Abstract
Introduction
Accurate data on maternal mortality are essential for assessing progress towards Sustainable Development Goals (SDG).The aim of the study was to determine the incidence and causes of maternal deaths in Georgia, then explore the potential for improvement of quality of maternal health care. The study’s secondary aims were to identify the level of underreporting of maternal deaths in Georgian vital statistics over 1 year (2012) and to compare these results with previous data from 2006. The study findings allow to support the country in developing evidence-based policies and tracking progress towards meeting SDG targets.
Methods
A national Reproductive Age Mortality Survey (RAMOS) was conducted in Georgia in 2014–15. Multiple data sources were used to identify deaths of women aged 15–49 years between January and December 2012. All deaths in women of reproductive age were investigated through verbal autopsy (VA) diagnoses. Deaths in women during pregnancy or one-year postpartum were further investigated by conducting interviews and medical record reviews at the last medical facility which provided health care for the woman during her fatal condition. A specialist panel reviewed these cases and assigned underlining causes of deaths.
Results
We found that 98% of deaths among women of reproductive age were registered by Georgia's civil registration and vital statistics system (CRVS). A total of 918 deaths met the study inclusion criteria. Thirty-six (4.1%) women died during pregnancy or within one-year postpartum. Among these 36 deaths, 23 (63.8%) were maternal deaths, 15 early (either during pregnancy or 42 days postpartum) and eight late (43–365 days postpartum) deaths (65.2% vs 34.8%). The remaining 13 of 36 deaths were coincidental deaths. Fourteen maternal deaths were reported by official statistics and nine deaths were not included in these statistics. Thus, the underreporting rate was 39%. Direct obstetric causes accounted for 73.9% (n=17) of maternal deaths, whereas 26.1% (n=6) were indirect. The leading causes of direct maternal deaths were infection (21.7%), hemorrhage (17.4%), pulmonary embolism (13.0%), and pregnancy-induced hypertension (8.7%). The RAMOS study calculated a maternal mortality ratio (early maternal deaths) of 26.3 per 100,000 live births compared with the official figure of 22.8 per 100,000 live births.
Discussions
Registration of early maternal deaths significantly improved since last survey in 2008, while indirect and late maternal deaths continue to be unrecognized, as reflected in official Georgian statistics. The difference between RAMOS study findings and officially reported maternal mortality rates is minimal, showing improvements in detection of maternal deaths by the national maternal mortality surveillance system. The greatest number of direct obstetric deaths occur in the first week postpartum, which likely reflects deficiencies in quality of care.
Keywords: maternal mortality, maternal death, incidence, causes, underreporting of maternal deaths, verbal autopsy, reproductive age mortality study
Introduction
Maternal mortality data and tracking the causes of maternal death are two principal indicators of overall maternal health and markers of the health system performance.1,2
Since adoption of the Millennium Development Goals (MDG), significant progress has been made in reducing maternal deaths. Globally, maternal mortality ratios have been almost halved between 1990 and 2015.3 Unfortunately, achievement of the MDG target to reduce the rate of maternal mortality by three-quarters by 2015 fell short globally, including in Georgia. To reassert the importance of this unfinished agenda of maternal mortality reduction, Sustainable Development Goals (SDGs) adopted by the United Nations (UN) set a target to reduce the global maternal mortality ratio (MMR) to less than 70 per 100 000 live births. The national target for maternal mortality in the SDG framework is that all countries should reduce their MMR by at least two-thirds from the 2010 baseline and achieve equity in maternal mortality levels for vulnerable populations at the subnational level.4,5
Georgia, situated in the South Caucasus, is a lower-middle-income country with a population of 3.7 million.6 Since its independence in 1991, Georgia has gone through considerable political, economic and social turmoil. Despite recent economic growth, poverty remains the key economic and social issue for the country.7
Routine data on maternal mortality in Georgia comes from the national vital registration statistics (CRVS). The maternal mortality ratio in Georgia fell from 49/100 000 in 2000 to 21/100 000 live births in 2010.8 An increased share of poorly defined causes of death among all reported mortality in Georgia from 2007, reaching 55% in 2010, was observed.9 Reliance on hand delivery system for registration of deaths, poor completion of vital documents, inadequate quality control measures, lack of appreciation on the public health importance of proper death certification by the medical professionals were among key problems in the mortality measurement. The high proportion of ill-defined causes and significant difference between official statistics and international surveys has created uncertainty for policymakers about the actual level and trends of maternal mortality in the country.
Periodic population-based studies, such as RAMOS or census-based mortality studies are valid alternatives to relying solely on routine data sources to measure maternal mortality. These studies also provide a source of more detailed information about the actual circumstances of maternal deaths.10 The first Reproductive Age Mortality Study in Georgia was conducted in 2008 (RAMOS08). It attempted to determine the true levels of maternal mortality in Georgia in 2006.11 The study showed that national statistics significantly underestimated maternal mortality. Also, both underreporting and misclassification of causes of deaths were major issues in maternal mortality measurement. Only 84% of deaths in women of reproductive age (WRA) were registered, and 65% of all maternal deaths went unreported. In terms of the main causes of maternal deaths, hemorrhage, pregnancy-induced hypertension and sepsis were the leading causes identified by RAMOS08.
From 2010 and onward, Georgia implemented several important initiatives to improve maternal deaths registration and surveillance. Georgia civil registration reform introduced regulations and interventions (eg, a monetary penalty for responsible bodies for failing to report death events, electronic medical death certificates [as opposed to paper] and a pregnancy checkbox on the death certificate) to improve maternal death registration. The Georgian Statistics Office began to match maternal death certificates to birth and fetal death certificates. The National Centers for Diseases Control and Public Health (NCDC & PH) introduced active surveillance of maternal mortality by incorporating WRA deaths into integrated electronic disease surveillance system (IEDSS) and implementing the verbal autopsy methodology to review all pregnancy-related deaths. On the medical side, specific protocols, guidelines and training programs for the management of common causes of maternal deaths were developed and implemented. Despite these improvements, accurate reporting on the cause of death reporting remains a challenge.
A second RAMOS was conducted in Georgia in 2014 (RAMOS14), using retrospective 2012 data. In this study, we assessed the magnitude of maternal mortality and its causes, enabling comparison to the similar survey conducted in 2008, based on 2006 data. We also investigated progress and accuracy of the official statistics in reporting maternal deaths. Findings from the study were used as the basis for the development of an action plan and policies to further reduce preventable maternal deaths, improve maternal death reporting and enhance quality of care.
Materials and Methods
Definition of Terms
We used the definition of maternal death and underlying causes of death classification from the World Health Organization (WHO) Application International Statistical Classification of Diseases and Related Health Problems, 10th Revision (ICD – 10) to deaths during pregnancy, childbirth and puerperium: ICD MM.12 WHO defines maternal death as
the death of a woman while pregnant or within 42 days of termination of pregnancy (0-42 days postpartum), irrespective of the duration and site of the pregnancy, from any cause related to or aggravated by the pregnancy or its management, but not from accidental or incidental causes.
We also identified late maternal deaths defined as delayed deaths occurring between 6 weeks (42 days) and 1-year postpartum.
All maternal deaths were included and classified based on their causes as either direct or indirect.
Direct obstetric deaths are the ones resulting from obstetric complications of the pregnant state (ie, pregnancy, labor and the puerperium), from interventions, omissions of- or incorrect treatment, or from a chain of events resulting from any of the above. Indirect obstetric deaths are those resulting from a previous existing disease or one that developed during pregnancy and which was not due to direct obstetric causes but was aggravated by physiologic effects of pregnancy. Coincidental deaths are those deaths that occur during pregnancy, childbirth or the puerperium (42 days) but that are not by definition considered maternal deaths.
The national RAMOS was conducted between March 2014 and January 2015. The target population for the RAMOS study included all women aged 15 to 49 with a permanent residence in Georgia, and who died in 2012. The year 2012 was selected as the most recent year for which full and error-checked databases were available at the initiation of the study.
The research methodology involved investigation of all causes of death to WRA. There were three phases of data collection: death identification, personal interviews with relatives of deceased women using a verbal autopsy (VA) questionnaire and medical record review at the last health facility that provided care for the woman during her fatal condition in pregnancy or 1 year after childbirth. The RAMOS questionnaire contains additional-specific questions about circumstances that may have led to death among women aged 15–49, including cancer and other chronic diseases, intentional or unintentional injuries, and conditions related to or aggravated by pregnancy and its management. The instrument was developed based on questionnaires used in pregnancy mortality studies and surveillance systems conducted by the Centers for Diseases Control (CDC) in the United States and Latin America combined with elements from the WHO verbal autopsy tool.13 A comprehensive history of use of health-care services prior to death had been added to capture barriers to appropriate and timely care and to facilitate needed improvements in the health system.
Multiple data sources were used to identify potentially eligible WRA cases. These included: 1) the CRVS mortality electronic dataset; 2) routine health statistics and surveillance data from NCDC&PH; 3) hospital and ambulance service registers electronic datasets; 4) regional death registers; and 5) community informants contacted during the field investigation.
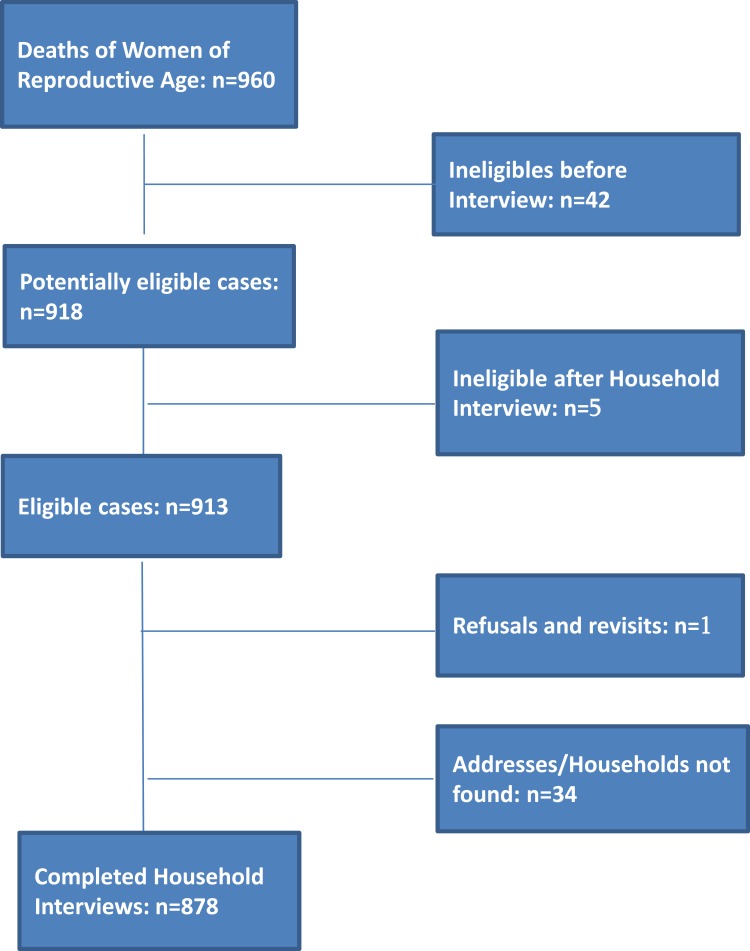
Of the 913 eligible deaths to WRA in 2012, verbal autopsies (VA) were completed for 878 (96.2%) deaths (Figure 1).
Figure 1.
Case identification (2014–15) of study eligible maternal deaths Reproductive Age Mortality Study: Georgia, 2014.
This second step, VA interviews with family members or caregivers of deceased women, was conducted through household visits. Information about signs and symptoms prior to death was collected through VA questionnaires by trained female interviewers with medical background.
Completed questionnaires were assessed independently by two expert clinicians who were approved by the Ministry of Labor, Health and Social Affairs. They assigned the most probable underlying cause of death based on the international standard death certificate and International Classification of Diseases, 10th revision (ICD-10). Discrepancies in coding were resolved by a third clinician who provided the final coding of each questioned case.
Deaths during pregnancy or one-year postpartum were further investigated by a review of hospital medical records. A multidisciplinary panel of medical experts experienced in classification of pregnancy-related causes of death categorized pregnancy-related deaths as maternal deaths, “direct” or “indirect”, or as “co-incidental”. The panel also defined one main cause of death and assessed contributing circumstances and commented on preventability.
Data analysis was performed using SPSS version 21.0. Data were analyzed using simple descriptive methods with frequencies and cross-tabulations. Patterns of misclassification between CRVS, VA diagnoses and multidisciplinary panel of medical experts were further analyzed by cross-tabulating these data.
Ethical Approval
The study protocol was approved by the Georgian Institutional Review Boards of NCDC & PH (IRB 2017-035 and 2019-013) and the Regional Committees for Medical and Health Research Ethics South East Norway (2015/1352). Written informed consent was obtained from all respondents (family members or caretakers of the deceased women) prior to interviews.
Results
Characteristics of the Maternal Death Study Population
In 2012, 57,031 live births were registered in Georgia. A total of 36 pregnancy-related deaths was identified. Among these, 23 (63.9%) deaths were classified as maternal, directly or indirectly caused by pregnancy and 13 (36.1%) as deaths from co-incidental causes. Of the 23 maternal deaths, 15 (65.2%) were early and eight (34.8%) were late.
Of the 23 maternal deaths, about half of the women were 20–29 years of age at the time of death; 39% were 30–39 years; and 13% were 40 years or older. We found the highest age-specific maternal mortality ratio in the older age groups (35–39 and 40–44), and the lowest in age group25–29 (Table 1).
Table 1.
Distribution of Live Birth by Age Groups and Age-Specific MMRs in Georgia in 2012
| Age Groups | Distribution of Birth by Maternal Age | Age-Specific MMRs per 100 000 Live Births | |
|---|---|---|---|
| N (%) | N | Ratio | |
| 15–19 | 5662 (9.9) | 0 | 0.0 |
| 20–24 | 19,571 (34.4) | 8 | 40.9 |
| 25–29 | 16,833 (29.6) | 3 | 17.8 |
| 30–34 | 9734 (17.1) | 5 | 51.4 |
| 35–39 | 4131 (7.2) | 4 | 96.8 |
| 40–44 | 980 (1.7) | 3 | 306.1 |
| 45–49 | 91 (0.2) | 0 | 0.0 |
| Total | 57,002 (100) | 23 | |
Time of Death
A total of 23.1% of the women died during pregnancy, 30.4% of the maternal deaths occurred during the first postpartum week, and 13.0% within 8–42 days postpartum. The remaining 34.8% were late maternal deaths, occurring 43–365 days postpartum.
Pregnancy Outcomes
Among the 23 maternal deaths, 52.2% (n=12) followed delivery of a live birth and 8.7% (n=2) occurred after a stillbirth, while 13% (n=3) were still pregnant at the time of death. 17.4% women (n=4) died after early fetal loss (three miscarriages, one ectopic pregnancy) and two after induced abortions (Table 2).
Table 2.
Pregnancy Outcome in Pregnancy-Related Deaths in Georgia, 2012
| RAMOS Classification | Total | Outcome of Pregnancy | ||||
|---|---|---|---|---|---|---|
| Induced Abortion | Other Fetal Loss* | Stillbirth | Pregnant | Livebirth | ||
| Maternal deaths n (%) | 23 | 2 (8.7) | 4 (17.4) | 2 (8.7) | 3 (13.0) | 12 (52.2) |
| Direct obstetric death | 17 | 1 | 3 | 1 | 3 | 9 |
| Indirect obstetric death | 6 | 1 | 1 | 1 | 0 | 3 |
| Coincidental deaths | 13 | 2 | 1 | 0 | 3 | 7 |
| All Pregnancy-related deaths | 36 | 4 | 5 | 2 | 6 | 19 |
Note: *Ectopic pregnancy or miscarriage.
Causes of Deaths
Direct obstetric deaths constituted 73.9% (n=17) of all maternal deaths. The four leading causes were sepsis, hemorrhage, pulmonary embolism and preeclampsia/eclampsia (Table 3).
Table 3.
Causes of Maternal Deaths in Georgia in 2012 by the Time of Death
| All Maternal Deaths | Early Maternal Deaths (0–42 Days pp) | Late Maternal Deaths (43–365 Days pp) | |
|---|---|---|---|
| N=23 (%) | N=15 (%) | N=8 (%) | |
| Direct Causes | |||
| Sepsis | 6 (26.1) | 5 (33.3) | 1 (12.5) |
| Hemorrhage | 3 (13.0) | 3 (20.0) | 0 (0.0) |
| Embolism | 3 (13.0) | 1 (6.7) | 2 (25.2) |
| PIH | 2 (8.7) | 2 (13.3) | 0 (0.0) |
| Other direct | 3 (13.0) | 3 (20.0) | 0 (0.0) |
| Indirect causes | 6 (26.1) | 1 (6.7) | 5 (62.5) |
| Total | 23 (100.0) | 15 (100.0) | 8 (100.0) |
Other direct causes of deaths were sudden death (n=1), unanticipated complication of anesthesia during delivery (n=1) and complication following intrauterine fetal death (IUFD) at term (n=1).
Among the indirect maternal death causes, cancer was the most common (n=3), whereas tuberculosis, bacterial meningitis and postpartum suicide resulted in one death each (Table 3).
The 13 (36.1%) coincidental deaths (i.e., causes unrelated to pregnancy) were transport accidents (n=2) and other accidents (n=4), cancer, representing brain and retroperitoneal tumors (n=6), and liver cirrhosis (n=1).
Mode of Delivery
Of the 14 deceased women whose pregnancies resulted in a live birth or stillbirth, 57.1% (n=8) delivered by Cesarean section (CS) and 42.9% (n=6) had assisted vaginal deliveries. Four CSs were performed due to previous CS, one for pre-existing medical condition, one for preeclampsia, one due to obstructed labor and one without any medical indication. Of all CSs, 37.5% (n=3) were followed by post-operative infections, 25% (n=2) by postpartum embolism, and one was related to complication of anesthesia.
Maternal Deaths Underreporting
Fourteen (60.9%) of the 23 maternal deaths documented in this study were officially recognized by the Georgian vital registration system as maternal. Only one of the eight late maternal deaths was reported in official statistics. Additionally, two early maternal deaths went unrecognized by the official statistics (Table 4).
Table 4.
Death Reported in the RAMOS14 Study and the Official Maternal Mortality Statistics Deaths to Currently or Recently Pregnant Women Aged 15–49
| RAMOS Classification | Pregnancy-Related Deaths | Maternal Deaths | ||||
|---|---|---|---|---|---|---|
| RAMOS | Officially Reported | RAMOS | Officially Reported | |||
| N | N | % | N | N | % | |
| Total | 36 | 36 | (100.0%) | 23 | 14 | (60.9%) |
| Early deaths (0–42 days pp) | 20 | 13 | (65.0%) | 15 | 13 | (86.7%) |
| Direct obstetric deaths | 14 | 12 | (85.7%) | 14 | 12 | (85.7%) |
| Indirect obstetric deaths | 1 | 1 | (100.0%) | 1 | 1 | (100.0%) |
| Coincidental deaths | 5 | 0* | † | † | † | † |
| Late deaths (43–365 days pp) | 16 | 13 | (81.3%) | 8 | 1 | (12.5%) |
| Direct obstetric deaths | 3 | 1‡ | (33.3%) | 3 | 1‡ | (33.3%) |
| Indirect obstetric deaths | 5 | 0 | † | 5 | 0 | † |
| Coincidental deaths | 8 | 12* | (150.0%) | † | † | † |
| Other§ | 0 | 10 | † | † | † | † |
Notes: *Virtually all death certificates of WRA lacked the pregnancy status specified (empty pregnancy check-box); late coincidental deaths identified by data matching include six late maternal deaths classified by RAMOS14. †Not applicable. ‡Reclassified as indirect in RAMOS14. § Pregnancy-related deaths to women aged 15–49 identified by RAMOS14 including 3 maternal deaths.
Incidence and Reporting
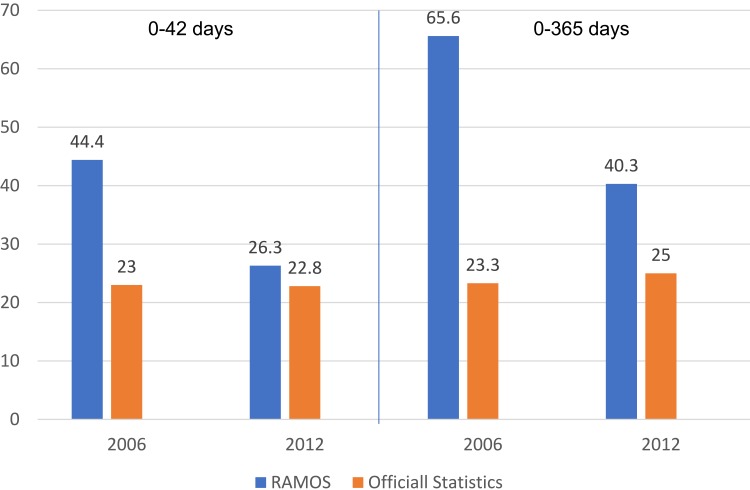
We found an overall MMR (early and late maternal deaths) for 2012 of 40.3 per 100 000 live births, which is a 38.5% reduction compared to the MMR of 65.6 per 100 000 live births in 2006.11 Early maternal mortality declined by 40.8%, from 44.4 per 100 000 live births in 2006 to 26.3 per 100 000 in 2012 (Figure 2).
Figure 2.
Maternal Mortality Ratios (MMR) per 100,000 Live Births RAMOS 2008 and RAMOS 2014 and Official Reports of Maternal Deaths in 2012 and 2006.
Discussion
This paper presents the nationwide maternal mortality data from Georgia in 2012 (named RAMOS14). We found both a decreased incidence and an improvement in reporting maternal deaths as compared with 2006 (named RAMOS08) findings. This trend reflects similar trends in the WHO European Region. Over the past decade, many countries in the WHO European Region have made substantial progress in reducing maternal mortality. The average-estimated maternal mortality ratio for the region decreased by more than half from an average 33 maternal deaths per 100 000 live births in 2000 to 16 in 2015.9 Despite a decreasing trend, the estimated number of maternal deaths in 2015, in Georgia is much higher than the WHO European region estimate. The estimate-36 maternal deaths per 100,000 live births is concerning.9 Georgia has the highest maternal death rate among the Black Sea and neighboring countries.9,14 On the other hand, it is important to note that countries with accurate maternal mortality surveillance systems and continuous audits report higher MMR than countries without these implemented initiatives. Thus, it is plausible that the very low mortality ratios in some countries may be due to systemic underreporting of maternal deaths, lack of national registers, or problems with completeness of ascertainment or suboptimal procedures for coding rather than higher quality services.
In our study, the age group 25–29 had the lowest MMR and it increased with increasing age. The age-group 40–44 had an almost a 20-fold increased risk of MMR compared to the 25–29 age group. Advancing age is associated with increased adverse outcomes in pregnant women. According to most studies, a maternal age of 35 years is the threshold for a significant increased maternal morbidity and mortality and our findings are in accordance to these studies.15
More women with pregnancy-related deaths lived in urban areas than in rural areas 60.9% vs 39.1%. The clear majority were married at the time of death. Women with medium and low socioeconomic status were at higher risk for maternal death. One-third of maternal deaths occurred among women living at subsistence or below subsistence levels. The high probability of not receiving care among women living in households with the lowest wealth quintile and having poor health outcomes are well documented and our findings are in line with these studies.16,17
All 36 pregnancy-related deaths included in our study were officially reported in the vital registration system, whereas only 85.7% were reported in 2006.11 This represents a significant improvement in death registration coverage in Georgia, reported by WHO as well (98%).15 The improvement is most likely due to major reforms implemented in the CRVS described earlier and implemented because of results of the first RAMOS.
Georgia’s official statistics reported a MMR of 22.8 compared to findings of 26.3 in RAMOS14. This is a relatively small gap between the official statistic and RAMOS14 (Figure 2). The trend of minimal difference between official statistic and estimations via special studies continued to be present in 2015, when official statistics reported 30 maternal deaths per 100 000 live births compared to the estimated maternal mortality of 36 female deaths per 100,000 live births.3
Similarly, to other European countries, reporting direct maternal causes in Georgia is comparatively more accurate than reporting on indirect causes or late maternal deaths. Our study shows that 39.1% of maternal deaths went unreported. Similar underreporting of maternal deaths was found in Austria and the Netherlands where overall underreporting was 38% and 33%, respectively.18,19 However, we documented remarkable improvement since 2006, when 64.5% of maternal deaths went unreported. These significant improvements in Georgia in the systems for registering the deaths of women of reproductive age and maternal death identification were made because the country implemented statewide reforms, as described in the introduction. The results of our study indicate importance of periodic assessments of the quality of routine mortality statistics for reporting maternal deaths and related public health actions. Our study also highlights that comparison between countries should not be restricted to maternal rates published by the national offices responsible for death statistics, but also verified by special studies.
Causes of Maternal Deaths
The finding that 79.3% of maternal deaths had direct obstetric causes is in line with data from 2006 (RAMOS08). Sepsis and hemorrhage were the leading obstetric causes of death, followed by embolism and preeclampsia, which points to issues of quality of hospital care. In contrast, major contributors to maternal deaths in high-income countries in Europe are preeclampsia, cardiac disease and thromboembolism.20
Although nationally in Georgia, the rate of hospital deliveries is 99%, quality of care improvements must still be made to reduce maternal deaths. Efforts to improve the quality of care at the hospital level were accelerated beginning in 2010. These efforts included: nationwide training of multidisciplinary teams of providers dealing with obstetric emergencies; development of evidence-based clinical practice guidelines and enforcement of their implementation by the government health authorities; monitoring of hospital clinical quality indicators; and near-miss maternal case reviews. Despite these efforts, our findings highlight an ongoing need to further improve the quality of care at the point-of-care detection as well as gaps in appropriate management of major pregnancy-related events. Most women (90%) had at least four antenatal visits. Indirect causes of maternal deaths in our study suggest weaknesses at the primary care level. In addition, preconception care, which plays a critical role in timely detection of diseases such as cancer and tuberculosis, is lacking.
Although recommended by the WHO model, postpartum care after discharge from the hospital in Georgia is focused on health and development of the newborn, while woman typically receive no or little follow-up. Our study findings related to timing of death, highlights a need to strengthen postpartum follow up and to reinforce the role of family doctors in postpartum care, especially improvement of early identification of complications and timely referral for specialized care.
It is noteworthy that our research (RAMOS14) revealed only one direct maternal death related to unsafe induced abortion performed by the woman herself after 12 weeks of gestation. This is an improvement compared to the three direct maternal deaths caused by unsafe medical abortions found by RAMOS08. Improved safe abortion practices and post-abortion care may be a factor in the relatively low death rate from induced abortion. On the other hand, the study showed an increase in maternal deaths associated with miscarriages compared to the previous study, where no miscarriage-associated maternal deaths were reported.
Despite an increase in contraceptives use by married women or in union from 41% in 1999 to 53% in 2010, 65% of married women still have a potential demand for contraception.21 Improved access to contraceptive commodities and safe and effective use will likely help reduce the risk of maternal death posed by unintended pregnancies and adverse pregnancy outcomes in women with poor health.22
Caesarian sections (CS) were performed in 57% of the women who died. The increased CS rate from 20.7% (2006) to 36.7% (2012) is worrying, but also in line with international trends.23,24 CS performed for maternal request, medicolegal reasons, provider and patient-driven medicalization of birth are possible explanations of the rise in Georgia, repeatedly reported in studies from many countries.8,25,26 While RAMOS08 found that the majority (95%) of CSs were emergency and life-saving, RAMOS14 revealed that only a quarter of CSs were emergency interventions performed on the same day the women initially presented for care. In our study maternal mortality was 14.0 per 100,000 live births for CS and 12.3 per 100,000 for vaginal births. This finding is in line with other studies from developed countries where the chance of dying from a CS is rare, but it’s a little higher than a vaginal delivery.27
The potential benefits of CS in saving maternal and infant lives in emergency situations have been accepted globally in medical practice, but there is no evidence showing the benefits of CS for women or infants when surgery is not medically indicated. On the contrary, CS carries an increased risk of maternal mortality and severe acute morbidity and increases complication rates in subsequent pregnancies.28 CS complications, such as sepsis and thromboembolism, were two of three leading causes of maternal deaths in our study.
No maternal deaths identified in the study had been followed by an autopsy. The practice of postmortem examinations needs to be improved to help physicians determine the primary and underlying causes of death. The low uptake of postmortem examination has not been widely studied in Georgia. Likely factors found in other studies encompass not only cultural, but professional and organizational.29
Conclusions
In our study, we found a significant improvement in death registration coverage, decreased incidence and an improvement in reporting maternal deaths as compared with previous, RAMOS08 findings. The improvement in reporting was particularly prominent for early maternal deaths. The causes of deaths were mainly direct with sepsis as the number one diagnose.
Our study supports the hypothesis that a well-organized vital registration system is important for policy-making and to drive decisions on quality of care in maternal health. However, a vital registration system alone cannot address issues of misclassification of maternal deaths. Therefore, active surveillance, routine nationwide data linkage and audits are essential.
The major strides made over the last decade in Georgia to improve maternal and neonatal care have had a real impact, when measured in the reduction of maternal deaths. While still above the European Union (EU) average, the maternal mortality rate in Georgia has fallen. Despite this progress, we suggest that maternal health and survival remain high on the public agenda and that effective reforms continue to promote the goal of ending preventable maternal deaths in Georgia.
Acknowledgments
The authors thank the US International Development Agency for funding this study and Letten Foundation for providing financial support for this work. The authors are also grateful to the staff of NCDC&PH and all other contributors to this study. Our gratitude to Nancy Pendarvis Harris for editorial support as well. The funding agency had no role in the study design and data collection, data analysis, or preparation of this manuscript.
Ethics and Consent Statement
Georgian Institutional Review Boards of NCDC & PH (IRB 2017-035 and 2019-013) and the Regional Committees for Medical and Health Research Ethics South East Norway (2015/1352).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
- 1.Alexander S, Wildman K, Zhang W, Langer M, Vutuc C, Lindmark G. Maternal health outcomes in Europe. Eur J Obstet Gynecol Reprod Biol. 2003;111(Suppl 1):S78–S87. doi: 10.1016/j.ejogrb.2003.09.008 [DOI] [PubMed] [Google Scholar]
- 2.Wildman K, Bouvier-Colle MH. Maternal mortality as an indicator of obstetric care in Europe. BJOG. 2004;111(2):164–169. doi: 10.1046/j.1471-0528.2003.00034.x-i1 [DOI] [PubMed] [Google Scholar]
- 3.Bongaarts J, WHO, UNICEF, UNFPA, World Bank Group, and United Nations Population Division. Trends in Maternal Mortality: 1990 to 2015. Geneva: World Health Organization; 2015:726. [Google Scholar]
- 4.Seventieth session of the United Nations General Assembly. Transforming our world: the 2030 agenda for sustainable development. Resolution adopted by the General Assembly; September 25, 2015; Available from: https://sustainabledevelopment.un.org/post2015/transformingourworld. Accessed June25, 2019.
- 5.World Health Organization. Strategies towards ending preventable maternal mortality (EPMM). 2015. Available from: https://www.who.int/reproductivehealth/topics/maternal_perinatal/epmm/en/Accessed. Accessed June25, 2019.
- 6.The World Bank. Data: Georgia. Washington, 2019. Available from: https://data.worldbank.org/country/georgia. Accessed June26, 2019.
- 7.Richardson E, Berdzuli N. Georgia: health system review. Health Syst Transit. 2017;19(4):1–90. [PubMed] [Google Scholar]
- 8.Ministry of Labour, Health and Social Affairs of Georgia. National Center for Disease Control and Public Health. Health Care Statistical Yearbook. Georgia; 2014. Available from: http://ncdc.ge/Handlers/GetFile.ashx?ID=989ce699-9b6b-4bb8-96df-70c7125595a6. Accessed July16, 2019. [Google Scholar]
- 9.World Health Organization. European Health Information Gateway. Copenhagen: WHO Regional Office for Europe; 2019. Available from:https://gateway.euro.who.int/en/hfa-explorer/. Accessed September2, 2019. [Google Scholar]
- 10.Mgawadere F, Kana T, van den Broek N. Measuring maternal mortality: a systematic review of methods used to obtain estimates of the maternal mortality ratio (MMR) in low- and middle-income countries. Br Med Bull. 2017;121(1):121–134. doi: 10.1093/bmb/ldw056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Serbanescu F, Teft M, Shakhnazarova M, GA: Division of Reproductive Health, Centers for Disease Control, et al. Reproductive Age Mortality Study, Georgia 2008–Part II: maternal mortality. 2009.
- 12.World Health Organization. The WHO Application of ICD-10 to Deaths During Pregnancy, Childbirth and Puerperium: ICD MM. Geneva: World Health Organization; 2012. [Google Scholar]
- 13.Campbell O, Ronsmans C Verbal autopsies for maternal deaths. World Health Organization workshop held at the London School of Hygiene and Tropical Medicine; January10–13, 1994; London (UK); 1995. Available from: https://apps.who.int/iris/bitstream/handle/10665/61029/WHO_FHE_MSM_95.15.pdf?sequence=1. Accessed September2, 2019. [Google Scholar]
- 14.World Health Organization. Maternal Mortality in 1990-2015. Georgia: Global Health Observatory (GHO) Available from:: http://www.who.int/gho/maternal_health/countries/geo.pdf. Accessed September2, 2019. [Google Scholar]
- 15.Pollard K. MBRRACE-UK report launch December 2016. Pract Midwife. 2017;20(2):30–32. [PubMed] [Google Scholar]
- 16.Gwatkin DR, Rutstein S, Johnson K, Suliman E, Wagstaff A, Amouzou A. Socio-economic differences in health, nutrition, and population within developing countries: an overview. Niger J Clin Pract. 2007;10(4):272–282. [PubMed] [Google Scholar]
- 17.Goldenberg RL, McClure EM. Disparities in interventions for child and maternal mortality. Lancet (London, England). 2012;379(9822):1178–1180. doi: 10.1016/S0140-6736(12)60474-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karimian-Teherani D, Haidinger G, Waldhoer T, Beck A, Vutuc C. Under-reporting of direct and indirect obstetrical deaths in Austria, 1980-98. Acta Obstet Gynecol Scand. 2002;81(4):323–327. doi: 10.1034/j.1600-0412.2002.810408.x [DOI] [PubMed] [Google Scholar]
- 19.Bouvier-Colle MH, Mohangoo AD, Gissler M, et al. What about the mothers? An analysis of maternal mortality and morbidity in perinatal health surveillance systems in Europe. BJOG. 2012;119(7):880–9; discussion 90. doi: 10.1111/j.1471-0528.2012.03330.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vangen S, Bodker B, Ellingsen L, et al. Maternal deaths in the Nordic countries. Acta Obstet Gynecol Scand. 2017;94:1112–1119. doi: 10.1111/aogs.13172 [DOI] [PubMed] [Google Scholar]
- 21.Serbanescu F, Egnatashvili V, Ruiz A, Suchdev D. Reproductive Health Survey in Georgia, 2010. Summary Report. Atlanta (GA, USA): Georgian National Center for Disease Control and Centers for Disease Control andPrevention; 2011. Available from: https://stacks.cdc.gov/view/cdc/8251. Accessed September2, 2019. [Google Scholar]
- 22.Berdzuli N, Pestvenidze E, Lomia N, Stray-Pedersen B. A maternal death from self-induced medical abortion: a call for action. Eur J Contracept Reprod Health Care. 2017;22(5):393–395. doi: 10.1080/13625187.2017.1390080 [DOI] [PubMed] [Google Scholar]
- 23.Boerma T, Ronsmans C, Melesse DY, et al. Global epidemiology of use of and disparities in caesarean sections. Lancet (London, England). 2018;392(10155):1341–1348. doi: 10.1016/S0140-6736(18)31928-7 [DOI] [PubMed] [Google Scholar]
- 24.Molina G, Weiser TG, Lipsitz SR, et al. Relationship between cesarean delivery rate and maternal and neonatal mortality. JAMA. 2015;314(21):2263–2270. doi: 10.1001/jama.2015.15553 [DOI] [PubMed] [Google Scholar]
- 25.Torloni MR, Betran AP, Montilla P, et al. Do Italian women prefer cesarean section? Results from a survey on mode of delivery preferences. BMC Pregnancy Childbirth. 2013;13:78. doi: 10.1186/1471-2393-13-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Angeja AC, Washington AE, Vargas JE, Gomez R, Rojas I, Caughey AB. Chilean women’s preferences regarding mode of delivery: which do they prefer and why? BJOG. 2006;113(11):1253–1258. doi: 10.1111/bjo.2006.113.issue-11 [DOI] [PubMed] [Google Scholar]
- 27.Clark SL, Belfort MA, Dildy GA, Herbst MA, Meyers JA, Hankins GD. Maternal death in the 21 century: causes,prevention, and relationship to cesarean delivery. Am J Obstetr Gynecol. 2008;199(1):36.e1–36.e5. doi: 10.1016/j.ajog.2008.03.007 [DOI] [PubMed] [Google Scholar]
- 28.Sandall J, Tribe RM, Avery L, et al. Short-term and long-term effects of caesarean section on the health of women and children. Lancet (London, England). 2018;392(10155):1349–1357. doi: 10.1016/S0140-6736(18)31930-5 [DOI] [PubMed] [Google Scholar]
- 29.Lewis C, Hill M, Arthurs OJ, Hutchinson C, Chitty LS, Sebire NJ. Factors affecting uptake of postmortem examination in the prenatal, perinatal and paediatric setting. Int J Obstetr Gynecol. 2018;125:172–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Seventieth session of the United Nations General Assembly. Transforming our world: the 2030 agenda for sustainable development. Resolution adopted by the General Assembly; September 25, 2015; Available from: https://sustainabledevelopment.un.org/post2015/transformingourworld. Accessed June25, 2019.
- World Health Organization. Strategies towards ending preventable maternal mortality (EPMM). 2015. Available from: https://www.who.int/reproductivehealth/topics/maternal_perinatal/epmm/en/Accessed. Accessed June25, 2019.
- The World Bank. Data: Georgia. Washington, 2019. Available from: https://data.worldbank.org/country/georgia. Accessed June26, 2019.