Abstract
Purpose
To describe two cases of retinal artery occlusion followed by contralateral amaurosis fugax associated with eosinophilic granulomatosis with polyangiitis (EGPA, formerly known as Churg-Strauss syndrome).
Observations
Case 1 is a 57 year-old male who presented with transient vision loss in the right eye two weeks after a cilioretinal artery occlusion in the left eye. Evaluation eventually led to a diagnosis of EGPA. The patient was treated with high-dose steroids followed by systemic immunomodulatory therapy. Vision in the right eye recovered to 20/20 with no further episodes of vision loss. Case 2 is a 55 year-old male with a known diagnosis of EGPA who presented with transient vision loss in the right eye four weeks after a central retinal artery occlusion of the left eye. This patient also successfully recovered vision in the right eye after treatment with high-dose steroids following a change in his systemic immunomodulatory therapy.
Conclusions and Importance
While ANCA-vasculitides are an uncommon cause of retinal artery occlusion and amaurosis fugax, it is important that they remain in the differential diagnosis, as good visual outcomes can be achieved with prompt initiation of appropriate therapies.
Keywords: Retinal artery occlusion, Amaurosis fugax, Vasculitis, ANCA
1. Introduction
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides are rare diseases associated with necrotizing inflammation of small and medium-sized blood vessels. This group of diseases includes granulomatosis with polyangiitis (GPA, formerly known as Wegener's granulomatosis), microscopic polyangiitis (MPA), and eosinophilic granulomatosis with polyangiitis (EGPA, formerly known as Churg-Strauss syndrome).1 Two main patterns of ANCA antibodies are commonly described, a cytoplasmic staining pattern (cANCA, usually directed against proteinase-3/PR-3) and a perinuclear pattern (pANCA, usually directed against myeloperoxidase/MPO). Patients with GPA are most commonly PR3-ANCA positive, while patients with MPA and EGPA tend to be MPO-ANCA positive.2
Ocular involvement amongst the ANCA-associated vasculitides is most commonly seen in patients with GPA, and is relatively rare in EGPA. Ocular manifestations may include idiopathic orbital inflammation, episcleritis/scleritis, and ischemic vasculitis.3 A recent literature review4 of the ophthalmic findings in EGPA found 10 cases of central retinal artery occlusion (CRAO) and two cases of amaurosis fugax associated with EGPA. There were no cases of retinal artery occlusion (RAO) followed by contralateral amaurosis fugax. Herein we present two such cases.
2. Findings
2.1. Case 1
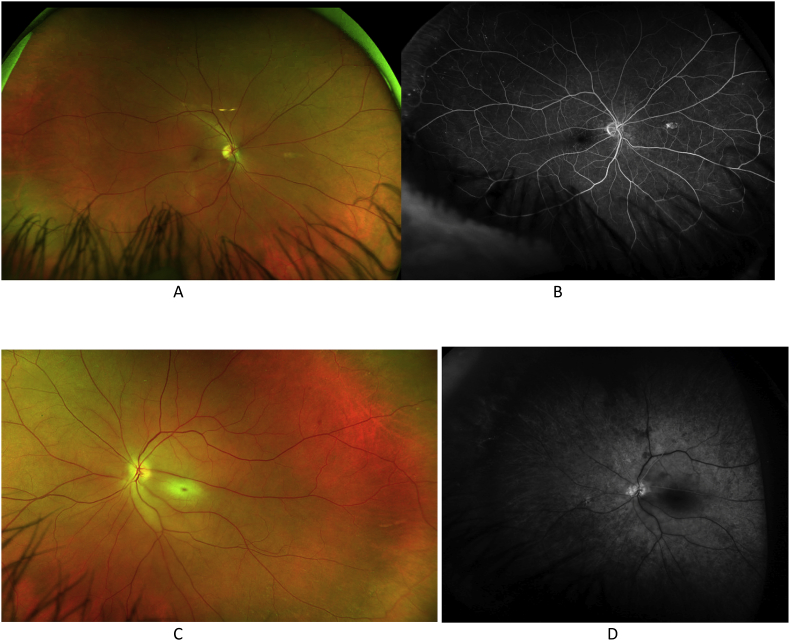
A 57 year-old male presented to the emergency department (ED) with several episodes of transient vision loss in his right eye occurring over the prior 24 hours. The patient had been diagnosed with a cilioretinal artery occlusion of the left eye two weeks prior at an outside hospital, where he was also found to have Raynaud's phenomenon, digital ischemia, peripheral neuropathy, and a pulmonary nodule on chest imaging. An evaluation for embolic phenomenon was negative, including CT angiography of the head, neck, and chest, as well as echocardiography. Infectious work-up was also negative. Biopsy of the pulmonary nodule showed focal necrosis and reactive changes. Initial ophthalmic exam of the right eye in the ED was unremarkable. However repeat exam during an episode of vision loss while still in the ED showed severe involution of the superior retinal arterioles. Visual acuity in the left eye was stable at light perception. Further testing in the ED revealed eosinophilia and hematuria. Given the patient's presentation and the negative evaluation for embolic and infectious etiologies, there was high suspicion for an underlying vasculitic process. The patient was started on intravenous methylprednisolone 1 g daily for 3 days, along with intravenous heparin. Subsequent testing was positive for a pANCA antibody, confirmatory MPO antibody, and peripheral eosinophilia, leading to the diagnosis of EGPA. The patient was transitioned to an oral prednisone taper, and initiated on combination induction therapy with cyclophosphamide and mepolizumab. The patient also remained on anticoagulation with oral coumadin for concurrent digital ischemia. He was discharged with stable 20/20 visual acuity in the right eye. Fundus photography and fluorescein angiography were obtained after the initiation of this immunomodulatory therapy, with normal findings in the right eye (Fig. 1A and B) and central retinal whitening with non-perfusion in the left eye (Fig. 1C and D). At nine months follow-up, he had suffered no recurrent episodes of vision loss.
Fig. 1.
Case 1. The right eye with normal fundus photography (1A) and fluorescein angiography at 5 minutes (1B). The left eye with central retinal whitening on fundus photography (1C) and central retinal non-perfusion on fluorescein angiography at 5 minutes (1D).
2.2. Case 2
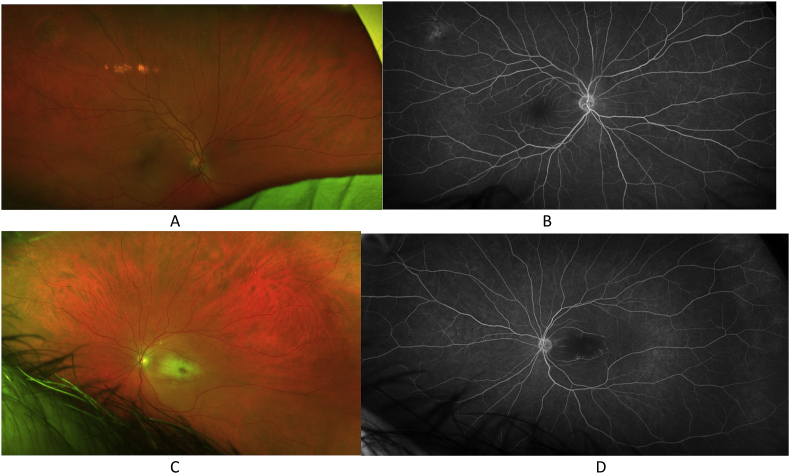
A 55 year-old male with a known history of EGPA and a recent change in immunomodulatory therapy (changed from benralizumab to dupilumab due to persistent symptoms on benralizumab) presented to an outside ophthalmologist with acute vision loss in the left eye due to a CRAO. Work-up with echocardiography was negative at that time. The patient was evaluated in our retina clinic one week later with examination of the left eye showing hand motion visual acuity, retinal whitening with a cherry red macular spot (Fig. 2C), and delayed choroidal filling on fluorescein angiography (Fig. 2D), suggesting a component of ophthalmic artery occlusion. The right eye had normal visual acuity, examination findings, and fluorescein angiography with the exception of a chorioretinal scar in the nasal mid-periphery.
Fig. 2.
Case 2. The right eye with normal fundus photography with the exception of an chorioretinal scar in the nasal mid-periphery, and normal fluorescein angiography at 5 minutes. The left eye with fundus photography showing retinal whitening and a cherry-red spot (2C) and fluorescein angiography at 21 seconds showing delayed choroidal filling (2D). (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Three weeks later, the patient presented to the ED with an acute decrease in vision in the right eye. He denied any other systemic symptoms in association with the vision loss. He was immediately given 1 g of intravenous methylprednisolone, with return of normal vision in the right eye in approximately 30 minutes. Examination of the right eye in the ED following symptom resolution was stable with 20/20 visual acuity and unremarkable dilated fundus exam. The patient was continued on 1 g daily of intravenous methylprednisolone for three days, then transitioned to oral prednisone with a slow taper directed by rheumatology. Induction therapy with cyclophosphamide was initiated, with transition to azathioprine maintenance therapy alongside reslizumab. At four months of follow-up, he had not suffered further episodes of vision loss. Repeat fundus photography (Fig. 2A) and fluorescein angiography (Fig. 2B) at that time were unchanged.
3. Discussion
We present two cases of EGPA complicated by RAO and subsequent contralateral amaurosis fugax. EGPA is a small and medium-vessel vasculitis that is associated with upper and lower respiratory-tract disease, peripheral neuropathy, eosinophilia, and MPO-ANCA positivity.5 Studies have shown a prevalence of arterial occlusions ranging from 3.1% to 18.7% in patients with EGPA.6 Vascular occlusions within the central nervous system (CNS) are even less common, with one study finding only 5.2% of EGPA patients have CNS involvement of any kind.7 Although rare, ophthalmic involvement in EGPA can occur, including orbital inflammation, cranial neuropathy, optic neuropathy, and ischemic vasculitis.8 CRAO appears to be the most common intra-ocular finding in patients with EGPA; a recent literature review identified 10 cases of CRAO and two cases of amaurosis fugax associated with EGPA.4 Both cases of amaurosis fugax attained good visual recovery, one patient to 20/20 and one patient to 20/60, after initiation of high-dose corticosteroids.9,10
To our knowledge, there have been no previous cases reported in the literature of initial RAO in one eye followed by amaurosis fugax in the contralateral eye associated with EGPA. Both of our patients had full visual recovery in the eye with amaurosis fugax without further episodes of vision loss after initiation of high-dose corticosteroids and immunomodulatory therapy. In one patient, EGPA was diagnosed at the onset of ocular manifestions, while the second patient presented with ocular systems in the setting of established disease.
4. Conclusions
Our cases highlight the importance of maintaining a broad differential diagnosis when encountering sequential retinal ischemia. A thorough medical history and review of systems should be obtained to evaluate for symptoms suggestive of vasculitis, such as giant cell arteritis and ANCA-associated vasculitides.
Patient consent
Consent to publish the case series was not obtained. This paper does not contain any personal information that could identify the patients.
Funding
No funding or grant support.
Authorship
All authors attest that they meet the current ICMJE criteria for authorship.
Declaration of competing interest
No financial disclosure or conflicting relationship exists for any author.
Acknowledgments
None.
References
- 1.Jennette J.C., Falk R.J., Bacon P.A. Revised international Chapel Hill consensus conference nomenclature of vasculitides. Arthritis Rheum. 2012;65:1–11. doi: 10.1002/art.37715. 2013. [DOI] [PubMed] [Google Scholar]
- 2.Cornec D., Cornec-Le Gall E., Fervenza F.C. ANCA-associated vasculitis – clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol. 2016;12:570–579. doi: 10.1038/nrrheum.2016.123. [DOI] [PubMed] [Google Scholar]
- 3.Takanashi T., Uchida S., Arita M. Orbital inflammatory pseudotumor and ischemic vasculitis in Churg-Strauss syndrome: report of two cases and review of the literature. Ophthalmol Times. 2001;208:1129–1133. doi: 10.1016/s0161-6420(01)00557-7. [DOI] [PubMed] [Google Scholar]
- 4.Akella S.S., Schlachter D.M., Black E.H. Ophthalmic eosinophilic granulomatosis with polyangiitis (Churg-Strauss syndrome): a systematic review of the literature. Ophthalmic Plast Reconstr Surg. 2019;35:7–16. doi: 10.1097/IOP.0000000000001202. [DOI] [PubMed] [Google Scholar]
- 5.Noth I., Strek M.E., Leff A.R. Churg-Strauss syndrome. Lancet. 2003;361:587–594. doi: 10.1016/S0140-6736(03)12518-4. [DOI] [PubMed] [Google Scholar]
- 6.Ames P.R., Margaglione M., Mackie S. Eosinophilia and thrombophilia in Churg Strauss syndrome: a clinical and pathogenetic overview. Clin Appl Thromg Hemost. 2010;16:628–636. doi: 10.1177/1076029609348647. [DOI] [PubMed] [Google Scholar]
- 7.Comarmond C., Pagnoux C., Khellaf M. Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): clinical characteristics and long-term follow-up of the 383 patients enrolled in the French Vaculitis Study Group cohort. Arthritis Rheum. 2013;65:270–281. doi: 10.1002/art.37721. [DOI] [PubMed] [Google Scholar]
- 8.Watkins A.S., Kempen J.H., Choi D. Ocular disease in patients with ANCA-positive vasculitis. J Ocul Bio Dis Inform. 2009;3:12–19. doi: 10.1007/s12177-009-9044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carmichael J., Conron M., Beynon H. Churg-Strauss syndrome presenting with visual loss. Rheumatol (Oxford) 2000;39:1433–1434. doi: 10.1093/rheumatology/39.12.1433. [DOI] [PubMed] [Google Scholar]
- 10.Alberts A.R., Lasonde R., Ackerman K.R. Reversible monocular blindness complicating Churg-Strauss syndrome. J Rheumatol. 1994;21:363–365. [PubMed] [Google Scholar]