Abstract
Background
Total mesorectal excision (TME) gives excellent oncological results in rectal cancer treatment, but patients may experience functional problems. A novel approach to performing TME is by single‐port transanal minimally invasive surgery. This systematic review evaluated the functional outcomes and quality of life after transanal and laparoscopic TME.
Methods
A comprehensive search in PubMed, the Cochrane Library, Embase and the trial registers was conducted in May 2019. PRISMA guidelines were used. Data for meta‐analysis were pooled using a random‐effects model.
Results
A total of 11 660 studies were identified, from which 14 studies and six conference abstracts involving 846 patients (599 transanal TME, 247 laparoscopic TME) were included. A substantial number of patients experienced functional problems consistent with low anterior resection syndrome (LARS). Meta‐analysis found no significant difference in major LARS between the two approaches (risk ratio 1·13, 95 per cent c.i. 0·94 to 1·35; P = 0·18). However, major heterogeneity was present in the studies together with poor reporting of functional baseline assessment.
Conclusion
No differences in function were observed between transanal and laparoscopic TME.
A novel surgical technique for total mesorectal excision (TME) is single‐port transanal minimally invasive surgery. This systematic review aimed to evaluate the functional outcomes and quality of life after transanal total mesorectal excision (TaTME) and compare them with those of laparoscopic TME (LapTME). No differences between TaTME and LapTME were seen in terms of impaired functioning and reduced quality of life after rectal cancer surgery. However, major heterogeneity was present in the studies together with poor reporting of functional baseline assessment.

Comparable function
Antecedentes
La escisión total del mesorrecto (total mesorectal excision, TME) proporciona excelentes resultados oncológicos en el tratamiento del cáncer de recto, pero los pacientes pueden presentar trastornos funcionales. Un abordaje novedoso para realizar la TME es mediante cirugía transanal mínimamente invasiva de puerto único. En esta revisión sistemática se evaluaron los resultados funcionales y la calidad de vida después de TME transanal (TaTME) y TME laparoscópica (LapTME).
Métodos
En mayo de 2019 se realizó una búsqueda exhaustiva en las bases de datos de Pubmed, Biblioteca Cochrane, EMBASE y en los registros de ensayos clínicos. Se utilizaron las guías PRISMA. Los datos para el metaanálisis se agruparon utilizando un modelo de efectos aleatorios.
Resultados
Se identificaron un total de 11.660 estudios, de los cuales se incluyeron 14 estudios y 6 resúmenes de congresos con 846 pacientes (599 TaTME/247 LapTME). Un número sustancial de pacientes presentó trastornos funcionales consistentes con el síndrome de resección anterior baja (low anterior resection syndrome, LARS). El metaanálisis no encontró diferencias significativas en los porcentajes de LARS grave entre los dos abordajes (razón de oportunidades, odds ratio, OR 1,13; i.c. del 95% 0,94‐1,35; P = 0,18). Sin embargo, los estudios globalmente presentaron una gran heterogeneidad, así como una deficiente información sobre la evaluación funcional basal.
Conclusión
No se observaron diferencias en la función entre TaTME y LapTME.
Introduction
Total mesorectal excision (TME) is the standard surgical treatment for rectal cancer, with excellent long‐term local recurrence‐free and overall survival rates1. Over time, advances in technology led to a shift from open to laparoscopic surgery owing to favourable short‐term outcomes such as less pain, reduced blood loss and improved recovery time2, 3, 4, 5, 6. However, quality of life (QoL) and functional outcomes were not significantly improved by the laparoscopic approach7, 8. The latest developments are the robotic and the transanal approach. The latter, called transanal TME (TaTME) has been developed to overcome surgical difficulties experienced during distal pelvic dissection, especially in men with a narrow pelvis, a low tumour and a high BMI9. Long‐term results of randomized studies are awaited, especially since the Norwegian moratorium on TaTME owing to an unexpectedly high local recurrence rate10.
Although many studies have investigated functional bowel dysfunction after laparoscopic low anterior resection11, 12, 13, little is known about these functional sequelae after TaTME and their impact on QoL. The most common postoperative complaints, such as incontinence, urgency and frequent bowel movement, are described as low anterior resection syndrome (LARS). This syndrome has a severe adverse effect on QoL14, 15, 16. Known risk factors for the development of LARS are a low level of anastomosis, poor preoperative function and neoadjuvant chemoradiotherapy17, 18, 19, 20. With the TaTME technique, surgeons might choose a lower anastomosis for technical rather than oncological reasons, and urethral injuries are more likely21. Concerns regarding functional outcomes after TaTME have been expressed. This meta‐analysis was conducted to compare functional outcomes and QoL after TaTME and laparoscopic TME (LapTME).
Methods
This review was conducted in accordance with PRISMA guidelines22, 23, with an a priori developed review protocol (PROSPERO; CRD42019126975). A comprehensive search was undertaken in PubMed, Embase, the Cochrane database and the trial registers. The full search strategy is available in Appendix S1 (supporting information).
Two reviewers performed the selection process and reviewed all included studies. Discrepancies were resolved through discussion. The following inclusion criteria were applied: patients with rectal cancer who underwent TaTME and received any assessment of functional outcome or QoL. If a study also included patients who underwent LapTME, this group was used as a comparator for the TaTME group. All study designs with a population of ten or more patients were included. No filters for language or date were used. Studies were excluded if they evidently contained the same data, or were letters to the editor or expert opinions. If reported, the time from ileostomy closure to the evaluation of functional outcome was included. Quality assessment was performed by using the Newcastle–Ottawa Scale for observational studies24 and the Cochrane quality assessment tool for randomized trials25.
Analysis
Basic descriptive statistics were used to summarize patient characteristics and outcome data. A meta‐analysis was performed if sufficient studies and adequate data were available. The Mantel–Haenszel method was used for dichotomous data. A random‐effects model was used and checked using a fixed‐effect model. If the requested data were not available, mean(s.d.) values were calculated for overall analysis, if possible26. A meta‐analysis of P values was performed in comparative studies of QoL data evaluated by the European Organization for Research and Treatment of Cancer (EORTC) questionnaires27. The Cochrane handbook 6 was used as a guideline for this analysis28. No funnel plots were presented, owing to the limited number of studies available for meta‐analysis28. Analyses were performed using Review Manager version 5.3.5 (Nordic Cochrane Centre, The Cochrane Collaboration, Copenhagen, Denmark), and Microsoft Excel® (Microsoft, Redmond, Washington, USA) for the meta‐analysis that combined P values.
Results
Study selection
The search was performed in May 2019 and returned 11 660 articles after removal of duplicates from which left 8572 studies. After exclusion of irrelevant articles, 90 potentially relevant studies and 39 potentially relevant trials were assessed further. Eventually 14 studies and six conference abstracts were included (Fig. 1)9, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47. Studies were excluded for the following reasons: did not investigate TaTME (11), did not provide functional/QoL data (34), included fewer than ten patients (2) or other reasons (62).
Figure 1.
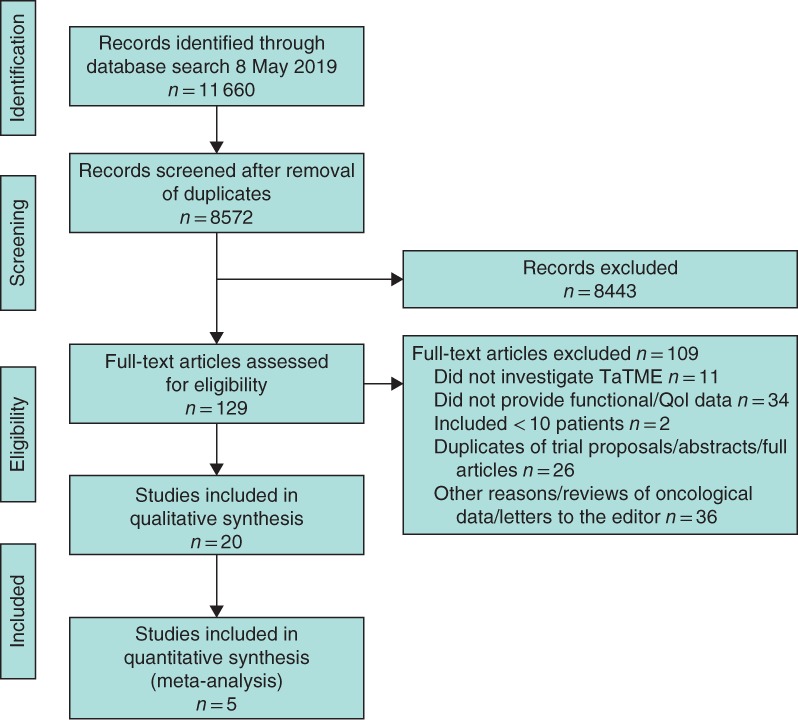
PRISMA diagram showing selection of articles for review TaTME, transanal total mesorectal excision; QoL, quality of life.
Study characteristics and quality control
Six retrospective (3 cross‐sectional, 2 cohort, 1 case–control) and 14 prospective (11 cohort, 2 cross‐sectional, 1 RCT) studies were included (Table S1 , supporting information). The studies included 599 patients who underwent TaTME. A total of 247 patients who underwent LapTME were identified as a control group to compare with patients who underwent TaTME. Duration of follow‐up after surgery varied from 3 to 75 months. Seven studies included a baseline measurement in the study design. In the majority of studies, the tumour was located in the lower and middle rectum (tumour height 3·7–7·1 cm). Mean temporary ileostomy rates were 92·2 per cent in the TaTME group compared with 88·1 per cent in the LapTME group. Some 61·5 per cent of the patients received neoadjuvant treatment before TaTME compared with 70·8 per cent before LapTME. The height of anastomosis was not reported systematically, but was significantly lower after TaTME in the study of Mosquera and colleagues45. Other comparative studies showed no relevant differences in tumour height or site (mid, low, high).
Four of the included studies were of high quality based on the Newcastle–Ottawa Scale, scoring at least 7 points (Table S2 , supporting information). Overall quality was acceptable, except that baseline measurements were not frequently reported and relatively few studies presented a comparator LapTME group. The only RCT was of good quality, except for an unclear risk of selective reporting.
Bowel dysfunction
Thirteen studies assessed bowel dysfunction by measuring the LARS score (Table 1), and five compared LARS scores after TaTME versus LapTME. Meta‐analysis showed no significant differences in the incidence of major LARS between the procedures (Fig. 2). Sensitivity analyses excluding studies with follow‐up of less than 12 months (risk ratio (RR) 1·15, 95 per cent c.i. 0·93 to 1·43) and studies with significant differences in baseline characteristics between TaTME and LapTME groups (RR 1·08, 0·89 to 1·32) showed no differences in bowel dysfunction outcomes between procedures.
Table 1.
Bowel dysfunction as measured by low anterior resection score
| Reference | No. of patients | Duration of follow‐up (months) | Total LARS score | No LARS | Minor LARS | Major LARS |
|---|---|---|---|---|---|---|
| Bjoern et al.29 | 49 TaTME | 22·7 (10·3)* | 26·2 (10·3)* | 17 (35) | 15 (31) | 17 (35) |
| 36 LapTME | 75·1 (17·6)* | 20·6 (14·5)* (P = 0·054) | 16 (44) | 8 (22) | 12 (33) | |
| Veltcamp Helbach et al.30 | 27 TaTME | 20·0 (6·6–44·4)† | 27·7 (13·3)* | 7 (26) | 4 (15) | 16 (59) |
| 27 LapTME | 59·5 (39·7–82·0)† | 24·0 (10·5)* (P = 0·267) | 11 (41) | 8 (30) | 8 (30) | |
| Turrado‐Rodriguez et al.31 | 80 TaTME | 37·6‡ | n.r. | 31 (39) | 49 (61) | |
| Rubinkiewicz et al.32 | 25 TaTME | Baseline | 5 (0–12)§ | n.r. | n.r. | n.r. |
| 6 | 32 (30–37)§ | 0 (0) | 4 (16) | 21 (84) | ||
| Reali et al.33 | 29 TaTME | Baseline | n.r. | 11 (38) | 13 (45) | 5 (17) |
| 24 | n.r. | 8 (28) | 15 (52) | 6 (21) | ||
| Mora et al.34 | 16 TaTME | 6 | n.r. | 3 (19) | 3 (19) | 10 (63) |
| 15 LapTME | 6 | n.r. | 4 (27) | 2 (13) | 9 (60) | |
| Koedam et al.37 | 30 TaTME | Baseline | 15·4 (7·3, 23·5)¶ | 16 (53) | 10 (33) | 4 (13) |
| 1 | 35·7 (32·9, 38·6)¶ | 0 (0) | 6 (20) | 24 (80) | ||
| 6 | 21·7 (13·6, 29·9)¶ | 14 (47) | 6 (20) | 10 (33) | ||
| Hanke et al.38 | 31 TaTME | 3 | 25‡ | n.r. | n.r. | n.r. |
| 17 | 6 | 21‡ | n.r. | n.r. | n.r. | |
| 13 | 9 | 18‡ | n.r. | n.r. | n.r. | |
| 10 | 12 | 10‡ | n.r. | n.r. | n.r. | |
| 7 | 18 | 10‡ | n.r. | n.r. | n.r. | |
| 4 | 24 | 2·5‡ | n.r. | n.r. | n.r. | |
| Pontallier et al.40 | 38 TaTME | >12 | 36 (12–42)† | n.r. | n.r. | 31 (82) |
| 34 LapTME | 37 (12–42)† (P = 0·977) | n.r. | n.r. | 26 (76) | ||
| Kneist et al.41 | 10 TaTME | Baseline | n.r. | 9 (90) | 1 (10) | 0 (0) |
| 3 | 28 (9–38)† | 3 (30) | 3 (30) | 4 (40) | ||
| 6 | 26 (9–32)† | 4 (40) | 5 (50) | 1 (10) | ||
| Keller et al.44 | 61 TaTME | Baseline | 23·0 (9·7)* | 22 (40) | 20 (36) | 13 (24) |
| 12 | 25·6 (8·0)* | n.r. | n.r. | n.r. | ||
| Leão et al.46 | 20 TaTME | 1 | 32·7# | (14) | (7) | (79) |
| 3 | n.r. | (23) | (8) | (69) | ||
| 6 | n.r. | (38) | (23) | (38) | ||
| 12 | 19·5# | (50) | (40) | (10) | ||
| Dou et al.47 | 54 TaTME | 17·2 (12·1–30·4)† | n.r. | n.r. | n.r. | 26 (48) |
| 53 LapTME | n.r. | n.r. | n.r. | 22 (42) |
Values in parentheses are percentages unless indicated otherwise; values are
mean(s.d.)
median (range)
median
median (i.q.r.)
mean (95 per cent c.i.)
mean. LARS, low anterior resection syndrome; TaTME, transanal total mesorectal excision
LapTME, laparoscopic total mesorectal excision; n.r., not reported. P values are shown for TaTME versus LapTME.
Figure 2.
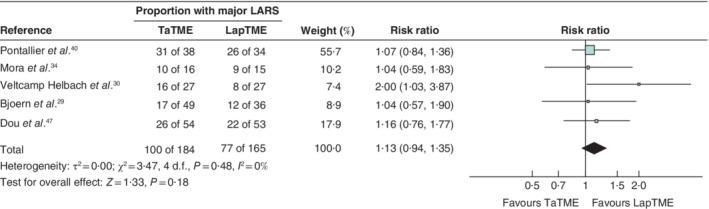
Meta‐analysis of the prevalence of major low anterior resection syndrome after transanal versus laparoscopic total mesorectal excision A Mantel–Haenszel random‐effects model was used for meta‐analysis. Risk ratios are shown with 95 per cent confidence intervals. The longest follow‐up data for each study were used. If a study favours laparoscopic total mesorectal excision (LapTME), fewer patients experienced major low anterior resection syndrome (LARS) in this group. TaTME, transanal total mesorectal excision.
Bjoern and colleagues29 reported no significant difference in LARS scores after TaTME compared with LapTME (P = 0·054) (Table 1). For the subcategories clustering of stools (P = 0·017) and faecal urgency (P = 0·032), a significant disadvantage for TaTME was found. Koedam and co‐workers37 reported significantly worse LARS scores 1 month after TaTME surgery, but did not note a significant difference at 6 months compared with baseline scores. A significant increase in LARS scores was demonstrated after surgery in all studies33. However, these scores returned to baseline values in the majority of studies33, 44, 46.
Continence
Eleven studies used the Wexner score to assess the level of continence (Table 2); two others36, 44 used the Vaizey or Kirwin score. All studies that performed a preoperative assessment of function32, 39, 41 confirmed that no major preoperative deviations in Wexner score were present. Summarizing data that reported Wexner scores at specific times (3, 6, 9, 12, 18, 24 months) showed a median Wexner score at 3 months of 9 (range 1–20)38, 41, 43. At 6 months, median scores ranged from 3 to 738, 41, 43. Rouanet et al.9 recorded a median Wexner score of 11 after 12 months. Tuech and colleagues42 reported that three of 52 patients received a colostomy owing to faecal incontinence after a minimum of 12 months of conservative therapy. Three studies40, 43, 45 compared TaTME with LapTME and none of them reported significant differences.
Table 2.
Continence status as measured by Wexner score
| Reference | No. of patients | Duration of follow‐up (months) | Wexner score* | Wexner score > 10 (major incontinence) |
|---|---|---|---|---|
| Turrado‐Rodriguez et al.31 | 80 TaTME | 37·6(17·7)† | 10(5)† | n.r. |
| Rubinkiewicz et al.32 | 25 TaTME | Baseline | 0 (0–2) | n.r. |
| 6 | 11 (8–12) | n.r. | ||
| Hanke et al.38 | 31 TaTME | 3 | 9 | n.r. |
| 17 | 6 | 6 | n.r. | |
| 13 | 9 | 4 | n.r. | |
| 10 | 12 | 2 | n.r. | |
| 7 | 18 | 4 | n.r. | |
| 4 | 24 | 0 | n.r. | |
| Elmore et al.39 | 12 TaTME | Baseline | n.r. | n.r. |
| 6 | 3 (1–8) | n.r. | ||
| Pontallier et al.40 | 38 TaTME | > 12 | 9 (2–20) | 16 (42) |
| 34 LapTME | 10 (3–20)(P = 0·932) | 14 (41) (P = 0·936) | ||
| Kneist et al.41 | 10 TaTME | Baseline | 1 (0–7) | 0 (0) |
| 3 | 9 (1–20) | 4 (40) | ||
| 6 | 7 (0–15) | 3 (30) | ||
| Tuech et al.42 | 52 TEAP | > 12 | 4 (3–12) | 7 (13) > 7 points |
| De'Angelis et al.43 | 32 TaTME | 3 | 9 (3–15) | 10 (32) |
| 32 LapTME | 3 | 10·5 (4–19) (P = 0·115) | 16 (50) | |
| Rouanet et al.9 | 30 TEAP | 12 | 11 | n.r. |
| Keller et al.44 | 61 TaTME | Baseline | n.r. | n.r. |
| 12 | n.r. | n.r. | ||
| Leão et al.46 | 20 TaTME | 1 | 10·3 | n.r. |
| 20 | 3 | 7·9 | n.r. | |
| 20 | 6 | 4·6 | n.r. | |
| 8 | 12 | 2·8 | n.r. |
Values in parentheses are percentages unless indicated otherwise
values are median (range), except
mean(s.d.). TaTME, transanal total mesorectal excision; n.r., not reported; LapTME, laparoscopic total mesorectal excision; TEAP, transanal endoscopic proctectomy. P values are shown for TaTME versus LapTME.
Urogenital dysfunction
The International Index of Erectile Function (IIEF/IIEF‐5), International Prostate Symptom Score (IPPS) and Female Sexual Function Index (FSFI) were used to evaluate urogenital dysfunction after TaTME (Table 3).
Table 3.
Urogenital dysfunction as measured by International Index of Erectile Function and International Prostate Symptom Score
| Reference | No. of patients | Duration of follow‐up (months) | IIEF score | Patients with erectile dysfunction (IIEF score ≤ 21) | IPSS | IPSS quality‐of‐life score | IPSS category |
|---|---|---|---|---|---|---|---|
| Bjoern et al.29 | 37 TaTME | 22·7(10·3)* | n.r. | n.r. | 6·7(7·4)* | Score 1/2/3/4/5/6/7: 22/7/7/0/0/1/0 | No: 6 (16)Mild: 17 (46)Moderate: 12 (32)Severe: 2 (5) |
| 20 LapTME | 75·1(17·6)* | n.r. | n.r. | 10·1(8·2)* (P = 0·060) | Score 1/2/3/4/5/6/7: 8/7/0/3/1/0/1 (P = 0·01) | No: 1 (5) Mild: 9 (45) Moderate: 8 (40) Severe: 2 (10) (P = 0·236) | |
| Veltcamp Helbach et al.30 | 14 TaTME | 20·0 (6·6–44·4)† | n.r. | n.r. | 8(6·6)* | n.r. | No/mild: 7 (50) Moderate: 7 (50) Severe: 0 (0) |
| 18 LapTME | 59·5 (39·7–82·0)† | n.r. | n.r. | 6·7(6·3)* (P = 0·582) | n.r. | No/mild: 12 (67) Moderate: 5 (28) Severe: 1 (6) (P = 0·277) | |
| Pontallier et al.40 | 21 TaTME | 38 (15–39)† Functional assessment >12 | 17·5 (5–25)† | 14 (67) | 5·5 (0–23)† | 1 (0–6)† | IPPS >10: 21% |
| 16 LapTME | 7 (5–21)† (P = 0·119) | 15 (93) (P = 0·108) | 3·5 (0–27)† (P = 0·821) | 1 (0–5)† (P = 0·967) | IPPS >10: 21% (P = 0·961) | ||
| Kneist et al.41 | 10 TaTME | ||||||
| 9 | Baseline | n.r. | n.r. | 5 (0–31)† | 1 (0–4) | No/mild: 6 (67) Moderate: 2 (22) Severe 1 (11) | |
| 9 | 3 | n.r. | n.r. | 3 (1–20)† | n.r. | No/mild: 7 (78) Moderate: 1 (11) Severe: 1 (11) | |
| 9 | 6 | n.r. | n.r. | n.r. | n.r. | No/mild: 7 (78) Moderate: 1 (11) Severe: 1 (11) | |
| 6 | 9 | n.r. | n.r. | n.r. | n.r. | No/mild: 5 (83) Moderate: 1 (17) Severe: 0 (0) | |
| Keller et al.44 | TaTME 61 | ||||||
| 50 | Baseline | 19·3(5·9)* | n.r. | 6·3(5·0)* | 1·3(1·4) | n.r. | |
| 50 | 12 | 17·6(6·4)* | n.r. | 5·9(4·7)* | 1·4(1·2) | n.r. |
Values in parentheses are percentages unless indicated otherwise; values are
mean(s.d.)
median (range). The International Prostate Symptom Score (IPSS) ranges from 0 to 35, with categories no/mild (0–7), moderate (8–19) and severe (20–35) complaints. IIEF, International Index of Erectile Function; TaTME, transanal total mesorectal excision; n.r., not reported/not reported correctly; LapTME, laparoscopic total mesorectal excision. P values are shown for TaTME versus LapTME.
Urogenital function in men
Foo and colleagues35 noted that erectile function in 23 men worsened significantly after surgery (P = 0·002) but returned to baseline after 6 months (P = 0·142). Pontallier and co‐workers40 did not find any significant differences in IIEF scores (P = 0·119) or category of erectile dysfunction (IIEF 21 or less; P = 0·108). Regarding urological function, Foo et al.35 showed no significant differences in scores measured at baseline, and 3 and 6 months after surgery. In studies that compared TaTME with LapTME29, 30, 40, there were no significant difference in IPPS scores between procedures (Table 3). Bjoern and co‐workers29 reported a significant effect on the IPPS QoL score in favour of TaTME (P = 0·01).
Urogenital function in women
Pontallier and colleagues40 reported sexual dysfunction in two of five women after TaTME and in two of three in the LapTME group. Turrado‐Rodriguez and co‐workers31 reported sexual dysfunction in 17 of 26 women after TaTME and concluded that these outcomes were similar to those of LapTME.
Quality‐of‐life assessment
Four different QoL questionnaires were used, namely the EuroQol Five Dimensions (EQ‐5D™; EuroQol Group, Rotterdam, the Netherlands), EORTC QLQ‐C30, QLQ‐CR29 and Faecal Incontinence Quality of Life scale (FIQL) questionnaire. The QLQ‐CR38 is also frequently used for colorectal cancer, but not in the studies included in the present review. EQ‐5D™ data are known to correlate weakly with changes in defaecation pattern48, and are shown in Table S3 (supporting information).
Faecal Incontinence Quality of Life scale
Only one study46 included the FIQL, and reported baseline scores of 4·0 (lifestyle, coping/behaviour, embarrassment) and 4·4 (depression/self‐perception). A decrease in QoL scores was seen 1 and 3 months after surgery (lifestyle 2·1–2·4, coping 2·5–3·5, depression 2·2–2·5, embarrassment 2·0–3·2), but scores returned to baseline within 1 year after TaTME (lifestyle 3·8, other scores 3·9).
EORTC QLQ‐C30
Two studies presented QoL scores over time (Table S4 , supporting information). Keller and colleagues44 reported that emotional function increased significantly after 1 year compared with preoperative measurements (P ≤ 0·01). Koedam and co‐workers37 described a significant decrease in QoL (P = 0·012), physical functioning (P = 0·001), role functioning (P = 0·001), fatigue (P = 0·002) and general pain (P = 0·001). After 6 months, these effects disappeared, except for social functioning (P = 0·013) and anal pain (P = 0·013), which remained significantly worse than at baseline.
Three studies29, 30, 34 compared TaTME with LapTME. Veltcamp Helbach and colleagues30 reported scores for role functioning (89·5 versus 80·2; P = 0·042), fatigue (12 versus 26·5; P = 0·021) and faecal incontinence (2·4 versus 14·8; P = 0·032) in favour of LapTME. A discrepancy between studies was found for the domain emotional functioning; scores favouring LapTME were reported by Bjoern et al.29 (83·51 versus 87·07; P = 0·041), whereas Mora and colleagues34 described better scores for TaTME (89·58 versus 77·38; P = 0·031). Functional scores for diarrhoea were in favour of LapTME in the study of Bjoern and co‐workers29 (17·69 versus 4·62; P = 0·009). In a meta‐analysis combining significance levels, no statistically significant differences were found between QoL subdomains for the comparative studies (Table S4 , supporting information).
EORTC QLQ‐CR29
Buttock pain (P = 0·01) and faecal incontinence (P = 0·03) were significantly worse in the TaTME group29, 30. Scores on all other scales were comparable, including flatulence and sexual function. Mora et al.34 described more abdominal pain and a bloated feeling in the LapTME group. A meta‐analysis combining significance levels showed no significant differences between the QoL subdomains for the comparative studies (Table S4 , supporting information).
Discussion
The present review investigated the impact of TaTME on functional outcomes and QoL. A significant proportion of patients who underwent TaTME experienced impaired postoperative bowel function. These complaints appeared to be present equally in patients treated by transanal and laparoscopic approaches.
A potential advantage of TaTME is that it allows construction of a (low) anastomosis in patients in whom abdominoperineal resection would previously have been necessary32. However, since the introduction of TaTME, concerns have been raised about postoperative function and QoL owing to factors such as the low anastomosis, urethral injuries, insertion of the transanal platform and anal stretch21, 49. Anal stretch and dilatation carries a potential risk of damaging the sphincter complex during transanal surgery. Previous studies of transanal endoscopic microsurgery (TEM) showed that controlled anal dilatation caused significant decreases in resting and voluntary contraction pressures, but had no influence on Wexner scores indicating clinical incontinence50, or long‐term QoL after TEM51.
To the extent that the included studies allow, given their follow‐up and quality, TaTME appears to be similar to LapTME in terms of functional outcomes. Potential risk factors for functional outcomes after TaTME were not investigated in this review. In a meta‐analysis regarding major LARS, no significant differences were found between LapTME and TaTME (RR 1·13, 95 per cent c.i. 0·94 to 1·35). In several non‐comparative studies that analysed TaTME only, variations in outcomes were found that could be explained by patient characteristics. In the study of Bjoern and colleagues29, scores for the subcategories clustering of stools and faecal urgency reached statistical significance not in favour of TaTME, but it is important to note that this study failed to report several important patient characteristics (such as preoperative function) and showed a significant difference in the timing of questionnaires. Although LARS scores were impaired after TaTME, only a few patients were reported who underwent complete disconnection of the anastomosis and construction of colostomy owing to faecal incontinence42. Male erectile function worsened after surgery but returned to baseline within 6 months35. No differences in sexual function for women31 or urological function for men29, 30, 40 were described between the two approaches.
Discrepancies in results were found between studies that used the EORTC questionnaires to measure QoL. Emotional functioning scores favoured LapTME in the study by Bjoern and co‐workers29 but were reported to favour TaTME by Mora et al.34. A difference in follow‐up was suggested as an explanatory factor because median follow‐up was 22·7 months for TaTME but 75·1 months for LapTME in the Bjoern study. The duration of follow‐up was also suggested to explain the differences in individual domains described by Veltcamp Helbach et al.30 (role function, fatigue and faecal incontinence in favour of LapTME). Overall, QoL and global health status were comparable between the TaTME and LapTME groups. In terms of buttock pain29 and faecal incontinence30, QoL was worse after TaTME. It is remarkable that these QoL deteriorations were not detected by the functional assessment tools used in these studies.
Overall, reporting of the included studies was complete, except for the conference abstracts that were obviously restricted in reporting, and some did not report all QoL domains34. A wide variety of adequate and valid questionnaires were used to assess QoL and functional outcomes52, 53. The overall quality of evidence was moderate, owing to considerable heterogeneity, lack of baseline measurements and relatively small sample sizes. The heterogeneity may have been the result of wide selection criteria, but these were specifically chosen to allow review of all available functional TaTME data. Additional treatment, preoperative function, height of the tumour and anastomosis, and differences in follow‐up times were important factors contributing to heterogeneity and the interpretation of functional outcomes20. Height of anastomosis was not reported systematically, but was significantly lower among patients who underwent TaTME in the study of Mosquera and colleagues45. In other comparative studies, no relevant differences in tumour height (in centimetres) and site (mid, low, high) were found. Six of eight studies properly described the rate of neoadjuvant therapy, and generally patients in the TaTME group underwent neoadjuvant therapy less frequently, yet this difference was not statistically significant.
The main limitation of this study is the lack of large RCTs. The majority of the studies were heterogeneous comparative studies and only seven of 20 reported preoperative baseline measurements. In addition, the surgeon's learning curve was reported poorly54. These limitations make it difficult to reach firm conclusions. However, it is important to draw attention to the oncological concerns surrounding TaTME: an unexpected pattern of recurrences early after TaTME resulted in a moratorium in Norway10. Several studies55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65 are currently investigating different aspects of transanal methods of TME surgery. The COLOR III trial66 is comparing TaTME with LapTME in a large cohort that should provide decisive data about the safety of TaTME.
Supporting information
Appendix S1 Search strategy per database (Pubmed, EMBASE, Cochrane Library and the WHO and http://clinicaltrial.gov trial registers).
Acknowledgements
The authors thank Professor K. Gurusamy from University College London for the teaching of his systematic review course and helpful insights in conducting this systematic review. Preregistration of this study was undertaken, which included an analysis plan for the primary outcome measures (PROSPERO; CRD42019126975).
Disclosure: The authors declare no conflict of interest.
References
- 1. Brouwer NPM, Bos ACRK, Lemmens VEPP, Tanis PJ, Hugen N, Nagtegaal ID et al An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer 2018; 143: 2758–2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Veldkamp R, Kuhry E, Hop WC, Jeekel J, Kazemier G, Bonjer HJ et al.; COlon cancer Laparoscopic or Open Resection Study Group (COLOR). Laparoscopic surgery versus open surgery for colon cancer: short‐term outcomes of a randomised trial. Lancet Oncol 2005; 6: 477–484. [DOI] [PubMed] [Google Scholar]
- 3. Kang SB, Park JW, Jeong SY, Nam BH, Choi HS, Kim DW et al Open versus laparoscopic surgery for mid or low rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): short‐term outcomes of an open‐label randomised controlled trial. Lancet Oncol 2010; 11: 637–645. [DOI] [PubMed] [Google Scholar]
- 4. Jeong SY, Park JW, Nam BH, Kim S, Kang SB, Lim SB et al Open versus laparoscopic surgery for mid‐rectal or low‐rectal cancer after neoadjuvant chemoradiotherapy (COREAN trial): survival outcomes of an open‐label, non‐inferiority, randomised controlled trial. Lancet Oncol 2014; 15: 767–774. [DOI] [PubMed] [Google Scholar]
- 5. Jayne DG, Thorpe HC, Copeland J, Quirke P, Brown JM, Guillou PJ. Five‐year follow‐up of the Medical Research Council CLASICC trial of laparoscopically assisted versus open surgery for colorectal cancer. Br J Surg 2010; 97: 1638–1645. [DOI] [PubMed] [Google Scholar]
- 6. Bonjer HJ, Deijen CL, Abis GA, Cuesta MA, van der Pas MH, de Lange‐de Klerk ES et al.; COLOR II Study Group. A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 2015; 372: 1324–1332. [DOI] [PubMed] [Google Scholar]
- 7. Andersson J, Abis G, Gellerstedt M, Angenete E, Angerås U, Cuesta MA et al Patient‐reported genitourinary dysfunction after laparoscopic and open rectal cancer surgery in a randomized trial (COLOR II). Br J Surg 2014; 101: 1272–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Andersson J, Angenete E, Gellerstedt M, Angerås U, Jess P, Rosenberg J et al Health‐related quality of life after laparoscopic and open surgery for rectal cancer in a randomized trial. Br J Surg 2013; 100: 941–949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rouanet P, Mourregot A, Azar CC, Carrere S, Gutowski M, Quenet F et al Transanal endoscopic proctectomy: an innovative procedure for difficult resection of rectal tumors in men with narrow pelvis. Dis Colon Rectum 2013; 56: 408–415. [DOI] [PubMed] [Google Scholar]
- 10. Larsen SG, Pfeffer F, Korner H; Norwegian Colorectal Cancer Group . Norwegian moratorium on transanal total mesorectal excision. Br J Surg 2019; 106: 1120–1121. [DOI] [PubMed] [Google Scholar]
- 11. Chen TY, Wiltink LM, Nout RA, Meershoek‐Klein Kranenbarg E, Laurberg S, Marijnen CA et al Bowel function 14 years after preoperative short‐course radiotherapy and total mesorectal excision for rectal cancer: report of a multicenter randomized trial. Clin Colorectal Cancer 2015; 14: 106–114. [DOI] [PubMed] [Google Scholar]
- 12. Emmertsen KJ, Laurberg S; Rectal Cancer Function Study Group . Impact of bowel dysfunction on quality of life after sphincter‐preserving resection for rectal cancer. Br J Surg 2013; 100: 1377–1387. [DOI] [PubMed] [Google Scholar]
- 13. Dulskas A, Miliauskas P, Tikuisis R, Escalante R, Samalavicius NE. The functional results of radical rectal cancer surgery: review of the literature. Acta Chir Belg 2016; 116: 1–10. [DOI] [PubMed] [Google Scholar]
- 14. Vironen JH, Kairaluoma M, Aalto AM, Kellokumpu IH. Impact of functional results on quality of life after rectal cancer surgery. Dis Colon Rectum 2006; 49: 568–578. [DOI] [PubMed] [Google Scholar]
- 15. Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL. Anterior resection syndrome. Lancet Oncol 2012; 13: e403–e408. [DOI] [PubMed] [Google Scholar]
- 16. Camilleri‐Brennan J, Ruta DA, Steele RJ. Patient generated index: new instrument for measuring quality of life in patients with rectal cancer. World J Surg 2002; 26: 1354–1359. [DOI] [PubMed] [Google Scholar]
- 17. Croese AD, Lonie JM, Trollope AF, Vangaveti VN, Ho YH. A meta‐analysis of the prevalence of low anterior resection syndrome and systematic review of risk factors. Int J Surg 2018; 56: 234–241. [DOI] [PubMed] [Google Scholar]
- 18. Bregendahl S, Emmertsen KJ, Lous J, Laurberg S. Bowel dysfunction after low anterior resection with and without neoadjuvant therapy for rectal cancer: a population‐based cross‐sectional study. Colorectal Dis 2013; 15: 1130–1139. [DOI] [PubMed] [Google Scholar]
- 19. Ekkarat P, Boonpipattanapong T, Tantiphlachiva K, Sangkhathat S. Factors determining low anterior resection syndrome after rectal cancer resection: a study in Thai patients. Asian J Surg 2016; 39: 225–231. [DOI] [PubMed] [Google Scholar]
- 20. Mir SA, Chowdri NA, Parray FQ, Mir PA, Bashir Y, Nafae M. Sphincter‐saving surgeries for rectal cancer: a single center study from Kashmir. South Asian J Cancer 2013; 2: 227–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sylla P, Knol JJ, D'Andrea AP, Perez RO, Atallah SB, Penna M et al.; International taTME Urethral Injury Collaborative. Urethral injury and other urologic injuries during transanal total mesorectal excision: an international collaborative study. Ann Surg 2019; [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 22. Panic N, Leoncini E, de Belvis G, Ricciardi W, Boccia S. Evaluation of the endorsement of the preferred reporting items for systematic reviews and meta‐analysis (PRISMA) statement on the quality of published systematic review and meta‐analyses. PLoS One 2013; 8: e83138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Med 2009; 6: e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M et al The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non‐Randomized Studies in Meta‐Analysis; 2000. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed 4 June 2019].
- 25. Sterne JAC, Savović J, Page MJ, Elbers RG, Blencowe NS, Boutron I et al RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 26. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol 2005; 5: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Becker BJ. Combining significance levels In Handbook of Research Synthesis, Cooper H, Hedges L. (eds). Russell Sage: New York, 1994; 215–230. [Google Scholar]
- 28. Higgins JPT, Green S. Cochrane Handbook for Systematic Reviews of Interventions. John Wiley & Sons: Chichester, 2011. [Google Scholar]
- 29. Bjoern MX, Nielsen S, Perdawood SK. Quality of life after surgery for rectal cancer: a comparison of functional outcomes after transanal and laparoscopic approaches. J Gastrointest Surg 2019; 23: 1623–1630. [DOI] [PubMed] [Google Scholar]
- 30. Veltcamp Helbach M, Koedam TWA, Knol JJ, Velthuis S, Bonjer HJ, Tuynman JB et al Quality of life after rectal cancer surgery: differences between laparoscopic and transanal total mesorectal excision. Surg Endosc 2019; 33: 79–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Turrado‐Rodriguez V, Torroella AT, De Lacy Oliver F, Guarner Piquet P, Otero‐Pineiro A, Martin‐Perez B et al Functional outcomes after tatme: retrospective analysis of quality of life and pelvic function. Dis Colon Rectum 2018; 61: e222. [Google Scholar]
- 32. Rubinkiewicz M, Zarzycki P, Czerwińska A, Wysocki M, Gajewska N, Torbicz G et al A quest for sphincter‐saving surgery in ultralow rectal tumours – a single‐centre cohort study. World J Surg Oncol 2018; 16: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Reali C, Keller DS, Penna M, Hompes R. Low anterior resection syndrome: are we getting to the bottom of the problem with TaTME? Colorectal Dis 2018; 20(Suppl 4): 41. [Google Scholar]
- 34. Mora L, Zarate A, Serra‐Aracil X, Pallisera A, Serra S, Navarro‐Soto S. [Functional impairment and quality of life after rectal cancer surgery.] Cir Cir 2018; 86: 140–147. [DOI] [PubMed] [Google Scholar]
- 35. Foo CC, Tsang J, Lam W, Law WL, Lo O. A comparative study on the sexual and urinary functions after transanal total mesorectal excision and low anterior resection for rectal cancer. Colorectal Dis 2018; 20(Suppl 4): 35.28795776 [Google Scholar]
- 36. Lelong B, Meillat H, Zemmour C, Poizat F, Ewald J, Mege D et al Short‐ and mid‐term outcomes after endoscopic transanal or laparoscopic transabdominal total mesorectal excision for low rectal cancer: a single institutional case–control study. J Am Coll Surg 2017; 224: 917–925. [DOI] [PubMed] [Google Scholar]
- 37. Koedam TW, van Ramshorst GH, Deijen CL, Elfrink AK, Meijerink WJ, Bonjer HJ et al Transanal total mesorectal excision (TaTME) for rectal cancer: effects on patient‐reported quality of life and functional outcome. Tech Coloproctol 2017; 21: 25–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hanke LI, Kauff DW, Lang H, Kneist W. Ano(neo‐)rectal function after transanal total mesorectal excision (TaTME) for primary rectal cancer. Eur Surg Res 2017; 58(Suppl 1): 39. [Google Scholar]
- 39. Elmore U, Vignali A, Cossu A, De Nardi P, Lemma M, Parise P et al Transanal total mesorectal excision for rectal cancer. Preliminary experience. Surg Endosc 2017; 31(Suppl 1): S364. [Google Scholar]
- 40. Pontallier A, Denost Q, Van Geluwe B, Adam JP, Celerier B, Rullier E. Potential sexual function improvement by using transanal mesorectal approach for laparoscopic low rectal cancer excision. Surg Endosc 2016; 30: 4924–4933. [DOI] [PubMed] [Google Scholar]
- 41. Kneist W, Wachter N, Paschold M, Kauff DW, Rink AD, Lang H. Midterm functional results of taTME with neuromapping for low rectal cancer. Tech Coloproctol 2016; 20: 41–49. [DOI] [PubMed] [Google Scholar]
- 42. Tuech JJ, Karoui M, Bridoux V, Manceau G, Kianifard B, Michot FA. A step towards NOTES total mesorectal excision for rectal cancer: endoscopic trans anal proctectomy (ETAP). Surg Endosc 2013; 1: S28. [DOI] [PubMed] [Google Scholar]
- 43. de'Angelis N, Portigliotti L, Azoulay D, Brunetti F. Transanal total mesorectal excision for rectal cancer: a single center experience and systematic review of the literature. Langenbecks Arch Surg 2015; 400: 945–959. [DOI] [PubMed] [Google Scholar]
- 44. Keller DS, Reali C, Spinelli A, Penna M, Di Candido F, Cunningham C et al Patient‐reported functional and quality‐of‐life outcomes after transanal total mesorectal excision. Br J Surg 2019; 106: 364–366. [DOI] [PubMed] [Google Scholar]
- 45. Mosquera C, Licardie E, Bravo D, Madarro C, Rosales J, Romero JA et al Fecal incontinence after surgical treatment of middle–low rectal cancer. Laparoscopic low anterior resection versus tatme. Surg Endosc 2019; 33(Suppl 1): S281. [Google Scholar]
- 46. Leão P, Santos C, Goulart A, Caetano AC, Sousa M, Hogemann G et al TaTME: analysis of the evacuatory outcomes and EUS anal sphincter. Minim Invasive Ther Allied Technol 2019; 28: 332–337. [DOI] [PubMed] [Google Scholar]
- 47. Dou R, Sun W, Luo S, Hou Y, Zhang C, Kang L. [Comparison of postoperative bowel function between patients undergoing transanal and laparoscopic total mesorectal excision.] Zhonghua Wei Chang Wai Ke Za Zhi 2019; 22: 246–254. [PubMed] [Google Scholar]
- 48. Deutekom M, Terra MP, Dobben AC, Dijkgraaf MG, Felt‐Bersma RJ, Stoker J et al Selecting an outcome measure for evaluating treatment in fecal incontinence. Dis Colon Rectum 2005; 48: 2294–2301. [DOI] [PubMed] [Google Scholar]
- 49. Buchs NC, Nicholson GA, Ris F, Mortensen NJ, Hompes R. Transanal total mesorectal excision: a valid option for rectal cancer? World J Gastroenterol 2015; 21: 11 700–11 708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mora López L, Serra Aracil X, Hermoso Bosch J, Rebasa P, Navarro Soto S. Study of anorectal function after transanal endoscopic surgery. Int J Surg 2015; 13: 142–147. [DOI] [PubMed] [Google Scholar]
- 51. D'Ambrosio G, Picchetto A, Campo S, Palma R, Panetta C, De Laurentis F et al Quality of life in patients with loco‐regional rectal cancer after ELRR by TEM versus VLS TME after nChRT: long‐term results. Surg Endosc 2019; 33: 941–948. [DOI] [PubMed] [Google Scholar]
- 52. Zerillo JA, Schouwenburg MG, van Bommel ACM, Stowell C, Lippa J, Bauer D et al.; Colorectal Cancer Working Group of the International Consortium for Health Outcomes Measurement (ICHOM). An international collaborative standardizing a comprehensive patient‐centered outcomes measurement set for colorectal cancer. JAMA Oncol 2017; 3: 686–694. [DOI] [PubMed] [Google Scholar]
- 53. COMET Initiative . Core Outcome Measures in Effectiveness Trials Search term: colorectal cancer. http://www.comet-initiative.org/ [accessed 2 June 2019].
- 54. Koedam TWA, Veltcamp Helbach M, van de Ven PM, Kruyt PM, van Heek NT, Bonjer HJ et al Transanal total mesorectal excision for rectal cancer: evaluation of the learning curve. Tech Coloproctol 2018; 22: 279–287. [DOI] [PubMed] [Google Scholar]
- 55. ClinicalTrials.gov . Rectal Surgery Evaluation Trial (RESET) https://clinicaltrials.gov/show/NCT03574493 [accessed 2 June 2019].
- 56. ClinicalTrials.gov . Trans‐anal Versus Laparoscopic TME for Mid and Low Rectal Cancer (MansTaTME) https://clinicaltrials.gov/show/NCT03242187 [accessed 2 June 2019].
- 57. ClinicalTrials.gov . Laparoscopic Assisted Transanal Resection of Rectal Cancer With Total Mesorectal Excision https://clinicaltrials.gov/show/NCT03171298 [accessed 2 June 2019].
- 58. ClinicalTrials.gov . Transanal Minimal Invasive Surgery Versus Conventional Laparoscopic Surgery for Rectal Cancer https://clinicaltrials.gov/show/NCT02252250 [accessed 2 June 2019].
- 59. Serra‐Aracil X, Mora L, Pericay C, Delgado S, Targarona E, Vallribera F et al Phase III multicenter, prospective, controlled, randomized trial to evaluate the safety and efficacy of treatment of rectal cancer T2–T3S (superficial) N0, M0 with preoperative chemoradiotherapy and transanal endoscopic microsurgery versus total mesorectal excision. Preliminary results. Dis Colon Rectum 2015; 58: e323–e324. [Google Scholar]
- 60. ClinicalTrials.gov . Laparoscopic Assisted Transanal Total Mesorectal Excision for Rectal Cancer in Low Site (LATERAL‐01) https://clinicaltrials.gov/show/NCT03253302 [accessed 2 June 2019].
- 61. ClinicalTrials.gov . Transanal Total Mesorectal Excision for Rectal Cancer on Anal Physiology + Fecal Incontinence https://clinicaltrials.gov/ct2/show/NCT03283540 [accessed 2 June 2019].
- 62. ClinicalTrials.gov . Multicenter Phase II Study of Transanal TME (taTME) https://clinicaltrials.gov/ct2/show/NCT03144765 [accessed 2 June 2019].
- 63. ClinicalTrials.gov . Physical Exercise for Colorectal Cancer Patients After Transanal Total Mesorectal Excision https://clinicaltrials.gov/ct2/show/NCT03120104 [accessed 2 June 2019].
- 64. Luo S, Wang J, Kang L, Chen W. Transanal versus laparoscopic total mesorectal excision for low rectal cancer: a multicenter randomized phase III clinical trial (TaLaR trial) protocol. J Clin Oncol 2017; 35(Suppl 1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Lacy AM, Espin E, Biondo S, Fernandez‐Hevia M, Tasende M, Jimenez M et al Transanal total mesorectal excision versus laparoscopic total mesorectal excision: a randomized study comparing 30‐day postoperative morbidity. Colorectal Dis 2014; 3: 106. [Google Scholar]
- 66. Deijen CL, Velthuis S, Tsai A, Mavroveli S, de Lange‐de Klerk ES, Sietses C et al COLOR III: a multicentre randomised clinical trial comparing transanal TME versus laparoscopic TME for mid and low rectal cancer. Surg Endosc 2016; 30: 3210–3215. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1 Search strategy per database (Pubmed, EMBASE, Cochrane Library and the WHO and http://clinicaltrial.gov trial registers).


