Abstract
Our ability to focus on goal‐relevant aspects of the environment is critically dependent on our ability to ignore or inhibit distracting information. One perspective is that distractor inhibition is under similar voluntary control as attentional facilitation of target processing. However, a rapidly growing body of research shows that distractor inhibition often relies on prior experience with the distracting information or other mechanisms that need not rely on active representation in working memory. Yet, how and when these different forms of inhibition are neurally implemented remains largely unclear. Here, we review findings from recent behavioral and neuroimaging studies to address this outstanding question. We specifically explore how experience with distracting information may change the processing of that information in the context of current predictive processing views of perception: by modulating a distractor's representation already in anticipation of the distractor, or after integration of top‐down and bottom‐up sensory signals. We also outline directions for future research necessary to enhance our understanding of how the brain filters out distracting information.
Keywords: attention, inhibition, predictive processing, brain, EEG
Here, we address the outstanding question how the brain suppresses distracting information. We review findings from recent behavioral and neuroimaging studies that suggest that distractor inhibition is not under voluntary control, but relies on experience with the distracting information. Building on predictive processing views of perception, we discuss how experience with distracting information may reduce distractor processing and interference: by modulating a distractor's representation in advance, or after integration of top‐down and bottom‐up sensory signals.

Introduction
Our daily visual surroundings, like city crossroads, contain a multitude of objects, most of which are irrelevant to our current goals. To flexibly navigate such complex environments, ignoring visual distractions (e.g., advertisement billboards) is arguably equally important as focusing on goal‐relevant information (e.g., surrounding cars). Yet, while over the past several decades, much has been learned about how selective attention can facilitate neural processing of goal‐relevant information,1, 2, 3 the mechanisms that underlie suppression of visual distractions at the neural level remain relatively poorly understood.
Facilitation and inhibition have long been envisioned as two sides of the same coin.4, 5 Yet, a rapidly growing body of work indicates that distractor suppression is not unitary and reveals itself in different guises, likely reflecting multiple underlying neural architectures and processes.6, 7 This diversity is also hard to reconcile with influential attentional theories (e.g., biased competition) that only regard suppression as resulting from inhibitory influences arising from competitive interactions between neural populations.1, 8 Instead, it is now evident that inhibition can also be accomplished through neural mechanisms that are (in part) independent from well‐characterized facilitative attention mechanisms.6, 9, 10
Here, we review recent work on the neural mechanisms underlying inhibition in selective attention, and specifically address the outstanding question: how and under what conditions can the brain, if at all, suppress distractors before they capture attention? In this, we dissociate between proposed preparatory mechanisms that suppress distractor features in advance9, 11, 12 and suppressive mechanisms that come about in response to distracting sensory input.7, 10, 13 While the long‐standing view has been that inhibition of distracting or irrelevant sensory information is under direct, volitional control similar to attention to goal‐relevant aspect of the environment, it is becoming increasingly clear that suppression is not under direct top‐down control and can be implemented via a multitude of underlying mechanisms, each tuned to specific circumstances.6, 9, 14 Recent behavioral studies, for example, have shown that the ability to ignore distracting information often strongly depends on learning based on previous experiences with the distracting information.15, 16 Yet, what factors determine the ability to inhibit distractor information and which specific set of neural mechanisms arise under different circumstances remain topics of active debate.6, 9, 10, 14
In addressing this outstanding issue, we will discuss evidence from key behavioral and neuroimaging studies in humans and some animal studies in light of different ideas on distractor inhibition.6, 9, 10, 17 The picture that emerges from this is that how the brain deals with distracting information is not simply determined by whether or not information is relevant versus irrelevant to the task at hand, but influenced by many factors, including previous experience with the distracting information (expectations), whether suppression is feature based or space based, target‐distractor similarity, the level at which distracting information can be suppressed, and distractor salience. Building on the notion of expectation‐dependent distractor suppression,9 we specifically discuss different ways in which experience with distracting information may change the neural representation and processing of that information in the context of current predictive processing accounts of perception.18, 19 In these accounts, expectations derived from past experience and grounded in statistical regularities in the environment strongly shape sensory information processing and thereby perception, but it is unclear if and how predictive processing is affected by the distracting or irrelevant nature of encountered information. Expectations could result in tuning toward or away from the expected distractor features (Fig. 1A and B), or they could not be expressed in changes in anticipatory tuning but exert their effects via synaptic plasticity (Fig. 1C). We will also discuss recent findings that show that alpha‐band oscillations may play an important role in facilitating processing of goal‐relevant information, by suppressing noise20 or stabilizing the representation of the attended stimulus.21, 22 We explore the possibility that alpha oscillations could similarly modulate the representation of distracting information. We end by discussing important avenues for future research.
Figure 1.
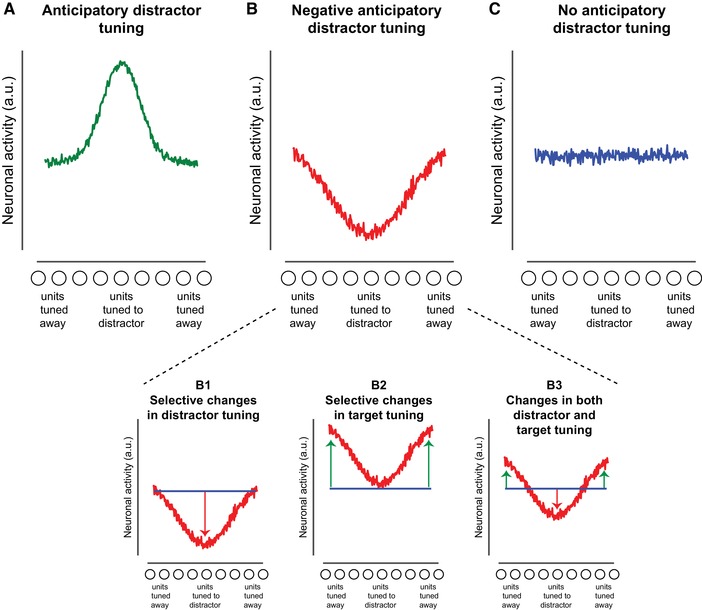
Three different scenarios of how expectations may modulate the representation of distracting information in anticipation of new sensory input (i.e., anticipatory distractor tuning). The tuning curves reflect selectivity of population‐level neural activity to a particular feature (e.g., location or orientation). Expectations about upcoming distractor information may result in anticipatory tuning (A) toward the expected distractor feature or (B) away from the expected distractor feature resulting in negative anticipatory distractor tuning. The subplots of figure B illustrate different scenarios that may all produce negative tuning slopes: as consequence of reduced anticipatory tuning to the distractor (panel B1), of shifting anticipatory tuning away from the expected distractor to nondistractor features/locations (panel B2), or a combination of both (panel B3). The horizontal blue line in each subplot indicates the baseline situation of no expectation. (C) Alternatively, distractor expectations may not be evident in anticipatory neural activity (firing) patterns.
Preparatory distractor suppression
It is currently actively debated whether distractor inhibition is under similar flexible top‐down control as target facilitation and depends on representation in working memory.6, 9, 14 A large body of work shows a close relationship between selective attention and visual working memory, such that attention is directed toward visual information that is actively represented in working memory.1, 23 Indeed, many studies have demonstrated that the content of working memory automatically biases attention toward matching incoming information, even when that information is known to be detrimental to performance at the current task.24, 25 It has been proposed that information in working memory about anticipated distracting input can also be represented as a “rejection template” such that matching input will be strategically inhibited.11, 12 In contrast to the notion of direct working memory–based distractor suppression, it has also been suggested that suppression of distracting information may rely on mechanisms that render the representation of the target in working memory maximally distinct from that of the distractor, thereby indirectly decreasing the chance of distractor selection.17, 26 In yet another account, distractor inhibition may bypass representation in working memory altogether, relying on expectation‐dependent suppression.9 Below, we discuss these different accounts of preparatory inhibition in more detail in light of the empirical literature to address the outstanding question if distractor inhibition can be implemented in advance, and if so, how and under what conditions?
Direct preparatory distractor suppression
It has been proposed that information in working memory about anticipated distracting input can be represented as a rejection template, such that matching distracting input will be strategically inhibited.11, 12, 14 This notion of direct top‐down attentional inhibition is challenged by behavioral studies showing that cues informing about the upcoming distractor location or feature carry no behavioral benefit15, 27, 28 or even hamper performance, when this information varies from trial to trial.29 These findings suggest that if anything, distractor templates in working memory result in increased distractibility and a prerequisite for successful preparatory inhibition may therefore be that the underlying mechanisms bypass working memory maintenance. However, there is also evidence that distractor foreknowledge can actually be used to selectively avoid or inhibit matching distractors11, 12, 30, 31 or specific distractor locations.32, 33 Based on behavioral measures alone, however, it is hard to establish if these observed behavioral benefits of distractor foreknowledge are indeed driven by an advance inhibitory template or instead rely on a postdistractor inhibition mechanism.7, 13 To unequivocally establish the existence of inhibitory templates and direct, active preparatory suppression, we need to turn to measures of brain activity. The dominant view in the cognitive neuroscience literature over the past two decades or so has been that just like a frontoparietal control network can enhance processing of goal‐relevant information by biasing task‐relevant sensory regions in advance, this control network can also inhibit sensory processing of distracting information in advance, and that alpha‐band oscillations implement this top‐down attentional bias.4, 34
It is specifically proposed that alpha‐band oscillations implement direct, top‐down inhibition via the suppression of activity of sensory regions representing the task‐irrelevant or distracting information.4, 34 Indeed, alpha oscillatory activity has been functionally linked to reduced cortical excitability,35 and there is abundant evidence from spatial attention studies that prestimulus alpha‐band activity decreases over visual regions contralateral to attended target locations and/or increases over ipsilateral visual regions that represent the task‐irrelevant hemifield.36, 37, 38, 39 Several studies have also related increases in prestimulus alpha‐band activity to anticipatory suppression of nonspatial visual features.40, 41, 42 These modulations in anticipatory alpha activity have generally been taken to reflect the release of inhibition of task‐relevant visual networks and enhanced the inhibition of task‐irrelevant visual networks. However, as also pointed out recently by Foster and Awh,43 evidence in support of the notion that alpha oscillations implement active, top‐down distractor suppression is ambiguous. That is, the vast majority of attention studies reporting increased preparatory alpha activity over irrelevant visual regions used task designs in which foreknowledge about the distractor location or features was accompanied by foreknowledge about the target location or features, such as bilateral displays,36, 37, 38 rendering it possible that observed effects could also simply reflect attending away or secondary inhibition related to attention to the target.9 The same is true for studies that used cues that signaled the likelihood of upcoming distraction, but also provided information about the upcoming target, and examined changes in other measures of preparatory activity, such as the cue‐evoked LDAP ERP component.44, 45
To unambiguously establish a role for alpha‐oscillations in preparatory inhibition, it is not only important that the process of interest is selectively manipulated, but also that this cannot lead to changes in how attention is directed toward task‐relevant information. In the spatial domain, one approach is to cue participants about the upcoming distracting information without making target information explicit, which could be accomplished by using visual search tasks with multiple stimulus locations. Of the substantial body of work linking alpha‐band activity to spatial suppression, however, only a single study actually examined alpha‐modulations in response to distractor location cues that were not also informative about the upcoming target location.46 In this study, prestimulus alpha‐band activity tracked the anticipated location when the target was cued, but not when the distractor was cued. Rather than reflecting direct top‐down inhibition, observed changes in prestimulus alpha activity reported previously could thus also be a secondary consequence of top‐down target facilitation.
Although alpha oscillations have predominantly been linked to spatially specific suppression, as noted above, there is also evidence that alpha‐suppression mechanisms operate in much the same manner during nonspatial selection.40, 41, 42 However, here too, observed effects cannot be unambiguously interpreted as active preparatory inhibition because knowledge about the upcoming irrelevant nonspatial feature was also always accompanied by knowledge about the relevant nonspatial feature. Moreover, the one study to date that specifically modulated distractor interference and examined whether alpha oscillations contribute to feature‐based, preparatory distractor suppression investigated changes in alpha activity with respect to the location of the distractor stimulus. In this study by de Vries et al.,47 lateralized posterior alpha power did not dissociate between laterally presented colors in memory, which were cued to either be a target or a distractor in subsequent searches, further arguing against the idea that human observers can set up an inhibitory template in advance. Yet, although lateralized alpha power did not help to dissociate between targets and distractors, overall nonlateralized power over the visual cortex was higher when observers were anticipating a distractor versus a target (see also Ref. 48). Similarly, using functional magnetic resonance imaging (fMRI), another study observed less activation in large parts of the visual cortex in response to distractor color cues relative to neutral or target cues,49 without this decrease being distinctive for specific distractor features.50 Together, these studies suggest that behavioral benefits observed as a function of distractor foreknowledge may be related to nonspecific suppression of sensory activity, possibly in an attempt to filter out anticipated distractors. Yet, they do not support the notion of direct top‐down preparatory distractor feature‐specific inhibition.
The previous suggests that distractor suppression may rely on nonspecific sensory inhibition. Indeed, benefits elicited by target location cues that concurrently signal distractor probability are increased on high relative to low probable distractor trials.51, 52, 53 In such contexts, when visual distraction is expected, but information about the upcoming distractor is nonspecific, the visual system may prepare to inhibit perceptual processing as whole via increased posterior alpha power.48 Also, in a more recent fMRI study, preparatory BOLD activity increased in the middle frontal gyrus in frequent relative to infrequent distractor blocks, a signal increase that was subsequently accompanied by attenuation of signal processing in the occipital cortex.54 Together, these latter studies suggest that nonspecific suppressive mechanisms can be flexibly induced in anticipation of distractors, although the neural mechanisms underlying this form of suppression remain largely speculative. Alternatively, knowing that external distraction is likely to occur can simply also modulate arousal or response readiness, and affect performance through mechanisms unrelated to sensory inhibition per se. Future neuroimaging studies are necessary to determine how nonspecific inhibition facilitates performance.
Thus, to date, there is very little neural evidence in support of the notion that distractor foreknowledge is associated with direct top‐down inhibition of activity in sensory regions representing the anticipated distractor location or feature. This may suggest, as discussed in the below, that distractor suppression relies on mechanisms that bypass working memory representation or only becomes evident after integration of bottom‐up sensory input with top‐down influences. However, given that only a handful of studies examined markers of active preparatory suppression, it is premature to conclude that direct top‐down inhibition in either the spatial or feature domain is not possible. One intriguing possibility is that in contrast to preactivating an attentional template, preparatory suppression is cognitively demanding and therefore limited to contexts of especially difficult searches.55 Future studies that examine the effect of search difficulty on distractor inhibition at the neural level are necessary to test the idea that advance distractor suppression may selectively occur in cognitively demanding situations, and whether alpha contributes to this.
Indirect preparatory distractor suppression
While it is currently unclear whether templates for rejection can be implemented in a voluntary, top‐down manner, there is a general consensus that repeated encounters with visual distractors reduce their propensity to capture attention. Below, we discuss whether such learned inhibition, which does not seem to depend on working memory, is evident in changes in preparatory neural activity (or firing) in regions representing the distractor. First, however, we review the recent evidence showing that distractor interference can also be reduced by rendering the representation of the target in working memory maximally distinct from that of the distractor, thereby indirectly decreasing the chance of distractor selection.17
Indirect distractor suppression through template‐to‐distractor distinctiveness
Distractor interference is considerably reduced when observers can define a clear attentional set that is accurately tuned to target‐defining features. This is elegantly demonstrated by studies that either encourage searching for a target that is unique in a specific feature dimension, without making the exact feature explicit (e.g., color; singleton‐detection mode), and studies that do allow for a precise attentional template (e.g., red; feature‐search mode).56, 57 Distractor interference is greatly reduced when attentional (target) templates are feature specific, but not when they are not.56, 57, 58 Although challenged by some,59 this dissociation indicates that distractors, even when similar to the target, can be more efficiently ignored when the search allows for goal‐directed feature selection. Intriguingly, recent evidence indicates that the (neural) representation of the target template in working memory does not necessarily need to be a veridical copy of the target, but is a highly dynamic representation that can be flexibly adapted to also incorporate distractor information.26, 60, 61, 62 That is, the target template representation can be strategically shifted off‐veridical to optimize the ability to distinguish targets from distractors and thereby improve attentional selectivity.17 Fine‐tuning of the target template in relation to distractor features may take place in the lateral prefrontal cortex due to the abundance of neurons with mixed selectivity in this area. A recent study demonstrated that information in working memory in monkey's lateral prefrontal cortex reorganized into a different pattern of activity upon distractor presentation, whereas the same code remained stable in frontal eye fields (FEFs).63
A challenge for future work will be to establish how such code morphing or template‐to‐distractor distinctiveness17 may help, albeit indirectly, the suppression of distractor processing, especially when targets and distractors are highly similar at the feature level. In particular, it is still unclear whether only the target representation is adjusted, or whether the representation of both targets and distractors can be adapted. To summarize, one way to suppress distracting information may be to optimize the distinctiveness of target versus distractor representations, thereby indirectly reducing the ability of distracting information to capture attention.
Expectation suppression
A form of inhibition that does not seem to rely on representation in working memory and as such may also prevent distractor capture is suppression driven by previous experiences64 or statistical learning.65 A growing body of work indicates that just like target selection is shaped by stimulus probabilities,66, 67 statistical regularities both in the nonspatial29, 68, 69 and spatial domain16, 65, 70, 71, 72, 73 modulate distractor interference. For example, although color singletons often capture attention,74 performance costs become reliable smaller when the color singleton recurs on subsequent searches.69 Similarly, in search tasks, subjects are faster in responding to targets when a distractor is presented more often at one of the search locations, that is, when its location has become predictable. Crucially, this benefit of distractor predictability cannot be explained by more attention to the remaining possible target locations73 or mere priming.75, 76 Of further note, observers are typically unaware of the unequal probability of distractor occurrences across display locations, indicating that this form of suppression relies on implicit learning mechanisms, that is, is not dependent on working memory.16, 65 In direct support of the notion that statistical distractor learning does not rely on working memory, Gao and Theeuwes76 recently showed that learning to suppress distracting information was not affected by the load of a concurrent working memory task.
One recent proposal is that this form of learned inhibition relies on expectation suppression,9, 46 consistent with predictive processing models, that have recently gained a lot of scientific traction and stature.77 In these models, the brain continuously generates predictions about incoming sensory input based on learned regularities in the environment, and what is being fed up the hierarchy is not sensory input per se, but rather the mismatch between the brain's a priori predictions and the incoming input, or so‐called prediction errors. In this framework, processing of any expected stimulus, whether relevant or irrelevant, is thus suppressed (explained away), which should reduce distractor processing and interference. These informed predictions, which need not necessarily be conscious, provide an elegant solution to profit from the abundant statistical regularities that we encounter in our daily environments.18, 19 An important outstanding question is how distractor inhibition via expectation suppression is neurally implemented. Specifically, it is unclear whether learned inhibition is implemented already in anticipation of distracting input through modulation of activity in visual regions representing the distracting information, or whether it operates via synaptic plasticity and thus only becomes apparent once distractor knowledge can be integrated with bottom‐up sensory input.
The question whether learned inhibition can be preparatory mirrors an ongoing debate in the predictive processing literature, which centers on the question if expectations exert their influence already in advance78, 79 or alternatively, only become apparent after stimulus presentation.80, 81 In line with the notion that the brain continuously generates expectations about upcoming sensory input based on learned regularities in the environment,78, 79 expectations have been associated with changes in prestimulus sensory activity. For example, a recent magnetoencephalography study showed expectation‐dependent sensory templates already before stimulus onset.82 Expectations (stimulus likelihood) and attention (stimulus relevance) also interact, such that top‐down biasing, as reflected in prestimulus alpha‐lateralization, is most pronounced when targets are also most likely occur at the cued, task‐relevant location.83 Thus, expectations may modulate activity in corresponding sensory regions in advance to facilitate goal‐directed behavior. Nevertheless, it is still debated how early expectations modulate stimulus processing, with several recent electroencephalography (EEG) studies suggesting that expectations may primarily affect later stages of information processing.83, 84 In addition, how distractor‐specific expectations may help resolve interference at the neural level remains unclear, as very few neuroimaging studies have so far investigated how distractor learning helps to filter out visual distractions.
Two recent EEG studies examined whether learning about the likely upcoming location of a distractor stimulus was associated with enhanced prestimulus alpha‐band activity over contralateral visual regions and provided mixed results. In line with the notion of top‐down predictive processing, and the notion of alpha as inhibition, one study demonstrated increased alpha‐band activity contralateral to high probability distractor locations already in anticipation of search display onset.85 By contrast, in the study by Noonan et al.,46 as discussed above, alpha‐band modulations were only observed in response to target cues, but not in response to distractor cues, not even when the cued distractor location was fixed in a block of trials (see also Ref. 78). One potential explanation for this apparent discrepancy in findings is that in the first study, the shapes and colors of targets and distractors randomly alternated across trials, whereas targets and distractors had fixed identities in the second study. In the second study, distractor learning could thus occur at the feature level, whereas in the first study, it could only occur based on spatial regularities. Alpha‐band oscillations may thus specifically implement learning‐related space‐based suppression. Indeed, recent behavioral studies suggest that the locus of suppression is flexible, and that how distractor location learning changes the representation of distracting information depends on whether the targets and distractors can be identified based on separate features and/or dimensions (e.g., color for distractors and orientation for targets) or not. For example, it has been shown that when the target and distractor cannot be dissociated on the basis of dimension‐specific information, target processing is also slowed down at high probably distractor locations,16, 65, 86 suggesting that inhibition is implemented at a higher‐level spatial priority or master saliency map.71 Yet, when the defining dimensions of targets and distractors not only differ, but are also predictable, responses to targets at high probability distractor locations no longer slow down, suggestive of nonspatial or dimension‐specific inhibition.71, 75 Note, however, that typically, in these tasks, both the dimension of the target and the distractor are known in advance, rendering it possible that observed effects simply reflect greater attention to the target dimension rather than inhibition of the distractor dimension per se. Future work is also necessary to establish whether preparatory alpha‐band suppression is indeed specific to conditions where the inhibitory set can be implemented at the spatial priority map, and if and how statistical learning about distractor features may affect advance distractor representation.
The studies discussed in the above looked at changes in level of preparatory activity. There are now many demonstrations that the information content of neural activity can be disconnected from the overall amount of neural activity,87 rendering it possible that even in the absence of any changes in the overall level of prestimulus activity, distractor expectations change the quality of the sensory representation of the distracting information. We consider three ways in which expectations about upcoming distracting information may change the representation of that information (Fig. 1). First, just like expectations about upcoming target information may increase the representational content of neural activity, as discussed above, expectations about upcoming distractor information may also result in anticipatory distractor tuning (Fig. 1A). If the distractor is already represented in the preparatory neural code, it may subsequently elicit a weaker response (i.e., prediction error), thereby reducing distractor interference. In this scenario, expectations silence information processing regardless of its task relevance. However, in some predictive processing accounts, attention is proposed to regulate the relative influence of prior expectations by controlling the weight of, or precision assigned to, prediction errors.18 In this way, otherwise small prediction errors triggered by expected task‐relevant stimuli can be assigned greater value, reversing expectation‐dependent suppression.88 A second possibility is thus that distractor learning suppresses anticipatory tuning to expected distractor features by downregulating the weights on corresponding sensory units, resulting in negative anticipatory distractor tuning, effectively cancelling distractor processing (Fig. 1B). Note that in this latter scenario it is important to establish in empirical studies whether negative tuning, if observed, is selectively driven by distractor expectations, as a negative tuning slope may also solely arise as a consequence of shifting sensory tuning away from the expected distractor location/features or some combination of both reduced tuning to the expected distractor location/features and enhanced tuning toward less likely distractor locations/features17 (Fig. 1B, panels B1–B3). A third, and last possibility, discussed in more detail below, is that distractor expectations are not associated with any changes in anticipatory tuning (Fig. 1C) and are only expressed upon distractor presentation.
In a recent study, we addressed the outstanding question as to whether and how distractor expectations may change the sensory representation of distracting information.76 Specifically, combining EEG and inverted encoding modeling,89 we investigated if distractor location learning, induced by keeping the distractor location stable over trials (i.e., four repetitions), was associated with changes in anticipatory spatial tuning to the distractor location. While distractor location foreknowledge was associated with clear behavioral benefits, it did not result in any changes in preparatory spatial tuning to the distractor location (Fig. 2A; bottom row). Note, however, that, although highly variable across participants and thus far from statistically robust, there was a hint of negative tuning prior to search display onset. It is possible that the limited number of repetitions was only sufficient for a small subset of participants to instantiate negative anticipatory tuning and that with more opportunity to learn, negative distractor tuning may have become reliable. It is also possible that strategies differed across participants. In contrast, and in line with previous studies of spatial attention,90, 91, 92 repeating the target location was associated with reliable spatial tuning to the target location in advance of stimulus presentation (Fig. 2A; top row). Thus, we found a dissociation in that only expectations about upcoming relevant information, not about upcoming irrelevant information, were associated with changes in the representational content of visual activity prior to stimulus presentation. The lack of changes in preparatory tuning to distractor features may support the notion that distractor expectations exert their effects only once confronted with the distracting information (Fig. 1C). It is notable in this respect, as described in more detail below, that distractor learning was associated with changes in postdistractor processing.
Figure 2.
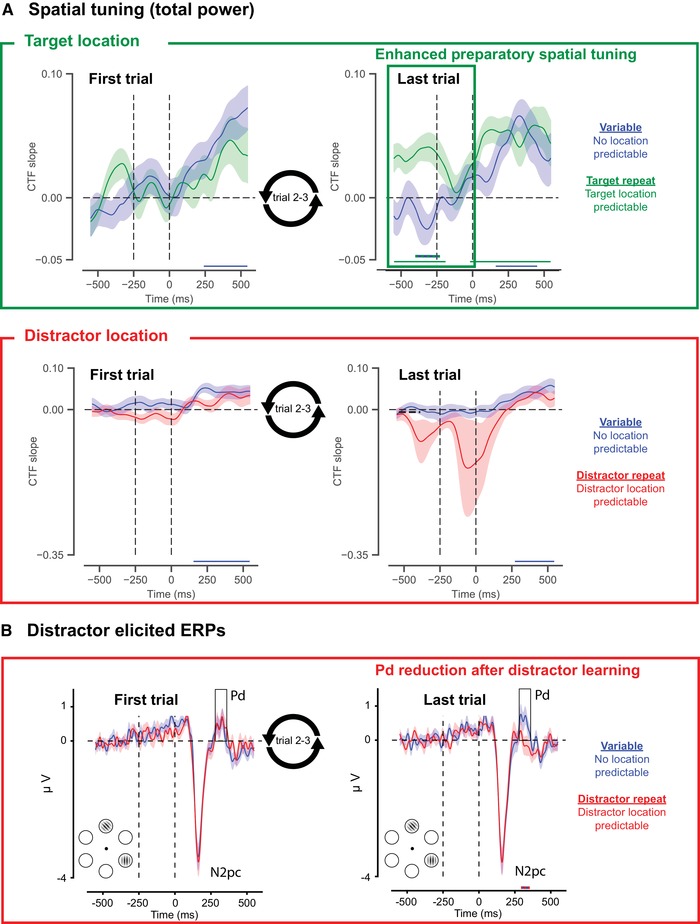
Summary of results of our recent EEG study76 examining how distractor learning influences distractor processing. (A) Slopes of channel tuning functions (CTFs) tuned to the target location (top; green) and the distractor location (bottom; red), estimated based on total alpha power. While target location learning, induced by keeping the target location fixed across a block of trials, resulted in anticipatory tuning toward the expected target location, no such anticipatory tuning was observed after distractor location repetition. That is, no evidence was obtained for a change in anticipatory tuning to the distractor location in the last versus the first trials of the block. (B) Difference waveforms (contralateral–ipsilateral) revealing the N2pc and Pd elicited by distractors in the first trial and in the last trial of a block in which either the location of the distractor was repeated or varied across trials. As the figure shows, the Pd elicited by expected distractors (last trial distractor‐repeat condition) was greatly reduced in amplitude compared to distractors that occurred at a nonpredictable location (e.g., last trial variable condition). Double‐colored thick lines in all plots indicate time points with a significant difference between the respective conditions after cluster correction (P < 0.05).
Based on the evidence reviewed above, we conclude that there is currently very little evidence in support of preparatory inhibition, as indexed by changes in the pattern of activity in visual regions representing the distracting information in anticipation of upcoming distracting input, even after statistical learning. As discussed below, however, this does not necessarily imply that distractors always capture attention, as growing evidence indicates that distractors can be filtered out preattentively.
Postdistractor inhibition: preattentive and reactive inhibition
A parallel, but largely separate debate in the literature, next to the question as to whether it is possible to prepare the system in advance to suppress sensory processing of distracting information and whether this relies on representation in working memory, has concerned the question whether it is possible to suppress distractors before they capture attention. This debate has primarily focused on suppression of physically salient distractors (e.g., a uniquely colored object among homogenously colored search items) by examining whether they can be suppressed before they capture attention (i.e., preattentively),10, 93 or only reactively, after attentional selection.7, 13
At first sight, distractor suppression in the absence of attentional selection may appear paradoxical as one would assume that to filter out a specific item, that item must first, at least to some degree, be selected. This ignoring paradox is evident in the search and destroy hypothesis advocated by Moher and Egeth,13 which argues that distractors automatically capture attention and then only subsequently (i.e., reactively) are suppressed. Recently, however, it was postulated that even salient distractors that generate a strong bottom‐up saliency signal can be suppressed before they capture attention, preattentively.10, 93 Although in the original conception of this so‐called signal‐suppression hypothesis, it was assumed that the inhibitory processes are implemented by direct top‐down control, more recent conceptions also consider a role for statistical learning as discussed above.10, 93 To dissociate between these competing hypotheses, behavioral studies have used capture probe paradigms, where probe displays are randomly intermixed with search displays. A lower probability of reporting probes embedded within singleton distractors has been taken as evidence in support of the signal suppression hypothesis.94, 95 Yet, the same pattern of behavioral results can be explained by reactive suppression in the sense that attention is first captured by the singleton distractor (if only for the briefest moment) and subsequently immediately suppressed.96
To bypass the ambiguities of behavioral evidence, researchers have turned to two event‐related potentials in the electrophysiological (EEG) signal: the N2pc and the Pd. These typically occur around 200 ms poststimulus onset, and reflect attentional selection97, 98, 99 and suppression,94, 100 respectively. The Pd, a transient positivity contralateral to the distractor, is especially relevant as its amplitude inversely scales with behavioral measures of distractor interference.101, 102, 103 It is selectively elicited by distractors, and is independent from other lateralized components reflective of attentional selection, such as the N2pc76, 93, 100, 104 and N1pc.104 Accordingly, the Pd has been proposed to reflect a mechanism that prevents or terminates the allocation of attention toward a salient distractor.10, 105
Although in many cases the Pd follows the N2pc76, 102, 106, 107, 108 or N1pc104 in the ERP waveform as predicted by the ignoring paradox, growing evidence indicates that salient distractors can also be inhibited (as evidenced by a Pd) in the absence of any neural evidence for attentional selection (e.g., the absence of an N2pc).10 Notably, in the majority of these studies, the experimental design allowed for statistical learning, either because the target and distractor identities were fixed (i.e., same color/shape) across trials,94, 101, 109, 110 or because there was a high probability distractor location.85 This suggests that experience with distracting information is necessary for preattentive suppression of distracting information. However, salient distractors have also been found to elicit a Pd in the absence of an N2pc in ERP studies with search display configurations that did not allow for the formation of distractor‐specific expectations (e.g., when the location and color of the target and singleton distractor vary from trial to trial),93 or when search displays were not presented until response, but only briefly,106 and when analysis was limited to fast trials only.111 These findings are difficult to reconcile with the notion of expectation‐dependent distractor suppression and suggest that salient distractors can also be filtered out preattentively in other ways. Indeed, distractor inhibition may also be based on feature discontinuity23, 112, 113 or global, dimension‐independent salience.10, 114 Note that such feature‐unspecific distractor inhibition should also not be associated with a precise distractor template in working memory, which could thereby reduce the chance of attentional capture. An important question for future research is how distractor feature‐specific and ‐unspecific inhibition are differentially implemented at the neural level, and the extent to which they are dependent on statistical learning.
It is typically assumed that preattentive suppression relies on proactive mechanisms set up before stimulus presentation.115 However, as discussed above, there is currently little evidence in support of preparatory distractor–specific sensory inhibition at the neural level. In this respect, it is important to note that the majority of studies examining preattentive suppression are agnostic about the underlying neural mechanisms as they only focused on distractor‐evoked responses, such as the Pd. Rather than modulating activity in regions representing the distracting information, however, distractor learning could also change synaptic efficiency within these regions, analogous to long‐term visual recognition memory116 and activity‐silent coding in working memory.117 Synaptic memory traces provide a more efficient coding scheme than active suppression through inhibition and could explain longer lasting effects of learning on distractor interference. The notion of synaptic plasticity as a mechanism underlying statistical learning can also be reconciled with proposals that expectations exert their influence only after the bottom‐up stimulus has been initially processed, during later stages of sensory processing.83, 118
Recent nonhuman primate work suggests that the FEFs and lateral intraparietal (LIP) cortex play an important role in integrating top‐down expectations with bottom‐up input in feature‐specific inhibition.119, 120, 121 While typically responses in these areas increase as a function of saliency,122, 123 as the animals learn to ignore salient distractors, evoked responses to those distractors become smaller than responses elicited by nonsalient distractors. In a study by Cosman and colleagues,119 the suppressed FEF response, which was observed once the learned to‐be‐ignored distractor no longer incurred a behavioral cost, was furthermore followed by a scalp‐recorded Pd‐like component, suggesting that FEFs play an important role in implementing inhibition. In combination with previous work showing that V4 responses initially do not differentiate between targets and distractors,124 these findings support the notion that distractor inhibition can be instantiated after distractor learning, once the to‐be‐suppressed stimulus is physically presented and bottom‐up information can be integrated with top‐down influences. The FEF and LIP may play a critical role in this.
Notably, several recent studies in humans have associated distractor learning with decreases in the amplitude of the Pd ERP component, indicative of a reduced distractor inhibition.76, 125, 126 In our recent study,76 for example, the Pd was virtually eliminated when the location of the distractor was learned, in the absence of any changes in early visual processing, as reflected in the amplitude of the early visual‐evoked P1 and N1 ERP components, or any modulations of the N2pc (Fig. 2C). This finding may suggest that the brain no longer considered distractors as distractors, once it had learned that they could be safely ignored. As noted above and shown in Figure 2A, in our study, we also observed no anticipatory spatial tuning toward expected distractor locations. These findings may thus provide further support for the idea that distractor learning is expressed postdistractor through integration of top‐down influences and bottom‐up information, just like target learning118 possibly through changes in synaptic efficiency.117
In sum, the evidence reviewed above indicates that although suppression of physically salient distractors often occurs reactively, other mechanisms may intervene to filter out salient distractors preattentively, such that capture is entirely prevented. Such inhibition of physically salient distractors on the basis of individual visual features in particular seems dependent on prior experience with the distracting information, to prevent working memory template–driven attentional capture. Future research will need to more precisely establish how such distractor inhibition is neurally implemented and to what extent this depends on the level at which suppression operates: based on spatial information and/or features, feature discontinuity23 or global, dimension‐independent salience.114 Few studies have also so far investigated if other types of physically salient stimuli (e.g., sudden onsets) can be suppressed. Recent behavioral work suggests that distraction by sudden onsets can be eliminated through mere passive viewing,127 indicative of latent learning mechanisms or habituation. How learning through passive viewing may be related to statistical learning/predictive processing is another important avenue for future research.6 Finally, information about upcoming distraction can also be nonspecific, but nevertheless used to prepare the system more globally, for example, through global suppression of sensory processing.
Inhibition in task‐relevant networks
So far, we have discussed neural mechanisms underlying inhibition of distracting information and pointed out the relative lack of support for the notion that alpha oscillations implement direct top‐down inhibition (see also Ref. 43). This is not to say that alpha oscillations do not functionally reflect inhibition. Notably, a growing body of work suggests that alpha oscillations play an important role in biasing visual regions toward processing of task‐relevant information. That is, while the evidence for alpha‐band oscillatory activity in direct, top‐down inhibition is ambiguous, as discussed above, many studies have now shown that the focus of attention can be decoded from the pattern of alpha‐band EEG activity even before stimulus presentation.90, 91, 92 These and other findings support the notion that one important role for alpha oscillations may be to enhance signal‐to‐noise ratio within task‐relevant regions by suppressing noise.20 Suppression of neuronal activity within task‐relevant sensory regions through alpha‐band oscillations could, when at an intermediate level, suppress activity of neurons with low activity to begin with, but not of neurons with high activity to begin with (i.e., those representing the attended, task‐relevant information), thereby increasing the signal‐to‐noise ratio. Rather than playing a role in inhibition per se, alpha oscillations may thus enhance tuning to task‐relevant features in visual regions by selectively suppressing the activity of neurons tuned to other features. Interestingly, and furthermore in line with this possibility, recent studies have related increased alpha‐band activity to more stable visual percepts, leading to the proposal that alpha oscillations may not signal inhibition of cortical activity per se, but stabilization of the current configuration of neuronal activity.21, 22 From this perspective, the observed distractor location learning–related increase in prestimulus alpha activity in the study by Wang and colleagues85 discussed above could also denote sharper representation of distracting information (Fig. 1A), not preparatory inhibition of expected distractor information. This is a radically different interpretation, and more work is necessary to establish to what extent alpha‐band oscillations implement preparatory inhibition of distracting information (cf. Fig. 1B) or allow for a more precise representation of the distracting information (cf. Fig. 1A).
Next to enhancing signal‐to‐noise, alpha oscillations may also facilitate processing of task‐relevant information by creating periods of optimal information processing or “pulsed” inhibition.128 It has been proposed that oscillatory alpha activity operates in a phasic manner, alternating between phases of relatively greater inhibition and relatively reduced inhibition/greater excitability.129, 130 Indeed, it has been shown that at certain phases of the alpha cycle visual‐evoked ERPs are larger and stimulus detection ability is higher than at other phases.131, 132 Yet, it is still unclear whether this alignment in time of the most optimal alpha phase with incoming input is under voluntary control.133 Moreover, this mechanism is only useful in situations in which the timing of goal‐relevant information can be predicted with relatively high precision.134 Furthermore, alpha phase may only modulate processing of at‐threshold visual stimuli, not of clearly visible stimuli that evoke a strong bottom‐up response.135 Finally, prestimulus alpha phase does not influence auditory processing and detection.136 Thus, pulsed inhibition through alpha phase may be specific to the visual domain and only of value in very specific conditions. An interesting question for future research is nevertheless whether alpha phase can also be adjusted when the timing of distracting information is highly predictable.
To summarize, inhibition implemented by alpha‐band oscillations may play a critical role in task‐relevant sensory networks by suppression noise and creating optimal time windows for information processing through pulsed inhibition. An important question is whether alpha oscillations play a similar role within task‐irrelevant visual networks. Specifically, future research should clarify whether alpha‐band oscillations may reduce distractor interference by allowing the brain to more precisely represent the distracting information in the same way as they may sharpen target representations, or through preparatory inhibition of distractor representations.
Conclusions and future directions
Until very recently, it was typically assumed in the cognitive neuroscience literature that inhibition in selective attention is under similar flexible control as the selection of relevant information.4, 137 The evidence reviewed here, however, demonstrates that the neural mechanisms underlying distractor inhibition differ, at least to a large extent, from the ones that guide attention in space or along other feature dimensions. While attention can flexibly bias visual regions in advance to boost processing of goal‐relevant information,138, 139 distractor foreknowledge often hampers performance, unless a defining distractor property (e.g., its location or color) becomes predictable through statistical learning.6, 9, 10, 65 Yet, how such learned inhibition is neurally implemented remains an important outstanding question for future research. Based on notions of predictive processing,18, 19 we outlined several ways in which expectations about upcoming distracting information grounded in statistical learning may modulate the sensory representation of distracting information and distractor processing (Fig. 1), which require further investigation. While there is now abundant evidence that salient distractors can be filtered out preattentively,10 the majority of these studies did not examine potential changes in anticipatory activity. It therefore remains unclear whether learned inhibition modulates activity in regions representing the distracting information in advance, or alternatively, exerts its effect in interaction with the bottom‐up input, for example, through synaptic plasticity.
Few studies that did examine effects of distractor learning on prestimulus activity only manipulated spatial probability and provided mixed results, with the one study reporting a role for prestimulus alpha‐band activity,85 whereas another study did not.46 This apparent discrepancy can be explained by assuming a flexible locus of distractor suppression, which either in case of only spatial regularities operates at the level of spatial priority maps, or in case of additional feature expectations operates at so‐called conspicuity maps coding specific feature dimensions.140 Given the tight link between alpha‐band activity and spatial attention,141 suppression operating at spatial priority maps likely relies on modulations of alpha oscillations. More work, however, is necessary to determine the functional significance of alpha‐band oscillatory activity, especially since recent work indicates that alpha oscillations may not signal top‐down inhibition of cortical activity per se, but stabilization of the current configuration of neuronal activity,21, 22 possibly through enhancing signal‐to‐noise.20 Distractor expectation–dependent increases in prestimulus alpha activity thus may not necessarily reflect preparatory inhibition (Fig. 1B), but could also denote a more precise anticipatory representation of distracting information (Fig. 1A), which has very different theoretical implications. With the recent advance of new encoding techniques that use the topographic distribution of M/EEG signals to track how spatial attention is deployed via so‐called spatial tuning functions,90, 92 we can now address this outstanding issue. In a first study, however, we did not observe any learning‐related changes in anticipatory tuning to an expected distractor location59 (Fig. 2A). Encoding techniques also allow for reconstructions of nonspatial information,142 so that future work can establish whether feature‐based suppression can (also) be localized within anticipatory activation patterns, and how spatial and feature distractor foreknowledge may interact to reduce distractor interference. This work will be essential for furthering our understanding of how expectations may be differentially implemented depending on the relevance or irrelevance of information.
Further studies are also necessary to gain a better understanding of the brain regions and networks that play a key role in distractor learning and statistical learning, more generally. Distractor learning likely depends on the hippocampus and subcortical regions, specifically the basal ganglia and thalamus. The hippocampus records the relations between aspects of an experience, such as its sensory components encoded by the neocortex, by providing a spatial and temporal context, and stores this representation of the experience into long‐term memory.143, 144, 145, 146, 147 Notably, visual and memory systems are reciprocally connected148 raising the possibility that the hippocampus is the source of expectation‐based influences on visual processing. Indeed, recent fMRI work shows that representations in the hippocampus code predicted shapes.149 The basal ganglia150 and pulvinar151 have also been associated with implicit learning, predictive processing, and distractor filtering.152 Lastly, effects of distractor learning may be expressed in priority maps in the frontal and parietal cortex,16, 153 as also suggested by studies in nonhuman primates.119, 120, 121 Plastic changes in priority maps of space in the frontoparietal cortex can account for the fact that attentional priority at a given location is increased or decreased depending on whether that location is associated with a target or a distractor, and observations from behavioral studies that also processing of targets presented at a likely distractor location is impaired,16, 65, 71 at least when targets and distractors cannot be distinguished at the dimension level.75 How cortical and subcortical regions interact to implement learned inhibition is another important outstanding question for future studies.
That distractor filtering capitalizes on experience with the distractor information agrees with characteristics of habituation, the progressive attenuation of the amplitude of responses to repeated sensory stimulation which is not caused by sensory adaptation or motor fatigue.154 Although, thus far, habituation has especially been shown to account for reduced distractor interference following repeated exposure to visual onset distractors,155, 156, 157 its defining characteristics resonate with the idea that only prediction errors capture attention.6 Just as in predictive coding models where the brain is continuously trying to predict new sensory input based on previous experiences, in habituation models, sensory input is also compared against stored representations based on expected frequency and the context.158 Intriguingly, a habituated response to visual onsets can even be instantiated during passive viewing,127 indicating that distractor filtering can emerge independent from any attentional biases implemented by task set. It is well established that habituation effects disappear spontaneously over time when the inducing stimulus is withheld.159 Indeed, a recent study reported that in case of short‐term habituation (across 100 trials/16 min), 16 min of distractor removal was sufficient to observe a recovery of attentional capture by the distractor.156 Yet, it was also shown that effects of longer experience with the distractors were still visible 24 and 48 h later, in line with the notion that distractor learning can have postsynaptic effects that have long‐term effects on attentional selection.160 An important avenue for future research is to determine effects of much longer distractor learning, for example, across multiple days or weeks, on distractor processing at the neural level.
To conclude, selective attention critically relies on the ability to suppress distracting information. The evidence reviewed here suggests that the brain can proactively inhibit distracting information through integration of bottom‐up input and top‐down influences, but this ability appears to strongly depend on previous experience with the distracting information, at least when implemented at the feature level. Additional research is necessary to establish how distractor learning affects distractor processing, through preparatory suppression of activity in regions representing the distractor or synaptic plasticity, and to identify the specific brain networks involved.
Competing interests
The authors declare no competing interests.
Acknowledgment
This work was supported by a European Research Council (ERC) starting Grant (679399) to H.A.S.
Contributor Information
Dirk van Moorselaar, Email: dirkvanmoorselaar@gmail.com.
Heleen A. Slagter, Email: h.a.slagter@vu.nl.
References
- 1. Desimone, R. & Duncan J.. 1995. Neural mechanisms of selective visual attention. Annu. Rev. Neurosci. 18: 193–222. [DOI] [PubMed] [Google Scholar]
- 2. Buschman, T.J. & Kastner S.. 2015. From behavior to neural dynamics: an integrated theory of attention. Neuron 88: 127–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reynolds, J.H. & Heeger D.J.. 2009. The normalization model of attention. Neuron 61: 168–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jensen, O. & Mazaheri A.. 2010. Shaping functional architecture by oscillatory alpha activity: gating by inhibition. Front. Hum. Neurosci. 4: 186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Gazzaley, A. & Nobre A.C.. 2012. Top‐down modulation: bridging selective attention and working memory. Trends Cogn. Sci. 16: 129–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Chelazzi, L. , Marini F., Pascucci D. & Turatto M.. 2019. Getting rid of visual distractors: the why, when, how and where. Curr. Opin. Psychol. 29: 135–147. [DOI] [PubMed] [Google Scholar]
- 7. Geng, J.J. 2014. Attentional mechanisms of distractor suppression. Curr. Dir. Psychol. Sci. 23: 147–153. [Google Scholar]
- 8. Kastner, S. & Ungerleider L.G.. 2001. The neural basis of biased competition in human visual cortex. Neuropsychologia 39: 1263–1276. [DOI] [PubMed] [Google Scholar]
- 9. Noonan, M.P. , Crittenden B.M., Jensen O. & Stokes M.G.. 2018. Selective inhibition of distracting input. Behav. Brain Res. 355: 36–47. [DOI] [PubMed] [Google Scholar]
- 10. Gaspelin, N. & Luck S.J.. 2018. The role of inhibition in avoiding distraction by salient stimuli. Trends Cogn. Sci. 22: 79–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arita, J.T. , Carlisle N.B. & Woodman G.F.. 2012. Templates for rejection: configuring attention to ignore task‐irrelevant features. J. Exp. Psychol. Hum. Percept. Perform. 38: 580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Woodman, G.F. & Luck S.J.. 2007. Do the contents of visual working memory automatically influence attentional selection during visual search? J. Exp. Psychol. Hum. Percept. Perform. 33: 363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Moher, J. & Egeth H.E.. 2012. The ignoring paradox: cueing distractor features leads first to selection, then to inhibition of to‐be‐ignored items. Attent. Percept. Psychophys. 74: 1590–1605. [DOI] [PubMed] [Google Scholar]
- 14. Geng, J.J. , Won B.‐Y. & Carlisle N.B.. 2019. Distractor ignoring: strategies, learning, and passive filtering. Curr. Dir. Psychol. Sci. 26: 606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wang, B. & Theeuwes J.. 2018. How to inhibit a distractor location? Statistical learning versus active, top‐down suppression. Attent. Percep. Psychophys. 80: 1–11. [DOI] [PubMed] [Google Scholar]
- 16. Ferrante, O. , Patacca A., Di Caro V., et al 2018. Altering spatial priority maps via statistical learning of target selection and distractor filtering. Cortex 102: 67–95. [DOI] [PubMed] [Google Scholar]
- 17. Geng, J.J. & Witkowski P.. 2019. Template‐to‐distractor distinctiveness regulates visual search efficiency. Curr. Opin. Psychol. 29: 119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Friston, K. 2009. The free‐energy principle: a rough guide to the brain? Trends Cogn. Sci. 13: 293–301. [DOI] [PubMed] [Google Scholar]
- 19. Rao, R.P. 2005. Bayesian inference and attentional modulation in the visual cortex. Neuroreport 16: 1843–1848. [DOI] [PubMed] [Google Scholar]
- 20. Klimesch, W. 2012. Alpha‐band oscillations, attention, and controlled access to stored information. Trends Cogn. Sci. 16: 606–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Clayton, M.S. , Yeung N. & Cohen Kadosh R.. 2019. Electrical stimulation of alpha oscillations stabilizes performance on visual attention tasks. J. Exp. Psychol. Gen. 148: 203. [DOI] [PubMed] [Google Scholar]
- 22. Piantoni, G. , Romeijn N., Gomez‐Herrero G., et al 2017. Alpha power predicts persistence of bistable perception. Sci. Rep. 7: 5208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wolfe, J.M. 1994. Guided search 2.0: a revised model of visual search. Psychon. Bull. Rev. 1: 202–238. [DOI] [PubMed] [Google Scholar]
- 24. Olivers, C.N. , Peters J., Houtkamp R. & Roelfsema P.R.. 2011. Different states in visual working memory: when it guides attention and when it does not. Trends Cogn. Sci. 15: 327–334. [DOI] [PubMed] [Google Scholar]
- 25. van Moorselaar, D. , Theeuwes J. & Olivers C.N.. 2014. In competition for the attentional template: can multiple items within visual working memory guide attention? J. Exp. Psychol. Hum. Percept. Perform. 40: 1450. [DOI] [PubMed] [Google Scholar]
- 26. Navalpakkam, V. & Itti L.. 2007. Search goal tunes visual features optimally. Neuron 53: 605–617. [DOI] [PubMed] [Google Scholar]
- 27. Becker, M.W. , Hemsteger S. & Peltier C.. 2015. No templates for rejection: a failure to configure attention to ignore task‐irrelevant features. Vis. Cogn. 23: 1150–1167. [Google Scholar]
- 28. Beck, V.M. & Hollingworth A.. 2015. Evidence for negative feature guidance in visual search is explained by spatial recoding. J. Exp. Psychol. Hum. Percept. Perform. 41: 1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Cunningham, C.A. & Egeth H.E.. 2016. Taming the white bear: initial costs and eventual benefits of distractor inhibition. Psychol. Sci. 27: 476–485. [DOI] [PubMed] [Google Scholar]
- 30. Park, S. , Kim M.‐S. & Chun M.M.. 2007. Concurrent working memory load can facilitate selective attention: evidence for specialized load. J. Exp. Psychol. Hum. Percept. Perform. 33: 1062. [DOI] [PubMed] [Google Scholar]
- 31. Carlisle, N.B. & Nitka A.W.. 2019. Location‐based explanations do not account for active attentional suppression. Vis. Cogn. 27: 305–306. [Google Scholar]
- 32. Munneke, J. , Van der Stigchel S. & Theeuwes J.. 2008. Cueing the location of a distractor: an inhibitory mechanism of spatial attention? Acta Psychol. (Amst.) 129: 101–107. [DOI] [PubMed] [Google Scholar]
- 33. Chao, H.‐F. 2010. Top‐down attentional control for distractor locations: the benefit of precuing distractor locations on target localization and discrimination. J. Exp. Psychol. Hum. Percept. Perform. 36: 303. [DOI] [PubMed] [Google Scholar]
- 34. Foxe, J.J. & Snyder A.C.. 2011. The role of alpha‐band brain oscillations as a sensory suppression mechanism during selective attention. Front. Psychol. 2: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Haegens, S. , Nácher V., Luna R., et al 2011. Α‐Oscillations in the monkey sensorimotor network influence discrimination performance by rhythmical inhibition of neuronal spiking. Proc. Natl. Acad. Sci. USA 108: 19377–19382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Worden, M.S. , Foxe J.J., Wang N. & Simpson G.V.. 2000. Anticipatory biasing of visuospatial attention indexed by retinotopically specific‐band electroencephalography increases over occipital cortex. J. Neurosci. 20: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Thut, G. , Nietzel A., Brandt S.A. & Pascual‐Leone A.. 2006. Α‐Band electroencephalographic activity over occipital cortex indexes visuospatial attention bias and predicts visual target detection. J. Neurosci. 26: 9494–9502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kelly, S.P. , Lalor E.C., Reilly R.B. & Foxe J.J.. 2006. Increases in alpha oscillatory power reflect an active retinotopic mechanism for distracter suppression during sustained visuospatial attention. J. Neurophysiol. 95: 3844–3851. [DOI] [PubMed] [Google Scholar]
- 39. Rihs, T.A. , Michel C.M. & Thut G.. 2007. Mechanisms of selective inhibition in visual spatial attention are indexed by α‐band EEG synchronization. Eur. J. Neurosci. 25: 603–610. [DOI] [PubMed] [Google Scholar]
- 40. Snyder, A.C. & Foxe J.J.. 2010. Anticipatory attentional suppression of visual features indexed by oscillatory alpha‐band power increases: a high‐density electrical mapping study. J. Neurosci. 30: 4024–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Jokisch, D. & Jensen O.. 2007. Modulation of gamma and alpha activity during a working memory task engaging the dorsal or ventral stream. J. Neurosci. 27: 3244–3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Romei, V. , Thut G., Mok R.M., et al 2012. Causal implication by rhythmic transcranial magnetic stimulation of alpha frequency in feature‐based local vs. global attention. Eur. J. Neurosci. 35: 968–974. [DOI] [PubMed] [Google Scholar]
- 43. Foster, J.J. & Awh E.. 2019. The role of alpha oscillations in spatial attention: limited evidence for a suppression account. Curr. Opin. Psychol. 29: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Munneke, J. , Heslenfeld D.J., Usrey W.M., et al 2011. Preparatory effects of distractor suppression: evidence from visual cortex. PLoS One 6: e27700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Couperus, J. & Mangun G.R.. 2010. Signal enhancement and suppression during visual–spatial selective attention. Brain Res. 1359: 155–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Noonan, M.P. , Adamian N., Pike A., et al 2016. Distinct mechanisms for distractor suppression and target facilitation. J. Neurosci. 36: 1797–1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. de Vries, I.E. , Savran E., van Driel J. & Olivers C.N.. 2019. Oscillatory mechanisms of preparing for visual distraction. J. Cogn. Neurosci. 31: 1873–1894. [DOI] [PubMed] [Google Scholar]
- 48. Bonnefond, M. & Jensen O.. 2012. Alpha oscillations serve to protect working memory maintenance against anticipated distracters. Curr. Biol. 22: 1969–1974. [DOI] [PubMed] [Google Scholar]
- 49. Reeder, R.R. , Olivers C.N. & Pollmann S.. 2017. Cortical evidence for negative search templates. Vis. Cogn. 25: 278–290. [Google Scholar]
- 50. Reeder, R.R. , Olivers C.N., Hanke M. & Pollmann S.. 2018. No evidence for enhanced distractor template representation in early visual cortex. Cortex 108: 279–282. [DOI] [PubMed] [Google Scholar]
- 51. Awh, E. , Matsukura M. & Serences J.T.. 2003. Top‐down control over biased competition during covert spatial orienting. J. Exp. Psychol. Hum. Percept. Perform. 29: 52. [DOI] [PubMed] [Google Scholar]
- 52. Serences, J.T. , Yantis S., Culberson A. & Awh E.. 2004. Preparatory activity in visual cortex indexes distractor suppression during covert spatial orienting. J. Neurophysiol. 92: 3538–3545. [DOI] [PubMed] [Google Scholar]
- 53. Ruff, C.C. & Driver J.. 2006. Attentional preparation for a lateralized visual distractor: behavioral and fMRI evidence. J. Cogn. Neurosci. 18: 522–538. [DOI] [PubMed] [Google Scholar]
- 54. Marini, F. , Demeter E., Roberts K.C., et al 2016. Orchestrating proactive and reactive mechanisms for filtering distracting information: brain–behavior relationships revealed by a mixed‐design fMRI study. J. Neurosci. 36: 988–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Conci, M. , Deichsel C., Müller H.J. & Töllner T.. 2019. Feature guidance by negative attentional templates depends on search difficulty. Vis. Cogn. 27: 317–326. [Google Scholar]
- 56. Bacon, W.F. & Egeth H.E.. 1994. Overriding stimulus‐driven attentional capture. Percept. Psychophys. 55: 485–496. [DOI] [PubMed] [Google Scholar]
- 57. Leber, A.B. & Egeth H.E.. 2006. It's under control: top‐down search strategies can override attentional capture. Psychon. Bull. Rev. 13: 132–138. [DOI] [PubMed] [Google Scholar]
- 58. Lamy, D. , Leber A. & Egeth H.E.. 2004. Effects of task relevance and stimulus‐driven salience in feature‐search mode. J. Exp. Psychol. Hum. Percept. Perform. 30: 1019. [DOI] [PubMed] [Google Scholar]
- 59. Theeuwes, J. 2004. Top‐down search strategies cannot override attentional capture. Psychon. Bull. Rev. 11: 65–70. [DOI] [PubMed] [Google Scholar]
- 60. Geng, J.J. , DiQuattro N.E. & Helm J.. 2017. Distractor probability changes the shape of the attentional template. J. Exp. Psychol. Hum. Percept. Perform. 43: 1993. [DOI] [PubMed] [Google Scholar]
- 61. Yu, X. & Geng J.J.. 2019. The attentional template is shifted and asymmetrically sharpened by distractor context. J. Exp. Psychol. Hum. Percept. Perform. 45: 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Becker, S.I. , Folk C.L. & Remington R.W.. 2010. The role of relational information in contingent capture. J. Exp. Psychol. Hum. Percept. Perform. 36: 1460. [DOI] [PubMed] [Google Scholar]
- 63. Parthasarathy, A. , Herikstad R., Bong J.H., et al 2017. Mixed selectivity morphs population codes in prefrontal cortex. Nat. Neurosci. 20: 1770–1779. [DOI] [PubMed] [Google Scholar]
- 64. Gao, Y. & Theeuwes J.. 2019. Learning to suppress a distractor is not affected by working memory load. Psychon. Bull. Rev. 10.3758/s13423-019-01679-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang, B. & Theeuwes J.. 2018. Statistical regularities modulate attentional capture. J. Exp. Psychol. Hum. Percept. Perform. 44: 13. [DOI] [PubMed] [Google Scholar]
- 66. Geng, J.J. & Behrmann M.. 2005. Spatial probability as an attentional cue in visual search. Percept. Psychophys. 67: 1252–1268. [DOI] [PubMed] [Google Scholar]
- 67. Jiang, Y.V. , Swallow K.M., Rosenbaum G.M. & Herzig C.. 2013. Rapid acquisition but slow extinction of an attentional bias in space. J. Exp. Psychol. Hum. Percept. Perform. 39: 87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Stilwell, B.T. , Bahle B. & Vecera S.P.. 2019. Feature‐based statistical regularities of distractors modulate attentional capture. J. Exp. Psychol. Hum. Percept. Perform. 45: 419. [DOI] [PubMed] [Google Scholar]
- 69. Vatterott, D.B. & Vecera S.P.. 2012. Experience‐dependent attentional tuning of distractor rejection. Psychon. Bull. Rev. 19: 871–878. [DOI] [PubMed] [Google Scholar]
- 70. Goschy, H. , Bakos S., Müller H.J. & Zehetleitner M.. 2014. Probability cueing of distractor locations: both intertrial facilitation and statistical learning mediate interference reduction. Front. Psychol. 5: 1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Sauter, M. , Liesefeld H.R., Zehetleitner M. & Müller H.J.. 2018. Region‐based shielding of visual search from salient distractors: target detection is impaired with same‐but not different‐dimension distractors. Attent. Percept. Psychophys. 80: 622–642. [DOI] [PubMed] [Google Scholar]
- 72. Reder, L.M. , Weber K., Shang J. & Vanyukov P.M.. 2003. The adaptive character of the attentional system: statistical sensitivity in a target localization task. J. Exp. Psychol. Hum. Percept. Perform. 29: 631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Failing, M. , Wang B. & Theeuwes J.. 2019. Spatial suppression due to statistical regularities is driven by distractor suppression not by target activation. Attent. Percept. Psychophys. 81: 1405–1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Theeuwes, J. 1992. Perceptual selectivity for color and form. Percept. Psychophys. 51: 599–606. [DOI] [PubMed] [Google Scholar]
- 75. Zhang, B. , Allenmark F., Liesefeld H.R., et al 2019. Probability cueing of singleton‐distractor locations in visual search: priority‐map‐or dimension‐based inhibition? J. Exp. Psychol. Hum. Percept. Perform. 45: 1146–1163. [DOI] [PubMed] [Google Scholar]
- 76. van Moorselaar, D. & Slagter H.A.. 2019. Learning what is irrelevant or relevant: expectations facilitate distractor inhibition and target facilitation through distinct neural mechanisms. Cold Spring Harb. Lab. 10.1101/565069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Friston, K. 2005. A theory of cortical responses. Philos. Trans. R. Soc. B Biol. Sci. 360: 815–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Fiser, A. , Mahringer D., Oyibo H.K., et al 2016. Experience‐dependent spatial expectations in mouse visual cortex. Nat. Neurosci. 19: 1658. [DOI] [PubMed] [Google Scholar]
- 79. Bell, A.H. , Summerfield C., Morin E.L., et al 2016. Encoding of stimulus probability in macaque inferior temporal cortex. Curr. Biol. 26: 2280–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Bar, M. , Kassam K.S., Ghuman A.S., et al 2006. Top‐down facilitation of visual recognition. Proc. Natl. Acad. Sci. USA 103: 449–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Rao, R.P. & Ballard D.H.. 1999. Predictive coding in the visual cortex: a functional interpretation of some extra‐classical receptive‐field effects. Nat. Neurosci. 2: 79. [DOI] [PubMed] [Google Scholar]
- 82. Kok, P. , Mostert P. & De Lange F.P.. 2017. Prior expectations induce prestimulus sensory templates. Proc. Natl. Acad. Sci. USA 114: 10473–10478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Alilović, J. , Timmermans B., Reteig L.C., et al 2019. No evidence that predictions and attention modulate the first feedforward sweep of cortical information processing. Cereb. Cortex 29: 2261–2278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Rungratsameetaweemana, N. , Itthipuripat S., Salazar A. & Serences J.T.. 2018. Expectations do not alter early sensory processing during perceptual decision making. J. Neurosci. 38: 5632–5648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Wang, B. , van Driel J., Ort E. & Theeuwes J.. 2019. Anticipatory distractor suppression elicited by statistical regularities in visual search. J. Cogn. Neurosci. 31: 1535–1548. [DOI] [PubMed] [Google Scholar]
- 86. Failing, M. , Feldmann‐Wustefeld T., Wang B., et al 2019. Statistical regularities induce spatial as well as feature‐specific suppression. J. Exp. Psychol. Hum. Percept. Perform. 45: 1291–1303. [DOI] [PubMed] [Google Scholar]
- 87. Kok, P. , Jehee J.F. & De Lange F.P.. 2012. Less is more: expectation sharpens representations in the primary visual cortex. Neuron 75: 265–270. [DOI] [PubMed] [Google Scholar]
- 88. Feldman, H. & Friston K.. 2010. Attention, uncertainty, and free‐energy. Front. Hum. Neurosci. 4: 215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Foster, J.J. , Sutterer D.W., Serences J.T., et al 2015. The topography of alpha‐band activity tracks the content of spatial working memory. J. Neurophysiol. 115: 168–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Samaha, J. , Sprague T.C. & Postle B.R.. 2016. Decoding and reconstructing the focus of spatial attention from the topography of alpha‐band oscillations. J. Cogn. Neurosci. 28: 1090–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. van Moorselaar, D. , Foster J.J., Sutterer D.W., et al 2018. Spatially selective alpha oscillations reveal moment‐by‐moment trade‐offs between working memory and attention. J. Cogn. Neurosci. 30: 256–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Foster, J.J. , Sutterer D.W., Serences J.T., et al 2017. Alpha‐band oscillations enable spatially and temporally resolved tracking of covert spatial attention. Psychol. Sci. 28: 929–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sawaki, R. & Luck S.J.. 2010. Capture versus suppression of attention by salient singletons: electrophysiological evidence for an automatic attend‐to‐me signal. Attent. Percept. Psychophys. 72: 1455–1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Gaspelin, N. & Luck S.J.. 2018. Combined electrophysiological and behavioral evidence for the suppression of salient distractors. J. Cogn. Neurosci. 30: 1265–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Won, B.‐Y. , Kosoyan M. & Geng J.J.. 2019. Evidence for second‐order singleton suppression based on probabilistic expectations. J. Exp. Psychol. Hum. Percept. Perform. 45: 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Chang, S. , Cunningham C.A. & Egeth H.E.. 2018. The power of negative thinking: paradoxical but effective ignoring of salient‐but‐irrelevant stimuli with a spatial cue. Vis. Cogn. 10.1080/13506285.2018.1541950. [DOI] [Google Scholar]
- 97. Luck, S.J. 2012. Electrophysiological correlates of the focusing of attention within complex visual scenes: N2pc and related ERP components In The Oxford Handbook of Event‐Related Potential Components. Luck S.J. & Kappenman E.S., Eds: 329–360. Oxford University Press. [Google Scholar]
- 98. Eimer, M. 2014. The neural basis of attentional control in visual search. Trends Cogn. Sci. 18: 526–535. [DOI] [PubMed] [Google Scholar]
- 99. Luck, S.J. & Hillyard S.A.. 1994. Electrophysiological correlates of feature analysis during visual search. Psychophysiology 31: 291–308. [DOI] [PubMed] [Google Scholar]
- 100. Hickey, C. , Di Lollo V. & McDonald J.J.. 2009. Electrophysiological indices of target and distractor processing in visual search. J. Cogn. Neurosci. 21: 760–775. [DOI] [PubMed] [Google Scholar]
- 101. Gaspar, J.M. & McDonald J.J.. 2014. Suppression of salient objects prevents distraction in visual search. J. Neurosci. 34: 5658–5666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Liesefeld, H.R. , Liesefeld A.M., Töllner T. & Müller H.J.. 2017. Attentional capture in visual search: capture and post‐capture dynamics revealed by EEG. Neuroimage 156: 166–173. [DOI] [PubMed] [Google Scholar]
- 103. Weaver, M.D. , van Zoest W. & Hickey C.. 2017. A temporal dependency account of attentional inhibition in oculomotor control. Neuroimage 147: 880–894. [DOI] [PubMed] [Google Scholar]
- 104. Donohue, S.E. , Bartsch M.V., Heinze H.‐J., et al 2018. Cortical mechanisms of prioritizing selection for rejection in visual search. J. Neurosci. 38: 4738–4748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Sawaki, R. , Geng J.J. & Luck S.J.. 2012. A common neural mechanism for preventing and terminating the allocation of attention. J. Neurosci. 32: 10725–10736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kiss, M. , Grubert A., Petersen A. & Eimer M.. 2012. Attentional capture by salient distractors during visual search is determined by temporal task demands. J. Cogn. Neurosci. 24: 749–759. [DOI] [PubMed] [Google Scholar]
- 107. Hilimire, M.R. & Corballis P.M.. 2014. Event‐related potentials reveal the effect of prior knowledge on competition for representation and attentional capture. Psychophysiology 51: 22–35. [DOI] [PubMed] [Google Scholar]
- 108. Sawaki, R. & Luck S.J.. 2013. Active suppression after involuntary capture of attention. Psychon. Bull. Rev. 20: 296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Jannati, A. , Gaspar J.M. & McDonald J.J.. 2013. Tracking target and distractor processing in fixed‐feature visual search: evidence from human electrophysiology. J. Exp. Psychol. Hum. Percept. Perform. 39: 1713. [DOI] [PubMed] [Google Scholar]
- 110. Burra, N. & Kerzel D.. 2013. Attentional capture during visual search is attenuated by target predictability: evidence from the N2pc, Pd, and topographic segmentation. Psychophysiology 50: 422–430. [DOI] [PubMed] [Google Scholar]
- 111. McDonald, J.J. , Green J.J., Jannati A. & Di Lollo V.. 2013. On the electrophysiological evidence for the capture of visual attention. J. Exp. Psychol. Hum. Percept. Perform. 39: 849. [DOI] [PubMed] [Google Scholar]
- 112. Müller, H.J. , Heller D. & Ziegler J.. 1995. Visual search for singleton feature targets within and across feature dimensions. Percept. Psychophys. 57: 1–17. [DOI] [PubMed] [Google Scholar]
- 113. Müller, H.J. , Reimann B. & Krummenacher J.. 2003. Visual search for singleton feature targets across dimensions: stimulus‐ and expectancy‐driven effects in dimensional weighting. J. Exp. Psychol. Hum. Percept. Perform. 29: 1021. [DOI] [PubMed] [Google Scholar]
- 114. Treisman, A.M. & Gelade G.. 1980. A feature‐integration theory of attention. Cognit. Psychol. 12: 97–136. [DOI] [PubMed] [Google Scholar]
- 115. Gaspelin, N. & Luck S.J.. 2019. Inhibition as a potential resolution to the attentional capture debate. Curr. Opin. Psychol. 29: 12–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116. Cooke, S.F. , Komorowski R.W., Kaplan E.S., et al 2015. Visual recognition memory, manifested as long‐term habituation, requires synaptic plasticity in V1. Nat. Neurosci. 18: 262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Stokes, M.G. 2015. ‘Activity‐silent’ working memory in prefrontal cortex: a dynamic coding framework. Trends Cogn. Sci. 19: 394–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Rao, V. , DeAngelis G.C. & Snyder L.H.. 2012. Neural correlates of prior expectations of motion in the lateral intraparietal and middle temporal areas. J. Neurosci. 32: 10063–10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Cosman, J.D. , Lowe K.A., Zinke W., et al 2018. Prefrontal control of visual distraction. Curr. Biol. 28: 414–420. e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Ipata, A.E. , Gee A.L., Gottlieb J., et al 2006. LIP responses to a popout stimulus are reduced if it is overtly ignored. Nat. Neurosci. 9: 1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Suzuki, M. & Gottlieb J.. 2013. Distinct neural mechanisms of distractor suppression in the frontal and parietal lobe. Nat. Neurosci. 16: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Kusunoki, M. , Gottlieb J. & Goldberg M.E.. 2000. The lateral intraparietal area as a salience map: the representation of abrupt onset, stimulus motion, and task relevance. Vision Res. 40: 1459–1468. [DOI] [PubMed] [Google Scholar]
- 123. Thompson, K.G. & Bichot N.P.. 2005. A visual salience map in the primate frontal eye field. Prog. Brain Res. 147: 249–262. [DOI] [PubMed] [Google Scholar]
- 124. Ogawa, T. & Komatsu H.. 2004. Target selection in area V4 during a multidimensional visual search task. J. Neurosci. 24: 6371–6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Heuer, A. & Schubö A.. 2019. Cueing distraction: electrophysiological evidence for anticipatory active suppression of distractor location. Psychol. Res. 10.1007/s00426-019-01211-4. [DOI] [PubMed] [Google Scholar]
- 126. Hickey, C. , Weaver M., Kadel H. & van Zoest W.. 2017. How do we ignore salient distractors? J. Vis. 17: 205. [Google Scholar]
- 127. Turatto, M. , Bonetti F., Pascucci D. & Chelazzi L.. 2018. Desensitizing the attention system to distraction while idling: a new latent learning phenomenon in the visual attention domain. J. Exp. Psychol. Gen. 147: 1827. [DOI] [PubMed] [Google Scholar]
- 128. Mazaheri, A. & Jensen O.. 2010. Rhythmic pulsing: linking ongoing brain activity with evoked responses. Front. Hum. Neurosci. 4: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. VanRullen, R. & Koch C.. 2003. Is perception discrete or continuous? Trends Cogn. Sci. 7: 207–213. [DOI] [PubMed] [Google Scholar]
- 130. Valera, F.J. , Toro A., John E.R. & Schwartz E.L.. 1981. Perceptual framing and cortical alpha rhythm. Neuropsychologia 19: 675–686. [DOI] [PubMed] [Google Scholar]
- 131. Busch, N.A. , Dubois J. & VanRullen R.. 2009. The phase of ongoing EEG oscillations predicts visual perception. J. Neurosci. 29: 7869–7876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Mathewson, K.E. , Gratton G., Fabiani M., et al 2009. To see or not to see: prestimulus α phase predicts visual awareness. J. Neurosci. 29: 2725–2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Van Diepen, R. , Foxe J.J. & Mazaheri A.. 2019. The functional role of alpha‐band activity in attentional processing: the current zeitgeist and future outlook. Curr. Opin. Psychol. 29: 229–238. [DOI] [PubMed] [Google Scholar]
- 134. Samaha, J. , Bauer P., Cimaroli S. & Postle B.R.. 2015. Top‐down control of the phase of alpha‐band oscillations as a mechanism for temporal prediction. Proc. Natl. Acad. Sci. USA 112: 8439–8444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. van Diepen, R.M. , Cohen M.X., Denys D. & Mazaheri A.. 2015. Attention and temporal expectations modulate power, not phase, of ongoing alpha oscillations. J. Cogn. Neurosci. 27: 1573–1586. [DOI] [PubMed] [Google Scholar]
- 136. VanRullen, R. , Zoefel B. & Ilhan B.. 2014. On the cyclic nature of perception in vision versus audition. Philos. Trans. R. Soc. B Biol. Sci. 369: 20130214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Zanto, T.P. , Rubens M.T., Thangavel A. & Gazzaley A.. 2011. Causal role of the prefrontal cortex in top‐down modulation of visual processing and working memory. Nat. Neurosci. 14: 656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138. Battistoni, E. , Stein T. & Peelen M.V.. 2017. Preparatory attention in visual cortex. Ann. N.Y. Acad. Sci. 1396: 92–107. [DOI] [PubMed] [Google Scholar]
- 139. Posner, M.I. 1980. Orienting of attention. Q. J. Exp. Psychol. 32: 3–25. [DOI] [PubMed] [Google Scholar]
- 140. Liesefeld, H.R. & Müller H.J.. 2019. Distractor handling via dimension weighting. Curr. Opin. Psychol. 29: 160–167. [DOI] [PubMed] [Google Scholar]
- 141. Bae, G.‐Y. & Luck S.J.. 2018. Dissociable decoding of spatial attention and working memory from EEG oscillations and sustained potentials. J. Neurosci. 38: 409–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142. Smout, C.A. , Tang M.F., Garrido M.I. & Mattingley J.B.. 2019. Attention promotes the neural encoding of prediction errors. PLoS Biol. 17: e2006812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143. Squire, L.R. & Zola‐Morgan S.. 1991. The medial temporal lobe memory system. Science 253: 1380–1386. [DOI] [PubMed] [Google Scholar]
- 144. Eichenbaum, H. 2000. A cortical–hippocampal system for declarative memory. Nat. Rev. Neurosci. 1: 41. [DOI] [PubMed] [Google Scholar]
- 145. Kraus, B.J. , R.J. Robinson, II , White J.A., et al 2013. Hippocampal “time cells”: time versus path integration. Neuron 78: 1090–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146. McGaugh, J.L. 2000. Memory—a century of consolidation. Science 287: 248–251. [DOI] [PubMed] [Google Scholar]
- 147. O'Keefe, J. & Dostrovsky J.. 1971. The hippocampus as a spatial map: preliminary evidence from unit activity in the freely‐moving rat. Brain Res. 34: 171–175. [DOI] [PubMed] [Google Scholar]
- 148. Murray, E.A. , Bussey T.J. & Saksida L.M.. 2007. Visual perception and memory: a new view of medial temporal lobe function in primates and rodents. Annu. Rev. Neurosci. 30: 99–122. [DOI] [PubMed] [Google Scholar]
- 149. Kok, P. & Turk‐Browne N.B.. 2018. Associative prediction of visual shape in the hippocampus. J. Neurosci. 38: 6888–6899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150. Seger, C.A. 2006. The basal ganglia in human learning. Neuroscientist 12: 285–290. [DOI] [PubMed] [Google Scholar]
- 151. Strumpf, H. , Mangun G.R., Boehler C.N., et al 2013. The role of the pulvinar in distractor processing and visual search. Hum. Brain Mapp. 34: 1115–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152. Nakajima, M. , Schmitt L.I. & Halassa M.M.. 2019. Prefrontal cortex regulates sensory filtering through a basal ganglia‐to‐thalamus pathway. Neuron 103: 445–458.e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153. Zelinsky, G.J. & Bisley J.W.. 2015. The what, where, and why of priority maps and their interactions with visual working memory. Ann. N.Y. Acad. Sci. 1339: 154–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154. Sokolov, E.N. 1963. Higher nervous functions: the orienting reflex. Annu. Rev. Physiol. 25: 545–580. [DOI] [PubMed] [Google Scholar]
- 155. Pascucci, D. & Turatto M.. 2015. The distracting impact of repeated visible and invisible onsets on focused attention. J. Exp. Psychol. Hum. Percept. Perform. 41: 879. [DOI] [PubMed] [Google Scholar]
- 156. Turatto, M. & Pascucci D.. 2016. Short‐term and long‐term plasticity in the visual‐attention system: evidence from habituation of attentional capture. Neurobiol. Learn. Mem. 130: 159–169. [DOI] [PubMed] [Google Scholar]
- 157. Bonetti, F. & Turatto M.. 2019. Habituation of oculomotor capture by sudden onsets: stimulus specificity, spontaneous recovery and dishabituation. J. Exp. Psychol. Hum. Percept. Perform. 45: 264. [DOI] [PubMed] [Google Scholar]
- 158. Turatto, M. , Bonetti F. & Pascucci D.. 2018. Filtering visual onsets via habituation: a context‐specific long‐term memory of irrelevant stimuli. Psychon. Bull. Rev. 25: 1028–1034. [DOI] [PubMed] [Google Scholar]
- 159. Thompson, R.F. 2009. Habituation: a history. Neurobiol. Learn. Mem. 92: 127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160. Rankin, C.H. , Abrams T., Barry R.J., et al 2009. Habituation revisited: an updated and revised description of the behavioral characteristics of habituation. Neurobiol. Learn. Mem. 92: 135–138. [DOI] [PMC free article] [PubMed] [Google Scholar]


