Abstract
Background:
Postoperative delirium and postoperative cognitive dysfunction (POCD) are common after cardiac surgery and contribute to an increased risk of postoperative complications, longer length of stay, and increased hospital mortality. Cognitive training (CT) may be able to durably improve cognitive reserve in areas deficient in delirium and POCD and, therefore, may potentially reduce the risk of these conditions. We sought to determine the feasibility and potential efficacy of a perioperative CT program to reduce the incidence of postoperative delirium and POCD in older cardiac surgery patients.
Methods:
Randomized controlled trial at a single tertiary care center. Participants included 45 older adults age 60–90 undergoing cardiac surgery at least 10 days from enrollment. Participants were randomly assigned in a 1:1 fashion to either perioperative CT via a mobile device or a usual care control. The primary outcome of feasibility was evaluated by enrollment patterns and adherence to protocol. Secondary outcomes of postoperative delirium and POCD were assessed using the Confusion Assessment Method and the Montreal Cognitive Assessment, respectively. Patient satisfaction was assessed via a postoperative survey.
Results:
Sixty-five percent of eligible patients were enrolled. Median (interquartile range [IQR]) adherence (as a percentage of prescribed minutes played) was 39% (20%–68%), 6% (0%–37%), and 19% (0%–56%) for the preoperative, immediate postoperative, and postdischarge periods, respectively. Median (IQR) training times were 245 (136–536), 18 (0–40), and 122 (0–281) minutes for each period, respectively. The incidence of postoperative delirium (CT group 5/20 [25%] versus control 3/20 [15%]; P = .69) and POCD (CT group 53% versus control 37%; P = .33) was not significantly different between groups for either outcome in this limited sample. CT participants reported a high level of agreement (on a scale of 0–100) with statements that the program was easy to use (median [IQR], 87 [75–97]) and enjoyable (85 [79–91]). CT participants agreed significantly more than controls that their memory (median [IQR], 75 [54–82] vs 51 [49–54]; P = .01) and thinking ability (median [IQR], 78 [64–83] vs 50 [41–68]; P = .01) improved as a result of their participation in the study.
Conclusions:
A CT program designed for use in the preoperative period is an attractive target for future investigations of cognitive prehabilitation in older cardiac surgery patients. Changes in the functionality of the program and enrichment techniques may improve adherence in future trials. Further investigation is necessary to determine the potential efficacy of cognitive prehabilitation to reduce the risk of postoperative delirium and POCD.
As many as 50% of cardiac surgery patients willbe diagnosed with postoperative delirium.1,2 Characterized by an acute onset and fluctuating course of inattention, disorientation, and reduced arousal, delirium can increase the risk of postoperative complications, lengthen hospital length of stay, and is associated with increased hospital mortality.3 While often misperceived as a temporary cognitive disturbance, postoperative delirium is associated with an increased likelihood of long-term impairment known as “postoperative cognitive decline (POCD).”4,5 Many potential pharmacological and procedural interventions to prevent postoperative delirium have been proposed through compelling mechanistic studies; however, the majority of clinical investigations into these proposed interventions have shown limited and inconsistent benefit.6
Cognitive reserve is considered a potentially modifiable protective factor against the development of postoperative delirium and POCD.6 Although classically thought of as the result of an accumulation of cognitive skills over a lifetime, currently there is ongoing debate as to whether cognitive reserve may be amenable to modification over the lifespan.7–9 Recently, multiple software programs have been created that promise to durably improve cognitive reserve in older persons after short-term use. To date, the limited published data regarding these programs have shown that 10–20 hours of training can lead to sustained improve-ments on tests of attention and processing speed, cognitive domains commonly affected in postoperative delirium and POCD.10,11 While observational data suggest that increased participation in cognitive activities in the preoperative period is associated with a reduction in the risk of postoperative delirium, it is unclear whether this finding is due to a training effect or reflects a marker of increased cognitive reserve at baseline.12
Given the numerous risk factors for perioperative acute brain injury after cardiac surgery, it is possible that some loss of cognitive reserve is unavoidable. An approach that focuses on building reserve in domains most affected in the postoperative period may allow better tolerance of this injury, similar to the theory behind physical prehabilitation.13 Therefore, we hypothesize that “cognitive prehabilitation” could prevent postoperative delirium and POCD in older cardiac surgery patients. Before a large-scale efficacy trial of cognitive prehabilitation can be performed in this population, however, significant questions regarding interest and adherence need to be addressed. The objectives of this study are to evaluate the feasibility of a perioperative cognitive training (CT) program in older cardiac surgical patients and estimate effect sizes of postoperative delirium and POCD to inform the conduct of future investigations.
METHODS
The Prevention of Early Postoperative Decline (PEaPoD) study was a randomized, controlled feasibility trial at a single center. This study was approved by the Beth Israel Deaconess Medical Center Institutional Review Board (IRB #P000145), and written informed consent was obtained from all subjects participating in the trial. The trial was registered before patient enrollment at clinicaltrials.gov (NCT02908464; principal investigator: B.P.O.’G.; date of registration: September 21, 2016). The protocol has previously been published.14 Written informed consent was obtained from all individuals before initiation of study procedures. This article adheres to applicable Consolidated Standards of Reporting Trials (CONSORT) guidelines.
Inclusion and Exclusion Criteria
Patients were eligible for inclusion if they were between 60 and 90 years of age, scheduled to undergo cardiac surgery ≥10 days from enrollment, and had an educational level of at least high school or the equivalent. Exclusion criteria consisted of a history of psychiatric illness associated with an increased risk of postoperative delirium or POCD such as anxiety or depression, stroke, epilepsy, Parkinson or Alzheimer disease, other forms of cognitive decline, inability to speak or understand English, or presence of significant visual impairment.15,16 After informed consent, a baseline cognitive assessment was performed using the Montreal Cognitive Assessment (MoCA). If the patient achieved a score indicating the presence of severe baseline cognitive impairment (<10), they were subsequently withdrawn.
Intervention and Control
After enrollment and baseline testing, participants were randomly assigned in a 1:1 fashion using a permuted block of size 4 to either a CT group or usual care control. CT consisted of a mobile software application (Lumosity; Lumos Labs, Inc) featuring programs designed to train users in the cognitive domains of memory, attention, problem solving, flexibility, and processing speed. Each program automatically adjusts the difficulty of the subsequent level to maintain a balance between cognitive challenge and enjoyment. Participants in the CT group were instructed to train for 2 separate 15-minute sessions per day, from the day of enrollment until 4 weeks after surgery including the immediate postoperative period. During each session, participants were asked to select ;≥1 game from each of the 5 available cognitive domains. After study enrollment and assignment to the intervention group, an in-person training session was performed by an unblinded investigator. Subsequently, unblinded investigators were available in person and via telephone to participants to address technical issues and to review training performance. A Wi-Fi-enabled iPad locked to the CT program was provided to each participant in the intervention group and returned at the end of the study. A Wi-Fi connection was necessary for the transmission of gameplay data but was only permitted through the hospital’s protected network to safeguard vulnerable patient data, such as an Internet Protocol address. Raw gameplay data were provided via Lumos Labs, Inc to investigators to subsequently be analyzed for adherence to the prescribed study protocol.
Outcomes
The primary outcome of PEaPoD was feasibility, determined according to the criteria of recruitment and adherence. In addition, a postoperative survey was performed to assess patient satisfaction. Adequate recruitment was defined as enrollment of ;≥50% of eligible patients, reflective of efficient screening and approach procedures as well as sufficient patient interest. Adherence was quantified according to time trained for each perioperative period, namely the preoperative, immediate postoperative (from surgery until hospital discharge), and postdischarge periods. The postoperative satisfaction survey was conducted electronically using a Research Electronic Data Capture (REDCap) survey administered at an in-person postoperative cardiac surgery clinic visit, typically occurring 1 month after hospital discharge.17 Participants who did not complete the survey at this visit were approached via email. Survey questions were both structured and open-ended. Quantitative scales were used to assess the level of agreement with prompted statements, using slide bars with visible anchors of “strongly disagree” (0), “neutral” (50), and “strongly agree” (100). Full details of the postoperative survey are outlined in Supplemental Digital Content, Survey, http://links.lww.com/AA/C944.
Secondary outcomes included the incidence of postoperative delirium and POCD. Postoperative delirium was assessed daily by blinded study members with the Confusion Assessment Method (CAM) or CAM-intensive care unit (CAM-ICU) as appropriate.18,19 Delirium assessments were completed daily up to the day of hospital discharge or hospital day 7 (inclusive), whichever came first. POCD was evaluated by blinded study members using the MoCA.20 Currently no unified test or battery has been agreed upon for the detection of POCD. The MoCA was chosen due to its high degree of sensitivity and specificity in the detection of mild cognitive impairment in older persons.21,22 In addition, the MoCA was chosen for practical considerations, including its ability to be administered quickly and our group’s familiarity in using the MoCA for other protocols.1,23 A full MoCA was given on the day of enrollment, on the day of surgery, and on the day of hospital discharge. A telephonic MoCA (t-MoCA) was administered at 1, 3, and 6 months postoperatively. Additional data including patient demographics and intensive care unit and hospital length of stay were abstracted from medical records. It should be noted that POCD at discharge, as we have defined it in this study, would be classified as “delayed neurocognitive recovery” using a newly recommended nomenclature for describing perioperative cognitive disorders.24
Statistical Analysis
Continuous data are presented as mean ± standard deviation or median (interquartile range [IQR]) for variables not normally distributed. Categorical data are presented as frequencies and proportions. Standardized differences between groups are presented for characteristics at baseline.
Feasibility was analyzed using descriptive statistics of enrollment and adherence. Adherence was defined as the proportion of minutes spent training over the total minutes required per protocol (minutes trained/[30 × number of available days]). Additional descriptive statistics including median gameplay times per period are also reported. A post hoc analysis was performed to assess adherence among only participants who trained for >60 minutes preoperatively.
Postoperative delirium incidence was defined as the proportion of subjects with the presence of delirium by CAM criteria on ≥1 postoperative day. The incidence of POCD was defined as a 1 standard deviation decrease in MoCA score at discharge as compared to baseline. The one standard deviation threshold used was to coincide with definitions from previous trials of POCD and current recommendations on defining POCD or delayed neurocognitive recovery.24,25 The standard deviation used was calculated from the scores in our sample. Differences in the incidence of postoperative delirium and POCD are presented as proportions and analyzed with a χ2 test. Differences in the raw MoCA and t-MoCA scores at each time point were analyzed using parametric t test or Mann-Whitney U tests, as appropriate. Differences in the discharge MoCA scores in each cognitive domain were assessed in a similar fashion during a post hoc exploratory analysis. In the event that differences between groups persisted after baseline, multivariable logistic regression was performed, accounting for those variables for which appreciable differences were observed in the standardized difference at baseline.26 Assessment of patient satisfaction was assessed between groups with the use of a t test or Mann-Whitney U test, as appropriate. SAS 9.4 was utilized for all analyses with 2-sided P values of <.05 considered statistically significant. A sample size of 45 patients was chosen to support a thorough assessment of study feasibility and collection of outcome data to inform future effect size calculations. Due to the small sample size, this trial has very low power to be able to accurately detect any clinically important differences between groups.
RESULTS
Patients were enrolled between September 2016 and July 2018, with final follow-up completed in January 2019. A total of 523 patients were screened, of which 69 met eligibility criteria. Forty-five of the eligible 69 patients (65%) were enrolled. Of those enrolled, the median (IQR) age was 70 years of age (64–75 years of age) and 73% were male (Table 1). No clinically meaningful differences were observed with regard to the baseline demographics, medical comorbidities, or surgical characteristics between the CT and control arms. Participants were enrolled a median (IQR) of 30 days (19–38 days) before surgery. Of note, 60% of patients in the CT group underwent coronary artery bypass grafting as opposed to 40% in the control group (P = .21). In addition, only 40% of patients underwent aortic valve replacement in the CT group as compared to 65% of controls (P = .11).
Table 1.
Patient Characteristics at Baseline
| CT Group N = 20 | Control Group N = 20 | Standardized Difference | |
|---|---|---|---|
| Age, y | 70 ± 6 | 69 ± 7 | 0.070 |
| Male sex | 14 (70) | 15 (75) | −0.112 |
| Height, inches | 68 ± 4 | 68 ± 3 | 0.078 |
| Weight, pounds | 200 ± 41 | 188 ± 37 | 0.302 |
| Body mass index | 30 ± 6 | 28 ± 5 | 0.313 |
| Language status | |||
| Native born English speaker | 17 (85) | 19 (95) | −0.338 |
| Foreign born, English is a second language | 3 (15) | 1 (5) | 0.338 |
| Education level | |||
| High school graduate or equivalent | 1 (5) | 5 (25) | −0.583 |
| Some college, associate’s degree | 4 (20) | 2 (10) | 0.283 |
| Bachelor’s degree | 8 (40) | 5 (25) | 0.324 |
| Master’s degree | 5 (25) | 4 (20) | 0.120 |
| Doctoral degree | 2 (10) | 4 (20) | −0.283 |
| Comorbidities | |||
| Hyperlipidemia | 16 (80) | 15 (75) | 0.120 |
| Hypertension | 14 (70) | 15 (75) | −0.112 |
| Obesity | 11 (55) | 7 (35) | 0.410 |
| Solid tumor nonmetastatic | 6 (30) | 6 (30) | 0.000 |
| Arrhythmia | 8 (40) | 6 (30) | 0.211 |
| Congestive heart failure | 6 (30) | 6 (30) | 0.000 |
| Peripheral vascular disease | 6 (30) | 4 (20) | 0.232 |
| Diabetes without complications | 5 (25) | 3 (15) | 0.252 |
| Chronic obstructive pulmonary disease/asthma | 3 (15) | 3 (15) | 0.000 |
| Moderate or severe renal disease | 3 (15) | 2 (10) | 0.152 |
| Diabetes mellitus with end-organ damage | 2 (10) | 2 (10) | 0.000 |
| Lymphoma or multiple myeloma | 0 (0) | 3 (15) | −0.594 |
| Ulcer disease | 2 (10) | 0 (0) | 0.471 |
| Connective tissue disease | 0 (0) | 1 (5) | −0.324 |
| Solid tumor metastatic | 0 (0) | 1 (5) | −0.324 |
| Chronic pain | 0 (0) | 1 (5) | −0.324 |
| Moderate or severe liver disease | 0 (0) | 1 (5) | −0.324 |
| Surgical characteristics | |||
| Preoperative days | 33 (19, 38) | 26 (20, 40) | −0.068 |
| Bypass time, min | 82 (69, 90) | 74 (65, 88) | 0.178 |
| Coronary artery bypass graft | 12 (60) | 8 (40) | 0.408 |
| Aortic valve replacement | 8 (40) | 13 (65) | −0.517 |
| Aortic surgery | 2 (10) | 2 (10) | 0.000 |
| Mitral valve replacement | 0 (0) | 1 (5) | −0.324 |
| Mitral valve repair | 6 (30) | 3 (15) | 0.365 |
| Tricuspid valve repair | 3 (15) | 0 (0) | 0.594 |
| Other | 0 (0) | 3 (15) | −0.594 |
Data are presented as mean ± standard deviation, median (quartile 1, quartile 3), or n (%) depending on type and distribution. The standardized difference evaluates a difference in group means or prevalence in units of standard deviation. Values <0.1 are considered to represent a negligible difference. Abbreviation: CT, cognitive training.
Primary Outcome: Feasibility
As stated above, 65% of eligible patients who were approached by study staff consented to participate (Figure 1). Patients most commonly cited the time commitment (21%), lack of interest in the research study (21%), and a desire to not use an iPad (17%) as their primary reason for declining consent. Among the 45 randomly assigned participants, 5 patients’ participation was terminated and they were, therefore, excluded from subsequent analyses. Two patients randomly assigned to the CT group withdrew before surgery and were excluded from future outcome analyses. Two patients randomly assigned to the control group were found ineligible after randomization but before surgery and were also excluded from analyses. One additional patient in the control group withdrew immediately after surgery, such that no cognitive outcomes (POCD or delirium) could be assessed. This patient was subsequently withdrawn from further analysis. The remaining CT group patient withdrew from the study at the 6-month time period after completing all assessments; therefore, their data were included and analyzed.
Figure 1.
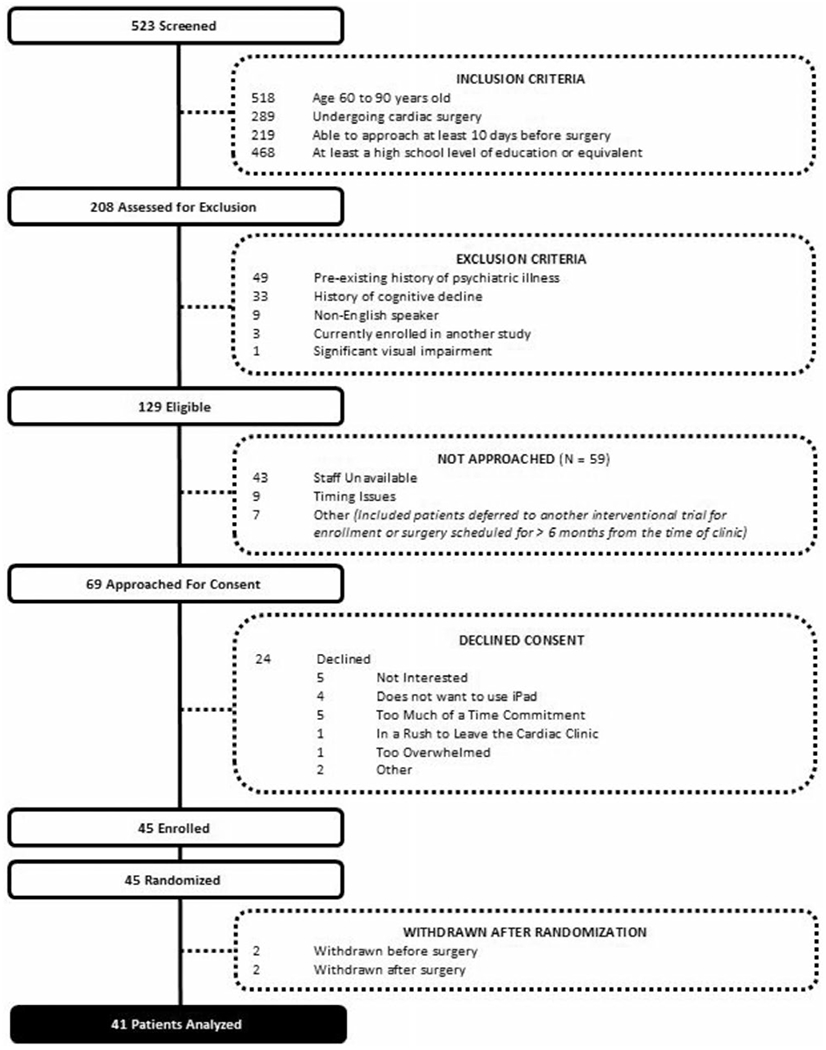
CONSORT diagram. Participant eligibility, enrollment, and follow-up are depicted. CONSORT indicates Consolidated Standards of Reporting Trials.
Analysis of automated gameplay reports revealed that time spent training varied greatly by study period. The median (IQR) adherence for each period was 39% (20%–68%), 6% (0%–37%), and 19% (0%–56%), respectively. For reference, a participant enrolled 10 days before surgery would be expected to train for 300 minutes to achieve 100% adherence. This corresponded to median (IQR) training times of 245 minutes (136536 minutes), 18 minutes (0–40 minutes), and 122 minutes (0–281 minutes) for the preoperative, immediate postoperative, and postdischarge periods, respectively (Figure 2). On the days in which patients used the CT program, they completed a median (IQR) of 10 (5–14) games per day. A post hoc analysis including only participants who trained for >60 minutes preoperatively resulted in an updated median (IQR) preoperative adherence of 51% (37%–78%) and median (IQR) preoperative gameplay time of 457 minutes (231–834 minutes).
Figure 2.
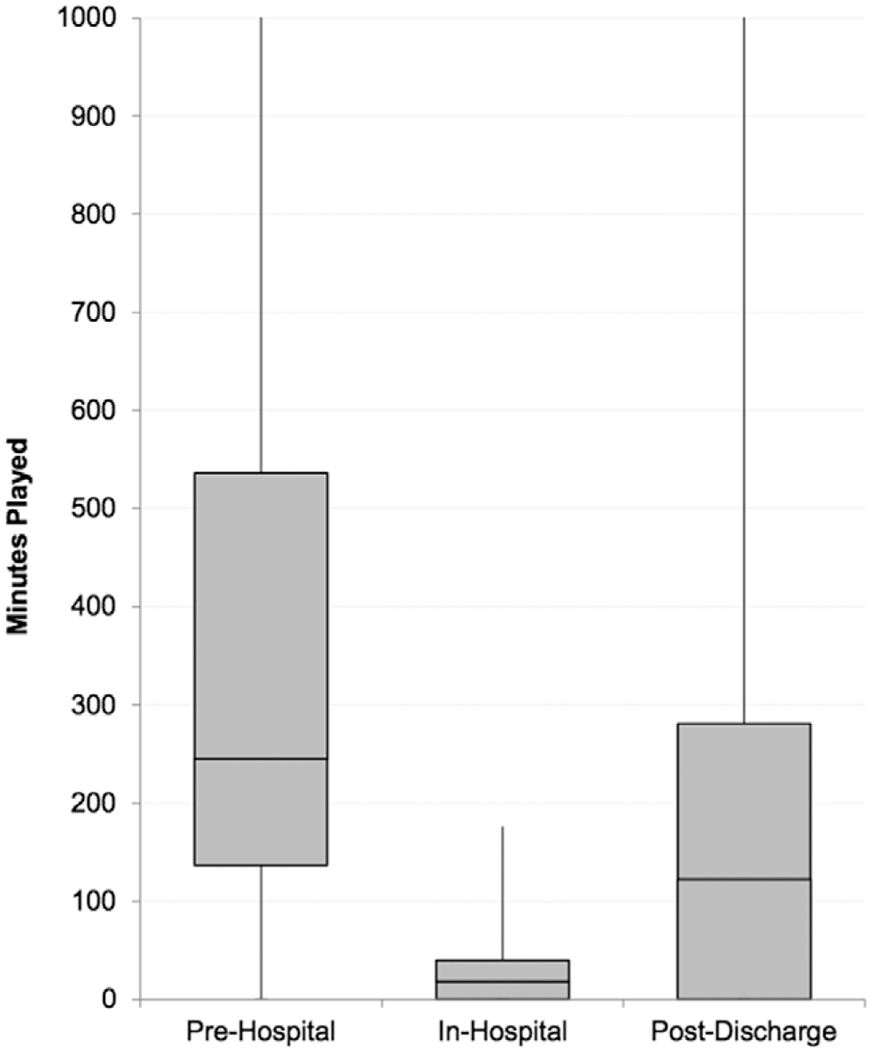
CT time per perioperative period. The median training times for CT participants for each defined perioperative period are depicted. CT indicates cognitive training.
Secondary Outcomes
Delirium and POCD.
The overall incidence of postoperative delirium was 20% (Table 2). In the CT group, 25% (5/20) were delirious as compared to 15% in the control group (3/20; P = .69; Table 2). The overall incidence of POCD at discharge was 44%. No statistically significant difference was observed in the incidence of POCD between groups (CT group 53% vs 37%; P = .33). Post hoc analysis of POCD excluding patients who had been categorized as having postoperative delirium resulted in updated incidences of 50% vs 29.4% (CT versus control; P = .23). No positive CAM scores occurred on the day of the discharge assessment in either group for subjects categorized as having POCD. Post hoc exploratory analysis of discharge MoCA scores in each cognitive domain did not reveal any differences within any category. The median MoCA score was not found to be significantly different between groups at any time point through the follow-up period of 6 months (Figure 3). Of note, 3 patients experienced ≥1 standard deviation improvement in their cognitive performance at discharge. This included 2 patients (10%) in the control group who increased their baseline MoCA score by 4 points and 1 patient (5%) in the CT group who increased their baseline MoCA score by 3 points. Median (IQR) intensive care unit length of stay (2.2 days [2.0–3.5 days] vs 2.3 days [2.1–3.1 days]; P = .62) and hospital length of stay (7 days [6–10 days] vs 6 days [6–8 days]; P = .30) did not differ significantly between groups.
Table 2.
Secondary Outcomes
| CT Group N = 20 | Control Group N = 20 | P | |
|---|---|---|---|
| In-hospital deliriuma | 5 (25) | 3 (15) | 0.69 |
| Baseline MoCA score (0–30, 30 best) | 25.0 (24.0, 27.0) n = 19 | 26.0 (24.0, 27.0) n = 19 | – |
| Preoperative MoCA score (0–30, 30 best) | 25.0 (23.0, 26.0) n = 18 | 26.0 (24.0, 27.0) n = 18 | 0.49 |
| Discharge MoCA score (0–30, 30 best) | 23.5 (21.0, 26.0) n = 18 | 24.0 (22.5, 25.0) n = 20 | 0.74 |
| Visuospatial/executive subtotal (maximum score 5) | 5.0 (4.0, 5.0) | 5.0 (4.0, 5.0) | 0.97 |
| Naming subtotal (maximum score 3) | 3.0 (3.0, 3.0) | 3.0 (2.0, 3.0) | 0.62 |
| Attention subtotal (maximum score 6) | 5.0 (4.0, 6.0) | 6.0 (5.0, 6.0) | 0.33 |
| Language subtotal (maximum score 3) | 2.0 (1.0, 3.0) | 1.0 (1.0, 2.0) | 0.62 |
| Abstraction subtotal (maximum score 2) | 2.0 (1.0, 2.0) | 2.0 (1.5, 2.0) | 0.67 |
| Delayed recall subtotal (maximum score 5) | 1.0 (0.0, 3.0) | 2.0 (1.0, 3.0) | 0.68 |
| Orientation subtotal (maximum score 6) | 6.0 (6.0, 6.0) | 6.0 (6.0, 6.0) | 0.52 |
| Postoperative cognitive decline at discharge | 9 (53) n = 17 | 7 (37) n = 19 | 0.33 |
| Postoperative t-MoCA scores (0–22, 22 best), mo | |||
| 1 | 20.0 (18.0, 21.0) n = 17 | 19.0 (18.0, 21.0) n = 19 | 0.71 |
| 3 | 20.0 (19.0, 21.0) n = 11 | 21.0 (20.0, 21.0) n = 13 | 0.41 |
| 6 | 20.0 (19.0, 22.0) n = 13 | 20.5 (18.0, 21.0) n = 14 | 0.84 |
| ICU length of stay, d | 2.2 (2.0, 3.5) | 2.3 (2.1, 3.1) | 0.62 |
| Hospital length of stay, d | 7.0 (6.0, 9.5) | 6.0 (6.0, 8.0) | 0.30 |
Data are presented as mean ± standard deviation, median (quartile 1, quartile 3), or n (%) depending on type and distribution. Differences in the incidence of delirium and postoperative cognitive decline at discharge were analyzed with χ2 tests. Differences in length of stay, MoCA, and t-MoCA scores (and their components) were analyzed with Mann-Whitney U tests.
Abbreviations: CT, cognitive training; ICU, intensive care unit; MoCA, Montreal Cognitive Assessment; t-MoCA, telephonic Montreal Cognitive Assessment.
Defined as present or absent on any day from postoperative day 1 to postoperative day 7.
Figure 3.
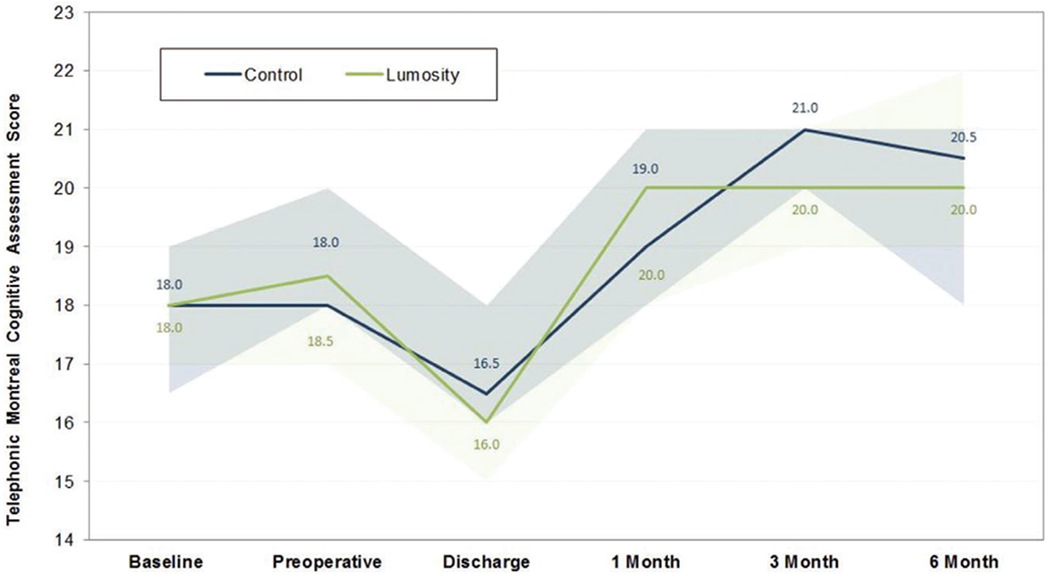
MoCA scores over time. Changes in participants’ cognitive trajectories are reported stratified by group. Scores for the baseline, preoperative, and discharge assessments shown here have been calculated using the same components as the telephonic MoCA maximum score (22), to allow comparisons across the range of perioperative assessments using a uniform scale. MoCA indicates Montreal Cognitive Assessment.
Postoperative Survey.
Results of the postoperative survey are presented in Table 3. Participants in the CT group reported a high level of agreement with statements indicating that “the CT program was easy to use” (median [IQR] agreement, 87 [75–97]) and “I enjoyed playing the training games” (85 [79–91]). When compared to controls, CT group participants reported significantly higher median agreement with statements indicating that their memory (75 [54–82] vs 51 [49–54]; P = .01) and thinking ability (78 [64–83] vs 50 [41–68]; P = .01) improved because of their participation in the study. Participants in both groups reported high median levels of satisfaction with the study overall (90 [78–94] vs 74 [67–87]; P = .06). Participants in the control group agreed that they would be interested in a brain training program if they were to undergo another major surgery (median [IQR], 70 [50–86]). When asked whether preparation or recovery from cardiac surgery should include some form of CT, 89% of the control group participants responded “yes.” These 2 questions were not asked of the CT group. When asked in a multiple-choice format, participants in the CT group identified the most frequent reasons for not using the CT program. Frequent responses included “I didn’t have enough energy” (33%), “I forgot” (28%), “the frequency of games was too often” (22%), “too overwhelmed by surgery and/or recovery” (22%), “I had difficulty focusing” (17%), and “I was too busy” (17%). Sixty-one percent of CT participants responded that they would not have been able to participate in this study if they were not provided with an iPad.
Table 3.
Postoperative Survey
| Control Group | CT Group | P Value | |
|---|---|---|---|
| Reported agreement with the following statements:a | |||
| The program was easy to use | … | 87 (75, 97) | … |
| I enjoyed playing the games | … | 85 (79, 91) | … |
| I think my memory improved because of my participation in this study | 51 (49, 54) | 75 (54, 82) | 0.01 |
| I think my thinking ability improved because of my participation in this study | 50 (41, 68) | 78 (64, 83) | 0.01 |
| Overall satisfaction | 74 (67, 87) | 90 (78, 94) | 0.06 |
| I would be interested in a brain training program if I was going to have another major surgery | 70 (50, 86) | … | … |
| Preparation and recovery from cardiac surgery should include some form of brain training program (yes/no) | 17 (89) | … | … |
Data are presented as mean ± standard deviation, median (quartile 1, quartile 3), or n (%) depending on type and distribution. Differences between groups were analyzed with Mann-Whitney U tests.
Abbreviation: CT, cognitive training.
0: strongly disagree, 50: neutral, 100: strongly agree.
DISCUSSION
PEaPoD is the first trial to evaluate the feasibility and potential efficacy of a CT program to prevent postoperative delirium and POCD in older cardiac surgical patients. Our results indicate substantial interest within this population to participate in a perioperative CT program. Analysis of gameplay data revealed that training adherence varies widely by perioperative period. Perhaps not surprisingly, the immediate postoperative period was a time in which not a lot of training was achieved. The potential reasons for this are numerous and some were clearly reflected in our postoperative survey. Pain, weakness, and complications such as respiratory failure, infection, or delirium itself are all potential reasons why one’s ability to participate in CT may be reduced in the immediate postoperative period. In addition, once discharged from the hospital, returning to work or normal life routine may make the use of a CT program a secondary concern.
With regard to cognitive prehabilitation before surgery, our finding that patients in the CT group spent a median of 4 hours training in the preoperative period is encourageing, but this falls short of the 10 hours presumed to be the effective “dose” of CT.27 Furthermore, our estimate is also heavily influenced by the number of available preoperative days for each participant, which varied widely both within and between groups. However, there are ways in which adherence could potentially be improved in a future trial. The inclusion of a run-in phase could allow for identification and dropout of individuals who are unlikely to adhere well to the prescribed regimen, although excluding patients who have difficulty adhering to CT due to cognitive or behavioral reasons could have potential unintended consequences, if such patients are vulnerable to developing POCD. The use of devices with cellular data connectivity may allow for real-time analysis of gameplay, automated reminders, targeted coaching, and more customizable training packages than what were used in this study. Even with these improvements, however, expecting perfect adherence may not be realistic because previous investigations have shown that it is very difficult to achieve >50% compliance with patient-led interventions to prevent certain chronic medical conditions.28,29 In fact, our post hoc analysis of adherence of only those patients who trained for >60 minutes preoperatively only resulted in an updated median preoperative adherence of 50%.
The rates of postoperative delirium and POCD did not differ significantly between groups in our study. Furthermore, there were no significant changes evident in MoCA scores at any time point before or after surgery, or within categories of cognitive domains tested. These findings are likely the product of measurements made within a small sample size. It is possible, however, that the intervention is not effective or that our measures of cognitive dysfunction after surgery were not specific enough to detect potential benefits in cognitive domains such as processing speed. Our findings that patients in the CT group agreed significantly more strongly than their counterparts with statements suggesting that their memory and thinking ability improved is heavily affected by bias, but could possibly reflect that an aspect of their cognition has improved that we were unable to detect with our current methods.
It should be noted that the incidence of postoperative delirium was found to be higher in the intervention group in a recently published trial of preoperative CT, although the authors noted a high degree of early dropout and low regimen completion rates, and this result was also not statistically significant.30 The completion of the Neurobics trial evaluating the potential benefit of preoperative CT before noncardiac, nonneurological surgery (NCT02230605) may provide additional insights regarding the potential protective versus harmful effect of a preoperative application-based CT program.
PEaPoD has several limitations, most notably that this trial was not powered to detect differences between groups in clinical outcomes. Due to the preexisting uncertainties of the interest and ability to adhere to a mobile, tablet-based application in older persons, our group thought it was prudent to address feasibility concerns before committing to a large randomized controlled efficacy trial. The main limitation of the CT program was that adherence and overall training time varied greatly by patient and by perioperative period, limiting insight into its potential efficacy. Focusing future efforts on the ideal candidates and the times they are most likely to adhere to training, along with more customizable and responsive software may enable targeted applications of perioperative CT programs. In addition, our results are limited by the single-center nature of the trial design; therefore, we may not be able to generalize these findings to patients in other institutions or settings.
Our findings suggest that patients presenting for elective cardiac surgery may be most likely to adhere to a training program in the preoperative period and have sufficient lead in time for such an intervention. When taking these data into account and combining the high degree of patient interest seen in PEaPoD, we believe that a future trial of cognitive prehabilitation before cardiac surgery in this population is likely to be feasible. On the other hand, given the difficulties evident in adherence to a behavioral intervention and the lack of efficacy signal seen in this feasibility study, it is possible that this approach may be challenging to truly evaluate in a large-scale efficacy trial. Cognitive prehabilitation may serve as an innovative, patient-led, minimal-risk intervention to prevent postoperative delirium and POCD and potentially enable a more complete recovery after cardiac surgery. Given these considerations, additional investigation into this emerging field and its possible extension to other surgical populations is warranted.
Supplementary Material
KEY POINTS.
Question:
Is a perioperative cognitive training program designed to potentially reduce the risk of postoperative delirium and postoperative cognitive decline (POCD) feasible in the older cardiac surgery population?
Findings:
Participants demonstrated a high degree of interest but varying ability to adhere to a perioperative cognitive training program, and the incidence of delirium and POCD was not significantly different between groups.
Meaning:
The preoperative period is an attractive target of a cognitive prehabilitation intervention to reduce postoperative delirium and POCD, but steps need to be taken to improve protocol adherence before a larger-scale efficacy trial can be performed in the older cardiac surgery population.
ACKNOWLEDGMENTS
The authors thank the patients who participated in prevention of early postoperative decline (PEaPoD), as well as the nurses, midlevel providers, and cardiac surgery clinic staff including Erica Dillon at BIDMC for their logistical support to help complete the trial. In addition, the authors thank the Center for Anesthesia Research Excellence within the Department of Anesthesia at BIDMC who supported enrollment, protocol implementation, and regulatory compliance throughout this trial.
Funding: The conduct of this trial was performed with support from a BIDMC Department of Anesthesia Internal Research Award (John Hedley-White Award, via Harvard Medical School’s Eleanor and Miles Shore Foundation). A portion of this work was conducted while Dr B.P.O.’G. was a member of the Harvard Anesthesia National Institutes of Health (NIH) T32 program (NIH T32GM007592-38). Research subscriptions to the training program were provided free of charge by Lumos Labs, Inc.
The sponsor had no role in the design/conduct of the study, data collection or analysis, interpretation of the data, manuscript preparation and review, or decision to submit the manuscript for publication.
GLOSSARY
- CAM
Confusion Assessment Method
- CONSORT
Consolidated Standards of Reporting Trials
- CT
cognitive training
- ICU
intensive care unit
- IQR
interquartile range
- IRB
Institutional Review Board
- MoCA
Montreal Cognitive Assessment
- PEaPoD
Prevention of Early Postoperative Decline
- POCD
postoperative cognitive decline
- REDCap
Research Electronic Data Capture
- t-MoCA
telephonic MoCA
Footnotes
DISCLOSURES
Name: Brian P. O’Gara, MD, MPH.
Contribution: This author helped with study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript.
Conflicts of Interest: Brian P. O’Gara is a consultant for Sedana Medical.
Name: Ariel Mueller, MA.
Contribution: This author helped with study concept and design, acquisition of subjects and data, analysis and interpretation of data, and preparation of manuscript.
Conflicts of Interest: None.
Name: Doris Vanessa I. Gasangwa, BS.
Contribution: This author helped with acquisition of subjects and data and preparation of manuscript.
Conflicts of Interest: None.
Name: Melissa Patxot, BS.
Contribution: This author helped with acquisition of subjects and data and preparation of manuscript.
Conflicts of Interest: None.
Name: Shahzad Shaefi, MD, MPH.
Contribution: This author helped with study concept and design and preparation of manuscript.
Conflicts of Interest: None.
Name: Kamal Khabbaz, MD.
Contribution: This author helped with acquisition of subjects and preparation of manuscript.
Conflicts of Interest: None.
Name: Valerie Banner-Goodspeed, MPH.
Contribution: This author helped with study concept and design and preparation of manuscript.
Conflicts of Interest: None.
Name: Alvaro Pascal-Leone, MD, PhD.
Contribution: This author helped with study concept and design, analysis and interpretation of data, and preparation of manuscript.
Conflicts of Interest: Alvaro Pascal-Leone is on the scientific advisory board for multiple companies related to transcranial stimulation, visual rehabilitation, and induction of gamma wave activity.
Name: Edward R. Marcantonio, MD, SM.
Contribution: This author helped with study concept and design, analysis and interpretation of data, and preparation of manuscript
Conflicts of Interest: None.
Name: Balachundhar Subramaniam, MD, MPH.
Contribution: This author helped with study concept and design, analysis and interpretation of data, and preparation of manuscript.
Conflicts of Interest: None.
This manuscript was handled by: Robert Whittington, MD.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s website (www.anesthesia-analgesia.org).
Clinical Trial Registration: Clinicaltrials.gov # NCT02908464 https://clinicaltrials.gov/ct2/show/NCT02908464.
REFERENCES
- 1.Subramaniam B, Shankar P, Shaefi S, et al. Effect of intravenous acetaminophen vs placebo combined with propofol or dexmedetomidine on postoperative delirium among older patients following cardiac surgery. JAMA. 2019;321:686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rudolph JL, Jones RN, Levkoff SE, et al. Derivation and validation of a preoperative prediction rule for delirium after cardiac surgery. Circulation. 2009;119:229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet . 2014;383:911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. [DOI] [PubMed] [Google Scholar]
- 5.Inouye SK, Marcantonio ER, Kosar CM, et al. The short-term and long-term relationship between delirium and cognitive trajectory in older surgical patients. Alzheimers Dement. 2016;12:766–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger M, Terrando N, Smith SK, Browndyke JN, Newman MF, Mathew JP. Neurocognitive function after cardiac surgery: from phenotypes to mechanisms. Anesthesiology. 2018;129:829–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Perneczky R, Kempermann G, Korczyn AD, et al. Translational research on reserve against neurodegenerative disease: consen-sus report of the international conference on cognitive reserve in the dementias and the Alzheimer’s Association Reserve, resilience and protective factors professional interest area working groups. BMC Med. 2019;17:47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cabeza R, Albert M, Belleville S, et al. Maintenance, reserve and compensation: the cognitive neuroscience of healthy ageing. Nat Rev Neurosci. 2018;19:701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stern Y Cognitive reserve. Neuropsychologia. 2009;47:2015–2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anguera JA, Boccanfuso J, Rintoul JL, et al. Video game training enhances cognitive control in older adults. Nature. 2013;501:97–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rebok GW, Ball K, Guey LT, et al. Ten-year effects of the ACTIVE cognitive training trial on cognition and everyday functioning in older adults. J Am Geriatr Soc. 2014;62:16–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tow A, Holtzer R, Wang C, et al. Cognitive reserve and postoperative delirium in older adults. J Am Geriatr Soc. 2016;64:1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Furze G, Dumville JC, Miles JNV, Irvine K, Thompson DR, Lewin RJP. “Prehabilitation” prior to CABG surgery improves physiccal functioning and depression. Int J Cardiol. 2009;132:51–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O’Gara B, Marcantonio ER, Pascual-Leone A, et al. Prevention of Early Postoperative Decline (PEaPoD): protocol for a randomized, controlled feasibility trial. Trials. 2018;19:676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung JM, Sands LP, Mullen EA, Wang Y, Vaurio L. Are preoperative depressive symptoms associated with postoperative delirium in geriatric surgical patients? J Gerontol A Biol Sci Med Sci. 2005;60:1563–1568. [DOI] [PubMed] [Google Scholar]
- 16.Avidan MS, Maybrier HR, Abdallah AB, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet . 2017;390:267–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. [DOI] [PubMed] [Google Scholar]
- 19.Ely E, Inouye SK, Bernard GR, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286:2703–2710. [DOI] [PubMed] [Google Scholar]
- 20.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. [DOI] [PubMed] [Google Scholar]
- 21.Gold S, Forryan S. Postoperative cognitive decline: a current problem with a difficult future. Trends Anaesth Crit Care. 2019;24:49–58. [Google Scholar]
- 22.Luis CA, Keegan AP, Mullan M. Cross validation of the Montreal cognitive assessment in community dwelling older adults residing in the Southeastern US. Int J Geriatr Psychiatry. 2009;24:197–201. [DOI] [PubMed] [Google Scholar]
- 23.Shaefi S, Marcantonio ER, Mueller A, et al. Intraoperative oxygen concentration and neurocognition after cardiac surgery: study protocol for a randomized controlled trial. Trials. 2017;18:600–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Evered L, Silbert B, Knopman DS, et al. Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery-2018. Anesthesiology. 2018;129:872–879. [DOI] [PubMed] [Google Scholar]
- 25.Berger M, Nadler JW, Browndyke J, et al. Postoperative cognitive dysfunction: minding the gaps in our knowledge of a common postoperative complication in the elderly. Anesthesiol Clin. 2015;33:517–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Willis SL, Tennstedt SL, Marsiske M, et al. Long-term effects of cognitive training on everyday functional outcomes in older adults. JAMA. 2006;296:2805–2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gay P, Weaver T, Loube D, Iber C. Evaluation of positive airway pressure treatment for sleep related breathing disorders in adults. Sleep. 2006;29:381–401. [DOI] [PubMed] [Google Scholar]
- 29.Foster JM, Usherwood T, Smith L, et al. Inhaler reminders improve adherence with controller treatment in primary care patients with asthma. J Allergy Clin Immunol. 2014;134:1260.e1263–1268.e1263. [DOI] [PubMed] [Google Scholar]
- 30.Vlisides PE, Das AR, Thompson AM, et al. Home-based cognitive prehabilitation in older surgical patients: a Feasibility Study. J Neurosurg Anesthesiol. 2018;31:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


