Abbreviations
- b‐GGT
big GGT
- cDNA
complementary DNA
- f‐GGT
free GGT
- GGT
γ‐glutamyltransferase
- GGT1
γ‐glutamyltransferase 1
Assay of γ‐glutamyltransferase (GGT) activity is a widely used test to indicate and monitor liver and biliary tract injury. We observed dominant inheritance of highly elevated plasma GGT levels, designated GGTemia, in two unrelated families. Neither clinical symptoms nor alterations of GGT substrates were associated with GGTemia. A plasma GGT fraction pattern distinguishes this trait from common liver diseases. Heterozygous γ‐glutamyltransferase 1 (GGT1) mutations that disrupt the GGT1 transmembrane domain were identified. We establish GGTemia as a benign condition; GGT1 mutation testing can prevent repeated and invasive diagnostic workup in such patients.
Case Series
Dominant inheritance of isolated GGT plasma elevations in the range of 2,500‐9,600 U/L (reference 9‐55 U/L)—GGTemia—was observed in two families (Fig. 1A); part of family 1 was reported.1 The index patients were identified by routine testing; congenital onset and lifelong persistence of this trait were suggested by observing GGTemia in a 4‐year‐old and a 70‐year‐old patient. GGTemia was not associated with clinical complaints (Supporting Information). The persistently high plasma GGT activity was not associated with altered levels of known GGT substrates: we observed normal levels of amino acids, ammonia, and cysteinyl leukotrienes in plasma, total glutathione in whole blood, and leukotriene E4 in urine.
Figure 1.
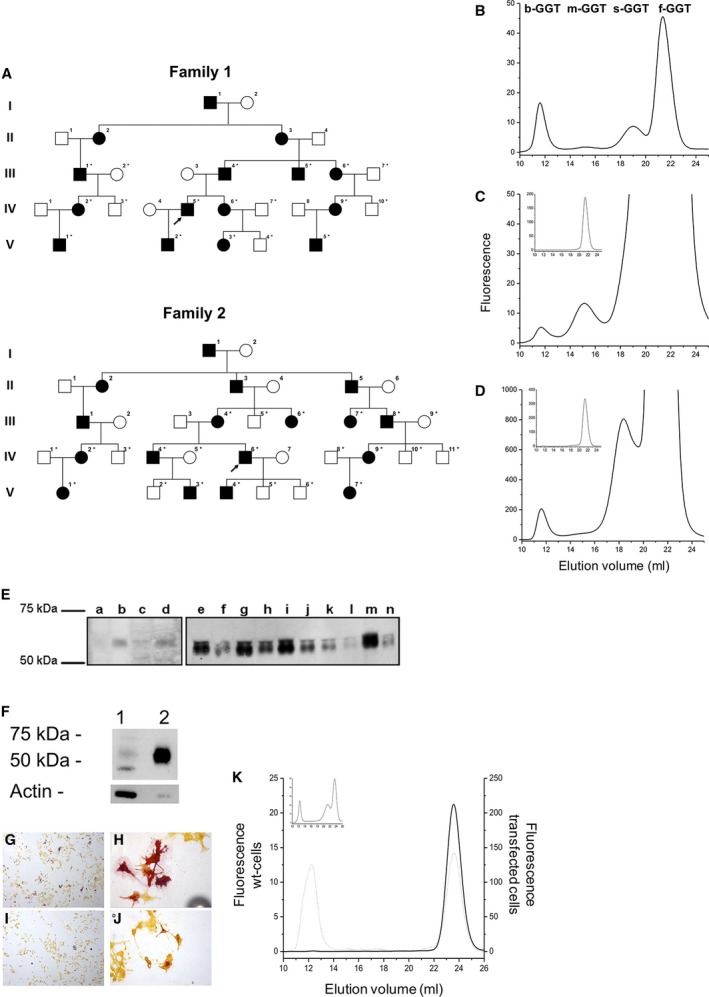
Dominantly inherited GGTemia in two unrelated families shows a characteristic pattern of GGT plasma fractions, an increase of plasma GGT protein, and loss from expressing cells. (A) Family 1 is from Italy, and family 2 is from Slovakia. Squares denote male individuals, circles denote female individuals, filled symbols indicate GGTemia trait carriers. Asterisks denote individuals available for mutation segregation analysis. Arrows denote individuals in whom whole‐genome sequencing was performed. (B‐D) GGT fraction analysis: (B) control, (C) GGTemia (inserted graph: 100‐fold diluted serum), (D) GGTemia and alcoholism (inserted graph: 100‐fold diluted serum). (E) GGT1 serum western blot analysis in family 1. Lanes a‐d, control members of the family (exposition time 30 minutes); lanes e‐n, GGTemia (exposition 3 minutes). (F) Cell homogenate western blot analysis. Lane 1, c21‐Leu15Arg (70 μg protein, 0.6 mU GGT activity); lane 2, c21 wild type (8 μg protein, 0.6 mU GGT activity). (G,H) Cytochemical staining for GGT activity is present in cells transfected with wild‐type GGT1 cDNA (magnification ×20 and ×40) and absent in cells transfected with mutated GGT cDNA (I, J; magnification ×20 and ×40). (K) GGT activity in culture media of cells transfected with wild‐type (dotted line) or mutated (continuous line) GGT cDNA. The inserted graph shows a normal serum sample. Abbreviations: m‐GGT, medium GGT; s‐GGT, small GGT; wt, wild type.
An unprecedented GGT fraction pattern was found in GGTemia: all four recognized GGT fractions were present; big‐GGT (b‐GGT) activity values were in the reference range, but medium, small, and free GGT (f‐GGT) activities were highly elevated over the higher reference limits,2 with f‐GGT activity representing about 97% of total activity (Supporting Table S1; Fig. 1B,C). A mixed pattern of GGTemia and alcoholism was seen in one individual (Fig. 1D).
We first mapped the trait locus, and whole‐genome sequencing identified heterozygous GGT1 variants in families 1 and 2: a missense mutation, c.44T>G (p.Leu15Arg), and an inframe deletion, c.28_54del (p.Leu10_Val18del) (Supporting Fig. S1). These variants were considered pathogenic as they (1) segregated with GGTemia in families, (2) were absent from control populations, and (3) were predicted to affect protein structure (Supporting Information). GGT1 complementary DNA (cDNA) sequencing and real‐time expression analyses revealed equal expression of mutant and wild‐type alleles, indicating that GGTemia is not caused by increased transcription. However, immunoblotting showed a highly increased serum GGT protein amount in patients (Fig. 1E).
The inframe deletion removes half of the N‐terminal GGT1 transmembrane domain, and transient expression of the p.Leu15Arg mutation in c21 cells resulted in nearly absent cell‐attached GGT1 expression and higher GGT activity in the culture medium compared with wild type (Fig. 1F), as was confirmed by cytochemical staining of cells expressing mutant (Fig. 1G,H) and wild type (Fig. 1I,J) GGT1. c21‐p.Leu15Arg cells secreted almost exclusively f‐GGT, while wild‐type cells secreted similar quantities of b‐GGT and f‐GGT (Fig. 1K).
Discussion
We show that GGTemia is a benign condition that represents a differential diagnosis of elevated GGT plasma levels. The identified mutations disrupt the GGT1 transmembrane domain so that the enzymes retain their activities but are not attached anymore to the plasma membrane. GGT1 mutation and plasma GGT fraction analyses should be considered in patients with unexplained elevation of GGT levels. Prior to our study, only one pathogenic GGT1 variant was reported, associated with total loss of GGT activity and intellectual disability.3 The presence of a GGT1 wild‐type copy in GGTemia likely precludes any symptoms of GGT deficiency.
The GGT1 protein is conserved in all prokaryotes and eukaryotes, emphasizing its critical role in all organisms; and serum GGT levels have been linked to an array of chronic diseases.4 Plasma GGT is supposed to originate mostly from liver, and a distinct plasma GGT fraction pattern is observed for GGTemia and several liver diseases.2 b‐GGT is contained in vesicles, which are shed from the plasma membrane in both bile and plasma5 and carry GGT as a marker of its cellular origin. The lower‐weight GGT fractions supposedly arise from modifications of the b‐GGT fraction.5 f‐GGT represents a free‐soluble form of the enzyme that lacks the N‐terminal anchoring peptide, which is primarily produced from the mutant alleles in GGTemia.
Author Contributions
A.D.G., M.F., S.R., and A.R.J. were responsible for study concept and design; S.R., M.D., E.R.‐D., G.Z., P.P.P., T.M., and A.R.J. were responsible for patient recruitment and characterization; R.R., A.C., S.S.‐B., E.M., D.H., and A.R.J. were responsible for data acquisition and analysis; A.P., A.P., M.E., H.W., H.Z., H.T., P.P.P., and T.M. were responsible for data analysis and conceptual advice; A.R.J. was responsible for manuscript preparation; all authors provided input and critical revision to the manuscript and approved the final version.
Supporting information
Supported by Jubiläumsfonds der Österreichischen Nationalbank (16678) and the Department of Innovation, Research and University of the Autonomous Province of Bolzano/Bozen, Italy.
Potential conflict of interest: Nothing to report.
References
- 1. Bibas M, Zampa G, Procopio A, Guaitolini R. High serum gamma‐glutamyltransferase concentrations in a family. N Engl J Med 1994;330:1832‐1833. [DOI] [PubMed] [Google Scholar]
- 2. Franzini M, Fornaciari I, Fierabracci V, Elawadi HA, Bolognesi V, Maltinti S, et al. Accuracy of b‐GGT fraction for the diagnosis of non‐alcoholic fatty liver disease. Liver Int 2012;32:629‐634. [DOI] [PubMed] [Google Scholar]
- 3. Darin N, Leckstrom K, Sikora P, Lindgren J, Almen G, Asin‐Cayuela J. Gamma‐glutamyl transpeptidase deficiency caused by a large homozygous intragenic deletion in GGT1. Eur J Hum Genet 2018;26:808‐817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kunutsor SK. Gamma‐glutamyltransferase‐friend or foe within? Liver Int 2016;36:1723‐1734. [DOI] [PubMed] [Google Scholar]
- 5. Fornaciari I, Fierabracci V, Corti A, Aziz Elawadi H, Lorenzini E, Emdin M, et al. Gamma‐glutamyltransferase fractions in human plasma and bile: characteristic and biogenesis. PLoS One 2014;9:e88532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


