Summary
Although 18F‐fluorodeoxyglucose positron emission tomography (18F‐FDG PET) is commonly used for initial staging and therapeutic response evaluation in aggressive lymphomas, its prognostic utility for mantle cell lymphoma (MCL) is controversial. Therefore, we retrospectively evaluated the correlations of interim PET (iPET) and end‐of‐treatment PET (ePET) response with survival outcomes in 89 consecutive advanced MCL patients treated with frontline R‐CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine and prednisolone). iPET positivity was strongly associated with inferior five‐year overall survival (OS) [hazard ratio (HR) 7·84, P < 0·0001] and poor five‐year progression‐free survival (PFS) (HR 3·34, P < 0·0001). OS and PFS were more favourable in the order early metabolic responder (iPETneg → ePETneg), delayed responder (iPETpos → ePETneg), loss‐metabolic responder (iPETneg → ePETpos), and never‐metabolic responder (iPETpos → ePETpos). In the autologous haematopoietic stem cell transplantation (auto‐HSCT)‐fit subgroup, OS was more favourable in the order early metabolic responders, delayed metabolic responders, and non‐metabolic responders, with a marginal trend toward statistical significance (HR 3·41, P = 0·051), and PFS was significantly superior in early metabolic responders (HR 4·43, P = 0·002). In a group that was ineligible for auto‐HSCT, OS and PFS were significantly superior in early metabolic responders. Our results suggested that iPET is of prognostic value and an independent predictor of survival in MCL patients receiving frontline R‐CHOP. Therefore, prospective clinical trials of iPET‐guided treatment strategies for these patients are warranted.
Keywords: mantle cell lymphoma, interim 18F‐FDG PET, prognosis, R‐CHOP, treatment response
Mantle cell lymphoma (MCL) is a challenging subtype of non‐Hodgkin lymphoma (NHL), accounting for 3–6% of all NHL cases and having a poor prognosis due to an undesirable disease course and aggressive clinical behaviour. The majority of MCL patients are diagnosed with advanced stage disease and commonly present with extranodal involvement, including bone marrow, skin, or the gastrointestinal tract (Abrahamsson et al., 2014). However, the results of conventional chemotherapy are disappointing, and this disease entity shows low sensitivity to conventional chemotherapeutic regimens. The high relapse rate commonly results in disseminated refractory lymphoma (Cheah et al., 2016), and the remission duration is short at approximately three years with an overall survival (OS) of 3–4 years (Freytes et al., 2012).
To improve the poor prognosis of MCL, a more effective therapeutic strategy was established involving induction chemotherapy, and major clinical trials over the last decade have focused on improving frontline treatment of MCL. The therapeutic approach was adjusted according to the risk profile of each patient, based on their biological age, comorbidities, and general performance status (Smolewski et al., 2015). Young and fit patients with MCL are considered suitable for dose‐intensive therapeutic approaches, followed (in responsive patients) by autologous haematopoietic stem cell transplantation (auto‐HSCT) (Dreyling et al., 2014b). Elderly or young patients in poor health are prescribed less aggressive regimens without auto‐HSCT. Accordingly, and due also to the availability of rituximab, steady improvements in the survival outcomes of patients with advanced MCL have been seen over the past several years.
Although auto‐HSCT is considered after frontline chemotherapy according to the therapeutic guidelines for MCL (Dreyling et al., 2014b), in many cases it has not been adopted. In daily clinical practice, optimal frontline therapy is performed using the R‐CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone) regimen (Cheminant et al., 2015). However, methods for early prediction of R‐CHOP‐related outcomes have not been thoroughly evaluated in patients with MCL.
Our study focused on identifying and validating factors predicting therapeutic outcomes in MCL patients treated with a standard frontline R‐CHOP regimen. We were interested not only in well‐known biological factors, but also in the relationship between 18F‐fluorodeoxyglucose positron emission tomography‐computed tomography (18F‐FDG PET‐CT) findings and therapeutic efficacy. The revised (2007) International Working Group (IWG) criteria for malignant lymphoma included 18F‐FDG PET‐CT findings, and this modality is now considered essential for predicting therapeutic response in cases of Hodgkin lymphoma and diffuse large B‐cell lymphoma (Cheson et al., 2007; Juweid et al., 2007; Cheson et al., 2014; Lamonica et al., 2017). Although MCL is an FDG‐avid NHL subtype, PET‐CT is still not strongly recommended for therapeutic assessment due to inconsistent findings in the literature (Brepoels et al., 2008; Mato et al., 2012; Barrington & Johnson, 2017; Barrington & Kluge, 2017; Lamonica et al., 2017).
Therefore, we examined the prognostic impact of the FDG‐PET‐assessed treatment response in MCL patients. The prognostic value of interim PET (iPET) and end‐of‐treatment PET (ePET)‐assessed treatment response, indexed by the Deauville five‐point scale, as well as other biological factors, was retrospectively analysed in a homogenous MCL cohort undergoing treatment with a standard R‐CHOP regimen, with long‐term follow‐up duration.
Patients and methods
Patients
Consecutive adult patients diagnosed with MCL from January 2007 to January 2018 at a single centre (Lymphoma‐Myeloma Department, Catholic Hematology Hospital, Seoul, Korea) were screened. All biopsy specimens had been histopathologically confirmed as CD20‐positive, cyclin D1‐positive, and non‐blastoid subtype B‐cell MCL, according to the current World Health Organization classification, by lymphoma‐specific pathologists of the Catholic University Lymphoma Group (CULG). Among the specimens, those from patients with stage II bulky masses or Ann Arbor stage III–IV disease were identified, after which uniformly treated MCL patients undergoing a standard dose of frontline R‐CHOP regimen were selected for analysis. All patient’s 18F‐FDG PET‐CT interpretations were double‐checked by two different nuclear medicine specialists among CULG members. The investigation was extended to October 2018 to ensure a minimum follow‐up duration of six months. Clinical data including demographic information, initial or salvage chemotherapy, and therapeutic response to initial or salvage chemotherapy were retrospectively extracted from the patients’ electronic medical records. The study was approved by the Institutional Review Board of Seoul St. Mary’s Hospital of the Catholic University of Korea in accordance with the Declaration of Helsinki.
Frontline therapeutic strategy
All patients received the first three cycles of R‐CHOP (rituximab 375 mg/m2 day 1, cyclophosphamide 750 mg/m2 day 1, doxorubicin 50 mg/m2 day 1, vincristine 1·4 mg/m2 day 1, and prednisolone 40 mg/m2 days 1–5) at three‐week intervals as frontline chemotherapy (Jardin et al., 2010). Continuation of R‐CHOP or transition to another salvage chemotherapy was determined based on the interim therapeutic response. Under the European Society for Medical Oncology (ESMO) and National Comprehensive Cancer Network (NCCN) guidelines, young and fit patients showing a favourable response to R‐CHOP were administered upfront high‐dose chemotherapy followed by autologous stem cell rescue. Autologous stem cell mobilization was performed after six cycles of R‐CHOP, as well as a response evaluation for patients in whom auto‐HSCT was planned. For auto‐HSCT, the conditioning regimen was a combination of busulfan (2·4 mg/kg/day for three consecutive days), melphalan (40 mg/m2 per day for two days), and thiotepa (200 mg/m2 per day for two consecutive days) in our centre (Lee et al., 2010; Yoon et al., 2019).
Young responders to frontline R‐CHOP who were not suitable for upfront auto‐HSCT and elderly patients (≥65 years) completed six full cycles of R‐CHOP chemotherapy followed by regular monitoring until disease progression.
Efficacy of R‐CHOP assessed using 18F‐FDG PET‐CT
All patients with MCL underwent PET‐CT diagnosis prior to treatment, during chemotherapy, and as a final evaluation of the response to R‐CHOP. A diagnostic pretreatment PET‐CT scan was performed within four weeks before initiation of R‐CHOP. The iPET‐CT was performed after the third cycle of R‐CHOP. An ePET scan was performed within eight weeks of completion of R‐CHOP. The Deauville scale (DS) was used to measure 18F‐FDG‐uptake in PET‐CT (Meignan et al., 2012). FDG uptake in the hottest residual mass was compared with uptake in the liver. PET positivity was defined as a DS 4 (FDG uptake moderately higher than in the liver) or DS 5 (FDG uptake markedly higher than in the liver). A DS ≤3 (DS 1, no uptake; DS 2, uptake no more than that in the mediastinum; DS 3, uptake greater than that in the mediastinum but no more than that in the liver) was defined as PET negativity.
Statistical analysis
Progression‐free survival (PFS) was defined as the time from the end of frontline R‐CHOP chemotherapy to the time of documented disease progression/recurrence or death. OS was calculated from the time of R‐CHOP initiation to death from any cause. Patients with documented disease progression or death or who were lost to follow‐up were censored from further analysis. Surviving patients were censored on the last day of follow‐up. All R‐CHOP‐related categorical variables are expressed as proportions and were compared by the chi‐square test and Fischer’s exact test. For continuous variables, the median and range were calculated and compared between two groups using the Mann–Whitney U‐test. All patients were classified using the International Prognostic Index (IPI) and four Mantle Cell Lymphoma International Prognostic Index (MIPI) scoring systems (standard MIPI, simplified MIPI, biologic MIPI, and combined MIPI) (Hoster et al., 2008). PFS and OS rates were calculated using the Kaplan–Meier survival method and log‐rank analysis. Univariate and multivariate Cox regression analyses were performed to determine the independent factors affecting PFS or OS. Cumulative incidence estimates of relapse were calculated, with relapse or death from other causes defined as competitive events using the Gray test for the univariate analyses and the Fine–Gray method for the multivariate analyses. Multivariate models for predicting OS and PFS were developed, including parameters significant at P < 0·05. A risk score was assigned to each parameter based on respective standardized β‐coefficients, where the lowest score had a value of 1. Using a gradation of 0·25 (i.e., 0·25, 0·5, 1·0, etc.), other values were rounded to the nearest gradation according to their standardized β‐coefficient values. All statistical analyses were performed using R software (version 3.2.0; R Core Team, R Foundation for Statistical Computing, Vienna, Austria) and the EZR graphical user interface (Saitama Medical Center, Jichi Medical University, Saitama, Japan) (Kanda, 2013).
Results
Clinical characteristics
A total of 126 patients diagnosed with MCL were initially identified; 36 patients were excluded due to being treated by a frontline rituximab plus Ara‐C (cytarabine)‐based regimen (n = 16), loss to follow‐up (n = 6), receiving no treatment due to frailty (n = 4), or the unavailability of ePET data (n = 10). A total of 90 patients treated with frontline R‐CHOP were included in the analysis. All 90 patients underwent baseline 18F‐FDG PET, iPET, and ePET. One patient whose disease progression was confirmed during an early examination of ePET‐CT, due to suspected progressive disease after the fifth cycle of R‐CHOP, was excluded from this analysis. Therefore, 89 patients who were administered a full cycle of frontline R‐CHOP combined with the complete number of 18F‐FDG PET‐CT scans were analysed in this study (Fig 1). All relevant biological information and therapeutic results of the enrolled patients, including 18F‐FDG PET data, were available for analysis. The baseline clinical characteristics of the MCL patients, including staging and MCL risk classification, are shown in Table 1.
Figure 1.
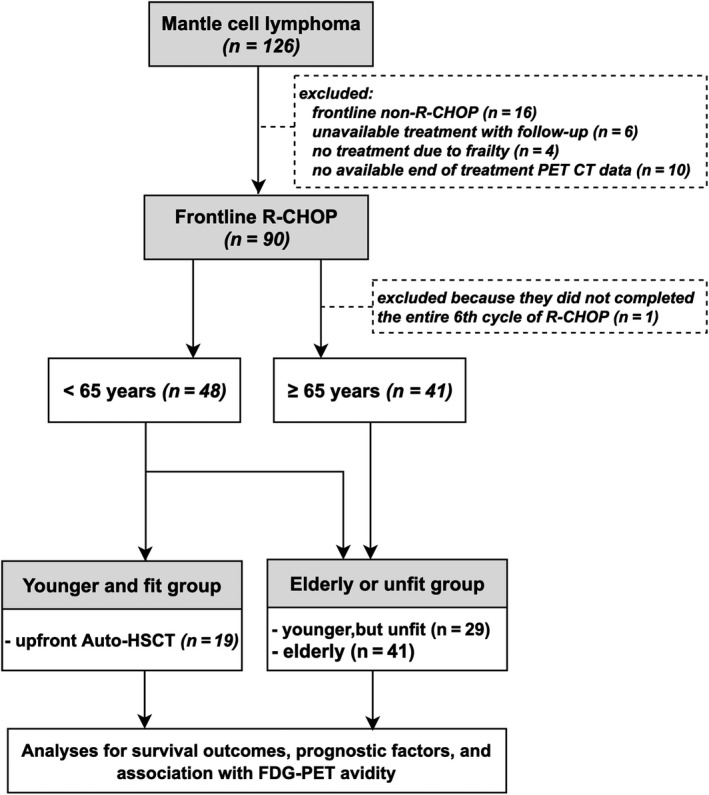
Flow diagram of mantle cell lymphoma (MCL) patients receiving frontline rituximab, cyclophosphamide, doxorubicin, vincristine, prednisolone (R‐CHOP) therapy.
Table 1.
Baseline characteristics of mantle cell lymphoma patients (n = 89) with frontline R‐CHOP.
| Characteristics at diagnosis | Number of patients (%) |
|---|---|
| Gender | |
| Male | 72 (81) |
| Female | 17 (19) |
| Age, median, years (range) | 64 (26–84) |
| ≥65 | 41 (46) |
| Pathological subtype | |
| Classic | 87 (98) |
| Small cell variant | 2 (2) |
| Ann‐Arbor stage | |
| II* | 8 (9) |
| III | 18 (20) |
| IV | 63 (71) |
| ECOG performance status | |
| 0 | 33 (37) |
| 1 | 44 (50) |
| 2 | 8 (9) |
| 3 | 4 (4) |
| Presence of B symptoms | 33 (37) |
| LDH, median (range) | 395 (158–5196) |
| Elevated (>450 U/l) | 34 (38) |
| WBC (×109/l), median (range) | 6·9 (1·9–60·3) |
| Ki‐67 index | |
| <30% | 56 (63) |
| ≥30% | 33 (37) |
| Presence of BM involvement | 55 (61) |
| Upfront auto‐HSCT | 19 (21) |
| IPI | |
| Low | 20 (22) |
| Low‐intermediate | 27 (30) |
| High‐intermediate | 22 (25) |
| High | 20 (22) |
| Standard MIPI | |
| Low | 37 (42) |
| Intermediate | 23 (26) |
| High | 29 (32) |
| Simplified MIPI | |
| Low | 38 (43) |
| Intermediate | 31 (35) |
| High | 20 (22) |
| Biological MIPI | |
| Low | 20 (22) |
| Intermediate | 21 (24) |
| High | 48 (54) |
| Combined MIPI | |
| Low | 23 (26) |
| Low‐intermediate | 31 (35) |
| High‐intermediate | 21 (23) |
| High | 14 (16) |
ECOG, Eastern Cooperative Oncology Group; LDH, lactate dehydrogenase; WBC, white blood cells; BM, bone marrow; HSCT, haematopoietic stem transplantation; IPI, International Prognostic Index; MIPI, Mantle Cell Lymphoma International Prognostic Index.
All cases with Ann‐Arbor stage II had a huge mass (≥10 cm).
Survival outcomes according to 18F‐FDG PET
All 89 patients underwent a baseline PET‐CT scan before initiation of frontline R‐CHOP, in addition to iPET followed by ePET‐CT during R‐CHOP chemotherapy. FDG avidity on pretreatment PET‐CT was noted in 94% of MCL patients (n = 84), with a median maximum standardized uptake value (SUV) of 9·2 (range: 2·1–42·5) at the initial diagnosis. The PET avidity at each time point was significantly correlated with the clinical outcomes (Table SI, Fig 2A, 2); patients with iPET positivity had poorer five‐year OS [hazard ratio (HR), 7·84; 95% confidence interval (CI): 2·39–25·66, P < 0·0001] and poorer five‐year PFS (HR, 3·34; 95% CI: 1·88–5·93, P < 0·0001) compared with those without iPET positivity. In addition, positivity on ePET‐CT was significantly associated with inferior five‐year OS (HR, 6·04; 95% CI: 2·88–12·65, P < 0·0001) as well as poor five‐year PFS (HR, 4·76; 95% CI: 2·77–8·18, P < 0·0001) (Fig 2C, 2).
Figure 2.
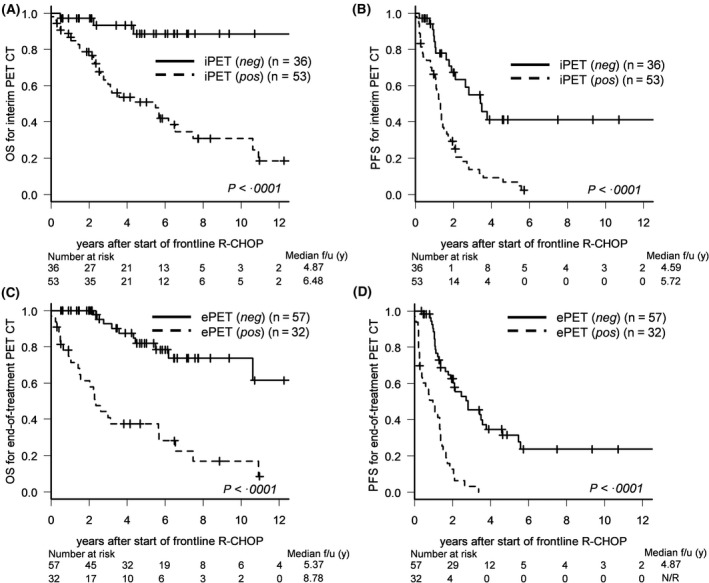
Survival outcomes according to interim positron emission tomography (iPET) and end‐of‐treatment positron emission tomography‐computed tomography (ePET‐CT). (A) Overall survival (OS) and (B) progression‐free survival (PFS) according to interim PET response. (C) OS and (D) PFS according to ePET.
Survival outcomes according to serial changes in 18F‐FDG PET positivity
The results outlined above indicated that PET avidity at each independent time point during chemotherapy was significantly related to survival outcome. Based on these results, we examined whether survival outcomes were affected by serial dynamic changes in PET avidity during frontline R‐CHOP chemotherapy.
Based on the metabolic changes in PET‐CT during R‐CHOP chemotherapy, our cohort was subdivided into the following groups: metabolic responders, including early metabolic responders (iPET‐negative → ePET‐negative) and delayed metabolic responders (iPET‐positive → ePET‐negative); and non‐metabolic responders, including loss‐metabolic responders (iPET‐negative → ePET‐positive) and never‐metabolic responders (iPET‐positive → ePET‐positive). OS and PFS were best in early metabolic responders, and the poorest outcomes were seen in never‐metabolic responders: OS rates at five years were 94% (95% CI: 68–99), 67% (95% CI: 40–84), 33% (95% CI: 10–62), and 38% (95% CI: 20–55) in early metabolic responders, delayed metabolic responders, loss‐metabolic responders, and never‐metabolic responders respectively (Fig 3A). PFS rates at three years were 61% (95% CI: 40–77), 27% (95% CI: 10–46), 0%, and 4% (95% CI: 2–15) respectively (Fig 3B).
Figure 3.
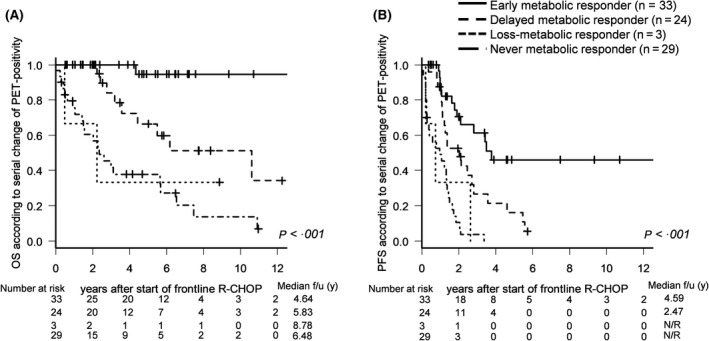
Survival outcomes according to serial changes in PET response. (A) OS and (B) PFS according to PET response in early metabolic responders, delayed metabolic responders, loss‐metabolic responders, and never‐metabolic responders during frontline R‐CHOP.
Survival analysis of young/fit versus elderly/unfit auto‐HSCT subgroups
As the survival outcome interpretation of metabolic responsiveness according to PET‐CT could be affected by the intensity of therapy, such as auto‐HSCT, the relationship between FDG avidity and survival outcomes was analysed separately in subgroups with and without auto‐HSCT.
In the subgroup receiving upfront auto‐HSCT (n = 19), OS was more favourable in the order of early metabolic responders, delayed metabolic responders, and non‐metabolic responders, with a marginal trend toward statistical significance (HR, 3·41; 95% CI: 0·83–14·05, P = 0·051; Fig 4A, Figure S1). PFS was significantly superior in early metabolic responders (HR, 4·43; 95% CI: 1·64–11·95, P = 0·002; Fig 4A, Figure S2). In the other auto‐HSCT subgroup of young/unfit or elderly patients (n = 70), OS and PFS were significantly superior in the order of early metabolic responders, delayed metabolic responders, and non‐metabolic responders (HR, 4·49; 95% CI: 2·39–8·46, P < 0·0001 for OS; HR, 2·56; 95% CI: 1·75–3·74, P < 0·0001 for PFS; Fig 4B, Figure S3).
Figure 4.
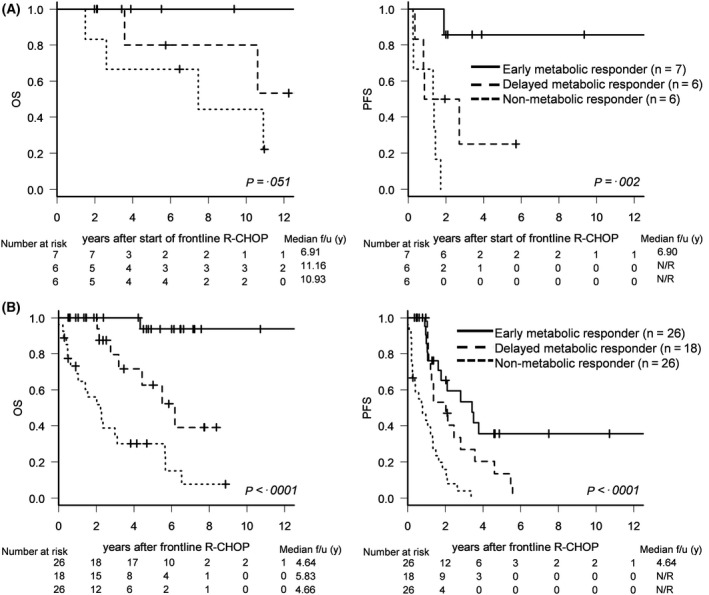
Survival outcomes according to serial changes in PET response. (A) OS and PFS according to serial changes in PET positivity in the young and fit autologous haematopoietic stem transplantation (auto‐HSCT) subgroup (n = 19). (B) OS and PFS according to serial changes in PET positivity in the unfit or elderly auto‐HSCT subgroup (n = 70).
Analyses of prognostic factors affecting survival outcomes after frontline R‐CHOP
The prognostic factors affecting survival outcome were analysed according to baseline biological risk factors. Using factors that were significant in univariate analysis (Table SI), multivariate analysis was performed. To exclude confounding effects due to duplicated or collinear factors, multivariate analysis was performed using only the biological Mantle Cell Lymphoma International Prognostic Index (b‐MIPI) score, which showed the highest HR in univariate analysis. As a result, advanced b‐MIPI (HR, 1·24; 95% CI: 0·18–8·70, P = 0·028 for intermediate risk; and HR, 8·59; 95% CI: 2·05–35·90, P = 0·003 for high risk), iPET positivity (HR, 3·74; 95% CI: 1·05–13·36, P = 0·042), as well as ePET positivity (HR, 4·41; 95% CI: 1·92–10·14, P < 0·001) were independently associated with poor OS in the multivariate analysis (Table SII). In addition, poor PFS‐related independent factors were similar to those for OS: advanced b‐MIPI (HR, 1·16; 95% CI: 0·73–1·79, P = 0·543 for intermediate risk; HR, 1·91; 95% CI: 0·91–4·03, P = 0·021 for high risk), iPET positivity (HR, 2·05; 95% CI: 1·05–4·01, P = 0·035), and ePET positivity (HR, 3·45; 95% CI: 1·89–6·31, P < 0·001) (Table SII).
PET as a putative prognostic factor during systemic chemotherapy
Multivariate analysis revealed that b‐MIPI, iPET, and ePET status were important independent prognostic factors for survival outcome (Table SII). A risk score for each patient was derived by summing the scores of the b‐MIPI, iPET, and ePET parameters, which together constitute a modified MIPI scoring system based on β‐coefficients (Table 2).
Table 2.
Components of our modified prognostic scoring system: b‐MIPI, iPET, and ePET scores.
| Factors | OS | PFS | ||||||
|---|---|---|---|---|---|---|---|---|
| β‐coefficients (95% CI) | P‐value | log HR | Score | β‐coefficients (95% CI) | P‐value | log HR | Score | |
| Biological MIPI | ||||||||
| Low | 0 (reference) | 0 | 0 | 0 (reference) | 0 | 0 | ||
| Intermediate | 1·24 (0·18–8·70) | 0·828 | 0 | 0 | 1·16 (0·73–1·79) | 0·543 | 0 | 0 |
| High | 8·59 (2·05–35·90) | 0·003 | 0·840 | 0·75 | 1·91 (0·91–4·03) | 0·090 | 0·217 | 0·25 |
| Interim PET‐CT | ||||||||
| Negative | 0 (reference) | 0 | 0 | 0 (reference) | 0 | 0 | ||
| Positive | 3·74 (1·05–13·36) | 0·042 | 0·480 | 0·5 | 2·05 (1·05–4·01) | 0·035 | 0·248 | 0·25 |
| End‐of‐treatment PET | ||||||||
| Negative | 0 (reference) | <0·001 | 0 | 0 | 0 (reference) | <0·001 | 1 | 0 |
| Positive | 4·41 (1·92–10·14) | 0·551 | 0·5 | 3·45 (1·89–6·31) | 0·473 | 0·5 | ||
OS, overall survival; PFS, progression‐free survival; CI, confidence interval; HR, hazard ratio; MIPI, Mantle Cell Lymphoma International Prognostic Index; b‐MIPI, biological MIPI; PET‐CT, positron emission tomography‐computed tomography.
The five‐year OS was 100% (median not reached), 70·7% (median, 10·5 years; 95% CI: 51–84%), and 15·8% (median, 1·42 years; 95% CI: 4–35%) in the low‐risk group (score range: 0), intermediate‐risk group (score range: 0·5–1·25), and high‐risk group (score range: 1·75) respectively according to the modified scoring system (Fig 2A2). In addition, the three‐year PFS was 75·5% (median not reached; 95% CI: 47–90%), 23·7% (median, 1·4 years; 95% CI: 12–38%), and 0% (median, 0·4 years) in the low‐risk group (score range: 0), intermediate‐risk group (score range: 0·25–0·75), and high‐risk group (score range: 1·0) respectively, using the modified scoring system (Fig 2B2). Overall OS and PFS based on our modified scoring system were more accurate than those based on the conventional b‐MIPI‐based system (HR, 2·81; 95% CI: 1·83–4·31, P = 0·00000122 vs. HR, 9·05; 95% CI 4·67–17·53, P = 0·00000002 for OS and HR, 1·62; 95% CI: 1·22–2·16, P = 0·00244 vs. HR, 3·92; 95% CI: 2·54–6·07, P = 0·0000000008 for DFS, Fig 5), and differences among the risk groups were clearer using our modified scoring system.
Figure 5.
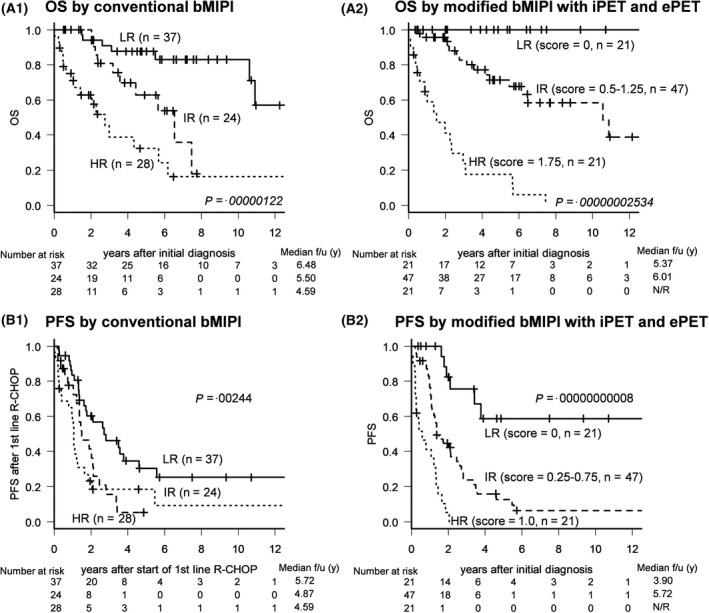
Modified biologic Mantle Cell Lymphoma International Prognostic Index (b‐MIPI) scoring system. The modified scoring system based on biologic MIPI (b‐MIPI), interim PET (iPET), and end‐of‐treatment (ePET) scores (A2, B2) had higher prognostic accuracy than the conventional b‐MIPI‐based system (A1, B1).
As another method of data validation, receiver operator characteristic (ROC) curves were compared for paired samples: the ROC curve of the modified scoring system illustrated that it had better diagnostic capability than the conventional system, with a significant improvement in predictive accuracy for both OS [area under curve (AUC), 0·902 vs. 0·736 respectively, P = 0·00007, Fig 6A) and PFS (AUC, 0·843 vs. 0·620 respectively, P = 0·00002, Fig 6B).
Figure 6.
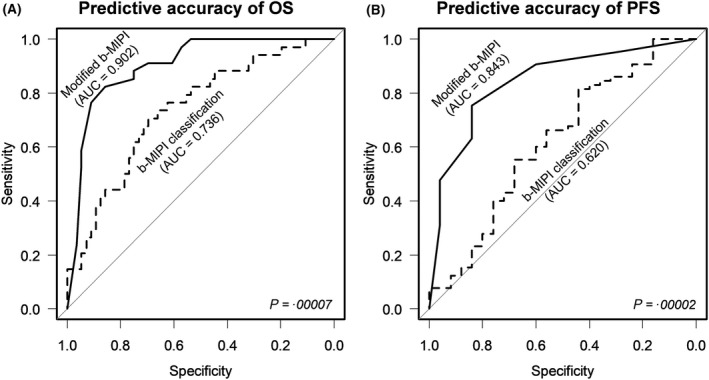
Comparison of receiver operating characteristic (ROC) curves between the conventional and modified b‐MIPI‐based scoring systems. (A) Predictive capability for OS and (B) PFS based on area under the curve (AUC) analysis.
Discussion
The results of this study demonstrated that 18F‐FDG PET status can independently predict the prognosis of patients with MCL during or after frontline chemotherapy. In the analysis of serial changes in PET‐CT response during frontline chemotherapy, early metabolic responders showed superior OS and PFS than delayed metabolic responders and non‐metabolic responders. In survival analysis of young and fit versus elderly or unfit auto‐HSCT subgroups, patients in both subgroups with iPET or ePET negativity showed superior survival outcomes.
Positron emission tomography‐computed tomography is recommended for assessing the therapeutic response in almost all subtypes of lymphoma (Romaguera et al., 2005; Geisler et al., 2012; Dreyling et al., 2014a). However, in the case of MCL, the prognostic value of 18F‐FDG PET‐CT is still a matter of debate, as the degree of 18F‐FDG avidity for MCL remains unclear (Karam et al., 2009; Hosein et al., 2011; Kedmi et al., 2014). In addition, studies using PET imaging to assess MCL have been non‐uniform due to a lack of prospective data and heterogeneous treatment strategies.
Taking these points into consideration, along with the low incidence rate of MCL, our study was unique in that we investigated the correlation between cross‐sectional PET‐CT data and survival outcome, as well as between serial changes in PET response and survival outcome, in a large series of MCL patients treated uniformly with frontline the full cycle of R‐CHOP.
Most previous studies on the relationship between PET‐CT data and lymphoma used continuous variables, such as the maximum SUV (SUVmax) by body weight or body surface area. In our cohort, the SUVmax had adequate accuracy for predicting survival outcomes (Table SIII). Although methods based on continuous variables obviously have objectivity, categorical (positive vs. negative) PET data derived using a DS measurement system may be better due to more convenient diagnosis, reasonable reproducibility, and flexibility in a real‐world clinical environment (Barrington et al., 2014; Cheson et al., 2014). In addition, several validation studies confirmed that response assessments based on the DC method could predict outcomes and demonstrated excellent interobserver agreement (Biggi et al., 2013; Itti et al., 2013). Although the major purpose of this study was not to compare the efficacy of DS and the SUVmax method, our study indirectly confirmed that the DS calculation method may be sufficient to determine survival outcomes independently in multivariate analyses.
Our results also suggested that measurement of consecutive serial changes in PET response was useful for predicting patient survival outcomes. In detail, early metabolic responders showed a more favourable PFS rate than delayed metabolic responders and non‐metabolic responders. To examine the correlation between PET response and survival outcome, we devised a modified b‐MIPI‐based scoring system that added PET results to the conventional b‐MIPI system. The b‐MIPI classification system includes the Ki‐67 index as a biological marker and is an excellent prognostic tool widely used for MCL patients; we confirmed this in our cohort. Interestingly, our modified scoring system accurately distinguished MCL patient risk groups. Thus, while the b‐MIPI can be used as an objective measure of disease status at initial diagnosis, the iPET component of our modified scoring system could be useful as a biological indicator. Therefore, the evaluation system combined with PET results is expected to show enhanced predictive value, where PET‐CT is important for evaluating tumour metabolic burden in real‐time as a non‐invasive imaging modality.
Among our entire cohort of 126 patients diagnosed with MCL, at least 90 patients (>70%) were treated with frontline R‐CHOP. Several patients had been treated with aggressive, cytarabine‐based induction regimens, which are used as primary chemotherapy regimens in Western countries (Smolewski et al., 2015). However, most of our patients failed to complete the entire cycle of chemotherapy in our institute due to frequent chemotherapy‐related infections or haematological adverse events. Most patients were treated with frontline R‐CHOP because it is a less aggressive regimen than those based on cytarabine. In relation to this, several studies have reported that post‐treatment PET evaluation may be useful for predicting prognosis, especially in patients receiving the bendamustine/rituximab (BR) regimen or combined rituximab/bendamustine/low‐dose cytarabine regimen as less intensive chemotherapy regimens due to ineligibility for an intensive regimen or auto‐HSCT (Visco et al., 2013; Lamonica et al., 2017). However, these studies focused mainly on the utility for ePET‐CT. Moreover, Mato et al., (2012) also reported an association between post‐treatment PET positivity and inferior PFS, with a trend toward inferior OS, in MCL patients treated with frontline dose‐intensive chemotherapy [R‐hyperCVAD (cyclophosphamide, vincristine, adriamycin, and dexamethasone) regimen]. In contrast to our study’s outcome, their results did not support the prognostic utility of PET‐CT in interim treatment evaluation. Considering these previous results, our study only included patients receiving frontline R‐CHOP regimen. Therefore, our results should be confirmed in future prospective studies using other aggressive frontline chemotherapy regimens, such as the cytarabine‐based or rituximab plus bendamustine regimen.
Dreyling et al., (2014b) recommended upfront auto‐HSCT in selected MCL patients to improve survival outcomes, where a tailored therapeutic approach is important to enhance survival outcomes. Our subgroup analysis of the correlation between FDG metabolic responders and auto‐HSCT showed that PFS was significantly improved by upfront auto‐HSCT in those showing an early metabolic response (Figure S4A). However, although patients with auto‐HSCT had more favourable conditions (young age or fewer comorbidities), OS and PFS did not differ by auto‐HSCT among the delayed metabolic responders (Figure S4B). These results indirectly indicated that auto‐HSCT consolidation treatment is the most appropriate approach for early metabolic responders. On the other hand, in delayed metabolic responders who transitioned from iPET‐positive to ePET‐negative status, auto‐HSCT may not improve the survival outcome. Taken together, these results suggest that the patient group appropriate for auto‐HSCT should be selected more specifically; it is possible that early metabolic responders may be more suitable for auto‐HSCT and the delayed responders more suitable for close observation after frontline chemotherapy or maintenance therapy. However, it is important to verify our results based on a future prospective study.
This study has some limitations that should be considered. First, it was a retrospective, single‐institution study without prospective surveillance or follow‐up, so bias and unmeasured confounding factors may have influenced our results. Second, the statistical results regarding auto‐HSCT should be interpreted with caution because of the small number of patients. Third, by selecting only patients who performed iPET and ePET‐CT, not all patients who received R‐CHOP chemotherapy were included; thus, selection bias was unintentionally introduced.
At this time, iPET‐guided treatment for MCL remains debatable and cannot be considered a standard care in daily practice. However, our results suggested that FDG avidity, at the midpoint of treatment and after completion of frontline chemotherapy, may be a significant independent prognostic factor for OS and PFS, while dynamic serial changes in PET response provide accurate prognostic predictions, especially in treatment‐naïve MCL patients receiving frontline R‐CHOP. These findings of iPET‐driven strategies may provide novel interpretation methods and integrative treatment algorithms, providing useful clinical practice insights; the findings warrant further prospective clinical investigations.
Conflict of interest
The authors have no conflict of interest to declare.
Author contributions
This manuscript was read and approved by all authors, whose individual contributions are as follows: Y‐WJ and S‐GC contributed to the study conception and design, data acquisition, analysis, and interpretation, and drafting of the manuscript. Y‐WJ, J‐HO, K‐SP, and S‐GC contributed to revising the manuscript. J‐HY, GJM, S‐SP, K‐SE and C‐KM enhanced the intellectual content. S‐GC approved the final content of this manuscript.
Supporting information
Fig S1. OS according to the time of PET‐CT in (A) upfront auto‐HSCT and (B) non‐upfront auto‐HSCT.
Fig S2. Survival outcomes of the auto‐HSCT fit subgroup according to FDG avidity on PET after frontline R‐CHOP (n = 19).
Fig S3. Survival outcomes of the elderly/auto‐HSCT unfit subgroup according to FDG avidity on PET after frontline R‐CHOP (n = 70).
Fig S4. Survival outcomes of metabolic responders according to auto‐HSCT status. (A) PFS, but not OS, was different between the auto‐HSCT and non‐auto‐HSCT early metabolic responders. (B) OS and PFS did not differ according to the auto‐HSCT status in delayed metabolic responders.
Table SI. Univariate analysis of prognostic factors affecting survival outcome after frontline R‐CHOP.
Table SII. Multivariate analysis of prognostic factors affecting survival outcome after frontline R‐CHOP.
Table SIII. Prediction of survival outcome based on the SUVmax.
Acknowledgements
This research has been funded by a grant (HI14C3417) from the Korea Healthcare Technology R&D Project, Ministry for Health, Welfare, and Family Affairs, Republic of Korea and the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education, Science, and Technology.
References
- Abrahamsson, A. , Albertsson‐Lindblad, A. , Brown, P.N. , Baumgartner‐Wennerholm, S. , Pedersen, L.M. , D'Amore, F. , Nilsson‐Ehle, H. , Jensen, P. , Pedersen, M. , Geisler, C.H. & Jerkeman, M. (2014) Real world data on primary treatment for mantle cell lymphoma: a Nordic Lymphoma Group observational study. Blood, 124, 1288–1295. [DOI] [PubMed] [Google Scholar]
- Barrington, S.F. & Johnson, P.W.M. (2017) (18)F‐FDG PET/CT in lymphoma: has imaging‐directed personalized medicine become a reality? Journal of Nuclear Medicine, 58, 1539–1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington, S.F. & Kluge, R. (2017) FDG PET for therapy monitoring in Hodgkin and non‐Hodgkin lymphomas. European Journal of Nuclear Medicine and Molecular Imaging, 44, 97–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrington, S.F. , Mikhaeel, N.G. , Kostakoglu, L. , Meignan, M. , Hutchings, M. , Mueller, S.P. , Schwartz, L.H. , Zucca, E. , Fisher, R.I. , Trotman, J. , Hoekstra, O.S. , Hicks, R.J. , O'Doherty, M.J. , Hustinx, R. , Biggi, A. & Cheson, B.D. (2014) Role of imaging in the staging and response assessment of lymphoma: consensus of the International Conference on Malignant Lymphomas Imaging Working Group. Journal of Clinical Oncology, 32, 3048–3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biggi, A. , Gallamini, A. , Chauvie, S. , Hutchings, M. , Kostakoglu, L. , Gregianin, M. , Meignan, M. , Malkowski, B. , Hofman, M.S. & Barrington, S.F. (2013) International validation study for interim PET in ABVD‐treated, advanced‐stage hodgkin lymphoma: interpretation criteria and concordance rate among reviewers. Journal of Nuclear Medicine, 54, 683–690. [DOI] [PubMed] [Google Scholar]
- Brepoels, L. , Stroobants, S. , De Wever, W. , Dierickx, D. , Vandenberghe, P. , Thomas, J. , Mortelmans, L. , Verhoef, G. & De Wolf‐Peeters, C. (2008) Positron emission tomography in mantle cell lymphoma. Leukaemia & Lymphoma, 49, 1693–1701. [DOI] [PubMed] [Google Scholar]
- Cheah, C.Y. , Seymour, J.F. & Wang, M.L. (2016) Mantle cell lymphoma. Journal of Clinical Oncology, 34, 1256–1269. [DOI] [PubMed] [Google Scholar]
- Cheminant, M. , Robinson, S. , Ribrag, V. , Le Gouill, S. , Suarez, F. , Delarue, R. & Hermine, O. (2015) Prognosis and outcome of stem cell transplantation for mantle cell lymphoma. Expert Review of Hematology, 8, 493–504. [DOI] [PubMed] [Google Scholar]
- Cheson, B.D. , Pfistner, B. , Juweid, M.E. , Gascoyne, R.D. , Specht, L. , Horning, S.J. , Coiffier, B. , Fisher, R.I. , Hagenbeek, A. , Zucca, E. , Rosen, S.T. , Stroobants, S. , Lister, T.A. , Hoppe, R.T. , Dreyling, M. , Tobinai, K. , Vose, J.M. , Connors, J.M. , Federico, M. , Diehl, V. & International Harmonization Project on Lymphoma (2007) Revised response criteria for malignant lymphoma. Journal of Clinical Oncology, 25, 579–586. [DOI] [PubMed] [Google Scholar]
- Cheson, B.D. , Fisher, R.I. , Barrington, S.F. , Cavalli, F. , Schwartz, L.H. , Zucca, E. , Lister, T.A. , Alliance, Australasian Leukaemia and Lymphoma Group , Eastern Cooperative Oncology Group , European Mantle Cell Lymphoma Consortium , Italian Lymphoma Foundation , European Organisation for Research , Treatment of Cancer/Dutch Hemato‐Oncology Group , Grupo Español de Médula Ósea , German High‐Grade Lymphoma Study Group , German Hodgkin's Study Group , Japanese Lymphorra Study Group , Lymphoma Study Association , NCIC Clinical Trials Group , Nordic Lymphoma Study Group , Southwest Oncology Group & United Kingdom National Cancer Research Institute (2014) Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non‐Hodgkin lymphoma: the Lugano classification. Journal of Clinical Oncology, 32, 3059–3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyling, M. , Ferrero, S. & Hermine, O. (2014a) How to manage mantle cell lymphoma. Leukemia, 28, 2117–2130. [DOI] [PubMed] [Google Scholar]
- Dreyling, M. , Ferrero, S. , Vogt, N. , Klapper, W. & European Mantle Cell Lymphoma Network (2014b) New paradigms in mantle cell lymphoma: is it time to risk‐stratify treatment based on the proliferative signature? Clinical Cancer Research, 20, 5194–5206. [DOI] [PubMed] [Google Scholar]
- Freytes, C.O. , Zhang, M.J. , Carreras, J. , Burns, L.J. , Gale, R.P. , Isola, L. , Perales, M.A. , Seftel, M. , Vose, J.M. , Miller, A.M. , Gibson, J. , Gross, T.G. , Rowlings, P.A. , Inwards, D.J. , Pavlovsky, S. , Martino, R. , Marks, D.I. , Hale, G.A. , Smith, S.M. , Schouten, H.C. , Slavin, S. , Klumpp, T.R. , Lazarus, H.M. , van Besien, K. & Hari, P.N. (2012) Outcome of lower‐intensity allogeneic transplantation in non‐Hodgkin lymphoma after autologous transplantation failure. Biology of Blood and Marrow Transplantation, 18, 1255–1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geisler, C.H. , Kolstad, A. , Laurell, A. , Jerkeman, M. , Raty, R. , Andersen, N.S. , Pedersen, L.B. , Eriksson, M. , Nordstrom, M. , Kimby, E. , Bentzen, H. , Kuittinen, O. , Lauritzsen, G.F. , Nilsson‐Ehle, H. , Ralfkiaer, E. , Ehinger, M. , Sundstrom, C. , Delabie, J. , Karjalainen‐Lindsberg, M.L. , Brown, P. , Elonen, E. & Nordic Lymphoma, G. (2012) Nordic MCL2 trial update: six‐year follow‐up after intensive immunochemotherapy for untreated mantle cell lymphoma followed by BEAM or BEAC + autologous stem‐cell support: still very long survival but late relapses do occur. British Journal of Haematology, 158, 355–362. [DOI] [PubMed] [Google Scholar]
- Hosein, P.J. , Pastorini, V.H. , Paes, F.M. , Eber, D. , Chapman, J.R. , Serafini, A.N. , Alizadeh, A.A. & Lossos, I.S. (2011) Utility of positron emission tomography scans in mantle cell lymphoma. American Journal of Hematology, 86, 841–845. [DOI] [PubMed] [Google Scholar]
- Hoster, E. , Dreyling, M. , Klapper, W. , Gisselbrecht, C. , van Hoof, A. , Kluin‐Nelemans, H.C. , Pfreundschuh, M. , Reiser, M. , Metzner, B. , Einsele, H. , Peter, N. , Jung, W. , Wormann, B. , Ludwig, W.D. , Duhrsen, U. , Eimermacher, H. , Wandt, H. , Hasford, J. , Hiddemann, W. , Unterhalt, M. , German Low Grade Lymphoma Study Group (GLSG) , European Mantle Cell Lymphoma Network (2008) A new prognostic index (MIPI) for patients with advanced‐stage mantle cell lymphoma. Blood, 111, 558–565. [DOI] [PubMed] [Google Scholar]
- Itti, E. , Meignan, M. , Berriolo‐Riedinger, A. , Biggi, A. , Cashen, A.F. , Vera, P. , Tilly, H. , Siegel, B.A. , Gallamini, A. , Casasnovas, R.O. & Haioun, C. (2013) An international confirmatory study of the prognostic value of early PET/CT in diffuse large B‐cell lymphoma: comparison between Deauville criteria and DeltaSUVmax. European Journal of Nuclear Medicine and Molecular Imaging, 40, 1312–1320. [DOI] [PubMed] [Google Scholar]
- Jardin, F. , Jais, J.P. , Molina, T.J. , Parmentier, F. , Picquenot, J.M. , Ruminy, P. , Tilly, H. , Bastard, C. , Salles, G.A. , Feugier, P. , Thieblemont, C. , Gisselbrecht, C. , de Reynies, A. , Coiffier, B. , Haioun, C. & Leroy, K. (2010) Diffuse large B‐cell lymphomas with CDKN2A deletion have a distinct gene expression signature and a poor prognosis under R‐CHOP treatment: a GELA study. Blood, 116, 1092–1104. [DOI] [PubMed] [Google Scholar]
- Juweid, M.E. , Stroobants, S. , Hoekstra, O.S. , Mottaghy, F.M. , Dietlein, M. , Guermazi, A. , Wiseman, G.A. , Kostakoglu, L. , Scheidhauer, K. , Buck, A. , Naumann, R. , Spaepen, K. , Hicks, R.J. , Weber, W.A. , Reske, S.N. , Schwaiger, M. , Schwartz, L.H. , Zijlstra, J.M. , Siegel, B.A. , Cheson, B.D. & Imaging Subcommittee of International Harmonization Project in Lymphoma (2007) Use of positron emission tomography for response assessment of lymphoma: consensus of the Imaging Subcommittee of International Harmonization Project in Lymphoma. Journal of Clinical Oncology, 25, 571–578. [DOI] [PubMed] [Google Scholar]
- Kanda, Y. (2013) Investigation of the freely available easy‐to‐use software 'EZR' for medical statistics. Bone Marrow Transplantation, 48, 452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karam, M. , Ata, A. , Irish, K. , Feustel, P.J. , Mottaghy, F.M. , Stroobants, S.G. , Verhoef, G.E. , Chundru, S. , Douglas‐Nikitin, V. , Oliver Wong, C.Y. & Brepoels, L.M. (2009) FDG positron emission tomography/computed tomography scan may identify mantle cell lymphoma patients with unusually favorable outcome. Nuclear Medicine Communications, 30, 770–778. [DOI] [PubMed] [Google Scholar]
- Kedmi, M. , Avivi, I. , Ribakovsky, E. , Benyamini, N. , Davidson, T. , Goshen, E. , Tadmor, T. , Nagler, A. & Avigdor, A. (2014) Is there a role for therapy response assessment with 2‐[fluorine‐18] fluoro‐2‐deoxy‐D‐glucose‐positron emission tomography/computed tomography in mantle cell lymphoma? Leukaemia & Lymphoma, 55, 2484–2489. [DOI] [PubMed] [Google Scholar]
- Lamonica, D. , Graf, D.A. , Munteanu, M.C. & Czuczman, M.S. (2017) 18F‐FDG PET for measurement of response and prediction of outcome to relapsed or refractory mantle cell lymphoma therapy with bendamustine‐rituximab. Journal of Nuclear Medicine, 58, 62–68. [DOI] [PubMed] [Google Scholar]
- Lee, S.C. , Kim, S.J. , Lee, D.H. , Kim, W.S. , Suh, C. & Won, J.H. (2010) Excessive toxicity of once daily i.v. BU, melphalan and thiotepa followed by auto SCT on patients with non‐Hodgkin's lymphoma. Bone Marrow Transplantation, 45, 801–802. [DOI] [PubMed] [Google Scholar]
- Mato, A.R. , Svoboda, J. , Feldman, T. , Zielonka, T. , Agress, H. , Panush, D. , Miller, M. , Toth, P. , Lizotte, P.M. , Nasta, S. , Goldberg, S. , Chong, E. , Schuster, S. , Pecora, A.L. & Goy, A. (2012) Post‐treatment (not interim) positron emission tomography‐computed tomography scan status is highly predictive of outcome in mantle cell lymphoma patients treated with R‐HyperCVAD. Cancer, 118, 3565–3570. [DOI] [PubMed] [Google Scholar]
- Meignan, M. , Gallamini, A. , Itti, E. , Barrington, S. , Haioun, C. & Polliack, A. (2012) Report on the third international workshop on interim positron emission tomography in lymphoma held in Menton, France, 26–27 September 2011 and Menton 2011 consensus. Leukaemia & Lymphoma, 53, 1876–1881. [DOI] [PubMed] [Google Scholar]
- Romaguera, J.E. , Fayad, L. , Rodriguez, M.A. , Broglio, K.R. , Hagemeister, F.B. , Pro, B. , McLaughlin, P. , Younes, A. , Samaniego, F. , Goy, A. , Sarris, A.H. , Dang, N.H. , Wang, M. , Beasley, V. , Medeiros, L.J. , Katz, R.L. , Gagneja, H. , Samuels, B.I. , Smith, T.L. & Cabanillas, F.F. (2005) High rate of durable remissions after treatment of newly diagnosed aggressive mantle‐cell lymphoma with rituximab plus hyper‐CVAD alternating with rituximab plus high‐dose methotrexate and cytarabine. Journal of Clinical Oncology, 23, 7013–7023. [DOI] [PubMed] [Google Scholar]
- Smolewski, P. , Witkowska, M. & Robak, T. (2015) Treatment options for mantle cell lymphoma. Expert Opinion on Pharmacotherapy, 16, 2497–2507. [DOI] [PubMed] [Google Scholar]
- Visco, C. , Finotto, S. , Zambello, R. , Paolini, R. , Menin, A. , Zanotti, R. , Zaja, F. , Semenzato, G. , Pizzolo, G. , D'Amore, E.S. & Rodeghiero, F. (2013) Combination of rituximab, bendamustine, and cytarabine for patients with mantle‐cell non‐Hodgkin lymphoma ineligible for intensive regimens or autologous transplantation. Journal of Clinical Oncology, 31, 1442–1449. [DOI] [PubMed] [Google Scholar]
- Yoon, J.H. , Min, G.J. , Park, S.S. , Jeon, Y.W. , Lee, S.E. , Cho, B.S. , Eom, K.S. , Kim, Y.J. , Lee, S. , Kim, H.J. , Min, C.K. , Lee, J.W. & Cho, S.G. (2019) Autologous hematopoietic cell transplantation using dose‐reduced intravenous busulfan, melphalan, and thiotepa for high‐risk or relapsed lymphomas. Bone Marrow Transplantation, 54, 330–333. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. OS according to the time of PET‐CT in (A) upfront auto‐HSCT and (B) non‐upfront auto‐HSCT.
Fig S2. Survival outcomes of the auto‐HSCT fit subgroup according to FDG avidity on PET after frontline R‐CHOP (n = 19).
Fig S3. Survival outcomes of the elderly/auto‐HSCT unfit subgroup according to FDG avidity on PET after frontline R‐CHOP (n = 70).
Fig S4. Survival outcomes of metabolic responders according to auto‐HSCT status. (A) PFS, but not OS, was different between the auto‐HSCT and non‐auto‐HSCT early metabolic responders. (B) OS and PFS did not differ according to the auto‐HSCT status in delayed metabolic responders.
Table SI. Univariate analysis of prognostic factors affecting survival outcome after frontline R‐CHOP.
Table SII. Multivariate analysis of prognostic factors affecting survival outcome after frontline R‐CHOP.
Table SIII. Prediction of survival outcome based on the SUVmax.


