Abstract
Introduction
Women with inherited platelet receptor defects (IPRD) may have an increased risk of heavy menstrual bleeding (HMB) and postpartum haemorrhage (PPH).
Aim
To present a systematic overview of the literature on the prevalence and management of menstrual and obstetrical bleeding in women with IPRD.
Methods
Electronic databases were searched for original patient data on the prevalence and management of HMB and PPH in women with known IPRD or who were being investigated for IPRD.
Results
Sixty‐nine papers (61 case reports/series and 8 cohort studies) were included. Overall, studies were rated as ‘poor quality’. The included cohort studies reported HMB in 25% (13/52) of women with Bernard‐Soulier syndrome and in 22.1% (34/154) of women with Glanzmann thrombasthenia. In total, 164 deliveries in women with IPRD were described. Excessive bleeding occurred in 16.9% (11/65) of deliveries described in the largest cohort. PPH occurred in 63.2% (55/87) of deliveries described in case reports/series. PPH occurred in 73.7% (14/19) of deliveries that were not covered by prophylaxis compared with 54.2% (32/59) of deliveries that were (OR = 2.36, 95% CI 0.75‐7.40). Neonatal bleeding complications were reported in 10.0% (8/80) of deliveries. In all (6/6) deliveries with neonatal bleeding complications wherein the presence of alloantibodies was investigated, either antiplatelet or anti‐HLA antibodies were detected.
Discussion/Conclusion
Menstrual and particularly obstetrical bleeding problems frequently occur in women with IPRD, based on small case reports and series of poor quality. International collaboration, preferably on prospective studies, is needed to improve clinical management of women‐specific bleeding in IPRD.
Keywords: Bernard‐Soulier syndrome, Glanzmann thrombasthenia, gynaecological bleeding, heavy menstrual bleeding, inherited platelet receptor defects, obstetrical bleeding, postpartum haemorrhage
1. INTRODUCTION
Inherited platelet receptor defects (IPRD) are a subgroup of inherited platelet disorders (IPD) characterized by qualitative or quantitative deficiencies in platelet membrane receptors.1 The group comprises six disorders: Glanzmann thrombasthenia (GT), Bernard‐Soulier syndrome (BSS), platelet‐type von Willebrand disease (PT‐VWD), ADP receptor (P2Y12) defects, thromboxane A2 receptor (TxA2) defects and glycoprotein VI (GPVI) deficiency. These receptor defects cause impaired primary haemostasis at the site of a blood vessel injury, often leading to a bleeding diathesis.1 In Western countries, the population prevalence of GT and BSS is approximately 1 in 1 000 000.2 Prevalence of the other IPRD is unknown, but less than a hundred affected individuals with TxA2 and P2Y12 deficiency worldwide have been reported in the literature, unlikely reflecting the true prevalence.3 PT‐VWD and TxA2 defects are inherited in an autosomal dominant pattern, whereas GT, BSS, P2Y12 defects and GPVI deficiency are all autosomal recessive disorders. GT and BSS are more common in populations with a high incidence of consanguineous marriages, such as some communities in Iran.3
Clinical manifestations are diverse and may vary from recurrent mucocutaneous bleeding such as epistaxis, gingival bleeding, petechiae and abnormal bruising to less common but potentially fatal manifestations such as intracranial and postpartum haemorrhage (PPH).3 Moreover, affected women often suffer from heavy menstrual bleeding (HMB).4, 5, 6 HMB in women with IPRD can be challenging to manage and has been associated with a reduced quality of life.7, 8 Furthermore, women appear to have an increased risk of develop PPH. Previous reviews on pregnancy outcomes in women with GT and BSS report a PPH prevalence of 34% and 33%, respectively.9, 10 These numbers are generally higher than the population prevalence of PPH, which is approximately 19%.11, 12 Neonates are at increased risk of bleeding, such as intracranial haemorrhage and subsequent death, due to placental transfer of maternal antiplatelet antibodies directed against foetal thrombocytes.3 Thus, pregnancy in women with IPRD can cause severe maternal and neonatal morbidity and even mortality.
Accurate data on the prevalence of HMB and PPH in women with IPRD are lacking, and clinical guidelines on the management are currently based on empirical evidence, as the separate IPRD are rare and randomized controlled trials have not been performed.13 In 2006, the UKHCDO formulated guidelines for pregnancy in women with severe platelet disorders, including GT and BSS.3 Most of these guidelines were based on expert opinion and case reports. In a review paper by Grainger et al (2018), these guidelines were re‐evaluated, yet no consistent approach to the prevention or treatment of bleeding complications is provided.2
The aim of this paper is to present an overview of the literature on the prevalence and management of menstrual and obstetrical bleeding in women with IPRD to provide a complete literature background aiding clinical decision‐making.
2. METHODS
2.1. Protocol and registration
The protocol details for this systematic review can be found in the International Prospective Register of Systematic Reviews (registration number CRD42018115116).14 This review was conducted in accordance with the Preferred Reporting Items for Systematic Review and Meta‐Analyses (PRISMA) statement and Cochrane methods.15, 16 The database search covered all IPD, but due to the amount of data, the authors decided to focus on IPRD in this review.
2.2. Review questions
This systematic review addresses the following research questions: (a) What has been published on the prevalence of HMB in women with IPRD? (b) What has been published on the prevalence of pregnancy‐related bleeding in women with IPRD reported in literature? (c) Which management strategies have been published on women with IPRD and HMB? and (d) Which management strategies have been published on women with IPRD and pregnancy‐related bleeding?
2.3. Inclusion and exclusion criteria
Studies were considered eligible for inclusion if they had an observational or interventional study design and included women already diagnosed with IPRD or women with HMB or PPH who were investigated for platelet receptor defects. Only studies in English or Dutch, concerning the prevalence and management of HMB and pregnancy‐related bleeding and containing original patient data, were included. Abstracts, posters and articles without full‐text access as well as articles that merely mentioned the prevalence of IPRD in a group of women with HMB were excluded.
2.4. Data sources and search strategy
The electronic bibliographic databases PubMed, The Cochrane Library, Embase and CINAHL were searched up to the 16th of January 2019. A combination of search terms and MeSH/Emtree terms related to IPD, HMB and pregnancy was used (Appendix S1). No search limits were applied.
2.5. Study selection
Search results were merged using Mendeley Reference Management Software, and duplicate references were removed using the Mendeley Deduplicate Tool and by hand. Two reviewers (PS and MP) independently screened the titles and abstracts to identify potentially relevant articles. Subsequently, full‐text papers were retrieved and assessed on eligibility by two independent reviewers (PS and MP). Whenever the full text of an article was unobtainable, the corresponding author was contacted once. Any difference of opinion between the reviewers concerning study selection was resolved by consulting a third reviewer (KG). Cross‐referencing was conducted in the included studies and relevant reviews.
2.6. Data extraction
One reviewer (PS) extracted data from the included articles using a standardized data collection form (Appendix S2). A second reviewer (MP) double‐checked all articles for accuracy of data extraction. HMB was defined as a Pictorial Blood Loss Assessment Chart (PBAC) score > 100, reflecting at least one menstrual cycle.17 In the absence of PBAC scores, the author's definition of HMB was used. Primary PPH was defined as estimated blood loss ≥ 500 mL occurring within 24 hours of delivery, and severe PPH was defined as estimated blood loss ≥ 1000 mL.18 Secondary PPH was defined as excessive bleeding requiring medical attention between 24 hours and three months after delivery.18 Treatment of HMB or PPH could consist of desmopressin (DDAVP), iron supplements, hormonal treatment, antifibrinolytics, uterotonic agents, blood products, surgery or other interventions (eg fibrinogen concentrates, local compression devices and crystalloids). Prophylactic treatment for delivery and the postpartum period included measures taken to prevent PPH and could consist of blood products (erythrocytes, platelets, plasma, coagulation factors, plasmapheresis), recombinant Factor VIIa (rFVIIa), DDAVP or other preventive measures (eg steroids, uterotonic agents, antifibrinolytics, fibrinogen concentrate and operative and invasive procedures such as preventive hysterectomy and embolization). Study design was based upon the following criteria: a cohort study in case all eligible patients during a certain time period were included in the study, and a case series if patient selection was not described.19 Any doubt regarding the extraction of data was resolved by consulting a third reviewer (KG).
2.7. Quality assessment
One reviewer (PS) assessed the quality of each included study through an adjusted Chambers scale, and each assessment was checked by a second reviewer (MP) (Appendix S3).20 This adjusted version of the Chambers scale including only criteria relevant to the included study designs was used to provide an illustrative reflection of the quality of small case series. Studies were rated as ‘good’, ‘satisfactory’ or ‘poor’. Any doubt regarding quality assessment was resolved by consulting a third reviewer (KG).
2.8. Data synthesis
The quantitative results were described separately for case reports and series and cohort studies. A descriptive overview was provided for prevalence and (prophylactic) treatment of HMB in women with IPRD and for deliveries and the postpartum period in women with IPRD. Due to the limited amount of data on the different subtypes of IPRD, it was not possible to perform any subgroup analyses. A narrative synthesis on menstruation includes patient and menstruation characteristics and treatment of HMB. A narrative synthesis on pregnancy includes patient and pregnancy characteristics, prophylaxis for delivery, PPH and neonatal outcome.
3. RESULTS
A total of 1261 articles were screened after duplicate removal and addition of eleven articles identified through cross‐reference searching (Figure 1). One hundred and thirty‐two articles were examined in full text, of which 69 were eventually included in this systematic review. These comprised 61 case reports and series 13, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 62, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 78, 79, 80 and eight cohort studies.4, 81, 82, 83, 84, 85, 86, 87 The exact number of women with HMB is uncertain due to incomplete reporting in one article.87 In total, 164 deliveries in women with IPRD were described.
Figure 1.
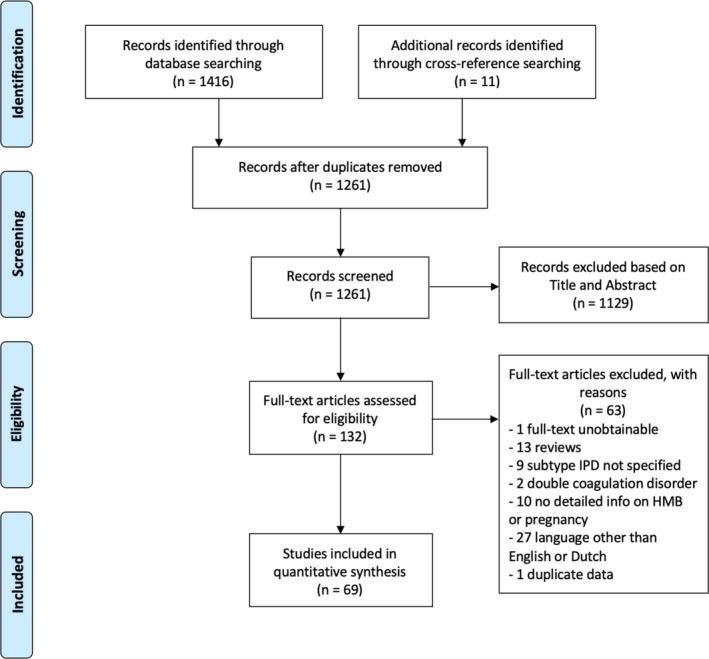
PRISMA flow diagram of study selection
3.1. Quality assessment
A summary of the quality assessment of the included studies according to the study population size is provided in Table 1. A complete overview of the quality assessment of each included study is provided in Appendix S4. None of the included studies were considered of high quality. Three studies (4.3%) were rated as ‘satisfactory’ and the remaining 66 studies (95.7%) were rated as ‘poor’. Main limitations were seen across the entire quality assessment including the lack of diagnostic criteria, inadequate reporting of the outcome measures and the absence of reporting on prognostic/confounding factors.
Table 1.
Risk of bias assessment of small, medium and large studies using the adjusted Chambers scale
| Study population sizea | Percentage of studies in which criterium is fulfilled (% yes) | |||||||
|---|---|---|---|---|---|---|---|---|
| Number of studies | 1. Were diagnostic criteria adequately reported? | 2. Was the selected population representative of that seen in normal practice? | 3. Was the outcome measure adequately reported?b | 4. Was loss to follow‐up reported or explained? | 5. Were those included at baseline sufficiently long followed up?c | 6. Were patients recruited prospectively? | 7. Did the study report relevant prognostic/confounding factors? | |
| Small (n = 1‐10) | 57 | 1.8 | 1.8 | 36.8 | 0.0 | 0.0 | 0.0 | 33.3 |
| Medium (n = 11‐50) | 6 | 66.7 | 50.0 | 33.3 | 0.0 | 83.3 | 50.0 | 16.7 |
| Large (n = 51‐382) | 6 | 83.3 | 100.0 | 33.3 | 0.0 | 100.0 | 50.0 | 33.3 |
Overall quality rating: good (if the answer is ‘yes’ to all criteria); satisfactory (if the answer is ‘yes’ to 5‐6 criteria); poor (if the answer is ‘yes’ to less than 5 criteria). In this review, overall quality of included studies is ‘poor’.
References: small studies,20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51, 52, 53, 54, 55, 56, 57, 58, 59, 60, 61, 63, 64, 65, 66, 67, 68, 69, 70, 71, 72, 73, 74, 75, 76, 77, 79 medium studies,13, 38, 62, 78, 83, 86 large studies.4, 80, 81, 82, 84, 85
Refers to all patients included in a study.
Meaning blood loss in mL for postpartum hemorrhage (PPH) and PBAC scores for heavy menstrual bleeding (HMB).
At least three months for PPH and two consecutive menstrual cycles for HMB.
3.2. Narrative synthesis of results on menstruation
3.2.1. Data from case reports and series
A total of 32 women with at least one episode of HMB were described in 18 case reports and series. These included 18 women with GT (56.3%) and 14 women with BSS (43.8%). No data were available on PT‐VWD, TxA2, P2Y12 and GPVI deficiency. Mean age (±SD) was 19 years (7.67) in the 21 cases with available information. Median age at diagnosis of IPRD was 15 years (range 14 months – 50 years) in the 17 women for whom this was documented. HMB had been present since menarche in 80% (16/20) of cases in which the timing of the first manifestation of HMB was reported.
None of the individual cases used PBAC scores to diagnose HMB. HMB was defined as blood loss > 80 mL per menstrual cycle in 9.4% (3/32) of individual cases. Menstrual bleeding was described as ‘mild’ or ‘moderate’ in 9.4% (3/32) of women, described as ‘prolonged’, ‘severe’, ‘excessive’ or ‘unrestrainable’ in 65.6% (21/32) of women. In the remaining 15.6% (5/32) of cases, no other description than ‘meno‐ or metrorrhagia’ was given.
All included women received treatment for HMB (Figure 2). Most frequently prescribed treatments were blood products, hormonal therapy and antifibrinolytics, in 87.5% (28/32), 84.4% (27/32) and 53.1% (17/32) of women, respectively. Blood products consisted of red blood cell transfusions in 22 women, platelet transfusions in twenty women, whole blood transfusions in five women, plasma transfusions in four women and for one woman, the type of transfusion was unspecified. Two women were treated with DDAVP. They did not experience any adverse events such as hyponatremia due to this treatment. Surgery for HMB was performed in 21.9% (7/32) of women and mainly included thermal endometrial ablation and examination under anaesthesia (EUA) with uterine tamponade. Three (9.4%) women also received other forms of therapy including uterotonic agents, steroids and IVIG. No deaths due to HMB were reported.
Figure 2.
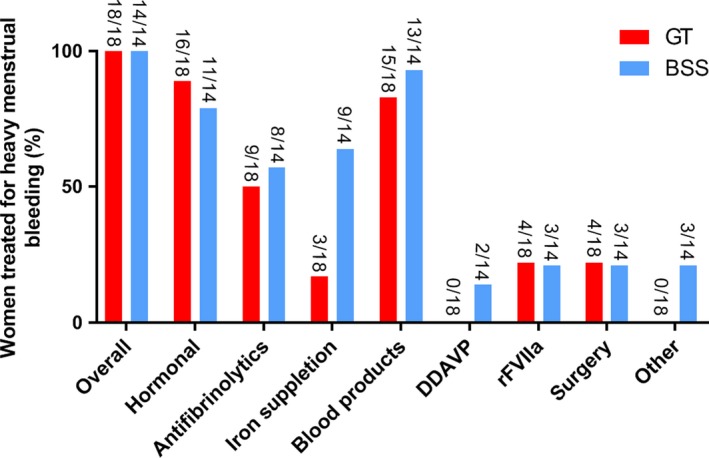
Treatment of heavy menstrual bleeding according to the subtype of inherited platelet receptor defect. GT, Glanzmann thrombasthenia; BSS, Bernard‐Soulier syndrome; rFVIIa, recombinant Factor VIIa; DDAVP, desmopressin; Other = steroids and immunoglobulins
3.2.2. Data from cohort studies
Seven cohort studies with information about menstruation described a total of 189 women with GT and 58 women with BSS (Table 2).
Table 2.
Cohort studies on heavy menstrual bleeding in women with inherited platelet receptor defects
| Author, year | Study design, study population, HMB definition | Results | HMB treatment | |
|---|---|---|---|---|
| HMB in women with inherited platelet receptor defects | Awidi (1992)87 |
Prospective cohort study 25 women with GT HMB definition unknown |
HMB incidence unclear | 48% (12/25) of women together received 140 units of blood and 80 units of platelets for treatment of HMB during a total follow‐up of 145 years. In 57.1% (4/7) of women that used OCPs, HMB was successfully managed, whereas in 42.9% (3/7), the need for transfusions was unchanged |
| Vijapurkar (2009)86 |
Prevalence of HMB among Indian women with rare bleeding disorders HMB defined as > 80 mL blood loss per menstruation lasting more than seven days |
HMB was reported in 66.7% (2/3) of women with BSS and in 77.3% (17/22) of women with GT. In two women, HMB led to iron deficiency anaemia despite the use of OCPs | Management mainly included tranexamic acid and OCPs. Both women with BSS received platelet and blood transfusions in combination with OCPs. 11.8% (2/17) of women required more than a hundred units of platelets and whole blood transfusions over the course of more than twenty years, one of whom was later efficaciously treated with endometrial ablation. | |
| Toogeh (2010)82 |
Retrospective cohort study 49 Iranian women with BSS HMB definition unknown |
22.4% (11/49) of women had had at least one episode of HMB. | Not described | |
| Toogeh (2004) 4 |
Retrospective cohort study 132 Iranian women of reproductive age diagnosed with GT HMB definition unknown |
12.9% (17/132) of women had had at least one episode of HMB | Not described | |
| Inherited platelet receptor defects in women with HMB | Kushwaha (2017)83 |
Prospective study 104 women who presented at the emergency department with complaints of menorrhagia in whom local pelvic pathology and hormonal disorders had been eliminated as a cause HMB definition not provided |
Identified seven women with GT (6.7%) and one woman with BSS (1.0%) | Not described |
| Cakı Kılıç (2013) 85 |
Sixty women of reproductive age women who presented at the gynaecology and haematology outpatient and emergency care units HMB defined as > 80 mL blood loss per menstrual cycle |
Identified two women with GT (3.3%) and three women with BSS (5.0%) | Detailed information on HMB treatment was not available, except that all included women received oral tranexamic acid and none of them underwent endometrial ablation or hysterectomy | |
| Hutspardol (2010) 84 |
28 adolescents with a history of menorrhagia HMB defined as > 80 mL blood loss per menstrual cycle |
One woman with GT (3.6%) and two women with BSS (7.1%) | HMB was successfully treated using a combination of OCPs and tranexamic acid |
Abbreviations: GT, Glanzmann thrombasthenia; BSS, Bernard‐Soulier syndrome; HMB, heavy menstrual bleeding; OCP, oral contraceptive pills.
Four cohort studies investigated the prevalence of HMB in women with IPRD.4, 82, 86, 87 Overall, 25% (13/52) of women with BSS experienced at least one episode of HMB 82, 86 and 22.1% (34/154) of women with GT experienced HMB,4, 86 but the exact number was uncertain due to incomplete reporting in one article.87 Awidi et al and Vijapurkar et al report the following blood product use: 48% (12/25) of women together received 140 units of blood and 80 units of platelets for treatment of HMB during a total follow‐up of 145 patient years and 11.8% (2/17) of women required more than a hundred units of platelets and whole blood transfusions over the course of more than twenty years, respectively.86, 87
Three cohort studies investigated the prevalence of IPRD in women with HMB.83, 84, 85 Out of 104 women who presented at the emergency department in India with complaints of menorrhagia not caused by local pelvic pathology or hormonal disorders, 6.7% (7/104) was diagnosed with GT and 1.0% (1/104) with BSS (Table 2).
3.3. Narrative synthesis of results on pregnancy
3.3.1. Narrative synthesis of data from case reports and series
A total of 90 deliveries were described in 45 case reports and series in 66 women with IPRD. In 37.9% (25/66) of women, diagnosis had been made before pregnancy, in 6.1% (4/66) during pregnancy and in 6.1% (4/66) after pregnancy. For the remaining 50.0% (33/66) of women, the timing of diagnosis in relation to pregnancy was not stated. 10.6% (7/66) of women were known to be born to consanguineous parents. Bleeding history before pregnancy was documented in 84.8% (56/66) of women, and a bleeding tendency was reported in 96.4% (54/56) of women. The most common bleeding symptoms were epistaxis (48.1%), menorrhagia (46.3%), easy bruising (22.2%) and gingival bleeding (13.0%). Antenatal bleeding was reported in 41.2% (21/30) of pregnancies where the antepartum period was described. The mean maternal age at delivery was 28 years ± 5.08 SD. Information on alloantibody formation (against HLA and/or the lacking glycoprotein) was available in 43.3% (39/90) of deliveries, and they were present in 66.7% (26/39). The mode of delivery was reported in 92.2% (83/90) of deliveries and comprised 39.8% (33/83) normal vaginal deliveries, 7.2% (6/83) assisted vaginal deliveries (3 forceps deliveries, 2 vacuum extractions and 1 unspecified instrumental delivery) and 53.0% (44/83) caesarean sections.
Peri‐ and postpartum prophylactic treatment
Information on prophylactic therapy administration was available in 88.9% (80/90) of individual cases. Overall, 75.0% (60/80) of deliveries were covered by some kind of prophylaxis and 25.0% (20/80) of deliveries were not (Figure 3). The included papers did not describe the reason for prophylaxis usage and, therefore, we could not distillate any differences between the group which received prophylactic treatment and the group which did not. Blood products were most often used as prophylaxis (85.0%, 51/60), followed by rFVIIa (15.0%, 9/60) and DDAVP (1.7%, 1/60). Blood products consisted of platelet transfusions in 47 cases, platelet plus red blood cell transfusions in two cases and platelet‐rich plasma in two other cases. Other prophylactic therapies were given in 53.3% (32/60) of deliveries and included intravenous immunoglobulin (IVIG), steroids, oxytocin, antifibrinolytics and in one case a caesarean hysterectomy. In the latter case, this preventive measure was taken because the patient had antiglycoprotein IB/IX antibodies, but there were no suitable platelets available if life‐threatening bleeding would occur.67
Figure 3.
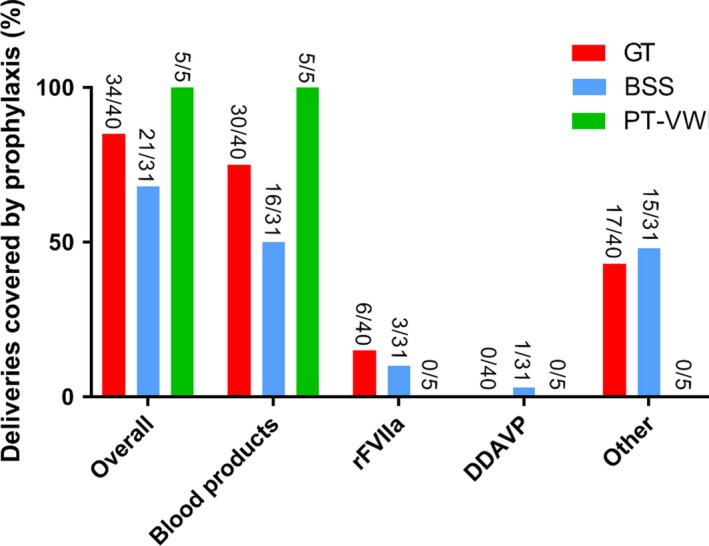
Prophylaxis administration according to the subtype of inherited platelet receptor defect. GT, Glanzmann thrombasthenia; BSS, Bernard‐Soulier syndrome; PT‐VWD, platelet‐type von Willebrand disease; TxA2, thromboxane A2 receptor defects; P2Y12, ADP receptor defects. Prophylactic treatment for delivery is defined as measures taken to prevent PPH and could consist of blood products (erythrocytes, platelets, plasma, coagulation factors, plasmapheresis), recombinant Factor VIIa (rFVIIa), desmopressin (DDAVP), or other measures. Other = preventive hysterectomy, antifibrinolytics, uterotonic agents, fibrinogen concentrate and steroids
Postpartum haemorrhage
Information on the prevalence of PPH was available in 96.7% (87/90) of individual cases (Figure 4). In the 27.8% (25/90) of deliveries in which estimated blood loss during delivery was mentioned, median blood loss was 866 mL with a range of 50‐2000 mL.23, 25, 27, 30, 32, 34, 35, 36, 37, 38, 39, 40, 41, 42, 44, 48, 49, 54, 56, 76 In all twenty of these articles, the method of blood loss estimation (eg weighted or visual estimation) was not specified. Other studies defined PPH as blood loss ≥ 500 mL without further specifying the exact amount of blood loss or elaboration on the definition of PPH.13, 22, 24, 26, 28, 29, 31, 33, 43, 45, 46, 50, 51, 52, 53, 55, 57, 58, 65, 77, 78, 79, 80 Overall, PPH occurred in 63.2% (55/87) of deliveries. Severe PPH (≥ 1000 mL) occurred in 11.5% (10/87) of deliveries. Primary PPH was reported in 52.7% (29/55), secondary PPH in 47.3% (26/55), and in fifteen deliveries (27.3%), the timing of PPH was not stated. Both primary and secondary PPH were reported in 27.3% (15/55) of deliveries.
Figure 4.
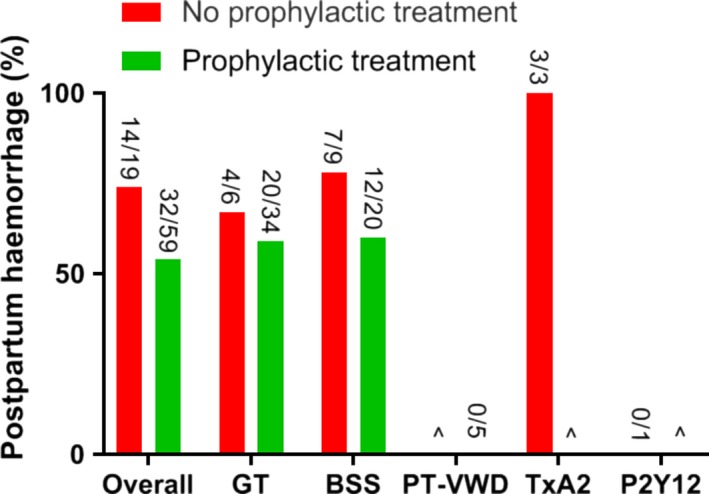
Prevalence of PPH according to the subtype of inherited platelet receptor defect and use of prophylactic treatment. ^No data available. GT, Glanzmann thrombasthenia; BSS, Bernard‐Soulier syndrome; PT‐VWD, platelet‐type von Willebrand disease; TxA2, thromboxane A2 receptor defects; P2Y12, ADP receptor defects. Prophylactic treatment for delivery is defined as measures taken to prevent PPH and could consist of blood products (erythrocytes, platelets, plasma, coagulation factors, plasmapheresis), recombinant Factor VIIa (rFVIIa), desmopressin (DDAVP), or other measures (preventive hysterectomy, antifibrinolytics, uterotonic agents, fibrinogen concentrate and steroids).
PPH occurred in 73.7% (14/19) of deliveries that were not covered by prophylaxis compared with 54.2% (32/59) of deliveries that were (odds ratio (OR) = 2.36, 95% confidence interval (CI): 0.75‐7.40, P = .14). For caesarean sections and vaginal deliveries, PPH incidence was 64.7% (22/34) and 71.9% (23/32), respectively (OR = 0.72, 95% CI: 0.25‐2.04, P = .53).
Treatment of PPH was described in 94.8% (55/58) of deliveries (Figure 5). The most common therapies included blood products (83.9%, 47/55), uterotonics (45.7%, 21/55) and antifibrinolytics (23.9%, 11/55). No thrombotic complications or maternal deaths due to PPH were reported in any of the individual cases.
Figure 5.
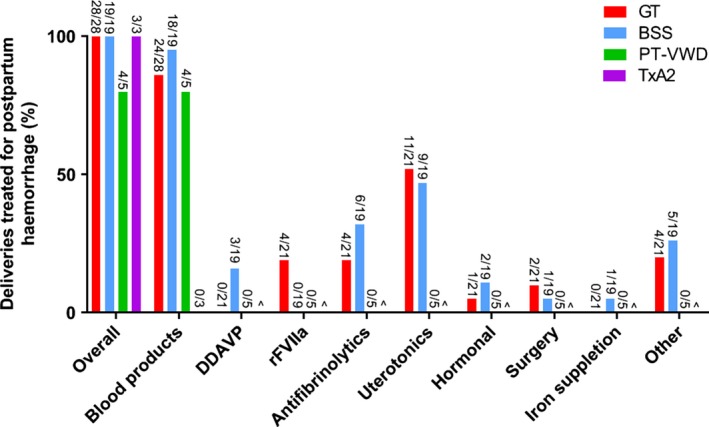
Treatment of postpartum haemorrhage according to the subtype of inherited platelet receptor defect. ^No data available. GT, Glanzmann thrombasthenia; BSS, Bernard‐Soulier syndrome; PT‐VWD, platelet‐type von Willebrand disease; TxA2, thromboxane A2 receptor defects; rFVIIa, recombinant Factor VIIa; DDAVP, desmopressin. Other = fibrinogen concentrates, local compression devices and crystalloids
Neonatal outcome
Neonatal bleeding complications were reported in 10.0% (8/80; maternal diagnosis being GT n = 42, BSS n = 29, PT‐VWD n = 5, TxA2 n = 3 and P2Y12 n = 1) of deliveries with data on neonatal outcome. These included one foetal death due to severe third trimester gastrointestinal bleeding (maternal BSS),79 one in utero foetal death due to intracranial haemorrhage (maternal GT),38 one neonatal death six hours after delivery due to antepartum intracranial haemorrhage (maternal BSS),21 one neonatal death 48 hours after delivery due to haemolytic disease of the newborn (maternal GT),47 one neonate suffering from bruising after vaginal delivery (maternal GT) 41 and three neonates that developed petechiae and ecchymoses shortly after caesarean sections (maternal BSS in all three cases).26 In 6/8 deliveries with neonatal bleeding complications, the presence of alloantibodies was investigated, and in all six, either antiplatelet or anti‐HLA antibodies were present. None of these neonatal bleeding complications occurred during an instrumental delivery. Screening for postpartum neonatal thrombocytopenia was reported in 27 deliveries, of which the results were ‘normal’ or ‘no thrombocytopenia’ in fifteen neonates. A platelet count (range 0‐307.000/µL, seven neonates with a platelet count of < 100.000/µL) was provided in twelve neonates.
3.3.2. Narrative synthesis of data from cohort studies
Three cohort studies with information on postpartum blood loss described a total of 74 deliveries.4, 81, 82 These included 41 women with BSS and two women with PT‐VWD. The exact number of women with GT is uncertain because of incomplete reporting, yet seven deliveries in women with GT were included.4
Noris et al (2014) conducted a retrospective, multicentre study evaluating 339 pregnancies in 181 women with different forms of inherited thrombocytopenia.81 They adapted two definitions of excessive bleeding at delivery: ‘excessive bleedings requiring blood transfusion’ (EBBT), which included transfusion of platelets and/or red blood cells, and ‘all excessive bleedings’ (AEB), which included all blood loss deemed larger than normal by the treating physician. Out of 21 deliveries in thirteen women with biallelic BSS (bBSS), EBBT was reported in 4.7% (1/21, 95% CI 0.1‐23.8) and AEB in 14.2% (3/21, 95% CI 3‐36.3). In 24 women with monoallelic BSS (mBSS), EBBT and AEB occurred in 2.5% (1/39, 95% CI 0.1‐13.4) and 12.8% (5/39, 95% CI 4.2‐27.4) of deliveries, respectively. Out of five deliveries in two women with PT‐VWD, 20% (1/5, 95% CI 0.5‐71.6) was complicated by EBBT and 60% (3/5, 95% CI 14.6‐94.7) by AEB. 38.2% (21/55) of neonates were affected by either bBSS, mBSS or PT‐VWD; one of them (with mBSS) had postpartum bleeding symptoms in the form of petechiae.
A large single‐centre retrospective study by Toogeh et al (2004) reported nine pregnancies in 131 investigated Iranian women of reproductive age with GT.4 Two out of nine pregnancies ended in spontaneous abortion, and one out of six caesarean sections was complicated by bleeding. No bleeding complications were reported in the one vaginal delivery. No information on prophylactic treatment or neonatal outcome was provided.
Another retrospective study by Toogeh et al (2010) described four pregnancies in 49 investigated Iranian women with BSS.82 Two (100%) vaginal deliveries were complicated by bleeding requiring a hysterectomy, while the remaining two pregnancies ended in spontaneous abortion.
4. DISCUSSION
In this paper, we reviewed all published literature on the prevalence and management of menstrual and obstetrical bleeding in women with IPRD. This review provides sufficient data to assume an increased incidence of HMB in these women and highlights a markedly increased risk of peripartum maternal and neonatal bleeding.
The included cohort studies reported HMB in 25% (13/52) of women with BSS and in 22.1% (34/154) of women with GT. These numbers are higher than previously reported, yet previous HMB definitions are unclear. It is difficult to determine whether HMB can solely be attributed to the existing receptor defect, or whether gynaecological risk factors (eg uterine fibroids or ovulatory dysfunction) play a role too.88 Nonetheless, the high rates of HMB observed in our study vastly exceed the estimated population prevalence of 8%‐10% and call for increased awareness of HMB in women with IPRD.89 More importantly, the high rate of transfusion of blood products to treat HMB, greatly burdens women with IPRD and the use of blood products is associated with high costs and risks, and should therefore be minimized.86, 87 The significant use of blood products may be explained by the lengthy period in which the included studies were published. Still, we believe greater effort should be made to move away from the use of blood products, where possible, to decrease the chance of alloantibody formation. For example, to prevent severe anemia due to HMB or PPH by timely administering hormonal treatment, antifibrinolytics, iron supplementation, rFVIIa and uterotinics. Currently, the optimal clinical management in this respect remains to be elucidated. In addition, HMB often required specifically platelets, with resultant high rates of alloimmunization, which in turn increases the risk of foetal complications in future pregnancies. Unfortunately, the majority of included cohort studies did not describe HMB treatment in detail, such as the use of rFVIIa, hormonal treatment and antifibrinolytics. Hence, we cannot draw any firm conclusions regarding the optimal management of HMB in women with IPRD.
Overall, PPH occurred in 63.2% (55/87) of deliveries in individual cases, which is vastly higher than previously reported in systematic reviews of pregnancy in GT and BSS.9, 10 On the contrary, excessive bleeding occurred in only 16.9% (11/65) of deliveries described in the largest included cohort (n = 39 women).81 This difference may be explained by the effect of reporting and selection bias in case reports and series and the definition used by the authors of the mentioned cohort study (for example, abnormal bleeding based on the obstetrician's judgement). Visual estimation of blood loss has been proven to underestimate blood loss.90 Known risk factors for PPH such as uterus atony, a prolonged second stage of labour, previous caesarean section and retained placenta, are potential confounders that may have affected our results, yet have been infrequently described.12 Our data show the ongoing risk of PPH for women receiving prophylactic treatment of 54.2% (32/59), compared with a PPH incidence of 73.7% (14/19) in the non‐prophylactic group, with both incidences being much higher than currently seen in the general population.91 Although the number of included deliveries is small, these data indicate that more women might benefit from prophylactic treatment and that current prophylaxis might be insufficient to prevent PPH. Furthermore, we observed a high rate of secondary PPH (47.3%, 26/55). These findings are in accordance with previously published results and highlight the need for optimizing peripartum management strategies.9, 10 The difference in management strategies for vaginal deliveries and caesarean sections, as recommended by the UK guidelines, is not evident from the included literature in this review.3 The heterogeneity of the included study population, the few deliveries per type of treatment strategy and unknown obstetric confounders, makes subpopulation analysis and subsequent recommendations unreliable. For example, rFVIIa was mentioned as prophylaxis for PPH in only a few cases. The results of the current review are further limited given that the data were either relatively outdated, based on anecdotal evidence 9, 10 or lack objective uniform definitions of PPH.13, 81
This systematic review utilizes a thorough literature search in various online databases without publication date restrictions. The main limitations of this review relate to the low quality of evidence and incomplete reporting. The majority of included studies were small case reports and series of low quality, susceptible to reporting and selection bias, more commonly seen in rare diseases. The included cohort studies were of higher quality, but generally less comprehensive in describing HMB and PPH management than the case reports and series. Moreover, most studies did not use objective or identical outcome measurements of HMB and PPH, such as PBAC scores and total blood loss in millilitres. Lastly, in some cases it was hard to distinguish whether a certain measure was used to prevent or treat PPH.
Our findings support current clinical guidelines that women with IPRD should be managed by a multidisciplinary team.3 Considering the remarkably high percentage (66.7%, 26/39 deliveries) of maternal alloimmunization in our study, and its strong association with neonatal bleeding complications, we underline the importance of compatible HLA‐matched platelets and monitoring of alloantibody titres during pregnancy to prevent platelet refractoriness and foetal alloimmune thrombocytopenia. Furthermore, we would like to highlight the importance of optimization of prophylactic treatment, for example through continued administration of tranexamic acid and closer observation during the postpartum period. More women who currently do not receive prophylactic treatment might benefit from it.
We call for international collaboration to systematically gather data on the management of menstrual and obstetrical bleeding in women with IPRD. Large prospective studies using uniform objective definitions are needed to evaluate the true safety and efficacy of different management strategies to lay the foundation for evidence‐based clinical guidelines.
5. CONCLUSION
Literature on the management of menstrual and obstetrical bleeding in women with IPRD mainly comprises small case reports and series of poor quality. This review provides sufficient data to assume an increased incidence of HMB associated with an undesirable high consumption of blood products. There is a markedly increased risk of PPH, which is lowered with prophylactic haemostatic therapy, but still high. Neonates appear to be at risk of bleeding complications if antiplatelet or anti‐HLA antibodies are present. Large prospective studies are required to lay the foundation for evidence‐based guidelines on the management of HMB and delivery to reduce morbidity and mortality among women with IPRD and their newborns.
DISCLOSURES
The authors stated that they had no interests which might be perceived as posing a conflict or bias.
Supporting information
ACKNOWLEDGEMENTS
MC Punt designed and performed the research, analysed and interpreted the data and wrote the manuscript. PCE Schuitema performed the research, analysed and interpreted the data and wrote the manuscript. KPM van Galen designed the research, analysed and interpreted the data and critically reviewed the manuscript. KWM Bloemenkamp and ICL Kremer Hovinga interpreted the data and critically reviewed the manuscript. All authors gave their consent to the final version of the manuscript.
Punt MC, Schuitema PCE, Bloemenkamp KWM, Kremer Hovinga ICL, van Galen KPM. Menstrual and obstetrical bleeding in women with inherited platelet receptor defects—A systematic review. Haemophilia. 2020;26:216–227. 10.1111/hae.13927
REFERENCES
- 1. Kirchmaier C, Pillitteri D. Diagnosis and Management of Inherited Platelet Disorders. Transfus Med Hemother. 2010;37(5):237‐246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grainger J, Thachil J, Will A. How we treat the platelet glycoprotein defects; Glanzmann thrombasthenia and Bernard Soulier syndrome in children and adults. Br J Haematol. 2018;182(5):621‐632. [DOI] [PubMed] [Google Scholar]
- 3. Bolton‐Maggs PHB, Chalmers EA, Collins PW, et al. A review of inherited platelet disorders with guidelines for their management on behalf of the UKHCDO. Br J Haematol. 2006. Dec;135(5):603‐633. [DOI] [PubMed] [Google Scholar]
- 4. Toogeh G, Sharifian R, Lak M, Safaee R, Artoni A, Peyvandi F. Presentation and pattern of symptoms in 382 patients with Glanzmann thrombasthenia in Iran. Am J Hematol. 2004. Oct;77(2):198‐199. [DOI] [PubMed] [Google Scholar]
- 5. George J, Caen J, Nurden A. Glanzmann’s thrombasthenia: the spectrum of clinical disease. Blood. 1990. Apr 1;75(7):1383‐1395. [PubMed] [Google Scholar]
- 6. Ray S, Ray A. Non‐surgical interventions for treating heavy menstrual bleeding (menorrhagia) in women with bleeding disorders. Cochrane Database Syst Rev. 2016; 11:1465‐1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kadir R, Edlund M, von Mackensen S. The impact of menstrual disorders on quality of life in women with inherited bleeding disorders. Haemophilia. 2010;16(5):832‐839. [DOI] [PubMed] [Google Scholar]
- 8. Karlsson T, Marions L, Edlund M. Heavy menstrual bleeding significantly affects quality of life. Acta Obs Gynecol Scand. 2014;93(1):52‐57. [DOI] [PubMed] [Google Scholar]
- 9. Siddiq S, Clark A, Mumford A. A systematic review of the management and outcomes of pregnancy in Glanzmann thrombasthenia. Haemophilia. 2011. Sep;17(5):e858–e869. [DOI] [PubMed] [Google Scholar]
- 10. Peitsidis P, Datta T, Pafilis I, Otomewo O, Tuddenham EGD, Kadir RA. Bernard Soulier syndrome in pregnancy: a systematic review. Haemophilia. 2010. Jul;16(4):584‐591. [DOI] [PubMed] [Google Scholar]
- 11. Zwart J, Richters J, Ory F, de Vries J, Bloemenkamp K, van Roosmalen J. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population‐based study of 371,000 pregnancies. BJOG. 2008;115(7):842‐850. [DOI] [PubMed] [Google Scholar]
- 12. Bais J, Eskes M, Pel M, Bonsel G, Bleker O. Postpartum haemorrhage in nulliparous women: incidence and risk factors in low and high risk women: A Dutch population‐based cohort study on standard (≥500 ml) and severe (≥1000 ml) postpartum haemorrhage. Eur J Obs Gynecol Reprod Biol. 2004;115(2):166‐172. [DOI] [PubMed] [Google Scholar]
- 13. Civaschi E, Klersy C, Melazzini F, et al. Analysis of 65 pregnancies in 34 women with five different forms of inherited platelet function disorders. Br J Haematol. 2015. Aug;170(4):559‐563. [DOI] [PubMed] [Google Scholar]
- 14. National Institute for Health Research . PROSPERO International prospective register of systematic reviews [Internet]. Available from: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID%253D115116%26sa=D%26ust=1553638683494000%26usg=AFQjCNHkgPyatfDQ-90jrj15Wn7ZQsh9mA. Accessed January 16, 2019.
- 15. Moher D, Liberati A, Tetzlaff J, Altman D, PRISMA Group . Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Higgings J. Cochrane Handbook for Systematic Reviews of Interventions. Version 5. The Cochrane Collaboration; [Internet]; 2011. Available from: http://www.cochrane-handbook.org. Accessed November 1, 2018. [Google Scholar]
- 17. Higham J, O’Brien P, Shaw R. Assessment of menstrual blood loss using a pictorial chart. Br J Obs Gynaecol. 1990;97(8):734‐739. [DOI] [PubMed] [Google Scholar]
- 18. World Health Organisation . WHO Guidelines for the Management of Postpartum Haemorrhage and Retained Placenta. Geneva: World Health Organisation; 2009. [PubMed] [Google Scholar]
- 19. Dekkers O, Egger M, Altman D, Vandenbroucke J. Distinguishing case series from cohort studies. Ann Intern Med. 2012;156(1):37‐40. [DOI] [PubMed] [Google Scholar]
- 20. Chambers D, Rodgers M, Woolacott N. Not only randomized controlled trials, but also case series should be considered in systematic reviews of rapidly developing technologies. J Clin Epidemiol. 2009;62(12):1253–1260.e4. [DOI] [PubMed] [Google Scholar]
- 21. Fujimori K, Ohto H, Honda S, Sato A. Antepartum diagnosis of fetal intracranial hemorrhage due to maternal Bernard‐Soulier syndrome. Obstet Gynecol. 1999. Nov;94(5 Pt 2):817‐819. [DOI] [PubMed] [Google Scholar]
- 22. Greinacher A, Pötzsch B, Kiefel V, White JG, Müller‐Berghaus G, Mueller‐Eckhardt C. Evidence that DDAVP transiently improves hemostasis in Bernard‐Soulier syndrome independent of von Willebrand‐Factor. Ann Hematol. 1993;67(3):149‐150. [DOI] [PubMed] [Google Scholar]
- 23. Kriplani A, Singh B, Sowbernika R, Choudhry V. Successful pregnancy outcome in Bernard‐Soulier syndrome. J Obstet Gynaecol Res. 2005. Feb;31(1):52‐56. [DOI] [PubMed] [Google Scholar]
- 24. Rahimi G, Rellecke S, Mallmann P, Nawroth F. Course of pregnancy and birth in a patient with Bernard‐Soulier syndrome – a case report. J Perinat Med. 2005;33(3):264‐266. [DOI] [PubMed] [Google Scholar]
- 25. Zafar S, Sultana S, Iqbal W, et al. Pregnancy outcome in Bernard‐Soulier syndrome complicated by preeclampsia. J Turkish‐German Gynecol Assoc. 2007;8(3):324‐326. [Google Scholar]
- 26. Uotila J, Tammela O, Makipernaa A. Fetomaternal platelet immunization associated with maternal Bernard‐Soulier syndrome. Am J Perinatol. 2008. Apr;25(4):219‐223. [DOI] [PubMed] [Google Scholar]
- 27. Palsson R, Vidarsson B, Gudmundsdottir B, et al. Complementary effect of fibrinogen and rFVIIa on clotting ex vivo in Bernard‐Soulier syndrome and combined use during three deliveries. Platelets. 2014;25(5):357‐362. [DOI] [PubMed] [Google Scholar]
- 28. Anwer A, Hanley J, Kumarendran K. Proposed management of pregnancy and labour in an inherited platelet disorder, Glanzmann’s thrombasthenia. J Obstet Gynaecol (Lahore). 2007. May;27(4):421‐423. [DOI] [PubMed] [Google Scholar]
- 29. Bayraktaroglu T, Colak N, Nalcaci M, Yenerel MN. Sheehan’s syndrome associated with Glanzmann’s Thrombasthenia: case report and literature review. Exp Clin Endocrinol Diabetes. 2008. Oct;116(9):549‐553. [DOI] [PubMed] [Google Scholar]
- 30. Bell J‐A, Savidge G. Glanzmann’s thrombasthenia proposed optimal management during surgery and delivery. Clin Appl Thromb Hemost. 2003. Apr;9(2):167‐170. [DOI] [PubMed] [Google Scholar]
- 31. Caen J, Castaldi P, Leclerc J, et al. Congenital bleeding disorders with long bleeding time and normal platelet count I. Am J Med. 1966;41:4‐26. [DOI] [PubMed] [Google Scholar]
- 32. Peaceman A, Katz A, Laville M. Bernard‐Soulier syndrome complicating pregnancy: a case report. Obstet Gynecol. 1989. Mar;73(3 Pt 2):457‐459. [PubMed] [Google Scholar]
- 33. Capuzzo E, Polatti F, Zara C. Glanzmann’s thrombasthenia and puerperium. Int J Gynaecol Obstet. 1997. Jun;57(3):313‐314. [DOI] [PubMed] [Google Scholar]
- 34. Dede M, Ural A, Yenen M, Mesten Z, Baser I. Glanzmann’s thrombasthenia in two pregnant females. Am J Hematol. 2007. Apr;82(4):330‐331. [DOI] [PubMed] [Google Scholar]
- 35. Ito K, Yoshida H, Hatoyama H, et al. Antibody removal therapy used successfully at delivery of a pregnant patient with Glanzmann’s thrombasthenia and multiple anti‐platelet antibodies. Vox Sang. 1991;61(1):40‐46. [DOI] [PubMed] [Google Scholar]
- 36. Kale A, Bayhan G, Yalinkaya A, Yayla M. The use of recombinant factor VIIa in a primigravida with Glanzmann’s thrombasthenia during delivery. J Perinat Med. 2004;32(5):456‐458. [DOI] [PubMed] [Google Scholar]
- 37. Kashyap R, Kriplani A, Saxena R, Takkar D, Choudhry V. Pregnancy in a patient of Glanzmann’s thrombasthenia with antiplatelet antibodies. J Obstet Gynaecol Res. 1997. Jun;23(3):247‐250. [DOI] [PubMed] [Google Scholar]
- 38. Leticee N, Kaplan C, Lemery D. Pregnancy in mother with Glanzmann’s thrombasthenia and isoantibody against GPIIb‐IIIa: Is there a foetal risk? Eur J Obstet Gynecol Reprod Biol. 2005. Aug;121(2):139‐142. [DOI] [PubMed] [Google Scholar]
- 39. Malhotra N, Chanana C, Deka D. Pregnancy in a patient of Glanzmann’s thromasthenia. Indian J Med Sci. 2006. Mar;60(3):111‐113. [PubMed] [Google Scholar]
- 40. Monte S, Lyons G. Peripartum management of a patient with Glanzmann’s thrombasthenia using Thrombelastograph. Br J Anaesth. 2002. May;88(5):734‐738. [DOI] [PubMed] [Google Scholar]
- 41. Payne PR. Glanzmann’s thrombasthenia and pregnancy. Proc R Soc Med. 1970. Jan;63(1):56‐57. [PMC free article] [PubMed] [Google Scholar]
- 42. Sherer D, Lerner R. Glanzmann’s thrombasthenia in pregnancy: a case and review of the literature. Am J Perinatol. 1999;16(6):297‐301. [DOI] [PubMed] [Google Scholar]
- 43. Saade G, Homsi R, Seoud M. Bernard‐Soulier syndrome in pregnancy; a report of four pregnancies in one patient, and review of the literature. Eur J Obstet Gynecol Reprod Biol. 1991. Jul;40(2):149‐152. [DOI] [PubMed] [Google Scholar]
- 44. Sundqvist S, Nilsson I, Svanberg L, Cronberg S. Pregnancy and parturition in a patient with severe Glanzmann’s thrombasthenia. Scand J Haematol. 1981. Sep;27(3):159‐164. [DOI] [PubMed] [Google Scholar]
- 45. Leibowitz G, Salameh M, Gomori MJ, Reubinoff CA, Rosler A, Gross DJ. Thrombasthenia–a rare cause of Sheehan’s syndrome. Acta Obstet Gynecol Scand. 1995. Jul;74(6):482‐484. [DOI] [PubMed] [Google Scholar]
- 46. Al‐Ramahi M, Abbadi A. Glanzmann’s thrombasthenia and pregnancy. Jordan Med J. 2009;43(2):134‐136. [Google Scholar]
- 47. Jallu V, Pico M, Chevaleyre J, Vezon G, Kunicki TJ, Nurden AT. Characterization of an antibody to the integrin β3 subunit (GP IIIa) from a patient with neonatal thrombocytopenia and an inherited deficiency of GP IIb‐IIIa complexes in platelets (Glanzmann’s thrombasthenia). Hum Antibodies Hybridomas. 1992. Apr;3(2):93‐106. [PubMed] [Google Scholar]
- 48. Magudapathi C, Kannan S. Glanzmann’s thrombasthenia complicating pregnancy. J Obstet Gynaecol India. 2014. Dec;64(Suppl 1):3‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wijemanne A, Watt‐Coote I, Austin S. Glanzmann thrombasthenia in pregnancy: optimising maternal and fetal outcomes. Obstet Med. 2016. Dec;9(4):169‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sharma P, Gogia S, Zafar M, Saraf A, Bhargava M. Glanzmann thrombasthenia in pregnancy: the value of a probing bleeding history. Clin Appl Thromb. 2012;18(1):110‐112. [DOI] [PubMed] [Google Scholar]
- 51. Bozkurt N, Guler I, Ozturk M, Utkan ZE, Yalcin MM, Karakaya C. Live birth following an intracytoplasmic sperm injection in a patient with Glanzmann thrombasthenia. J Obstet Gynaecol (Lahore). 2016;36(2):273‐274. [DOI] [PubMed] [Google Scholar]
- 52. Domschke C, Strowitzki T, Huth‐Kuehne A, Staritz P, Sohn C, Schuetz F. Successful in vitro fertilization and pregnancy in Glanzmann thrombasthenia. Haemophilia. 2012. Sep;18(5):e380–e381. [DOI] [PubMed] [Google Scholar]
- 53. Nurden P, Lanza F, Bonnafous‐Faurie C, Nurden A. A second report of platelet‐type von Willebrand disease with a Gly233Ser mutation in the GPIBA gene. Thromb Haemost. 2007. Feb;97(2):319‐321. [PubMed] [Google Scholar]
- 54. Osmanagaoglu M, Osmanagaoglu S, Bozkaya H. Bernard‐Soulier syndrome and pregnancy: a case report. Internet J Gynecol Obstet. 2004;4(2):1‐5. [Google Scholar]
- 55. O’Connor D, Lester W, Willoughby S, Wilde J. Pregnancy in platelet‐type VWD: a case series. Thromb Haemost. 2011. Aug;106(2):386‐387. [DOI] [PubMed] [Google Scholar]
- 56. Grover N, Boama V, Chou M. Pseudo (Platelet‐type) von Willebrand disease in pregnancy: a case report. BMC Pregnancy Childbirth. 2013. Jan;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Sanchez‐Luceros A, Woods A, Bermejo E, et al. PT‐VWD posing diagnostic and therapeutic challenges – small case series. Platelets. 2017. Jul;28(5):484‐490. [DOI] [PubMed] [Google Scholar]
- 58. Santoro C, Rago A, Biondo F, et al. Prevalence of allo‐immunization anti‐HLA and anti‐integrin alphaIIbbeta3 in Glanzmann Thromboasthenia patients. Haemophilia. 2010. Sep;16(5):805‐812. [DOI] [PubMed] [Google Scholar]
- 59. Shlebak A, Poles A, Manning R, et al. A novel homozygous c.800C>G substitution in GP1BA exon 2 in a Kuwaiti family with Bernard‐Soulier syndrome. Acta Haematol. 2015;134(3):193‐198. [DOI] [PubMed] [Google Scholar]
- 60. Hossain N, Shamsi T, Feroz A. Successful management of acute catastrophic juvenile vaginal bleeding in Glanzmann’s thromboasthenia by uterine tamponade: a case report and review of the literature. Case Rep Hematol. 2012;2012:530908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Rajpurkar M, O’Brien S, Haamid F, Cooper D, Gunawardena S, Chitlur M. Heavy menstrual bleeding as a common presenting symptom of rare platelet disorders: illustrative case examples. J Pediatr Adolesc Gynecol. 2016. Dec;29(6):537‐541. [DOI] [PubMed] [Google Scholar]
- 62. Yi B, Karabulut A, Kabukcu S, Sari I, Keskin A. Intensive menstrual bleeding successfully treated with recombinant factor VIIa in Glanzmann thrombasthenia. Clin Appl Thromb Hemost. 2010. Aug;17(4):320‐322. [DOI] [PubMed] [Google Scholar]
- 63. Almeida A, Khair K, Hann I, Liesner R. The use of recombinant factor VIIa in children with inherited platelet function disorders. Br J Haematol. 2003;121(3):477‐481. [DOI] [PubMed] [Google Scholar]
- 64. Kaleelrahman M, Minford A, Parapia L. Use of recombinant factor VIIa in inherited platelet disorders. Br J Haematol. 2004. Apr;125(1):95‐96. [DOI] [PubMed] [Google Scholar]
- 65. Khalil A, Seoud M, Tannous R, Usta I, Shamseddine A. Bernard‐Soulier syndrome in pregnancy: case report and review of the literature. Clin Lab Haematol. 1998. Apr;20(2):125‐128. [DOI] [PubMed] [Google Scholar]
- 66. Ozelo M, Svirin P, Larina L. Use of recombinant factor VIIa in the management of severe bleeding episodes in patients with Bernard‐Soulier syndrome. Ann Hematol. 2005. Nov;84(12):816‐822. [DOI] [PubMed] [Google Scholar]
- 67. Hacihanefioglu A, Tarkun P, Gonullu E. Use of recombinant factor VIIa in the management and prophylaxis of bleeding episodes in two patients with Bernard–Soulier syndrome. Thromb Res. 2007;120(3):455‐457. [DOI] [PubMed] [Google Scholar]
- 68. Sharma J, Buckshee K, Sharma S. Puberty menorrhagia due to Bernard Soulier syndrome and its successful treatment by “Ovral” hormonal tablets. Aust N Z J Obstet Gynaecol. 1991. Nov;31(4):369‐370. [DOI] [PubMed] [Google Scholar]
- 69. Lu M, Yang X. Levonorgestrel‐releasing intrauterine system for treatment of heavy menstrual bleeding in adolescents with Glanzmann’s Thrombasthenia: illustrated case series. BMC Womens Health. 2018. Feb;18(1):45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bhatt A, Chenoy R. Menorrhagia due to Bernard‐Soulier syndrome and its successful treatment by thermal balloon endometrial ablation. BJOG. 2001. Jun;108(6):667‐668. [DOI] [PubMed] [Google Scholar]
- 71. Markovitch O, Ellis M, Holzinger M, Goldberger S, Beyth Y. Severe juvenile vaginal bleeding due to Glanzmann’s thrombasthenia: case report and review of the literature. Am J Hematol. 1998. Mar;57(3):225‐227. [DOI] [PubMed] [Google Scholar]
- 72. Jimenez J, Martin I, de La Fuente L, et al. Severe menorrhagia due to Glanzmann thrombasthenia treated with hydrothermal ablation. J Am Assoc Gynecol Laparosc. 2000. May;7(2):265‐267. [DOI] [PubMed] [Google Scholar]
- 73. Hossain N, Farzana T, Khan N, Shamsi T, James A. Adolescent menorrhagia due to platelet function disorder. J Pak Med Assoc. 2010. Feb;60(2):127‐129. [PubMed] [Google Scholar]
- 74. Aydinok Y, Egemen A, Balkan C. Menorrhagia due to abnormalities of the platelet function: evaluation of two young patients. Pediatr Int. 2007. Feb;49(1):106‐108. [DOI] [PubMed] [Google Scholar]
- 75. Savoia A, Pastore A, De Rocco D, et al. Clinical and genetic aspects of Bernard‐Soulier syndrome: searching for genotype/phenotype correlations. Haematologica. 2011;96(3):417‐423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Prabu P, Parapia L. Bernard‐Soulier syndrome in pregnancy. Clin Lab Haematol. 2006. Jun;28(3):198‐201. [DOI] [PubMed] [Google Scholar]
- 77. Macêdo MB, Brito JDMM, Macêdo PDS, Brito JA. Primigravida with Bernard‐Soulier syndrome: a case report case reports. BMC Res Notes. 2015;8(1):1‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Michalas S, Malamitsi‐Puchner A, Tsevrenis H. Pregnancy and delivery in Bernard‐Soulier syndrome. Acta Obstet Gynecol Scand. 1984;63(2):185‐186. [DOI] [PubMed] [Google Scholar]
- 79. Peng T, Kickler T, Bell W, Haller E. Obstetric complications in a patient with Bernard‐Soulier syndrome. Am J Obstet Gynecol. 1991. Aug;165(2):425‐426. [DOI] [PubMed] [Google Scholar]
- 80. Heslop H, Hickton C, Laird E, Tait J, Doig J, Beard E. Twin pregnancy and parturition in a patient with the Bernard Soulier syndrome. Scand J Haematol. 1986. Jul;37(1):71‐73. [DOI] [PubMed] [Google Scholar]
- 81. Noris P, Schlegel N, Klersy C, et al. Analysis of 339 pregnancies in 181 women with 13 different forms of inherited thrombocytopenia. Haematologica. 2014. Aug;99(8):1387‐1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Toogeh G, Keyhani M, Sharifian R, Safaee R, Emami A, Dalili H. A study of bernard‐soulier syndrome in Tehran, Iran. Arch Iran Med. 2010;13(6):549‐551. [PubMed] [Google Scholar]
- 83. Kushwaha R, Kumar A, Mishra KL, Sankhwar PL, Singh R. Haemostatic disorder in women with unexplained menorrhagia: a tertiary care centre experience from Northern India. J Clin Diagn Res. 2017. May;11(5):EC46–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Hutspardol S, Sirachainan N, Soisamrong A, Atchararit N, O‐Prasertsawat P, Chuansumrit A. Hemostatic defects in Thai adolescents with menorrhagia. J Med Assoc Thai. 2010. Apr;93(4):436‐442. [PubMed] [Google Scholar]
- 85. Caki Kilic S, Sarper N, Zengin E, Aylan GS. Screening bleeding disorders in adolescents and young women with menorrhagia. Turkish J Hematol. 2013. Jun;30(2):168‐176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Vijapurkar M, Mota L, Shetty S, Ghosh K. Menorrhagia and reproductive health in rare bleeding disorders: a study from the Indian subcontinent. Haemophilia. 2009. Jan;15(1):199‐202. [DOI] [PubMed] [Google Scholar]
- 87. Awidi A. Delivery of infants with Glanzmann thrombasthenia and subsequent blood transfusion requirements: a follow‐up of 39 patients. Am J Hematol. 1992. May;40(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 88. Hapangama D, Bulmer J. Pathophysiology of heavy menstrual bleeding. Womens Heal (Lond). 2016;12(1):3‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Fraser I. Menorrhagia–a pragmatic approach to the understanding of causes and the need for investigations. Br J Obs Gynaecol. 1994;101(Suppl 11):3‐7. [DOI] [PubMed] [Google Scholar]
- 90. Stafford I, Dildy G, Clark S, Belfort M. Visually estimated and calculated blood loss in vaginal and cesarean delivery. Am J Obs Gynecol. 2008;199(5):e1–7. [DOI] [PubMed] [Google Scholar]
- 91. van Stralen G, von Schmidt auf Altenstadt JF, Bloemenkamp KWM, van Roosmalen J, Hukkelhoven CWPM. Increasing incidence of postpartum hemorrhage: the Dutch piece of the puzzle. Acta Obstet Gynecol Scand. 2016;95(10):1104‐1110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


