Abstract
Among extramedullary manifestations of multiple myeloma, testicular localization is exceptional. A scrotal mass in this context poses diagnostic and therapeutic challenges given the aesthetic, psychological and reproductive impact of surgery.
Authors report a case of testicular plasmocytoma seven years after remission from multiple myeloma. The treatment consisted of left inguinal orchidectomy. Diagnosis needed the recourse to immunohistochemistry.
Diagnostic modalities, therapeutic options and evolutive eventualities will be discussed.
Extra-medullar localization is exceptionally reported in extramedullary multiple melanoma. Management depends on the concomitant or distant character of hemopathy diagnosis and the disease evolutive history.
Keywords: Testicular neoplasms, Multiple myeloma, Recurrence, Orchiectomy
Introduction
Extramedullary localizations of multiple myeloma include three entities. In addition to extraosseous plasmocytoma and distant plasma cells clonal infiltrations from the bone marrow, extramedullary plasmocytoma are defined as extramedullary tissue masses, responding histologically to the primitive medullar infiltration.1
Testicular plasmocytoma are rarely reported. The modalities of medical and surgical management, often discussed collegially, depend on the disease evolutionary status, on treatment history and the deadline compared with regression if the recurrence is isolated.
Clinical case
It's about a 75 years old patient, with a history of testicular cancer in his nephew at the age of 35. He was diagnosed in 2011 with igA lambda multiple myeloma with 7% of dystrophic plasma cells in the myelogram. Initial assessment found lytic bone lacunas on the standard radiography and plasmacytic tissue infiltration at the fine-needle aspiration of the 7th right rib, without associated visceral lesions. Proteinuria and the circulating free light chain were negative. He had initially 4 Velcade-Dexamethasone cures, followed by Mephalan at a dose of 200mg/m2 of body surface, and peripheral stem cells autograft in September 2011. The response was favorable with complete remission.
Seven years later, a left scrotal mass was observed by the patient, with no clinical manifestations. Examination found a left testicle indurated in its lower pole.
Scrotal Ultrasound showed an extensively modified left echopattern, with hypervascular heterogeneous postero-inferior mass of 28 × 17 × 15 mm. The right testicle was without abnormalities.
Plasmatic lactate dehydrogenase (LDH) and total HCG were in the normal values. Alfa-fetoprotein was two and a half times the normal. Protein electrophoresis found an IgA Lambda monoclonal peak at 3, 5 g/l.
PET- SCAN didn't show hyperfixation, outside a suspect left testicular hypermetabolism.
A left orchidectomy by inguinal incision, with pedicle first clamping, was performed. The operative exploration showed an intratesticular tumor.
Macroscopically, the testicle measured 40 × 28 × 25 mm, with a cord of 75 mm of length. The tumor, of light beige appearance, measured 32 × 24 mm (Fig. 1).
Fig. 1.
Macroscopic aspect.
The tumor, of light beige appearance, measured 32 × 24 mm.
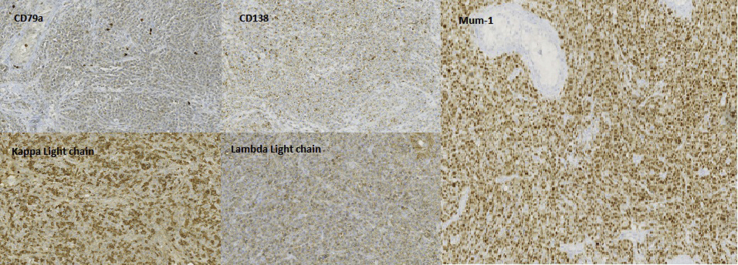
Histologically, tumor cells were voluminous, with large-sized central nuclei and nucleolus. Mitoses were frequent. There persisted within tumor proliferation some atrophic peripheric semniferous tubules. The rete testis and the tunica albuginea were focally invaded (Fig. 2). In immunohistochemistry, tumor proliferation was of plasma cell phenotype, expressing CD138, CD79a, Mum1, with a monotypic kappa light chain. Expression of CD20, CD30, CD56 and of LMP-1 was negative (Fig. 3).
Fig. 2.
Microscopic aspect: Hematoxyllin-eosin coloration.
Voluminous tumor cells, large central nuclei and nucleolus. Frequent mitoses were. Persistance of some atrophic semniferous tubules. Focal invasion of the rete testis and the tunica albuginea.
Fig. 3.
Immuno-histo-chemical study. Postivie
reactions to CD79a, CD138, Mum-1, kappa and lambda light chains.
Immunochemistry allowed to eliminate seminoma in its anaplastic form, retaining the diagnosis of testicular plasma cell localization of multiple myeloma recurrence in its plasmablastic form.
Six weeks later, lactate dehydrogenase (LDH) and total HCG dosage was normal and the rate of Alfa-fetoprotein was still two times the normal.
Electrophoresis of plasma proteins concluded to the persistence of a monoclonal IgA Lambda at 5, 3 g/l. The search for circulating free light chains was negative.
Myelogram by sternal puncture found 2% of dysmorphic plasma cells. Plasma cells population was evaluated at less than 5% of cells on the bone marrow biopsy.
Given these findings, it was decided to supervise closely and initiate an adapted treatment in case of clinical or biological progression.
TEP-Scanner, six months later, showed moderate uptake on multiple bone sites. Protein electrophoresis showed an IgA Lambda peak at 31 g/l. The Kappa and Lambda free light chains were respectively at 12, 04 mg/l and 47, 42/l. The myelogram found plasma cells at 28%, whose phenotype was that of monotypic Lambda population at 97%. Immunohistochemistry was positive for CD28 and negative for CD19 and CD27. FISH analysis didn't find any FGFR3-IgH, P53 or chromosome 1 abnormalities.
After multidisciplinary concentration, it was decided to start a Daratumumab, Lenalidomide and Dexamethasone association, given the biological and radiological progression of the disease.
Discussion
Multiple melanoma, defined as medullar plasma cells proliferation, can generalize towards exramedullary localizations in the third of cases. They are associated to an unfavourable prognosis and a terminal stage of disease.
Solitary plasmocytoma, by definition extramedullary, are observed in the absence of systemic disease. Often, the prognosis remains favorable, with no evidence of systemic disease in the long term.
Testicular plasmocytoma are estimated at 0,03% to 0,1% of testicular tumors.1
Bilateral forms at the time of diagnosis are exceptional. Only two cases are reported in English literature.2
Concerning pathogenesis of testicular localizations, some authors refer to the role of low temperature in the scrotum as a factor favoring resistance of malignant tumor cells to the initial systemic treatment.3
From a molecular point of view, the most consensual hypothesis of medullary myeloma cells dissemination resides in the expression of adhesion molecules, namely CD138. The lack of expression of other molecules such as CD56 favors also dissemination. Interaction with growth factors induces extramedullary dissemination of malignant cells.4
Important fact, and even after transplant of stem cells, certain cells can be resistant to the cytotoxic effect of the donor lymphocyte T. This phenomenon can explain extramedullary recurrence following transplant.5
The treatment is not consensual, and should be discussed on a case by case basis. It depends essentially on the patient general state, and the solitary or diffuse character of the myeloma disease.
In the case we report, myeloma recurrence, occurring seven years from the stem cells autograft was revealed by testicular localization. Orchidectomy was performed, as it was not possible to rule out malignant testicular primary tumor, and the slight elevation of Alfa-fetoprotein. Surveillance was initially justifiable, given the absence of clinical manifestations, and the presence of minimal para-clinical abnormalities. As the patient presented, during follow-up, biological, cytological and radiological progression, the myeloma disease systemic treatment was decided.
Conclusion
Malignant hemopathy can rarely be located at the testicles, with an unfavourable prognosis. Histological diagnosis before surgery is difficult, given the impossibility to rule out a primitive tumor and the contraindication to testicular biopsy. Frozen examination is an option, if conservative surgery is planned.
Medical management is multidisciplinary, considering the disease evolutive status. A particular attention should be brought to fertility, taking into account the radical character of surgery and the impact of associated treatments.
Acknowledgments
Alaa Abdelwahab, Constance Le Goux, Elodie Le Bihan and Jacques Vargaftig provided help in medical management of the case and in collecting medical data.
References
- 1.Chica G., Johnson D.E., Ayala A.G. Plasmacytoma of testis presenting as primary testicular tumor. Urology. 1978;11:90. doi: 10.1016/0090-4295(78)90213-3. [DOI] [PubMed] [Google Scholar]
- 2.Narayanan G., Joseph R., Soman L.V. Bilateral synchronous plasmacytoma of the testis. Proc (Bayl Univ Med Cent) 2016;29:196–197. doi: 10.1080/08998280.2016.11929415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valentini C.G., Bozzoli V., Fianchi L. Primary plasma cell leukemia followed by testicular plasmacytoma. Int J Hematol. 2011;93:224–227. doi: 10.1007/s12185-010-0745-z. [DOI] [PubMed] [Google Scholar]
- 4.Walker J.D., Kaczmarski R.S. Survival of twenty-two months in a patient with primary plasma cell leukaemia treated with melphalan and prednisolone. Postgrad Med. 1988;64(749):232–235. doi: 10.1136/pgmj.64.749.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Perez-Simon J.A., Sureda A., Fernandez-Aviles F. Reducedintensity conditioning allogeneic transplantation is associated with a high incidence of extramedullary relapses in multiple myeloma patients. Leukemia. 2006;20:542–545. doi: 10.1038/sj.leu.2404085. [DOI] [PubMed] [Google Scholar]