Abstract
Aims
Higher intakes of fruits and vegetables, and vitamin C are associated with improved periodontal healing post‐scaling and root planing (SRP). This study determined if this association was sustained at 3–4 years post‐SRP, and if flavonoid intake is associated with periodontal health. Whether reduced probing depth (PD) is sustained and whether PD is correlated with salivary IL‐1β, IL‐6 and CRP at 3–4 years post‐SRP were also studied.
Materials and Methods
Clinical periodontal outcomes, dietary intakes and salivary markers of inflammation were measured in patients (n = 43, 23 females, 37–93 years) who had undergone SRP 3–4 years earlier and had been part of a periodontal maintenance programme.
Results
Flavonoid intake was inversely associated with PD (p = .042) and salivary IL‐1β concentration (p = .015) after adjustment for multiple confounders. When changes in PD were considered, the association of flavonoid intake with reduced PD became borderline significant (p = .051) but persisted for IL‐1β (p = .018). PD at 3–4 years and 2–4 months post‐SRP was similar. There was a positive correlation between PD and salivary IL‐1β (p = .005) but not with salivary CRP and IL‐6.
Conclusion
Higher flavonoid intake is associated with lower IL‐1β. Also, regular supportive periodontal therapy maintained the improved PD at 3–4 years post‐SRP regardless of smoking status.
Keywords: diet, flavonoids, probing depth, salivary biomarkers, scaling and root planing
Clinical Relevance.
Scientific rationale for the study: Higher fruit and vegetable intake, a source of flavonoids, is associated with improved periodontal healing at 8–12 weeks post‐SRP. Whether this association is sustained at 3–4 years post‐SRP, and if flavonoid intake is also associated with periodontal health was studied.
Principal findings: Higher flavonoid intake is associated with reduced probing depth and lower salivary IL‐1β at 3–4 years post‐SRP among patients that receive regular periodontal maintenance. These associations persisted when other confounders were considered.
Practical implications: Flavonoids, at levels present in foods, is associated with lower salivary IL‐1β which may be a marker of a diet that supports periodontal health.
1. INTRODUCTION
Initial treatment of periodontal disease involves non‐surgical scaling and root planing (SRP). Following initial SRP, a periodontal maintenance programme is recommended to maintain periodontal health and prevent the recurrence and progression of periodontitis in order to avoid tooth loss and to maintain or improve the probing depths (PDs) achieved following SRP (Axelsson & Lindhe, 1981; Becker, Berg, & Becker, 1979; Chambrone, Chambrone, Lima, & Chambrone, 2010; Chambrone & Chambrone, 2006; Lindhe & Nyman, 1984; McGuire, 1991). A previous study, on which this study is based, reported that a diet higher in fruits and vegetables, beta‐carotene, vitamin C, alpha‐tocopherol and fish oils (specifically the long‐chain omega‐3 fatty acids, eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA)) was positively associated with periodontal healing after SRP (Dodington, Fritz, Sullivan, & Ward, 2015). This positive association with fruits and vegetables may be due to higher intakes of flavonoids with known antioxidant and anti‐inflammatory properties; of particular interest is higher intakes of flavonoids as well as higher intakes of fruits and vegetables. Another study investigated the effect of supplementation with fruit and vegetable concentrate, with and without a berry concentrate, on periodontal outcomes after SRP (Chapple et al., 2012). At 2 months after SRP, patients receiving the fruit and vegetable concentrate had a greater reduction in PD >4 mm compared to patients that had received placebo capsules. However, this effect was not sustained at 5 and 8 months post‐SRP. Interestingly, the addition of berry concentrate—that would have been a rich source of flavonoids—did not result in a similar benefit compared to placebo at 2, 5 or 8 months post‐SRP. The authors provide several explanations for the findings: small sample size, potential low bioavailability or interactions with other components in the supplement and/or low adherence (Chapple et al., 2012).
A recent systematic review has reported the effect of green tea catechins (the main flavonoids in green tea) to assist with healing of the periodontium after SRP (Gartenmann et al., 2019). Specifically, this review reported that the mean PD was significantly reduced in patients who had SRP and received green tea catechin in a carboxymethyl‐cellulose gel or as a hydroxypropyl cellulose strip compared to patients who underwent SRP alone. However, the authors noted that further study is needed to confirm a relationship due to concern about risk of bias within studies as well as the significant heterogeneity among studies (Gartenmann et al., 2019). Nonetheless, epigallocatechin gallate (EGCG), a main flavonoid catechin present in green tea extract, may be a component that mediates potential positive effects. Using a mouse osteoblast‐osteoclast co‐culture cell model, EGCG has been shown to inhibit the expression of matrix metalloproteinase‐9 by osteoblasts and to also reduce the formation of osteoclasts (Yun et al., 2004). Higher intakes of specific fruits or citrus flavonoids—without periodontal therapy—have also been shown to result in better periodontal health: reduced gingival inflammation due to higher intakes of kiwis (Graziani et al., 2018) or reduced subgingival periodontal pathogens with supplementation of citrus flavonoids along with vitamin C and calcium (Amaliya, Laine, Loos, & Van der Velden, 2015).
Saliva contains locally produced mediators which can be used as biomarkers of periodontal health (Giannobile et al., 2009). Salivary biomarkers of inflammation such as IL‐1β, IL‐6, matrix metalloproteinase‐8 and C‐reactive protein (CRP) have all been used as clinical indicators of periodontitis (Herr et al., 2007; Miller, King, Langub, Kryscio, & Thomas, 2006; Pederson et al., 1995) and have been shown to decline post‐SRP (Sexton et al., 2011). Whether dietary intakes of flavonoids and other dietary components are associated with these biomarkers is less clear.
The main study objective was to determine if there was an association between a sustainable improvement in periodontal treatment outcomes at 3–4 years post‐SRP and higher consumption of fruit and vegetables, vitamin C and total flavonoids. These clinical outcomes included reduced PD, fewer areas of bleeding on probing (BOP), plaque score and levels of salivary markers of inflammation (IL‐1β, IL‐6 and CRP). Secondary objectives were to determine if reduced PD observed 2–4 months post‐SRP is sustained at 3–4 years post‐SRP and if PD is correlated with salivary IL‐1β, IL‐6 and CRP. We hypothesized that patients who consume more fruit, vegetables, vitamin C and total flavonoids would have better periodontal health and show a correlation with a lower level of salivary markers of inflammation. Additionally, as research has demonstrated the effectiveness in periodontal health from periodontal maintenance appointments following SRP, we hypothesized that PDs observed at 2–4 months post‐SRP would be sustained 3–4 years post‐SRP. Greater PDs were also anticipated to be associated with higher levels of inflammatory salivary markers.
2. MATERIALS AND METHODS
2.1. Study population and design
The study took place at a Periodontal Wellness and Implant Surgery Clinic, Fonthill, Ontario, Canada between January 2017 to December 2017 and was a follow‐up study to a previous investigation in which 111 patients with moderate to severe periodontal disease had undergone SRP. Specifically, the relationship between healing and dietary intake was evaluated at 8–12 weeks post‐SRP in this previous study (Dodington et al., 2015). Sixty‐nine of these patients had continued with periodontal maintenance treatment at the specialty clinic and were invited to participate in the study. Of the 69 patients invited to participate, 43 patients provided informed consent (Figure 1). Patients were contacted 1 week prior to their scheduled periodontal maintenance appointment to confirm their appointment and invite them to the study. Patients who agreed were scheduled 30 min prior to their appointment. At the time of the follow‐up appointment, patients provided written consent and the following data were collected: clinical outcomes (PD, BOP, plaque score), salivary levels of inflammatory markers (IL‐1β, IL‐6 and CRP) and dietary intakes by food frequency questionnaire (FFQ; Figure 1). The study protocol was approved by the human research ethics board at Brock University in St. Catharines, Ontario, Canada and registered with clinicaltrials.gov as NCT03073174.
Figure 1.
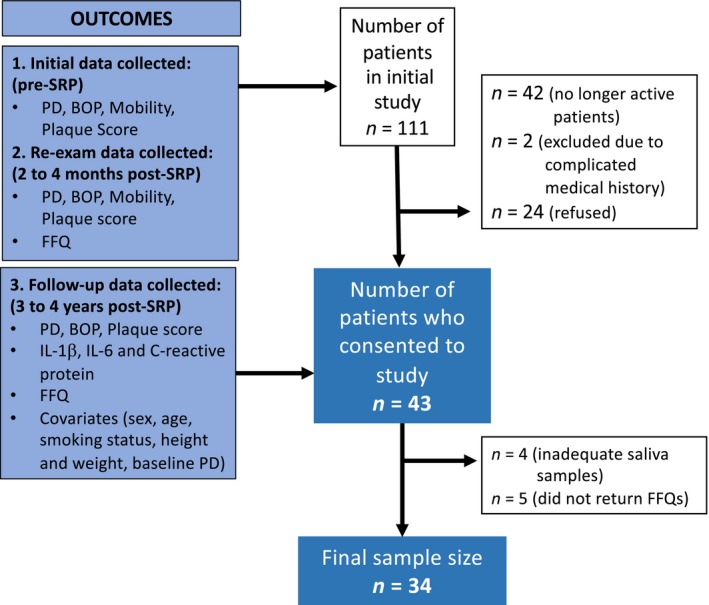
Recruitment and final sample size flow chart for smoking and non‐smoking adults undergoing non‐surgical periodontal therapy. Outcomes measured and reasons for patients not being included in final sample size are identified
2.2. Periodontal examination
To coincide with the original study, the follow‐up periodontal examination included measurements of PD, BOP, tooth mobility and plaque score. These outcomes were measured by Registered Dental Hygienists using a periodontal probe (UNC‐15, Hu‐Friedy). Hygienists were calibrated to use 25 N of pressure when measuring PD. On all present teeth and implants, six PD sites were measured (mesiobuccal, buccal, distobuccal, mesiolingual, lingual and distolingual). PD was again used as a main outcome to assess the health of the tooth and evaluate the current periodontal status. BOP was measured after PD measurements at six sites per tooth to address areas of acute inflammation. BOP was recorded as a per cent of sites that bled upon provocation. Visual inspection was also used to assess a patient's plaque score. Plaque scores are represented by the percentage of teeth with plaque and biofilm visually present over the total number of teeth present (Silberman et al., 1998). Tooth mobility was assessed and categorized based on visual movement in millimetres (Laster, Laudenbach, & Stoller, 1975). The periodontal measurements were collected and compared at three time points: prior to SRP (pre‐SRP), 2–4 months following SRP at the patient's re‐examination (post‐SRP), and at the periodontal maintenance appointment after consent was obtained for this study (follow‐up, 3–4 years after initial SRP).
2.3. Dietary assessment using a food frequency questionnaire
At the follow‐up appointment, patients completed the 2014 Block FFQ to estimate the intakes of food and nutrients over the previous year. An earlier version of this questionnaire was previously validated (Boucher et al., 2006). This FFQ contains a list of 127 foods and beverages with an additional category of dietary intake to adjust for precision in fat, protein, carbohydrates, sugars and whole‐grain content intake. The analysis of the FFQ also includes the determination of total flavonoid intake from foods and beverages (flavonoid values were developed from the USDA data released from NHANES 2007–2008 and updated based on the expanded USDA flavonoid database from September 2014). Portion size as well as the frequency of consumption for each food item was recorded (never, a few times a year, once per month, 2–3 times per month, once per week, 2 times per week, 3–4 times per week, 5–6 times per week or every day). Images of portion sizes were provided to improve patient recollection of portions. All FFQs were checked for completeness, and missing values were interpreted as no intake.
2.4. Saliva collection
A saliva sample was obtained at the follow‐up appointment. Saliva was collected and analysed as per the guidelines from Salimetrics. The collection began with patient's rinsing with 85 ml of water for 1 min, 10 min prior to collection. For saliva sample collection, patients were instructed to tilt their chin downwards in order to facilitate an unstimulated passive drool. The saliva was collected through the SalivaBio Collection Aid (Salimetrics) into the polypropylene vial. Saliva flow rate was calculated for each patient by measuring the volume of sample collected by the time required. We aimed to collect approximately 2 ml of saliva per donor and provided patients with up to 10 min to provide the sample. 200 microlitres (μL) of saliva was immediately pipetted from the polypropylene vial into separate vials. The samples were stored at −80°C until analyses were performed.
2.5. Assessment of covariates
Information on the patient's sex, age and smoking status was collected from their updated medical history form. Patient height and weight measurements were obtained at the time of saliva collection to calculate their body mass index (BMI; in kg/m2). The change in PD from baseline to follow‐up was also used.
2.6. Statistical analysis
Patient characteristics and clinical outcomes were analysed by independent sample t test or one‐way ANOVA, followed by Tukey pairwise comparisons for continuous variables and chi‐square test for categorical variables. Salivary IL‐1β, IL‐6 and CRP concentrations were log transformed to normalize their distribution. Dietary intakes were energy adjusted and standardized to a 2000 Kcal diet according to the residual method (Willett, Howe, & Kushi, 1997). Associations were investigated using bivariate Pearson correlation coefficients or multiple linear regression. Statistical analysis was performed using SPSS version 20 (SPSS Inc.), and statistical significance was defined as p < .05.
3. RESULTS
3.1. Patient characteristics
Recruitment for the study is shown in Figure 1. From the 111 patients who completed both the pre‐ and post‐SRP visits from the initial study, 42 were no longer patients at the clinic leaving 69 patients who were invited to participate in the study. Two patients were excluded due to medical complications and were unable to provide saliva samples or complete personal oral hygiene and 24 declined to participate. Forty‐three patients gave informed consent and participated in the study and completed the follow‐up study visit. Of these 43 study patients, 39 were able to provide adequate saliva samples and 38 returned completed dietary questionnaires. Therefore, our final sample size with completed saliva samples and questionnaires was 34. Based on data from the original study, there were no significant differences in patient characteristics or clinical outcomes between those who participated in the follow‐up study and those that did not (Table 1).
Table 1.
Patient characteristics and clinical outcomes of patients from the original study who did or did not participate in the follow‐up studya, b
|
Participating Patients n = 43 |
Non‐Participating Patients n = 68 |
p Valuec | |
|---|---|---|---|
| Age (years) | 58 ± 12 | 56 ± 11 | .40 |
| Sex (n females) | 23 (54%) | 36 (53%) | .96 |
| Smokers (n) | 11 (26%) | 20 (29%) | .66 |
| BMI (kg/m2) | 28.6 ± 5.8 | 28.8 ± 5.0 | .86 |
| Probing depth (# sites ≥4 mm) | |||
| Pre‐SRP | 92 ± 38 | 92 ± 43 | .99 |
| Post‐SRP | 14 ± 13 | 18 ± 17 | .30 |
| Bleeding on probing (# sites) | |||
| Pre‐SRP | 71 ± 45 | 77 ± 52 | .56 |
| Post‐SRP | 5 ± 7 | 10 ± 21 | .19 |
| Plaque score (% teeth) | |||
| Pre‐SRP | 65 ± 31 | 74 ± 27 | .15 |
| Post‐SRP | 29 ± 24 | 39 ± 25 | .07 |
| Mobility (# teeth) | |||
| Pre‐SRP | 4 ± 5 | 5 ± 6 | .56 |
Data are from the original study (Dodington et al., 2015) as the majority of patients not participating in the follow‐up study were no longer patients at the specialty clinic.
All values are mean ± SD for continuous variables and counts (%) for categorical variables.
Differences among groups were calculated by independent sample t test.
3.2. Periodontal health is maintained during long‐term follow‐up
Periodontal outcomes from all three visits are listed in Table 1 and depicted in Figure 2. Outcomes for baseline and post‐SRP were previously reported in our prior publication (Dodington et al., 2015) and are shown in this paper for comparison with the clinical outcomes measured at 3–4 years post‐SRP. Probing depth and BOP were significantly reduced (p < .001) compared to baseline at both the 2‐ to 4‐month follow‐up and the 3‐ to 4‐year follow‐up visits. Notably, there was no difference in these outcomes between the 2‐ to 4‐month follow‐up and the 3‐ to 4‐year follow‐up indicating that periodontal health was maintained over the course of many years. Plaque scores were also reduced (p < .001) at both follow‐up time points; however, there was a small increase in plaque score between the 2‐ to 4‐month and the 3‐ to 4‐year time points (p < .05). There was a trend for decreased tooth mobility at the 3‐ to 4‐year follow‐up compared to baseline; however, it did not reach statistical significance.
Figure 2.
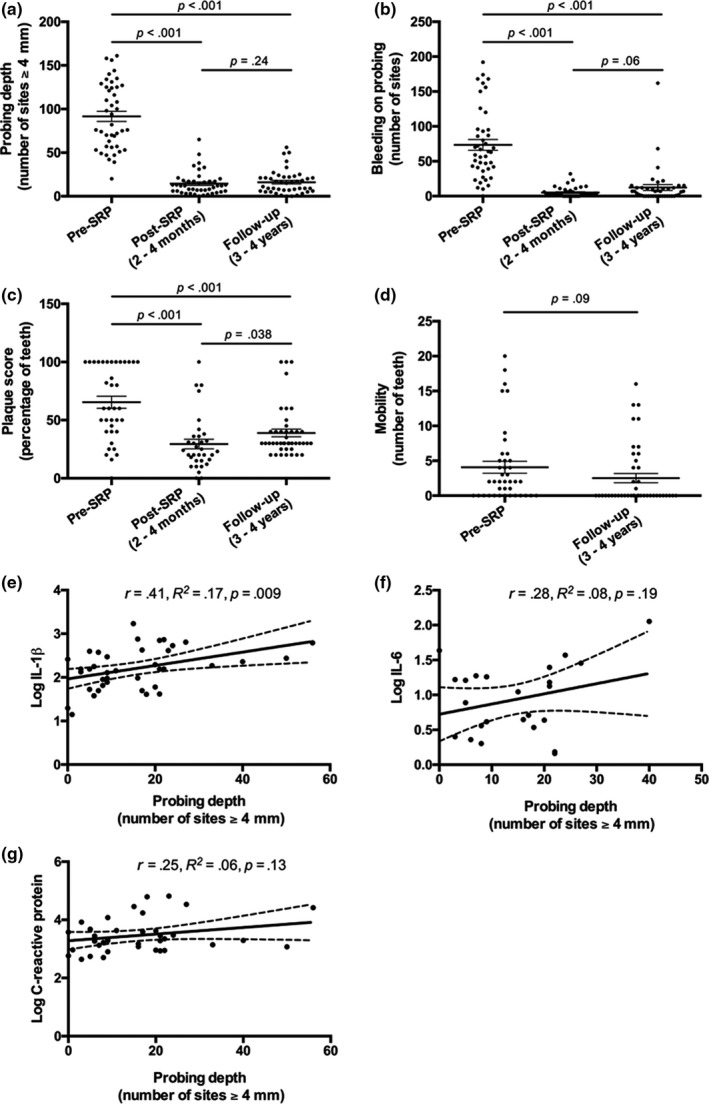
Periodontal health is maintained during long‐term follow‐up, and salivary IL‐1β is positively associated with periodontal PD. Clinical assessment of PD (a), BOP (b), plaque score (c) and mobility (d) pre‐SRP, post‐SRP and at long‐term follow‐up, 3–4 years post‐SRP. Probing depth plotted against log‐transformed concentrations of salivary IL‐1β (e), IL‐6 (f) and CRP (g) at follow‐up, 3–4 years post‐SRP. Differences among groups were calculated by one‐way ANOVA followed by Tukey pairwise comparisons. For a, b, c and d, each data point represents an individual patient and lines represent mean ± SEM. For e, f and g, all statistical associations are by Pearson correlations and solid lines indicates line of best fit and dashed lines indicate the 95% confidence interval
3.3. Salivary IL‐1β is positively associated with periodontal probing depth
Next, we investigated whether salivary inflammatory markers were associated with PD measured at the follow‐up visit. Figure 2 depicts log‐transformed salivary IL‐1β, IL‐6 and CRP levels plotted against PD. The number of sites with PD ≥ 4 mm was significantly correlated with salivary IL‐1β levels (r = 0.41, p = .009, n = 39) but not with salivary levels of IL‐6 (r = 0.29, p = .19, n = 24) or CRP (r = 0.25, p = .13, n = 38). Of note, there were 15 patients that had salivary IL‐6 levels below the limit of detection and one patient with CRP levels below the limit of detection.
3.4. Total flavonoid intake is inversely associated with probing depth and salivary IL‐1β concentrations
We investigated the association between select dietary intakes and periodontal outcomes at the follow‐up time point (3–4 years post‐SRP). We first investigated correlations with fruit and vegetable intake, and vitamin C as these were the most strongly associated with reductions in PD in our previous study (Dodington et al., 2015). In addition, we investigated associations with flavonoid intake, which have been hypothesized to promote some of the periodontal health with fruit, vegetable and tea intake. Fruit and vegetable intake, and vitamin C intake were not significantly associated with PD or salivary IL‐1β levels (Table 2). However, there was a strong inverse relationship between total flavonoid intake and both PD (r = −0.36, p = .03, n = 36) and salivary IL‐1β concentrations (r = −0.49, p = .003, n = 33). This relationship is graphically represented in Figure 3 where total flavonoid intake is plotted against PD and salivary IL‐1β concentrations.
Table 2.
Correlations between periodontal health 3–4 years after SRP and dietary intakes of fruit and vegetables, vitamin C and total flavonoidsa
|
Dietary Intakes Mean ± SD (range) |
Probing Depth n = 36 |
Salivary IL−1β n = 33 |
|||
|---|---|---|---|---|---|
| r | p Value | r | p Value | ||
| Fruit and Vegetable (servings) | 3.8 ± 1.2 (1.4–6.6) | 0.01 | .96 | −0.20 | .25 |
| Vitamin C (mg) | 147 ± 62 (49–348) | 0.16 | .35 | −0.22 | .23 |
| Total Flavonoids (mg) | 276 ± 279 (61–1321) | −0.36 | .03 | −0.49 | .003 |
Pearson correlation coefficient (r) shown.
Figure 3.
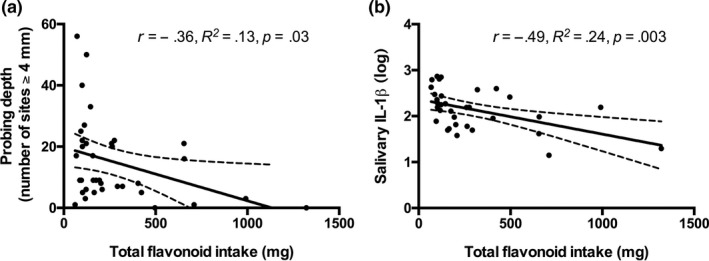
Total flavonoid intake is inversely associated with PD and salivary IL‐1β concentrations. Total flavonoid intake plotted against PD (a) and log‐transformed concentrations of salivary IL‐1β (b) at follow‐up, 3–4 years post‐SRP. Statistical associations were assessed using Pearson correlation. Solid line indicates line of best fit, and dashed line indicates its 95% confidence interval
Next, we wanted to see if this relationship persisted after controlling for potential confounders. We performed multiple linear regression analysis to control for age, sex, smoking status and bleeding on probing as a marker of dental hygiene (Table 3, Model 2). Total flavonoid intake remained significantly associated with PD and salivary IL‐1β concentrations after adjustment for these confounders (Table 3). Each gram of flavonoid intake was associated with a reduction in PD at 18 sites (95% CI: 1, 35) and a reduction in salivary IL‐1β by 0.71 log units (95% CI: 0.15, 1.28). With adjustment for the change in PD (Table 3, Model 3), the relationship between flavonoid intake and PD was marginally not significant (p = .051) but remained significant for flavonoid intake and salivary IL‐1β concentrations.
Table 3.
Multiple linear regressions of probing depth and salivary IL‐1β by total flavonoid intake, age, sex, smoking status, bleeding on probing and change in probing deptha
|
Probing Depth, n = 36 (# of sites ≥ 4 mm) |
Salivary IL−1β, n = 33 (log) |
|||||
|---|---|---|---|---|---|---|
| B (95% CI) | β | p | B (95% CI) | β | p | |
| Model 1 | ||||||
| Flavonoid Intake (g) | −17 (−33, −2) | −0.36 | .030 | −0.76 (−1.25, −0.27) | −0.49 | .003 |
| Model 2 | ||||||
| Flavonoid Intake (g) | −18 (−35, −1) | −0.38 | .042 | −0.71 (−1.28, −0.15) | −0.47 | .015 |
| Age (10 years) | −1 (−5, 3) | −0.12 | .51 | −0.04 (−0.18, 0.09) | −0.12 | .50 |
| Sex (female) | −3 (−11, 6) | −0.10 | .55 | −0.10 (−0.40, 0.20) | −0.11 | .51 |
| Smoker | 8 (−3, 19) | 0.24 | .17 | 0.02 (−0.36, 0.41) | 0.02 | .90 |
| BOP (# sites) | −0.04 (−0.20, 0.12) | −0.09 | .61 | 0.00 (−0.01, 0.02) | 0.05 | .78 |
| Model 3 | ||||||
| Flavonoid Intake (g) | −17 (−34, 0) | −0.35 | .051 | −0.71 (−1.28, −0.13) | −0.46 | .018 |
| Age (10 years) | 0 (−5, 4) | −0.02 | .91 | −0.03 (−0.17, 0.11) | −0.09 | .66 |
| Sex (female) | −2, (−11, 6) | −0.09 | .59 | −0.10 (−0.40, 0.21) | −0.11 | .52 |
| Smoker | 8 (−3, 19) | 0.23 | .17 | 0.02 (−0.37, 0.41) | 0.02 | .93 |
| BOP (# sites) | −0.07 (−0.23, 0.10) | −0.14 | .41 | 0.001 (−0.012, 0.015) | 0.04 | .83 |
| Change PD (# sites) | 0.11 (−0.04, 0.27) | 0.27 | .14 | 0.001 (−0.004, 0.007) | 0.10 | 0.58 |
Non‐standardized (B) and standardized (β) regression coefficients shown.
4. DISCUSSION
A major finding from this study was the association between higher total flavonoid intake and both reduced PD and lower salivary IL‐1β while there were no significant correlations between intake of fruit and vegetables nor vitamin C and clinical outcomes. The relationship between flavonoid intake and PD was, however, weakened with the inclusion of the change in PD between pre‐ and post‐treatment resulting in a borderline significant result. This may be due to the fact that baseline PD is such a strong predictor of PD after SRP, which we have previously shown in this cohort (Dodington et al., 2015). Interestingly, flavonoid intake showed a stronger relationship with salivary IL‐1β, which persisted after adjusting for confounders including change in PD. While flavonoid intake was not measured in the previous study by Dodington et al. (2015), there was an association between fewer number of sites with PD < 4 mm and consumption of at least five servings of fruit and vegetables per day (Dodington et al., 2015). This association may not be present in the present study as the patients were in a maintenance programme and thus clinically stable rather than in a healing phase. Thus, at the 3‐ to 4‐year follow‐up, patients had stable periodontal health, and the association of PD with fruit and vegetables, and vitamin C may be attenuated. Nonetheless, that there was a significant association between higher flavonoid intakes and lower salivary IL‐1β, a marker of inflammation, suggests that sources of flavonoids beyond those consumed in fruits and vegetables may be important for maintaining periodontal health. While the original study showed a relationship between periodontitis and fruit and vegetables, beta‐carotene, vitamin C, alpha‐tocopherol, EPA, and DHA intakes in non‐smokers but not smokers, this follow‐up study did not demonstrate that smoking status modified the relationship. Though, there is a non‐significant trend (p = .17) that current smoking is associated with on average 8 more sites ≥4 mm compared to non‐smokers. The lack of a statistically significant association may be due to the lower number of patients who were current smokers (11 vs. 23) but this difference between the two studies was not statistically significant.
Interestingly, only total flavonoid intake and not flavonoid intake from specific foods was associated with periodontal health and lower salivary IL‐1β. Flavonoids are abundant in a variety of foods such as fruits and vegetables, and also tea, coffee and wine (Yao et al., 2004) and within our population commonly consumed foods containing higher levels of flavonoids included citrus and berries such as strawberries and blackberries. When tea intake was independently examined against PD and BOP, no significant relationships were identified, and this may suggest that flavonoids with different biological activity and present in a range of foods are needed for periodontal health.
A secondary objective of this study was to confirm that periodontal maintenance appointments at the clinic can sustain the improved clinical outcomes observed at 2–4 months post‐SRP through to 3–4 years post‐SRP. Similar to previous studies, we found that PD and BOP sites were both significantly reduced at 2–4 months as well as at 3–4 years post‐SRP compared to pre‐SRP (Axelsson & Lindhe, 1981; Becker et al., 1979; Chambrone & Chambrone, 2006; Lindhe & Nyman, 1984; McGuire, 1991). A prospective study from 2004 reported that among 550 subjects that were followed for 30 years, only 21 teeth were lost during periodontal maintenance phase when the majority of teeth prior to SRP had poor prognoses (Axelsson & Lindhe, 1981). In the present study, we observed a decrease in plaque scores at both follow‐up time points compared to baseline. This may be a result of patient compliance to oral hygiene instruction as well frequent and routine periodontal maintenance appointments in which oral hygiene instruction could also be reinforced (Axelsson & Lindhe, 1981; Kakudate, Morita, Sugai, & Kawanami, 2009).
We also included measurement of markers of inflammation in saliva that may associate with periodontitis within this sample population. Our study found that PD was significantly correlated with IL‐1β levels, but there was no significant association with salivary levels of IL‐6 or CRP. That the relationship between salivary IL‐1β and PD, compared to salivary CRP and IL‐6, is stronger suggests that even when periodontal health is stable, IL‐1β may be a sensitive marker of periodontal health. Because salivary biomarkers of inflammation detected in chronic periodontitis are often significantly higher than in individuals with no active periodontitis (D'Aiuto et al., 2004), it suggests that lower IL‐1β may be an informative measure of inflammation even when periodontal health is categorized as stable.
An initial limitation of the study included the relatively small sample size due to the fact that this study recruited from the original patient population (Dodington et al., 2015). Use of an FFQ to measure dietary intake has been shown to overestimate dietary intakes; however, we utilized this method as patients had to recall their dietary patterns retrospectively (Shu et al., 2004). We also do not know how dietary intake may have varied between the post‐SRP and follow‐up appointment and could potentially explain the differing associations between diet and periodontal outcomes. Additionally, when assessing salivary biomarkers, we focused on IL‐1β, IL‐6 and CRP as these inflammatory markers have been associated with existing periodontitis (D'Aiuto et al., 2004; Miller et al., 2006; Pederson et al., 1995; Ridker, Hennekens, Buring, & Rifai, 2000). This may be a potential limitation as higher levels of other salivary biomarkers such as matrix metalloproteinase‐8 have been shown to be associated with a higher risk of periodontitis (Miller et al., 2006). Additionally, we only had salivary samples from one time point during the maintenance phase; thus, no comparisons in saliva were possible with other time points. Strengths of this study included patient compliance with periodontal maintenance and questionnaires. Additionally, including the collection of saliva at the 3‐ to 4‐year post‐SRP time point allows for future examination of inflammatory markers prospectively, and such follow‐up will further identify if IL‐1β continues to align with PD. This could also help identify the potential usefulness in monitoring periodontal health. Lastly, the inclusion criteria of patients at the pre‐SRP appointment ensured that individuals had similar periodontal health prior to SRP. Thus, findings of this study are more applicable to patients with moderate to severe periodontitis.
In conclusion, our findings suggest there is an association between greater flavonoid intake and reduced salivary IL‐1β in patients undergoing periodontal maintenance at a specialty office. Without inclusion of baseline PD, there is also an association between greater flavonoid intake and reduced PD. Whether this is a cause and effect relationship between higher flavonoid intake and reduced PD or with reduced salivary IL‐1β requires future study. Moreover, there were no significant differences in PD and BOP between post‐SRP and at the 3–4‐year follow‐up, suggesting periodontal health is stable with routine periodontal maintenance appointments at a speciality office. Future studies are needed to investigate the role of diet in long‐term maintenance of periodontal health post‐SRP.
CONFLICT OF INTEREST
The authors declare that there are no conflicts of interest in this study.
ACKNOWLEDGEMENTS
T.V.S. completed this research, in part, through the Dental Hygiene Periodontal Residency Program at the Periodontal Wellness and Implant Surgery Clinic, Fonthill, ON. W.E.W. holds a Canada Research Chair in Bone and Muscle Development.
Sparrow TV, Dodington DW, Yumol JL, Fritz PC, Ward WE. Higher intakes of flavonoids are associated with lower salivary IL‐1β and maintenance of periodontal health 3–4 years after scaling and root planing. J Clin Periodontol. 2020;47:461–469. 10.1111/jcpe.13263
REFERENCES
- Amaliya, A. , Laine, M. L. , Loos, B. G. , & Van der Velden, U. (2015). Java project on periodontal diseases: Effect of vitamin C/calcium threonate/citrus flavonoids supplementation on periodontal pathogens, CRP and HbA1c. Journal of Clinical Periodontology, 42(12), 1097–1104. 10.1111/jcpe.12478 [DOI] [PubMed] [Google Scholar]
- Axelsson, P. , & Lindhe, J. (1981). Effect of controlled oral hygiene procedures on caries and periodontal disease in adults. Results after 6 years. Journal of Clinical Periodontology, 8(3), 239–248. [DOI] [PubMed] [Google Scholar]
- Becker, W. , Berg, L. , & Becker, B. E. (1979). Untreated periodontal disease: A longitudinal study. Journal of Periodontology, 50(5), 234–244. [DOI] [PubMed] [Google Scholar]
- Boucher, B. , Cottericho, M. , Kreiger, N. , Nadalin, V. , Block, T. , & Block, G. (2006). Validity and reliability of the Block98 food‐frequency questionnaire in a sample of Canadian women. Public Health Nutrition, 9(1), 84–93. [DOI] [PubMed] [Google Scholar]
- Chambrone, L. , Chambrone, D. , Lima, L. A. , & Chambrone, L. A. (2010). Predictors of tooth loss during long‐term periodontal maintenance: A systematic review of observational studies. Journal of Clinical Periodontology, 37(7), 675–684. 10.1111/j.1600-051X.2010.01587.x [DOI] [PubMed] [Google Scholar]
- Chambrone, L. A. , & Chambrone, L. (2006). Tooth loss in well‐maintained patients with chronic periodontitis during long‐term supportive therapy in Brazil. Journal of Clinical Periodontology, 33(10), 759–764. 10.1111/j.1600-051X.2006.00972.x [DOI] [PubMed] [Google Scholar]
- Chapple, I. L. , Milward, M. R. , Ling‐Mountford, N. , Weston, P. , Carter, K. , Askey, K. , … Matthews, J. B. (2012). Adjunctive daily supplementation with encapsulated fruit, vegetable and berry juice powder concentrates and clinical periodontal outcomes: A double‐blind RCT. Journal of Clinical Periodontology, 39(1), 62–72. 10.1111/j.1600-051X.2011.01793.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Aiuto, F. , Parkar, M. , Andreou, G. , Suvan, J. , Brett, P. M. , Ready, D. , & Tonetti, M. S. (2004). Periodontitis and systemic inflammation: Control of the local infection is associated with a reduction in serum inflammatory markers. Journal of Dental Research, 83(2), 156–160. 10.1177/154405910408300214 [DOI] [PubMed] [Google Scholar]
- Dodington, D. W. , Fritz, P. C. , Sullivan, P. J. , & Ward, W. E. (2015). Higher intakes of fruits and vegetables, beta‐carotene, vitamin C, alpha‐tocopherol, EPA, and DHA are positively associated with periodontal healing after nonsurgical periodontal therapy in nonsmokers but not in smokers. Journal of Nutrition, 145(11), 2512–2519. 10.3945/jn.115.211524 [DOI] [PubMed] [Google Scholar]
- Gartenmann, S. J. , Weydlich, Y. V. , Steppacher, S. L. , Heumann, C. , Attin, T. , & Schmidlin, P. R. (2019). The effect of green tea as an adjunct to scaling and root planing in non‐surgical periodontitis therapy: A systematic review. Clinical Oral Investigations, 23(1), 1–20. 10.1007/s00784-018-2684-7 [DOI] [PubMed] [Google Scholar]
- Giannobile, W. V. , Beikler, T. , Kinney, J. S. , Ramseier, C. A. , Morelli, T. , & Wong, D. T. (2009). Saliva as a diagnostic tool for periodontal disease: Current state and future directions. Periodontology 2000, 50(1), 52–64. 10.1111/j.1600-0757.2008.00288.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graziani, F. , Discepoli, N. , Gennai, S. , Karapetsa, D. , Nisi, M. , Bianchi, L. , … Van der Velden, U. (2018). The effect of twice daily kiwifruit consumption on periodontal and systemic conditions before and after treatment: A randomized clinical trial. Journal of Periodontology, 89(3), 285–293. 10.1002/JPER.17-0148 [DOI] [PubMed] [Google Scholar]
- Herr, A. E. , Hatch, A. V. , Throckmorton, D. J. , Tran, H. M. , Brennan, J. S. , Giannobile, W. V. , & Singh, A. K. (2007). Microfluidic immunoassays as rapid saliva‐based clinical diagnostics. Proceedings of the National Academy of Sciences of the United States of America, 104(13), 5268–5273. 10.1073/pnas.0607254104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kakudate, N. , Morita, M. , Sugai, M. , & Kawanami, M. (2009). Systematic cognitive behavioral approach for oral hygiene instruction: A short‐term study. Patient Education and Counseling, 74(2), 191–196. 10.1016/j.pec.2008.08.014 [DOI] [PubMed] [Google Scholar]
- Laster, L. , Laudenbach, K. W. , & Stoller, N. H. (1975). An evaluation of clinical tooth mobility measurements. Journal of Periodontology, 46(10), 603–607. 10.1902/jop.1975.46.10.603 [DOI] [PubMed] [Google Scholar]
- Lindhe, J. , & Nyman, S. (1984). Long‐term maintenance of patients treated for advanced periodontal disease. Journal of Clinical Periodontology, 11(8), 504–514. [DOI] [PubMed] [Google Scholar]
- McGuire, M. K. (1991). Prognosis versus actual outcome: A long‐term survey of 100 treated periodontal patients under maintenance care. Journal of Periodontology, 62(1), 51–58. 10.1902/jop.1991.62.1.51 [DOI] [PubMed] [Google Scholar]
- Miller, C. S. , King, C. P. Jr , Langub, M. C. , Kryscio, R. J. , & Thomas, M. V. (2006). Salivary biomarkers of existing periodontal disease: A cross‐sectional study. Journal of the American Dental Association, 137(3), 322–329. [DOI] [PubMed] [Google Scholar]
- Pederson, E. D. , Stanke, S. R. , Whitener, S. J. , Sebastiani, P. T. , Lamberts, B. L. , & Turner, D. W. (1995). Salivary levels of alpha 2‐macroglobulin, alpha 1‐antitrypsin, C‐reactive protein, cathepsin G and elastase in humans with or without destructive periodontal disease. Archives of Oral Biology, 40(12), 1151–1155. [DOI] [PubMed] [Google Scholar]
- Ridker, P. M. , Hennekens, C. H. , Buring, J. E. , & Rifai, N. (2000). C‐reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. New England Journal of Medicine, 342(12), 836–843. 10.1056/NEJM200003233421202 [DOI] [PubMed] [Google Scholar]
- Sexton, W. M. , Lin, Y. , Kryscio, R. J. , Dawson, D. R. 3rd , Ebersole, J. L. , & Miller, C. S. (2011). Salivary biomarkers of periodontal disease in response to treatment. Journal of Clinical Periodontology, 38(5), 434–441. 10.1111/j.1600-051X.2011.01706.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu, X. O. , Yang, G. , Jin, F. , Liu, D. , Kushi, L. , Wen, W. , … Zheng, W. (2004). Validity and reproducibility of the food frequency questionnaire used in the Shanghai Women's Health Study. European Journal of Clinical Nutrition, 58(1), 17–23. 10.1038/sj.ejcn.1601738 [DOI] [PubMed] [Google Scholar]
- Silberman, S. L. , Le Jeune, R. C. , Serio, F. G. , Devidas, M. , Davidson, L. , & Vernon, K. (1998). A method for determining patient oral care skills: The University of Mississippi Oral Hygiene Index. Journal of Periodontology, 69(10), 1176–1180. 10.1902/jop.1998.69.10.1176 [DOI] [PubMed] [Google Scholar]
- Willett, W. C. , Howe, G. R. , & Kushi, L. H. (1997). Adjustment for total energy intake in epidemiologic studies. American Journal of Clinical Nutrition, 65(4), 1220S–1228S. 10.1093/ajcn/65.4.1220S [DOI] [PubMed] [Google Scholar]
- Yao, L. H. , Jiang, Y. M. , Shi, J. , Tomas‐Barberan, F. A. , Datta, N. , Singanusong, R. , & Chen, S. S. (2004). Flavonoids in food and their health benefits. Plant Foods for Human Nutrition, 59(3), 113–122. [DOI] [PubMed] [Google Scholar]
- Yun, J. H. , Pang, E. K. , Kim, C. S. , Yoo, Y. J. , Cho, K. S. , Chai, J. K. , … Choi, S. H. (2004). Inhibitory effects of green tea polyphenol (‐)‐epigallocatechin gallate on the expression of matrix metalloproteinase‐9 and on the formation of osteoclasts. Journal of Periodontal Research, 39(5), 300–307. 10.1111/j.1600-0765.2004.00743.x [DOI] [PubMed] [Google Scholar]


