Abstract
Grasslands are subject to considerable alteration due to human activities globally, including widespread changes in populations and composition of large mammalian herbivores and elevated supply of nutrients. Grassland soils remain important reservoirs of carbon (C) and nitrogen (N). Herbivores may affect both C and N pools and these changes likely interact with increases in soil nutrient availability. Given the scale of grassland soil fluxes, such changes can have striking consequences for atmospheric C concentrations and the climate. Here, we use the Nutrient Network experiment to examine the responses of soil C and N pools to mammalian herbivore exclusion across 22 grasslands, under ambient and elevated nutrient availabilities (fertilized with NPK + micronutrients). We show that the impact of herbivore exclusion on soil C and N pools depends on fertilization. Under ambient nutrient conditions, we observed no effect of herbivore exclusion, but under elevated nutrient supply, pools are smaller upon herbivore exclusion. The highest mean soil C and N pools were found in grazed and fertilized plots. The decrease in soil C and N upon herbivore exclusion in combination with fertilization correlated with a decrease in aboveground plant biomass and microbial activity, indicating a reduced storage of organic matter and microbial residues as soil C and N. The response of soil C and N pools to herbivore exclusion was contingent on temperature – herbivores likely cause losses of C and N in colder sites and increases in warmer sites. Additionally, grasslands that contain mammalian herbivores have the potential to sequester more N under increased temperature variability and nutrient enrichment than ungrazed grasslands. Our study highlights the importance of conserving mammalian herbivore populations in grasslands worldwide. We need to incorporate local‐scale herbivory, and its interaction with nutrient enrichment and climate, within global‐scale models to better predict land–atmosphere interactions under future climate change.
Keywords: carbon sequestration, exclosure, fertilization, global change, grazing, herbivory, nutrient dynamics, nutrient enrichment, Nutrient Network (NutNet), soil microorganisms
In this study, we use the Nutrient Network experiment to examine the responses of soil C and N pools to mammalian herbivore exclusion across 22 grasslands, under unfertilized and fertilized conditions. We show that the impact of herbivore exclusion on soil C and N pools depends on fertilization; the highest soil C and N pools were found in grazed (+H) and fertilized plots (+F). Our study highlights the importance of conserving mammalian herbivore populations in grasslands worldwide and incorporating local‐scale herbivory within global‐scale models to better predict land–atmosphere interactions under future climate change.

1. INTRODUCTION
Grasslands cover 30% of the terrestrial earth surface (White, Murray, & Rohweder, 2000) and their soils are important reservoirs of carbon (C) and nitrogen (N; Jobbagy & Jackson, 2000). Grasslands are subject to considerable ongoing alterations due to human activities (IPCC, 2014), including changes in populations and composition of grazing mammalian herbivores (Dirzo et al., 2014; Hempson, Archibald, & Bond, 2017; Ripple et al., 2015; Svenning et al., 2016). As nearly all natural grasslands co‐evolved with some degree of grazing (Axelrod, 1985; Janis, Damuth, & Theodor, 2002; Souttie, Reynold, & Batello, 2005) and mammalian herbivores are major drivers of grassland functioning (Blair, Nippert, & Briggs, 2014), changes in their populations or composition are expected to have important consequences for C and N fluxes and pools (McSherry & Ritchie, 2013; Pineiro, Paruelo, Oesterheld, & Jobbagy, 2010; Zhou et al., 2017). Given the scale of grassland soil fluxes, such changes can have striking consequences for atmospheric C concentrations and the climate; losses of soil C could exacerbate climate change whereas increased soil C sequestration may mitigate it (Crowther et al., 2016; Lu et al., 2013). As ecosystem C sequestration is constrained by nutrients (Crowther et al., 2019), and in particular N, changes in soil N are tightly linked to C feedbacks between land and atmosphere (Hungate, Dukes, Shaw, Luo, & Field, 2003).
Herbivores influence the fluxes of C and N into and out of the soil locally, thereby determining soil C and N pools (Figure 1). Herbivores can alter C and N inputs to the soil by changing the quantity and quality of organic inputs (e.g. plant litter, herbivore dung), by decreasing biological fixation through the consumption of legumes, or through changes in soil conditions, such as temperature and moisture (Bardgett & Wardle, 2003; Pastor, Dewey, Naiman, McInnes, & Cohen, 1993; Pineiro et al., 2010), which in turn impact soil microbial communities and activity (Bardgett & Wardle, 2010). As herbivore‐induced changes in soil C and N fluxes occur simultaneously (Figure 1), considerable uncertainty exists regarding the net effect of herbivores on soil C and N pools, and which local C and N fluxes are most important in driving this herbivore effect. The direction and magnitude of the impact of herbivores on soil C and N have been shown to be contingent on environmental conditions, such as climate (e.g. temperature, rainfall), soil properties (e.g. fertility, texture) and site productivity (e.g. plant biomass; McSherry & Ritchie, 2013; Milchunas & Lauenroth, 1993; Pineiro et al., 2010; Schrama et al., 2013; Tanentzap & Coomes, 2012; Zhou et al., 2017). So far, however, studies on the effect of herbivores on soil C and N pools have not accounted for the effect of human‐induced increases in grassland soil nutrient availability, that may arise due to atmospheric N deposition or the use of artificial fertilizers (Asner et al., 2001; Galloway et al., 2004). This makes it difficult to incorporate the role of herbivores in global models predicting land–atmosphere interactions under future climate change.
Figure 1.
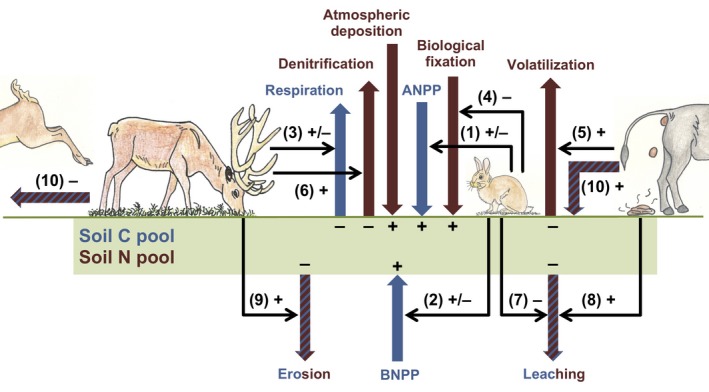
Conceptual framework showing how mammalian herbivores can influence soil C and N pools by their impact on C and N inputs to and outputs from the soil. The blue arrows are the main C fluxes and the brown arrows the main N fluxes, while the arrows shaded both blue and brown indicate both C and N fluxes. The thinner black arrows indicate the impact aboveground mammalian herbivores can have on these fluxes. Herbivores can modify C inputs to the soil by changing aboveground and belowground net primary production (ANPP and BNPP; arrows 1 and 2; Frank et al., 2002; Milchunas & Lauenroth, 1993; Pineiro et al., 2010; Ziter & MacDougall, 2013), thereby changing soil influx of litter and root exudates. C fluxes from the soil can be modified by herbivores via impacts on soil respiration rates and decomposition of organic matter (arrow 3), by changing the quantity and/or quality of organic inputs (dung, urine, plant litter), or through changes in soil conditions, such as temperature and moisture (Bardgett & Wardle, 2003; Pastor et al., 1993; Pineiro et al., 2010), and soil microbial communities and activity (Bardgett & Wardle, 2010). N input fluxes can be modified as herbivores generally reduce the biomass of N2‐fixing legumes (arrow 4; Ritchie & Tilman, 1995; Ritchie, Tilman, & Knops, 1998). They also may increase N losses by stimulating volatilization (arrow 5) via urine and dung deposition (Frank & Evans, 1997; Pineiro, Paruelo, Jobbagy, Jackson, & Oesterheld, 2009), denitrification (arrow 6) and surface runoff as a result of trampling‐induced soil compaction (Schrama et al., 2013), leaching (arrow 7) of mineral nutrients from urine and dung patches or soil erosion (arrow 9; Neff, Reynolds, Belnap, & Lamothe, 2005; Pei, Fu, & Wan, 2008; Steffens, Kolbl, Totsche, & Kogel‐Knabner, 2008; Steinauer & Collins, 2001). In contrast, C and N may be retained under herbivory (arrow 7) through greater plant root allocation (Derner, Boutton, & Briske, 2006; Pineiro et al., 2009; Reeder, Schuman, Morgan, & Lecain, 2004) and higher soil microbial activity (Lange et al., 2015). Herbivores can locally remove or add C and N (arrow 10), by feeding on plant biomass in one area, while depositing dung and/or urine in another (Giese et al., 2013; Singer & Schoenecker, 2003; Van Uytvanck, Milotic, & Hoffmann, 2010)
Under increased nutrient inputs, the impact of herbivores on plant production (De Mazancourt, Loreau, & Abbadie, 1998; Ziter & MacDougall, 2013) and thereby C inputs into the soil may become more positive. Also, the capacity of grasses to regrow after herbivory may increase (Hawkes & Sullivan, 2001), allowing sustained C inputs belowground (Bardgett & Wardle, 2003; Hamilton & Frank, 2001; Olff & Ritchie, 1998), but only if grazing pressure is not too high (Zhou et al., 2017). In addition, attraction of herbivores to nutrient‐rich sites (van der Graaf, Stahl, Veen, Havinga, & Drent, 2007; van der Waal et al., 2011) will increase local biomass removal and waste inputs. Finally, increased nutrient availability may alleviate stoichiometric constraints for microorganisms (van Groenigen et al., 2017) resulting in greater microbial activity. Recent studies indicate that greater microbial acitivity might not only increase litter decomposition rates and C respiration, but alternatively increase C transfer into slow‐cycling forms of C (i.e. microbial necromass; Lange et al., 2015; Sokol & Bradford, 2019), thereby further increasing the potential for C sequestration under grazing. Hence, under future more eutrophied conditions it is likely that herbivores will promote soil C and N storage in grasslands.
Here, we quantify the responses of soil C and N pools to the exclusion of mammalian herbivores in 22 grasslands distributed across the globe, under both ambient and elevated nutrient supply. These sites are part of the Nutrient Network (NutNet) distributed experiment, which was established to examine the combined effects of nutrient addition and herbivore exclusion on ecosystem processes in grasslands worldwide (Borer, Grace, Harpole, MacDougall, & Seabloom, 2017). This collaborative experimental network uses a consistent and standardized methodology and experimental design. We use the experiment to: (a) test the responses of grassland soil C and N pools to herbivore exclusion and fertilization, (b) relate these responses to changes in plant biomass and soil microbial properties, and (c) examine if these responses are driven by environmental variables including climate, soil properties and site productivity.
2. METHODS
2.1. Site selection
For this study, we used sites from the Nutrient Network (http://www.nutnet.org; Borer, Harpole, et al., 2014), where each site consisted of three blocks of four treatment plots of 5 m × 5 m. Each block contained the following four treatment plots: (a) with herbivores and without fertilization (the unfenced control plot), (b) without herbivores and without fertilization (the fenced plot), (c) with herbivores and with fertilization (the fertilized plot), (d) without herbivores and with fertilization (the fenced and fertilized plot). At each block, two plots received no nutrients, while two plots received nitrogen (N), phosphorus (P) and potassium (K) plus micronutrients (μ) to alleviate all forms of nutrient limitation (Fay et al., 2015). The nutrient treatment involved annual application of 10 g m−2 year−1 N, P and K as time‐released urea [(NH2)2CO], triple‐super phosphate [Ca(H2PO4)2] and potassium sulphate [K2SO4] respectively. Once, at the start of the experiment, 100 g/m2 of μ mix of Fe (15%), S (14%), Mg (1.5%), Mn (2.5%), Cu (1%), Zn (1%), B (0.2%) and Mo (0.05%) was applied to all fertilized plots. At each block, one unfertilized and one fertilized plot were unfenced and subject to variable grazing by the contemporary suite of mammalian herbivores present per site (Table S2). The other unfertilized and fertilized plot were fenced (2.30 m high) to exclude aboveground mammalian herbivores (>c. 50 g). More details on the experimental set‐up and nutrient sources are available in Borer, Harpole, et al. (2014).
For this study, 22 NutNet sites were included (Table S1) as they each met two conditions: (a) soil property data had been collected (i.e. C and N concentrations, bulk density) in each of the four treatments for a minimum of 2 years of treatment applications (25 sites); and (b) mammalian herbivores were present in the sites and excluded by the fences (three sites were excluded by this criterion because herbivores were small, rare or absent). Hence, sites that only had herbivores with a body weight <c. 50 g (e.g. voles, mice, rats, squirrels, gophers) were not included. The mammalian herbivores in the selected sites ranged from domestic ungulates such as sheep to wild ungulates such as deer and wild macropods like kangaroos (for an overview of all herbivore species see Table S2). The majority of the herbivores in our sites were grazers or mixed‐feeders, no strict browsers were present, and therefore we describe herbivory in terms of ‘grazing’. Our study sites represent a wide range of herbaceous ecosystems including prairie, montane grassland, shrub steppe, alpine grassland and savanna. The sites also encompassed a wide range of environmental gradients including mean annual temperature (MAT; 0.1–18.2°C), mean annual precipitation (MAP; 246–1,877 mm) and total soil N concentration (0.06%–1.2%).
2.2. Soil C and N pools
After 2–4 years (3.5 years on average) of experimental nutrient addition and herbivore exclusion (Table S1), two soil cores (2.5 cm diameter at 10 cm depth) were collected from each plot after plant litter and vegetation were removed. The soil cores were sieved (2 mm) and homogenized per plot, air‐dried and analysed for total C and N content (Costech ESC 4010 Elemental Analyzer). At three sites where pH > 7.5, soil samples were pretreated with 0.1 M hydrochloric acid to remove carbonates (cdpt.us, hart.us, shps.us). In each plot, an additional intact soil core was collected, for which the volume of the core and fresh and dry weight of the soil were determined to estimate soil bulk density (for more details, see Data S1). To calculate soil C and N pools (kg/m2) in the top 10 cm, we multiplied values of soil bulk density with C and N concentrations for each plot. Grasslands store their greatest proportion of soil C and N near the soil surface (Crowther et al., 2016; Jobbagy & Jackson, 2001), but that does not preclude effects at depths that our sampling approach cannot account for.
We present the effect of the exclusion of mammalian herbivores (>50 g) as the log response ratio (RR) = ln(fenced/unfenced). We calculated separate RRs for the unfertilized and the fertilized (NPKμ) plots within each block per site. If RR = 0 herbivore exclusion had no effect on soil C or N, while RR < 0 indicates that herbivore exclusion decreased soil C or N and RR > 0 indicates that herbivore exclusion increased soil C or N.
2.3. Local controls of the impact of herbivore exclusion on soil C and N
We used data on the impact of herbivore exclusion on aboveground and belowground plant biomass and soil microbial properties collected at the plot‐level, to examine if changes in these local controls were related to the impact of herbivore exclusion on soil C and N (see Figure 1 for details on these potential mechanisms). Total aboveground biomass of all plants was clipped at peak biomass within two 1 m × 0.1 m strips. Aboveground biomass was sorted to live (current year's growth) and dead (previous years’ growth) biomass (or further into functional groups such as legumes), dried to a constant mass and weighed to the nearest 0.01 g. To accurately reflect the flux of biomass inputs to the soil from the start of the experiment up until the year the soil cores were collected, we calculated cumulative plant biomass for each plot by summing the annual harvested biomass over the years since the start of the experiment. This parameter therefore takes into account that longer running experiments might have greater C and N inputs from vegetation, possibly contributing to more pronounced changes in soil C and N.
Directly following aboveground biomass collection, five soil cores were collected from the clipped area, homogenized, and a subsample of the soil (c. 60 g) was used to estimate belowground plant biomass to a depth of 10 cm (Cleland et al., 2019). The subsample was suspended in water, and roots were captured with fine sieves and hand‐picked. Picked roots were dried at 40°C for 72 hr (to constant mass) and weighed to calculate dry root biomass per unit area.
In 2015, 12 of the 22 sites (Table S1) contributed samples for an additional research project focusing on soil microbial properties. At these sites three additional soil cores (5 cm diameter at 12 cm depth) were collected from each plot in 2015 to estimate microbial activity and biomass. Soils were sieved (2 mm) and homogenized per plot. An O2‐microcompensation system (Scheu, 1992) was used to measure the respiratory response of soil microbes in two separate steps using approximately 5.5 g of fresh soil. In a first step, basal respiration was determined as a measure of soil microbial activity (μl O2 hr−1 g−1 soil dry weight) without the addition of any substrate. In a second step, the maximal respiratory response to the addition of glucose solution (4 mg glucose/g soil dry weight dissolved in distilled water) allowed us to estimate soil microbial biomass (μg Cmic/g soil dry weight; Anderson & Domsch, 1978).
Herbivore exclusion effects on all vegetation (live, dead and total aboveground biomass, legume biomass, root biomass) and microbial properties (activity, biomass) were estimated using log response ratios as RR = ln(fenced/unfenced), using both the unfertilized and fertilized plots separately in each block per site. Correlation analyses between all local controls were performed and when variables were strongly correlated (Pearson's r > .7), one of them was excluded to limit the impact of multicollinearity (see correlation Table S3). Threshold‐based preselection with a suggested threshold of .7 is an appropriate method to deal with collinearity between variables (Dormann et al., 2013). Hence, we excluded the impact of herbivore exclusion on total aboveground plant biomass (live + dead), as this variable was highly correlated with the impact of herbivore exclusion on live biomass. Thus, we retained four—or six in the subset of sites including microbial data—candidate local controls.
2.4. Environmental drivers of the impact of herbivore exclusion on soil C and N
We used data on climate and several vegetation and soil properties at the site‐level as candidate environmental drivers of the impact of herbivore exclusion on soil C and N. We selected six climate variables from the WorldClim database (version 1.4; Hijmans, Cameron, Parra, Jones, & Jarvis, 2005) that summarized the mean and seasonality of temperature and rainfall and their seasonal synchrony (Seabloom et al., 2013): (a) MAT (°C), (b) temperature seasonality (SD of temperature among months; TEMP_VAR), (c) mean annual range in temperature (°C; ANN_TEMP_RANGE), (d) mean temperature of wettest quarter (°C; TEMP_WET_Q), (e) MAP (mm), and (f) precipitation seasonality (coefficient of variation in precipitation among months; MAP_VAR). These global climate data were interpolated at high spatial resolution from weather/meteorological stations with 10–30 years of data (Hijmans et al., 2005). To determine the annual atmospheric N deposition (kg N ha−1 year−1) for each site, we used modelled rates based on existing measurements using a global three‐dimensional chemistry‐transport model (TM3, The Oak Ridge National Laboratory Distributed Active Archive Center; http://daac.ornl.gov/). The model provides sufficient spatial resolution (50 × 50 km grid cells) to distinguish site‐level variation in annual N deposition among our sites (Borer, Seabloom, et al., 2014).
We included data on aboveground plant biomass, soil N and soil texture at each site before the NutNet experimental treatments were established (pretreatment data at year 0), to get an accurate measure of the in situ productivity, soil fertility and texture before herbivore presence and site fertility were manipulated. We calculated mean values for the unfertilized and fertilized treatment per block at each site, by averaging the values of the unfenced and fenced treatment plots per fertilization treatment. Pretreatment soil N concentrations were missing for the fertilized plots in two sites (mtca.au and sgs.us), so means were based on the unfertilized plots (assuming no large differences between plots in year 0). Soil texture (% sand, silt and clay) was measured in one plot per block using the hydrometer method (Ashworth, Keyes, Kirk, & Lessard, 2001). Based on correlation analyses between all environmental drivers we excluded mean annual range in temperature, as this variable was highly correlated with temperature seasonality (Table S4). We therefore retained 10 candidate environmental drivers.
2.5. Statistical analyses
We excluded one block of data from the site smith.us because of missing soil data on the fertilized (+NPKμ) treatment, which precluded calculation of the response ratio. Additionally, one block in sage.us was dropped because of an extremely low bulk density value in its unfenced control plot (0.04 g/cm3 compared to a mean of 0.66 g/cm3 for this treatment in the other two blocks) due to waterlogging (95.9% soil moisture) and was therefore considered to be an outlier. All statistical analyses were therefore performed on data from 252 plots in 63 blocks (three blocks per site except for bldr.us, smith.us and sage.us) in 22 sites (Table S1).
To examine the impact of herbivore exclusion and fertilization on soil C and N pools, we used linear mixed models (LMMs) with block nested within site as random effect. In addition, we performed one sample t tests on our RRs to examine the impact of the exclusion of herbivores on soil C and N pools and C:N ratio under unfertilized and fertilized conditions. If the 95% confidence interval values of the RRs did not overlap with zero, there was a significant decrease or increase with herbivore exclusion. We also tested if treatment duration affected the RRs using an LMM, with number of treatment years as fixed predictor and site ID as a random effect. We found no significant impact of treatment duration on the responses of soil C and N pools to herbivore exclusion (LMM, F 1,20 = 0.65, p = .431 for C and F 1,20 = 1.30, p = .268 for N), allowing us to pool the data across treatment years, even though the sites differed in the number of years that the treatments were applied.
We used multi‐model inference (Burnham & Anderson, 2002; Richards, Whittingham, & Stephens, 2011) to examine (a) which local controls over soil C and N were responsible for changes in soil C and N pools due to herbivore exclusion, and (b) which across‐site environmental drivers affected the impact of herbivore exclusion on soil C and N pools. For this, we modelled the effects of our predictor variables (either the local controls or the environmental variables) on the C and N response ratios with a full LMM with site ID as a random effect. The models also included fertilization as a fixed factor to observe any significant interactions between fertilization and local controls/environmental drivers. Multi‐model inference uses model averaging based on Akaike's information criterion (AIC) to arrive at consistent parameter estimates of the most important explanatory variables in the full LMM, by averaging a set of top models which share similarly high levels of parsimony. We defined the top models as those that fell within 4 AIC units of the model with the lowest AIC value (Richards et al., 2011). We standardized our regression predictors through centering and dividing by 2 SD (Gelman, 2008), which resulted in variance inflation factors <5 of all predictors, indicating low collinearity among them (Dormann et al., 2013).
We assessed the response of soil C and N to herbivore exclusion with and without the impact of herbivore exclusion on legume biomass (local control) and with and without soil texture (environmental driver) as we missed data on these variables at several sites. These variables had no significant effect on the response of soil C and N to herbivore exclusion, and to maintain the largest spatial extent possible, we only present models without these variables. Additionally, we ran a separate model on the subset of sites including microbial data (12 of 22 sites; Table S1), and results of this model are also presented.
3. RESULTS
Fertilization led to significant increases in soil C and N pools, but only when herbivores were not excluded (LMM, F 3,186 = 6.46, p < .001 for C and F 3,186 = 7.12, p < .001 for N; Figure 2). In fertilized plots, this led to herbivore exclosures having C pools that were on average 61.0 g m−2 year−1 (2.2%/year) smaller and N pools that were on average 3.7 g m−2 year−1 (1.7%/year) smaller (p = .005 for C and p = .01 for N; Figure 3a,b) than in grazed plots. Without fertilization, herbivore exclusion had no effect on soil C and N pools (p = .14 for C and p = .11 for N). Herbivore exclusion had no impact on soil C:N ratio under unfertilized or fertilized conditions (Figure 3c). The responses of soil C and N concentrations to herbivore exclusion and fertilization showed similar patterns as the C and N pools (p = .02 for C and p = .04 for N under fertilized conditions), while there was no effect on soil bulk density (Figure S1).
Figure 2.
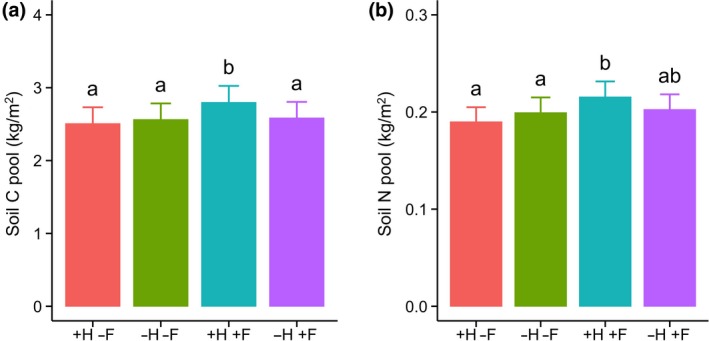
Effect of herbivore exclusion (+H: herbivores present; −H: herbivores excluded) and fertilization (+F: fertilized with NPKµ; −F: unfertilized) on soil C (a) and N pools (b). Shown are sample means ± SE. Different letters indicate significant differences among the treatment means
Figure 3.
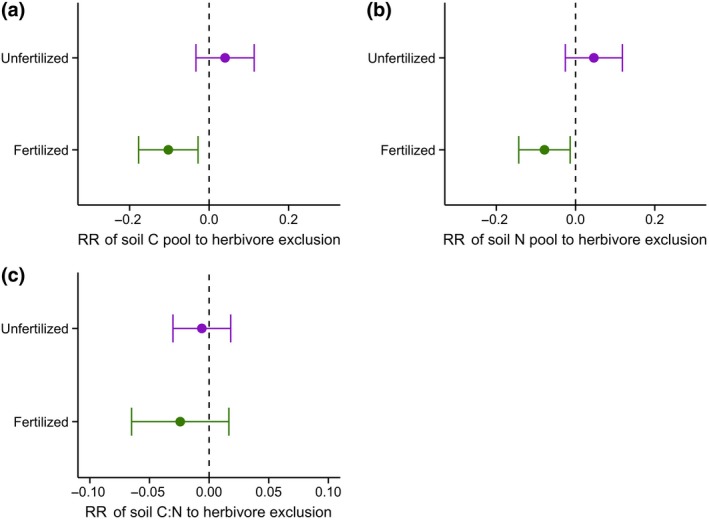
Log response ratios of soil C pool (a), N pool (b) and C:N ratio (c) to herbivore exclusion calculated as RR = ln(fenced/unfenced) for unfertilized (purple) and fertilized (NPKμ) plots (green). If response ratio (RR) = 0 herbivore exclusion had no effect on the variable, while RR < 0 herbivore exclusion decreased the variable and RR > 0 herbivore exclusion increased the variable. Graphs show the mean RRs across all 22 sites (n = 63 per fertilization treatment), where points represent the mean RR and error bars represent the range of 95% confidence intervals. The vertical dashed line was drawn at RR = 0 and responses are considered significant if error bars do not overlap with zero
The between‐site variation in the responses of soil C and N pools to herbivore exclusion was positively, albeit weakly, correlated with changes in aboveground live plant biomass (Figure 4a,b; Table S5; model‐averaged R 2 for all predictor variables = .09 for C and .12 for N); when herbivore exclusion increased plant biomass, it also increased soil C and N pools, and when herbivore exclusion decreased plant biomass, it also decreased soil C and N pools (Figure S2a,b). Fertilization did not have an impact on this relationship (i.e. no significant interaction). We also found a positive correlation with changes in microbial activity; when exclusion of herbivores increased microbial activity it also increased soil C pools and tended to increase soil N pools (it did significantly increase soil N concentrations; Figure 4b,c; Figure S2c; Table S5). This relationship was again not impacted by fertilization. Our results differed slightly for the analyses with and without the microbial data, possibly because only a subset of sites collected soil microbial data. We detected no overall effect of herbivore exclusion on aboveground live plant biomass or microbial activity (Figure S3). We did not find any detectable relationship between responses of soil C and N pools to herbivore exclusion and changes in dead plant biomass or root biomass (Figure 4; Table S5).
Figure 4.
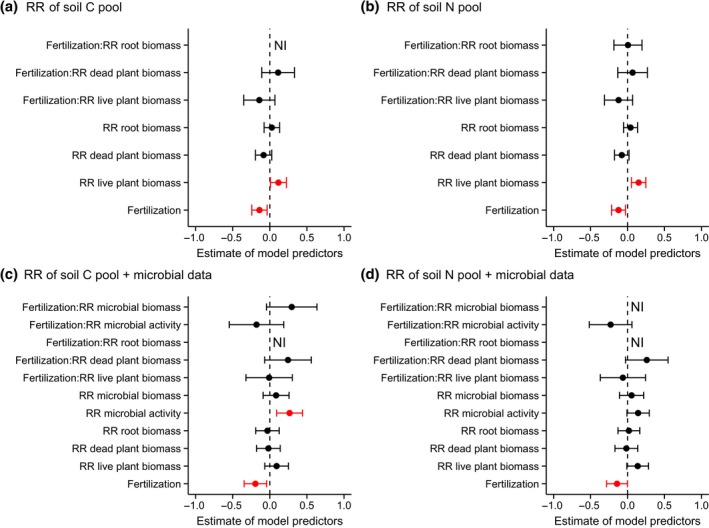
Plots showing the parameter estimates of the potential local controls explaining the response ratios (RR) of soil C (a, c) and N pools (b, d) to herbivore exclusion. The parameters are response ratios of plant biomass (live, dead and root) and microbial properties (biomass, activity) to herbivore exclusion. Parameter estimates were generated by multi‐model inference, which uses model averaging to arrive at consistent parameter estimates of the most important explanatory variables. Models included fertilization as a fixed factor (under fertilization the effect of herbivore exclusion is negative; also see Figure 3) and interactions are presented as ‘Fertilization:other parameter’. Models were run without (22 sites, n = 126; a, b) and with (12 sites, n = 67; c, d) microbial data. Points represent the mean value of the model predictor while error bars represent the range of 95% confidence intervals. Predictors are considered significant if error bars do not overlap with zero and are coloured red. NI indicates the variable was not included in the set of top models
Across sites, variation in temperature, precipitation, N deposition, aboveground plant biomass or soil fertility had minimal predictive power in explaining the variation in the impact of herbivore exclusion on soil C (model‐averaged R 2 = .09) or N pools (model‐averaged R 2 = .12; Figure 5; Table S6). However, the soil N response to herbivore exclusion was related to several climate variables in interaction with fertilization, indicating that these relationships were controlled by nutrient addition. Significant interactions were observed between fertilization and temperature seasonality (TEMP_VAR), and between fertilization and mean temperature of wettest quarter (TEMP_WET_Q; Figure 5b; Table S6). These interactions indicate that, in fertilized plots, the decrease of soil N pools upon herbivore exclusion increased with temperature variability among months (TEMP_VAR; Figure S4a), while it decreased with temperature in the wettest quarter (TEMP_WET_Q; Figure S4b). Additionally, the impact of herbivore exclusion on soil C and N concentrations became increasingly negative with increasing MAT, and the same pattern was observed for soil C and N pools, although it was not significant (Table S6).
Figure 5.
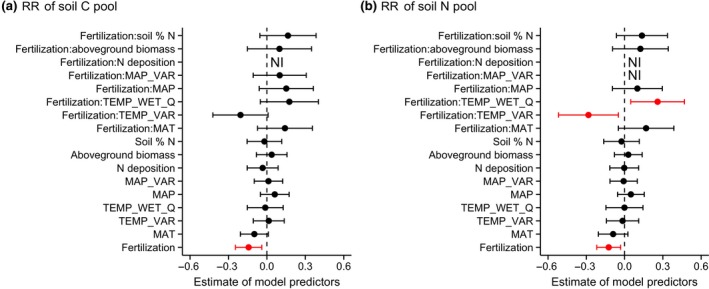
Plots showing the parameter estimates of the potential environmental drivers explaining the response ratios (RR) of soil C (a) and N pools (b) to herbivore exclusion. Parameter codes are: MAT, mean annual temperature; TEMP_VAR, temperature seasonality; TEMP_WET_Q, mean temperature of wettest quarter; MAP, mean annual precipitation; MAP_VAR, precipitation seasonality; aboveground biomass and soil % N are measures of in situ productivity and soil fertility. See Section 2 for more details. Parameter estimates were generated by multi‐model inference, which uses model averaging to arrive at consistent parameter estimates of the most important explanatory variables. Models included fertilization as a fixed factor and interactions are presented as ‘Fertilization:other parameter’. Points represent the mean value of the model predictor while error bars represent the range of 95% confidence intervals. Predictors are considered significant if error bars do not overlap with zero and are coloured red. NI indicates the variable was not included in the set of top models
4. DISCUSSION
Overall, nutrient availability explained most strongly the responses of soil C and N pools to the exclusion of mammalian herbivores: under ambient nutrient conditions, we observed no effect of herbivore exclusion, but under elevated nutrient supply (fertilization with NPKμ) pools were smaller upon herbivore exclusion (Figure 3). This means that fertilized plots that were grazed had the highest soil C and N pools (Figure 2). These results demonstrate that nutrient availability is a limiting factor in grazer‐induced C and N sequestration in grasslands across a broad range of environmental conditions. As soil C and N covaried in their response to herbivore exclusion, the soil C:N ratio was less variable (Figure 3c), suggesting that soil C:N ratio is well‐constrained in our grassland sites regardless of herbivory and nutrient availability. Indeed, the average ratio of 13.0 ± 0.2 was very similar to the average C:N ratio of 13.8 ± 0.4 across 75 grasslands on a larger scale (Cleveland & Liptzin, 2007), indicating stoichiometric balance despite variation in total soil C and N.
Previous clipping experiments combined with nutrient addition demonstrated an increase in grassland soil C storage, which was related to increased belowground production (Frank, Kuns, & Guido, 2002; Ziter & MacDougall, 2013; Figure 1). However, this mechanism was not associated with C storage in our grassland sites (Figure 4a), although this might be because our biomass measures did not capture root turnover (Frank et al., 2002). Alternatively, herbivores might be attracted to the fertilized plots due to increased aboveground plant biomass (Fay et al., 2015) and forage quality (La Pierre & Smith, 2015), resulting in increases in C and N inputs through dung and urine (van der Graaf et al., 2007; van der Waal et al., 2011; Figure 1). Carbon contained in herbivore dung can then be incorporated into the soil due to the activities of invertebrates, such as dung beetles or termites, thus allowing little C to volatize (Ritchie, 2014). Moreover, in fertilized and grazed plots, plants are likely to regrow better after herbivory (Hawkes & Sullivan, 2001), allowing for increased root exudation and C inputs belowground (Bardgett & Wardle, 2003; Hamilton & Frank, 2001; Figure 1). Grazing also may increase the photosynthetic rates of the regrowth and residual plant biomass, especially under high nutrient supply, leading to higher relative growth rates and increased productivity (Frank & McNaughton, 1993; McNaughton, 1985). Although we observed an increase in aboveground plant biomass due to fertilization, the extent of this increase did not differ between exclosures and grazed plots (Figure S3). It is possible that increased biomass removal by herbivores in the fertilized and grazed plots in combination with increased plant growth resulted in an overall net effect of zero. To confirm this, we would need to estimate annual net primary productivity and biomass consumption in our NutNet plots (e.g. using moveable exclosures; McNaughton, Milchunas, & Frank, 1996).
Our findings suggest that when herbivore exclusion increased aboveground plant biomass, this was related to an increase in soil C and N pools, while when herbivore exclusion decreased biomass, for example because of the disappearance of compensatory plant growth responses due to the absence of grazing, it might decrease plant inputs into the soil and thereby C and N pools (Figures 1 and 4a,b; Figure S2a,b; Frank, McNaughton, & Tracy, 1998; Milchunas & Lauenroth, 1993; Pineiro et al., 2010). In addition, we found that a decrease in microbial activity due to herbivore exclusion, potentially mediated via shifts in litter and dung inputs and root turnover (Ziter & MacDougall, 2013), was associated with a decreased soil C pool (Figure 4c; Figure S2c). Our results are in line with recent studies showing that decreased microbial activity results in reduced storage of microbial residues as soil C, suggesting that microbial activity could serve as a proxy for C transfer into slow‐cycling forms of C (Lange et al., 2015; Sokol & Bradford, 2019). Changes in legume biomass due to herbivore exclusion did not have an impact on the response of soil N, suggesting that the decrease of N input by legumes through selective feeding by herbivores might not be very common in grasslands (Ritchie & Tilman, 1995; Figure 1). Even though herbivore‐induced changes in aboveground plant biomass and microbial activity may affect changes in soil C and N pools, the amount of variation that these factors explained was relatively low (<25%; Table S5). This highlights the limitation of the local factors we were able to measure and suggests that other local (unmeasured) factors, e.g. volatilization and leaching (Figure 1), are likely important in explaining impacts of herbivore exclusion on soil C and N. Moreover, the microbial activity as measured here might not be the best index of C balance in the soil; to further unravel the role of microbes in driving the responses of soil C and N to herbivore exclusion additional measurements on microbial carbon use efficiency and microbial residues in the soil organic matter are recommended (Geyer, Kyker‐Snowman, Grandy, & Frey, 2016; Manzoni et al., 2018).
Fertilization not only controlled the responses of soil C and N to herbivore exclusion, but also modified the relationship of soil N response to interannual temperature variability (Figure 5b). Our results indicate that, with increased temperature variability, grasslands that contain herbivores have the potential to sequester more N under nutrient enrichment. Additionally, in warmer grasslands (higher MAT) the presence of herbivores could increase soil C and N sequestration (Table S6). In contrast, herbivore presence may stimulate losses of C and N in colder sites, which are already more likely to lose a considerable amount of C to the atmosphere as a result of global warming (Crowther et al., 2016). This relationship with temperature could be related to a shift from C3‐ to C4‐dominated grasslands, whereby grazing increases soil C in C4‐dominated grasslands likely due to an increase in belowground production of root C and/or mycorrhizae (McSherry & Ritchie, 2013). We however did not find an effect of grass type on the response of soil C to herbivore exclusion, which might be related to the disproportionate distribution of the grass types among our sites (Figure S5). Our study highlights the importance of conserving herbivore populations in grasslands, as the capacity of herbivores to enable soils to store more C and N is likely to become stronger under global change (i.e. under nutrient enrichment, increased temperature and interannual temperature variability). Additionally, the presence of herbivores might make grasslands better able to withstand climate change, as increases in soil C and N storage could result in increases in productivity and soil water‐holding capacity (Teague et al., 2011).
Studies have acknowledged that herbivore type and grazing intensity are important factors in determining the effects of herbivore exclusion on soil C and N pools (Bakker, Olff, Boekhoff, Gleichman, & Berendse, 2004; Pastor, Cohen, & Hobbs, 2006; Zhou et al., 2017). We did not have data on the density of the different herbivore species in our sites, however we could estimate grazing intensity (see Seabloom et al., 2013). We found little evidence that this grazing intensity estimate explained responses of soil C or N to herbivore exclusion (Figure S6). Future data collection on herbivores at each site using standardized methods will support further insight into the ecosystem effects of different types and densities of herbivores. However, we stress that despite the high among‐site variability in herbivore species (Table S2) and proxied grazing intensity (Figure S7), and hence variability in responses of soil C and N to herbivore exclusion across sites (Figure S8), we detected a significant overall decrease in soil C and N under fertilized conditions when herbivores were excluded (Figure 3). This points to a consistent, general effect that rises above the ‘noise’ from among‐site variation in herbivores. We captured these net changes in C and N dynamics over a relatively short time‐period (c. 3.5 years), and expect these changes to become greater with time (Fornara & Tilman, 2012). Additionally, our results are likely to be a conservative representation of soil C and N pools as we only sampled to a depth of 10 cm, while, for example, C accumulation can be substantial below these depths (Jobbagy & Jackson, 2000). At larger spatial scales the interactive effects of herbivores and nutrient enrichment remain uncertain. If grasslands were enriched with nutrients on a landscape‐scale, for instance through increased atmospheric deposition, the effect of herbivore attraction to local patches might likely disappear, but the overall carrying capacity of the ecosystem for herbivores could increase (i.e. increase in quantity and quality of forage; Parsons, Rowarth, Thornley, & Newton, 2011), potentially resulting in an increase of herbivore impacts that could facilitate soil C and N sequestration.
Here, we show that nutrient enrichment in concert with herbivore grazing is likely to increase total organic matter storage in grasslands, especially where herbivore exclusion is associated with a decrease in aboveground live biomass and/or decreased microbial activity. The broad‐scale experiment enabled us to examine the environmental contingencies of these soil C and N responses, suggesting that these effects might be greatest in warmer regions, and will increase with climate change. This work highlights the importance of incorporating local‐scale herbivory, and its interaction with nutrient enrichment and climate, within global‐scale models predicting land–atmosphere interactions under future climate change (Crowther et al., 2019; Schmitz et al., 2014; Thebault, Mariotte, Lortie, & MacDougall, 2014; Tylianakis, Didham, Bascompte, & Wardle, 2008).
AUTHOR CONTRIBUTIONS
This specific study was designed by Judith Sitters, E.R.J.W., E.S.B., T.W.C. and G.F.V. The NutNet experiment was coordinated by E.T.B., S.E.H. and E.W.S. Statistical analyses were performed by Judith Sitters and E.R.J.W., with additional data and analytical contributions from E.E.C., N.E., C.R. and Julia Siebert. Data collection was performed by P.B.A., J.D.B., L.B., E.T.B., E.E.C., N.E., J.M.H.K., R.L.M., J.L.M., S.M.P., C.R., A.C.R., M.S., E.W.S. and Julia Siebert. Judith Sitters and E.R.J.W. wrote the first draft of the manuscript and all authors contributed substantially to revisions. See Table S7 for details.
Supporting information
ACKNOWLEDGEMENTS
This work was generated using data from the Nutrient Network (http://www.nutnet.org) experiment, funded at the site‐scale by individual researchers. Coordination and data management have been supported by funding to E.T.B. and E.W.S. from the National Science Foundation Research Coordination Network (NSF‐DEB‐1042132) and Long Term Ecological Research (NSF‐DEB‐1234162 to Cedar Creek LTER) programs, and the Institute on the Environment (DG‐0001‐13). We also thank the Minnesota Supercomputer Institute for hosting project data and the Institute on the Environment for hosting Network meetings. Judith Sitters was financially supported by Strategic Resources of the Netherlands Institute of Ecology (NIOO‐KNAW) and the Research Foundation Flanders (FWO), grant 12N2615N. E.R.J.W. was funded by a NWO‐RUBICON grant (the Netherlands Organisation for Scientific Research, contract no. 019.181EN.01). G.F.V. was supported by a VENI grant from the Netherlands Organisation for Scientific Research (NWO), grant 863.14.013. N.E. and Julia Siebert acknowledge support by the German Centre for Integrative Biodiversity Research (iDiv) Halle‐Jena‐Leipzig, funded by the German Research Foundation (FZT 118). This is publication number 6912 of the Netherlands Institute of Ecology (NIOO‐KNAW). Author contributions are listed in Table S7 and data contributors are listed in Table S8.
Sitters J, Wubs ERJ, Bakker ES, et al. Nutrient availability controls the impact of mammalian herbivores on soil carbon and nitrogen pools in grasslands. Glob Change Biol. 2020;26:2060–2071. 10.1111/gcb.15023
Judith Sitters and E. R. Jasper Wubs should be considered joint first author.
DATA AVAILABILITY STATEMENT
Data associated with this study are available from the Dryad Data Repository https://doi.org/10.5061/dryad.wstqjq2gw.
REFERENCES
- Anderson, J. , & Domsch, K. (1978). A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biology and Biochemistry, 10, 215–221. 10.1016/0038-0717(78)90099-8 [DOI] [Google Scholar]
- Ashworth, J. , Keyes, D. , Kirk, R. , & Lessard, R. (2001). Standard procedure in the hydrometer method for particle size analysis. Communications in Soil Science and Plant Analysis, 32(5–6), 633–642. 10.1081/css-100103897 [DOI] [Google Scholar]
- Asner, G. P. , Townsend, A. R. , Riley, W. J. , Matson, P. A. , Neff, J. C. , & Cleveland, C. C. (2001). Physical and biogeochemical controls over terrestrial ecosystem responses to nitrogen deposition. Biogeochemistry, 54(1), 1–39. 10.1023/a:1010653913530 [DOI] [Google Scholar]
- Axelrod, D. I. (1985). Rise of the grassland biome, central North America. Botanical Review, 51(2), 163–201. 10.1007/bf02861083 [DOI] [Google Scholar]
- Bakker, E. S. , Olff, H. , Boekhoff, M. , Gleichman, J. M. , & Berendse, F. (2004). Impact of herbivores on nitrogen cycling: Contrasting effects of small and large species. Oecologia, 138(1), 91–101. 10.1007/s00442-003-1402-5 [DOI] [PubMed] [Google Scholar]
- Bardgett, R. D. , & Wardle, D. A. (2003). Herbivore‐mediated linkages between aboveground and belowground communities. Ecology, 84(9), 2258–2268. 10.1890/02-0274 [DOI] [Google Scholar]
- Bardgett, R. D. , & Wardle, D. A. (2010). Aboveground‐belowground linkages: Biotic interactions, ecosystem processes, and global change. Oxford, UK: Oxford University Press. [Google Scholar]
- Blair, J. , Nippert, J. , & Briggs, J. (2014). Grassland ecology In Monson R. (Ed.), Ecology and the environment (pp. 389–423). New York, NY: Springer. [Google Scholar]
- Borer, E. T. , Grace, J. B. , Harpole, W. S. , MacDougall, A. S. , & Seabloom, E. W. (2017). A decade of insights into grassland ecosystem responses to global environmental change. Nature Ecology & Evolution, 1(5), 10.1038/s41559-017-0118 [DOI] [PubMed] [Google Scholar]
- Borer, E. T. , Harpole, W. S. , Adler, P. B. , Lind, E. M. , Orrock, J. L. , Seabloom, E. W. , & Smith, M. D. (2014). Finding generality in ecology: A model for globally distributed experiments. Methods in Ecology and Evolution, 5(1), 65–73. 10.1111/2041-210x.12125 [DOI] [Google Scholar]
- Borer, E. T. , Seabloom, E. W. , Gruner, D. S. , Harpole, W. S. , Hillebrand, H. , Lind, E. M. , … Yang, L. H. (2014). Herbivores and nutrients control grassland plant diversity via light limitation. Nature, 508(7497), 517–520. 10.1038/nature13144 [DOI] [PubMed] [Google Scholar]
- Burnham, K. , & Anderson, D. (2002). Model selection and multimodel inference: A practical information‐theoretic approach. New York, NY: Springer‐Verlag. [Google Scholar]
- Cleland, E. E. , Lind, E. M. , DeCrappeo, N. M. , DeLorenze, E. , Wilkins, R. A. , Adler, P. B. , … Seabloom, E. W. (2019). Belowground biomass response to nutrient enrichment depends on light limitation across globally distributed grasslands. Ecosystems, 22(7), 1466–1477. 10.1007/s10021-019-00350-4 [DOI] [Google Scholar]
- Cleveland, C. C. , & Liptzin, D. (2007). C:N:P stoichiometry in soil: Is there a “Redfield ratio” for the microbial biomass? Biogeochemistry, 85(3), 235–252. 10.1007/s10533-007-9132-0 [DOI] [Google Scholar]
- Crowther, T. W. , Riggs, C. , Lind, E. M. , Borer, E. T. , Seabloom, E. W. , Hobbie, S. E. , … Routh, D. (2019). Sensitivity of global soil carbon stocks to combined nutrient enrichment. Ecology Letters, 22(6), 936–945. 10.1111/ele.13258 [DOI] [PubMed] [Google Scholar]
- Crowther, T. W. , Todd‐Brown, K. E. O. , Rowe, C. W. , Wieder, W. R. , Carey, J. C. , Machmuller, M. B. , … Bradford, M. A. (2016). Quantifying global soil carbon losses in response to warming. Nature, 540(7631), 104–109. 10.1038/nature20150 [DOI] [PubMed] [Google Scholar]
- De Mazancourt, C. , Loreau, M. , & Abbadie, L. (1998). Grazing optimization and nutrient cycling: When do herbivores enhance plant production? Ecology, 79(7), 2242–2252. 10.1890/0012-9658(1998)079[2242:Goancw]2.0.Co;2 [DOI] [Google Scholar]
- Derner, J. D. , Boutton, T. W. , & Briske, D. D. (2006). Grazing and ecosystem carbon storage in the North American Great Plains. Plant and Soil, 280(1–2), 77–90. 10.1007/s11104-005-2554-3 [DOI] [Google Scholar]
- Dirzo, R. , Young, H. S. , Galetti, M. , Ceballos, G. , Isaac, N. J. B. , & Collen, B. (2014). Defaunation in the Anthropocene. Science, 345(6195), 401–406. 10.1126/science.1251817 [DOI] [PubMed] [Google Scholar]
- Dormann, C. F. , Elith, J. , Bacher, S. , Buchmann, C. , Carl, G. , Carré, G. , … Lautenbach, S. (2013). Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography, 36(1), 27–46. 10.1111/j.1600-0587.2012.07348.x [DOI] [Google Scholar]
- Fay, P. A. , Prober, S. M. , Harpole, W. S. , Knops, J. M. H. , Bakker, J. D. , Borer, E. T. , … Yang, L. H. (2015). Grassland productivity limited by multiple nutrients. Nature Plants, 1(7), 10.1038/nplants.2015.80 [DOI] [PubMed] [Google Scholar]
- Fornara, D. A. , & Tilman, D. (2012). Soil carbon sequestration in prairie grasslands increased by chronic nitrogen addition. Ecology, 93(9), 2030–2036. 10.1890/12-0292.1 [DOI] [PubMed] [Google Scholar]
- Frank, D. A. , & Evans, R. D. (1997). Effects of native grazers on grassland N cycling in Yellowstone National Park. Ecology, 78(7), 2238–2248. 10.1890/0012-9658(1997)078[2238:eongog]2.0.co;2 [DOI] [Google Scholar]
- Frank, D. A. , Kuns, M. M. , & Guido, D. R. (2002). Consumer control of grassland plant production. Ecology, 83(3), 602–606. 10.2307/3071865 [DOI] [Google Scholar]
- Frank, D. A. , & McNaughton, S. J. (1993). Evidence for the promotion of aboveground grassland production by native large herbivores in Yellowstone National Park. Oecologia, 96(2), 157–161. 10.1007/BF00317727 [DOI] [PubMed] [Google Scholar]
- Frank, D. A. , McNaughton, S. J. , & Tracy, B. F. (1998). The ecology of the Earth’s grazing ecosystems. BioScience, 48(7), 513–521. 10.2307/1313313 [DOI] [Google Scholar]
- Galloway, J. N. , Dentener, F. J. , Capone, D. G. , Boyer, E. W. , Howarth, R. W. , Seitzinger, S. P. , … Vorosmarty, C. J. (2004). Nitrogen cycles: Past, present, and future. Biogeochemistry, 70(2), 153–226. 10.1007/s10533-004-0370-0 [DOI] [Google Scholar]
- Gelman, A. (2008). Scaling regression inputs by dividing by two standard deviations. Statistics in Medicine, 27(15), 2865–2873. 10.1002/sim.3107 [DOI] [PubMed] [Google Scholar]
- Geyer, K. M. , Kyker‐Snowman, E. , Grandy, A. S. , & Frey, S. D. (2016). Microbial carbon use efficiency: Accounting for population, community, and ecosystem‐scale controls over the fate of metabolized organic matter. Biogeochemistry, 127(2–3), 173–188. 10.1007/s10533-016-0191-y [DOI] [Google Scholar]
- Giese, M. , Brueck, H. , Gao, Y. Z. , Lin, S. , Steffens, M. , Kögel‐Knabner, I. , … Han, X. G. (2013). N balance and cycling of Inner Mongolia typical steppe: A comprehensive case study of grazing effects. Ecological Monographs, 83(2), 195–219. 10.1890/12-0114.1 [DOI] [Google Scholar]
- Hamilton, E. W. , & Frank, D. A. (2001). Can plants stimulate soil microbes and their own nutrient supply? Evidence from a grazing tolerant grass. Ecology, 82(9), 2397–2402. 10.1890/0012-9658(2001)082[2397:CPSSMA]2.0.CO;2 [DOI] [Google Scholar]
- Hawkes, C. V. , & Sullivan, J. J. (2001). The impact of herbivory on plants in different resource conditions: A meta‐analysis. Ecology, 82(7), 2045–2058. 10.1890/0012-9658(2001)082[2045:tiohop]2.0.co;2 [DOI] [Google Scholar]
- Hempson, G. P. , Archibald, S. , & Bond, W. J. (2017). The consequences of replacing wildlife with livestock in Africa. Scientific Reports, 7, 17196 10.1038/s41598-017-17348-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hijmans, R. J. , Cameron, S. E. , Parra, J. L. , Jones, P. G. , & Jarvis, A. (2005). Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology, 25(15), 1965–1978. 10.1002/joc.1276 [DOI] [Google Scholar]
- Hungate, B. A. , Dukes, J. S. , Shaw, M. R. , Luo, Y. Q. , & Field, C. B. (2003). Nitrogen and climate change. Science, 302(5650), 1512–1513. 10.1126/science.1091390 [DOI] [PubMed] [Google Scholar]
- IPCC . (2014). Climate change 2014: Synthesis report In Core Writing Team , Pachauri R. K., & Meyer L. A. (Eds.), Contribution of working groups I, II and III to the fifth assessment report of the Intergovernmental Panel on Climate Change. Geneva, Switzerland: IPCC, 151 pp. [Google Scholar]
- Janis, C. M. , Damuth, J. , & Theodor, J. M. (2002). The origins and evolution of the North American grassland biome: The story from the hoofed mammals. Palaeogeography Palaeoclimatology Palaeoecology, 177(1–2), 183–198. 10.1016/s0031-0182(01)00359-5 [DOI] [Google Scholar]
- Jobbagy, E. G. , & Jackson, R. B. (2000). The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecological Applications, 10(2), 423–436. 10.2307/2641104 [DOI] [Google Scholar]
- Jobbagy, E. G. , & Jackson, R. B. (2001). The distribution of soil nutrients with depth: Global patterns and the imprint of plants. Biogeochemistry, 53(1), 51–77. 10.1023/a:1010760720215 [DOI] [Google Scholar]
- La Pierre, K. J. , & Smith, M. D. (2015). Functional trait expression of grassland species shift with short‐ and long‐term nutrient additions. Plant Ecology, 216(2), 307–318. 10.1007/s11258-014-0438-4 [DOI] [Google Scholar]
- Lange, M. , Eisenhauer, N. , Sierra, C. A. , Bessler, H. , Engels, C. , Griffiths, R. I. , … Gleixner, G. (2015). Plant diversity increases soil microbial activity and soil carbon storage. Nature Communications, 6, 10.1038/ncomms7707 [DOI] [PubMed] [Google Scholar]
- Lu, M. , Zhou, X. , Yang, Q. , Li, H. , Luo, Y. , Fang, C. , … Li, B. O. (2013). Responses of ecosystem carbon cycle to experimental warming: A meta‐analysis. Ecology, 94(3), 726–738. 10.1890/12-0279.1 [DOI] [PubMed] [Google Scholar]
- Manzoni, S. , Čapek, P. , Porada, P. , Thurner, M. , Winterdahl, M. , Beer, C. , … Way, D. (2018). Reviews and syntheses: Carbon use efficiency from organisms to ecosystems – Definitions, theories, and empirical evidence. Biogeosciences, 15(19), 5929–5949. 10.5194/bg-15-5929-2018 [DOI] [Google Scholar]
- McNaughton, S. J. (1985). Ecology of a grazing ecosystem – The Serengeti. Ecological Monographs, 55(3), 259–294. 10.2307/1942578 [DOI] [Google Scholar]
- McNaughton, S. J. , Milchunas, D. G. , & Frank, D. A. (1996). How can net primary productivity be measured in grazing ecosystems? Ecology, 77(3), 974–977. 10.2307/2265518 [DOI] [Google Scholar]
- McSherry, M. E. , & Ritchie, M. E. (2013). Effects of grazing on grassland soil carbon: A global review. Global Change Biology, 19(5), 1347–1357. 10.1111/gcb.12144 [DOI] [PubMed] [Google Scholar]
- Milchunas, D. G. , & Lauenroth, W. K. (1993). Quantitative effects of grazing on vegetation and soils over a global range of environments. Ecological Monographs, 63(4), 327–366. 10.2307/2937150 [DOI] [Google Scholar]
- Neff, J. C. , Reynolds, R. L. , Belnap, J. , & Lamothe, P. (2005). Multi‐decadal impacts of grazing on soil physical and biogeochemical properties in southeast Utah. Ecological Applications, 15(1), 87–95. 10.1890/04-0268 [DOI] [Google Scholar]
- Olff, H. , & Ritchie, M. E. (1998). Effects of herbivores on grassland plant diversity. Trends in Ecology & Evolution, 13(7), 261–265. 10.1016/s0169-5347(98)01364-0 [DOI] [PubMed] [Google Scholar]
- Parsons, A. , Rowarth, J. , Thornley, J. , & Newton, J. (2011). Primary production of grasslands, herbage accumulation and use and impacts of climate change In Lemaire G., Hodgson J., & Chabbi A. (Eds.), Grassland productivity and ecosystem services (pp. 3–18). Wallingford, UK: CAB International. [Google Scholar]
- Pastor, J. , Cohen, Y. , & Hobbs, N. T. (2006). The role of large herbivores in ecosystem nutrient cycles In Dannell R., Bergstrom R., Duncan P., & Pastor J. (Eds.), Large herbivore ecology, ecosystem dynamics and conservation (pp. 289–319). Cambridge: Cambridge University Press. [Google Scholar]
- Pastor, J. , Dewey, B. , Naiman, R. J. , McInnes, P. F. , & Cohen, Y. (1993). Moose browsing and soil fertility in the boreal forests of Isle Royale National Park. Ecology, 74(2), 467–480. 10.2307/1939308 [DOI] [Google Scholar]
- Pei, S. F. , Fu, H. , & Wan, C. G. (2008). Changes in soil properties and vegetation following exclosure and grazing in degraded Alxa desert steppe of Inner Mongolia, China. Agriculture Ecosystems & Environment, 124(1–2), 33–39. 10.1016/j.agee.2007.08.008 [DOI] [Google Scholar]
- Pineiro, G. , Paruelo, J. M. , Jobbagy, E. G. , Jackson, R. B. , & Oesterheld, M. (2009). Grazing effects on belowground C and N stocks along a network of cattle exclosures in temperate and subtropical grasslands of South America. Global Biogeochemical Cycles, 23, 10.1029/2007gb003168 [DOI] [Google Scholar]
- Pineiro, G. , Paruelo, J. M. , Oesterheld, M. , & Jobbagy, E. G. (2010). Pathways of grazing effects on soil organic carbon and nitrogen. Rangeland Ecology & Management, 63(1), 109–119. 10.2111/08-255.1 [DOI] [Google Scholar]
- Reeder, J. D. , Schuman, G. E. , Morgan, J. A. , & Lecain, D. R. (2004). Response of organic and inorganic carbon and nitrogen to long‐term grazing of the shortgrass steppe. Environmental Management, 33(4), 485–495. 10.1007/s00267-003-9106-5 [DOI] [PubMed] [Google Scholar]
- Richards, S. A. , Whittingham, M. J. , & Stephens, P. A. (2011). Model selection and model averaging in behavioural ecology: The utility of the IT‐AIC framework. Behavioral Ecology and Sociobiology, 65(1), 77–89. 10.1007/s00265-010-1035-8 [DOI] [Google Scholar]
- Ripple, W. J. , Newsome, T. M. , Wolf, C. , Dirzo, R. , Everatt, K. T. , Galetti, M. , … Van Valkenburgh, B. (2015). Collapse of the world’s largest herbivores. Science Advances, 1, e1400103 10.1126/sciadv.1400103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, M. E. (2014). Plant compensation to grazing and soil carbon dynamics in a tropical grassland. Peerj, 2, 10.7717/peerj.233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie, M. E. , & Tilman, D. (1995). Responses of legumes to herbivores and nutrients during succession on a nitrogen‐poor soil. Ecology, 76(8), 2648–2655. 10.2307/2265835 [DOI] [Google Scholar]
- Ritchie, M. E. , Tilman, D. , & Knops, J. M. H. (1998). Herbivore effects on plant and nitrogen dynamics in oak savanna. Ecology, 79(1), 165–177. 10.2307/176872 [DOI] [Google Scholar]
- Scheu, S. (1992). Automated measurement of the respiratory response of soil microcompartments – Active microbial biomass in earthworm feces. Soil Biology & Biochemistry, 24(11), 1113–1118. 10.1016/0038-0717(92)90061-2 [DOI] [Google Scholar]
- Schmitz, O. J. , Raymond, P. A. , Estes, J. A. , Kurz, W. A. , Holtgrieve, G. W. , Ritchie, M. E. , … Wilmers, C. C. (2014). Animating the carbon cycle. Ecosystems, 17(2), 344–359. 10.1007/s10021-013-9715-7 [DOI] [Google Scholar]
- Schrama, M. , Veen, G. F. , Bakker, E. S. , Ruifrok, J. L. , Bakker, J. P. , & Olff, H. (2013). An integrated perspective to explain nitrogen mineralization in grazed ecosystems. Perspectives in Plant Ecology Evolution and Systematics, 15(1), 32–44. 10.1016/j.ppees.2012.12.001 [DOI] [Google Scholar]
- Seabloom, E. W. , Borer, E. T. , Buckley, Y. , Cleland, E. E. , Davies, K. , Firn, J. , … Yang, L. (2013). Predicting invasion in grassland ecosystems: Is exotic dominance the real embarrassment of richness? Global Change Biology, 19(12), 3677–3687. 10.1111/gcb.12370 [DOI] [PubMed] [Google Scholar]
- Singer, F. J. , & Schoenecker, K. A. (2003). Do ungulates accelerate or decelerate nitrogen cycling? Forest Ecology and Management, 181, 189–204. 10.1016/s0378-1127(03)00133-6 [DOI] [Google Scholar]
- Sokol, N. W. , & Bradford, M. A. (2019). Microbial formation of stable soil carbon is more efficient from belowground than aboveground input. Nature Geoscience, 12(1), 46–53. 10.1038/s41561-018-0258-6 [DOI] [Google Scholar]
- Souttie, J. , Reynold, S. , & Batello, C. (2005). Grasslands of the world. Rome, Italy: Food and Agriculture Organization. [Google Scholar]
- Steffens, M. , Kolbl, A. , Totsche, K. U. , & Kogel‐Knabner, I. (2008). Grazing effects on soil chemical and physical properties in a semiarid steppe of Inner Mongolia (PR China). Geoderma, 143(1–2), 63–72. 10.1016/j.geoderma.2007.09.004 [DOI] [Google Scholar]
- Steinauer, E. M. , & Collins, S. L. (2001). Feedback loops in ecological hierarchies following urine deposition in tallgrass prairie. Ecology, 82(5), 1319–1329. 10.1890/0012-9658(2001)082[1319:FLIEHF]2.0.CO;2 [DOI] [Google Scholar]
- Svenning, J.‐C. , Pedersen, P. B. M. , Donlan, C. J. , Ejrnæs, R. , Faurby, S. , Galetti, M. , … Vera, F. W. M. (2016). Science for a wilder Anthropocene: Synthesis and future directions for trophic rewilding research. Proceedings of the National Academy of Sciences of the United States of America, 113(4), 898–906. 10.1073/pnas.1502556112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanentzap, A. J. , & Coomes, D. A. (2012). Carbon storage in terrestrial ecosystems: Do browsing and grazing herbivores matter? Biological Reviews, 87(1), 72–94. 10.1111/j.1469-185X.2011.00185.x [DOI] [PubMed] [Google Scholar]
- Teague, W. R. , Dowhower, S. L. , Baker, S. A. , Haile, N. , DeLaune, P. B. , & Conover, D. M. (2011). Grazing management impacts on vegetation, soil biota and soil chemical, physical and hydrological properties in tall grass prairie. Agriculture Ecosystems & Environment, 141(3–4), 310–322. 10.1016/j.agee.2011.03.009 [DOI] [Google Scholar]
- Thebault, A. , Mariotte, P. , Lortie, C. J. , & MacDougall, A. S. (2014). Land management trumps the effects of climate change and elevated CO2 on grassland functioning. Journal of Ecology, 102(4), 896–904. 10.1111/1365-2745.12236 [DOI] [Google Scholar]
- Tylianakis, J. M. , Didham, R. K. , Bascompte, J. , & Wardle, D. A. (2008). Global change and species interactions in terrestrial ecosystems. Ecology Letters, 11(12), 1351–1363. 10.1111/j.1461-0248.2008.01250.x [DOI] [PubMed] [Google Scholar]
- van der Graaf, A. J. , Stahl, J. , Veen, G. F. , Havinga, R. M. , & Drent, R. H. (2007). Patch choice of avian herbivores along a migration trajectory – From Temperate to Arctic. Basic and Applied Ecology, 8(4), 354–363. 10.1016/j.baae.2006.07.001 [DOI] [Google Scholar]
- van der Waal, C. , Kool, A. , Meijer, S. S. , Kohi, E. , Heitkönig, I. M. A. , de Boer, W. F. , … de Kroon, H. (2011). Large herbivores may alter vegetation structure of semi‐arid savannas through soil nutrient mediation. Oecologia, 165(4), 1095–1107. 10.1007/s00442-010-1899-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Groenigen, J. W. , van Kessel, C. , Hungate, B. A. , Oenema, O. , Powlson, D. S. , & van Groenigen, K. J. (2017). Sequestering soil organic carbon: A nitrogen dilemma. Environmental Science & Technology, 51(9), 4738–4739. 10.1021/acs.est.7b01427 [DOI] [PubMed] [Google Scholar]
- Van Uytvanck, J. , Milotic, T. , & Hoffmann, M. (2010). Nitrogen depletion and redistribution by free‐ranging cattle in the restoration process of mosaic landscapes: The role of foraging strategy and habitat proportion. Restoration Ecology, 18, 205–216. 10.1111/j.1526-100X.2009.00599.x [DOI] [Google Scholar]
- White, R. , Murray, S. , & Rohweder, M. (2000). Pilot analysis of global ecosystems: Grassland ecosystems technical report. Washington, DC: World Resources Institute. [Google Scholar]
- Zhou, G. , Zhou, X. , He, Y. , Shao, J. , Hu, Z. , Liu, R. , … Hosseinibai, S. (2017). Grazing intensity significantly affects belowground carbon and nitrogen cycling in grassland ecosystems: A meta‐analysis. Global Change Biology, 23(3), 1167–1179. 10.1111/gcb.13431 [DOI] [PubMed] [Google Scholar]
- Ziter, C. , & MacDougall, A. S. (2013). Nutrients and defoliation increase soil carbon inputs in grassland. Ecology, 94(1), 106–116. 10.1890/11-2070.1 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data associated with this study are available from the Dryad Data Repository https://doi.org/10.5061/dryad.wstqjq2gw.


