Abstract
Introduction
The diagnostic trajectory of patients with increased bleeding tendency can be very costly and time‐consuming. In addition, previous studies have shown that half of these patients remain without final diagnosis despite all efforts.
Aim
This study aimed to improve insight into the current diagnostic process of these patients.
Methods
A total of 117 adult patients, referred to an academic hospital because of being suspected to have an increased bleeding tendency, were included. Different parameters were compared between patients receiving final diagnosis, patients without final diagnosis but a high Tosetto bleeding assessment tool (BAT) score (classified as bleeding of unknown cause, or BUC) and a control group consisting of patients without final diagnosis and a low BAT score.
Results
The BAT score was significantly higher in patients in the BUC group as compared to patients reaching final diagnosis (8.1 vs 4.9). Interestingly, the two subcategories most prevalently increased were surgery and post‐partum haemorrhage‐associated bleeding (surgery: 2.1 vs 1.1; post‐partum haemorrhage: 0.7 vs 0.0). Laboratory screening results were more often abnormal in patients reaching final diagnosis compared to patients remaining without diagnosis and a high BAT score (n = 32 (78%) vs n = 14 (46%), 95% CI 1.5‐12), especially concerning the PFA (=27 (66%) vs n = 10 (33%), 95% CI 1.4‐10) and von Willebrand factor activity levels (n = 11 (27%) vs n = 1 (3%), 95% CI 1.3‐91).
Conclusion
Isolated high bleeding score on surgical or post‐partum bleeding correlates with a lower chance of receiving final diagnosis. Withholding extensive haemostatic testing should be considered. Better screening and confirmative haemostatic assays are still needed.
Keywords: coagulation factors, fibrinolysis, haemostasis disorders, platelet function disorder
1. INTRODUCTION
In clinical practice, diagnosing patients with a suspected bleeding diathesis can be a challenge. Establishing a definitive diagnosis enables clinicians to provide patients with appropriate treatment without unnecessary consumption of factor concentrates and limits bleeding complications.
To unravel the patients’ potential bleeding disorder, patients undergo a stepwise diagnostic route. This starts with an extensive bleeding history with integration of a bleeding assessment tool (BAT), proper analysis of family history and use of medication, like non‐steroidal anti‐inflammatory drugs and supplements. The International Society of Thrombosis and Haemostasis‐BAT (ISTH‐BAT) and several modified and easy to perform bleeding assessment tools like the Tosetto bleeding score are currently the most frequently used instruments for determining whether a bleeding disorder is likely.1, 2, 3 The following step is a laboratory work up to detect abnormalities in primary or secondary haemostasis. As screening assays such as the platelet function analyser (PFA), the prothrombin time (PT) and activated partial thromboplastin time (aPTT) lack specificity, it is essential to perform confirmation assays to establish final diagnosis if screening assays are abnormal. Confirmation assays are also required in case of high BAT scores with no abnormalities in screening assays due to the lack of sensitivity. These assays include individual coagulation factor levels in case of prolonged PT and APTT, or platelet function, VWF and fibrinolysis assays.4, 5
Previous studies indicated that 28%‐42% of patients referred for a bleeding tendency to a tertiary haematological clinic received a final diagnosis.6, 7, 8, 9, 10, 11, 12, 13 However, a significant group of patients remained without diagnosis. This may be due to a lack of sensitive and specific assays that adequately determine pathological alterations in the haemostatic balance, or due to a lack of specific bleeding assessment tools. In addition, a proportion of patients with an increased BAT might not have a congenital bleeding disorder but an acquired cause of bleeding, such as post‐partum haemorrhage due to uterus atony or bleeding due to use of medication.14, 15, 16 In addition, a correlation between bleeding symptoms and hypermobility syndrome/Ehlers‐Danlos syndrome (EDS) is also described.17
This study aimed to improve insight in current clinical practice of patients suspected to have an increased bleeding tendency, and furthermore our ability to provide them with a correct haemostatic diagnosis. We analysed the diagnostic pathway of patients referred to a Dutch haemophilia treatment centre (HTC), suspected to have an increased bleeding tendency. Additionally, their clinical and laboratory phenotypes were compared to determine differences between patients receiving diagnosis and patients remaining without diagnosis.
2. PATIENTS AND METHODS
2.1. Patients
This study included patients suspected to have an increased bleeding tendency but without established final diagnosis, consecutively referred to the Radboud HTC from 1 January 2016 through 1 July 2017.
At the end of their diagnostic trajectory, patients were divided into three groups: (a) patients receiving final diagnosis; (b) patients without final diagnosis and a high BAT score (≥3 for men and ≥5 for women), classified as bleeding of unknown cause (BUC); and (c) patients without final diagnosis and a low BAT (<3 for men and <5 for women). Patients from group 1 and 2 were studied in more detail. Patients in group three were considered to have no haemostatic disorder and assigned as control group.
2.2. Data collection
Data were collected retrospectively from the electronic patient records and only obtained from patients who had indicated that their data could be used for research purposes. Demographic information, referral type and results of the diagnostic trajectory were collected (Table 1). At first, all patients underwent screening assays. Depending on the results of these screening assays and the BAT score, the diagnostic trajectory was extended. An overview of this diagnostic process is presented in Figure 1. Confirmatory assays were performed sequentially. In case of a high BAT score without abnormal screening assays, first platelet function assays were performed, afterwards fibrinolysis assays were performed if the platelet assays did not yield a diagnosis. In case of an abnormal screening test, associated confirmatory assays were done.
Table 1.
Characteristics of referred patients
| Group 1: Final diagnosis n = 41 | Group 2: No final diagnosis and high BATc n = 30 | Group 3: No final diagnosis and low BATc n = 46 | Total n = 117 | |
|---|---|---|---|---|
| Gender (n (%))b | ||||
| Male | 13 (32%) | 3 (10%) | 7 (15%) | 23 (20%) |
| Female | 28 (68%) | 27 (90%) | 39 (85%) | 94 (80%) |
| Age (median in years) | 40 (IQR 27) | 38 (IQR 31) | 35 (IQR 25) | 37 (IQR 28) |
| Type of referral (n (%))b | ||||
| First‐line health care | 19 (46%) | 13 (43%) | 13 (43%) | 45 (38%) |
| Second‐line health care | 22 (54%) | 17 (57%) | 33 (72%) | 72 (62%) |
| BATb | ||||
| Bleeding score (median) | 5.0 (IQR 8) | 7.0 (IQR 4) | 2.0 (IQR 2) | 4.0 (IQR 5) |
| Epistaxis (n (%)) | 8 (20%) | 11 (37%) | 5 (11%) | 24 (21%) |
| Cutaneous (n (%)) | 25 (61.0%) | 23 (77%) | 28 (61%) | 76 (65%) |
| Minor wounds (n (%)) | 18 (44%) | 21 (70%) | 10 (22%) | 49 (42%) |
| Oral cavity (n (%)) | 6 (15%) | 5 (17%) | 5 (11%) | 16 (14%) |
| GI bleeding (n (%)) | 5 (12%) | 8 (27%) | 6 (13%) | 19 (16%) |
| Tooth extraction (n (%)) | 11 (27%) | 14 (47%) | 1 (2%) | 26 (22%) |
| Surgery (n (%)) | 16 (39%) | 21 (70%) | 3 (7%) | 40 (34%) |
| Menorrhagia (n (%))a | 16 (57%) | 21 (78%) | 24 (62%) | 61 (65%) |
| Postpartum haemorrhage (n (%))a | 6 (21%) | 14 (52%) | 10 (26%) | 30 (32%) |
| Muscle haematomas (n (%)) | 4 (10%) | 0 (0%) | 1 (2%) | 5 (4%) |
| Haemarthrosis (n (%)) | 4 (10%) | 2 (7%) | 1 (2%) | 7 (6%) |
| CNS bleeding (n (%)) | 2 (5%) | 0 (0%) | 2 (4%) | 4 (3%) |
| Family history (n (%))b | ||||
| First‐degree relative | ||||
| Positive | 15 (37%) | 1 (3%) | 12 (26%) | 28 (24%) |
| Symptoms | 10 (24%) | 10 (33%) | 12 (26%) | 32 (27%) |
| Negative | 16 (39%) | 19 (63%) | 22 (48%) | 57 (49%) |
| Second‐degree relative | ||||
| Positive | 6 (15%) | 1 (3%) | 9 (20%) | 16 (14%) |
| Symptoms | 9 (22%) | 10 (33%) | 7 (15%) | 26 (22%) |
| Negative | 26 (63%) | 19 (63%) | 30 (56%) | 75 (64%) |
| Third‐degree relative | ||||
| Positive | 3 (7%) | 0 (0%) | 5 (11%) | 8 (7%) |
| Symptoms | 3 (7%) | 3 (10%) | 41 (89%) | 47 (40%) |
| Negative | 35 (85%) | 27 (90%) | 0 (0%) | 62 (53%) |
Women only.
Percentages are pertaining to the group concerned.
Low BAT: for men Score < 3, for women < 5; high BAT: for men ≥ 3, for women ≥ 5.
Figure 1.
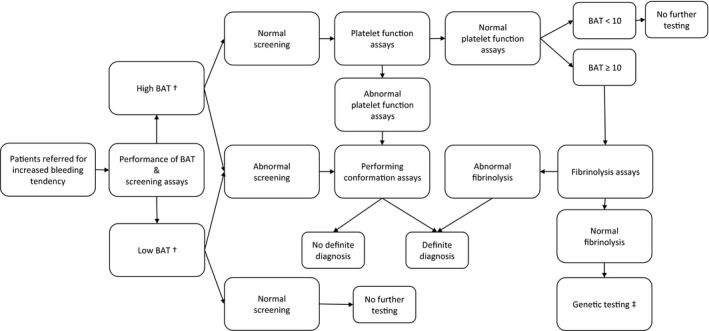
Flow chart of diagnostic process of patients referred for increased bleeding tendency
Laboratory results were categorized as follows:
Screening panel: including aPTT, PT, fibrinogen level, VWF ristocetin cofactor activity, VWF antigen level and PFA.
Confirmation tests:
Panel 1: Platelet function assays, consisting of aggregation tests in whole blood based on impedance with high and low collagen concentrate, arachidonic acid, adenosine diphosphate (ADP) and analysis of alpha and delta granules constituents such as ADP, ATP, ATP/ADP ratio, beta‐thromboglobulin and platelet factor 4 secretion, and flow cytometry to assess the presence of the activation markers CD36, GPIIbIIIa and P‐selectin and platelet receptors GPIIb, GPIIIa, GPIb and GPIX.
Panel 2: von Willebrand factor parameters, consisting of VWF ristocetin cofactor activity, VWF antigen level, collagen III binding assay, factor VIII binding assay (only when indicated) and VWF multimers.
Panel 3: Coagulation factors, consisting of activity levels of FII, FV, FVII, FVIII‐one stage and chromogenic FVIII, FIX, FX, FXI, FXII and FXIII,
Panel 4: Fibrinolysis parameters, consisting of the euglobulin clot lysis test before and after venous occlusion induced by a tourniquet, plasminogen activator inhibitor‐1 (PAI) activity and antigen level and alfa2‐antiplasmin activity level.
Test results were considered abnormal if results were beyond the defined normal range of our haematological laboratory. Final diagnosis was only given when test results were abnormal at least twice. For diagnosing von Willebrand disease (vWD), this meant at least two low vWF levels, because of the risk of falsely raised or lowered values when measured only once under inflammatory conditions.
Platelet dysfunction was defined as a confirmed abnormality of at least one aggregation test and/or abnormal secretion marker and/or dysfunctional activation marker and/or receptor. A fibrinolytic disorder was defined as at least an abnormal PAI‐1 activity and antigen level, and or increased ratio of the euglobulin clot lysis time (>5.5).
2.2.1. BAT
To evaluate differences in bleeding symptoms, the Tosetto bleeding score was used. The Tosetto bleeding score evaluates bleeding symptoms based on several subcategories: epistaxis, cutaneous bleeding, minor wounds, oral cavity, gastro‐intestinal bleeding, tooth extraction, surgery, menorrhagia, post‐partum haemorrhage, muscle haematomas, haemarthrosis and central nervous system bleeding. These subcategories are scored on a scale of −1 (or 0) up to 4, with a minimum score of −2 for men and −3 for women and a maximum score of 37 for men and 45 for women.1, 18
2.2.2. Family history
Information on the first‐, second‐ and third‐degree family history was collected. This was categorized as (a) positive: at least one family member is diagnosed with a haemostatic disorder; (b) symptoms only: no family member is diagnosed with a haemostatic disorder but at least one family member displays symptoms of a bleeding disorder; or (c) negative: no family member is diagnosed with a haemostatic disorder, nor does any family member display symptoms of a bleeding disorder.
2.3. Statistics
Statistical analyses were done using SPSS version 22. Continuous variables were compared using the Mann‐Whitney U test. Results are given by P‐values. P‐values < .05 were considered statistically significant. Univariate binomial logistic regression models were performed to evaluate the strength of the individual (binomial) variables. Results of the binomial logistic regression models are given by the estimated odds ratios (OR) with 95% confidence intervals (CI). No correction for multiplicity was applied because of the exploratory character of this study.
3. RESULTS
3.1. Characteristics of the subjects
From 1 January 2016 through 1 July 2017, one hundred and thirty‐one consecutive adult patients were referred to the Radboud HTC because of being suspected to have an increased bleeding tendency. Nine patients had a preliminary diagnostic trajectory at the time of analysis and were therefore excluded. Another five patients were excluded during the analysis because their bleeding tendency appeared drug related (Figure 2).
Figure 2.
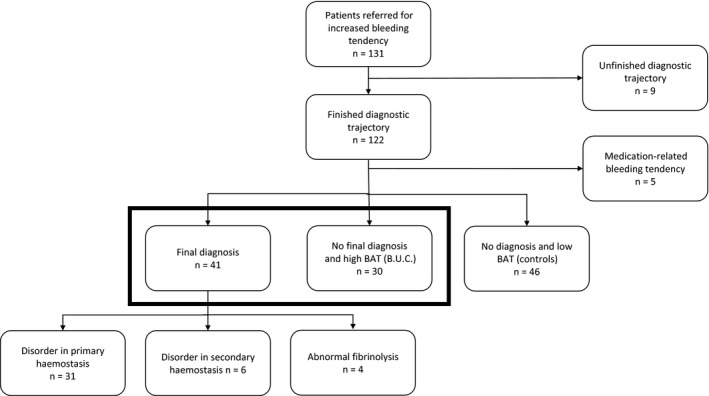
Flow chart of patients inclusion. Both groups at the bottom of the flow chart in bold are studied in more detail
A total of 117 patients remained after exclusion of unfinished testing and iatrogenic bleeding upon medication use and were included into the study. Demographics are shown in Table 1.
Ninety‐four patients (80%) were female, and 23 patients (20%) were men. Median age of the cohort was 37.0 years (range: 18‐68 years). Forty‐five (38%) patients were referred by first‐line healthcare professionals, while the majority (n = 72, 62%) was referred by second‐line healthcare professionals. Referral could be due to a variety of underlying reasons, such as previous bleeding episodes (n = 82), family members with a known haemostatic disorder (n = 29), or because of abnormal haemostatic screening assays (n = 6), for example in the preoperative trajectory.
With respect to their clinical phenotype, cutaneous bleeding was the most frequent reported bleeding symptom reported by 65% of all patients. Central nervous system bleeding (n = 4, 3.4%), muscle haematomas (n = 5, 4.3%) and haemarthrosis (n = 7, 6.0%) were reported with low frequencies (Table 1.).
A total of 41 patients received final diagnosis at the end of the diagnostic trajectory: 31 had a disorder in primary haemostasis, 6 had a disorder of secondary haemostasis and 4 had an abnormal fibrinolysis. Thirty patients had a high BAT score but did not receive final diagnosis since laboratory parameters could not confirm a haemostatic disorder. The remaining 46 patients had a low BAT score and no abnormalities in their laboratory parameters that confirmed the presence of a disorder in haemostasis (control group) (Figure 2).
3.2. Clinical phenotype
3.2.1. Gender and age
When comparing the group receiving final diagnosis and the BUC group, the chance of reaching final diagnosis was higher in men than in women (OR = 4.2, 95% CI 1.1‐16). Age did not correlate to reaching final diagnosis (P = .7) (Table 1).
3.2.2. BAT
The Tosetto BAT score was significantly higher in patients classified as BUC (group 2) compared to the group of patients reaching final diagnosis (group 1) (8.1 vs 4.9, P = .002) (Table 1). The control group scored significantly lower as compared to the group reaching final diagnosis (2.2 vs 4.9, P = .009).
Further analyses showed that, specifically, the individual BAT items surgery and post‐partum haemorrhage were significantly higher in the BUC group as compared to group of patients reaching final diagnosis. Concerning the individual item surgery, the average Tosetto bleeding score was 1.1 in group 1 and 2.1 in group 2 (P = .008) and 0.2 in controls. For the individual item post‐partum haemorrhage, the average score was 0.0 in group 1 and 0.7 in group 2 (P = .008) and 0.2 in controls (Figure 3). No significant difference was observed between controls and the patient group reaching final diagnosis.
Figure 3.
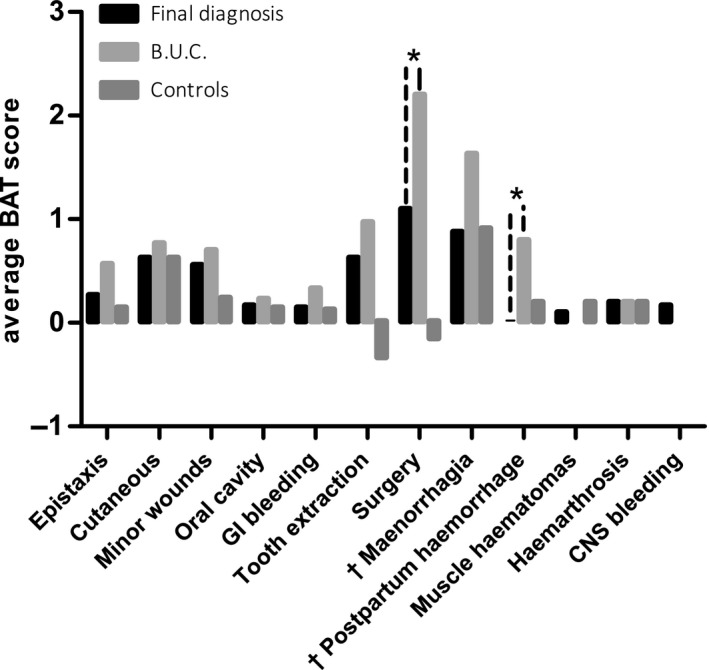
Individual BAT score parameter in the subgroups. * Significance between the group reaching a final diagnoses and the B.U.C. group. Significance between the other groups are not shown. † Women only
3.2.3. Family history
The number of patients having a family history with a haemostatic diagnosis was significantly higher in the group reaching final diagnosis compared to the group of patients classified as BUC (n = 24 (59%) vs n = 2 (7%), OR = 9.0, 95% CI 1.9‐43). Therefore, a positive family history with a haemostatic disorder was associated with a higher percentage of patients with a definitive diagnosis.
Further analyses showed that the number of patients with a diagnosis of a bleeding disorder in a first‐degree relative was significantly higher in the group reaching final diagnosis, compared to the BUC group (n = 15 (37%) vs n = 1 (3%), OR = 17, 95% CI 2.1‐136). The number of patients having a second‐degree relative with a positive family history was also higher in the group reaching final diagnosis compared to the BUC group (n = 6 (15%) vs n = 1 (3%), OR = 5.0, 95% CI 0.6‐44), although this difference was not statistically significant. Consequently, a first‐degree family member with an established bleeding disorder is a strong predictor for having a haemostatic disorder.
3.3. Laboratory phenotype
Patients receiving final diagnosis had significantly more often abnormal results in assays of the screening panel (n = 32 (78%) vs n = 14 (46%), OR = 4.1, 95% CI 1.5‐12, n = 16 (35%) in controls). For each individual assay, the results are as follows: PFA (n = 27 (66%) vs n = 10 (33%), OR = 3.9, 95% CI 1.4‐10, n = 12 (26%) in controls); APTT (n = 11 (27%) vs n = 6 (20%), OR = 1.3, 95% CI 0.4‐4.2, n = 7 (15%) in controls); PT (n = 4 (10%) vs n = 2 (7%), OR = 1.4, 95% CI 0.3‐8.5, n = 1 (2%) in controls); von Willebrand factor activity levels (n = 11 (27%) vs n = 1 (3%), OR = 11.0, 95% CI 1.3‐91, n = 4 (9%) in controls) (Figure 4).
Figure 4.
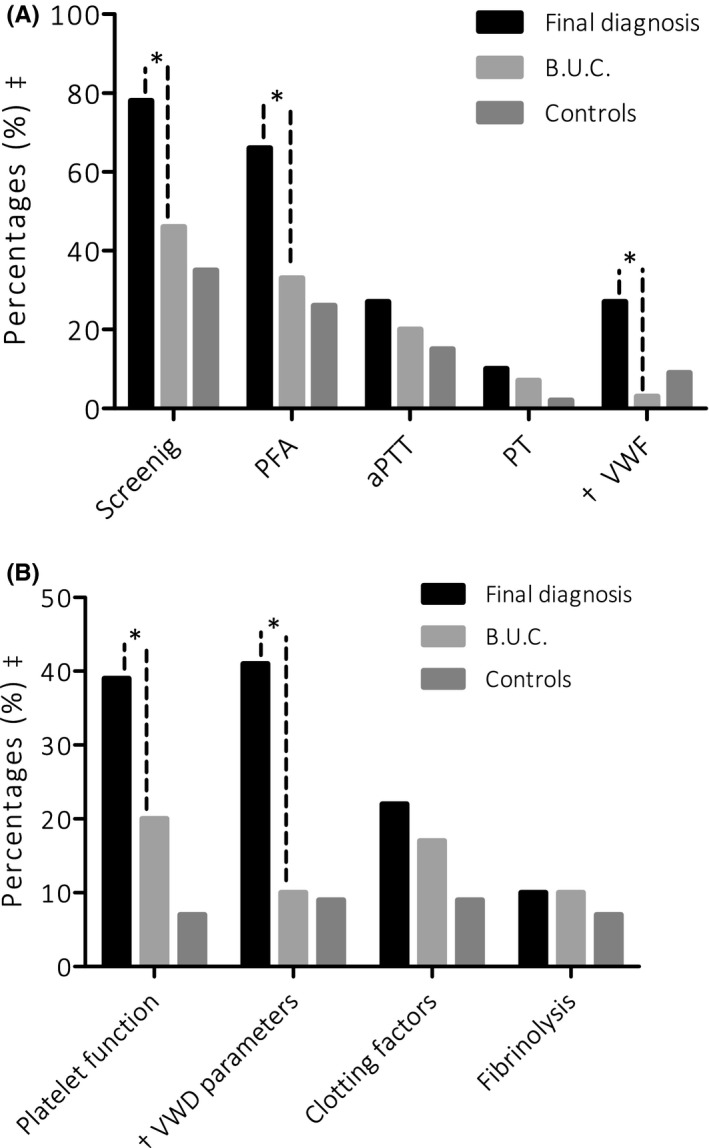
Percentage of patients with an abnormal diagnostic test. A, tests results on screening assays, (B) results on confirmation assays. * Significance between the group reaching a final diagnosis and the B.U.C. group. Significance between the other groups are not shown. ++ Percentages are pertaining the group concerned. † VWD is also part of our screening panel. Moreover, an extended VWF panel is used for confirmation of von Willebrand Disease
Results of the confirmation assay (panel 1 to 4) showed an increased frequency of abnormal platelet function assay (n = 16 (39%) vs n = 6 (20%), OR = 6.0, 95% CI 1.7‐21, n = 3 (7%) in controls) and abnormal von Willebrand factor parameters (n = 17 (41%) vs n = 3 (10%), OR = 6.7, 95% CI 1.7‐26, n = 4 (9%) in controls) in the group of patients receiving final diagnosis compared to the patients assigned to the BUC group. Abnormal coagulation factor levels were not significantly different in both groups, nor were abnormal fibrinolysis parameters.
Eventually, 13 patients were diagnosed with vWD (low‐normal VWF with blood type 0 (n = 5), type 1 (n = 4), type 2M (n = 2), type 3 (n = 1) and carrier type 3 (n = 1)), 18 patients with mild/severe platelet function disorder (PFD)(abnormalities found in; aggregation (n = 5), secretion (n = 8), activation marker (n = 7), thrombocyte receptor (n = 2)), 4 patients with a single coagulation factor deficiency (FVIII (n = 2), FX (n = 1) and FXI (n = 1)), 4 patients with hyperfibrinolysis and 2 women appeared to be a haemophilia carrier.
4. DISCUSSION
This retrospective observational study showed that patients without final diagnosis but a high BAT had a higher BAT score than patients receiving final diagnosis and controls. Interestingly, the two subcategories most prevalently increased appeared surgery and post‐partum haemorrhage‐associated bleeding.
It illustrates that isolated blood loss due to surgical procedures or post‐partum haemorrhage can have a large effect on the BAT score and the possibility to be diagnosed with a well‐defined haemostatic disorder. This suggests that a high BAT score primarily due to surgical or post‐partum bleeding, in the absence of other identifiable bleeding symptoms, is probably due to these circumstances instead of a haemostatic disorder. Consequently, this suggest withholding further testing in these cases. This change in approach to a bleeding patient would first be validated in a future study in which an algorithm would be implemented, that withholds further haemostatic testing based on several arguments, like a high BAT score on isolated items or the type of surgery or method of delivery. The most notorious interventions at young age that lead to excessive blood loss in case of an underlying haemostatic disorder are tonsillectomies and circumcisions.19, 20, 21 In case of delivery, the risk of excessive blood loss is increased during a caesarean section compared to a vaginal deliver.22
In addition, in our study, men appeared to have a higher chance of achieving final diagnosis. This might be explained by the absence of post‐partum haemorrhage and menorrhagia and therefore perhaps a more representing bleeding score, supporting the hypotheses above. Finally, post‐partum haemorrhage and menorrhagia are frequently described in healthy women as well.23 In addition, the Tosetto BAT was initially developed to diagnose vWD.2, 24, 25, 26 It was not until later that physicians started to incorporate the BAT into their standard anamnesis. However, this tool might not be as usable in screening for other haemostatic disorders as previously thought.27 Another disadvantage of BAT scores is that these scores increase in time and are not based on time intervals. Longitudinal BAT scores may overcome this.28
In previous studies, measuring PFA was found to be an insensitive screenings assay, especially for patients with mild PFD’s.27, 29 It was considered not very useful since the PFA cannot differentiate between different PFD’s and vWD. Even though this disadvantage remains, in our study a prolonged PFA correlated with a definitive diagnosis. Patients who had a prolonged PFA were two times more likely to receive final diagnosis as compared to patients with a normal PFA (n = 27 (55%) vs n = 14 (23%). This was found to be statistically significant. About half of the patients (n = 27) with a prolonged PFA were diagnosed with a haemostatic disorder; 41% (n = 11) appeared to have vWD and 44% (n = 12) with a PFD. The remaining 15% (n = 4) appeared to have a coagulation factor deficiency (n = 2) or hyperfibrinolysis (n = 2). It was therefore found that the PFA is associated with a primary haemostasis abnormality; however, the PFA is not able to discriminate between the vWD and PFD.
Furthermore, a positive family history (particularly a first‐degree relative) also correlated with a definitive diagnosis, emphasizing the importance of the family history.
Our diagnostic process led to a final diagnosis in 58% of patients referred, which is in line with previous reports.6, 7, 8, 9, 10, 11, 12, 13 Current laboratory assays have proven to be useful in detecting coagulation disorders. Even though, a significant challenge remains when it comes to diagnosing mild bleeding disorders or combined haemostatic disorders of primary and secondary haemostasis. This results in a time‐consuming diagnostic process, which still leaves a major group of patients without a definite diagnosis. In addition, undergoing the full diagnostic trajectory is cumbersome and costly. The control group had the lowest cost with an average of around 2000 euro per patient. Average costs of the diagnostic trajectory for one patient in the group reaching final diagnosis were approximately 65% higher. The BUC group had the highest costs (80% higher) per patient for performing all assays in our centre. Eight patients in the BUC group had only high scores on surgery or PPH. This corresponds with 28 800 euro, which points to a significant burden of health costs.
The applicability of performing screening assays in our study is in line with previous studies. Seventy‐eight per cent of the patients receiving final diagnosis had a positive screening assay. The other two groups had only a positive screening assay result in less than half of the cases. Interestingly, the difference between these two groups was only 12 per cent (no final diagnosis but high BAT: 47% vs no final diagnosis and low BAT 35%).
Furthermore, in one‐third of the patients with a low BAT and no diagnosis, a positive screening result was observed suggesting that there is room for improvement with respect to the current screening assay panel. Whether improvements can be reached with global assays like thrombin and plasmin generation assays 30, 31 still needs to be determined. Most of them are still not incorporated in the diagnostic work up, as these assays are still not properly validated or standardized. The expectation, however, is that these assays are more sensitive and can differentiate whether the abnormality is due to alterations in initiation, propagation or termination of coagulation, fibrinolysis and even in platelet activation.
Recently, several studies described the value of next‐generation sequencing.5, 32, 33, 34 In our clinic, whole exome sequencing (WES) was recently incorporated in the diagnostic trajectory of patients without a definite diagnosis but with a high bleeding score (cut‐off BAT score ≥ 10).35 The possibility of analysing many genes at once is a huge advantage.34 Performing WES analysis can potentially increase the number of patients receiving a definite diagnosis. However, WES is still not widely accepted as a diagnostic tool and it can only be of additional value if better functional screening and confirmation tests are being developed to approve the WES data.34
A limitation of this study was the use of the Tosetto BAT instead of the ISTH‐BAT, which is currently the internationally accepted bleeding assessment tool.1, 3, 18 Due to time constraints, the Tosetto BAT is used in our practice. A crucial difference is the negative score (−1) on the Tosetto BAT if no bleeding occurred after surgery or delivery. This would be scored a zero on the ISTH‐BAT.1, 18 Since both assessment tools score on similar categories, any effect is limited.18 Another limitation is that the collagen binding assay was not included in the screenings panel, which might have led to the possibility that we have missed type 2M vWD patients.
In conclusion, our study reveals the clinical and laboratory characteristics of patients referred to the haematology department of a tertiary referral centre with an increased bleeding tendency. Increased, isolated high bleeding score on surgical or post‐partum bleeding predisposes to the inability to achieve a final haemostatic diagnosis. This challenges the intuition that an underlying bleeding disorder classically is unmasked by the haemostatic challenge of delivery or surgery.
With this in mind, extensive haemostatic testing can be avoided in these cases. Further research validating an algorithm eliminating further testing in those with an increased BAT on the basis of PPH or surgery related, would be interesting. An improved implementation of the diagnostic trajectory can lead to reduced healthcare cost, which is an ultimate opportunity in the current healthcare system. Furthermore, better screening and confirmation haemostatic assays are still needed. Global haemostatic assays and genetic sequencing techniques can play an important role in the near future and introducing these diagnostics in clinical research is of essential importance.
ACKNOWLEDGEMENTS
SAM Zegers collected the data and was responsible for the data analysis. She has also contributed in the interpretation of the data and led in writing the manuscript. J. Saes and Y. Smit contributed in the data analysis, as well as the interpretation of the data. They also critically reviewed the manuscript. C. van Duren and TJ Schuijt critically reviewed the manuscript and contributed to the analysis of the data. WL van Heerde contributed in the data analysis, as well as the interpretation of the data. He also contributed in writing the manuscript. SEM Schols designed the project and contributed in the data analysis, as well as the interpretation of the data. She also contributed in writing the manuscript. The authors have no competing interests.
Zegers SAM, Smit Y, Saes JL, et al. Diagnostic work up of patients with increased bleeding tendency. Haemophilia. 2020;26:269–277. 10.1111/hae.13922
REFERENCES
- 1. Elbatarny M, Mollah S, Grabell J, et al. Normal range of bleeding scores for the ISTH‐BAT: adult and pediatric data from the merging project. Haemophilia. 2014;20(6):831‐835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rodeghiero F, Castaman G, Tosetto A, et al. The discriminant power of bleeding history for the diagnosis of type 1 von Willebrand disease: an international, multicenter study. J Thromb Haemost. 2005;3(12):2619‐2626. [DOI] [PubMed] [Google Scholar]
- 3. Rydz N, James PD. The evolution and value of bleeding assessment tools. J Thromb Haemost. 2012;10(11):2223‐2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Quiroga T, Mezzano D. Is my patient a bleeder? A diagnostic framework for mild bleeding disorders. Hematology Am Soc Hematol Educ Program. 2012;2012:466‐474. [DOI] [PubMed] [Google Scholar]
- 5. Sivapalaratnam S, Collins J, Gomez K. Diagnosis of inherited bleeding disorders in the genomic era. Br J Haematol. 2017;179(3):363‐376. [DOI] [PubMed] [Google Scholar]
- 6. Parkin JD, Smith IL, O'Neill AI, Ibrahim KM, Butcher LA. Mild bleeding disorders. A clinical and laboratory study. Med J Aust. 1992;156(9):614‐617. [PubMed] [Google Scholar]
- 7. Bolton‐Maggs P, Wilkinson LS. Mild bleeding disorders: review of 120 patients. Clin Lab Haematol. 1984;6(3):247‐256. [PubMed] [Google Scholar]
- 8. Agren A, Wiman B, Stiller V, et al. Evaluation of low PAI‐1 activity as a risk factor for hemorrhagic diathesis. J Thromb Haemost. 2006;4(1):201‐208. [DOI] [PubMed] [Google Scholar]
- 9. Podda GM, Bucciarelli P, Lussana F, Lecchi A, Cattaneo M. Usefulness of PFA‐100 testing in the diagnostic screening of patients with suspected abnormalities of hemostasis: comparison with the bleeding time. J Thromb Haemost. 2007;5(12):2393‐2398. [DOI] [PubMed] [Google Scholar]
- 10. Posan E, McBane RD, Grill DE, Motsko CL, Nichols WL. Comparison of PFA‐100 testing and bleeding time for detecting platelet hypofunction and von Willebrand disease in clinical practice. Thromb Haemost. 2003;90(3):483‐490. [DOI] [PubMed] [Google Scholar]
- 11. Wuillemin WA, Gasser K, Zeerleder SS, Lammle B. Evaluation of a Platelet Function Analyser (PFA‐100) in patients with a bleeding tendency. Swiss Med Wkly. 2002;132(31–32):443‐448. [DOI] [PubMed] [Google Scholar]
- 12. Gebhart J, Hofer S, Panzer S, et al. High proportion of patients with bleeding of unknown cause in persons with a mild‐to‐moderate bleeding tendency: Results from the Vienna Bleeding Biobank (VIBB). Haemophilia. 2018;24(3):405‐413. [DOI] [PubMed] [Google Scholar]
- 13. Relke N, Kuthiala S, Grabell J, Hopman WM, James P. The bleeding score: Useful in predicting spontaneous bleeding events in adults with bleeding of unknown cause? Haemophilia. 2019. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Bateman BT, Berman MF, Riley LE, Leffert LR. The epidemiology of postpartum hemorrhage in a large, nationwide sample of deliveries. Anest Analg. 2010;110(5):1368‐1373. [DOI] [PubMed] [Google Scholar]
- 15. James AH, McLintock C, Lockhart E. Postpartum hemorrhage: when uterotonics and sutures fail. Am J Hematol. 2012;87(Suppl 1):S16‐22. [DOI] [PubMed] [Google Scholar]
- 16. van Rein N, Biedermann JS, van der Meer FJM, et al. Major bleeding risks of different low‐molecular‐weight heparin agents: a cohort study in 12 934 patients treated for acute venous thrombosis. J Thromb Haemost. 2017;15(7):1386‐1391. [DOI] [PubMed] [Google Scholar]
- 17. Jesudas R, Chaudhury A, Laukaitis CM. An update on the new classification of Ehlers‐Danlos syndrome and review of the causes of bleeding in this population. Haemophilia. 2019;25(4):558‐566. [DOI] [PubMed] [Google Scholar]
- 18. Rodeghiero F, Tosetto A, Abshire T, et al. ISTH/SSC bleeding assessment tool: a standardized questionnaire and a proposal for a new bleeding score for inherited bleeding disorders. J Thromb Haemost. 2010;8(9):2063‐2065. [DOI] [PubMed] [Google Scholar]
- 19. Warad D, Hussain FT, Rao AN, Cofer SA, Rodriguez V. Haemorrhagic complications with adenotonsillectomy in children and young adults with bleeding disorders. Haemophilia. 2015;21(3):e151‐e155. [DOI] [PubMed] [Google Scholar]
- 20. Kulkarni R, Presley RJ, Lusher JM, et al. Complications of haemophilia in babies (first two years of life): a report from the Centers for Disease Control and Prevention Universal Data Collection System. Haemophilia. 2017;23(2):207‐214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Di Capua M, Livia Burzo M, Di Palo M, et al. Predicting early and delayed bleedings in children who undergo adeno‐tonsillectomy surgery. Is it really possible? Haemophilia. 2014;20(6):e438‐e440. [DOI] [PubMed] [Google Scholar]
- 22. Lee CA, Chi C, Pavord SR, et al. The obstetric and gynaecological management of women with inherited bleeding disorders–review with guidelines produced by a taskforce of UK Haemophilia Centre Doctors' Organization. Haemophilia. 2006;12(4):301‐336. [DOI] [PubMed] [Google Scholar]
- 23. Mauer AC, Khazanov NA, Levenkova N, et al. Impact of sex, age, race, ethnicity and aspirin use on bleeding symptoms in healthy adults. J Thromb Haemost. 2011;9(1):100‐108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pathare A, Al Omrani S, Al Hajri F, Al Obaidani N, Al Balushi B, Al FK. Bleeding score in Type 1 von Willebrand disease patients using the ISTH‐BAT questionnaire. International journal of laboratory hematology. 2017. [DOI] [PubMed] [Google Scholar]
- 25. Tosetto A, Castaman G, Rodeghiero F. Assessing bleeding in von Willebrand disease with bleeding score. Blood Rev. 2007;21(2):89‐97. [DOI] [PubMed] [Google Scholar]
- 26. Bowman ML, James PD. Bleeding scores for the diagnosis of von willebrand disease. Semin Thromb Hemost. 2017;43(5):530‐539. [DOI] [PubMed] [Google Scholar]
- 27. Moenen F, Vries MJA, Nelemans PJ, van Rooy KJM, Vranken J, Verhezen PWM. Screening for platelet function disorders with Multiplate and platelet function analyzer. Platelets. 2017;30:1‐7. [DOI] [PubMed] [Google Scholar]
- 28. Borhany M, Fatima N, Abid M, Shamsi T, Othman M. Application of the ISTH bleeding score in hemophilia. Transfus Apher Sci. 2018;57(4):556‐560. [DOI] [PubMed] [Google Scholar]
- 29. Favaloro EJ. Clinical utility of the PFA‐100. Semin Thromb Hemost. 2008;34(8):709‐733. [DOI] [PubMed] [Google Scholar]
- 30. van Geffen M, van Heerde WL. Global haemostasis assays, from bench to bedside. Thromb Res. 2012;129(6):681‐687. [DOI] [PubMed] [Google Scholar]
- 31. Ilich A, Key NS. Global assays of fibrinolysis. Int J Lab Hematol. 2017;39(6):e142‐e143. [DOI] [PubMed] [Google Scholar]
- 32. Lentaigne C, Freson K, Laffan MA, Turro E, Ouwehand WH; BRIDGE‐BPD Consortium and the ThromboGenomics Consortium . Inherited platelet disorders: toward DNA‐based diagnosis. Blood. 2016;127(23):2814‐2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lee EJ, Dykas DJ, Leavitt AD, et al. Whole‐exome sequencing in evaluation of patients with venous thromboembolism. Blood Adv. 2017;1(16):1224‐1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Leinoe E, Zetterberg E, Kinalis S, et al. Application of whole‐exome sequencing to direct the specific functional testing and diagnosis of rare inherited bleeding disorders in patients from the Oresund Region. Scandinavia. 2017;179(2):308‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saes JL, Simons A, de Munnik SA, et al. Whole exome sequencing in the diagnostic workup of patients with a bleeding diathesis. Haemophilia. 2018;25:127‐135. [DOI] [PubMed] [Google Scholar]


