Abstract
Background
The cardiovascular safety profile of biologic therapies used for psoriasis is unclear.
Objectives
To compare the risk of major cardiovascular events (CVEs; acute coronary syndrome, unstable angina, myocardial infarction and stroke) in patients with chronic plaque psoriasis treated with adalimumab, etanercept or ustekinumab in a large prospective cohort.
Methods
Prospective cohort study examining the comparative risk of major CVEs was conducted using the British Association of Dermatologists Biologics and Immunomodulators Register. The main analysis compared adults with chronic plaque psoriasis receiving ustekinumab with tumour necrosis‐α inhibitors (TNFi: etanercept and adalimumab), whilst the secondary analyses compared ustekinumab, etanercept or methotrexate against adalimumab. Hazard ratios (HRs) with 95% confidence intervals (CIs) were calculated using overlap weights by propensity score to balance baseline covariates among comparison groups.
Results
We included 5468 biologic‐naïve patients subsequently exposed (951 ustekinumab; 1313 etanercept; and 3204 adalimumab) in the main analysis. The secondary analyses also included 2189 patients receiving methotrexate. The median (p25–p75) follow‐up times for patients using ustekinumab, TNFi, adalimumab, etanercept and methotrexate were as follows: 2.01 (1.16–3.21), 1.93 (1.05–3.34), 1.94 (1.09–3.32), 1.92 (0.93–3.45) and 1.43 (0.84–2.53) years, respectively. Ustekinumab, TNFi, adalimumab, etanercept and methotrexate groups had 7, 29, 23, 6 and 9 patients experiencing major CVEs, respectively. No differences in the risk of major CVEs were observed between biologic therapies [adjusted HR for ustekinumab vs. TNFi: 0.96 (95% CI 0.41–2.22); ustekinumab vs. adalimumab: 0.81 (0.30–2.17); etanercept vs. adalimumab: 0.81 (0.28–2.30)] and methotrexate against adalimumab [1.05 (0.34–3.28)].
Conclusions
In this large prospective cohort study, we found no significant differences in the risk of major CVEs between three different biologic therapies and methotrexate. Additional studies, with longer term follow‐up, are needed to investigate the potential effects of biologic therapies on incidence of major CVEs.
Short abstract
Linked Commentary: K. Kridin and A.D. Cohen. J Eur Acad Dermatol Venereol 2020; 34: 668–669. https://doi.org/10.1111/jdv.16345.
Introduction
Psoriasis is a common, chronic inflammatory skin disease affecting over 125 million people worldwide.1 The prevalence of psoriasis varies between countries (0.91–8.5%), and recent estimates suggest that almost 3% of the UK population are affected by the disease.2, 3 Cardiovascular (CV) comorbidities are common among patients with psoriasis.4 Moreover, CV risk factor screening of adult patients with psoriasis in primary care has found a high proportion of patients being sub‐optimally treated for known CV risk factors.5 This can contribute to an increased risk of major CV events (CVEs) in patients with psoriasis.
Biologic therapies are increasingly used for the treatment of moderate–severe psoriasis, but their CV safety profile is still unclear. In recent years, concerns have been raised regarding an increased CV risk due to the use of anti‐interleukin (IL)‐12/23 agents after a number of major adverse CVEs s [MACEs; myocardial infarction (MI), cerebrovascular accident or CV death] occurred in patients receiving briakinumab [anti‐IL‐12/23 agent; Five patients experiencing major adverse CVEs (onset ranged from 21–55 days) during the induction phase and two patients experiencing the events on day 131 and 225 during the maintenance phase] which in part resulted in the discontinuation of the development of this treatment.6, 7, 8 A recent meta‐analysis of randomized controlled trials (RCTs) suggested that there was no significant difference in the risk of MACEs between licensed biologic therapies and placebo.9 However, the risks were examined over short periods (10–30 weeks) and participants included in RCTs tend to have fewer comorbidities than psoriasis patients in a real‐world setting.9, 10 Several cohort studies have examined the impact of biologic therapies on CVEs in patients with psoriasis involving a range of different reference treatments including non‐biologic, non‐systemic therapies (topical therapy, phototherapy and climate therapy) or methotrexate.11, 12, 13, 14, 15 These therapies are typically recommended for patients before receiving biologic therapies. To assess the association between CVEs and treatments, participants in treatment and reference groups should have a similar severity of psoriasis since this may influence the development of CVEs.16 Ideally, biologic therapies should be directly compared.
The objectives of this study were to directly compare the risk of major CVEs (acute coronary syndrome, unstable angina, MI and stroke) in adult patients with chronic plaque psoriasis under routine care treated with adalimumab, etanercept or ustekinumab in a large prospective cohort using the British Association of Dermatologists Biologics and Immunomodulators Register (BADBIR).
Methods
The BADBIR is a large prospective cohort study examining the long‐term safety of biologic therapies in patients with psoriasis. It compares a cohort of psoriasis patients treated with biologic therapies and a cohort of those treated with conventional systemic therapies (e.g. methotrexate). Data have been collected on patients with moderate–severe psoriasis being treated at 160 secondary care dermatology centres across the UK and the Republic of Ireland since September 2007. BADBIR was approved by the NHS Research Ethics Committee North West England (reference 07/MRE08/9) in March 2007, and all patients have provided written informed consent for participation. Further details regarding study design of BADBIR has been published previously.17
Baseline assessments
Baseline data collected at enrolment include patient demographic characteristics, comorbidities, anthropometric data, drug therapies and clinical data such as type and severity of psoriasis (Psoriasis Area and Severity Index; PASI) by healthcare professionals using an online database, whilst lifestyle information such as smoking and alcohol consumption was collected directly from patients using a questionnaire.
Follow‐up assessments
Data are collected every 6 months for the first 3 years and then annually. These include information on changes in drug therapies, measures of disease severity, hospitalization and details of adverse events (AEs) including the outcomes of interest of this study. Patient death details are derived from the BADBIR register via linkage with the Office of National Statistics mortality records. AEs are coded using the Medical Dictionary for Regulatory Activities (MedDRA) system.18
Study population and exposure
Patients who enrolled in the BADBIR from September 2007 to October 2016 and had at least 6 months of follow‐up data following initiation of treatment were selected for this study. Biologic‐naïve patients aged at least 18 years old with chronic plaque psoriasis who had no prior history of major CVEs were selected for the inclusion in this cohort study. For the main analysis, patients receiving the first‐line originator anti‐IL‐12/23 agent (ustekinumab) were compared with TNFi (etanercept or adalimumab) as the reference group. For the secondary analyses, patients receiving first‐line adalimumab (the referent group) were compared with ustekinumab, etanercept or methotrexate.
Outcome of interest and ascertainment
The outcome of interest was fatal or non‐fatal major CVEs [acute coronary syndrome, unstable angina, MI or stroke (Table S1, Supporting Information) provides the relevant MedDRA outcome codes]. All relevant MedDRA codes or descriptions of events were identified by WR. Both codes and descriptions were independently reviewed by a clinician with extensive experience in managing CV disease (MKR) in order to ascertain the final outcome of the study. To validate all serious outcomes, the BADBIR staff members asked study sites to confirm these events. Moreover, patients experiencing acute coronary syndrome, unstable angina and MI were also collected information on cardio marker, electrocardiogram, previous history of CV diseases, the use of thrombolysis and angioplasty and cardiac intervention, whilst patients experiencing stroke were also collected information on type of stroke, computed tomography scan and history of thrombolysis and atrial fibrillation in order to confirm these events.
Data analysis
Patients were observed from the date of receiving therapy to developing the first major CVE or the earliest date of change in treatment (changing to other biologic therapy in the biologic cohorts or starting a biologic therapy in the methotrexate cohort); end of recorded data in the BADBIR; death; or end of the study follow‐up (30 September 2016). Discontinuation of treatment was defined as a gap in a regimen for more than 90 days. We examined the risk of major CVEs occurring over two periods: (i) whilst exposed to treatment; and (ii) extending the exposure effect window until 90 days after the last dose. Planned secondary analyses included direct comparisons between the individual biologic therapies and users of methotrexate.
Descriptive statistics were used to analyse baseline patient characteristics. Frequency (%) and median values [25th percentile (p25)‐75th percentile (p75)] were calculated for categorical and continuous variables, respectively. To control for imbalances in patient characteristics between cohorts, we calculated an exposure‐specific propensity score as the predicted probability of receiving the treatment of interest conditional upon the subjects’ baseline covariates using logistic regression models for the primary analysis and multinomial logistic regression models for the sensitivity analyses. We included the following covariates: baseline PASI (the score which was before and closest to the start of the treatment exposures within 6 months), smoking status (ever/never), current alcohol drinking (yes/no), alcohol consumption (units/week), obesity (≥30 kg/m2), age, gender, history of psoriatic arthritis (PsA), hypertension, diabetes, dyslipidemia, angina, previous treatment with ciclosporin, acitretin, fumaric acid esters and methotrexate. Covariate balance between the cohorts before and after propensity score overlap weighting was assessed using the expected percentage bias which is the difference in the outcome owing to the imbalance between each covariate taking into account the strength of the association between each covariate and the outcome. A maximum bias of 5% in either direction was considered an acceptable threshold. After generating propensity scores, overlap weights which were proportional to the probability of patients being assigned to the reference groups were calculated for only patients having predicted probabilities within the common support range. The common support range was defined as propensity scores of the treated groups overlapping the propensity scores of the reference groups.
Multiple imputation was used to address missing data on baseline PASI score, smoking status, current alcohol drinking, alcohol consumption and obesity using chained equations of 20 cycles to reduce bias. This method preserved the variability and uncertainty of missing data and avoids the loss of patients due to missing data and bias when compared with complete case analysis.19 The imputation model consisted of exposures, start year of exposure, log of censoring time for the outcome occurring during drug therapy; and during the extended window period, and whether patients experienced the outcomes during drug therapy; and during the extended window period, history of other heart diseases, concomitant drug therapies including ciclosporin, acitretin, fumaric acid esters and methotrexate; and the other covariates included in the propensity score model for the main analysis whilst the sensitivity analyses did not include concomitant methotrexate.
For each comparison (ustekinumab vs. TNFi for the primary analysis; and ustekinumab, etanercept or methotrexate vs. adalimumab for the secondary analyses) and for all outcomes, we calculated incidence rates (IRs), IR ratios, unadjusted, age and sex adjusted and overlap weighed hazard ratio (HRs) with 95% confidence intervals (CIs). We assessed the proportional hazards assumption by examining Schoenfeld residuals and confirming that it was not violated. All analyses were performed using Stata 14 (StataCorp LP, College Station, Texas, USA).
Results
A total of 5468 patients were included in the main analysis [anti‐IL‐12/23 agent (ustekinumab): 951 and TNFi (adalimumab and etanercept): 4517; Fig. 1]. Patients in the ustekinumab group were more likely to be obese, but less likely to have either a history of PsA, currently drink alcohol or concomitantly receive methotrexate therapy, as shown in Table 1. The median (p25 and p75) follow‐up times for patients taking individual therapies were as follows: ustekinumab 1.76 (0.92–2.96) years and TNFi 1.69 (0.81–3.10) years for the analysis of events occurring during drug therapy; and ustekinumab 2.01 (1.16–3.21) years and TNFi 1.93 (1.05–3.34) years for the analysis of events occurring during the extended exposure window period.
Figure 1.
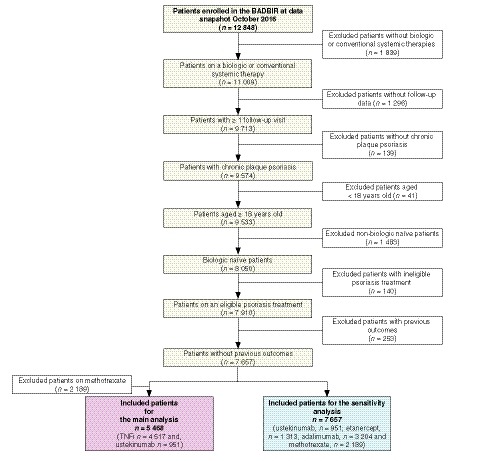
Patient selection.
Table 1.
Baseline characteristics of patients receiving anti‐interleukin‐12/23 agent (ustekinumab) or TNFi (etanercept and adalimumab)
| Characteristics | Ustekinumab | TNFi |
|---|---|---|
| Number of patients (N = 5468) | 951 | 4517 |
| Age (years; N = 5468) | 45 (35–54) (n = 951) | 44 (35.2–53) (n = 4517) |
| Sex, male (N = 5468) | 590 (62.0) (n = 951) | 2645 (58.6) (n = 4517) |
| Ethnicity, white (N = 5461) | 853 (89.7) (n = 951) | 4157 (92.2) (n = 4510) |
| BMI (kg/m2; N = 4983) | 30.3 (26.2–35.7) (n = 851) | 29.4 (25.9–33.8) (n = 4132) |
| Obese (BMI ≥30 kg/m2) | 441 (51.8) (n = 851) | 1922 (46.5) (n = 4132) |
| Ever smoke (yes/no; N = 4885) | 599 (66.6) (n = 899) | 2541 (63.8) (n = 3986) |
| Disease durations (years; N = 5417) | 19 (11–30) (n = 943) | 20 (12–29) (n = 4474) |
| PASI score (N = 4833) | 14.6 (11.2–19.2) (n = 845) | 14.1 (11.0–19.3) (n = 3988) |
| DLQI (N = 2949) | 18 (12–24) (n = 460) | 18 (13–24) (n = 2489) |
| Comorbidities | ||
| No comorbidities | 315 (33.1) | 1356 (30.0) |
| Psoriatic arthritis | 134 (14.1) | 1035 (22.9) |
| Hypertension | 241 (25.3) | 1103 (24.4) |
| Diabetes mellitus | 98 (10.3) | 357 (7.9) |
| Dyslipidemia | 98 (10.3) | 435 (9.6) |
| Angina | 20 (2.1) | 57 (1.3) |
| Other heart diseases | 23 (2.4) | 80 (1.8) |
| Other comorbidities | 512 (53.8) | 2422 (53.6) |
| Current alcohol drinking ( N = 4899) | 593 (65.7) (n = 903) | 2854 (71.4) (n = 3996) |
| Alcohol units per week in patients consuming alcohol (N = 3382) | 8 (3–15) (n = 584) | 9 (3–16) (n = 2798) |
| Previous treatment of conventional systemic therapies | ||
| Methotrexate | 667 (70.1) | 3124 (69.2) |
| Ciclosporin | 540 (56.8) | 2585 (57.2) |
| Acitretin | 399 (42.0) | 2008 (44.5) |
| Fumaric acid esters | 165 (17.4) | 879 (19.5) |
| Concomitant therapies during drug therapy | ||
| Methotrexate | 120 (12.6) | 909 (20.1) |
| Ciclosporin | 71 (7.5) | 455 (10.1) |
| Acitretin | 28 (2.9) | 163 (3.6) |
| Fumaric acid esters | 13 (1.4) | 79 (1.8) |
| Concomitant therapies during active use of the exposure or window period | ||
| Methotrexate | 121 (12.7) | 946 (20.9) |
| Ciclosporin | 74 (7.8) | 491 (10.9) |
| Acitretin | 29 (3.1) | 179 (4.0) |
| Fumaric acid esters | 13 (1.4) | 87 (1.9) |
Data are n (%) or median (25th percentile‐75th percentile).
BMI, body mass index; DLQI, Dermatology Life Quality Index; PASI, Psoriasis Area Severity Index; TNFi, tumour necrosis factor‐α inhibitors.
Seven patients in the ustekinumab group experienced a major CVE during treatment with no additional patients experiencing such an outcome within 90 days after the last dose. For the TNFi cohort, 24 and 29 patients experienced major CVEs during drug therapy and during the extended exposure window period, respectively. The median times to onset of the major CVEs in both groups were about 1 year during either drug therapy or the extended exposure window period (Table 2).
Table 2.
Incidence rates and incidence rate ratios among patients receiving anti‐interleukin‐12/23 agent (ustekinumab) or TNFi (etanercept and adalimumab)
| Ustekinumab | TNFi | |
|---|---|---|
| Outcome during drug therapy | ||
| Total patient‐years | 1936.56 | 9757.22 |
| Patient‐years of follow‐up (median, p25–p75) | 1.76 (0.92–2.96) | 1.69 (0.81–3.10) |
| Number of major cardiovascular events | 7 | 24 |
| Incidence rate per 1000 patient‐years (95% CI) | 3.61 (1.72–7.58) | 2.46 (1.65–3.67) |
| Incidence rate ratio | 1.47 (0.53–3.52) | Reference |
| Duration between the start of exposure to development of the outcome (years; median, p25–p75; only patients experiencing the outcome) | 1.06 (0.59–1.94) | 1.19 (0.50–2.14) |
| Outcome during drug therapy plus grace period (90 days) | ||
| Total patient‐years | 2167.61 | 10 858.90 |
| Patient‐years of follow‐up (median, p25–p75) | 2.01 (1.16–3.21) | 1.93 (1.05–3.34) |
| Number of major cardiovascular events | 7 | 29 |
| Incidence rate per 1000 patient‐years (95% CI) | 3.23 (1.54–6.77) | 2.67 (1.86–3.84) |
| Incidence rate ratio | 1.21 (0.45–2.82) | Reference |
| Duration between the start of exposure to development of the outcome (years; median, p25–p75; only patients experiencing the outcome) | 1.06 (0.59–1.94) | 1.06 (0.47–1.98) |
95% CI, 95% confidence interval; p25–p75, 25th percentile‐75th percentile; TNFi, tumour necrosis factor‐α inhibitors.
Incidence rates of major cardiovascular events
The IRs of major CVEs associated with ustekinumab therapy for both periods were numerically but not statistically significantly higher than those associated with TNFi. Crude IRs (95% CI) in the ustekinumab and TNFi groups were 3.61 (1.72–7.58) and 2.46 (1.65–3.67) per 1000 patient‐years, respectively, for the outcome during drug therapy; and 3.23 (1.54–6.77) and 2.67 (1.86–3.84) per 1000 patient‐years, respectively, for the extended exposure window period (Table 2).
Comparative risks of major cardiovascular events
The unadjusted and age‐sex adjusted HRs showed no difference in the risk of major CVEs between patients treated with ustekinumab and TNFi therapies. In the propensity score‐adjusted analysis, there was similarly no difference in the risk of major CVEs occurring during both periods (Fig. 2a). The baseline characteristics of the treatment cohorts were comparable after applying the overlap weights using the propensity score method as shown in Fig. 3.
Figure 2.
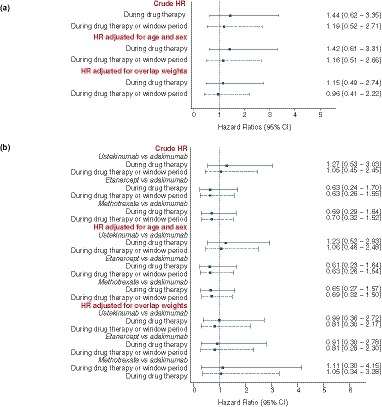
Crude and adjusted hazard ratios (95% confidence interval) for major cardiovascular events associated with different psoriasis therapies. (a) Comparison of anti‐interleukin‐12/23 agent (ustekinumab) with tumour necrosis factor‐α inhibitors (referent group). (b) Comparisons of ustekinumab, etanercept or methotrexate with adalimumab (referent group).
Figure 3.
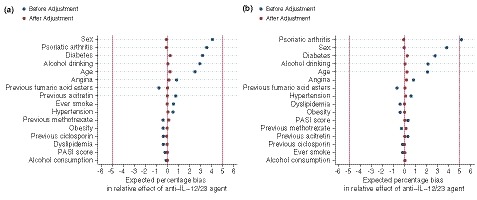
Distribution of confounders between anti‐interleukin‐12/23 agent (ustekinumab) and tumour necrosis factor‐α inhibitors (referent) patients before creating propensity score and after overlap weighting by propensity score. (a) Outcomes occurring during drug therapy. (b) Outcomes occurring during drug therapy plus grace period (90 days).
Secondary analyses comparing the risk of major CVEs associated with individual therapies
A total of 7657 patients were included in the secondary analyses (ustekinumab, 951; etanercept, 1313; methotrexate, 2189 and: adalimumab, 3204). The proportions of patients with PsA in the ustekinumab (14.1%) and methotrexate (8.9%) groups were lower than in the adalimumab (23.3%) or etanercept (21.9%) groups, as shown in Table S2 (Supporting Information). The ustekinumab, etanercept and adalimumab cohorts had longer durations of follow‐up than the methotrexate group (Table 3).
Table 3.
Incidence rates and incidence rate ratios among patients receiving ustekinumab, etanercept, methotrexate or adalimumab
| Ustekinumab | Etanercept | Methotrexate | Adalimumab | |
|---|---|---|---|---|
| Outcome during drug therapy | ||||
| Total patient‐years | 1936.56 | 2905.99 | 3650.81 | 6851.23 |
| Patient‐years of follow‐up (median, p25–p75) | 1.76 (0.92–2.96) | 1.67 (0.69–3.20) | 1.18 (0.59–2.29) | 1.69 (0.84–3.07) |
| Number of major cardiovascular events | 7 | 5 | 7 | 19 |
| Incidence rate per 1000 patient‐years (95% CI) | 3.61 (1.72–7.58) | 1.72 (0.72–4.13) | 1.92 (0.91–4.02) | 2.77 (1.77–4.35) |
| Incidence rate ratio | 1.30 (0.46–3.24) | 0.62 (0.18–1.72) | 0.69 (0.25–1.72) | Reference |
| Incidence rate ratio | 1.89 (0.56–6.30) | 0.90 (0.22–3.28) | Reference | 1.45 (0.58–4.07) |
| Duration between the start of exposure to development of the outcome (years; median, p25–p75; only patients experiencing the outcome) | 1.06 (0.59–1.94) | 1.29 (1.08–1.82) | 0.99 (0.86–1.60) | 0.90 (0.46–2.29) |
| Outcome during drug therapy plus grace period (90 days) | ||||
| Total patient‐years | 2167.61 | 3226.03 | 4185.94 | 7632.87 |
| Patient‐years of follow‐up (median, p25–p75) | 2.01 (1.16–3.21) | 1.92 (0.93–3.45) | 1.43 (0.84–2.53) | 1.94 (1.09–3.32) |
| Number of major cardiovascular events | 7 | 6 | 9 | 23 |
| Incidence rate per 1000 patient‐years (95% CI) | 3.23 (1.54–6.77) | 1.86 (0.84–4.14) | 2.15 (1.12–4.13) | 3.01 (2.00–4.53) |
| Incidence rate ratio | 1.07 (0.39–2.58) | 0.62 (0.21–1.56) | 0.71 (0.29–1.60) | Reference |
| Incidence rate ratio | 1.50 (0.48–4.53) | 0.87 (0.25–2.72) | Reference | 1.40 (0.62–3.44) |
| Duration between the start of exposure to development of the outcome (years; median, p25–p75; only patients experiencing the outcome) | 1.06 (0.59–1.94) | 1.19 (1.06–1.82) | 0.99 (0.86–1.60) | 0.90 (0.44–2.29) |
95% CI, 95% confidence interval; p25–p75, 25th percentile‐75th percentile.
During drug therapy, major CVEs occurred in 7, 5, 7 and 19 patients receiving ustekinumab, etanercept, methotrexate and adalimumab, respectively; during the extended exposure window period, major CVEs occurred in 7, 6, 9 and 23 patients, respectively. The IRs associated with exposure to ustekinumab were numerically higher than those associated with adalimumab and methotrexate but these differences were not significant. The median times to onset of major CVEs in all groups and analyses were about 1 year but etanercept had the longest onset of major CVEs compared with the other groups (Table 3).
The proportionality test for all comparisons and both analysis times showed no violation of the proportional hazard assumptions. Moreover, the expected percentage bias achieved a good balance in all analyses, after adjusted for overlap weights by propensity score (Figs S1–S3, Supporting Information).
There were no significant differences in the risk for major CVE occurring during drug therapy or the extended exposure window period when patients using ustekinumab, etanercept or methotrexate were compared with those using adalimumab as shown in (Fig. 2b).
Discussion
In this large prospective cohort study, we found no significant differences in the risk of major CVEs between biologic therapies in adult patients with chronic plaque psoriasis. Moreover, the risk of major CVEs for methotrexate was not significantly different from adalimumab. These findings are derived from propensity score‐adjusted models taking into account a range of important CV risk factors. Our findings were consistent for separate analyses comparing the risk of major CVEs both during therapy and for an extended exposure window period.
Earlier observational studies had a number of differences which make comparison with our study difficult: notably, different comparators and definitions of CV outcomes, including participants with prior CVEs in the studies, and not controlling for some important CV risk factors11, 12, 13, 14, 15 (Table S3, Supporting Information). The results of these previous studies suggested benefits of biologic therapies in relation to risk of CV outcomes. One study suggested that TNFi‐treated patients (adalimumab, etanercept and infliximab; n = 9148) had a significantly lower risk of composite and individual CVEs (MI; stroke or transient ischaemic attack; or unstable angina) when compared with those treated with methotrexate (n = 8581).11 In addition, two cohort studies suggested that TNFi (n1 = 1463 and n2 = 11 410) significantly decreased the risk of major adverse CVEs when compared with topical therapies (n = 13 112) and the risk of major CVEs (MI; stroke or transient ischaemic attack; or unstable angina; which is different definition than we used in this current study) when compared with phototherapy (n = 12 433).14, 15 Another study defined CVEs as composite MI, stroke and CV death. It found a significantly lower risk of CVEs in TNFi (n = 959) and methotrexate (n = 3564)‐treated groups, whilst the risk in those treated with ustekinumab (n = 178) was similar to those using other therapies (topical, phototherapy and climate therapy; n = 3961).13 Since the sample size of the ustekinumab group was very small in this earlier study, it is unlikely that any difference in the risk of CVEs would be detected for this comparison. In line with our findings, an earlier cohort study found that patients treated with biologic therapies (including ustekinumab, adalimumab, etanercept, alefacept and efalizumab; n = 7682 at enrolment) had a similar risk of CVEs (non‐fatal‐MI, non‐fatal‐stroke and CV death) when compared to those treated with non‐biologic agents (n = 5576 at enrolment).12 A recent large cohort study compared ustekinumab (n = 9071) with TNFi (adalimumab, etanercept, infliximab, certolizumab or golimumab; n = 50 957) among patients with psoriasis or PsA to examine the risk of atrial fibrillation or major adverse CVEs.20 In line with our findings, this study found no significant difference in the risk of CVEs outcomes. Of related interest, two RCTs examining the impact of adalimumab (TNFi) on aortic vascular inflammation in patients with moderate–severe psoriasis also reported that adalimumab did not improve aortic vascular inflammation after 52 weeks of treatment.21, 22
Power calculation is used to inform how well we could characterize nature in the future given in a certain situation and statistical study design.23 Since this study explored the relationship between major CVEs and biologic therapies for the treatment of psoriasis using the real data and there was no significant difference in the risk of major CVEs for all comparisons, it is pointless to calculate post hoc power calculation. It does not yield additional insights.23
Our study has several important strengths. Firstly, we reduced potential bias by using a new‐user study design for the biologic cohorts24 and propensity score techniques for examining the impact of biologic therapies on risk of major CVEs. The propensity score technique adequately controlled for measured CV confounders between comparison groups. Secondly, we excluded patients who had experienced prior major CVEs to further minimize bias.
We also acknowledge some study limitations. First, although we controlled for measured confounders including the most important CV risk factors, we cannot exclude the effects of residual confounding due to other unmeasured variables such as physical activity and dietary factors. The propensity score technique cannot address this limitation. Second, some aspects of CV risk factor management may be specific to this national cohort, and therefore, the results may not be generalizable to patients managed in different healthcare systems. Third, the small numbers of major CVEs and participants and the limited follow‐up may have had an impact on the power for these analyses as seen in HRs with 95% CIs. Moreover, the impact of biologic therapies on the risk of major CVEs may change over time. Our findings may serve as hypothesis generating for future studies. Therefore, continued surveillance of the risk of major CVEs in more patients with plaque psoriasis with longer follow‐up is needed.
Conclusion
Overall, we found no difference in the risk of major CVEs between etanercept, adalimumab and ustekinumab in adult patients with moderate–severe plaque psoriasis following short‐to‐medium‐term exposure. The impact of biologic therapies or methotrexate on the risk of major CVEs in patients with psoriasis may take longer to manifest. Thus, future comparative studies with longer follow‐up and additional data on CV risk factors will be helpful for continued surveillance of major CVEs in patients with psoriasis exposed to biologic therapies.
Supporting information
Table S1. Potential adverse event terms
Table S2. Baseline characteristics of patients receiving ustekinumab, etanercept, methotrexate or adalimumab.
Table S3. Cohort studies examining the association between biologic therapies and cardiovascular events.
Figure S1. Distribution of confounders between ustekinumab and adalimumab (referent) patients before creating propensity score and after overlap weighting by propensity score.
Figure S2. Distribution of confounders between etanercept and adalimumab (referent) patients before creating propensity score and after overlap weighting by propensity score.
Figure S3. Distribution of confounders between methotrexate and adalimumab (referent) patients before creating propensity score and after overlap weighting by propensity score.
Acknowledgements
The authors acknowledge the substantial contribution of the BADBIR team to the administration of the project in particular the database manager Mr Hassan Ali, for his advice and support. BADBIR acknowledges the support of the National Institute for Health Research (NIHR) through the clinical research networks and its contribution in facilitating recruitment into the registry. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the BADBIR, NIHR, NHS or the Department of Health. The authors are grateful to the members of the Data Monitoring Committee (DMC): Dr Robert Chalmers, Dr Carsten Flohr (Chair), Dr Richard Weller and David Prieto‐Merino and the BADBIR Steering Committee (in alphabetical order): Prof Jonathan Barker, Ms Marilyn Benham (CEO of BAD), Prof David Burden (Chair), Mr Ian Evans, Prof Christopher Griffiths, Dr Sagair Hussain, Prof Brian Kirby, Ms Linda Lawson, Dr Kathleen McElhone, Dr.Tess McPherson, Dr Kayleigh Mason, Dr Ruth Murphy, Dr Caroline Owen, Prof Anthony Ormerod, Ms. Eleanor Pearson, Prof Nick Reynolds, Mr. Josh Richards, Prof Catherine Smith and Prof Richard Warren. We acknowledge the enthusiastic collaboration of all of the dermatologists and specialist nurses in the U.K. and the Republic of Ireland who provided the data. The principal investigators at the participating sites at the time of data cut‐off are listed on the following website: http://www.badbir.org. Finally, we also thank Zenas Z.Z. Yiu, Salwa S. Zghebi, Hayley Gorton and Ireny Y.K. Iskandar for advice during the course of the study. BADBIR is coordinated by the University of Manchester and funded by the British Association of Dermatologists (BAD). The BAD receives income from Pfizer, AbbVie, Almirall, Amgen, Celgene, Eli Lilly, Hexal AG, Janssen, Leo, Novartis, Samsung Bioepis, Sandoz and UCB for providing pharmacovigilance services. This income finances a separate contract between the BAD and the University of Manchester who coordinate BADBIR. All decisions concerning analysis, interpretation and publication are made independently of any industry contribution.
Conflict of interest
KJM has received honorarium from Eli Lilly and Janssen. KM has been a speaker for Janssen and Eli Lilly. ADB has been reimbursed for work as a lecturer, consultant and researcher for Abbvie, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen Cilag, Novartis and UCB. MKR served on advisory boards and has received research grants and/or honorarium from GSK, MSD, Novo Nordisk and Roche Diabetes Care. RBW has been a consultant and/or speaker and/or has received research grants for Abbvie, Almirall, Amgen, Boehringer Ingelheim, Celgene, Eli Lilly, Janssen, LEO Pharma, Novartis, Pfizer, Sanofi, Xenoport and UCB Pharma. CEMG has received honoraria and/or research grants from Abbvie, Almirall, Amgen, Celgene, Eli Lilly, GSK‐Stiefel, Janssen, LEO Pharma, MSD, Nestle Skin Health, Novartis, Pfizer, Sandoz and UCB Pharma. DMA has received research grants from Abbvie, Almirall, Celgene, Eli Lilly, Novartis, UCB and the Leo Foundation. Other authors have no conflict of interest.
Funding source
WR is funded by the Royal Thai Government to undertake her PhD at the University of Manchester. This study is part of the PhD programme. CEMG, DMA and RBW are funded in part by the Medical Research Council (MR/L011808/1). CEMG is also a National Institute for Health Research Senior Investigator. The interpretation of the findings and decision to publish the work are those of the authors alone.
Contributor Information
W. Rungapiromnan, Email: watcharee.rungapiromnan@manchester.ac.uk.
BADBIR Study Group:
Jonathan Barker, Marilyn Benham, Fiona Browne, Ian Evans, Sagair Hussain, Brian Kirby, Linda Lawson, Tess McPherson, Ruth Murphy, Caroline Owen, Anthony Ormerod, Eleanor Pearson, Nick Reynolds, Josh Richards, and Catherine Smith
References
- 1. National Psoriasis Foundation . Statistics, 2018. URL https://www.psoriasis.org/content/statistics (last accessed: 21 September 2018). [Google Scholar]
- 2. Parisi R, Symmons DPM, Griffiths CEM, Ashcroft DM. Global epidemiology of psoriasis: a systematic review of incidence and prevalence. J Invest Dermatol 2013; 133: 377–385. [DOI] [PubMed] [Google Scholar]
- 3. Springate DA, Parisi R, Kontopantelis E, Reeves D, Griffiths CEM, Ashcroft DM. Incidence, prevalence and mortality of patients with psoriasis: a U.K. population‐based cohort study. Br J Dermatol 2017; 176: 650–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Miller IM, Ellervik C, Yazdanyar S, Jemec GBE. Meta‐analysis of psoriasis, cardiovascular disease, and associated risk factors. J Am Acad Dermatol 2013; 69: 1014–1024. [DOI] [PubMed] [Google Scholar]
- 5. Rutter MK, Kane K, Lunt M et al Primary care‐based screening for cardiovascular risk factors in patients with psoriasis. Br J Dermatol 2016; 175: 348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gordon KB, Langley RG, Gottlieb AB et al A phase III, randomized, controlled trial of the fully human IL‐12/23 mAb briakinumab in moderate‐to‐severe psoriasis. J Invest Dermatol 2012; 132: 304–314. [DOI] [PubMed] [Google Scholar]
- 7. Krueger GG, Langley RG, Leonardi C et al A human interleukin‐12/23 monoclonal antibody for the treatment of psoriasis. N Engl J Med 2007; 356: 580–592. [DOI] [PubMed] [Google Scholar]
- 8. Grogan K. Abbott Withdraws Briakinumab Applications in USA, Europe, 2011. URL https://web.archive.org/web/20110417091156/http://www.pharmatimes.com/article/11-01-17/Abbott_withdraws_briakinumab_applications_in_USA_Europe.aspx. (last accessed: 31 August 2018). [Google Scholar]
- 9. Rungapiromnan W, Yiu ZZN, Warren RB, Griffiths CEM, Ashcroft DM. Impact of biologic therapies on risk of major adverse cardiovascular events in patients with psoriasis: systematic review and meta‐analysis of randomized controlled trials. Br J Dermatol 2017; 176: 890–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Mason KJ, Barker JNWN, Smith CH et al Comparison of drug discontinuation, effectiveness, and safety between clinical trial eligible and ineligible patients in BADBIR. JAMA Dermatol 2018; 154: 581–588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Wu JJ, Guérin A, Sundaram M, Dea K, Cloutier M, Mulani P. Cardiovascular event risk assessment in psoriasis patients treated with tumor necrosis factor‐α inhibitors versus methotrexate. J Am Acad Dermatol 2017; 76: 81–90. [DOI] [PubMed] [Google Scholar]
- 12. Gottlieb AB, Kalb RE, Langley RG et al Safety observations in 12095 patients with psoriasis enrolled in an international registry (PSOLAR): experience with infliximab and other systemic and biologic therapies. J Drugs Dermatol 2014; 13: 1441–1448. [PubMed] [Google Scholar]
- 13. Ahlehoff O, Skov L, Gislason G et al Cardiovascular outcomes and systemic anti‐inflammatory drugs in patients with severe psoriasis: 5‐year follow‐up of a Danish nationwide cohort. J Eur Acad Dermatology Venereol 2015; 29: 1128–1134. [DOI] [PubMed] [Google Scholar]
- 14. Wu JJ, Joshi AA, Reddy SP et al Anti‐inflammatory therapy with tumor necrosis factor inhibitors is associated with reduced risk of major adverse cardiovascular events in psoriasis. J Eur Acad Dermatology Venereol 2018; 32: 1320–1326. [DOI] [PubMed] [Google Scholar]
- 15. Wu JJ, Sundaram M, Cloutier M et al The risk of cardiovascular events in psoriasis patients treated with tumor necrosis factor–α inhibitors versus phototherapy: an observational cohort study. J Am Acad Dermatol 2018; 79: 60–68. [DOI] [PubMed] [Google Scholar]
- 16. Armstrong EJ, Harskamp CT, Armstrong AW. Psoriasis and major adverse cardiovascular events: a systematic review and meta‐analysis of observational studies. J Am Heart Assoc 2013; 2: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Burden AD, Warren RB, Kleyn CE et al The British Association of Dermatologists’ Biologic Interventions Register (BADBIR): design, methodology and objectives. Br J Dermatol 2012; 166: 545–554. [DOI] [PubMed] [Google Scholar]
- 18. Bousquet C, Lagier G, Lillo‐Le Louët A, Le Beller C, Venot A, Jaulent MC. Appraisal of the MedDRA conceptual structure for describing and grouping adverse drug reactions. Drug Saf 2005; 28: 19–34. [DOI] [PubMed] [Google Scholar]
- 19. Sterne JAC, White IR, Carlin JB et al Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. Br Med J 2009; 338: b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lee MP, Desai RJ, Jin Y, Brill G, Ogdie A, Kim SC. Association of ustekinumab vs TNF inhibitor therapy with risk of atrial fibrillation and cardiovascular events in patients with psoriasis or psoriatic arthritis. JAMA Dermatol 2019; 155: 700–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Bissonnette R, Harel F, Krueger JG et al TNF‐α antagonist and vascular inflammation in patients with psoriasis vulgaris: a randomized placebo‐controlled study. J Invest Dermatol 2017; 137: 1638–1645. [DOI] [PubMed] [Google Scholar]
- 22. Mehta NN, Shin DB, Joshi AA et al Effect of 2 psoriasis treatments on vascular inflammation and novel inflammatory cardiovascular biomarkers. Circ Cardiovasc Imaging 2018; 11: e007394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hoenig JM, Heisey DM. The abuse of power: the pervasive fallacy of power calculations for data analysis. Am Stat 2001; 55: 19–24. [Google Scholar]
- 24. Ray WA. Evaluating medication effects outside of clinical trials: new‐user designs. Am J Epidemiol 2003; 158: 915–920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Potential adverse event terms
Table S2. Baseline characteristics of patients receiving ustekinumab, etanercept, methotrexate or adalimumab.
Table S3. Cohort studies examining the association between biologic therapies and cardiovascular events.
Figure S1. Distribution of confounders between ustekinumab and adalimumab (referent) patients before creating propensity score and after overlap weighting by propensity score.
Figure S2. Distribution of confounders between etanercept and adalimumab (referent) patients before creating propensity score and after overlap weighting by propensity score.
Figure S3. Distribution of confounders between methotrexate and adalimumab (referent) patients before creating propensity score and after overlap weighting by propensity score.


