Abstract
Background
The recent emphasis on value‐based health care (VBHC) is thought to provide new opportunities for shared decision‐making (SDM) in the Netherlands, especially when using patient‐reported outcome measures (PROMs) in routine medical encounters. It is still largely unclear about how PROMs could be linked to SDM and what we expect from clinicians in this respect.
Aim
To describe approaches and lessons learned in the fields of SDM and VBHC implementation that converge in using PROMs in medical encounters.
Approach
Based on input from three Dutch forerunner case examples and available evidence about SDM and VBHC, we describe barriers and facilitators regarding the use of PROMs and SDM in the medical encounter. Barriers and facilitators were structured according to a conversational model that included monitoring and managing, team talk, option talk, choice talk, and decision talk. Key lessons learned and recommendations were synthesized.
Results
The use of individual, N = 1 PROMs scores in the medical encounter has been largely achieved in the forerunner projects. Conversation on monitoring and managing is relatively well implemented, and option talk to some extent, unlike team talk, and decision talk. Aggregated PROMs information describing outcomes of treatment options seemed to be scarcely used. Experienced barriers largely corresponded to what is known from the literature, eg, perceived lack of time and lack of tools summarizing the options. Some concerns were identified about increasing health care consumption as a result of using PROMs and SDM in the medical encounter.
Conclusion
Successful implementation of SDM within VBHC initiatives may not be self‐evident, even though individual, N = 1 PROMs scores are being used in the medical encounter. Education and staff resources on meso and macro levels may facilitate the more time‐consuming SDM aspects. It seems fruitful to especially target team talk and choice talk in redesigning clinical pathways.
Keywords: patient‐reported outcome measures, routine outcome monitoring, shared decision‐making, value‐based health care
1. INTRODUCTION
Shared decision‐making (SDM) has become the preferred conversation model between clinicians and patients in health care, explicitly involving patients in decision‐making about diagnostic and treatment options.1, 2, 3, 4, 5 In SDM, patients and clinicians work together to select tests and treatments, using the best available evidence with incorporation of patients' values and preferences.3, 6 This requires cooperation and activation of both clinicians and patients. Despite being so heavily embraced by scientific scholars and policymakers, formal implementation of SDM in practice has proven to be challenging.3, 7, 8, 9, 10 One key barrier seems to be the perceived time needed to practice SDM,11, 12, 13 addressing both risk communication and clarification of patients' values.12, 14, 15 Although clinicians generally support SDM, they are also often hesitant to practice it and often revert to a more authoritarian conversational model.10, 16, 17, 18
In the Netherlands, the recent emphasis on value‐based health care (VBHC) in health policy is thought to provide new opportunities for SDM, especially by using information based on patient‐reported outcome measures (PROMs) in routine medical encounters. VBHC is a health care delivery model organized around patients' needs,19 aimed at improving outcomes that matter to patients/populations while optimizing resource utilization.20, 21, 22 PROMs are standardized questionnaires for patients to measure how they experience their health or quality of life, such as the SF‐36 and EQ‐5D.23, 24 In mental health care, this same type of questionnaires is used in a process called ROM (routine outcome monitoring), typically throughout the treatment process to monitor patients’ condition in line with the symptoms being treated.25, 26 Three types of value have been distinguished in VBHC22: (a) personal value—the delivery of services informed by what matters to the individual patient via SDM; (b) technical value—determined by how well resources are used within services for each purpose, favouring the right intervention for the right patient at the right moment; and (c) allocative value—determined by how assets are allocated to services for different purposes, thus maximizing health benefits at a population level. For a patient with knee arthrosis, the choice between being able to kneel in the garden vs having a knee prosthesis and less pain may be a matter of personal value; performing a technically optimal surgery and discharging the patient as soon as he can take care of himself at home may be a matter of technical value; identifying the patients that gain most quality‐adjusted life years out of the many with arthrosis, if only a limited number of surgeries can be performed, may be a matter of allocative value.
VBHC could provide an additional route to promote SDM implementation,27, 28 especially when and where teams of professionals redesign health care pathways bottom‐up.29 Convincing evidence30, 31, 32, 33, 34, 35, 36 shows that SDM delivers triple value as outlined above and thus fits the imperative of VBHC. SDM is known to increase patients' awareness and understanding of available options and the quality of decision‐making,30, 31 which help to positively impact patient outcomes and health care resource utilization.32, 33, 34, 35, 36 In the Netherlands, formal institutions such as the Dutch Healthcare Institute and Netherlands Federation of University Medical Centres (NFU) mention SDM as a key element of VBHC and outcomes‐oriented health care.8, 37, 38
There is, however, little clarity about how PROMs can be linked to SDM in the medical encounter and what we should expect from clinicians in this respect. Individual, N = 1, PROMs scores could be used to prepare patients and clinicians for patient activation in general, as well as for value clarification in particular.3, 39 Aggregated PROMs scores could be used to inform patients more completely about benefits and harms of options. All in all, for treatment choices being made, it is important to have insight not only into clinical outcomes derived from clinical registries (eg, survival rates) but also into quality of life as reported by patients.40 In theory, use of PROMs in the medical encounter could thus contribute to the delivery of value.32, 41 Figure 1 presents a conceptual model integrating these suggested elements. Figure 2 shows examples of individual, N = 1, and aggregated PROMs scores.
Figure 1.
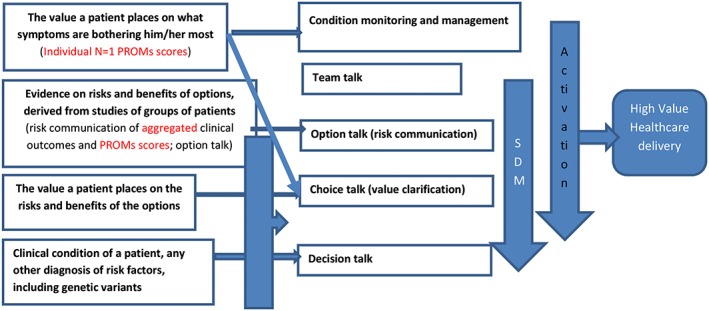
Suggested elements of SDM, use of PROMs and VBHC
Figure 2.
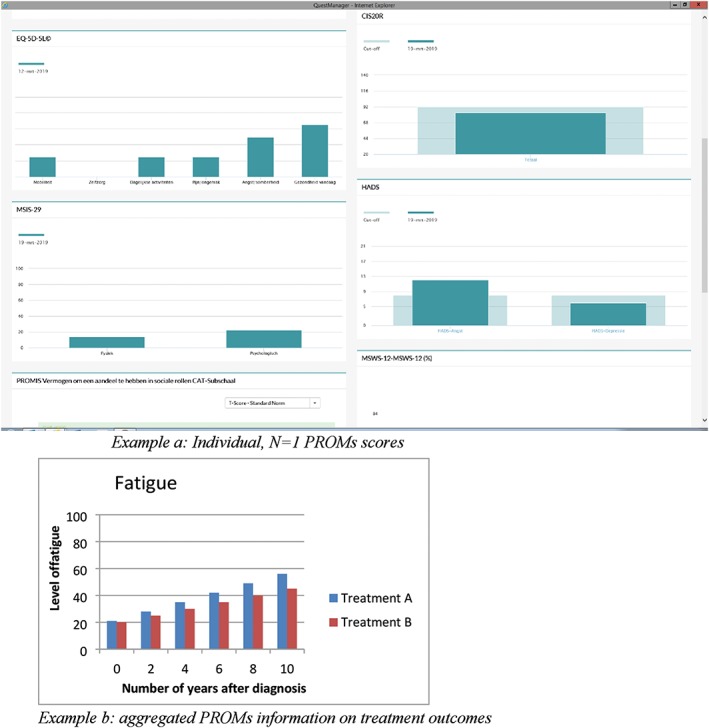
Example of individual, N = 1 PROMs scores (derived from the MS clinic in Amsterdam UMC; example a) and aggregated PROMs information on treatment outcomes (hypothetical; example b)
However, the use of PROMs and SDM both regularly meet with hesitance and scepticism from clinicians.7, 29, 40, 42 Furthermore, VBHC primarily focuses on optimizing outcomes, while SDM mainly addresses the process of patient‐clinician interaction (although the link with outcomes is increasingly made43). It therefore seems worthwhile to synthesize lessons learned so far in this emerging arena. This paper aims to describe approaches and lessons learned in the fields of SDM and VBHC implementation that converge in using PROMs in medical encounters. We did this by (a) summarizing available evidence about SDM and VBHC (including barriers and facilitators); (b) descriptive evidence collected from leading representatives at three forerunner projects (multiple sclerosis [MS] outpatient clinic; lung cancer care unit; outpatient specialist mental health care); and (c) synthesis on key lessons learned and recommendations to successfully implement PROMs and SDM in the medical encounter at the micro, meso, and macro levels.
2. SDM IMPLEMENTATION
SDM is usually implemented using two approaches, namely, training of professionals and development and dissemination of decision aids or option grids.3, 29 In SDM training, typically, a conversational model is offered to professionals, breaking down the conversation into different steps. This paper used the four steps as described by Stiggelbout et al.,3 in turn influenced by the work of Elwyn and colleagues44, 45: team talk (indicating that the patient has a choice and offering assistance in this choice as a professional); option talk (communicating about treatment options and their risks and benefits); choice talk (inviting the patient to express his or her preferences and underlying values and weighing options against those preferences [note that this is sometimes also labelled as “preference talk”]); and decision talk (involving [the preferences of] the patient in the eventual shared treatment decision). In several SDM models, it is also emphasized that patients' understanding of their condition and its management should be gauged in a first step.46, 47 We have therefore included this as well, called “management and monitoring.”
Many hurdles for SDM implementation have been described.11, 29, 48 Legaré and Witteman48 concluded that the three most common barriers among clinicians are “perceived time constraints, perceptions that SDM cannot be applied because of patients' characteristics, and the nature of the clinical situation.” It has been demonstrated that clinicians themselves think they are already involving patients in decisions,49 while observations in practice suggest they do not.9 There is no robust evidence that SDM costs more time than usual care.30, 31, 48 Studies consistently show that patients themselves opt for the SDM model.50, 51, 52, 53 However, less is clear about how SDM relates to the nature of the clinical situation.5, 8, 29 Besides organizational‐ and system‐level barriers, such as lack of fit to existing workflows of professionals7, 11 and lack of clinical guidelines emphasizing equipoise of options,29 there may be barriers related to each phase of the conversational model, hindering SDM at the micro level of the medical encounter.
As said, one of the most apparent barriers for professionals is the experienced lack of time. While it has been argued that “this is the most frequently cited barrier for any change in clinical practice”,48 it is not refuted that time during the medical encounter with patients is usually limited.13 It may be expected that clinicians allocate their sparse time to option talk instead of team or choice talk,15, 45 because information provision about the chosen option is legally required in light of informed consent procedures.54 However, Kunneman and colleagues55 showed the importance of team talk; if no choice awareness was fostered, the subsequent SDM steps were poorly executed. Choice talk, which is not legally required, is a task that most doctors will be especially unfamiliar with, probably due to lack of training. Box 1 shows how clinical specialties may vary in addressing values related to patient preferences, prior to introducing SDM or VBHC.
Currently, it may remain unclear for clinicians what type of values need to be discussed in SDM. Asking what treatment patients prefer is different from asking about broad motivation goals in life,56, 57 and discussion of motivations obviously requires time, especially with patients with low health literacy.15 However, if patient values remain unexplored and no assistance is provided in preference construction, patients will likely more often agree to treatment they do not actually prefer, leading to silent misdiagnoses and overtreatment.2
3. VBHC IMPLEMENTATION
VBHC implementation can occur top‐down (ie, based on external incentives) and bottom‐up (ie, based on motivations of professionals/teams/departments). In practice, often mixtures are observed. Figure 3 describes common components of VBHC implementation.
Figure 3.

Key components of VBHC
3.1. Defining and measuring outcomes that matter to patients
One important step is usually to define and measure outcomes that matter to patients,27, 58, 59 which make outcomes, prioritized and experienced by patients transparent. These are used to ensure patients' needs are met and, in turn, to improve health care delivery.60 It has been argued that outcomes reflecting patients “priorities were traditionally infrequently measured in clinical practice, compared to clinical outcomes such as survival rates”.61, 62 Another problem was that definitions used often varied between institutions or countries, hindering comparisons. Therefore, the development and collection of standard outcome sets have gained ground.58, 63, 64, 65, 66, 67
3.2. Organization of care around the patient or patient group
Organization of care around the patient or patient group is used to bring together the full range of providers and professionals around the patient and his/her needs.68 Patient journeys (ie, maps of the journey from the patient's perspective) are often being made transparent, to identify problems experienced by patients and improve delivery of services accordingly.69 Another key element is that each professional involved should add optimal value by performing those tasks best suiting his/her expertise at the optimal moment. Therefore, interdisciplinary coordination of care combined with effective communication is essential, especially in shifts and referral situations.70, 71, 72 Increasingly, patients are empowered to monitor and treat their own disease, implying that they need to be included in care coordination and communication. When patient information is translated from hospital settings to primary or community care, an essential question is how to optimally share this information to guarantee high value, while also optimally using patients' active involvement.
3.3. Use of PROMS in medical encounters with patients
Currently, the main rationale for collecting PROMs is to enable evaluation and comparison at an aggregate level. Yet it is increasingly recognized that PROMS should also be discussed with patients in routine medical encounters.42, 64, 73 Such use has been theorized to (a) increase patients' motivation to complete PROMs60; (b) improve management and monitoring of patients' problems25, 74, 75, 76; (c) improve doctor‐patient communication25, 77; and (d) improve SDM and informed patient decisions.. 39, 40, 42, 74 PROMs are typically made available in the medical encounter through electronic health records and dashboards,78, 79 displaying individual, N = 1, PROMs scores of patients over time. There is not much experience yet on the merits of using PROMs in medical encounters, but the mere collection of PROMs is known to increase awareness of patients' concerns80, 81 and to enhance patient‐provider communication.25, 81 Use of PROMs in medical encounters is a task that most clinicians are unfamiliar with,42, 82 and clinicians may perceive that it will take time.42, 74 It may also be problematic that not all systems allow clinicians to actively monitor and discuss symptoms or to refer patients to other facilities, ie, in stepped care models.83, 84 There may also be concerns about overconsumption, as a result of more active monitoring, although there is evidence that activating patients is generally associated with lower costs, rather than higher costs.41
3.4. Evaluation and comparison
The ultimate aim of PROMs collection is to learn from outcomes at the level of the individual patient and patient groups to improve value of health care delivery.25, 60, 85 These data can help professionals, teams, or departments to evaluate whether their care met the needs of their patients and to compare performance to other groups within and outside their institution.86 When teams start to work in a different way, they can monitor the patients' experiences over time.
4. FORERUNNER CASE EXAMPLES FROM THE NETHERLANDS
Amsterdam UMC at the VUmc campus in the Netherlands has made commitment to VBHC, including the triple value framework, as a model for health care delivery. The rationale was to manage health care based on outcomes that matter to patients most and, by doing so, to deliver better patient and population outcomes while optimizing resource utilization. This meant a shift from focusing just on the technical value of interventions to also including personal and allocative value to ensure that care being delivered met the needs of the patients and populations the Amsterdam UMC is accountable to. Different pilot projects have been conducted, eg, at the multiple sclerosis (MS) clinic, the gender clinic, the nephrology department, and the lung cancer care unit. Within each pilot, the standard approach was to establish a multi‐stakeholder group, including patients, to design the VBHC concept including a core outcomes set, care standard, and patient journey; develop the VBHC pathway, including collection of PROMs and clinical outcomes, ICT infrastructure with PROMs dashboards; training professionals in SDM and team collaboration; implement outcomes measurement and try to learn and improve based on insights garnered; and create better insight into costs. For this paper, input from the MS clinic and lung cancer care unit was collected.
In Dutch mental health care, routine outcome monitoring (ROM) was broadly implemented in a SDM context for patients with psychiatric disorders such as depression, anxiety, bipolar, psychotic, and personality disorders. The standard approach was to implement ROM tailored to the patient group in routine clinical practice; develop a conversation model in SDM using ROM as a personalized source of information (Table 1, 39); and support clinicians with training and booster sessions to apply the SDM model using ROM as a source of information.
Table 1.
SDM‐ROM model used in Dutch mental health care: shared decision‐making using routine outcome monitoring as an information source (Steps 1 to 5).
| 1. Introduction |
✓ Refer to expectations about shared process. ✓ Discuss which role the patient desires in decision‐ making. ✓ Connect with patient's wishes and goals. “What does he/she want to achieve in treatment?'’ ✓ Explain about ROM as an information source. |
| 2. Give meaning to ROM |
✓ Discuss ROM outcomes ✓ Steps: Identify, Understand, Appreciate, Act. |
| 3. Explore options | ✓ Discuss options, advantages and disadvantages, in a neutral manner. |
| 4. Weigh options | ✓ Weigh advantages and disadvantages: “What's important for you?'’ |
| 5. Shared Decision |
✓ Can a choice be made? ✓ Together, select most appropriate option. ✓ Make follow‐up appointments. |
Table 2 describes the approaches used to implement PROMs and SDM in the medical encounter in three Dutch forerunner case examples, two from Amsterdam UMC and one in Dutch mental health care. Experienced and/or foreseen barriers, facilitators, and blind spots are also described. This information was collected by asking the clinicians who lead the pilots in Amsterdam UMC (B.d.J. and A.B.) and the leading representative for implementation in mental health care (M.M.) to provide descriptive evidence.
Table 2.
SDM and use of PROMs/ROM in routine medical encounters: experiences of Amsterdam UMC at the VUMC campus and Dutch specialist mental health care
| General characteristics |
Pilot 1: MS
Number of patients included thus far: 25
Number of centers involved: 1 (Amsterdam MC center)
Number of different disciplines involved: 6 (neurologists, rehabilitation clinicians, MS nurses, psychologists, physiotherapists, and welfare workers).
Period: one year |
Pilot 2: Lung cancer
No patients or institutions included yet. The aim is to include 3 institutions.
Number of different disciplines involved: needs to be defined. In any case, pulmonologists, thoracic surgeons, radiotherapists, psychologists, and nurses involved.
Period: one year |
Pilot 3: Mental health care Number of patients included thus far: unknown
Number of centers involved: 21
Number of different disciplines involved: 3 (Clinicians, psychologists, and nurses).
Period: one year |
| General approach |
Approach: MS patients, together with health professionals, created a list of 25 outcome parameters, based on validated questionnaires, related to symptoms associated with MS.
Before the annual check‐up at the MS center, the patient receives online questionnaires resulting in a digital PROMs list accessible for patient and health care providers after filling out. At the end of the questionnaires, the patient can select topics he/she wants to focus on in the annual check‐up, which informs the composition of health care providers that will join the interdisciplinary outpatient evaluation during the annual check‐up. |
Approach: LC patients, together with health professionals, create a list of outcome, related clinical outcomes, PROMS and PREMS.
PROMS will be measured digitally every three 3 months as part of a clinical study in all stages of lung cancer. In this study, patients will also monitor 12 common alarming symptoms (e.g., fever, coughing up blood) on a weekly basis. If feasible, this will be extended to all patients with LC. In addition, a dashboard will be constructed with information on all outcome parameters that can be used for internal improvement of care, but also for discussion with patients in routine medical encounters. |
Approach: Outcome parameters measured by ROM instruments and frequency of measuring was tailored to the patient group and determined by multidisciplinary teams of clinicians and patient representatives Outcome parameters are: symptoms (i.e., depression, anxiety, psychotic complaints, and personality traits), quality of life, social, and personal recovery (participation in society and live the life patients choose). The frequency of measuring: ‐ In short ‐term treatment: during intake, treatment (at least every three 3 months) and at the end of treatment; ‐ In long ‐term treatment: during intake, treatment (at least once a year) and at the end of treatment. ROM is planned before treatment evaluation. Patients fill out the ROM‐questionnaires digitally at home or at the mental health care organization. Clinicians are provided with a SDM‐ROM conversation model (see Table 1). |
|
Monitoring and management: Discussing individual, N = 1, PROMs scores with patient before, during, and after care delivery including trends over time |
Approach: During the interdisciplinary outpatient clinic, the PROMs scores (N = 1) are visualized on a dashboard. The idea is that in addition to common physical symptoms (eg, deterioration in walking and seeing), less visible symptoms (eg, pain, fatigue, and sexual problems), and/or symptoms as prioritized by patients, are being monitored and managed. |
Approach: During routine medical encounters, the PROMs scores (N = 1) and clinical outcomes are visualized on a dashboard. Both the weekly registered 12 symptoms and the PROMS monitored every 3 months can be used in discussions about management and treatment. Both types of information are thought to provide input for decisions about (continuation of) treatment and for clinical evaluations of potential cancer recurrence. In general, PROMs will be used as input to discuss with patients what they want to achieve with treatment. |
Approach: ROM results (N = 1) are visualized on a dashboard. Patients get insight into their own ROM results by a patient portal or by the clinician (who can make a print out for the patient). During treatment encounters, patients are asked what they want to achieve in treatment (see step 1 in Table 1), and patients and clinicians give meaning to ROM results together and use ROM as an input to evaluation of current treatment (see step 2 in Table 1). |
|
Barriers: Accessible and good working technology platform |
Barriers: The time needed for nurses to send questionnaires at the right time
Accessible and good working technology platform |
Barriers: Currently patients are dependent on the (attitude of the) clinician for the feedback of ROM‐results, because implementation of patient portals for viewing results independently is in an initial phase. |
|
|
Facilitators: Support of the management team of the hospital
Advanced visualization of the bars and graphs of the PROMs scores (N = 1) |
Facilitators: Support of the management team of the hospital |
Facilitators: Development and implementation of ROM together with clinicians and patient representatives. Support of management and secretary (for logistics and planning). |
|
|
Blind spots: Professionals, especially neurologists, tend to focus on physical limitations and less on non‐visible complaints, like cognition, fatigue, and pain. The question is to what extent the other symptoms are sufficiently addressed in the patient's visit to the Neurology outpatient clinic.
There is no specific approach on how to support patients in discussing and interpreting PROMs results with their clinicians |
Blind spots: Professionals may focus on the 12 symptoms for rapid diagnostics and not so much on the use of PROMs for SDM.
There is no specific approach on how to support patients in discussing and interpreting PROMs results with their clinicians |
Blind spots: There is no specific approach on how to support patients in discussing and interpreting ROM results with their clinicians, and how they can use these results in SDM. |
|
| SDM step 1: Team talk (Indicating that the patient has a choice and offering assistance in this choice as a professional) |
Approach: Patients are invited to select topics he/she wants to focus on in the annual check. There is no specific strategy yet to subsequently address patient participation in the decisions to be made about treatment and management. |
Approach: For stage I non‐small cell LC, patients are routinely informed about the equipoise of options (surgery versus stereotactic ablative radiotherapy [(SABR)]) by the pulmonologist, and invited to participate in the treatment decision.
For advanced stages of LC, there is no equipoise from a medical perspective (since treatment is associated with longer survival than no‐treatment). However, the option of no treatment is routinely mentioned and especially patients with a relatively bad condition are offered assistance in making a decision. |
Approach: Clinicians invite patients to share their expectations about a shared process and the role they would fulfill. ROM outcomes are referred to as one of the information sources (see Step 1 in Table 1). |
| Barriers: Lack of time. |
Barriers: Stage I non‐small cell LC: Dependence of the pulmonologist to recognize equipoise of options and to also address this issue in multidisciplinary meeting.
Advanced stages of LC: Rapid progress in the scientific literature about the outcomes of treatment, difficult for professionals to keep up and judge whether there is equipoise and how to include patients in the decision.
Lack of time.
Lack of training of professionals in SDM. |
Barriers: Insufficient awareness among clinicians of the importance of team talk (step 1 in Table 1). |
|
| Facilitators: The basic concept of a routine encounter with the complete team with different specialties including the patient/care giver. Advanced visualization of the bars and graphs of the PROMs scores (N = 1). | Facilitators: Advanced visualization of the PROMS scores in dashboards, which will give input about patients' complaints and concerns. As such, this information could shift the discussion from a focus on survival to a focus on quality of life, and thus on SDM. | Facilitators: Team training, booster sessions in the conversation model SDM using ROM. | |
|
Blind spots: The facility to work with a whole team of caregivers instead of one individual may be more expensive than thought.
It may be that using PROMs and SDM may lead to additional diagnostics and therapies being chosen.
It may be that most professionals tend to focus on the management of the discussed symptoms, and less so on the discussion about patient participation in the decisions to be made. |
Blind spots: It may be that clinicians will continue to aim at treatment and skip the stage of team talk, because from a medical perspective, there is no equipoise of options (for advanced stages of LC). However, for patients this is likely to be different. A question is how patients will be invited to express their concerns based on the PROMs scores. |
Blind spots: Insufficient awareness among patients that they can participate in decision‐making. Clinicians do not pay enough attention to this first step. Too little personalized support for patients to participate in decision ‐making. |
|
| SDM step 2: Option talk (Communicating about treatment options and their risks and benefits (i.e., risk communication) e.g,. using option grids or PDAs) |
Approach: Based on the individual, N = 1, PROMs scores combined with additional discussion during interdisciplinary outpatient clinic evaluation, the different treatment/management options are discussed with the team and patient/care giver. The non‐treatment option is also routinely discussed. There is currently a lack of formal decision aids or option grids, but when these will become available (with the release of the new clinical guideline), the idea is that they will be adopted in this stage of option talk. |
Approach: For stage I non‐small cell LC, patients are routinely informed about the options and their main benefits and risks (surgery versus stereotactic ablative radiotherapy [(SABR)]). Patients are given the web link to a decision aid that provides this info.
For advanced stages of LC, there is no standard approach to communicate about treatment versus no‐treatment and the associated benefits and harms. Professionals base their advice on the evidence in the medical literature.
Options outside the clinic are routinely discussed based on PROMs scores, such a visit to a medical psychologist or a dietician. |
Approach: Based on the individual, N = 1, ROM results and other sources of information (http://www.ggzstandaarden.nl; http://www.thuisarts.nl; local clinical pathways), options with their risks and benefits are discussed with the patient, in a neutral manner. (See step 3 Explore options [(conversation model SDM using ROM, Table 1)]). |
|
Barriers: Insufficient knowledge among professionals and patients about all potential treatment/management options, also in primary care.
Lack of decision aids or option grids that facilitate comparison of different options, time needed to wait for a new release of clinical guidelines.
No access yet to aggregated PROMs scores, so information about benefits and harms of treatment options is incomplete. |
Barriers: Lack of decision aids or option grids that facilitate comparison of treatment versus no‐treatment for advanced stages of LC.
No access yet to aggregated PROMs scores for advanced LC stages, so information about benefits and harms of treatment options is incomplete.
Lack of training of professionals in SDM and the importance of option talk. |
Barriers: Teams have insufficient overview about all options inside and outside the mental health care organization.
Lack of decision aids or option grids that facilitate comparison of different options. Development of prediction models are is in an initial phase; as a result, limited knowledge about benefits, harms, and its probabilities.
When new evidence is available: time needed to refer to it in quality standards and to implement these insights in clinical practice. |
|
|
Facilitators: Training of professionals in SDM.
Training of professionals in how to collaborate as a team. |
Facilitators: Stage I non‐small cell LC: availability of a decision aid for patients.
In oncology, professionals have a long tradition of referral to other healthcare providers such as psychologists or dieticians. There is, thus, a good overview of options outside the medical field. |
Facilitators: Training, booster sessions and supervision of clinicians in SDM.
Availability of quality standards, which describe all treatment options in mental health care from the perspective of patients, are is published nationally. (http://www.ggzstandaarden.nl) Patients could prepare by reading the patient version (http://www.thuisarts.nl). Clinicians could use the standards to get an overview of treatment options. |
|
|
Blind spots: There is no (comparative) information on costs of different treatment/management options, which is often important for patients in decision‐making. |
Blind spots: There is incomplete information about benefits and harms of treatment options, especially experienced quality of life (aggregated PROMs scores).
Limited insight among clinicians into the importance and difficulty of exploring treatment options in a neutral way. |
Blind spots: Limited insight among clinicians into the importance and difficulty of exploring treatment options in a neutral way. |
|
| SDM step 3: Choice talk (Inviting the patient to express his or her preferences and underlying values (value clarification), and discussing the trade‐off between those values, e.g., using value clarification methods or PDAs) |
Approach: During the interdisciplinary outpatient clinic, the patient and his/her care givers are routinely invited to express their preferences and values between the different treatment options. The idea is that the individual, N = 1, PROMs scores and their answers in the questionnaire will help patients in expressing those preferences and values, and this in choice talk. |
Approach: During medical encounters before treatment (or no‐treatment) is chosen, patients are routinely asked the questions that they find important in their current life and what they aim for by treatment. The idea is that the individual, N = 1, PROMs scores will help patients in answering those questions. |
Approach: During treatment encounter, the clinician invites the patient to weigh the risks and benefits of options, based on the starting question “What is important for you?' The idea is that the individual, N = 1, ROM results will help patients in choice talk by answering this question. (See step 4 Weigh options ([conversation model SDM using ROM, Table 1)]). |
|
Barriers: Lack of decision aids that facilitate more thorough value clarification and preference construction.
Insufficient training of professionals in eliciting/diagnosing patients' values and preferences. |
Barriers: Lack of decision aids that facilitate more thorough value clarification and preference construction.
Insufficient training of professionals in eliciting/diagnosing patients' values and preferences. |
Barriers: Lack of invitation by professionals and lack of (personalized) tools (i.e., decision aid material) that can support patients in participating in choice talk more actively. |
|
| Facilitators: Training in SDM and developing communication tools |
Facilitators: It is expected that the individual, N = 1, PROMs scores will prepare patients for choice talk. |
Facilitators: Training and booster sessions in SDM for professionals.
The availability of individual, N = 1, ROM results, visualized in graphics, for patients, that could prepare them for choice talk. |
|
| Blind spots: SDM training often tends to focus on option talk and less so on choice talk. It seems easy to ask patients for their preferences but real assistance in preference construction is lacking. | Blind spots: It seems easy to ask patients for their preferences but real assistance in preference construction is lacking. It may be that patients' preferences are heavily influenced by their doctors' advice. |
Blind spots: The individual, N = 1, ROM results can be more actively used as a source of information to prepare for choice talk (see step 2 in Table 1).
Lack of decision aids and if a decision aid is available for a specific psychiatric disorder, value clarification is often not yet embedded. |
|
| SDM step 4: Decision talk (Involving the (preferences of) the patient in the treatment decision) | Approach: At the end of the annual check‐up, the team including the patient/care giver decide together which additional diagnostics and/or therapeutic options will be chosen |
Approach: For stage I non‐small cell LC, the pulmonologist, together with the patient, decides about treatment after the patient has used the decision aid and indicated his/her treatment preference and underlying reasons.
For advanced stages of LC, the pulmonologist typically gives a treatment recommendation (treatment/no treatment), after being informed about the context and treatment goals of the patient. |
Approach: The clinician and patient discuss whether a choice can be made, select the most appropriate option and make follow‐up appointments. (See step 5 Shared decision [(conversation model SDM using ROM, Table 1)]). |
| Barriers: All barriers described in the previous steps. |
Barriers: Lack of time to truly have a good conversation about what preferences of the patient can mean for the decision.
For advances stages of LC: lack of positive attitude towards SDM among medical professionals, due to a lack of experienced equipoise.
All barriers described in the previous steps. |
Barriers: Clinicians' expectations that a good conversation about which option fits best, takes much time, especially when relatives of the patient are involved. |
|
| Facilitators: Sufficient training in SDM and in team collaboration, and all other facilitators described in the previous steps. | Facilitators: The availability of PROMs scores that can better prepare medical professionals and patients for a discussion about quality of life (harms of treatment), and for SDM. All facilitators described in the previous steps. |
Facilitators: Training of professionals in SDM. All facilitators described in the previous steps. |
|
| Blind spots: Costs. It is uncertain whether and to what extent this new annual check‐up in which patients are explicitly involved in treatment decisions will create additional therapeutic sessions and whether this will be less or more expensive in the long run. | Blind spots: Training in SDM and how to deal with the question of equipoise; clarifying the decisions that are suitable for SDM. |
Blind spots: How to involve relatives of patients in decision‐making in short term treatment (in long term treatment involvement of relatives is already more common).
Does the patient need more time to make a decision?
Making follow up appointments. |
4.1. Monitoring and management
In all three case examples, the standard approach is to start the medical encounter with patients by using visualized individual, N = 1, PROMs scores in dashboards. Such dashboards can be made available through and before the consultation, depending on used technology. In the MS pilot, patients are explicitly asked before the consultation what topics they want to focus on. Common questions asked in in the medical encounter include what symptoms or complaints are bothering patients most and what they want to achieve in potential treatment. Support of management teams and adequate ICT structures are mentioned as key facilitators of using individual, N = 1, PROMs for monitoring and management. Experienced barriers relate to technology problems and lack of time of supporting personnel to get PROMs results in the consultation on time. A blind spot mentioned is a lack of knowledge on how to support patients in correct interpretation of PROMs scores as well as in using them in the conversation. Because it was acknowledged that professionals may naturally focus on clinical and physical aspects/complaints, an important question is how to guarantee that PROMs information is actually being addressed.
4.2. Team talk
Compared with this rather clear management and monitoring, the other steps of the conversational model, starting with team talk, are less well organized. Symptoms bothering the patient are thought to shift the discussion towards quality of life considerations into decisions about management and treatment. In some cases (eg, MS, mental health care), it is also explicitly expected that dashboards will increase choice awareness among clinicians and patients. However, there is no specific approach yet regarding how to actually engage patients in such team talk. In some areas (eg, MS and advanced LC stages), a current barrier/blind spot may be that clinicians continue to prioritize clinical outcomes (eg, survival) above PROMs and give treatment advice accordingly. Especially when there is no clear equipoise of options concerning survival, team talk seems to be largely skipped. So although monitoring and management based on PROMs puts the patient's perspective more central, the conversational model for decision‐making still often remains authoritarian.
4.3. Option talk
Apparent from the projects was that clinicians feel a need to share existing options with patients, both concerning health care providers/facilities (eg, MS and LC) and management and treatment options (eg, MS, LC, mental health care). Individual, N = 1, PROMs scores are thought to give direction for the options to be discussed. For example, if lung cancer patients have a bad condition and many complaints, the need to discuss the nontreatment option seems harder for clinicians. In the MS clinic, treatment and provider options are now more routinely discussed by the interdisciplinary team before consultations. Although option talk does seem to take place, the three projects also described lack of overview of all existing options for the specific patient groups, for example, regarding transmural care, rehabilitation, and primary care. This corresponds to previous findings in the Dutch context29 that clinicians perceive a lack of adequate (patient) information on available options.
Despite options being discussed with patients, less emphasis is placed on informing patients about the exact risks and benefits of those options. This seems to be largely due to a perceived lack of time, a lack of decision aids, or insufficient awareness of those tools that could help in risk communication. Alternatively, it may be that clinicians do know about available decision aids, but think they are not suitable for informing their individual patients, due to a lack of personalized estimates.5, 87, 88 In this respect, aggregated PROMs information on the level of treatment options is not routinely available and/or used in the three projects. Facilitators for option talk are training in SDM, training in team collaboration (contributing to better knowledge/overview of options), and availability of clinical guidelines or quality standards describing all treatment options and the importance of SDM.
4.4. Choice talk
Patients are routinely asked about what they consider important, but there are no specific approaches yet to preference construction based on trade‐offs between patients' values. Experienced facilitators focus on the availability of individual, N = 1, PROMs scores, that could prepare both patients and professionals for discussion of patient values. Barriers are that SDM training did not include sufficient time for the topic of value clarification and a lack of decision aids that invite patients earlier to bring forward what they care about. More generally, if professionals do not feel the need to practice SDM, because there is no clear equipoise of options or simply because they are unfamiliar with SDM, patients' preferences are described to be heavily influenced by clinicians' advice in the projects.
4.5. Decision talk
When there is equipoise of options, such as for early‐stage lung cancer, decision talk is often explicitly initiated, mostly after patients have completed a decision aid. When there is less equipoise, or when SDM is not fully embraced among teams, it often remains unclear to what extent decision talk is practiced. In the MS example, where patients are invited to an interdisciplinary consultation involving multiple professionals, more awareness now exists among professionals that there are decisions to be made with the patient. At the end of the annual check‐up, the MS team explicitly involves the patient in those decisions. Experienced barriers in the three projects mainly relate to a perceived lack of time to practice decision talk. Interesting in this context was a concern of professionals about how to involve patients' relatives. A blind spot described is that decision talk may need additional appointments, therapeutic sessions, or treatment options, which may result in increasing health care consumption and costs. However, choosing to postpone or refrain from (invasive) treatments may also reduce costs at a later stage.
5. SYNTHESIS
5.1. Micro level
Based on the description of the representatives of the three projects, the use of individual, N = 1, PROMs for management and monitoring of the condition of the patient can be adopted without much difficulty by clinicians. Based on this notion and corresponding to what is known about using PROMs in the medical encounter,25, 80, 81, 85, 89 professionals' awareness of patients' concerns seems to increase. However, when drilling down to the other SDM steps, it seems to be less evident to apply those individual scores in order to involve patients in decision‐making about their options. In line with this, aggregated PROMs information about outcomes of options in terms of quality of life seems to be scarcely used. This latter finding corresponds to a recent study,40 showing that professionals stressed the opportunity to monitor changes in individual PROMs over time but not so much aggregated PROMs to make treatment decisions.
Option talk appeared to be practiced to some extent, but the other SDM steps (team talk, choice talk, and decision talk) were more difficult to achieve. The mere availability of PROMs, although helpful, is not sufficient for achieving SDM in VBHC initiatives. Team talk should be emphasized as a key step in training of professionals, because lack of team talk typically leads to suboptimal implementation of the subsequent steps.55 More attention is also needed for the integration of PROMs in choice talk. This talk about the trade‐offs between patients' values is a core element of both SDM and VBHC's “personal value”, but also known to be difficult to apply.15 Yet training of professionals in choice talk will probably not provide the magic bullet, given their limited time for the consultation.13 The use of decision aids as well as patient activation tools (eg, Ask 3 Questions Campaign90) could further enable clinicians practicing choice talk and probably also boost the integration of aggregated PROMs scores.40 Although patients' health literacy was not explicitly addressed in the project descriptions, the difficulty of explaining PROMs information and activating patients in using information was mentioned. It is known that just providing numbers will not help patients in decision‐making.91, 92, 93 To help patients with correct interpretation of PROMs information in the consultation, risk communication guidelines from the SDM field may be helpful.91, 94, 95, 96
5.2. Meso level
At the level of the department or organization, one key lesson is to continue to invest in training health care staff in SDM, not only in broad principles but also in the steps of the conversational model and the collaboration of the (multidisciplinary) team in trying to achieve this. In this training, attention should be devoted to the importance of SDM in delivering high value for patients, the employment of available PROMs and decision aids, and avoiding the silent misdiagnosis of patients' preferences and overtreatment.2 In general, it also seems important to clearly instruct what SDM entails, because previous studies suggest the concept is often unclear for clinicians.29, 49
Another key insight is the need to allocate time for staff to explicitly deliver SDM and all of its steps. This means that more staff will likely need to be hired and/or innovations need to be adopted that increase the productivity and efficiency of the teams. Especially with regard to routine administrative and logistics tasks that take so much of clinicians' time it is important to gain time to spend on SDM. Nurses or physician assistants could be trained in choice talk and using decision aids. When teams are reorganizing local care pathways around patients' needs within VBHC implementation, the following aspects could be addressed: (a) definition of the aim(s) of using PROMs in the medical encounter; (b) identification of possible preference‐based decisions (ie, equipoise of options); (c) definition of responsibilities and tasks of team members during the patient's journey (including initiation of team talk and who communicates what during the journey29; and (d) based on a‐c, decisions about allocation of time and effort. As noted by Pieterse et al13 and also described for the MS pilot, SDM often already starts before the actual consultation with patients, and this could provide a valuable perspective in redesigning care pathways.
Clinicians' beliefs about equipoise of options seem to be especially important to target at an early stage. The term “clinical equipoise' is usually referred to as a form of uncertainty among medical experts over whether one treatment is more beneficial to the patient than another treatment. In the SDM context, equipoise usually means a state of equilibrium, and this can also be the result of trials showing comparable clinical outcomes of two options (ie, mostly survival). However, it is known that evaluation of treatment options is a subjective process, and this is true for both clinicians, researchers and patients.97, 98 While a clinical trial may support the notion of equipoise between option A and B, clinicians or groups of clinicians may continue to believe that A is better than B.18, 97 Moreover, patients themselves may weigh evidence differently, especially when they attach importance to nonclinical outcomes, and the increasing use of PROMs may widen the scope of outcomes considered. The relationship between equipoise and patient characteristics influencing the effectiveness of treatments is also interesting, ie, on an aggregate level, two treatments may be comparable, but this may not be the case for an individual patient. This all leads to the question of how we define and construct notions of equipoise and how to act when different stakeholders' beliefs on equipoise differ. The notion of team talk in the SDM conversational model may help health care staff to more routinely discuss this.
With respect to innovations, a recommendation is to invest in technology that enables professionals' use of PROMs and decision aids, preferably linked to electronic health records and clinical guidelines.40 If equipoise of options does not pop‐up somewhere for professionals during the patient's journey, it is no wonder that patients are left with short descriptions of options, potentially biased towards clinicians' preferences. Smartphrases for clinicians and patients stating that there is a choice, followed by lists of available options or decision aids seem to have potential to achieve this. However, as already argued by Elwyn et al,7 implementation of such tools should ideally be done through thorough needs assessments, to deal with professional/organizational resistance to those tools.
We realize that for the described recommendations, tough trade‐offs and resource allocation decisions are required from departments or organizations. Stakeholders will thus need to understand costs/benefits associated with these investments. As apparent from the case examples, there are concerns about whether using PROMs and SDM in the medical encounter might result in more care consumption. Insight is therefore needed to see how costs/benefits compare and how these relate to other areas organizations need to invest in. Another option might be to identify lower value activities that can be disinvested from (eg, standard visits to clinics when patients have no concerns), so that resources can be funnelled to the initiatives above. The general principles that will facilitate these processes are to look at long to medium term instead of just short‐term outcomes and to include SDM‐linked outcomes sets.99 SDM can yield return on investment, but it may take longer to realize the returns relative to interventions predicated in the biomedical model. For example, as evidenced from the Dutch examples in this manuscript, fully implementing SDM first requires a one‐time up‐front investment to train staff in the processes and procedures linked to SDM and second requires a change in care pathways—overlaid onto both of these steps is culture change required within the provider setting to allow, facilitate, and actively promote SDM such that it becomes “business as usual.' SDM will likely yield return on investment once fully integrated into the culture and practice of organizations, mainly by patients' commitment to (less‐invasive) treatment. Initial savings will offset the initial one‐time up‐front investment, thus allowing for subsequent savings to actually generate a return on investment.
5.3. Macro level
At the system level, there need to be policy drivers and centrally coordinated initiatives to support key aspects of SDM, especially team talk and choice talk.8, 29 In the Netherlands, there is already investment from central institutions into SDM implementation programmes and use of PROMs in medical encounters.37 One underexposed aspect, however, is the availability of subsidized good‐quality training programmes on SDM across the country. Financial incentives may be needed to facilitate the use of SDM training and decision aids7; in the Dutch context, these financial incentives would also need to be delivered through health insurance companies.29 However, one macro level aspect that should be acknowledged is that professionals are not only expected to work with PROMs and SDM but also within organizational systems such as stepped care.84 Eventually, this might lead to questions about the freedom that professionals have to use PROMs and SDM, when they also need to achieve technical and allocative/population value.
Those dilemmas and accompanied learning objectives related to both SDM and other aspects of high value health care delivery could be more explicitly incorporated into medical curricula, to become part of clinicians' routine thinking and practice. In the Netherlands, SDM learning objectives have been adopted in medical curricula to some extent, but this implementation has not reached its full potential.8, 29 In line with the conceptualization of Montori,100 and the Dutch Council for Health and Society,5 we believe that it is SDM and sensitivity to patients' values and context (or “personal value') that should be the main objective of training future clinicians and not merely outcomes over costs.
Another lesson learned is that central investment in high‐quality and standardized decision aids, option grids, and quality standards as is now practiced in the Netherlands101 will continue to be of great importance. We recommend to also include preferred presentation formats of dashboards in those quality standards. An important question is whether current decision aids adequately take account of value clarification, as studies have not yet revealed best practices.57, 102 In addition, investment in research into getting SDM into practice seems necessary, as well as in building better technology to integrate PROMs and SDM into routine practice and into value (outcomes/cost) derived from the routine use of SDM in practice to show to what extent value is being delivered.
6. STRENGTHS AND LIMITATIONS
Combining analysis of literature on SDM and VBHC with insights from forerunner projects allowed us to assess current trends in using PROMs and SDM and exigencies of implementing it in practice. The comparison of different initiatives yielded insights from a range of disciplines and health care practices, including somatic and mental health care. Limitations are the fact that reports from the Amsterdam UMC projects were obtained from one leading representative per project, so potentially conflicting views could not be ascertained. Furthermore, the projects started relatively recently, so insights may still further develop or change.
7. CONCLUSION
The use of individual, N = 1, PROM scores in the medical encounter seems to be relatively easily adopted by clinicians, mainly for monitoring and management of the patient's condition. In theory, this information could greatly enhance choice talk (ie, value clarification) in SDM, but training of professionals seems necessary. Easy access to information about the existence of preference‐based decisions and to aggregated PROMs scores could give a further boost to SDM. Education, and staff resources on meso and macro levels may facilitate the more time‐consuming SDM aspects.
FUNDING
The work described in this paper did not receive any funding.
ACKNOWLEDGEMENTS
The contribution of C.v.E., A.J., M.C. and M.G. for this research was supported by the PRECeDI project (Personalized PREvention of Chronic Diseases) funded by the European Union's Horizon 2020 research and innovation programme MSCA‐RISE‐2014: Marie Skłodowska‐Curie Research and Innovation Staff Exchange (grant agreement number 645740). O.D. was local advisor on shared decision‐making to secondees from this programme.
Damman OC, Jani A, de Jong BA, et al. The use of PROMs and shared decision‐making in medical encounters with patients: An opportunity to deliver value‐based health care to patients. J Eval Clin Pract. 2020;26:524–540. 10.1111/jep.13321
Correction added on 6 February 2020, after first online publication: Figures 2 and 3 were mistakenly switched. These have been corrected in this version.
REFERENCES
- 1. Barry MJ, Edgman‐Levitan S. Shared decision making—the pinnacle of patient‐centred care. NEJM. 2012;366:780‐781. [DOI] [PubMed] [Google Scholar]
- 2. Mulley AG, Trimble C, Elwyn G. Stop the silent misdiagnosis: patients' preferences matter. BMJ. 2012;8:345, e6572. [DOI] [PubMed] [Google Scholar]
- 3. Stiggelbout AM, Pieterse AH, De Haes JC. Shared decision making: concepts, evidence, and practice. Patient Educ Couns. 2015;98(10):1172‐1179. [DOI] [PubMed] [Google Scholar]
- 4. Hoffman TC, Montori VM, DelMar C. The connection between evidence‐based medicine and shared decision making. JAMA. 2014;312(13):1295‐1296. [DOI] [PubMed] [Google Scholar]
- 5. Council for Health and Society (Raad voor Volksgezondheid en Samenleving, RVS) . No evidence without context; Den Haag: 2017.
- 6. Elwyn G, Laitner S, Coulter A, Walker E, Watson P. Thomson R. Implementing shared decision making in the NHS. BMJ. 2010;341:c5146. [DOI] [PubMed] [Google Scholar]
- 7. Elwyn G, Scholl I, Tiebohl C, et al. Many miles to go … : a systematic review of the implementation of patient decision support interventions into routine clinical practice. BMC Med Inform Decis Mak. 2013;13(Suppl2):S14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Van der Weijden T, Post H, Brand PLP, et al. Shared decision making, a buzz‐word in the Netherlands, the pace quickens towards nationwide implementation …. Z Evid Fortbild Qual Gesundhwes. 2017;123‐124. 69‐74 [DOI] [PubMed] [Google Scholar]
- 9. Couët N, Desroches S, Robitaille H, et al. Assessments of the extent to which health‐care providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect. 2015;18(4):542‐561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. De Mik SML, Stubenrouch FE, Balm R, Ubbink DT. Systematic review of shared decision‐making in surgery. Br J Surg. 2018;105(13):1721‐1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Legaré F, Ratte S, Gravel K, Graham ID. Barriers and facilitators to implementing shared decision making in clinical practice: update of a systematic review of health professionals' perceptions. Patient Educ Couns. 2008;73:526‐535. [DOI] [PubMed] [Google Scholar]
- 12. Sanders ARJ, Bensing JM, Magnée T, Verhaak P, de Wit NJ. The effectiveness of shared decision‐making followed by positive reinforcement on physical disability in the long‐term follow‐up of patients with nonspecific low back pain in primary care: a clustered randomised controlled trial. BMC Fam Pract. 2018;19(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pieterse AH, Stiggelbout AM. Montori VM. JAMA: Shared decision making and the importance of time; 2019. Apr 19. [DOI] [PubMed] [Google Scholar]
- 14. Lenzen SA, Daniëls R, Van Bokhoven MA, Van der Weijden T, Beurskens A. What makes it so difficult for nurses to coach patients in shared decision making? A process evaluation. Int J Nurs Stud. 2018;80:1‐11. [DOI] [PubMed] [Google Scholar]
- 15. Stans SEA, Dalemans RJP, Roentgen UR, Smeets HWH, Beurskens AJHM. Who said dialogue conversations are easy? The communication between communication vulnerable people and health‐care professionals: a qualitative study. Health Expect. 2018;21(5):848‐857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Karnieli‐Miller O, Eisikovitz Z. Physician as partner or salesman? Shared decision‐making in real‐time encounters. Soc Sci Med. 2009;69(1):1‐8. [DOI] [PubMed] [Google Scholar]
- 17. Kunneman M, Stiggelbout AM, Marijnen CA, Pieterse AH. Probabilities of benefit and harms of preoperative radiotherapy for rectal cancer: what do radiation oncologists tell and what do patients understand? Patient Educ Couns. 2015;98(9):1092‐1098. [DOI] [PubMed] [Google Scholar]
- 18. Hopmans W, Zwaan L, Senan S, et al. Differences between pulmonologists, thoracic surgeons and radiation oncologists in deciding on the treatment of stage I non‐small cell lung cancer: a binary choice experiment. Radiother Oncol. 2015;115(3):361‐366. [DOI] [PubMed] [Google Scholar]
- 19. Porter ME, Lee TH. From Volume to value in health care: the work begins. JAMA. 2016;316(10):1047‐1048. [DOI] [PubMed] [Google Scholar]
- 20. Porter ME. What is value in healthcare? N Engl J Med. 2010;363(26):2477‐2481. [DOI] [PubMed] [Google Scholar]
- 21. Berwick DM, Nolan TW, Whittington J. The triple aim: care, health, and cost. Health Aff. 2008;27(3):759‐769. [DOI] [PubMed] [Google Scholar]
- 22. Jani A, Jungmann S, Gray M. Shifting to triple value healthcare: reflections from England. Z Evid Fortbild Qual Gesundhwes. 2018;130:2‐7. [DOI] [PubMed] [Google Scholar]
- 23. Lins L, Carvalho FM. SF‐36 total score as a single measure of health‐related quality of life: scoping review. SAGE Open Med. 2016;4:2050312116671725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ‐5D‐5L: mapping the EQ‐5D‐5L to EQ‐5D‐3L Value Sets. Value Health. 2012;15(5):708‐715. [DOI] [PubMed] [Google Scholar]
- 25. Carlier IV, Meuldijk D, Van Vliet IM, Van Fenema E, Van der Wee NJ, Zitman FG. Routine outcome monitoring and feedback on physical or mental health status: evidence and theory. J Eval Clin Pract. 2012;18(1):104‐110. [DOI] [PubMed] [Google Scholar]
- 26. De Beurs E, Hollander den‐Gijsman ME, Van Rood YR, et al. Routine outcome monitoring in the Netherlands: practical experiences with a web‐based strategy for the assessment of treatment outcome in clinical practice. Clin Psychol Psychother. 2011;18(1):1‐12. [DOI] [PubMed] [Google Scholar]
- 27. Jani A, Gray M. Outcomes as a foundation for designing and building population healthcare systems in England. BMJ Outcomes Article Collection. 2015;16. [Google Scholar]
- 28. Spatz ES, Elwyn G, Moulton BW, Volk RJ, Frosch DL. Shared decision making as part of value based care: new U.S. policies challenge our readiness. Z Evid Fortbild Qual Gesundhwes. 2017;123‐124:104‐108. [DOI] [PubMed] [Google Scholar]
- 29. Van Veenendaal H, Van der Weijden T, Ubbink DT, Stiggelbout AM, Van Mierlo LA, Hilders CGJM. Accelerating implementation of shared decision‐making in the Netherlands: an exploratory investigation. Patient Educ Couns. 2018;101(12):2097‐2104. [DOI] [PubMed] [Google Scholar]
- 30. Stacey D, Légaré F, Col NF, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2014;1:CD001431. [DOI] [PubMed] [Google Scholar]
- 31. Stacey D, Légaré F, Lewis K, et al. Trevena L Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;4:CD001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Veroff D, Marr A, Wennberg DE. Enhanced support for shared decision making reduced costs of care for patients with preference‐sensitive conditions. Health Aff. 2013;32(2):285‐293. [DOI] [PubMed] [Google Scholar]
- 33. Joosten EAG, DeFuentes‐Merillas L, de Weert GH, Sensky T, van der Staak CP, de Jong CA. Systematic review of the effects of shared decision making on patient satisfaction, treatment adherence and health status. Psychother Psychosom. 2008;77:219‐226. [DOI] [PubMed] [Google Scholar]
- 34. Fiks AG, Mayne S, Localio AR, Alessandrini EA, Guevara JP. Shared decision‐making and health care expenditures among children with special health care needs. Pediatrics. 2012;129(1):99‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Cantor SB, Rajan T, Linder SK, Volk RJ. A framework for evaluating the cost‐effectiveness of patient decision aids: a case study using colorectal cancer screening. Prev Med. 2015;77:168‐173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Arterburn D, Wellman R, Westbrook E, et al. Introducing decision aids at Group Health was linked to sharply lower hip and knee surgery rates and costs. Health Aff. 2012;31(9):2094‐2104. [DOI] [PubMed] [Google Scholar]
- 37. Zorginstituut . Meer patiëntregie door meer uitkomstinformatie in 2022. Zorginstituut/Diemen; 2018.
- 38. NFU . Bouwstenen voor werken aan waardegedreven zorg. https://nfukwaliteit.nl/pdf/Bouwstenen_voor_werken_aan_waardegedreven_zorg.pdf
- 39. Metz MJ, Franx GC, Veerbeek MA, de Beurs E, van der Feltz‐Cornelis CM, Beekman AT. Shared decision making in mental health care using routine outcome monitoring as a source of information: a cluster randomised controlled trial. BMC Psychiatry. 2015;15:313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Damman OC, Verbiest MEA, Vonk SI, et al. Using PROMs during routine medical consultations: the perspectives of people with Parkinson's disease and their health professionals. Health Expect. 2019; in press;22(5):939‐951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hibbard JH, Greene J, Overton V. Patients with lower activation associated with higher costs; delivery systems should know their patients' ‘scores’. Health Aff. 2013;32(2):216‐222. [DOI] [PubMed] [Google Scholar]
- 42. Santana MJ, Haverman L, Absolom K, et al. Training clinicians in how to use patient‐reported outcome measures in routine clinical practice. Qual Life Res. 2015;24(7):1707‐1718. [DOI] [PubMed] [Google Scholar]
- 43. Clayman ML, Bylund CL, Chewning B, Makoul G. The impact of patient participation in health decisions within medical encounters: a systematic review. Med Decis Making. 2016;36(4):427‐452. [DOI] [PubMed] [Google Scholar]
- 44. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Elwyn G, Durand MA, Song J, et al. A three‐talk model for shared decision making: multistage consultation process. BMJ. 2017;359:j4891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Makoul G, Clayman ML. An integrative model of shared decision making in medical encounters. Patient Educ Couns. 2006;60(3):301‐312. [DOI] [PubMed] [Google Scholar]
- 47. McCaffery KJ, Smith SK, Wolf M. The challenge of shared decision making among patients with lower literacy: a framework for research and development. Med Decis Making. 2010;30(1):35‐44. [DOI] [PubMed] [Google Scholar]
- 48. Legaré F, Witteman H. Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Aff. 2013;32(2):276‐284. [DOI] [PubMed] [Google Scholar]
- 49. Joseph‐Williams N, Lloyd A, Edwards A, et al. Implementing shared decision making in the NHS: lessons from the MAGIC programme. BMJ. 2017;357:j1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nl Patiëntenfederatie. Rapport meldactie Samen Beslissen. Utrecht; 2014.
- 51. Oostendorp LJ, Ottevanger PB, van de Wouw AJ, et al. Patients' preferences for information about the benefits and risks of second‐line palliative chemotherapy and their oncologist's awareness of these preferences. J Cancer Educ. 2016;31(3):443‐448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kiesler DJ, Auerbach SM. Optimal matches of patient preferences for information, decision‐making and interpersonal behavior: evidence, models and interventions. Patient Educ Couns. 2006;61(3):319‐341. [DOI] [PubMed] [Google Scholar]
- 53. Hopmans W, Damman OC, Senan S, Hartemink K, Smit E, Timmermans DR. A patient perspective on shared decision making in stage I non‐small cell lung cancer: a mixed methods study. BMC Cancer. 2015;15(1):959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lindor RA, Kunneman M, Hanzel M, Schuur JD, Montori VM, Sadosty AT. Liability and informed consent in the context of shared decision making. Acad Emerg Med. 2016;23(12):1428‐1433. [DOI] [PubMed] [Google Scholar]
- 55. Kunneman M, Branda ME, Hargraves I, Pieterse AH, Montori VM. Fostering choice awareness for shared decision making: a secondary analysis of video‐recorded clinical encounters. Mayo Clin Proc Innov Qual Outcomes. 2018;2(1):60‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Schwartz SH. Universals in the content and structure of values: theoretical advances and empirical tests in 20 countries. Adv Exp Soc Psychol. 1992;25:1‐65. [Google Scholar]
- 57. Witteman HO, Scherer LD, Gavaruzzi T, et al. Design features of explicit values clarification methods: a systematic review. Med Decis Making. 2016;36(4):453‐471. [DOI] [PubMed] [Google Scholar]
- 58. Black N. Patient reported outcome measures could help transform healthcare. BMJ. 2013;346:f167. [DOI] [PubMed] [Google Scholar]
- 59. Browne JP, Cano SJ, Smith S. Using patient‐reported outcome measures to improve health care: time for a new approach. Med Care. 2017;55(10):901‐904. [DOI] [PubMed] [Google Scholar]
- 60. Van Der Wees PJ, Nijhuis‐Van Der Sanden MW, Ayanian JZ, Black N, Westert GP, Schneider EC. Integrating the use of patient‐reported outcomes for both clinical practice and performance measurement: views of experts from 3 countries. Milbank Q. 2014;92(4):754‐775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Wu AW, Snyder C, Clancy CM, Steinwachs DM. Adding the patient perspective to comparative effectiveness research. Health Aff. 2010;29(10):1863‐1871. [DOI] [PubMed] [Google Scholar]
- 62. Anker SD, Agewall S, Borggrefe M, et al. The importance of patient‐reported outcomes: a call for their comprehensive integration in cardiovascular clinical trials. Eur Heart J. 2014;35(30):2001‐2009. [DOI] [PubMed] [Google Scholar]
- 63. Alonso J, Bartlett SJ, Rose M, et al. The case for an international patient‐reported outcomes measurement information system (PROMIS®) initiative. Health Qual Life Outcomes. 2013;20;11:210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Øvretveit J, Zubkoff L, Nelson EC, Frampton S. Lehmann Knudsen, Zimlichman E. Using patient‐reported outcome measurement to improve patient care. International J Qual Health Care. 2017;29(6):874‐879. [DOI] [PubMed] [Google Scholar]
- 65. McNamara RL, Spatz ES, Kelley TA, Stowell CJ, Beltrame J, Heidenreich P, Tresserras R, Jernberg T, Chua T, Morgan L, Panigrahi B, Rosas Ruiz A, Rumsfeld JS, Sadwin L, Schoeberl M, Shahian D, Weston C, Yeh R, Lewin J. Standardized outcome measurement for patients with coronary artery disease: consensus from the International Consortium for Health Outcomes Measurement (ICHOM). J Am Heart Assoc 2015;4(5): pii: e001767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Nijagal MA, Wissig S, Stowell C, et al. Standardized outcome measures for pregnancy and childbirth, an ICHOM proposal. BMC Health Serv Res. 2018;18(1):953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Verberne WR, Das‐Gupta Z, Allegretti AS, et al. Development of an international standard set of value‐based outcome measures for patients with chronic kidney disease: a report of the International Consortium for Health Outcomes Measurement (ICHOM) CKD Working Group. Am J Kidney Dis. 2019;73(3):372‐384. [DOI] [PubMed] [Google Scholar]
- 68. Ouwens M, Wollersheim H, Hermens R, Hulscher M, Grol R. Integrated care programmes for chronically ill patients: a review of systematic reviews. International J Qual Health Care. 2005;17(2):141‐146. [DOI] [PubMed] [Google Scholar]
- 69. Trebble TM, Hansi N, Hydes T, Smith MA, Baker M. Process mapping the patient journey: an introduction. BMJ. 2010;341:c4078. [DOI] [PubMed] [Google Scholar]
- 70. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831‐841. 284 [DOI] [PubMed] [Google Scholar]
- 71. Atwal A, Caldwell K. Do multidisciplinary integrated care pathways improve interprofessional collaboration? Scand J Caring Sci. 2002;16(4):360‐367. [DOI] [PubMed] [Google Scholar]
- 72. Hansson A, Svensson A, Ahlström BH, Larsson LG, Forsman B, Alsén P. Flawed communications: health professionals' experience of collaboration in the care of frail elderly patients. Scand J Public Health. 2018;46(7):680‐689. [DOI] [PubMed] [Google Scholar]
- 73. Greenhalgh J, Dalkin S, Gooding K, et al. Functionality and feedback: a realist synthesis of the collation, interpretation and utilisation of patient‐reported outcome measures data to improve patient care. NIHR Journals Library: Southampton (UK); 2017. Jan. [PubMed] [Google Scholar]
- 74. Greenhalgh J, Abhyankar P, McClusky S, Takeuchi E, Velikova G. How do doctors refer to patient‐reported outcome measures (PROMS) in oncology consultations? Qual Life Res. 2013;22(5):939‐950. [DOI] [PubMed] [Google Scholar]
- 75. Mathias SD, Fifer SK, Mazonson PD, Lubeck DP, Buesching DP, Patrick DL. Necessary but not sufficient: the effect of screening and feedback on outcomes of primary care patients with untreated anxiety. J Gen Intern Med. 1994;9(11):606‐615. [DOI] [PubMed] [Google Scholar]
- 76. Guo T, Xiang YT, Xiao L, et al. Measurement‐based care versus standard care for major depression: a randomized controlled trial with blind raters. Am J Psychiatry. 2015;172(10):1004‐1013. [DOI] [PubMed] [Google Scholar]
- 77. Detmar SB, Muller MJ, Schornagel JH, Wever LD, Aaronson NK. Health related quality of life assessments and patient‐physician communication. JAMA. 2002;288(23):3027‐3034. [DOI] [PubMed] [Google Scholar]
- 78. Jensen RE, Snyder CF, Abertnethy AP, et al. Review of electronic patient‐reported outcomes systems used in cancer clinical care. J Oncol Pract. 2013;10:215‐222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Hartzler AL, Izard JP, Dalkin BL, Mikles SP, Gore JL. Design and feasibility of integrating personalized PRO dashboards into prostate cancer care. J Am Med Inform Assoc. 2016;23(1):38‐47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Marshall S, Haywood K, Fitzpatrick R. Impact of patient‐reported outcome measures on routine practice: a structured review. J Eval Clin Pract. 2006;12(5):559‐568. [DOI] [PubMed] [Google Scholar]
- 81. Yang LY, Manhas DS, Howard AF, Olson RA. Patient‐reported outcome use in oncology: a systematic review of the impact on patient‐clinician communication. Support Care Cancer. 2018;26(1):41‐60. [DOI] [PubMed] [Google Scholar]
- 82. Boyce MB, Browne JP, Greenhalgh J. The experiences of professionals with using information from patient‐reported outcome measures to improve the quality of healthcare: a systematic review of qualitative research. BMJ Qual Saf. 2014;23(6):508‐518. [DOI] [PubMed] [Google Scholar]
- 83. Van Straten A, Hill J, Richards DA, Cuijpers P. Stepped care treatment delivery for depression: a systematic review and meta‐analysis. Psychol Med. 2015;45(2):231‐246. [DOI] [PubMed] [Google Scholar]
- 84. Von Korff M, Glasgow RE, Sharpe M. Organising care for chronic illness. BMJ. 2002;325:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Metz MJ, Veerbeek MA, Franx GC, van der Feltz‐Cornelis CM, de Beurs E, Beekman ATF. A National Quality Improvement Collaborative for the clinical use of outcome measurement in specialised mental healthcare: results from a parallel group design and a nested cluster randomised controlled trial. BJPsych Open. 2017;3(3):106‐112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kampstra NA, Zipfel N, van der Nat PB, Westert GP, van der Wees PJ, Groenewoud AS. Health outcomes measurement and organizational readiness support quality improvement: a systematic review. BMC Health Serv Res. 2018;18(1):1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Krakow EF, Hemmer M, Wang T, et al. Tools for the precision medicine era: how to develop highly personalized treatment recommendations from cohort and registry data using Q‐learning. Am J Epidemiol. 2017;186(2):160‐172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Kidwell KM. SMART designs in cancer research: past, present, and future. Clin Trials. 2014;11(4):445‐456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Metz MJ, Veerbeek MA, Twisk JWR, van der Feltz‐Cornelis CM, de Beurs E, Beekman ATF. Shared decision‐making in mental health care using routine outcome monitoring: results of a cluster randomised‐controlled trial. Soc Psychiatry Psychiatr Epidemiol. 2019;54(2):209‐219. [DOI] [PubMed] [Google Scholar]
- 90. Shepherd HL, Barratt A, Trevena LJ, et al. Three questions that patients can ask to improve the quality of information physicians give about treatment options: a cross‐over trial. Patient Educ Couns. 2011;357:379‐385. [DOI] [PubMed] [Google Scholar]
- 91. Fagerlin A, Zikmund‐Fisher BJ, Ubel PA. Helping patients decide: ten steps to better risk communication. J Natl Cancer Inst. 2011;103(19):1436‐1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Peters E, Dieckmann NF, Västfjäll D, Mertz CK, Slovic P, Hibbard JH. Bringing meaning to numbers: the impact of evaluative categories on decisions. J Exp Psychol Appl. 2009;15(3):213‐227. [DOI] [PubMed] [Google Scholar]
- 93. Stubenrouch FE, Baumann M, Legemate DA, Ubbink DT. A web‐based application to communicate benefits and risks of surgical treatments. Surg Technol Int. 2017. 25;30:31‐37. [PubMed] [Google Scholar]
- 94. McCaffery KJ, Holmes‐rovner M, Smith SK, et al. Addressing health literacy in patient decision aids. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Trevena LJ, Zikmund‐Fisher BJ, Edwards A, et al. Presenting quantitative information about decision outcomes: a risk communication primer for patient decision aid developers. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Tolbert E, Brundage M, Bantug E, Blackford AL, Smith K. Snyder C; and PRO Data Presentation Stakeholder Advisory Board. Picture this: presenting longitudinal patient‐reported outcome research study results to patients. Med Decis Making. 2018;38(8):994‐1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Ubel PA, Silbergleit R. Behavioral equipoise: a way to resolve ethical stalemates in clinical research. Am J Bioeth. 2011;11(2):1‐8. [DOI] [PubMed] [Google Scholar]
- 98. Veatch RM. The irrelevance of equipoise. J Med Philos. 2007;32:167‐183. [DOI] [PubMed] [Google Scholar]
- 99. Toupin‐April K, Barton JL, Fraenkel L, et al. OMERACT development of a core domain set of outcomes for shared decision‐making interventions. J Rheumatol. 2019; in press;46(10):1409‐1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Montori VM. Turning away from industrial health care toward careful and kind care. Acad Med. 2019;94(6):768‐770. [DOI] [PubMed] [Google Scholar]
- 101. Van der Weijden T, Dreesens D, Faber MJ, et al. Developing quality criteria for patient‐directed knowledge tools related to clinical practice guidelines. A development and consensus study. Health Expect. 2019;22(2):201‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. De Angst IB, Weernink MG, Kil PJ, van Til JA, Cornel EB, Takkenberg JJ. Development and usability testing of a multi‐criteria value clarification methods for patients with localized prostate cancer. Health Informatics J. 2019. in press [DOI] [PubMed] [Google Scholar]


