scholar]
- 25.Murashige T., Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol. Plantarum. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- 26.Faria D.V., Simão M.J., Cipriano R., Werner E.T., Soares T.C.B., Aoyama E.M., Lima-Gontijo A.B.P. In vitro morphogenesis and micropropagation of Aechmea ramosa var. ramosa Mart. ex Schult. f. (Bromeliaceae) from leaf explants. In Vitro Cell. Dev. Biol.-Plant. 2018;54:530–536. doi: 10.1007/s11627-018-9907-0. [DOI] [Google Scholar]
- 27.Regalado J.J., Carmona-Martín E., López-Granero M., Jiménez A., Castro P., Encina C.L. Micropropagation of Asparagus macrorrhizus, a Spanish endemic species in extreme extinction risk. Plant Cell Tissue Organ Cult. 2018;132:573–578. doi: 10.1007/s11240-017-1346-9. [DOI] [Google Scholar]
- 28.Lakshmi S.R., Parthibhan S., Sherif N.A., Kumar T.S., Rao M.V. Micropropagation, in vitro flowering, and tuberization in Brachystelma glabrum Hook.f., an endemic species. In Vitro Cell. Dev. Biol.-Plant. 2017;53:64–72. doi: 10.1007/s11627-017-9803-z. [DOI] [Google Scholar]
- 29.Nair L.G., Seeni S. In vitro multiplication of Calophyllum apetalum (Clusiaceae), an endemic medicinal tree of the Western Ghats. Plant Cell Tissue Organ Cult. 2003;75:169–174. doi: 10.1023/A:1025001214995. [DOI] [Google Scholar]
- 30.Chavan J.J., Nalawade A.S., Gaikwad N.B., Gurav R.V., Dixit G.B., Yadav S.R. An efficient in vitro regeneration of Ceropegia noorjahaniae: An endemic and critically endangered medicinal herb of the Western Ghats. Physiol. Mol. Biol. Plants. 2014;20:405–410. doi: 10.1007/s12298-014-0236-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abraham S., Augustine J., Thomas T.D. Asymbiotic seed germination and in vitro conservation of Coelogyne nervosa A. Rich. an endemic orchid to Western Ghats. Physiol. Mol. Biol. Plants. 2012;18:245–251. doi: 10.1007/s12298-012-0118-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Arrabal R., Amancio F., Carneiro L.A., Neves L.J., Mansur E. Micropropagation of endangered endemic Brazilian bromeliad Cryptanthus sinuosus (L.B. Smith) for in vitro preservation. Biodivers. Conserv. 2002;11:1081–1089. doi: 10.1023/A:1015860804695. [DOI] [Google Scholar]
- 33.Pence V.C., Finke L.R., Chaiken M.F. Tools for the ex situ conservation of the threatened species, Cycladenia humilis var. jonesii. Conserv. Physiol. 2017;5:cox053. doi: 10.1093/conphys/cox053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jarda L., Cristea V., Halmagyi A., Palada M. In vitro culture initiation and cryopreservation of endemic taxa Dianthus giganteus ssp. banaticus. Acta Hortic. 2011;918:153–159. doi: 10.17660/ActaHortic.2011.918.18. [DOI] [Google Scholar]
- 35.Markovic M., Grbić M., Djukic M. Micropropagation of Endangered and Decorative Species Dianthus pinifolius Sibth. et Sm. Braz. Arch. Biol. Technol. 2016;59:e16150320. doi: 10.1590/1678-4324-2016150320. [DOI] [Google Scholar]
- 36.Prameela J., Ramakrishnaiah H., Deepalakshmi A.P., Kumar N.N., Radhika R.N. Micropropagation and assessment of genetic fidelity of Henckelia incana: An endemic and medicinal Gesneriad of South India. Physiol. Mol. Biol. Plants. 2015;21:441–446. doi: 10.1007/s12298-015-0314-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ambrožič-Dolinšek J., Ciringer T., Kaligarič M. Micropropagation of the Narrow Endemic Hladnikia pastinacifolia (Apiaceae) Acta Bot. Croat. 2016;75:244–252. doi: 10.1515/botcro-2016-0028. [DOI] [Google Scholar]
- 38.Coste A., Halmagyi A., Keul A., Deliu C., Coldea G., Hurdu B. In vitro propagation and cryopreservation of Romanian endemic and rare Hypericum species. Plant Cell. Tissue Organ Cult. 2012;110:213–226. doi: 10.1007/s11240-012-0144-7. [DOI] [Google Scholar]
- 39.Castro M., Belo A., Afonso A., Zavattieri A. Micropropagation of Juniperus navicularis, an endemic and rare species from Portugal SW coast. Plant Growth Regul. 2011;65:223–230. doi: 10.1007/s10725-011-9590-1. [DOI] [Google Scholar]
- 40.Khater N., Benbouza H. Preservation of Juniperus thurifera L.: A rare endangered species in Algeria through in vitro regeneration. J. For. Res. 2019;30:77–86. doi: 10.1007/s11676-018-0628-3. [DOI] [Google Scholar]
- 41.Mata-Rosas M., Jiménez-Rodríguez A. Somatic embryogenesis and organogenesis in Magnolia dealbata Zucc. (Magnoliaceae), an endangered, endemic Mexican species. HortScience. 2006;41:1329. doi: 10.21273/HORTSCI.41.5.1325. [DOI] [Google Scholar]
- 42.Ramírez-Mosqueda M.A., Cruz-Cruz C.A., Atlahua-Temoxtle J., Bello-Bello J.J. In vitro conservation and regeneration of Laelia anceps Lindl. S. Afr. J. Bot. 2019;121:219–223. doi: 10.1016/j.sajb.2018.11.010. [DOI] [Google Scholar]
- 43.Alfonso D., Cicatelli A., Guarino F., Rodríguez D., Castiglione S. In vitro propagation of Leucocroton havanensis Borhidi (Euphorbiaceae): A rare serpentine-endemic species of Cuba. Plant Biosyst. 2018;152:649–656. doi: 10.1080/11263504.2017.1311961. [DOI] [Google Scholar]
- 44.Mendonça D., Luna S., Bettencourt S., Lopes M.S., Monteiro L., Neves J.D., Monjardino P., Machado A.C. In vitro propagation of Picconia azorica (Tutin) Knobl. (Oleaceae) an Azorean endangered endemic plant species. Acta Physiol. Plant. 2015;37:47. doi: 10.1007/s11738-015-1797-8. [DOI] [Google Scholar]
- 45.Gonçalves S., Martins N., Romano A. Micropropagation and conservation of endangered species Plantago algarbiensis and P. almogravensis. Biol. Plantarum. 2009;53:774–778. doi: 10.1007/s10535-009-0142-8. [DOI] [Google Scholar]
- 46.Kartsonas E., Papafotiou M. Mother plant age and seasonal influence on in vitro propagation of Quercus euboica Pap., an endemic, rare and endangered oak species of Greece. Plant Cell Tissue Organ Cult. 2007;90:111–116. doi: 10.1007/s11240-007-9232-5. [DOI] [Google Scholar]
- 47.San José M.C., Santiago M.T., Cernadas M.J., Montenegro R., Mosteiro F., Corredoira E. Biotechnological efforts for the propagation of Quercus lusitanica Lam., an endangered species. Trees-Struct. Funct. 2017;31:1571–1581. doi: 10.1007/s00468-017-1570-2. [DOI] [Google Scholar]
- 48.Al-Qurainy F., Mohammad N., Khan S., Alansi S., Tarroum M., Alameri A.A., Gaafar A.-R.Z., Al-Shameri A. Rapid plant regeneration, validation of genetic integrity by ISSR markers and conservation of Reseda pentagyna an endemic plant growing in Saudi Arabia. Saudi J. Biol. Sci. 2017;25:111–116. doi: 10.1016/j.sjbs.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuenca S., Amo-Marco J.B. In vitro propagation of two Spanish endemic species of Salvia through bud proliferation. In Vitro Cell. Dev. Biol.-Plant. 2000;36:225–229. doi: 10.1007/s11627-000-0042-2. [DOI] [Google Scholar]
- 50.Coelho N., Gonçalves S., González-Benito M.E., Romano A. Establishment of an in vitro propagation protocol for Thymus lotocephalus, a rare aromatic species of the Algarve (Portugal) Plant Growth Regul. 2012;66:69–74. doi: 10.1007/s10725-011-9630-x. [DOI] [Google Scholar]
- 51.Gonçalves S., Fernandes L., Romano A. High-frequency in vitro propagation of the endangered species Tuberaria major. Plant Cell Tissue Organ Cult. 2010;101:359–363. doi: 10.1007/s11240-010-9683-y. [DOI] [Google Scholar]
- 52.Carra A., Catalano C., Badalamenti O., Carimi F., Pasta S., Motisi A., Abbate L., Bella F., Fazan L., Kozlowski G., et al. Overcoming sexual sterility in conservation of endangered species: The prominent role of biotechnology in multiplication of Zelkova sicula (Ulmaceae), a relict tree at the brink of extinction. Plant Cell Tissue Organ Cult. 2019;137:139–148. doi: 10.1007/s11240-019-01558-x. [DOI] [Google Scholar]
- 53.Reed B.M., Gupta S., Uchendu E.E. In Vitro Genebanks for Preserving Tropical Biodiversity. In: Normah M., Chin H., Reed B., editors. Conservation of Tropical Plant Species. Springer; New York, NY, USA: 2013. p. 77. Chapter 5. [Google Scholar]
- 54.Iriondo J.M., Pérez C. Micropropagation and in vitro storage of Centaurium rigualii Esteve (Gentianaceae) Isr. J. Plant Sci. 1996;44:115–123. doi: 10.1080/07929978.1996.10676640. [DOI] [Google Scholar]
- 55.Martin K.P., Pradeep A.K. Simple strategy for the in vitro conservation of Ipsea malabarica an endemic and endangered orchid of the Western Ghats of Kerala, India. Plant Cell Tissue Organ Cult. 2003;74:197–200. doi: 10.1023/A:1023971625994. [DOI] [Google Scholar]
- 56.Benson E.E. Cryopreservation of Phytodiversity: A Critical Appraisal of Theory & Practice. Crit. Rev. Plant Sci. 2008;27:141–219. doi: 10.1080/07352680802202034. [DOI] [Google Scholar]
- 57.Matsumoto T. Cryopreservation of plant genetic resources: Conventional and new methods. Rev. Agric. Sci. 2017;5:13–20. doi: 10.7831/ras.5.13. [DOI] [Google Scholar]
- 58.Harding K. Genetic integrity of cryopreserved plant cells: A review. CryoLetters. 2004;25:3–22. [PubMed] [Google Scholar]
- 59.Walters C., Wheeler L., Stanwood P.C. Longevity of cryogenically stored seeds. Cryobiology. 2004;48:229–244. doi: 10.1016/j.cryobiol.2004.01.007. [DOI] [PubMed] [Google Scholar]
- 60.Fuller B.J. Cryoprotectants: The essential antifreezes to protect life in the frozen state. CryoLetters. 2004;25:375–388. [PubMed] [Google Scholar]
- 61.Reed B.M. Plant Cryopreservation: A Practical Guide. Springer; New York, NY, USA: 2008. [Google Scholar]
- 62.Ciringer T., Martín C., Šajna N., Kaligarič M., Ambrožič-Dolinšek J. Cryopreservation of an endangered Hladnikia pastinacifolia Rchb. by shoot tip encapsulation-dehydration and encapsulation-vitrification. In Vitro Cell. Dev. Biol.-Plant. 2018;54:565–575. doi: 10.1007/s11627-018-9917-y. [DOI] [Google Scholar]
- 63.Coelho N., Gonçalves S., González-Benito M.E., Romano A. Germination and cryopreservation tolerance of seeds from the rare aromatic species Thymus lotocephalus. Sci. Hortic. 2012;145:84–86. doi: 10.1016/j.scienta.2012.07.031. [DOI] [Google Scholar]
- 64.Gonçalves S., Fernandes L., Pérez-García F., González-Benito M.E., Romano A. Germination requirements and cryopreservation tolerance of seeds of the endangered species Tuberaria major. Seed Sci. Technol. 2009;37:480–484. doi: 10.15258/sst.2009.37.2.22. [DOI] [Google Scholar]
- 65.Hmeljevski K.V., Freitas L., Domingues R., Pereira A.R., Cancio A.S., Andrade A.C.S., Machado M.A., Viccini L.F., Forzza R.C. Conservation assessment of an extremely restricted bromeliad highlights the need for population-based conservation on granitic inselbergs of the Brazilian Atlantic Forest. Flora. 2014;209:250–259. doi: 10.1016/j.flora.2014.03.004. [DOI] [Google Scholar]
- 66.Voronkova N.M., Kholina A.B. Conservation of Endemic Species from the Russian Far East Using Seed Cryopreservation. Biol. Bull. 2010;37:496–501. doi: 10.1134/S1062359010050092. [DOI] [PubMed] [Google Scholar]
- 67.Ferrari E., Colombo R., Faria R., Takane R. Cryopreservation of seeds of Encholirium spectabile Martius ex Schultes f. by the vitrification method. Rev. Cienc. Agron. 2016;47:172–177. doi: 10.5935/1806-6690.20160020. [DOI] [Google Scholar]
- 68.Almerekova S., Mukhitdinova Z., Ydyrys A., Mukhitdinov N., Курманбаева М., Tynybekov B., Inelova Z., Akhmetova A. The effect of cryopreservation on seed germination of the endangered, rare, endemic and medicinal plant Ferula iliensis Krasn. ex Korov. J. Biotechnol. 2016;231:S40. doi: 10.1016/j.jbiotec.2016.05.158. [DOI] [Google Scholar]
- 69.Civatti L.M., Marchi M.N.G., Bellintani M.C. Cryopreservation of cacti seeds of three ornamental species endemic to the state of Bahia, Brazil. Seed Sci. Technol. 2015;43:284–290. doi: 10.15258/sst.2015.43.2.08. [DOI] [Google Scholar]
- 70.Coelho N., González-Benito M.E., Romano A. Approaches for the cryopreservation of Plantago algarbiensis, a rare endemic species of the Algarve. CryoLetters. 2014;35:521–529. [PubMed] [Google Scholar]
- 71.Dodonova A.S., Gavrilkova H.A., Ishmuratova M., Tleukenova S.U., Pogossyan G.P. Analysis of the effects of cryopreservation conditions on germination ability of Tanacetum ulutavicum seeds. Res. J. Pharm. Biol. Chem. Sci. 2016;7:698–703. [Google Scholar]
- 72.Walters C., Wesley-Smith J., Crane J., Hill L.M., Chmielarz P., Pammenter N.W., Berjak P. Cryopreservation of Recalcitrant (i.e. Desiccation-Sensitive) Seeds. In: Reed B.M., editor. Plant Cryopreservation: A Practical Guide. Springer; New York, NY, USA: 2008. p. 465. Section II. Chapter 18. [Google Scholar]
- 73.Wesley-Smith J., Berjak P., Pammenter N.W., Walters C. Intracellular ice and cell survival in cryo-exposed embryonic axes of recalcitrant seeds of Acer saccharinum: An ultrastructural study of factors affecting cell and ice structures. Ann. Bot. 2014;113:695–709. doi: 10.1093/aob/mct284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Radha R.K., Decruse S.W., Krishnan P.N. Cryopreservation of excised embryonic axes of Nothapodytes nimmoniana (Graham) Mebberly—A vulnerable medicinal tree species of the Western Ghats. Indian J. Biotechnol. 2010;9:435–437. doi: 10.1007/s11240-014-0683-1. [DOI] [Google Scholar]
- 75.Ajeeshkumar S., Decruse S.W. Fertilizing Ability of Cryopreserved Pollinia of Luisia macrantha, an Endemic Orchid of Western Ghats. CryoLetters. 2013;34:20–29. [PubMed] [Google Scholar]
- 76.Martín C., Iriondo J., González-Benito M.E., Pérez C. The use of tissue culture techniques in the conservation of plant biodiversity. Agro Food Ind. Hi-Tech. 1998;9:37–40. [Google Scholar]
- 77.Kaczmarczyk A., Funnekotter B., Menon A., Phang P., Al-Hanbali A., Bunn E., Mancera R. Current Issues in Plant Cryopreservation. In: Katkov I., editor. Current Frontiers in Cryobiology. IntechOpen Ltd.; London, UK: 2012. p. 417. Chapter 14. [Google Scholar]
- 78.Kaczmarczyk A., Funnekotter B., Turner S., Bunn E., Bryant G., Hunt T., Mancera R. Development of cryopreservation for Loxocarya cinerea—An endemic Australian plant species important for post-mining restoration. CryoLetters. 2013;34:508–519. [PubMed] [Google Scholar]
- 79.Lin L., Yuan B., Wang D., Li W. Cryopreservation of adventitious shoot tips of Paraisometrum mileense by droplet vitrification. CryoLetters. 2014;35:22–28. [PubMed] [Google Scholar]
- 80.Marco-Medina A., Casas J.L., González-Benito M.E. Comparison of vitrification and encapsulation-dehydration for cryopreservation of Thymus moroderi shoot tips. CryoLetters. 2010;31:301–309. [PubMed] [Google Scholar]
- 81.Marco-Medina A., Casas J.L., Swennen R., Panis B. Cryopreservation of Thymus moroderi by droplet vitrification. CryoLetters. 2010;31:14–23. [PubMed] [Google Scholar]
- 82.Coelho N., González-Benito M.E., Romano A. Cryopreservation of shoot tips from the rare endemic species Tuberaria major. Acta Physiol. Plant. 2014;36:3333–3336. doi: 10.1007/s11738-014-1678-6. [DOI] [Google Scholar]
- 83.Turner S., Krauss S.L., Bunn E., Senaratna T., Dixon K.W., Tan B., Touchell D.H. Genetic fidelity and viability of Anigozanthos viridis following tissue culture, cold storage and cryopreservation. Plant Sci. 2001;161:1099–1106. doi: 10.1016/S0168-9452(01)00519-2. [DOI] [Google Scholar]
- 84.Turner S., Senaratna T., Touchell D., Bunn E., Dixon K., Tan B. Stereochemical arrangement of hydroxyl groups in sugar and polyalcohol molecules as an important factor in effective cryopreservation. Plant Sci. 2001;160:489–497. doi: 10.1016/S0168-9452(00)00420-9. [DOI] [PubMed] [Google Scholar]
- 85.Danova K., Nikolova-Damianova B., Denev R., Markovska Y. Impact of pre-culture on short- and long-term in vitro recovery of the biosynthetic potential and enzymatic and non-enzymatic antioxidant defense of Hypericum rumeliacum Boiss. after cryostorage. Plant Growth Regul. 2012;68:447–457. doi: 10.1007/s10725-012-9733-z. [DOI] [Google Scholar]
- 86.Turner S., Tan B., Senaratna T., Bunn E., Dixon K., Touchell D. Cryopreservation of the Australian species Macropidia fuliginosa (Haemodoraceae) by vitrification. CryoLetters. 2000;21:379–388. [PubMed] [Google Scholar]
- 87.Choi C.-H., Popova E., Lee H., Park S.-U., Ku J., Kang J.-H., Kim H.-H. Cryopreservation of Endangered Wild Species, Aster altaicus var. uchiyamae Kitam, Using Droplet-Vitrification Procedure. CryoLetters. 2019;40:113–122. [PubMed] [Google Scholar]
- 88.Barraco G., Sylvestre I., Iapichino G., Engelmann F. Investigating the cryopreservation of nodal explants of Lithodora rosmarinifolia (Ten.) Johnst., a rare, endemic Mediterranean species. Plant Biotechnol. Rep. 2013;7:141–146. doi: 10.1007/s11816-012-0241-4. [DOI] [Google Scholar]
- 89.Menon A., Funnekotter B., Kaczmarczyk A., Bunn E., Turner S., Mancera R.L. Cryopreservation of Lomandra sonderi (Asparagaceae) shoot tips using droplet-vitrification. CryoLetters. 2012;33:259–270. [PubMed] [Google Scholar]
- 90.Ozudogru E.A., Kaya E. Cryopreservation of Thymus cariensis and T. vulgaris shoot tips: Comparison of three vitrification-based methods. CryoLetters. 2012;33:363–375. [PubMed] [Google Scholar]
- 91.Coelho N., González-Benito M.E., Martín C., Romano A. Cryopreservation of Thymus lotocephalus shoot tips and assessment of genetic stability. CryoLetters. 2014;35:119–128. [PubMed] [Google Scholar]
- 92.González-Benito M.E., Nunez-Moreno Y., Martín C. A protocol to cryopreserve nodal explants of Antirrhinum microphyllum by encapsulation-dehydration. CryoLetters. 1998;19:225–230. [Google Scholar]
- 93.González-Benito M.E., Pérez C. Cryopreservation of nodal explants of an endangered plant species (Centaurium rigualii Esteve) using the encapsulation-dehydration method. Biodivers. Conserv. 1997;6:583–590. doi: 10.1023/A:1018337429589. [DOI] [Google Scholar]
- 94.Funnekotter B., Bunn E., Mancera R.L. Cryo-mesh: A simple alternative cryopreservation protocol. CryoLetters. 2017;38:155–159. [PubMed] [Google Scholar]
- 95.Funnekotter B., Whiteley S., Turner S., Bunn E., Mancera R. Evaluation of the New Vacuum Infiltration Vitrification (VIV) Cryopreservation Technique for Native Australian Plant Shoot Tips. CryoLetters. 2015;36:104–113. [PubMed] [Google Scholar]
- 96.Filipin E., Zitta C., Schmidt E., Randi A. Pleopeltis lepidopteris Langsd. & Fisch. (Polypodiaceae), an endemic fern from Brazilian “restingas”: Viability of spores under different storage conditions. Braz. J. Bot. 2017;40:59–65. doi: 10.1007/s40415-016-0332-5. [DOI] [Google Scholar]
- 97.Reed B.M., Uchendu E. Controlled Rate Cooling. In: Reed B.M., editor. Plant Cryopreservation: A Practical Guide. Springer; New York, NY, USA: 2008. p. 77. Section I. Chapter 5. [Google Scholar]
- 98.Wang Q., Li P., Batuman O., Gafny R., Mawassi M. Effect of benzyladenine on recovery of cryopreserved shot tips of grapevine and citrus cultured in vitro. CryoLetters. 2003;24:293–302. [PubMed] [Google Scholar]
- 99.Sakai A., Kobayashi S., Oiyama I. Cryopreservation of nucellar cells of navel orange (Citrus sinensis Osb. var. brasiliensis Tanaka) by vitrification. Plant Cell Rep. 1990;9:30–33. doi: 10.1007/BF00232130. [DOI] [PubMed] [Google Scholar]
- 100.Sakai A., Hirai D., Niino T. Development of PVS-Based Vitrification and Encapsulation-Vitrification Protocols. In: Reed B.M., editor. Plant Cryopreservation: A Practical Guide. Springer; New York, NY, USA: 2008. p. 33. Section I. Chapter 3. [Google Scholar]
- 101.Sakai A., Engelmann F. Vitrification, encapsulation-vitrification and droplet-vitrification: A review. CryoLetters. 2007;28:151–172. [PubMed] [Google Scholar]
- 102.Normah M.N., Sulonga N., Reed B.M. Cryopreservation of shoot tips of recalcitrant and tropical species: Advances and strategies. Cryobiology. 2019;87:1–14. doi: 10.1016/j.cryobiol.2019.01.008. [DOI] [PubMed] [Google Scholar]
- 103.Engelmann F., Gonzalez-Arnao M.T., Wu Y., Escobar R. The Development of Encapsulation Dehydration. In: Reed B.M., editor. Plant Cryopreservation: A Practical Guide. Springer; New York, NY, USA: 2008. p. 59. Section I. Chapter 4. [Google Scholar]
- 104.Reed B.M. Plant cryopreservation: A continuing requirement for food and ecosystem security. In Vitro Cell. Dev. Biol.-Plant. 2017;53:285–288. doi: 10.1007/s11627-017-9851-4. [DOI] [Google Scholar]
- 105.Kartha K.K., Leung N.L., Mroginski L.A. In vitro growth responses and plant regeneration from cryopreserved meristems of Cassava (Manihot esculenta Crantz) Zeitschrift Pflanzenphysiol. 1982;107:133–140. doi: 10.1016/S0044-328X(82)80099-8. [DOI] [Google Scholar]
- 106.Panis B., Piette B., Swennen R. Droplet vitrification of apical meristems: A cryopreservation protocol applicable to all Musaceae. Plant Sci. 2005;168:45–55. doi: 10.1016/j.plantsci.2004.07.022. [DOI] [Google Scholar]
- 107.Yamamoto S., Rafique T., Priyantha W.S., Fukui K., Matsumoto T., Niino T. Development of a cryopreservation procedure using aluminium cryo-plates. CryoLetters. 2011;32:256–265. [PubMed] [Google Scholar]
- 108.Niino T., Thwin W., Watanabe K., Nohara N., Rafique T., Yamamoto S., Fukui K., Arizaga M.V., Castillo-Martínez C., Matsumoto T., et al. Cryopreservation of mat rush lateral buds by air dehydration using aluminum cryo-plate. Plant Biotechnol. 2014;31:281–287. doi: 10.5511/plantbiotechnology.14.0624a. [DOI] [Google Scholar]
- 109.Nadarajan J., Pritchard H.W. Biophysical characteristics of successful oilseed embryo cryoprotection and cryopreservation using vacuum infiltration vitrification: An innovation in plant cell preservation. PLoS ONE. 2014;9:e96169. doi: 10.1371/journal.pone.0096169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Funnekotter B., Mancera R.L., Bunn E. Advances in understanding the fundamental aspects required for successful cryopreservation of Australian flora. In Vitro Cell. Dev. Biol.-Plant. 2017;53:289–298. doi: 10.1007/s11627-017-9850-5. [DOI] [Google Scholar]
- 111.Teixeira A.S., González-Benito M.E., Molina-García A.D. Determination of glassy state by cryo-SEM and DSC in cryopreservation of mint shoot tips by encapsulation– dehydration. Plant Cell Tissue Organ Cult. 2014;119:269–280. doi: 10.1007/s11240-014-0531-3. [DOI] [Google Scholar]
- 112.Funnekotter B., Sortey A., Bunn E., Turner S., Mancera R. Influence of abiotic stress preconditioning on antioxidant enzymes in shoot tips of Lomandra sonderi (Asparagaceae) prior to cryostorage. Aust. J. Bot. 2016;64:260–268. doi: 10.1071/BT16006. [DOI] [Google Scholar]
- 113.Marco-Medina A., Casas J.L. RAPD and phytochemical analysis of Thymus moroderi plantlets after cryopreservation. CryoLetters. 2013;34:119–127. [PubMed] [Google Scholar]
- 114.Corlett R.T. Plant diversity in a changing world: Status, trends, and conservation needs. Plant Divers. 2016;38:10–16. doi: 10.1016/j.pld.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pence V.C. The Application of Biotechnology for the Conservation of Endangered Plants. In: Benson E.E., editor. Plant Conservation Biotechnology. Taylor & Francis; London, UK: 1999. p. 227. Part 2. Chapter 15. [Google Scholar]
- 116.Coelho N., Martín C., González-Benito M.E., Romano A. Estimation of genetic diversity in seedlings of Plantago algarbiensis, an endangered species endemic to the south of Portugal in risk of global extinction. Braz. J. Bot. 2016;40:257–264. doi: 10.1007/s40415-016-0340-5. [DOI] [Google Scholar]
- 117.Niazian M. Application of genetics and biotechnology for improving medicinal plants. Planta. 2019;249:953–973. doi: 10.1007/s00425-019-03099-1. [DOI] [PubMed] [Google Scholar]
- 118.Sipes S., Wolf P. Clonal Structure and Patterns of Allozyme Diversity in the Rare Endemic Cycladenia humilis var. Jonesii (Apocynaceae) Am. J. Bot. 1997;84:401–409. doi: 10.2307/2446013. [DOI] [PubMed] [Google Scholar]
- 119.Torres E., Iriondo J.M., Pérez C. Genetic structure of an endangered plant, Antirrhinum microphyllum (Scrophulariaceae): Allozyme and RAPD analysis. Am. J. Bot. 2003;90:85–92. doi: 10.3732/ajb.90.1.85. [DOI] [PubMed] [Google Scholar]
- 120.Kholina A., Koren O., Zhuravlev Y. Genetic structure and differentiation of populations of the tetraploid species Oxytropis chankaensis (Fabaceae) Russ. J. Genet. 2009;45:70–80. doi: 10.1134/S1022795409010104. [DOI] [PubMed] [Google Scholar]
- 121.Šajna N., Kavar T., Šustar-Vozlič J., Kaligarič M. Population Genetics of the Narrow Endemic Hladnikia Pastinacifolia RCHB. (Apiaceae) Indicates Survival in Situ During the Pleistocene. Acta Biol. Cracov. Bot. 2012;54:84–96. doi: 10.2478/v10182-012-0009-8. [DOI] [Google Scholar]
- 122.Trindade H., Sena I., Gonçalves S., Romano A. Genetic diversity of wild populations of Tuberaria major (Cistaceae), an endangered species endemic to the Algarve region (Portugal), using ISSR markers. Biochem. Syst. Ecol. 2012;45:49–56. doi: 10.1016/j.bse.2012.06.028. [DOI] [Google Scholar]
- 123.Tang A.-J., Zhou R., Ge X.-J., Wu W. Development of 11 Microsatellite Loci for an Endangered Herb, Paraisometrum mileense (Gesneriaceae), in Southwest China. Appl. Plant Sci. 2013;1:apps.1200133. doi: 10.3732/apps.1200133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ferreira V., Matos M., Correia S., Martins N., Gonçalves S., Romano A., Pinto-Carnide O. Genetic diversity of two endemic and endangered Plantago species. Biochem. Syst. Ecol. 2013;51:37–44. doi: 10.1016/j.bse.2013.08.003. [DOI] [Google Scholar]
- 125.Shivaprakash K.N., Ramesha B.T., Uma Shaanker R., Dayanandan S., Ravikanth G. Genetic Structure, Diversity and Long Term Viability of a Medicinal Plant, Nothapodytes nimmoniana Graham. (Icacinaceae), in Protected and Non-Protected Areas in the Western Ghats Biodiversity Hotspot. PLoS ONE. 2014;9:e112769. doi: 10.1371/journal.pone.0112769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gonçalves-Oliveira R., Wöhrmann T., Iseppon A., Krapp F., Alves M., Wanderley M., Weising K. Population genetic structure of the rock outcrop species Encholirium spectabile (Bromeliaceae): The role of pollination vs. seed dispersal and evolutionary implications. Am. J. Bot. 2017;104:868–878. doi: 10.3732/ajb.1600410. [DOI] [PubMed] [Google Scholar]