Abstract
Background
Using synthetic antibiotic‐eluting envelope (ABE) is an effective intervention for prevention of cardiovascular implantable electronic device (CIED) infection. The biologic extracellular‐matrix envelope (ECME), may offer potential advantages over the synthetic ABE. To further minimize the risk of infection, the ECME can be hydrated in gentamicin prior to CIED implantation. We aimed to evaluate the efficacy and pharmacokinetics (PK) of gentamicin containing ECME in an animal model.
Methods
For all experiments, the ECME was hydrated in gentamicin (40 mg/Ml) (treatment) for 2 min. In vitro antimicrobial efficacy against six different bacterial species was assessed. In vivo experiments were conducted using a rabbit model of CIED pocket infection. Serum and ECM gentamicin concentrations were measured. Five different organisms were inoculated into the device pocket of control (ECME hydrated in 0.9% saline) and treatment groups. Macroscopic appearance and colony forming units from CIED, ECME, and tissue were determined.
Results
No bacteria were recovered from any culture after 12 h of exposure to the gentamicin containing ECME. Serum gentamicin levels dropped below the limit of quantification at 15 h after implant. Gentamicin concentration in the ECME remained relatively stable for up to 7 days. Signs of clinical infection were observed in the control but not in the treatment group. In the presence of gentamicin, statistically significant reduction was demonstrated across all tested bacterial species.
Conclusions
In this preclinical animal infection model, gentamicin containing ECME was highly effective in reducing bacterial burden in the implant pocket, while systemic exposure after implantation remained low.
Keywords: CIED pocket infection, efficacy, gentamicin extracellular matrix envelope, pharmacokinetics, prevention
1. INTRODUCTION
Cardiac implantable electronic devices (CIED), including permanent pacemakers, cardioverter‐defibrillators, and cardiac resynchronization therapy devices, have become critical in the management of heart failure and life‐threatening arrhythmias. With expanding technologies and indications, implantation rates continue to increase. 1 However, the number of postprocedural complications, 2 including infection, has also grown in paralell. 1 , 3 , 4 , 5 Interestingly, the rate of CIED infection has outpaced that of device implantation. 1 , 3 , 4 A possible explanation for this observation may be increasing number of device reinterventions following initial implantation, which further increases the risk of CIED infection. 6 , 7 , 8
CIED infections are associated with significant short‐ and long‐term mortality, and are costly to treat. 6 , 9 Thus, development and implementation of effective strategies for prevention of these infections are of critical importance. 10 However, only a limited number of evidence‐based interventions are currently available. As majority of infections develop at the time of device implantation, these strategies are directed at decreasing the bacterial burden at the implant site and include perioperative antistaphylococcal antibiotic prophylaxis, 11 and aseptic techniques. More recently, adjunctive use of a synthetic absorbable antibiotic‐eluting envelope (ABE) was shown to significantly reduce the incidence of CIED pocket infections. 12 , 13 With such approach, the pulse generator is placed into the ABE, stabilizing the device within the surgical pocket; while gradually delivering antibiotics over a period of days.
A second type of CIED envelope constructed from a biologic material is currently available for clinical use (ECM Aziyo Biologics; Silver Spring, MD) (Figure 1). This envelope consists of a multilaminate sheet of decellularized, noncrosslinked, lyophilized extracellular matrix (ECM), derived from porcine small intestinal submucosa. This biological scaffold provides a substrate for better tissue integration, vascular ingrowth, and more rapid clearance of bacteria. 14 , 15 , 16 , 17 , 18 , 19 , 20 , 21 Furthermore, some studies suggest intrinsic bactericidal activity. Over time, the ECM envelope remodels into a vascularized pocket that may facilitate implant removal or revision when required. Given its theoretical lessened foreign body response and resistance to infection, the ECM envelope may offer plausible advantages over the synthetic ABE.
FIGURE 1.

Extracellular matrix envelope
To further minimize the risk of CIED infection, this biologic ECM envelope can be hydrated in a gentamicin solution prior to implantation. Gentamicin is an aminoglycoside with broad‐spectrum bactericidal activity including the most common organisms identified in CIED infections including Staphylococcus spp. and aerobic Gram‐negative organisms. 22 , 23 , 24 , 25 When given as monotherapy, gentamicin has also demonstrated in vitro efficacy against established Staphyloccocal biofilms. 26 However, there are major limitations associated with systemic use of gentamicin therapy including the potential development of nephrotoxicity and otoxocity. 27 Local gentamicin delivery to the site that potentially harbors pathogens, may overcome some of these risks. Earlier studies have demonstrated reduced infection rates with local application of gentamicin containing collagen sponge during CIED implantation. 28 , 29 Furthermore, Deering et al 30 demonstrated that after hydration of ECM envelope in different antibiotics solution, elution was observed for a period of up to 6 days. In this same study, subsequent experiments performed using an animal model of cardiac device pocket infection showed that the released antibiotics (vancomycin or gentamicin) substantially reduced colonization by two strains of Staphylococcus on CIED devices. However, in these initial studies, only two strains of Gram‐positive bacteria were tested in a semiquantitative animal model and pharmacokinetic (PK) studies were not conducted.
In this study, we aimed to evaluate the efficacy and PK of gentamicin containing ECM envelope using an animal model of cardiac device pocket infection.
2. METHODS
2.1. In vitro efficacy of gentamicin containing ECM envelope
The modified ASTM E‐2315 Method (American Society for Tests and Materials, “Standard Guide for Assessment Activity Using a Time‐Kill Procedure”) was used to assess the antimicrobial efficacy against three Gram‐positive species (methicillin‐resistant Staphylococcus aureus (MRSA) ATCC‐33591, S. aureus ATCC‐6538, and Staphylococcus epidermidis ATCC‐51625) and three Gram‐negative species (Escherichia coli ATCC‐8739, Pseudomonas aeruginosa ATCC‐9027, and Serratia marcescens ATCC‐14756). Small‐size ECM envelopes were used for the in vitro experiments. ECM envelopes were hydrated in 20 mL of a 40 mg/mL gentamicin solution (Fresenius Kabi, Lake Zurich, IL) for 2 min. ECM envelopes hydrated in normal saline (0.9%) were used as controls.
The envelopes were incubated in 50 mL of bacterial culture on a rotary shaker (VWR, Radnor, PA, USA) for 24 h. The initial bacterial concentration was approximately 1 × 106 colony forming units (CFU)/mL. At 3, 6, 12, and 24 h, an aliquot of 1 milliliter was removed from the culture. Serial dilutions and neutralization with Dey/Engley (D/E) broth filtration were performed before the aliquot was plated and incubated. The bacterial concentrations were calculated as CFU/mL at each time point, and log10(CFU/mL) was reported. When no colonies were reported (zero CFU count), this was expressed as log10(CFU/mL) <1.0. Log reduction (treatment versus time = 0) was calculated by comparing the bacterial concentrations in the treatment group at each time point to the initial inoculated concentration (time = 0). Alternatively, log reduction (treatment versus control) was calculated by comparing the bacterial concentrations in the treatment group (gentamicin) to the control group (saline).
2.2. Animal experiments
All animal studies conformed to the Guide for the Care and Use of Laboratory Animals, and the surgical procedures and animal care were conducted at an independent research organization (Wuxi Apptec, Saint Paul, MN) in compliance to Good Laboratory Practice. 31 Institutional Animal Care and Use Committee approved all animal study protocols prior to study initiation (WuXi AppTec IACUC Protocol Number 16–549A).
2.3. Serum gentamicin concentrations
For this study, 22 New Zealand White rabbits were implanted with a CIED placed into a small‐size ECM envelope in a subcutaneous pocket on the left or right dorsal side. Prior to implantation, as with in vitro studies, the ECM envelope was hydrated in 20 mL of a 40 mg/mL gentamicin solution for 2 min.
To measure the serum gentamicin concentration, postimplantation, four blood samples from the rabbit models were obtained at 15 min; 1, 3, 6, 10, 15 h; and 7 days (Supporting Information Table S1).
Serum gentamicin concentration was analyzed by noncompartmental method. The maximum serum concentration (C max) and the time to reach max level (T max) were determined directly from the serum concentration‐time curve. The log‐linear phase was illustrated by the log serum concentration‐time plot, with its slope obtained by linear regression for derivation of the terminal rate constant kβ, and the terminal half‐life (T 1/2 β). The tissue absorption phase α with its rate constant kα and the absorption half‐life (T 1/2 α) were estimated by the method of residuals. The area under serum concentration‐time curve from time 0 to infinity ([AUC]0∞) was obtained by applying the linear trapezoidal rule up to C max, and log‐trapezoidal rule after C max. Extrapolation to T ∞ was calculated by using the predicted concentration at the last observed time point where [AUC]∞ = Cl ast/kβ. Then [AUC]0∞ was estimated by the sum of [AUC]t0 tlast and [AUC]∞.
2.4. ECM envelope gentamicin concentration
Next, we measured the gentamicin concentration in the ECM envelopes by explanting them at different termination time points (1, 6, 15, 24, 48, 72 h and 7 days) postimplantation. A reverse‐phase liquid chromatography‐mass spectrometry (LC/MS) method was developed to assay gentamicin concentrations in rabbit serum and the envelope. For gentamicin measurement, the ECM envelope was homogenized before LC/MS. Chromatographic separation was achieved with PFP propyl column (Waters Corporation, Milford, MA), and the analytes were ionized by positive electrospray and detected by selected reaction monitoring with a mass spectrometer capable of multiple reaction monitoring (Thermo TSQ; Thermo Fisher Scientific, Waltham, MA). Data acquisition and processing was performed using Thermo Xcalibur software (Thermo Fisher Scientific). Gentamicin C1, C1a, and C2 were measured by the LC/MS method, and total gentamicin was calculated as the sum of the three components.
2.5. Efficacy of gentamicin containing ECM envelope
To analyze the antibacterial performance of gentamicin containing ECM envelope, in vivo experiments were conducted using an established New Zealand White rabbit cardiac device pocket infection model. 26 , 27 In this study, five different species of bacteria, three Gram‐positive [MRSA (ATCC 33591) (Supporting Information Table S6), S. aureus (ATCC 29213) (Supporting Information Table S2), S. epidermidis (ATCC 35984) (Supporting Information Table S3)]and two Gram‐negative [E. coli (ATCC 25922) (Supporting Information Table S4) and P. aeruginosa (ATCC 25922) (Supporting Information Table S5)], were tested in five separate experiments.
For each bacterial strain, three groups of animals were included. The treatment group (n = 5) received bacterial inoculation and gentamicin ECM envelope (20 mL, 40 mg/mL, Fresenius Kabi); the control group (n = 5) received bacterial inoculation and ECM envelope hydrated in normal saline (20 mL, 0.9%); and the environment control group (n = 5) received ECM envelope hydrated in normal saline (20 mL, 0.9%) without bacterial inoculation. For these experiments, medium‐size ECM envelopes were used.
As previously described, 32 , 33 implant pockets were created dorsally, one on each side of the midline. For all groups, one CIED was placed inside of one ECM envelope and both materials were placed in the subcutaneous pocket. Immediately after implantation, 1.0 mL of bacterial inoculation was delivered into each individual subcutaneous pocket through a catheter tunneled subcutaneously into the pocket via a separate incision, followed by a 1.0 mL saline flush to ensure all inoculum was delivered. The inoculation concentration was determined in a series of dosing studies for each species to ensure a sustained infection for at least 7 days, characterized by >104 CFUs of bacterial recovery at implant removal. The inoculation concentrations were determined to be 1 × 109, 5 × 103, 1 × 107, 1 × 103, and 2 × 104 CFU/mL for MRSA, S. aureus, S. epidermidis, E. coli, and P. aeruginosa, respectively.
After 7 days, animals were euthanized, and the implants and surrounding tissue were explanted. Macroscopic examination of the appearance of the device and pocket was performed, and evaluated for signs of erythema, swelling, exudate or purulent‐like material on the device surface and envelope.
We then separately assessed for viable bacteria using a vortexing/sonication and culture procedure. CIED and ECM envelopes were placed in 45‐mL sonication buffer (0.5% Tween‐80 with Dey/Engley [D/E] neutralizing broth). Tissue samples were collected from the surrounding tissue pocket and then minced and placed in a separate container with 25 mL of sonication buffer. Device and tissue samples were weighed separately.
The samples were vortexed for 15 s and sonicated for 5 min. Sonicant solutions were serially diluted 10−1, 10−2, 10−3, and higher if necessary, and the undiluted and diluted samples were plated on Trypticase soy agar plates. Plates or media selective for the specific strain of bacteria used in this study were utilized. All plates were cultured for up to 48 h at 37°C. Plates were examined for presence of colonies. Colonies were counted and recorded. Resulting colonies were identified for bacterial strain via Gram stain, colony morphology, and analytical profile index test strips. In the event of unexpected culture results, bacterial samples were sent for analysis by DNA identification.
Two‐sided nonparametric Mann‐Whitney‐Wilcoxon tests were performed using R to compare total bacterial recovery between treatment group (gentamicin hydrated) and control group (normal saline hydrated).
3. RESULTS
3.1. Complete in vitro bacterial elimination by gentamicin containing ECM envelope
A complete elimination (100% kill) of cultured microorganisms by gentamicin containing ECM envelopes was observed for all six microorganisms tested. No bacteria (0 CFU/mL) were recovered from any culture after 12 h of exposure to the gentamicin containing ECM envelope. Escherichia coli was killed completely within 3 h; S. epidermidis, MRSA, P. aeruginosa, and S. marcescens were killed completely within 6 h; and S. aureus was killed completely within 12 h.
As compared to the initial inoculate, the gentamicin containing ECM envelope achieved 100% (>5‐log) reduction for all six strains of bacteria within 12 h (Table 1). When compared to the control samples, the gentamicin containing ECM envelopes achieved 100% (>6‐log) reduction for all strains of bacteria, except for E. coli (Table 2). The maximum log‐reduction achieved for E. coli was >2.1 due to the marked antimicrobial activity observed in the control group. However, the gentamicin containing ECM envelope still achieved a 100% reduction of E. coli as compared to the control samples.
TABLE 1.
Log reduction of bacterial counts at different time points as compared to the initial inoculate
| Log reduction treatment vs T = 0 | ||||||
|---|---|---|---|---|---|---|
| Time (h) | S. aureus | S. epidermidis | MRSA | E. coli | P. aeruginosa | S. marcescens |
| 3 | 3.9 | 5.5 | 4.1 | >5.4 | 5.8 | 5.1 |
| 6 | 5.4 | >5.5 | >5.6 | >5.4 | >5.8 | >5.1 |
| 12 | >5.5 | >5.5 | >5.6 | >5.4 | >5.8 | >5.1 |
| 24 | >5.5 | >5.5 | >5.6 | >5.4 | >5.8 | >5.1 |
When 0 CFU were recovered from the treatment group (complete kill), Log10 (CFU/mL) <1.0 was used to calculate the log reduction.
TABLE 2.
Log reduction of bacterial counts at different time points as compared to control samples
| Log reduction treatment vs control | ||||||
|---|---|---|---|---|---|---|
| Time (h) | S. aureus | S. epidermidis | MRSA | E. coli | P. aeruginosa | S. marcescens |
| 3 | 3.3 | 4.5 | 3.1 | >2.1 | 3.3 | 4.1 |
| 6 | 6.0 | >7.6 | >4.8 | >0.7 | >4.3 | >4.9 |
| 12 | >7.9 | >8.1 | >7.8 | >0.4 | >6.8 | >6.2 |
| 24 | >8.3 | >8.5 | >8.5 | >2.0 | >7.5 | >8.2 |
When 0 CFU were recovered from the treatment group (complete kill), Log10 (CFU/mL) < 1.0 was used to calculate the log reduction.
3.2. Low risk of systemic exposure to gentamicin
The serum gentamicin concentrations in rabbit following implantation of ECM envelope at each time point are presented in Table 3. Following subcutaneous implant of the ECM envelope, serum gentamicin levels increased to 34.53 ± 12.18 µg/mL within 15 min, peaked at 1 h (59.96 ± 23.60 µg/mL), and then declined, with the serum gentamicin level dropping below the limit of quantification at 15 h after implant. The three gentamicin isomers C1, C2, and C1a have very similar PK profiles after subcutaneous delivery of the drug by ECM envelope in this rabbit model. Results of the noncompartmental analysis of gentamicin serum levels were pooled and averaged for samples (n = 4) at each time point (Table 4).
TABLE 3.
Serum gentamicin concentrations in animal model of cardiac device pocket infection for 7 days postimplant
| n | Total gentamicin a (µg/mL) | ||
|---|---|---|---|
| Time | Mean | SD | |
| 15 min | 4 | 34.53 | 12.18 |
| 1 h | 4 | 59.96 | 23.60 |
| 3 h | 4 | 30.95 | 3.46 |
| 6 h | 4 | 6.14 | 1.16 |
| 10 h | 4 | 2.26 | 0.49 |
| 15 h‐7 days | 4 | <LLOQ | ‐ |
C1a 0.378 µg/mL; C2 0.729 µg/mL; LLOQ, lower Limit of Quantification; LLOQ for aGentamicin C1: 0.613 µg/mL; SD, standard deviation.
TABLE 4.
Estimated pharmacokinetic parameters of gentamicin in rabbit serum following implantation of ECM envelope
| Parameters | Value |
|---|---|
| C max | 59.96 mg/L |
| T max | 1.00 h |
| Kβ | 0.41 h−1 |
| Kα | 4.47 h−1 |
| T β 1/2 | 1.67 h |
| Tα 1/2 | 0.15 h |
| [AUC]0 10h | 88.90 mg•h/L |
| [AUC]0 ∞ | 93.07 mg•h/L |
[AUC]0 10h, area under serum concentration‐time curve from time 0 to 10 h; [AUC]0 ∞, area under serum concentration‐time curve from time 0 to infinity; Cmax, maximum serum concentration; Kβ, terminal rate constant; Kα, absorption rate constant; Tβ ½, terminal half‐life; Tα ½, absorption half‐life; Tmax: time to maximum concentration.
3.3. Gentamicin concentration in the ECM envelope remains stable for up to 7 days
Gentamicin concentration in the ECM envelope expressed as microgram per gram of explanted device is shown in Table 5. At the first time point (1 h), gentamicin level was 18.45 mg/g, subsequently fell to 2.21 mg/g at 6 h and to 52.71 µg/g at 24 h. After 24 h, gentamicin level remained relatively stable at 5‐8 µg/g for up to 7 days.
TABLE 5.
Gentamicin concentration in explanted ECM envelopes
| Total gentamicin (µg/g) | |||
|---|---|---|---|
| Time | n | Mean | SD |
| 1 h | 3 | 18450.45 | 3426.10 |
| 6 h | 3 | 2208.36 | 1797.08 |
| 15 h | 3 | 143.67 | 95.48 |
| 24 h | 3 | 52.71 | 20.85 |
| 48 h | 3 | 8.24 | 1.81 |
| 72 h | 3 | 7.08 | 1.27 |
| 7 days | 4 | 5.60 | 3.08 |
SD, standard deviation.
As previously mentioned, the ECM envelope is made of porcine small intestinal submucosa; therefore, following hydration, cellular infiltration, and fluid absorption during the implant period, the density of the explanted envelope is similar to soft tissue and approximately 1 g/mL. The gentamicin measured in microgram per gram in the explanted device could be translated directly to gentamicin concentration (µg/mL). For most microbial species that are susceptible to gentamicin, the in vitro minimal inhibitory concentrations (MIC) are approximately 1 to 2 µg/mL. 22 , 34 , 35 Therefore, the gentamicin concentration in the ECM envelopes was maintained well above the MIC for most clinically relevant bacterial species for at least 7 days, as shown in Figure 2.
FIGURE 2.
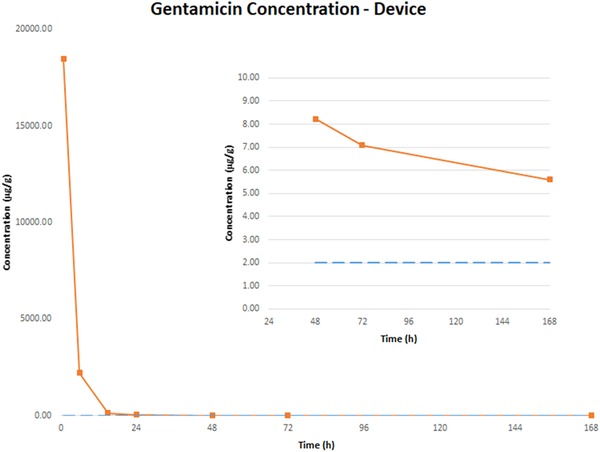
Gentamicin concentration in ECM envelopes following implantation
3.4. Significant reductions in bacterial colonization by gentamicin ECM envelope
In all studies, no target microorganisms were recovered from any sample in the environmental control group, indicating excellent asepsis and elimination of cross‐contamination. Representative gross photographs of devices at removal are shown in Figure 3. Signs of clinical infection were observed in the control (saline) group, but not in the gentamicin group.
FIGURE 3.
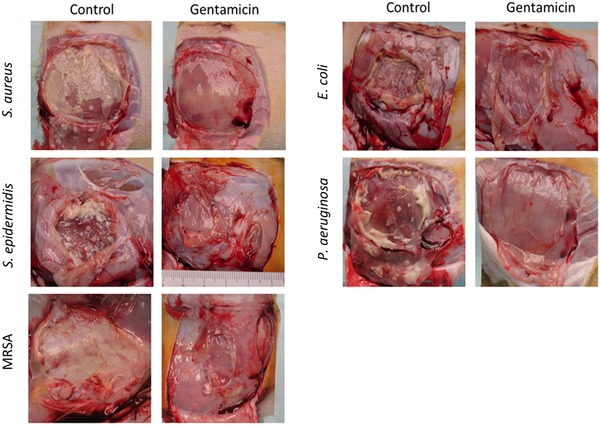
Representative gross necropsy photographs at 7 days
The results of quantitative bacterial colony counts are presented in Table 6. The average CFUs recovered from CIED, ECM envelope, and surrounding tissue and the average total CFU recovery from implant sites were calculated. In the presence of gentamicin containing ECM envelope, statistically significant reductions (P = .017 for S. epidermidis, P < .001 for all other bacteria tested) of >4‐log were demonstrated across all tested bacterial species.
TABLE 6.
Quantitative bacterial colony counts from animal studies recovered at day 7
| Group | # of samples with recovery | Average recovery (log10 CFU) | ||||||
|---|---|---|---|---|---|---|---|---|
| CIED | Envelope | Tissue | CIED | Envelope | Tissue | Average total recovery (log10 CFU) | ||
| S. aureus | Control | 10/10 | 10/10 | 10/10 | 7.84 | 8.70 | 7.32 | 8.79 |
| Treatment | 0/10 | 0/10 | 0/10 | 0 | 0 | 0 | 0 | |
| Log reduction | 7.84 | 8.70 | 7.32 | 8.79 | ||||
| S. epidermidis | Control | 10/10 | 10/10 | 10/10 | 5.40 | 6.38 | 4.28 | 6.43 |
| Treatment | 2/8 | 2/8 | 3/8 | 1.51 | 2.24 | 1.16 | 2.26 | |
| Log reduction | 3.89 | 4.14 | 3.12 | 4.17 | ||||
| MRSA | Control | 10/10 | 10/10 | 10/10 | 8.26 | 8.18 | 9.29 | 9.36 |
| Treatment | 0/10 | 0/10 | 0/10 | 0 | 0 | 0 | 0 | |
| Log reduction | 8.26 | 8.18 | 9.29 | 9.36 | ||||
| E. coli | Control | 10/10 | 10/10 | 10/10 | 7.97 | 8.29 | 8.91 | 9.12 |
| Treatment | 0/10 | 0/10 | 0/10 | 0 | 0 | 0 | 0 | |
| Log reduction | 7.97 | 8.29 | 8.91 | 9.12 | ||||
| P. aeruginosa | Control | 10/10 | 10/10 | 10/10 | 6.61 | 7.48 | 5.62 | 7.55 |
| Treatment | 0/10 | 0/10 | 0/10 | 0 | 0 | 0 | 0 | |
| Log reduction | 6.61 | 7.48 | 5.62 | 7.55 | ||||
Note. The bold values represent those with a >3 log reduction as above.
A ≥3‐log reduction constitutes a bactericidal effect whereas a <3‐log reduction is defined as a bacteriostatic effect.
4. DISCUSSION
The results of the current study demonstrate that gentamicin, delivered locally via ECM envelope, may minimize infection risk by rapidly reaching bactericidal levels in the implant pocket, with effective in vitro and in vivo killing of key bacterial species, and with minimal systemic exposure. In addition, macroscopic examination of the gentamicin containing ECM envelopes demonstrated no evidence of clinical infection when compared to the control groups.
4.1. Advantages of local gentamicin delivery
With regard to local delivery, preclinical studies of gentamicin have demonstrated concentration‐dependent killing of Gram‐negative and, at least partially, concentration‐dependent killing of Gram‐positive bacteria such as S. aureus. 22 This property means that higher concentrations of gentamicin result in significantly faster bacterial killing, even against organisms with low susceptibility or resistance to this agent. This relationship was demonstrated by a clinical study of sternal wound infection, which reported that treatment of wound infection using a collagen implant impregnated with gentamicin was effective, even when antibiotic‐resistant S. epidermidis was present, presumably due to high local concentrations of gentamicin. 36 Indeed, the clinical efficacy of local delivery of gentamicin for prevention of surgical site infection has been demonstrated across multiple indications, including cardiac, orthopedic, and gastrointestinal procedures, without risk of systemic exposure. 28 , 29 , 37 , 38 , 39 , 40 The very high local gentamicin concentrations achieved in this study suggest that bacterial killing should occur rapidly for key bacterial species.
While prolonged high serum levels promote the development of adaptive resistance to gentamicin, high peak drug concentrations at the surgical site and low systemic exposure may be protective against the development of resistant bacteria. 41 The first dose of an aminoglycoside appears to have the greatest bactericidal efficacy, as adaptive resistance among bacteria may develop following frequent and/or repeated exposure to antimicrobials. 42 , 43 In keeping with these properties, local administration of gentamicin via ECM envelope provides high early tissue concentrations of antibiotic, maximizing bacterial killing, and possibly minimizing risk for the development of resistance.
The advantage of high local gentamicin concentrations was evidenced by the time‐kill studies in our investigation, which demonstrated 3.9‐ to 5.8‐log reductions in bacterial colonies within 3 h for all tested bacterial species. Within 6 h, 100% killing was achieved for all bacterial species, with the exception of S. aureus (5.4‐log reduction at 6 h). The amount of gentamicin absorption and release in vitro were previously studied by Deering et al, 30 and the gentamicin concentration in our time‐kill experiment was approximately 1.5 mg/mL. This concentration is approximately 1000 higher than the MIC for susceptible species. Although this concentration cannot be achieved by traditional intravenous or intramuscular delivery of gentamicin, it can be easily achieved in vivo via ECM envelope, as demonstrated in the PK experiments (∼18.5 mg/mL at 1 h and 2.2 mg/mL at 6 h).
In the in vivo experiments evaluating the efficacy of gentamicin containing ECM envelope, complete (100%) reduction in bacterial colonies was achieved for S. aureus, MRSA, E. coli, and P. aeruginosa; and a greater than 4‐log reduction for S. epidermidis at the end of 7‐day study. A ≥3‐log reduction constitutes a bactericidal effect whereas a <3‐log reduction is defined as a bacteriostatic effect. 44 It has been reported that even a bacteriostatic effect may be effective in the treatment of Gram‐positive bacterial infections. 45 Moreover, an in vitro pharmacodynamics model evaluating the effect of gentamicin on Staphylococcus spp. demonstrated that a 3‐log kill (99.9% reduction in CFU/mL) was achieved across a six‐fold range of concentrations (note that the effectiveness between concentration groups was not statistically different). 34 Combined, these studies suggest that a 3‐log reduction is the clinically relevant threshold for supporting antimicrobial claims for an implant material coated or impregnated with gentamicin. Previous in vitro elution study 30 and also current in vivo PK study demonstrated that the gentamicin was released from the ECM envelope for at least 7 days. Therefore, the local antibiotic concentration was maintained above MIC level for a relatively long period of time, which cannot be simply achieved by antibiotic wash during the procedure.
Although CIED infections due to Gram‐negative bacteria represent a small proportion of cases, they mainly present as pocket infections and majority are caused by P. aeruginosa. 46 In a randomized clinical trial evaluating the efficacy of ABE, when analyzing the microbiology of breakthrough pocket infections, a sizable number of those cases was due to Gram‐negative bacteria, predominantly Pseudomonas spp. 47 Therefore, gentamicin containing ECM envelope may in theory be more effective than rifampin/minocycline combination in preventing pocket infections due to Gram‐negative bacteria.
Importantly, the results in animal studies may not necessarily translate to humans, and therefore, further clinical studies are needed to confirm efficacy of gentamicin containing ECM envelope in reducing the risk of major CIED infections.
4.2. Low risk of systemic exposure to gentamicin
Importantly, in the rabbits evaluated in the current study, serum gentamicin levels peaked early after implantation of the ECM envelope (∼1 h), then rapidly declined to below the lower limit of detection (and below the recommended safety trough concentration of 2 mg/L 27 after 15 h, suggesting that the risk of systemic exposure to gentamicin is low following implantation of the gentamicin containing ECM envelope.
The noncompartmental analysis identified PK parameters of serum gentamicin that are consistent with previous reports, including a terminal half‐life of approximately 2 h, indicating that systemically absorbed gentamicin is rapidly cleared from the blood. 48 These findings align with previous clinical studies, in which gentamicin delivered via collagen ECM or directly to the surgical site reached concentrations in local tissues that exceeded 300 mg/L, whereas systemic exposure was limited to approximately 1‐2 mg/L. 36 , 49 It is worth noting that the blood volume of a rabbit is approximately 3% of the blood volume of a human (∼0.15 L in rabbits and 5 L in humans). 50 , 51 Therefore, the concentration of gentamicin expected in the systemic circulation of a patient implanted with one gentamicin containing ECM envelope would be much lower than the levels observed in this rabbit study, and probably undetectable. However, low systemic exposure to gentamicin may be more relevant in older patients and those with pre‐existing renal failure. Studies in human subjects are needed to further determine the safety of gentamicin containing ECM envelope in these patients.
4.3. Limitations
The translation of experiments conducted in animal models into clinical practice requires further investigation. However, the animal model of cardiac device pocket infection here described has been used earlier to investigate the safety and efficacy of minocycline/rifampin synthetic ABE. 32 Notably, the results of such animal experiments were later confirmed in a prospective randomized clinical trial. 12
Although microscopic analysis of the biofilm present on the device surfaces was not performed in this study, vortexing/sonication is considered a surrogate. Conventional swabs and tissue cultures of the pocket would also be valuable to further confirm the results obtained by vortexing/sonication.
5. CONCLUSIONS
The results of this preclinical animal infection model demonstrate high, sustained local gentamicin concentrations and excellent bacterial killing in vivo following implantation of the gentamicin containing ECM envelope, with a low risk of systemic exposure to gentamicin.
CONFLICT OF INTEREST
Dr. Sohail reports receiving funds from TYRX Inc. and Medtronic for prior research unrelated to this study administered according to a sponsored research agreement between Mayo Clinic and study sponsor that prospectively defined the scope of the research effort and corresponding budget; and honoraria/consulting fees from Medtronic Inc. and Aziyo Biologics, Inc.
Research Grant: Medtronic (significant ‐ $40K)
Honoraria: Medtronic (significant $20K), and Aziyo Biologics (modest $5K)
John Catanzaro, MD: Honoraria/Consulting fee: Aziyo Biologics, Inc. (<US$10K).
All other authors: None. The authors report no conflicts of interest.
Supporting information
Table S1. Animal study design for pharmacokinetic study
Table S2. Study design of Staphylococcus aureus bacterial colonization study
Table S3. Study design of S. epidermidis bacterial colonization study
Table S4. Study design of E. coli bacterial colonization study
Table S5. Study design of P. aeruginosa bacterial colonization study
Table S6. Study groups of MRSA bacterial colonization study
Sohail MR, Esquer Garrigos Z, Elayi CS, Xiang K, Catanzaro JN. Preclinical evaluation of efficacy and pharmacokinetics of gentamicin containing extracellular‐matrix envelope. Pacing Clin Electrophysiol. 2020;43:341–349. 10.1111/pace.13888
REFERENCES
- 1. Voigt A, Shalaby A, Saba S. Continued rise in rates of cardiovascular implantable electronic device infections in the United States: temporal trends and causative insights. Pacing Clin Electrophysiol. 2010;33:414‐419. [DOI] [PubMed] [Google Scholar]
- 2. Shakya S, Matsui H, Fushimi K, Yasunaga H. In‐hospital complications after implantation of cardiac implantable electronic devices: analysis of a national inpatient database in Japan. J Cardiol. 2017;70:405‐410. [DOI] [PubMed] [Google Scholar]
- 3. Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter‐defibrillators: calendar year 2009—a world society of arrhythmia's project. Pacing Clin Electrophysiol. 2011;34:1013‐1027. [DOI] [PubMed] [Google Scholar]
- 4. Greenspon AJ, Patel JD, Lau E, et al. 16‐year trends in the infection burden for pacemakers and implantable cardioverter‐defibrillators in the United States 1993 to 2008. J Am Coll Cardiol. 2011;58:1001‐1006. [DOI] [PubMed] [Google Scholar]
- 5. Dai M, Cai C, Vaibhav V, et al. Trends of cardiovascular implantable electronic device infection in 3 decades: a population‐based study. JACC Clin Electrophysiol. 2019;5:1071‐1080. [DOI] [PubMed] [Google Scholar]
- 6. Rohacek M, Baddour LM. Cardiovascular implantable electronic device infections: associated risk factors and prevention. Swiss Med Wkly. 2015;145:w14157. [DOI] [PubMed] [Google Scholar]
- 7. Lekkerkerker JC, van Nieuwkoop C, Trines SA, et al. Risk factors and time delay associated with cardiac device infections: Leiden device registry. Heart. 2009;95:715‐720. [DOI] [PubMed] [Google Scholar]
- 8. Romeyer‐Bouchard C, Da Costa A, Dauphinot V, et al. Prevalence and risk factors related to infections of cardiac resynchronization therapy devices. Eur Heart J. 2010;31:203‐210. [DOI] [PubMed] [Google Scholar]
- 9. Sohail MR, Eby EL, Ryan MP, Gunnarsson C, Wright LA, Greenspon AJ. Incidence, treatment intensity, and incremental annual expenditures for patients experiencing a cardiac implantable electronic device infection: evidence from a large us payer database 1‐year post implantation. Circ Arrhythm Electrophysiol. 2016;9:e003929. [DOI] [PubMed] [Google Scholar]
- 10. Baddour LM, Epstein AE, Erickson CC, et al, American Heart Association Rheumatic Fever E, Kawasaki Disease C, Council on Cardiovascular Disease in Y, Council on Cardiovascular S, Anesthesia, Council on Cardiovascular N, Council on Clinical C, Interdisciplinary Council on Quality of C and American Heart A. Update on cardiovascular implantable electronic device infections and their management: a scientific statement from the American Heart Association. Circulation. 2010;121:458‐477. [DOI] [PubMed] [Google Scholar]
- 11. de Oliveira JC, Martinelli M, Nishioka SAD, et al. Efficacy of antibiotic prophylaxis before the implantation of pacemakers and cardioverter‐defibrillators results of a large, prospective, randomized, double‐blinded, placebo‐controlled trial. Circ‐Arrhythmia Elec. 2009;2:29‐34. [DOI] [PubMed] [Google Scholar]
- 12. Tarakji KG, Mittal S, Kennergren C, et al. Antibacterial envelope to prevent cardiac implantable device infection. N Engl J Med. 2019;381:1782‐1784. [DOI] [PubMed] [Google Scholar]
- 13. Krahn AD, Longtin Y, Philippon F, et al. Prevention of arrhythmia device infection trial: the PADIT trial. J Am Coll Cardiol. 2018;72:3098‐3109. [DOI] [PubMed] [Google Scholar]
- 14. Badylak SF, Freytes DO, Gilbert TW. Reprint of: extracellular matrix as a biological scaffold material: structure and function. Acta Biomater. 2015;23:S17‐26. [DOI] [PubMed] [Google Scholar]
- 15. Cavallo JA, Greco SC, Liu J, Frisella MM, Deeken CR, Matthews BD. Remodeling characteristics and biomechanical properties of a crosslinked versus a non‐crosslinked porcine dermis scaffolds in a porcine model of ventral hernia repair. Hernia. 2015;19:207‐218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fallon AM, Goodchild TT, Cox JL, Matheny RG. In vivo remodeling potential of a novel bioprosthetic tricuspid valve in an ovine model. J Thorac Cardiovasc Surg. 2014;148:333‐340 e1. [DOI] [PubMed] [Google Scholar]
- 17. Brown BN, Londono R, Tottey S, et al. Macrophage phenotype as a predictor of constructive remodeling following the implantation of biologically derived surgical mesh materials. Acta Biomater. 2012;8:978‐987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wolf MT, Carruthers CA, Dearth CL, et al. Polypropylene surgical mesh coated with extracellular matrix mitigates the host foreign body response. J Biomed Mater Res A. 2014;102:234‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Medberry CJ, Tottey S, Jiang H, Johnson SA, Badylak SF. Resistance to infection of five different materials in a rat body wall model. J Surg Res. 2012;173:38‐44. [DOI] [PubMed] [Google Scholar]
- 20. Milburn ML, Holton LH, Chung TL, et al. Acellular dermal matrix compared with synthetic implant material for repair of ventral hernia in the setting of peri‐operative Staphylococcus aureus implant contamination: a rabbit model. Surg Infect (Larchmt). 2008;9:433‐442. [DOI] [PubMed] [Google Scholar]
- 21. Brennan EP, Reing J, Chew D, Myers‐Irvin JM, Young EJ, Badylak SF. Antibacterial activity within degradation products of biological scaffolds composed of extracellular matrix. Tissue Eng. 2006;12:2949‐2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tam VH, Kabbara S, Vo G, Schilling AN, Coyle EA. Comparative pharmacodynamics of gentamicin against Staphylococcus aureus and Pseudomonas aeruginosa . Antimicrob Agents Chemother. 2006;50:2626‐2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Salmeri M, Sorbello MG, Mastrojeni S, et al. Infections of cardiovascular implantable electronic devices: 14 years of experience in an Italian hospital. Infez Med. 2016;24:131‐136. [PubMed] [Google Scholar]
- 24. Wang R, Li X, Wang Q, Zhang Y, Wang H. Microbiological characteristics and clinical features of cardiac implantable electronic device infections at a tertiary hospital in China. Front Microbiol. 2017;8:360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stokkou S, Tammer I, Zibolka S, Grabau C, Geginat G. Impact of minimal inhibitory concentration breakpoints on local cumulative bacterial susceptibility data and antibiotic consumption. BMC Res Notes. 2014;7:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dall GF, Tsang SJ, Gwynne PJ, et al. Unexpected synergistic and antagonistic antibiotic activity against Staphylococcus biofilms. J Antimicrob Chemother. 2018;73:1830‐1840. [DOI] [PubMed] [Google Scholar]
- 27. Gentamicin [prescribing information] . Lake Zurich, IL: Fresenius Kabi; 2015. https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/062366s033lbl.pdf. [Google Scholar]
- 28. Koerber SM, Turagam MK, Winterfield J, Gautam S, Gold MR. Use of antibiotic envelopes to prevent cardiac implantable electronic device infections: a meta‐analysis. J Cardiovasc Electrophysiol. 2018;29:609‐615. [DOI] [PubMed] [Google Scholar]
- 29. Futyma PR, Gluszczyk R, Ciapala K, Futyma M. Zero device‐related infections in 4285 patient‐years of follow‐up after cardiac implantable electronic device replacement combined with topic gentamicin‐collagen sponge application. EP Europace. 2017;19:iii306. [Google Scholar]
- 30. Deering TF, Chang C, Snyder C, Natarajan SK, Matheny R. Enhanced antimicrobial effects of decellularized extracellular matrix (CorMatrix) with added vancomycin and gentamicin for device implant protection. Pacing Clin Electrophysiol. 2017;40:615‐623. [DOI] [PubMed] [Google Scholar]
- 31. Food and Drug Administration . Good Laboratory Practice for Nonclinical Laboratory Studies. 21 CFR. Part 58.
- 32. Hansen LK, Brown M, Johnson D, Palme Ii DF, Love C, Darouiche R. In vivo model of human pathogen infection and demonstration of efficacy by an antimicrobial pouch for pacing devices. Pacing Clin Electrophysiol. 2009;32:898‐907. [DOI] [PubMed] [Google Scholar]
- 33. Hansen LK, Berg K, Johnson D, Sanders M, Citron M. Efficacy of local rifampin/minocycline delivery (AIGIS(RX)(R)) to eliminate biofilm formation on implanted pacing devices in a rabbit model. Int J Artif Organs. 2010;33:627‐635. [DOI] [PubMed] [Google Scholar]
- 34. Schafer JA, Hovde LB, Rotschafer JC. Consistent rates of kill of staphylococcus aureus by gentamicin over a 6‐fold clinical concentration range in an in vitro pharmacodynamic model (IVPDM). J Antimicrob Chemother. 2006;58:108‐111. [DOI] [PubMed] [Google Scholar]
- 35. Zhuang L, He Y, Xia H, Liu Y, Sy SK, Derendorf H. Gentamicin dosing strategy in patients with end‐stage renal disease receiving haemodialysis: evaluation using a semi‐mechanistic pharmacokinetic/pharmacodynamic model. J Antimicrob Chemother. 2016;71:1012‐1021. [DOI] [PubMed] [Google Scholar]
- 36. Leyh RG, Bartels C, Sievers HH. Adjuvant treatment of deep sternal wound infection with collagenous gentamycin. Ann Thorac Surg. 1999;68:1648‐1651. [DOI] [PubMed] [Google Scholar]
- 37. Friberg O, Svedjeholm R, Soderquist B, Granfeldt H, Vikerfors T, Kallman J. Local gentamicin reduces sternal wound infections after cardiac surgery: a randomized controlled trial. Ann Thorac Surg. 2005;79:153‐161. [DOI] [PubMed] [Google Scholar]
- 38. Kepa K, Krzych L, Krejca M. Gentamicin‐containing collagen implant reduces sternal wound complications after cardiac surgery: a retrospective analysis. Int J Surg. 2015;13:198‐206. [DOI] [PubMed] [Google Scholar]
- 39. Kowalewski M, Pawliszak W, Zaborowska K, et al. Gentamicin‐collagen sponge reduces the risk of sternal wound infections after heart surgery: meta‐analysis. J Thorac Cardiovasc Surg. 2015;149:1631‐1640. [DOI] [PubMed] [Google Scholar]
- 40. Friberg O, Dahlin LG, Kallman J, Kihlstrom E, Soderquist B, Svedjeholm R. Collagen‐gentamicin implant for prevention of sternal wound infection: long‐term follow‐up of effectiveness. Interact Cardiovasc Thorac Surg. 2009;9:454‐458. [DOI] [PubMed] [Google Scholar]
- 41. Pagkalis S, Mantadakis E, Mavros MN, Ammari C, Falagas ME. Pharmacological considerations for the proper clinical use of aminoglycosides. Drugs. 2011;71:2277‐2294. [DOI] [PubMed] [Google Scholar]
- 42. Kashuba AD, Bertino JS, Jr , Nafziger AN. Dosing of aminoglycosides to rapidly attain pharmacodynamic goals and hasten therapeutic response by using individualized pharmacokinetic monitoring of patients with pneumonia caused by Gram‐negative organisms. Antimicrob Agents Chemother. 1998;42:1842‐1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xiong YQ, Caillon J, Kergueris MF, et al. Adaptive resistance of Pseudomonas aeruginosa induced by aminoglycosides and killing kinetics in a rabbit endocarditis model. Antimicrob Agents Chemother. 1997;41:823‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Barry AL, Craig WA, Nadler H, Reller LB, Sanders CC, Swanson JM. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline. Wayne, PA: Clinical and Laboratory Standards Institute; 1999. [Google Scholar]
- 45. Pankey GA, Sabath LD. Clinical relevance of bacteriostatic versus bactericidal mechanisms of action in the treatment of Gram‐positive bacterial infections. Clin Infect Dis. 2004;38:864‐870. [DOI] [PubMed] [Google Scholar]
- 46. Esquer Garrigos Z, George MP, Vijayvargiya P, et al. Clinical presentation, management, and outcomes of cardiovascular implantable electronic device infections due to Gram‐negative versus Gram‐positive bacteria. Mayo Clin Proc. 2019;94:1268‐1277. [DOI] [PubMed] [Google Scholar]
- 47. Muhammad R, Sohail M, Ralph CoreyG, et al. Reduced CIED infections with an antibacterial envelope: microbiologic analysis of the WRAP‐IT study. Open Forum Infectious Diseases. 2019;6:S16. [Google Scholar]
- 48. Turnidge J. Pharmacodynamics and dosing of aminoglycosides. Infect Dis Clin North Am. 2003;17:503‐528. [DOI] [PubMed] [Google Scholar]
- 49. Friberg O, Jones I, Sjoberg L, Soderquist B, Vikerfors T, Kallman J. Antibiotic concentrations in serum and wound fluid after local gentamicin or intravenous dicloxacillin prophylaxis in cardiac surgery. Scand J Infect Dis. 2003;35:251‐254. [DOI] [PubMed] [Google Scholar]
- 50. Baby PM, Kumar P, Kumar R, et al. A novel method for blood volume estimation using trivalent chromium in rabbit models. Indian J Plast Surg. 2014;47:242‐248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Sharma R, Sharma S. Physiology, Blood Volume. Treasure Island, FL: StatPearls; 2019. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Animal study design for pharmacokinetic study
Table S2. Study design of Staphylococcus aureus bacterial colonization study
Table S3. Study design of S. epidermidis bacterial colonization study
Table S4. Study design of E. coli bacterial colonization study
Table S5. Study design of P. aeruginosa bacterial colonization study
Table S6. Study groups of MRSA bacterial colonization study


