ABSTRACT
Background and objective
Indacaterol/glycopyrronium (IND/GLY) 110/50 μg once daily (q.d.) has demonstrated greater improvements in lung function, patient‐reported outcomes and lower exacerbation rates versus mono long‐acting muscarinic antagonists (LAMA) in chronic obstructive pulmonary disease (COPD) patients. However, data are limited on initial treatment with IND/GLY 110/50 μg q.d. versus mono LAMA in COPD patients, not previously on maintenance treatment with long‐acting bronchodilators (LABD).
Methods
A pooled analysis of ARISE, SHINE and SPARK trials was conducted to evaluate the efficacy of IND/GLY 110/50 μg q.d. versus open‐label (OL) tiotropium (TIO) 18 μg q.d. and GLY 50 μg q.d. in COPD patients, not on maintenance treatment with LABD at study entry (LABD‐naïve). Efficacy was assessed after 24/26 weeks of treatment.
Results
In total, 998 LABD‐naïve patients were included (IND/GLY: 353; OL TIO: 328; GLY: 317). Patients treated with IND/GLY 110/50 μg q.d. experienced greater improvements in trough forced expiratory volume in 1 s (FEV1) versus OL TIO 18 μg q.d. (least squares mean treatment difference (Δ): 0.086 L) and GLY 50 μg q.d. (Δ: 0.080 L) after 24/26 weeks. Improvements in electronic diary (eDiary) symptom scores, transition dyspnoea index (TDI) focal score, St George's Respiratory Questionnaire (SGRQ) total score and rescue medication use were also greater with IND/GLY versus OL TIO and GLY. Greater proportion of patients achieved minimal clinically important difference in trough FEV1, TDI and SGRQ with IND/GLY versus OL TIO and GLY.
Conclusion
LABD‐naïve patients treated with IND/GLY 110/50 μg q.d. achieved improvements in lung function, daily symptoms, dyspnoea, health‐related quality of life and rescue medication use versus those who received single LAMA.
Keywords: bronchodilator‐naïve, chronic obstructive pulmonary disease, glycopyrronium, indacaterol–glycopyrronium combination, tiotropium
Data are limited on initial treatment with indacaterol/glycopyrronium (IND/GLY) versus mono long‐acting muscarinic antagonist (LAMA) in long‐acting bronchodilator (LABD)‐naïve chronic obstructive pulmonary disease (COPD) patients. This pooled analysis of ARISE, SHINE and SPARK trials demonstrated improvements with IND/GLY in lung function, daily symptoms, dyspnoea, health‐related quality of life and rescue medication use versus tiotropium or GLY in LABD‐naïve COPD patients.
See related https://onlinelibrary.wiley.com/doi/10.1111/resp.13703

INTRODUCTION
Inhaled bronchodilators provide improvements in lung function, reduce symptoms and exacerbations and are therefore the mainstay of pharmacological management of chronic obstructive pulmonary disease (COPD).1, 2 The Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2019 recommends initial treatment with a single bronchodilator—long‐acting β2‐agonist (LABA) or long‐acting muscarinic antagonist (LAMA) for GOLD group B and LAMA for group C patients.2 However, many COPD patients receiving long‐acting bronchodilator (LABD) monotherapy continue to experience significant symptoms and poor quality of life, and therefore a dual bronchodilator therapy (LABA/LAMA) is recommended for follow‐up treatment in these patients.2
Treatment with LABA/LAMA is recommended based on its superior results versus standard of care therapy with LAMA monotherapy or LABA/inhaled corticosteroid (ICS), and lower risk of development of pneumonia versus ICS‐containing treatment.3, 4, 5 Dual bronchodilator therapy with fixed‐dose LABA/LAMA has demonstrated improvements in lung function and health‐related quality of life, and has reduced the usage of rescue medication in patients with prior maintenance therapy with a single bronchodilator.6, 7
Once‐daily (q.d.) indacaterol/glycopyrronium (IND/GLY) is a fixed‐dose combination (FDC) of a LABA, IND 110 μg and a LAMA, GLY 50 μg, approved in over 90 countries (excluding the United States) for the maintenance treatment of patients with COPD.8 IND/GLY 110/50 μg q.d. has demonstrated greater improvements in lung function, exacerbations and patient‐reported outcomes (PRO) versus tiotropium (TIO) 18 μg q.d. (open‐labelled in many trials) and GLY 50 μg q.d. in the Indacaterol and GlycopyrroNium bromide clInical sTudiEs (IGNITE) trial programme.9 TIO 18 μg q.d. and GLY 50 μg q.d. are well‐established LAMA in the management of COPD10 and have demonstrated improvements in lung function, exacerbations, breathlessness, exercise capacity and PRO versus placebo, LABA and LAMA in clinical trials.11, 12, 13, 14, 15, 16, 17, 18, 19
Limited data are available on initial treatment with LABA/LAMA versus single LAMA in COPD patients, who were not previously on maintenance treatment with a LABD. The objective of this post hoc pooled analysis of the ARISE, SHINE and SPARK trials is to evaluate the efficacy of IND/GLY 110/50 μg q.d. versus open‐label (OL) TIO 18 μg q.d. and GLY 50 μg q.d. in COPD patients who were not on maintenance treatment with a LABD at study entry (LABD‐naïve).
METHODS
Study design
This is a pooled post hoc analysis of data from the ARISE (NCT01285492), SHINE (NCT01202188) and SPARK (NCT01120691) studies. ARISE20 was a 52‐week, multicentre, OL, parallel‐group, active‐controlled study that randomized (3:1) Japanese patients to either IND/GLY 110/50 μg q.d. or OL TIO 18 μg q.d. SHINE3 was a 26‐week, multicentre, double‐blind, parallel‐group, placebo‐ and active‐controlled study that randomized (2:2:2:2:1) patients to either IND/GLY 110/50 μg q.d., IND 150 μg q.d., GLY 50 μg q.d., OL TIO 18 μg q.d. or placebo. SPARK4 was a 64‐week, multicentre, double‐blind, parallel‐group study that randomized (1:1:1) patients to either IND/GLY 110/50 μg q.d., GLY 50 μg q.d. or OL TIO 18 μg q.d. IND/GLY 110/50 μg q.d., IND 150 μg q.d. and GLY 50 μg q.d. were delivered via the Breezhaler device (Novartis, Basel, Switzerland) and OL TIO 18 μg q.d. was delivered via the HandiHaler device in the above‐mentioned studies.
Patients with moderate‐to‐severe COPD were enrolled in SHINE and ARISE studies, and severe‐to‐very severe COPD patients were enrolled in the SPARK study. Patients treated with ICS at baseline continued its use when LABA/LAMA or LAMA treatment was started. Considering the different durations of these studies, this pooled analysis was performed after 24/26 weeks of treatment.
All the studies were approved by the Independent Ethics Committee or Institutional Review Boards of each participating centre and were conducted in accordance with the International Conference on Harmonization Guidelines for Good Clinical Practice and the Declaration of Helsinki. All patients provided their informed consent for inclusion in the studies.
Patients
The analysis included LABD‐naïve patients, that is those patients who were not on maintenance treatment with a LABD (LABA, LAMA, LABA/ICS or LABA/ICS + LAMA) at baseline/study entry. Key eligibility criteria are tabulated in Table 1A,B. Detailed study methodology and patient criteria were reported previously.3, 4, 20
Table 1.
(A) Key inclusion criteria. (B) Key exclusion criteria
| (A) | |
|---|---|
| ARISE and SHINE studies | SPARK study |
| Men and women aged ≥40 years with moderate‐to‐severe COPD according to the GOLD 200821 criteria | Men and women aged ≥40 years with severe‐to‐very severe COPD according to the GOLD 200821 criteria |
| Post‐bronchodilator FEV1 with ≥30% and <80% of predicted normal |
Post‐bronchodilator FEV1 with <50% of predicted normal |
| Post‐bronchodilator FEV1/FVC < 0.70 |
Post‐bronchodilator FEV1/FVC < 0.70 |
| Smoking history of ≥10 pack‐years | Smoking history of ≥10 pack‐years |
| History of ≥1 COPD exacerbation in the previous year that required treatment with systemic corticosteroids and/or antibiotics |
| (B) |
|---|
| ARISE, SHINE and SPARK studies |
| COPD exacerbation that required treatment with antibiotics and/or systemic corticosteroids and/or hospitalization in the 6 weeks prior to screening |
| History of asthma |
| Blood eosinophil count >600/mm3 at the start of run‐in period |
COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 s; forced vital capacity; GOLD, Global Initiative for Chronic Obstructive Lung Disease.
Assessments
This pooled analysis compared the efficacy of IND/GLY 110/50 μg q.d. versus OL TIO 18 μg q.d. and GLY 50 μg q.d. in LABD‐naïve patients using the efficacy endpoints that were common to all the studies except for electronic diary (eDiary) total symptom score, which was not evaluated in the ARISE study.
Improvement in trough forced expiratory volume in 1 s (FEV1) and proportion of patients achieving clinically meaningful improvement of ≥100‐mL22 increase in trough FEV1 were evaluated after 24–26 weeks of treatment. Change in daily total symptom scores were collected through eDiary23 at Week 24/26. Treatment effect on breathlessness was evaluated by change from baseline at Week 24/26 in transition dyspnoea index (TDI) focal score24 and proportion of patients achieving minimal clinically important difference (MCID) of ≥1‐point improvement in the score. Improvement in health status was assessed by change from baseline in St George's Respiratory Questionnaire (SGRQ) total score and proportion of patients achieving MCID of ≥4‐unit reduction in the score25 at Week 24/26. Change from baseline in rescue medication use (number of puffs per day) was evaluated during 24/26 week of treatment. Exacerbations were evaluated only in the SPARK study, and were not assessed in this pooled analysis. Assessments were performed at Week 26 in ARISE and SHINE studies, and at Week 24 in the SPARK study.3, 4, 20
Statistical analysis
All analyses were performed in the full analysis set, which consisted of all randomized patients who received at least one dose of medication. Patients included in this analysis were not on maintenance treatment with a LABD (LABA, LAMA, LABA/ICS or LABA/ICS + LAMA) at baseline/study entry. Responder analyses were performed using the logistic regression models, and treatment differences were evaluated using appropriate analysis of covariance (ANCOVA) model. Both the logistic regression and ANCOVA model included fixed effects of treatment, baseline covariates as appropriate (FEV1, FEV1 reversibility components for analyses related to FEV1; SGRQ total score for SGRQ; TDI focal score for TDI; daily total symptom score for symptoms and average number of puffs for rescue medication), baseline ICS use, baseline smoking status, country and the study. The centre was considered as a random effect nested within country.
RESULTS
Study population
In total, 998 LABD‐naïve patients (IND/GLY: 353; OL TIO: 328; GLY: 317) were included in this pooled analysis. Baseline demographics and clinical characteristics were comparable between the treatment groups (Table 2). Most patients were men and more than half of the patients experienced severe airflow limitation.
Table 2.
Baseline demographics and clinical characteristics (full analysis set)
| Characteristic | IND/GLY 110/50 μg q.d. (n = 353) | OL TIO 18 μg q.d. (n = 328) | GLY 50 μg q.d. (n = 317) |
|---|---|---|---|
| Age (years) | 63.4 ± 9.33 | 63.0 ± 8.95 | 62.1 ± 9.42 |
| Men, n (%) | 283 (80.2) | 263 (80.2) | 239 (75.4) |
| BMI (kg/m2) | 24.6 ± 5.19 | 25.0 ± 5.72 | 25.3 ± 5.95 |
| Current smoker, n (%) | 145 (41.1) | 135 (41.2) | 139 (43.8) |
| Estimated number of pack‐years | 43.9 ± 25.99 | 43.5 ± 26.10 | 42.7 ± 23.66 |
| Duration of COPD (years) | 5.8 ± 5.97 | 6.4 ± 5.56 | 6.0 ± 5.29 |
| Severity of airflow limitation†, n (%) | |||
| Mild (GOLD 1) | 0 | 0 | 2 (0.6) |
| Moderate (GOLD 2) | 132 (37.4) | 116 (35.4) | 121 (38.2) |
| Severe (GOLD 3) | 196 (55.5) | 175 (53.4) | 162 (51.1) |
| Very severe (GOLD 4) | 25 (7.1) | 37 (11.3) | 32 (10.1) |
| ICS users at baseline, n (%) | 135 (38.2) | 132 (40.2) | 141 (44.5) |
| COPD exacerbation(s) in the previous year, n (%) | |||
| 0 | 168 (47.6) | 152 (46.3) | 152 (47.9) |
| 1 | 151 (42.8) | 142 (43.3) | 138 (43.5) |
| ≥2 | 34 (9.6) | 34 (10.4) | 27 (8.5) |
Data are presented as mean ± SD unless otherwise specified.
Defined according to GOLD 2008.
BMI, body mass index; COPD, chronic obstructive pulmonary disease; GLY, glycopyrronium; GOLD, Global Initiative for Chronic Obstructive Lung Disease; ICS, inhaled corticosteroid; IND, indacaterol; OL, open‐label; q.d., once daily; TIO, tiotropium.
Lung function
IND/GLY 110/50 μg q.d., OL TIO 18 μg q.d. and GLY 50 μg q.d. showed clinically relevant improvement in trough FEV1 of >100 mL from baseline (0.194, 0.108 and 0.114 L, respectively). Greater improvements in trough FEV1 were seen with IND/GLY 110/50 μg q.d. versus OL TIO 18 μg q.d. and GLY 50 μg q.d. after 24/26 weeks of treatment (Fig. 1).
Figure 1.

Treatment difference with IND/GLY versus OL TIO and GLY for trough FEV1 after 24/26 weeks of treatment (full analysis set). Data are presented as LSM ± SE. Error bars represent SE values. Δ, LSM treatment difference; FEV1, forced expiratory volume in 1 s; GLY, glycopyrronium 50 μg q.d.; IND, indacaterol 110 μg q.d.; LSM, least squares mean; OL, open‐label; q.d., once daily; TIO, tiotropium 18 μg q.d.
Daily total symptom score and dyspnoea
Improvements in daily total symptom score after 24/26 weeks of treatment were greater with IND/GLY 110/50 μg q.d. compared with OL TIO 18 μg q.d. and GLY 50 μg q.d. (Fig. 2A).
Figure 2.

Treatment difference with IND/GLY versus OL TIO and GLY for (A) daily total symptom score and (B) TDI focal score after 24/26 weeks of treatment (full analysis set). Daily total symptom scores were not assessed in the ARISE study. Data are presented as LSM ± SE. Error bars represent SE values. Δ, LSM treatment difference; GLY, glycopyrronium 50 μg q.d.; IND, indacaterol 110 μg q.d.; LSM, least squares mean; OL, open‐label; q.d., once daily; TDI, transition dyspnoea index; TIO, tiotropium 18 μg q.d.
All the evaluated treatments improved dyspnoea, as is evident from clinically relevant improvement in TDI focal score from baseline. Improvements in TDI focal score after 24/26 weeks of treatment were numerically greater with IND/GLY 110/50 μg q.d. compared with TIO 18 μg q.d. and GLY 50 μg q.d. (Fig. 2B).
Health status and rescue medication use
After 24/26 weeks of treatment, clinically relevant improvements from baseline in health status (reduction in the SGRQ total score) were observed with IND/GLY 110/50 μg q.d., OL TIO 18 μg q.d. and GLY 50 μg q.d. Improvement in health status was found to be numerically greater with IND/GLY 110/50 μg q.d. compared with OL TIO 18 μg q.d. and GLY 50 μg q.d. (Fig. 3A).
Figure 3.

Treatment difference with IND/GLY versus OL TIO and GLY for (A) SGRQ total score after 24/26 weeks of treatment and (B) in rescue medication use (full analysis set). Data are presented as LSM ± SE. Error bars represent SE values. Δ, LSM treatment difference; GLY, glycopyrronium 50 μg q.d.; IND, indacaterol 110 μg q.d.; LSM, least squares mean; OL, open‐label; q.d., once daily; SGRQ, St George's Respiratory Questionnaire; TIO, tiotropium 18 μg q.d.
IND/GLY 110/50 μg q.d. reduced daily rescue medication use during 24/26 weeks of treatment versus OL TIO 18 μg q.d. and GLY 50 μg q.d. (Fig. 3B).
Responder analysis
The proportion of patients achieving MCID of ≥100 mL improvement in trough FEV1 was greater with IND/GLY 110/50 μg q.d. than OL TIO 18 μg q.d. and GLY 50 μg q.d. after 24/26 weeks of treatment. At Week 24/26, there was a numerical difference between proportion of patients achieving a ≥4‐unit reduction in the SGRQ total score (MCID) IND/GLY 110/50 μg q.d. versus OL TIO 18 μg q.d. and GLY 50 μg q.d. Furthermore, a numerical difference was also observed in the proportion of patients who achieved clinically meaningful improvement in TDI focal score with IND/GLY 110/50 μg q.d. compared with OL TIO 18 μg q.d. and GLY 50 μg q.d. (Fig. 4).
Figure 4.
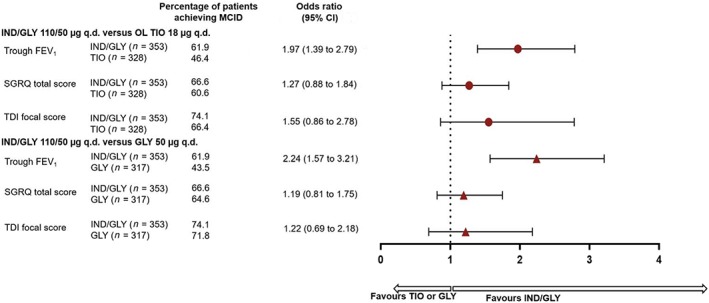
Proportion of patients achieving MCID for trough FEV1, SGRQ total score and TDI focal score with IND/GLY, OL TIO and GLY at Week 24/26 (full analysis set). FEV1, forced expiratory volume in 1 s; GLY, glycopyrronium 50 μg q.d.; IND, indacaterol 110 μg q.d.; MCID, minimal clinically important difference; OL, open‐label; q.d., once daily; SGRQ, St George's Respiratory Questionnaire; TDI, transition dyspnoea index; TIO, tiotropium 18 μg q.d.
DISCUSSION
This post hoc analysis of pooled data from ARISE, SHINE and SPARK studies compared the efficacy of LABA/LAMA (IND/GLY) versus LAMA (TIO and GLY) in LABD‐naïve COPD patients. The results of this analysis showed that dual bronchodilation with IND/GLY improved trough FEV1 compared with LAMA monotherapies (TIO and GLY) in LABD‐naïve patients. Improvement in lung function with IND/GLY was complemented by improvements in daily symptoms, dyspnoea, health‐related quality of life and rescue medication use compared with TIO and GLY. Furthermore, a higher proportion of patients on IND/GLY achieved a clinically meaningful improvement in trough FEV1 (≥100 mL), SGRQ total score (≥4 units) and TDI focal score (≥1 unit) versus TIO and GLY.
Disease severity and study duration are important considerations while interpreting results of a clinical trial in COPD patients.26, 27 Unlike exacerbations (that were not evaluated in this pooled analysis), efficacy outcomes evaluated in this pooled analysis respond quickly to treatment28 and 24/26 weeks present an ideal time period for their assessment. Patients with moderate‐to‐severe COPD were enrolled in SHINE and ARISE trials, while patients with severe‐to‐very severe COPD were included in the SPARK trial. This pooled analysis included patients across the range of COPD severities who can benefit from dual LABD. It should also be noted that SPARK study enrolled patients with history of ≥1 exacerbation in the previous year.4 The improvement in efficacy outcomes with IND/GLY versus OL TIO and GLY in LABD‐naïve patients is in line with the results observed in overall population in the above‐mentioned studies, and also with data from the IGNITE trial programme.9
Results from this pooled analysis are consistent with a post hoc analysis of two 12‐week OTEMTO studies, where TIO/olodaterol (TIO/OLO) 5/5 μg q.d. demonstrated improvements in trough FEV1, SGRQ total score and TDI versus TIO 5 μg q.d. (all treatments via the Respimat device; Boehringer Ingelheim, Ingelheim, Germany) in treatment‐naïve patients. However, it should be noted that these studies were of a 12‐week duration in patients with moderate‐to‐severe COPD,29 while our post hoc analysis included studies of at least 26 weeks' duration, and COPD severity ranged from moderate‐to‐very severe. Similarly, in a post hoc analysis from TONADO studies, TIO/OLO 5/5 μg q.d. and 2.5/5 μg q.d. improved trough FEV1 versus TIO 5 μg q.d. (all treatments via the Respimat device) in treatment‐naïve patients with moderate‐to‐very severe COPD.7 Other PRO, however, were not assessed in the post hoc analysis of TONADO studies. In another pooled analysis of three 24‐week randomized trials, umeclidinium/vilanterol (UMEC/VI) 62.5/25 μg q.d. (via Ellipta device; GlaxoSmithKline, Middlesex, UK) provided improvement in trough FEV1, SGRQ for COPD (SGRQ‐C) total score and rescue medication use versus TIO 18 μg q.d. (via HandiHaler device; Boehringer Ingelheim, Ingelheim, Germany) in maintenance‐naïve COPD patients. TDI and symptom scores were not evaluated in this pooled analysis.6
A large proportion of COPD patients receive suboptimum treatment.30, 31 Previous studies have suggested that early initiation of maintenance therapy may provide long‐term benefits.32, 33 An OL study in Japanese COPD patients demonstrated improvements in lung function and quality of life with guideline‐based pharmacotherapy in treatment‐naïve patients versus those who received prior COPD treatment.34 This further highlights the importance of selection of initial therapy in COPD patients.
LAMA, LABA/LAMA and LABA/ICS are widely used maintenance therapies in COPD. GOLD 2019 recommends LAMA monotherapy as initial treatment in the majority of COPD patients; however, many patients remain symptomatic on monotherapy, and LABA/LAMA is recommended in these patients.2, 35, 36 On the other hand, use of ICS in COPD is associated with side effects—pneumonia, diabetes, osteoporosis and mycobacterial infections.37, 38, 39, 40 Furthermore, as per GOLD 2019 update, initial treatment with LABA/ICS may be the first choice only for COPD patients with history of asthma or with blood eosinophil counts ≥300 cells/μL.2 LABA/LAMA combinations, particularly IND/GLY, have shown improvements in lung function, PRO, rescue medication use and exacerbations versus monocomponents, placebo and well‐established COPD treatments including LABA/ICS.9, 26 Considering the above‐mentioned aspects, a rationale for dual bronchodilators as first‐line maintenance therapy in COPD patients is emerging. Data from this post hoc analysis and other pooled analyses6, 7, 29 further support this rationale.
Safety evaluations were not performed in this pooled analysis; however, the safety profile of all treatments is well established.10, 41 A systematic review and meta‐analysis by Rodrigo et al. showed comparable safety profile between LABA/LAMA and LAMA.42 In particular, IND/GLY has demonstrated comparable safety profile as its monocomponents and TIO.41 A real‐world study using the UK Clinical Practice Research Datalink database showed that adding a second LABD does not increase the risk of most cardiovascular events.43 To the best of our knowledge, no clinical trials have evaluated safety of adding a second LABD to existing one in patients with COPD.
The current analysis has certain strengths and limitations. The most important strength is that we compared the efficacy of dual bronchodilation with IND/GLY 110/50 μg q.d. versus mono LAMA in a relatively large population, with a wide range of COPD severity, to answer a clinically relevant question. Also, the post hoc analysis demonstrated greater improvements with IND/GLY 110/50 μg q.d. versus OL TIO 18 μg q.d. and GLY 50 μg q.d., whereas previous similar analyses have considered only TIO as comparator.
The limitation of this evaluation was that this was a post hoc analysis and was not powered for comparison between the treatment groups. Due to its post hoc nature, the authors do not claim statistical significance between treatments groups for any of the parameters described in this analysis. Prospective studies in LABD‐naïve patients are required to validate these outcomes. Exacerbations were evaluated only in the SPARK study, and therefore these were not assessed in this pooled analysis. Comparison with TIO was open‐labelled in all the studies included in this pooled analysis. Lastly, this analysis was done by pooling data from three studies and then selecting those patients who were not on maintenance treatment with a LABD at baseline/study entry. This led to an unbalanced distribution of LABD‐naïve patients across studies, which can be expected from such post hoc analyses.
In conclusion, this post hoc analysis has shown that in COPD patients who were not receiving LABD at study entry, the introduction of IND/GLY 110/50 μg q.d. provided improvements in lung function, daily symptoms, dyspnoea, health‐related quality of life and rescue medication use compared with LAMA monotherapy. Given the safety of LABA/LAMA combinations such as IND/GLY, the results of the current analysis suggest that initial therapy with two bronchodilators may be considered in LABD‐naïve symptomatic COPD patients.
Disclosure statement
A part of this analysis was previously presented at the Annual Congress of the European Respiratory Society 2018 and the Thoracic Society of Australia and New Zealand 2019. S.M. reported lecture and advisory fees from Novartis Pharma and AstraZeneca; lecture fees and grants from Boehringer Ingelheim and Fukuda Life Tech; advisory fees from GlaxoSmithKline; grants from Eisai Pharmaceutical, Otsuka Pharmaceutical and Fuji Film Medical; and lecture fees from Astellas Pharmaceutical, Kyorin Pharmaceutical and Meiji Seika Pharma. H.Y., P.O. and P.G. are employees of the study sponsor. P.O. holds shares of Novartis Pharma AG. K.K. was an employee of Novartis at the time of the conduct of this analysis. J.A.W. has not received any speaker or consulting fees since January 2015. She has received research grants from AstraZeneca, Boehringer Ingelheim, GlaxoSmithKline, Novartis and Johnson and Johnson.
Author contributions
Conceptualization: K.K., P.O., P.G., H.Y. Data curation: P.G. Formal analysis: P.G. Funding acquisition: K.K., P.O. Investigation: P.G. Methodology: P.G. Project administration: K.K., P.O. Resources: K.K., P.O. Software: P.G. Supervision: K.K., P.O., P.G., S.M., H.Y., J.A.W. Validation: P.G. Visualization: S.M., H.Y., K.K., P.O., P.G., J.A.W. Writing—original draft: H.Y., K.K., P.O., P.G., S.M., J.A.W. Writing—review and editing: S.M., H.Y., K.K., P.O., P.G., J.A.W.
Abbreviations
- Δ
LSM treatment difference
- ANCOVA
analysis of covariance
- eDiary
electronic diary
- FEV1
forced expiratory volume in 1 s
- FVC
forced vital capacity
- GLY
glycopyrronium
- GOLD
Global Initiative for Chronic Obstructive Lung Disease
- ICS
inhaled corticosteroid
- IGNITE
Indacaterol and GlycopyrroNium bromide clInical sTudiEs
- IND
indacaterol
- LABA
long‐acting β2‐agonist
- LABD
long‐acting bronchodilator
- LAMA
long‐acting muscarinic antagonist
- LSM
least squares mean
- MCID
minimal clinically important difference
- OL
open‐label
- OLO
olodaterol
- PRO
patient‐reported outcome
- q.d.
once daily
- SGRQ
St George's Respiratory Questionnaire
- TDI
transition dyspnoea index
- TIO
tiotropium
Supporting information
Visual Abstract ‘IND/GLY’ versus ‘TIO’ or ‘GLY’ in long‐acting bronchodilator‐naïve COPD patients: A pooled analysis.
Acknowledgements
Under the direction of authors, Vatsal Vithlani and Hasitha Shilpa Anantharaju (professional medical writers; Novartis) assisted in the preparation of this article in accordance with the third edition of Good Publication Practice guidelines. Medical writing support was funded by the study sponsor. The authors would also like to thank Dr Alexander J. Mackay for his contribution to the design of this analysis and interpretation of the data.
Muro, S , Yoshisue, H , Kostikas, K , Olsson, P , Gupta, P , Wedzicha, JA . Indacaterol/glycopyrronium versus tiotropium or glycopyrronium in long‐acting bronchodilator‐naïve COPD patients: A pooled analysis. Respirology. 2020;25:393–400. 10.1111/resp.13651
(Associate Editor: Frits Franssen; Senior Editor: Fanny Ko)
Data availability statement
Novartis as the study sponsor is committed to sharing with qualified external researchers, access to patient‐level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with the applicable laws and regulations.
REFERENCES
- 1. Thomas M, Halpin DM, Miravitlles M. When is dual bronchodilation indicated in COPD? Int. J. Chron. Obstruct. Pulmon. Dis. 2017; 12: 2291–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2019. [Accessed 16 Nov 2018.] Available from URL: http://goldcopd.org/gold-reports/
- 3. Bateman ED, Ferguson GT, Barnes N, Gallagher N, Green Y, Henley M, Banerji D. Dual bronchodilation with QVA149 versus single bronchodilator therapy: the SHINE study. Eur. Respir. J. 2013; 42: 1484–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wedzicha JA, Decramer M, Ficker JH, Niewoehner DE, Sandstrom T, Taylor AF, D'Andrea P, Arrasate C, Chen H, Banerji D. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double‐blind, parallel‐group study. Lancet Respir. Med. 2013; 1: 199–209. [DOI] [PubMed] [Google Scholar]
- 5. Wedzicha JA, Banerji D, Chapman KR, Vestbo J, Roche N, Ayers RT, Thach C, Fogel R, Patalano F, Vogelmeier CF; FLAME Investigators . Indacaterol‐glycopyrronium versus salmeterol‐fluticasone for COPD. N. Engl. J. Med 2016; 374: 2222–34. [DOI] [PubMed]
- 6. Maleki‐Yazdi MR, Singh D, Anzueto A, Tombs L, Fahy WA, Naya I. Assessing short‐term deterioration in maintenance‐naive patients with COPD receiving umeclidinium/vilanterol and tiotropium: a pooled analysis of three randomized trials. Adv. Ther. 2017; 33: 2188–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ferguson GT, Flezar M, Korn S, Korducki L, Gronke L, Abrahams R, Buhl R. Efficacy of tiotropium + olodaterol in patients with chronic obstructive pulmonary disease by initial disease severity and treatment intensity: a post hoc analysis. Adv. Ther. 2015; 32: 523–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ultibro Breezhaler: Summary of Product Characteristics. [Accessed 16 Nov 2018.] Available from URL: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/002679/WC500151255.pdf
- 9. Banerji D, Fogel R, Patalano F. Indacaterol/glycopyrronium: a dual bronchodilator for COPD. Drug Discov. Today 2018; 23: 196–03. [DOI] [PubMed] [Google Scholar]
- 10. D'Urzo AD, Kardos P, Wiseman R. Practical considerations when prescribing a long‐acting muscarinic antagonist for patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2018; 13: 1089–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tashkin DP, Celli B, Senn S, Burkhart D, Kesten S, Menjoge S, Decramer M; UPLIFT Study Investigators . A 4‐year trial of tiotropium in chronic obstructive pulmonary disease. N. Engl. J. Med. 2008; 359: 1543–54.18836213 [Google Scholar]
- 12. Bateman ED, Tashkin D, Siafakas N, Dahl R, Towse L, Massey D, Pavia D, Zhong NS. A one‐year trial of tiotropium Respimat plus usual therapy in COPD patients. Respir. Med. 2010; 104: 1460–72. [DOI] [PubMed] [Google Scholar]
- 13. Cooper CB, Celli BR, Jardim JR, Wise RA, Legg D, Guo J, Kesten S. Treadmill endurance during 2‐year treatment with tiotropium in patients with COPD: a randomized trial. Chest 2013; 144: 490–7. [DOI] [PubMed] [Google Scholar]
- 14. Yohannes AM, Willgoss TG, Vestbo J. Tiotropium for treatment of stable COPD: a meta‐analysis of clinically relevant outcomes. Respir. Care 2011; 56: 477–87. [DOI] [PubMed] [Google Scholar]
- 15. Vogelmeier C, Hederer B, Glaab T, Schmidt H, Rutten‐van Molken MP, Beeh KM, Rabe KF, Fabbri LM; POET‐COPD Investigators . Tiotropium versus salmeterol for the prevention of exacerbations of COPD. N. Engl. J. Med. 2011; 364: 1093–103. [DOI] [PubMed] [Google Scholar]
- 16. Wise RA, Anzueto A, Cotton D, Dahl R, Devins T, Disse B, Dusser D, Joseph E, Kattenbeck S, Koenen‐Bergmann M et al; TIOSPIR Investigators . Tiotropium Respimat inhaler and the risk of death in COPD. N. Engl. J. Med 2013; 369: 1491–501. [DOI] [PubMed]
- 17. D'Urzo A, Ferguson GT, van Noord JA, Hirata K, Martin C, Horton R, Lu Y, Banerji D, Overend T. Efficacy and safety of once‐daily NVA237 in patients with moderate‐to‐severe COPD: the GLOW1 trial. Respir. Res. 2011; 12: 156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kerwin E, Hebert J, Gallagher N, Martin C, Overend T, Alagappan VK, Lu Y, Banerji D. Efficacy and safety of NVA237 versus placebo and tiotropium in patients with COPD: the GLOW2 study. Eur. Respir. J. 2012; 40: 1106–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beeh KM, Singh D, Di Scala L, Drollmann A. Once‐daily NVA237 improves exercise tolerance from the first dose in patients with COPD: the GLOW3 trial. Int. J. Chron. Obstruct. Pulmon. Dis. 2012; 7: 503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Asai K, Hirata K, Hashimoto S, Fukuchi Y, Kitawaki T, Ikeda K, Fogel R, Banerji D. Efficacy and safety of indacaterol/glycopyrronium in Japanese patients with COPD: pooled analysis of SHINE and ARISE. Respir. Investig. 2016; 54: 428–35. [DOI] [PubMed] [Google Scholar]
- 21. Global Initiative for Chronic Obstructive Lung Disease . Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease 2008.
- 22. Donohue JF. Minimal clinically important differences in COPD lung function. COPD 2015; 2: 111–24. [DOI] [PubMed] [Google Scholar]
- 23. Kulich K, Keininger DL, Tiplady B, Banerji D. Symptoms and impact of COPD assessed by an electronic diary in patients with moderate‐to‐severe COPD: psychometric results from the SHINE study. Int. J. Chron. Obstruct. Pulmon. Dis. 2015; 10: 79–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mahler DA, Weinberg DH, Wells CK, Feinstein AR. The measurement of dyspnea. Contents, interobserver agreement, and physiologic correlates of two new clinical indexes. Chest 1984; 85: 751–8. [DOI] [PubMed] [Google Scholar]
- 25. Jones PW. St. George's Respiratory Questionnaire: MCID. COPD 2005; 2: 75–9. [DOI] [PubMed] [Google Scholar]
- 26. Miravitlles M, Baek S, Vithlani V, Lad R. Optimal bronchodilation for COPD patients: are all long‐acting beta(2)‐agonist/long‐acting muscarinic antagonists the same? Tuberc. Respir. Dis. (Seoul) 2018; 81: 198–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Friedman LM, Furberg CD, DeMets D, Reboussin DM, Granger CB. Fundamentals of Clinical Trials. xxx, Springer International Publishing, 2005. [Google Scholar]
- 28. Calverley PM, Postma DS, Anzueto AR, Make BJ, Eriksson G, Peterson S, Jenkins CR. Early response to inhaled bronchodilators and corticosteroids as a predictor of 12‐month treatment responder status and COPD exacerbations. Int. J. Chron. Obstruct. Pulmon. Dis. 2016; 11: 381–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Singh D, Gaga M, Schmidt O, Bjermer L, Gronke L, Voss F, Ferguson GT. Effects of tiotropium + olodaterol versus tiotropium or placebo by COPD disease severity and previous treatment history in the OTEMTO(R) studies. Respir. Res. 2016; 17: 73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Takahashi T, Ichinose M, Inoue H, Shirato K, Hattori T, Takishima T. Underdiagnosis and undertreatment of COPD in primary care settings. Respirology 2003; 8: 504–8. [DOI] [PubMed] [Google Scholar]
- 31. Zaas D, Wise R, Wiener C; Longcope Spirometry Investigation Team . Airway obstruction is common but unsuspected in patients admitted to a general medicine service. Chest 2014; 125: 106–11. [DOI] [PubMed] [Google Scholar]
- 32. Tantucci C, Modina D. Lung function decline in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 2012; 7: 95–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Troosters T, Sciurba FC, Decramer M, Siafakas NM, Klioze SS, Sutradhar SC, Weisman IM, Yunis C. Tiotropium in patients with moderate COPD naive to maintenance therapy: a randomised placebo‐controlled trial. NPJ Prim. Care Respir. Med. 2014; 24: 14003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Setoguchi Y, Izumi S, Nakamura H, Hanada S, Marumo K, Kurosaki A, Akata S. Survey to determine the efficacy and safety of guideline‐based pharmacological therapy for chronic obstructive pulmonary disease patients not previously receiving maintenance treatment. Expert Opin. Pharmacother. 2015; 16: 2271–81. [DOI] [PubMed] [Google Scholar]
- 35. Price D, West D, Brusselle G, Gruffydd‐Jones K, Jones R, Miravitlles M, Rossi A, Hutton C, Ashton VL, Stewart R et al Management of COPD in the UK primary‐care setting: an analysis of real‐life prescribing patterns. Int. J. Chron. Obstruct. Pulmon. Dis. 2014; 9: 889–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Dransfield MT, Bailey W, Crater G, Emmett A, O'Dell DM, Yawn B. Disease severity and symptoms among patients receiving monotherapy for COPD. Prim. Care Respir. J. 2011; 20: 46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kew KM, Seniukovich A. Inhaled steroids and risk of pneumonia for chronic obstructive pulmonary disease. Cochrane Database Syst. Rev. 2014; 3: CD010115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Brode SK, Campitelli MA, Kwong JC, Lu H, Marchand‐Austin A, Gershon AS, Jamieson FB, Marras TK. The risk of mycobacterial infections associated with inhaled corticosteroid use. Eur. Respir. J. 2017; 50: pii: 1700037. [DOI] [PubMed] [Google Scholar]
- 39. Suissa S, Kezouh A, Ernst P. Inhaled corticosteroids and the risks of diabetes onset and progression. Am. J. Med. 2010; 123: 1001–6. [DOI] [PubMed] [Google Scholar]
- 40. Gonzalez AV, Coulombe J, Ernst P, Suissa S. Long‐term use of inhaled corticosteroids in COPD and the risk of fracture. Chest 2018; 153: 321–8. [DOI] [PubMed] [Google Scholar]
- 41. Wedzicha JA, Dahl R, Buhl R, Schubert‐Tennigkeit A, Chen H, D'Andrea P, Fogel R, Banerji D. Pooled safety analysis of the fixed‐dose combination of indacaterol and glycopyrronium (QVA149), its monocomponents, and tiotropium versus placebo in COPD patients. Respir. Med. 2014; 108: 1498–507. [DOI] [PubMed] [Google Scholar]
- 42. Rodrigo GJ, Price D, Anzueto A, Singh D, Altman P, Bader G, Patalano F, Fogel R, Kostikas K. LABA/LA MA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta‐analysis. Int. J. Chron. Obstruct. Pulmon. Dis. 2017; 12: 907–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Suissa S, Dell'Aniello S, Ernst P. Concurrent use of long‐acting bronchodilators in COPD and the risk of adverse cardiovascular events. Eur. Respir. J. 2017; 49: 1602245. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Visual Abstract ‘IND/GLY’ versus ‘TIO’ or ‘GLY’ in long‐acting bronchodilator‐naïve COPD patients: A pooled analysis.
Data Availability Statement
Novartis as the study sponsor is committed to sharing with qualified external researchers, access to patient‐level data and supporting clinical documents from eligible studies. These requests are reviewed and approved by an independent review panel on the basis of scientific merit. All data provided are anonymized to respect the privacy of patients who have participated in the trial in line with the applicable laws and regulations.


