Abstract
Background and purpose
The efficacy of galcanezumab, a monoclonal antibody for migraine prevention, has been demonstrated in two pivotal trials in patients with episodic migraine.
Methods
EVOLVE‐1 and EVOLVE‐2 were identical phase 3, randomized, double‐blind, placebo‐controlled studies in patients with episodic migraine. Mean migraine headache days per month at baseline was 9. Patients were randomized 2:1:1 to monthly injections of placebo, galcanezumab 120 mg/240 mg during the 6‐month double‐blind treatment period. Key efficacy outcomes were assessed in subgroups amongst patients for whom, previously, for efficacy and/or safety/tolerability reasons (i) one or more (≥1) preventives failed, (ii) two or more (≥2) preventives failed and (iii) preventives were never used, or used but not failed (no prior failure).
Results
In an integrated analysis of EVOLVE studies, galcanezumab 120 mg/240 mg versus placebo led to larger overall mean (SE) reductions in monthly migraine headache days across 6 months in patients with prior preventive failures (P < 0.001): ≥1 failure: 120 mg: −4.0 (0.4); 240 mg: −4.2 (0.5); placebo: −1.3 (0.4); ≥2 failures: 120 mg: −3.1 (0.7); 240 mg: −3.8 (0.8); placebo: −0.5 (0.6). Similar results were observed amongst patients with no prior failure, but the placebo response was larger: 120 mg: −4.7 (0.2); 240 mg: −4.5 (0.2); placebo: −3.0 (0.2) (P < 0.001 versus placebo). Significant improvements were observed with galcanezumab versus placebo for ≥50% and ≥75% reduction in monthly migraine headache days.
Conclusion
In patients with episodic migraine treated with galcanezumab, those with ≥1 or ≥2 prior preventive failures had significantly larger improvements, versus placebo, in efficacy outcomes. Similar results were observed in patients with no prior failure, with a larger placebo response.
Keywords: episodic migraine, galcanezumab, LY2951742, phase 3, preventive failure
Introduction
According to the latest estimates from the Global Burden of Disease Study, migraine is the second highest cause of disability worldwide 1. In the 15–49‐year age group, it is the number one cause of disability globally, accounting for 8.2% of the total years lived with disability 2. Global estimates show that, amongst patients with ≥4 migraine headache days per month and with ≥1 preventive treatment failure, the burden of migraine is substantial on several aspects of daily living and healthcare resource utilization, and this increased in line with the number of preventive treatment failures 3. Patients with episodic migraine experience headache <15 days a month 4. Most commonly used preventive treatments for episodic migraine include medicines originally developed for hypertension, depression and epilepsy; these are also recommended by evidence‐based guidelines 5. Developed for other indications 6, current oral preventive treatments are associated with high discontinuation rates 7, 8 that can result in many patients relying solely on acute medications to manage their disease. However, overuse of acute medications may put patients at risk of disease progression towards chronic migraine 9, with higher disability and economic burden 10, 11. Patients who have failed prior preventives for efficacy and/or safety/tolerability reasons form an important subgroup with unmet need for prevention 3 and for whom it is important to assess the efficacy of new preventives.
Galcanezumab, a humanized monoclonal antibody against calcitonin‐gene‐related peptide (CGRP), belongs to a novel class of injectable antibodies designed specifically for migraine prevention 12. The efficacy and safety of monthly injections of galcanezumab in patients with episodic migraine have been demonstrated in two identically designed phase 3 randomized clinical trials (RCTs) with 6‐month treatment periods (EVOLVE‐1 and EVOLVE‐2) 13, 14. In these studies, galcanezumab 120 mg/240 mg versus placebo significantly reduced the mean number of monthly migraine headache days. In these RCTs, more than 60% of patients had taken migraine preventives previously. Patients previously exposed to preventives, where they have not responded or had poor tolerability, have an unmet need requiring new therapies. Therefore, the objectives of this subgroup analysis were to evaluate key efficacy outcomes in a pooled analysis of EVOLVE studies amongst patients with (i) one or more (≥1) prior preventive failures, (ii) two or more (≥2) prior preventive failures and (iii) no prior preventive failures (never used or used but not failed). Failure was defined as stopping treatment for efficacy and/or safety/tolerability reasons.
Methods
Study design and patients
The study designs and inclusion/exclusion criteria of the EVOLVE‐1 (NCT02614183) and EVOLVE‐2 (NCT02614196) studies have been described in detail previously 13, 14 and are detailed in Appendix S1. The EVOLVE studies comprised a 6‐month double‐blind treatment period during which patients experiencing 4–14 migraine headache days per month were randomized 2:1:1 to receive monthly subcutaneous injections of placebo and galcanezumab 120 mg (a loading dose of 240 mg) or 240 mg. A key exclusion criterion was failure to respond for efficacy reasons over the patient’s lifetime to three or more adequately dosed preventive treatments from different medication classes with level A or B evidence based on American Academy of Neurology/American Headache Society treatment guidelines 15 (details in Appendix S1). Of note, this criterion is different from the definition of treatment failures for the subgroup analyses as described subsequently.
Appropriate institutional review boards at study sites reviewed and approved study protocols. The studies were conducted per good clinical practice and Declaration of Helsinki guidelines. Patients provided written informed consent before undergoing study procedures.
Study objectives, measures and assessments
The primary objective in EVOLVE studies was to evaluate whether galcanezumab 120mg/240mg is superior versus placebo in overall mean change from baseline in the number of monthly migraine headache days during the double‐blind treatment period (months 1–6). A migraine headache day was a calendar day on which migraine headache or probable migraine headache (lasting ≥ 30 min and with features meeting the International Classification of Headache Disorders, 3rd edition beta version criteria [16]) occurred. Key secondary outcomes included here are mean proportions of patients with ≥50%, ≥75% and 100% reduction from baseline in monthly migraine headache days. Subgroup analyses of the above outcome measures were conducted for the following subgroups of patients:
patients with one or more (≥1) prior preventive failures,
patients with two or more (≥2) prior preventive failures and
patients with no prior preventive failures; this included patients who had never taken a preventive previously (treatment naïve) and patients who had taken a preventive but for whom the treatment had not failed;
patients with one prior preventive failure (results in Appendix S1).
In these analyses, the number of preventive failures refers to the number of individual medications (not medication classes) failed in the past 5 years. Migraine preventive medications taken in the past 5 years and the reasons for stopping them were collected. Failure of a prior preventive treatment was defined as cessation of treatment for efficacy (‘nonresponse’ or ‘inadequate response’) or safety/tolerability‐related reasons. Definition of failure did not include need for treatment with an optimal dose or for a specific duration. The list of preventives from investigative sites was further restricted to medications identified in treatment guidelines as having been investigated for preventive use, irrespective of level of evidence 5, 15. These criteria are summarized in Appendix S1. All data reported are based on these criteria.
Statistical analyses
The full analysis set, based on the intent‐to‐treat principle, included all patients who received at least one dose of the study drug. Data from the EVOLVE studies were pooled for subgroup analyses. Restricted maximum likelihood based mixed models with repeated measures and generalized linear mixed models were used to conduct subgroup analyses for repeated continuous and binary measures, respectively. Overall mean change from baseline, i.e. average mean change from baseline across months 1–6, was estimated from these models. Response rates were calculated as the mean percentage of responders using a categorical, pseudo‐likelihood‐based repeated‐measures analysis assessing overall response rate across months 1–6. Treatment‐by‐subgroup interactions were included in all models.
Results
Baseline demographics and disease characteristics
Patient disposition, baseline demographics and disease characteristics for individual studies have been described in detail previously 13, 14; Table 1 provides a summary by number of prior failures from the integrated analysis set. Table 2 shows the distribution of patients by the number of prior treatment failures. A majority of preventive treatment failures for any reason were for topiramate followed by propranolol and amitriptyline (Table 3). Across the two studies, the most common reason for failure was efficacy.
Table 1.
Patient demographics and baseline disease characteristics, and distribution of patients by number of prior treatment failures
| Population by number of prior failures |
PBO N = 894 |
GMB 120 mg N = 444 |
GMB 240 mg N = 435 |
Overall N = 1773 |
|
|---|---|---|---|---|---|
| n (%) | 0 | 672 (75.2) | 316 (71.2) | 331 (76.1) | 1319 (74.4) |
| ≥1 | 222 (24.8) | 128 (28.8 | 104 (23.9) | 454 (25.6) | |
| ≥2 | 92 (10.3) | 51 (11.5) | 45 (10.3) | 188 (10.6) | |
| Age, years, mean (SD) | 0 | 41.8 (11.5) | 40.1 (11.7) | 40.1 (11.4) | 41.0 (11.5) |
| ≥1 | 42.0 (10.9) | 42.9 (10.9) | 41.9 (10.6) | 42.3 (10.8) | |
| ≥2 | 43.8 (10.2) | 43.0 (11.6) | 43.8 (9.9) | 43.6 (10.5) | |
| Gender (female), % | 0 | 82.9 | 82.6 | 81.9 | 82.6 |
| ≥1 | 89.2 | 91.4 | 91.4 | 90.3 | |
| ≥2 | 88.0 | 90.2 | 86.7 | 88.3 | |
| Duration of migraine disease, years, mean (SD) | 0 | 20.3 (12.3) | 19.6 (12.2) | 19.0 (11.6) | 19.8 (12.1) |
| ≥1 | 21.3 (13.2) | 22.9 (12.5) | 21.8 (13.1) | 21.8 (13) | |
| ≥2 | 22.9 (13.6) | 22.5 (12.9) | 24.8 (13.7) | 23.2 (13.4) | |
| Migraine headache days per month, mean (SD) | 0 | 9.1 (3.0) | 9.0 (3.0) | 9.0 (2.9) | 9.1 (3.0) |
| ≥1 | 9.2 (2.9) | 9.5 (2.8) | 9.4 (2.9) | 9.3 (2.8) | |
| ≥2 | 9.1 (3.0) | 9.3 (2.8) | 9.9 (2.8) | 9.3 (2.9) | |
| Migraine headache days per month with acute medication use, mean (SD) | 0 | 7.4 (3.5) | 7.2 (3.5) | 7.3 (3.3) | 7.3 (3.5) |
| ≥1 | 7.8 (3.2) | 8.0 (3.5) | 7.9 (3.1) | 7.9 (3.3) | |
| ≥2 | 7.6 (3.4) | 8.2 (3.3) | 8.3 (3.1) | 7.9 (3.3) | |
| MSQ RF‐R, mean (SD)a | 0 | 52.8 (15.6) | 52.8 (15.4) | 49.7 (17.1) | 52.0 (16.0) |
| ≥1 | 50.1 (15.5) | 49.8 (15.4) | 52.1 (14.8) | 50.5 (15.3) | |
| ≥2 | 49.7 (15.2) | 51.9 (14.5) | 55.1 (14.9) | 51.6 (15.0) |
GMB, galcanezumab; MSQ RF‐R, Role Function‐Restrictive domain score of the Migraine‐Specific Quality of Life Questionnaire version 2.1; PBO, placebo.
For MSQ RF‐R domain scores: PBO, N = 887; GMB 120 mg, N = 443; GMB 240 mg, N = 430; total population 1760.
Table 2.
Distribution of patients by number of prior treatment failures in EVOLVE studies
|
PBO N = 894 |
GMB 120 mg N = 444 |
GMB 240 mg N = 435 |
Overall N = 1773 |
|
|---|---|---|---|---|
| Prior preventive treatment, % | 62.1 | 65.3 | 61.8 | 62.8 |
| Failure of ≥1 medication | 24.8 | 28.8 | 23.9 | 25.6 |
| Failure of ≥2 medications | 10.3 | 11.5 | 10.3 | 10.6 |
| Failure of ≥2 medication classes | 8.6 | 10.8 | 9.2 | 9.3 |
| Failure of ≥3 medications | 4.0 | 6.8 | 4.1 | 4.7 |
| Failure of ≥3 medication classes | 3.1 | 6.3 | 3.0 | 3.9 |
GMB, galcanezumab; PBO, placebo.
Table 3.
Reasons for discontinuation of previous migraine prevention therapy in patients with ≥1 prior preventive failure in EVOLVE studies (N = 453)
|
Inadequate efficacy n (%) |
No efficacy n (%) |
Safety/tolerability n (%) |
Overall n (%) |
|
|---|---|---|---|---|
| Antiepileptic | 199 (43.9) | 54 (11.9) | 46 (10.2) | 280 (61.8) |
| Topiramate | 181 (40.0) | 39 (8.6) | 32 (7.1) | 246 (54.3) |
| Valproate | 28 (6.2) | 10 (2.2) | 12 (2.6) | 49 (10.8) |
| Gabapentin | 8 (1.8) | 4 (0.9) | 0 (0.0) | 12 (2.6) |
| Zonisamide | 4 (0.9) | 3 (0.7) | 3 (0.7) | 10 (2.2) |
| Pregabalin | 1 (0.2) | 3 (0.7) | 3 (0.7) | 6 (1.3) |
| Ergenyl® chrono | 2 (0.4) | 1 (0.2) | 2 (0.4) | 5 (1.1) |
| Beta blocker | 95 (21.0) | 32 (7.1) | 19 (4.2) | 145 (32.0) |
| Propranolol | 64 (14.1) | 16 (3.5) | 14 (3.1) | 94 (20.8) |
| Metoprolol | 15 (3.3) | 9 (2.0) | 6 (1.3) | 30 (6.6) |
| Nadolol | 9 (2.0) | 4 (0.9) | 1 (0.2) | 14 (3.1) |
| Antidepressant | 100 (22.1) | 25 (5.5) | 25 (5.5) | 140 (30.9) |
| Amitriptyline | 67 (14.8) | 16 (3.5) | 12 (2.6) | 92 (20.3) |
| Nortriptyline | 16 (3.5) | 4 (0.9) | 1 (0.2) | 21 (4.6) |
| Venlafaxine | 7 (1.5) | 2 (0.4) | 6 (1.3) | 15 (3.3) |
| Duloxetine | 2 (0.4) | 3 (0.7) | 2 (0.4) | 6 (1.3) |
| Escitalopram | 2 (0.4) | 1 (0.2) | 3 (0.7) | 6 (1.3) |
| Calcium channel blocker | 26 (5.7) | 13 (2.9) | 10 (2.2) | 48 (10.6) |
| Flunarizine | 19 (4.2) | 9 (2.0) | 8 (1.8) | 35 (7.7) |
| Verapamil | 3 (0.7) | 3 (0.7) | 1 (0.2) | 7 (1.5) |
| Botulinum toxin type A | 16 (3.5) | 16 (3.5) | 0 | 31 (6.8) |
| Angiotensin II antagonists | 10 (2.2) | 6 (1.3) | 2 (0.4) | 18 (4.0) |
| Supplements | 16 (3.5) | 1 (0.2) | 0 (0.0) | 17 (3.8) |
| Magnesium | 9 (2.0) | 1 (0.2) | 0 (0.0) | 10 (2.2) |
| Riboflavin | 5 (1.1) | 0 (0.0) | 0 (0.0) | 5 (1.1) |
| Antihistamines | 9 (2.0) | 0 (0.0) | 3 (0.7) | 12 (2.6) |
| Pizotifen | 9 (2.0) | 0 (0.0) | 3 (0.7) | 12 (2.6) |
| Muscle relaxant | 6 (1.3) | 2 (0.4) | 0 (0.0) | 8 (1.8) |
| Tizanidine | 5 (1.1) | 1 (0.2) | 0 (0.0) | 6 (1.3) |
| NSAIDs | 3 (0.7) | 3 (0.7) | 1 (0.2) | 7 (1.5) |
| Antipsychotic | 2 (0.4) | 0 (0.0) | 0 (0.0) | 2 (0.4) |
| ACE inhibitors | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
| Ergot alkaloids | 0 (0.0) | 1 (0.2) | 0 (0.0) | 1 (0.2) |
| Triptan | 1 (0.2) | 0 (0.0) | 0 (0.0) | 1 (0.2) |
ACE, angiotensin‐converting enzyme; NSAID, non‐steroidal anti‐inflammatory drugs.
A full list of drugs with reasons for failure is presented in Appendix S1. Individual medications included here are those that were failed by >1% of patients for efficacy and/or safety/tolerability reasons. Medications identified in the treatment guidelines as having been investigated for preventive use 5, 15 were used to restrict the list of preventives reported by the investigative sites.
Reductions in monthly migraine headache days
In an integrated analysis of EVOLVE studies, amongst patients who failed ≥1 or ≥2 prior preventives, treatment with galcanezumab 120 mg/240 mg versus placebo led to significantly (P < 0.001) larger overall reductions from baseline in monthly migraine headache days over the 6‐month period. Least squares (LS) mean change (standard error, SE) in prior failure subgroups were as follows: ≥1 prior failure: galcanezumab 120 mg: −4.04 (0.43); galcanezumab 240 mg: −4.21 (0.46); placebo: −1.30 (0.37); ≥2 prior failures: galcanezumab 120 mg: −3.06 (0.74); galcanezumab 240 mg: −3.83 (0.80); placebo: −0.46 (0.64) (Fig. 1a, b). Similar results were observed in patients with no prior failures but the placebo response was larger [LS mean change (SE): galcanezumab 120 mg: −4.72 (0.24); galcanezumab 240 mg: −4.46 (0.23); placebo: −3.02 (0.20); Fig. 1c]. In all three subgroups, treatment with galcanezumab 120 mg/240 mg versus placebo led to significantly (P < 0.05) larger reductions from baseline in the number of monthly migraine headache days in each month (months 1–6) of the treatment period (Fig. 1a–c).
Figure 1.
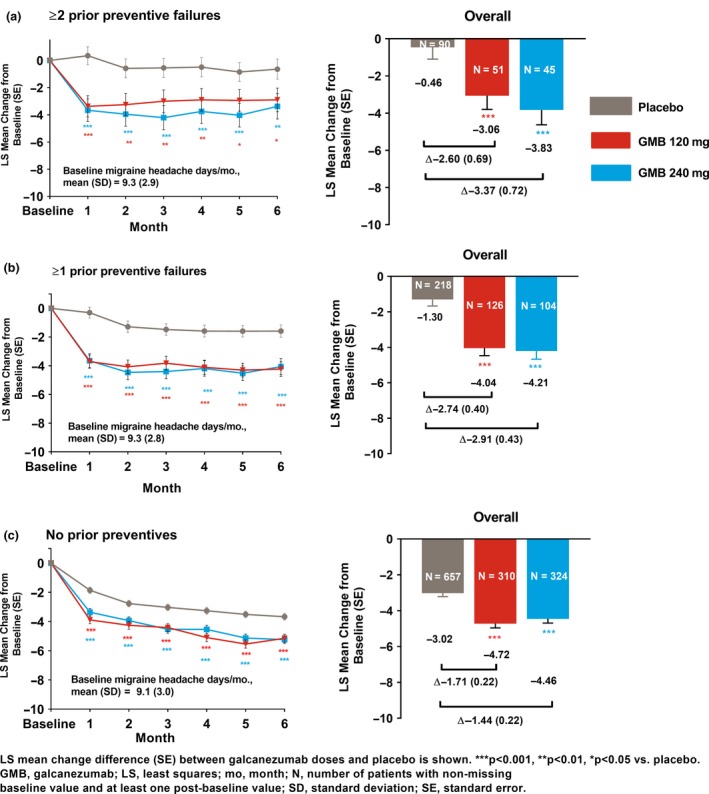
Monthly and overall LS mean changes from baseline in the number of migraine headache days per month during the treatment period. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Differences in overall reductions in migraine headache days between galcanezumab dose groups and placebo during the 6‐month period were larger in patients with prior failures (≥1 or ≥2 prior failures) versus patients with no prior failures (Fig. 1). A larger placebo response in patients with no prior failure appeared to drive the smaller differences in this subgroup. Subgroup‐by‐treatment interactions were significant (P < 0.05) for both galcanezumab doses versus placebo for comparison of the subgroup with ≥1 prior failure versus no prior failure. This interaction was significant only for galcanezumab 240 mg versus placebo for comparison of the subgroup with ≥2 prior failures versus no prior failure.
Percentage of ≥50% and ≥75% responders for migraine headache days
Among patients with ≥1 or ≥2 prior preventive failures, significantly (P < 0.001) greater mean proportions of patients achieved ≥50% and ≥75% reduction in monthly migraine headache days on an average month in the galcanezumab dose groups versus placebo (Figs 2 and 3). At months 3 and 6, the estimated proportions of patients with ≥50% or ≥75% response were significantly (P < 0.01) greater with galcanezumab doses versus placebo. Similar results were observed in the subgroup with no prior failure (P < 0.001, galcanezumab versus placebo); however, there was a larger placebo response in this subgroup (Figs 2 and 3). For 100% response, the analysis model did not converge due to the small number of patients with 100% response.
Figure 2.
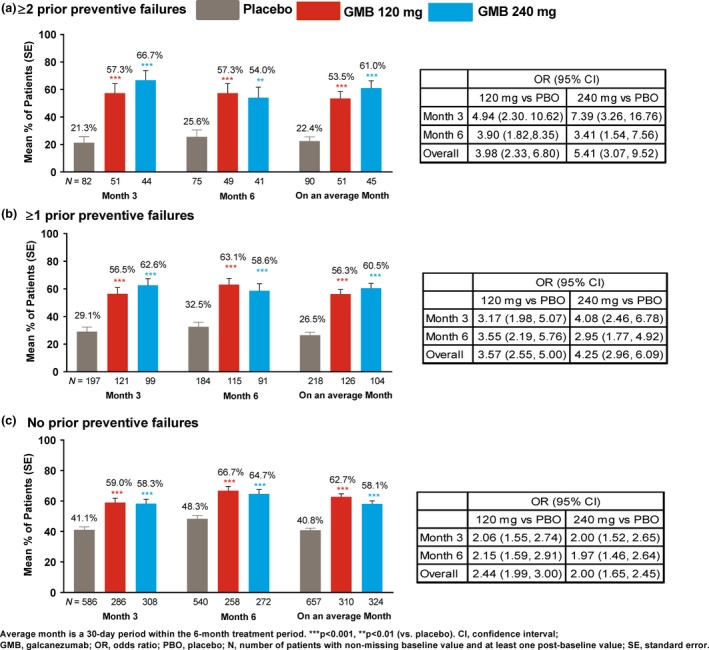
Mean percentage of patients with ≥50% reduction from baseline in monthly migraine headache days. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Figure 3.
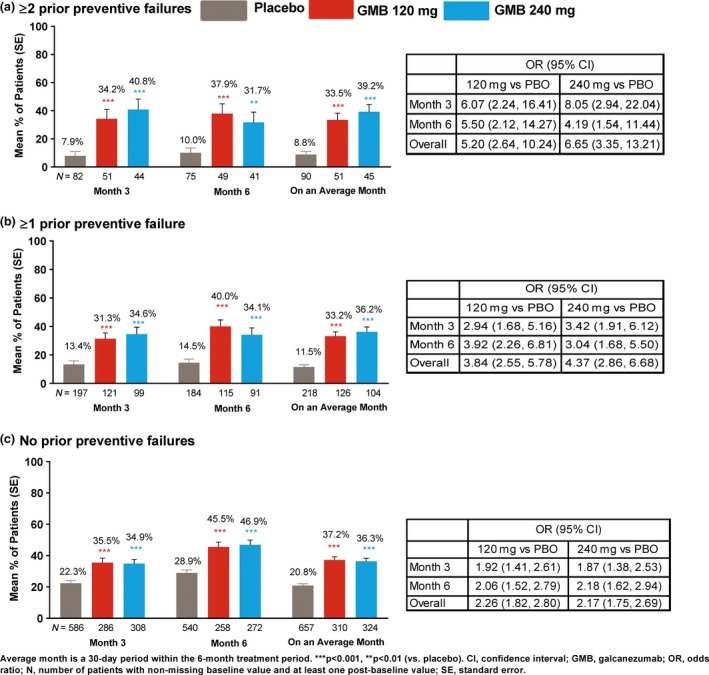
Mean percentage of patients with ≥75% reduction from baseline in monthly migraine headache days. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Other outcomes and results
Similar results were observed amongst patients with ≥1 or ≥2 prior preventive failures and with no prior failure for reductions in migraine headache days with acute medication use and improvements in patient functioning per the Role Function‐Restrictive domain score of the Migraine‐Specific Quality of Life Questionnaire version 2.1 (MSQ‐RFR) (Appendix S1). Results in the subgroup of patients with one prior failure were consistent with results observed in the subgroups with ≥1 or ≥2 prior failures across outcomes (Appendix S1).
Discussion
Results from this subgroup analysis demonstrate that galcanezumab 120 and 240 mg versus placebo is efficacious across multiple end‐points in patients with episodic migraine irrespective of their history of ≥1 or ≥2 prior preventive failures for efficacy and/or safety/tolerability reasons. A large proportion of patients (25.6%) had ≥1 prior preventive failure; this is consistent with the high discontinuation rates associated with the current standard of care 7, 15, 16. During the 6‐month treatment period, patients with ≥1 or ≥2 prior failures experienced significantly greater overall reductions in the number of migraine headache days per month, and ≥50% and ≥75% response rates with galcanezumab versus placebo. These improvements were also consistently observed in the subgroup with no prior failures, although the reductions versus placebo were lower. In all three subgroups, significant reductions in migraine headache days were observed with both galcanezumab doses versus placebo at each month of the 6‐month period.
Findings from this subgroup analysis are important given the recently published European Headache Federation and American Headache Society treatment guidelines that recommend initiating treatments targeting the CGRP pathway when multiple criteria are met, including the inability to tolerate or an inadequate response to at least two common oral preventives 16, 17. Results from the overall populations in phase 3 RCTs targeting the CGRP pathway demonstrated efficacy and safety/tolerability in a broader population with migraine; however, the guidelines noted the need for cost‐effective care and therefore restricted access. The study population herein consisted of patients who met the headache frequency and moderate disability thresholds described in the guidelines. Therefore, these results will be valuable for prescribing physicians and healthcare policy makers who want to understand efficacy outcomes in the population with episodic migraine with at least one or at least two prior preventive treatment failures. The EVOLVE studies excluded adequately dosed non‐responders, for efficacy reasons, to ≥3 different preventive medication classes with level A or B evidence 15. However, as failure in daily practice includes intolerability issues, our analysis was able to include patients who failed at least three preventive treatments as well.
Differences in responses to all outcomes between galcanezumab and placebo were larger in the prior preventive failure subgroups (≥1 or ≥2 prior failures) compared to the subgroup with no prior preventive failures. These results are consistent with findings from phase 3 studies of erenumab (a monoclonal antibody against CGRP receptor) in patients with episodic migraine 18, 19, a phase 3 study of fremenezumab (a monoclonal antibody to CGRP 20) and recently presented prospective data with galcanezumab 21. In this subgroup analysis and in erenumab studies, larger differences in patients with prior failure appear to be driven by the lower placebo response in this subgroup. When entering an RCT, patients with prior failures may have lower expectations from the study drug than patients who have never received prior preventives or have received but not failed previously 22, 23. This subgroup analysis was not powered to detect statistically significant differences between the galcanezumab doses. However, there were no notable differences between galcanezumab dose groups for any of the outcome measures across subgroups, which is consistent with results seen in overall populations 13, 14.
Overall mean changes from baseline in monthly migraine headache days (months 1–6) amongst galcanezumab‐treated patients were similar across the overall population 13, 14 and in the subgroups presented here. This demonstrates the fairly consistent nature of the results for the primary outcome across the overall population and in patients with various histories of preventive failure. Similarly, comparable results were noted for the secondary outcome measures discussed here. These results are also similar to those reported with erenumab 140 mg in a phase 3b study in patients with episodic migraine and 2–4 prior failures 18 the latter data being consistent with a phase 3 study of erenumab 70/140 mg in patients with episodic migraine over months 4–6 19, 24.
In this subgroup analysis, in patients with various histories of preventive failure, proportions of patients achieving ≥75% overall reduction in migraine headache days were significantly greater versus placebo. In patients with episodic migraine, ≥50% reduction in monthly migraine headache days is recognized as being clinically meaningful 25. Evaluating clinical meaningfulness is important, considering that many patients with migraine demonstrate inadequate response to current preventive therapies and/or experience intolerable side effects, thereby making it difficult for them to remain on medication long enough to receive sufficient and sustained therapeutic benefit.
The strengths of this subgroup analysis in patients with episodic migraine include that these are findings from two large, global, multi‐centre, phase 3 RCTs. Failure of prior preventives within subgroups was based on multiple reasons related to both efficacy and safety/tolerability with inadequate or no response being the most common reasons. The list of medications associated with prior failure was restricted to medications identified in treatment guidelines as having been investigated for preventive use. Efficacy in subgroups was demonstrated not only for migraine frequency‐related end‐points but also for other outcomes. Further, the results are consistent with those in the overall population and in patients with one prior failure. Limitations include that the sample sizes of the subgroups presented here are limited. The definition of failure in the subgroups had no adequate dose or duration requirement. Moreover, patients with failures of ≥3 preventive classes (level A or B evidence, adequate dose) over their lifetime were excluded from studies. A study designed specifically to examine the effects of galcanezumab in patients with episodic or chronic migraine with 2–4 prior preventive failures (NCT03559257) 21 may address this.
In conclusion, the findings of this subgroup analysis in patients with episodic migraine are clinically relevant considering that the subgroups chosen here are consistent with the expected target population for galcanezumab based on current treatment guidelines. Galcanezumab with its mechanism of action targeting the pathophysiology of migraine and favourable efficacy may offer an effective treatment option for patients with episodic migraine who have failed prior preventives.
Disclosure of conflicts of interest
DDR, JHF, AT‐H, VLS, SG, SKA are employees of Eli Lilly and Company, and/or its subsidiaries, and may be minor stock holders. PJG reports grants and personal fees from Amgen and Eli Lilly and Company; personal fees from Alder Biopharmaceuticals, Allergan, Autonomic Technologies Inc., Biohaven Pharmaceuticals Inc., Dr Reddy’s Laboratories, Electrocore LLC, eNeura, Impel Neuropharma, MundiPharma, Novartis, Teva Pharmaceuticals, Trigemina Inc., WL Gore; personal fees from MedicoLegal work, Massachusetts Medical Society, Up‐to‐Date, Oxford University Press and Wolters Kluwer; and a patent, Magnetic stimulation for headache, assigned to eNeura without fee. GMT reports grants and consultancy or industry support from Eli Lilly and Company, Amgen, Novartis, Teva Pharmaceuticals, and independent support from Netherlands Organization for Scientific Research (NWO), European Community, Dutch Heart Foundation, Dutch Brain Foundation.
Supporting information
Appendix S1. Methods.
Acknowledgements
Studies sponsored by Eli Lilly and Company. Trial registration: NCT02614183, NCT02614196.
See Letter to the editor by I. ‐H. Huang on page https://doi.org/10.1111/ene.14143
References
- 1. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017; 390: 1211–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. GBD Headache Collaborators . Global, regional, and national burden of migraine and tension‐type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 2018; 17: 954–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Martelletti P, Schwedt TJ, Lanteri‐Minet M, et al My Migraine Voice survey: a global study of disease burden among individuals with migraine for whom preventive treatments have failed. J Headache Pain 2018; 19: 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Headache Classification Committee of the International Headache Society . The international classification of headache disorders, 3rd edition. Cephalalgia 2018; 38: 1–211. [DOI] [PubMed] [Google Scholar]
- 5. Evers S, Afra J, Frese A, et al EFNS guideline on the drug treatment of migraine – revised report of an EFNS task force. Eur J Neurol 2009; 16: 968–981. [DOI] [PubMed] [Google Scholar]
- 6. Charles A. Migraine. N Engl J Med 2017; 377: 1698–1699. [DOI] [PubMed] [Google Scholar]
- 7. Blumenfeld AM, Bloudek LM, Becker WJ, et al Patterns of use and reasons for discontinuation of prophylactic medications for episodic migraine and chronic migraine: results from the Second International Burden of Migraine Study (IBMS‐II). Headache 2013; 53: 644–655. [DOI] [PubMed] [Google Scholar]
- 8. Hepp Z, Bloudek LM, Varon SF. Systematic review of migraine prophylaxis adherence and persistence. J Manag Care Pharmacy 2014; 20: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schwedt TJ. Chronic migraine. BMJ 2014; 348: g1416–g1416. [DOI] [PubMed] [Google Scholar]
- 10. Blumenfeld AM, Varon SF, Wilcox TK, et al Disability, HRQoL and resource use among chronic and episodic migraineurs: results from the International Burden of Migraine Study (IBMS). Cephalalgia 2011; 31: 301–315. [DOI] [PubMed] [Google Scholar]
- 11. Lanteri‐Minet M, Duru G, Mudge M, et al Quality of life impairment, disability and economic burden associated with chronic daily headache, focusing on chronic migraine with or without medication overuse: a systematic review. Cephalalgia 2011; 31: 837–850. [DOI] [PubMed] [Google Scholar]
- 12. Ong JJY, Wei DY, Goadsby PJ. Recent advances in pharmacotherapy for migraine prevention: from pathophysiology to new drugs. Drugs 2018; 78: 411–437. [DOI] [PubMed] [Google Scholar]
- 13. Stauffer VL, Dodick DW, Zhang Q, et al Evaluation of galcanezumab for the prevention of episodic migraine: the EVOLVE‐1 randomized clinical trial. JAMA Neurol 2018; 75: 1080–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Skljarevski V, Matharu M, Millen BA, et al Efficacy and safety of galcanezumab for the prevention of episodic migraine: results of the EVOLVE‐2 phase 3 randomized controlled clinical trial. Cephalalgia 2018; 38: 1442–1454. [DOI] [PubMed] [Google Scholar]
- 15. Silberstein SD, Holland S, Freitag F, et al Evidence‐based guideline update: Pharmacologic treatment for episodic migraine prevention in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology and the American Headache Society. Neurology 2012; 78: 1337–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sacco S, Bendtsen L, Ashina M, et al European Headache Federation guideline on the use of monoclonal antibodies acting on the calcitonin gene related peptide or its receptor for migraine prevention. J Headache Pain 2019; 20: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. American Headache Society . The American Headache Society position statement on integrating new migraine treatments into clinical practice. Headache 2019; 59: 1–18. [DOI] [PubMed] [Google Scholar]
- 18. Reuter U, Goadsby PJ, Lanteri‐Minet M, et al Efficacy and tolerability of erenumab in patients with episodic migraine in whom two‐to‐four previous preventive treatments were unsuccessful: a randomised, double‐blind, placebo‐controlled, phase 3b study. Lancet 2018; 392: 2280–2287. [DOI] [PubMed] [Google Scholar]
- 19. Goadsby PJ, Paemeleire K, Broessner G, et al Efficacy and safety of erenumab (AMG334) in episodic migraine patients with prior preventive treatment failure: a subgroup analysis of a randomized, double‐blind, placebo‐controlled study. Cephalalgia 2019; 39: 817–826. [DOI] [PubMed] [Google Scholar]
- 20. Ferrari MD, Diener HC, Ning X, et al Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double‐blind, placebo‐controlled, phase 3b trial. Lancet 2019; 365: 695–699. [DOI] [PubMed] [Google Scholar]
- 21. Mulleners WM, Kim BK, Lainez MJ, et al A randomized, placebo‐controlled study of galcanezumab in patients with treatment‐resistant migraine: double‐blind results from the CONQUER study. Cephalalgia 2019; 39: IHC‐OR‐042. [Google Scholar]
- 22. Finniss DG, Benedetti F. Mechanisms of the placebo response and their impact on clinical trials and clinical practice. Pain 2005; 114: 3–6. [DOI] [PubMed] [Google Scholar]
- 23. Diener HC, Schorn CF, Bingel U, et al The importance of placebo in headache research. Cephalalgia 2008; 28: 1003–1011. [DOI] [PubMed] [Google Scholar]
- 24. Goadsby PJ, Reuter U, Hallstrom Y, et al A controlled trial of erenumab for episodic migraine. N Engl J Med 2017; 377: 2123–2132. [DOI] [PubMed] [Google Scholar]
- 25. Tfelt‐Hansen P, Pascual J, Ramadan N, et al Guidelines for controlled trials of drugs in migraine: third edition. A guide for investigators. Cephalalgia 2012; 32: 6–38. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Methods.


