Abstract
Objective
To evaluate the efficacy of ubrogepant on patient‐reported functional disability, satisfaction with study medication, and global impression of change.
Background
Ubrogepant is a small‐molecule, oral calcitonin gene‐related peptide receptor antagonist indicated for the acute treatment of migraine. In 2 phase 3 trials (ACHIEVE I and II), ubrogepant demonstrated efficacy vs placebo on the 2 co‐primary endpoints of headache pain freedom and absence of the most bothersome migraine‐associated symptom at 2 hours post dose for the 50 and 100 mg doses. Patient‐reported outcomes, such as functional disability, satisfaction, and patient global impression of change, can provide additional evidence of the efficacy of an acute treatment for migraine on clinically meaningful and patient‐relevant outcomes.
Methods
ACHIEVE I and ACHIEVE II were multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group, single‐attack trials in adults (18‐75 years) with migraine. In ACHIEVE I, participants were randomized 1:1:1 to placebo or ubrogepant 50 or 100 mg; in ACHIEVE II, participants were randomized 1:1:1 to placebo or ubrogepant 25 or 50 mg to treat a migraine attack with moderate or severe headache pain. Participants rated ability to perform daily activities on the Functional Disability Scale, before dosing and at 1, 2, 4, and 8 hours after the initial dose; satisfaction with study medication at 2 and 24 hours; and impression of overall change in migraine on the Patient Global Impression of Change scale at 2 hours. In prespecified analyses for each trial, each outcome was compared between each ubrogepant dose group and the relevant placebo group. Data were pooled from the ubrogepant 50 mg and placebo groups of the 2 trials in a post hoc analysis.
Results
In ACHIEVE I, 559 participants were randomized to placebo, 556 to ubrogepant 50 mg, and 557 to ubrogepant 100 mg; in ACHIEVE II, 563 were randomized to placebo, 561 to ubrogepant 25 mg, and 562 to ubrogepant 50 mg. At 2 hours post dose, significantly higher proportions of ubrogepant‐treated participants vs placebo‐treated participants reported being able to function normally (ACHIEVE I: ubrogepant 50 mg, 40.6% [171/421], P = .0012 vs placebo; ubrogepant 100 mg, 42.9% [192/448], P < .0001 vs placebo; placebo, 29.8% [136/456]; ACHIEVE II: ubrogepant 25 mg, 42.6% [185/434], P = .0015 vs placebo; ubrogepant 50 mg, 40.5% [188/464], P = .0118 vs placebo; placebo, 34.2% [156/456]; pooled 50 mg, 40.6% [359/885], vs pooled placebo, 32.0% [292/912]; P < .0001), were satisfied/extremely satisfied with study medication (ACHIEVE I: 50 mg, 36.3% [147/405], P < .0001 vs placebo; 100 mg, 35.8% [149/416], P = .0002 vs placebo; placebo, 24.1% [104/432]; ACHIEVE II: 25 mg, 35.1% [141/402], P = .0018 vs placebo; 50 mg, 37.8% [163/431], P < .0001 vs placebo; placebo, 24.8% [106/427]; pooled ubrogepant 50 mg, 37.1% [310/836], vs pooled placebo, 24.5% [210/859]; P < .0001), and indicated that their migraine was much/very much better on the Patient Global Impression of Change scale (ACHIEVE I: 50 mg, 34.4% [103/299], P = .0006 vs placebo; 100 mg, 34.3% [102/297], P = .0009 vs placebo; placebo, 22.0% [69/313]; ACHIEVE II: 25 mg, 34.1% [124/364], P < .0001 vs placebo; 50 mg, 33.4% [131/392], P = .0002 vs placebo; placebo, 20.7% [78/376]; pooled 50 mg, 33.9% [234/691], vs pooled placebo, 21.3% [147/689]; P < .0001).
Conclusions
A significantly higher proportion of participants treated with ubrogepant were able to function normally, were satisfied with the study medication, and reported clinically meaningful improvement compared with those receiving placebo. The results reinforce the potential benefits of ubrogepant on patient‐centered outcomes in the acute treatment of migraine.
Keywords: migraine, headache, calcitonin gene‐related peptide, patient‐reported outcomes, quality of life, functional disability
Abbreviations
- CGRP
calcitonin gene‐related peptide
- FDS
Functional Disability Scale
- HRQoL
health‐related quality of life
- mITT
modified intention‐to‐treat
- NSAID
nonsteroidal antiinflammatory drug
- PGIC
Patient Global Impression of Change
- PRO
patient‐reported outcome
Introduction
The defining symptoms of a migraine attack, which include headache pain, photophobia, phonophobia, and nausea, are often incapacitating.1, 2 More than half of people with migraine require bed rest during acute attacks.1, 3 The negative impact on physical and emotional functioning, and on participation in social and leisure activities, can lead to a considerable burden on health‐related quality of life (HRQoL), both during and between migraine attacks.4, 5, 6, 7, 8 Migraine‐associated disability can result in absence from work, as well as lost productivity while at work, which contributes to a substantial economic burden on the individuals, employers, and society.4, 9, 10, 11, 12 Migraine was shown to be the second leading cause of years lost to disability, according to the 2016 Global Burden of Disease Study.13
Many people with migraine have treatment management gaps, including high levels of migraine‐associated disability and dissatisfaction with the efficacy and/or tolerability of their current acute treatment regimen.14, 15 Currently available medications for the acute treatment of migraine attacks may be ineffective or poorly tolerated in some patients and are contraindicated in people with certain cardiovascular comorbidities.14, 16, 17, 18 The recently conducted 2017 Migraine in America Symptoms and Treatment Study demonstrated that 95.8% of respondents with migraine (inclusion criteria: at least 3 monthly headache days in the past 3 months and at least 1 headache day in the past 30 days) who were currently taking an oral acute prescription medication for headache had at least 1 unmet need; the most common attack‐related unmet needs were rapid onset (65.3%) and disability (55.6%).15 New migraine‐specific acute medications that reduce headache‐related disability may provide more treatment options with increased satisfaction for people with migraine.
Calcitonin gene‐related peptide (CGRP) is a neuropeptide that is involved in the pathophysiology of migraine.19, 20 Gepants are small‐molecule, CGRP receptor antagonists primarily developed for the acute treatment of migraine.21 Ubrogepant is the first oral gepant approved for the acute treatment of migraine with or without aura in adults.22 In the phase 3 ACHIEVE I and II trials, the percentages of participants with headache pain freedom 2 hours post dose were significantly superior with ubrogepant 25 mg (20.7%), 50 mg (19.2‐21.8%), and 100 mg (21.2%) vs placebo (11.8‐14.3%; P ≤ .01). In addition, the percentages of participants reporting absence of the most bothersome migraine‐associated symptom (photophobia, phonophobia or nausea) at 2 hours were significantly greater for ubrogepant 50 mg (38.6‐38.9%) and 100 mg (37.7%) than placebo (27.4‐27.8%; P ≤ .01), but not for ubrogepant 25 mg (34.1%). These benefits were achieved with good tolerability; the incidence of treatment‐emergent adverse events was similar in the ubrogepant and placebo groups.23, 24
Given the extensive burden and disability associated with migraine, patient‐reported outcome (PRO) measures of functional disability and satisfaction are important for understanding the holistic treatment effect of an acute treatment. We hypothesized that treatment with ubrogepant is superior to placebo on improvement of functional disability and satisfaction with study treatment. Here, we report the results of the PRO measures evaluated in ACHIEVE I and in ACHIEVE II, including assessments of functional disability, satisfaction with medication, and the participant’s global impression of change.
Methods
Trial Design and Participants
The designs of ACHIEVE I (ClinicalTrials.gov, NCT02828020) and ACHIEVE II (NCT02867709; funded by Allergan plc), including complete inclusion and exclusion criteria, have been published elsewhere.23, 24 Both trials were multicenter, randomized, double‐blind, placebo‐controlled, parallel‐group, single‐attack phase 3 trials conducted in the United States (ACHIEVE I: 89 sites; ACHIEVE II: 99 sites). Eligible participants were 18‐75 years of age, had a history of migraine with or without aura for at least 1 year consistent with a diagnosis according to the International Classification of Headache Disorders, 3rd edition (beta version) criteria,25 and must have experienced 2‐8 migraine attacks with moderate to severe headache pain in each of the 3 months before screening. Participants with a current diagnosis of chronic migraine were excluded; however, participants with a previous diagnosis of chronic migraine who were currently experiencing fewer than 15 headache days per month while taking concomitant preventive treatment were allowed to enroll in the trial.
In ACHIEVE I, participants were randomized 1:1:1 to placebo, ubrogepant 50 mg, or ubrogepant 100 mg; in ACHIEVE II, participants were randomized 1:1:1 to placebo, ubrogepant 25 mg, or ubrogepant 50 mg. In each trial, randomization was stratified by previous response to triptans and current use of concomitant preventive medication for migraine. Participants took their assigned study medication as soon as possible (no later than 4 hours after headache onset) to treat a qualifying migraine attack, defined as a migraine attack with moderate to severe headache pain accompanied by at least 1 migraine‐associated symptom (photophobia, phonophobia, or nausea). The treated migraine headache must have been new (ie, no other headache had occurred within 48 hours, and the headache was not a recurrence of a previous migraine headache) and not already resolving on its own. An optional second dose of study medication or a rescue medication (eg, acetaminophen, nonsteroidal antiinflammatory drug [NSAID], opioid, antiemetic, triptan) was allowed for the treatment of moderate or severe headache pain starting from 2 to 48 hours after the initial dose. For those who opted to take a second dose of study medication, participants in the ubrogepant groups were randomized to receive either placebo or ubrogepant (dose same as initial dose) for the optional second dose, whereas all participants in the placebo group received placebo for the optional second dose. The protocols were approved by institutional review boards. All participants provided written informed consent before initiation of trial procedures. The protocols and all amendments have been published.23, 24
Assessments
The co‐primary and secondary efficacy outcomes of ACHIEVE I and II have been published.23, 24 This article reports the results of prespecified efficacy analyses of additional PRO measures for each treatment group from each trial – namely, the Functional Disability Scale (FDS), participant satisfaction with study medication, and Patient Global Impression of Change (PGIC) scale. The results of a post hoc analysis of pooled data for a comparison of the pooled ubrogepant 50 mg group vs the pooled placebo group are also presented.
Participants rated their ability to perform daily activities on the FDS, a single‐item, 4‐point response scale ranging from 0 (“no disability, able to function normally”) to 3 (“severely impaired, cannot do all or most things, bed rest may be necessary”)26 before dosing and at 1, 2, 4, and 8 hours after initial dose. Responders were defined as participants who provided a score of 0 (no disability, able to function normally) on the FDS. Satisfaction with study medication was rated on a single‐item, 7‐point response scale ranging from “extremely satisfied” (0) to “extremely dissatisfied” (6) at 2 and 24 hours after dosing. Responders were defined as participants who reported scores of 1 (satisfied) or 0 (extremely satisfied). Content validity of the FDS and satisfaction with study medication was established in a separate qualitative study based on concept elicitation and cognitive interviews in participants with episodic migraine and chronic migraine. Quantitative analyses based on clinical trial data supported the convergent validity of the FDS and satisfaction measures and will be reported elsewhere.
Participants rated their impression of overall change in their migraine at 2 hours after the initial dose compared with immediately before taking the study medication using the PGIC, a single‐item, 7‐point rating scale with responses ranging from “very much better” (0) to “very much worse (6).” Responders were defined as participants who rated the PGIC as 0 (very much better) or 1 (much better). Participants completed all efficacy assessments in an electronic diary. The responder definitions for the FDS, satisfaction measures, and the PGIC were based on qualitative interpretation of the respective response options.
Statistical Analysis
Each trial had a target sample size of 550 randomized participants per treatment group to provide at least 85% power to detect treatment differences between each ubrogepant dose and placebo for the co‐primary efficacy outcomes.23, 24 The modified intention‐to‐treat (mITT) population included all randomized participants who received at least 1 dose of study medication, recorded baseline migraine headache severity, and reported at least 1 post dose migraine headache severity rating or migraine‐associated symptom outcome at or before 2 hours after the initial dose. The proportion of participants in the mITT population reporting “no disability, able to function normally” on the FDS before dosing and at 1, 2, 4, and 8 hours after initial dose was analyzed using a logistic regression model, with categorical terms for treatment group, historical triptan response (triptan responder, triptan insufficient responder, or triptan naive), use of medication for migraine prevention (yes/no), and baseline headache severity (moderate or severe), with baseline functional disability score included as a covariate. The last observation carried forward approach was applied to impute missing post‐baseline values. The proportion of participants reporting satisfaction with study medication (“satisfied” or “extremely satisfied”) at 2 and 24 hours post initial dose and the proportion responding “much better” or “very much better” on the PGIC at 2 hours were analyzed using a logistic regression model, with categorical terms for treatment group, historical triptan response, use of medication for migraine prevention, and baseline headache severity. For each of these PROs, responder rates in the 25, 50, and 100 mg doses were evaluated vs the relevant placebo group in each trial (prespecified planned analyses). Responder rates in the pooled ubrogepant 50 mg group were evaluated vs the pooled placebo group in post hoc pooled analyses. All statistical tests were 2‐sided tests performed at the 5% level of significance (ie, P < .05). Multiple testing was not considered because the PRO endpoints were not primary or secondary endpoints. All analyses were conducted using SAS version 9.3 (SAS Institute Inc).
Results
Participant Characteristics
In ACHIEVE I, 1672 participants were randomly assigned to either placebo (n = 559), ubrogepant 50 mg (n = 556), or ubrogepant 100 mg (n = 557; Fig. 1).23 In ACHIEVE II, 1686 participants were randomized to either placebo (n = 563), ubrogepant 25 mg (n = 561), or ubrogepant 50 mg (n = 562; Fig. 1).24 The mITT population from ACHIEVE I included 456 participants in the placebo group, 423 in the ubrogepant 50 mg group, and 448 in the ubrogepant 100 mg group. In ACHIEVE II, the mITT population included 456 participants in the placebo group, 435 in the ubrogepant 25 mg group, and 464 in the ubrogepant 50 mg group. The pooled mITT population included 912 participants in the pooled placebo group and 887 in the pooled ubrogepant 50 mg group.
Figure 1.

Participant disposition. aThe mITT population used for efficacy analyses included all randomized participants who received at least 1 dose of study medication, recorded baseline migraine headache severity, and reported at least one post dose migraine headache severity rating or migraine‐associated symptom outcome at or before 2 hours after the initial dose.
mITT = modified intention‐to‐treat. [Color figure can be viewed at https://wileyonlinelibrary.com]
Demographics and baseline clinical characteristics are summarized in the Table 1. Across the trials and treatment arms, the mean age was 40‐41 years; most participants were female (86.2‐91.2%) and white (79.6‐85.7%). Approximately one‐quarter (22.3‐25.0%) of participants reported current use of a preventive migraine medication. Immediately before taking trial medication for a qualifying migraine attack (ie, attack baseline), participants reported the presence of migraine‐associated symptoms of photophobia (87.3‐92.2%), phonophobia (74.5‐81.1%), and/or nausea (56.0‐65.3%). The most bothersome migraine‐associated symptom was most frequently photophobia (53.7‐59.1%), followed by phonophobia (19.4‐29.8%) and nausea (16.4‐22.4%). The distribution of FDS scores at baseline was similar between treatment groups (Table). At attack baseline, 28.1‐32.7% of participants reported moderate or severe functional disability on the FDS and 36.3‐40.5% reported mild functional impairment.
Table 1.
| ACHIEVE I23 | ACHIEVE II24 | Pooled | ||||||
|---|---|---|---|---|---|---|---|---|
| Placebo (n = 456) | Ubrogepant 50 mg (n = 423) | Ubrogepant 100 mg (n = 448) | Placebo (n = 456) | Ubrogepant 25 mg (n = 435) | Ubrogepant 50 mg (n = 464) | Placebo (n = 912) | Ubrogepant 50 mg (n = 887) | |
| Age (years), mean (SD)† | 41 (12) | 40 (12) | 40 (12) | 41 (12) | 41 (12) | 41 (12) | 41 (12) | 40 (12) |
| Sex, n (%) | ||||||||
| Female | 407 (89.3) | 380 (89.8) | 386 (86.2) | 402 (88.2) | 393 (90.3) | 423 (91.2) | 809 (88.7) | 803 (90.5) |
| Male | 49 (10.7) | 43 (10.2) | 62 (13.8) | 54 (11.8) | 42 (9.7) | 41 (8.8) | 103 (11.3) | 84 (9.5) |
| Race, n (%) | ||||||||
| White | 391 (85.7) | 349 (82.5) | 372 (83.0) | 363 (79.6) | 365 (83.9) | 379 (81.7) | 754 (82.7) | 7728 (82.1) |
| Black or African American | 51 (11.2) | 58 (13.7) | 63 (14.1) | 75 (16.4) | 60 (13.8) | 77 (16.6) | 126 (13.8) | 135 (15.2) |
| Asian | 6 (1.3) | 7 (1.7) | 4 (0.9) | 7 (1.5) | 6 (1.4) | 2 (0.4) | 13 (1.4) | 9 (1.0) |
| American Indian or Alaska Native | 3 (0.7) | 3 (0.7) | 4 (0.9) | 3 (0.7) | 0 | 2 (0.4) | 6 (0.7) | 5 (0.6) |
| Native Hawaiian or Other Pacific Islander | 2 (0.4) | 1 (0.2) | 3 (0.7) | 1 (0.2) | 1 (0.2) | 1 (0.2) | 3 (0.3) | 2 (0.2) |
| Multiple‡ | 3 (0.7) | 5 (1.2) | 2 (0.4) | 7 (1.5) | 3 (0.7) | 3 (0.6) | 10 (1.1) | 8 (0.9) |
| Hispanic or Latino ethnicity, n (%) | 48 (10.5) | 49 (11.6) | 46 (10.3) | 84 (18.4) | 85 (19.5) | 97 (20.9) | 132 (14.5) | 146 (6.5) |
| BMI (kg/m2), mean (SD) | 29.9 (7.5) | 30.1 (8.0) | 30.5 (8.1) | 29.9 (7.7) | 29.7 (7.1) | 30.6 (7.6) | 29.9 (7.6) | 30.3 (7.8) |
| On concomitant preventive migraine medication,§ n (%) | 106 (23.2) | 96 (22.7) | 100 (22.3) | 111 (24.3) | 100 (23.0) | 116 (25.0) | 217 (23.8) | 212 (23.9) |
| Characteristics of treated migraine attack,¶ n (%) | ||||||||
| Headache pain severity | ||||||||
| Moderate | 287 (62.9) | 260 (61.5) | 288 (64.3) | 258 (56.6) | 257 (59.1) | 289 (62.3) | 545 (59.8) | 549 (61.9) |
| Severe | 169 (37.1) | 163 (38.5) | 160 (35.7) | 198 (43.4) | 178 (40.9) | 175 (37.7) | 367 (40.2) | 338 (38.1) |
| Presence of migraine‐associated symptoms | ||||||||
| Photophobia | 416 (91.2) | 390 (92.2) | 391 (87.3) | 404 (88.6) | 399 (91.7) | 420 (90.5) | 820 (89.9) | 810 (91.3) |
| Phonophobia | 362 (79.4) | 315 (74.5) | 360 (80.4) | 370 (81.1) | 353 (81.1) | 374 (80.6) | 732 (80.3) | 689 (77.7) |
| Nausea | 292 (64.0) | 237 (56.0) | 274 (61.2) | 279 (61.2) | 284 (65.3) | 297 (64.0) | 571 (62.6) | 534 (60.2) |
| Vomiting | 26 (5.7) | 27 (6.4) | 18 (4.0) | 22 (4.8) | 19 (4.4) | 21 (4.5) | 48 (5.3) | 48 (5.4) |
| Most bothersome migraine‐associated symptom | ||||||||
| Photophobia | 254 (55.7) | 248 (58.6) | 246 (54.9) | 245 (53.7) | 257 (59.1) | 265 (57.1) | 499 (54.7) | 513 (57.8) |
| Phonophobia | 98 (21.5) | 82 (19.4) | 116 (25.9) | 136 (29.8) | 102 (23.4) | 115 (24.8) | 234 (25.7) | 197 (22.2) |
| Nausea | 102 (22.4) | 90 (21.3) | 86 (19.2) | 75 (16.4) | 75 (17.2) | 83 (17.9) | 177 (19.4) | 173 (19.5) |
| Missing | 2 (0.4) | 3 (0.7) | 0 | 0 | 1 (0.2) | 2 (0.1) | 2 (0.2) | 4 (0.5) |
| FDS score at baseline | ||||||||
| 0‐No disability | 135 (29.6) | 139 (33.0) | 134 (29.9) | 146 (32.0) | 134 (30.9) | 135 (29.1) | 281 (30.8) | 274 (30.9) |
| 1‐Mildly impaired | 172 (37.7) | 153 (36.3) | 179 (40.0) | 182 (39.9) | 167 (38.5) | 188 (40.5) | 354 (38.8) | 341 (38.4) |
| 2‐Moderately impaired | 113 (24.8) | 102 (24.2) | 103 (23.0) | 104 (22.8) | 95 (21.9) | 113 (24.4) | 217 (23.8) | 215 (24.2) |
| 3‐Severely impaired | 36 (7.9) | 27 (6.4) | 32 (7.1) | 24 (5.3) | 38 (8.8) | 28 (6.0) | 60 (6.6) | 55 (6.2) |
| Missing | 0 | 2 | 0 | 0 | 1 | 0 | 0 | 2 |
BMI = body mass index; FDS = functional disability scale; mITT = modified intention‐to‐treat; SD = standard deviation.
Age on date of informed consent.
Participants who reported ≥2 races, including participants who reported white and ≥1 other race.
Recorded at time of randomization.
Recorded by participant before taking trial medication.
Patient‐Reported Outcome Measures
1. Functional Disability Scale
In ACHIEVE I, the proportions of participants reporting that they had no disability (ie, able to function normally) were significantly greater in the ubrogepant 50 mg and ubrogepant 100 mg groups than in the placebo group at 2 hours (ubrogepant 50 mg, 40.6% [171/421], P = .0012 vs placebo; ubrogepant 100 mg, 42.9% [192/448], P < .0001 vs placebo; placebo, 29.8% [136/456]), 4 hours (50 mg, 60.6% [255/421], P < .0001 vs placebo; 100 mg, 60.7% [272/448], P < .0001 vs placebo; placebo, 45.2% [206/456]), and 8 hours (50 mg, 78.0% [329/422], P < .0001 vs placebo; 100 mg, 75.0% [336/448], P = .0002 vs placebo; placebo, 63.3% [290/456]; Fig. 2a) post initial dose. In ACHIEVE II, rates of normal function were significantly greater in the ubrogepant 25 mg and the ubrogepant 50 mg groups vs placebo at 2 hours (ubrogepant 25 mg, 42.6% [185/434], P = .0015 vs placebo; ubrogepant 50 mg, 40.5% [188/464], P = .0118 vs placebo; placebo, 34.2% [156/456]), 4 hours (25 mg, 60.1% [261/434], P < .0001 vs placebo; 50 mg, 60.8% [282/464], P < .0001 vs placebo; placebo, 47.6% [217/456]), and 8 hours (25 mg, 73.5% [319/434], P < .0001 vs placebo; 50 mg, 74.8% [347/464], P < .0001 vs placebo; placebo, 62.1% [283/456]; Fig. 2b). Participant‐reported rates of normal functional ability were also significantly higher in the pooled ubrogepant 50 mg group than in the pooled placebo group at 2 hours (pooled ubrogepant 50 mg, 40.6% [359/885]; pooled placebo, 32.0% [292/912]; P < .0001), 4 hours (60.7% [537/885] vs 46.4% [423/912]; P < .0001), and 8 hours (76.3% [676/886] vs 62.8% [573/912]; P < .0001; Fig. 2c). At 2 hours and beyond, the odds of reporting normal functioning were nearly twice as high with ubrogepant 50 or 100 mg compared with placebo (Fig. 2a,b) in ACHIEVE I and II.
Figure 2.

Proportion of participants reporting ability to function normally on the Functional Disability Scale in (a) ACHIEVE I, (b) ACHIEVE II, and (c) the pooled analysis of ACHIEVE I and II. *P ≤ .01. P values are based on logistic regression with treatment group, baseline severity, historical triptan response, and use of medication for migraine prevention as factors and baseline value as a covariate. Missing values post baseline were imputed using the last observation carried forward approach. [Color figure can be viewed at https://wileyonlinelibrary.com]
2. Satisfaction With Study Medication
In ACHIEVE I, the percentage of participants reporting that they were “satisfied” or “extremely satisfied” with study medication was significantly greater in the ubrogepant 50 mg group and the ubrogepant 100 mg group than in the placebo group at 2 hours (ubrogepant 50 mg, 36.3% [147/405], P < .0001 vs placebo; ubrogepant 100 mg, 35.8% [149/416], P = .0002 vs placebo; placebo, 24.1% [104/432]) and 24 hours (50 mg, 61.6% [233/378], P < .0001 vs placebo; 100 mg, 59.0% [230/390], P < .0001 vs placebo; placebo, 35.3% [140/397]) after the initial dose (Fig. 3a). In ACHIEVE II, satisfaction rates were significantly higher with ubrogepant 25 mg and ubrogepant 50 mg than placebo at 2 hours (25 mg, 35.1% [141/402], P = .0018 vs placebo; 50 mg, 37.8% [163/431], P < .0001 vs placebo; placebo, 24.8% [106/427]) and 24 hours (25 mg, 55.1% [217/411], P < .0001 vs placebo; 50 mg, 60.8% [253/416], P < .0001 vs placebo; placebo, 39.4% [162/411]; Fig. 3b). Satisfaction was also significantly greater in the pooled ubrogepant 50 mg group vs the pooled placebo group at 2 hours (37.1% [310/836] vs 24.5% [210/859]; P < .0001) and 24 hours (61.2% [486/794] vs 37.4% [302/808]; P < .0001; Fig. 3c). In ACHIEVE I, the odds of reporting satisfaction with study medication at 24 hours were nearly 3 times higher in the ubrogepant 50 and 100 mg groups compared with placebo (Fig. 3a). In ACHIEVE II, the odds of reporting satisfaction with study medication at 24 hours were nearly 2.5 times higher with ubrogepant 50 mg than placebo (Fig. 3b).
Figure 3.
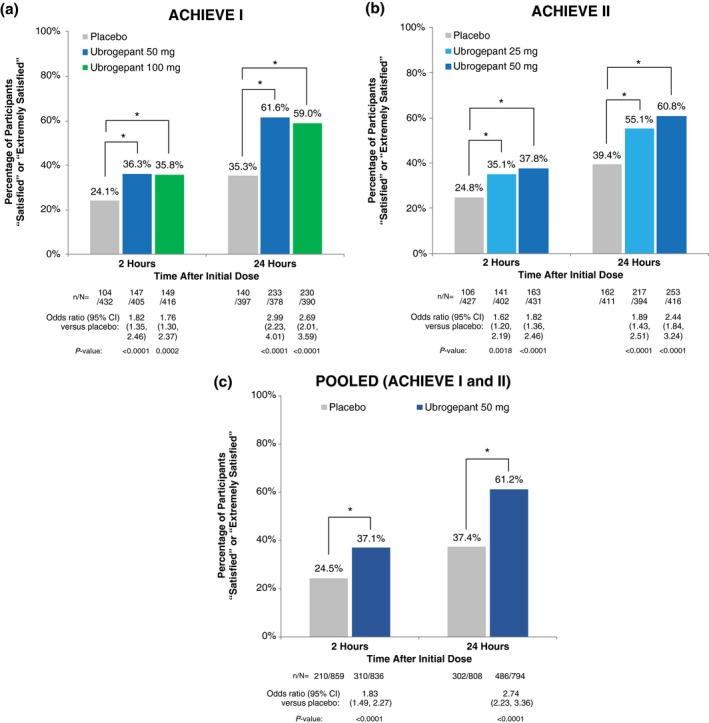
Proportion of participants “satisfied” or “extremely satisfied” with study medication at 2 and 24 hours after initial dose in (a) ACHIEVE I, (b) ACHIEVE II, and (c) the pooled analysis of ACHIEVE I and II. *P < .002. P values are based on logistic regression with treatment group, baseline severity, historical triptan response, and use of medication for migraine prevention as factors. [Color figure can be viewed at https://wileyonlinelibrary.com]
3. Patient Global Impression of Change
At 2 hours after the initial dose, the proportions of participants reporting that the overall change in their migraine was “much better” or “very much better” on the PGIC were significantly greater in each ubrogepant group than in the placebo group in ACHIEVE I (50 mg, 34.4% [103/299], P = .0006 vs placebo; 100 mg, 34.3% [102/297], P = .0009 vs placebo; placebo, 22.0% [69/313]; Fig. 4a) and in ACHIEVE II (25 mg, 34.1% [124/364], P < .0001 vs placebo; 50 mg, 33.4% [131/392], P = .0002 vs placebo; placebo, 20.7% [78/376]; Fig. 4b). The PGIC responder rate was also significantly higher in the pooled ubrogepant 50 mg group (33.9% [234/691]) than in the pooled placebo group (21.3% [147/689]; P < .0001; Fig. 4c). At 2 hours, in ACHIEVE I and II, the odds of reporting the overall change in migraine as “better” or “very much better” was nearly twice as high in the ubrogepant 50 and 100 mg groups compared with the placebo groups (Fig. 4a,b).
Figure 4.

Proportion of participants reporting that their migraine was “much better” or “very much better” on the Patient Global Impression of Change scale at 2 hours after initial dose in (a) ACHIEVE I, (b) ACHIEVE II, and (c) the pooled analysis of ACHIEVE I and II. *P ≤ .0009. P values are based on logistic regression, with treatment group, baseline severity, historical triptan response, and use of medication for migraine prevention as factors. [Color figure can be viewed at https://wileyonlinelibrary.com]
Safety
Treatment‐emergent adverse events in ACHIEVE I and ACHIEVE II have been previously published23, 24 and are summarized in Tables S1 and S2, respectively. The incidence of adverse events was similar in the ubrogepant and the placebo groups in each trial.
Discussion
Ideally, a medication for the acute treatment of migraine should provide rapid and consistent relief of pain and associated symptoms while restoring the ability to function, without causing adverse events or the need for rescue treatment.27, 28, 29 Treatment with ubrogepant vs placebo showed significant improvements in freedom from pain and the most bothersome migraine‐associated symptom (co‐primary endpoints) and was well tolerated in the pivotal ACHIEVE trials.23, 24 As reported herein, ubrogepant also restored and maintained participants’ ability to function in daily activities, as demonstrated by the significantly higher proportions of ubrogepant‐treated participants who reported normal function on the FDS at 2, 4, and 8 hours after the initial dose, compared with placebo‐treated participants. The rate of normal function increased over time in a pattern that mirrored increasing rates of pain freedom up to 8 hours after the initial dose.23, 30 Of note, the rate of normal function was consistently higher than the rate of pain freedom at each time point.23, 30 In comparison to pain free rates, pain relief rates at 2 hours post dose were higher with ubrogepant at 50 mg (60.7%) and 100 mg (61.4 vs 49.1% with placebo) in ACHIEVE I23 and at 25 mg (60.5%) and 50 mg (62.7 vs 48.2% with placebo) in ACHIEVE II.24 These results suggest that the pain relief achieved with ubrogepant can reduce migraine‐associated disability when used for the acute treatment of migraine attacks and that restoration of functional ability can occur before freedom from pain.
Despite the array of currently available options for the acute treatment of migraine, many patients report being dissatisfied with their treatment because of side effects or suboptimal effectiveness.14, 31, 32 In the current trial, significantly higher proportions of participants who took ubrogepant reported being “satisfied” or “extremely satisfied” with study medication, compared with those who took placebo. At 24 hours post dose, 61% of participants in the pooled ubrogepant 50 mg group were satisfied, compared with 37% in the pooled placebo group. The significantly higher proportion of patients reporting improvement in migraine based on global impression of change (PGIC) with ubrogepant over placebo further complements previously reported positive results on efficacy measures of pain and migraine‐associated symptoms. Together, these data support the benefit of ubrogepant in reducing the burden of migraine. Comparing the current outcomes with those reported in clinical trials of other acute medications has challenges due to some inherent differences in the measures used and other differences in trial design.33, 34, 35, 36
Current acute treatments for migraine with established efficacy (eg, triptans, ergotamine derivatives, NSAIDs, opioids, and combination analgesic medications) should be used with caution or may need to be avoided in patients with specific comorbidities or contraindications.29 Ubrogepant, with its novel mechanism of action, represents a promising new acute treatment option that could provide benefit beyond the current migraine‐specific acute treatments. The favorable efficacy and tolerability profile of ubrogepant in the ACHIEVE trials23, 24 suggests that it may be a treatment option for patients who are inadequately managed on currently available treatments or for those who have contraindications to or intolerable side effects from other treatments.
Strengths and Limitations
Strengths of the ACHIEVE trials include their double‐blind, placebo‐controlled design and the collection of multiple measures of drug efficacy, including PROs that assessed multiple perspectives of drug efficacy (eg, pain, migraine‐associated symptoms, functional disability, satisfaction, and patient‐reported global impression of change). Most notably, the improvements in PROs of functional disability over time, satisfaction with study medication at 2 and 24 hours, and being “much better” or “very much better” based on the PGIC compared with placebo were replicated in both ACHIEVE trials. The effect of ubrogepant could be different in participants with migraine attacks of mild pain severity compared with attacks of moderate to severe pain severity. However, in keeping with regulatory and clinical trial guidelines of the International Headache Society,37 the trial design required participants to treat only moderate‐to‐severe attacks, so PRO efficacy data for mild migraine attacks are not available from the current trial. Additional analyses of potential interactions between baseline FDS severity and treatment response may be informative. The duration of follow‐up for the FDS was limited to 1‐8 hours after the initial dose. Ubrogepant has an elimination half‐life of approximately 5‐7 hours.22 Assessments of PRO measures through 48 hours and longer after treatment and during real‐world use can better capture the full extent of the impact of ubrogepant on functional disability and HRQoL. Additional PRO measures that further evaluate improvement in terms of work productivity or HRQoL in future phase 4 trials and real‐world effectiveness studies can provide more insights into the impact of this novel acute treatment.
Conclusions
In ACHIEVE I and II, ubrogepant significantly improved rates of freedom from and relief of migraine headache pain at 2 hours post initial dose vs placebo. In each trial and in a pooled analysis of the 2 trials, significantly higher proportions of ubrogepant‐treated patients reported being able to function normally at 2, 4, and 8 hours after the initial dose. Compared with placebo, ubrogepant‐treated patients also reported significantly higher rates of satisfaction with study medication and global improvement in migraine. The results of these patient‐centered outcomes are clinically meaningful and reinforce the potential benefits of ubrogepant in the acute treatment of migraine.
1. Statement of Authorship
Category 1
(a) Conception and Design
Anand R. Shewale, Hema N. Viswanathan, Richard B. Lipton, David W. Dodick
(b) Acquisition of Data
Anand R. Shewale, Hema N. Viswanathan, Sihui Zhao, Sung Yu, Joel Trugman
(c) Analysis and Interpretation of Data
Rashmi B. Halker Singh, Hema N. Viswanathan, Jessica Ailani, Anand R. Shewale, Sihui Zhao, Sung Yu, Joel Trugman, Richard B. Lipton, David W. Dodick
Category 2
(a) Drafting the Manuscript
Jessica Ailani, Richard B. Lipton, David W. Dodick
(b) Revising It for Intellectual Content
Rashmi B. Halker Singh, Jessica Ailani, Hema N. Viswanathan, Anand Shewale, Sihui Zhao, Sung Yu, Joel Trugman, Richard B. Lipton, David W. Dodick
Category 3
(a) Final Approval of the Completed Manuscript
Rashmi B. Halker Singh, Anand R. Shewale, Jessica Ailani, Hema N. Viswanathan, Sihui Zhao, Sung Yu, Joel Trugman, Richard B. Lipton, David W. Dodick
Supporting information
Acknowledgments
Thank you to all the participants and investigators who participated in these studies. These trials were sponsored by Allergan plc, Dublin, Ireland. Medical writing and editorial support were provided by Lela Creutz, PhD, and Lisa Feder, PhD, of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, and funded by Allergan plc. The opinions expressed in this article are those of the authors. The authors received no honorarium/fee or other form of financial support related to the development of this article.
Conflict of Interest: David W. Dodick reports the following conflicts: Personal fees: AEON, Alder BioPharmaceuticals, Allergan, Amgen, Amzak Health, Association of Translational Medicine, Autonomic Technologies, Axsome, Biohaven, Charleston Laboratories, Clexio, Daniel Edelman Inc., Dr Reddy’s Laboratories/Promius, electroCore LLC, Eli Lilly, eNeura, Equinox, Foresite Capital, Impel, Ipsen, Neurolief, Nocira, Novartis, Oppenheimer, Pieris, PSL Group Services, Revance, Salvia, Satsuma, Sun Pharma (India), Supernus, Teva, Theranica, University Health Network, Upjohn (Division of Pfizer), Vedanta, WL Gore, XoC, Zosano, and ZP Opco; Speaking fees: Amgen, Eli Lilly, Lundbeck, and Novartis Canada; Speakers bureaus: None; CME fees or royalty payments: Academy for Continued Healthcare Learning, Cambridge University Press, Catamount, Chameleon, Global Access Meetings, Global Life Sciences, Global Scientific Communications, Haymarket, HealthLogix, Medicom Worldwide, MedLogix Communications, Mednet, Miller Medical, Oxford University Press, PeerView, Universal Meeting Management, UpToDate (Elsevier), WebMD Health/Medscape, and Wolters Kluwer Health; Stock options: Aural Analytics, Epien, Healint, King‐Devick Technologies, Matterhorn, Nocira, Ontologics, Precon Health, Second Opinion/Mobile Health, and Theranica; Consulting without fee: Aural Analytics, Epien, Healint, Second Opinion/Mobile Health; Board of Directors: Epien, King‐Devick Technologies, Matterhorn, Ontologics, and Precon Health; Patent: 17189376.1‐1466:vTitle: Botulinum Toxin Dosage Regimen for Chronic Migraine Prophylaxis without fee; Research funding: American Migraine Foundation, Henry Jackson Foundation, PCORI, and US Department of Defense; Professional society fees or reimbursement for travel: American Academy of Neurology, American Brain Foundation, American Headache Society, American Migraine Foundation, Canadian Headache Society, and International Headache Society. Richard B. Lipton, MD, serves on the editorial boards of Neurology and Cephalalgia and as senior advisor to Headache. He receives research support from the NIH. He also receives support from the Migraine Research Foundation and the National Headache Foundation. He has reviewed for the NIA and NINDS; serves as consultant, advisory board member, or has received honoraria from Alder, Allergan, Amgen, Dr. Reddy’s, Electrocore, Eli Lilly, eNeura Therapeutics, GlaxoSmithKline, Merck, Novartis, Teva, and Vedanta. He receives royalties from Wolff’s Headache, 8th Edition (Oxford University Press, 2009) and Informa. He holds stock options in eNeura Therapeutics and Biohaven. Jessica Ailani, MD, reports the following conflicts from within the past 48 months: Allergan (consulting, speaking, clinical trial), Amgen (consulting, speaking), Alder (consulting, speaking), Avanir (speaking), Biohaven (consulting, clinical trial), Electrocore (consulting, speaking), Eli Lilly and Company (consulting, speaking, clinical trial), Neurodiem (honoraria), Neurology live (honoraria), Medscape (consulting), WebMD (honoraria), Promius (consulting, speaking), Impel (consulting), Revance (consulting), Satsuma (consulting, clinical trial), Zosano (consulting, clinical trial), Aptus (consulting), Miller Medical Communications (consulting), Alpha sites consulting (consulting), American Migraine Foundation (clinical trial), Theranica (clinical trial), Current Pain and Headache Reports (editor). Rashmi B. Halker Singh, MD, has received honoraria from WebMD/Medlink, Amgen, Biohaven, and Allergan. Anand R. Shewale, PhD, is a full‐time employee and stockholder of Allergan plc. Sihui Zhao, PhD, is a full‐time employee and stockholder of Allergan plc. Joel M. Trugman, MD, is a full‐time employee and stockholder of Allergan plc. Sung Yun Yu, BA, is a full‐time employee and stockholder of Allergan plc. Hema N. Viswanathan, PhD, is a full‐time employee and stockholder of Allergan plc.
Funding: This study was sponsored by Allergan plc, Dublin, Ireland.
ClinicalTrials.gov identifiers: ACHIEVE I, NCT02828020; ACHIEVE II, NCT02867709.
References
- 1. Lipton RB, Bigal ME, Diamond M, Freitag F, Reed ML, Stewart WF. Migraine prevalence, disease burden, and the need for preventive therapy. Neurology. 2007;68:343‐349. [DOI] [PubMed] [Google Scholar]
- 2. Headache Classification Committee of the International Headache Society . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 3. Brandes JL. Global trends in migraine care: Results from the MAZE survey. CNS Drugs. 2002;16(Suppl. 1):13‐18. [DOI] [PubMed] [Google Scholar]
- 4. Lanteri‐Minet M, Duru G, Mudge M, Cottrell S. Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: A systematic review. Cephalalgia. 2011;31:837‐850. [DOI] [PubMed] [Google Scholar]
- 5. Bussone G, Usai S, Grazzi L, Rigamonti A, Solari A, D'Amico D. Disability and quality of life in different primary headaches: Results from Italian studies. Neurol Sci. 2004;25(Suppl. 3):S105‐S107. [DOI] [PubMed] [Google Scholar]
- 6. Blumenfeld AM, Varon SF, Wilcox TK, et al. Disability, HRQoL and resource use among chronic and episodic migraineurs: Results from the International Burden of Migraine Study (IBMS). Cephalalgia. 2011;31:301‐315. [DOI] [PubMed] [Google Scholar]
- 7. Leonardi M, Raggi A, Bussone G, D'Amico D. Health‐related quality of life, disability and severity of disease in patients with migraine attending to a specialty headache center. Headache. 2010;50:1576‐1586. [DOI] [PubMed] [Google Scholar]
- 8. Freitag FG. The cycle of migraine: Patients' quality of life during and between migraine attacks. Clin Ther. 2007;29:939‐949. [DOI] [PubMed] [Google Scholar]
- 9. Serrano D, Manack AN, Reed ML, Buse DC, Varon SF, Lipton RB. Cost and predictors of lost productive time in chronic migraine and episodic migraine: Results from the American Migraine Prevalence and Prevention (AMPP) study. Value Health. 2013;16:31‐38. [DOI] [PubMed] [Google Scholar]
- 10. Hawkins K, Wang S, Rupnow M. Direct cost burden among insured US employees with migraine. Headache. 2008;48:553‐563. [DOI] [PubMed] [Google Scholar]
- 11. D'Amico D, Grazzi L, Curone M, et al. Difficulties in work activities and the pervasive effect over disability in patients with episodic and chronic migraine. Neurol Sci. 2015;36(Suppl. 1):9‐11. [DOI] [PubMed] [Google Scholar]
- 12. Raggi A, Giovannetti AM, Quintas R, et al. A systematic review of the psychosocial difficulties relevant to patients with migraine. J Headache Pain. 2012;13:595‐606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lipton RB, Buse DC, Serrano D, Holland S, Reed ML. Examination of unmet treatment needs among persons with episodic migraine: Results of the American Migraine Prevalence and Prevention (AMPP) study. Headache. 2013;53:1300‐1311. [DOI] [PubMed] [Google Scholar]
- 15. Lipton RB, Munjal S, Buse DC, et al. Unmet acute treatment needs from the 2017 Migraine in America Symptoms and Treatment Study. Headache. 2019;59:1310‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lipton RB, Hutchinson S, Ailani J, Reed ML, Fanning KM. Patterns and characterization of acute prescription headache medication use: Results from the CaMEO study [poster] Presented at: Annual Scientific Meeting of the American Headache Society; June 28‐July 1, 2018; San Francisco, CA. [Google Scholar]
- 17. Lipton RB, Reed ML, Kurth T, Fanning KM, Buse DC. Framingham‐based cardiovascular risk estimates among people with episodic migraine in the US population: Results from the American Migraine Prevalence and Prevention (AMPP) Study. Headache. 2017;57:1507‐1521. [DOI] [PubMed] [Google Scholar]
- 18. Marmura MJ, Silberstein SD, Schwedt TJ. The acute treatment of migraine in adults: The American Headache Society evidence assessment of migraine pharmacotherapies. Headache. 2015;55:3‐20. [DOI] [PubMed] [Google Scholar]
- 19. Goadsby PJ, Holland PR, Martins‐Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: A disorder of sensory processing. Physiol Rev. 2017;97:553‐622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Edvinsson L, Warfvinge K. Recognizing the role of CGRP and CGRP receptors in migraine and its treatment. Cephalalgia. 2019;39:366‐373. [DOI] [PubMed] [Google Scholar]
- 21. Charles A, Pozo‐Rosich P. Targeting calcitonin gene‐related peptide: A new era in migraine therapy. Lancet. 2019;394:1765‐1774. [DOI] [PubMed] [Google Scholar]
- 22. Ubrelvy [package insert]. Madison, NJ: Allergan USA, Inc.; 2019. [Google Scholar]
- 23. Dodick DW, Lipton RB, Ailani J, et al. Ubrogepant for the treatment of migraine. N Engl J Med. 2019;381:2230‐2241. [DOI] [PubMed] [Google Scholar]
- 24. Lipton RB, Dodick DW, Ailani J, et al. Effect of ubrogepant versus placebo on pain and the most bothersome associated symptom in the acute treatment of migraine: The ACHIEVE II randomized clinical trial. JAMA. 2019;322:1887‐1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Headache Classification Committee of the International Headache Society . The International Classification of Headache Disorders, 3rd edition (beta version). Cephalalgia. 2013;33:629‐808. [DOI] [PubMed] [Google Scholar]
- 26. Cady RK, McAllister PJ, Spierings EL, et al. A randomized, double‐blind, placebo‐controlled study of breath powered nasal delivery of sumatriptan powder (AVP‐825) in the treatment of acute migraine (The TARGET Study). Headache. 2015;55:88‐100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Silberstein SD. Practice parameter: Evidence‐based guidelines for migraine headache (an evidence‐based review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology. 2000;55:754‐762. [DOI] [PubMed] [Google Scholar]
- 28. Matchar DB, Young WB, Rosenberg JH, et al. Evidence‐based guidelines for migraine headache in the primary care setting: Pharmacological management of acute attacks. Am Acad Neurol. 2000. Available at: http://www.aan.com/professionals/practice/pdfs/gl0087.pdf. Accessed March 20, 2015. [Google Scholar]
- 29. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache. 2019;59:1‐18. [DOI] [PubMed] [Google Scholar]
- 30. Lipton RB, Dodick DW, Ailani J, et al. Efficacy, safety, and tolerability of ubrogepant for the acute treatment of migraine: Results from a single attack phase III study, ACHIEVE II [abstract PS111LB]. Headache. 2018;58:1315‐1316. [Google Scholar]
- 31. Williams GS. Triptan use and discontinuation: Results from the MAST study. Neurol Rev. 2018;26:30. [Google Scholar]
- 32. Bigal M, Rapoport A, Aurora S, Sheftell F, Tepper S, Dahlof C. Satisfaction with current migraine therapy: Experience from 3 centers in US and Sweden. Headache. 2007;47:475‐479. [DOI] [PubMed] [Google Scholar]
- 33. Colman SS, Brod MI, Krishnamurthy A, Rowland CR, Jirgens KJ, Gomez‐Mancilla B. Treatment satisfaction, functional status, and health‐related quality of life of migraine patients treated with almotriptan or sumatriptan. Clin Ther. 2001;23:127‐145. [DOI] [PubMed] [Google Scholar]
- 34. Landy SH, Cady RK, Nelsen A, White J, Runken MC. Consistency of return to normal function, productivity, and satisfaction following migraine attacks treated with sumatriptan/naproxen sodium combination. Headache. 2014;54:640‐654. [DOI] [PubMed] [Google Scholar]
- 35. Lainez MJ, Galvan J, Heras J, Vila C. Crossover, double‐blind clinical trial comparing almotriptan and ergotamine plus caffeine for acute migraine therapy. Eur J Neurol. 2007;14:269‐275. [DOI] [PubMed] [Google Scholar]
- 36. Cady RK, Munjal S, Cady RJ, Manley HR, Brand‐Schieber E. Randomized, double‐blind, crossover study comparing DFN‐11 injection (3 mg subcutaneous sumatriptan) with 6 mg subcutaneous sumatriptan for the treatment of rapidly‐escalating attacks of episodic migraine. J Headache Pain. 2017;18:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tfelt‐Hansen P, Block G, Dahlof C, et al. Guidelines for controlled trials of drugs in migraine: Second edition. Cephalalgia. 2000;20:765‐786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


