Key Points
Question
Is the implementation of an unplanned extubation (UE) bundle, as part of a national quality improvement initiative, associated with a reduction in UEs in critically ill neonates, infants, and children?
Findings
In this quality improvement study of neonates, infants, and children from 43 participating children’s hospitals, use of the UE bundle was associated with a 24.1% reduction in UEs. Importantly, there was also a 36.6% reduction in cardiovascular collapse associated with UE's across the cohort.
Meaning
A widespread quality improvement initiative with bundle implementation led to a meaningful and sustained reduction in pediatric UEs and morbidity associated with these events.
This quality improvement study assesses the association of a multicenter quality improvement initiative targeting all intubated neonatal and pediatric patients with a reduction in unplanned extubations and morbidity associated with unplanned extubation events.
Abstract
Importance
Unplanned extubations (UEs) in children contribute to significant morbidity and mortality, with an arbitrary benchmark target of less than 1 UE per 100 ventilator days. However, there have been no multicenter initiatives to reduce these events.
Objective
To determine if a multicenter quality improvement initiative targeting all intubated neonatal and pediatric patients is associated with a reduction in UEs and morbidity associated with UE events.
Design, Setting, and Participants
This multicenter quality improvement initiative enrolled patients from pediatric, neonatal, and cardiac intensive care units (ICUs) in 43 participating children’s hospitals from March 2016 to December 2018. All patients with an endotracheal tube requiring mechanical ventilation were included in the study.
Interventions
Participating hospitals implemented a quality improvement bundle to reduce UEs, which included standardized anatomic reference points and securement methods, protocol for high-risk situations, and multidisciplinary apparent cause analyses.
Main Outcomes and Measures
The main outcome measures for this study included bundle compliance with each factor tested and UE rates on the center level and on the cohort level.
Results
Among the 43 children’s hospitals, the quality improvement initiative was associated with an aggregate 24.1% reduction in UE events, from a baseline rate of 1.135 UEs per 100 ventilator days to 0.862 UEs per 100 ventilator days. Across ICU settings studied, the pediatric ICU and neonatal ICU demonstrated centerline shifts, with an absolute reduction in events of 20.6% (from a baseline rate of 0.729 UEs per 100 ventilator days to 0.579 UEs per 100 ventilator days) and 17.6% (from a baseline rate of 1.555 UEs per 100 ventilator days to 1.282 UEs per 100 ventilator days), respectively. Most UEs required reintubation within 1 hour (mean of 120 of 206 events per month [58.3%]), followed by UEs that did not require reintubation (mean of 78 of 206 events per month [37.9%]) and UEs that resulted in cardiovascular collapse (mean of 8 of 206 events per month [3.9%]). Cardiovascular collapse events represented the most significant consequence of UE studied, and the collaborative reduced these UE events by 36.6%, from a study baseline rate of 0.041 UEs per 100 ventilator days to 0.026 UEs per 100 ventilator days.
Conclusions and Relevance
This multicenter quality improvement initiative was associated with a reduction in UEs across different pediatric populations in diverse settings. A significant reduction in event rate and rate of harm (cardiovascular collapse) was observed, which was sustained over the time course of the intervention. This quality improvement process and UE bundle may be considered standard of care for pediatric hospitals in the future.
Introduction
Unplanned extubation (UE) is defined as any dislodgement of an endotracheal tube from the trachea that is not intentional or ordered by a health care professional. Adult studies have demonstrated that UEs are associated with harm, including increased days of mechanical ventilation, increased hospital stay,1 increased ventilator-associated pneumonia, and increased mortality.2 Since the initial pediatric case report of UE in a single neonate,3 studies remain limited to mainly single-center analyses. Additional studies are largely descriptive and aim to describe causative factors associated with events, including sedation, secretions, restraints, and bedside procedures.4,5,6
Although there are few large multicenter studies of UE in pediatrics, significant morbidity has been described, including cardiovascular collapse and cardiac arrest in up to 20% of those with UE events7,8 and reintubation rates ranging from 49% to 69% in all patients experiencing UE.7,8,9,10 Pediatric UEs are also associated with increased costs and length of stay.11
While harm associated with pediatric UE is well demonstrated, a broader, more comprehensive understanding of UE in pediatrics is lacking because event description and analysis has been limited to single intensive care unit (ICU) environments. Critically ill patients requiring mechanical ventilation are at risk of UE regardless of patient age or the type of ICU care delivered (pediatric ICU [PICU], neonatal ICU [NICU], or cardiac ICU [CICU]), and efforts to reduce UE should be comprehensive and consider all patient factors.
Despite a growing body of single-center observational studies of UE and quality improvement studies to reduce UE,12,13,14,15,16 to our knowledge, no pediatric multicenter efforts to reduce UE have been published to date. We describe a multi-institutional national quality improvement intervention led by Children’s Hospitals Solutions for Patient Safety (SPS) across 43 volunteer hospitals to reduce UEs in all hospitalized patients with an aim to reduce the absolute rate of UEs by 40% over a 2-year period.
Methods
Development of Testable Factors
Because evidence is scarce regarding the appropriate interventions to reduce UE in pediatrics, we used the multihospital collaboration methods recommended by SPS to determine the standardized elements of the UE bundle for testing in cohort hospitals.17 We assembled a group of 10 national experts from 8 centers, including physicians, nurses, and respiratory therapists from PICUs, NICUs, and CICUs, for a 2-day, in-person design meeting to discuss the intent and strategy of improvement efforts and to determine definitions and factors for testing. Following review of relevant literature and small-scale successes, iterative discussion was used to prioritize factors and justify responses. Finally, we performed a simplified failure modes and effects analysis to gain consensus, prioritize the top factors for active improvement, and identify future factors.18,19 These discussions were organized into a key driver diagram (eAppendix 1 in the Supplement) and factor definitions (Table). The SPS quality improvement work was reviewed by the Cincinnati Children’s Institutional Review Board and considered exempt. All data were deidentified, and informed consent was waived.
Table. Unplanned Extubation (UE) Quality Improvement Bundle Factors and Definitions.
| Factor | Definition |
|---|---|
| Standard elements | |
| Standardized anatomic reference points and securement methods |
|
| Protocol for high-risk situations | Repositioning occurs with 2 licensed clinicians (having 1 dedicated to hold the tube during movement and repositioning) during high-risk situations, including:
|
| Recommended elements | |
| Multidisciplinary ACA |
|
Abbreviation: ACA, apparent cause analysis.
Three factors were implemented by participating institutions. Required elements of each factor were defined (Table), and centers were encouraged to use local data and experience, collected through apparent cause analysis (ACA) (eAppendix 2 in the Supplement) of each event, to expand on the high-risk situations designated factor.
Operational Definition and Participants
Unplanned extubation was defined as any dislodgement of an endotracheal tube from the trachea that was not intentional. Tracheostomy tube dislodgement was not included. The study population included all mechanically ventilated patients with an endotracheal tube cared for in a participating hospital, including in the emergency department, operative or procedural suites, radiology department, and inpatient units. Exclusion criteria included any patient with a tracheostomy tube and any UE event occurring outside the hospital during transport. Events were classified into 3 levels of harm: no reintubation was defined as the patient not requiring reintubation within 1 hour of the UE event, reintubation as the patient requiring reintubation within 1 hour of the UE event, and cardiovascular collapse as the patient requiring cardiopulmonary resuscitation or bolus epinephrine following the UE event.
Methodology
The SPS leadership oversaw identification of the factors for UE reduction. Each participating hospital was asked to implement these factors by using local quality improvement methods, such as the Model for Improvement20 or Lean Six Sigma,21 and leverage the structure and resources provided by the collaborative to accelerate and sustain improvement. No protected health information was collected or transmitted. Data use is governed within the Hospital Participant Agreement between SPS and the hospital participating in the SPS network. At the collaborative level, improvement strategies were designed using the Model for Improvement, including aims, metrics, and key drivers. Hospitals were encouraged to establish local improvement teams for UE and instructed to target 80% compliance to the factors being tested. Hospitals identified team leaders for UE, assembled multidisciplinary teams from each unit, and planned, implemented, and measured tests of change designed to reduce UE focused on reliably implementing the factors. Based on the all teach, all learn approach, multiple opportunities for multidisciplinary collaboration across the network were offered, including semiannual in-person learning sessions, monthly webinars with presentations by cohort hospitals focused on high-reliability principles, discussions on a password-protected website, and monthly data review. Algorithms for accurate data extraction from electronic health records were shared on collaborative learning calls to ensure consistency in data collection of ventilator days across participant sites. The SPS support staff consisted of quality consultants, project managers, and a data analyst who provided guidance and analytics support to the network.
Data Submission
Each hospital submitted monthly hospital-acquired condition outcome data for an 8-month preliminary data period (March 1, 2016, to October 31, 2016) and an 11-month implementation period (November 1, 2016, to September 30, 2017). Only hospitals that submitted baseline outcomes data with at least 80% compliance were included in the analysis. The cohort was longitudinally tracked through the end of December 31, 2018, to measure for additional improvement.
Outcome Measures
Participating hospitals submitted monthly outcome data. The required data elements included total UE events, UE events by ICU location, and UE by severity.
Process Measures
Participating hospitals submitted monthly compliance data for each factor and ACA data. Hospitals were instructed to submit at least 30 factor compliance audits per month for each factor. Factor compliance was defined as adherence to each respective factor definition (Table). Hospital teams were encouraged to measure process reliability using visual observations and kamishibai cards.
Cohort hospitals used a variety of methods to implement the factors, which included high-reliability strategies ranging from education to checklists to risk-stratification tools. For implementation of standardized anatomic reference points and securement methods, teams developed job aids, representative images, and textual descriptions. Several units created practice stations for team members to practice taping standards and performed individual competency assessments. For implementation of the protocol for high-risk situations, teams developed bedside tools and processes around frequent high-risk procedures to reinforce the need for 2 licensed health care professionals. For the ACA, a standardized template was created by SPS that could be adapted by individual institutions (eAppendix 2 in the Supplement). Apparent cause analysis information was collected on paper or electronically based on institutional preference. Compliance to bundle elements was audited using a combination of bedside observations and auditing tools.
Setting
Participating hospitals were asked to implement the bundle and all factors in all intensive care environments, including the CICU, NICU, and PICU. Hospitals were also asked to implement efforts in other areas where intubated patients are cared for or transported to, including the emergency department, radiology department, and operating room.
Statistical Analysis
The study design followed the analytical approach applied for pioneer cohorts described by Lyren et al17 for improvement bundle development. The 3 selected factors were the standardized anatomic reference points and endotracheal tube securement method, a high-risk protocol for moving intubated patients, and ACA following UE events (Table). Overall, baseline (preintervention) and study period (postintervention) outcomes were measured for each factor, and the impact of reliability to a tested factor for each hospital was studied for its association with the outcome using response plots and analysis of covariance (ANCOVA).17
Each factor was analyzed independently at the network level to understand the degree to which reliable implementation of the factor influenced overall results. To aid in this analysis, hospital data were placed in reliability groups as described by Lyren et al.17 The 3 groups were (1) not measuring the factor, (2) measuring factors with less than 80% reliability, and (3) measuring factors with 80% reliability or more. For the purposes of this study, we identified a fourth group of hospitals that were reliable at baseline. This last group accounted for hospitals that had already implemented the factor intervention with at least 80% reliability during the baseline and study periods.
The ANCOVA analysis examined statistical differences between the 4 analysis groups for each factor in regard to baseline and study period rates. Response plots measured the degree of improvement from baseline to study period as rates for each group. Results from ANCOVA tests identified the association of each group with the study period rate while statistically controlling for the baseline rate for each group. Lastly, the least square means displayed the mean response for each group at the average value(s) of their baseline. To detect statistically significant changes in outcomes related to the interventions, Shewhart control U-charts were used to track monthly outcomes data. Improvement was recognized by following special-cause rules of a run of 8 or more points in a row less than the centerline.22 The 8-point rule is a probability-based rule generally corresponding to a P value less than .01.23 All analyses were conducted using SAS version 9.4 (SAS Institute).
Results
Aggregate UE
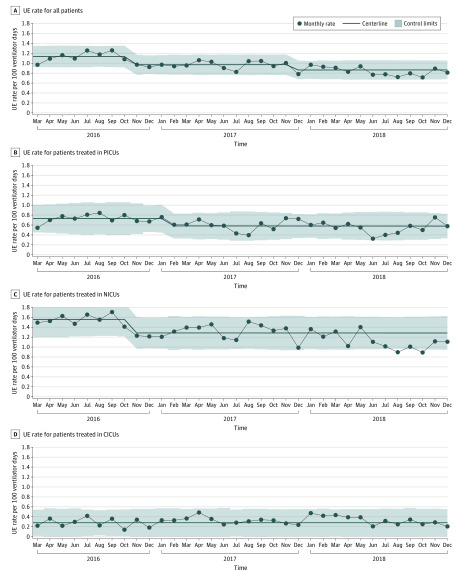
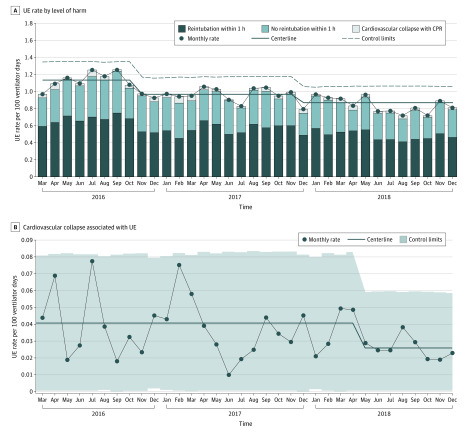
A total of 54 SPS network hospitals joined the UE cohort. Of these, 43 hospitals submitted outcomes data with at least 80% reliability and were included in this analysis. There was an aggregate 24.1% reduction in UE events across the study cohort, from a baseline rate of 1.135 UEs per 100 ventilator days to 0.862 UEs per 100 ventilator days (Figure 1). Of the 3 distinct ICU settings studied, the PICU and NICU demonstrated centerline shifts, with an absolute reduction in events of 20.6% (from a baseline rate of 0.729 UEs per 100 ventilator days to 0.579 UEs per 100 ventilator days) and 17.6% (from a baseline rate of 1.555 UEs per 100 ventilator days to 1.282 UEs per 100 ventilator days), respectively (Figure 1). The baseline UE rate across patients treated in the CICU was 0.281 UEs per 100 ventilator days without a noted centerline shift during the study period (Figure 1). Of all UE events, the highest percentage of UE occurred in the NICU (142 of 206 events [68.9%]), followed by the PICU (46 of 206 events [22.3%]), CICU (10 of 206 events [4.9%]), and other settings (ie, outside the ICU; 8 of 206 events [3.9%]). Most UEs required reintubation within 1 hour (mean of 120 of 206 events per month [58.3%]), followed by UEs that did not require reintubation (mean of 78 of 206 events per month [37.9%]) and UEs that resulted in cardiovascular collapse (mean of 8 of 206 events per month [3.9%]). Cardiovascular collapse events represented the most significant consequence of UE for which data were collected, and the collaborative reduced these UE events by 36.6%, from a study baseline rate of 0.041 UEs per 100 ventilator days to 0.026 UEs per 100 ventilator days, with an associated centerline shift. Extrapolating the rate into an average number of patients per month demonstrates that 3 patients per month avoided cardiovascular collapse (Figure 2).
Figure 1. Unplanned Extubation (UE) Rates by Setting.
Statistical process control charts of UE rates. A, There was an aggregate 24.1% reduction in UE events among all patients, from a baseline rate of 1.135 UEs per 100 ventilator days to 0.862 UEs per 100 ventilator days. B, There was an absolute reduction in UE events of 20.6% among patients treated in the pediatric intensive care unit (PICU), from a baseline rate of 0.729 UEs per 100 ventilator days to 0.579 UEs per 100 ventilator days. C, There was an absolute reduction in UE events of 17.6% among patients treated in the neonatal ICU (NICU), from a baseline rate of 1.555 UEs per 100 ventilator days to 1.282 UEs per 100 ventilator days. D, The UE rate of 0.281 UEs per 100 ventilator days did not change over the study period among patients treated in the cardiac ICU (CICU). Centerline shift was determined by the Shewhart rule of 8 or more points below the centerline. The centerline shift began in November 2016.
Figure 2. Harm Associated With Unplanned Extubations (UEs).
A, Level of harm associated with UE categorized by reintubation within 1 hour, no reintubation within 1 hour, and cardiovascular collapse and rate over time. B, Statistical process control chart of cardiovascular collapse associated with UE. There was an absolute reduction of 36.6%, from a study baseline rate of 0.041 UEs per 100 ventilator days to 0.026 UEs per 100 ventilator days. Centerline shift was determined by the Shewhart rule of 8 or more points below the centerline. The centerline shift began in November 2016.
Factor Analysis
Each tested factor was analyzed to ascertain its relative contribution to the overall results.
Standardized Anatomic Reference Points and Securement Methods
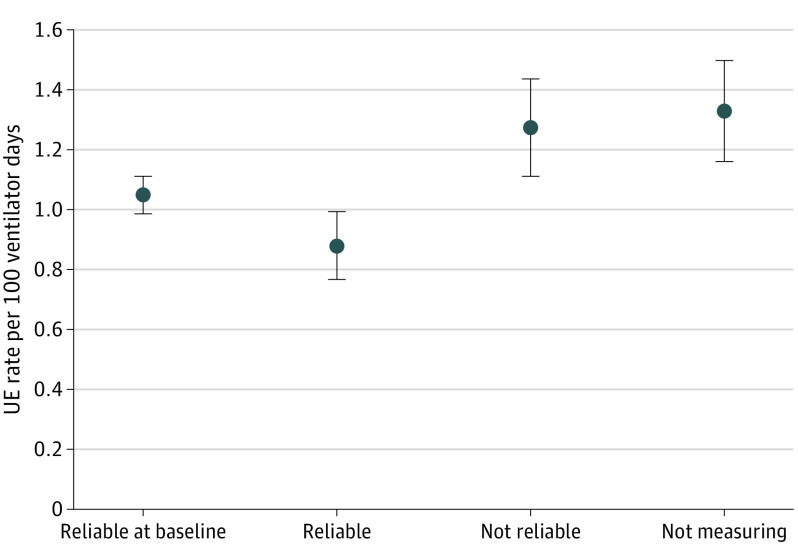
Comparing baseline rates to study period rates (September 2016 to December 2018) for the factor standardized anatomic reference points and securement methods, sites that measured compliance and had high reliability to the factor had an absolute reduction in UE rates of 26.9%, from a baseline rate of 0.930 UEs per 100 ventilator days to 0.680 UEs per 100 ventilator days. These sites also had the greatest reduction in their least square means (Figure 3).
Figure 3. Standardized Anatomic Reference Points and Securement Methods.
Analysis of covariance of statistical differences between each of the 4 analytic groups. The 4 groups included hospitals that (1) had already implemented the factor intervention with at least 80% reliability before the study period, (2) measured factors with 80% reliability or more, (3) measured factors with less than 80% reliability, and (4) did not measure factors during the baseline and study periods. Sites measuring compliance and reliable to the factor had the greatest reduction in unplanned extubation (UE) rates and least square means.
Protocol for High-Risk Situations
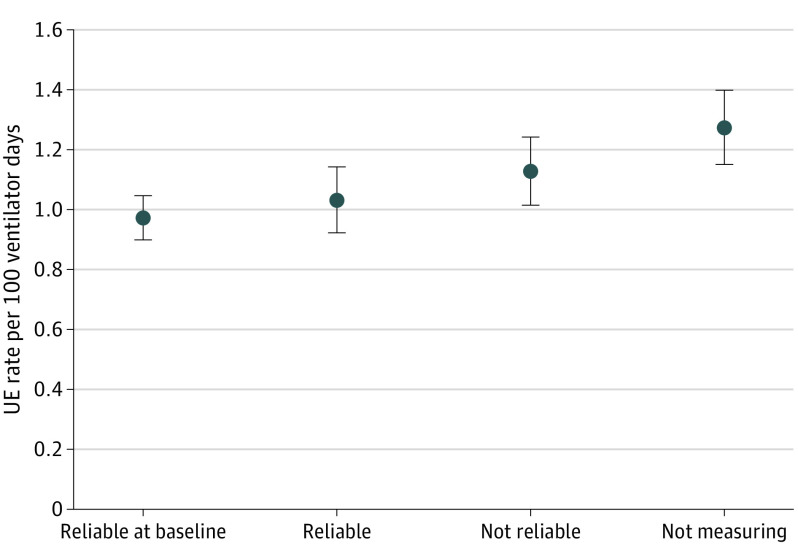
Comparing baseline rates with study period rates (September 2016 to December 2018) for the factor high-risk protocol, sites that measured compliance and had high reliability to the factor had an absolute reduction in UE rates of 30.3%, from a baseline rate of 1.247 UEs per 100 ventilator days to 0.869 UEs per 100 ventilator days. These sites also had the greatest reduction in their least square means (Figure 4).
Figure 4. Protocol for High-Risk Situations.
Analysis of covariance of statistical differences between each of the 4 analytic groups. The 4 groups included hospitals that (1) had already implemented the factor intervention with at least 80% reliability before the study period, (2) measured factors with 80% reliability or more, (3) measured factors with less than 80% reliability, and (4) did not measure factors during the baseline and study periods. Sites measuring compliance and reliable to the factor had the greatest reduction in unplanned extubation (UE) rates and least square means.
Multidisciplinary ACA
Comparison of multidisciplinary ACA rates over time was performed (September 2016 to December 2018). There were insufficient statistical data to support the factor of multidisciplinary ACA (data not shown).
Discussion
To our knowledge, SPS successfully implemented the first multicenter quality improvement initiative to date to reduce UEs for all intubated patients in participating children’s hospitals. Overall, we report a 24.1% absolute reduction in UEs across the collaborative, with an absolute reduction in associated cardiovascular collapse of 36.6%. Of patients with UE, 3.9% of patients experienced cardiovascular collapse. Centers with the highest bundle compliance and those with the most sustained compliance demonstrated the most significant reduction in UEs over time.
The Institute for Healthcare Improvement has endorsed bundled care as an important tool for improving outcomes. Single-center reports have shown an association of adherence to standardized bundled elements of care with a reduction in UEs.12 Similarly, in a single-center tertiary NICU, Merkel et al24 demonstrated that bundle development coupled with quality improvement using plan-do-study-act cycles led to significant reductions in UE. We report a 24.1% reduction in UE for patients intubated in the PICU, NICU, and CICU across 43 institutions following implementation of 2 bundle factors and the use of ACA reviews. The reduction of rates in response plots, significant findings in the ANCOVA analysis, and decreased rates after the study period validate the importance of these results. Our analysis shows that standardized anatomic reference points and securement methods and the high-risk protocol contribute significantly to UE reduction. While there was a lack of statistical evidence to support the results of the ACA, the ACA helped institutions to identify areas for improvement, and we recommend using it as part of the bundle. The demonstrated improvement in this collaborative has also been sustained over time, which is comparable with and exceeds that of smaller single-center quality improvement initiatives.
The bundle was not difficult to implement in any ICU despite unique patient characteristics and risk factors. Based on the types of cares performed for intubated patients and the activities occurring around the time of UE, patients treated in the PICU, NICU, or CICU were not anticipated to differ significantly, and bundle elements or interventions were chosen that could be applied to all patients. Further, in many institutions, nurses, respiratory therapists, and trainees often rotate through multiple ICUs, and occasionally, attending physicians also care for patients in different ICUs. By standardizing the bundle across ICU environments, there is increased opportunity for consistency in care of the intubated patient, which likely outweighs patient-specific risk factors for UE. Individual ICUs and institutions were encouraged to standardize endotracheal tube securement per unit, as preferences may differ, for example, between the use of tape or securement devices, particularly when addressing skin integrity issues in premature neonates.
Two prior studies identified cardiac arrest in 2% of events,9,10 while additional studies identified cardiovascular collapse (defined as cardiopulmonary resuscitation or administration of epinephrine) in up to 20% of events.7,8 Cardiovascular collapse following UE is significantly more likely in younger patients, with an odds ratio of cardiovascular collapse as high as 3.4 in infants younger than 6 months.7,8 In-hospital cardiac arrest is associated with mortality as high as 30%, making the prevention of cardiovascular collapse an important outcome measure.25 We report the prevention of at least 3 episodes of cardiovascular collapse per month.
The success and scope of our work highlights the value and impact of multidisciplinary collaboration. The foundation of quality improvement initiatives is engagement from all stakeholders and commitment across disciplines to embrace new initiatives and often changes in practice. Prior studies have reported the importance of quality improvement to reduce UEs. Using structured education and plan-do-study-act cycles, Sadowski et al13 reported a reduction from 1.5 UEs to 0.8 UEs per 100 airway days over a 5-year study period. Studying the impact of continuous quality improvement in a 5-bed academic PICU, da Silva et al16 reported a reduction of UEs from a rate of 2.9 UEs per 100 ventilator days to 0.6 UEs per 100 ventilator days. While our work does not assess patient-level variables, the broad reduction in UEs in 43 centers was achievable because of multidisciplinary collaboration, data sharing, and broad adoption of quality improvement efforts. The ability to adapt improvement efforts for local context also promoted successful implementation of the factors and improvement success. While all participating hospitals followed the framework of the factor, each institution differed in their implementation methods. The empowerment of local teams to adapt the bundle to their environment was an important part of the success of this work.
The data we report are similar to the only other multicenter analysis of pediatric UE, which sought to determine risk factors for pediatric UE across 11 institutions.9 Unplanned extubation rates varied by PICU site, from 0.3 UEs per 100 ventilator days to 2.1 UEs per 100 ventilator days. Rates of UE in NICUs have also been variable but even higher, based on rates described in single-center studies, with rates varying between 0.6 UEs per 100 ventilator days and 4.5 UEs per 100 ventilator days.15,24,26,27,28,29 Our work highlights the broad applicability of this bundle and demonstrates the value of standardized bundled care while providing a framework for future multicenter quality improvement initiatives. By including 43 hospitals and PICUs, NICUs, and CICUs, we attempted to minimize the effects of individual unit size, population differences, and any significant changes in a small number of ICUs. The study also extends over a 2-year time span, longer than any other reported multi-institutional effort to date, to our knowledge, which minimizes the effects of special-cause variations across the collaborative.
Limitations
This study has a number of limitations. First, patient-specific factors and demographic characteristics were not collected in this work, which limits the ability to understand specific associations between patient factors and UE events. We found that UE events occurred with the greatest frequency in the NICU and the least frequency in the CICU, likely reflecting differences in the patient population, physiology, and management strategies. Patients treated in NICUs are the smallest patients, and their short tracheal length makes endotracheal positioning vulnerable to dislodgement. Furthermore, the use of sedative infusions in the NICU is lower than that of the PICU or CICU. These 2 factors together make patients treated in the NICU particularly vulnerable to events. Postoperative patients represent a large percentage of patients in the CICU, and current practice emphasizes early extubation, which may contribute to a reduction in ventilator days, thereby reducing the risk exposure for UEs. Second, in an effort to encourage participation and reporting, we did not collect data on mortality associated with UE events. It is difficult to temporally associate UE with death, and to our knowledge, no pediatric studies assessing harm from UE have identified immediate mortality.7,8,9,10 Third, because the intervention was implemented at the same time across hospitals, it is not possible to separate the effect of a temporal trend from the effect of the intervention. However, this limitation is minimized by the participation of a large number of hospitals that vary in size and location over a 2-year period.
Conclusions
The suggested benchmark of 1 UE per 100 ventilator days has been widely accepted.30 While ongoing work must be done to achieve a rate of 0 UEs, we demonstrate that a benchmark rate of 1 UEs per 100 ventilator days is too high, and adoption of this UE bundle as standard of care across all ICU settings may help reduce rates well below 1 UE per 100 ventilator days. Unplanned extubation rates should be evaluated based on the population in question because rates vary by ICU type. Broadly suggesting a benchmark rate underestimates the magnitude of improvement, which is possible with collaborative multidisciplinary quality improvement, as demonstrated in this work. Eliminating UE and the harm associated with these events remains achievable, and all ICUs should strive for rates at or near 0 UEs per 100 ventilator days with the implementation of this bundle. While rates in patients treated in the NICU are higher than those treated in the PICU or CICU, the value of this quality improvement intervention is evident by the 24.1% reduction in UE events and its broad applicability across a large number of hospitals, practice settings, and patient populations. Further, the reduction shown was independent of UE rate at the start of participation in this quality improvement collaborative, clearly underscoring the ability of a unit and, more broadly, an institution to improve on an already low UE rate. The power of collaboration and the all teach, all learn approach as the bedrock of SPS31 likely plays an important role in the results achieved.
eAppendix 1. Unplanned extubations key driver diagram.
eAppendix 2. Unplanned extubation real-time ACA event investigation.
References
- 1.Krinsley JS, Barone JE. The drive to survive: unplanned extubation in the ICU. Chest. 2005;128(2):560-566. doi: 10.1378/chest.128.2.560 [DOI] [PubMed] [Google Scholar]
- 2.Gao F, Yang LH, He HR, et al. The effect of reintubation on ventilator-associated pneumonia and mortality among mechanically ventilated patients with intubation: a systematic review and meta-analysis. Heart Lung. 2016;45(4):363-371. doi: 10.1016/j.hrtlng.2016.04.006 [DOI] [PubMed] [Google Scholar]
- 3.Todres ID, deBros F, Kramer SS, Moylan FM, Shannon DC. Endotracheal tube displacement in the newborn infant. J Pediatr. 1976;89(1):126-127. doi: 10.1016/S0022-3476(76)80946-8 [DOI] [PubMed] [Google Scholar]
- 4.Little LA, Koenig JC Jr, Newth CJ. Factors affecting accidental extubations in neonatal and pediatric intensive care patients. Crit Care Med. 1990;18(2):163-165. doi: 10.1097/00003246-199002000-00007 [DOI] [PubMed] [Google Scholar]
- 5.Marcin JP, Rutan E, Rapetti PM, Brown JP, Rahnamayi R, Pretzlaff RK. Nurse staffing and unplanned extubation in the pediatric intensive care unit. Pediatr Crit Care Med. 2005;6(3):254-257. doi: 10.1097/01.PCC.0000160593.75409.6B [DOI] [PubMed] [Google Scholar]
- 6.Scott PH, Eigen H, Moye LA, Georgitis J, Laughlin JJ. Predictability and consequences of spontaneous extubation in a pediatric ICU. Crit Care Med. 1985;13(4):228-232. doi: 10.1097/00003246-198504000-00004 [DOI] [PubMed] [Google Scholar]
- 7.Klugman D, Berger JT, Spaeder MC, Wright A, Pastor W, Stockwell DC. Acute harm: unplanned extubations and cardiopulmonary resuscitation in children and neonates. Intensive Care Med. 2013;39(7):1333-1334. doi: 10.1007/s00134-013-2932-x [DOI] [PubMed] [Google Scholar]
- 8.Lucas da Silva PS, Fonseca MCM. Incidence and risk factors for cardiovascular collapse after unplanned extubations in the pediatric ICU. Respir Care. 2017;62(7):896-903. doi: 10.4187/respcare.05346 [DOI] [PubMed] [Google Scholar]
- 9.Fitzgerald RK, Davis AT, Hanson SJ; National Association of Children’s Hospitals and Related Institution PICU Focus Group Investigators . Multicenter analysis of the factors associated with unplanned extubation in the PICU. Pediatr Crit Care Med. 2015;16(7):e217-e223. doi: 10.1097/PCC.0000000000000496 [DOI] [PubMed] [Google Scholar]
- 10.Al-Abdwani R, Williams CB, Dunn C, et al. Incidence, outcomes and outcome prediction of unplanned extubation in critically ill children: an 11 year experience. J Crit Care. 2018;44:368-375. doi: 10.1016/j.jcrc.2017.12.017 [DOI] [PubMed] [Google Scholar]
- 11.Roddy DJ, Spaeder MC, Pastor W, Stockwell DC, Klugman D. Unplanned extubations in children: impact on hospital cost and length of stay. Pediatr Crit Care Med. 2015;16(6):572-575. doi: 10.1097/PCC.0000000000000406 [DOI] [PubMed] [Google Scholar]
- 12.Kaufman J, Rannie M, Kahn MG, et al. An interdisciplinary initiative to reduce unplanned extubations in pediatric critical care units. Pediatrics. 2012;129(6):e1594-e1600. doi: 10.1542/peds.2011-2642 [DOI] [PubMed] [Google Scholar]
- 13.Sadowski R, Dechert RE, Bandy KP, et al. Continuous quality improvement: reducing unplanned extubations in a pediatric intensive care unit. Pediatrics. 2004;114(3):628-632. doi: 10.1542/peds.2003-0735-L [DOI] [PubMed] [Google Scholar]
- 14.Popernack ML, Thomas NJ, Lucking SE. Decreasing unplanned extubations: utilization of the Penn State Children’s Hospital Sedation Algorithm. Pediatr Crit Care Med. 2004;5(1):58-62. doi: 10.1097/01.CCM.0000105305.95815.91 [DOI] [PubMed] [Google Scholar]
- 15.Loughead JL, Brennan RA, DeJuilio P, Camposeo V, Wengert J, Cooke D. Reducing accidental extubation in neonates. Jt Comm J Qual Patient Saf. 2008;34(3):164-170, 125. [DOI] [PubMed] [Google Scholar]
- 16.da Silva PS, de Aguiar VE, Neto HM, de Carvalho WB. Unplanned extubation in a paediatric intensive care unit: impact of a quality improvement programme. Anaesthesia. 2008;63(11):1209-1216. doi: 10.1111/j.1365-2044.2008.05628.x [DOI] [PubMed] [Google Scholar]
- 17.Lyren A, Dawson A, Purcell D, Hoffman JM, Provost L. Developing evidence for new patient safety bundles through multihospital collaboration. J Patient Saf. Published online January 31, 2019. doi: 10.1097/PTS.0000000000000564 [DOI] [PubMed] [Google Scholar]
- 18.Institute for Healthcare Improvement Quality Improvement Essentials Toolkit. Accessed November 20, 2019. http://www.ihi.org/resources/Pages/Tools/Quality-Improvement-Essentials-Toolkit.aspx
- 19.DeRosier J, Stalhandske E, Bagian JP, Nudell T. Using health care failure mode and effect analysis: the VA National Center for Patient Safety’s prospective risk analysis system. Jt Comm J Qual Improv. 2002;28(5):248-267, 209. [DOI] [PubMed] [Google Scholar]
- 20.Langley GJ, Moen RD, Nolan KM, Nolan TW, Norman CL, Provost LP. The Improvement Guide: A Practical Approach to Enhancing Organizational Performance. Jossey-Bass; 2009. [Google Scholar]
- 21.Pande PS, Neuman RP, Cavanagh RR. The Six Sigma Way: How GE, Motorola, and Other Top Companies are Honing Their Performance. McGraw-Hill Education; 2000. [Google Scholar]
- 22.Provost LP, Murray S. The Health Care Data Guide: Learning from Data for Improvement. Jossey-Bass; 2011. [Google Scholar]
- 23.Wheeler TA, Davis JT, Brilli RJ. The aggregate point rule for identifying shifts on p charts and u charts. Pediatr Qual Saf. 2018;3(5):e103. doi: 10.1097/pq9.0000000000000103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Merkel L, Beers K, Lewis MM, Stauffer J, Mujsce DJ, Kresch MJ. Reducing unplanned extubations in the NICU. Pediatrics. 2014;133(5):e1367-e1372. doi: 10.1542/peds.2013-3334 [DOI] [PubMed] [Google Scholar]
- 25.Bhanji F, Topjian AA, Nadkarni VM, et al. ; American Heart Association’s Get With the Guidelines–Resuscitation Investigators . Survival rates following pediatric in-hospital cardiac arrests during nights and weekends. JAMA Pediatr. 2017;171(1):39-45. doi: 10.1001/jamapediatrics.2016.2535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Silva PS, Reis ME, Aguiar VE, Fonseca MC. Unplanned extubation in the neonatal ICU: a systematic review, critical appraisal, and evidence-based recommendations. Respir Care. 2013;58(7):1237-1245. doi: 10.4187/respcare.02164 [DOI] [PubMed] [Google Scholar]
- 27.Fontánez-Nieves TD, Frost M, Anday E, Davis D, Cooperberg D, Carey AJ. Prevention of unplanned extubations in neonates through process standardization. J Perinatol. 2016;36(6):469-473. doi: 10.1038/jp.2015.219 [DOI] [PubMed] [Google Scholar]
- 28.Powell BM, Gilbert E, Volsko TA. Reducing unplanned extubations in the NICU using lean methodology. Respir Care. 2016;61(12):1567-1572. doi: 10.4187/respcare.04540 [DOI] [PubMed] [Google Scholar]
- 29.Crezeé KL, DiGeronimo RJ, Rigby MJ, Carter RC, Patel S. Reducing unplanned extubations in the NICU following implementation of a standardized approach. Respir Care. 2017;62(8):1030-1035. doi: 10.4187/respcare.04598 [DOI] [PubMed] [Google Scholar]
- 30.Lucas da Silva PS, de Carvalho WB. Unplanned extubation in pediatric critically ill patients: a systematic review and best practice recommendations. Pediatr Crit Care Med. 2010;11(2):287-294. doi: 10.1097/PCC.0b013e3181b80951 [DOI] [PubMed] [Google Scholar]
- 31.Lyren A, Coffey M, Shepherd M, Lashutka N, Muething S; SPS Leadership Group . We will not compete on safety: how children’s hospitals have come together to hasten harm reduction. Jt Comm J Qual Patient Saf. 2018;44(7):377-388. doi: 10.1016/j.jcjq.2018.04.005 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix 1. Unplanned extubations key driver diagram.
eAppendix 2. Unplanned extubation real-time ACA event investigation.